Back to Journals » International Journal of Nanomedicine » Volume 19
Bioactive Materials That Promote the Homing of Endogenous Mesenchymal Stem Cells to Improve Wound Healing
Authors Jiang Z, Chen L, Huang L, Yu S, Lin J, Li M , Gao Y, Yang L
Received 5 January 2024
Accepted for publication 23 April 2024
Published 30 July 2024 Volume 2024:19 Pages 7751—7773
DOI https://doi.org/10.2147/IJN.S455469
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kamakhya Misra
Ziwei Jiang,* Lianglong Chen,* Lei Huang, Shengxiang Yu, Jiabao Lin, Mengyao Li, Yanbin Gao, Lei Yang
Department of Burns, Nanfang Hospital, Southern Medical University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lei Yang, Department of Burns, Nanfang Hospital, Southern Medical University, Jingxi Street, Baiyun District, Guangzhou, 510515, People’s Republic of China, Tel +86-20-6164-1841, Email [email protected]
Abstract: Endogenous stem cell homing refers to the transport of endogenous mesenchymal stem cells (MSCs) to damaged tissue. The paradigm of using well-designed biomaterials to induce resident stem cells to home in to the injured site while coordinating their behavior and function to promote tissue regeneration is known as endogenous regenerative medicine (ERM). ERM is a promising new avenue in regenerative therapy research, and it involves the mobilizing of endogenous stem cells for homing as the principal means through which to achieve it. Comprehending how mesenchymal stem cells home in and grasp the influencing factors of mesenchymal stem cell homing is essential for the understanding and design of tissue engineering. This review summarizes the process of MSC homing, the factors influencing the homing process, analyses endogenous stem cell homing studies of interest in the field of skin tissue repair, explores the integration of endogenous homing promotion strategies with cellular therapies and details tissue engineering strategies that can be used to modulate endogenous homing of stem cells. In addition to providing more systematic theories and ideas for improved materials for endogenous tissue repair, this review provides new perspectives to explore the complex process of tissue remodeling to enhance the rational design of biomaterial scaffolds and guide tissue regeneration strategies.
Keywords: endogenous stem cell homing, in situ tissue regeneration, biomimetic design, wound healing, bioactive materials
Introduction
As the primary barrier against the external environment, the skin is vulnerable to various deleterious factors, which can result in different types of skin lesions and damage.1 However, chronic, acute, massive, and complex wounds often struggle to heal2 as they rely on the patient’s self-repairing ability, which can bring severe physical and psychological burdens to patients.3 Therefore, a variety of therapies have been developed to accelerate wound healing. These therapies usually involve traditional treatments with wound dressings, antibiotics, the removal of necrotic tissue, as well as the performance of skin grafts if necessary.4 It is often performed together with emerging therapies and using biophysical modalities such as electrical stimulations5 or shock waves.6 In addition, it comprises tissue engineering therapies that rely on prefabricated biomaterials,7 ex vivo expanded cells, and selected growth factors.8
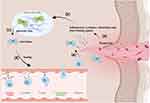
The term homing, known as recruitment in tissue repair and regeneration contexts, typically refers to the ability of a cell to be recruited and find its way to the site of an injury.9 In natural healing, after platelets are activated, platelet-derived factors are released to induce the homing of innate immune cells to the damaged tissues. After this, the recruited immune cells create a favorable microenvironment and secrete paracrine factors at the injury site, where both resident and circulating reparative cell populations are recruited. For example, progenitors and stem cells are subsequently involved in tissue repair and the regeneration of new tissue (Figure 1).10,11 However, natural endogenous regenerative processes, in many cases, are often too limited for completing wound repair.12 As such, a substantial number of stem cell therapies have thus been developed.13 Bone marrow mesenchymal stem cells (BMSCs) are one of the most intensively studied candidates for cell therapy.14 Despite this, however, transplanted MSCs have poor efficacy due to inhospitable wound microenvironments and low homing efficiency.15 In addition, in vitro cell transplantation has to overcome high economic costs and significant market regulatory hurdles while requiring meticulous handling at all stages of stem cell acquisition, processing, and transplantation.16 Consequently, it is crucial to improve homing efficiency.
Endogenous stem cell homing refers to the transport of endogenous mesenchymal stem cells (MSCs) to damaged tissues.15 Endogenous regenerative medicine (ERM) is defined as the paradigm of using well-designed biomaterials to coax the homing of resident stem cells toward injured sites, orchestrating their behaviors and functions to promote tissue regeneration.17,18 Endogenous regenerative medicine is a promising new avenue in regenerative therapy research, which focuses on the mobilization of endogenous stem cells for homing. By understanding the MSC homing process, we are able to modulate endogenous MSC homing by interfering with MSC-influencing factors, which promotes more resident MSCs in the stem cell niche to reach the site of injury and participate in tissue repair without the need for exogenous implantation of MSC cells. Promoting more endogenous MSCs to reach the site of injury or facilitating more targeted and increased secretion of already reached MSCs by carrying key homing factors, or carefully designing the physical properties, 3D structure and microstructure of the material is the main means to promote endogenous homing. Compared with cell transplantation, promoting the homing of endogenous MSCs to increase tissue self-repair can avoid the risk of direct application; moreover, it is safer, simpler, more practical, and more economical.19 Indeed, the enhancement of endogenous stem cell homing can be applied not only directly, but also as an adjunctive strategy to optimise the efficacy of cellular therapies, where homing is a critical step after transplantation of cellular material. Achieving efficient and optimal cell homing at the target site is also an ongoing challenge in cell transplantation technology.20
Undoubtedly, ERM is a promising new avenue in regenerative therapy research, and the mobilization of endogenous stem cell homing is its mainstay. In this review, we summarize the notable research on endogenous stem cell homing in the field of skin tissue repair. We also identify the tissue engineering strategies currently available for regulating the stem cell homing process. This article outlines the progress that has been made in this study area and the obstacles that still to be overcome. We hope to inspire more tissue engineers, biomaterials scientists, and surgical clinicians to actively engage in this field and provide practical solutions to clinical issues.
How Mesenchymal Stem Cells Homing
Homing is usually divided into systematic homing and non-system homing.21 In systemic homing, MSCs are endogenously recruited or administered into the bloodstream, and they then must go through a multi-step process to leave the blood circulation and migrate to the damaged area. In non-systemic homing, MSCs are locally transplanted into the target tissue, and they are then directed to the site of injury by a chemokine gradient. Systemic homing can be divided into five steps: the initial tethering of selectins, chemokine-mediated activation, integrin-promoted arrest, matrix remodeler-assisted diapedesis or transmigration, and chemokine gradient-guided extravascular migration (Figure 2).22
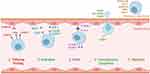 |
Figure 2 The process of the systematic homing of mesenchymal stem cells (MSCs). Created with BioRender.com. |
Tethering
The first step, tethering and rolling, is contributed to by the selectins that are expressed by endothelial cells.23 Only if they are already arrested or trapped could MSCs adhere to the endothelium; meanwhile, in intact vessels, MSCs would be rare under normal circulatory conditions.24 The selectin that primarily contributes to bolting and rolling of MSC is the P selectin. The CD44 expressed by MSCs catches onto the selectins and causes the cell to start rolling along the vasculature wall.23 The glycoprotein CD44 is expressed on the surface of many different cells, and it acts as a ligand to adhere to cells through other molecules, including hyaluronic acid.25 Its role as a ligand for P-selectin has also been reported. Therefore, P-selectin may be a ligand for the P-selectin that are expressed by MSCs. Galectin-1 or CD24 may also serve as ligands for P-selectin on MSCs.26,27 The selectin used by MSCs has yet to be well understood, whereby several studies have suggested that the interaction of mesenchymal stem cells with endothelial cells is mediated by P-selectin.26 Other studies have shown that E-selectin and L-selectin are not expressed in MSC cell membranes.28 Their interaction with the vessel wall is not apparent. One study found that, by blocking the E-selectin on endothelial cells, the adhesion of BMSCs was not reduced.29 However, other researchers have found that the CD44 on MSCs interacts with E-selectin and L-selectin.30 Several adhesion molecules, thus far, have been identified as being connected with MSC transendothelial migration. These include particularly late antigen-4 (VLA-4), ICAM-1,31 VCAM-1, and P-selectin.32 Antonín Sedlá ř et al found that the interaction between Galectin-3 and integrins mediates the cell–matrix adhesion in endothelial cells and mesenchymal stem cells.33 Rüster et al proposed that P-selectin and α4β1-integrin/VCAM-1 play important roles in the venule recruitment to mouse mesenchymal stem cells.29 Under the current concept of homing, tissues will need to recruit circulating mesenchymal stem cells from the bloodstream to ensure an efficient delivery to the damaged site. To this end, the surface of mesenchymal stem cells has many different adhesion molecules, and these molecules are also shared with leukocytes. These adhesion molecules include CD29 (β1-integrin), CD24, CD49a-f (α1-α6 integrin),34 and CD44, although CD24 was not found in other studies.35 The role and mechanism of selectin in MSC homing are not yet clear, and there is still controversy over whether CD24 is an adhesive molecule that plays a role in this process. In the application of tissue engineering, there are currently almost no biomaterials targeting selectins to promote endogenous homing. This may be due to the unclear mechanism and insufficient impact of selectins on homing efficiency, which makes the application or engineering of selectins not an ideal way for endogenous regenerative medicine.
