Back to Journals » ImmunoTargets and Therapy » Volume 13
Addition of Immune Checkpoint Inhibitor Showed Better Efficacy for Infiltrative Hepatocellular Carcinoma Receiving Hepatic Arterial Infusion Chemotherapy and Lenvatinib: A Multicenter Retrospective Study
Authors Wang W, Li R, Li H, Wang M, Wang J, Wang X , Zhou Q
Received 26 March 2024
Accepted for publication 13 August 2024
Published 19 August 2024 Volume 2024:13 Pages 399—412
DOI https://doi.org/10.2147/ITT.S470797
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Flavio Salazar-Onfray
Wei Wang,1,* Ruixia Li,2,* Hui Li,3 Murong Wang,3 Juncheng Wang,4 Xiaohui Wang,5 Qunfang Zhou6
1Department of General Surgery, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning, People’s Republic of China; 2Department of Thyroid Surgery, The First Affiliated Hospital of Sun Yat-Sen University, Guangzhou, Guangdong, People’s Republic of China; 3Department of Minimally Invasive Interventional Radiology, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, People’s Republic of China; 4Department of Liver Surgery, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, People’s Republic of China; 5Department of Hepatobiliary Surgery, Hunan Provincial People’s Hospital, Changsha, Hunan, People’s Republic of China; 6Department of Interventional Radiology, Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaohui Wang, Department of Hepatobiliary Surgery, Hunan Provincial People’s Hospital, Changsha, Hunan Province, 410005, People’s Republic of China, Tel +86-0731-83928052, Email [email protected] Qunfang Zhou, Department of Interventional Radiology, Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China, Tel +86-010-66939123, Email [email protected]
Purpose: The prognosis of infiltrative hepatocellular carcinoma (HCC) is dismal. Hepatic arterial infusion chemotherapy (HAIC) plus Lenvatinib (Len) and immune checkpoint inhibitor (ICI) have shown promising results for HCC. However, this three combination therapy on infiltrative HCC is unknown. In this study, we compared HAIC plus lenvatinib (Len) and programmed cell death protein-1 (PD-1) inhibitor with HAIC plus Len for infiltrative HCC.
Patients and Methods: This multi-center cohort study included patients with infiltrative HCC who received HAIC combined with Len (HAIC+Len group, n = 173) or HAIC combined with Len and PD-1 inhibitor (HAIC+Len+ICI group, n = 128) as the first-line treatment from January 2019 to December 2021. To balance any intergroup differences, one-to-one propensity score matching (PSM) was applied. Overall survival (OS) and progression-free survival (PFS) were compared between the two groups.
Results: After PSM, the median OS was 14.1 ± 1.0 and 16.1 ± 1.4 months in the HAIC+Len and HAIC+Len+ICI groups, respectively. The median PFS was 4.6 ± 0.4 months in the HAIC+Len group and 7.5 ± 0.8 months in the HAIC+Len+ICI group. The HAIC+Len+ICI group showed significantly better OS (hazard ratio [HR], 0.66; 95% CI, 0.49– 0.90; P = 0.008) and PFS (HR, 0.53; 95% confident index [CI], 0.40– 0.70; P < 0.001) compared with the HAIC+Len group. Subgroup analysis revealed that for OS in HCC without metastasis, the addition of PD-1 inhibitor was not significant (HR, 0.68; 95% CI, 0.43– 1.07; P = 0.091). No difference was observed in OS between low (2– 3 cycles) and high (4– 6 cycles) level of HAIC cycles (HR, 0.99; 95% CI, 0.67– 1.44; P = 0.938).
Conclusion: The HAIC+Len+ICI group had a longer PFS and OS compared with the HAIC+Len group, demonstrating an acceptable safety profile. This triple combination strategy may be an alternative treatment for infiltrative HCC management.
Plain Language Summary: The evidence of HAIC plus Len and PD-1 inhibitors for infiltrative HCC is limited. There was no study to evaluate the efficacy of HAIC combined with Len and PD-1 inhibitors for infiltrative HCC. In this study, we found that HAIC plus Len and PD-1 inhibitor (HAIC+Len+ICI) was associated with longer progression-free survival and overall survival than HAIC plus Len combination (HAIC+Len) for patient with infiltrative HCC. In addition, OS in patients with metastasis was improved with HAIC+Len+ICI treatment. OS in patients without metastasis, addition of PD-1 inhibitor after HAIC and Len was not beneficial. What’s more, three cycles of HAIC are adequate, especially for patients with high tumor burden, especially with main branch portal vein tumor thrombus (PVTT). Our research provides new evidence that HAIC+Len+ICI treatment significantly improved the OS and PFS of infiltrative HCC patients compared with those who received HAIC+Len treatment. It provides a strong reference for clinical treatment.
