Back to Journals » International Journal of Women's Health » Volume 16
Analysis of Risk Factors for Secondary Endometrial Cancer-Related Death: A SEER-Based Study
Authors Miao L, Feng S, Ding B, Zhang K, Ding Y, Shen Y
Received 19 March 2024
Accepted for publication 18 July 2024
Published 31 July 2024 Volume 2024:16 Pages 1303—1313
DOI https://doi.org/10.2147/IJWH.S469642
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Lianjie Miao, Songwei Feng, Bo Ding, Ke Zhang, Yue Ding, Yang Shen
Department of Obstetrics and Gynecology, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, People’s Republic of China
Correspondence: Yang Shen, Department of Obstetrics and Gynecology, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, Jiangsu, 210096, People’s Republic of China, Email [email protected]
Purpose: The aim of this study was to analyze the survival of patients with endometrial cancer diagnosed after a prior cancer and identify risk factors of endometrial cancer death in this population.
Methods: Totally 1371 women diagnosed with second primary endometrial cancer (SPEC) between 2004 and 2015 were identified using the SEER database. Clinicopathological characteristics were collected, and Fine and Gray regression model was employed to assess the impact of treatment for the first primary cancer (FPC) and SPEC on the mortality of endometrial cancer patients. After propensity score matching (PSM), patients diagnosed with single primary endometrial cancer and SPEC between 2004 and 2015 were included as the second cohort. Kaplan-Meier and Cox survival risk models were used to assess the influence of previous cancer history on survival.
Results: Patients previously diagnosed as lung cancer exhibited the lowest overall survival (OS). A diagnostic interval of ≥ 3 years was significantly associated with higher mortality from SPEC compared with that < 3 years. Surgical treatment for SPEC was linked to a reduced risk of endometrial cancer-specific mortality (ECSM) and non-ECSM. Conversely, radiotherapy and chemotherapy were associated with an increased risk of ECSM. The 1-, 3-, and 5-year OS rates of patients with SPEC were significantly lower than those with single primary endometrial cancer whether before or after PSM. Univariate and multivariate analyses further demonstrated that endometrial cancer, either as FPC or SPEC, was independently associated with an increased risk for endometrial cancer-specific survival (ECSS) and OS.
Conclusion: Chemotherapy and radiotherapy for SPEC can elevate the risk of ECSM. Whether as FPC or SPEC, endometrial cancer is demonstrated to be a significant independent risk factor for ECSS and OS.
Keywords: second primary cancer, endometrial cancer, risk factor, overall survival, SEER database
Introduction
The global incidence of cancer has surged in recent decades due to a combination of a growing and aging population, advances in early cancer detection, and improved cancer treatments. In 2020, there were 19.3 million new cases of cancer worldwide, resulting in 10 million deaths.1 In 2023, the United States (US) was projected to have about 1.9 million new cases of cancer and 0.6 million cancer-related deaths.2 Notably, endometrial cancer will be the second most prevalent cancer in women after breast cancer.3 Despite the continuous increase in cancer incidence, the 5-year survival rate for all cancers in the US has improved significantly since the mid-1970s, with nearly two-thirds of cancer survivors living for more than 5 years after their initial diagnosis, which increases the risk of developing a second primary cancer (SPC).2,4 Based on established SEER criteria and previous literature, an SPC was defined as a distinct cancer that arises in a new organ or tissue at least two months after the diagnosis of the initial primary cancer, affects over 10% of young adult cancer patients and approximately 25% of older adult cancer patients.5,6 Currently, the etiology of SPC remains uncertain. It is reported that the occurrence of SPC is potentially associated with genetic susceptibility, negative effects caused by the first primary cancer (FPC) treatment, environmental influences and lifestyle choices.7–9
Several studies have explored the risk of SPC in patients with multiple cancer types, including ovarian, testicular, breast, and gastrointestinal cancers.10–14 However, there is limited information about the characteristics of the FPC related to different types of SPC, which hinders the formulation of early screening strategies.15 A previous study has demonstrated that the prior cancer history had an adverse effect on the overall survival (OS) of patients with primary gastric cancer.16 A study on the impact of previous malignancies on breast cancer revealed that breast cancer as a SPC was associated with a reduced survival, particularly in hormone receptor-positive women. Furthermore, breast cancer-related treatment could lower the risk of breast cancer-specific mortality.17 Nevertheless, it remains unknown regarding the factors influencing overall and cancer-specific survival in endometrial cancer patients with prior cancer history.
