Back to Journals » International Journal of Nanomedicine » Volume 19
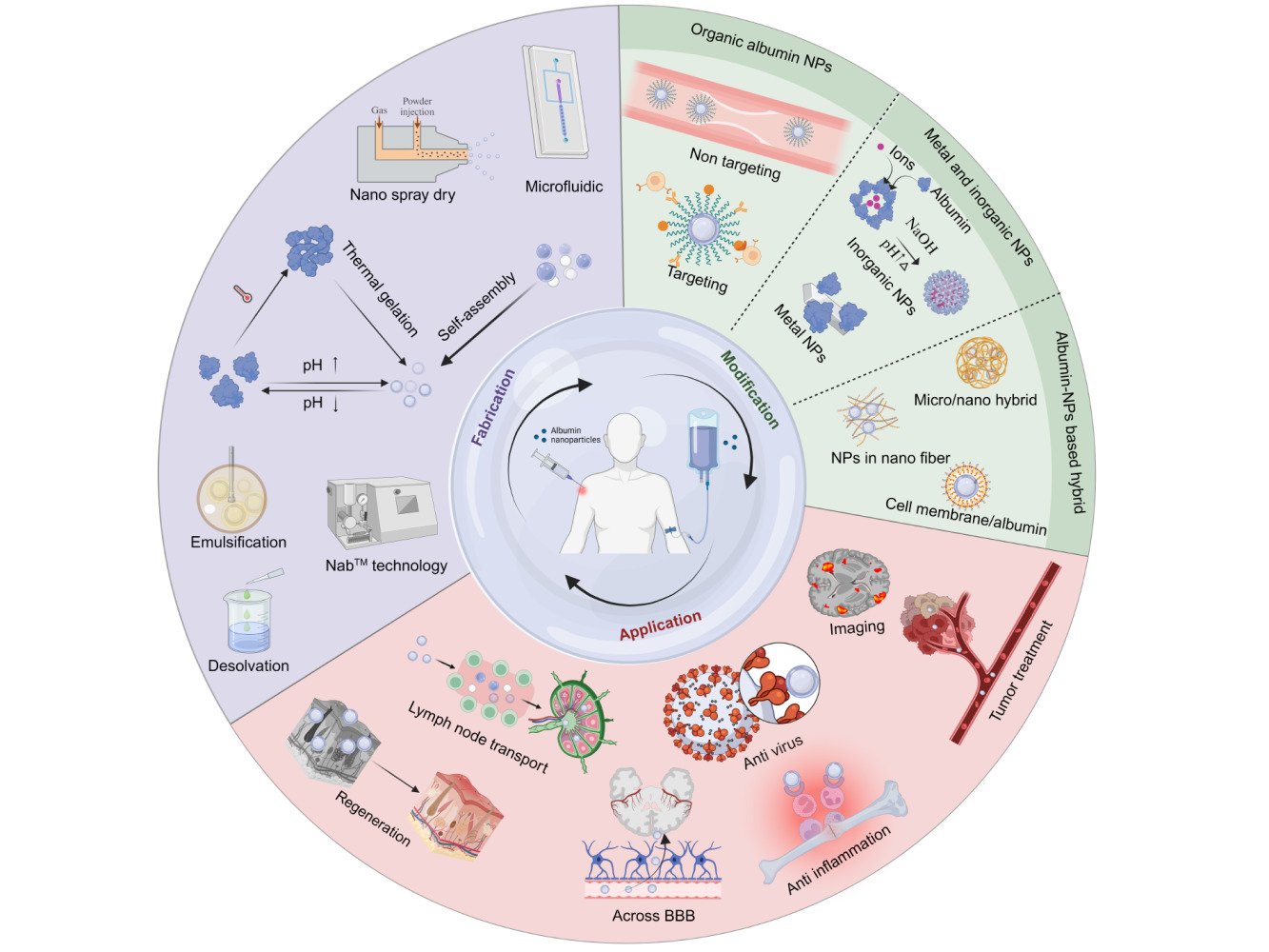
Albumin Nanoparticle-Based Drug Delivery Systems
Authors Qu N, Song K , Ji Y , Liu M, Chen L, Lee RJ, Teng L
Received 8 March 2024
Accepted for publication 28 May 2024
Published 10 July 2024 Volume 2024:19 Pages 6945—6980
DOI https://doi.org/10.2147/IJN.S467876
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. RDK Misra
Na Qu,1 Ke Song,2 Yating Ji,1 Mingxia Liu,1 Lijiang Chen,1 Robert J Lee,3,4 Lesheng Teng3,5
1School of Pharmacy, Liaoning University, Shenyang, 110036, People’s Republic of China; 2MERLN Institute for Technology-Inspired Regenerative Medicine, Maastricht University, Maastricht, 6229 ER, the Netherlands; 3School of Life Sciences, Jilin University, Changchun, 130023, People’s Republic of China; 4College of Pharmacy, The Ohio State University, Columbus, OH, 43210, USA; 5State Key Laboratory of Long-Acting and Targeting Drug Delivery System, Yantai, 264000, People’s Republic of China
Correspondence: Lesheng Teng, Email [email protected]
Abstract: Nanoparticle-based systems are extensively investigated for drug delivery. Among others, with superior biocompatibility and enhanced targeting capacity, albumin appears to be a promising carrier for drug delivery. Albumin nanoparticles are highly favored in many disease therapies, as they have the proper chemical groups for modification, cell-binding sites for cell adhesion, and affinity to protein drugs for nanocomplex generation. Herein, this review summarizes the recent fabrication techniques, modification strategies, and application of albumin nanoparticles. We first discuss various albumin nanoparticle fabrication methods, from both pros and cons. Then, we provide a comprehensive introduction to the modification section, including organic albumin nanoparticles, metal albumin nanoparticles, inorganic albumin nanoparticles, and albumin nanoparticle-based hybrids. We finally bring further perspectives on albumin nanoparticles used for various critical diseases.
Plain Language Summary: Albumin appears to be a promising carrier for drug delivery with superior biocompatibility and enhanced targeting capacity. This review focuses on the importance of albumin nanoparticles in drug delivery and concludes the recent fabrication techniques to prepare albumin nanoparticles, the modification strategies to require functional albumin nanoparticles, and critical applications of albumin nanoparticles in various diseases. The aim of this review is to help readers understand the significant potential of albumin nanoparticles in drug delivery.
Keywords: albumin nanoparticle, drug delivery, fabrication, modification, application
Graphical Abstract:
Introduction
Nanoparticle delivery systems have attracted much attention as a drug delivery strategy. It has the advantages of unique drug targeting, slow controlled release features, and protection function, especially in the transfer of hydrophobic drugs. Nanotechnology Initiative (NNI) defines nanotechnology as a size of approximately 1 to 100 nanometers (nm), but it can be extended to 1000 nanometers over a wide range.1 The ideal nanoparticle carrier should have the following properties: specific targeting, drug release controllability, carrier non-toxic, and biodegradable.
Albumin is a very soluble protein, which is consisting of 585 amino acid residues. The relative molecular weight of albumin is 66,500 Da, and it can maintain biological activity under conditions of pH 4–9, 40% ethanol, or heating at 60 °C for 10 hours.2 The three-dimensional structure of albumin, including hydrophilic and hydrophobic domains and charged amino acids, enables it to deliver drugs with different physicochemical properties. Meanwhile, the amino and carboxyl groups on the surface of albumin provide binding sites for polymers or ligands.3 In addition, albumin is more suitable as a carrier for drug delivery owing to its ready availability, nontoxicity, biodegradability, immunogenicity, and preferential accumulation and uptake in tumors and inflamed tissue.4 Many therapeutic drugs and metabolic compounds in blood plasma are transported by human serum albumin (HSA), one of the smallest proteins and abundant in blood plasma.5 HSA affects the drug metabolism process and therapeutic effect in vivo, which has attracted researchers to explore the mechanism and influencing factors of drug albumin binding.6–8 Large amount of clinical trials on albumin-based nanoformulations have been described elsewhere.9 Here, we listed marked albumin nanodrug in Figure 1. In fact, it has been a long history for albumin being used for drug delivery, reported as early as 1960s, initially as a diagnostic agent.10 Nano-sized albumin drug carrier emerged in 1970s11 and 1980s,12 whereas the latter one was developed into Nanocoll®, [99mTc]-labeled nanocolloidal albumin, approved for oncology rheumatology in 1995.13 Some other albumin nanoformulations were developed during 1990s, including hydrocortisone-albumin, which was reported for treating eye inflammation,14 and methotrexate–albumin conjugate, which was conducted in a Phase I trial for cancer treatment.15 The latter one was reported capable of rheumatoid arthritis treatment in 2003.16 Among others, due to the fast development of nanoparticle albumin bound (nab) technology, one useful and proven drug delivery platform that poorly water-soluble active pharmaceutical ingredients can be encapsulated into nanoparticles,17 Abraxane® (nab-paclitaxel) was approved by the Food and Drug Administration (FDA) in 2005. It showed the advantage of avoiding solvents/solubilizers during the formulation process, high tolerated dose, long drug residence time in the tumor, short infusion time, and it reduced risk of hypersensitivity reactions. Abraxane® (~130nm) is considered to dissociate into a 10 nm albumin bound paclitaxel (PTX) complex during intravenous administration18 and widely used for clinical tumor treatment due to its accumulation in tumors, increasing the concentration of PTX in the tumor stroma and enhancing its anti-tumor activity.19 The successful launch of Abraxane®, is a milestone in the development of albumin drug delivery systems and is also seen as a turning point in nanomedicine. In 2007, just two years after its launch, annual sales reached approximately $300 million. Based on the nab technology, Fyarro® (nab-sirolimus) was approved in November 2021.20 Fyarro® is able to inhibit the mammalian target of rapamycin (mTOR), which controls key cellular processes such as cell survival, growth, and proliferation, and is often dysregulated in human cancers. Compared to oral mTOR inhibitors, intravenous administration of nab-sirolimus demonstrated higher intratumoral accumulation, stronger mTOR inhibition, and higher tumor growth inhibition. Fyarro® was approved for the treatment of unresectable or metastatic malignant perivascular epithelial cell tumors (PEComa) in adults.21 Besides, Nanozora®, an albumin-binding nanobody directed against human TNF-α, was approved in Japan for the treatment of rheumatoid arthritis in 2022. Other drug formulations that bind to albumin in vitro or in vivo are currently at different clinical stages.22
 |
Figure 1 Marketed albumin nanodrug products. |
Albumin nanoparticles as drug delivery systems have been mainly used for drug delivery and targeted therapies in the last two decades. Figure 2 shows the numbers of publications in PubMed combining the key word “albumin nanoparticle” with different applications from 2004 to 2023, with anti-tumor being a major area of research. However, with the increasing health impact of inflammatory diseases globally, research on albumin nanoparticles in the field of anti-inflammation is on the rise. Great potential of using albumin nanoparticles for different applications remains covered. Therefore, in this review, we first discuss various albumin nanoparticle fabrication methods, from both pros and cons. Then, modification strategies used for the functionalization or stabilization of albumin nanoparticle will be introduced, including organic albumin nanoparticles (targeted and non-targeted modification with organic building blocks), metal albumin nanoparticles, inorganic albumin nanoparticles, and albumin nanoparticle-based hybrids. Finally, further application on albumin nanoparticles used for various critical diseases will be discussed.
 |
Figure 2 Number of peer-reviewed publications from 2004 to 2023 combining key words “albumin nanoparticle” and different applications (PubMed database). |
Albumin
Human Serum Albumin (HSA)
HSA is abundant in plasma protein (35–50 g/L human serum), which has the inherent characteristics of ligand binding, wide tissue distribution, and long cycle half-life. There is a total of 35 cysteine residues within the HSA molecule, of which 34 residues form 17 disulfide bonds with each other. These 17 disulfide bonds make the structure of proteins more stable and can be modified under certain conditions.23,24 The three structural domains I, II, and III of Albumin have flexibility. Further, the major three structural domains and their subdomains stabilized with disulfide bonds in the albumin molecule offer the different binding sites of the drug, which can not only bind to multiple compounds to improve the solubility of hydrophobic compounds, but also bind to specific ligands and antibody molecules to achieve targeted drug accumulation.7 There are two ways for drugs to bind to albumin: one is by covalent bond, and the other is by hydrophobic embedding in the albumin hydrophobic cavity. For Abraxane®, PTX and albumin are combined by hydrophobic interaction.8,25
Bovine Serum Albumin (BSA)
BSA and HSA exhibit high homology in structure, making them widely studied as model proteins. BSA is a water-soluble spherical protein containing a polypeptide chain composed of 583 amino acid residues,26,27 with an isoelectric point of ~4.7. It makes up approximately 60% all proteins animal serum.28 BSA has a unique hydrophobic region in its molecular structure27,29 and is also used to study the interaction between albumin and drugs.30,31 The properties of rich, low cost, easy to purify, unusual ligand-binding properties, and widely accepted characteristics in the pharmaceutical industry (easy process ability and scalability) have been widely used in drug delivery systems.32,33 BSA is rich in functional groups, such as carboxyl and amino groups, and has a strong affinity34 with metal nanoparticles. It is often used as a coating agent for metal nanoparticles to improve their colloidal stability and biocompatibility, and endow them with the characteristics of easy functionalization and low toxicity, which is conducive to the application of metal nanoparticles in biomedicine and other fields.35,36 Compared to HSA, the limitation of BSA is its potential immunogenic response.33
Preparation Techniques
Desolvation
The preparation of HSA nanoparticles by desolvation is reproducible and relatively simple.37 This method has a short preparation cycle without surfactants and widespread applications in the encapsulation of hydrophilic and hydrophobic drugs.2 In this method, acetone or ethanol as a desolvation agent is added to the aqueous solution of albumin with the condition of a constant stirring and it continues until the solution becomes turbidity.38,39 The effect of desolvating agents is to gradually alter the tertiary structure of albumin, leading to phase separation and aggregation of proteins.40,41 A crosslinking agent, such as a glutaraldehyde solution, is necessary to stabilize the unstable particles. In order to completely crosslink the amino acid residues in the protein, the suspension is stirred continuously. The guanidino side chains in the arginine residues and amino moieties in lysine residues of albumin are solidified with the aldehyde group of glutaraldehyde by a condensation reaction.42–44 And then, the nanoparticles are purified with centrifugation. The preparation process is shown in Figure 3A. Langer et al38 confirmed that the rate of ethanol addition, the pH of the solution and the concentration of the albumin had an impact on the particle size of nanoparticles in this method. The smaller nanoparticles were formed with the smaller the ethanol addition rate and the high pH levels achieved proper particle size. At an albumin concentration of 50mg/mL, the smallest particles were obtained.
A tubing pump is an essential instrument to carefully control desolvation agent addition speed. Some studies reported manually adding ethanol using a syringe. Bax et al45 optimized the design of the device and reported a method for simplifying the program by controlling the addition of ethanol to the device. It is important to note that, in this study, N-(3-Dimethylaminopropyl)-N-ethyl carbodiimide hydrochloride (EDC) as a cross-linker was used, which is a zero space cross-linker and can be easily removed instead of glutaraldehyde, resulting in reducing the preparation time of nanoparticles to 3 hours. Although reports of albumin nanoparticles used for drug delivery have used glutaraldehyde for stabilization, in vivo studies have shown that residual aldehydes have a certain toxicity, which restricts their use considerably.46,47 Luna-Valdez et al48 reported the formation of nanoparticles from wheat bran water extract by a cold setting desolvation approach, which avoided using glutaraldehyde (toxic) and organic solvent. Besides, physical crosslinking methods include drying, heating and ultraviolet radiation27 and other potential crosslinking agents such as glucose49 are developed.
Emulsification
The emulsion-solvent evaporation method is more suitable for producing nanoparticles with a smaller size and lower polydispersity index, and it is a reliable method with good repeatability and amplification potential.50,51 Compared to pH-condensation technology or microfluidic methods, this method is less time-consuming, less complex, and uses fewer chemicals. An albumin solution (aqueous phase) is stirred with a non-aqueous solution (oily phase) containing an appropriate amount of emulsifier to obtain a crude emulsion. The organic solvents such as dichloromethane or chloroform are usually used as oil phase. The emulsion can be homogenized by ultrasonic treatment or homogenization. Then, the emulsion droplets are solidified by chemical crosslinking or heating deformation, and finally, the residual organic solvent is removed to collect albumin nanoparticles. This method is suitable for hydrophobic drug encapsulation by binding with hydrophobic cavities on HSA molecules. However, the use of toxic organic solvents is major setbacks of this method.52 Besides, surfactants are required for emulsion stabilization. Figure 3B shows the preparation process. Alfagih et al53 prepared nanoparticles encapsulating model antigen and BSA via double emulsion solvent evaporation. In addition, by adding chitosan hydrochloride (CHL) into the outer phase of the lotion solvent, a hybrid cation CHL nanoparticle was formed, which led to surface adsorption on the nanoparticle. It may be used to transport proteins to the lungs for immune stimulation applications such as vaccines.
Nab Technology
Nab technology can mostly not alter the physiological properties of HSA among these techniques.54 Nab technology (Figure 3C) is an albumin nanoparticle preparation technology that uses albumin as a matrix and stabilizer. Under high shear forces, an oil phase containing an aqueous insoluble drug and an albumin-containing aqueous phase are mixed to prepare an O/W emulsion in which the drug is in the absence of any conventional surfactant or any polymer core. First, the drug is dissolved in an organic solvent (usually chloroform, dichloromethane) at a high concentration as an oil phase. Secondly, albumin is dissolved in an aqueous medium to obtain an aqueous phase. Then, the oil and water phases mix under high-pressure homogenization, followed by vacuum evaporation of the solvent quickly to obtain a colloidal dispersion system composed of ultra-fine nanoparticles. The cavitation during homogenization makes the free sulfhydryl group of albumin cross-linked to form a disulfide bond. During the process, the drug is wrapped inside the nanoparticles, preserving the physiological properties of HSA. Compared with the traditional preparation method, nab technology has no conventional surfactants or any polymer core and no special infusion equipment.9 Furthermore, the albumin acts as a lyophilization agent without adding the other conventional freeze-dried protective agent. He et al55 assessed the risk of nab-PTX-related adverse events (AEs) compared with traditional taxanes in various primary solid organ malignancies. Although nab-PTX increases the risk of general hematological and non-hematological AEs, allergic reactions are significantly reduced, and the neurotoxicity is easier to recover. Compared to the traditional PTX, lower doses of nab-PTX administered weekly had better tolerance. However, it still carries ocular adverse effects.56 A significant decrease in visual acuity was observed in patients receiving nab-PTX treatment. In clinical, when the treatment cannot be stopped due to the patient’s general condition, the effective alternative treatment is topical dorzolamide or steroidal treatment.