Keywords: Infiltrative hepatocellular carcinoma, hepatic arterial infusion chemotherapy, Len, PD-1 inhibitor, Prognosis
Introduction
HCC is the sixth most common malignancy and ranks third in cancer-related deaths worldwide.1 HCC presents with varying morphologic subtypes, and most tumors present with single or multiple encapsulated nodule patterns, with 8%–18% appearing as diffuse infiltrative patterns.2 The definition of infiltrative HCC is a tumor that shows an ill-defined border, no evidence of convex margination, and no typical enhancement pattern by imaging.3 Most of the data have advanced HCC.4 As such, studies reporting infiltrative HCC are limited because it has not received significant attention as well as the small sample size.3 Because of the large tumor size, diffuse features, and the presence of portal vein tumor thrombus (PVTT), infiltrative HCC is relatively intractable to treat and usually concomitant with serious complications.3
Currently, the recommended option for infiltrative HCC is systemic therapy or combined with local regional therapy.5–7 However, patient prognosis varies. In a study of infiltrative HCC underwent transarterial chemoembolization (TACE) for infiltrative HCC with a median OS of 5.7 months.5 Owen et al reported a median OS of 16.2 months for transcatheter arterial radioembolization of infiltrative HCC in 18 patients.8 Len is one of the first-line systemic therapies for advanced HCC and is effective and well-tolerated;9 however, efficacy is limited for Len alone. HAIC, with oxaliplatin, 5-fluorouracil and leucovorin, improves the prognosis of advanced HCC and is recommended as an alternative therapy in Asia.10–12 HAIC combined with systemic therapy showed superior outcomes than systemic therapy alone in advanced HCC.10 Thus, the combination of systemic therapy with local therapy is promising and necessary for infiltrative HCC. Studies have proven that HAIC combined with Len and programmed cell death protein-1 (PD-1) inhibitor, as one of the immune checkpoint inhibitor (ICI), has demonstrated efficacy and safety in advanced HCC.13,14
The oxaliplatin and fluorouracil in HAIC induce tumor cell death, which releases tumor antigens and proinflammatory cytokines, which fosters an immune-activated tumor environment, which facilitates dendritic cell and immune cells migration and maturation.15 Len induces vascular normalization and enhances the efficacy of the infiltrating immune cells.4,16 PD-1 inhibitor activates cytotoxic T lymphocyte function, thereby providing a more favorable antitumor activity.17 These potential mechanisms might explain why this triple regimen showed significant improvements in advanced HCC.18 However, these triple combination regimens have not been fully evaluated in infiltrative HCC. Therefore, we conducted this multicenter analysis to compare the effectiveness of HAIC, Len, and PD-1 inhibitors versus HAIC and Len on the prognosis of patients with infiltrative HCC.
Material and Methods
Patients and Study Design
Consecutive patients diagnosed with infiltrative HCC from January 2019 to December 2021 were retrospectively reviewed at Chinese PLA General Hospital, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Jinzhou Medical University, Sun Yat-sen University Cancer Center, The First Affiliated Hospital of Sun Yat-sen University. The study was centrally approved by the ethics committee of these five centers and conducted according to the guidelines of the Declaration of Helsinki.19 Informed consent was waived because the study was retrospective.
HCC was diagnosed by imaging studies [contrast-enhanced computed tomography [CT] and/or magnetic resonance imaging (MRI)] in accordance with the American Association for the Study of Liver Disease guidelines20 or pathology. Images were conducted within two weeks before HAIC, and images were reviewed and evaluated independently by two experienced radiologists. Infiltrative HCC was defined as nonencapsulated arterial phase hyperenhancement, multifocal washout in the portal phase hyperenhancement, and noncircular, ill-defined margin (Figure S1).8
Patients meeting the following criteria were included: (1) primary infiltrative HCC according to MRI or CT imaging characteristics; (2) Child–Pugh class A or B, and Eastern Cooperative Oncology Group (ECOG) status of 0 or 1; (3) HAIC as initial treatment; (4) patients received Len with or without PD-1 inhibitor following HAIC; (5) no history of other malignancies; (6) no tumor thrombus in the atrium or vena cava. Patients were excluded: (1) HCC with tumor capsule; (2) TACE as initial treatment; (3) sorafenib or other systemic therapy with or without PD-1 inhibitor following HAIC; (4) incomplete tumor imaging data; (5) lost to follow-up after treatment within three months. The flow chart for patient selection is presented in Figure 1.
 |
Figure 1 Flow chart of patient’ selection. |
Treatment and Assessment of Response
The HAIC procedure was performed by an experienced radiologist with more than five years of experience. The microcatheter was inserted into the proper hepatic artery according to the tumor location and affected hepatic segments. After the patient returned to the sickroom, the FOLFOX-based regimen was intra-arterially administered through the microcatheter. The FOLFOX regimen was as follows: 85 or 135 mg/m2 oxaliplatin from hours 0 to 2 on day 1, 400 mg/m2 leucovorin from hours 2 to 4 on day 1, and 400 mg/m2 fluorouracil bolus at hour 5 on day 1, and 2400 mg/m2 fluorouracil over 46 h on days 1 and 2. HAIC was repeated every 3–4 weeks. Patients received 2–6 cycles of HAIC. We divided patients into two levels according to HAIC cycles. The low level was HAIC ≤ 3 cycles, and the high level was HAIC > 3 cycles. The discontinuation of HAIC depended on tumor response and patient’s condition and choice. After HAIC was discontinued, patients continued to receive Len or Len plus PD-1 inhibitor therapy between the two groups as maintenance therapy.