In this study, we analyzed the risk factors in patients with second primary endometrial cancer (SPEC) related death utilizing the data from Surveillance, Epidemiology, and End Results (SEER) database. This information will help us understand the clinicopathological features of SPEC, including the type of previous cancer and the diagnostic interval. These insights are crucial for guiding long-term surveillance and developing effective treatment strategies aimed at reducing mortality rates among individuals with endometrial cancer.
Materials and Methods
Data Source
The SEER database, a population-based cancer registry sponsored by the National Cancer Institute, collects comprehensive data on patient demographic characteristics, cancer incidence, treatment, and survival. For our study, two distinct patient cohorts from the SEER database were utilized for separate analyses. We utilized SEER*Stat version 8.4.2 software to extract the patient information from the SEER database based on Incidence - SEER Research Data, 17 Registries (covering approximately 26.5% of the US population according to the 2020 Census data). The flowchart is shown in Supplementary Figure 1. The patient information had been researched by the United States Department of Health and Human Services. The data is publicly available and de-identified after permission. Therefore, the research did not require participant consent and was exempted by the ethics committee of Zhongda Hospital, Southeast University.
Inclusion and Exclusion Criteria of the First Cohort
Inclusion criteria: (1) Female patients initially diagnosed with endometrial cancer between January 2004 and December 2015; (2) Patients with pathologically confirmed primary tumors and subsequent diagnosis of SPEC. Exclusion criteria: (1) Patients with endometrial cancer as the FPC; (2) Patients with missing information; (3) Patients with previous non-malignant tumors; (4) Patients with an interval of less than 2 months between the occurrence of both primary cancers; and (5) Patients diagnosed only through a death certificate or through an autopsy.
In accordance with the above criteria, we conducted screening using the Case Listing and Person Selection modules in the SEER database. Subsequently, a total of 1371 patients with SPEC were ultimately included in the study. The demographic, clinical, and pathological data extracted from the SEER database included race, age, marital status, tumor differentiation, American Joint Committee on Cancer (AJCC, 6th edition) staging, disease staging, histology, surgical treatment, radiotherapy, chemotherapy, survival time, and cause of death.
Variable Definitions
According to ICD-O-3 morphology codes (8140, 8210, 8255, 8260, 8262, 8323, 8380, 8480, 8481, 8570, and 8574; 8310, 8441, 8460, and 8461), histological subtypes were mainly classified as type I known as endometrial adenocarcinoma (endometrial adenocarcinoma, endometrioid, mucinous adenocarcinoma, and squamous differentiated adenocarcinoma) and type II (clear cell carcinoma and serous carcinoma), and the remaining were as others. According to the SEER summary staging criteria, disease staging at the time of diagnosis was classified as local, regional, and distant, and regional and distant cancers were defined as non-local cancers. The survival data in the dataset were recorded in months, including the time between the diagnosis of prior cancer and endometrial cancer, as well as the duration between the diagnosis of endometrial cancer and either death or the last follow-up. The survival status of each patient was classified as either alive, dead due to endometrial cancer, previous cancer, or other causes.
Inclusion and Exclusion Criteria of the Second Cohort
Inclusion criteria: (1) Female patients initially diagnosed as endometrial cancer between January 2004 and December 2015. (2) Patients with pathologically confirmed single primary endometrial cancer (PEC) and those with multiple primary cancers with SPEC secondary to the FPC. Exclusion criteria were the same as the first cohort.
A total of 71,376 patients with endometrial cancer were eligible for the second cohort, comprising 70,005 patients with single PEC and 1371 patients with SPEC (Supplementary Figure 1). To mitigate the confounding bias of the included cases, propensity score matching (PSM) analysis with 1:1 nearest-neighbor matching was performed on all the clinicopathological characteristics affecting the prognosis, except the primary diagnosis. Endometrial cancer-specific survival (ECSS) was defined as the period from the diagnosis of endometrial cancer to the time of death attributable to endometrial cancer. OS was defined as the duration from the diagnosis of endometrial cancer to death resulting from any cause.
