Back to Journals » Clinical Ophthalmology » Volume 18
Assessment of a Clinical Test for Detection of Alteration in Visual Perception Due to Astigmatism
Authors Gal E, Gispets J, Wilkins A, Zyroff M, Netanya E, Gantz L
Received 1 November 2023
Accepted for publication 18 December 2023
Published 7 March 2024 Volume 2024:18 Pages 723—733
DOI https://doi.org/10.2147/OPTH.S447627
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Eyal Gal,1,2 Joan Gispets,2 Arnold Wilkins,3 Meira Zyroff,1 Efrat Netanya,1 Liat Gantz1
1Department of Optometry and Vision Science, Hadassah Academic College, Jerusalem, Israel; 2University Vision Centre, Universitat Politècnica de Catalunya, Terrassa, Barcelona, Spain; 3Department of Psychology, University of Essex, Colchester, UK
Correspondence: Liat Gantz; Eyal Gal, Department of Optometry and Vision Science, Hadassah Academic College, 37 Hanevi’im St, Jerusalem, Jerusalem, 9101001, Israel, Email [email protected]; [email protected]
Purpose: Astigmatism blurs the retinal image of a circular spot along a particular orientation rendering it an elliptical shape. Astigmatic patients demonstrate adaptation to residual astigmatic blur that may affect their discrimination between oval and circular targets. The Wilkins Egg and Ball Test (WEBT) was created to detect altered visual perception due to residual astigmatic blur by discriminating a circle within a row of oval elements. This prospective, cross-sectional study examined the utility of WEBT in detecting uncorrected residual astigmatism on the perception of form symmetry in astigmatic and keratoconic participants as well as normal participants with induced astigmatism at four primary meridians.
Methods: The mean search time (sT) and number of errors (noE) of 33 non-astigmatic controls (mean age: 24± 5, range: 18– 43, 6 males), 23 astigmatic participants (mean age: 36± 12, range: 18– 43, 6 males) and 13 keratoconic participants (N=22 eyes, mean age: 36± 12, range: 18– 58, 6 males) were measured under baseline, and 2.00 DC induced cylinder at four primary meridians, and for uncorrected, spherical-correction only, and fully corrected conditions, respectively. Mean sT and noE were converted to Z-scores, combined for each condition, and compared using repeated measures ANOVA with post-hoc analysis.
Results: Combined Z-scores for the controls were significantly worse (p< 0.001) for all induced cylinder conditions. The induced 180° condition was significantly better than 45° and 90° conditions (p=0.04), but not the 135° condition. For both astigmatic and keratoconic cohorts, Z-scores of the uncorrected condition were significantly worse than the fully corrected condition (both p< 0.01), but the fully corrected and spherical-only conditions did not differ significantly (p=0.06 and p=0.05, respectively).
Conclusion: In accommodating young adults, WEBT detected altered visual perception due to overall blur, and moderate-high amounts of uncorrected induced astigmatism and keratoconus, but is not useful as a tool for detection of altered visual perception due to small residual astigmatic blur.
Keywords: astigmatism, Wilkins egg and ball test, visual search, keratoconus, search time, number of errors
A Letter to the Editor has been published for this article.
A Response to Letter by Dr Shuja has been published for this article.