Information regarding the initiation, completion of treatment, and adverse events (AEs) during treatment was systematically collected. The prescription dosage of Len was 12 mg (body weight ≥60 kg) or 8 mg (body weight <60 kg) orally once a day. The first use of Len was within 7 days of HAIC initiation. For PD-1 inhibitor administration in HAIC+Len+ICI group, the PD-1 inhibitor (including sintilimab, toripalimab, and camrelizumab) was applied according to the drug instruction. The first use of PD-1 inhibitor was within 7 days after HAIC initiation. AEs during the treatment were recorded according to the National Cancer Institute Common Terminology Criteria for Adverse Events 4.0.21 For grade 1–2 of AEs, patients were alleviated after accepting symptomatic treatment or dose reduction. For grade 3–4 of AEs, patients were temporarily stopped from drug administration until the AEs were alleviated.
Follow-Up and Definitions
Patients were evaluated every 3–4 weeks after HAIC treatment. Each follow-up visit consisted of image examination, and laboratory tests including the measurement of alpha-fetoprotein (AFP), albumin, bilirubin, aspartate transaminase, and alanine transaminase (ALT) levels. The follow-up period was terminated on June 30, 2023. Tumor response was evaluated by imaging and according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v 1.1).22 Briefly, the complete response (CR) was defined as the disappearance of arterial enhancement in the tumor. Partial response (PR) was defined as ≥30% reduction in the diameter of the tumors. Progressive disease (PD) was defined as at least a 20% increase in the sum of the diameters of the targeted tumors or the appearance of a new lesion. Stable disease (SD) neither met the criteria for CR, PR, or PD. The objective response rate (ORR) was the sum of CR and PR, and the disease control rate (DCR) was the sum of CR, PR, and SD.
The primary endpoint for the study was OS, defined as the time from the date of accepting HAIC to the date when patients died or the last follow-up. The secondary endpoints included PFS, tumor response and safety. PFS was defined as the time from the date of accepting HAIC to tumor progression or the last follow-up. Patients lost to follow-up were considered censored at the last observation date. Tumor stage was assessed by systemic imaging.
Hepatitis was based on a history of chronic HBV infection and/or positive hepatitis B virus RNA testing. Cirrhosis was diagnosed based on five imaging parameters (irregular or nodular liver surface, blunt liver edge, liver parenchymal abnormalities, liver morphological changes and manifestations of portal hypertension) on CT or MRI, and patients were usually infected with chronic hepatitis infection.23,24 Portal hypertension (PH) was defined as the presence of gastroesophageal varices or splenomegaly (diameter longer than 12 cm) combined with thrombocytopenia (platelet count less than 100 × 109/L).25 Antivirus drugs for patients with HBV infections were entecavir and tenofovir. The albumin–bilirubin (ALBI) grade was used to assess liver function.26 Portal vein tumor thrombus (PVTT) type was determined according to Cheng’s criteria27 as follows: type I, tumor thrombus involving the segmental branches of the portal vein or higher; type II, tumor thrombus involving the right/left portal vein; type III, tumor thrombus involving the main portal vein; and type IV, tumor thrombus involving the superior mesenteric vein. Metastasis was diagnosed by two experienced radiologists, which involved a new extrahepatic nodule showing enhancement by imaging with primary HCC in the liver.
Statistical Analysis
To evaluate the differences between the two groups, the Pearson χ2 test and Fisher’s exact test were used to compare categorical variables. The survival curves of OS and PFS in the entire cohort, the PSM cohort, and subgroup analysis were analyzed by the Kaplan–Meier method with the Log rank test. All statistical tests were two-sided, and P < 0.05 was considered statistically significant. Univariate and multivariate Cox regression were used to determine the survival factors. Variables with P values less than 0.05 in the univariate analysis were subjected to the multivariate Cox regression.
