Back to Journals » Journal of Inflammation Research » Volume 17
Associations of HDL-C and ApoA-I with Mortality Risk in PCI Patients Across Different hsCRP Levels
Authors Yan K, Li J, Zhu P, Tang X, Li Y, Yang Y, Gao R, Yuan J, Zhao X
Received 20 February 2024
Accepted for publication 25 June 2024
Published 4 July 2024 Volume 2024:17 Pages 4345—4359
DOI https://doi.org/10.2147/JIR.S465015
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Ning Quan
Kailun Yan, Jiawen Li, Pei Zhu, Xiaofang Tang, Yulong Li, Yuejin Yang, Runlin Gao, Jinqing Yuan, Xueyan Zhao
National Clinical Research Center for Cardiovascular Diseases, State Key Laboratory of Cardiovascular Disease, Fu Wai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100037, People’s Republic of China
Correspondence: Xueyan Zhao; Jinqing Yuan, National Clinical Research Center for Cardiovascular Diseases, State Key Laboratory of Cardiovascular Disease, Fu Wai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100037, People’s Republic of China, Tel +86-10-88322451, Fax +86-10-68351786, Email [email protected]; [email protected]
Purpose: The association between high-density lipoprotein cholesterol (HDL-C) and apolipoprotein A-I (ApoA-I) and cardiovascular risk in patients with coronary artery disease remains inconsistent. Recent investigations indicated potential dysfunctionality of HDL under inflammation. This study endeavors to explore whether the inflammatory status modifies the effects of HDL-C and ApoA-I on cardiovascular risk in individuals with percutaneous coronary intervention (PCI).
Patients and Methods: Consecutive 10,724 PCI patients at Fuwai hospital in 2013 were enrolled. Inflammation status was defined by high-sensitivity C-reactive proteins (hsCRP) ≥ 2 mg/L. The study endpoint was cardiac mortality.
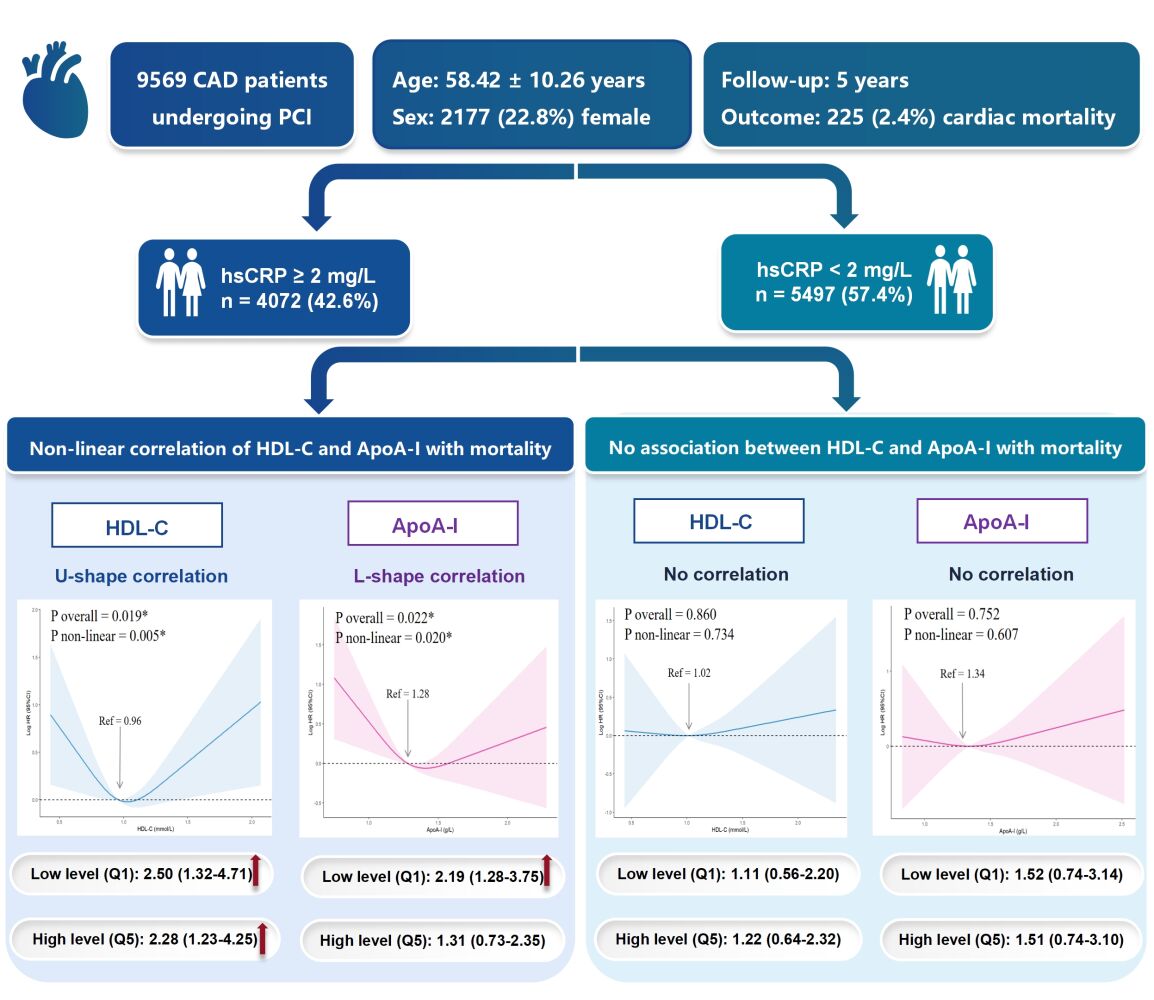
Results: Among 9569 PCI patients eventually included, 225 (2.4%) cardiac mortality happened during 5 years. In hsCRP ≥ 2 mg/L group, an U-shaped curve was observed for HDL-C and multivariate Cox regression showed that elevated risks of cardiac mortality correlated to both the lowest quintile (hazard ratio [HR], 2.50; 95% confidence interval [CI], 1.32– 4.71) and the highest quintile of HDL-C (HR, 2.28; 95% CI, 1.23– 4.25). However, an L-shaped curve existed in ApoA-I, indicating only the lowest quintile level of ApoA-I was associated with an increased cardiac mortality risk (HR, 2.19; 95% CI, 1.28– 3.75). Nevertheless, in hsCRP < 2 mg/L group, no significant correlations between HDL-C and ApoA-I and cardiac mortality risk were identified (both P > 0.05).
