Back to Journals » ClinicoEconomics and Outcomes Research » Volume 16
Clinical and Economic Impact of Early Diagnosis of Chronic Kidney Disease in General Practice: The Endorse Study
Authors Pesce F, Bruno GM , Colombo GL , Di Matteo S, Maurizi AR, Mongelli V, Mele S, Narici L, Bianchi S , Bonomini M, Castellano G, De Nicola L, Gambaro G, Grandaliano G, La Manna G , Pani A, Ranghino A, Gesualdo L
Received 28 May 2024
Accepted for publication 29 July 2024
Published 5 August 2024 Volume 2024:16 Pages 547—555
DOI https://doi.org/10.2147/CEOR.S470728
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Samer Hamidi
Francesco Pesce,1 Giacomo Matteo Bruno,2 Giorgio Lorenzo Colombo,2 Sergio Di Matteo,3 Anna Rita Maurizi,4 Valentina Mongelli,4 Silvia Mele,5 Lavinia Narici,5 Stefano Bianchi,6 Mario Bonomini,7 Giuseppe Castellano,8 Luca De Nicola,9 Giovanni Gambaro,10 Giuseppe Grandaliano,11 Gaetano La Manna,12 Antonello Pani,13 Andrea Ranghino,14 Loreto Gesualdo15
1Division of Renal Medicine, “Fatebenefratelli Isola Tiberina—Gemelli Isola”, Rome, 00186, Italy; 2Department of Drug Sciences, University of Pavia, Pavia, Italy; 3S.A.V.E. Studi Analisi Valutazioni Economiche, Milan, Italy; 4Cardiovascular, Renal and Metabolism Medical Affairs, AstraZeneca, Milan, Italy; 5Value & Access, AstraZeneca, Milan, Italy; 6Nephrology and Dialysis Unit, Department of Internal Medicine, ASL Toscana Nordovest, Regione Toscana, Livorno, Italy; 7Department of Medicine, Section of Nephrology and Dialysis, G. D’Annunzio University, Chieti, 66013, Italy; 8UOC of Nephrology, Dialysis, and Kidney Transplant, IRCCS Foundation Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; 9Department of Advanced Medical and Surgical Sciences, Nephrology and Dialysis Unit, University Vanvitelli, Naples, Italy; 10Division of Nephrology and Dialysis, Department of Medicine, University of Verona, Verona, Italy; 11Department of Translational Medicine and Surgery, Università Cattolica Sacro Cuore, Rome, Italy; 12Nephrology Dialysis and Kidney Transplant Unit, IRCCS Azienda Ospedaliero-Universitaria Di Bologna, Bologna, Italy; 13Department of Nephrology and Dialysis, G. Brotzu Hospital, Cagliari, Italy; 14Nephrology, Dialysis and Kidney Transplant Unit, Ospedali Riuniti Ancona, Ancona, Italy; 15Nephrology, Dialysis and Transplantation Unit, Department of Precision and Regenerative Medicine and Ionian Area (Dimepre-J), University of Bari “aldo Moro”, Bari, Italy
Correspondence: Giorgio Lorenzo Colombo, Email [email protected]
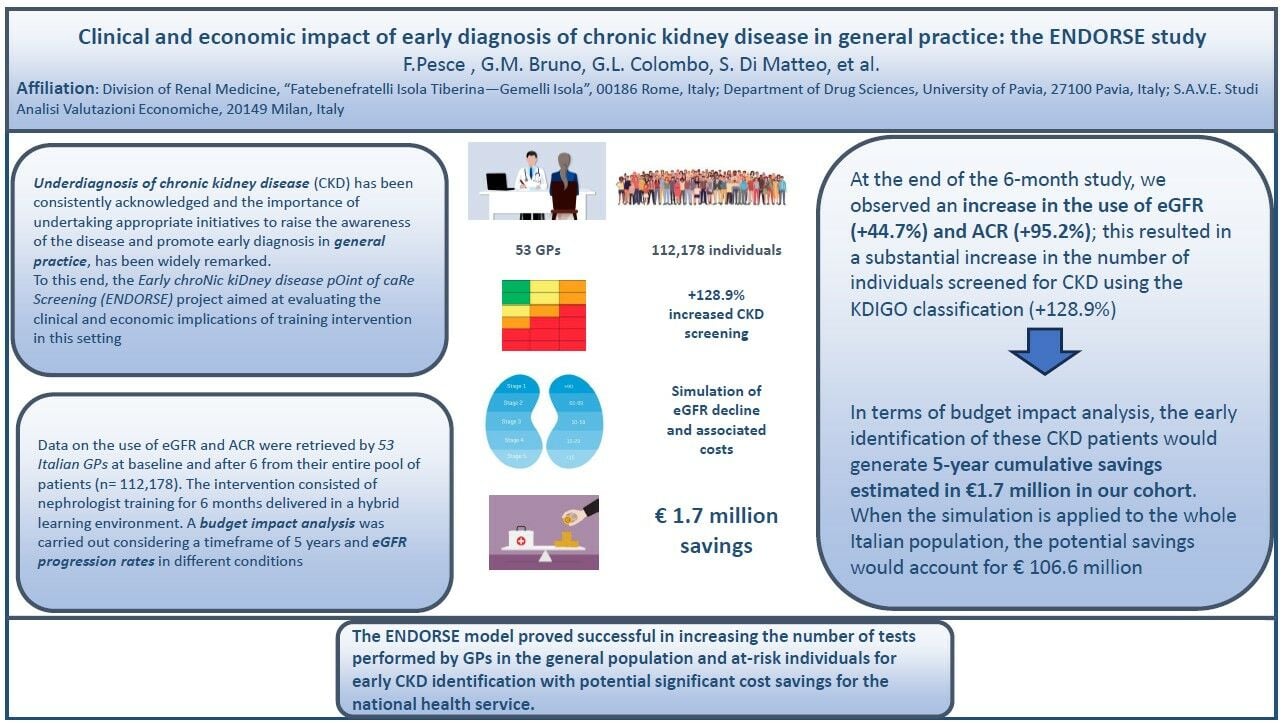
Introduction: The underdiagnosis of chronic kidney disease (CKD) remains a significant public health concern. The Early chroNic kiDney disease pOint of caRe Screening (ENDORSE) project aimed to evaluate the clinical and economic implications of a targeted training intervention for general practitioners (GPs) to enhance CKD awareness and early diagnosis.