Activation
The second step, activation—which is generally in response to inflammatory signals—is facilitated by chemokine receptors. In MSC trafficking, the chemokines released from endothelial cells and tissues can promote the activation of ligands that are involved in multiple homing processes, including the adhesion, chemotaxis, and migration of MSCs in target tissues. Stromal cell-derived factor (SDF)-1 expressed on endothelial cells is critical for this step.36,37 SDF-1 is the ligand to the chemokine receptor CXCR4, and it is considered expressed by MSCs.38–40 Indeed, it has been researched that CXCR7 is also expressed in MSCs, which similarly binds SDF-1 to promote the homing to various tissues.41–43 No matter how the specific receptors and ligands interact, the effect of the activation step is to multiply the affinity of the integrins’ extracellular domain by inducing conformational changes; furthermore, these integrins are essential for cell arrest.44,45 Chemokines that mediate the activation step, such as monocyte chemoattractant protein-1 (MCP-1) or SDF-1, increase the affinity of integrins, thus leading to cell arrest. Integrin arrest is most likely primarily mediated by CD49d (α4β1) via its binding to VCAM-1 (CD106) on endothelial cells.46 Certain studies have reported the expression of chemokine receptors in MSCs, including CCR1, CCR2, CCR4, CCR6, CCR7, CCR8, CCR9, CCR10, CXCR1, CXCR2, CXCR3, CXCR4, CXCR5, and CXCR6; moreover, they have noted the functional roles of some of the chemokine receptors during the MSC migrating process.44,47–49 Other studies have shown that, during the movement of MSCs from bone marrow, the SDF-1 / CXCR4 axis also plays an essential role.50–52 The chemokines released from tissues may lead the CXCR4 receptor in the cell to move to the cell surface, which facilitates the migration of MSCs to their destinations. For example, Andong Zhao et al have shown that the SDF-1/CXCR4 signaling pathway guides systemically transplanted bone marrow mesenchymal stem cell migration toward the lesion site.51 Although SDF-1/CXCR4 is the most well-studied chemokine–chemokine receptor axis, according to recent findings, other signaling interactions are also important mediators of stem cell homing, such as CCL27-CCR10 and CCL21-CCR7.53–55 Chemokines are the most commonly used homing factors in endogenous regenerative medicine to bind material applied to the wound site, and by applying chemokines to provide navigational signals to MSCs in the surrounding stem cell niches to mobilise more and more distant MSCs to homing towards the damaged site.56
Arrest
The third step is the arrest, which is facilitated by integrins. VLA-4 (integrin a4b1) expressed by MSCs becomes activated in response to chemokines such as SDF-1. Following activation, the integrin VLA-4 binds to the VCAM-1 on endothelial cells.15 Steingen et al reported that, instead of undergoing full diapedesis, MSCs could use the VLA-4/VCAM-1 molecule to migrate through non-activated endothelia; furthermore, they are inclined to integrate with the endothelial layer.57 Just like the interactions of intercellular adhesion molecule (ICAM) and vascular cell adhesion molecule (VCAM)-1), the adhesion molecules on the endothelial surface interact with the integrins expressed in MSC cell membranes, which then lead to the formation of transmigration wells and docking structures. These positions are sites that are rich in VCAM-1 molecules, ICAM-1, proteins, and cytoskeleton components like α-actinin.28 However, anti-VCAM-1 and anti-VLA-4 antibodies cannot entirely block the transendothelial migration of MSCs; As such, other integrins that are also involved in this process can be assumed.57 MSCs have been shown to express varieties of receptors that are associated with adherence to extracellular matrix proteins and intercellular contacts, such as integrins α1~α5, αv, β1, β3, and β4, together with other adhesive molecules, ie, ICAM-1, ICAM-3, VCAM-1, and CD166.28 Engineering integrins and their receptors are also commonly used methods to promote endogenous homing. By designing peptide segments with high affinity for integrins or integrin ligands, they promote the residence of MSCs in target tissues, thereby promoting endogenous regeneration.58,59
Transmigration
In the next step, diapedesis or transmigration, MSCs must trans-cellularly travel through the endothelial cell layer and, later, the basement membrane. To accomplish this, MSCs break down the endothelial basement membrane by secreting membrane matrix metalloproteinases (MMPs).60 Various other proteins regulate the maturation and activity of MMPs, of which, most prominently, are the tissue inhibitors of metalloproteinases (TIMPs). Inflammatory cytokines induce the expression of these remodeling enzymes, and the expressed remodeling enzymes then serve as a signal for migration into harmed tissue.61 For instance, TNF-α and IL1β stimulate MSCs to produce matrix metalloproteinases (MMPs), as well as to trigger the activation of chemotaxis through the extracellular matrix.62,63 Proteolytic enzymes-MMPs play an essential role in MSC migration by regulating the degradation of the extracellular matrix.64 Different MMPs and their signaling pathways affect MSC differentiation, migration, proliferation, and angiogenesis. Migration and invasion into the damaged tissues of MSCs are facilitated by the expression of MMP-2, CXCR4, and MT1-MMP.65,66 MMP is a key influence on cell migration, and MMP overexpression can enhance cell invasiveness. Some materials will carry bioactive factors to promote MMP overexpression to accelerate wound healing.67
Migration
The final step is led by chemotactic signals that are set free in response to tissue damage. MSCs must migrate to the site of injury through the interstitium. The damaged tissues release specific factors, such as chemoattractants, to promote the adhesion, migration, and homing of marrow mesenchymal stem cells in the affected areas. In response to the factors regulated under inflammation, MSCs can migrate toward inflamed tissues.68–70 Inflammatory cytokines, such as IL-6, IL-8, IL-1β, and TNF-α, are included. Numerous growth factors, eg, the vascular endothelial-derived growth factor-A, epidermal growth factor, platelet-derived growth factor (PDGF-AB), fibroblast growth factor, transforming growth factor-β1, hepatocyte growth factor, insulin-like growth factor (IGF1).47,70–73 Additionally, this includes, to a lesser extent, the chemokines RANTES, MDC, and SDF-1.66,74 MSCs migrate to these signals and thus to the damaged tissue. The creation of a suitable microenvironment at the wound site by the material can attract more MSCs to the target tissue, and the combination of chemokines, inflammatory cytokines, and a variety of growth factors with well-designed materials can help more MSCs to migrate to the damaged site to participate in tissue repair.56
Factors Influencing the Homing Process
The use of tissue engineering to promote endogenous wound healing is an emerging direction in wound repair. To promote MSC homing through bioactive materials, we must first know what factors affect MSC homing (Figure 3). Adhesion molecules, chemokines, pro-inflammatory cytokines, growth factors, and the MMPs involved in the natural homing process are the main influencing factors of homing. The exogenous application of key homing factors can provide more attraction, as well as more stable and durable navigation clues for MSCs to guide endogenous MSCs to their destination (Table 1). Adhesion molecules are mainly involved in rolling and transendothelial migration during homing.28 It has been confirmed that the adhesion molecules involved in homing are as follows: VCAM-1, ICAM-1, ICAM-2, P selectin, CD24, CD44, CD29 (β1-integrin), CD49a-f (α1-α6 integrin), and CD51/61. The main ones involved in the chemotactic process are chemokines and their receptors, pro-inflammatory cytokines, and growth factors.75 Chemokines and their receptors mainly include CXCR1-6, CCR1/2/4/6/8/9, MCP-1, MIP-1α, and SDF-1. Pro-inflammatory cytokines such as IL-3, IL-6, IL-8, IL-1β, TGF-β, IGF-1, and TNF-α, as well as growth factors such as VEGF, PDGF, EGF, FGF, HGF, IGF, and SCF, are released from the site of injury to provide chemotaxis signals for MSCs to attract them to the designated location. The action of metalloproteases allows MSCs to cross the endothelial basement membrane during homing, and the MMPs that have been studied with respect to this are mainly MMP-1 and MMP-2.65
 |
Figure 3 Factors affecting the homing of MSCs. Created with BioRender.com. |
 |
Table 1 Factors Influencing Endogenous MSC Homing |
In addition to the influence of various homing factors, certain internal factors can also regulate the homing process. For instance, intracellular ceramide kinases regulate BM–MSC migration, while the inhibition of ceramide kinases inhibits migration.82 Lin-Hua Jiang et al proposed that ATP-induced Ca2+ signaling can regulate MSC migration.83 Histologic origin can also affect the homing ability of MSCs. MSCs (DPSCs) are isolated from the pulp exhibit top-notch osteogenic potential, as well as the highest proliferative capacity, when compared to fat and bone marrow sources.97 Platelets have also been found to promote the adhesion ability of MSCs to homing.84 Physical factors in the body can also affect the homing process, such as hemodynamic shear stress,85 cyclic tensile strain,86 and the diameter of blood vessels.87 And endogenous electric fields (EFs) can be harnessed by MSCs for directed migration.88
The exogenous factors affecting homing mainly include culture conditions, various pretreatments, mechanical stretching, exogenous magnetic fields, and ultrasounds with different parameters. Culture conditions can affect the expression of cell surface markers. A 3D culture can better adapt MSCs to their cell niche environment than 2D cultures, thus enhancing their paracrine signaling activity.89 H2O2 pretreatment, cytokines (such as interleukin (IL)-6 and hepatocyte growth factor), and hypoxia conditions98 can stimulate the expression of the chemokine receptor (CXCR) 4 receptor. In addition, collagen structure can regulate the behavior of MSCs. For instance, collagen has a more advanced collagen structure than gelatin, which can promote biological cell–matrix interaction by regulating MMP activity, thereby promoting MSC diffusion and proliferation.99 Experiments by Xiao Liang et al show that mechanical stretching can promote MSC transdifferentiation and homing.96 Magnetic targeting technology has been proven to improve the delivery of MSCs to targets. Using selected magnetic nanoparticles to magnetize MSCs, MSCs can be promoted via differentiation and secretion, or through improving targeting under an external static or dynamic magnetic field.100–104 Other studies have shown that combining a magnetic field and electrospun scaffold can produce directional synergy, which can be utilized to promote the cartilage differentiation of MSCs.105 Ultrasound is a physical signal that is often enhanced to promote homing and accelerate MSC secretion; moreover, different types of ultrasounds can play different roles. Yanni He et al recruited endogenous BM-MSCs using the sound capture force generated by pulsed ultrasound (p-US) irradiation.90 Dongmei Ye et al found that a shortwave in high-frequency electrotherapy can upregulate the expression of HIF-1, CXCR-4, and SDF-1 in osteoblastic culture media when promoting the process of endogenous MSC chemotaxis.94 Experiments by Rebecca M. Lorsung et al show that cavitation forces or acoustic radiation from low-intensity pulsed ultrasound (LIPUS) can shape the microenvironment that supports MSC tropism.91
Biomaterials for Skin Tissue Regeneration Using MSC Endogenous Homing
Promoting the homing of endogenous stem cells to accelerate repair and regeneration has been widely used in many fields, including the reconditioning of bone,106 cartilage,107 tendons,108 the heart,109 lungs,110 the endometrium,111 teeth,112 and other tissues and organs. In contrast, the application in promoting skin wound healing has yet to be explored thoroughly. Mesenchymal stem cells in a static state reside in the central or surrounding stem cell niches. When injured, the MSCs transition from a quiescent state to an alert phase, which then migrate to the injured site through homing to participate in the in situ repair of tissues (Figure 1).113,114 It is a vital part of endogenous regenerative medicine to design bioactive materials for diverse targets in different stages of homing to promote stem cell homing (Table 2). In tissue regeneration, we must advance more mesenchymal stem cells to set out from the niche, to assist more coordinated cell movement during migration, and stimulate the paracrine secretion of mesenchymal stem cells at the wound (Figure 4).115–119 In addition, there has been evidence that in vitro-transplanted cells trigger endogenous cell recruitment in the process of inducing tissue regeneration, which indicates that endogenous homing is not only an event in ERM. Cell therapy and endogenous regeneration can also promote and assist each other.120
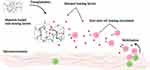 |
Figure 4 Bioactive materials loaded with various homing factors to promote MSC homing in order to aid skin wound repair. Created with BioRender.com. |
 |
Table 2 Bioactive Materials That Modulate Different Physicochemical Factors to Promote Homing of MSCs |
Promotion of MSC Leaving
Making more stem cells leave the stem cell niche is undoubtedly an effective means through which to promote endogenous homing. In the ERM field, increasing the attraction of the destination (the wound site) to the stem cells in the stem cell niche is the research focus. The essential way through which to increase the attraction of the destination is to make homing signals more visible to the stem cells, make the stem cells more sensitive to homing factors, and make the navigation clues more stable and lasting. Making stem cells more visible to homing signals depends on applying various homing factors. Using homing factors alone or combined with tissue engineering materials can make stem cells receive more homing signals, which then promotes endogenous homing.56 When combined with cell therapy, MSCs are often pretreated with homing factors or placed in various biomaterials (such as hydrogels and sponge scaffolds) that combine homing elements or other substances that can promote homing. They are then implanted into the body as a whole. Making stem cells more sensitive to homing factors is mainly conducted in cell therapy. Through genetic engineering technology, exogenous MSCs have been gene edited to improve their homing efficiency after transplantation into a body. Making navigation clues more stable and lasting is usually carried out simultaneously by applying homing factors. Advanced slow-release technology controls the release concentration of homing factors in the optimal range.