Conclusion: In PCI patients with hsCRP ≥ 2 mg/L. both low and high HDL-C levels correlated with higher cardiac mortality risk (U-shaped), while only low ApoA-I levels were linked to elevated risk (L-shaped). However, in patients with hsCRP < 2 mg/L, neither HDL-C nor ApoA-I levels were associated with higher cardiac mortality risk. These findings shed light on the importance of considering inflammation status, particularly hsCRP levels, in managing HDL-C and ApoA-I levels, and suggest targeting elevated ApoA-I levels as a potential therapeutic approach for PCI patients with hsCRP ≥ 2 mg/L.
Keywords: apolipoprotein A-I, high-density lipoprotein-cholesterol, inflammation, mortality, percutaneous coronary intervention
Graphical Abstract:
Introduction
Previous studies have focused on the protective role of high-density lipoproteins (HDLs) in coronary artery disease (CAD). HDL particles are made up of a bunch of lipids and proteins, represented by HDL-cholesterol (HDL-C) and apolipoprotein A-I (ApoA-I). Epidemiological studies from the general population found an inverse relationship between HDL-C and cardiovascular risk, leading to the belief that HDL-C is the crucial protective component of HDL particles.1,2 However, recent studies raised doubts about the protective role of HDL-C in CAD patients, showing that patients with high HDL-C levels may also have increased cardiovascular risk.3 As a result, increasing studies suggest that HDL-C, as the cholesterol component of HDL particles, may not fully reflect the structural and functional complexity of HDL particles.4 ApoA-I, the main protein constituent inside HDL particles, has been shown to perform various beneficial functions, including anti-inflammatory, antioxidant, and cholesterol efflux activities.5 Consequently, recent researchers are shifting focus to the impact of ApoA-I on cardiovascular risk and its potential as a novel therapeutic target in the CAD patients.6–8
Inflammation is strongly correlated with adverse outcomes in CAD patients undergoing percutaneous coronary intervention (PCI).9 High-sensitivity C-reactive protein (hsCRP) is a widely used biomarker of systematic inflammation because of its convenient measurement. In CAD patients undergoing PCI, hsCRP levels ≥ 2 mg/L indicate residual inflammatory risk and have been consistently associated with poor prognosis.10,11 In addition, clinical trials targeting anti-inflammatory therapy have classified CAD patients with hsCRP ≥ 2 mg/L as having persistent inflammation.12
More importantly, recent studies have found that hsCRP interacted with lipid metabolism in CAD patients undergoing PCI, especially the lipoprotein(a)-associated ischemic risk was significantly amplified when patients with hsCRP ≥ 2 mg/L.13,14 Given the controversial evidence regarding HDL-C in CAD patients, whether inflammation also interacts with HDL-related lipid metabolism and thereby affects the relationship between HDL-C, ApoA-I, and cardiovascular risk has not been explored. Previously, research has found that although HDL particles have anti-inflammatory effects, their composition and functionality are significantly altered in inflammatory conditions, such as autoimmune diseases.15 For example, in patients with rheumatoid arthritis and systemic lupus erythematosus, HDL particles exhibit increased levels of the inflammatory protein serum amyloid A, which replaces normal ApoA-I on the HDL particles, and show decreased levels of the antioxidant enzyme paraoxonase-1, resulting in pro-inflammatory HDL particles.16,17 Additionally, anti-HDL and anti-ApoA-I autoantibodies are detectable in these autoimmune disease patients, linking to disease activity and increased cardiovascular events.18,19 However, in real-world CAD patients undergoing PCI, it remains unclear whether inflammation also alters the function of HDL particles, specifically influencing their main components HDL-C and ApoA-I, and their relationship with cardiovascular risk.
Currently, despite receiving guideline-recommended secondary prevention strategies, CAD patients undergoing PCI still face a high risk of ischemic recurrence, leading to efforts to identify novel treatment targets.20 Existing guidelines primarily focus on managing low-density lipoprotein cholesterol levels, but due to recent conflicting evidence regarding HDL-C, there are no clear recommendations for managing HDL-C and ApoA-I levels.21,22 Therefore, in the era of precision medicine, it is crucial to utilize real-world data to explore whether inflammatory status alters the relationship between HDL-C, ApoA-I, and cardiac mortality risk in CAD patients undergoing PCI, as understanding these interactions may bridge the knowledge gap in managing HDL-C and ApoA-I levels in clinical practice, potentially improving prognoses for PCI patients. We aim to use real-world data from a sizable cohort to investigate the association between HDL-C, ApoA-I, and cardiac mortality risk in CAD patients undergoing PCI under different hsCRP levels. The hypothesis is that inflammation (indicated as hsCRP ≥ 2 mg/L) could significantly modify the association of HDL-C and ApoA-I with cardiac mortality in PCI patients.
Materials and Methods
Study Population
This prospective observational study was conducted at Fuwai Hospital (National Center for Cardiovascular Diseases, Beijing, China), and included 10,724 consecutive individuals who underwent PCI between January 2013 and December 2013. Participants with missing data on hsCRP, HDL-C, or ApoA-I, as well as those lost to follow-up, were excluded from the final analysis (refer to Figure 1).