In a recent study, the palmitate albumin nanoparticles loaded with PTX were prepared based on the nab technology: anhydrous ethanol and chloroform are used as the solvents for emulsification and homogenization under high pressure without surfactants. Albumin was not easily denatured at low temperatures during the preparation process. Finally, the organic solvents were removed through ultrafiltration.57 In 2016, Furedi et al58 prepared Voriconazole nanoparticles (VCZ-NPs) by nab technology. The concentration of HSA in water was controlled at 2% because during high-pressure homogenization processes, higher HSA concentration can cause sample foaming in the samples, resulting in unstable and unpredictable VCZ concentration. An acceptable PDI value (below 0.3) for VCZ-NPs was obtained under the condition of six homogenization cycles at 1800 bar. And the optimized particles met the requirements for intravenous administration with an average particle size of 81.2 ± 1 nm. Furthermore, VCZ-NPs showed a good encapsulated concentration of 69.7 ± 4.2% and increased the water solubility of VCZ greatly.
pH-Condensation
The pH-condensation method (Figure 3D) is to dissolve the drug in an HSA solution at room temperature and incubate it in the dark with the adjusted pH value. The solution is stirred or sonicated to accelerate the coagulation process of albumin followed by crosslink with glutaraldehyde. Then, HSA nanoparticles (HSA-NPs) are obtained by centrifugation, washing, and freeze-drying. However, it is not convenient to control the particle size of the nanoparticles by adjusting the pH value. It usually controls the particle size by adjusting the salt concentration or adding other organic solvents to obtain uniform particle size and spherical nanoparticles.
Lin et al34 reported the preparation of HSA-NPs with approximately 100 nm in diameter using the pH-condensation method without surfactants. The particles were prepared by dropping acetone into an HSA aqueous solution with a pH of 7–9, then cross-linking with glutaraldehyde and purification by gel permeation chromatography. The study showed that as the pH value of the HSA solution increased, the particle size decreased, which was clear as the ionization of HSA increased, and the repulsion of HSA molecules occurred during particle formation. HSA nanoparticles with sizes between 90 and 250 nm were obtained through the control of the pH and the addition of acetone. Merodio et al43 added ganciclovir and a cross-linking agent in an albumin aqueous solution. Then, the pH of the solution was adjusted to the isoelectric point of the protein. The aqueous phase is washed with ethanol (Vwater/Vethanol=1:2) to obtain albumin nanoparticles. The main drawback of this method was that the pH value was adjusted under salt-free conditions, while the glass electrode had limited reliability, especially in high protein concentrations.
Thermal Gelation
In brief, protein conformational changes and unfolding can be induced by heating the albumin solution, subsequent protein–protein interactions (hydrogen bonding, electrostatic and hydrophobic interactions, and disulfide sulfhydryl exchange reactions) and aggregation of albumin particles. This method avoids the potential toxicity caused by adding organic solvent. The properties of the obtained nanoparticles depend on the process conditions, such as pH, protein concentration, and ionic strength.9 However, this method is not suitable for heat-sensitive drugs. Figure 3E shows the preparation process. Li et al59 proposed a novel self-assembly method through thermal-driven to prepare BSA-NPs. BSA and vanillin (non-toxic, as a crosslinking agent) were dissolved in deionized water at 37 °C. Then, the solution was incubated at 70 °C for 2 hours to form BSA-NPs. During the heating process, a large number of covalent bonds were formed, such as amide and disulfide bonds between BSA molecules, which greatly improved the stability of nanoparticles.
Nano Spray Drying
Spray drying is a mature method of producing dry powder from liquid phases commonly used in the pharmaceutical industry with the advantage of integrated particle formation and drying.60,61 In this method, particle formation occurs in a continuous, single-step process. Moreover, through the simple operation of process parameters or configuration changes, the ideal particle properties, such as particle size, flow property, and bulk density, can be adjusted. Compared with liquid formulations, the solid products through this method have the advantages of physicochemical properties and stability.62,63 Therefore, nano spray drying may be a general and commercially feasible technology for preparing peptide and protein drugs. A conventional spray drying process includes the following steps: Liquid raw materials are atomized into small spray droplets, and then contact with hot dry gas at high temperature to evaporate water. When water evaporates from a droplet, it forms a solid product and recovers the powder from the dry gas.64,65 However, albumin is prone to deformation and inactivation.66 The preparation process is shown in Figure 3F.
However, due to the product loss in the drying chamber wall and the separation of fine particles by the cyclone separator (<2 μm), the capacity of spray drying is low, and the yield of traditional spray drying on a laboratory scale is not optimal (20–70%). In order to improve the technology and expand the application range, B ü chi (Switzerland Labortechnik AG) has developed the fourth laboratory-scale spray dryer B-90 (following previous generations).67 This equipment is suitable for producing fine particles (300 nm – 5 μm) at a satisfactory, even a small amount of sample (milligrams).68 The latest development in technology and the successful launch of the nano spray dryer B–90 have produced submicron spray drying particles.69 Although the nano spray dryer B–90 has significant advantages, the mini spray dryer B–290 with dual fluid nozzles can more directly expand the process to the initial pilot stage, and then to the industrial production stage. The feed flow rate is 1.41 kg/h, much higher than the previous 0.60 kg/h, which greatly improves production efficiency.
Self-Assembly
Self-assembly technology (Figure 3G) increases the hydrophobicity of albumin molecules through certain methods, such as the reduction of disulfide bonds within protein molecules by the addition of denaturants (β-mercaptoethanol, dithiothreitol and cysteine),27 and the reduction of primary amine groups on protein surfaces by the addition of lipophilic drugs.70 The drug molecules can combine with the hydrophobic region of albumin under stirring conditions, thus inducing the formation of albumin nanoparticles. Self-assembly technology mainly forms a clear and stable structure through non-covalent interaction between molecules. Nanoparticles have the characteristic of small particle size and good flexibility due to the solidification of nano micelles. However, this method is only used for lipophilic drugs and has difficulties in scaling up.71 In addition, the addition of reducing agents has incidences of potential toxicity. In the self-assembly method, glutathione (GSH) can be used as a reducing agent to prepare a redox-sensitive albumin nanoparticle. GSH disrupts disulfide bonds in albumin molecules, exposing hydrophobic cavities and binding with hydrophobic drugs, which then re-oxidize to form disulfide bonds. This nanoparticle can rapidly release drugs in tumor tissue, as tumor tissue result in higher levels of GSH compared to normal tissue.72,73 Safavi et al74 reported a strategy for preparing BSA nanoparticles loaded curcumin (CCM-BSA-NPs) by self-assembly with high ionic strength of buffer solution instead of reducing agents at room temperature. Compared to CCM-BSA-NPs with DTT, the CCM-BSA-NPs had better antioxidant and more effective biological activity. Their group also synthesized piperine-loaded HSA-NPs (PIP-HSA-NPs) with self-assembly and desolvation methods, respectively. The results indicated that the self-assembled nanoparticles had significantly higher drug encapsulation efficiency and drug loading efficiency than the particles obtained by the desolvation method. And the self-assembly method maintained the secondary structure of HSA. Besides, the NPs by self-assembly method exhibited more cumulative drug release, making them have better therapeutic effects on tumor cells.71
Microfluidic Technology
Recently, studies have shown that microfluidic systems can accelerate clinical translation of nanoparticles due to their ability to generate nanoparticles in a well-controlled and reproducible manner.75 The microfluidic device can produce nanoparticles from milliliters or even several liters by viscously mixing nanofluids. Compared to the traditional preparation methods, the liquid is in the laminar flow state in the microfluidic chip, and the flow rate of the liquid is consistent, with a high mixing efficiency. Therefore, the size distribution of nanoparticles is uniform, with good repeatability and high efficiency.76 The preparation process is shown in Figure 3H, where the aqueous phase and the organic phase flow into the mixture from multiple inlet ports on the chip. Samples are collected within specified time intervals. Nanoparticles with different requirements were prepared by adjusting the flow rate and the shape of the chip. For example, Sun et al77 prepared cabazitaxel-HSA NPs with an inverted W-type microfluidic chip through microfluidic technology without any cross-linking agent and toxic solvents. The nanoparticle showed a higher efficiency and higher drug loading than that prepared by the traditional preparation.
Albumin Nanoparticles Modification
Organic Albumin Nanoparticles
Albumin is typically negatively charged at a pH of 7 (PI=4.7),78–80 which makes it carry different types of chemotherapy drugs. Although albumin nanoparticles reduce drug side effects and improve the curative effect, because of the antibody targeting or protein adsorption in plasma, easily results in the mononuclear macrophage system identification and consumption, thus, the nanoparticles are cleared in the systemic circulation.81,82 In this section, we briefly introduce albumin-based organic nanoparticles for efficient delivery. The high content of carboxyl and amine groups and different binding sites offer a great possibility that albumin nanoparticles can be easily coated with different polymers or ligands, through covalent coupling to change surface properties (such as PEG modification to improve the hydrophilic, avoid the macrophage phagocytosis), binding with ligands (such as folic acid modification of tumor cells with rich folate receptors targeting, immune antibody interacts with antigen (antibody-mediated system)), and other measures to realize the active target of drugs.
Non-Targeted Modification
Circulating albumin nanoparticles can passively accumulate in tumor tissue through enhanced penetration and retention (EPR). In addition, the properties of nanoparticles can be controlled by changing particle size, surface coating, and binding with ligands to achieve tissue-specific targeting. These approaches enable the capacity of HSA NPs avoiding the uptake of macrophages mainly located in the reticuloendothelial system (RES) of the liver.83 Table 1 shows the examples of albumin nanoparticles modified with non-targeted modification polymers.84–99
 |
Table 1 The Examples of Albumin Nanoparticles Modified with Non-Targeted Modification Polymers |
Polyethylene Glycol (PEG)
Since the albumin nanoparticles are cleared in a few seconds or several minutes after intravenous administration, they cannot be applied to tumor therapy. PEG is a hydrophilic, non-toxic, non-immunogenic Polymer material, and has good biocompatibility.100,101 It can be covalently linked to albumin to modify nanoparticles. Studies have shown that albumin nanoparticles coated with PEG could reduce the recognition of RES, resulting in a prolonged plasma-circulating half-life.102 Then, they can passively penetrate malignant tissue, where they are retained and accumulated to improve therapeutic efficacy. In addition, PEG also can be used as a linker to connect albumin and ligands, making it targeted.103,104
Fahrländer et al84 developed several PEGylated HSA NPs and the PEGylation extent was characterized and quantified. Actually, this study aims to develop a quantitative PEG method and monitor the effect of different PEG reagents on the amount of PEG attached to the surface of HSA NPs. PEGylation is a potential way to improve the blood circulation time of nanoparticles. Results confirmed that modifying HSA NPs covalently with a suitable PEG derivative could enhance plasma circulation in vivo. In Sharma’s study, 5-FU conjugated PEG-HSA-NPs were developed to improve the pharmacokinetic of 5-FU and therapeutic profiles. The results indicated that the IC50 value of PEG-modified nanoparticles was significantly lower than that of unmodified nanoparticles, and the half-life was significantly prolonged.105
Chitosan
Chitosan is a cationic, alkaline polysaccharide polymer, which has unique biological activity. It has excellent biocompatibility and biodegradability, less toxic, meantime low prices.106 The deacetylation levels and molecular weight of chitosan have a certain impact on its toxicity. Lower toxicity is related to less molecular weight.107 Chitosan-coated albumin nanoparticles have been studied for delivery of DNA,108 the antitumor drug,90 the nose to brain drug,109 and the ocular drug.88 The surface charge of nanoparticles has a significant impact on its interaction and absorption with the cell membrane.107 Positively charged nanoparticles demonstrate an enhanced internalization on account of the ionic interactions with the negative-charged cytomembrane, and then they are able to escape from lysosomes. So, positive-charged nanoparticles exhibit perinuclear localization, whereas the negatively charged and neutral nanoparticles prefer to colocalize with lysosomes.110,111 Modified by chitosan coating, albumin nanoparticles can shift the original negative charges to positive charges, which exhibits the potential for drug delivery.112 The use of water-soluble chitosan modification can effectively improve the surface hydrophilicity of nanoparticles and adjust the surface charge to near neutrality, thus avoiding the adsorption of plasma protein and macrophage phagocytosis, and extending the cycle time of the nanoparticles in the blood.113,114 However, excessive positive charge brings greater toxicity. In Esfandyari-Manesha’s studies, chitosan-coated HSA-NPs showed more cytotoxic effects than the negatively charged HSA-NPs.112
Polyethylenimine (PEI)
PEI is a cationic polymer, which can provide positive charges to the albumin nanoparticles’ surface to facilitate the transport of negatively charged small molecules as well as facilitate transport across the Blood-Brain Barrier (BBB).96,115
Mohammad-Beigi et al78 developed polyethyleneimine-coated HSA (PEI-HSA) NPs applied to different neurodegenerative disorders. The aggregation of the 140-residue protein α-synuclein (αSN) shows an important role in the pathogenesis of these diseases. However, αSN could interact strongly with PEI-HSA NPs, resulting in significant changes in the secondary structure of αSN. These findings emphasized the potential role of PEI-HSA NPs in reducing the pathogenicity of αSN in vivo. In 2016, the group prepared cationic PEI-HSA NPs loaded with the antioxidant gallic acid (GA) through adsorption which could inhibit αSN aggregation. They concluded that PEI-HSA-GA NP is a potential delivery system for transporting GA to the brain.116
PEI is also an effective carrier for nucleic acid delivery.117 Rahimi et al92 developed PEI-coated BSA NPs (BSA-PEI NPs) for efficient delivery of CRISPR/Cas9 system in both ribonucleoprotein and DNA (px458 plasmid) forms. Through electrostatic interactions, PEI with a positive charge binds to the surface of BSA and increases cellular uptake.
Polycation/Polyanion Deposition
In cancer-targeting therapy, layer-by-layer nanoparticles (LbL-NPs) displayed their growing important positions, which were prepared by depositing polycation and polyanion on the surface of colloids.118 LbL NPs offer unique benefits, including improved stability of nanocarrier in circulation, controlled release of drug, and storage stability. The electrostatic effect is the main driving force for multi-layer assembly, and other forces include hydrophobic interaction, hydrogen bond, covalent bond and so on. Ruttala et al97 reported that polyarginine and poly(ethylene glycol)-block-poly (L-aspartic acid) deposit alternately onto an albumin conjugate for the formation of the LbL-based nanoparticle albumin-bound PTX (nab-PTX). The colloidal stability was improved because of the presence of polyamino acids, which prevented the dissociation of nab-PTX even in the case of high dilution.
Targeted Modification
In addition to passive targeting, the active targeting of nanoparticles further improves the therapeutic effect of tumors. Tumor cells differ from normal cells in their biology and physiology and generally express specific markers on the cell surfaces that can be exploited for drug-targeting approaches. The clinical treatment of the tumor is not ideal; the main reason is that anti-tumor drugs produce a variety of serious adverse reactions. Receptor-mediated targeting agents are therefore developed to deliver the drug to tumor cells specifically, reduce damage to normal cells, and reduce side effects.119 Active targeting can be achieved by a specific modification (Figure 4). The ligand modified on the surface of the nanoparticle has specific targeting and high affinity, which leads to specific targeting of the tumor cells.120,121 Table 2 shows the examples of albumin nanoparticles modified with targeted ligands.122–141
 |
Table 2 The Examples of Albumin Nanoparticles Modified with Targeted Ligands |
Hyaluronic Acid (HA)
Surface antigen differentiation group 44 (CD44) is a widely distributed glycoprotein on the cell surface.142,143 As one of the important receptors of hyaluronic acid, CD44 is overexpressed on the surface of malignant cells, it therefore can be used as an active target by HA and CD44 receptor, mediating tumor-targeted therapy.144 The active amino group on the surface of albumin nanoparticles can be coupled with hyaluronic acid to achieve the targeting modification.
Huang et al145 prepared HA-coated FITC-Cx43 MP-HSA NPs, while Connexins (Cx) are transmembrane proteins and Cx43 mimetic peptide (Cx43 MP) has the effect to reduce inflammation, edema, and vascular leakage. Experimental results showed that HA-coated HSA NPs led to higher cellular uptake in vitro and enhanced in vivo tissue penetration through ligand–receptor interactions between CD44 receptors and HA, as compared to uncoated HSA NPs. In addition, HA-coated HSA NPs were also a sustained-release system with better cell biocompatibility, which reduced injection frequency in clinical practice.
Folic Acid (FA)
Elevated expression of the folate receptor (FR) was found in tumor cells.146 Based on the research, 97% of investigated ovarian carcinomas overexpressed FR. Hence, FA becomes an attractive targeting molecule besides its small molecular weight (Mw441.4 g/mol), low immunogenicity, as well as its good biological compatibility. Albumin nanoparticles decorated by FA can activate targeting to tumor cells, which helps to reduce drug side effects, and improve therapeutic effect.147–149 Usually, EDC and N-hydroxy succinimide (NHS) are used to activate the carboxyl group of folic acid and prepare an active ester intermediate (FA-NHS), which can couple with albumin.
Chen et al150 prepared a FA-conjugated HSA magnetic nanoparticle (FA-CDDP/HSA MNP). Characterization indicates that FA-CDDP/HSA MNPs could be prepared successfully with an average size of 79 nm. Wang et al151 developed FA-decorated HSA loaded with nanohydroxycamptothecin (nHCPT) nanoparticles (FA-HSA-nHCPT-NPs), and determined the antitumor activity of FA-HSA-nHCPT-NPs adopting the MCF-7 cells with FR overexpressed on the surface. In the meantime, BALB/c mice were inoculated with human MCF-7 cells in situ and ectopic, respectively. FA-HSA-nHCPT-NPs showed more effective antitumor activity than that of raw HCPT. Meanwhile, the concentration of HCPT in tissue distribution analysis was much higher in the mice treated with FA-HSA-nHCPT-NPs. The group of FA-HSA-nHCPT-NPs also showed a higher tumor inhibitory rate than the raw HCPT. In conclusion, FA-HSA-nHCPT NPs can serve as a feasible delivery system with significant targeting effects on tumors. The examples above indicate that HAS-NPs can play a different role by modifying different polymer materials in cancer treatment. Based on previous research,152 our group developed FR-targeted HSA NPs of cabazitaxel (FA-NPs-CTX) by a self-assembly method. FA-NPs CTX did not affect the drug release of NPs and has good biocompatibility and physicochemical properties, such as sustained release and colloidal stability. Compared with FA-unmodified NPs-CTX, FA-NPs-CTX showed a greater inhibitory effect on tumor cells overexpressing FR in the cytotoxicity experiments.153
Transferrin (Tf)
Endocytosis mediated by membrane Tf receptors (TfR) is an effective cellular uptake pathway during the administration of anticancer drugs. The TfR is overexpressed on a great many tumor cells,154 so that Tf and the antibodies against the TfR have been widely studied. Due to the presence of TfR in the BBB, Tf-coupled solid lipid nanoparticles can enhance drug delivery to the brain.155 The mechanism by which nanoparticles bound to Tf enhance therapeutic efficacy seems to be that cells have a higher absorption of drugs.133,156
Ulbrich et al133 prepared the Tf-coupled HSA NPs and the TfR-antibody-coupled HSA NPs. NHS-PEG-MAL was used as a cross-linking agent to couple transferrin or TfR-mAb to connect HSA-NPs. Loperamide-loaded HSA NPs coupled with transferrin or TfR-mAb had a strong anti-nociceptive effect, while IgG2a-modified HSA NPs could not transport the drug through the BBB. Therefore, these TfR mAb or Tf-mediated nanoparticles are effective carriers for drugs to cross the BBB.