The microenvironment is critical to cell function and activity. Different microenvironments have different effects on the biological functions of MSCs. The differentiation potential of MSCs is highly dependent on microenvironmental soluble factors, including cytokines (TNF-α and IL-6), growth factors (VEGF, TGF-β, IGF-1, and FGF), and hormones (estrogen, parathyroid hormones, and growth hormones).141 Under normal conditions, cells live in the complex and dynamic extracellular matrix (ECM), which contains various complete microenvironment clues that determine cell behavior and function. The natural matrix has a great potential in promoting cell homing, and it does not require additional engineering to play a beneficial role.142 Relying on the mixtures of the natural ingredients they contain, acellular matrices and acellular tissue have been directly used as biomaterial scaffolds.143 The biomaterial components used to attract stem cells to guide in situ regeneration mostly drew inspiration from ECM components, and they mainly rely on mimicking one or a few components of the ECM. Substitutes for its applications mainly include proteins (gelatin,144 collagen,145 silk fibroin,146 etc.) and polysaccharides (hyaluronic acid,147 starch,148 chitosan,149 etc.). Scientists have applied one or more ECM components into hydrogels or sponge scaffolds, which are then placed into the wound to promote endogenous homing and to help skin regeneration. The bionic design that simulates the ECM, which is simultaneously combined with navigation hints for the specific aspects of the MSC homing process and small molecule drug delivery, is also a primary research direction of bionic technology and regenerative medicine. For example, in one study, horseradish peroxidase (HRP)-catalyzed sprayable gelatin hydrogels (GHs) were used as a platform to direct the carrying of two chemokines (in the process of the in situ crosslinking for endogenous cell recruitment in diabetes wounds150): interleukin-8 (IL-8) and macrophage inflammatory protein-3a (MIP-3a) hydrogel excipients. In diabetes wounds, MSC migration is inhibited and chemotaxis is insufficient.151 In comparison, the local application of these two in situ hydrogels with chemokines for MSC recruitment can improve the wound’s local microenvironment, as well as promote the chemotaxis process in MSC homing, thus promoting the wound healing of diabetes. van de Kamp et al loaded collagen and silk fibroin with a chemoattractant for MSCs and the hepatocyte growth factor (HGF). The experimental results showed endogenous MSCs were recruited from the local environment after subcutaneous implantation.145 In addition to the well-known growth factors and chemokines that can be used in tissue engineering to promote homing, certain particular substances can also play a role in recruiting endogenous MSCs, such as substance P, as well as the bioceramics that can provide bioactive ions such as Ca, Mg, and Si.152 Graphene oxide has also been employed to construct nucleo-shell microfiber array hydrogels with chemokines (SDF-1α), which can effectively recruit and stimulate the neuroid differentiation of BMSCs.153
We already know the basic process of MSC homing and its various homing factors. How one is to introduce the key factors involved in the homing process into tissue engineering is a problem worth exploring. To play a more stable role in damaged parts, the homing factor is combined with various materials to promote its steady and lasting release. Electrospinning technology and 3D printing technology are focused on154 because they can not only simulate the tissue structure,155 such as the blood vessel structure,156 but they can also emulate the natural tissue (such as pores and microchannels) in the microstructure.157
It is beyond doubt that more MSC homing can be promoted by combining navigation clues that enhance endogenous homing with bioactive materials, as well as by making them release at a constant speed and for a long time. However, not all homing factors in vivo are suitable for direct application to tissue engineering. For example, although SDF-1α is one of the most widely studied potent homing factors (as a high molecular weight protein), it is not easily processed or introduced into the support material. Additionally, SDF-1α is easily degraded by the proteolytic enzymes activated when tissues are damaged, such as matrix metalloproteinase (MMP-2) and endogenous CD26/dipeptidyl peptidase IV (DPP-IV). To solve this problem, researchers have used small molecular weight inhibitors to block and inactivate the enzyme, as well as designed anti-protease (MMP-2 and DPP-IV/CD26) cleaved SDF-1α peptides; these, in turn, have produced SDF-1 molecules that have high stability. Used alone, it can enhance endogenous cell recruitment to repair myocardia.158 It has been shown that SDF-1α peptide can be incorporated into synthetic grafts to promote cell growth and tissue repair. For example, Muhammad Shafiq et al synthesized SDF-1α-derived peptide and heparin-tethered poly (L-lactide-co-ε-caprolactone) (PLCL) copolymer by coupling SDF-1α-derived peptide and heparin to eight-arm star-shaped PLCL copolymers, as well as by making a double-layer tubular scaffold with electrospinning technology. It was implanted subcutaneously in rats to observe its effect in the promotion of regenerating skin blood vessels. The results showed that PLCL copolymers can provide a stable local gradient of chemokines in scaffold material, which is beneficial for attracting and enriching endogenous cells. The number of blood vessels, stem cells, and α-SMA positive cells in SDF-1/ heparin grafts was found to be significantly higher than that found in the control group.156 Zhen Xu et al designed a microgel array patch that encapsulates SDF-1, which can be customized with its concentration gradient and is capable of generating a long-lasting concentration gradient of signaling molecules that promotes MSC recruitment, thereby recruiting endogenous stem cells for the treatment of skin damage.159 Yucong Li et al designed a bioactive microsphere (MESS) capable of endogenous stem cell recruitment and induction based on hBMSCs secretion and supramolecular hydrogel. Oxidised alginate (o-alg), tris(hydroxymethyl)aminomethane (THAM) and gelatin were the basic components of the hydrogel microspheres. And the biophysical stimulation by pulsed electromagnetic field (PEMF) was utilised to enhance the quality and bioactivity of MESS after injection into the body, constituting PEMF-enhanced MESS (PE-MESS). The hydrogel injection was implanted subcutaneously on the back of rats, and it was observed that significantly more MSCs were recruited in the MESS hydrogel compared with the other groups.In addition, after the application of the pulsed electromagnetic field, significantly more MSCs were attracted and migrated within the PE-MESS hydrogel, and a large number of MSCs were aggregated around the PE-MESS. This suggests that both engineered MESS and PEMF have desirable stem cell chemotaxis effects and that the combined application maximises the promotion of stem cell aggregation around the microspheres (Figure 5).12
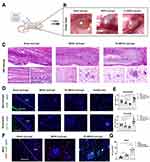 |
Figure 5 An oxidised alginate-tris (hydroxymethyl)aminomethane (THAM)/gelatin hydrogel as a scaffold combined with an hBMSCs-derived bioactive microsphere system as a bioactive supplement promotes endogenous MSC recruitment under pulsed electromagnetic fields (PEMF). (A) Hydrogel injection was implanted subcutaneously in the back of rats. (B) Gross condition of the hydrogel microsphere system 7 days after implantation with (C) H&E staining. (D and E) IF staining and semi-quantitative analysis of CD68 and CD11b. (F and G) Dual-labelled IF staining of CD44 and CD90, and semi-quantitative analysis of double-positive cells for CD44 and CD90 recruited to the hydrogel. *p < 0.05, **p < 0.01. Reprinted with permission from Li Y, Li L, Wang M, et al. O-alg-THAM/gel hydrogels functionalized with engineered microspheres based on mesenchymal stem cell secretion recruit endogenous stem cells for cartilage repair. Bioact Mater. 2023;28:255–272.12 |
Making cells more sensitive to homing signals depends on gene technology, which usually requires operating cells in vitro. Therefore, it is mainly used in cell therapy to improve homing after cell transplantation. Durand et al transfected BMSCs with CXCR4 mRNA, thus creating BMSCs that overexpress CXCR4. This increased the directed cell migration of the MSCs, as well as altering their cytokine secretion.160 Shuhong Kuang used genetic engineering techniques to produce MSCs that are overexpressed by C-motif chemokine receptor 2 (CCR2), which enhances the targeted migration and immunomodulatory potential of MSCs in vitro in response to the C-C motif chemokine ligand 2 (CCL2).161
Promotion of More Efficient Cell Migration
Cell migration is a highly dynamic physiological process that is regulated by biochemical factors, physical factors, and is primarily driven by the cytoskeleton.162 Its biochemical factors mainly include guide cues, bonding ligands, and matrix degradability.163 Its physical factors mainly include the ECM’s stiffness, elasticity, and viscosity, as well as the ECM’s topology, pore size, etc.164 The migration of cells in one-, two-, and three-dimensional environments have been described as different processes.165 Broadly speaking, when migration occurs, the cytoskeleton—including the microtubules, intermediate filament, and actin microfilament—is rearranged under the control of an extensive signaling cascade; next, the cell is polarized, and then the cell migration is completed by actin that is protruding forward, adhesion to the surrounding matrix, and actin contraction.166 Cell-to-matrix interactions and cell-to-cell interactions play a crucial role in migration. Tissue engineering technology can realize the regulation of cell migration through intervention in the ECM environment, restriction of the mechanical properties of the matrix, and a careful design of material topology. TNF-α,78 IL-6,77 and other inflammatory factors chemokines (such as SDF-1α,167 matrix metalloproteinases,168 integrin169 and engineered integrin-ligands132) are the usual methods of tissue engineering that control cell migration by interfering with biochemical elements.
In addition to regulating chemical factors, the design of the physical properties of a matrix is also a means of constructing skin substitutes that promote cell migration. An eligible wound implant should promote endogenous cell recruitment and allow cells to pass freely. Furthermore, 3D printing technology is typically used to construct ingen microstructures (such as pores and microchannels), and it is specially designed for hardness gradients.170–173 Electrospinning, advanced foaming techniques, and various engineering methods for hydrogel or scaffold structures can also create rich pore and microchannel networks, or it can provide dynamic and controllable mechanical properties to aid cell migration.174–179 Electric fields were found to be able to adjust the hierarchical microstructure of materials. By adjusting the hierarchical microstructure of the silk fibroin matrix in an electric field, researchers have created an anisotropic porous scaffold that provides suitable mechanical signals for migration in skin healing.177 Researchers have also discovered certain naturally excellent fibrin arrangements that can be used to provide more explicit physical and mechanical clues for cell migration. Kimberly Nellenbach et al found that the fibrin network of neonates had a higher fiber arrangement; through in vivo and in vitro experiments, they showed that, compared with adult fibrin scaffolds, fibrin scaffolds from neonates can enhance wound healing results.180 Degang Yang et al introduced gradient channels in tissue engineering scaffolds.181 With the help of 3D printing technology, the spatial connection of different nanocomposite hydrogel slurries, which change by changing the concentration of the nanomaterials, is formed in order to construct a gradual gradient nanocomposite hydrogel and to control cell migration.182 The combination of various components can also provide smoother passages for migration or for specially tailored hardness. Hossein Ravanbakhsh et al reported a glycol chitosan hydrogel loaded with carbon nanotubes, which promoted cell recruitment and migration relative to carrier-free hydrogels.183 Miriam Dietrich et al introduced uniaxial strains in a matrix, thus creating local anisotropic hardness by embedding microstructured photopolymerizable hydrogel strips in channel slides to guide cell migration.184 Jiawen Li et al adjusted the lamellar spacing and micro-ridge spacing of radial sponges with the ice template method, and they found that denser lamellae and micro-ridges could promote L929 cells and HUVECs to achieve a more ordered arrangement, as well as more efficient cell migration, thus promoting wound healing (Figure 6).185 However, cell migration in wound healing has been explored more in terms of fibroblasts, keratinocytes, and vascular endothelial cells, and little research has been done on how materials can be used to promote the harmonious migration of MSCs to improve homing efficiency. Even though the factors affecting cell migration are largely the same, migration-friendly materials for MSCs still need to be developed.