Before PCI, all patients received aspirin and P2Y12 inhibitors. For those who had not been on antiplatelet therapy, a loading dose of 300 mg aspirin, along with either 300 mg clopidogrel or 180 mg ticagrelor, was administered. PCI was performed by interventional cardiologists who were blinded to the study protocol. The revascularization procedures followed contemporary practice guidelines23,24 and incorporated the expert judgment and preferences of interventional cardiologists. Following the PCI procedure, patients were prescribed an ongoing daily regimen of 100 mg aspirin, in conjunction with either 75 mg clopidogrel per day or 90 mg ticagrelor twice daily, for a minimum duration of 1 year.
All procedures involving human participants were conducted with informed consent. This study received approval from the Fu Wai Hospital Ethics Committee (Approval Number: 2013-449) and was conducted in accordance with the principles outlined in the Declaration of Helsinki.
Laboratory Test
Blood samples were collected within the first 24 hours of admission, following an overnight fasting period, and analyzed at Fuwai Hospital’s core laboratory using standard biochemical techniques. Using the LABOSPECT 008 analyzer (HITACHI, Japan), hsCRP levels were determined via the particle enhanced immunoturbidimetric assay (Ultrasensitive CRP kit; Aidian Oy, Espoo, Finland), with intra- and interassay imprecisions having coefficients of variation <7% and <12%, respectively. HDL-C levels were measured using a chemically modified enzyme method (Determiner L HDL kit; Kyowa Medex Co., Ltd., Tokyo, Japan). ApoA-I and apolipoprotein B levels were assessed using the immunoturbidimetric method (Apolipoprotein A1 FS and Apolipoprotein B FS multi-purpose kits; DiaSys Diagnostic Systems GmbH, Holzheim, Germany). Triglyceride levels were analyzed using the GPO-PAP method (TG Assay Kit; Biosino Bio-Technology and Science Incorporation, Beijing, China). Low-density lipoprotein cholesterol was measured using a selective solubilization method (Low Density Lipid Cholesterol Test Kit; Kyowa Medex Co., Ltd., Tokyo, Japan). Serum creatinine was measured with the sarcosine oxidase method (Weihai Weigao Biotech Co., Ltd., Shandong, China), and the estimated glomerular filtration rate (eGFR) was calculated using the Cockcroft–Gault formula. Based on the widely used cutoff point in clinical practice and cardiovascular research, inflammatory status was defined by hsCRP ≥ 2 mg/L in this study.10,12
Clinical Endpoint and Follow-Up
The clinical endpoint was cardiac mortality. A five-year follow-up period was chosen to capture long-term outcomes and ensure comparability with existing cardiovascular studies, providing a robust dataset to observe cardiac mortality post-PCI. Follow-up procedures encompassed 30 days, 6 months, 1 year, 2 years, and 5 years of interviews over the phone or visits to the clinic, demonstrating a robust 91.5% follow-up rate at the conclusion of the 5-year period. Time to event was computed as the period from the PCI intervention to the incidence of cardiac mortality or the date of disconnection from subsequent follow-up, whichever took place first. To maintain data quality, rigorous measures were adopted, such as investigator training, the utilization of blinded questionnaires, and the recording of telephone interactions. To ensure accuracy and consistency, two independent cardiologists meticulously analyzed and adjudicated all adverse events, settling any disagreements by agreement.
Statistical Analysis
Except for patients with missing baseline data for hsCRP, HDL-C, and ApoA-I, who were excluded from the analysis (as mentioned in the flowchart Figure 1), all other data in the study were complete. A power analysis using the pwr package in R software confirmed that with 9569 participants, a medium effect size (Cohen’s d = 0.5), and a significance level of 0.05, the study has a statistical power of 1, indicating adequate sample. Continuous variables with a normal distribution were presented as mean ± standard deviation and assessed using the Student’s t-test. Categorical variables were conveyed as numbers and percentages and compared through the Pearson Chi-square test or Fisher’s exact test. Patients were categorized into groups based on hsCRP levels (≥ 2 mg/L and < 2 mg/L), and all analytical procedures were employed both across the entire cohort and within distinct hsCRP subgroups. The HDL-C and ApoA-I were investigated as continuous and categorical (quintile) variables. To provide very low and very high levels of data granularity that included probable non-linear connections, quintile classification was adopted. Quintiles were determined by identifying the 20th, 40th, 60th, and 80th percentiles of HDL-C and ApoA-I levels in each group. These percentiles were then transformed into quintile ranges (Q1-Q5) to categorize the data for analysis across the overall population, hsCRP ≥ 2 mg/L, and hsCRP < 2 mg/L groups. The quintile ranges were defined as follows: Q1: ≤20th percentile; Q2: >20th to ≤40th percentile; Q3: >40th to ≤60th percentile; Q4: >60th to ≤80th percentile; Q5: >80th percentile. The specific values and range are represented in the Supplementary Table 1.
Firstly, on a continuous scale, the linear or non-linear correlations between the continuous levels of HDL-C and ApoA-I and cardiac mortality were determined using restricted cubic splines (RCS) in the overall cohort, hsCRP ≥ 2 mg/L group, and hsCRP < 2 mg/L group, respectively. The median levels of HDL-C and ApoA-I in each group were used as the reference point.
