Back to Journals » Journal of Inflammation Research » Volume 17
Breviscapine Reduces Sepsis-Induced Acute Lung Injury by Targeting CASP8 to Regulate Neutrophil Apoptosis and Inflammation
Authors Song J, Zhang J, Shi J, Pan X, Mo D
Received 16 November 2023
Accepted for publication 23 July 2024
Published 1 August 2024 Volume 2024:17 Pages 5161—5176
DOI https://doi.org/10.2147/JIR.S446345
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Jia Song,1 Jiancheng Zhang,2 Jun Shi,2 Xuming Pan,2 Dayu Mo3
1Department of General Practice, The Second Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, People’s Republic of China; 2Department of Emergency, The Second Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, People’s Republic of China; 3Department of Education, The Second Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, People’s Republic of China
Correspondence: Dayu Mo, Department of Education, The Second Affiliated Hospital of Zhejiang Chinese Medical University, No. 318 Chaowang Road, Hangzhou, Zhejiang, 310005, People’s Republic of China, Tel +86-13857197190, Email [email protected]
Background: Breviscapine has been demonstrated to have beneficial effects in ameliorating acute lung injury (ALI), yet its potential therapeutic value and molecular mechanisms in sepsis-induced ALI remain unexplored.
Methods: We utilized network pharmacology approach to identify the potential targets and mechanisms of breviscapine in treating sepsis-induced ALI. To construct a murine model of sepsis, we performed cecal ligation and puncture (CLP). Hematoxylin and eosin (HE) staining and enzyme-linked immunosorbent assay (ELISA) were employed to respectively determine the pathologic changes and levels of inflammatory factors. Neutrophil count and total protein level in bronchoalveolar lavage fluid (BALF) were detected by corresponding kit. Additionally, we utilized flow cytometry, immunofluorescence, Western blotting, and real-time reverse transcription PCR (qRT-PCR) to detect cell apoptosis, protein expression, and gene expression. Finally, we used ELISA kits to detect the activity of myeloperoxidase (MPO) and caspase-8 (CASP8).
Results: Breviscapine was revealed to target 81 potential proteins in the treatment of sepsis-induced ALI, while CASP8 was the most important one as demonstrated by network analysis. In vivo experiments demonstrated that breviscapine effectively reduced the severity of sepsis-induced ALI and inflammation, and significantly suppressed neutrophil infiltration in the lung tissues of CLP mice and promoted neutrophil apoptosis in the peripheral blood. In vitro experiments revealed that lipopolysaccharide (LPS)-induced neutrophil apoptosis was inhibited, and the expression and activity of CASP8 were down-regulated. Breviscapine intervention markedly up-regulated the expression and activity of CASP8, consequently activating neutrophil apoptosis and inhibiting inflammatory response by activating the NF-κB signaling pathway.
Conclusion: Breviscapine is remarkably effective in improving sepsis-induced ALI, and its mechanism of action may be to induce neutrophil apoptosis, inhibit inflammatory overreaction and reduce its infiltration in pulmonary tissues by up-regulating the expression and activity of CASP8.
Keywords: Breviscapine, acute lung injury, sepsis, network pharmacology, neutrophil
Introduction
Sepsis is a complex and potentially deadly condition that arises when the body’s immune system overreacts to an infection, and it represents a substantial worldwide health challenge associated with considerable morbidity and mortality.1 Characterized by leukocytosis, hyperthermia and hypotension, sepsis is a disorder manifested by diffuse alveolar destruction and acute respiratory distress syndrome that regularly accompanied by acute lung injury (ALI).2 Sepsis-associated ALI is a frequently encountered clinical issue with increased mortality and morbidity due to its complex pathophysiological mechanisms. In recent years, extensive research has been carried out to further elucidate the underlying mechanisms of ALI and to discover its potential therapeutic targets. Measures such as preventing the occurrence of sepsis, providing supportive care, and using antibiotics, vasopressors, and other medications to support blood pressure, maintain oxygen levels, and reduce inflammation are conductive to the treatment of sepsis-induced ALI.3 Additionally, extracorporeal membrane oxygenation (ECMO) is a complex and resource-intensive treatment option for patients with severe sepsis-induced ALI.4
Breviscapine is a phenanthrene lignan derived from the Chinese herbal medicine, Erigeron Breviscapus. It has been studied extensively thanks to its anti-inflammatory, antioxidant, anti-atherosclerosis, and anti-platelet aggregation activities.5 Studies have shown that continuous infusion of breviscapine prior to cardiopulmonary bypass can decrease the levels of procalcitonin and neutrophil elastase, leading to a reduction in systemic inflammation and lung protection in pediatric patients undergoing open heart surgery.6 Besides, breviscapine has been reported to reduce ALI caused by left heart ischemic reperfusion in rats, possibly through the inhibition of ICAM-1 and IL-18 expression,7 and improve hypoxia-induced hypercoagulable state and ameliorate alveolar septal thickening.8 Despite these promising findings on the potential therapeutic impacts of breviscapine, further investigation is required to elucidate its molecular mechanisms of action and clinical applications.
Network pharmacology integrates multiple disciplines such as traditional pharmacology, systems biology and network science. It has been developed for studying the entire process of drug-disease interactions, from drug molecules to clinical targets, from disease mechanisms to drug clinical efficacy, and from drug design to clinical application.9 Its main feature is to study the holistic effect of drugs on diseases from a network perspective. This study intends to investigate the efficacy of breviscapine on sepsis-associated ALI and discover the underlying mechanism of actions through network pharmacology and experimental validations.
