Back to Journals » Drug Design, Development and Therapy » Volume 18
Combinational Antitumor Strategies Based on the Active Ingredients of Toad Skin and Toad Venom
Authors Tian H, Zhao F, Yue BS, Zhai BT
Received 17 April 2024
Accepted for publication 25 July 2024
Published 9 August 2024 Volume 2024:18 Pages 3549—3594
DOI https://doi.org/10.2147/DDDT.S469832
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Huan Tian,1 Feng Zhao,1 Bao-Sen Yue,1 Bing-Tao Zhai2– 5
1Department of Pharmacy, Xi’an Hospital of Traditional Chinese Medicine, Xi’an, People’s Republic of China; 2College of Pharmacy, Shaanxi University of Chinese Medicine, Xi’an, People’s Republic of China; 3State Key Laboratory of Research & Development of Characteristic Qin Medicine Resources (Cultivation), Xi’an, People’s Republic of China; 4Shaanxi Key Laboratory of Chinese Medicine Fundamentals and New Drugs Research, Xi’an, People’s Republic of China; 5Shaanxi Collaborative Innovation Center of Chinese Medicinal Resources Industrialization, Xi’an, People’s Republic of China
Correspondence: Bing-Tao Zhai, Email [email protected]
Abstract: A multidrug combination strategy is an important mean to improve the treatment of cancer and is the mainstream scheme of clinical cancer treatment. The active ingredients of traditional Chinese medicine, represented by toad skin and toad venom, have the advantages of high efficiency, low toxicity, wide action and multiple targets and have become ideal targets in combined treatment strategies for tumors in recent years. Toad skin and toad venom are traditional Chinese animal medicines derived from Bufo bufo gargarizans Cantor or Bufo melanostictus Schneider that have shown excellent therapeutic effects on the treatment of various cancers and cancer pain as adjuvant antitumor drugs in clinical practice. The involved mechanisms include inducing apoptosis, arresting the cell cycle, inhibiting cell proliferation, migration and invasion, inhibiting tumor angiogenesis, reversing the multidrug resistance of tumor cells, and regulating multiple signaling pathways and targets. Moreover, a multidrug combination strategy based on a nanodelivery system can realize the precise loading of the active ingredients of toad skin or toad venom and other antitumor drugs and carry drugs to overcome physiological and pathological barriers, complete efficient enrichment in tumor tissues, and achieve targeted delivery to tumor cells and the controlled release of drugs, thus enhancing antitumor efficacy and reducing toxicity and side effects. This article reviewed the clinical efficacy and safety of the combination of toad skin and toad venom with chemotherapeutic drugs, targeted drugs, analgesics and other drugs; evaluated the effects and mechanisms of the combination of toad skin and toad venom with chemotherapy, targeted therapy, radiotherapy or hyperthermia, traditional Chinese medicine, signaling pathway inhibitors and other therapies in cell and animal models; and summarized the codelivery strategies for the active ingredients of toad skin and toad venom with chemotherapeutic drugs, small-molecule targeted drugs, monoclonal antibodies, active ingredients of traditional Chinese medicine, and photodynamic and photothermal therapeutic drugs to provide a basis for the rational drug use of toad skin and toad venom in the clinic and the development of novel drug delivery systems.
Keywords: toad skin, toad venom, combinational antitumor strategies, codelivery strategies, cancer, cancer pain
Introduction
For decades, cancer has been a leading cause of death.1 For many years, chemotherapy has been regarded as the main method of tumor treatment. However, the long-term use of chemotherapy drugs, which are based on a single antitumor mechanism, easily destroys the human immune system, causing multidrug resistance (MDR) and serious adverse reactions.2 In recent years, with the rise of targeted therapy, immunotherapy, photodynamic therapy (PDT), traditional Chinese medicine (TCM), and interventional therapy, cancer patients have an increasing number of treatment options, and combination therapy has become the standard strategy for cancer treatment. Combination therapy with anticancer drugs targeting different targets can achieve synergistic effects by regulating multiple pathways of abnormal cells and has many advantages, such as reducing adverse drug reactions, delaying the occurrence of MDR, and improving the antitumor immune response.3–5 At present, diverse combined antitumor drug strategies include combinations of chemotherapy drugs and targeted drugs, combinations of chemotherapy drugs and immunotherapy drugs, combinations of targeted drugs and immunotherapy drugs, combinations of active ingredients of TCM and chemotherapy drugs, and combinations of active ingredients of TCM and targeted drugs.6–9 Among them, the active ingredients of TCM have a wide range of sources, low toxicity and high safety, can exert an antitumor effect through multiple targets and multiple pathways, and show unique advantages in reducing drug resistance, regulating the tumor microenvironment, promoting immune function recovery, reducing recurrence and metastasis, and improving the long-term prognosis. Therefore, in recent years, an increasing number of researchers have focused on the active ingredients of TCM for combined application to prolong the survival of cancer patients.10
The toad is the whole body of Bufo bufo gargarizans Cantor or Bufo melanostictus Schneider. The head, tongue, skin, secretions of the posterior ear glands and skin glands, liver and gallbladder are all medicinal parts. The head is the Chinese medicine toad head, which has the effects of eliminating malnutrition and digesting the retained food and can be used to treat infantile malnutrition. The tongue is the Chinese medicine toad tongue, which detoxifies and removes furuncles and can be used to treat furuncles. The dried skin lacking the viscera is the Chinese medicine toad skin, which removes heat and toxic material, induces diuresis to remove edema, and can be used to treat ulcers, pyogenic infections, scrofula, eczema, infantile malnutrition, abdominal distension, and chronic tracheitis. The dry secretions of the gland behind the ear and the gland of the skin constitute the traditional Chinese medicine toad venom, which has the effects of detoxifying, relieving pain and inducing resuscitation and can be used for carbuncles and furuncles, sore throat, heat stroke, dizziness, diarrhea, acute filthy disease, and vomiting. The liver is used in the Chinese medicine toad liver, which detoxifies and disperses knots, removes furuncles and relieves swelling, and can be used to treat ulcers, furuncles, sores, snake bites and measles. The gallbladder is the Chinese medicine toad bile, which relieves cough and reduces sputum, detoxifies and disperses knots, and can be used to treat tracheitis, infantile aphonia, early lymph node tuberculosis and nose furuncle.11,12 Currently, dried toad skin has been developed as capsule (Huachansu capsule), tablet (Huachansu tablet), oral liquid (Huachansu oral liquid) and injection (Huachansu injection) for treating intermediate and advanced tumors, chronic hepatitis B and other diseases.13–15 Toad venom was also developed as an injection (Toad venom injection) for treating acute and chronic suppurative infections and is used as an adjuvant antitumor drug.16 Moreover, various preparations containing dried toad skin and toad venom, including Tianchan capsule, Kang Ai Ping pill, Xianchan tablet, Shenchan Xiaojie capsule, Chanwu Babu ointment, Chanwu gel ointment, Delisheng injection, Hupo Zhitong ointment, Hechan tablet, Jinpu capsule, Compound toad venom ointment, Jiawei Xihuang pill, Xiaojin pill, Tianfoshen oral liquid, Toad venom Zhentong gel ointment, Toad venom ingot, Toad venom Zhentong ointment and Compound toad venom pill, have also shown excellent therapeutic effects on the treatment of lung, liver, stomach and other cancers, as well as various types of cancer pain, as adjuvant antitumor drugs in clinical practice. Table 1 summarizes the marketed antitumor products containing toad skin or toad venom in China.