Monoclonal Antibodies (mAb)
Tumor-specific ligands can be coupled to the surface of nano-carrier systems to achieve targeted drug delivery.157 Among them, mAbs have great potential. Hasan et al developed a targeted delivery system of anti-human epidermal growth factor receptor 2 (anti-HER2) mAbs using polyethylene glycol albumin nanoparticles for breast cancer cells. A new mAb (1F2) targeting the HER2 extracellular domain connected to the surface of albumin nanoparticles was proved to have the highest cell uptake rate.158
Wagner et al136 covalently coupled DI17E6 to the surface of HSA NPs loaded with doxorubicin. DI17E6 is a humanized and de-immunized mAb against av integrin, which can inhibit the growth of melanoma in vitro and in vivo, and can inhibit angiogenesis by interfering with avb3 integrin. They demonstrated that compared to free doxorubicin, targeted HSA NPs loaded with the cell-inhibitory drug doxorubicin showed increased cytotoxic activity. In addition, the modification of DI17E6 also improved the therapeutic effect of the nanoparticles.
Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)
TRAIL belongs to the Tumor necrosis factor (TNF) receptor superfamily, which is a type II membrane protein.159,160 In humans, death receptors (DR4 or DR5) overexpress on neoplastic cells, while healthy normal cells do not significantly express death receptors.161 Therefore, TRAIL is considered a safe and selective anticancer and apoptotic drug with minimal toxicity to normal cells. The combination of chemotherapy drugs and TRAIL is a method for treating cancer. Due to the positive charge of TRAIL, it usually combines with HSA through electrostatic adsorption, as HSA is negatively charged under neutral pH conditions.139
Min et al139 developed a formulation of PTX-bound HSA NPs with embedded TRAIL (TRAIL/PTX HSA-NP) to treat pancreatic cancer. They investigated a series of characteristics of the nanoparticles, including surface morphologies, particle sizes, zeta potentials, loading efficiency, cytotoxicity, and apoptotic activity. Moreover, TRAIL-coupled nanoparticles showed stronger anti-tumor activity. Choi et al141 designed and prepared HSA-NPs with surface modification with TRAIL, meanwhile containing doxorubicin. The results showed that the nanoparticles had significant cytotoxicity in most types of tumor cells with general or drug-resistant characteristics, especially on CAPAN-1 cells, which are human pancreatic cancer cells. These studies have proved that it is a useful targeting agent that can affect tumor cells in various tissues and organs.141 Recently, the regulation of TRAIL-mediated exogenous apoptosis pathways in vitro through nanoparticles has also been reported as a strategy for cancer treatment. In Ahmed’s162 studies, F. cretica methanolic extracts based albumin nanoparticles, liposomes and silver nanoparticles were prepared. F. cretica albumin and silver nanoparticles upregulated the in vitro TRAIL, DR4, DR5, and FADD gene expression in Hep-2 cell lines and exhibited high anticancer activity.
Metal and Inorganic Albumin Nanoparticles
Metal and inorganic nanoparticles provide intrinsic advantages to biomedical fields due to their unique physical properties, controllable structure, and diverse surface chemistry. Metal nanoparticles are wildly applied to inhibit bacterial tolerance growth with expansion of the range of antimicrobial activity163 and antitumor treatment which can combine with radio- and chemotherapy.164 However, the low hydrophilicity of metal nanoparticles can lead to aggregation and their therapeutic potential remains to be justified. Inorganic nanoparticles can be divided into metal-based and non-carbon sources. Their physiochemical properties and released metal ions/trace elements contribute to various therapeutics without acute toxicity. Functionalized inorganic albumin nanoparticles can be generated with inorganic NPs through chemical conjugation, coating, or biomineralization. The coupling of albumin and inorganic nanoparticles makes full use of the benefits of albumin (high hydrophilicity, large amount of binding sites, and performance as a reduction reagent) in photothermal therapy and immunotherapy.165–167 In this section, we outline the main metal albumin NPs (gold, silver, copper) and main inorganic albumin nanoparticles (magnetic albumin and albumin-template nanoparticles). We further elaborate on their applications in drug delivery and biomedical treatment (Figure 5A).
 |
Figure 5 (A) Illustration of albumin metal nanoparticles fabrication and application; (B) Docking studies of C1–C3 and HSA using Autodock. a) The overall structure of the HSA complex; b) C1–C3 located within the hydrophobic pocket in subdomain IIA of HSA; c) the interaction mode between C1–C3 (showing stick representation) and HSA (cartoon form).168 (License Number: 5778641204720. Reprinted from Journal of Inorganic Biochemistry, 153, Gou Y, Zhang Z, J. Q, et al. Folate-functionalized human serum albumin carrier for anticancer copper(II) complexes derived from natural plumbagin, 13–22. Copyright 2015 with permission from Elsevier.168 (C) a) Synthetic process and therapeutic mechanism of BSA-IrO2 NPs. b) TEM image and photograph (inset) of BSA-IrO2 NPs. c) HAADF-STEM image and EDX elemental mapping. d) UV/Vis absorption spectrum of BSA-IrO2 NPs in aqueous solution. (Reprinted with permission from Zhen W, Liu Y, Lin L, et al. BSA-IrO(2): catalase-like nanoparticles with high photothermal conversion efficiency and a high X-ray absorption coefficient for anti-inflammation and antitumor theranostics. Angew Chem Int Ed Engl. 2018;57(32):10309–10313.© 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.166 Abbreviations: FRET, fluorescence resonance energy transfer; PTT, Photothermal therapy; MRI, magnetic resonance imaging; BSA, bovine serum albumin; PDT, Photodynamic Therapy; PAT, Photoacoustic tomography; CT, Computed Tomography; CAT-like, Catalase-like; ROS, reactive oxygen species. |
Silver Albumin Nanoparticles
Among metal nanoparticles, silver nanoparticles (AgNPs) are widely used due to their excellent antimicrobial and photoelectron behavior.169–172 Unmodified AgNPs will form aggregates due to their poor stability in aqueous media. In Korolev’s study,173 AgNPs were modified by the addition of either HSA or Tween-80 (Polysorbate-80) to increase the stability of AgNPs in aqueous suspensions. AgNPs with Tween-80 showed significant hemolysis incubation, while non-modified and albumin-coated AgNPs had minimal hemolytic activity. The cytotoxicity of the albumin-coated AgNPs on human adipose-tissue-derived mesenchymal stem cells was lower than that of the native and Tween-80-covered AgNPs. Meanwhile, albumin-coated AgNPs and non-modified AgNPs were equally effective in terms of bactericidal activity against pathogens. The targeting abilities, cellular uptake, and toxicity of nanoparticles can be influenced by a protein coating,174 which provides a new strategy for drug delivery. In earlier research, the protein coating may decrease the toxicity and cellular uptake of AgNPs.175 Recently, Park et al176 studied the role of protein coatings on the toxicity of AgNPs to liver hepatocellular carcinoma (HepG2) cells. The protein on the surface forms a protective coating that inhibits AgNPs dissolution. However, HSA-coated AgNPs are more toxic to HepG2 cells than uncoated AgNPs, and that the mechanism of toxicity cannot be simply explained by AgNP dissolution. The observed differences in toxicity reactions may be caused by various factors, including the size and concentration of nanoparticles, different cell types, exposure time, and the selection of toxicity measurements.
There are also scholars using silver nanoparticles of quantum dot immunoassay on HSA competitive fluorescence immunoassay. If the antibody-loaded CdSe quantum dots are aggregated with HSA-coated silver nanoparticles, fluorescence resonance energy transfer (FRET) will be caused by the distance between the two nanoparticles being reduced enough.177 In this case, the Ab-QD fluorescence detector is overcome. However, if HSA is added to Ab-QD, their surfaces will be blocked, and they will no longer be polymerized with HSA-AgNPs. The decrease in fluorescence intensity (peak 570 nm) was negatively correlated with HSA concentration in the sample. HSA concentration in the range of 30–600 ng/mL can be determined with this method. The data show that anisotropic silver nanoparticles and quantum dots as energy donors can be successfully applied to various competitive immunoassays for sensitive detection of any analyte based on resonance energy transfer.
Gold Albumin Nanoparticles
Gold and silver nanoparticles are hotspots in chemistry, condensed matter physics, nanomaterials, and clinical immunology research in recent years.178–181 The AuNP albumin system could be used in various biomedical areas, including photo-thermal therapy, drug delivery, diagnostics, theranostics and immunoassays.182 AuNPs could be capped with albumin molecules or encapsulated into albumin nano capsules to deliver drugs to target sites.183 Mocan et al184 used BSA bound to gold nanoparticles (GNPs) as active vectors to target liver cells with laser treatment. Compared with GNPs, BSA-GNPs showed their high affinity to liver cancer cells. Murawala et al185 provided methotrexate (MTX) loaded Au-BSA NPs, which were extremely stable under strong electrolyte and pH conditions. And Au-BSA NPs showed better proficiency in inhibiting human breast cancer cells (MCF-7) compared with free MTX. Chen et al186 designed albumin-based cisplatin-conjugated AuNPs with radiation therapy (RT), in which Au can increase RT-induced immunogenic cell death and potentiate the abscopal antitumor immunity. Compared with cisplatin, albumin AuNPs loaded cisplatin showed reduced side effects which can be safely administered concurrently with ablative RT.
Copper Albumin Nanoparticles
The interaction of metal compounds with HSA applied in anti-cancer research is increasing. According to the research, HSA can increase the solubility of the metal compound copper(II), and enhance its anti-tumor effect187,188 Fluorescence spectra and molecular docking show that the Cu(II) complex binds to the IIA subdomain of HSA (Figure 5B).168 Cu(II) compounds produce intracellular reactive oxygen species (ROS) in tumor cells, and the complexes of HSA and copper(II) compounds are, to some extent, able to selectivity accumulate in cancer cells compared to the individual Cu(II) complex, but do not improve the normal cell cytotoxicity levels in vitro. Gou et al189 prepared a component by the reaction of CuCl2/ Cu(NO3)2/CuBr2 with HL to obtain a novel phenoxy-bridged binuclear copper(II) compound C3. (C3) and mononuclear copper compounds (C1 and C2) showed stronger anticancer effects. The HSA nano carrier functionalized with folic acid (FA) was combined with Cu(II) to study its anti-cancer properties and mechanisms. FA-HSA-metal drug complexes exhibited high cytotoxicity to tumor cells with high expression of FA to a certain extent and showed the inhibition of the activity of proliferative Bcl-2 family proteins and CDK1/cyclin B1.
Jiang et al190 developed a Cu(II) reagent based on the specific residue of HSA NPs, which was used for multitargeted tumor microenvironment (TME) to inhibit cisplatin resistance. The structure of the HSA-Cu complex indicated that two Cu compound (C4) molecules bind and hide in the hydrophobic cavities of the IB and IIA subdomains of HSA, forming a stable HSA-C4 complex that effectively protected C4 from damage by endogenous ions/macromolecules/compounds. In addition, HSA NPs could serve as an effective carrier for C4 and transport them to tumor tissue.
Magnetic Iron Oxide Albumin Nanoparticles
Magnetic nanoparticles, especially Fe3O4 nanoparticles, are widely studied nanomaterials with special magnetic properties, easily synthesized, and low toxicity.191–193 It has been reported that there is a great potential in biomedical, biological separation, DNA hybridization, and detection.194–197 However, the characteristic of being easily oxidized by pure Fe3O4 may affect magnetic properties. In order to overcome this problem, various coatings have been added to its surface, but due to their toxicity, resulting in low biocompatibility and high immunogenicity. BSA protein can be immobilized on the surface of magnetic nanoparticles by physical adsorption or covalent binding. Among them, covalent conjugation can make the BSA coated magnetic nanoparticles more stability. He et al198 used BSA as a biosensor to protect Fe3O4 nanoparticles. In addition, iron oxide nanoparticles loaded with cytotoxic drugs were injected intravenously into blood vessels, and accumulated in the tumor tissue through a gradient magnetic field, the drug bioavailability effectively improved and side effects reduced, respectively, in MDT. Zaloga et al199 proposed a method for magnetic drug targeting with superparamagnetic iron oxide nanoparticles (SEON) coated with lauric acid (LA) and BSA (SEON LA-BSA). It is necessary to retain protein on the particle surface for drug adsorption by using anchoring molecules such as octanoate, which is a common stabilizer applied in clinically approved formulations. However, the protein was difficult to achieve the current good manufacturing practice (cGMP) standard, because octanoate could block albumin-binding sites, reducing the binding efficiency of protein and lauric acid loaded superparamagnetic iron oxide nanoparticles. Thereby, Zaloga et al200 presented a new SEON LA-HSA formulation that used pure HSA and octanoate-stabilized HSA as excipients and compared it with SEON LA-BSA. As a result, the structure of the core-shell structure surrounding the iron oxide nanoparticles was confirmed by frozen fracture transmission electron microscopy. Through the characterization of reproducibility of the synthesis, chemical structure, biocompatibility, cellular uptake, stability of the drug adsorption, and kinetics of drug release, SEON LA-HSA demonstrated feasibility for local drug delivery. In order to further investigate the adsorption and desorption mechanisms of drugs on SEON LA−HSA, in 2018, Zaloga et al201 prepared Mitoxantrone loaded SEON LA-HSA. The results showed that most likely the drug is located on the outer organic shell through the electrostatic adsorption.
In another study, Ostroverkhov et al202 developed photosensitizer loading on HSA-coated magnetic nanoparticles (PS loading HSA-coated MNPs) which could use non-invasive magnetic resonance imaging (MRI) to track drug accumulation in dynamics. The PS loading HSA-coated MNPs provided up to 17% of injection dose/g to tumors, accurately tracking drug accumulation in malignant tumors, and could be used to adjust irradiation time to achieve the most significant tumor growth inhibition in photodynamic therapy (PDT).
Albumin-Template Inorganic Nanoparticles via Biomineralization
Chemical grafting has been the main strategy to bind protein/peptide to inorganic particles for decades, but harsh synthetic conditions ineluctably mask protein/peptide bioactivity. As an alternative, biomineralization is a mild way to produce hard materials with highly ordered structures. By combining protein/peptide (organic phase) and inorganic ions, inorganic nucleation is first determined via preassembled organic macromolecules, then the crystal grows under the control of molecular recognition at the organic/inorganic interface; thereafter, the mineralized subunits are regulated and assembled through organic molecules and different forces (eg, hydrogen bonding interaction, electrostatic interaction, polarity).203
Among all biomineralization proteins/peptides, albumin has been extensively employed as a biotemplate to collect inorganic albumin nanoparticles. The good performance of albumin as a biotemplate results from its biocompatibility and lack of immunogenicity, as well as its covalent binding and reducing capability (eg, 35 Cys residues and 21 Tyr resides in BSA). The general albumin biomineralization process includes first adsorption or conjugation of ions to albumin through the affinity of the free thiol group, carboxyl and amino groups, and then sequester ions to form albumin-coated metal oxide nanoclusters by increasing pH value with NaOH. Catalase-like BSA-IrO2 NPs were prepared using such biomineralization method, which is promising to overcome tumor hypoxia by cleaving H2O2 for O2 generation, and further protect normal tissues against H2O2-induced inflammatory cytokines (Figure 5C (a–d)).166 Another common example is inorganic BSA-MnO2 nanoparticles.204–206 Chen et al206 prepared multicomponent HSA-MnO2-Ce6&Pt nanoparticles for combined tumor photodynamic and chemotherapy, permitting stimuli-responses of nanoparticles to acidic and hypoxia tumor microenvironment, releasing small size albumin-drug complex for intratumoral permeabilization. These biomineralized inorganic albumin nanoparticles can further combine with other inorganic nanoparticles, for instance, mesoporous silica nanoparticles, to make full use of advantages of different materials.204,207 A similar strategy has also been seen in nanodots for in-vivo fluorescence/photoacoustic and photothermal imaging.165,208
Other Inorganic Albumin Nanoparticles
Other inorganic albumin nanoparticles are also popular but are not our focus in this review, considering their limited number of reports. For example, zinc oxide nanoparticles (ZnO NPs) attract considerable concern due to their unique UV filtration properties, antifungal, antibacterial, and photochemical activity.209 S Sudheer Khan et al analyzed the toxicity of ZnO NPs with or without BSA. The results indicated that BSA-coated ZnO NPs decreased the toxic effect and could be more amenable to applications in bioengineering and biotechnology.210
Albumin Nanoparticle-Based Hybrids
As we have shown, organic or inorganic functionalization on albumin nanoparticles can bring them multiple passive, targeting, or photothermal ability. In this section, instead of discussing physiochemical modification on albumin itself, we give a short review of the bottom-up manufacturing strategy to make albumin nanoparticles-based hybrids. This section is divided into first biomimetic albumin hybrids with cell membrane camouflage, and second albumin nanoparticle hybrid scaffold.