Secondly, the cumulative incidences of cardiac mortality over time of HDL-C and ApoA-I quintiles were generated using the Kaplan–Meier curves, and the differences between quintiles were compared using the Log rank test. Further, univariate (Model 1) and multivariate Cox regression model (Model 2) were performed to evaluate the associations of HDL-C and ApoA-I quintiles with cardiac mortality in overall cohort and distinct hsCRP groups. Multivariate Cox regression model (Model 2) was adjusted for positive variables in the univariate model for cardiac mortality, including age, chronic obstructive pulmonary disease, diabetes, hypertension, previous myocardial infarction, prior PCI, prior coronary artery bypass grafting, target vessel, eGFR < 90 mL/min, calcium channel blocker use (refer to Supplementary Table 2). The reference values were the quintiles of HDL-C and ApoA-I with the lowest percentage of cardiac death. Hazard ratios (HR) and 95% confidence interval (CI) were calculated. Sensitivity analysis was performed to further investigate the impact of demographic and lifestyle factors (model 3), comorbidities (model 4), clinical diagnosis and revascularization information (model 5), important lipid variables (model 6), and medications (model 7) (details refer to Supplementary Table 3). We also performed subgroup analysis on patients with acute coronary syndrome (ACS) and chronic coronary syndrome (CCS) to assess the impact of inflammation on the association between HDL-C, ApoA-I, and cardiovascular mortality in these cohorts. A two-sided P-value < 0.05 was regarded as statistically significant. R software (4.2.2) and SPSS software version 26.0 (IBM Corp., Armonk, New York, USA) were used for all analyses in this study.
Results
Baseline Characteristics
Following a 5-year follow-up and the elimination of patients who fulfilled the excluded criteria, 9569 individuals were finally enrolled in the analysis (Figure 1). The average age was 58.42 ± 10.26 years, with 2177 (22.8%) females and 4072 (42.6%) patients presenting with hsCRP ≥ 2 mg/L. The median HDL-C level was 1.00 mmol/L (range: 0.13–2.78 mmol/L) and the median ApoA-I level was 1.31 g/L (range: 0.43–2.94 g/L). Among overall PCI patients, 42.3% were diagnosed with the three-vessel disease (with or without the involvement of the left main artery [LM]), 32.5% had two-vessel involvement (with or without LM involvement), 25.0% had single-vessel disease (with or without LM involvement), and only 0.2% had isolated LM involvement without other vessels affected. The mean number of stents implanted per patient in the overall cohort was 1.81 ± 1.11 and 94.4% of patients using drug-eluting stents. During 5 years of follow-up, 225 (2.4%) cardiac mortality occurred.
As indicated in Table 1, patients who experienced cardiac mortality were older; more likely to have chronic obstructive pulmonary disease, diabetes and hypertension; had a higher proportion of previous myocardial infarction, prior PCI and prior coronary artery bypass grafting. They also had higher rates of three-vessel disease (with or without LM), elevated levels of hsCRP, and a higher ratio of patients with an estimated glomerular filtration rate < 90 mL/min. Moreover, they were more likely to be prescribed calcium channel blockers during hospitalization.
 |
Table 1 Baseline Characteristic of Patients with and without Cardiac Mortality |
Baseline characteristics stratified by hsCRP levels were shown in Supplementary Table 4.
Non-Linear Correlation Between Continuous HDL-C, ApoA-I Levels and Cardiac Mortality
Within the RCS analysis, HDL-C and ApoA-I were examined as continuous variables to explore the relationship with cardiac mortality (Figure 2).
In the overall cohort, there was a non-linear correlation of HDL-C levels (P for non-linear = 0.013) and ApoA-I levels (P for non-linear = 0.009) with cardiac mortality. Lower HDL-C and ApoA-I levels significantly increased the cardiac mortality risk.
Among the hsCRP ≥ 2 mg/L group, both HDL-C levels (P for non-linear = 0.005) and ApoA-I levels (P for non-linear = 0.020) exhibited a non-linear correlation with cardiac mortality. For HDL-C, a clear U-shaped curve was identified, demonstrating that both extremely low and high HDL-C levels considerably increased the risk of cardiac death. For ApoA-I, an L-shaped curve was found, with only very low ApoA-I levels associating with a higher risk of cardiac mortality but not in very high ApoA-I levels.
However, within the hsCRP ≥ 2 mg/L group, there were no apparent associations between cardiac mortality and continuous HDL-C or ApoA-I levels (both P > 0.05).
Quintiles HDL-C and ApoA-I and Cardiac Mortality in the Overall Cohort
Among the overall cohort, patients were further divided into HDL-C quintiles and ApoA-I quintiles, respectively.
HDL-C: Kaplan-Meier curves showed no significant differences between HDL-C quintiles (Log-rank P = 0.180) (Figure 3). Nevertheless, multivariate Cox regression analysis (Model 2) revealed a notably elevated risk of cardiac mortality only in patients belonging to the lowest HDL-C quintile (Q1: 0.13–0.81 mmol/L) compared to the reference group which had the lowest event rate (Q4: 1.07–1.23 mmol/L) (HR: 1.80; 95% CI 1.18–2.76; P = 0.007) (Figure 4).
 |
Figure 3 Kaplan-Meier curves of cumulative incidence for cardiac mortality. The quintile levels in each group were represented in Supplementary Table 3. Abbreviations: ApoA-I, apolipoprotein A-I; HDL-C, high-density lipoprotein-cholesterol; hsCRP, high-sensitivity C reactive proteins. Note: *P values indicating statistical significance. |
ApoA-I: Kaplan-Meier curves revealed a significant survival difference across ApoA-I quintiles (Log-rank P = 0.034) (Figure 3). Subsequent multivariate Cox regression analysis (Model 2) showed a substantially greater risk of cardiac death only for individuals in the lowest ApoA-I quintile (Q1: 0.43–1.15 g/L) compared to the reference group which had the lowest event rate (Q2: 1.16–1.26 g/L) (HR: 1.81; 95% CI 1.19–2.75; P = 0.005) (Figure 4).