Introduction
Astigmatism is one of the most common refractive errors managed in clinical practice.1 The presence of uncorrected astigmatism can negatively impact performance2 in clinical visual measures such as visual acuity,3 contrast sensitivity,4 and functional tasks such as reading, computer work, and driving.3 The exact influence of uncorrected astigmatism on visual function depends on the specific task, the interaction with other co-existing ocular aberrations, the spherical refractive state of the eye, higher-order aberrations, pupil size, and the accommodative state of the eye.4 Nevertheless, each diopter of uncorrected astigmatism has been estimated to reduce high contrast visual acuity by 1.5 lines and low contrast visual acuity by 1.4 lines.5 Furthermore, a spherical equivalent correction with residual uncorrected astigmatic refractive error has been shown to negatively impact contrast sensitivity, depending on the amount of residual uncorrected astigmatism.6 In children, ocular residual astigmatism may increase aberrations in the visual system, causing blurred vision, and even amblyopia, depending on its magnitude and orientation.7 Large amounts of uncorrected astigmatism in childhood can cause meridional amblyopia.8
Visual acuity is affected differently by overall defocus as opposed to astigmatic blur, especially at low blur strengths.9 Further, the minimum magnitude of postoperative astigmatism that negatively affects patients’ visual acuity, especially in pseudophakic patients with no accommodation, is unclear.10
Patients with habitual uncorrected astigmatism appear to be naturally adapted to their astigmatism, with their visual acuity less impaired by induced astigmatic blur compared to non-astigmatic controls with similar amounts of induced astigmatic blur.11 Astigmatic patients have also been found to be adapted to their residual astigmatic correction. This adaptation changes the perceived “neutral point” in images containing elements with horizontal and vertical elements, even after a brief period of adaptation.12 This perceptual adaptation may not be apparent in standard clinical measures such as visual acuity and contrast sensitivity. For clinical use as well as research studies examining visual quality for varying compensations in clinical populations such as post-surgical outcomes of intraocular lenses13 and fitting toric contact lenses,14 it may be useful to examine perceptual alterations due to residual uncorrected astigmatism.
Furthermore, keratoconic patients with good visual acuity that exhibit larger higher-order aberrations compared with normal control participants have been shown to have only mild correlations between their contrast sensitivity and their ocular higher-order aberrations.15 In other words, keratoconic patients with good visual acuity may have altered visual perception that current clinical tests, such as contrast sensitivity, visual acuity, and even higher-order aberrations, may not be able to detect. Therefore, it is useful to have a test that measures their perceptual function.
Astigmatism blurs the retinal image of a spot along a particular orientation. If one of the two line foci is on the retina, the image of an object point would result in a retinal image in the shape of a line. If the accommodative response causes the circle of least confusion to fall on the retina, the image of an object point would result in a retinal image in the shape of a circle. Otherwise, at any other foci plane, the object point would result in an elliptical shape on the retina.16 The clock dial test for the measurement of astigmatism is based on variations in the perception of oval shapes. It displays lines of varying axes (orientations) that vary in their widths.17 Similarly, the two-circle grid chart for the measurement of astigmatism that was introduced in 1930’s18 and again recently19 displays circles of varying orientations.
Induced astigmatic blur reduces the response times on the developmental eye movement test (DEM) which examines fixational and saccadic eye movements during reading and non-reading tasks, on the Digit Symbol Substitution test (DSST) which examines general information processing speeds, and on the Trail-Making Tests A and B (TMT A and TMT B) which examine motor speed, visual attention, mental flexibility, and motor function.20 However, these tests are not specifically designed to detect perceptual alterations produced by residual uncorrected astigmatism. Shape search reaction times have been shown to be reduced with overall blur,21 but the effect of astigmatic blur on the reaction times of shape search tasks has not been previously evaluated.
The Wilkins Egg and Ball Test (WEBT, Figure 1) was developed as a test to examine alterations in visual perception due to residual astigmatic blur. Zemax ray tracing software was applied to examine the effects of 2.00 DC of astigmatism on retinal images of circular and oval shapes (see Figure 2). Each point comprising the object is affected by astigmatism such that it becomes smeared or extended horizontally. As a consequence, the resulting overall shape no longer appears as a circle, but is extended either in the horizontal or the vertical meridians. For example, in Figure 2, the image on column “circle” and row “2.00X90deg” appears as an oval extended in the horizontal meridian rather than a circle. The oval shape in the column “oval1” and row “2.00X180deg” appears extended horizontally and is therefore more similar to a circle. Based on this simulation, a patient with residual astigmatism should encounter difficulty when asked to detect a circular image, as it is perceived to be elliptical. Therefore, the WEBT comprises rows of ten elements, nine randomly oriented ellipses, and one circular element. In the WEBT, the observer is asked to detect the circular element within the row.
The main goal of this prospective, cross-sectional study was to examine the utility of the WEBT in detecting uncorrected residual astigmatism due to its influence on the perception of form symmetry. We hypothesized that retinal image alteration due to residual uncorrected astigmatism and/or adaptation to the axis of astigmatic blur would disadvantage participants with residual uncorrected astigmatism in this task because blur would increase the similarity between circles and ellipses. If found to be a sensitive method for measuring perceptual deficits due to residual uncorrected astigmatic blur, the Wilkins Egg and Ball Test would be a new tool for research purposes.