Cell Membrane Camouflaged Albumin Nanohybrids
Biomimetic albumin hybrid can be achieved by albumin/vaccine complex,211 albumin/living cell conjugation,212 or cell membrane camouflage. Compared with other two methods, cell membrane camouflage is easier for fabrication with higher availability. A variety of cell membranes, derived from different cell origins, including cancer cells,213 red blood cells (RGB),214 and stem cells,215 have been exploited for nanoparticle encapsulation. Surprisingly, few reports were described for cell membrane camouflaged albumin nanoparticles, which may result from the intrinsic properties in albumin of great biocompatibility and lack of immunity. However, introducing cell membranes to albumin nanoparticles contributes to albumin stability and targeting capacity towards tumor treatment.216 Cao et al217 described a macrophage membrane camouflaged albumin nanohybrids. In this study, they displayed that compared to albumin nanoparticles, improved tumor targetability, and enhanced cellular uptake efficiency are found in albumin nanohybrids. Additionally, the presence of macrophage membranes induced more specific accumulation at the tumor site and stronger antitumor efficacy. Wen et al218 prepared cRGD-modified RBC membranes to encapsulate gefitinib-loaded albumin nanoparticles. Such RBC membrane-camouflaged albumin nanohybrid demonstrated a prolonged drug release of less than 70% in 120 h, lower RAW 264.7 macrophages, and higher tumor cell uptake. The in vivo SPECT imaging showed that compared to only BSA nanoparticles, a significant increase of nanoparticle accumulation at the tumor site was found after cell membrane camouflage, illustrating a high targeting efficiency of cell membrane/albumin nanohybrid. As cell membrane isolation and modification techniques rapidly progress, it emerges as a promising strategy that employs cell membranes, exosomes, or secreted vesicles to prepare albumin-based nanohybrid for tumor targeting, inflammation treatment, and tissue repair.
Albumin Hybrid Scaffolds
Controlled local drug delivery is required for tissue engineering scaffold. The superior drug loading efficiency and biosecurity permit excellent performance of albumin nanoparticles in both systematic and local drug delivery. BSA/bone morphology protein type 2 (BMP-2) nanocomplex formulation is widely used to stabilize BMP-2.219,220 Liu et al219 conjugated BSA/BMP-2 nanoparticles to poly-l-lysine functionalized graphene oxide (GO-PLL/BMP-2) by electrostatic attraction, showing a prolonged BMP-2 release over 14 days and significantly increased alkaline phosphatase activity. Nano-in-nano engineering, in which drug-loaded albumin nanoparticles are embedded in electrospun nanofibers, emerges as a promising approach for efficient tissue repair.89,220,221 In this approach, polymers such as chitosan are often performed as a coating on the albumin surface for drug capping, with polymeric nanofiber used as another capping layer. In addition to nano-in-nano engineering, micro/nano engineering demonstrates successful applications. Recently, porous poly (l-lactic acid) (PLLA)/BSA micro/nano particles had been introduced to deliver recombinant human soluble TNF receptor type II (rhs TNFRII) for nucleus pulposus regeneration (Figure 6A–C).222 In this study, rhs TNFRII was loaded in BSA, with further chitosan coating, then the BSA nanoparticles were introduced into hollow PLLA microparticles for in-situ injection. Without an apparent burst release, the loading drug was released to around 79.85% at day 35. Histological staining suggested that more residual NPs were found in the micro/nano group, with a lower TNF-α expression. The authors also emphasized that this achievement was attributed to a proper microenvironment for drug delivery.
 |
Figure 6 (A) Fabrication and alkaline hydrolysis of PLLA microspheres. (B) Preparation and grafting of BNP loaded with rhsTNFRII. (C) The transplantation of MS-BNP.Reprinted with permission from Y. X, Gu Y, Cai F, et al. Metabolism balance regulation via antagonist-functionalized injectable microsphere for nucleus pulposus regeneration. Adv Funct Mater. 2020;30(52):2006333. © 2020 Wiley-VCH GmbH.222 Abbreviations: PLLA, poly(l-lactic acid); W/O, oil-in-water; PVA, Polyvinyl alcohol; BSA, bovine serum albumin; BNP, bovine serum albumin nanoparticles; MS-BNP, antagonist-functionalized injectable porous microspheres; Rhs TNF RII, recombinant human soluble tumor necrosis factor (TNF) receptor type II; TNF- α, tumor necrosis factor α. |
Albumin Nanoparticle Application
Anti-Tumor Therapy
In some studies, the long circulating half-life of ~19 days of albumin is associated with Megalin Cubilin receptor-mediated renal rescue223 and its binding to endothelial and epithelial cell cycle neovascularized Fc receptors (FcRn).224 HSA level is maintained by FcRn through the intracellular sorting mechanism that protects from lysosomal degradation:225 HSA is internalized into cells through pinocytosis and then combines with FcRn at low pH within the acidic endosome, recycles to the extracellular chambers and dissociates at a physiological pH, which avoids degradation in the lysosome.226 However, other non-receptor bond plasma components are degraded (Figure 7).
 |
Figure 7 Recycling model of HSA through binding FcRn receptors. Abbreviations: FcRn, the neonatal Fc receptor; HSA, human serum albumin. |
The enhanced tumor accumulation of HSA NP is due to the EPR effect passively mediated uptake enhancement. In brief, the extensive angiogenesis of tumor tissue can lead to leakage of the vascular system, and larger molecules can enter the tumor stroma through the vascular system. Another pathway is to rely on active receptor transport. The most well-known albumin receptor, gp60, a 60 kDa glycoprotein (albondin), which mediates the endocytosis of natural HSA in endothelial cells, is widely expressed and involved in vascular endothelial cells except for the brain. Albumin combined with gp60 causes internalization and transcytosis: Albumin binds gp60 receptors and activates caveolin-1 and cell phagocytosis through endothelial cells.74,227–229 Besides, gp18 and 30 can be found in a wide variety of cells, such as macrophages, fibroblasts, and brain cells. However, gp18 and gp30 do not preferentially bind to native albumin and only have affinity for modified albumin. The entered albumin drug complex binds to the overexpressed secretory acidic cysteine-rich protein (SPARC) in tumor cells, which promotes drug entry into the tumor cells.229 However, few clinical studies have suggested that the concentration of SPARC in tumor tissue has no significant impact on treatment efficacy.230 Figure 8 shows the HSA-NPs delivery strategy.
 |
Figure 8 HSA-NPs delivery strategy. Abbreviations: HSA-NPs, human serum albumin nanoparticles; gp60, glycoprotein 60; SPARC, secretory acidic cysteine-rich protein. |
K-Ras is one of the driver genes for pancreatic cancer, and tumor cells with mutated K-Ras exhibit high levels of endocytosis. HSA is its main source of nutrition.231 Lu et al232 prepared BSA – polycaprolactone (PCL) nanoparticles loaded with albendazole (ABZ) for the treatment of pancreatic cancer by desolvation method, and in the cytotoxicity experiment, the IC50 value of BSA-PCL nanoparticles was 1.32 ± 0.35 μg/mL, and the IC50 value of free ABZ was 3.17 ± 0.36 μg/mL, which was about 2.4 times the concentration of BSA-PCL nanoparticles, indicating that the uptake of BSA-PCL nanoparticles by cancer cells was higher than that of free ABZ. In this literature, it is shown that the optimal particle size of albumin nanoparticles is between 100 and 200 nm, and it may be that the larger the particle size, the larger the area where the nanoparticles contact the cell membrane. Tumor-associated macrophages (TAMs) are the most abundant immune cells in the tumor microenvironment (TME), which promote tumor growth and metastasis. Therefore, eliminating TME can achieve anti-tumor efficacy.233 Previous studies have shown that palmitic acid (PA)-modified albumin can target the scavenger receptor-A of polarized macrophages such as TAM.234 Feng et al235 prepared palmitic acid-modified HSA NPs (PSA NPs) loaded with pirubicin (THP) to achieve double targeting of tumor cells and TAMs, and the inhibition rate of THP-PSA NPs on tumor volume in mice reached 81.0%, without causing damage to normal tissues. The mechanism of action is shown in Figure 9.
 |
Figure 9 THP-PSA NPs mechanism of action.235 (Reprint with permission from Feng J, Xiang L, Fang C, et al. Dual-targeting of tumor cells and tumor-associated macrophages by palmitic acid modified albumin nanoparticles for antitumor and antimetastasis therapy. ACS Appl Mater Interfaces. 2022;14(13):14887–14902. Copyright (2022) American Chemical Society.235 Abbreviations: THP-PSA NPs, palmitic acid-modified human serum albumin nanoparticles; M2-TAMs, M2-tumor-associated macrophages; IL-10, interleukin-10; TGF- β1, transforming growth factor-β1; IL-12p70, interleukin-12p70; TNF- α, tumor necrosis factor α; IFN- γ, Interferon γ; MDSCs, Myeloid-derived suppressor cells. |
Anti-Virus Therapy
In 2020, the rapid spread of severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) worldwide was associated with reduced albumin levels, cytokine levels, etc., and more recently, there have been reports of a complete recovery in patients with severe COVID-19 infused with immune serum rich in albumin, antibodies, and plasma proteins.236 The mechanism of action should be similar to that of convalescent plasma, ie, albumin prevents histone H4-mediated platelet activation and aggregation.237 Park et al238 proposed drug-loaded albumin nanoparticles as therapeutic agents to address the clinical outcomes observed in patients with severe SARS-CoV-2. Polyethylene glycolated nanoparticle albumin binding can alter the pharmacokinetic behavior of ginsenoside soap, such as reducing clearance and prolonging blood circulation time. Analysis of plasma samples from COVID-19 patients revealed decreased serum albumin levels, increased coagulation and NETosis associated with elevated histone H4, and severe cytokine storms and inflammation in blood and lung tissue. Therefore, albumin was chosen as a vehicle for ginsenoside delivery to simultaneously address blood clotting, NETosis, and cytokine storms. It is hypothesized that another effect of albumin is to inhibit platelet aggregation, pulmonary hemorrhage, and endothelial necrosis by binding to extracellular histones secreted by viral infections in patients with severe COVID-19. Steroid glycosides, found in ginsenosides, can inhibit severe inflammation and prevent death from hypotension due to septic shock in critically ill patients.239 HSA can combine a variety of endogenous and exogenous compounds, these properties can affect the distribution and efficacy of compounds in the body, HSA can store many compounds and can greatly increase the solubility of compounds.240 Li et al241 studied the interaction of ribavirin and lamivudine with HSA using fluorescence spectroscopy and X-ray crystallography, and the X-ray structure showed that ribavirin, lamivudine and lamivudine bind to the IIA subregion of HSA mainly through the formation of hydrogen bonds and hydrophobic interaction forces, which provided guidance for the subsequent albumin delivery system of ribavirin and lamivudine.
Anti-Inflammatory Therapy
Inflammatory diseases are caused by the adhesion of polymorphonuclear neutrophils to the lining of the circulatory system or vascular endothelium, and transmigration of the uncontrolled neutrophils.242 Therefore, it is a useful therapeutic strategy to target neutrophils through nanodrug delivery systems.243 Albumin nanoparticles are reported to be internalized by neutrophils and have effects on acute inflammation244 and chronic inflammation.245 BSA nanoparticles can internalize into neutrophils through Fcγ receptors on the surface of neutrophils. Recently, Liu et al44 prepared an RGD peptides-modified BSA NPs loading with Celastrol (CLT) (CBR NPs) using a desolvation method to induce apoptosis of circulating inflammatory neutrophils to treat rheumatoid arthritis, while CBR NPs could decrease the toxic side effects of CLT. The results showed that CBR NPs effectively inhibit the recruitment of inflammatory neutrophils in inflammatory joints. Besides, neutrophils could be like “a bus” to deliver therapeutic nanoparticles across the blood vessel barrier for inflammatory treatment. Zhu et al245 reported BSA NPs loaded with glucose oxidase (BSA-GOx-NPs) to treat endometriosis, which is a chronic inflammatory disease, by the neutrophil hitchhiking strategy. BSA-GOx-NPs internalized by neutrophils in vivo and neutrophils consistently enriched in ectopic lesions. GOx could be released to deplete glucose and induce apoptosis. The results provided a promising direction for the treatment of endometriosis.
Across BBB
BBB is a major challenge in brain cancer treatment, and the characteristics of brain microvascular endothelial cells are high expression of tight junction proteins and poor cross cell endocytosis. The BBB is not a static barrier, in fact, there is a large exchange of substances on the BBB through nutrient transporters,246 transporters in the brain barrier can be used for the delivery of brain drugs. Albumin is an important source of nutrition for the body, but it is excluded by the brain, due to the rapid growth of tumors and active metabolism, it is very thirsty for nutrients, in this case, the intake of albumin in tumor tissues will be greatly increased and used as an energy source. Albumin-binding proteins, such as SPARC and gp 60, are the main mechanisms by which tumors take up albumin,247 and SPARC is overexpressed in brain tumors. Lin et al248 provided a green method for the synthesis of BBB-penetrating albumin nanoparticles with the ability to co-encapsulate different drugs and does not require cross-linking agents (Figure 10). The hydrophobic drugs PTX and fenreta amine produce a synergistic effect to induce albumin self-assembly, forming double-loaded nanoparticles. The albumin nanoparticles were modified by the cell-penetrating peptide LMWP (low molecular weight protamine), which offers a promising pathway of biomimetic targeted administration of brain tumors in combination therapy. T807 is a novel tau positron emission tomography agent for Alzheimer’s disease that has a low molecular weight and can effectively cross the BBB. Hahn et al249 prepared erythrocyte membrane (ETm) coating and T807-modified HSA nanoparticles (T807-ETm/HSA NPs), red blood cell membrane coating can prolong the circulation time of nanoparticles in the blood, and T807 ligand can target the brain. In mouse in vivo imaging experiments, T807-ETm/HSA NPs had the largest distribution in the brain, so this vector can cross the BBB and be used to transport drugs to treat brain diseases.
 |
Figure 10 Schematic diagram of Albumin NP entering the BBB.248 (Reprint with permission from Lin T, Zhao P, Jiang Y, et al. Blood-brain-barrier-penetrating albumin nanoparticles for biomimetic drug delivery via albumin-binding protein pathways for antiglioma therapy. ACS Nano. 2016;10(11):9999–10012. Copyright (2016) American Chemical Society.248 Abbreviations: NP, nanoparticle; BBB, Blood-Brain-Barrier; LMWP, low molecular weight protamine; Gp60, glycoprotein 60; SPARC, secretory acidic cysteine-rich protein; PTX, paclitaxel; 4-HPR, fenreta amine. |
Lymph Node (LN) Transport
LNs are attractive therapeutic targets for treating various unmet clinical needs. However, due to the anatomical structure and nature of LNs, high concentrations of therapeutic agents in these tissues are difficult to achieve through the administration of free drugs or conventional formulations. Albumin-hitchhiking approach is attributed to the drug/vaccines targeting towards LNs and enhancing immunogenicity (Figure 11). Antigens-bond albumin is taken to the lymphatic vessels and then flowed into the LN until it has been processed to the surface of dendritic cells.250 Zhang et al251 designed a novel HSA nanoformulation loaded with an immunosuppressant tacrolimus (TAC) (TAC-HSA-NPs), which had high lymphatic targeting efficiency to further enhance the efficacy of lymphatic immunosuppression. Wherein, hydrophobic TAC triggers the self-assembly of albumin and enhances the adhesion between HSA molecules without crosslinking. TAC-HSA NP could target and drain LNs via cell surface Fc γ receptors and inhibit the proliferation of immune cells via cell surface Fc γ receptors, which was considered consistent with the reported targeted delivery of albumin nanoparticles to inflammation site infiltrated with adherent neutrophil.242 In order to further improve the efficiency of LN targeting, albumin nanoparticles coupled with antibodies that could recognize lymphocytes or LN vasculature have also been widely studied. Tumor-draining lymph nodes (tdLNs), including Tregs and MDSCs, are the first site of metastasis and abound with immunosuppressive factors, which play a crucial role in generating and regulating tumor-related immune responses.252 They experience antigen priming during lymphatic drainage of tumor-associated antigens (TAAs). Albumin nanoparticles modified with TAAs targeted tdLNs by lymphatic drainage as an anticancer vaccine will also be a field worth studying.
 |
Figure 11 Albumin-hitchhiking approach to target vaccines to the lymph node and enhance immunogenicity.250 Reprinted from Journal of Controlled Release, 327, Abdallah M, Mullertz OO, Styles IK, et al. Lymphatic targeting by albumin-hitchhiking: applications and optimisation, 117-128. Copyright 2020 with permission from Elsevier.250 |
Besides, sentinel lymph node (SLN) resection is one of the traditional methods for tumor treatments. SLNs are the first regional LNs that receive lymph flow from primary tumors. The detection of SLN is crucial for estimating tumor staging and treatment decisions. SLN localization has been used for the diagnosis of solid tumor metastasis. 99mTc-labeled Mannose-based HSA (MSA), which binds to a blue dye, has been reported as an SLN imaging agent by binding to macrophages in LNs.253 Indocyanine green loaded HSA (ICG-HSA) nanoparticles which radiolabeled with technetium-99 m (99mTc) were prepared to locate SLNs and inhibit tumor metastasis. The photothermal treatment of SLN enhanced the inhibitory effect on lung metastasis in mice and significantly prolonged their survival time.254
Immunotherapy combined with chemotherapy is one of the current options for cancer treatment. LNs play a crucial role in initiating the progression and metastasis of cancer.255 The therapeutic strategies for regulating the immune suppression microenvironment of LNs are receiving increasing attention. Various immune modulators have been developed to reshape the tumor immunosuppressive microenvironment and improve treatment effectiveness. However, currently, most immune modulators have limited accumulation in tumor sites or LNs after intravenous or oral administration, which hinders their clinical efficacy. Albumin nanoparticles show great potential in the delivery of immune modulators. Song et al256 developed an albumin nanoparticle to encapsulate PTX and PI3Kγ inhibitors which increased the drug delivery to macrophages in both tumors and LNs.