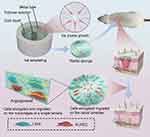 |
Figure 6 Radial sponges with denser lamellae and microridges that can be adjusted for lamellar spacing and microridge spacing with the ice template method, thus achieving a more ordered arrangement of cells, greater elongation, and greater migration. Reprinted with permission from Li J, Xiao L, Gao S, et al. Radial sponges facilitate wound healing by promoting cell migration and angiogenesis. Adv Healthc Mater. 2023;12(11):e2202737. © 2023 Wiley-VCH GmbH.185 |
Promotion of MSC Paracrine Secretion
Studies have shown that MSCs mainly improve the proliferation and survival of target cells in a paracrine manner.186 By enhancing the paracrine effects of each MSC that reaches the wound site, the MSC can be made to better perform their therapeutic role, thereby promoting skin wound healing. This approach has usually been combined with cellular therapy in the past. One method pretreats or co-cultures exogenous MSCs to increase their biological function after implantation. The studies by Chenyang Liu et al showed that MSCs that are pretreated with the pro-inflammatory factors IFN-γ and TNF-α exhibited a significant amplification in the secretome. This pretreated MSC supernatant of human umbilical cord-derived MSCs (UC-MSCs) is known as S-IT MSCs, and they can accelerate wound healing at the wound site by promoting macrophage migration and M2 polarization.187 Another experiment showed that the exosomes of bone marrow-derived MSCs (BMSCs) that were pretreated by deferoxamine (DFO-Exos) activate the PI3K/AKT signaling pathway in wound repair by the miR-126-mediated downregulation of PTEN, thereby stimulating cutaneous angiogenesis in vitro.188 Prakoeswa et al co-cultured different doses of resveratrol with MSCs, and they found that resveratrol could promote the proliferation of mesenchymal stem cells that are derived from adult and fetal tissues, as well as the secretion of wound healing-related growth factors in a dose-dependent manner.189 In addition to applying educated MSCs individually to the treatment, cell therapy could combine with bioactive materials. Then, the complex can be implanted at the wound site to promote MSC secretion at the wound site. Meihua Gong et al developed a dopamine-methacrylate hyaluronic acid (DA-MeHA) as an effective carrier for stem cells in skin regeneration therapy. Adipose stem cells (ADSCs) from DA-MeHA hydrogels can secrete higher levels of growth factors.190 Murphy et al reported an engineered fibrin hydrogel that was taken as a carrier of MSC spheroids; in addition, it increased the secretion of VEGF and PGE2 from MSC spheroids, enhanced the macrophage polarization at the wound site, stimulated endothelial cell proliferation, and promoted angiogenesis.191 Jiujiang Zeng et al designed a PNIPAM-based porous hydrogel crosslinked to disulfide bonds. This porous hydrogel becomes a solution within 25 minutes of adding glutathione at 15 °C, and it gels again within 80 seconds at 33 °C. At 37 °C, the pore size is 300 μm, and the ASCs inoculated into the porous hydrogel spontaneously aggregate inside the wells to form galore spheres. This hydrogel was used to prepare stem cell spheroids that were easy to collect, and the researchers observed a marked upregulation of paracrine levels in ASC spheroids within the hydrogel.192
Nowadays, researchers in the field of tissue engineering have increasingly focused on the issue of the secretory function of MSCs in target tissues, and more and more well-designed tissue-engineered materials can be realized to promote the secretion of endogenous MSCs instead of complex in vitro treatments in conjunction with cellular therapies. The physical properties (eg matrix stiffness,136 viscoelasticity, porosity, cell adhesion capacity, etc.) and microstructures (eg construction of two- or three-dimensional structures,138 3D-printed fibrous scaffolds to design morphology,193 electrostatic spinning to design fiber arrangement,140 etc.) of the materials are being designed to influence cell-matrix and cell-cell interactions and thus direct the secretion of MSCs. In addition biochemical cues provided by bioactive materials (proteins, peptides, and some small molecules) are also able to influence the secretory profile of MSCs,194 and providing suitable physicochemical cues for MSCs at wound sites by means of tissue engineering is a promising development in the field of endogenous regenerative medicine. Aeolus Vilar et al showed that substrate stiffness modulates MSC paracrine activity and therapeutic potential, with 0.2 kPa substrate increasing IL-6 secretion in MSC, and TIMP-2, OPG, sTNFR1, and MCP-1 secretion elevated in MSC on 100 kPa substrate (Figure 7).76 The study of Ni Su et al showed that the fibrous structure of the scaffold can regulate the paracrine function of MSCs. Compared with cells that are cultured on microplates, MSCs on electrospinning fibers produce higher levels of anti-inflammatory and proangiogenic cytokines.195 Ruiying Huang et al prepared a polycaprolactone and bacterial cellulose scaffold using a bio-3D printing system, a fibrous scaffold with a morphology that modulates the paracrine function of Ad-MSC in skin tissue regeneration. By regulating cell-material interactions, MSC paracrine secretion was promoted thereby accelerating wound healing.193 Meifei Lian et al developed spongy scaffolds with a layered structure and interconnected pores using a low-temperature deposition model (LDM) printing technique capable of facilitating cell-material interactions to promote MSC adhesion, retention, survival and inward growth. Protein function assays indicated that after using this scaffold downstream AKT, adhesion patch kinase (FAK) and yes-associated protein (YAP) signalling may paracrine the mechanotransduction pathway required for MSC paracrine secretion through which the layered porous structure stimulates MSC paracrine action (Figure 8).137
 |
Figure 7 Secretome analysis of MSCs on soft and stiff substrates. (A) The pi value analysis plot identified five associated proteins: OPG, IL-6, MCP-1, TIMP-2 and sTNFR1. (B) The bars show the relative levels of proteins determined by Pi value analysis. (C) Bioinformatics analyses of the biological processes associated with these candidate proteins were performed, and the biological processes with a high proportion of differentially expressed proteins were plotted in the form of bubble plots. Reprinted with permission from A. Vilar et al, Substrate mechanical properties bias MSC paracrine activity and therapeutic potential, Acta Biomaterialia, vol. 168, pp. 144-158, © (copyright 2023).76 |
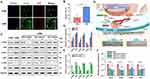 |
Figure 8 Molecular mechanisms by which sponge scaffolds with hierarchical structure and interconnected pores modulate paracrine secretion by mesenchymal stem cells. (A) Representative immunofluorescence staining images of YAP (red), F-actin (green) and nuclei (blue). (B) Quantitative analysis of nuclear YAP (%) of MSCs cultured for 24 h on FDM- and LDM- printed scaffolds. (C) Representative Western blot images and semi-quantitative analysis of FAK, AKT and YAP signaling pathway protein expression in MSCs cultured on both scaffolds (D) and (E) MSCs cultured on LDM-printed sponges containing FAK inhibitors. Activation of FAK and downstream AKT and YAP pathways was observed in MSCs cultured on LDM printed sponges. (F) Inhibitory effects of FAK, AKT and YAP on paracrine factor expression in MSCs cultured on LDM printed sponges were found by RT-PCR analysis. The key role of FAK and its downstream AKT and YAP signaling in the regulation of paracrine function of MSCs by LDM sponge was verified. (G) Schematic diagram showing the regulation of MSC paracrine function by the porous structure of LDM printed sponge through FAK and downstream AKT and YAP-dependent mechanotransduction pathways. *p < 0.05, **p < 0.01. Reprinted from Biomaterials, 274, Lian M, Sun B, Han Y, et al. A low-temperature-printed hierarchical porous sponge-like scaffold that promotes cell-material interaction and modulates paracrine activity of MSCs for vascularized bone regeneration, 120841. Copyright 2021 with permission from Elsevier.137 |
In addition to promoting the secretion of MSCs in target tissues, a cell-free therapy with MSC secretion products as the core has also attracted increasing attention. Exosomes are globular lipid bilayer vesicles that are secreted by MSCs with a diameter of 30–150 nanometers.196 MSC-released exosomes can reach target cells through circulation or paracrines, and they are then internalized by recipient cells through surface molecule-mediated endocytosis, ligand–receptor interactions, membrane–recipient cell fusion, micropinocytosis, or phagocytosis.197 Exosomes have a high skin penetration rate because they are a natural bilayer lipid sphere.197,198 This feature allows exosomes to be administered topically at the wound site.199
Moreover, exosomes have a certain chemotaxis, and they can migrate to the lesion area that is a certain distance from the administration area.200 To skin lesions, exosomes promote healing in various ways, such as regulating macrophage polarization,201 promoting blood vessel formation,202 enhancing reepithelialization,203 promoting collagen deposition, and the secretion of target cell growth factors.204 A study by Cooper et al showed that human ADMSC-derived exosomes promote the migration of human skin fibroblasts (HDFs) to accelerate skin ischemic wound healing.205 Biomaterials offer a more flexible form for exosome applications. The combination with biomaterials can compensate for some of the shortcomings of the application of exosomes alone, such as the viability of exosomes, the release of exosomes, etc. The Pluronic F-127 hydrogel reported by Yang Zhou et al, which is injectable, biocompatible, and heat-sensitive, was used to encapsulate allogeneic human adipose mesenchymal stem cell exosomes (hADSCs-Exos). This hydrogel–exosome complex was applied topically to full-thickness skin wounds in mice; following this, the PF-127/hADSCs-Exos complex was observed to maintain the biological activity of the hADSCs-Exos when compared to hADSCs Exos alone, as well as seen to improve the efficiency of exosome delivery and optimize the performance of hADSCs-Exos.206 Xinrong Geng et al designed a novel bone marrow mesenchymal stem cell-derived exosome (MSC-Exo) that was loaded carboxyethyl chitosan (CEC)-aldehyde carboxymethylcellulose (DCMC) hydrogel (MSC-Exos@CEC-DCMC HG) for the purpose of chronic diabetes wound healing.207 These experiments provide a new paradigm for harnessing mesenchymal stem cell homing for the purpose of accelerating wound healing.
Conclusions and Future Perspectives
The mobilization of endogenous stem cell homing is a safe and reliable method for enhancing tissue repair, and it is currently excelling in the treatment of stress urinary incontinence,208 repair of myocardial infarction,209 and the repair of bone and cartilage injuries;12,210,211 however, it has had few applications in the field of skin wound repair. In combination with tissue engineering materials, the main signaling molecule that has been applied to mobilize MSCs for endogenous homing is SDF-1; however, there are many more key molecules involved in the homing process that may be applied to the wound site in combination with biologically active materials in order to influence the efficiency of homing, such as inflammatory cytokines and growth factors. A variety of physical stimuli can also promote endogenous homing for MSCs, such as exogenous magnetic fields, ultrasound, and mechanical stretching. Moreover, combining the appropriate physical stimuli during material application may further improve the efficiency of homing. In addition to the key homing factors loaded by bioactive materials, as well as externally applied physical stimuli, the physicochemical properties of the material itself can also influence the attraction of the wound site to the MSCs in the stem cell niche. Biomaterials that adequately mimic the extracellular matrix are more capable of promoting endogenous homing, such as materials that are made from natural proteins, as well as polysaccharides that are components of the extracellular matrix. Whether the design of the topographical features of wound dressings, which modulate cell behavior and promote the migration of fibroblasts and vascular endothelial cells, can similarly promote the migration of nesting MSCs during the mobilization of endogenous wound repair is a question worth exploring. However, in any case, the design of the dressing’s microscopic morphology can indeed influence the cellular motor behavior at the wound site, which can thus promote endogenous wound healing.185
The current mobilization of endogenous stem cells to home in and to promote tissue repair tends to be a single-process facilitation, such as through promoting more MSCs from nearby stem cell niches, facilitating faster migration, or promoting paracrine secretion that is more conducive to wound healing. These type of approaches are conducted rather than a synergistic multi-process, multi-perspective mobilization (such as the ability to increase both the homing signals given by the wound site as a guide while designing the physical properties of the material to promote the differentiation of the MSCs that reach their destination, as well as in promoting the differentiation of the MSCs that reach their destination). MSC differentiation and secretion occur at the destination. The development of mobilized materials for a multifunctional endogenous repair is a promising direction for creating more effective regenerative therapies. The mobilization of endogenous stem cells for wound repair is not subject to the regulatory constraints of stem cell transplantation strategies, and it is able to meet the feasibility requirements for large-scale clinical translation. However, the design of scaffolding materials with spatio-temporal properties, as well as drug-carrying bioactive materials with appropriate concentration gradients (or with appropriate micromorphology and mechanical properties to modulate the behavior of stem cells at the wound site in terms of their regeneration, functional manifestation, and differentiation), is still a huge challenge.
The research findings of this review have a broad impact on the field of regenerative medicine. Firstly, it provides a more systematic theory and approach for improving materials for endogenous tissue repair, which is crucial for understanding and designing tissue engineering. Secondly, this review provides a new perspective for exploring the complex process of tissue remodeling, which is of great significance for improving the rational design of biomaterial scaffolds and guiding tissue regeneration strategies. In addition, this review also emphasizes that mobilizing endogenous stem cell homing is a safe and reliable method for enhancing tissue repair, which performs well in the treatment of stress urinary incontinence, myocardial infarction repair, bone and cartilage injury repair, and plays an increasingly important role in skin tissue repair. Compared to other tissue repair fields, there is still a lack of research on promoting endogenous MSC homing through materials for skin wound repair, which requires more attention from tissue engineers. Finally, this review proposes the development of mobilization materials for multifunctional endogenous repair, which is a promising direction for creating more effective skin regeneration therapies. Overall, the research findings of this review are of great significance for the research and application of skin tissue regeneration medicine.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (82372526 and 82302797), the Natural Science Foundation of Guangdong Province (2023A1515012970 and 2020A1515010107), the National Key R&D Program of China (2018YFE0194300), the Guangdong Province Key Field R&D Program Project (2020B1111150001), and the Science and Technology Innovation Project of Guangdong Province (2018KJYZ005).
Disclosure
The authors declare no conflicts of interest.
