Back to Journals » International Journal of Nanomedicine » Volume 19
Cancer Immunotherapy with “Vascular-Immune” Crosstalk as Entry Point: Associated Mechanisms, Therapeutic Drugs and Nano-Delivery Systems
Authors Jiang Z, Fang Z, Hong D, Wang X
Received 5 March 2024
Accepted for publication 4 July 2024
Published 19 July 2024 Volume 2024:19 Pages 7383—7398
DOI https://doi.org/10.2147/IJN.S467222
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Jie Huang
Zhijie Jiang, Zhujun Fang, Dongsheng Hong, Xiaojuan Wang
Department of Clinical Pharmacy, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, 310000, People’s Republic of China
Correspondence: Xiaojuan Wang, Email [email protected]
Abstract: Tumor vessels characterized by abnormal functions and structures hinder the infiltration and immune antigen presentation of immune cells by inducing the formation of an immunosuppressive microenvironment (“cold” environment). Vascular-targeted therapy has been proven to enhance immune stimulation and the effectiveness of immunotherapy by modulating the “cold” microenvironment, such as hypoxia and an acidic microenvironment. Notably, a therapeutic strategy based on “vascular-immune” crosstalk can achieve dual regulation of tumor vessels and the immune system by reprogramming the tumor microenvironment (TME), thus forming a positive feedback loop between tumor vessels and the immune microenvironment. From this perspective, we discuss the factors of tumor angiogenesis and “cold” TME formation. Building on this foundation, some vascular-targeted therapeutic drugs will be elaborated upon in detail to achieve dual regulation of tumor vessels and immunity. More importantly, we focus on cutting-edge nanotechnology in view of “vascular-immune” crosstalk and discuss the rational fabrication of tailor-made nanosystems for efficiently enhancing immunotherapy.
Keywords: immunotherapy, nano-delivery systems, anti-angiogenic therapy, “vascular-immune” crosstalk, tumor vessels
Graphical Abstract:
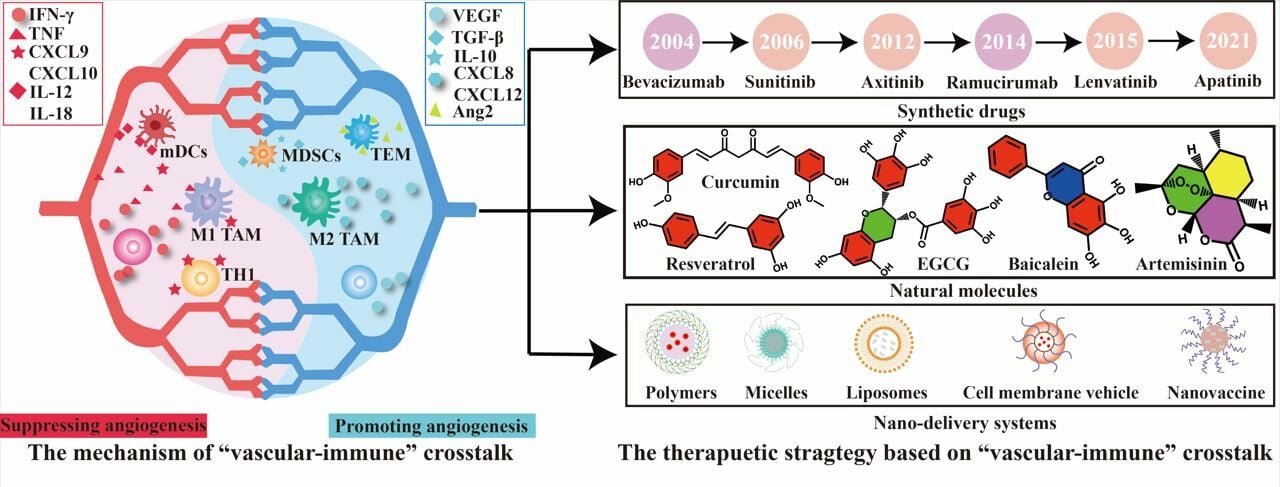
Introduction
Cancer is the leading cause of mortality worldwide. The International Agency for Research on Cancer (IARC) predicts that the annual number of cancer cases will increase from approximately 18.1 million in 2018 to 27.5 million in 2040.1 Cancer immunotherapy has gradually become a powerful treatment for solid tumors, with the aim of enhancing the activation of the immune system to kill cancer cells. Immunotherapeutic agents, such as immune checkpoint inhibitors (ICIs) and tumor vaccines that can reactivate dysfunctional and/or exhausted T cells, have been proven to be effective against multiple cancers.2,3 However, 50–80% of cancer patients do not benefit from ICIs and many experience severe adverse events.4,5 The characterized microenvironment of immunosuppressive cancer, lack of immune cell infiltration, and low T cell activity are responsible for the low immune response.6 In addition, immunosuppressive tumor microenvironment (“cold” TME), characterized by hypoxia, low pH, also reduces the effectiveness of immunotherapy via inhibiting T-cell function and recruiting immunosuppressive cells.7,8
Recent research has revealed that tumor vessels are an important factor in triggering the formation of a “cold” environment. Firstly, tumor cells release pro-angiogenic factors to promote tumor angiogenesis with structural and functional abnormalities. These abnormal tumor vessels further promote tumor hypoxia owing to uneven oxygen delivery and further reprogramming of tumor metabolism.9 Subsequently, its metabolites, such as lactate, are released by tumor cells and accumulate in the TME, exacerbating the formation of a “cold” TME and inducing resistance to tumor immunotherapy.10 In addition, tumor vessels are also reported to hinder immune effector cells infiltration and the delivery of therapeutic drugs.11,12 As the “cold” TME worsens, immunosuppressive cells release a series of bioactive molecules, such as cytokines, chemokines, which in turn affect the process of tumor angiogenesis. They can also regulate tumor angiogenesis by directly acting on endothelial cells (ECs) or activating signaling pathways related to tumor angiogenesis.13 And increasing clinical research supports the substantial enhancement of overall survival (OS) and overall response rate (ORR) with the combination of ICIs and antiangiogenic therapy.14,15 Furthermore, preclinical and clinical research also found low-dose anti-angiogenic drugs could induce vascular normalization by augmenting ECs coverage within vascular tissue and pericytes, which increase the infiltration of immune effector cells into tumors and convert the intrinsically “cold” TME to “hot” one.16,17 In summary, the reciprocal modulation between immune cells and tumor vessels (“vascular-immune” crosstalk) is vital in enhancing the effectiveness of cancer immunotherapy, and understand “vascular-immune” crosstalk will be essential for developing potent combination cancer immunotherapies.
In this review, we elaborate on the immunosuppressive mechanisms of tumor vessels, therapeutic drugs, clinical treatments, and nano-delivery strategies based on the “vascular-immune” pathway. In addition, this review discusses the associated mechanisms and feasible therapeutic drugs based on the “vascular-immune” effect. Notably, drug delivery nanosystems customized for tumor vessels have been elaborated and discussed in detail using cutting-edge nanotechnology. Finally, the future prospects and potential breakthroughs of these antiangiogenic agents and nanosystems are briefly discussed.