 |
Table 1 Marketed Antitumor Products Containing Toad Skin or Toad Venom in China |
In addition to traditional preparations of toad skin and toad venom, the advantages of the active ingredients of toad skin and toad venom in the treatment of cancer are increasingly prominent with the advancement of separation technology and the study of drug action mechanisms. The chemical components of toad skin and toad venom are mainly bufodienolides, indole alkaloids, steroids and other compounds. Among them, the monomeric components of bufadienolides, such as bufalin, cinobufagin, bufotalin, cinobufotalin, gamabufotalin, and arenobufagin, are the main active antitumor components (Figure 1). A number of studies have confirmed that these compounds can exert antitumor effects through multiple targets and multiple pathways and have the advantages of enhancing efficacy, reducing toxicity, reversing tumor MDR, reducing cancer pain, etc., when combined with chemotherapy, radiotherapy, targeted therapy and other treatment methods.17–20 Although combination therapeutic strategies are helpful for better treating cancer to a certain extent, the abovementioned active ingredients have the disadvantages of low bioavailability and poor solubility in water, and free drug combinations cannot solve problems such as the low drug-targeting ability, significant harmful and adverse off-target effects, and the inconsistent pharmacokinetic characteristics and tissue distributions of different drugs.21,22 With the rapid development of nanomedicine, nanodelivery systems have shown great potential in overcoming the shortcomings of existing antitumor multidrug delivery strategies. Nanodrug delivery systems can accurately regulate the flexible loading of multiple components of drugs and deliver drugs to specific tissues, organs, and even cells and intracellular structures, thereby enhancing the therapeutic effects and reducing adverse reactions. In addition, by constructing biomimetic drug delivery systems or introducing targeted molecules and stimulus-responsive groups into the codelivery vector, the duration of the drug delivery system in the blood circulation can be extended, and the enrichment of the drug delivery system at the tumor site and the internalization by specific cells can be improved. Moreover, the drug delivery system can respond to endogenous (pH, hypoxia, and redox) or exogenous (light and heat) stimuli at the tumor site to achieve rapid drug release in the lesion area.23,24 In recent years, codelivery systems loaded with multiple active ingredients of toad skin or toad venom and other antitumor drugs have been developed to further enhance the antitumor effects, reduce adverse reactions, and overcome MDR.
 |
Figure 1 The source of toad skin and toad venom and the structure of their related active ingredients. |
Therefore, based on the research progress on the use of toad skin and toad venom in combined antitumor therapy, this article first reviews the clinical efficacy and safety of toad skin- and toad venom-related preparations with chemotherapy drugs, targeted drugs, analgesics and transarterial chemoembolization (TACE) interventional therapy and summarizes the meta-analysis results of toad skin- and toad venom-related preparations as adjuvant therapies for cancer and cancer pain. Subsequently, the antitumor effects and mechanisms of toad skin- and toad venom-related preparations and active ingredients with chemotherapy, targeted therapy, radiotherapy, hyperthermia, Chinese herbal therapy, signaling pathway inhibitors, and other therapies in cells and animal models are evaluated. Finally, the combination antitumor strategies of the active ingredients of toad skin and toad venom combined with chemotherapy drugs, small-molecule targeted drugs, monoclonal antibodies, active ingredients of TCM, photodynamic therapy drugs and photothermal therapy drugs based on nanoplatforms are summarized and serve as a foundation for the clinical combination of toad skin and toad venom and the construction of novel drug delivery systems.
Clinical Combination Therapy Strategies
Clinical Efficacy and Safety of Cancer Treatment
Many studies have indicated that the use of toad skin or toad venom-related preparations as adjunct treatments for cancer could enhance therapeutic effects, improve patients’ quality of life (QOL) or immunological response, or alleviate the incidence of side effects. For example, the Huachansu tablet combined with TACE is effective at prolonging progression-free survival (PFS) and overall survival (OS) in patients with unresectable hepatocellular carcinoma (HCC).25 The use of Huachansu capsule in combination with zoledronic acid therapy can result in a more favorable therapeutic outcome and an improved QOL in the treatment of metastatic bone tumors.26 Huachansu injection combined with the CEMT regimen (carboplatin, etoposide, methotrexate, and thalidomide) achieved better clinical effects on patients with relapsed or refractory multiple myeloma.27 Toad venom injection combined with apatinib can improve the disease control rate (DCR), reduce the incidence of neutropenia and fatigue symptoms, decrease the level of a tumor marker (CA199), and increase immune indices (CD3+, CD4+, CD8+, CD4+/CD8+, IgG, IgA, and IgM) in patients with advanced gastric cancer.28
Moreover, when combined with Kang Ai Ping pill, capecitabine tablets have a beneficial therapeutic effect on rectal cancer, and can improve the objective response rate (ORR), DCR and QOL; reduce the levels of tumor markers (CEA, CA242, and CA199); and enhance the immune function of patients.29 Xianchan tablets combined with chemotherapy (paclitaxel, calcium folinate and cisplatin) have significant efficacy in the care of elderly esophageal cancer patients and can improve the DCR, QOL, serum tumor markers and immune function indicators without increasing adverse reactions.30 Delisheng injection combined with the XELIRI regimen (irinotecan + capecitabine) has a better clinical curative effect on liver metastases of colon cancer. It can improve patient survival, reduce the blood toxicity of chemotherapy, and protect the body’s immune function.31 Hechan tablets combined with the DP regimen (docetaxel + cisplatin) have good curative effects on non-small cell lung cancer (NSCLC), and can decrease the levels of tumor markers, improve the QOL, PFS and immune function of patients, and reduce side effects (such as decreased white blood cell count, damage to renal function, damage to liver function, and diarrhea).32 Tianfoshen oral liquid combined with a GP regimen (gemcitabine + cisplatin) can improve the immune functions of elderly patients with advanced NSCLC.33 In addition, the Toad venom injection + TC regimen, Toad venom injection + TP regimen, Kang Ai Ping pill + mFOLFOX6 regimen + trastuzumab, Xianchan tablet + docetaxel, Xianchan tablet + mFOLFOX6 regimen + trastuzumab, and Xianchan tablet + GP regimen could all enhance the therapeutic effects, improve patients’ QOL or immune system, or reduce the risk of side effects.34–39 The clinical efficacy and safety of toad skin or toad venom-related preparations as adjunct treatments for cancer are shown in Table 2.
 |
Table 2 Clinical Efficacy and Safety of Toad Skin or Toad Venom-Related Preparations as Adjunct Treatments for Cancer |
Clinical Efficacy and Safety of Treating Cancer Pain
Cancer patients are deeply affected by cancer pain resulting from either cancer therapy or the malignancy itself. Effective pain treatment is an important step in improving the QOL of cancer patients. Presently, the three-step analgesic method is the standard treatment scheme for cancer pain, but it has large adverse impacts and dependence readily occurs.40 Recently, numerous investigations have revealed that toad skin- and toad venom-related preparations, especially Huachansu preparations, combined with three-step analgesia can improve the total effective rate of cancer pain treatment, reduce the onset time of pain relief, extend the duration of pain relief, reduce the daily dosage of opioids, improve QOL, and lessen the risk of adverse reactions. For example, the combination of Huachansu capsules with sustained-release oxycodone hydrochloride tablets could significantly improve the analgesic effect, reduce the VAS score, increase the KPS score, shorten the onset time, prolong the duration of analgesia, and reduce the dose of oxycodone hydrochloride.41,42 The Huachansu capsule + fentanyl transdermal patch combination increased the effective rate of analgesia, reduced the VAS score and estazolam dosage, and improved the QOL of patients.43 The ability of the Huachansu capsule combined with zoledronic acid to relieve osseous metastasis pain in prostate cancer patients is significant and can effectively decrease the levels of prostate-specific antigen (PSA) and free PSA (f-PSA) and improve patients’ QOL.44 In addition, the Huachansu capsule + three-step analgesic ladder could enhance the analgesic effect by increasing the level of β-endorphin in patients’ peripheral blood and decreasing the level of 5-hydroxytryptamine.45 Intervention with Huachansu and opioids in patients with opium intolerance with incomplete analgesia can reduce breakthrough pain, decrease the rate of long-acting opioid increase, and improve sleep and mood.46 Huachansu injection + acetaminophen oxycodone effectively alleviated pain; decreased the occurrence of negative reactions, including drowsiness, constipation, dysuria, nausea and vomiting; and was effective at treating patients suffering from moderate to severe cancer-related visceral pain.47 Huachansu capsule + sustained-release morphine hydrochloride tablets could significantly improve the analgesic effect, shorten the onset time, prolong the duration of analgesia, and reduce the incidence of side effects.48 Huachansu injection + sodium ibandronate injection can significantly improve the treatment effect and QOL of patients with metastatic bone cancer pain.49 Moreover, the clinical efficacy of Tianchan capsule + sustained-release oxycodone hydrochloride tablets in the management of cancer-related pain is remarkable, as they can not only effectively relieve cancer pain but also improve QOL, with good safety.50,51 Cancer pain can also be effectively managed with the traditional three-step analgesic ladder + acupoint sticking with Chanwu gel ointment. This treatment can also lower the dosage of three-step analgesics and minimize side effects.52 In addition, other studies have indicated the therapeutic effectiveness and safety of Huachansu + the three-step analgesic ladder.53–58 The clinical efficacy and safety of toad skin- or toad venom-related preparations as adjunct treatments for cancer pain are shown in Table 3.