References
1. Jiang Z, Zheng Z, Yu S, et al. Nanofiber scaffolds as drug delivery systems promoting wound healing. Pharmaceutics. 2023;15(7). doi:10.3390/pharmaceutics15071829
2. Liu M, Wei X, Zheng Z, et al. Recent advances in nano-drug delivery systems for the treatment of diabetic wound healing. Int J Nanomed. 2023;18:1537–1560. doi:10.2147/ijn.S395438
3. Wei X, Li M, Zheng Z, et al. Senescence in chronic wounds and potential targeted therapies. Burns Trauma. 2022;10. doi:10.1093/burnst/tkab045
4. Zhao Y, Wang M, Liang F, Li J. Recent strategies for enhancing the therapeutic efficacy of stem cells in wound healing. Stem Cell Res Ther. 2021;12(1):588. doi:10.1186/s13287-021-02657-3
5. Konstantinou E, Zagoriti Z, Pyriochou A, Poulas K. Microcurrent stimulation triggers MAPK Signaling and TGF-beta 1 release in fibroblast and osteoblast-like cell lines. Cells. 2020;9(9):1924. doi:10.3390/cells9091924
6. Chen R-F, Yang M-Y, Wang C-J, Wang C-T, Kuo Y-R. Proteomic analysis of peri-wounding tissue expressions in extracorporeal shock wave enhanced diabetic wound healing in a streptozotocin-induced diabetes model. Int J Mol Sci. 2020;21(15):5445. doi:10.3390/ijms21155445
7. Da Silva J, Leal EC, Carvalho E, Silva EA. Innovative functional biomaterials as therapeutic wound dressings for chronic diabetic foot ulcers. Int J Mol Sci. 2023;24(12):9900. doi:10.3390/ijms24129900
8. Shakhakarmi K, Seo J-E, Lamichhane S, Thapa C, Lee S. EGF, a veteran of wound healing: highlights on its mode of action, clinical applications with focus on wound treatment, and recent drug delivery strategies. Arch. Pharmacal Res. 2023;46(4):299–322. doi:10.1007/s12272-023-01444-3
9. Yan W, Chen Y, Guo Y, et al. Irisin promotes cardiac homing of intravenously delivered MSCs and protects against ischemic heart injury. Adv Sci. 2022;9(7):2103697. doi:10.1002/advs.202103697
10. Xia H, Li X, Gao W, et al. Tissue repair and regeneration with endogenous stem cells. Nature Rev Mater. 2018;3(7):174–193. doi:10.1038/s41578-018-0027-6
11. Cancedda R, Bollini S, Descalzi F, Mastrogiacomo M, Tasso R. Learning from mother nature: innovative tools to boost endogenous repair of critical or difficult-to-heal large tissue defects. Front Bioeng Biotechnol. 2017;5:28. doi:10.3389/fbioe.2017.00028
12. Li Y, Li L, Wang M, et al. O-alg-THAM/gel hydrogels functionalized with engineered microspheres based on mesenchymal stem cell secretion recruit endogenous stem cells for cartilage repair. Bioact Mater. 2023;28:255–272. doi:10.1016/j.bioactmat.2023.05.003
13. Wu X, Huang D, Xu Y, Chen G, Zhao Y. Microfluidic templated stem cell spheroid microneedles for diabetic wound treatment. Adv Mater. 2023;35:2301064. doi:10.1002/adma.202301064
14. Guo L, Du J, Yuan D-F, et al. Optimal H2O2 preconditioning to improve bone marrow mesenchymal stem cells’ engraftment in wound healing. Stem Cell Res Ther. 2020;11(1):434. doi:10.1186/s13287-020-01910-5
15. Chen Y, Li Y, Lu F, Dong Z. Endogenous bone marrow-derived stem cell mobilization and homing for in situ tissue regeneration. Stem Cells. 2023;41(6):541–551. doi:10.1093/stmcls/sxad026
16. Kaushik K, Das A. TWIST1-reprogrammed endothelial cell transplantation potentiates neovascularization-mediated diabetic wound tissue regeneration. Diabetes. 2020;69(6):1232–1247. doi:10.2337/db20-0138
17. Wu R-X, Xu X-Y, Wang J, He X-T, Sun -H-H, Chen F-M. Biomaterials for endogenous regenerative medicine: coaxing stem cell homing and beyond. Appl Mater Today. 2018;11:144–165. doi:10.1016/j.apmt.2018.02.004
18. Yang Z, Li H, Tian Y, et al. Biofunctionalized structure and ingredient mimicking scaffolds achieving recruitment and chondrogenesis for staged cartilage regeneration. Front Cell Dev Biol. 2021;9:655440. doi:10.3389/fcell.2021.655440
19. Li X, Wang Y, Shi L, et al. Magnetic targeting enhances the cutaneous wound healing effects of human mesenchymal stem cell-derived iron oxide exosomes. J Nanobiotechnology. 2020;18(1):113. doi:10.1186/s12951-020-00670-x
20. Li M, Jiang Y, Hou Q, Zhao Y, Zhong L, Fu X. Potential pre-activation strategies for improving therapeutic efficacy of mesenchymal stem cells: current status and future prospects. Stem Cell Res Ther. 2022;13(1):146. doi:10.1186/s13287-022-02822-2
21. Nitzsche F, Muller C, Lukomska B, Jolkkonen J, Deten A, Boltze J. Concise review: MSC adhesion cascade-insights into homing and transendothelial migration. Stem Cells. 2017;35(6):1446–1460. doi:10.1002/stem.2614
22. Ullah M, Liu DD, Thakor AS. Mesenchymal stromal cell homing: mechanisms and strategies for improvement. iScience. 2019;15:421–438. doi:10.1016/j.isci.2019.05.004
23. Alghamdi A, Tamra A, Rakhmatulina A, et al. Nanoscopic characterization of cell migration under flow using optical and electron microscopy. Anal. Chem. 2023;95(3):1958–1966. doi:10.1021/acs.analchem.2c04222
24. Dollet PE, Hsu MJ, Ambroise J, et al. Evaluation of strategies aimed at improving liver progenitor cell rolling and subsequent adhesion to the endothelium. Cell Transplantation. 2020;29:0963689720912707. doi:10.1177/0963689720912707
25. Zhou L, Hao Q, Sugita S, et al. Role of CD44 in increasing the potency of mesenchymal stem cell extracellular vesicles by hyaluronic acid in severe pneumonia. Stem Cell Res Ther. 2021;12(1):293. doi:10.1186/s13287-021-02329-2
26. Suila H, Hirvonen T, Kotovuori A, et al. Human umbilical cord blood-derived mesenchymal stromal cells display a novel interaction between P-selectin and galectin-1. Scand J Immunol. 2014;80(1):12–21. doi:10.1111/sji.12179
27. Bailey AM, Lawrence MB, Shang H, Katz AJ, Peirce SM. Agent-based model of therapeutic adipose-derived stromal cell trafficking during ischemia predicts ability to roll on P-selectin. PLoS Comput Biol. 2009;5(2):e1000294. doi:10.1371/journal.pcbi.1000294
28. Szydlak R. Biological, chemical and mechanical factors regulating migration and homing of mesenchymal stem cells. World J Stem Cells. 2021;13(6):619–631. doi:10.4252/wjsc.v13.i6.619
29. Ruster B, Gottig S, Ludwig RJ, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108(12):3938–3944. doi:10.1182/blood-2006-05-025098
30. Thankamony SP, Sackstein R. Enforced hematopoietic cell E- and L-selectin ligand (HCELL) expression primes transendothelial migration of human mesenchymal stem cells. Proc Natl Acad Sci U S A. 2011;108(6):2258–2263. doi:10.1073/pnas.1018064108
31. Li X, Wang Q, Ding L, et al. Intercellular adhesion molecule-1 enhances the therapeutic effects of MSCs in a dextran sulfate sodium-induced colitis models by promoting MSCs homing to murine colons and spleens. Stem Cell Res Ther. 2019;10(1):267. doi:10.1186/s13287-019-1384-9
32. Schäfer R, Schwab M, Siegel G, et al. Modulating endothelial adhesion and migration impacts stem cell therapies efficacy. EBioMedicine. 2020;60:102987. doi:10.1016/j.ebiom.2020.102987
33. Sedlar A, Trávníčková M, Bojarová P, et al. Interaction between galectin-3 and integrins mediates cell-matrix adhesion in endothelial cells and mesenchymal stem cells. Int J Mol Sci. 2021;22(10):5144. doi:10.3390/ijms22105144
34. Cui L-L, Nitzsche F, Pryazhnikov E, et al. Integrin α4 overexpression on rat mesenchymal stem cells enhances transmigration and reduces cerebral embolism after intracarotid injection. Stroke. 2017;48(10):2895–2900. doi:10.1161/strokeaha.117.017809
35. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–2749. doi:10.1634/stemcells.2007-0197
36. Meng Z, Feng G, Hu X, Yang L, Yang X, Jin Q. SDF Factor-1α promotes the migration, proliferation, and osteogenic differentiation of mouse bone marrow mesenchymal stem cells through the wnt/β-catenin pathway. Stem Cells Dev. 2021;30(2):106–117. doi:10.1089/scd.2020.0165
37. Lau TT, Wang DA. Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expert Opin Biol Ther. 2011;11(2):189–197. doi:10.1517/14712598.2011.546338
38. Sun Y, Fang Y, Li X, et al. A static magnetic field enhances the repair of osteoarthritic cartilage by promoting the migration of stem cells and chondrogenesis. J Orthopaedic Transl. 2023;39:43–54. doi:10.1016/j.jot.2022.11.007
39. Chatterjee T, Lewis TL, Arora I, et al. Sex-based disparities in leukocyte migration and activation in response to inhalation lung injury: role of SDF-1/CXCR4 signaling. Cells. 2023;12(13):1719. doi:10.3390/cells12131719
40. Sui M, Li T, Lu H, et al. SOCS3 inhibits the mesenchymal stromal cell secretory factor SDF-1-mediated improvement of islet function in non-obese diabetic mice. Stem Cell Res Ther. 2023;14(1):172. doi:10.1186/s13287-023-03347-y
41. Ngamsri K-C, Jans C, Putri RA, et al. Inhibition of CXCR4 and CXCR7 is protective in acute peritoneal inflammation. Front Immunol. 2020;11:2020-March–10. doi:10.3389/fimmu.2020.00407
42. Ghadge SK, Messner M, Seiringer H, et al. Loss of smooth muscle SDF-1/CXCL12 leads to cardiac hypertrophy and aortic valve stenosis. Eur Heart J. 2020;41(Supplement_2). doi:10.1093/ehjci/ehaa946.3630
43. Song B, Chen D, Liu Z, et al. Stromal cell-derived factor-1 exerts opposing roles through CXCR4 and CXCR7 in angiotensin II-induced adventitial remodeling. Biochem Biophys Res Commun. 2022;594:38–45. doi:10.1016/j.bbrc.2022.01.030
44. Lin TH, Liu -H-H, Tsai T-H, et al. CCL2 increases αvβ3 integrin expression and subsequently promotes prostate cancer migration. Biochim Biophys Acta. 2013;1830(10):4917–4927. doi:10.1016/j.bbagen.2013.06.033
45. Constantin G, Majeed M, Giagulli C, et al. Chemokines trigger immediate beta2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity. 2000;13(6):759–769. doi:10.1016/s1074-7613(00)00074-1
46. Choi JH, Lee YB, Jung J, Hwang SG, Oh IH, Kim GJ. Hypoxia inducible factor-1alpha regulates the migration of bone marrow mesenchymal stem cells via integrin alpha 4. Stem Cells Int. 2016;2016:7932185. doi:10.1155/2016/7932185
47. Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24(4):1030–1041. doi:10.1634/stemcells.2005-0319
48. Ringe J, Strassburg S, Neumann K, et al. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem. 2007;101(1):135–146. doi:10.1002/jcb.21172
49. Von Lüttichau I, Notohamiprodjo M, Wechselberger A, et al. Human Adult CD34 − progenitor cells functionally express the chemokine receptors CCR1, CCR4, CCR7, CXCR5, and CCR10 but Not CXCR4. Stem Cells Dev. 2005;14(3):329–336. doi:10.1089/scd.2005.14.329
50. Vitale E, Rossin D, Perveen S, et al. Silica nanoparticle internalization improves chemotactic behaviour of human mesenchymal stem cells acting on the SDF1α/CXCR4 axis. Biomedicines. 2022;10(2):336. doi:10.3390/biomedicines10020336
51. Zhao A, Chung M, Yang Y, Pan X, Pan Y, Cai S. The SDF-1/CXCR4 signaling pathway directs the migration of systemically transplanted bone marrow mesenchymal stem cells towards the lesion site in a rat model of spinal cord injury. Current Stem Cell Res Therap. 2023;18(2):216–230. doi:10.2174/1574888X17666220510163245
52. Ling L, Hou J, Liu D, et al. Important role of the SDF-1/CXCR4 axis in the homing of systemically transplanted human amnion-derived mesenchymal stem cells (hAD-MSCs) to ovaries in rats with chemotherapy-induced premature ovarian insufficiency (POI. Stem Cell Res Ther. 2022;13(1):79. doi:10.1186/s13287-022-02759-6