Regeneration
The Regeneration of complex structures after injury requires different processes, such as wound healing, cell death, dedifferentiation, and stem cell (or progenitor cell) proliferation. Tissue regeneration is related to the apoptotic program and inflammation.257 Research on model organisms has begun to characterize the source of regenerated cells, determine their efficacy, and determine the molecules required for supplementary events.258 Albumin nanoparticles have shown great potential for application in the delivery of regeneration promoters in regenerating bone tissue,259–261 wound healing,262–264 repairing spinal cord injury,265,266 and so on. Lin et al89 provided a nanofiber scaffold containing chitosan-stabilized BSA nanoparticles for controlling the delivery of dual drugs to create an osteogenic microenvironment for bone regeneration. The nanoparticles were prepared through desolvation with BSA as the nano carrier to maintain the bioactivity of drugs. Then, a chitosan stable layer was prepared by the electrostatic self-assembly method. The results indicated that the scaffold promoted the differentiation of osteoblasts, which could provide a new approach for designing functional biomaterials for bone tissue engineering that can be used in regenerative medicine. The basic fibroblast growth factor (bFGF) is one of the key components that promotes dermal cell proliferation and migration for wound healing. However, due to the rapid decomposition of bFGF in physiological microenvironments, its unstable nature greatly limits its clinical application. As shown in Figure 12, Son et al262 presented the bFGF-loaded HSA NPs (HSA-bFGF NPs) to improve their stability with a simple desolvation and crosslinking method. Due to the short half-life of bFGF, the strong cross-linking of bFGF and HSA to form nanoparticles is basically beneficial for the long-term supplementation of bioactive bFGF in tissue regeneration. The HSA-bFGF NPs retained the complete characteristics of the bFGF protein, especially improving tissue regeneration in terms of microstructure and functional recovery in vivo.
 |
Figure 12 Schematic illustrations depicting cellular delivery of bFGF, followed by wound healing cascades of HSA-bFGF NPs. Reprint with permission from Son B, Kim M, Won H, et al. Secured delivery of basic fibroblast growth factor using human serum albumin-based protein nanoparticles for enhanced wound healing and regeneration. J Nanobiotechnol. 2023;21(1):310. http://creativecommons.org/licenses/by/4.0/262. Abbreviations: bFGF, the basic fibroblast growth factor; HSA, human serum albumin; TGF- β, transforming growth factor-β; MEK, mitogen-activated protein kinase; PI3K, Phosphatidylinositol 3-kinase; ERK, extracellular regulated protein kinases; AKT, protein kinase B. |
Outlook
Albumin is an attractive protein for preparing nanoparticles with potential therapeutic applications. However, current reports indicate that various albumin-based nanocomplexes are usually synthesized by strategies such as electrostatic adsorption, chemical conjugation and biomineralization.267 These methods may lead to the presence of multiple components in the complexes, and there is a lack of comprehensive studies on the biocompatibility and toxicity of these complexes, which has seriously hindered the further development and widespread clinical application of albumin-based complexes. In response to these problems, measures could also be taken to focus on the reduction of impurities and more comprehensive toxicity testing of the complexes during the process of design and synthesis. The use of glutaraldehyde as a crosslinking agent or UV irradiation9 for crosslinking during the preparation of albumin nanoparticles can increase the potential toxicity of the obtained albumin nanoparticles. Therefore, some natural cross linking agents such as tannic acid, ascorbic acid, municipal acid, and glucose268 have been studied. Besides, for the drug-loaded albumin nanoparticles, physical instability and premature release during a long-term storage are still the challenges. Further, whether albumin nanoparticles can maintain uniform and stable particle size and high drug loading in large-scale production and the reproducibility are urgent issues that need to be considered in the clinical translation process. Careful design and formulation optimization are necessary. Albumin as a drug carrier also has the limitations, such as albumin variability, high cost, limited sources of HSA, and mild immune reactions when BSA is used for injection. Some studies have reported that proteins isolated from other species can also be used as drug delivery carriers. For example, soybean protein-based nanoparticles are considered as potential natural delivery carriers because of their structural characteristics. Gong et al269 enumerated a variety of preparation methods for soy protein-based nanoparticles and described the improvement of functional properties of bioactives after encapsulation. It provides a theoretical basis and reference for the subsequent preparation of novel soy protein nanoparticles. Besides, it offers a potential pathway for the application of soy proteins and natural bioactives in the food and pharmaceutical fields. Silk protein has been widely used as scaffolds for vascular, skin, bone, cartilage and neural tissues.270 Silk fibroin nanoparticles (SFN) have the advantages of simple preparation method, good biocompatibility, good degradation, and easy chemical modification. Lozano-Perez et al271 prepared SFN loaded with hydrophobic platinum precursor (PtBz), which successfully improved the delivery of insoluble PtBz and showed high selectivity for tumor cells, reducing the killing of normal cells.
Albumin has potential in delivering antibacterial, analgesic and antifungal drugs as well as active substances for obesity prevention. In future studies, more attention could be paid on chemical modifications of albumin, which could broaden the application of albumin nanoparticles. Besides, albumin nanoparticles can passively target tumor sites through the EPR effect; however, albumin is also an essential protein for tumor maintenance and growth. Therefore, how the administration of albumin formulations affects the development of tumors and the changes in the immune environment of albumin carriers after treatment of tumors, as well as the effects of subsequent tumor metastasis and recurrence are the interesting studies.272 The field of nanomedicine is attracting more and more attention as it offers efficient and smart therapeutic options for the treatment of cancer, inflammatory and other diseases. In this field, albumin nanoparticles are attracting attention due to their high affinity for hydrophobic drugs, surface modification possibilities and high loading capacity, which allows us to overcome the formidable hurdles posed by many of the compounds currently on the market.9 Although there is a lot of research on nanotechnology, some synthetic polymers, metal nanoparticles, etc. are limited in clinical applications due to their own toxicity. With the continuous development of the biomedical field and the increase of application demand, the market potential of albumin nanoparticles will be further released, and it is expected to become an area with greater market potential and commercial application prospects. Especially with the rise of personalized medicine and precision therapy, the targeted delivery advantage of albumin nanoparticles will be more valued. We expect albumin nanoparticles to be promising drug delivery systems in the clinical development of disease.
Acknowledgments
This research was supported by the Doctoral Start-up Foundation of Liaoning Province (CN) [Grant Number 2021-BS-083].
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Bharali DJ, Khalil M, Gurbuz M, Simone TM, Mousa SA. Nanoparticles and cancer therapy: a concise review with emphasis on dendrimers. Int J Nanomed. 2009;4:1–7.
2. Lei C, Liu XR, Chen QB, et al. Hyaluronic acid and albumin based nanoparticles for drug delivery. J Control Release. 2021;331:416–433. doi:10.1016/j.jconrel.2021.01.033
3. Shen X, Liu X, T. L, et al. Recent advancements in serum albumin-based nanovehicles toward potential cancer diagnosis and therapy. Front Chem. 2021;9:746646. doi:10.3389/fchem.2021.746646
4. Tincu Iurciuc CE, Andritoiu CV, Popa M, Ochiuz L. Recent advancements and strategies for overcoming the blood-brain barrier using albumin-based drug delivery systems to treat brain cancer, with a focus on glioblastoma. Polymers. 2023;15(19):3969.
5. Tao C, Chuah YJ, Xu C, Wang DA. Albumin conjugates and assemblies as versatile bio-functional additives and carriers for biomedical applications. J Mater Chem B. 2019;7(3):357–367. doi:10.1039/C8TB02477D
6. Laurini E, Marson D, Posocco P, Fermeglia M, Pricl S. Structure and binding thermodynamics of viologen-phosphorous dendrimers to human serum albumin: a combined computational/experimental investigation. Fluid Phase Equilibria. 2016;422:18–31. doi:10.1016/j.fluid.2016.02.014
7. Fehske KJ, Muller WE, Wollert U. The location of drug binding sites in human serum albumin. Biochem Pharmacol. 1981;30(7):687–692. doi:10.1016/0006-2952(81)90151-9
8. Thirupathi Kumara Raja S, Prakash T, Gnanamani A. Redox responsive albumin autogenic nanoparticles for the delivery of cancer drugs. Colloids Surf B Biointerfaces. 2017;152:393–405. doi:10.1016/j.colsurfb.2017.01.044
9. Spada A, Emami J, Tuszynski JA, Lavasanifar A. The uniqueness of albumin as a carrier in nanodrug delivery. Mol Pharm. 2021;18(5):1862–1894. doi:10.1021/acs.molpharmaceut.1c00046
10. Loken MK, Staab EV, Shea AW. 131I Colloidal albumin as an agent for scanning liver and spleen. Investigat Radiol. 1966;1(4):295–300. doi:10.1097/00004424-196607000-00019
11. Oppenheim RC, Stewart NF. The manufacture and tumor cell uptake of nanoparticles labelled with fluorescein isothiocyanate. Drug Dev Tndustr Pharm. 1979;5(6):563–571. doi:10.3109/03639047909055680
12. Munz DL. Bone marrow imaging: basic concepts and clinical results. Nuklearmediziner. 1984;7(4):251–268.
13. O’Brien LM, Duffin R, Millar AM. Preparation of 99mTc-Nanocoll for use in sentinel node localization: validation of a protocol for supplying in unit-dose syringes. Nucl Med Commun. 2006;27(12):999–1003. doi:10.1097/MNM.0b013e328010642f
14. Zimmer AK, Maincent P, Thouvenot P, Kreuter J. Hydrocortisone delivery to healthy and inflamed eyes using a micellar polysorbate-80 solution or albumin nanoparticles. Int J pharm. 1994;110(3):211–222. doi:10.1016/0378-5173(94)90243-7
15. Hartung G, Stehle G, Sinn H, et al. Phase I trial of methotrexate-albumin in a weekly intravenous bolus regimen in cancer patients. Clin Cancer Res. 1999;5(4):753–759.
16. Wunder A, Müller-Ladner U, Stelzer EHK, et al. Albumin-based drug delivery as novel therapeutic approach for rheumatoid arthritis. J Immunol. 2003;170(9):4793–4801. doi:10.4049/jimmunol.170.9.4793
17. Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother. 2006;7(8):1041–1053. doi:10.1517/14656566.7.8.1041
18. Wang Z, Liu Y, S. L, et al. Bioinspired nanocomplexes comprising phenolic acid derivative and human serum albumin for cancer therapy. Nano Lett. 2022;22(24):10040–10048. doi:10.1021/acs.nanolett.2c03763
19. Ezhilarasan D, Lakshmi T, Mallineni SK. Nano-based targeted drug delivery for lung cancer: therapeutic avenues and challenges. Nanomedicine. 2022;17(24):1855–1869. doi:10.2217/nnm-2021-0364
20. Adick A, Hoheisel W, Schneid S, et al. Challenges of nanoparticle albumin bound (nab) technology: comparative study of Abraxane(R) with a newly developed albumin-stabilized itraconazole nanosuspension. Eur J Pharm Biopharm. 2023;193:129–143. doi:10.1016/j.ejpb.2023.10.022
21. Mann JE. Sirolimus protein-bound particles (Fyarro™). Pharm Forum. 2022. doi:10.1097/01.cot.0000854104.90358.c3
22. Nian Q, J. L, Han Z, et al. SPARC in hematologic malignancies and novel technique for hematological disease with its abnormal expression. Biomed Pharmacother. 2022;153:113519. doi:10.1016/j.biopha.2022.113519
23. Raghav A, Ahmad J, Alam K, Khan AU. New insights into non-enzymatic glycation of human serum albumin biopolymer: a study to unveil its impaired structure and function. Int J Biol Macromol. 2017;101:84–99. doi:10.1016/j.ijbiomac.2017.03.086
24. Maciazek-Jurczyk M, Janas K, Pozycka J, et al. Human serum albumin aggregation/fibrillation and its abilities to drugs binding. Molecules. 2020;25(3):618. doi:10.3390/molecules25030618
25. Tao HY, Wang RQ, Sheng WJ, Zhen YS. The development of human serum albumin-based drugs and relevant fusion proteins for cancer therapy. Int J Biol Macromol. 2021;187:24–34. doi:10.1016/j.ijbiomac.2021.07.080
26. Malarkani K, Sarkar I, Selvam S. Denaturation studies on bovine serum albumin-bile salt system: bile salt stabilizes bovine serum albumin through hydrophobicity. J Pharm Anal. 2018;8(1):27–36. doi:10.1016/j.jpha.2017.06.007
27. Lamichhane S, Lee S. Albumin nanoscience: homing nanotechnology enabling targeted drug delivery and therapy. Arch Pharm Res. 2020;43(1):118–133. doi:10.1007/s12272-020-01204-7
28. Holm NK, Jespersen SK, Thomassen LV, et al. Aggregation and fibrillation of bovine serum albumin. Biochim Biophys Acta. 2007;1774(9):1128–1138. doi:10.1016/j.bbapap.2007.06.008
29. Abboud R, Charcosset C, Greige-Gerges H. Interaction of triterpenoids with human serum albumin: a review. Chem Phys Lipids. 2017;207:260–270. doi:10.1016/j.chemphyslip.2017.05.011
30. Peng W, Ding F, Jiang YT, Peng YK. Bioavailability and activity of natural food additive triterpenoids as influenced by protein. J Agric Food Chem. 2014;62(10):2271–2283. doi:10.1021/jf4049512
31. Van de Sande L, Cosyns S, Willaert W, Ceelen W. Albumin-based cancer therapeutics for intraperitoneal drug delivery: a review. Drug Deliv. 2020;27(1):40–53. doi:10.1080/10717544.2019.1704945
32. Solanki R, Rostamabadi H, Patel S, Jafari SM. Anticancer nano-delivery systems based on bovine serum albumin nanoparticles: a critical review. Int J Biol Macromol. 2021;193(Pt A):528–540. doi:10.1016/j.ijbiomac.2021.10.040
33. Karimi M, Bahrami S, Ravari SB, et al. Albumin nanostructures as advanced drug delivery systems. Expert Opin Drug Deliv. 2016;13(11):1609–1623. doi:10.1080/17425247.2016.1193149
34. Lin W, Coombes AG, Davies MC, Davis SS, Illum L. Preparation of sub-100 nm human serum albumin nanospheres using a pH-coacervation method. J Drug Target. 1993;1(3):237–243. doi:10.3109/10611869308996081
35. Kim D, Amatya R, Hwang S, et al. BSA-silver nanoparticles: a potential multimodal therapeutics for conventional and photothermal treatment of skin cancer. Pharmaceutics. 2021;13(4):575.
36. Jaiswal VD, Pangam DS, Dongre PM. Biophysical study of cisplatin loaded albumin-gold nanoparticle and its interaction with glycans of gp60 receptor. Int J Biol Macromol. 2023;231:123368. doi:10.1016/j.ijbiomac.2023.123368
37. Steinhauser IM, Langer K, Strebhardt KM, Spankuch B. Effect of trastuzumab-modified antisense oligonucleotide-loaded human serum albumin nanoparticles prepared by heat denaturation. Biomaterials. 2008;29(29):4022–4028. doi:10.1016/j.biomaterials.2008.07.001
38. Langer K, Balthasar S, Vogel V, et al. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int J Pharm. 2003;257(1–2):169–180. doi:10.1016/S0378-5173(03)00134-0
39. Rubino OP, Kowalsky R, Swarbrick J. Albumin microspheres as a drug-delivery system - relation among turbidity ratio, degree of cross-linking, and drug-release. Pharm Res. 1993;10(7):1059–1065. doi:10.1023/A:1018979126326
40. Jahanban-Esfahlan A, Dastmalchi S, Davaran S. A simple improved desolvation method for the rapid preparation of albumin nanoparticles. Int J Biol Macromol. 2016;91:703–709. doi:10.1016/j.ijbiomac.2016.05.032
41. Storp B, Engel A, Boeker A, Ploeger M, Langer K. Albumin nanoparticles with predictable size by desolvation procedure. J Microencapsul. 2012;29(2):138–146. doi:10.3109/02652048.2011.635218
42. Meziani MJ, Sun YP. Protein-conjugated nanoparticles from rapid expansion of supercritical fluid solution into aqueous solution. J Am Chem Soc. 2003;125(26):8015–8018. doi:10.1021/ja030104k
43. Merodio M, Arnedo A, Renedo MJ, Irache JM. Ganciclovir-loaded albumin nanoparticles: characterization and in vitro release properties. Eur J Pharm Sci. 2001;12(3):251–259. doi:10.1016/S0928-0987(00)00169-X
44. Liu S, Liu M, Xiu J, et al. Celastrol-loaded bovine serum albumin nanoparticles target inflamed neutrophils for improved rheumatoid arthritis therapy. Acta Biomater. 2024;174:345–357. doi:10.1016/j.actbio.2023.11.028
45. Bax DV, Nair M, Weiss AS, et al. Tailoring the biofunctionality of collagen biomaterials via tropoelastin incorporation and EDC-crosslinking. Acta Biomater. 2021;135:150–163. doi:10.1016/j.actbio.2021.08.027
46. Weiner J, Widman S, Golek Z, Tranquilli M, Elefteriades JA. Role of bovine serum albumin-glutaraldehyde glue in the formation of anastomatic pseudoaneurysms. J Cardiac Surg. 2011;26(1):76–81. doi:10.1111/j.1540-8191.2010.01162.x
47. Wegrzynowska-Drzymalska K, Mylkie K, Nowak P, et al. Dialdehyde starch nanocrystals as a novel cross-linker for biomaterials able to interact with human serum proteins. Int J Mol Sci. 2022;23(14):7652. doi:10.3390/ijms23147652
48. Luna-Valdez JG, Balandran-Quintana RR, Azamar-Barrios JA, et al. Structural and physicochemical characterization of nanoparticles synthesized from an aqueous extract of wheat bran by a cold-set gelation/desolvation approach. Food Hydrocoll. 2017;62:165–173. doi:10.1016/j.foodhyd.2016.07.034
49. Niknejad H, Mahmoudzadeh R. Comparison of different crosslinking methods for preparation of docetaxel-loaded albumin nanoparticles. Iran J Pharm Res. 2015;14(2):385–394.