Quintiles HDL-C and ApoA-I and Cardiac Mortality in hsCRP ≥ 2 mg/L Group
HDL-C: Significant cumulative survival differences were observed between HDL-C quintiles in Kaplan-Meier curves (Log-rank P = 0.035) (Figure 3). Multivariate Cox regression (Model 2) found that compared to patients in the reference quintile with the lowest risk (Q4: 1.03–1.17 mmol/L), patients in the two lowest HDL-C quintiles (Q1: 0.13–0.78 mmol/L and Q2: 0.79–0.90 mmol/L) both had a substantially increased risk of cardiac death, with adjusted HR of 2.50 (95% CI 1.32–4.71; P = 0.005) and 2.34 (95% CI 1.23–4.45; P = 0.009), respectively. Importantly, in comparison with the reference quintile (Q4: 1.03–1.17 mmol/L), patients in the highest HDL-C quintile (Q5: 1.18–2.71 mmol/L) exhibited a 2.28-fold increased risk of cardiac death (HR: 2.28; 95% CI 1.23–4.25; P = 0.009) (Figure 5).
ApoA-I: Kaplan-Meier curves did not show a significant cumulative survival difference across ApoA-I quintiles (Log-rank P = 0.066) (Figure 3). Nonetheless, multivariable Cox regression (Model 2) demonstrated that when compared to those at the lowest risk (Q4: 1.35–1.49 g/L), only patients in the lowest ApoA-I quintile (Q1: 0.43–1.12 g/L) had a 2.19 times greater risk of cardiac mortality (HR: 2.19; 95% CI 1.28–3.75; P = 0.004) (Figure 5).
Quintiles HDL-C and ApoA-I and Cardiac Mortality in hsCRP < 2 mg/L Group
HDL-C: No significant cumulative survival differences across HDL-C quintiles were found in Kaplan-Meier curves (Log-rank P = 0.578) (Figure 3). In the Cox regression analysis, none of the HDL-C quintiles were linked to an increased risk of cardiac death (all P > 0.05) (Figure 6).
ApoA-I: Kaplan-Meier curves showed that the cumulative survival of the ApoA-I quintiles did not differ significantly (Log-rank P = 0.261) (Figure 3). Furthermore, as shown by the multivariate Cox regression analysis, none of the ApoA-I quintiles were linked to a higher risk of cardiac death (all P > 0.05) (Figure 6).
Sensitivity Analysis
After further adjusting for demographic and lifestyle factors (Model 3), comorbidities (Model 4), clinical diagnosis and revascularization information (Model 5), important lipid variables (Model 6), and medications (Model 7) in the multivariate Cox regression models, the results remained consistent for the overall cohort (Supplementary Table 5), the hsCRP ≥ 2 mg/L group (Supplementary Table 6), and the hsCRP < 2 mg/L group (Supplementary Table 7).
Subgroup Analysis
In the ACS subgroup, concomitant with the presence of hsCRP ≥ 2 mg/L, the extremes in HDL levels—both very low (Q1 and Q2) and very high (Q5)—were associated with an elevated risk of cardiac mortality. Furthermore, only significantly low levels of ApoA-I (Q1) were found to be associated with an increased risk of cardiac mortality (Supplementary Figure 1).
Conversely, in the CCS subgroup, no significant associations were observed between either HDL-C or ApoA-I levels and cardiac mortality, regardless of hsCRP levels (Supplementary Figure 1).
Discussion
The primary findings of this large-sample real-world study, which followed CAD patients receiving PCI for 5 years, were as follows: i) Among the overall cohort, only lower HDL-C and ApoA-I levels were associated with elevated cardiac mortality. ii) In the hsCRP ≥ 2 mg/L group, HDL-C exhibited a pronounced U-shaped association with cardiac mortality, suggesting that both very low and very high HDL-C levels are associated with increased cardiac mortality risk; while ApoA-I exhibited an L-shaped correlation with risk of cardiac mortality, indicating that only extremely low ApoA-I levels are linked to elevated risk. iii) However, in the hsCRP < 2 mg/L group, neither HDL-C nor ApoA-I levels were linked to cardiac mortality. This is the first study to reveal potential differences in the association of HDL-C and ApoA-I levels with cardiac mortality based on baseline hsCRP levels.
HDL-C, Inflammation and Outcomes
There remains a heated debate about the protective role of HDL-C currently. Our research validated the risk of low HDL-C levels in the PCI population, and only patients with inflammation showed a U-shaped correlation between HDL-C levels and cardiac death.
Previous epidemiological studies showed a linearly negative relationship between HDL-C and cardiovascular events among the general population without known CAD,2,25,26 providing a hypothesis for elevating HDL-C levels to reduce cardiovascular risk. Nevertheless, clinical trials attempt to increase HDL-C values failed to improve the clinical outcome.27,28 Besides, recent study enrolling CAD participants found a U-shaped correlation between HDL-C and survival, indicating that both very low and very high HDL-C could be harmful.3 More complicated than the U-shaped association is the fact that the relationship between HDL-C levels and unfavourable outcomes could be affected by various factors such as ethnicity and diabetes.29,30 Therefore, there is no consistent conclusion about the relationship between HDL-C and prognosis and more importantly, it is unclear whether these inconsistent results are influenced by other important factors, especially inflammation. Given the strong association between inflammation and the risk of coronary events, it is crucial to explore whether the contradictory evidence regarding HDL-C is related to inflammation, a question that has not been adequately addressed in real-world studies.
The present study provides a unique perspective compared with previous research and fills the gap in understanding the interaction between inflammation and HDL-C in CAD patients undergoing PCI. Our study goes a step further by demonstrating that the clinical significance of HDL-C is amplified by the presence of inflammation risk. Specifically, the hazard of very high or low HDL-C levels only existed in individuals with inflammation, but not in those without inflammation.