53. Hocking AM. The role of chemokines in mesenchymal stem cell homing to wounds. Adv Wound Care. 2015;4(11):623–630. doi:10.1089/wound.2014.0579
54. Geraldo LH, Garcia C, Xu Y, et al. CCL21-CCR7 signaling promotes microglia/macrophage recruitment and chemotherapy resistance in glioblastoma. Cell Mol Life Sci. 2023;80(7):179. doi:10.1007/s00018-023-04788-7
55. Pennycuick A, Teixeira VH, AbdulJabbar K, et al. Immune surveillance in clinical regression of preinvasive squamous cell lung cancer. Cancer Discovery. 2020;10(10):1489–1499. doi:10.1158/2159-8290.Cd-19-1366
56. Hopman RK, DiPersio JF. Advances in stem cell mobilization. Blood Rev. 2014;28(1):31–40. doi:10.1016/j.blre.2014.01.001
57. Steingen C, Brenig F, Baumgartner L, Schmidt J, Schmidt A, Bloch W. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J Mol Cell Cardiol. 2008;44(6):1072–1084. doi:10.1016/j.yjmcc.2008.03.010
58. Pistone A, Iannazzo D, Espro C, et al. Tethering of Gly-Arg-Gly-Asp-Ser-Pro-Lys Peptides on Mg-Doped Hydroxyapatite. Engineering. 2017;3(1):55–59. doi:10.1016/J.ENG.2017.01.007
59. Hao D, Ma B, He C, et al. Surface modification of polymeric electrospun scaffolds via a potent and high-affinity integrin α4β1 ligand improved the adhesion, spreading and survival of human chorionic villus-derived mesenchymal stem cells: a new insight for fetal tissue engineering. J Mater Chem B. 2020;8(8):1649–1659. doi:10.1039/c9tb02309g
60. Burk J, Sassmann A, Kasper C, Nimptsch A, Schubert S. Extracellular matrix synthesis and remodeling by mesenchymal stromal cells is context-sensitive. Int J Mol Sci. 2022;23(3):1758. doi:10.3390/ijms23031758
61. ter Mors B, Spieler V, Merino Asumendi E, Gantert B, Lühmann T, Meinel L. Bioresponsive cytokine delivery responding to matrix metalloproteinases. ACS Biomater Sci Eng. 2023. doi:10.1021/acsbiomaterials.2c01320
62. Zhang R-F, Zhang B, Chang-Jiang W, Jin J-Y. Labelling matrix metalloproteinases. Curr Med Chem. 2023;30(40):4569–4585. doi:10.2174/0929867330666230113121728
63. Laronha H, Carpinteiro I, Portugal J, et al. Challenges in matrix metalloproteinases inhibition. Biomolecules. 2020;10(5):717. doi:10.3390/biom10050717
64. De Becker A, Van Hummelen P, Bakkus M, et al. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica. 2007;92(4):440–449. doi:10.3324/haematol.10475
65. Almalki SG, Agrawal DK. Effects of matrix metalloproteinases on the fate of mesenchymal stem cells. Stem Cell Res Ther. 2016;7(1):129. doi:10.1186/s13287-016-0393-1
66. Ponte AL, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25(7):1737–1745. doi:10.1634/stemcells.2007-0054
67. Yen JH, Chio WT, Chuang CJ, Yang HL, Huang ST. Improved wound healing by naringin associated with MMP and the VEGF pathway. Molecules. 2022;27(5):1695. doi:10.3390/molecules27051695
68. Szydlak R. Mesenchymal stem cells’ homing and cardiac tissue repair. Acta Biochim Pol. 2019;66(4):483–489. doi:10.18388/abp.2019_2890
69. Ahmadian Kia N, Bahrami AR, Ebrahimi M, et al. Comparative analysis of chemokine receptor’s expression in mesenchymal stem cells derived from human bone marrow and adipose tissue. J Mol Neurosci. 2011;44(3):178–185. doi:10.1007/s12031-010-9446-6
70. Szydlak R, Majka M, Lekka M, Kot M, Laidler P. AFM-based analysis of wharton’s jelly mesenchymal stem cells. Int J Mol Sci. 2019;20(18):4351. doi:10.3390/ijms20184351
71. Fox JM, Chamberlain G, Ashton BA, Middleton J. Recent advances into the understanding of mesenchymal stem cell trafficking. Br J Haematol. 2007;137(6):491–502. doi:10.1111/j.1365-2141.2007.06610.x
72. Kortesidis A, Zannettino ACW, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105(10):3793–3801. doi:10.1182/blood-2004-11-4349
73. Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 2016;7(1):7. doi:10.1186/s13287-015-0271-2
74. Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278(4):369–395. doi:10.1111/joim.12395
75. Nam D, Park A, Dubon MJ, et al. Coordinated regulation of mesenchymal stem cell migration by various chemotactic stimuli. Int J Mol Sci. 2020;21(22):8561. doi:10.3390/ijms21228561
76. Vilar A, Hodgson-Garms M, Kusuma GD, et al. Substrate mechanical properties bias MSC paracrine activity and therapeutic potential. Acta Biomater. 2023;168:144–158. doi:10.1016/j.actbio.2023.06.041
77. Casson J, O’Kane S, Smith C-A, Dalby MJ, Berry CC. Interleukin 6 plays a role in the migration of magnetically levitated mesenchymal stem cells spheroids. Appl Sci. 2018;8(3):412. doi:10.3390/app8030412
78. Bai X, Xi J, Bi Y, et al. TNF -α promotes survival and migration of MSC s under oxidative stress via NF -κB pathway to attenuate intimal hyperplasia in vein grafts. J Cell Mol Med. 2017;21(9):2077–2091. doi:10.1111/jcmm.13131
79. Garg A, Khan S, Luu N, et al. TGFβ 1 priming enhances CXCR3 -mediated mesenchymal stromal cell engraftment to the liver and enhances anti-inflammatory efficacy. J Cell Mol Med. 2023;27(6):864–878. doi:10.1111/jcmm.17698
80. Chen MS, Lin CY, Chiu YH, Chen CP, Tsai PJ, Wang HS. IL-1β-induced matrix metalloprotease-1 promotes mesenchymal stem cell migration via PAR1 and G-protein-coupled signaling pathway. Stem Cells Int. 2018;2018:3524759. doi:10.1155/2018/3524759
81. Zhang Y, Yan J, Xu H, et al. Extremely low frequency electromagnetic fields promote mesenchymal stem cell migration by increasing intracellular Ca2+ and activating the FAK/Rho GTPases signaling pathways in vitro. Stem Cell Res Ther. 2018;9(1):143. doi:10.1186/s13287-018-0883-4
82. Yu J, Kim HM, Kim KP, Son Y, Kim MS, Park KS. Ceramide kinase regulates the migration of bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2019;508(2):361–367. doi:10.1016/j.bbrc.2018.11.154
83. Jiang LH, Mousawi F, Yang X, Roger S. ATP-induced Ca(2+)-signalling mechanisms in the regulation of mesenchymal stem cell migration. Cell Mol Life Sci. 2017;74(20):3697–3710. doi:10.1007/s00018-017-2545-6
84. Holmes HL, Wilson B, Goerger JP, et al. Facilitated recruitment of mesenchymal stromal cells by bone marrow concentrate and platelet rich plasma. PLoS One. 2018;13(3):e0194567. doi:10.1371/journal.pone.0194567
85. Mehdi Maneshi M, Sachs F, Zonglu Hua S. Shear stress stimulated MSC activities: direct changes of membrane tension or cytoskeletal stress? Biophys. J. 2017;112(3):76. doi:10.1016/j.bpj.2016.11.455
86. Carroll SF, Buckley CT, Kelly DJ. Cyclic tensile strain can play a role in directing both intramembranous and endochondral ossification of mesenchymal stem cells. Front Bioeng Biotechnol. 2017;5:73. doi:10.3389/fbioe.2017.00073
87. Kharazi AZ, Atari M, Vatankhah E, Javanmard SH. A nanofibrous bilayered scaffold for tissue engineering of small-diameter blood vessels. Polym Adv Technol. 2018;29(12):3151–3158. doi:10.1002/pat.4437
88. Iwasa SN, Babona-Pilipos R, Morshead CM. Environmental factors that influence stem cell migration: an “electric field”. Stem Cells Int. 2017;2017:4276927. doi:10.1155/2017/4276927
89. Kusuma G, Li A, Zhu D, et al. Engineering mesenchymal stem cell paracrine activity with 3D culture. Cytotherapy. 2020;22(5):S51. doi:10.1016/j.jcyt.2020.03.064
90. He Y, Li F, Jiang P, et al. Remote control of the recruitment and capture of endogenous stem cells by ultrasound for in situ repair of bone defects. Bioact Mater. 2023;21:223–238. doi:10.1016/j.bioactmat.2022.08.012
91. Lorsung RM, Rosenblatt RB, Cohen G, Frank JA, Burks SR. Acoustic radiation or cavitation forces from therapeutic ultrasound generate prostaglandins and increase mesenchymal stromal cell homing to murine muscle. Front Bioeng Biotechnol. 2020;8:870. doi:10.3389/fbioe.2020.00870
92. Xu H, Huang Y, Zhang F, et al. Ultrasonic microbubbles promote mesenchymal stem cell homing to the fibrotic liver via upregulation of CXCR4 expression. Cell Div. 2024;19(1):7. doi:10.1186/s13008-023-00104-8
93. Sun Z, Cai Y, Chen Y, et al. Ultrasound-targeted microbubble destruction promotes PDGF-primed bone mesenchymal stem cell transplantation for myocardial protection in acute Myocardial Infarction in rats. J Nanobiotechnology. 2023;21(1):481. doi:10.1186/s12951-023-02204-7
94. Ye D, Chen C, Wang Q, Zhang Q, Li S, Liu H. Short-wave enhances mesenchymal stem cell recruitment in fracture healing by increasing HIF-1 in callus. Stem Cell Res Ther. 2020;11(1):382. doi:10.1186/s13287-020-01888-0
95. Abdelrahman SA, Raafat N, Abdelaal GMM, Aal SMA. Electric field-directed migration of mesenchymal stem cells enhances their therapeutic potential on cisplatin-induced acute nephrotoxicity in rats. Naunyn Schmiedebergs Arch Pharmacol. 2023;396(6):1077–1093. doi:10.1007/s00210-022-02380-7
96. Liang X, Huang X, Zhou Y, Jin R, Li Q. Mechanical stretching promotes skin tissue regeneration via enhancing mesenchymal stem cell homing and transdifferentiation. Stem Cells Transl Med. 2016;5(7):960–969. doi:10.5966/sctm.2015-0274
97. Mucientes A, Herranz E, Moro E, et al. Influence of mesenchymal stem cell sources on their regenerative capacities on different surfaces. Cells. 2021;10(2):481. doi:10.3390/cells10020481
98. Yang Y, Lee EH, Yang Z. Hypoxia-conditioned mesenchymal stem cells in tissue regeneration application. Tissue Eng Part B Rev. 2022;28(5):966–977. doi:10.1089/ten.TEB.2021.0145
99. Ni Y, Tang Z, Yang J, et al. Collagen structure regulates MSCs behavior by MMPs involved cell-matrix interactions. J Mater Chem B. 2018;6(2):312–326. doi:10.1039/c7tb02377d
100. Silva LHA, Silva SM, Lima ECD, et al. Effects of static magnetic fields on natural or magnetized mesenchymal stromal cells: repercussions for magnetic targeting. Nanomedicine. 2018;14(7):2075–2085. doi:10.1016/j.nano.2018.06.002
101. Li X, Wei Z, Zhang W, et al. Anti-inflammatory effects of magnetically targeted mesenchymal stem cells on laser-induced skin injuries in rats. Int J Nanomed. 2020;15:5645–5659. doi:10.2147/ijn.S258017
102. Manjua AC, Cabral JMS, Portugal CAM, Ferreira FC. Magnetic stimulation of the angiogenic potential of mesenchymal stromal cells in vascular tissue engineering. Sci Technol Adv Mater. 2021;22(1):461–480. doi:10.1080/14686996.2021.1927834
103. Manjua AC, Cabral JMS, Ferreira FC, Portugal CAM. Magnetic field dynamic strategies for the improved control of the angiogenic effect of mesenchymal stromal cells. Polymers. 2021;13(11). doi:10.3390/polym13111883
104. Ma Y, Yang J, Hu Y, Xia Z, Cai K. Osteogenic differentiation of the MSCs on silk fibroin hydrogel loaded Fe(3)O(4)@PAA NPs in static magnetic field environment. Colloids Surf B Biointerfaces. 2022;220:112947. doi:10.1016/j.colsurfb.2022.112947
105. Celik C, Franco-Obregón A, Lee EH, Hui JH, Yang Z. Directionalities of magnetic fields and topographic scaffolds synergise to enhance MSC chondrogenesis. Acta Biomater. 2021;119:169–183. doi:10.1016/j.actbio.2020.10.039
106. Lienemann PS, Vallmajo‐Martin Q, Papageorgiou P, et al. Smart hydrogels for the augmentation of bone regeneration by endogenous mesenchymal progenitor cell recruitment. Adv Sci. 2020;7(7):1903395. doi:10.1002/advs.201903395