The Aberrant Tumor Vessels and Poor Drug Delivery
Under physiological conditions, endothelial progenitor cells are locally stimulated, recruited, and differentiated into mature vascular endothelial cells, which ultimately form blood vessels.18 Tumor angiogenesis begins with locally damaged endothelial basement membranes, and pro-angiogenic factors induce ECs migration and promote ECs proliferation, thus forming capillary loops and new basement membranes.19 Tumor angiogenesis is simultaneously regulated by pro- and anti-angiogenic factors, and its balance is disrupted during tumor progression.20 Overexpression of pro-angiogenic factors such as vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), and angiopoietin (Ang) in the TME is responsible for tumor angiogenesis. These molecules induce ECs migration and attach to vessels by binding to receptors on ECs, thus forming structurally and functionally abnormal tumor vessels.21 VEGF, a key protein in angiogenesis, is responsible for maintaining the activity of the immature vasculature and accelerating vascular permeability by binding to VEGF receptors (VEGFR-1 and −2), which impairs vascular perfusion and elevates interstitial hydraulic pressure.22 In addition, TGF-β regulates the migratory and invasive activities of ECs by increasing the expression of metalloproteases that degrade extracellular matrix (ECM) such as MMP-2 and MMP-9.23 Ang is closely related to the development of mature blood vessels, endothelial sprouting, vessel wall remodeling, and the recruitment of mural cells.24 Under the common effect of these factors, the perivascular cells of tumor vessels are detached or absent, causing a reduction in vascular integrity, incoherent perfusion, and vessel immaturity, thus forming hyperleaky tumor vessels. It has been reported that hyperleaky tumor vessels not only restrict the delivery of oxygen and elevate interstitial fluid pressure (IFP) but also facilitate the dissemination and metastasis of tumor cells. In addition, it reduces the efficacy of immunotherapy by hindering the entry of antitumor therapeutics and immune cells from the bloodstream into tumor tissue.25 Moreover, other adverse effects on tumor vessels, such as inflammation, hypoxia, low pH, and poor delivery of therapeutic drugs, are important reasons for reducing tumor immunogenicity and promoting immunosuppression.26
The accumulation of drugs at the tumor site needs to undergo intra-circulatory transport and span the vascular wall and interstitial spaces, which is hindered by the tumor vessels. Tumor vessels are characterized by high leakage, large gaps in defective ECs, chaotic microvascular branches, and irregular arrangements, which affect blood flow behavior, vascular perfusion, and hydrostatic and osmotic pressure gradients.27 Diffusion and convection across discrete endothelial intercellular spaces, the main pathways of vascular-mediated drug transport, are dually hindered by a leaky coronary system and interstitial ECM, thus resulting in inefficient drug distribution throughout the tumor.28 In addition, the enhanced permeability and retention effect (EPR effect) was also the other transport pathway, while diffusion barriers composed of dense cellular and stromal components impede transport through the interstitium.29 Interestingly, the ECM, which consists of fibrin, glycoprotein (GP), proteoglycan (PG), and glycosaminoglycan (GAG), provides structural support, promotes ECs migration, and prevents the entry of macromolecular drugs into the TME. The cross-linking of ECM proteins (eg, collagen, elastin, and fibronectin) forms a numerous and highly cohesive fibrous network that significantly inhibits their activity and diffusion efficiency by trapping drugs around tumor vessels.30,31 ECM proteins induce cancer-associated fibroblasts (CAFs) to synthesize matrix proteins, which impairs the deep penetration of macromolecular drugs by increasing tumor stiffness.32 In addition, a hypoxic environment upregulates the expression of drug resistance genes (eg, P-gp, MDR, MRP1) and increases drug efflux to enhance drug resistance in tumor cells.33,34 In summary, tumor vessels hinder multiple aspects of therapeutic drugs, including drug delivery, penetration, and sensitivity.
The Vascular-Immune Crosstalk
A complete immune response chain is necessary for effective cancer immunotherapy; however, it encounters an immunosuppressive TME caused by abnormal tumor vessels (Figure 1). Additionally, immune cells exhibit pro-angiogenic activities that regulate tumor angiogenesis. Thus, reciprocal modulation exists between immune cells and tumor vessels. Understanding this interplay is crucial to achieve sustained immune activation and effective immunotherapeutic outcomes. In this section, we will elaborate on the effects of “vascular-immune” crosstalk from different perspectives, including immune cell infiltration, immune response, and tumor vessels.
Effect on Immune Cells Infiltration
Tumor vessels usually impair the adhesion between immune cells and ECs and upregulate the expression of immunosuppressive molecules to form a barrier, making it difficult for them to reach the tumor site. ECs can prevent immune cell adhesion through intracellular sequestration or transcriptional repression of endothelial adhesion molecules (EAMs), whereas post-inflammatory ECs impair EAM sequestration via inducing P-selectin expression and pro-inflammatory cytokine activation.35 These pathways activate the gene expression of EAMs (eg, E-selectin, ICAM-1 and VCAM-1) and secretion of chemokines, which further affect the migration of immune cells. E-selectin and P-selectin are involved in leukocyte capture and rolling, while ICAM-1, ICAM-2, and VCAM-1 are involved in the subsequent steps of rolling, crawling, arrest, and transendothelial migration.36,37 However, the expression of multiple EAM genes in tumor-associated endothelial cells (TECs) is inhibited by vascular-related factors, including pro-angiogenic factors, inflammatory factors, and chemokines, which reduce T cell-endothelial cell interactions. For example, immune cell adhesion is initiated by ECs activation via tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), resulting in the upregulation of adhesion molecules and chemokine production.38,39 bFGF and VEGF inhibit proinflammatory cytokine-induced ECs adhesion by inhibiting the expression of ICAM-1, VCAM-1 and E-selectin.40 The death mediator FAS ligand (FASL) is usually found in the vasculature of solid tumors, and upregulated by VEGF-A, which selectively kills effector T cells, resulting in a lack of intratumoral CD8+ T cells.41 In addition, TECs can upregulate multiple inhibitory molecules, including galectin 1 (Gal-1) and endothelin B receptor (ETBR), creating an endothelial barrier for immune cells to infiltrate tumor tissue. The ETBR plays a pivotal role in maintaining vascular homeostasis. Its binding to endothelin-1 reduces the adhesion between T cells and ECs, thereby decreasing the homing of T cells.42 Gal-1 is overexpressed in the neovascularization of tumors and specifically upregulated in TECs to induce T-cell apoptosis. Additionally, research has noted a substantial reduction in T lymphocyte recruitment with significant expression of Gal-1.43
Effect on Immune Response
The anti-tumor immune response is a complex and coordinated physiological process in which the body combats tumor cells and involves immune recognition, antigen presentation, immune cell activation, and immune effects. However, heterogeneous oxygen delivery to tumor vessels induces the formation of a highly hypoxic TME, restraining the effectiveness of the immune response. The vascular-mediated hypoxic microenvironment contributes to immunosuppression through several mechanisms. Firstly, hypoxia induces immune escape of tumor cells by increasing the expression of immunosuppressive molecules such as programmed cell death ligand 1 (PD-L1). Recent research has shown that PD-L1 expressed by tumor cells can induce the loss of cytotoxic activity in T cells by binding to its PD-1 receptor, which is a crucial pathway for immune evasion.44 PD-L1 expressed by TECs can directly inhibit the inactivation of T cells at the vascular site by binding to PD-1, which is stimulated by inflammatory cytokines (IFN-γ and TNF-α).45 Secondly, hypoxia alters tumor metabolism and upregulates the secretion of immunosuppressive metabolites, predisposing immune cells to an immunosuppressive state.46,47 Recent studies have shown that hypoxia can activate hypoxia-inducible factor 1-alpha (HIF-1α), which further enhances the expression of pyruvate dehydrogenase kinase 1 (PDK1), leading to the reduced conversion of pyruvate to acetyl-CoA and increased lactate production. Lactate acidifies the TME, forming a low-pH environment, which consequently suppresses proliferation and weakens the anti-tumor function of tumor-infiltrating cells (TILs) by blocking the export of lactate from CD8+ T cells and impairing adenosine triphosphate (ATP) production.48,49 Lactate also inhibits the activation and proliferation of T cells and induces M2-like polarization of tumor-associated macrophages (TAM).50 It is worth mentioning that hypoxia also upregulates the activity of indoleamine 2.3-dioxygenase (IDO), which catalyzes the degradation of tryptophan to kynurenine (Kyn), inducing the formation of FoxP3+ T-regs and tolerogenic dendritic cells (tolDCs) by binding aryl hydrocarbon nuclear receptors(AHR).51,52 Finally, hypoxia promotes the secretion of immunosuppressive factors that worsen the immunosuppressive microenvironment by inducing the secretion of immunomodulatory molecules from the TECs. VEGF-A inhibits the function of T cells and upregulates the immune resistance of tumor cells by inducing the accumulation and proliferation of myeloid-derived suppressor cells (MDSCs), which can induce the transformation of M2 macrophages and regulatory T cells (Tregs) by secreting cytokines (such as IL-10 and TGF-β), downregulating cell adhesion factors, promoting immune cell extravasation, and depleting nutrients (such as L-arginine and cystine).53 Ang2 promotes Tregs infiltration and inhibits CTL activation by recruiting M2 TAMs and Tie-2-expressing monocytes/macrophages (TEM).54 TGF-β silences tumor immune surveillance via downregulating T cell receptor (TCR) expression and reducing intracellular Ca2+ signaling and transcription factor expression.55
Effect on Tumor Vessels
The role of immune cells in regulating tumor vessels is a complex and multi-level process that involves a variety of cell types and molecular signals. Activated CD8+ T cells can secrete IFN-γ to downregulate the expression of delta-like protein 4 in ECs, thus inhibiting Notch signaling, which is a critical requirement for sprouting angiogenesis. CD4+ T helper 1 (TH1) cells are related to pericyte coverage of tumor vessels, and loss of CD4 TH1 cells causes vascular abnormalities.56 The accumulation of Tregs at tumor sites has been demonstrated to promote the release of angiogenic cytokines by suppressing the activity of immune cells such as CD4+ effector TH1 cells.57 Tumor-associated immune cells participate in angiogenesis. For example, TAMs, which share many features with M2-like macrophages, produce VEGF-A and chemokines (CXCL8, CXCL12, and TNFα) to activate and recruit ECs, which are responsible for the induction of sprouting, tube formation, and maturation of new vessels.58 Interestingly, M1-like macrophages release anti-angiogenic cytokines such as IL-12, IL-18, and TNF-α to suppress sprouting angiogenesis.59
The Therapeutic Strategy Based on “Vascular-Immune” Crosstalk
Based on the immune-regulatory effect of tumor vessels, anti-angiogenic therapy has been widely combined with immunotherapy for the treatment of diverse cancers such as breast cancer, melanoma, and lung cancer. Currently, anti-angiogenic drugs are categorized into synthetic and natural molecular drugs, both of which exert dual regulatory effects on the “vascular-immune” system. This review summarizes several representative classes of natural products and synthetic antiangiogenic drugs influencing the “vascular-immune” relationship, emphasizing the promising therapeutic potential of combination therapy targeting the “vascular-immune” interplay.