Methods
Ethics Statement
All participants provided written informed consent. All protocols met the tenets of the Declaration of Helsinki and had been approved by the Hadassah Academic College Ethics Committee.
Participants
The prospective, cross-sectional study consisted of three cohorts: non-astigmatic control participants in which astigmatism was induced, astigmatic participants with 1.00 DC of astigmatism or greater, and keratoconic participants. The latter group was included as keratoconus is characterized by steepening and distortion, apical thinning,22 central scarring,23 and a cone-shaped protrusion of the cornea24 resulting in myopia, and a large irregular astigmatism.25,26 The inclusion criteria included ages ranging between 18 and 65, monocular best corrected LogMAR visual acuity of at least 0.00 for the non-astigmatic cohort, and 0.22 for the astigmatic and keratoconic cohorts, a spectacle cylindrical refraction of 0.75 DC or lower for the non-astigmatic cohort, and at least 1.00 DC for the astigmatic cohort. Keratoconic participants had an Amsler-Krumeich stage 2 or lower. Participants with amblyopia, uncontrolled systemic conditions, and ocular pathology (other than keratoconus for those in the keratoconus cohort), and those unable to identify the numbers within the circular or elliptical elements in the WEBT described below, were excluded.
Procedures
First, inclusion criteria were verified. After responding to a questionnaire regardingexclusion criteria including pre-existing ambylopia, past ocular surgery for strabismus, or uncontrolled systemic conditions. Subsequently, their distance visual acuity (6 m) was measured with the habitual correction using the View-M Chart system (VLC 1900, Korea). If the distance visual acuity following the over-refraction evaluation did not meet inclusion criteria, the participant was excluded. The habitual correction was measured with a lensometer (Charops CLM-7000, Huvitz, Korea).
For keratoconic participants, an over-refraction was performed and the best corrected visual acuity was measured. In addition, tomography (anterior and posterior corneal curvature) and pachymetry were recorded using the VX130 Analyzer (Luneau, FR), and the scarring was assessed using a slit-lamp biomicroscope (HS-500, Huvitz, Korea).
The Wilkins Egg and Ball Test
The WEBT (Figure 1) is a printed test that contains four pages, each with ten rows consisting of a circle randomly positioned among nine ellipses. The dimensions of the round elements are 1.0 cm × 1.0 cm, and the elliptical elements are 1.0 cm × 1.1 cm. The stroke width of the elements is 1 point. The mean horizontal and vertical inter-element spacing from center to center is 1.25 cm and 1.5 cm, respectively.
The test was placed on a table in a fully lit room, and the participants were asked to perform the test at a viewing distance of 40 cm, which was verified using a meterstick.
For each page presented, participants were asked to identify the circular element in each of 10 rows containing 10 elements by stating its number.
The pages were presented at random and non-sequentially to minimize memorization. All participants performed the test monocularly. The eye tested in the control and astigmatic cohorts was selected randomly, whereas both eyes of keratoconic participants were examined, as it is an asymmetric disease.23 For each trial, response time (in seconds) from the instruction to begin until the round element was detected in the last row (row 10) of the page, was measured using a stopwatch. In addition, the examiner counted and recorded the number of errors for each page.
Three cohorts participated in the experiment as described below.
Non-Astigmatic Control Participants
These participants were examined under five experimental conditions: fully corrected with their habitual correction and with a +2.00 DC cylindrical lens placed at 180, 90, 45, and 135 degree axes in front of the eye. Each condition was measured twice such that a total of 10 trials were measured.
Astigmatic Participants and Participants with Keratoconus
These participants were examined under three experimental conditions: with fully habitual near refractive correction, with only the spherical component of the refractive correction in a trial frame (ie, cylindrical component was uncorrected), and without any refractive correction (ie, fully uncorrected). Note that the spherical-component only condition was a condition in which just the spherical part of the prescription (in minus-cylinder form) was inserted in the trial frame. This is not the same as the spherical equivalent correction. When considering the interval of Sturm, in patients that are not accommodating, correcting only the spherical-component in the minus-cylinder form brings one of the foci lines onto the retina, while the other focal line is in front of the retina. Thus, in these patients, this ensures that in the spherical-correction only condition, there is astigmatic blur on the retina from the focal line that is in front of the retina.27
Each condition was measured twice such that a total of six trials were measured.