 |
Table 3 Clinical Efficacy and Safety of Toad Skin or Toad Venom-Related Preparations as Adjunct Treatments for Cancer Pain |
Systematic Review and Meta-Analysis of Cancer Treatment and Pain Relief
Many systematic reviews and meta-analyses have reported that toad skin- or toad venom-related preparations are effective and safe as complementary therapies for cancer and cancer pain. For example, a meta-analysis was conducted on the efficacy and safety of Huachansu injection combined with chemotherapy in the treatment of lung cancer using Review Manager 5.3 software. A total of 21 clinical studies involving 1735 patients with lung cancer were included. The results showed that Huachansu injection did not cause additional side effects in lung cancer patients, such as hematological and gastrointestinal toxicity, cardiotoxicity, hepatotoxicity, or nephrotoxicity, when it was used in combination with chemotherapy. Instead, it could significantly improve the ORR, DCR, and QOL, and alleviate pain.59 Another meta-analysis of the efficacy and safety of Huachansu capsules combined with first-line platinum-based chemotherapy for advanced NSCLC was conducted using Review Manager 5.3 and Stata 15.1 software. A total of 19 clinical studies involving 1564 patients were included. In patients with stage III/IV NSCLC, Huachansu capsule significantly improved the ORR and 1- and 2-year survival rates, increased the percentages of CD3+ cells and CD4+ cells, increased the ratio of CD4+/CD8+ cells, and minimized toxicity, including thrombocytopenia, leukopenia and vomiting, when combined with first-line chemotherapy based on platinum.60 Some researchers have also conducted a meta-analysis of the efficacy and safety of Huachansu injection combined with chemotherapy in the treatment of gastric cancer using Review Manager 5.3 and Stata 13.0 software. A total of 14 randomized controlled trials (RCTs) with 976 participants were included. Compared with traditional chemotherapy alone, Huachansu injection + chemotherapy was related to better outcomes in terms of increasing the ORR, improving the performance status, and alleviating leukopenia in patients diagnosed with gastric cancer.61 Moreover, some researchers have systematically investigated the safety and efficacy of the combination of TACE and Huachansu injection for patients with advanced HCC using Review Manager 5.3 and Stata 12.0 software. A total of 27 RCTs involving 2079 patients were included in this analysis. Huachansu injection combined with TACE prolonged the 1-, 1.5-, 2-, and 3-year OS; improved the ORR, DCR, and QOL; enhanced immune function and liver function (CD3+, CD4+, CD4+/CD8+, natural killer cell, total bilirubin, alanine aminotransferase [ALT], aspartate aminotransferase [AST] levels); and did not cause serious adverse events in patients diagnosed with advanced HCC.62 Furthermore, one meta-analysis was conducted on the efficacy and safety of toad venom injection combined with chemotherapy/radiotherapy for tumor patients using Review Manager 5.0.24 software. Six studies involving 369 participants (192 participants in the treatment group and 177 participants in the control group) were included. Toad venom injection has a certain effect on increasing the KPS functional score, improving the clinical syndrome, reducing the toxicity of chemotherapy and radiotherapy, and enhancing immune function in patients with tumors.63 In addition, two meta-analyses were conducted on the efficacy and safety of Huachansu combined with a three-step analgesic ladder in the treatment of cancer pain using Review Manager 5.3/5.4 software. One study included 10 RCTs with 1293 patients (648 patients in the test group and 645 patients in the control group), and the other included 18 RCTs with 2088 patients (1046 patients in the test group and 1042 patients in the control group). Huachansu + the three-step analgesic ladder could greatly increase the overall effectiveness of cancer pain management, reduce the onset time and extend the duration of pain relief, reduce the daily dosage of opioids, improve QOL, and reduce the risk of adverse reactions (constipation, nausea and vomiting, dizziness, drowsiness, and anorexia).64,65 Additionally, other systematic reviews and meta-analyses have shown that Huachansu is effective and safe as a complementary therapy for cancer.66–72 The meta-analyses of the use of toad skin- or toad venom-related preparations as adjunct treatments for cancer are shown in Table 4.
 |
Table 4 Meta-Analyses of the Use of Toad Skin or Toad Venom-Related Preparations as Adjunct Treatments for Cancer |
Combined Antitumor Strategies Based on in vitro and in vivo Models
Combined with Chemotherapy
Enhancing the Effect of Chemotherapy
Toad skin, toad venom and its main active ingredients, such as gamabufotalin, arenobufagin, bufalin and cinobufagin, combined with chemotherapy drugs, such as cisplatin, arsenite (AsIII), gemcitabine, paclitaxel, temozolomide, doxorubicin (DOX) and hydroxycampothecin, could strengthen the therapeutic effect on tumors by arresting the cell cycle, triggering apoptosis and autophagic death in cells, and preventing the growth, migration, and invasion of cells in different types of cancer.
The combination of 100 µg/mL Huachansu injection and 1 µg/mL cisplatin increased the apoptosis of OS732 cells by upregulating Fas expression.73 The combination of Huachansu (aqueous extract) and DOX could increase HCC cell apoptosis via Fas- and mitochondria-mediated pathways.74 In pancreatic cancer, gemcitabine combined with bufalin suppressed cell proliferation and promoted cell apoptosis by triggering the apoptosis signal-regulating kinase 1 (ASK1)/c-Jun N-terminal kinase (JNK) pathway.75 By suppressing the integrin α2β5/focal adhesion kinase (FAK) pathway, the combination of bufalin and paclitaxel more effectively inhibited the growth of xenograft tumors and inhibited the proliferation of cervical cancer cells. The tumor weight inhibition rate of the combined group was 75.2%, which was significantly higher than that of the groups treated with bufalin alone (46.8%) or paclitaxel alone (41.3%).76 Additionally, bufalin increased the apoptosis of glioma stem-like cells induced by temozolomide by triggering the mitochondrial pathway.77 In castration‑resistant prostate cancer, bufalin (0.6 or 0.8 mg/kg) + hydroxycampothecin (2 mg/kg) greatly reduced the tumor volume (tumor inhibition rate: 81.26±9.19% or 92.99±3.96%) and induced higher levels of cell apoptosis by increasing the expression of Bcl-2-like protein 4 (Bax), p53, programmed cell death 4 (PDCD4) and glycogen synthase kinase 3beta (GSK‑3β) and reducing the expression of B-cell lymphoma-extra large (Bcl‑xl) and phosphorylated protein kinase B (p-Akt) compared with bufalin or hydroxycampothecin alone.78 When combined with cisplatin, bufalin can reduce the cisplatin-induced activation of AKT under both normoxic and hypoxic conditions, which can limit cell growth and promote apoptosis in gastric cancer cells. Downstream molecules of AKT such as glycogen synthase kinase, mechanistic target of rapamycin (mTOR), ribosomal protein S6 kinase and eukaryotic translation initiation factor‑4E‑binding protein‑1 are likewise inhibited by bufalin.79 In addition, compared with cinobufagin or cisplatin treatment alone, the combination of these two agents significantly reduced cell migration and invasion, enhanced apoptosis and S-phase arrest in vitro, inhibited the growth and metastasis of xenograft tumors, and prolonged the survival rate in vivo by blocking the Notch pathway.80 Arsenite (AsIII) and gamabufotalin together had synergistic cytotoxic effects on U-87 cells by triggering necrosis, autophagic cell death, and G2/M arrest.81 Arenobufagin also sensitized U-87 cells to AsIII-mediated cytotoxicity. The synergistic effect was partially attributed to the induction of apoptosis, necrosis, and G2/M-phase arrest, as well as autophagic cell death and the regulation of the Jagged1/Notch pathway.82
Reversing Chemotherapy Resistance
The active ingredients of toad skin and toad venom, such as bufalin, cinobufagin and bufotalin, can also reverse MDR by affecting the cell cycle and cell apoptosis, regulating cancer cell stemness and the polarization of M2 macrophages, inhibiting drug efflux pump activity, and decreasing the expression of drug resistance genes and proteins, among others.