The specific mechanisms of these findings are not yet defined. Previous research indicates that individuals with extremely high HDL-C levels frequently exhibit elevated large, lipid-rich HDL particles and reduced small, lipid-poor HDL particles (the small ones known for their protective capacity in effluxing cellular cholesterol).31,32 Consequently, in our study, a plausible explanation for the observed association between high HDL-C levels and increased mortality risk in inflammatory conditions may be attributed to the increased heterogeneity of HDL particles, particularly the rise in dysfunctional HDL particles. Previous studies pointed out that inflammation could impair the protective function of HDL particle by affecting its quantity and component and thus transforming it into a dysfunctional particle.33 Specifically, inflammation increases the triglyceride and cholesteryl ester content in HDL particles and elevates levels of the inflammation-related protein serum amyloid A, while reducing antioxidation enzymes like paraoxonase-1.5 These changes contribute to the formation of dysfunctional HDL particles, which lose their protective function and may even become harmful. Therefore, higher HDL-C levels in inflammation patients might indicated and related to the increased dysfunctional HDL particles in this study. Future research is warranted to further elucidate these mechanisms and better understand the complex relationship between HDL-C levels and mortality risk in inflammatory conditions.
ApoA-I, Inflammation and Outcomes
Since there is disagreement over the relevance of HDL-C levels in cardiovascular disease, researchers are shifting attention toward assessing HDL functionality beyond solely HDL-C concentration. The crucial molecule ApoA-I possesses the ability to carry out a number of biological functions for HDL particles. Our study demonstrated that inflammation could also influence the correlation between ApoA-I and cardiac mortality. More interestingly, unlike the U-shaped performance of HDL-C, only low ApoA-I level was the independent risk factor in patients with inflammation and high ApoA-I level was not.
The association between low ApoA-I on adverse outcomes has been explored in various populations. For the general population without CAD, the AMORIS study revealed an inverse correlation between ApoA-I levels and fatal myocardial infarction.34 Similarly, a meta-analysis of individuals on statin therapy discovered the inverse relationship between ApoA-I and major adverse cardiovascular events (MACE).8 As for CAD patients undergoing PCI, the only factor linked to a higher risk of cancer mortality was decreased ApoA-I levels.35 This was the first study to report the relationship between ApoA-I and cardiac death among PCI patients, which was basically in line with the above-mentioned studies, providing evidence of the hazard of low ApoA-I levels.
However, no research has examined the relationship between ApoA-I and cardiovascular risk under varying degrees of inflammation so far. The present study reported that decreased ApoA-I levels were linked to greater cardiac mortality only in PCI patients with inflammation, but not in those without inflammation. It is worth noting that, unlike the U-shaped pattern of HDL-C, no higher risk could be observed in high ApoA-I levels in individuals with inflammation.
Potential mechanisms for the different performances of high ApoA-I and HDL-C in inflammation might be attributed to their distinct role within the HDL particles. Compared with the cholesterol cargo HDL-C, which primarily reflects the cholesterol content within HDL particles and may not fully represent the protective function of HDL particles,4 ApoA-I, which is the main protein component of HDL particles, has been demonstrated to have multiple protective functions, such as anti-inflammation, antioxidant, and stimulating macrophage cholesterol efflux.5 Experimental studies confirmed that ApoA-I could carry out the anti-inflammatory function by means of stimulating macrophage cholesterol efflux via ATP-binding cassette transporter A1 and modulating inflammatory Toll-like receptor 4-dependent signaling in macrophages.36,37 In patients with inflammation, anti-ApoA-I antibody levels increase, with decreased levels of protective ApoA-I.18,19 This suggests that higher ApoA-I levels might indicate a greater protective capacity in patients with inflammation. Therefore, higher ApoA-I levels might be relatively safer in PCI patients with inflammation. The specific pathological mechanisms of ApoA-I in PCI patients with inflammation require further clarification.
Clinical Implications and Future Directions
In clinical practice and guidelines, there is no consensus regarding the optimal management of HDL-C and ApoA-I levels. Our study emphasizes the importance of focusing on the patient’s inflammatory status (especially those hsCRP ≥ 2 mg/L) when managing HDL-C and ApoA-I levels. Specifically, in patients with concurrent inflammation, maintaining HDL-C within a moderate range, neither too low nor too high, is crucial; while ensuring ApoA-I levels are not excessively low. In contrast, HDL-C and ApoA-I levels may not be a major issue in patients without inflammation. Our findings suggest personalized management of HDL-C and ApoA-I levels in PCI patients based on inflammatory status, in line with current trends toward personalized medicine.
Furthermore, our study found that in PCI patients with hsCRP ≥ 2 mg/L, elevated HDL-C levels were associated with increased mortality risk, whereas high ApoA-I levels were not. This finding implies the potential of elevating ApoA-I levels as a promising therapeutic target in PCI patients with hsCRP ≥ 2 mg/L. Given the negative outcomes of clinical trials focusing on increasing HDL-C levels, several clinical trials have extensively explored the potential benefit of elevating ApoA-I levels. However, the research results were inconsistent and it remains uncertain who will benefit most from this strategy. Apabetalon, a small molecule that could increase the endogenous production of ApoA-I,38 did not significantly reduce the risk of MACE among ACS patients.39 Nevertheless, a pooled analysis revealed that Apabetalone could significantly reduce MACE risk in CAD patients who had hsCRP levels > 2 mg/L.6 In conjunction with the findings from our real-world study, we highly assume that perhaps elevating the ApoA-I level rather than solely focusing on increasing HDL-C might potentially enhance the prognosis of PCI patients, particularly those who are at inflammation risk. Future large-scale randomized controlled trials and observational studies in different populations should be conducted to validate this hypothesis and further investigate the optimal management of ApoA-I and HDL-C levels in PCI patients.