50. Jenjob R, Phakkeeree T, Seidi F, Theerasilp M, Crespy D. Emulsion techniques for the production of pharmacological nanoparticles. Macromol Biosci. 2019;19(6):e1900063. doi:10.1002/mabi.201900063
51. Ranjan AP, Mukerjee A, Helson L, Vishwanatha JK. Scale up, optimization and stability analysis of Curcumin C3 complex-loaded nanoparticles for cancer therapy. J Nanobiotechnol. 2012;10(1):38. doi:10.1186/1477-3155-10-38
52. Verma ML, Dhanya BS, Sukriti, et al. Carbohydrate and protein based biopolymeric nanoparticles: current status and biotechnological applications. Int J Biol Macromol. 2020;154:390–412. doi:10.1016/j.ijbiomac.2020.03.105
53. Alfagih IM, Kaneko K, Kunda NK, et al. In vitro characterization of inhalable cationic hybrid nanoparticles as potential vaccine carriers. Pharmaceuticals. 2021;14(2):164. doi:10.3390/ph14020164
54. Wan X, Zheng XY, Pang XY, Zhang ZM, Zhang QZ. Incorporation of lapatinib into human serum albumin nanoparticles with enhanced anti-tumor effects in HER2-positive breast cancer. Colloids Surf B Biointerfaces. 2015;136:817–827. doi:10.1016/j.colsurfb.2015.10.018
55. F. H, Liu J, Shen X, et al. Adverse event profile for nanoparticle albumin-bound paclitaxel compared with solvent-based taxanes in solid-organ tumors: a systematic review and meta-analysis of randomized clinical trials. Ann Pharmacother. 2022;56(8):898–909. doi:10.1177/10600280211058385
56. Di Pippo M, Di Staso F, De Ponte C, Fragiotta S, Abdolrahimzadeh S. Nab-Paclitaxel related cystoid macular edema. Clin Ter. 2022;173(4):377–383. doi:10.7417/CT.2022.2449
57. Chen H, Huang S, Wang H, et al. Preparation and characterization of paclitaxel palmitate albumin nanoparticles with high loading efficacy: an in vitro and in vivo anti-tumor study in mouse models. Drug Deliv. 2021;28(1):1067–1079. doi:10.1080/10717544.2021.1921078
58. Furedi P, Kovacs K, Ludanyi K, Antal I, Klebovich I. Development and characterization of voriconazole loaded nanoparticles for parenteral delivery. Int J Pharm. 2016;510(1):159–163. doi:10.1016/j.ijpharm.2016.06.027
59. F. L, Yeh S, Shi Q, et al. A novel thermal-driven self-assembly method to prepare albumin nanoparticles: formation kinetics, degradation behavior and formation mechanism. AAPS Pharm Sci Tech. 2022;23(7):250. doi:10.1208/s12249-022-02407-5
60. Altin G, Gultekin-Ozguven M, Ozcelik B. Chitosan coated liposome dispersions loaded with cacao hull waste extract: effect of spray drying on physico-chemical stability and in vitro bioaccessibility. J Food Eng. 2018;223:91–98. doi:10.1016/j.jfoodeng.2017.12.005
61. Strojewski D, Krupa A. Spray drying and nano spray drying as manufacturing methods of drug-loaded polymeric particles. Polim Med. 2022;52(2):101–111. doi:10.17219/pim/152230
62. Al-Zoubi N, Partheniadis I, Aljaberi A, Nikolakakis I. Co-spray drying drugs with Aqueous Polymer Dispersions (APDs)-a systematic review. AAPS Pharm Sci Tech. 2022;23(5):140. doi:10.1208/s12249-022-02293-x
63. Pan LH, Chen LP, Wu CL, et al. Microencapsulation of blueberry anthocyanins by spray drying with soy protein isolates/high methyl pectin combination: physicochemical properties, release behavior in vitro and storage stability. Food Chem. 2022;395:133626. doi:10.1016/j.foodchem.2022.133626
64. Vehring R. Pharmaceutical particle engineering via spray drying. Pharm Res. 2008;25(5):999–1022. doi:10.1007/s11095-007-9475-1
65. Almansour K, Ali R, Alheibshy F, et al. Particle engineering by nano spray drying: optimization of process parameters with hydroethanolic versus aqueous solutions. Pharmaceutics. 2022;14(4):800. doi:10.3390/pharmaceutics14040800
66. Sristi, Fatima M, Sheikh A, et al. Recent advancement on albumin nanoparticles in treating lung carcinoma. J Drug Target. 2023;31(5):486–499. doi:10.1080/1061186X.2023.2205609
67. Heng D, Lee SH, Ng WK, Tan RB. The nano spray dryer B-90. Expert Opin Drug Deliv. 2011;8(7):965–972. doi:10.1517/17425247.2011.588206
68. Schmid K, Arpagaus C, Friess W. Evaluation of the Nano Spray Dryer B-90 for pharmaceutical applications. Pharm Dev Technol. 2011;16(4):287–294. doi:10.3109/10837450.2010.485320
69. Ozturk AA, Arpagaus C. Nano spray-dried drugs for oral administration: a review. Assay Drug Dev Technol. 2021;19(7):412–441. doi:10.1089/adt.2021.053
70. Jiang L, Y. X, Liu Q, et al. A nontoxic disulfide bond reducing method for lipophilic drug-loaded albumin nanoparticle preparation: formation dynamics, influencing factors and formation mechanisms investigation. Int J Pharm. 2013;443(1–2):80–86. doi:10.1016/j.ijpharm.2012.12.035
71. Abolhassani H, Shojaosadati SA. A comparative and systematic approach to desolvation and self-assembly methods for synthesis of piperine-loaded human serum albumin nanoparticles. Colloids Surf B Biointerfaces. 2019;184:110534. doi:10.1016/j.colsurfb.2019.110534
72. Saleh T, Soudi T, Shojaosadati SA. Redox responsive curcumin-loaded human serum albumin nanoparticles: preparation, characterization and in vitro evaluation. Int J Biol Macromol. 2018;114:759–766. doi:10.1016/j.ijbiomac.2018.03.085
73. Curcio M, Avena P, Cirillo G, et al. Functional albumin nanoformulations to fight adrenocortical carcinoma: a redox-responsive approach. Pharm Res. 2020;37(3):55. doi:10.1007/s11095-020-2775-4
74. Safavi MS, Shojaosadati SA, Yang HG, et al. Reducing agent-free synthesis of curcumin-loaded albumin nanoparticles by self-assembly at room temperature. Int J Pharm. 2017;529(1–2):303–309. doi:10.1016/j.ijpharm.2017.06.087
75. Zhang H, Yang J, Sun R, et al. Microfluidics for nano-drug delivery systems: from fundamentals to industrialization. Acta Pharm Sin B. 2023;13(8):3277–3299. doi:10.1016/j.apsb.2023.01.018
76. Minetti F, Mengatto LN, Laura Olivares M, Berli CLA. Generation of curcumin-loaded albumin nanoparticles by using off-The-shelf microfluidics driven by gravity. Food Res Int. 2022;162(Pt A):111984. doi:10.1016/j.foodres.2022.111984
77. Sun Y, Lee RJ, Meng F, et al. Microfluidic self-assembly of high cabazitaxel loading albumin nanoparticles. Nanoscale. 2020;12(32):16928–16933. doi:10.1039/C9NR10941B
78. Mohammad-Beigi H, Shojaosadati SA, Marvian AT, et al. Strong interactions with polyethylenimine-coated human serum albumin nanoparticles (PEI-HSA NPs) alter alpha-synuclein conformation and aggregation kinetics. Nanoscale. 2015;7(46):19627–19640. doi:10.1039/C5NR05663B
79. Mishra V, Heath RJ. Structural and biochemical features of human serum albumin essential for eukaryotic cell culture. Int J Mol Sci. 2021;22(16):8411. doi:10.3390/ijms22168411
80. Akbarzadehlaleh P, Mirzaei M, Mashahdi-Keshtiban M, Shamsasenjan K, Heydari H. PEGylated human serum albumin: review of PEGylation, purification and characterization methods. Adv Pharm Bull. 2016;6(3):309–317. doi:10.15171/apb.2016.043
81. Zeeshan F, Madheswaran T, Panneerselvam J, Taliyan R, Kesharwani P. Human serum albumin as multifunctional nanocarrier for cancer therapy. J Pharm Sci. 2021;110(9):3111–3117.
82. Jahanban-Esfahlan A, Ostadrahimi A, Jahanban-Esfahlan R, et al. Recent developments in the detection of bovine serum albumin. Int J Biol Macromol. 2019;138:602–617. doi:10.1016/j.ijbiomac.2019.07.096
83. Kunde SS, Wairkar S. Targeted delivery of albumin nanoparticles for breast cancer: a review. Colloids Surf B Biointerfaces. 2022;213:112422. doi:10.1016/j.colsurfb.2022.112422
84. Fahrlander E, Schelhaas S, Jacobs AH, Langer K. PEGylated human serum albumin (HSA) nanoparticles: preparation, characterization and quantification of the PEGylation extent. Nanotechnology. 2015;26(14):145103. doi:10.1088/0957-4484/26/14/145103
85. Mehtala JG, Kulczar C, Lavan M, Knipp G, Wei A. Cys34-PEGylated human serum albumin for drug binding and delivery. Bioconjug Chem. 2015;26(5):941–949. doi:10.1021/acs.bioconjchem.5b00143
86. Lee JE, Kim KL, Kim D, et al. Apatinib-loaded nanoparticles suppress vascular endothelial growth factor-induced angiogenesis and experimental corneal neovascularization. Int J Nanomed. 2017;12:4813–4822. doi:10.2147/IJN.S135133
87. Lee JE, Kim MG, Jang YL, et al. Self-assembled PEGylated albumin nanoparticles (SPAN) as a platform for cancer chemotherapy and imaging. Drug Deliv. 2018;25(1):1570–1578. doi:10.1080/10717544.2018.1489430
88. Radwan SE, El-Moslemany RM, Mehanna RA, et al. Chitosan-coated bovine serum albumin nanoparticles for topical tetrandrine delivery in glaucoma: in vitro and in vivo assessment. Drug Deliv. 2022;29(1):1150–1163. doi:10.1080/10717544.2022.2058648
89. Lin P, Zhang W, Chen D, et al. Electrospun nanofibers containing chitosan-stabilized bovine serum albumin nanoparticles for bone regeneration. Colloids Surf B Biointerfaces. 2022;217:112680. doi:10.1016/j.colsurfb.2022.112680
90. Razi MA, Wakabayashi R, Goto M, Kamiya N. Self-assembled reduced albumin and glycol chitosan nanoparticles for paclitaxel delivery. Langmuir. 2019;35(7):2610–2618. doi:10.1021/acs.langmuir.8b02809
91. Salim EI, Abd El Khalik EAM, Shalaby TI, Ali EMM. Synthesis, characterisation and enhanced apoptotic effect of gemcitabine-loaded albumin nanoparticles coating with chitosan. Arch Physiol Biochem. 2022;128(4):970–978. doi:10.1080/13813455.2020.1742165
92. Rahimi H, Zaboli KA, Thekkiniath J, et al. BSA-PEI nanoparticle mediated efficient delivery of CRISPR/Cas9 into MDA-MB-231 cells. Mol Biotechnol. 2022;64(12):1376–1387. doi:10.1007/s12033-022-00514-z
93. Kim DH, Kim KH, Kwon TY, et al. Antibacterial releasing titanium surface using albumin nanoparticle carriers. J Nanosci Nanotechnol. 2014;14(11):8422–8426. doi:10.1166/jnn.2014.9934
94. Zhang P, Li B, Du J, Wang Y. Regulation the morphology of cationized gold nanoparticles for effective gene delivery. Colloids Surf B Biointerfaces. 2017;157:18–25. doi:10.1016/j.colsurfb.2017.04.056
95. Tan Y, Xie L, Wang Z, et al. Epsilon-caprolactone modified polyethylenimine for highly efficient antigen delivery and chemical exchange saturation transfer functional MR imaging. Biomaterials. 2015;56:219–228. doi:10.1016/j.biomaterials.2015.03.049
96. Langiu M, Dadparvar M, Kreuter J, Ruonala MO, Hinderberger D. Human serum albumin-based nanoparticle-mediated in vitro gene delivery. PLoS One. 2014;9(9):e107603. doi:10.1371/journal.pone.0107603
97. Ruttala HB, Ramasamy T, Shin BS, et al. Layer-by-layer assembly of hierarchical nanoarchitectures to enhance the systemic performance of nanoparticle albumin-bound paclitaxel. Int J Pharm. 2017;519(1–2):11–21. doi:10.1016/j.ijpharm.2017.01.011
98. Kristo K, Szekeres M, Makai Z, et al. Preparation and investigation of core-shell nanoparticles containing human interferon-alpha. Int J Pharm. 2020;573:118825. doi:10.1016/j.ijpharm.2019.118825
99. Pulakkat S, Balaji SA, Rangarajan A, Raichur AM. Surface engineered protein nanoparticles with hyaluronic acid based multilayers for targeted delivery of anticancer agents. ACS Appl Mater Interfaces. 2016;8(36):23437–23449. doi:10.1021/acsami.6b04179
100. Pannuzzo M, Esposito S, Wu LP, et al. Overcoming nanoparticle-mediated complement activation by surface PEG pairing. Nano Lett. 2020;20(6):4312–4321. doi:10.1021/acs.nanolett.0c01011
101. Madan J, Pandey RS, Jain UK, et al. Sterically stabilized gelatin microassemblies of noscapine enhance cytotoxicity, apoptosis and drug delivery in lung cancer cells. Colloids Surf B Biointerfaces. 2013;107:235–244. doi:10.1016/j.colsurfb.2013.02.010
102. Huckaby JT, Lai SK. PEGylation for enhancing nanoparticle diffusion in mucus. Adv Drug Deliv Rev. 2018;124:125–139. doi:10.1016/j.addr.2017.08.010
103. Gao C, Gong W, Yang M, et al. T807-modified human serum albumin biomimetic nanoparticles for targeted drug delivery across the blood-brain barrier. J Drug Target. 2020;28(10):1085–1095. doi:10.1080/1061186X.2020.1777420
104. Geng T, Zhao X, Ma M, Zhu G, Yin L. Resveratrol-loaded albumin nanoparticles with prolonged blood circulation and improved biocompatibility for highly effective targeted pancreatic tumor therapy. Nanoscale Res Lett. 2017;12(1):437. doi:10.1186/s11671-017-2206-6
105. Sharma A, Kaur A, Jain UK, Chandra R, Madan J. Stealth recombinant human serum albumin nanoparticles conjugating 5-fluorouracil augmented drug delivery and cytotoxicity in human colon cancer, HT-29 cells. Colloids Surf B Biointerfaces. 2017;155:200–208. doi:10.1016/j.colsurfb.2017.04.020
106. Song X, Chen Y, Zhao G, et al. Effect of molecular weight of chitosan and its oligosaccharides on antitumor activities of chitosan-selenium nanoparticles. Carbohydr Polym. 2020;231:115689. doi:10.1016/j.carbpol.2019.115689
107. Rashki S, Asgarpour K, Tarrahimofrad H, et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr Polym. 2021;251:117108. doi:10.1016/j.carbpol.2020.117108
108. Karimi M, Avci P, Mobasseri R, Hamblin MR, Naderi-Manesh H. The novel albumin-chitosan core-shell nanoparticles for gene delivery: preparation, optimization and cell uptake investigation. J Nanopart Res. 2013;15(4):1651. doi:10.1007/s11051-013-1651-0
109. Piazzini V, Landucci E, D’Ambrosio M, et al. Chitosan coated human serum albumin nanoparticles: a promising strategy for nose-to-brain drug delivery. Int J Biol Macromol. 2019;129:267–280. doi:10.1016/j.ijbiomac.2019.02.005
110. Ouyang L, Shaik R, Xu R, Zhang G, Zhe J. Mapping surface charge distribution of single-cell via charged nanoparticle. Cells. 2021;10(6):1519. doi:10.3390/cells10061519
111. Bhattacharjee S. DLS and zeta potential - What they are and what they are not? J Control Release. 2016;235:337–351. doi:10.1016/j.jconrel.2016.06.017
112. Esfandyari-Manesh M, Mohammadi A, Atyabi F, et al. Specific targeting delivery to MUC1 overexpressing tumors by albumin-chitosan nanoparticles conjugated to DNA aptamer. Int J Pharm. 2016;515(1–2):607–615. doi:10.1016/j.ijpharm.2016.10.066
113. Othman SI, Alturki AM, Abu-Taweel GM, et al. Chitosan for biomedical applications, promising antidiabetic drug delivery system, and new diabetes mellitus treatment based on stem cell. Int J Biol Macromol. 2021;190:417–432. doi:10.1016/j.ijbiomac.2021.08.154
114. Naveed M, Phil L, Sohail M, et al. Chitosan oligosaccharide (COS): an overview. Int J Biol Macromol. 2019;129:827–843. doi:10.1016/j.ijbiomac.2019.01.192
115. Pereira P, Barreira M, Cruz C, et al. Brain-targeted delivery of Pre-miR-29b using lactoferrin-stearic acid-modified-chitosan/polyethyleneimine polyplexes. Pharmaceuticals. 2020;13(10):314. doi:10.3390/ph13100314
116. Mohammad-Beigi H, Morshedi D, Shojaosadati SA, et al. Gallic acid loaded onto polyethylenimine-coated human serum albumin nanoparticles (PEI-HSA-GA NPs) stabilizes alpha-synuclein in the unfolded conformation and inhibits aggregation. RSC Adv. 2016;6(88):85312–85323. doi:10.1039/C6RA08502D
117. Xue L, Yan Y, Kos P, Chen X, Siegwart DJ. PEI fluorination reduces toxicity and promotes liver-targeted siRNA delivery. Drug Deliv Transl Res. 2021;11(1):255–260. doi:10.1007/s13346-020-00790-9
118. Hasanzadeh M, Mokhtari F, Shadjou N, et al. Poly arginine-graphene quantum dots as a biocompatible and non-toxic nanocomposite: layer-by-layer electrochemical preparation, characterization and non-invasive malondialdehyde sensory application in exhaled breath condensate. Mater Sci Eng C Mater Biol Appl. 2017;75:247–258. doi:10.1016/j.msec.2017.02.025
119. An X, Zha D. Development of nanoparticle drug-delivery systems for the inner ear. Nanomedicine. 2020;15(20):1981–1993. doi:10.2217/nnm-2020-0198
120. Zhang M, Zou Y, Zuo C, et al. Targeted antitumor comparison study between dopamine self-polymerization and traditional synthesis for nanoparticle surface modification in drug delivery. Nanotechnology. 2021;32(30):305102.