Statistical Analysis
The demographic and clinicopathological characteristics of patients who succumbed to endometrial cancer were compared with those who died from FPC using Student’s t-test and chi-square test. Fine and Gray competing risk regression models were utilized to assess the association of treatments targeting the FPC and SPEC with the risk of endometrial cancer-specific mortality (ECSM) defined as the proportion of deaths caused specifically by endometrial cancer, and the death from prior cancer or other causes was set as a competing risk. Survival between the two groups in the second cohort was compared using Kaplan-Meier analysis. The factors influencing ECSS and OS were evaluated through univariate and multivariate Cox regression analyses. Additionally, subgroup analyses were conducted based on histological subtypes and AJCC staging. All statistical tests were two-sided, with a significance level of p<0.05. All statistical analyses were performed using SPSS software (version 25.0, IBM, USA) and R software (version 4.2.2, R Foundation for Statistical Computing).
Results
Patient Characteristics of the First Cohort
Of the 1371 patients, the most common first primary tumor site was the breast (60.6%), followed by colorectum (15.5%), gynecological cancers including ovary, cervix, vagina and vulva (5.5%), lymph (5.3%), kidney (4.2%), lung and bronchus (2.8%) and thyroid (1.5%). The highest number of patients diagnosed with a previous cancer was in the 60–64 year group (222 cases, 16.2%), while that of patients with diagnosis of SPEC was in the 65–70 year group (233 cases, 17.0%). The majority of patients were white (80.5%). Totally 1104 patients (80.5%) had a prior cancer diagnosis of AJCC staging I–II, while 1059 (77.2%) had a diagnosis of endometrial cancer of AJCC staging I–II. The median (interquartile range, IQR) interval between the two diagnoses was 34 months (IQR: 14–66 months). By the end of 2015, the median follow-up of patients was 76 months (IQR: 36–109 months), and 595 patients (43.4%) died during this period, among whom 203 (34.1%) died from endometrial cancer, 139 (23.4%) died from previous cancers, and 253 (42.5%) died from other causes (Table 1).
 |
Table 1 Summary Description of Demographic and Clinicopathological Factors (n=1371), n (%) |
Endometrial Cancer-Related Deaths in the First Cohort
There was a statistically significant difference in OS between patients with different primary tumor types (P = 0.026), with those having prior lung and bronchial cancers showing the shortest OS (Figure 1A). Patients were stratified based on the death cause. Notably, 34.1% of patients died from endometrial cancer, whereas 23.4% died from prior cancers. Patients with prior gynecological cancers had the lowest ECSM (14.9%), while those with previous lymphoma had the highest ECSM (37.1%). The proportion of patients who died from endometrial cancer was higher than those who died from prior breast cancer (36.8% vs 23.4%), colorectal cancer (26.4% vs 25.3%), lymphoma (37.1% vs 17.1%), and renal cancer (31.8% vs 22.7%). However, it was lower than prior gynecological malignancies (14.9% vs 63.0%), lung and bronchial cancers (29.2% vs 41.7%), and thyroid cancers (28.6% vs 42.9%) (all P<0.05) (Figure 1B). Additionally, the number of patients who died from endometrial cancer with the diagnostic interval of ≥3 years was significantly higher than those with the interval of <3 years (P < 0.001).
Association of Clinicopathological Characteristics with Endometrial Cancer Mortality in the First Cohort
The rates of type II (33.8% vs 10.1%), chemotherapy (36.2% vs 9.1%), and high-grade disease (40.7% vs 12.2%), III–IV AJCC staging (40.7% vs 12.2%), and non-local cancers (33.4% vs 11.1%) of endometrial cancer were significantly higher in patients who died from endometrial cancer compared with those who died from previous cancer (all P < 0.05). Conversely, no statistical differences were found regarding the age, marital status, race, radiotherapy, and surgery. Additionally, the patients who died from endometrial cancer experienced a longer duration between the two cancer diagnoses compared to those who died from previous cancer (48.6 vs 29.9 months, P < 0.001).