Limitations
This study has several Limitations. Firstly, the single-center observational design may introduce selection biases and restrict the ability to extrapolate our findings, as our patient population and clinical practices might not fully represent other settings. Replicating this study in other countries or regions would provide valuable insights into the potential effects of genetic diversity, local diets, and other potential confounders. Future multi-center studies with diverse populations are needed to validate our findings. Secondly, there was a lack of routine evaluation of hsCRP and lipid concentrations during follow-up. In the future, it is necessary to dynamically detect whether the fluctuating inflammatory and blood lipid indicators will have an impact on the result. Thirdly, the functional properties of ApoA-I and HDL-C were not assessed. Future investigations into their specific functions, particularly in the context of chronic inflammation, are warranted to better elucidate their distinct roles in cardiovascular risk. Fourth, this study did not collect data on the exact stent type and therapy variations, which might impact the results. Additionally, despite thorough adjustment for cardiovascular risk variables, there may still be unmeasured confounders that could influence the connection. Future research should address these limitations by gathering comprehensive data and more thoroughly considering potential residual unmeasured confounding factors to strengthen the validity of this study. Fifth, due to its widespread clinical use and strong association with adverse outcomes in CAD patients, hsCRP was considered the inflammation biomarker in the present study. Future studies should consider incorporating a broader range of inflammatory markers such as interleukin-6 and white blood cells to provide more comprehensive insights.
Conclusions
In this large cohort study, in patients with hsCRP ≥ 2 mg/L, both very low and very high HDL-C levels were associated with cardiac mortality (U-shaped correlation), while only low ApoA-I levels showed a correlation (L-shaped). In patients with hsCRP < 2 mg/L, neither HDL-C nor ApoA-I levels were associated with cardiac mortality. These findings suggest that future personalized strategies should consider inflammation status, especially hsCRP levels, when managing HDL-C and ApoA-I levels and highlight the potential of elevating ApoA-I levels as a promising therapeutic target in PCI patients with hsCRP ≥ 2 mg/L. Despite valuable insights, the observational design may limit the generalizability of our findings. Future research should explore the biological mechanisms and validate these findings through large clinical trials and multi-center studies.
Abbreviations
ACS, acute coronary syndrome; ApoA-I, apolipoprotein A-I; CAD, coronary artery disease; CCS, chronic coronary syndrome; CI, confidence interval; HDL, high-density lipoprotein; HDL-C, HDL-cholesterol; HR, hazard ratio; hsCRP, high-sensitivity C reactive proteins; LM, left main artery; MACE, major adverse cardiovascular events; PCI, percutaneous coronary intervention; RCS, restricted cubic splines.
Data Sharing Statement
Our datasets are accessible through the corresponding author upon reasonable request, subject to approval from the Institutional Review Board of the State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases. The availability is constrained by ethical considerations associated with the consent obtained from subjects at the initiation of the study.
Ethics Approval and Informed Consent
All procedures involving human participants were conducted with informed consent. Approval for this study was granted by the Fu Wai Hospital Ethics Committee (Approval Number: 2013-449), adhering to the principles of the Declaration of Helsinki.
Consent for Publication
All authors accept and confirm publication.
Acknowledgments
We express our gratitude to all the staff contributing to this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National High Level Hospital Clinical Research Funding (2023-GSP-GG-40); CS Optimizing Antithrombotic Research Fund (BJUHFCSOARF201801-06); the National Clinical Research Center for Cardiovascular Diseases, Fuwai Hospital, Chinese Academy of Medical Sciences (NCRC2020013); and CAMS Innovation Fund for Medical Sciences (CIFMS) (2023-I2M-1-002).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987–1003. doi:10.1016/s0195-668x(03)00114-3
2. Wilson PW, Abbott RD, Castelli WP. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arteriosclerosis. 1988;8(6):737–741. doi:10.1161/01.atv.8.6.737
3. Liu C, Dhindsa D, Almuwaqqat Z, et al. Association between high-density lipoprotein cholesterol levels and adverse cardiovascular outcomes in high-risk populations. JAMA Cardiol. 2022;7(7):672–680. doi:10.1001/jamacardio.2022.0912
4. von Eckardstein A, Nordestgaard BG, Remaley AT, Catapano AL. High-density lipoprotein revisited: biological functions and clinical relevance. Eur Heart J. 2022. doi:10.1093/eurheartj/ehac605
5. Rosenson RS, Brewer HB Jr, Ansell BJ, et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol. 2016;13(1):48–60. doi:10.1038/nrcardio.2015.124
6. Nicholls SJ, Ray KK, Johansson JO, et al. Selective BET protein inhibition with apabetalone and cardiovascular events: a pooled analysis of trials in patients with coronary artery disease. Am J Cardiovasc Drugs. 2018;18(2):109–115. doi:10.1007/s40256-017-0250-3
7. Michael Gibson C, Korjian S, Tricoci P, et al. Safety and tolerability of CSL112, a reconstituted, infusible, plasma-derived apolipoprotein A-I, after acute myocardial infarction: the AEGIS-I trial (ApoA-I Event Reducing in Ischemic Syndromes I). Circulation. 2016;134(24):1918–1930. doi:10.1161/circulationaha.116.025687
8. Boekholdt SM, Arsenault BJ, Hovingh GK, et al. Levels and changes of HDL cholesterol and apolipoprotein A-I in relation to risk of cardiovascular events among statin-treated patients: a meta-analysis. Circulation. 2013;128(14):1504–1512. doi:10.1161/circulationaha.113.002670