In methotrexate (MTX)-resistant human osteosarcoma U-2OS/MTX300 cells, bufalin promotes apoptosis and G2/M phase arrest via a p53-dependent pathway without influencing or being impacted by the expression of dihydrofolate reductase (DHFR).83 The chemosensitivity of BEL-7402/5-Fluorouracil (5-FU) cells to 5-FU was also increased by bufalin at a dose of 1 nM, with a reversal of 3.8-fold, which was comparable to that of 1 µM verapamil. This reversal of drug resistance was associated with suppressing drug efflux pump activity by downregulating multidrug resistance-associated protein 1 (MRP1), inducing cell G0/G1 phase arrest, increasing the Bax/Bcl-xl ratio to trigger apoptosis, and decreasing TS expression.84 In the vincristine (VCR)-resistant leukemia cell line K562/VCR, bufalin has the ability to reverse MDR, with reversal index values of 4.85, 6.94 and 14.77 at doses of 0.0002, 0.001 and 0.005 μM, respectively, by suppressing the expression of MRP1 and triggering apoptosis signaling through changes in the Bcl-xl/Bax ratio.85 By increasing p53 and p21 expression and decreasing the expression of Aurora A, cell division cycle 25 (CDC25), cyclin-dependent kinase 1 (CDK1), cyclin A, and cyclin B1, bufotalin causes G2/M phase arrest in multidrug-resistant HepG2 cells. Additionally, bufotalin causes apoptosis by regulating B-cell lymphoma (Bcl-2) and Bax, activating caspase-9/-3, inducing the cleavage of poly ADP-ribose polymerase (PARP), decreasing the mitochondrial membrane potential (ΔΨm), and increasing reactive oxygen species (ROS) and intracellular calcium levels. Moreover, bufotalin-induced apoptosis can be amplified in a synergistic manner by the Akt inhibitor LY294002 or an siRNA targeting Akt. Additionally, an in vivo test revealed that bufotalin markedly suppresses the proliferation of a xenograft R-HepG2 tumor model without causing splenic toxicity.86
Cinobufagin had no effect on the parental HCT116/oxaliplatin (L-OHP) (HCT116/L), LoVo/adriamycin (ADR), or Caco-2/ADR cells, but it greatly increased the sensitivity of these cells to L-OHP and DOX. With no appreciable in vivo toxicity, cinobufagin also increased the anticancer efficacy of DOX in a P-glycoprotein (P-gp)-overexpressing LoVo/ADR xenograft tumor model. Subsequent investigations revealed that cinobufagin reversed MDR by increasing apoptosis and the intracellular accumulation of DOX via the inhibition of P-gp-mediated efflux through the noncompetitive suppression of P-gp ATPase activity.87 In docetaxel (DCT)-resistant MCF-7 and MDA-MB-231 cell lines (MCF-7/DCTR and MDA-MB-231/DCTR), bufalin has the ability to overcome ATP-binding cassette subfamily B member 1 (ABCB1)-mediated DCT resistance by increasing DCT accumulation through a decrease in ABCB1 ATPase function and the inhibition of ABCB1 protein expression. Additionally, in an MDA-MB-231/DCTR xenograft tumor model, bufalin + DCT greatly reduced tumor growth and decreased ABCB1 expression.88 By suppressing nuclear factor erythroid 2-related factor 2 (Nrf2) and reducing the expression of heme oxygenase-1 (HO-1) and P-gp, bufalin can reverse MDR in K562/A02 cells by limiting DOX efflux. Furthermore, by activating the inositol-requiring enzyme 1a (IRE1a)/tumor necrosis factor receptor associated factor 2 (TRAF2)/JNK/caspase-12 pathway, bufalin can trigger endoplasmic reticulum (ER) stress and apoptosis in drug-resistant cells.89 In ABCB1-overexpressing HCT8/ADR, LoVo/ADR and HCT8/ABCB1 colorectal cancer cells, bufalin increased intracellular drug accumulation to increase chemosensitivity by limiting the transport function of ABCB1, suppressing the expression of the ABCB1 protein and regulating ABCB1 ATPase activity. In an HCT8/ADR xenograft tumor model, the efficacy of DOX was also significantly strengthened by bufalin, but no observable toxicity was induced.90 CD133-overexpressing cells exhibit increased chemoresistance and levels of Akt/nuclear factor-kappa B (NF-κB) signaling mediators and MDR1. By controlling cancer cell stemness by lowering multidrug resistance gene 1 (MDR1)/P-gp expression through the inhibition of CD133 expression, AKT phosphorylation, NF-κB/p65 nuclear translocation, and MDR1 translation in vitro and in vivo, bufalin could reverse MDR in colorectal cancer.91 By suppressing the stemness of colorectal cancer cells and decreasing the expression of stemness markers such as CD133, CD44, octamer-binding transcription factor 4 (OCT4), sex-determining region Y-box 2 (SOX2), and NANOG, as well as the ATP-binding cassette transporter G2 (ABCG2) protein, bufalin can overcome acquired cisplatin resistance.92 M2 macrophage polarization is one of the key factors of chemoresistance. According to Chen et al, bufalin inhibits macrophage migration inhibitory factor (MIF) release by targeting the steroid receptor coactivator 3 (SRC-3) protein, which in turn controls M2 macrophage polarization in chemoresistant cells. Moreover, they reported that the Huachansu capsule improved the antitumor effect of L-OHP both in vivo and in the clinic by controlling the polarization of M2 macrophages via the SRC-3/MIF pathway.93
Moreover, in lung adenocarcinomas, cinobufotalin reduces cisplatin resistance, migration and invasion. Mechanistically, cinobufotalin induces the expression of ENKUR by inactivating the phosphoinositide 3-kinase (PI3K)/AKT/c-Jun pathway. In addition to its interaction with myosin heavy chain 9 (MYH9), increased ENKUR also recruits β-catenin, which reduces MYH9 transcription induced by c-Jun. Downregulating MYH9 reduces ubiquitin-specific protease 7 (USP7) recruitment, ubiquitination-mediated c-Myc degradation, and epithelial‒mesenchymal transition (EMT) signaling, thus attenuating cisplatin resistance.94 Bufalin may mitigate cisplatin resistance in gastric cancer cells by decreasing AKT, GSK-3β, mTOR, S6 kinase (S6K), and eIF4E-binding protein 1 (4EBP1) activation.79 In addition, cinobufagin could increase the sensitivity of MNNG/HOS and U2OS cells to ADR, which was mediated by increased forkhead box O1 (FOXO1)-mediated transcription of the Fc fragment of the IgG binding protein (FCGBP) in osteosarcoma.95
Relieving Chemotherapy-Induced Cancer Pain
Approximately 80% of patients who receive cytostatic pharmacotherapy may experience chemotherapy-induced peripheral neuropathic pain (CIPNP), a serious side effect.96 As the number of cancer survivors quickly increases, avoiding and treating this adverse reaction becomes increasingly important. Some evidence has demonstrated that Huachansu + chemotherapy not only enhances anticancer effects but also alleviates cancer-related pain symptoms.