Propensity score-matching (PSM) analysis was used to reduce the effect of selection bias and potential confounding variables between the two groups. Propensity scores were estimated using a multivariate logistic regression model, by inserting the following variables: age, tumor size, tumor number, AFP, PVTT, ALBI grade, metastasis, and portal hypertension. Patients were matched 1:1 using the nearest neighbor method with a caliber of 0.05, and this matching process was described in our previous study.28
We progressively adjusted confounding variables to assess the robustness and potential risk factors. We included HAIC cycle, tumor size, tumor number, AFP, PVTT, metastasis, BCLC stage, and portal hypertension. The selection of confounders was based on the observed patient characteristics before treatment and expert considerations. A posthoc calculation revealed that a power of >80% was required for the primary outcome. The statistical analyses were performed using the SPSS software (version 22.0, SPSS Inc., Chicago, IL, USA) and R software for Windows (Version 3.6.4 http://www.r-project.org) and PASS software (version 2021, V21.0.3, NCSS, LLC).
Results
Baseline Characteristics
There were 1025 patients with HCC who received Len with or without PD-1 inhibitor after HAIC. A total of 376 patients had infiltrative HCC, and 301 patients were included for analysis based on the inclusion criteria. Of these, 173 patients were in HAIC+Len group, and 128 HAIC+Len+ICI group. There were 131 (75.7%) in HAIC+Len group and 81 (63.3%) in HAIC+Len+ICI group occurred death event. The median follow-up period in the HAIC+Len+ICI group was 16.8 (range: 5.5–45.9) months and 14.2 (range: 5.0–44.3) months in the HAIC+Len group. All the etiology of HCC was HBV infection. PSM analysis generated two new cohorts of 117 pairs of patients, and the characteristics of the two groups were balanced. Compared with the HAIC+Len group, the HAIC+Len+ICI group had a higher proportion of patients with tumor size ≤10 cm, tumor number ≤3, and portal hypertension in the entire cohort. After PSM, there were no significant differences between the two groups. The demographic data, etiology of liver disease, and tumor characteristics of the patients in the entire cohort and the matched cohort were listed in Table 1, and the SMD in the entire and PSM cohorts were presented in Table S1.
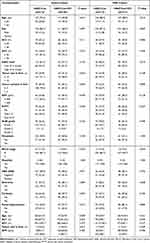 |
Table 1 Baseline Characteristics of Patients with Infiltrative HCC in Different Treatment Groups |
OS Analysis Between the HAIC+Len and HAIC+Len+ICI Groups
The median OS was 13.4 ± 0.9 and 16.7 ± 1.3 months in the HAIC+Len and HAIC+Len+ICI groups, respectively. For patients in the entire cohort, the 6-, 12-, and 24-month OS rates were 94.2%, 60.6%, and 22.9% in the HAIC+Len group, and 97.6%, 75.2%, and 29.0% in the HAIC+Len+ICI group, respectively. After PSM, the median OS was 14.1 ± 1.0 and 16.1 ± 1.4 months in the HAIC+Len and HAIC+Len+ICI groups, respectively. The 6-, 12-, and 24-month OS rates were 96.6%, 62.2%, and 19.8% in the HAIC+Len group, and 97.4%, 72.9%, and 31.3% in the HAIC+Len+ICI group, respectively. The HAIC+Len+ICI group had a better OS compared with the HAIC+Len group in the entire cohort (HR, 0.67; 95% CI, 0.49–0.92; P = 0.012) (Figure 2A) and in the PSM cohort (HR, 0.66; 95% CI, 0.49–0.90; P = 0.008) (Figure 2B).
Univariable and multivariable analysis of OS are listed in Table 2. Multivariable analysis after PSM revealed that HAIC+Len therapy (HR, 1.63; 95% CI, 1.18–2.26; P = 0.003), metastasis (HR, 1.73; 95% CI, 1.23–2.43; P = 0.002), BCLC stage (HR, 5.34; 95% CI, 2.30–12.37; P < 0.001), no antivirus therapy (HR, 1.43; 95% CI, 1.01–2.05; P = 0.049), and portal hypertension (HR, 1.90; 95% CI, 1.27–2.85; P = 0.002) were risk factors associated with poorer OS (Table 2). The OS benefits of HAIC+Len+ICI treatment were generally consistent across various clinical subgroups (Figure S2).
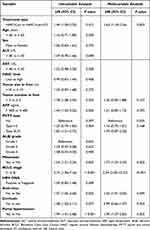 |
Table 2 Univariable and Multivariable Analysis of Prognostic Factors for OS in Infiltrative HCC After PSM |
Effect of Treatment on PFS
For patients in the entire cohort, the median PFS was 4.8 ± 0.4 months in the HAIC+Len group, and 7.5 ± 0.7 months in the HAIC+Len+ICI group, respectively. The 3-, 6-, and 12-month PFS rates were 82.1%, 35.8%, and 8.6% in the HAIC+Len group, and 91.4%, 59.9%, and 24.0% in the HAIC+Len+ICI group, respectively. After PSM, the median PFS was 4.6 ± 0.4 months in the HAIC+Len group, and 7.5 ± 0.8 months in the HAIC+Len+ICI group, respectively. The 3-, 6-, and 12-month PFS rates were 81.0%, 35.9%, and 11.1% in the HAIC+Len group, and 90.6%, 59.8%, and 26.1% in the HAIC+Len+ICI group, respectively. Compared with the HAIC+Len group, the HAIC+Len+ICI group had significantly better PFS in the entire cohort (HR, 0.51; 95% CI, 0.39–0.65; P < 0.001) (Figure 2C) and in the PSM cohort (HR, 0.53; 95% CI, 0.40–0.70; P < 0.001) (Figure 2D).