Synthetic Drugs
Small-molecule tyrosine kinase inhibitors (TKIs) and VEGF monoclonal antibodies are the main components of synthetic antiangiogenic drugs, many of which have been approved for clinical cancer treatment (Table 1). Sunitinib, an oral broad-spectrum TKIs, is used to treat renal cell carcinoma (RCC) through the inhibition of VEGFR-1, 2, and 3. In addition, sunitinib treatment demonstrated potent immune regulatory capabilities, including suppression of Tregs function, decreased numbers of MDSCs, and enhancement of immune cell infiltration. It also leads to a reduction in the expression of immune checkpoint receptors on CD4+ and CD8+ T cells.60,61 Preclinical and preliminary clinical findings have shown that apatinib not only efficiently activates natural killer (NK) cells but also enhances the expression of PD-L1 on tumor cells.62,63 Simultaneously, apatinib increases the proportion of CD4+ CD25+ T cells, which is recognized as a promising indicator of apatinib prognosis and contributes to the extension of progression-free survival (PFS) in patients.64 Axitinib has been developed as a selective inhibitor of VEGFRs 1–3, and is well-established as a second-line treatment for metastatic RCC.65 A preclinical study observed that after treatment with axitinib, immunosuppressive cells, including tumor-associated mast cells, TAM, and monocytic MDSCs, significantly decreased, enhancing the therapeutic efficacy of ICIs therapy.66 Lenvatinib, a multi-kinase inhibitor targeting VRGFR 1–3 and fibroblast growth factor receptor (FGFR 1–4), has been reported to reduce PD-L1 expression and Tregs infiltration in tumors. Lenvatinib enhances proteasomal degradation of PD-L1 and inhibits Tregs differentiation by blocking FGFR4, thereby enhancing the immune response to ICIs immunotherapy.67
 |
Table 1 Synthetic Drugs with Dual-Modulatory Effects on “Vascular-Immune” |
 |
Table 2 Natural Molecules with Dual-Modulatory Effects on “Vascular-Immune” |
 |
Table 3 Therapeutic Strategies of ICI Immunotherapy Combined with Antiangiogenic Therapy in Recent Years |
VEGF monoclonal antibody-based therapy has become one of the most important strategies for the treatment of solid tumors. Bevacizumab (Bev) is a recombinant humanized immunoglobulin G monoclonal antibody that targets VEGF-A and inhibits formation of the VEGF-A/VEGFR-2 complex. It increases the number of mature DCs and inhibits tumor infiltration by immunosuppressive cells.68 Bev has been reported to reduce the proportion of Tregs in the blood of metastatic CRC patients.69 Ramucirumab (Ram) is a human monoclonal antibody that specifically targets VEGFR2 by blocking its interactions with VEGF ligands. A clinical trial demonstrated that after treatment with Ram, there was an increase in the expression of PD-L1 on tumor cells and CD8+ T-cell infiltration in the TME. Concurrently, the number of Tregs and PD-1 expression in CD8+ T cells within TILs are significantly decreased.70
Natural Molecules
Plant molecules are safer than synthetic drugs and exhibit remarkable potential for treating angiogenesis-related diseases. Consequently, natural compounds are being explored as promising candidates for inhibiting pathological angiogenesis. Table 2 provides a summary of different natural extracts and molecules with “vascular-immune” dual regulatory functions, along with details on antiangiogenic targets and therapeutic mechanisms of immune regulation. Curcumin, a diketone compound extracted from the rhizome of Curcuma longa, exhibits anti-angiogenic effects by inhibiting VEGF expression, reducing extracellular VEGF secretion, and impeding VEGFR binding.71 Curcumin restores NF-κB activity and reactivates the TNF-α signaling pathway to reduce T cell apoptosis. Additionally, it inhibits Tregs function by hindering FOXP3 transcription, leading to downregulation of TGF-β and IL-10.72 Resveratrol (RSV), a non-flavonoid polyphenol organic compound, has antioxidant, anti-inflammatory, anticancer, and cardiovascular protective effects. In a study by Karina B Cullberg et al, it was observed that RSV has inhibitory effects on hypoxia-induced angiogenesis in a dose-dependent manner, including glucose transporter-1 (GLUT1), VEGF and IL-6.73,74 Yoolhee Yang et al found that RSV enhances the accumulation of CD8+ T cells and IFN-γ production.75,76 PGE2, a proangiogenic agent, induces angiogenesis by stimulating tumor cells to secrete CXCL1. This process directly triggers ECs migration, survival, proliferation, and tube formation as well as the phosphorylation and activation of fibroblast growth factor (FGF) receptor 1.89 Artemisinin (ART) has been reported to significantly decrease the production of PGE2. Additionally, it exhibits immune regulatory functions by activating T cells and inhibiting the proliferation of both Tregs and MDSCs.77,78 Baicalein, a bioactive flavonoid, exhibits potent inhibitory activity on the in vitro proliferation, migration, and tube formation of HUVEC while significantly impeding the growth of lung cancer.79 It also serves as a highly effective immune checkpoint regulator, which further downregulates IFN-γ-induced PD-L1 expression by reducing STAT3 activity, thus restoring the sensitivity of T cells to killing tumor cells.80 Epigallocatechin-3-Gallate (EGCG), the main constituent of green tea, not only directly blocks VEGF/VEGFR but also inhibits VEGF transcription via impeding the DNA binding activity of AP-1 and VEGF promoters.81 Additionally, Kengo Ogawa et al found that EGCG inhibited the transcriptional activity of the IDO promoter, IFN-stimulated response element, and IFN-γ activation sequence activated by STAT1 phosphorylation.82
Clinical Implications
Numerous preclinical studies have provided evidence that angiogenesis-induced immunosuppression can improve immunotherapy. Hence, the addition of anti-angiogenic agents to immunotherapies is currently considered an attractive treatment approach. In recent years, anti-angiogenic therapy has been shown to enhance the therapeutic effect of ICI immunotherapy, and related strategies are summarized in Table 3. Impower 150 (NCT02366143), an open-label, randomized, Phase 3 trial, evaluated the therapeutic effects of atezolizumab plus Bev plus carboplatin/paclitaxel chemotherapy in patients with metastatic NSCLC. This study indicated that combining atezolizumab with Bev-based treatment significantly improved the PFS and OS of patients with metastatic non-squamous NSCLC patients.84 In a study by CheckMate 9ER (NCT03141177), an open-label, randomized, phase 3 trial, previously untreated patients with advanced RCC were treated with sunitinib or nivolumab plus cabozantinib. Compared with sunitinib, nivolumab plus cabozantinib demonstrated improved efficacy in OS and PFS analyses, providing additional support for first-line treatment of advanced RCC.85 In addition, the combination of pembrolizumab and axitinib in patients with previously untreated advanced RCC significantly prolonged the final OS and PFS, as well as a higher objective response rate.86 Furthermore, different combinations of anti-angiogenic and immunotherapy agents are under evaluation, and novel therapeutic agents are continuously developed.87,88 Thus, more therapeutic strategies combining anti-angiogenic therapy and immunotherapy will appear in the future, and are expected to provide more effective and personalized treatment options for patients.