Statistical Analysis
The reaction time and number of errors were plotted against each other to examine the relationship between the variables. Due to their low correlation as described in the results, the reaction time and error scores were converted to Z-scores and combined for each condition. Z-scores enable performance comparison across multiple tests with varying distributions28 by ranking the individual performance relative to the mean and standard deviation of all scores of the fully corrected condition. Accordingly, a Z-score of zero is equivalent to the mean, and a Z-score of 1.00 is approximately one standard deviation above the mean. The Z-scores of the varying experimental conditions for each cohort were compared using a repeated measures ANOVA with post hoc analysis conducted to determine the significant pairwise comparisons.
P-values lower than 0.05 were considered significant. All statistical tests were calculated using SPSS statistical package version 25 (SPSS Inc, Chicago, IL).
Results
Participants
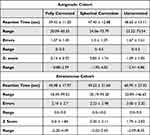
Three cohorts participated in this study. There were 32 participants in the non-astigmatic cohort, 23 participants in the astigmatic cohort, and 13 participants (22 eyes) in the keratoconic cohort. Their demographic information is tabulated in Table 1. The ages of the participants were not normally distributed and were compared using the Kruskal–Wallis test (H=18.59, p<0.001) which demonstrated that the ages of the three cohorts were significantly different, with the non-astigmatic cohort being younger than the other two cohorts.
 |
Table 1 Demographic Information for the Three Cohorts (Non-Astigmatic Controls, Astigmats, and Participants with Keratoconus) |
WEBT Results for Non-Astigmatic Control Participants
Correlations Between Reaction Time and Errors
The mean reaction time and number of errors for each condition for the non-astigmatic control cohort are tabulated in Table 2. To assess co-occurrence or a trade-off between reaction time and the number of errors,29 the relationship between the two outcome measures was examined using a Pearson correlation analysis after verifying that the outcome measures were normally distributed.30 The correlations for the induced cylinder at 45°90° 135° and 180° conditions were not significant (R=0.17, p=0.35; R=0.14, p=0.44, R=0.31, p=0.08, and R=0.20, p=0.27, respectively). However, the correlation for the baseline condition was positive and significant (R=0.47, p<0.008), indicating that reaction time and errors cannot be treated separately. Thus, a combined score of the two parameters was computed after calculating the Z-score of each outcome measure. After the Z-score of the reaction time and errors were computed, their sum was calculated yielding the combined Z-score for each condition for each participant. The combined Z-score was used in subsequent analyses. This strategy was also used for astigmatic and KC groups.
Z-scores and their ranges for each condition are shown in Table 3. Induced astigmatism significantly increased the Z score for all induced cylinder conditions (F(df4,128<0.01)=6.51, p<0.001, Figure 3).
Post-hoc analysis demonstrated that the Z-score for the baseline (fully corrected) condition (−0.30) was significantly lower than all induced cylinder conditions (p=0.001 for 45-degree, 90-degre, and 135-degree conditions, p=0.004 for the 180-degree condition). The Z scores for the 180-degree induced cylinder (0.93) and 135-degree-induced cylinder conditions (1.17) were not significantly different from each other (p=0.40). The 180-degree induced cylinder condition was significantly better than the induced cylinder at 45-degree and 90-degree conditions (both approximately 1.60, p-values= 0.045, and 0.042, respectively).
WEBT Results for Astigmatic Participants
The mean reaction time and number of errors for each condition for the participants with astigmatism are tabulated in Table 2, with Z-scores comparing between the conditions shown in Figure 4. There was a significant effect of condition (RM ANOVA, F(df=2,50)= 4.00, p< 0.05) with post hoc analysis demonstrating a significant difference between the uncorrected vs fully corrected conditions (p<0.05). There was no significant difference between the full correction and spherical correction conditions (p=0.06) or the uncorrected and spherical correction conditions (p=0.47).