Paclitaxel, a commonly used chemotherapy drug, often results in significant PNP with treatment. Ba et al investigated the effects of Huachansu on a rat model of paclitaxel-induced peripheral neuropathic pain (PIPNP). Compared with paclitaxel, a single intraperitoneal injection of Huachansu (2.5 g/kg) reduced mechanical and thermal hypersensitivity, hence alleviating preestablished PIPNP. Frequent intraperitoneal injections of Huachansu (1.25 and 2.5 g/kg) during PIPNP induction inhibited the progression of mechanical and thermal hypersensitivity induced by paclitaxel. This protective effect was linked to reduced spinal tumor necrosis factor-alpha (TNF-α) and interleukin-1beta (IL-1β) production, as well as the inhibition of transient receptor potential vanilloid 1 (TRPV1) upregulation and spinal astrocyte activation induced by paclitaxel. These results suggest that Huachansu may have therapeutic value in treating and preventing PIPNP.97
Colorectal carcinomas are commonly treated with L-OHP as the first-line treatment. Pain and sensory abnormalities may occur in up to 40% of cancer patients treated with platinum drugs. Hao et al studied the effects of Huachansu on a rat model of L-OHP-induced CIPNP. The findings indicated that, in contrast to L-OHP-treated rats, a single injection of 2.5 g/kg Huachansu had a short-term analgesic effect on preestablished CIPNP induced by L-OHP after 60 minutes, as evidenced by reduced mechanical and thermal hypersensitivity, whereas repeated Huachansu administration during CIPNP induction further inhibited CIPNP progression. The protective effect of Huachansu was linked to reduced activation of spinal astrocytes and TRPV1 upregulation produced by L-OHP in the dorsal root ganglia.98
Combined with Targeted Therapy
Enhancing the Effect of Targeted Therapy
The active ingredients of toad skin and toad venom, such as bufalin and cinobufagin, could produce sensitive therapeutic effects on HCC and lung cancer via targeted drugs such as gefitinib and sorafenib by inhibiting cell proliferation and migration, arresting the cell cycle, triggering cell apoptosis, and preventing tumor angiogenesis.
In PLC/PRF/5 and HepG-2 cells, bufalin can increase AKT phosphorylation to augment the sorafenib-mediated reduction in extracellular signal-regulated kinase (ERK) phosphorylation, thereby increasing the antitumor activity of sorafenib.99 Sorafenib + bufalin synergistically induced the apoptosis of PLC/PRF/5 and SMMC‑7721 cells by increasing the expression of Bax, Caspase 7 and PARP.100 Furthermore, by downregulating Akt/NF-κB signaling, bufalin and cinobufagin combined with sorafenib might synergistically suppress proliferation and trigger apoptosis and S phase arrest in HepG2 cells. Compared with single therapy, the expression levels of IκB, Bax, and Caspase 3/8 greatly increased, while the expression levels of p-Akt (Ser473), p-NF-κB p65, Bcl-2, proliferating cell nuclear antigen (PCNA), cyclin-dependent kinase 2 (CDK2) and cyclin A were obviously decreased in response to combination therapy.101,102
Bufalin + sorafenib could exert synergistic antiangiogenic effects on an intradermal HCC tumor model. In human umbilical vein endothelial cells (HUVECs), bufalin + sorafenib also increased apoptosis and suppressed proliferation, migration and blood vessel formation by decreasing the levels of angiogenin, platelet-derived growth factor-BB (PDGF-BB) and vascular endothelial growth factor (VEGF). Additional research has shown that bufalin can increase the antiangiogenic activity of sorafenib by modifying the AKT/VEGF pathway.103 Some researchers have also studied the synergistic anticancer activity of bufalin + sorafenib through the effects of the tumor vascular microenvironment on HCC cells and HUVECs. Bufalin + sorafenib strongly inhibited the migration and tubule formation of HUVECs and reduced the secretion of VEGF by HCC cells, and VEGF incubation reversed the suppression of HUVEC tube formation. In vivo tests also revealed that the mice administered bufalin + sorafenib had the lowest expression of VEGF in the subcutaneous HCC tumor model. Furthermore, HUVECs pretreated with the PI3K inhibitor PI103 presented lower levels of p-mTOR. Moreover, PI103 pretreatment inhibited HCC cell motility and HUVEC tube formation. The results showed that bufalin + sorafenib synergistically influence the tumor vascular milieu by modulating the mTOR/VEGF pathway, reducing VEGF release. A reduction in VEGF–vascular endothelial growth factor receptor (VEGFR) binding on vascular endothelial cells inhibited tumor angiogenesis.104
Bufalin + gefitinib markedly inhibited proliferation and induced apoptosis in H1975 cells by downregulating p-epidermal growth factor receptor (EGFR), p-Met, p-Akt and p-mTOR, indicating that the potential antitumor mechanism involves the inhibition of EGFR-Pl3K/Akt signaling.105 Cinobufotalin + gefitinib suppressed A549 cell viability, facilitated apoptosis, increased the generation of ROS and caused S phase arrest, as evidenced by the reductions in CDK2, cyclin A, and cyclin E levels and the increase in p21 levels. Furthermore, the downregulation of hepatocyte growth factor (HGF) and c-Met levels suggested that cinobufotalin may postpone the development of gefitinib resistance in lung cancer cells.106 Compared with sorafenib or bufalin therapy alone, sorafenib + bufalin also increased chromatin condensation and cell apoptosis by triggering increased expression of apoptotic protease activating factor-1 (APAF-1), caspase-3/-9, superoxide dismutase (SOD), Bax, and BCL2-associated agonist of cell death (Bad), decreased expression of Bcl-2 and catalase, and a reduced ΔΨm, thereby displaying stronger cytotoxic effects on NCI-H292 human lung cancer cells.107
Reversing Resistance to Targeted Therapy
Several studies have shown that targeted drugs such as gefitinib, sorafenib, afatinib or osimertinib combined with bufalin may help overcome acquired tumor resistance. For example, afatinib + bufalin obviously suppressed the proliferation of afatinib-resistant H1975 cells and prevented H1975 cell invasion. Western blot analysis revealed that the combination treatment significantly decreased the p⁃cMet, p⁃EGFR, p⁃ERK, p⁃AKT, snail and vimentin protein levels and increased the E⁃cadherin protein level in afatinib-resistant H1975 cells, indicating that the reversal of afatinib resistance in HGF-induced H1975 lung cancer cells by bufalin may be associated with blocking the EMT process and suppressing cMet/mitogen-activated protein kinase (MAPK)/ERK and cMet/PI3K/AKT signaling.108 In addition, by inhibiting sorafenib-mediated Akt activation, bufalin was able to overcome the intrinsic and acquired resistance of HCC cells to the drug. This effect might be attributed to bufalin-induced ER stress, which then decreases p-Akt levels, reduces cell proliferation, and triggers apoptosis. Furthermore, its action relies on the IRE1 pathway.109 Moreover, in gefitinib-resistant H460 cells, bufalin markedly suppressed cell adhesion, migration and invasion at concentrations ranging from 2.5 nM to 10 nM by reducing the expression of son of sevenless homolog 1 (SOS-1), matrix metalloproteinase-2 (MMP-2), Ras homolog gene family, member A (RhoA), urokinase-type plasminogen activator (uPA), p-FAK, p-ERK1/2, Ras, E-cadherin and tissue inhibitor of matrix metalloprotease 1 (TIMP1) after 24 or 48 h of treatment.110 Patients with EGFR-activating mutations in NSCLC are now treated with osimertinib as the conventional treatment; however, osimertinib-induced acquired resistance may be caused by elevated levels of myeloid cell leukemia-1 (MCL-1). Bufalin can suppress Ku70-mediated MCL-1 overexpression, hence restoring the susceptibility of resistant cells to osimertinib-induced growth inhibition and apoptosis.111 The mechanism of action of the active ingredients of toad skin and toad venom combined with chemotherapy and targeted therapy is shown in Figure 2.