Univariable and multivariable analysis of PFS were listed in Table S2. Multivariable Cox regression indicated that HAIC+Len therapy (HR, 2.06; 95% CI, 1.55–2.73; P < 0.001), ALT>40 U/L (HR, 1.39; 95% CI, 1.05–1.84; P = 0.020), metastasis (HR, 2.03; 95% CI, 1.51–2.73; P < 0.001), BCLC stage (HR, 2.36; 95% CI, 1.39–3.98; P = 0.001) and no antivirus therapy (HR, 1.62; 95% CI, 1.19–2.21; P = 0.002) were risk factors associated with poorer PFS (Table S2). The stratification analysis of PFS demonstrated that HAIC+Len+ICI treatment benefited from clinical subgroups (Figure S3).
Efficacy Evaluation
The tumor response was evaluated according to RECISTv1.1 and the results after PSM are presented in Table 3. For the 3-month evaluation, the ORR was 30.0% and 47.8%, whereas the DCR was 57.4% and 78.6% in the HAIC+Len and HAIC+Len+ICI groups, respectively. The proportion of CR, PR, SD, and PD in the two groups was different (P = 0.003) (Table 3). At the 6-month evaluation, there was one patient in the HAIC+Len group and five patients in the HAIC+Len+ICI group that achieved CR. The ORR were 15.4% and 47.0%, and the DCR were 29.1% and 51.3% in the HAIC+Len and HAIC+Len+ICI groups, respectively. Similarly, the proportions of CR, PR, SD, and PD in the two groups were different (P < 0.001) (Table 3).
 |
Table 3 Efficacy Outcomes in Patients with HCC in Different Treatment Groups According to RECIST v 1.1 Evaluation |
Sensitivity Analysis
The final adjusted HR value from the multivariable analysis was 0.41 (95% CI, 0.30–0.57) after progressively adjusting for confounders in the OS analysis (Table S3). The PSM model in the multivariable analysis of OS yielded an adjusted value of 0.59 (95% CI, 0.43–0.82) (Table S4). After progressively adjusting for variables in the PFS analysis, the adjusted HR from the multivariable analysis was 0.48 (95% CI, 0.36–0.62) (Table S3). The PSM model in the multivariable analysis of PFS yielded an adjusted HR of 0.48 (95% CI, 0.36–0.65) (Table S4). Data on OS with power (0.8408) and PFS with power (0.9882) conferred sufficient reliability to our results (Table S5).
Subgroup Analysis on the Prognosis of Metastasis
To further clarify the patients with extrahepatic metastasis on prognosis, we conducted a subgroup analysis based on metastasis in the PSM cohort. As shown in Table 1, there were 61 pairs of HCC without extrahepatic metastasis and 56 pairs with metastasis. The medium OS of HCC with metastasis was 12.7 ± 1.1 and 16.7 ± 3.1 months in the HAIC+Len group, and 14.8 ± 1.3 and 19.1 ± 2.5 months in the HAIC+Len+ICI group without metastasis. There was an obvious difference in OS between the two groups with metastasis (HR, 0.56; 95% CI, 0.36–0.87; P = 0.008) (Figure 3A). No statistically significant difference in OS was observed between the two groups without metastasis (HR, 0.68; 95% CI, 0.43–1.07; P = 0.091) (Figure 3B); however, PFS was significantly different between the two groups with metastasis (Figure S4A and B). In intragroup analysis, a significant difference in OS was observed for HCC with and without metastasis in the HAIC+Len group (HR, 0.43; 95% CI, 0.43–1.07; P < 0.001) (Figure 3C). However, the OS was not statistically significant for HCC with and without metastasis in the HAIC+Len+ICI group (HR, 0.67; 95% CI, 0.42–1.08; P = 0.096) (Figure 3D). PFS was significantly different in the intragroup analysis of the two groups (Figure S4C and D). The OS in HCC with metastasis was improved with HAIC+Len+ICI treatment, whereas for HCC without metastasis, the addition of PD-1 after HAIC and Len was not significant.