Methods: Data on estimated Glomerular Filtration Rate (eGFR) and Urinary Albumin-Creatinine Ratio (uACR) were collected by 53 Italian GPs from 112,178 patients at baseline and after six months. The intervention involved six months of hybrid training provided by 11 nephrologists, which included formal lectures, instant messaging support, and joint visits for complex cases.
Results: The results demonstrated a substantial increase in the use of eGFR (+44.7%) and uACR (+95.2%) tests. This led to a 128.9% rise in the number of individuals screened for CKD using the KDIGO classification, resulting in a 62% increase in CKD diagnoses. The intervention’s impact was particularly notable in high-risk groups, including patients with type 2 diabetes, hypertension, and heart failure.
Discussion: A budget impact analysis projected cumulative five-year savings of € 1.7 million for the study cohort. When these findings were extrapolated to the entire Italian CKD population, potential savings were estimated at € 106.6 million, highlighting significant cost savings for the national health service. The clinical simulation assumed that early diagnosed CKD patients would be treated according to current indications for dapagliflozin, which slows disease progression.
Conclusion: The ENDORSE model demonstrated that targeted training for GPs can significantly improve early CKD detection, leading to better patient outcomes and considerable economic benefits. This approach shows promise for broader implementation to address the underdiagnosis of CKD on a national and potentially international scale.
Keywords: eGFR, uACR, awareness, chronic kidney disease, general practice, economic impact, training intervention, early diagnosis
Graphical Abstract:
Introduction
Underdiagnosis of chronic kidney disease (CKD) has been consistently acknowledged, and the importance of undertaking appropriate initiatives to raise awareness of the disease and promote early diagnosis in general practice has been widely recognized.1,2 CKD, as a chronic condition characterized by a gradual loss of kidney function over time, is closely comorbid with other diseases such as hypertension, diabetes, and heart disease. These comorbidities often exacerbate the progression and severity of CKD, making early diagnosis and management even more critical. The presence of these comorbidities, with CKD as a pivotal factor, can significantly affect both the prognosis of the individual and healthcare expenditure.3
In the European Union (EU), 10% of the adult population is estimated to be affected by CKD, meaning more than 55 million individuals. As a result, CKD prevalence has remarkably outpaced other non-communicable diseases, including diabetes and cancer.4 This high prevalence underscores the urgent need for effective public health strategies and healthcare interventions to address CKD.