Nano-Delivery Systems Enhance Cancer Immunotherapy by Modulating “Vascular-Immune” Crosstalk
Although the combination of anti-angiogenesis and immunotherapy greatly enhances the efficiency of ICIs immunotherapy, only a few treatment options are clinically available. Furthermore, patients receiving long-term ICIs treatment may develop acquired drug resistance owing to defects in antigen presentation mechanisms and the depletion of new antigens.90 To further enhance the effectiveness of immunotherapy, novel immune drugs based on drug delivery systems (DDS) has been developed. In this section, we provide an overview of the use of DDS to reprogram the immune system to fight cancer. Regarding DDS, we present nanoconstruction methods and therapeutic mechanisms based on the tumor immune microenvironment (Figure 2).
 |
Figure 2 Nanosystems enhance cancer immunotherapy by multiple mechanisms. |
Nanosystems with “Vascular-Immune” Crosstalk for New Antigen Presentation Release
Tumor-associated antigens (TAAs) release is a crucial step in antigen presentation, however, an “cold” microenvironment and its immune evasion mechanisms result in limited antigen expression. Given the aim of promoting TAAs release in patients with solid tumors, extensive research has led to the development of nanomedicines for various immunotherapies. Immunogenic cell death (ICD) effect can induce the release of damage-associated molecular patterns (DAMPs) that are recognized by pattern recognition receptors (PRRs), thereby inducing the activation, differentiation, and maturation of antigen-presenting cells (APCs).91 Chemotherapeutic drugs and photosensitizer, act as ICD inducers, are often used in combination with ICIs in clinical practice.92,93 However, the long-term use of chemotherapeutic drugs, such as paclitaxel (PTX) and doxorubicin (DOX), is associated with cumulative toxicity, imposing a certain limit on the viability of this treatment strategy. Co-loading chemotherapeutic drugs and antiangiogenic drugs into nanosystems can enhance the therapeutic effects of ICD. Zhicheng et al designed a dual-targeting nanoparticle (PLA-PEG-ACUPA/TPP) to co-deliver DOX and Ingenol-3-angelate (I3A), an emerging antitumor drug with dual chemotherapeutic and anti-angiogenic effect (Figure 3A). Compared to DOX nanoparticle group, those treated with I3A/DOX nanoparticles led to increased levels of ICD proteins, such as calreticulin (CRT) and high-mobility group box 1 (HMGB1), along with enhanced T cells infiltration, effectively reversing the immunosuppressive microenvironment.94 In addition, the generation of reactive oxygen species (ROS) generated by photodynamic therapy (PDT) modulate immunity and anti-tumor responses, yet face limitations in hypoxic microenvironment. Combining with vascular normalization treatment was considered as a strategy to enhance the therapeutic effects of PDT. The team led by Jianliang S teams designed BSA-MHI148@SRF nanoparticles by using near-infrared photodynamic dye MHI148 and Sorafenib (SRF) (Figure 3B). These nanoparticles not only alleviated tumor oxygen consumption via inhibiting mitochondrial oxidative phosphorylation, but also enhanced oxygen perfusion through promoting vascular normalization, ultimately enhanced MHI148-induced ICD.95
 |
Figure 3 Nanosystems with “vascular-immune” crosstalk for new antigen presentation release. (A) the dual-targeting delivery system for the codelivery of I3A and DOX for chemoimmunotherapy. Reprinted with permission from Wang ZC, Sun C, Wu HJ, et al. Cascade targeting codelivery of ingenol-3-angelate and doxorubicin for enhancing cancer chemoimmunotherapy through synergistic effects in prostate cancer. Materials Today Bio. 2022 13:100189. Copyright © 2022.94 (B) The synthesis route and mechanism of BSA-MHI148@SRF nanoparticles mediated cascade two-stage re-oxygenation and immune re-sensitization strategy for enhanced PDT immunotherapy. Reprinted with permission from Zhou ZG, Chen JS, Liu Y, et al. Cascade two-stage tumor re-oxygenation and immune re-sensitization mediated by self-assembled albumin-sorafenib nanoparticles for enhanced photodynamic immunotherapy. Acta Pharm Sin B. 2022 12(11): 4204–4223. Copyright © 2022.95 (C) The pECM nanovaccine for nasopharyngeal carcinoma therapy via enhancing the formation of tertiary lymphoid structures (TLS). Reprinted with permission from Nanovaccines Fostering Tertiary Lymphoid Structure to Attack Mimicry Nasopharyngeal Carcinoma, ACS Nano 17(8) (2023) 7194–7206. Copyright (2023) American Chemical Society.96 (D) Combined strategy of mannose-LCP NP-based vaccine (a) and SUNb-PM to enhance anti-tumor immune response (b). Reprinted from J Control Release, 172(1), Xu ZH, Srinivas Ramishetti, Tseng Yu-Cheng, et al. Multifunctional nanoparticles co-delivering Trp2 peptide and CpG adjuvant induce potent cytotoxic T-lymphocyte response against melanoma and its lung metastasis, 259–265, Copyright (2013, a), with permission from Elsevier97 and J Control Release, 245, Huo MR, Zhao Y, Andrew Benson Satterlee, et al. Tumor-targeted delivery of sunitinib base enhances vaccine therapy for advanced melanoma by remodeling the tumor microenvironment, 81–94, Copyright (2017, b), with permission from Elsevier.98 |
Tumor vaccines can be derived from autologous tumor cells, tumor-associated antigens, tumor-specific antigens, or genetic vaccines, all of which activate CD4+ and CD8+ T-cells to generate an immune response. However, subcutaneous administration of tumor vaccines often faces challenges like low APC activity due to self-clearance and phagocytosis by other immune cells. Encapsulating antigens and adjuvants within nanoparticles improve vaccine delivery and retention in draining lymph nodes. This facilitates the controlled release of antigens and adjuvants, thereby enhancing antigen presentation levels.99 Zhenfu et al engineered a nanovaccine consisting of Epstein–Barr virus nuclear antigen 1 (EBNA1) and Mn2+/CpG bi-adjuvant (Figure 3C). These nanovaccines not only activate LT-α/β pathways to increase DCs maturation, but also facilitate T cells activation through the MHC-II antigen presentation pathway. More importantly, the nanovaccines promote normalization of tumor vessels and lymphatic vessels to increase the aggregation of tumor memory T cells, ultimately enhancing local immune responses significantly.96 In addition, Meirong et al developed a potent mannose-modified lipid calcium phosphate (LCP) NPs containing both tumor-specific antigen (tyrosinase-related protein 2 (Trp2) peptide) and adjuvant (CpG) (CpG/Trp2-LCP) and a targeted polymeric micelle loaded with sunitinib base (SUNb-PM) (Figure 3D). CpG/Trp2-LCP nanovaccine enhanced MHC I-restricted cytotoxic T-lymphocyte response by targeting DC cells, which was furtherly enhanced by combining SUNb-PM. They further studied the mechanism and found that SUNb-PM remodeled immune-suppressive microenvironment, such as the ratio of immune cells/immune-suppressive cells and tumor vessel, by inhibiting Stat3 and AKT signaling pathways, thus increasing the efficiency of MHC-I mediated antigen presentation.97,98
Nanosystems with “Vascular-Immune” Crosstalk for Antigen Presentation