Materials and Methods
Network Pharmacology
The molecular targets of breviscapine were acquired through the utilization of various databases and platforms: Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP), PubChem database, SwissTargetPrediction platform, and TargetNet platform. Genes associated with sepsis-induced ALI were identified from the Online Mendelian Inheritance in Man (OMIM), Comparative Toxicogenomics Database (CTD), GeneCards and DisGeNET databases. “Sepsis” and “acute lung injury” were used as keywords for relevant data retrieval. The candidate targets of breviscapine against sepsis-induced ALI were obtained by taking intersection of the targets of breviscapine and disease-related genes, and submitted to the STRING database (https://string-db.org/) to obtain the protein-protein interaction (PPI) data. The Cytoscape software was employed to generate and evaluate these PPI networks. In addition, the clusterprofiler package was utilized to perform functional enrichment analysis and identify enriched biological processes and molecular functions.10
Molecular Docking Analysis
A topological analysis was conducted on the networks to identify hub targets, followed by a molecular docking analysis. The target protein’s biostructure was obtained from the Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB PDB) repository, while that of breviscapine was downloaded from the PubChem. Afterwards, water molecules as well as organic solvents were removed from the biostructures of the proteins, and the grid box was generated for docking using the PyMOL software. The Autodock software was utilized to perform molecular docking analysis, wherein the Lamarckian Genetic algorithm was selected to conduct conformational search. The conformation with the highest aptitude was subsequently identified and chosen as the final docking conformation.
Animal Study
The Animal Center at Hangzhou Medical College provided male C57BL/6 mice aged 8–12 weeks and weighing between 20–25g for the study. All animal procedures were approved by the Institutional Animal Care and Use Committee of Zhejiang Centre for Laboratory Animals (ZJCLA-IACUC-20010219) and followed the National Institute of Health Guide for the Care and Use of Laboratory Animals. The mice were accommodated in standard plastic cages with husk bedding, and raised in SPF-grade animal rooms with a temperature of 25 ± 2 °C and a 12 h light dark cycle. Before the formal experiment, the mice were free to eat food and drink water. The mice were sorted into four groups at random: i) Control, ii) Bre, iii) CLP, and iv) Bre+CLP. The method of cecal ligation and puncture (CLP) was employed to generate septic mice in an experimental setting.11 The mice were anesthetized with isoflurane and had their abdomens shaved and cleaned with povidone iodine. A 1-cm incision was made to expose the cecum, which was then tied off with a silk suture and punctured with a needle to release fecal matter. The cecum was restored to its original position in the abdomen and the incision was sealed with sutures. Breviscapine suspension was prepared and administered orally (30 mg/kg) to Bre and CLP+ Bre groups, while the Control and CLP group received vehicle solution. After 20 h of oral administration, the mice were euthanized, peripheral blood and lung samples were gathered for further analysis.
Isolation and Culture of Bone Marrow-Derived Neutrophils (BMDNs)
To isolate BMDNs, femurs were removed from euthanized mice and their distal tips were excised. The bone marrow cells were rinsed with Hanks solution, filtered through the cell filter, and centrifuged. BMDNs were isolated using a commercial magnetic kit, and exposed to different conditions. To investigate the effect of breviscapine on the apoptosis of ALI neutrophils, the cells were divided into four groups: Control group, Bre group (80 μg/mL), LPS group (100 ng/mL), and LPS (100 ng/mL)+Bre (80 μg/mL) group. In order to explore the specific mechanism of regulation, cells were divided into four groups: LPS (100 ng/mL) group, LPS (100 ng/mL)+Bre (80 μg/mL) group, LPS (100 ng/mL)+Bre (80 μg/mL)+sh-NC group, and LPS (100 ng/mL)+Bre (80 μg/mL)+sh-CASP8 group.
Cell Transfection
LPS+Bre+sh-NC group (cells were transfected with sh-NC), LPS+Bre+sh-CASP8 group (cells were transfected with sh-CASP8). sh-CASP8/sh-NC was synthesized by GenePharma (Shanghai, China), Lipofectamine 2000 (Thermo Fisher Scientific, USA) was used as a dye aid.
Hematoxylin and Eosin (HE) Staining
After fixation in 4% paraformaldehyde for 24 h, the left lung lobes were embedded in paraffin and sectioned into 5 μm-thick slices. These sections were then stained with HE and viewed under a microscope. A pathologist, blinded to the experiment, evaluated the pulmonary histopathological changes according to a previously established assessment method12 and calculated the lung injury scores.
Measurement of Lung W/D Ratio
The pulmonary circulation was slowly flushed with 4 °C PBS buffer. The right lung was then isolated, quickly weighed, and the wet lung weight was recorded. The wet lungs were dried in an 80 °C oven for 72 h, then weighed again to record the dry lung weight. Finally, the W/D value was calculated.
Cell Counting and Protein Quantification
The collected bronchoalveolar lavage fluid (BALF) was centrifuged at 4 °C at 1200g for 10 min. The supernatant was collected for protein detection, and the remaining cells were resuspended for cell counting.