Combined with Radiotherapy or Hyperthermia
One of the most important factors influencing the radioresistance of glioblastoma cells is effective DNA damage repair. The Rad51 protein is essential for genomic stability and the regular cell cycle, as well as for homologous recombination (HR). Bufalin could increase the radiosensitivity of glioblastoma via the suppression of DNA damage repair by increasing phosphorylated histone H2AX (γ-H2AX) levels and decreasing Rad51 protein levels and HR efficiency.112 In addition, Huachansu has the ability to increase the effectiveness of radiotherapy in H460 cells. Huachansu can prolong the existence of radiation-induced γ-H2AX foci to inhibit DNA repair and increase radiation-induced apoptosis by increasing the levels of cleaved caspase-3 and cleaved PARP proteins and reducing the levels of the Bcl-2 and p53 proteins.113 Moreover, bufalin/cinobufotalin combined with radiotherapy/hyperthermia could significantly increase the degree of DNA fragmentation, decrease the ΔΨm, and increase the level of cleaved caspase-3 to increase cell apoptosis.114 The EMT is a complex biological mechanism that plays critical roles in tumor migration, invasion, and resistance to radiation. Bufalin was able to abrogate the radiation-induced EMT by suppressing the levels of vimentin, N-cadherin, Snail, and Slug and increasing the expression of E-cadherin through the targeting of Src signaling, thus achieving radiosensitization effects on NSCLC.115
Combined with Several Signaling Pathway Inhibitors
The AKT family of kinase enzymes, which includes AKT1, AKT2, and AKT3, is a crucial PI3K partner. In multiple myeloma (MM) plasma cells, AKT kinase is activated, which sensitizes the antiapoptotic pathway and is related to drug resistance. Although bufalin triggers cell apoptosis, it can also induce the phosphorylation of AKT, thereby preventing the drug from killing cells and promoting the development of therapeutic resistance. Some studies have shown that the combination of bufalin with the AKT inhibitor MK2206 can enhance the antitumor effect on MM. For example, bufalin combined with MK2206 increased cell apoptosis by downregulating p-Akt induced by bufalin and increasing the levels of cleaved PARP and cleaved caspase 3 in H929 human MM cells.116 In addition, MK2206 + bufalin synergistically inhibited the proliferation and triggered the apoptosis of MM cells by suppressing the AKT/mTOR signaling. This phenomenon was also observed after MM cells were cocultured with bone marrow stromal cells (BMSCs) and/or incubated with interleukin-6 (IL-6). The anti-MM effects of bortezomib-resistant cell lines (NCI-H929R and U266R) were comparable to those of the combination therapy. Furthermore, synergistic effects were also observed in MM xenografts in BALB-c mice, NOD-SCID mouse tumor models, and primary MM cells. In summary, MK2206 + bufalin considerably improved antitumor activity by blocking AKT/mTOR signaling, irrespective of MM cell susceptibility to bortezomib.117
In HepG2 and Huh-7 cells, cinobufagin triggers cytoprotective autophagy by increasing the levels of the microtubule-associated proteins 1A/1B light chain 3B (LC3B)-II, Beclin1 and autophagy-related gene (Atg)12-Atg5 to counteract its anticancer effects. Cinobufagin + the autophagy inhibitor MRT668921 was able to enhance the antiproliferative and proapoptotic effects by suppressing autophagy signaling through decreased expression of Bcl-2, PCNA, Beclin1, and Atg12-Atg5 and a decrease in the LC3B-II/LC3B-I ratio in vitro and in vivo.118
The Hedgehog signaling pathway is a highly conserved cellular signal transduction pathway that controls cell growth and survival in a variety of malignancies. Bufalin + hedgehog signaling inhibitors (GANT61 and cyclopamine) clearly suppressed proliferation; induced apoptosis and G2 + S phase arrest; inhibited adhesion, migration and invasion; and suppressed ECM degradation, the EMT process and tumor angiogenesis by regulating the expression of the hedgehog signaling proteins patched homolog 1 (Ptch1), GLI family zinc finger 1 (Gli1) and GLI family zinc finger 3 (Gli3) in HCC cells. Additionally, the combination treatment had the potential to increase E-cadherin expression by altering the Gli3 protein and decreasing the expression of downstream molecules, including MMP-2/9, β-catenin and VEGF, through effects on the Gli1 and Gli3 proteins.119
Combined with the Active Ingredients of TCM
Compared with monotreatment, cotreatment with bufalin and cinobufagin synergistically potentiated the anticancer effect on HepG2 cells. The combination therapy triggered apoptosis and cell cycle arrest by regulating metabolic pathways, including amino acid metabolism, energy metabolism, methionine metabolism and lipid metabolism, as well as glutathione (GSH) biosynthesis, as revealed by metabolomic and lipidomic profiling using liquid chromatography‒mass spectrometry (LC‒MS).120 The authors also showed that the intraperitoneal injection of the combination of 2 mg/kg bufalin and 4 mg/kg cinobufagin synergistically inhibited the growth of a HepG2-xenograft tumor model. A comprehensive approach based on MS-based lipidomics and matrix-assisted laser desorption ionization–mass spectrometry imaging (MALDI–MSI) has shown that cotreatment with bufalin and cinobufagin can regulate sphingolipid metabolism and glycerophospholipid metabolism by increasing the abundance of phosphatidylcholine (PC) and phosphatidylglycerol (PG), and decreasing the abundance of phosphatidylserine (PS) and phosphatidylethanolamine (PE), resulting in cell apoptosis triggered by mitochondria and the breakdown of tumor cell membranes. Additionally, altered lipid markers, which are key components of the tumor framework, are mostly found in nonnecrotic tumor regions.121
Combined with Other Therapies
Photodynamic therapy (TiO2) combined with bufalin enhanced the antitumor effect on human melanoma A375 cells by inhibiting cell proliferation, arresting the cell cycle at S phase, and inducing cell apoptosis by increasing ROS levels and the Bax/Bcl-2 ratio.122 In addition, high-intensity focused ultrasound (HIFU) combined with bufalin further suppressed proliferation and trigger apoptosis by increasing PARP expression, increasing activated caspase-3 and caspase-8 activation and decreasing Ki-67 expression in MiaPaCa2 and Panc-1 cells and in a MiaPaCa2 tumor model compared with single therapy.123 c-Myc, a proto-oncogene, is overexpressed in more than 50% of human malignancies and is associated with the formation and progression of several tumor types, including pancreatic cancer. siRNA-c-Myc + bufalin synergistically enhanced apoptosis, cell cycle arrest, migration and invasion by blocking hypoxia-inducible factor-1alpha (HIF-1a)/stromal cell-derived factor 1 (SDF-1)/C-X-C chemokine receptor type 4 (CXCR4) signaling in pancreatic cancer.124 The expression of microRNA-497 (miR-497) is decreased in colorectal cancer and is linked to increased tumor proliferation and metastasis and a poor prognosis. MiR-497 + bufalin markedly enhanced the anti-colorectal cancer effect by inhibiting tumor proliferation and metastasis.125 As a member of the TNF superfamily, the type II transmembrane protein known as TNF‑related apoptosis‑inducing ligand (TRAIL) can trigger programmed cell death. Bufalin + TRAIL greatly improved TRAIL‑mediated apoptosis and cell viability inhibition by upregulating death receptor 5 (DR5), downregulating the expression of cellular Fas‑associated death domain‑like interleukin‑1β‑converting enzyme inhibitory protein (cFLIP) and X‑linked inhibitor of apoptosis protein (XIAP), and activating caspase‑3, ‑8 and ‑9 in T24 cells.126 Table 5 summarizes the combination treatment strategies based on toad skin or toad venom-related active ingredients in vitro and in animal models.
 |
Table 5 Combination Treatment Strategies Based on Toad Skin or Toad Venom-Related Active Ingredients in vitro and in Animal Models |
 |
Table 6 Codelivery Systems Based on Toad Skin or Toad Venom-Related Active Ingredients |
Combined Antitumor Strategies Based on Drug Delivery Systems
Compared with the traditional “cocktail” therapy, the multidrug combination strategy for tumors based on a nanodelivery system can not only increase serum stability and biocompatibility, prolong the half-life in vivo, and improve the targeting ability and permeability of drugs at the tumor site but also realize the precise compatibility of combined drugs and specific stimulus-responsive release in the presence of external stimuli or the tumor microenvironment to enhance the therapeutic effect, reduce adverse reactions and resist MDR.149,150 Codelivery systems based on the active ingredients of toad skin and toad venom include codelivery systems for bufalin, gamabufotalin, and cinobufagin combined with chemotherapy drugs, small-molecule targeted drugs, monoclonal antibodies, the active ingredients of TCM, PDT and photothermal therapy (PTT) (Figure 3 and Table 6).