WEBT Results for Keratoconus Participants
The mean reaction time and number of errors for each condition for the cohort of keratoconus participants are tabulated in Table 2, with Z-scores comparing between the conditions shown in Figure 3. There was a significant effect of condition (RM ANOVA, F(df=2,42)= 16.65, p<0.01) with post-hoc analysis demonstrating that the difference was between the uncorrected and both the full and partially corrected conditions (p=0.001, p<0.001). There was no significant difference between the fully corrected and spherical correction conditions (p=0.05).
Discussion
This study examined if the WEBT could be a useful tool in detecting alterations in visual perception due to uncorrected astigmatism in three experimental cohorts. The WEBT was useful in detecting altered visual perception for uncorrected astigmatism in all meridians when astigmatism of 2.00 DC was induced. However, in the cohort of participants with clinical astigmatism, WEBT was useful in detecting altered visual perception only in fully uncorrected refractive error condition. When only the spherical component of refraction was corrected, the WEBT was not sensitive in detecting alterations in visual perception. This may be due to the fact that the cohort of normal participants is not accustomed to blurred perception, as opposed to astigmatic and keratoconic participants who may experience blurred vision more frequently and who adapt to their residual uncorrected astigmatism.31,32
This point could be addressed in a future experiment comparing the results of the test in two cohorts with similar magnitudes of astigmatism and with similar ocular wavefronts. The two cohorts being newly diagnosed vs long-standing astigmatism.
In the cohort of participants with keratoconus, WEBT was sensitive in discriminating alterations of visual perception in uncorrected astigmatism. Although it was not sensitive enough to discriminate between alterations in visual perception in the spherical-only corrected condition compared with full correction, the p-value approached significance (p=0.05) and could perhaps have been significant if a larger cohort had been recruited.
Correction of the spherical component significantly affected the performance only of the keratoconic cohort, possibly due to the fact that the mean spherical refractive error for keratoconics is much larger than for the other two cohorts.
One possible explanation for the differences in WEBT’s sensitivity to alterations in visual perception due to uncorrected astigmatic blur in the keratoconic cohort compared with the astigmatic cohort could be the amount of uncorrected cylindrical error, which is approximately 1.00 DC higher in the keratoconic cohort (−2.30 DC vs −3.19 DC).
Thus, WEBT, when used in young adult participants who were tested without cycloplegia, can be concluded as being sensitive to alterations in visual perception due to large amounts of uncorrected overall blur, but not sensitive enough to alterations in visual perception due to uncorrected astigmatic blur.
In the normal cohort, induced astigmatism at 45-degrees and 90-degrees impacted Z scores significantly higher than the 180-degree condition. This finding is similar to that of Hasegawa et al 6 who reported that induced against-the -rule astigmatism deteriorated the contrast sensitivity more than induced with-the- rule astigmatism.
Strengths and Limitations of the Study
Only subjects with mild-to-moderate KC participated in this study, as those with advanced KC could not read the WEBT in the uncorrected condition. Therefore, our conclusions are only applicable to mild-to-moderate KC patients.
Additionally, in the present study, the normal participants were significantly younger (12 years on average) than the astigmatic and KC groups, which could potentially affect the results. Younger patients have the ability to accommodate, which can influence the magnitude of astigmatism and its axis.33,34 Further, activation of accommodation in the spherical-only corrected condition affects the position of the image plane with respect to the interval of Sturm. In non-accommodating eyes, the spherical-only corrected condition is expected to bring one of the focal lines onto the retina, while the other focal line is in front of the retina. In accommodating eyes, the position of the lines of foci is affected by the amount of activated accommodation35 which is a limitation in the present investigation. However, changes in astigmatism due to accommodation have been shown to be clinically relevant for large accommodative demands exceeding 4.00 DS.34 Future investigations should compare these outcomes in participants without the ability to accommodate by use of cycloplegic agents and compare between cohorts of similar age distributions.
Finally, this study could have benefited from direct comparison of the results with existing clinical tests for detection of astigmatism, which would contextualize its utility and highlight potential advantages or limitations compared with the existing clinical tests.
Conclusions
The WEBT was found to be sensitive to altered visual perception due to overall uncorrected blur in accommodating young adults, in moderate-high amounts of uncorrected astigmatism in keratoconic patients, and when induced in non- astigmatic young adults. It cannot be recommended as a useful clinical or research tool for examining alterations in visual perception in cases with uncorrected residual astigmatism.