The presence of both tumor cells and TILs in tumor tissues implicates dysfunctional antigen presentation and recognition are primary factor limiting immunotherapy efficacy.100 DCs take up TAAs, digest them into immunogenic peptides, present them on MHC-peptide complexes, and activate T cells through TCR. Nanotechnology-based tumor vaccine, combined with anti-angiogenic therapy, has been applied to enhance DCs maturation and increase immunogenic peptides expression for improved tumor antigen presentation. For example, Ying et al utilized electrostatic forces to load tumor cell lysates onto polydopamine nanoparticles (NPs), creating TCLN nanovaccine (Figure 4A). This nanovaccine, in conjunction with an alginic hydrogel loaded with Endostar, effectively enhanced DC-mediated antigen presentation. In their study, the sustained release of Endostar from the alginic hydrogel inhibited tumor angiogenesis, significantly boosting CD8+ and CD4+ T cells number in the spleen, draining lymph nodes, and tumors. Precise delivery of the nanovaccine substantially enhanced DCs maturation and CTL lytic activity, as evidenced by increased MHC expression on DCs and upregulation of IL-12 levels within the tumor.101 Furthermore, TME-induced tumor metabolic reprogramming plays a negative regulatory role in the “vascular-immune” crosstalk. Specifically, metabolites released by tumor cells, such as lactate and Kyn, promote tumor vessels formation and inhibit DCs function.102,103 Notably, tumor microvascular density is positively correlated with IDO expression, which promotes luminal formation of ECs and tryptophan depletion.104 Based on this discovery, researchers designed an antigen carrier (BN@HM-OVA), a pH-sensitive antigen carrier co-loading NLG-919 and ovalbumin (OVA). This carrier, using poly (ethylene glycol)-hydrazide-poly(caprolactone) (PEG-hyd-PCL) copolymers and cationic poly (ethylene imine)-poly(caprolactone) (PEI-PCL) copolymers, released tumor antigens under acidic conditions to enhance DCs antigen presentation through two MHC-I pathways. Additionally, NLG-919 was precisely delivered to tumor cells, depleting tolDCs and inhibiting IDO activity to enhance ICIs immunotherapy.105
 |
Figure 4 Nanosystems with “vascular-immune” crosstalk for antigen presentation. (A) Construction of polydopamine nanovaccine and endostar alginate hydrogel to enhance anti-tumor immune response. Reprinted with permission from Yang Y, Wang N, Tian XX, et al. Synergy of Polydopamine Nanovaccine and Endostar Alginate Hydrogel for Improving Antitumor Immune Responses Against Colon Tumor. Int J Nanomedicine. 2022 17:4791–4805. Doi: 10.2147/IJN.S372048. Copyright © 2022.101 (B) Construction of hydrogel/nanoparticles to combine anti-angiogenesis therapy and immunotherapy. Reprinted from Acta Biomater, 153, Yang Af, Sheng SP, Bai Y, et al. Hydrogel/nanoparticles-mediated cooperative combination of antiangiogenesis and immunotherapy, 124–138, Copyright (2022), with permission from Elsevier.106 |
Macrophages, especially TAMs, also exhibit immunobiological functions, and their association with tumor progression has been observed. The STING pathway plays a critical role in the immune response to multiple cancer treatments, but faces challenges in systemic delivery.107 Kaiting et al developed a nanosystem targeting TAMs to enhance antigen processing and presentation and induce anti-tumor T cell responses. This nanosystem used cyclic dimeric adenosine monophosphate (CDA) polymerized with Zn(NO3)2 through coordination, surface-capped with 1.2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(polyethylene glycol) (DOPA) and encapsulated in DSPE-PEG2000 to form the ZnCDA NPs. This nanosystem enhanced tumor accumulation by disrupting ECs in the tumor vasculature and downregulated lysosomal enzyme-related genes in TAM by activating the endogenous STING signaling pathway, thereby delaying tumor antigen degradation and reinvigorating the anti-tumor activity of ICIs in immunologically “cold” pancreatic and glioma tumor models.108 Afeng et al constructed a hydrogel/nanomaterial system loaded with apatinib (Apa) and cytosine-phosphate-guanine (CpG) to enhance the TAM-mediated antigen presentation (Figure 4B). This nanosystem was prepared by cross-linking BSA and polyethyleneimine (PEI), then complexed with CpG via electrostatic adsorption to create Apa-loaded CpG NPs. These were incorporated into a hydrogel (GEL-Apa-CpG NP) by mixing with a solution of PEG-4000 and α-cyclodextrin (α-CD) containing aCD47. The obtained GEL-Apa-CpG NPs achieved a step-by-step controlled release of antiangiogenic and immunotherapeutic drugs. Notably, aCD47 activates the phagocytic capacity of TAM by binding to SIRPα, and CpG facilitated TAM-mediated antigen presentation through its interaction with the PPR Toll-like receptor 9 (TLR9). Apa-mediated vascular normalization increased immune effector cells in tumors, significantly improving the immune response.106
TILs consist of immune cells both effectors and suppressors, serving as indicators to assess immunotherapy efficacy.109 Vascular-targeted nanotechnology enhances the infiltration of immune effector by normalizing tumor vessels with low-dose antiangiogenic drugs, improving vascular function and the immune microenvironment, thus becoming a common strategy to enhance the efficacy of immunotherapy. Jing Y’s teams used a chemical linkage to graft low-molecular-weight heparin (LMWH), gambogic acid (GA), and F3 peptides to form vascular-targeted micelles (FLGs). These micelles induced vascular normalization, increasing immune effector cells (CD4+ and CD8+ T cells), and enhancing the therapeutic efficacy of CCR inhibitors.110 Nitric oxide (NO), synthesized in ECs, is a multifunctional signaling molecule that mediates angiogenesis and maintains vascular homeostasis and endothelial function. Yun-Chieh Sung et al constructed NanoNO by encapsulating DNIC [Fe(μ-SEt)2(NO)4] into PLGA liposomes with polyethylene glycol (PEG) modification. PLGA controls the sustained release of low-dose NanoNO from DNIC NPs to induce vascular normalization. This nanosystem reprogrammed immunosuppressive TAMs to an immunostimulatory phenotype and facilitated CD4+ and CD8+ T cell infiltration into the tumor tissue. Notably, DNIC also decreased the activity of the Sp1 transcription factor that binds to the PD-L1 promoter and mediates PD-L1 expression, thereby enhancing cancer vaccines efficacy.111
Conclusion
Increasing evidence suggests that cancer immunotherapy is encountering challenges, including the immune evasion mechanisms and heterogeneity of tumor, immune-related adverse events and resistance, ultimately affecting its clinical efficacy. Thus, continuous exploration of immune tolerance mechanisms and novel immunotherapy targets holds promise for addressing these limitations. In this review, abnormal tumor vessels were considered as critical factor in impeding the infiltration and functionality of immune cells and contributing to the development of an unfavorable TME. The interaction between the vascular and immune systems, known as “vascular-immune” crosstalk, highlights the significance of tumor vessels in the context of immunotherapy. Despite the promising results observed in clinical studies of “vascular-immune” therapy, this therapeutic strategy encounters various obstacles. Firstly, the diverse variations among individual patients pose a challenge in guaranteeing optimal benefits for each patient, underscoring the need for the development of an improved method to identify those likely to benefit from combined treatment. Furthermore, the therapeutic effects of combination therapy may decline over time. Hence, there is a pressing need for enhanced methods to determine the treatment timing to maximize the effectiveness of combination treatment. Additionally, both immunotherapy and anti-angiogenic therapy drugs can trigger a series of side effects and toxicities, and researchers can direct their attention towards understanding and effectively managing the potential adverse reactions arising from combination therapy. Finally, combining anti-angiogenic drugs with immunotherapy can be costly, potentially restricting patient access to this treatment option. Furthermore, certain novel therapeutic drugs might not be universally accessible across all geographic regions or healthcare facilities, posing challenges for patients seeking the latest treatment modalities.