Subgroup Analysis on the Prognosis of HAIC Cycles
The number of cycles of HAIC on the prognosis of infiltrative HCC was compared in the PSM cohort. Patients were divided into two groups based on HAIC cycle number (Table 1). No difference in OS was observed between low and high levels of cycle in the HAIC groups (HR, 0.99; 95% CI, 0.67–1.44; P = 0.938) (Figure 4A). Similarly, the PFS was also similar (HR, 0.92; 95% CI, 0.69–1.24; P = 0.531) (Figure 4B). There was also no difference in OS (Figure S5A) and PFS (Figure S5B) in the entire cohort between low and high levels of cycle. To determine the potential reasons for this, we assessed the 6-month liver function after the first HAIC cycle between the high and low groups. The results indicated that patients with a low number of HAIC cycles had significantly better liver function compared with those with a high number of HAIC cycles (Table S6).
 |
Figure 4 Kaplan–Meier curves of OS (A) and PFS (B) in patients receiving low and high levels of HAIC cycles in PSM cohort. |
Treatment-Related Adverse Events
Treatment-related deaths did not occur in this study. The main AEs were listed in Table S7. All 301 patients received 2–6 cycles of HAIC. The median duration of Len was 5.2 months (range, 3.0–23.0 months) in the HAIC+Len group and 8.5 months (range, 4.5–28.0 months) in the HAIC+Len+ICI group. The median duration of PD-1 inhibitors was 11.0 months (8.0–36.0 months).
For grade 1–2 of adverse events, patients were alleviated after accepting symptomatic treatment or dose reduction. For grade 3–4 of adverse events, patients were temporary stopped Len or PD-1 inhibitor administration until the adverse effects alleviated or disappeared, PD-1 inhibitor infusion and low dose of Len were continued if possible after recovery. For the AEs in HAIC, the 1–2 grades, patients received alleviative treatment in decreasing the AEs, and for 3–4 grades, HAIC was stopped by a multi-disciplinary decision.
Discussion
In the present study, HAIC+Len+ICI achieved a more promising outcome in both OS and PFS compared with HAIC+Len. These results provide valuable clinical evidence for the treatment of infiltrative HCC. The 6-, 12-, and 24-month OS rates were 97.6%, 75.2%, and 29.0% in the HAIC+Len+ICI group. The number of studies reporting infiltrative HCC was small. To added this gap, we conducted this multicenter study. Atezolizumab and Bevacizumab are the first-line recommended systemic therapy worldwide for infiltrative HCC.5 Although these two drug combinations is effective in prolonging OS, their effects are still unsatisfactory for macrovascular invasion and extrahepatic metastasis of infiltrative HCC. Therefore, combining different therapies and fully integrating the advantages are needed in the treatment of this subtype HCC. The median OS of HAIC+Len+ICI treatment was shorter than that OS of atezolizumab plus bevacizumab treatment (16.7 vs 19.2 months).29 The median PFS in the HAIC+Len+ICI was slightly longer compared with atezolizumab plus bevacizumab (7.5 vs 6.8 months).30 An et al reported that the 1- and 2-year OS rates were 38.2% and 8.4% for HAIC treatment of infiltrative HCC.31 Nisiewicz et al reported that the median OS following transarterial radioembolization of infiltrative HCC was 10.0 months.8 The survival following HAIC+Len+ICI treatment in the study was longer compared with the above studies. In addition, the baseline for patients in the present study was poorer compared with the IMbrave150 trial as well as studies by Nisiewicz and An.
We found that the HAIC combined with Len and PD-1 inhibitors were necessary for HCC with metastasis, whereas for HCC without metastasis, the two treatments showed no difference in OS. The underlying reasons may be explained as follows. First, for localized advanced HCC without extrahepatic metastasis, HAIC plus Len is an effective local therapy that may sufficiently control the limited tumors.32 This may be the reason that there was no difference for HCC without metastasis between the two groups. Second, HAIC as an effective locoregional therapy, could induce tumor cell necrosis which might create a favorite environment for ICI therapy.15 Thus, HAIC could enhance PD-1 inhibitor and Len in controlling the metastasis.17 Although, the LEAP-002 trial did not meet the prespecified significance for improved survival in advanced HCC. The results indicated that Len plus PD-1 inhibitor had better effective clinical activity compared with Len alone.33 We anticipate the second analysis of Len plus PD-1 inhibitor compared with Len for patients with a much poorer prognosis, such as beyond oligometastasis or infiltrative HCC.
For patients with infiltrative HCC, the use of HAIC can be beneficial;32 however, patients with infiltrative HCC usually present with high tumor burden and PVTT, and reserved liver function is vulnerable.8 HAIC can inevitably induce liver damage as well as hepatic failure.12 Thus, HAIC could be discontinued once the tumor is under control. Len or Len plus PD-1 inhibitor may be used as the systemic therapy in inhibiting tumor from progression.34 Thus, postoperative liver function can determine a patient's prognosis.35,36 In the present study, we demonstrated that OS was no different between high and low cycles of HAIC. Patients with a low HAIC cycle number had significantly better liver function compared with a high HAIC cycle number. Although adding HAIC+Len +ICI is beneficial for OS and PFS, this minimally therapy has the risk of damaging liver function. Thus, we recommended that three cycles of HAIC are adequate, particularly for patients with huge infiltrative HCC (tumor diameter >10 cm) with main branch PVTT.