Acknowledgment
Preliminary findings of this study were previously presented at the Academy for Research in Vision and Ophthalmology conference in 2021.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Parts of this study were funded by the Hadassah Academic College Conference and Research Board grant awarded to Eyal Gal.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Read SA, Vincent SJ, Collins MJ. The visual and functional impacts of astigmatism and its clinical management. Ophthalmic Physiol Opt. 2014;34(3):267–294. doi:10.1111/opo.12128
2. Vinas M, De Gracia P, Dorronsoro C, et al. Astigmatism impact on visual performance: meridional and adaptational effects. Opto Vision Sci. 2013;90:1430-–1442
3. Black AA, Wood JM, Colorado LH, Collins MJ. The impact of uncorrected astigmatism on night driving performance. Ophthalmic Physiol Opt. 2019;39(5):350–357. doi:10.1111/opo.12634
4. Remón L, Monsoriu JA, Furlan WD. Influence of different types of astigmatism on visual acuity. J Optom. 2017;10(3):141–148. doi:10.1016/j.optom.2016.07.003
5. Wolffsohn JS, Bhogal G, Shah S. Effect of uncorrected astigmatism on vision. J Cataract Refract Surg. 2011;37(3):454–460. doi:10.1016/j.jcrs.2010.09.022
6. Hasegawa Y, Hiraoka T, Nakano S, Okamoto F, Oshika T. Effects of astigmatic defocus on binocular contrast sensitivity. PLoS One. 2018;13(8):1–9. doi:10.1371/journal.pone.0202340
7. Chou YS, Tai MC, Chen PL, Lu DW, Chien KH. Impact of cylinder axis on the treatment for astigmatic amblyopia. Am J Ophthalmol. 2014;157(4):908–914.e1. doi:10.1016/j.ajo.2013.12.020
8. Mitchell DE, Freeman RD, Millodot M, Haegerstrom G. Meridional amblyopia: evidence for modification of the human visual system by early visual experience. Vision Res. 1973;13(3):535–I. doi:10.1016/0042-6989(73)90023-0
9. Atchison DA, Mathur A. Visual acuity with astigmatic blur. Optometry Vision Sci. 2011;88(7):798–805. doi:10.1097/OPX.0b013e3182186bc4
10. Pelouskova M, Schallhorn S, Hettinger K, Pelouskova M. Effect of residual astigmatism on uncorrected visual acuity and patient satisfaction in pseudophakic patients. J Cataract Refract Surg. 2021;47(8):991–998. doi:10.1097/j.jcrs.0000000000000560
11. de Gracia P, Dorronsoro C, Marin G, Hernández M, Marcos S. Visual acuity under combined astigmatism and coma: optical and neural adaptation effects. J Vis. 2011;11(2):1–11. doi:10.1167/11.2.5
12. Vinas M, Sawides L, de Gracia P, Marcos S. Perceptual adaptation to the correction of natural astigmatism. PLoS One. 2012;7(9). doi:10.1371/journal.pone.0046361
13. Kurna SA, Şengör T, M Ü. Success rates in the correction of astigmatism with toric and spherical soft contact lens fittings. Clin Ophthalmol. 2010;4(1):959–966. doi:10.2147/opth.s9464
14. Villegas EA, Alcon E, Rubio E, Marín JM, Artal P. Refractive accuracy with light-adjustable intraocular lenses. J Cataract Refract Surg. 2014;40(7):1075–1084. doi:10.1016/j.jcrs.2013.10.046
15. Shneor E, Piñero DP, Doron R. Contrast sensitivity and higher-order aberrations in Keratoconus subjects. Sci Rep. 2021;11(1):1–9. doi:10.1038/s41598-021-92396-5
16. Pujol J, Arjona M, Arasa J, Badia V. Influence of amount and changes in axis of astigmatism on retinal image quality. J Opt Soc America A. 1998;15(9):2514. doi:10.1364/josaa.15.002514
17. Perches S, Collados MV, Ares J. Retinal image simulation of subjective refraction techniques. PLoS One. 2016;11(3):1–15. doi:10.1371/journal.pone.0150204
18. Greene EB. Two circle grid charts for measuring visual acuity and astigmatism. Am J Ophthalmol. 1932;15(8):716–720. doi:10.1016/S0002-9394(32)91646-8
19. Hypothesis M. Striped circle visual acuity chart; a novel visual acuity chart based on the Landolt-C chart. Med Hypoth Dis Innova Ophthal. 2018;7(1):10–12.