Surgical treatment of patients with SPEC was associated with a reduced risk of ECSM [hazard ratio (HR): 0.260, 95% confidence interval (CI): 0.166–0.405, P < 0.001] and non-ECSM (HR: 0.231, 95% CI: 0.166–0.321, P < 0.001). However, radiotherapy and chemotherapy were associated with an increased risk of ECSM (HR: 1.357, 95% CI: 1.006–1.829, P = 0.045; HR: 5.035, 95% CI: 3.814–6.645, P < 0.001), but not non-ECSM. The site of the primary tumor in endometrial cancer patients was not significantly associated with ECSM or non-ECSM. The histological type II was also correlated with increased ECSM (HR: 3.958, 95% CI: 2.931–5.344, P < 0.001) and non-ECSM (HR: 1.659, 95% CI: 1.258–2.188, P < 0.001) in endometrial cancer patients (Supplementary Figure 2). Moreover, the FPC-related treatment was differentially associated with tumor-specific mortality in patients. Specifically, surgical treatment was associated with a decreased risk of ECSM (HR: 0.466, 95% CI: 0.290–0.748, P = 0.02) and non-ECSM (HR: 0.426, 95% CI: 0.306–0.593, P < 0.001). Radiotherapy was linked to a decreased risk of non-ECSM (HR: 0.647, 95% CI: 0.525–0.798, P < 0.001), but not ECSM. Additionally, chemotherapy did not demonstrate a significant association with either ECSM or non-ECSM.
Survival of Patients with Endometrial Cancer as Previous Cancer or SPC in the Second Cohort
We analyzed 70,005 patients who only had endometrial cancer and 1371 that had endometrial cancer and a prior cancer. The 1-, 3-, and 5-year OS rates for SPEC patients were significantly lower than those for PEC patients (5-year: 80.0% vs 68.1%, P < 0.001). However, the 1-, 3-, and 5-year ECSS rates (5-year: 87.1% vs 85.3%, P = 0.061) did not show a statistically significant difference when compared to PEC. After PSM was applied to balance the baseline characteristics of PEC and SPEC patients, the clinicopathological characteristic between the PEC and SPEC groups were not statistically significant (Table 2). The 1-, 3-, and 5-year OS rates for SPEC patients were significantly lower than those for PEC patients (5-year: 80.0% vs 68.1%, P < 0.001). However, no statistically significant difference was observed between the two groups for ECSS (5-year: 87.1% vs 85.3%, P = 0.175) (Table 3; Figure 2).
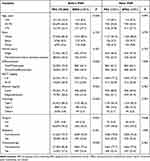 |
Table 2 Baseline Characteristics of Patients with PEC and SPEC, n (%) |
 |
Table 3 OS and ECSS of Patients with PEC and SPEC Before and After PSM |
Factors Associated with Survival in Patients Endometrial Cancer as a Single or SPC
Univariate and multivariate COX analyses were performed to determine the factors influencing survival. Based on the univariate analysis, age, race, marital status, tumor differentiation, AJCC staging, histological subtypes, disease staging, surgical treatment, chemotherapy, and radiotherapy were all found to be significant risk factors for both ECSS and OS (all P<0.001). In the multivariate analysis, age, race, tumor differentiation, AJCC staging, disease staging, status of endometrial cancer, surgical treatment, and radiotherapy were established as independent prognostic factors for both ECSS and OS. Interestingly, chemotherapy treatment was not an independent prognostic factor for OS (P =0.077) and ECSS (P =0.311) (Supplementary Table 1).
After stratification according to the subtype of endometrial cancer and AJCC staging, it was found that in type I, PEC and SPEC were both significant risk factors and independent prognostic factors for OS (P<0.001), but not for ECSS (P=0.14). However, a history of previous malignancies did not affect the OS (P=0.76) and cancer-specific survival (P=0.14) of patients with high-grade type I endometrial cancer.