9. Kalkman DN, Aquino M, Claessen BE, et al. Residual inflammatory risk and the impact on clinical outcomes in patients after percutaneous coronary interventions. Eur Heart J. 2018;39(46):4101–4108. doi:10.1093/eurheartj/ehy633
10. Everett BM. Residual inflammatory risk: a common and important risk factor for recurrent cardiovascular events. J Am Coll Cardiol. 2019;73(19):2410–2412. doi:10.1016/j.jacc.2019.02.056
11. Guedeney P, Claessen BE, Kalkman DN, et al. Residual inflammatory risk in patients with low LDL cholesterol levels undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2019;73(19):2401–2409. doi:10.1016/j.jacc.2019.01.077
12. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi:10.1056/NEJMoa1707914
13. Arnold N, Blaum C, Goßling A, et al. C-reactive protein modifies lipoprotein(a)-related risk for coronary heart disease: the BiomarCaRE project. Eur Heart J. 2024;45(12):1043–1054. doi:10.1093/eurheartj/ehad867
14. Yuan D, Wang P, Jia S, et al. Lipoprotein(a), high-sensitivity C-reactive protein, and cardiovascular risk in patients undergoing percutaneous coronary intervention. Atherosclerosis. 2022;363:109–116. doi:10.1016/j.atherosclerosis.2022.10.013
15. Wang Y, Lu S, Zhang G, et al. Anti-inflammatory effects of HDL in mice with rheumatoid arthritis induced by collagen. Front Immunol. 2018;9:1013. doi:10.3389/fimmu.2018.01013
16. Watanabe J, Charles-Schoeman C, Miao Y, et al. Proteomic profiling following immunoaffinity capture of high-density lipoprotein: association of acute-phase proteins and complement factors with proinflammatory high-density lipoprotein in rheumatoid arthritis. Arthritis Rheum. 2012;64(6):1828–1837. doi:10.1002/art.34363
17. Kim SY, Yu M, Morin EE, Kang J, Kaplan MJ, Schwendeman A. High-density lipoprotein in lupus: disease biomarkers and potential therapeutic strategy. Arthritis Rheumatol. 2020;72(1):20–30. doi:10.1002/art.41059
18. O’Neill SG, Giles I, Lambrianides A, et al. Antibodies to apolipoprotein A-I, high-density lipoprotein, and C-reactive protein are associated with disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2010;62(3):845–854. doi:10.1002/art.27286
19. Carbone F, Nencioni A, Mach F, Vuilleumier N, Montecucco F. Evidence on the pathogenic role of auto-antibodies in acute cardiovascular diseases. Thromb Haemost. 2013;109(5):854–868. doi:10.1160/TH12-10-0768
20. Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol. 2005;46(7):1225–1228. doi:10.1016/j.jacc.2005.07.006
21. Virani SS, Newby LK, Arnold SV, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2023;148(9):e9–e119. doi:10.1161/cir.0000000000001168
22. Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44(38):3720–3826. doi:10.1093/eurheartj/ehad191
23. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58(24):e44–e122. doi:10.1016/j.jacc.2011.08.007
24. Wijns W, Kolh P, Danchin N, et al. Guidelines on myocardial revascularization. Eur Heart J. 2010;31(20):2501–2555. doi:10.1093/eurheartj/ehq277
25. Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986;256(20):2835–2838.
26. Sharrett AR, Ballantyne CM, Coady SA, et al. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104(10):1108–1113. doi:10.1161/hc3501.095214
27. Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367(22):2089–2099. doi:10.1056/NEJMoa1206797
28. Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109–2122. doi:10.1056/NEJMoa0706628
29. Zakai NA, Minnier J, Safford MM, et al. Race-dependent association of high-density lipoprotein cholesterol levels with incident coronary artery disease. J Am Coll Cardiol. 2022;80(22):2104–2115. doi:10.1016/j.jacc.2022.09.027
30. Ishibashi T, Kaneko H, Matsuoka S, et al. HDL cholesterol and clinical outcomes in diabetes mellitus. Eur J Prev Cardiol. 2023;30(8):646–653. doi:10.1093/eurjpc/zwad029
31. Kontush A, de Faria EC, Chantepie S, Chapman MJ. Antioxidative activity of HDL particle subspecies is impaired in hyperalphalipoproteinemia: relevance of enzymatic and physicochemical properties. Arterioscler Thromb Vasc Biol. 2004;24(3):526–533. doi:10.1161/01.ATV.0000118276.87061.00
32. Asztalos BF, Horvath KV, Kajinami K, et al. Apolipoprotein composition of HDL in cholesteryl ester transfer protein deficiency. J Lipid Res. 2004;45(3):448–455. doi:10.1194/jlr.M300198-JLR200
33. Alwaili K, Bailey D, Awan Z, et al. The HDL proteome in acute coronary syndromes shifts to an inflammatory profile. Biochim Biophys Acta. 2012;1821(3):405–415. doi:10.1016/j.bbalip.2011.07.013
34. Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358(9298):2026–2033. doi:10.1016/s0140-6736(01)07098-2
35. Nishiyama H, Funamizu T, Iwata H, et al. Low apolipoprotein A1 was associated with increased risk of cancer mortality in patients following percutaneous coronary intervention: a 10-year follow-up study. Int J Cancer. 2022;151(9):1482–1490. doi:10.1002/ijc.34164
36. Yvan-Charvet L, Welch C, Pagler TA, et al. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118(18):1837–1847. doi:10.1161/circulationaha.108.793869
37. Smoak KA, Aloor JJ, Madenspacher J, et al. Myeloid differentiation primary response protein 88 couples reverse cholesterol transport to inflammation. Cell Metab. 2010;11(6):493–502. doi:10.1016/j.cmet.2010.04.006
38. Nicholls SJ, Gordon A, Johansson J, et al. Efficacy and safety of a novel oral inducer of apolipoprotein a-I synthesis in statin-treated patients with stable coronary artery disease a randomized controlled trial. J Am Coll Cardiol. 2011;57(9):1111–1119. doi:10.1016/j.jacc.2010.11.015
39. Ray KK, Nicholls SJ, Buhr KA, et al. Effect of apabetalone added to standard therapy on major adverse cardiovascular events in patients with recent acute coronary syndrome and type 2 diabetes: a randomized clinical trial. JAMA. 2020;323(16):1565–1573. doi:10.1001/jama.2020.3308
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