More importantly, although tailored nanosystems have shown effectiveness in enhancing the “vascular-immune” therapy, they have not yet received clinical approval, necessitating further research. Firstly, the distinguishing between various tumor types and stages during recruitment, animal model selection and treatment evaluation is needed, where the majority of clinical and animal studies focused on anti-angiogenesis and immune-related adverse reactions. Secondly, considering the mutational and heterogeneous nature of tumors, researchers can focus on drug concentrations of nanodrugs in different tumors, including mutants, to evaluate the tumor tissue specificity of nanosystems. Additionally, active agents that could trigger immune-related adverse reactions in non-target tissues need doubtlessly to be explored. Finally, clinical application considerations are necessary to facilitate translational research. All in all, the “vascular-immune” therapy remains a crucial and unmet clinical need, yet our current understanding of its underlying mechanisms only scratches the surface. Thus, subsequent research endeavors will concentrate on extensively exploring these mechanisms, refining clinical trial designs, and optimizing treatment strategies. We hope this review will inspire the design and fabrication of tailored drugs and nanosystems for “vascular-immune” therapy, propelling this amalgamated treatment approach into tangible clinical applications, thereby enhancing the treatment efficacy for individuals grappling with cancer.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No. 82003660), Zhejiang Provincial Natural Science Foundation of China (LQ23H300007), and the Zhejiang Pharmaceutical Association Hospital Pharmacy Special Scientific Research Funding Project (2019ZYY02).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Shen BJ, Lo WC, Lin HH. Global burden of tuberculosis attributable to cancer in 2019: global, regional, and national estimates. J Microbiol Immunol Infect. 2022;55(2):266–272. doi:10.1016/j.jmii.2021.02.005
2. Wu H, Fu X, Zhai Y, Gao S, Yang X, Zhai G. Development of effective tumor vaccine strategies based on immune response cascade reactions. Adv Healthc Mater. 2021;10(13):e2100299. doi:10.1002/adhm.202100299
3. Ruff SM, Manne A, Cloyd JM, Dillhoff M, Ejaz A, Pawlik TM. Current landscape of immune checkpoint inhibitor therapy for hepatocellular carcinoma. Curr Oncol. 2023;30(6):5863–5875. doi:10.3390/curroncol30060439
4. Sarmento-Ribeiro AB, Scorilas A, Goncalves AC, Efferth T, Trougakos IP. The emergence of drug resistance to targeted cancer therapies: clinical evidence. Drug Resist Updat. 2019;47:100646. doi:10.1016/j.drup.2019.100646
5. Dolladille C, Ederhy S, Sassier M, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. 2020;6(6):865–871. doi:10.1001/jamaoncol.2020.0726
6. Bonaventura P, Shekarian T, Alcazer V, et al. Cold tumors: a therapeutic challenge for immunotherapy. Front Immunol. 2019;10:168. doi:10.3389/fimmu.2019.00168
7. Wang JX, Choi SYC, Niu X, et al. Lactic acid and an acidic tumor microenvironment suppress anticancer immunity. Int J Mol Sci. 2020;21(21):8363. doi:10.3390/ijms21218363
8. Liu Z, Zhou Z, Dang Q, et al. Immunosuppression in tumor immune microenvironment and its optimization from CAR-T cell therapy. Theranostics. 2022;12(14):6273–6290. doi:10.7150/thno.76854
9. Wei X, Chen Y, Jiang X, et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol Cancer. 2021;20(1):7. doi:10.1186/s12943-020-01288-1
10. Hao X, Ren Y, Feng M, Wang Q, Wang Y. Metabolic reprogramming due to hypoxia in pancreatic cancer: implications for tumor formation, immunity, and more. Biomed Pharmacother. 2021;141:111798. doi:10.1016/j.biopha.2021.111798
11. Chauhan VP, Stylianopoulos T, Boucher Y, Jain RK. Delivery of molecular and nanoscale medicine to tumors: transport barriers and strategies. Annu Rev Chem Biomol. 2011;2(1):281–298. doi:10.1146/annurev-chembioeng-061010-114300
12. Ghosh M, Lenkiewicz AM, Kaminska B. The interplay of tumor vessels and immune cells affects immunotherapy of glioblastoma. Biomedicines. 2022;10(9):2292. doi:10.3390/biomedicines10092292
13. Missiaen R, Mazzone M, Bergers G. The reciprocal function and regulation of tumor vessels and immune cells offers new therapeutic opportunities in cancer. Semin Cancer Biol. 2018;52(Pt 2):107–116. doi:10.1016/j.semcancer.2018.06.002
14. Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. 2019;18(1):60. doi:10.1186/s12943-019-0974-6
15. McDermott DF, Huseni MA, Atkins MB, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24(6):749–757. doi:10.1038/s41591-018-0053-3
16. Luo X, Zou W, Wei Z, et al. Inducing vascular normalization: a promising strategy for immunotherapy. Int Immunopharmacol. 2022;112:109167. doi:10.1016/j.intimp.2022.109167
17. Yu P, Wang Y, Yuan D, Sun Y, Qin S, Li T. Vascular normalization: reshaping the tumor microenvironment and augmenting antitumor immunity for ovarian cancer. Front Immunol. 2023;14:1276694. doi:10.3389/fimmu.2023.1276694
18. Jain RK, Carmeliet P. SnapShot: tumor angiogenesis. Cell. 2012;149(6):1408–1408e1. doi:10.1016/j.cell.2012.05.025
19. Detmar M. Tumor angiogenesis. J Investig Dermatol Symp Proc. 2000;5(1):20–23. doi:10.1046/j.1087-0024.2000.00003.x
20. Li T, Kang G, Wang T, Huang H. Tumor angiogenesis and anti-angiogenic gene therapy for cancer. Oncol Lett. 2018;16(1):687–702. doi:10.3892/ol.2018.8733
21. Bhat SM, Badiger VA, Vasishta S, Chakraborty J, Joshi MB. 3D tumor angiogenesis models: recent advances and challenges. J Cancer Res Clin Oncol. 2021;147(12):3477–3494. doi:10.1007/s00432-021-03814-0
22. Patel SA, Nilsson MB, Le X, Cascone T, Jain RK, Heymach JV. Molecular Mechanisms and Future Implications of VEGF/VEGFR in Cancer Therapy. Clin Cancer Res. 2023;29(1):30–39. doi:10.1158/1078-0432.CCR-22-1366
23. Watabe T, Takahashi K, Pietras K, Yoshimatsu Y. Roles of TGF-beta signals in tumor microenvironment via regulation of the formation and plasticity of vascular system. Semin Cancer Biol. 2023;92:130–138. doi:10.1016/j.semcancer.2023.04.007
24. Cao Y, Sonveaux P, Liu S, et al. Systemic overexpression of angiopoietin-2 promotes tumor microvessel regression and inhibits angiogenesis and tumor growth. Cancer Res. 2007;67(8):3835–3844. doi:10.1158/0008-5472.CAN-06-4056
25. Majidpoor J, Mortezaee K. Angiogenesis as a hallmark of solid tumors - clinical perspectives. Cell Oncol. 2021;44(4):715–737. doi:10.1007/s13402-021-00602-3
26. Wang X, Zhang H, Chen X, et al. Overcoming tumor microenvironment obstacles: current approaches for boosting nanodrug delivery. Acta Biomater. 2023;166:42–68. doi:10.1016/j.actbio.2023.05.043
27. Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74(2–3):72–84. doi:10.1016/j.mvr.2007.05.003
28. Barua S, Mitragotri S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano Today. 2014;9(2):223–243. doi:10.1016/j.nantod.2014.04.008
29. Shi Y, van der Meel R, Chen X, Lammers T. The EPR effect and beyond: strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics. 2020;10(17):7921–7924. doi:10.7150/thno.49577
30. Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891–906. doi:10.1016/j.cell.2009.10.027
31. Thorne RG, Lakkaraju A, Rodriguez-Boulan E, Nicholson C. In vivo diffusion of lactoferrin in brain extracellular space is regulated by interactions with heparan sulfate. Proc Natl Acad Sci U S A. 2008;105(24):8416–8421. doi:10.1073/pnas.0711345105
32. Wang X, Zhang W, Zeng S, Wang L, Wang B. Collagenase type I and probucol-loaded nanoparticles penetrate the extracellular matrix to target hepatic stellate cells for hepatic fibrosis therapy. Acta Biomater. 2023;2023:1. doi:10.1016/j.actbio.2023.12.027
33. Dong Q, Zhou C, Ren H, et al. Lactate-induced MRP1 expression contributes to metabolism-based etoposide resistance in non-small cell lung cancer cells. Cell Commun Signal. 2020;18(1):167. doi:10.1186/s12964-020-00653-3