Discussion
The population of cancer survivors is rapidly increasing, leading to a growing number of patients with multiple primary or multi-organ cancers. It has been previously documented that SPC is significantly more prevalent in patients with certain cancers than in the general population; the number of cancer survivors in the US is increasing by 2% per year, and up to 18% of American cancers are likely to be second malignant tumors based on the SEER data.18 Nevertheless, the clinicopathological factors associated with fatality and survival in patients with SPEC have not been clarified.
In this population-based study, breast cancer, colorectal cancer, gynecological cancer, lymphoma, kidney cancer, lung cancer, and thyroid cancer were found to be the most prevalent pre-existing cancer types, which may be linked to the fact that breast, ovarian, colorectal, and endometrial cancers were all common malignancies with genetic predisposition. Survivors with breast and ovarian cancers often face a heightened risk of developing endometrial cancer as a SPC due to mutations in genes such as BRCA1 or BRCA2.19 Additionally, some breast cancer patients may receive estrogen receptor antagonists like tamoxifen to prevent cancer recurrence. While these antagonists can lower the risk of breast cancer recurrence, they can also have a weak estrogenic impact on the endometrium, potentially promoting abnormal proliferation and leading to the development of endometrial polyps, endometrial hyperplasia, and even endometrial cancer.20,21 Hereditary colorectal cancer, accounting for about 3% of all colorectal cancers, often manifests as Lynch syndrome, generally caused by germline mutations in DNA mismatch repair genes (MSH2, MLH1, MSH6, PMS2). Individuals with Lynch syndrome are not only prone to colorectal cancer but also at risk of other extracolonic malignancies, with endometrial cancer being the most common one.22 Consequently, regular gynecological examinations and prompt completion of relevant genetic screening are advisable for high-risk patients.
In this study, 34.1% of patients with SPEC succumbed to endometrial cancer, while only 23.4% died due to prior cancers, among whom patients with a history of lung cancer had the shortest OS, and those with prior breast, colorectal, kidney cancers and lymphoma were more susceptible to succumb to endometrial cancer. Patients with prior gynecological cancers exhibited the lowest ECSM, likely due to heightened awareness regarding reproductive health. Regular gynecological examinations among these patients facilitate early detection of endometrial lesions. Significantly more patients died from endometrial cancer when the diagnostic interval was ≥3 years compared to those diagnosed within <3 years. These observations suggest that endometrial cancer, as a SPC, represents the principal cause of mortality in women with a history of cancer. Importantly, SPEC was linked to decreased survival rates. Whether before or after PSM, the 1-, 3-, and 5-year OS rates of patients with SPEC were markedly lower than those with PEC. Univariate and multivariate analyses demonstrated that endometrial cancer, as the FPC or SPC, independently posed a risk for ECSS and OS. Notably, upon stratification by endometrial cancer subtypes and AJCC staging, it was determined that a history of prior malignancies did not affect OS and ECSS in patients with high-grade endometrial adenocarcinoma, consistent with the findings by Mohamed et al.23 Thus, it is important to carefully assess advanced endometrial adenocarcinoma patients with a history of malignancies in clinical trials to ensure that they are not deprived of potential therapeutic benefits.
The high incidence of endometrial cancer and mortality among cancer survivors may be attributable to genetic mutations and systemic treatments, including chemotherapy and radiotherapy for prior cancers.24–26 Patients with FPC who have received systemic treatments such as radiotherapy and chemotherapy may result in SPECs that are less sensitive and tolerant to radiotherapy and chemotherapy. As shown in our study, surgical treatment for endometrial cancer was associated with a reduced risk of ECSM and non-ECSM, but radiotherapy and chemotherapy were associated with an increased risk of ECSM. Therefore, the selection of chemoradiotherapy for SPEC should be carefully considered in the context of the patient’s overall condition. Recent studies have highlighted the potential benefits of sentinel lymph node (SLN) mapping in endometrial cancer treatment. According to Bogani et al, SLN mapping offers a minimally invasive alternative for assessing nodal status without increasing morbidity, compared to traditional lymphadenectomy. The authors indicate that SLN mapping is effective in identifying nodal disease, including micrometastases and isolated tumor cells, which might be missed by conventional pathological examination. This technique allows for more accurate staging and tailored postoperative treatments based on precise nodal involvement. In another significant study, Cuccu et al analyzed the five-year oncologic outcomes of SLN mapping in high-intermediate and high-risk endometrial cancer patients. Their multi-institutional retrospective study showed that SLN mapping did not negatively impact 5-year disease-free survival and overall survival when compared to systematic lymphadenectomy. This suggests that SLN mapping can be a viable option even for higher-risk patients, potentially reducing surgery-related complications and improving quality of life. While an increasing number of studies are investigating the use of sentinel lymph node mapping in patients with endometrial cancer, we were unable to include this factor in our analysis due to the absence of relevant data in the SEER database.27,28
The study has several limitations that should be acknowledged. First, as a retrospective study, it was susceptible to selection bias. Although efforts were made to minimize this bias through PSM, residual confounders cannot be entirely excluded. Second, the lack of detailed information on genetic or environmental factors and treatments may have impacted the outcomes. Additionally, the study only encompassed the data from cancer patients in the US, which may affect the generalization of the research results. In the future, patients from other regions will be included to comprehensively analyze and to further validate our results.