The economic burden of CKD is substantial and multifaceted, encompassing both direct and indirect costs. Direct costs are primarily associated with medical care, including hospitalizations, medications, and dialysis treatments. Indirect costs arise from loss of productivity, disability, and premature mortality. Patients with CKD incur 85% higher costs and 50% higher government subsidies than those without CKD.3,5 Although most of the costs per patient in the CKD population are related to end-stage renal disease (ESRD), earlier stages also generate certain costs, mainly by inducing cardiovascular events.6 Consequently, CKD is among the most expensive diseases for EU health systems, estimated to cost about €140 billion per year.
Early disease detection is crucial in mitigating the adverse outcomes of CKD and is significantly enhanced by better awareness among at-risk populations and healthcare professionals.3 However, in Western countries, awareness of CKD remains scarce in both the general population and among general practitioners (GPs).7,8 This lack of awareness leads to delayed diagnoses and treatment, worsening patient outcomes and increasing healthcare costs.
Early identification of CKD followed by risk stratification and treatment offers the potential to substantially reduce the morbidity and mortality associated with CKD and its related complications, such as cardiovascular disease.9 Given the severe implications of CKD for population health and the economic sustainability of healthcare systems globally, it is vital to establish and implement early-detection and prevention programs, starting in the primary care setting.
“The Disease Awareness Innovation Network (DANTE)” was a successful pilot study that tested whether targeted training for GPs by nephrologists could increase CKD awareness, thereby increasing the proportion of patients tested for estimated glomerular filtration rate (eGFR) and (Albumin-Creatinine Ratio) (uACR).10 The DANTE study reported a significant increase in the number of both tests and, consequently, in the number of people diagnosed with CKD, with remarkable results particularly in at-risk individuals with diabetes, hypertension, and heart failure.
Building on the success of the DANTE study, the ENDORSE (Early chroNic kiDney disease pOint of caRe Screening) project was developed to evaluate the clinical and economic impact of enhanced diagnostic performance in primary care on a larger scale. With ENDORSE, we strengthened the study framework by enrolling a greater number of GPs and analyzing data from over 100,000 individuals. Additionally, to simulate the potential economic benefits of early diagnosis, a budget impact analysis was carried out in the enrolled population with a timeframe of 5 years and varying eGFR decline rates.
Materials and Methods
Study Design
The primary endpoint of this single-arm non-randomized study was to evaluate the impact in primary care of targeted training and networking with nephrologists on CKD awareness in primary care. This was achieved by assessing the proportion of patients classified according to KDIGO in the general population and after stratification in at-risk patients for CKD. To this purpose, 53 GPs were trained by 11 nephrologists, one per region, across 11 Italian regions: Abruzzo, Campania, Emilia Romagna, Lazio, Liguria, Lombardia, Marche, Puglia, Sardegna, Sicilia, and Veneto. Although the limit in Italy is 1500 patients per GP, the average number of patients per GP in this cohort was 2065 due to a shortage of GPs in the country. Data were collected from the entire cohort of patients stored using the Millewin Digital Platform (see details in the supplementary material) in use by the GPs included in the study. The study analyzed for the presence of specific comorbidities, namely type 2 diabetes, hypertension, and heart failure, as well as the proportion of uACR and eGFR tests available at baseline (t0) and after 6 months (t6). Figure S1 illustrates the training and co-management plan. For each of the 11 Italian regions, one nephrologist delivered a formal 2-hour lecture in October 2021 to educate GPs on the significance of early detection of CKD, risk stratification based on available guidelines, and the rationale of the study. Additionally, an instant messaging group was established at the regional level to facilitate communication between each nephrologist and GPs specifically to discuss when to refer a patient based on certain patient characteristics. The co-management plan included a one-to-one joint visit between the nephrologist and GP upon request by a GP on a discretionary basis for specific comorbid cases. An online case discussion involving the nephrologist and GPs on a regional basis was planned after 6 months (April 2021).