Enzyme-Linked Immunosorbent Assay (ELISA)
Lung tissue homogenate was centrifuged at 20,000 g for 10 min, and the supernatant was collected. Commercially available ELISA kits (ab183218 for TNF-α, ab242452 for IL-1β, and ab242739 for IL-6, from Cambridge, MA, USA) were used to quantify the levels of immune reactivity for TNF-α, IL-1β, and IL-6.
Myeloperoxidase (MPO) Activity Assay
One hundred milligrams of lung tissue from the right lobe were homogenized using a suitable method, and the collected supernatant was detected using an MPO detection kit following the guidelines outlined by the manufacturer. The absorbance at 460 nm was used to measure enzymatic activity, which was determined using a 96-well microplate reader.
Confocal Microscopy
The expression levels and localization of Bcl-xL and Mcl-1 proteins in BALF cells were assessed using confocal microscopy. BALF cells were collected and immunostained with antibodies against Bcl-xL and Mcl-1, followed by labeling with Cy5-conjugated secondary antibodies. The cells were labeled with DAPI and imaged using an Olympus FV10i confocal microscope, which uses lasers to produce high-resolution images captured at different depths within a specimen, providing a 3D view of the sample. This allowed for precise detection of protein expression and localization within individual cells.
Lung Immunofluorescence
Immunofluorescence analysis was conducted on lung slices to evaluate the degree of neutrophil infiltration. The samples were left to incubate with anti-Ly6G antibody (1:300, 88876S, Cell Signaling Technology, USA) overnight at 4 °C, and then the secondary antibody was added for incubation at 37 °C for 2 h. To visualize the stained sections, The nuclei was stained for 10 min with DAPI, and then observed under a laser confocal microscope (Nikon Eclipse C1, Japan). The number of neutrophils stained with Ly6G was counted in six fields of view per section.
Flow Cytometry
Flow cytometry was employed to assess neutrophil apoptosis. We collected blood samples from mice, and isolated bone marrow-derived neutrophils for cell experiments. FITC-Annexin V and propidium iodide (PI) (V13242, Thermo Fisher Scientific, USA) were used to stain the cells, and an apoptosis detection kit was utilized to facilitate analysis. The resulting data was analyzed using the BD LSRFortessa™ flow cytometer, which allowed for precise quantification of apoptotic neutrophils in both experimental setting.
Western Blot Assay
Caspase-8 (CASP8) was selected for validation through Western blotting. Following cell protein extraction, the BCA method was used to determine protein concentration. An equal amount of protein were separated using SDS-PAGE, transferred onto a PVDF membrane, and then blocked with 5% skim milk for 2 h. Next, the membranes were incubated overnight at 4 ◦C with a primary antibody against CASP8 (Cat. No.: ab25901, diluted at 1:1000), phosphorylated NF-kB p65 (Cat. No.: ab76302, diluted at 1:1000), NF-kB p65 (Cat. No.: ab32536, diluted at 1:1000) and β-actin (Cat. No.: ab8227, diluted at 1:5000). The membranes were then incubated with a secondary antibody (diluted at 1:5000) for 2 h. Finally, the blots were visualized using the ECL detection system (Syngene, Cambridge, UK).
Real-Time Reverse Transcription PCR (qRT-PCR)
qRT-PCR was utilized to measure the level of CASP8 mRNA. RNA extraction kit (AM1931, Thermo Fisher Scientific, USA) was used to collect total RNA from each group of cells). Then, the collected RNA was subjected to purity assessment using a NanoDrop ND-2000, and reverse transcribed into cDNA using a reverse transcription kit rse transcriptase (Applied Biosystems, Foster City, CA), with the reaction procedure as follows: 37◦C for 15 min, 85◦C for 5 sec, and 4◦C for storage. Next, the HieffqPCR SYBRGreen Master Mix (No Rox) (H2208050, Yisheng Bio Technology Co., Ltd. Shanghai, China) kit was used to detect the relative expression level of CASP8 in each group of cells, with the reaction procedure as follows: predenaturation at 95◦C for 5 min (1 cycle), denaturation at 95◦C for 10 sec, annealing/extension at 60◦C for 30 sec (40 cycles). The primers of CASP8 and β-Actin were as follows: CASP8 Forward: AGAGTCTGTGCCCAAATCAAC, Reverse: GCTGCTTCTCTCTITGCTGAA; β-Actin: Forward: CGTGAAAAGATGACCCAGATCA, Reverse: TGGTACGACCA GAGGCATACAG. The 2−ΔΔCT method was adopted for the calculation of relative gene expression.
Detection of CASP8 Activity
The Caspase-Glo assay kit was utilized to detect the activity of CASP8 protein. Briefly, the cells were incubated on a 96-well plate for 24 h and then treated with DEVD or LETD. The cells were then incubated for 1 h and the activity of CASP8 was detected by a plate reader.
Statistical Analysis
The process of this study is shown in Figure 1. All data were expressed as mean ± standard deviation (SD), and statistically analyzed using GraphPad Prism The statistical analysis involved one-way analysis of variance (ANOVA) with the Student-Newman-Keuls’ (SNK) test for comparisons between multiple groups, and the Student’s t-test for comparisons between two groups. p < 0.05 was considered statistically significant.