 |
Figure 3 Schematic diagram of the classification of co-delivery systems based on active ingredients of toad skin and toad venom. |
Codelivery of Active Ingredients of Toad Skin/Toad Venom and Chemotherapy Drugs
Loading bufalin and paclitaxel onto nanocarriers can enhance their antitumor effects. Paclitaxel- and bufalin-loaded mPEG-cholic acid (CA)/D-a-tocopherol polyethylene glycol 1000 succinate (TPGS) polymer micelles (PTX/PCTm and BF/PCTm) were prepared for combined HCC treatment. The circulation time could be extended to 48 h in the liver and tumor by the PEG modification. Moreover, a liver-targeting ability could be achieved by the interaction of cholic acid groups and the Na+-taurocholate cotransporter polypeptide (NTCP) receptor. PTX/PCTm + BF/PCTm (PB/PCTm) could achieve a synergistic therapeutic effect on HepG2 cells. Moreover, in vivo tests revealed that PB/PCTm (5 mg/kg paclitaxel and 1 mg/kg bufalin) had the highest tumor suppression rate (82.29%) without obvious toxicity, which was significantly greater than that of Taxol® (41.17%), PTX/PCTm (58.26%) and BF/PCTm (73.54%) in a bioluminescence orthotopic HCC model.127
DOX + gamabufotalin synergistically inhibited the growth of MDA-MB-231 cells at a molar ratio of 10:1. Based on these findings, a DOX and gamabufotalin coloaded RGD-modified erythrocyte membrane-camouflaged graphene oxide quantum dots (GOQDs)-PEG-TAT@DOX@CS-6 biomimetic nanoplatform (GTDC@M-R NPs) was developed for the treatment of triple-negative breast cancer (TNBC). The tumor cell- and nucleus-targeting abilities of gamabufotalin and DOX were effectively enhanced by the TAT and RGD peptides. Moreover, this nanosystem could achieve the controlled release of drugs through a pH response. Approximately 26.5% of the DOX and 21.4% of the gamabufotalin were released from the GTDC@M-R NPs over 72 h at pH 7.4, while the release rates increased to 59.5% and 56.2% at pH 5.2, which effectively reduced drug release in the blood environment while increasing drug release in the tumor tissue to prevent side effects. In MDA-MB-231 cells, this nanosystem synergistically promoted apoptosis via Bax/Bcl-2 and p53 signaling and inhibited cell metastasis by downregulating VEGF and matrix metalloproteinase 9 (MMP-9) expression and inhibiting EMT activation. Moreover, in vivo experiments demonstrated that the accumulation of GTDC@M-R NPs at tumor locations was almost twice as high as that of GTDC NPs, along with a significant tumor inhibition rate (84.4%) and a high inhibition rate for lung metastatic nodules (80%) through the inhibition of chemotherapy-activated cyclooxygenase-2 (COX-2), MMP-9 and VEGF (Figure 4). Furthermore, the GTDC@M-R NPs significantly reversed the inflammatory reaction caused by the free drugs by decreasing white blood cell counts and increasing platelet counts, significantly reversed the negative effects of DOX on the liver (ALT and AST) and kidney (creatinine and blood urea nitrogen), and caused nearly no pathological changes in the main organs (heart, liver, spleen, and kidney).128
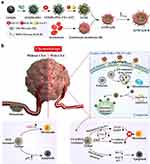 |
Figure 4 (a) Preparation scheme of RGD-modified erythrocyte membrane camouflaged GOQDs-PEG-TAT@DOX@CS-6 nanosystem (GTDC@M-R NPs). (b) Proposed mechanism of GTDC@M-R NPs mediated tumor ablation and metastasis inhibition, and GTDC@M-R NPs-mediated transfection of DOX and CS-6 in MDA-MB-231 cells. Reprinted from Acta Biomaterialia, Volume 113, Fan J, Liu B, Long Y, et al, Sequentially-targeted biomimetic nano drug system for triple-negative breast cancer ablation and lung metastasis inhibition, Pages 554–569, Copyright 2020, with permission from Elsevier.128 Abbreviations: GOQDs, Graphene oxide quantum dots; CS-6, Gamabufotalin; DOX, Doxorubicin; COX-2, Cyclooxygenase-2; VEGF, Vascular endothelial growth factor; MMP9, Matrix metalloproteinase 9; EMT, Epithelial-mesenchymal transition; Bcl-2, B-cell lymphoma; BAX, Bcl-2-like protein 4; NPs, Nanoparticles. |
In addition, a vitamin-E–succinate-grafted chitosan oligosaccharide (VES-CSO) and RGD-modified bufalin-loaded TPGS multifunctional mixed micelles (VeC/T-RGD MMs) were constructed via the emulsion solvent evaporation method to enhance L-OHP/DOX-resistant colon cancer treatment. Compared with free bufalin, VeC/T-RGD MMs reduced P-gp expression and increased apoptosis in LoVo/ADR cells. Moreover, these micelles notably suppressed tumor growth (65%) in the LoVo/ADR tumor-bearing model compared with free bufalin (22%). Moreover, these micelles effectively reversed pathological damage, including cardiomyocyte necrosis, connective tissue proliferation, neutrophil infiltration, liver cell necrosis, renal tubular necrosis, and apoptosis, caused by free bufalin. This improvement can be attributed to the enhanced permeability and retention (EPR) effect and RGD-mediated active targeting.129
Codelivery of Active Ingredients of Toad Skin/Toad Venom and Small-Molecule Targeted Drugs
Nintedanib is a potent inhibitor of several tyrosine kinases. It specifically binds to ATP-binding sites located inside the kinase domains of VEGFR 1–3, fibroblast growth factor receptor (FGFR) 1–4, platelet-derived growth factor receptor (PDGFR) α/β and c-Src. It can also regulate the tumor microenvironment to inhibit cancer cells. Coloaded bufalin and nintedanib multifunctional albumin submicrospheres (BF-ND-BUP-sMPs) were developed via coaxial-electrospray technology to synergistically enhance the effect of HCC therapy. The tumor targeting ability was effectively enhanced by modification with biguanide and ursodeoxycholic acid. An in vivo study revealed that the BF-ND-BUP-sMPs had the greatest tumor growth inhibition effect (84.2%) on the H22 cell xenograft model, which was significantly greater than that of the BF-BUP-sMPs (66.5%) and ND-BUP-sMPs (58.7%). The mechanism involved enhanced cell apoptosis and changes in the tumor microenvironment structure through the inhibition of angiogenesis, tumor-associated fibroblasts and stroma by decreasing the levels of CD31, alpha-smooth muscle actin (α-SMA) and collagen fibers. Moreover, these BF-ND-BUP-sMPs could significantly attenuate cardiac tissue lesions and inhibit inflammation in the myocardial interstitium caused by bufalin-induced cardiotoxicity, which can be attributed to the dual modification of biguanide and ursodeoxycholic acid.130 Moreover, the BF-ND-BUP-sMPs strengthened the tumor growth inhibitory effect on LLC cells. In vivo pharmacokinetic assays revealed that, compared with those of free bufalin and nintedanib, the t1/2 values of bufalin and nintedanib in BF-ND-BUP-sMPs were 5.6 and 2.4 times higher, the area under the curve (AUC)0-t was 4.5- and 2.1-fold higher, and the mean residence time (MRT) was 3.4 and 2.1 times higher, respectively, indicating that the albumin shell improved sMP stability in the circulatory system.131
By binding to the ATP site of the catalytic domain of VEGFR2, apatinib, a tyrosine kinase inhibitor, can prevent tumor cells from proliferating, metastasizing, invading, and promoting angiogenesis. Biomimetic coloaded apatinib and cinobufagin liposomes with pH-responsive properties (LP-R/C@AC NPs), which have the benefits of evading the immune system and specifically homologously targeting tumor cells, were developed to effectively treat gastric cancer. The hybrid membrane derived from red blood cells and HGC-27 gastric cancer cells extended the t1/2 and enhanced drug accumulation in solid and metastatic tumor tissues. The drug release assay revealed that approximately 82% of apatinib and 89% of cinobufagin were released from LP-R/C@AC NPs after 72 h of incubation at pH 5.2, whereas only approximately 44% and 52% of AP and CS-1 were released at pH 7.4, which could effectively decrease the toxicity and adverse reactions of free apatinib and cinobufagin. These liposomes can effectively fight cancer cells by blocking VEGFR2/signal transducer and activator of transcription 3 (STAT3) signaling through the induction of apoptosis, pyroptosis and autophagy; suppression of proliferation, angiogenesis and migration; and improvements in immunity and the hypoxic microenvironment. In addition, these liposomes efficiently suppressed tumor growth (86.78%), invasion and metastasis and improved tumor immunosuppression by inhibiting programmed death ligand-1 (PD-L1) and MMP-9 in an HGC-27 tumor-bearing nude BALB/c mouse model. Furthermore, the LP-R/C@AC NPs reversed the damage to the circulatory system caused by the free drugs and restored ALT levels to within the normal range. Moreover, other liver and kidney indicators and H&E-stained pathological sections also revealed the biosafety of the LP-R/C@AC NPs in vivo, which is necessary and critical for clinical application.132