Statistical Analysis
The statistical analysis was designed to evaluate the impact of the training intervention on CKD awareness and screening practices among GPs. Data were collected at two time points: baseline (t0) and six months after the intervention (t6). The primary outcomes measured were the changes in the usage rates of eGFR and uACR tests, and the increase in CKD diagnoses according to the KDIGO classification. Data were collected from 53 Italian GPs across 11 regions, covering a patient pool of 112,178 individuals. The dataset included demographic information, eGFR, and uACR test results, and the presence of comorbidities such as diabetes, hypertension, and heart failure. Descriptive statistics, including mean, median, and standard deviation for continuous variables, as well as frequencies and percentages for categorical variables, were calculated. To compare the mean differences in eGFR and uACR usage between t0 and t6, paired t-tests were conducted. Chi-square tests were employed to compare the proportion of patients screened for CKD at t0 and t6. Additionally, multiple logistic regression analysis was used to assess the association between the intervention and the likelihood of increased CKD screening, adjusting for potential confounders such as age, gender, and comorbid conditions. A budget impact model was developed to estimate the economic implications of increased CKD screening. This model included direct costs of CKD management, potential cost savings from early diagnosis and treatment with dapagliflozin, and the costs associated with dialysis. Sensitivity analyses were conducted to test the robustness of the budget impact model under different scenarios, including variations in treatment adherence and progression rates. The results demonstrated significant increases in the use of eGFR (+44.7%) and uACR (+95.2%) tests post-intervention. This led to a substantial rise in CKD screening (+128.9%) according to the KDIGO classification and a 62% increase in CKD diagnoses. The budget impact analysis projected cumulative five-year savings of €1.7 million for the study cohort, with potential national savings of €106.6 million. All statistical analyses were performed using SPSS and R. The budget impact analysis was conducted using a custom model developed in Microsoft Excel. A p-value of <0.05 was considered statistically significant for all tests. This comprehensive approach ensured the robustness and reliability of the study findings, providing clear insights into the clinical and economic benefits of targeted training interventions for early CKD detection.
Clinical Simulation and Economic Analysis
The clinical simulation was based on the rate of progressive decline of eGFR derived from Heerspink HJL and Vesga JI.11,12 In detail, the following estimates were used: an annual decrease of 0.60 mL/min/1.73m2 for non-diabetic patients treated with dapagliflozin; 1.99 mL/min/1.73m2 in diabetic treated patients; 1.34 mL/min/1.73m2 for non-diabetic non-treated patients; and 4.41 mL/min/1.73m2 for untreated patients with diabetes.
Next, the cohort of patients having both eGFR and uACR tests available at t6 (n=2335) was considered potentially available for screening, thus eligible for treatment and therefore used in subsequent analyses. Therefore, the results of the simulation assume that patients diagnosed with CKD are treated according to the current indications for dapagliflozin.
In order to evaluate the impact of an early diagnosis, which could slow the rate of progression of the disease and ultimately reduce healthcare costs, we ran a 5-years economic analysis both in the ENDORSE cohort and in the whole Italian population. To this aim, we used the results of the clinical simulation along with demographic and economic parameters as follows. The study was conducted on the Italian adult population (age ≥18) as of 01/01/2023 (n=49,786,127). Assuming a 10% diagnostic rate, we used the total estimated prevalence of CKD of 7% as reported in De Nicola L et al,13 to obtain the reference number of individuals with CKD (n = 348,503). Of these, 811 patients per million residents undergo dialysis, (n = 40,377 patients) (https://ridt.sinitaly.org/). Direct costs associated with CKD stages and dialysis costs (both direct and indirect) were obtained from the studies by Jommi et al and Cicchetti et al, respectively, and used as input (Table S1).14,15 In Table S2 detail of the number of tests according to comorbidities are described. Based on the Italian registry of Dialysis and Kidney Transplant, we used a prevalence of hemodialysis and peritoneal dialysis of 90% and 10%, respectively.