Conclusion
For SPEC, chemotherapy and radiotherapy can elevate the risk of ECSM. Regarding the status of endometrial cancer, it is associated with an increased risk of ECSS and OS.
Ethics Statement
The patient information had been researched by the United States Department of Health and Human Services. The data is publicly available and de-identified after permission. Therefore, the research did not require participant consent and was exempted by the ethics committee of Zhongda Hospital, Southeast University.
Acknowledgments
The authors thank the SEER database.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received support from the National Natural Science Foundation of China (No. 82072078), Jiangsu Provincial Commission of Health (M2022016), and High-level hospital platform construction (scientific research) project (CZXM-GSP-KY-2023GSPKY11).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Article. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. Article. CA Cancer J Clin. 2023;73(1):17–48. doi:10.3322/caac.21763
3. Miller KD, Nogueira L, Devasia T, et al. Cancer treatment and survivorship statistics, 2022. Article. CA Cancer J Clin. 2022;72(5):409–436. doi:10.3322/caac.21731
4. Wood ME, Vogel V, Ng A, Foxhall L, Goodwin P, Travis LB. Second malignant neoplasms: assessment and strategies for risk reduction. Review. J Clin Oncol. 2012;30(30):3734–3745. doi:10.1200/jco.2012.41.8681
5. Murphy CC, Gerber DE, Pruitt SL. Prevalence of prior cancer among persons newly diagnosed with cancer an initial report from the surveillance, epidemiology, and end results program. Article. JAMA Oncol. 2018;4(6):832–836. doi:10.1001/jamaoncol.2017.3605
6. Adjei Boakye E, Buchanan P, Hinyard L, et al. Trends in the risk and burden of second primary malignancy among survivors of smoking-related cancers in the United States. Int J Cancer. 2019;145(1):143–153. doi:10.1002/ijc.32101
7. Mensi C, Stella S, Dallari B, et al. Second primary cancers in a Population-Based Mesothelioma Registry. Article. Cancers. 2023;15(6):1746. doi:10.3390/cancers15061746
8. Elad S, Yarom N, Zadik Y, Davies A. Immunotherapy-related oral adverse effects: immediate sequelae, chronicity and secondary cancer. Article. Cancers. 2023;15(19):4781. doi:10.3390/cancers15194781
9. Tabuchi T, Ito Y, Ioka A, Nakayama T, Miyashiro I, Tsukuma H. Tobacco smoking and the risk of subsequent primary cancer among cancer survivors: a retrospective cohort study. Article. Ann Oncol. 2013;24(10):2699–2704. doi:10.1093/annonc/mdt279
10. Xu JQ, Huang C, Wu ZY, et al. Risk prediction of second primary malignancies in primary early-stage ovarian cancer survivors: a SEER-based national population-based cohort study. Front Oncol. 2022:12875489. doi:10.3389/fonc.2022.875489
11. Fu HC, Talluri S, Rai S, Liang LF, Trivedi J, Ankem MK. Identification of risk factors and prediction models for secondary malignant neoplasms (SMNs)-free survival and SMNs-specific survival in testicular cancer survivors. World J Urol. 2023;41(9):2413–2420. doi:10.1007/s00345-023-04515-8
12. Raymond JS, Hogue CJR. Multiple primary tumours in women following breast cancer, 1973–2000. Br J Cancer. 2006;94(11):1745–1750. doi:10.1038/sj.bjc.6603172
13. Shah BK, Khanal A, Hewett Y. Second primary malignancies in adults with gastric cancer - a US population-based study. Front Oncol. 2016;682. doi:10.3389/fonc.2016.00082