121. Gupta P, Garcia E, Sarkar A, et al. Nanoparticle based treatment for cardiovascular diseases. Cardiovasc Hematol Disord Drug Targets. 2019;19(1):33–44. doi:10.2174/1871529X18666180508113253
122. Shen Y, Li W. HA/HSA co-modified erlotinib-albumin nanoparticles for lung cancer treatment. Drug Des Devel Ther. 2018;12:2285–2292. doi:10.2147/DDDT.S169734
123. Y. H, Chen X, Y. X, et al. Hierarchical assembly of hyaluronan coated albumin nanoparticles for pancreatic cancer chemoimmunotherapy. Nanoscale. 2019;11(35):16476–16487. doi:10.1039/C9NR03684A
124. Brindisi M, Curcio M, Frattaruolo L, et al. CD44-targeted nanoparticles with GSH-responsive activity as powerful therapeutic agents against breast cancer. Int J Biol Macromol. 2022;221:1491–1503. doi:10.1016/j.ijbiomac.2022.09.157
125. Mohamed HI, El-Kamel AH, Hammad GO, Heikal LA. Design of targeted flurbiprofen biomimetic nanoparticles for management of arthritis: in vitro and in vivo appraisal. Pharmaceutics. 2022;14(1):140. doi:10.3390/pharmaceutics14010140
126. L. X, Jiang G, Chen H, et al. Folic acid-modified fluorescent dye-protein nanoparticles for the targeted tumor cell imaging. Talanta. 2019;194:643–648. doi:10.1016/j.talanta.2018.10.094
127. Hiremath CG, Kariduraganavar MY, Hiremath MB. Synergistic delivery of 5-fluorouracil and curcumin using human serum albumin-coated iron oxide nanoparticles by folic acid targeting. Prog Biomater. 2018;7(4):297–306. doi:10.1007/s40204-018-0104-3
128. Sinai Kunde S, Wairkar S. Folic acid anchored urchin-like raloxifene nanoparticles for receptor targeting in breast cancer: synthesis, optimisation and in vitro biological evaluation. Int J Pharm. 2022;623:121926. doi:10.1016/j.ijpharm.2022.121926
129. Dong Y, R. F, Yang J, et al. Folic acid-modified ginsenoside Rg5-loaded bovine serum albumin nanoparticles for targeted cancer therapy in vitro and in vivo. Int J Nanomed. 2019;14:6971–6988. doi:10.2147/IJN.S210882
130. Zhou Y, Zhou J, Wang F, Yang H. Polydopamine-based functional composite particles for tumor cell targeting and dual-mode cellular imaging. Talanta. 2018;181:248–257. doi:10.1016/j.talanta.2018.01.003
131. Pessoa B, Collado-Gonzalez M, Sandri G, Ribeiro A. Chitosan/albumin coating factorial optimization of alginate/dextran sulfate cores for oral delivery of insulin. Mar Drugs. 2023;21(3):179. doi:10.3390/md21030179
132. He YJ, Xing L, Cui PF, et al. Transferrin-inspired vehicles based on pH-responsive coordination bond to combat multidrug-resistant breast cancer. Biomaterials. 2017;113:266–278. doi:10.1016/j.biomaterials.2016.11.001
133. Ulbrich K, Hekmatara T, Herbert E, Kreuter J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood-brain barrier (BBB). Eur J Pharm Biopharm. 2009;71(2):251–256. doi:10.1016/j.ejpb.2008.08.021
134. Xiong J, Han S, Ding S, He J, Zhang H. Antibody-nanoparticle conjugate constructed with trastuzumab and nanoparticle albumin-bound paclitaxel for targeted therapy of human epidermal growth factor receptor 2-positive gastric cancer. Oncol Rep. 2018;39(3):1396–1404. doi:10.3892/or.2018.6201
135. Wei T, Xing H, Wang H, et al. Bovine serum albumin encapsulation of near infrared fluorescent nano-probe with low nonspecificity and cytotoxicity for imaging of HER2-positive breast cancer cells. Talanta. 2020;210:120625. doi:10.1016/j.talanta.2019.120625
136. Wagner S, Rothweiler F, Anhorn MG, et al. Enhanced drug targeting by attachment of an anti alphav integrin antibody to doxorubicin loaded human serum albumin nanoparticles. Biomaterials. 2010;31(8):2388–2398. doi:10.1016/j.biomaterials.2009.11.093
137. Nevala WK, Butterfield JT, Sutor SL, Knauer DJ, Markovic SN. Antibody-targeted paclitaxel loaded nanoparticles for the treatment of CD20(+) B-cell lymphoma. Sci Rep. 2017;7(1):45682. doi:10.1038/srep45682
138. Thao LQ, Byeon HJ, Lee C, et al. Doxorubicin-bound albumin nanoparticles containing a TRAIL protein for targeted treatment of colon cancer. Pharm Res. 2016;33(3):615–626. doi:10.1007/s11095-015-1814-z
139. Min SY, Byeon HJ, Lee C, et al. Facile one-pot formulation of TRAIL-embedded paclitaxel-bound albumin nanoparticles for the treatment of pancreatic cancer. Int J Pharm. 2015;494(1):506–515. doi:10.1016/j.ijpharm.2015.08.055
140. Cui W, Wang A, Zhao J, et al. Layer by layer assembly of albumin nanoparticles with selective recognition of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). J Colloid Interface Sci. 2016;465:11–17. doi:10.1016/j.jcis.2015.11.054
141. Choi SH, Byeon HJ, Choi JS, et al. Inhalable self-assembled albumin nanoparticles for treating drug-resistant lung cancer. J Control Release. 2015;197:199–207. doi:10.1016/j.jconrel.2014.11.008
142. Hassn Mesrati M, Syafruddin SE, Mohtar MA, Syahir A. CD44: a multifunctional mediator of cancer progression. Biomolecules. 2021;11(12):1850. doi:10.3390/biom11121850
143. Chen L, C. F, Zhang Q, et al. The role of CD44 in pathological angiogenesis. FASEB J. 2020;34(10):13125–13139. doi:10.1096/fj.202000380RR
144. Chen J, Meng J, X. L, et al. HA/CD44 regulates the T helper 1 cells differentiation by activating annexin A1/Akt/mTOR signaling to drive the pathogenesis of EAP. Front Immunol. 2022;13:875412. doi:10.3389/fimmu.2022.875412
145. Huang D, Chen YS, Rupenthal ID. Hyaluronic acid coated albumin nanoparticles for targeted peptide delivery to the retina. Mol Pharmaceut. 2017;14(2):533–545. doi:10.1021/acs.molpharmaceut.6b01029
146. Marko AJ, Borah BM, Siters KE, et al. Targeted nanoparticles for fluorescence imaging of folate receptor positive tumors. Biomolecules. 2020;10(12):1651. doi:10.3390/biom10121651
147. Kunjiappan S, Govindaraj S, Parasuraman P, et al. Design, in silico modelling and functionality theory of folate-receptor-targeted myricetin-loaded bovine serum albumin nanoparticle formulation for cancer treatment. Nanotechnology. 2020;31(15):155102. doi:10.1088/1361-6528/ab5c56
148. Ulbrich K, Michaelis M, Rothweiler F, et al. Interaction of folate-conjugated human serum albumin (HSA) nanoparticles with tumour cells. Int J Pharm. 2011;406(1–2):128–134. doi:10.1016/j.ijpharm.2010.12.023
149. Siri M, Ruocco MJF, Achilli E, et al. Effect of structure in ionised albumin based nanoparticle: characterisation, Emodin interaction, and in vitro cytotoxicity. Mater Sci Eng C Mater Biol Appl. 2019;103:109813. doi:10.1016/j.msec.2019.109813
150. Chen D, Tang Q, Xue W, et al. The preparation and characterization of folate-conjugated human serum albumin magnetic cisplatin nanoparticles. J Biomed Res. 2010;24(1):26–32. doi:10.1016/S1674-8301(10)60005-X
151. Wang WC, Liang H, Sun BH, et al. Pharmacokinetics and tissue distribution of folate-decorated human serum albumin loaded with nano-hydroxycamptothecin for tumor targeting. J Pharmaceut Sci. 2016;105(6):1874–1880. doi:10.1016/j.xphs.2016.03.016
152. N. Q, Lee RJ, Sun Y, et al. Cabazitaxel-loaded human serum albumin nanoparticles as a therapeutic agent against prostate cancer. Int J Nanomed. 2016;11:3451–3459. doi:10.2147/IJN.S105420
153. Sun Y, Zhao Y, Teng S, et al. Folic acid receptor-targeted human serum albumin nanoparticle formulation of cabazitaxel for tumor therapy. Int J Nanomed. 2019;14:135–148. doi:10.2147/IJN.S181296
154. Mojarad-Jabali S, Mahdinloo S, Farshbaf M, et al. Transferrin receptor-mediated liposomal drug delivery: recent trends in targeted therapy of cancer. Expert Opin Drug Deliv. 2022;19(6):685–705. doi:10.1080/17425247.2022.2083106
155. Stocki P, Szary J, Rasmussen CLM, et al. Blood-brain barrier transport using a high affinity, brain-selective VNAR antibody targeting transferrin receptor 1. FASEB J. 2021;35(2):e21172. doi:10.1096/fj.202001787R
156. Johnsen KB, Burkhart A, Thomsen LB, Andresen TL, Moos T. Targeting the transferrin receptor for brain drug delivery. Prog Neurobiol. 2019;181:101665. doi:10.1016/j.pneurobio.2019.101665
157. Stephen BJ, Suchanti S, Mishra R, Singh A. Cancer nanotechnology in medicine: a promising approach for cancer detection and diagnosis. Crit Rev Ther Drug Carrier Syst. 2020;37(4):375–405. doi:10.1615/CritRevTherDrugCarrierSyst.2020032634
158. Kouchakzadeh H, Shojaosadati SA, Tahmasebi F, Shokri F. Optimization of an anti-HER2 monoclonal antibody targeted delivery system using PEGylated human serum albumin nanoparticles. Int J Pharm. 2013;447(1–2):62–69. doi:10.1016/j.ijpharm.2013.02.043
159. Kim SH, Seung BJ, Bae MK, et al. Tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) loss in canine mammary carcinoma. Vet Comp Oncol. 2022;20(1):207–214. doi:10.1111/vco.12767
160. Yuan X, Gajan A, Chu Q, et al. Developing TRAIL/TRAIL death receptor-based cancer therapies. Cancer Metastasis Rev. 2018;37(4):733–748. doi:10.1007/s10555-018-9728-y
161. H. Y, Rong W, Yang J, et al. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL): a novel biomarker for prognostic assessment and risk stratification of acute pulmonary embolism. J Clin Med. 2022;11(13):3908.
162. Ahmed W, Mansoor Q, Ahmad MS, Zainab T, Shah MA. TRAIL mediated apoptosis ruling and anticancer trigger by fine-tuned nano spheres of Fagonia cretica methanolic extracts as novel cancer regime. Sci Rep. 2023;13(1):671. doi:10.1038/s41598-023-27441-6
163. Kotrange H, Najda A, Bains A, et al. Metal and metal oxide nanoparticle as a novel antibiotic carrier for the direct delivery of antibiotics. Int J Mol Sci. 2021;22(17):9596. doi:10.3390/ijms22179596
164. Kuchur OA, Tsymbal SA, Shestovskaya MV, et al. Metal-derived nanoparticles in tumor theranostics: potential and limitations. J Inorg Biochem. 2020;209:111117. doi:10.1016/j.jinorgbio.2020.111117
165. P. X, H. W, Wang D, et al. Ultra-small albumin templated Gd/Ru composite nanodots for in vivo dual modal MR/thermal imaging guided photothermal therapy. Adv Healthc Mater. 2018;7(19):e1800322. doi:10.1002/adhm.201800322
166. Zhen W, Liu Y, Lin L, et al. BSA-IrO(2): catalase-like nanoparticles with high photothermal conversion efficiency and a high X-ray absorption coefficient for anti-inflammation and antitumor theranostics. Angew Chem Int Ed Engl. 2018;57(32):10309–10313. doi:10.1002/anie.201804466
167. Zhu Y, Xue J, Chen W, et al. Albumin-biomineralized nanoparticles to synergize phototherapy and immunotherapy against melanoma. J Control Release. 2020;322:300–311. doi:10.1016/j.jconrel.2020.03.045
168. Gou Y, Zhang Z, J. Q, et al. Folate-functionalized human serum albumin carrier for anticancer copper(II) complexes derived from natural plumbagin. J Inorg Biochem. 2015;153:13–22. doi:10.1016/j.jinorgbio.2015.09.004
169. Sivolella S, Stellini E, Brunello G, et al. Silver nanoparticles in alveolar bone surgery devices. J Nanomater. 2012;2012:1–12. doi:10.1155/2012/975842:
170. Ratan ZA, Mashrur FR, Chhoan AP, et al. Silver nanoparticles as potential antiviral agents. Pharmaceutics. 2021;13(12):2034. doi:10.3390/pharmaceutics13122034
171. Tabaran AF, Matea CT, Mocan T, et al. Silver nanoparticles for the therapy of tuberculosis. Int J Nanomed. 2020;15:2231–2258. doi:10.2147/IJN.S241183
172. Jaiswal VD, Dongre PM. Biophysical interactions between silver nanoparticle-albumin interface and curcumin. J Pharm Anal. 2020;10(2):164–177. doi:10.1016/j.jpha.2020.02.004
173. Korolev D, Shumilo M, Shulmeyster G, et al. Hemolytic activity, cytotoxicity, and antimicrobial effects of human albumin- and polysorbate-80-coated silver nanoparticles. Nanomaterials. 2021;11(6):1484. doi:10.3390/nano11061484
174. Nierenberg D, Khaled AR, Flores O. Formation of a protein Corona influences the biological identity of nanomaterials. Rep Pract Oncol Radiother. 2018;23(4):300–308. doi:10.1016/j.rpor.2018.05.005
175. Monteiro-Riviere NA, Samberg ME, Oldenburg SJ, Riviere JE. Protein binding modulates the cellular uptake of silver nanoparticles into human cells: implications for in vitro to in vivo extrapolations? Toxicol Lett. 2013;220(3):286–293. doi:10.1016/j.toxlet.2013.04.022
176. Park HY, Chung C, Eiken MK, et al. Silver nanoparticle interactions with glycated and non-glycated human serum albumin mediate toxicity. Front Toxicol. 2023;5:1081753. doi:10.3389/ftox.2023.1081753
177. Ananth AN, Daniel SC, Sironmani TA, Umapathi S. PVA and BSA stabilized silver nanoparticles based surface-enhanced plasmon resonance probes for protein detection. Colloids Surf B Biointerfaces. 2011;85(2):138–144. doi:10.1016/j.colsurfb.2011.02.012
178. Singh P, Pandit S, Mokkapati V, et al. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int J Mol Sci. 2018;19(7):1979. doi:10.3390/ijms19071979
179. Jindal M, Nagpal M, Singh M, Aggarwal G, Dhingra GA. Gold nanoparticles- boon in cancer theranostics. Curr Pharm Des. 2020;26(40):5134–5151. doi:10.2174/1381612826666200701151403
180. Bloise N, Strada S, Dacarro G, Visai L. Gold Nanoparticles Contact with Cancer Cell: a Brief Update. Int J Mol Sci. 2022;23(14):7683. doi:10.3390/ijms23147683
181. Akasaka M, Nishi T, Niidome Y. Gold-silver and gold-palladium alloy nanoparticles as mass-probes for immunosensing. Anal Sci. 2021;37(9):1305–1307. doi:10.2116/analsci.21N001
182. Bolanos K, Kogan MJ, Araya E. Capping gold nanoparticles with albumin to improve their biomedical properties. Int J Nanomed. 2019;14:6387–6406. doi:10.2147/IJN.S210992
183. Nguyen VH, Lee BJ. Protein Corona: a new approach for nanomedicine design. Int J Nanomed. 2017;12:3137–3151. doi:10.2147/IJN.S129300
184. Mocan L, Matea C, Tabaran FA, et al. Selective ex vivo photothermal nano-therapy of solid liver tumors mediated by albumin conjugated gold nanoparticles. Biomaterials. 2017;119:33–42. doi:10.1016/j.biomaterials.2016.12.009
185. Murawala P, Tirmale A, Shiras A, Prasad BL. In situ synthesized BSA capped gold nanoparticles: effective carrier of anticancer drug methotrexate to MCF-7 breast cancer cells. Mater Sci Eng C Mater Biol Appl. 2014;34:158–167. doi:10.1016/j.msec.2013.09.004
186. Chen JL, Yang SJ, Pan CK, et al. Cisplatin and albumin-based gold-cisplatin nanoparticles enhance ablative radiation therapy-induced antitumor immunity in local and distant tumor microenvironment. Int J Radiat Oncol Biol Phys. 2023;116(5):1135–1149. doi:10.1016/j.ijrobp.2023.02.014
187. Ge EJ, Bush AI, Casini A, et al. Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat Rev Cancer. 2022;22(2):102–113. doi:10.1038/s41568-021-00417-2
188. Wittmann C, Bacher F, Enyedy EA, et al. Highly antiproliferative latonduine and indolo[2,3-c]quinoline derivatives: complex formation with Copper(II) markedly changes the kinase inhibitory profile. J Med Chem. 2022;65(3):2238–2261. doi:10.1021/acs.jmedchem.1c01740
189. Gou Y, Zhang Y, Qi JX, et al. Enhancing the copper(II) complexes cytotoxicity to cancer cells through bound to human serum albumin. J Inorg Biochem. 2015;144:47–55. doi:10.1016/j.jinorgbio.2014.12.012
190. Jiang M, Zhang Z, W. L, et al. Developing a Copper(II) agent based on His-146 and His-242 residues of human serum albumin nanoparticles: integration to overcome cisplatin resistance and inhibit the metastasis of nonsmall cell lung cancer. J Med Chem. 2022;65(13):9447–9458. doi:10.1021/acs.jmedchem.2c00698
191. Rahmani S, Durand JO, Charnay C, et al. Synthesis of mesoporous silica nanoparticles and nanorods: application to doxorubicin delivery. Solid State Sci. 2017;68:25–31. doi:10.1016/j.solidstatesciences.2017.04.003
192. Buzoglu L, Maltas E, Ozmen M, Yildiz S. Interaction of donepezil with human serum albumin on amine-modified magnetic nanoparticles. Colloids Surf A. 2014;442:139–145.