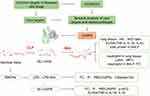 |
Figure 1 Flow chart for this study. |
Results
Potential Targets of Breviscapine in the Treatment of Sepsis-Induced ALI
Through database and software prediction, a total of 151 potential targets of breviscapine were identified, including 7 from PubChem database, 6 from TCMSP database, 100 from SwissTargetPrediction platform, and 54 from TargetNet platform, with multiple gene targets eliminated (Figure 2A). Subsequently, 3407 sepsis-related genes and 7731 ALI-related genes were retrieved from databases, respectively, and intersection was taken. As a result, 2630 genes related to sepsis-induced ALI were obtained. After taking intersection with the potential targets of breviscapine, 81 targets were identified for the treatment of sepsis-induced ALI (Figure 2B). Based on the STRING database, 482 protein interactions among the 81 proteins were retrieved, and a protein-proteins interaction network was built (Figure 2C). After performing MCODE analysis, a subnetwork consisting of 16 proteins and 115 interactions was identified, with more compact interactions (Figure 2D).
Biological Processes and Cellular Pathways Related to the Screened Potential Targets
We performed Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses to gain a deeper insight into the mechanisms of these potential targets. The analysis revealed significant enrichment of these targets in 1071 biological processes, 35 cellular components, and 59 molecular functions, as well as 245 KEGG pathways. The top 10 GO terms with the highest number of genes are shown in Figure 3A, which mainly involve regulation of cytokine production, MAPK cascade, and apoptotic signaling pathway. In addition, Figure 3B displays the top 10 KEGG pathways with the highest number of genes, encompassing apoptosis pathways and signal transduction-related pathways, such as the AGE-RAGE signaling pathway and the C-type lectin receptor signaling pathway. Supplementary Table 1 and Supplementary Table 2 summarized the details of the GO function and KEGG pathway of enrichment analysis.
 |
Figure 3 Enrichment analysis of the potential targets of breviscapine in treating sepsis-induced ALI. (A) GO terms. (B) KEGG pathways. |
Core Target and Molecular Docking Analysis
To identify the key targets of breviscapine for treating sepsis-induced ALI, a pathway-target network was constructed by selecting 10 disease-related pathways (Figure 4A). Topology analysis revealed that several targets displayed high degree values, indicating their potential involvement in multiple pathways and interaction with various proteins. Notably, CASP8, displayed the highest degree value and was connected to 7 pathways, suggesting that it might be the central target of breviscapine for treating sepsis-induced ALI. The results of molecular docking analysis demonstrated a strong binding affinity of breviscapine (−9.05 Kcal/mol) to CASP8, with the formation of 8 hydrogen bonds between them, namely Lys457, Asp455, Lys456, Asp454, Asn414, Trp420, and Lys453 (Figure 4B and C). The 2D image of molecular docking provided additional evidence of the broad Vander Waals interactions between breviscapine and CASP8 (Figure 4D). Thus, breviscapine may improve ALI by targeting CASP8.
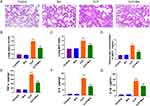
Breviscapine Alleviates ALI and Inflammation Induced by Sepsis
As shown in Figure 5A, mice in the CLP group displayed notable pathological changes, such as alveolar hemorrhage and substantial infiltration of inflammatory cells, which were significantly alleviated by breviscapine treatment. In comparison to the control group, administration of breviscapine alone did not result in significant differences in lung injury scores and lung W/D ratio, while the CLP group exhibited a marked elevation lung injury scores and lung W/D ratio. There was a significantly lower score and ratio in the Bre group than in the CLP group after post-CLP administration of breviscapine (Figure 5B and C). Furthermore, the CLP-operated mice had increased neutrophils in the BALF relative to the control-operated mice. Breviscapine inhibited this situation (Figure 5D). Additionally, further investigation into the levels of inflammatory factors revealed that the levels of TNF-α, IL-1β and IL-6 were substantially increased in the CLP group. The levels of inflammatory factors in mice treated with breviscapine alone were not significantly different from those of the control group. However, after post-CLP administration, the levels of TNF-α, IL-1β, and IL-6 in the lung tissues were significantly reduced compared to the CLP group (Figure 5E–G). These results demonstrate the efficacy of breviscapine in mitigating ALI and lung tissue inflammation caused by sepsis.
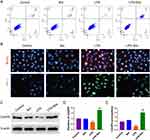
Breviscapine Inhibits Neutrophil Recruitment in Lung Tissues
Following the induction of ALI by CLP, breviscapine was administered to mice, and the accumulation of neutrophils in their lungs was detected using Ly6G staining. A considerable accumulation of neutrophils in the lungs of septic mice was indicated by the significant increase in Ly6G staining signal observed in the CLP group. Breviscapine treatment led to a significant reduction in neutrophil accumulation (Figure 6A). Neutrophil apoptosis in BALF was detected by flow cytometry. It was revealed that neutrophil apoptosis was markedly reduced in the CLP group, suggesting that CLP can extend the lifespan of neutrophils in ALI experimental model. Breviscapine was found to enhance neutrophil apoptosis following CLP (Figure 6B). To confirm these findings and quantify neutrophil accumulation in the lungs, MPO activity was measured in the three experimental groups. Compared to the control group, the lung tissue extract of the CLP group exhibited a higher level of MPO activity, which was significant reduced by breviscapine treatment (Figure 6C). The detection of neutrophil count in BALF also confirms this result again (Figure 6D). No significant effect on MPO activity was observed in normal mice treated with breviscapine. Collectively, these findings suggest that breviscapine treatment attenuates neutrophil accumulation in CLP-induced ALI.