Results
The ENDORSE project provided data on 109,487 individuals at t0 and 112,178 individuals at t6. The population characteristics are reported in Table 1. We categorized the cohort using eGFR and uACR (see details in Tables S3 and S4).
 |
Table 1 Characteristics of the Study Population |
At the end of the study, after 6 months of educational intervention and networking between nephrologists and GPs, we observed an increase by 44.7% and 95.2% in the number of tests of both eGFR and uACR, respectively. A significant increase in the number of patients that could be stratified using KDIGO classification was observed accordingly (see Table 2).
 |
Table 2 KDIGO Classification at T0 and T6 |
We found a 62% increase in CKD diagnosis at t6. The subgroup analysis for eGFR and uACR according to the subgroup of interest (type 2 diabetes, hypertension, and heart failure) is reported in Table 3.
 |
Table 3 Number of Tests According to Comorbidities |
As next step, we ran a 5-year clinical simulation of CKD progression (see Methods) considering two scenarios. In the first scenario we included only the subset of patients with both test at t0 (n=1020). Whereas in the second, more favorable scenario as influenced by the training intervention at the end of the study, we included all patients (n=2335). Patients that progressed to an eGFR < 5 mL/min were considered on dialysis.
The simulation was then extrapolated to reference population in Italy which consisted of 348,503 patients (see Methods). It was assumed that the distribution of patients across KDIGO stages for the reference population were comparable, except for patients on dialysis. The results of this 5-year clinical evolution simulation are reported in Tables S5 and S6.
Finally, combining the results of the clinical simulation with economic data we obtained the budget impact analysis for the ENDORSE cohort and for the population of reference (n=348,503). In Table 4 we report the 5-year economic analysis based on the costs applied to the clinical simulation performed on the ENDORSE cohort.
 |
Table 4 Economic Impact Based on a 5-Year Simulation for the ENDORSE Cohort |
Costs savings were reported due to the slower disease progression in patients who received an early diagnosis and therefore considered treated with dapagliflozin according to current indications. Dialysis accounted for approximately one-quarter of the total costs in both scenarios. Cumulative savings after 5 years from baseline accounted for 1.72 million euros (6.40%). The analysis was also carried out for the population of reference (Table S7). Consistently with the findings in the ENDORSE cohort, we estimated progressively increasing theoretical cost savings, cumulatively reaching 106.6 million euros.
Discussion
Chronic kidney disease (CKD) is a major global public health issue, affecting 700–840 million people worldwide and increasing in prevalence annually.16 The economic burden of CKD is significant, especially for dialysis patients.1,3,5,9,17 In Italy, healthcare spending related to ESRD alone reached €2.1 billion in 2010, accounting for 1.9% of the healthcare budget.14 The direct annual healthcare costs of dialysis patients range from €29,800 (peritoneal dialysis) to €43,800 (hemodialysis), with indirect costs estimated at €6650 per person/year.15 Direct healthcare costs span from €1169 (CKD-1) to €5453 (CKD-5) per patient per year, varying across Italian regions.14
Despite these figures, CKD awareness remains low among patients, policymakers, and healthcare professionals, as evidenced by limited serum creatinine testing by GPs.7 Given its burdensome consequences, promoting screening, prevention, and early diagnosis is crucial to tackle the disease in its early stages, slow its progression, and save money.3,10,18 Primary care services play a fundamental role in enhancing CKD diagnosis, as GPs must suggest appropriate screening, including eGFR and uACR tests.