107. Yang Z, Li H, Yuan Z, et al. Endogenous cell recruitment strategy for articular cartilage regeneration. Acta Biomater. 2020;114:31–52. doi:10.1016/j.actbio.2020.07.008
108. Cui J, Ning L-J, Wu F-P, et al. Biomechanically and biochemically functional scaffold for recruitment of endogenous stem cells to promote tendon regeneration. NPJ Regen Med. 2022;7(1):26. doi:10.1038/s41536-022-00220-z
109. Li Z, Shen D, Hu S, et al. Pretargeting and bioorthogonal click chemistry-mediated endogenous stem cell homing for heart repair. ACS Nano. 2018;12(12):12193–12200. doi:10.1021/acsnano.8b05892
110. McQualter JL. Endogenous lung stem cells for lung regeneration. Expert Opin Biol Ther. 2019;19(6):539–546. doi:10.1080/14712598.2019.1596256
111. Xin L, Zheng X, Chen J, et al. An acellular scaffold facilitates endometrial regeneration and fertility restoration via recruiting endogenous mesenchymal stem cells. Adv Healthc Mater. 2022;11:e2201680. doi:10.1002/adhm.202201680
112. Wang S, Niu Y, Jia P, et al. Alkaline activation of endogenous latent TGFβ1 by an injectable hydrogel directs cell homing for in situ complex tissue regeneration. Bioact Mater. 2022;15:316–329. doi:10.1016/j.bioactmat.2021.12.015
113. Rodgers JT, Schroeder MD, Ma C, Rando TA. HGFA is an injury-regulated systemic factor that induces the transition of stem cells into GAlert. Cell Rep. 2017;19(3):479–486. doi:10.1016/j.celrep.2017.03.066
114. Ferreira SA, Faull PA, Seymour AJ, et al. Neighboring cells override 3D hydrogel matrix cues to drive human MSC quiescence. Biomaterials. 2018;176:13–23. doi:10.1016/j.biomaterials.2018.05.032
115. Yin Y, Li X, He XT, Wu RX, Sun HH, Chen FM. Leveraging stem cell homing for therapeutic regeneration. J Dental Res. 2017;96(6):601–609. doi:10.1177/0022034517706070
116. Alaneme KK, Bodunrin MO. Self-healing using metallic material systems – a review. Appl Mater Today. 2017;6:9–15. doi:10.1016/j.apmt.2016.11.002
117. Wu R-X, Yin Y, He X-T, Li X, Chen F-M. Engineering a cell home for stem cell homing and accommodation. Adv. Biosyst. 2017;1(4):1700004. doi:10.1002/adbi.201700004
118. Hu X, Wang Y, Tan Y, et al. A difunctional regeneration scaffold for knee repair based on aptamer-directed cell recruitment. Adv Mater. 2017;29(15):1605235. doi:10.1002/adma.201605235
119. Tian B-M, He X-T, Xu X-Y, Li X, Wu R-X, Chen F-M. Advanced biotechnologies toward engineering a cell home for stem cell accommodation. Adv Mater Technol. 2017;2(11):1700022. doi:10.1002/admt.201700022
120. Dong F, Caplan AI. Cell transplantation as an initiator of endogenous stem cell-based tissue repair. Curr Opin Organ Transplant. 2012;17(6):670–674. doi:10.1097/MOT.0b013e328359a617
121. Mu C, Hu Y, Hou Y, et al. Substance P-embedded multilayer on titanium substrates promotes local osseointegration via MSC recruitment. J Mater Chem B. 2020;8(6):1212–1222. doi:10.1039/c9tb01124b
122. Fu Q, Song L, Li J, et al. Biodegradable nano black phosphorus based SDF1-α delivery system ameliorates Erectile Dysfunction in a cavernous nerve injury rat model by recruiting endogenous stem/progenitor cells. J Nanobiotechnology. 2023;21(1):487. doi:10.1186/s12951-023-02238-x
123. Dhavalikar P, Robinson A, Lan Z, et al. Review of integrin-targeting biomaterials in tissue engineering. Adv Healthc Mater. 2020;9:e2000795. doi:10.1002/adhm.202000795
124. Deng M, Mei T, Hou T, et al. RETRACTED ARTICLE: tGFβ3 recruits endogenous mesenchymal stem cells to initiate bone regeneration. Stem Cell Res Ther. 2017;8(1):258. doi:10.1186/s13287-017-0693-0
125. Lu J, Shen X, Sun X, et al. Increased recruitment of endogenous stem cells and chondrogenic differentiation by a composite scaffold containing bone marrow homing peptide for cartilage regeneration. Theranost Res Paper. 2018;8(18):5039–5058. doi:10.7150/thno.26981
126. Zhuang Y, Yang W, Zhang L, et al. A novel leptin receptor binding peptide tethered-collagen scaffold promotes lung injury repair. Biomaterials. 2022;291:121884. doi:10.1016/j.biomaterials.2022.121884
127. Al-Baadani MA, Xu L, Cai K, et al. Preparation of co-electrospinning membrane loaded with simvastatin and substance P to accelerate bone regeneration by promoting cell homing, angiogenesis and osteogenesis. Mater Today Bio. 2023;21:100692. doi:10.1016/j.mtbio.2023.100692
128. Li X, He L, Yue Q, et al. MiR-9-5p promotes MSC migration by activating β-catenin signaling pathway. Am J Physiol Cell Physiol. 2017;313(1):C80–C93. doi:10.1152/ajpcell.00232.2016
129. Jiang X, Xu C, Shi H, Cheng Q. PTH1-34 improves bone healing by promoting angiogenesis and facilitating MSCs migration and differentiation in a stabilized fracture mouse model. PLoS One. 2019;14(12):e0226163. doi:10.1371/journal.pone.0226163
130. Lu X, Han J, Xu X, et al. PGE2 promotes the migration of mesenchymal stem cells through the activation of FAK and ERK1/2 pathway. Stem Cells Int. 2017;2017:8178643. doi:10.1155/2017/8178643
131. Ishii M, Takahashi M, Murakami J, Yanagisawa T, Nishimura M. Vascular endothelial growth factor-C promotes human mesenchymal stem cell migration via an ERK-and FAK-dependent mechanism. Mol Cell Biochem. 2019;455(1–2):185–193. doi:10.1007/s11010-018-3481-y
132. Hu X, Roy SR, Jin C, et al. Control cell migration by engineering integrin ligand assembly. Nat Commun. 2022;13(1):5002. doi:10.1038/s41467-022-32686-2
133. Li Y, Zhang D, Chen M, et al. The novel miRNA N-72 regulates egf-induced migration of human amnion mesenchymal stem cells by targeting MMP2. Int J Mol Sci. 2018;19(5):1363. doi:10.3390/ijms19051363
134. Huang J, Wu J, Wang J, et al. Rock climbing-inspired electrohydrodynamic cryoprinting of micropatterned porous fiber scaffolds with improved MSC therapy for wound healing. Advanced Fiber Materials. 2023;5(1):312–326. doi:10.1007/s42765-022-00224-w
135. Xia P, Wang X, Wang Q, et al. Low-intensity pulsed ultrasound promotes autophagy-mediated migration of mesenchymal stem cells and cartilage repair. Cell Transplant. 2021;30:963689720986142. doi:10.1177/0963689720986142
136. Lin C, Xu K, He Y, et al. A dynamic matrix potentiates mesenchymal stromal cell paracrine function via an effective mechanical dose. Biomater Sci. 2020;8(17):4779–4791. doi:10.1039/d0bm01012j
137. Lian M, Sun B, Han Y, et al. A low-temperature-printed hierarchical porous sponge-like scaffold that promotes cell-material interaction and modulates paracrine activity of MSCs for vascularized bone regeneration. Biomaterials. 2021;274:120841. doi:10.1016/j.biomaterials.2021.120841
138. Carter K, Lee HJ, Na K-S, et al. Characterizing the impact of 2D and 3D culture conditions on the therapeutic effects of human mesenchymal stem cell secretome on corneal wound healing in vitro and ex vivo. Acta Biomater. 2019;99:247–257. doi:10.1016/j.actbio.2019.09.022
139. Qazi TH, Tytgat L, Dubruel P, Duda GN, Van Vlierberghe S, Geissler S. Extrusion Printed Scaffolds with varying pore size as modulators of MSC angiogenic paracrine effects. ACS Biomater Sci Eng. 2019;5(10):5348–5358. doi:10.1021/acsbiomaterials.9b00843
140. Kadir ND, Yang Z, Hassan A, Denslin V, Lee EH. Electrospun fibers enhanced the paracrine signaling of mesenchymal stem cells for cartilage regeneration. Stem Cell Res Ther. 2021;12(1):100. doi:10.1186/s13287-021-02137-8
141. Liu J, Gao J, Liang Z, et al. Mesenchymal stem cells and their microenvironment. Stem Cell Res Ther. 2022;13(1):429. doi:10.1186/s13287-022-02985-y
142. Snyder Y, Jana S. Strategies for development of decellularized heart valve scaffolds for tissue engineering. Biomaterials. 2022;288:121675. doi:10.1016/j.biomaterials.2022.121675
143. Chen F-M, Liu X. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci. 2016;53:86–168. doi:10.1016/j.progpolymsci.2015.02.004
144. Du J, Yao Y, Wang M, et al. Programmable building of radially gradient nanofibrous patches enables deployment, bursting bearing capability, and stem cell recruitment. Adv Funct Mater. 2022;32(8):2109833. doi:10.1002/adfm.202109833
145. van de Kamp J, Paefgen V, Wöltje M, et al. Mesenchymal stem cells can be recruited to wounded tissue via hepatocyte growth factor-loaded biomaterials. J Tissue Eng Regen Med. 2017;11(11):2988–2998. doi:10.1002/term.2201
146. Liang R, Shen X, Xie C, et al. Silk gel recruits specific cell populations for scarless skin regeneration. Appl Mater Today. 2021;23:101004. doi:10.1016/j.apmt.2021.101004
147. Li Q, Qi G, Lutter D, et al. Injectable peptide hydrogel encapsulation of mesenchymal stem cells improved viability, stemness, anti-inflammatory effects, and early stage wound healing. Biomolecules. 2022;12(9):1317. doi:10.3390/biom12091317
148. Kosaraju R, Rennert RC, Maan ZN, et al. Adipose-derived stem cell-seeded hydrogels increase endogenous progenitor cell recruitment and neovascularization in wounds. Tissue Eng Part A. 2016;22(3–4):295–305. doi:10.1089/ten.tea.2015.0277
149. Yao CH, Chen KY, Cheng MH, Chen YS, Huang CH. Effect of genipin crosslinked chitosan scaffolds containing SDF-1 on wound healing in a rat model. Mater Sci Eng C Mater Biol Appl. 2020;109:110368. doi:10.1016/j.msec.2019.110368
150. Yoon DS, Lee Y, Ryu HA, et al. Cell recruiting chemokine-loaded sprayable gelatin hydrogel dressings for diabetic wound healing. Acta Biomater. 2016;38:59–68. doi:10.1016/j.actbio.2016.04.030
151. Behm B, Babilas P, Landthaler M, Schreml S. Cytokines, chemokines and growth factors in wound healing. J Eur Acad Dermatol Venereol. 2012;26(7):812–820. doi:10.1111/j.1468-3083.2011.04415.x
152. Han Y, Li Y, Zeng Q, et al. Injectable bioactive akermanite/alginate composite hydrogels for in situ skin tissue engineering. J Mater Chem B. 2017;5(18):3315–3326. doi:10.1039/c7tb00571g
153. Zhang C, Yuan TJ, Tan MH, Xu XH, Huang YF, Peng LH. Smart graphene-based hydrogel promotes recruitment and neural-like differentiation of bone marrow derived mesenchymal stem cells in rat skin. Biomater Sci. 2021;9(6):2146–2161. doi:10.1039/d0bm01963a
154. Peng L-H, Xu X-H, Huang Y-F, et al. Self-adaptive all-in-one delivery chip for rapid skin nerves regeneration by endogenous mesenchymal stem cells. Adv Funct Mater. 2020;30(40):2001751. doi:10.1002/adfm.202001751
155. Zhang H, Wang Y, Zheng Z, et al. Strategies for improving the 3D printability of decellularized extracellular matrix bioink. Theranostics. 2023;13(8):2562–2587. doi:10.7150/thno.81785
156. Shafiq M, Kong D, Kim SH. SDF-1α peptide tethered polyester facilitates tissue repair by endogenous cell mobilization and recruitment. J Biomed Mater Res A. 2017;105(10):2670–2684. doi:10.1002/jbm.a.36130
157. Feng C, Zhang W, Deng C, et al. 3D printing of lotus root-like biomimetic materials for cell delivery and tissue regeneration. Adv Sci. 2017;4(12):1700401. doi:10.1002/advs.201700401
158. Segers VF, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116(15):1683–1692. doi:10.1161/circulationaha.107.718718