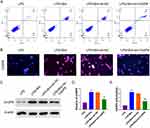
The Effect of Breviscapine on Neutrophil Apoptosis in ALI
Neutrophils were harvested from mouse bone marrow, cultured in vitro for 2 h, and then exposed to either LPS or LPS + Breviscapine. Results demonstrated that LPS stimulation reduced neutrophil apoptosis compared to the control group, while breviscapine had no noted effects. However, administration of LPS + Breviscapine resulted in a higher level of neutrophil apoptosis than LPS alone (Figure 7A). Neutrophils demonstrated enhanced expression of Bcl-xL and Mcl-1 in LPS group, which are two anti-apoptotic proteins that involved in activating the intrinsic apoptosis pathway, compared to control group. This was confirmed through immunostaining in Figure 7B. However, administration of breviscapine after LPS led to a significant decrease in Bcl-xL and Mcl-1 staining signals in neutrophils. Furthermore, CASP8 was found to have lower expression and weaker activity in the LPS group compared to the control group. Treatment with breviscapine alone did not bring about any noticeable changes in the expression or activity of CASP8. However, in comparison to the LPS group, CASP8 mRNA and protein were increased in the LPS + Breviscapine group, and its activity was significantly increased as shown in Figure 7C–E.
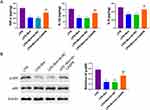
Breviscapine Targets CASP8 to Regulate Neutrophil Apoptosis
In order to further explore the mechanism of breviscapine targeting CASP8 to regulate the apoptosis of neutrophils in ALI, we interfered with the expression of CASP8 in the cells. Results demonstrated that breviscapine can significantly promote the apoptosis of neutrophils, and interfering with the expression of CASP8 can reverse this situation (Figure 8A). In addition, we also used experiments such as immunofluorescence, Western blot, qRT-PCR and Caspase-Glo assay kit to detect the expression of CASP8. It turned out that, in comparison to the LPS group, CASP8 increased in the LPS+Bre group. In comparison to the LPS+Bre+sh-NC group, CASP8 reduced in the LPS+Bre+sh-CASP8 group (Figure 8B–E). The above results show that breviscapine targets CASP8 to regulate neutrophil apoptosis in ALI.
Breviscapine Targets CASP8 to Regulate Inflammatory Signaling Pathway
We further explored the mechanism of Breviscapine regulating neutrophil inflammation in ALI by targeting CASP8. The results showed that breviscapine significantly reduced the production of neutrophil proinflammatory factors, and interfering with CASP8 expression reversed this situation (Figure 9A). In addition, we detected the expression of phosphorylated NF-κB p65 by Western blot. The results showed that breviscapine significantly reduced the expression of p-p65, but it did not affect p65. Compared with LPS+Bre+sh-NC group, the expression of p-p65 in LPS+Bre+sh-CASP8 group was significantly higher (Figure 9B). The above results showed that breviscapine targets CASP8 to regulate the NF-κB signaling pathway of ALI neutrophils, thus inhibiting the inflammatory response.
Discussion
In this research, the efficacy of breviscapine in mitigating CLP-induced ALI was demonstrated, and the potential mechanism of breviscapine in treating sepsis-induced ALI were also elucidated. Potential targets of breviscapine were identified and revealed to be significantly related to apoptosis. As neutrophil apoptosis is a crucial process in ALI, this study focused on the investigation of breviscapine’s effects on neutrophils in vitro and in vivo. Through network topology analysis, CASP8 was identified as the key mediator regulating breviscapine’s therapeutic effects on sepsis-induced ALI, as well as a critical protein involved in apoptosis.
Sepsis is responsible for causing at least 40% of ALI cases, with the lung being the earliest organ affected and having the highest incidence of sepsis-induced organ failure. Up to now, there is still no effective treatments available to prevent the fast progression of sepsis-induced acute lung injury. Breviscapine has shown therapeutic effects on lung injury, but whether breviscapine displays protective effects on pneumonia caused by sepsis remains to be ascertained. Herein, by establishing a mouse sepsis-ALI model via CLP, it was revealed that breviscapine could alleviate CLP-induced ALI in mice.
To investigate the mechanism of breviscapine in preventing CLP-induced ALI, potential targets of breviscapine were predicted using databases and software. By crossing these targets with the target genes of sepsis-induced ALI from multiple database sources, 81 overlapping genes were successfully screened out. Network analysis showed that CASP8, RELA, CASP3, PTGS2, MAPK14, BCL2L1, and TNF were key genes with high degree values. In vitro studies have shown that CASP 3 and 8 are essential for the regulation of apoptosis of neutrophils. Additionally, CASP8 activated by tissue protease D is a novel neutrophil-promoting apoptotic pathway, and is important in modulating the duration of innate immune response.13 CASP8 can be triggered by activation of some factors, including TNF-α, which triggers CASP8 activation via receptor-mediated signaling, ultimately leading to neutrophil apoptosis.14 We found that breviscapine promotes CASP8 expression and activity, and participates in the regulation of neutrophil apoptosis, which may be one of the important mechanisms underlying its protection against sepsis-induced ALI. CASP8 can also inhibit inflammation by inhibiting the pro-inflammatory process. CASP8 cleavage of RIPK1 helps to limit the production of pro-inflammatory cytokines mediated by nuclear factor kappa-B (NF-κB).15 As a ligand of toll like receptor 4 (TLR4), LPS can activate the NF-kB inflammatory signaling pathway downstream of it in neutrophils and promote the release of inflammatory cytokines.16 It has been proved17 that lung inflammation in mice can be alleviated and LPS-induced ALI can be improved by promoting neutrophil apoptosis and inhibiting the TLR4/NF-κB pathway.