34. Zhu J, Wang X, Su Y, et al. Multifunctional nanolocks with GSH as the key for synergistic ferroptosis and anti-chemotherapeutic resistance. Biomaterials. 2022;288:121704. doi:10.1016/j.biomaterials.2022.121704
35. Real E, Kaiser A, Raposo G, Amara A. Immature dendritic cells (DCs) use chemokines and intercellular adhesion molecule (ICAM)-1, but not DC-specific ICAM-3-grabbing nonintegrin, to stimulate CD4+ T cells in the absence of exogenous antigen. J Immunol. 2004;173(1):50–60. doi:10.4049/jimmunol.173.1.50
36. Dings RP, Vang KB, Castermans K, et al. Enhancement of T-cell-mediated antitumor response: angiostatic adjuvant to immunotherapy against cancer. Clin Cancer Res. 2011;17(10):3134–3145. doi:10.1158/1078-0432.CCR-10-2443
37. Singh V, Kaur R, Kumari P, Pasricha C, Singh R. ICAM-1 and VCAM-1: gatekeepers in various inflammatory and cardiovascular disorders. Clin Chim Acta. 2023;548:117487. doi:10.1016/j.cca.2023.117487
38. Mattila P, Majuri ML, Mattila PS, Renkonen R. TNF alpha-induced expression of endothelial adhesion molecules, ICAM-1 and VCAM-1, is linked to protein kinase C activation. Scand J Immunol. 1992;36(2):159–165. doi:10.1111/j.1365-3083.1992.tb03087.x
39. Xia P, Gamble JR, Rye KA, et al. Tumor necrosis factor-alpha induces adhesion molecule expression through the sphingosine kinase pathway. Proc Natl Acad Sci U S A. 1998;95(24):14196–14201. doi:10.1073/pnas.95.24.14196
40. Liu B, Gao J, Lyu BC, et al. Expressions of TGF-beta2, bFGF and ICAM-1 in lens epithelial cells of complicated cataract with silicone oil tamponade. Int J Ophthalmol. 2017;10(7):1034–1039. doi:10.18240/ijo.2017.07.03
41. Motz GT, Santoro SP, Wang LP, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20(6):607–615. doi:10.1038/nm.3541
42. Buckanovich RJ, Facciabene A, Kim S, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14(1):28–36. doi:10.1038/nm1699
43. Norling LV, Sampaio AL, Cooper D, Perretti M. Inhibitory control of endothelial galectin-1 on in vitro and in vivo lymphocyte trafficking. FASEB J. 2008;22(3):682–690. doi:10.1096/fj.07-9268com
44. Ding XC, Wang LL, Zhang XD, et al. The relationship between expression of PD-L1 and HIF-1alpha in glioma cells under hypoxia. J Hematol Oncol. 2021;14(1):92. doi:10.1186/s13045-021-01102-5
45. Tan J, Fan W, Liu T, et al. TREM2(+) macrophages suppress CD8(+) T-cell infiltration after transarterial chemoembolisation in hepatocellular carcinoma. J Hepatol. 2023;79(1):126–140. doi:10.1016/j.jhep.2023.02.032
46. Bai R, Li Y, Jian L, Yang Y, Zhao L, Wei M. The hypoxia-driven crosstalk between tumor and tumor-associated macrophages: mechanisms and clinical treatment strategies. Mol Cancer. 2022;21(1):177. doi:10.1186/s12943-022-01645-2
47. Li J, Qiao H, Wu F, et al. A novel hypoxia- and lactate metabolism-related signature to predict prognosis and immunotherapy responses for breast cancer by integrating machine learning and bioinformatic analyses. Front Immunol. 2022;13:998140. doi:10.3389/fimmu.2022.998140
48. Watson MJ, Vignali PDA, Mullett SJ, et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature. 2021;591(7851):645–651. doi:10.1038/s41586-020-03045-2
49. Zhang Y, Zhai Z, Duan J, et al. Lactate: the mediator of metabolism and immunosuppression. Front Endocrinol. 2022;13:901495. doi:10.3389/fendo.2022.901495
50. Mu X, Shi W, Xu Y, et al. Tumor-derived lactate induces M2 macrophage polarization via the activation of the ERK/STAT3 signaling pathway in breast cancer. Cell Cycle. 2018;17(4):428–438. doi:10.1080/15384101.2018.1444305
51. Engin AB, Engin A. Indoleamine 2,3-dioxygenase activity-induced acceleration of tumor growth, and protein kinases-related novel therapeutics regimens. Adv Exp Med Biol. 2021;1275:339–356. doi:10.1007/978-3-030-49844-3_13
52. Herbert A, Ng H, Jessup W, Kockx M, Cartland S, Thomas SR. Hypoxia regulates the production and activity of glucose transporter-1 and indoleamine 2,3-dioxygenase in monocyte-derived endothelial-like cells: possible relevance to infantile haemangioma pathogenesis. Br J Dermatol. 2011;164(2):308–315. doi:10.1111/j.1365-2133.2010.10086.x
53. Groth C, Hu X, Weber R, et al. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. 2019;120(1):16–25. doi:10.1038/s41416-018-0333-1
54. Skiba W, Suszczyk D, Pawlowska A, Wlodarczyk K, Panczyszyn A, Wertel I. Clinical Significance of Tie-2-expressing monocytes/macrophages and angiopoietins in the progression of ovarian cancer-state-of-The-Art. Cells. 2022;11(23):3851. doi:10.3390/cells11233851
55. Zhong T, Zhang W, Guo H, Pan X, Chen B, He Q. The regulatory and modulatory roles of TRP family channels in malignant tumors and relevant therapeutic strategies. Acta Pharm Sin B. 2022;12(4):1761–1780. doi:10.1016/j.apsb.2021.11.001
56. Tian L, Goldstein A, Wang H, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544(7649):250–254. doi:10.1038/nature21724
57. Sojka DK, Fowell DJ. Regulatory T cells inhibit acute IFN-gamma synthesis without blocking T-helper cell type 1 (Th1) differentiation via a compartmentalized requirement for IL-10. Proc Natl Acad Sci U S A. 2011;108(45):18336–18341. doi:10.1073/pnas.1110566108
58. Wenes M, Shang M, Di Matteo M, et al. Macrophage metabolism controls tumor blood vessel morphogenesis and metastasis. Cell Metab. 2016;24(5):701–715. doi:10.1016/j.cmet.2016.09.008
59. Watkins SK, Egilmez NK, Suttles J, Stout RD. IL-12 rapidly alters the functional profile of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. J Immunol. 2007;178(3):1357–1362. doi:10.4049/jimmunol.178.3.1357
60. He Y, Hung SW, Liang B, et al. Receptor tyrosine kinase inhibitor sunitinib as novel immunotherapy to inhibit myeloid-derived suppressor cells for treatment of endometriosis. Front Immunol. 2021;12:641206. doi:10.3389/fimmu.2021.641206
61. Finke JH, Rini B, Ireland J, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14(20):6674–6682. doi:10.1158/1078-0432.CCR-07-5212
62. Yang Y, Wang C, Sun H, Jiang Z, Zhang Y, Pan Z. Apatinib prevents natural killer cell dysfunction to enhance the efficacy of anti-PD-1 immunotherapy in hepatocellular carcinoma. Cancer Gene Ther. 2021;28(1–2):89–97. doi:10.1038/s41417-020-0186-7
63. Cai X, Wei B, Li L, et al. Apatinib enhanced anti-PD-1 therapy for colon cancer in mice via promoting PD-L1 expression. Int Immunopharmacol. 2020;88:106858. doi:10.1016/j.intimp.2020.106858
64. Luo HQ, He YF, Chen WJ, et al. Effect of apatinib on serum CD4+CD25+ T cells, NK Cells, and T cells subgroup in malignant tumor. Technol Cancer Res Treat. 2019;18:1533033819893667. doi:10.1177/1533033819893667
65. Umeyama Y, Shibasaki Y, Akaza H. Axitinib in metastatic renal cell carcinoma: beyond the second-line setting. Future Oncol. 2017;13(21):1839–1852. doi:10.2217/fon-2017-0104
66. Du Four S, Maenhout SK, De Pierre K, et al. Axitinib increases the infiltration of immune cells and reduces the suppressive capacity of monocytic MDSCs in an intracranial mouse melanoma model. Oncoimmunology. 2015;4(4):e998107 doi:10.1080/2162402X.2014.998107.
67. Yi C, Chen L, Lin Z, et al. Lenvatinib targets FGF Receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology. 2021;74(5):2544–2560. doi:10.1002/hep.31921
68. Elamin YY, Rafee S, Toomey S, Hennessy BT. Immune effects of bevacizumab: killing two birds with one stone. Cancer Microenviron. 2015;8(1):15–21. doi:10.1007/s12307-014-0160-8
69. Terme M, Pernot S, Marcheteau E, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73(2):539–549. doi:10.1158/0008-5472.CAN-12-2325
70. Tada Y, Togashi Y, Kotani D, et al. Targeting VEGFR2 with Ramucirumab strongly impacts effector/ activated regulatory T cells and CD8(+) T cells in the tumor microenvironment. J Immunother Cancer. 2018;6(1):106. doi:10.1186/s40425-018-0403-1
71. Bhandarkar SS, Arbiser JL. Curcumin as an inhibitor of angiogenesis. Adv Exp Med Biol. 2007;595:185–195. doi:10.1007/978-0-387-46401-5_7.