159. Xu Z, Wu J, Gong P, Chang C. Accelerate wound healing by microscale gel array patch encapsulating defined SDF-1α gradient. J Control Release. 2023;358:1–12. doi:10.1016/j.jconrel.2023.04.032
160. Durand N, Huang P, Zubair A. CXCR4-overexpressing mesenchymal stem cells exhibit increased cell migration and cytokine secretion. Cytotherapy. 2020;22(5):S80. doi:10.1016/j.jcyt.2020.03.131
161. Kuang S, He F, Liu G, et al. CCR2-engineered mesenchymal stromal cells accelerate diabetic wound healing by restoring immunological homeostasis. Biomaterials. 2021;275:120963. doi:10.1016/j.biomaterials.2021.120963
162. Seetharaman S, Etienne-Manneville S. Cytoskeletal crosstalk in cell migration. Trends Cell Biol. 2020;30(9):720–735. doi:10.1016/j.tcb.2020.06.004
163. Mishra AK, Campanale JP, Mondo JA, Montell DJ. Cell interactions in collective cell migration. Development. 2019;146(23). doi:10.1242/dev.172056
164. van Helvert S, Storm C, Friedl P. Mechanoreciprocity in cell migration. Nat Cell Biol. 2018;20(1):8–20. doi:10.1038/s41556-017-0012-0
165. Yamada KM, Sixt M. Mechanisms of 3D cell migration. Nat Rev Mol Cell Biol. 2019;20(12):738–752. doi:10.1038/s41580-019-0172-9
166. Infante E, Etienne-Manneville S. Intermediate filaments: integration of cell mechanical properties during migration. Front Cell Dev Biol. 2022;10:951816. doi:10.3389/fcell.2022.951816
167. Haji Mansor M, Najberg M, Contini A, et al. Development of a non-toxic and non-denaturing formulation process for encapsulation of SDF-1α into PLGA/PEG-PLGA nanoparticles to achieve sustained release. Eur J Pharm Biopharm. 2018;125:38–50. doi:10.1016/j.ejpb.2017.12.020
168. Ju S, Lim L, Ki YJ, Choi DH, Song H. Oxidative stress generated by polycyclic aromatic hydrocarbons from ambient particulate matter enhance vascular smooth muscle cell migration through MMP upregulation and actin reorganization. Part Fibre Toxicol. 2022;19(1):29. doi:10.1186/s12989-022-00472-z
169. Hight-Warburton W, Parsons M. Regulation of cell migration by α4 and α9 integrins. Biochem J. 2019;476(4):705–718. doi:10.1042/bcj20180415
170. Motealleh A, Dorri P, Schäfer AH, Kehr NS. 3D bioprinting of triphasic nanocomposite hydrogels and scaffolds for cell adhesion and migration. Biofabrication. 2019;11(3):035022. doi:10.1088/1758-5090/ab15ca
171. Siemsen K, Rajput S, Rasch F, et al. Tunable 3D hydrogel microchannel networks to study confined mammalian cell migration. Adv Healthc Mater. 2021;10(23):e2100625. doi:10.1002/adhm.202100625
172. Li C, Kuss M, Kong Y, et al. 3D printed hydrogels with aligned microchannels to guide neural stem cell migration. ACS Biomater Sci Eng. 2021;7(2):690–700. doi:10.1021/acsbiomaterials.0c01619
173. Liu J, Xu C, Xu Y, et al. Bioinspired scaffolds with hierarchical structures for tailored mechanical behaviour and cell migration. Ceram Int. 2020;46(15):24102–24109. doi:10.1016/j.ceramint.2020.06.189
174. Wang L, Wang C, Zhou L, et al. Fabrication of a novel Three-Dimensional porous PCL/PLA tissue engineering scaffold with high connectivity for endothelial cell migration. Eur. Polym. J. 2021;161:110834. doi:10.1016/j.eurpolymj.2021.110834
175. Wang X, Lin M, Kang Y. Engineering porous β-tricalcium phosphate (β-TCP) scaffolds with multiple channels to promote cell migration, proliferation, and angiogenesis. ACS Appl Mater Interfaces. 2019;11(9):9223–9232. doi:10.1021/acsami.8b22041
176. Xia J, Liu Z-Y, Han Z-Y, et al. Regulation of cell attachment, spreading, and migration by hydrogel substrates with independently tunable mesh size. Acta Biomater. 2022;141:178–189. doi:10.1016/j.actbio.2022.01.025
177. Lu G, Ding Z, Wei Y, Lu X, Lu Q, Kaplan DL. Anisotropic biomimetic silk scaffolds for improved cell migration and healing of skin wounds. ACS Appl Mater Interfaces. 2018;10(51):44314–44323. doi:10.1021/acsami.8b18626
178. Wu X, Huang W, Wu W-H, et al. Reversible hydrogels with tunable mechanical properties for optically controlling cell migration. Nano Res. 2018;11(10):5556–5565. doi:10.1007/s12274-017-1890-y
179. Yin X, Zhu X, Wang Z. Cell Migration Regulated by spatially controlled stiffness inside composition-tunable three-dimensional dextran hydrogels. Adv Mater Interfaces. 2021;8(17):2100494. doi:10.1002/admi.202100494
180. Nellenbach K, Nandi S, Peeler C, Kyu A, Brown AC. Neonatal fibrin scaffolds promote enhanced cell adhesion, migration, and wound healing in vivo compared to adult fibrin scaffolds. Cell Mol Bioeng. 2020;13(5):393–404. doi:10.1007/s12195-020-00620-5
181. Yang D, Zhao Z, Bai F, Wang S, Tomsia AP, Bai H. promoting cell migration in tissue engineering scaffolds with graded channels. Adv Healthc Mater. 2017;6(18). doi:10.1002/adhm.201700472
182. Motealleh A, Çelebi-Saltik B, Ermis N, Nowak S, Khademhosseini A, Kehr NS. 3D printing of step-gradient nanocomposite hydrogels for controlled cell migration. Biofabrication. 2019;11(4):045015. doi:10.1088/1758-5090/ab3582
183. Ravanbakhsh H, Bao G, Mongeau L. Carbon nanotubes promote cell migration in hydrogels. Sci Rep. 2020;10(1):2543. doi:10.1038/s41598-020-59463-9
184. Dietrich M, Le Roy H, Brückner DB, et al. Guiding 3D cell migration in deformed synthetic hydrogel microstructures. Soft Matter. 2018;14(15):2816–2826. doi:10.1039/c8sm00018b
185. Li J, Xiao L, Gao S, et al. Radial sponges facilitate wound healing by promoting cell migration and angiogenesis. Adv Healthc Mater. 2023;12(11):e2202737. doi:10.1002/adhm.202202737
186. Hu N, Zhang YY, Gu HW, Guan HJ. Effects of bone marrow mesenchymal stem cells on cell proliferation and growth factor expression of limbal epithelial cells in vitro. Ophthalmic Res. 2012;48(2):82–88. doi:10.1159/000331006
187. Liu C, Lu Y, Du P, et al. Mesenchymal stem cells pretreated with proinflammatory cytokines accelerate skin wound healing by promoting macrophages migration and M2 polarization. Regen Ther. 2022;21:192–200. doi:10.1016/j.reth.2022.06.009
188. Ding J, Wang X, Chen B, Zhang J, Xu J. Exosomes derived from human bone marrow mesenchymal stem cells stimulated by deferoxamine accelerate cutaneous wound healing by promoting angiogenesis. Biomed Res Int. 2019;2019:9742765. doi:10.1155/2019/9742765
189. Prakoeswa CRS, Rindiastuti Y, Wirohadidjojo YW, et al. ”Resveratrol promotes secretion of wound healing related growth factors of mesenchymal stem cells originated from adult and fetal tissues,”. Artif Cells Nanomed Biotechnol. 2020;48(1):1160–1167. doi:10.1080/21691401.2020.1817057
190. Gong M, Yan F, Yu L, Li F. A dopamine-methacrylated hyaluronic acid hydrogel as an effective carrier for stem cells in skin regeneration therapy. Cell Death Dis. 2022;13(8):738. doi:10.1038/s41419-022-05060-9
191. Murphy KC, Whitehead J, Zhou D, Ho SS, Leach JK. Engineering fibrin hydrogels to promote the wound healing potential of mesenchymal stem cell spheroids. Acta Biomater. 2017;64:176–186. doi:10.1016/j.actbio.2017.10.007
192. Zeng J, Chen X, Zhang J, et al. Stem cell spheroids production for wound healing with a reversible porous hydrogel. Mater Today Adv. 2022;15:100269. doi:10.1016/j.mtadv.2022.100269
193. Huang R, Wang J, Chen H, et al. The topography of fibrous scaffolds modulates the paracrine function of Ad-MSCs in the regeneration of skin tissues. Biomater Sci. 2019;7(10):4248–4259. doi:10.1039/c9bm00939f
194. Wechsler ME, Rao VV, Borelli AN, Anseth KS. Engineering the MSC Secretome: a Hydrogel Focused Approach. Adv Healthcare Mater. 2021;10(7):2001948. doi:10.1002/adhm.202001948
195. Su N, Gao PL, Wang K, Wang JY, Zhong Y, Luo Y. Fibrous scaffolds potentiate the paracrine function of mesenchymal stem cells: a new dimension in cell-material interaction. Biomaterials. 2017;141:74–85. doi:10.1016/j.biomaterials.2017.06.028
196. Riazifar M, Pone EJ, Lötvall J, Zhao W. Stem cell extracellular vesicles: extended messages of regeneration. Annu Rev Pharmacol Toxicol. 2017;57(1):125–154. doi:10.1146/annurev-pharmtox-061616-030146
197. Hu JC, Zheng CX, Sui BD, Liu WJ, Jin Y. Mesenchymal stem cell-derived exosomes: a novel and potential remedy for cutaneous wound healing and regeneration. World J Stem Cells. 2022;14(5):318–329. doi:10.4252/wjsc.v14.i5.318
198. Bi Y, Xia H, Li L, et al. Liposomal vitamin D3 as an anti-aging agent for the skin. Pharmaceutics. 2019;11(7):311. doi:10.3390/pharmaceutics11070311
199. Desmet E, Van Gele M, Lambert J. Topically applied lipid- and surfactant-based nanoparticles in the treatment of skin disorders. Expert Opin Drug Deliv. 2017;14(1):109–122. doi:10.1080/17425247.2016.1206073
200. Guo S, Perets N, Betzer O, et al. Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and tensin homolog siRNA repairs complete spinal cord injury. ACS Nano. 2019;13(9):10015–10028. doi:10.1021/acsnano.9b01892
201. Zhao H, Shang Q, Pan Z, et al. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. 2018;67(2):235–247. doi:10.2337/db17-0356
202. Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E. Mesenchymal stem cell exosomes induce proliferation and migration of normal and chronic wound fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev. 2015;24(14):1635–1647. doi:10.1089/scd.2014.0316
203. Ma T, Fu B, Yang X, Xiao Y, Pan M. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J Cell Biochem. 2019;120(6):10847–10854. doi:10.1002/jcb.28376
204. Zhang W, Bai X, Zhao B, et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp Cell Res. 2018;370(2):333–342. doi:10.1016/j.yexcr.2018.06.035
205. Cooper DR, Wang C, Patel R, et al. Human adipose-derived stem cell conditioned media and exosomes containing MALAT1 promote human dermal fibroblast migration and ischemic wound healing. Adv Wound Care. 2018;7(9):299–308. doi:10.1089/wound.2017.0775
206. Zhou Y, Zhang X-L, Lu S-T, et al. Human adipose-derived mesenchymal stem cells-derived exosomes encapsulated in pluronic F127 hydrogel promote wound healing and regeneration. Stem Cell Res Ther. 2022;13(1):407. doi:10.1186/s13287-022-02980-3
207. Geng X, Qi Y, Liu X, Shi Y, Li H, Zhao L. A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Biomater Adv. 2022;133:112613. doi:10.1016/j.msec.2021.112613
208. Yang L, Xie F, Li Y, et al. Chitin-based hydrogel loaded with bFGF and SDF-1 for inducing endogenous mesenchymal stem cells homing to improve stress urinary incontinence. Carbohydr Polym. 2023;319:121144. doi:10.1016/j.carbpol.2023.121144
209. Kong P, Dong J, Li W, et al. Extracellular matrix/glycopeptide hybrid hydrogel as an immunomodulatory niche for endogenous cardiac repair after myocardial infarction. Adv Sci. 2023;10(23):e2301244. doi:10.1002/advs.202301244
210. Zhou L, Xu J, Schwab A, et al. Engineered biochemical cues of regenerative biomaterials to enhance endogenous stem/progenitor cells (ESPCs)-mediated articular cartilage repair. Bioact Mater. 2023;26:490–512. doi:10.1016/j.bioactmat.2023.03.008
211. Dai W, Liu Q, Li S, et al. Functional injectable hydrogel with spatiotemporal sequential release for recruitment of endogenous stem cells and in situ cartilage regeneration. J Mater Chem B. 2023;11(18):4050–4064. doi:10.1039/d3tb00105a
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.