RELA encodes NF-κB, a ubiquitous transcription factor involved in various biological processes. Studies have indicated that NF-κB activation has a beneficial impact on ALI caused by H1N1.18 The PTGS2 gene is responsible for encoding the enzyme cyclooxygenase-2 (COX-2), which is increased in expression in reaction to diverse inflammatory stimuli, including LPS. Recent research suggests that the absence of COX-2 dampens LPS-induced inflammation, apoptosis, and ALI in adult mice. These findings indicated that COX-2 plays a dual role in inflammatory responses, both as a downstream participant and as a regulator through a feed-forward mechanism following LPS stimulation.19 MAPK14, an important MAP kinase, is an integrative point for multiple biochemical signals and participates in a range of cellular processes. Inhibition of MAPK14 expression and activation has been widely demonstrated to have a role in identifying ALI.20–22 The TNF-α signaling pathway has a two-fold role in controlling both cell survival and proliferation. However, aberrant activation of this pathway during ALI can induce an excessive cytokine response, ultimately culminating in extensive cellular apoptosis and organ dysfunction. However, optimal levels of TNF-α signal are required for tissue repair after acute injury. TNF-α can induce delayed neutrophil apoptosis and promote intestinal ischemia-reperfusion induced lung injury by activating JNK/FoxO3a pathway.23 The down-regulation of TNF-α has been demonstrated to alleviate ALI in rats with intestinal ischemia-reperfusion injury by up-regulating IL-10 expression.24 In addition, TNF-α blockers have shown potential as therapeutic drugs for ALI.25
Enrichment analysis indicated that the therapeutic effects of breviscapine on sepsis-induced ALI may involve several pathways. Notably, activation of the PI3K/Akt signaling pathway promotes the expression of heme oxygenase-1 (HO-1), which is crucial for mitochondrial quality control and attenuation of endotoxin-induced ALI.26 It is also involved in dendritic cell maturation and function to regulate ALI pathology.27 Additionally, it has been implicated in the protective effects of dexmedetomidine and methane on lung injury.28,29 Inhibition of p53 has been shown to reduce ALI caused by intestinal ischemia/reperfusion via suppression of ferroptosis,30 while regulation of p53 on NF-κB activity in inflammatory cells has been suggested as a potential therapeutic strategy for acute inflammatory diseases, such as ALI.31 Moreover, Interleukin-17A (IL-17A) is associated with the development of ALI through both SMAD-dependent and independent pathways, with curcumin being able to effectively modulate IL-17A signal transduction components, possibly providing anti-inflammatory effects on ALI.32 Furthermore, vascular endothelial growth factor (VEGF) may participate in the pathogenesis of LPS-induced ALI by affecting Flt-1 expression, and the downstream inhibition of VEGF signaling may contribute to the damage to tissue apoptosis and angiogenesis caused by LPS, which provides insight into the progression of LPS-induced ALI.33 Neutrophil apoptosis is a crucial process for maintaining neutrophil homeostasis. Delayed neutrophil apoptosis can exacerbate inflammation in septic patients,34 whereas promoting neutrophil apoptosis can effectively alleviate the inflammatory response.35 Herein, the impacts of breviscapine on neutrophil apoptosis and inflammatory pathway were assessed, which demonstrates its potential to ameliorate sepsis-induced ALI by inducing neutrophil apoptosis and inhibiting inflammatory reaction. To further investigate the role of these pathways in breviscapine therapy for sepsis-induced ALI, further research is necessary.
Despite the intriguing results of this study, some limitations should be taken into account. Firstly, the mechanism-based network pharmacology is still limited in the recognition of certain potential targets. Secondly, we only conducted analysis on neutrophils and CASP8, and further validation of other cellular effects of breviscapine has not yet been completed. Lastly, as breviscapine is available in multiple forms of administration, additional experimental assessment is required to accurately compare their efficacy.
Conclusion
This study applied the method of network pharmacology to discover therapeutic targets and signaling pathways for breviscapine in treating ALI induced by sepsis. Enrichment analysis suggested that apoptosis-related pathways and inflammation-related pathway may be the principal pharmacological mechanism of breviscapine in ALI. The findings were further supported by experiments on mice with CLP-induced ALI and LPS-induced neutrophils. Our data demonstrate that breviscapine can mitigate LPS-induced ALI, presumably by enhancing the intrinsic apoptosis pathway and activating NF-κB mediated inflammation pathway, which were regulated by CASP8. Nevertheless, whether there are other signaling pathways affecting the effectiveness of breviscapine deserves to be further studied.
Abbreviations
ALI, Acute lung injury; CLP, Cecal ligation and puncture; HE, Hematoxylin and eosin; ELISA, Enzyme-linked immunosorbent assay; BALF, Bronchoalveolar lavage fluid; qRT-PCR, Real-time reverse transcription PCR; CASP8, Caspase-8; LPS, Lipopolysaccharide; ECMO, Extracorporeal membrane oxygenation; TCMSP, Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform; OMIM, Online Mendelian Inheritance in Man; CTD, Comparative Toxicogenomics Database; PPI, Protein-protein interaction; RCSB PDB, Research Collaboratory for Structural Bioinformatics Protein Data Bank; BMDNs, Bone marrow-derived neutrophils; sh-CASP8, Short hairpin CASP8; BCA, Bicinchoninic acid; PBS, Phosphate-buffered saline; ANOVA, One-way analysis of variance; SNK, Student-Newman-Keuls’; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; TLR4, Toll like receptor 4; COX-2, Cyclooxygenase-2; HO-1, Heme oxygenase-1; VEGF, Vascular endothelial growth factor.