14. Jia HX, Li QG, Yuan J, Sun XD, Wu ZY. Second primary malignancies in patients with colorectal cancer: a population-based analysis. Oncologist. 2020;25(4):E651–E658. doi:10.1634/theoncologist.2019-0266
15. Ruan Z, Zhang Y, Li Z, et al. Characteristics and classification of first primary cancer patients with second primary cancer: a population-based cohort study. Article; Early Access. Clin Exp Med. 2023;23(8):5051–5062. doi:10.1007/s10238-023-01149-3
16. Chen Y, Sun R, Liu W. Impact of a previous cancer history on the overall survival of patients with primary gastric cancer: a SEER population-based study. Article. Ejso. 2022;48(10):2159–2165. doi:10.1016/j.ejso.2022.06.022
17. Ji F, Yang CQ, Li XL, et al. Risk of breast cancer-related death in women with a prior cancer. Aging. 2020;12(7):5894–5906. doi:10.18632/aging.102984
18. Smyth EC, Tarazona N, Peckitt C, et al. Exclusion of gastrointestinal cancer patients with prior cancer from clinical trials: is this justified? Clin Colorect Cancer. 2016;15(2):E53–E59. doi:10.1016/j.clcc.2015.11.003
19. de Jonge MM, Mooyaart AL, Vreeswijk MPG, et al. Linking uterine serous carcinoma to BRCA1/2-associated cancer syndrome: a meta-analysis and case report. Review. Eur J Cancer. 2017;72:215–225. doi:10.1016/j.ejca.2016.11.028
20. Conte P, Frassoldati A. Aromatase inhibitors in the adjuvant treatment of postmenopausal women with early breast cancer: putting safety issues into perspective. Article. Breast J. 2007;13(1):28–35. doi:10.1111/j.1524-4741.2006.00359.x
21. Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. Article. N Engl J Med. 2013;368(11):987–998. doi:10.1056/NEJMoa1209825
22. Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of lynch syndrome: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Article. Gastroenterology. 2014;147(2):502–526. doi:10.1053/j.gastro.2014.04.001
23. Mohamed HH, Sokkar MI, Afifi AM, Saad AM, Albarouki S, Al-Husseini MJ. Does a history of malignancy impact the survival of a subsequent endometrial adenocarcinoma? Should clinical trials eligibility criteria be revisited? Article. J Obstetrics Gynaecol. 2020;40(2):233–239. doi:10.1080/01443615.2019.1621808
24. Yu GH, Wei R, Li SF, et al. Risk and prognosis of second corpus uteri cancer after radiation therapy for pelvic cancer: a population-based analysis. Front Oncol. 2022:12957608. doi:10.3389/fonc.2022.957608
25. Rock CB, Chipman JJ, Parsons MW, et al. Second primary malignancies in diffuse large B-cell lymphoma survivors with 40 years of follow up: influence of chemotherapy and radiation therapy. Adv Radiat Oncol. 2022;7(6):101035. doi:10.1016/j.adro.2022.101035
26. Petersen GM, de Andrade M, Goggins M, et al. Pancreatic cancer genetic epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2006;15(4):704–710. doi:10.1158/1055-9965.Epi-05-0734
27. Bogani G, Giannini A, Vizza E, Di Donato V, Raspagliesi F. Sentinel node mapping in endometrial cancer. J Gynecol Oncol. 2024;35(1):e29. doi:10.3802/jgo.2024.35.e29
28. Cuccu I, Raspagliesi F, Malzoni M, et al. Sentinel node mapping in high-intermediate and high-risk endometrial cancer: analysis of 5-year oncologic outcomes. Eur J Surg Oncol. 2024;50(4):108018. doi:10.1016/j.ejso.2024.108018
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