193. Albalawi AE, Khalaf AK, Alyousif MS, et al. Fe3O4(@)piroctone olamine magnetic nanoparticles: synthesize and therapeutic potential in cutaneous leishmaniasis. Biomed Pharmacother. 2021;139:111566. doi:10.1016/j.biopha.2021.111566
194. Wang GK, Papasani MR, Cheguru P, Hrdlicka PJ, Hill RA. Gold-peptide nanoconjugate cellular uptake is modulated by serum proteins. Nanomed Nanotechnol Biol Med. 2012;8(6):822–832. doi:10.1016/j.nano.2011.10.007
195. Joshi P, Chakraborty S, Dey S, et al. Binding of chloroquine-conjugated gold nanoparticles with bovine serum albumin. J Colloid Interface Sci. 2011;355(2):402–409. doi:10.1016/j.jcis.2010.12.032
196. Qi X, Yao M, Jin M, Guo H. Application of magnetic resonance imaging based on Fe(3)O(4) nanoparticles in the treatment of cerebrovascular diseases. J Nanosci Nanotechnol. 2021;21(2):843–851. doi:10.1166/jnn.2021.18697
197. Yu X, Yang R, Wu C, Liu B, Zhang W. The heating efficiency of magnetic nanoparticles under an alternating magnetic field. Sci Rep. 2022;12(1):16055. doi:10.1038/s41598-022-20558-0
198. He CX, Xie MS, Hong F, et al. A highly sensitive glucose biosensor based on gold nanoparticles/bovine serum albumin/Fe3O4 Biocomposite nanoparticles. Electrochim Acta. 2016;222:1709–1715. doi:10.1016/j.electacta.2016.11.162
199. Zaloga J, Janko C, Nowak J, et al. Development of a lauric acid/albumin hybrid iron oxide nanoparticle system with improved biocompatibility. Int J Nanomed. 2014;9:4847–4866. doi:10.2147/IJN.S68539
200. Zaloga J, Pottler M, Leitinger G, et al. Pharmaceutical formulation of HSA hybrid coated iron oxide nanoparticles for magnetic drug targeting. Eur J Pharm Biopharm. 2016;101:152–162. doi:10.1016/j.ejpb.2016.01.017
201. Zaloga J, Feoktystov A, Garamus VM, et al. Studies on the adsorption and desorption of mitoxantrone to lauric acid/albumin coated iron oxide nanoparticles. Colloids Surf B Biointerfaces. 2018;161:18–26. doi:10.1016/j.colsurfb.2017.09.057
202. Ostroverkhov P, Semkina A, Naumenko V, et al. HSA-coated magnetic nanoparticles for MRI-guided photodynamic cancer therapy. Pharmaceutics. 2018;10(4):284. doi:10.3390/pharmaceutics10040284
203. Wang W, Liu X, Zheng X, Jin HJ, Li X. Biomineralization: an opportunity and challenge of nanoparticle drug delivery systems for cancer therapy. Adv Healthc Mater. 2020;9(22):e2001117. doi:10.1002/adhm.202001117
204. Fang J, Wang Q, Yang G, et al. Albumin-MnO(2) gated hollow mesoporous silica nanosystem for modulating tumor hypoxia and synergetic therapy of cervical carcinoma. Colloids Surf B Biointerfaces. 2019;179:250–259. doi:10.1016/j.colsurfb.2019.03.070
205. Prasad P, Gordijo CR, Abbasi AZ, et al. Multifunctional albumin-MnO(2) nanoparticles modulate solid tumor microenvironment by attenuating hypoxia, acidosis, vascular endothelial growth factor and enhance radiation response. ACS Nano. 2014;8(4):3202–3212. doi:10.1021/nn405773r
206. Chen Q, Feng L, Liu J, et al. Intelligent albumin-MnO2 nanoparticles as pH-/H2 O2 -responsive dissociable nanocarriers to modulate tumor hypoxia for effective combination therapy. Adv Mater. 2016;28(33):7129–7136. doi:10.1002/adma.201601902
207. D. L, Zhang T, Min C, et al. Biodegradable theranostic nanoplatforms of albumin-biomineralized nanocomposites modified hollow mesoporous organosilica for photoacoustic imaging guided tumor synergistic therapy. Chem Eng J. 2020;388:124253.
208. Yang T, Tang Y, Liu L, et al. Size-dependent Ag(2)S nanodots for second near-infrared fluorescence/photoacoustics imaging and simultaneous photothermal therapy. ACS Nano. 2017;11(2):1848–1857. doi:10.1021/acsnano.6b07866
209. Ambika S, Sundrarajan M. Green biosynthesis of ZnO nanoparticles using Vitex negundo L. extract: spectroscopic investigation of interaction between ZnO nanoparticles and human serum albumin. J Photochem Photobiol B. 2015;149:143–148. doi:10.1016/j.jphotobiol.2015.05.004
210. Janani B, Raju LL, Thomas AM, et al. Impact of bovine serum albumin - A protein Corona on toxicity of ZnO NPs in environmental model systems of plant, bacteria, algae and crustaceans. Chemosphere. 2021;270:128629. doi:10.1016/j.chemosphere.2020.128629
211. Zhu G, Lynn GM, Jacobson O, et al. Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nat Commun. 2017;8(1):1954. doi:10.1038/s41467-017-02191-y
212. Luo Y, Chen Z, Sun M, et al. IL-12 nanochaperone-engineered CAR T cell for robust tumor-immunotherapy. Biomaterials. 2022;281:121341. doi:10.1016/j.biomaterials.2021.121341
213. Yang R, J. X, Xu L, et al. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano. 2018;12(6):5121–5129. doi:10.1021/acsnano.7b09041
214. Gao W, Hu CM, Fang RH, et al. Surface functionalization of gold nanoparticles with red blood cell membranes. Adv Mater. 2013;25(26):3549–3553. doi:10.1002/adma.201300638
215. Bose RJ, Kim BJ, Arai Y, et al. Bioengineered stem cell membrane functionalized nanocarriers for therapeutic targeting of severe hindlimb ischemia. Biomaterials. 2018;185:360–370. doi:10.1016/j.biomaterials.2018.08.018
216. Cao Y, Yang Y, Feng S, Wan Y. Biomimetic cancer cell-coated albumin nanoparticles for enhanced colloidal stability and homotypic targeting of breast cancer cells. J Drug Delivery Sci Technol. 2022;75:103698. doi:10.1016/j.jddst.2022.103698
217. Cao X, Tan T, Zhu D, et al. Paclitaxel-loaded macrophage membrane camouflaged albumin nanoparticles for targeted cancer therapy. Int J Nanomed. 2020;15:1915–1928. doi:10.2147/IJN.S244849
218. Wen Q, Zhang Y, Muluh TA, et al. Erythrocyte membrane-camouflaged gefitinib/albumin nanoparticles for tumor imaging and targeted therapy against lung cancer. Int J Biol Macromol. 2021;193(Pt A):228–237. doi:10.1016/j.ijbiomac.2021.10.113
219. Liu F, Xue L, Xu L, et al. Preparation and characterization of bovine serum albumin nanoparticles modified by Poly-l-lysine functionalized graphene oxide for BMP-2 delivery. Mater Des. 2022;215:110479. doi:10.1016/j.matdes.2022.110479
220. L. L, Zhou G, Wang Y, et al. Controlled dual delivery of BMP-2 and dexamethasone by nanoparticle-embedded electrospun nanofibers for the efficient repair of critical-sized rat calvarial defect. Biomaterials. 2015;37:218–229. doi:10.1016/j.biomaterials.2014.10.015
221. Vijayan A, C KN, Vinod Kumar GS. ECM-mimicking nanofibrous scaffold enriched with dual growth factor carrying nanoparticles for diabetic wound healing. Nanoscale Adv. 2021;3(11):3085–3092. doi:10.1039/D0NA00926A
222. Y. X, Gu Y, Cai F, et al. Metabolism balance regulation via antagonist-functionalized injectable microsphere for nucleus pulposus regeneration. Adv Funct Mater. 2020;30(52):2006333.
223. Koral K, H. L, Ganesh N, et al. Akt recruits Dab2 to albumin endocytosis in the proximal tubule. Am J Physiol Renal Physiol. 2014;307(12):F1380–F1389. doi:10.1152/ajprenal.00454.2014
224. Viuff D, Antunes F, Evans L, et al. Generation of a double transgenic humanized neonatal Fc receptor (FcRn)/albumin mouse to study the pharmacokinetics of albumin-linked drugs. J Control Release. 2016;223:22–30. doi:10.1016/j.jconrel.2015.12.019
225. Rudnik-Jansen I, Howard KA. FcRn expression in cancer: mechanistic basis and therapeutic opportunities. J Control Release. 2021;337:248–257. doi:10.1016/j.jconrel.2021.07.007
226. Sleep D. Albumin and its application in drug delivery. Expert Opin Drug Deliv. 2015;12(5):793–812. doi:10.1517/17425247.2015.993313
227. Milici AJ, Watrous NE, Stukenbrok H, Palade GE. Transcytosis of albumin in capillary endothelium. J Cell Biol. 1987;105(6 Pt 1):2603–2612. doi:10.1083/jcb.105.6.2603
228. A MG, De alwis Weerasekera H, Pitre SP, et al. Photodynamic performance of zinc phthalocyanine in HeLa cells: a comparison between DPCC liposomes and BSA as delivery systems. J Photochem Photobiol B. 2016;163:385–390. doi:10.1016/j.jphotobiol.2016.09.002
229. Hassanin I, Elzoghby A. Albumin-based nanoparticles: a promising strategy to overcome cancer drug resistance. Cancer Drug Resist. 2020;3(4):930–946. doi:10.20517/cdr.2020.68
230. Ishima Y, Maruyama T, Otagiri M, Chuang VTG, Ishida T. The new delivery strategy of albumin carrier utilizing the interaction with albumin receptors. Chem Pharm Bull. 2022;70(5):330–333. doi:10.1248/cpb.c21-01024
231. Wang -Y-Y, Li L, Liu X-J, et al. Development of a novel multi-functional integrated bioconjugate effectively targeting K-Ras mutant pancreatic cancer. J Pharm Anal. 2022;12(2):232–242. doi:10.1016/j.jpha.2021.07.001
232. Lu H, Noorani L, Jiang Y, Du AW, Stenzel MH. Penetration and drug delivery of albumin nanoparticles into pancreatic multicellular tumor spheroids. J Mater Chem B. 2017;5(48):9591–9599. doi:10.1039/C7TB02902K
233. Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020;30(16):R921–R925. doi:10.1016/j.cub.2020.06.081
234. Gong T, Tan T, Zhang P, et al. Palmitic acid-modified bovine serum albumin nanoparticles target scavenger receptor-A on activated macrophages to treat rheumatoid arthritis. Biomaterials. 2020;258:120296. doi:10.1016/j.biomaterials.2020.120296
235. Feng J, Xiang L, Fang C, et al. Dual-targeting of tumor cells and tumor-associated macrophages by palmitic acid modified albumin nanoparticles for antitumor and antimetastasis therapy. ACS Appl Mater Interfaces. 2022;14(13):14887–14902. doi:10.1021/acsami.1c23274
236. Anderson J, Schauer J, Bryant S, Graves CR. The use of convalescent plasma therapy and remdesivir in the successful management of a critically ill obstetric patient with novel coronavirus 2019 infection: a case report. Case Rep Womens Health. 2020;27:e00221. doi:10.1016/j.crwh.2020.e00221
237. Lam FW, Cruz MA, Leung H-CE, et al. Histone induced platelet aggregation is inhibited by normal albumin. Thromb Res. 2013;132(1):69–76. doi:10.1016/j.thromres.2013.04.018
238. Park HH, Kim H, Lee HS, et al. PEGylated nanoparticle albumin-bound steroidal ginsenoside derivatives ameliorate SARS-CoV-2-mediated hyper-inflammatory responses. Biomaterials. 2021;273:120827. doi:10.1016/j.biomaterials.2021.120827
239. Paik SH, Kim HT, Chang SE. Severe bitemporal alopecia as a complication of the thread lift procedure. Dermatol Surg. 2019;45(7):983–986. doi:10.1097/DSS.0000000000001709
240. Yamasaki K, Chuang VT, Maruyama T, Otagiri M. Albumin-drug interaction and its clinical implication. Biochim Biophys Acta. 2013;1830(12):5435–5443. doi:10.1016/j.bbagen.2013.05.005
241. M. L, McAuley E, Zhang Y, et al. Comparison of binding characterization of two antiviral drugs to human serum albumin. Chem Biol Drug Des. 2014;83(5):576–582. doi:10.1111/cbdd.12270
242. Wang Z, Li J, Cho J, Malik AB. Prevention of vascular inflammation by nanoparticle targeting of adherent neutrophils. Nat Nanotechnol. 2014;9(3):204–210. doi:10.1038/nnano.2014.17
243. Bachmaier K, Stuart A, Singh A, et al. Albumin nanoparticle endocytosing subset of neutrophils for precision therapeutic targeting of inflammatory tissue injury. ACS Nano. 2022;16(3):4084–4101. doi:10.1021/acsnano.1c09762
244. Chu D, Gao J, Wang Z. Neutrophil-mediated delivery of therapeutic nanoparticles across blood vessel barrier for treatment of inflammation and infection. ACS Nano. 2015;9(12):11800–11811. doi:10.1021/acsnano.5b05583
245. Zhu S, Zhang J, Xue N, et al. Highly specific neutrophil-mediated delivery of albumin nanoparticles to ectopic lesion for endometriosis therapy. J Nanobiotechnol. 2023;21(1):81. doi:10.1186/s12951-023-01831-4
246. Geldenhuys WJ, Mohammad AS, Adkins CE, Lockman PR. Molecular determinants of blood-brain barrier permeation. Ther Deliv. 2015;6(8):961–971. doi:10.4155/tde.15.32
247. Merlot AM, Kalinowski DS, Richardson DR. Unraveling the mysteries of serum albumin—more than just a serum protein. Front Physiol. 2014;5. doi:10.3389/fphys.2014.00299
248. Lin T, Zhao P, Jiang Y, et al. Blood-brain-barrier-penetrating albumin nanoparticles for biomimetic drug delivery via albumin-binding protein pathways for antiglioma therapy. ACS Nano. 2016;10(11):9999–10012. doi:10.1021/acsnano.6b04268
249. Hahn A, Schain M, Erlandsson M, et al. Modeling strategies for quantification of in vivo (18)F-AV-1451 binding in patients with tau pathology. J Nucl Med. 2017;58(4):623–631. doi:10.2967/jnumed.116.174508
250. Abdallah M, Mullertz OO, Styles IK, et al. Lymphatic targeting by albumin-hitchhiking: applications and optimisation. J Control Release. 2020;327:117–128. doi:10.1016/j.jconrel.2020.07.046
251. Zhang Y, Pan J, H. L, et al. Albumin based nanomedicine for enhancing tacrolimus safety and lymphatic targeting efficiency. J Biomed Nanotechnol. 2019;15(6):1313–1324. doi:10.1166/jbn.2019.2777
252. Yang M, Li J, Gu P, Fan X. The application of nanoparticles in cancer immunotherapy: targeting tumor microenvironment. Bioact Mater. 2021;6(7):1973–1987. doi:10.1016/j.bioactmat.2020.12.010
253. Lee JY, Kim HY, Lee YS, Jeong JM. Naphthol Blue Black and (99m)Tc-labeled mannosylated human serum albumin ((99m)Tc-MSA) conjugate as a multimodal lymph node mapping nanocarrier. Sci Rep. 2018;8(1):13636. doi:10.1038/s41598-018-31933-1
254. Deng C, Zheng M, Xin J, An F. A nanoparticle composed of totally hospital-available drugs and isotope for fluorescence/SPECT dual-modal imaging-guided photothermal therapy to inhibit tumor metastasis. J Colloid Interface Sci. 2023;651:384–393. doi:10.1016/j.jcis.2023.07.163
255. Kim SY, Noh YW, Kang TH, et al. Synthetic vaccine nanoparticles target to lymph node triggering enhanced innate and adaptive antitumor immunity. Biomaterials. 2017;130:56–66. doi:10.1016/j.biomaterials.2017.03.034
256. Song Y, Bugada L, R. L, et al. Albumin nanoparticle containing a PI3Kgamma inhibitor and paclitaxel in combination with alpha-PD1 induces tumor remission of breast cancer in mice. Sci Transl Med. 2022;14(643):eabl3649. doi:10.1126/scitranslmed.abl3649
257. Nagashima M, Hitchcock PF. Inflammation regulates the multi-step process of retinal regeneration in Zebrafish. Cells. 2021;10(4):783. doi:10.3390/cells10040783
258. King RS, Newmark PA. The cell biology of regeneration. J Cell Biol. 2012;196(5):553–562. doi:10.1083/jcb.201105099
259. Akbari H, Askari E, Naghib SM, Salehi Z. Bovine serum albumin-functionalized graphene-decorated strontium as a potent complex nanoparticle for bone tissue engineering. Sci Rep. 2022;12(1):12336. doi:10.1038/s41598-022-16568-7
260. Zhang B, Li J, He L, Huang H, Weng J. Bio-surface coated titanium scaffolds with cancellous bone-like biomimetic structure for enhanced bone tissue regeneration. Acta Biomater. 2020;114:431–448. doi:10.1016/j.actbio.2020.07.024
261. Han L, Wang M, Sun H, et al. Porous titanium scaffolds with self-assembled micro/nano-hierarchical structure for dual functions of bone regeneration and anti-infection. J Biomed Mater Res A. 2017;105(12):3482–3492. doi:10.1002/jbm.a.36178
262. Son B, Kim M, Won H, et al. Secured delivery of basic fibroblast growth factor using human serum albumin-based protein nanoparticles for enhanced wound healing and regeneration. J Nanobiotechnol. 2023;21(1):310. doi:10.1186/s12951-023-02053-4
263. Shojania HR, Momeni-Moghaddam M, Hossini SE, Armin M, Omrani Bidi J. MicroRNA 155 downregulation by vitamin c-loaded human serum albumin nanoparticles during cutaneous wound healing in mice. Int J Low Extrem Wounds. 2019;18(2):143–152. doi:10.1177/1534734619842975
264. Li Y, Fu R, Duan Z, Zhu C, Fan D. Injectable hydrogel based on defect-rich multi-nanozymes for diabetic wound healing via an oxygen self-supplying cascade reaction. Small. 2022;18(18):e2200165. doi:10.1002/smll.202200165
265. Gao X, Han Z, Huang C, et al. An anti-inflammatory and neuroprotective biomimetic nanoplatform for repairing spinal cord injury. Bioact Mater. 2022;18:569–582. doi:10.1016/j.bioactmat.2022.05.026
266. Liu D, G. L, Shi B, et al. ROS-scavenging hydrogels synergize with neural stem cells to enhance spinal cord injury repair via regulating microenvironment and facilitating nerve regeneration. Adv Healthc Mater. 2023;12(18):e2300123. doi:10.1002/adhm.202300123
267. An FF, Zhang XH. Strategies for preparing albumin-based nanoparticles for multifunctional bioimaging and drug delivery. Theranostics. 2017;7(15):3667–3689. doi:10.7150/thno.19365
268. Amighi F, Emam-Djomeh Z, Labbafi-Mazraeh-Shahi M. Effect of different cross-linking agents on the preparation of bovine serum albumin nanoparticles. J Iran Chem Soc. 2020;17(5):1223–1235. doi:10.1007/s13738-019-01850-9
269. Gong H, Fu H, Zhang J, et al. Preparation of soybean protein-based nanoparticles and its application as encapsulation carriers of bioactive substances. Lwt. 2024;191:115680. doi:10.1016/j.lwt.2023.115680
270. Matsumoto A, Chen J, Collette AL, et al. Mechanisms of silk fibroin sol-gel transitions. J Phys Chem B. 2006;110(43):21630–21638. doi:10.1021/jp056350v
271. Lozano-Perez AA, Gil AL, Perez SA, et al. Antitumor properties of platinum(iv) prodrug-loaded silk fibroin nanoparticles. Dalton Trans. 2015;44(30):13513–13521. doi:10.1039/C5DT00378D
272. Zhang C, Yang K, Yang G. Design strategies for enhancing antitumor efficacy through tumor microenvironment exploitation using albumin-based nanosystems: a review. Int J Biol Macromol. 2024;258(Pt 2):129070. doi:10.1016/j.ijbiomac.2023.129070
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