The clinical results of the ENDORSE project in terms of increased CKD diagnosis align with previous studies.10 However, the increase in advanced stages observed between T0 and T6 is counterintuitive, as extending screening should bring out less severe asymptomatic cases. This may be due to GPs screening preferentially high-risk individuals rather than unselected individuals.19
A crucial question is: “What would happen if these patients were not diagnosed?”. This prompted us to set up the eGFR decline simulation and economic impact evaluation. Diagnosing more CKD patients incurs immediate costs, which depend on the disease stage and severity. However, prevention and early diagnosis should be weighed in the long run to determine the benefits. The second endpoint of the study was to investigate the association between early diagnosis and potential economic savings for the Italian national health system. Our findings demonstrate potential cost-saving benefits of programs aimed at enhancing early diagnosis, allowing timely treatment and slowing disease progression.3,6,9
The economic analysis presents intriguing results. The cumulative cost savings over five years would amount to over €1.7 million, equivalent to €147 per patient, with most savings resulting from dialysis prevention. When applied to the entire Italian CKD population, cumulative savings could exceed €106 million, mostly from patients not on dialysis. The difference between the two cohorts lies in the fact that in a GP setting, we expect to identify patients with less severe CKD stages at diagnosis, whereas the entire Italian population includes a higher proportion of advanced CKD stages, resulting in a higher prevalence of dialysis patients. This analysis takes a conservative approach, focusing on potential savings for those diagnosed with CKD. Extending the analysis to the entire Italian population, both costs and savings would be higher. In 2021, CKD generated direct costs of over €4 billion in Italy, representing 3.2% of the national health service budget.20 Our simulation aligns with this figure, particularly regarding annual dialysis cost estimates of €2–2.5 billion.15
This study has several limitations. The main limitation is that our study is not randomized, and we have only the intervention arm. Also, it is not possible to establish the antecedence of the intervention with respect to the observed outcome. Additionally, the screening was solely based on GPs’ decisions, and we analyzed aggregated data to evaluate the training’s overall impact on the entire population. Thus, the dataset did not allow for a specific evaluation of the subset of individuals who underwent screening intervention. The assumption of the distribution across KDIGO stages may not fully support generalizability. Finally, an important limitation is the relatively short six-month observation period. A longer period could provide a more comprehensive assessment of the intervention’s impact on CKD care costs. Additionally, our study did not include a control group, limiting the ability to definitively attribute changes solely to the educational intervention. Future studies should consider a randomized controlled design with a longer observation period to further validate these results and improve data generalizability.
In conclusion, this study highlights the importance of medical networking and training in increasing the number of CKD tests performed by GPs, with potential cost savings for the national health service.
Data Sharing Statement
Data are available upon request.
Ethical Statement
Patient consent was not required given the non-interventional/observational nature of the study and the fact that data were provided by the GPs to the investigators as pseudo-anonymous (hence, not allowing the study investigators to reconstruct the identity of study participants). Notice was not given to the local Ethics’ Committee/Institutional Review Board. The study complies with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by unconditional grants from AstraZeneca Italy.
Disclosure
FP received fees from AstraZeneca and GSK for lectures. LDN received fees from AstraZeneca, Astellas, Bayer and NovoNordisk for lectures and scientific consultations. GG received fees from AstraZeneca, Astellas, Alexion, GSK, Boehringer, Novartis and Bayer for lectures and scientific consultation. MB received fees from Astellas, AstraZeneca, GSK, Nipro and Travere Therapeutics for lectures and scientific consultations. GLM received payment or honoraria for lectures, presentation, speakers bureaus, eduational events - Support for attending meetings or and/or travel from Astellas, Hansa Biopharma, Travere, Vifor, Eli-Lily, GlaxoSmithKline, Alexion, Braun, and Boehringer Ingelheim. LG received research grants from Abionyx, Sanofi; consultancy fees from Baxter, Travere, AstraZeneca, GSK, Novartis, Chinook, Roche, Reata, Nestle, Otsuka, Gilead Science, Bayer, Vifor Fresenius; speaker fees from Bayer and Werfen. The authors report no other conflicts of interest in this work.
References
1. Carriazo S, Villalvazo P, Ortiz A. More on the invisibility of chronic kidney disease… and counting. Clin Kidney J. 2021;15(3):388–392. PMID: 35198154; PMCID: PMC8690216. doi:10.1093/ckj/sfab240
2. Glassock RJ, Warnock DG, Delanaye P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol. 2017;13(2):104–114. PMID: 27941934. doi:10.1038/nrneph.2016.163