20. Yang SH. The impact of simulated astigmatism on functional measures of visual performance. Facul Health. 2015;2015:1.
21. Musa A, Lane AR, Ellison A. The effects of induced optical blur on visual search performance and training. Q J Exp Psychol. 2022;75((2)):277–288. doi:10.1177/17470218211050280
22. Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297–319. doi:10.1016/S0039-6257(97)00119-7
23. Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28(4):293–322. doi:10.1016/0039-6257(84)90094-8
24. Zadnik K, Barr JT, Edrington TB, et al. Corneal scarring and vision in keratoconus: a baseline report from the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) study. Cornea. 2000;19(6):804–812. doi:10.1097/00003226-200011000-00009
25. Li Y, Meisler DM, Tang M, et al. Keratoconus diagnosis with optical coherence tomography pachymetry mapping. Ophthalmology. 2008;115(12):2159–2166. doi:10.1016/J.OPHTHA.2008.08.004
26. Gordon-Shaag A, Millodot M, Ifrah R, Shneor E. Aberrations and topography in normal, keratoconus-suspect, and keratoconic eyes. Optometry Vision Sci. 2012;89(4):411–418. doi:10.1097/OPX.0b013e318249d727
27. Prangen AD. The significance of Sturm’s interval in refraction. Am J Ophthalmol. 1941;24(4):413–422. doi:10.1016/S0002-9394(41)94130-2
28. Pettitt RW. Evaluating strength and conditioning tests with Z scores: avoiding common pitfalls. Strength Conditioning J. 2010;32(5):100–103. doi:10.1519/SSC.0b013e3181efe0c0
29. Rasmussen LS, Larsen K, Houx P, Skovgaard LT, Hanning CD, Moller JT. The assessment of postoperative cognitive function. Acta Anaes Scand. 2001;45(3):275–289. doi:10.1034/j.1399-6576.2001.045003275.x
30. Gilchrist JM, Allen PM, Monger L, Srinivasan K, Wilkins A. Precision, reliability and application of the Wilkins rate of reading test. Ophthalmic Physiol Opt. 2021;41(6):1198–1208. doi:10.1111/opo.12894
31. Sabesan R, Yoon G. Neural compensation for long-term asymmetric optical blur to improve visual performance in keratoconic eyes. Invest Ophthalmol Vis Sci. 2010;51(7):3835–3839. doi:10.1167/iovs.09-4558
32. Son S, Shim WM, Kang H, Lee J. Automatic compensation enhances the orientation perception in chronic astigmatism. Sci Rep. 2022;12(1):1–11. doi:10.1038/s41598-022-07788-y
33. Borsting E. Clinical management of binocular vision. Optometry Vision Sci. 2014;91(3):e86. doi:10.1097/opx.0000000000000214
34. Lara-Lacárcel F, Marín-Franch I, Fernández-Sánchez V, Riquelme-Nicolás R, López-Gil N. Objective changes in astigmatism during accommodation. Ophthalmic Physiol Opt. 2021;41(5):1069–1075. doi:10.1111/opo.12863
35. Raasch TW. Spherocylindrical refractive errors and visual acuity. Optometry Vision Sci. 1995;72(4):272–275. doi:10.1097/00006324-199504000-00008
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
A Novel Procedure for Keratoconus/Corneal Ectasia Treating Epithelial Compensation of Higher-Order Aberrations, Topographic Guided Ablation, and Corneal Cross Linking – The CREATE+CXL Protocol
Motwani M
Clinical Ophthalmology 2023, 17:1981-1992
Published Date: 13 July 2023
Descemetic Deep Anterior Lamellar Keratoplasty versus Penetrating Keratoplasty in Advanced Keratoconus: Comparison of Visual and Refractive Outcomes
Spadea L, Di Genova L, Trovato Battagliola E, Scordari S
Therapeutics and Clinical Risk Management 2024, 20:127-138
Published Date: 16 February 2024