This study has several limitations. First, as a retrospective study, selection bias existed in patients treated by PD-1 inhibitor, because it was the choice of doctors and patient’s tolerance and affordability. Second, the heterogeneous HAIC regime and patient’s response to Len or PD-1 inhibitor, and their combinations may make some sense to the outcomes. Third, although we have included data from multiple centers, more samples needed and future prospective studies are needed to validate the findings. Fourth, we compared HAIC+Len+ICI with HAIC+Len, future studies of comparing atezolizumab plus bevacizumab with HAIC+Len+ICI are also needed for treating infiltrative HCC. HAIC+Len+ICI compares with HAIC plus atezolizumab plus bevacizumab is also recommended. Fifth, although most of the patient in this study have an endpoint event, the short follow-up period might limit the study’s conclusions, the long-term follow-up is needed to strengthen the conclusion.
Conclusion
The results of the present study indicated that HAIC plus Len and PD-1 inhibitors were associated with longer OS and PFS compared with HAIC plus Len for infiltrative HCC. In addition, we found that OS in HCC with metastasis was improved with HAIC+Len+ICI treatment. The addition of PD-1 inhibitors after HAIC and Len in HCC without metastasis did not show survival improvement. In addition, we recommend that three cycles of HAIC are adequate, particularly for patients with high tumor burden and main branch PVTT.
Data Sharing Statement
Data are available from the authors upon reasonable request and with permission of four hospital authorities in China.
Ethics Statement
The Ethics Committee Board of the Chinese PLA General Hospital approved this retrospective study and waived the requirement for patient consent for this retrospective review. We solemnly promise that this study will strictly abide by relevant laws and regulations and will not disclose patient personal information and related information to any other personnel and organizations to ensure the security and confidentiality of patient information.
Acknowledgments
This research was funded by the National Natural Science Foundation of China (No. 82102082).
Disclosure
The abstract of our manuscript is available as a preprint: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4345805. We thank Fei Tuo, Xianhai Mao and Shaoqiang Li for reviewing and supporting this manuscript. The authors declare no conflicts of interest.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca a Cancer J Clinicians. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Yopp AC, Mokdad A, Zhu H, et al. Infiltrative hepatocellular carcinoma: natural history and comparison with multifocal, nodular hepatocellular carcinoma. Ann Surg Oncol. 2015;22(Suppl 3):S1075–1082. doi:10.1245/s10434-015-4786-7
3. Kneuertz PJ, Demirjian A, Firoozmand A, et al. Diffuse infiltrative hepatocellular carcinoma: assessment of presentation, treatment, and outcomes. Ann Surg Oncol. 2012;19(9):2897–2907. doi:10.1245/s10434-012-2336-0
4. He MK, Liang RB, Zhao Y, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Therapeut Adv Med Oncol. 2021;13:17588359211002720. doi:10.1177/17588359211002720
5. Han K, Kim JH, Yoon HM, et al. Transcatheter arterial chemoembolization for infiltrative hepatocellular carcinoma: clinical safety and efficacy and factors influencing patient survival. Korean J Radiol. 2014;15(4):464–471. doi:10.3348/kjr.2014.15.4.464
6. Huang J, Huang W, Zhan M, et al. Drug-eluting bead transarterial chemoembolization combined with FOLFOX-based hepatic arterial infusion chemotherapy for large or huge hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:1445–1458. doi:10.2147/jhc.s339379
7. Lu J, Zhang XP, Zhong BY, et al. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol. 2019;4(9):721–730. doi:10.1016/s2468-1253(19)30178-5
8. Nisiewicz MJ, Kapoor H, Fowler KJ, et al. Improved survival following transarterial radioembolization of infiltrative-appearance hepatocellular carcinoma. Abdom Radiol. 2021;46(5):1958–1966. doi:10.1007/s00261-020-02870-3
9. Huang A, Yang XR, Chung WY, et al. Targeted therapy for hepatocellular carcinoma. Signal Transduct Targeted Ther. 2020;5(1):146. doi:10.1038/s41392-020-00264-x
10. He M, Li Q, Zou R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5(7):953–960. doi:10.1001/jamaoncol.2019.0250
11. Chen S, Zhang K, Liu W, et al. Hepatic arterial infusion of oxaliplatin plus raltitrexed in patients with intermediate and advanced stage hepatocellular carcinoma: a Phase II, single-arm, prospective study. Eur. J. Cancer. 2020;134:90–98. doi:10.1016/j.ejca.2020.03.032
12. Li QJ, He MK, Chen HW, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized phase III trial. J Clin Oncol. 2022;40(2):150–160. doi:10.1200/jco.21.00608
13. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label Phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi:10.1016/s1470-2045(18)30351-6
14. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, Phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi:10.1016/s0140-6736(17)31046-2
15. Liu WM, Fowler DW, Smith P, et al. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br. J. Cancer. 2010;102(1):115–123. doi:10.1038/sj.bjc.6605465
16. Mei J, Tang YH, Wei W, et al. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma. Front Oncol. 2021;11:618206. doi:10.3389/fonc.2021.618206
17. Budimir N, Thomas GD, Dolina JS, et al. Reversing T-cell exhaustion in cancer: lessons learned from PD-1/PD-L1 immune checkpoint blockade. Cancer Immunol Res. 2022;10(2):146–153. doi:10.1158/2326-6066.cir-21-0515
18. Guan R, Zhang N, Deng M, et al. Patients with hepatocellular carcinoma extrahepatic metastases can benefit from hepatic arterial infusion chemotherapy combined with lenvatinib plus programmed Death-1 inhibitors. Int j Surg. 2024;29:10–97. doi:10.1097/js9.0000000000001378
19. Shrestha BM. The Declaration of Helsinki in relation to medical research: historical and current perspectives. J Nepal Health Res Council. 2012;10(22):254–257.
20. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
21. Chung AE, Shoenbill K, Mitchell SA, et al. Patient free text reporting of symptomatic adverse events in cancer clinical research using the national cancer institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). J Am Med Inform Assoc. 2019;26(4):276–285. doi:10.1093/jamia/ocy169
22. Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1-Update and clarification: from the RECIST committee. Eur. J. Cancer. 2016;62:132–137. doi:10.1016/j.ejca.2016.03.081
23. Coelho FF, Herman P, Kruger JAP, et al. Impact of liver cirrhosis, the severity of cirrhosis, and portal hypertension on the outcomes of minimally invasive left lateral sectionectomies for primary liver malignancies. Surgery. 2023;174(3):581–592. doi:10.1016/j.surg.2023.04.057
24. Kudo M, Zheng RQ, Kim SR, et al. Diagnostic accuracy of imaging for liver cirrhosis compared to histologically proven liver cirrhosis. A multicenter collaborative study. Intervirology. 2008;51(Suppl 1):17–26. doi:10.1159/000122595
25. Wang YY, Xiang BD, Ma L, et al. Development and validation of a nomogram to preoperatively estimate post-hepatectomy liver dysfunction risk and long-term survival in patients with hepatocellular carcinoma. Ann Surg. 2021;274(6):e1209–e1217. doi:10.1097/sla.0000000000003803
26. Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-The ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi:10.1200/jco.2014.57.9151
27. Wei X, Jiang Y, Zhang X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol. 2019;37(24):2141–2151. doi:10.1200/jco.18.02184
28. Wang XH, Duan WB, Liang W, et al. Efficacy of radiofrequency ablation following transarterial chemoembolisation combined with sorafenib for intermediate stage recurrent hepatocellular carcinoma: a retrospective, multicentre, cohort study. EClinicalMedicine. 2023;56:101816. doi:10.1016/j.eclinm.2022.101816
29. Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi:10.1016/j.jhep.2021.11.030
30. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. New England J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
31. An C, Zuo M, Li W, et al. Infiltrative hepatocellular carcinoma: transcatheter arterial chemoembolization versus hepatic arterial infusion chemotherapy. Front Oncol. 2021;11:747496. doi:10.3389/fonc.2021.747496
32. Lyu N, Wang X, Li JB, et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: a biomolecular exploratory, randomized, phase III trial (FOHAIC-1). J Clin Oncol. 2022;40(5):468–480. doi:10.1200/jco.21.01963
33. Llovet JM, Kudo M, Merle P, et al. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): a randomised, double-blind, Phase 3 trial. Lancet Oncol. 2023;24(12):1399–1410. doi:10.1016/s1470-2045(23)00469-2
34. Zheng K, Zhu X, Fu S, et al. Sorafenib plus hepatic arterial infusion chemotherapy versus sorafenib for hepatocellular carcinoma with major portal vein tumor thrombosis: a randomized trial. Radiology. 2022;303(2):455–464. doi:10.1148/radiol.211545
35. Poon RT, Fan ST, Lo CM, et al. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229(2):216–222. doi:10.1097/00000658-199902000-00009
36. Blüthner E, Bednarsch J, Malinowski M, et al. Dynamic liver function is an independent predictor of recurrence-free survival after curative liver resection for HCC - A retrospective cohort study. Int J Surg. 2019;71:56–65. doi:10.1016/j.ijsu.2019.08.033
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