Data Sharing Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics Approval
All animal procedures were approved by the Institutional Animal Care and Use Committee of Zhejiang Centre for Laboratory Animals (ZJCLA-IACUC-20010219).
The research conducted based on data from public databases in this study qualifies for exemption from ethical review according to item 1 and 2 of Article 32 of “the Measures for Ethical Review of Life Science and Medical Research Involving Human Subjects”, which was reviewed by the National Science and Technology Ethics Committee, approved by the State Council of China, and jointly promulgated by the National Health Commission, the Ministry of Education, the Ministry of Science and Technology and the State Administration of Traditional Chinese Medicine on Feb. 18, 2023, on the following circumstances:
- The research is conducted using legally obtained public data or data generated through observation and does not interfere with public behavior.
- Use of anonymized data for research.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Zhejiang Traditional Chinese Medicine Science and Technology Project (2023ZL440), and Zhejiang Medical and Health Science and Technology Project (2022KY926).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Gauer R, Forbes D, Boyer N. Sepsis: diagnosis and Management. Am Fam Physician. 2020;101(7):409–418.
2. Zhang J, Zheng Y, Wang Y, et al. YAP1 alleviates sepsis-induced acute lung injury via inhibiting ferritinophagy-mediated ferroptosis. Front Immunol. 2022;13:884362. doi:10.3389/fimmu.2022.884362
3. Dos Santos CC, Amatullah H, Vaswani CM, et al. Mesenchymal stromal (stem) cell therapy modulates miR-193b-5p expression to attenuate sepsis-induced acute lung injury. Eur Respir J. 2022;59(1):2004216. doi:10.1183/13993003.04216-2020
4. Mehaffey JH, Charles EJ, Schubert S, et al. In vivo lung perfusion rehabilitates sepsis-induced lung injury. J Thorac Cardiovasc Surg. 2018;155(1):440–448e2. doi:10.1016/j.jtcvs.2017.08.124
5. Wen L, He T, Yu A, et al. Breviscapine: a review on its phytochemistry, pharmacokinetics and therapeutic effects. Am J Chin Med. 2021;49(6):1369–1397. doi:10.1142/S0192415X21500646
6. Chen J, Zhao YH, Liu XL, et al. Effects of breviscapine on pulmonary inflammatory response and lung injury in children undergoing open heart surgery. J Asian Nat Prod Res. 2012;14(3):270–275. doi:10.1080/10286020.2011.652952
7. Wang Y, Ji M, Chen L, Wu X, Wang L. Breviscapine reduces acute lung injury induced by left heart ischemic reperfusion in rats by inhibiting the expression of ICAM-1 and IL-18. Exp Ther Med. 2013;6(5):1322–1326. doi:10.3892/etm.2013.1287
8. Huang JG, Xie M, Zhang X, He QY, He GY. 低氧致大鼠凝血功能异常对肺组织结构的改变及灯盏花素对其的拮抗作用 [Hypoxemia induced the changing structure of the lung tissue in SD rat though changing blood clotting and the effects of breviscapine’s intervention]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2014;45(4):567–571, 622. Chinese.
9. Li X, Wei S, Niu S, et al. Network pharmacology prediction and molecular docking-based strategy to explore the potential mechanism of Huanglian Jiedu Decoction against sepsis. Comput Biol Med. 2022;144:105389. doi:10.1016/j.compbiomed.2022.105389
10. Wu T, Hu E, Xu S, et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation. 2021;2(3):100141. doi:10.1016/j.xinn.2021.100141
11. Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4(1):31–36. doi:10.1038/nprot.2008.214
12. Su VY, Lin CS, Hung SC, Yang KY. Mesenchymal stem cell-conditioned medium induces neutrophil apoptosis associated with inhibition of the NF-kappaB pathway in endotoxin-induced acute lung injury. Int J Mol Sci. 2019;20(9):2208. doi:10.3390/ijms20092208
13. Conus S, Perozzo R, Reinheckel T, et al. Caspase-8 is activated by cathepsin D initiating neutrophil apoptosis during the resolution of inflammation. J Exp Med. 2008;205(3):685–698. doi:10.1084/jem.20072152
14. Fritsch M, Gunther SD, Schwarzer R, et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature. 2019;575(7784):683–687. doi:10.1038/s41586-019-1770-6
15. Han JH, Park J, Kang TB, Lee KH. Regulation of Caspase-8 Activity at the Crossroads of Pro-Inflammation and Anti-Inflammation. Int J Mol Sci. 2021;22(7). doi:10.3390/ijms22073318
16. Zhou GX, Liu ZJ. Potential roles of neutrophils in regulating intestinal mucosal inflammation of inflammatory bowel disease. J Dig Dis. 2017;18(9):495–503. doi:10.1111/1751-2980.12540