72. Wang Y, Lu J, Jiang B, Guo J. The roles of curcumin in regulating the tumor immunosuppressive microenvironment. Oncol Lett. 2020;19(4):3059–3070. doi:10.3892/ol.2020.11437
73. Cullberg KB, Olholm J, Paulsen SK, et al. Resveratrol has inhibitory effects on the hypoxia-induced inflammation and angiogenesis in human adipose tissue in vitro. Eur J Pharm Sci. 2013;49(2):251–257. doi:10.1016/j.ejps.2013.02.014
74. Hu WH, Chan GK, Duan R, et al. Synergy of ginkgetin and resveratrol in suppressing VEGF-induced angiogenesis: a therapy in treating colorectal cancer. Cancers. 2019;11(12):1828. doi:10.3390/cancers11121828
75. Yang Y, Paik JH, Cho D, Cho JA, Kim CW. Resveratrol induces the suppression of tumor-derived CD4+CD25+ regulatory T cells. Int Immunopharmacol. 2008;8(4):542–547. doi:10.1016/j.intimp.2007.12.006
76. Chen L, Yang S, Liao W, Xiong Y. Modification of antitumor immunity and tumor microenvironment by resveratrol in mouse renal tumor model. Cell Biochem Biophys. 2015;72(2):617–625. doi:10.1007/s12013-015-0513-z
77. Zhu XX, Yang L, Li YJ, et al. Effects of sesquiterpene, flavonoid and coumarin types of compounds from Artemisia annua L. on production of mediators of angiogenesis. Pharmacol Rep. 2013;65(2):410–420. doi:10.1016/S1734-1140(13)71016-8
78. Cao Y, Feng Y-H, Gao L-W, et al. Artemisinin enhances the anti-tumor immune response in 4T1 breast cancer cells in vitro and in vivo. Int Immunopharmacol. 2019;70:110–116. doi:10.1016/j.intimp.2019.01.041
79. Jiang X, Zhou J, Lin Q, et al. Anti-angiogenic and anticancer effects of baicalein derivatives based on transgenic zebrafish model. Bioorg Med Chem. 2018;26(15):4481–4492. doi:10.1016/j.bmc.2018.07.037
80. Ke M, Zhang Z, Xu B, et al. Baicalein and baicalin promote antitumor immunity by suppressing PD-L1 expression in hepatocellular carcinoma cells. Int Immunopharmacol. 2019;75:105824. doi:10.1016/j.intimp.2019.105824
81. Romano A, Martel F. The Role of EGCG in breast cancer prevention and therapy. Mini Rev Med Chem. 2021;21(7):883–898. doi:10.2174/1389557520999201211194445
82. Ogawa K, Hara T, Shimizu M, et al. Moriwaki, (-)-Epigallocatechin gallate inhibits the expression of indoleamine 2,3-dioxygenase in human colorectal cancer cells. Oncol Lett. 2012;4(3):546–550. doi:10.3892/ol.2012.761
83. Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82(12):1807–1821. doi:10.1016/j.bcp.2011.07.093
84. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi:10.1056/NEJMoa1716948
85. Motzer RJ, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): long-term follow-up results from an open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(7):888–898. doi:10.1016/S1470-2045(22)00290-X
86. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127. doi:10.1056/NEJMoa1816714
87. Kurtz J-E, Pujade-Lauraine E, Oaknin A, et al.; A.T.E.-o. Investigators. Atezolizumab combined with bevacizumab and platinum-based therapy for platinum-sensitive ovarian cancer: placebo-controlled randomized phase III ATALANTE/ENGOT-ov29 trial. J Clin Oncol. 2023;41(30):4768–4778. doi:10.1200/JCO.23.00529
88. Lu S, Wu L, Jian H, et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): first interim results from a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2022;23(9):1167–1179. doi:10.1016/S1470-2045(22)00382-5
89. Dada S, Demartines N, Dormond O. mTORC2 regulates PGE2-mediated endothelial cell survival and migration. Biochem Biophys Res Commun. 2008;372(4):875–879. doi:10.1016/j.bbrc.2008.05.154
90. Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375(9):819–829. doi:10.1056/NEJMoa1604958
91. Ahmed A, Tait SWG. Targeting immunogenic cell death in cancer. Mol Oncol. 2020;14(12):2994–3006. doi:10.1002/1878-0261.12851
92. Shiraishi Y, Kishimoto J, Shimose T, Toi Y, Sugawara S, Okamoto I. Phase II study of carboplatin/nab-paclitaxel/atezolizumab combination therapy for advanced nonsquamous non-small cell lung cancer patients with impaired renal function: RESTART trial. BMC Cancer. 2022;22(1):964. doi:10.1186/s12885-022-10056-x
93. Livingston MB, Jagosky MH, Robinson MM, et al. Phase II study of pembrolizumab in combination with doxorubicin in metastatic and unresectable soft-tissue sarcoma. Clin Cancer Res. 2021;27(23):6424–6431. doi:10.1158/1078-0432.CCR-21-2001
94. Wang Z, Sun C, Wu H, et al. Cascade targeting codelivery of ingenol-3-angelate and doxorubicin for enhancing cancer chemoimmunotherapy through synergistic effects in prostate cancer. Mater Today Bio. 2022;13:100189. doi:10.1016/j.mtbio.2021.100189
95. Zhou Z, Chen J, Liu Y, et al. Cascade two-stage tumor re-oxygenation and immune re-sensitization mediated by self-assembled albumin-sorafenib nanoparticles for enhanced photodynamic immunotherapy. Acta Pharm Sin B. 2022;12(11):4204–4223. doi:10.1016/j.apsb.2022.07.023
96. Wen Z, Liu H, Qiao D, et al. Nanovaccines fostering tertiary lymphoid structure to attack mimicry nasopharyngeal carcinoma. ACS Nano. 2023;17(8):7194–7206. doi:10.1021/acsnano.2c09619
97. Xu Z, Ramishetti S, Tseng Y-C, Guo S, Wang Y, Huang L. Multifunctional nanoparticles co-delivering Trp2 peptide and CpG adjuvant induce potent cytotoxic T-lymphocyte response against melanoma and its lung metastasis. J Control Release. 2013;172(1):259–265. doi:10.1016/j.jconrel.2013.08.021
98. Huo M, Zhao Y, Satterlee AB, Wang Y, Xu Y, Huang L. Tumor-targeted delivery of sunitinib base enhances vaccine therapy for advanced melanoma by remodeling the tumor microenvironment. J Control Release. 2017;245:81–94. doi:10.1016/j.jconrel.2016.11.013
99. Irvine DJ, Swartz MA, Szeto GL. Engineering synthetic vaccines using cues from natural immunity. Nat Mater. 2013;12(11):978–990. doi:10.1038/nmat3775
100. Yang K, Halima A, Chan TA. Antigen presentation in cancer - mechanisms and clinical implications for immunotherapy. Nat Rev Clin Oncol. 2023;20(9):604–623. doi:10.1038/s41571-023-00789-4
101. Yang Y, Wang N, Tian X, Wang X, Yang J. Synergy of polydopamine nanovaccine and endostar alginate hydrogel for improving antitumor immune responses against colon tumor. Int J Nanomed. 2022;20:4791–4805. doi:10.2147/IJN.S372048
102. Hirschhaeuser F, Sattler UG, Mueller-Klieser W. Lactate: a metabolic key player in cancer. Cancer Res. 2011;71(22):6921–6925. doi:10.1158/0008-5472.CAN-11-1457
103. Liu XH, Zhai XY. Role of tryptophan metabolism in cancers and therapeutic implications. Biochimie. 2021;182:131–139. doi:10.1016/j.biochi.2021.01.005
104. Wei L, Zhu S, Li M, Liu F, Wei F. High Indoleamine 2,3-dioxygenase is correlated with microvessel density and worse prognosis in breast cancer. Front Immunol. 2018;9:724. doi:10.3389/fimmu.2018.00724
105. Xie X, Feng Y, Zhang H, et al. Remodeling tumor immunosuppressive microenvironment via a novel bioactive nanovaccines potentiates the efficacy of cancer immunotherapy. Bioact Mater. 2022;16:107–119. doi:10.1016/j.bioactmat.2022.03.008
106. Yang A, Sheng S, Bai Y, et al. Hydrogel/nanoparticles-mediated cooperative combination of antiangiogenesis and immunotherapy. Acta Biomater. 2022;153:124–138. doi:10.1016/j.actbio.2022.09.060
107. Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461(7265):788–792. doi:10.1038/nature08476
108. Yang K, Han W, Jiang X, et al. Zinc cyclic di-AMP nanoparticles target and suppress tumours via endothelial STING activation and tumour-associated macrophage reinvigoration. Nat Nanotechnol. 2022;17(12):1322–1331. doi:10.1038/s41565-022-01225-x
109. Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4(1):59. doi:10.1186/s40425-016-0165-6
110. Deng Y, Jiang Z, Jin Y, et al. Reinforcing vascular normalization therapy with a bi-directional nano-system to achieve therapeutic-friendly tumor microenvironment. J Control Release. 2021;340:87–101. doi:10.1016/j.jconrel.2021.10.016
111. Sung YC, Jin PR, Chu LA, et al. Delivery of nitric oxide with a nanocarrier promotes tumour vessel normalization and potentiates anti-cancer therapies. Nat Nanotechnol. 2019;14(12):1160–1169. doi:10.1038/s41565-019-0570-3
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