3. Economist Impact. Chronic kidney disease. Driving change to address the urgent and silent epidemic in Europe; 2023.
4. Jager KJ, Kovesdy C, Langham R, Rosenberg M, Jha V, Zoccali C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019;96(5):1048–1050. PMID: 31582227. doi:10.1016/j.kint.2019.07.012
5. Damien P, Lanham HJ, Parthasarathy M, Shah NL. Assessing key cost drivers associated with caring for chronic kidney disease patients. BMC Health Serv Res. 2016;16(1):690. PMID: 28031020; PMCID: PMC5192586. doi:10.1186/s12913-016-1922-4
6. Marino C, Ferraro PM, Bargagli M, et al. Prevalence of chronic kidney disease in the Lazio region, Italy: a classification algorithm based on health information systems. BMC Nephrol. 2020;21(1):23. PMID: 31992222; PMCID: PMC6986004. doi:10.1186/s12882-020-1689-z
7. Minutolo R, De Nicola L, Mazzaglia G, et al. Detection and awareness of moderate to advanced CKD by primary care practitioners: a cross-sectional study from Italy. Am J Kidney Dis. 2008;52(3):444–453. PMID: 18468747. doi:10.1053/j.ajkd.2008.03.002
8. Ravera M, Noberasco G, Weiss U, et al. CKD awareness and blood pressure control in the primary care hypertensive population. Am J Kidney Dis. 2011;57(1):71–77. PMID: 21087817. doi:10.1053/j.ajkd.2010.08.022
9. Shlipak MG, Tummalapalli SL, Boulware LE, et al.; Conference Participants. The case for early identification and intervention of chronic kidney disease: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2021;99(1):34–47. PMID: 33127436. doi:10.1016/j.kint.2020.10.012
10. Pesce F, Pasculli D, Pasculli G, et al. ”The disease awareness innovation network” for chronic kidney disease identification in general practice. J Nephrol. 2022;35(8):2057–2065. PMID: 35701727; PMCID: PMC9584961. doi:10.1007/s40620-022-01353-6
11. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383(15):1436–1446. PMID: 32970396. doi:10.1056/NEJMoa2024816
12. Vesga JI, Cepeda E, Pardo CE, Paez S, Sanchez R, Sanabria RM. Chronic kidney disease progression and transition probabilities in a large preventive cohort in Colombia. Int J Nephrol. 2021;2021:8866446. PMID: 33868729; PMCID: PMC8032521. doi:10.1155/2021/8866446
13. De Nicola L, Donfrancesco C, Minutolo R, et al.; ANMCO-SIN Research Group. Prevalence and cardiovascular risk profile of chronic kidney disease in Italy: results of the 2008–12 National Health Examination Survey. Nephrol Dial Transplant. 2015;30(5):806–814. PMID: 25523453. doi:10.1093/ndt/gfu383
14. Jommi C, Armeni P, Battista M, et al.; IRIDE Study Group. The cost of patients with chronic kidney failure before dialysis: results from the IRIDE observational study. Pharmacoecon Open. 2018;2(4):459–467. PMID: 29623638; PMCID: PMC6249198. doi:10.1007/s41669-017-0062-z
15. Cicchetti A, Ruggeri M, Codella P, Ridolfi A. I costi socio-sanitari dell’insufficienza renale cronica. Farmacoeconomia E Percorsi Terapeutici. 2011;12:75–82.
16. Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2022;12(1):7–11. PMID: 35529086; PMCID: PMC9073222. doi:10.1016/j.kisu.2021.11.003
17. Ingrasciotta Y, Sultana J, Formica D, et al.; Italian Biosimilars Network (I-BioNetwork). Direct healthcare costs of chronic kidney disease management in Italy: what cost-savings can be achieved with higher biosimilar uptake and more appropriate use of erythropoiesis-stimulating agents? Pharmacoepidemiol Drug Saf. 2021;30(1):65–77. PMID: 33067914. doi:10.1002/pds.5152
18. Pergola PE, Pecoits-Filho R, Winkelmayer WC, et al. Economic Burden and health-related quality of life associated with current treatments for anaemia in patients with CKD not on dialysis: a systematic review. Pharmacoecon Open. 2019;3(4):463–478. PMID: 30968369; PMCID: PMC6861396. doi:10.1007/s41669-019-0132-5
19. Garofalo C, Borrelli S, De Stefano T, et al. 15-year-change of phenotype and prognosis in non-dialysis CKD patients referred to a nephrology clinic. Int Urol Nephrol. 2022;54(3):679–686. PMID: 34251604. doi:10.1007/s11255-021-02944-1
20. Mennini FS, Cabrera C, Card-Gowers J, et al. Inside CKD: projecting the economic burden of chronic kidney disease using patient-level microsimulation. Value Health. 2022;25(1):S73. doi:10.1016/j.jval.2021.11.341
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