17. Zhang C, Wang X, Wang C, et al. Qingwenzhike prescription alleviates acute lung injury induced by LPS via inhibiting TLR4/NF-kB pathway and NLRP3 inflammasome activation. Front Pharmacol. 2021;12:790072. doi:10.3389/fphar.2021.790072
18. Ling LJ, Lu Y, Zhang YY, et al. Flavonoids from Houttuynia cordata attenuate H1N1-induced acute lung injury in mice via inhibition of influenza virus and Toll-like receptor signalling. Phytomedicine. 2020;67:153150. doi:10.1016/j.phymed.2019.153150
19. Nelin LD, Jin Y, Chen B, Liu Y, Rogers LK, Reese J. Cyclooxygenase-2 deficiency attenuates lipopolysaccharide-induced inflammation, apoptosis, and acute lung injury in adult mice. Am J Physiol Regul Integr Comp Physiol. 2022;322(2):R126–R135. doi:10.1152/ajpregu.00140.2021
20. Ding L, Gao X, Yu S, Sheng L. miR-128-3p enhances the protective effect of dexmedetomidine on acute lung injury in septic mice by targeted inhibition of MAPK14. J Bioenerg Biomembr. 2020;52(4):237–245. doi:10.1007/s10863-020-09842-8
21. Li C, Liu JH, Su J, et al. LncRNA XIST knockdown alleviates LPS-induced acute lung injury by inactivation of XIST/miR-132-3p/MAPK14 pathway: XIST promotes ALI via miR-132-3p/MAPK14 axis. Mol Cell Biochem. 2021;476(12):4217–4229. doi:10.1007/s11010-021-04234-x
22. Zhang R, Chen L, Huang F, Wang X, Li C. Long non-coding RNA NEAT1 promotes lipopolysaccharide-induced acute lung injury by regulating miR-424-5p/MAPK14 axis. Genes Genomics. 2021;43(7):815–827. doi:10.1007/s13258-021-01103-1
23. Chen D, Chen C, Xiao X, Huang Z, Huang X, Yao W. TNF-alpha induces neutrophil apoptosis delay and promotes intestinal ischemia-reperfusion-induced lung injury through activating JNK/FoxO3a pathway. Oxid Med Cell Longev. 2021;2021:8302831. doi:10.1155/2021/8302831
24. Yang Z, Zhang XR, Zhao Q, et al. Knockdown of TNF‑alpha alleviates acute lung injury in rats with intestinal ischemia and reperfusion injury by upregulating IL‑10 expression. Int J Mol Med. 2018;42(2):926–934. doi:10.3892/ijmm.2018.3674
25. Lai WY, Wang JW, Huang BT, Lin EP, Yang PC. A novel TNF-alpha-targeting aptamer for TNF-alpha-Mediated acute lung injury and acute liver failure. Theranostics. 2019;9(6):1741–1751. doi:10.7150/thno.30972
26. Shi J, Yu J, Zhang Y, et al. PI3K/Akt pathway-mediated HO-1 induction regulates mitochondrial quality control and attenuates endotoxin-induced acute lung injury. Lab Invest. 2019;99(12):1795–1809. doi:10.1038/s41374-019-0286-x
27. Li R, Zou X, Huang H, et al. HMGB1/PI3K/Akt/mTOR signaling participates in the pathological process of acute lung injury by regulating the maturation and function of dendritic cells. Front Immunol. 2020;11:1104. doi:10.3389/fimmu.2020.01104
28. Li J, Chen Q, He X, et al. Dexmedetomidine attenuates lung apoptosis induced by renal ischemia-reperfusion injury through alpha(2)AR/PI3K/Akt pathway. J Transl Med. 2018;16(1):78. doi:10.1186/s12967-018-1455-1
29. Wang F, Wang F, Li F, et al. Methane attenuates lung ischemia-reperfusion injury via regulating PI3K-AKT-NFkappaB signaling pathway. J Recept Signal Transduct Res. 2020;40(3):209–217. doi:10.1080/10799893.2020.1727925
30. Li Y, Cao Y, Xiao J, et al. Inhibitor of apoptosis-stimulating protein of p53 inhibits ferroptosis and alleviates intestinal ischemia/reperfusion-induced acute lung injury. Cell Death Differ. 2020;27(9):2635–2650. doi:10.1038/s41418-020-0528-x
31. Liu G, Park YJ, Tsuruta Y, Lorne E, Abraham E. p53 Attenuates lipopolysaccharide-induced NF-kappaB activation and acute lung injury. J Immunol. 2009;182(8):5063–5071. doi:10.4049/jimmunol.0803526
32. Shaikh SB, Bhat SG, Bhandary YP. Curcumin attenuates IL-17A mediated pulmonary SMAD dependent and non-dependent mechanism during acute lung injury in vivo. Mol Biol Rep. 2020;47(7):5643–5649. doi:10.1007/s11033-020-05587-0
33. Jesmin S, Zaedi S, Islam AM, et al. Time-dependent alterations of VEGF and its signaling molecules in acute lung injury in a rat model of sepsis. Inflammation. 2012;35(2):484–500. doi:10.1007/s10753-011-9337-1
34. Wang JF, Wang YP, Xie J, et al. Upregulated PD-L1 delays human neutrophil apoptosis and promotes lung injury in an experimental mouse model of sepsis. Blood. 2021;138(9):806–810. doi:10.1182/blood.2020009417
35. Singhal A, Kumar S. Neutrophil and remnant clearance in immunity and inflammation. Immunology. 2022;165(1):22–43. doi:10.1111/imm.13423
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.