Lenvatinib is also a potent tyrosine kinase inhibitor that effectively suppresses the activity of several kinases, such as VEGFR 1–3, FGFR 1–4, KIT, PDGFR-α/β and RET. PEG- and folic acid (FA)-modified monodisperse mesoporous silica (mSiO2) nanoparticles coloaded lenvatinib and bufalin (Le/Bu@mSiO2-FA) were designed to synergistically enhance the therapeutic effect on cholangiocarcinoma (CCA). Compared with mSiO2, mSiO2-FA exhibited a superior intracellular drug delivery capacity in CCA 9810 cells. Additionally, Le/Bu@mSiO2-FA exhibited both slow release and pH-responsive drug release properties, which effectively prevented side effects related to the drug concentration and enhanced the therapeutic effect (72 h, pH = 7.4/5.5, the rate of bufalin release = 33.47/74.27%). Le/Bu@mSiO2-FA effectively inhibited the viability, migration, and invasion of 9810 cells. Furthermore, in the CCA tumor-bearing model, these nanoparticles dramatically decreased the tumor burden compared with lenvatinib or bufalin alone (Figure 5). Furthermore, no obvious changes in the ALT and AST contents and no obvious pathological damage to the heart, liver, spleen, lung, or kidney were observed, indicating that Le/Bu@mSiO2-FA has good biological safety, which may be due to FA-mediated active targeting and pH-responsive drug release.133
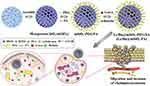 |
Figure 5 The synthesis process of Le/Bu@mSiO2-FA. Reproduced with permission from Ning Z, Zhao Y, Yan X, Hua Y, Meng Z. Flower-like composite material delivery of co-packaged lenvatinib and bufalin prevents the migration and invasion of cholangiocarcinoma. Nanomaterials-Basel. 2022;12(12):2048. Copyright 2022, MDPI AG. (https://creativecommons.org/licenses/by/4.0/).133 Abbreviations: TEOS, Tetraethyl orthosilicate; FA, Folic acid; TEA, Triethanolamine; BTES, bis[3-(triethoxysilyl)propyl] tetrasulfide; CTAB, Hexadecyltrimethylammonium bromide; PEG, Polyethylene glycol. |
Codelivery of Active Ingredients of Toad Skin/Toad Venom and Monoclonal Antibodies
T-cell–antigen-presenting cell interactions with anti-CD40 are crucial for the immune response. Anti-CD40 antibody-conjugated bufalin liposomes (BFLs) (anti-CD40-BFLs) were developed to enhance the effects of melanoma therapy and reduce systemic toxicity. Compared with free bufalin and anti-CD40, the anti-CD40-BFLs had the characteristics of sustained release. Compared with bufalin, the anti-CD40-BFLs had the strongest antitumor effect on B16 melanoma cells and tumor models. The enhanced therapeutic efficacy was partly due to bufalin-mediated apoptosis through caspase-dependent signaling; moreover, the interaction between anti-CD40 and CD40 adhesion molecules on antigen-presenting cells, such as dendritic cells, promoted the secretion of cytokines, leading to the generation of an ample number of cytotoxic T lymphocytes and a widespread immune response. Moreover, the administration of anti-CD40-BFLs resulted in no noticeable changes in body weight and notable decreases in the serum concentrations of TNF-α, IL-1β, IL-6, interferon-gamma (INF-γ), and alanine aminotransferase (ALT), indicating fewer adverse reactions.134
Codelivery of the Active Ingredients of Toad Skin/Toad Venom and the Active Ingredients of TCM
In China, local administration of realgar (tetraarsenic tetrasulfide) combined with toad venom is a frequent therapeutic approach for treating gynecological cancers, including ovarian and cervical cancer. Based on this information, a temperature-sensitive in situ gel coloaded with nanorealgar (NR) and toad venom-loaded solid lipid nanoparticles (TV-SLNs) (TV-SLNs/NR-TISG) was prepared for the treatment of cervical cancer via vaginal delivery. The coadministration of TV-SLNs and NR at a dosage ratio of 2:3 (w/w) resulted in the greatest inhibition of tumor cell proliferation. Following TV-SLN/NR treatment, HeLa cells exhibited more pronounced arrest in S and G2/M phases, whereas SKOV-3 cells exhibited greater arrest in G0/G1 phase than did the traditional powder group. The optimum formulation of TISG consisted of F127 (27.5%) and F68 (5%), resulting in gelation at a temperature of 33 ± 0.91 °C. Furthermore, this gel displayed good biocompatibility without inflammatory reactions in pathological sections when administered continuously for seven days, allowing the prolonged release of drugs by attachment to the vaginal mucosa.135 In addition, a coloaded bufalin, cinobufagin and resibufogenin solid dispersion was prepared via spray congealing. Three drugs were shown to be molecularly dispersed inside the matrix, and the solid dispersion significantly increased the dissolution rate of the drugs, which was four times faster than the release rate of the physical mixture. Moreover, the simultaneous release of three medicines from the matrix was accomplished as a result of the outstanding molecular dispersibility and solubilization capability of F127. Furthermore, under controlled conditions, the solid dispersion remained physically stable for a minimum of one month.136 The antitumor effect of solid dispersions needs to be further studied in the future.
Moreover, a cetuximab-conjugated immunoliposome loaded with bufalin and melittin was prepared to inhibit sorafenib resistance in HCC. The compound immune liposomes can effectively induce the complement-dependent cytotoxicity and antibody-dependent cell-mediated cytotoxicity of cetuximab. Compared with free drugs, the liposomes can inhibit cell proliferation and trigger apoptosis more effectively via the activation of ER signaling. An in vivo study also revealed that the liposomes had a stronger antitumor effect than free melitoxin/bufarin and could more effectively prolong the survival time of tumor-bearing nude mice. In addition, the H&E staining results for the liposomes revealed no significant hepatic or renal toxicity compared with that of melittin or melittin/bufalin, which may be attributed to the fact that cetuximab can actively target HER1 to reduce the accumulation of the drug in other organs.137 Based on the “unification of drugs and excipients” theory, toad skin extract (TSE) and Brucea javanica oil (BJO) coloaded nanoemulsions (TSE-BJO NEs) were also prepared. BJO not only is the main oil phase for the formation of nanoemulsions but also exerts a synergistic anti-liver cancer effect when combined with TSE. This nanoemulsion increased drug accumulation at the tumor site, extended the drug retention time, and resolved the issue of the fast clearance of hydrophobic active ingredients of toad skin in vivo. Moreover, the combined TSE-BJO NEs exerted synergistic antitumor effects by suppressing proliferation, arresting the cell cycle and triggering apoptosis in HepG2 cells and a HepG2 xenograft tumor model. In addition, the results of routine blood, liver and kidney indices and H&E staining of pathological sections all indicated that TSE-BJO NEs exhibited good biosafety.138
Codelivery of Active Ingredients of Toad Skin/Toad Venom and Photodynamic Therapy Drugs
PDT kills cancer cells through the generation of ROS; however, the hypoxic tumor microenvironment significantly limits its effectiveness. Since bufalin can significantly increase the effectiveness of PDT mediated by the photosensitizer meta-tetrahydroxyphenylchlorin (mTHPC) in treating colorectal cancer, VES-CSO/RGD-modified TPGS (TPGS-RGD) multifunctional nanoparticles were constructed to codeliver bufalin and mTHPC [mTHPCandBU@VES-CSO/TPGS-RGD nanoparticles (T-B@NPs)] and achieve synergistic colorectal cancer therapy. In vitro assays indicated that T-B@NPs increased the sensitivity of colorectal cancer cells to PDT via antiangiogenic effects and reduced hypoxia by targeting SRC-3/HIF-1α/VEGF signaling. In addition, T-B@NPs have a sustained drug release behavior that favors the passive accumulation of nanoparticles in tumor tissues. In vivo studies using a CT26 tumor-bearing BALB/c mouse model demonstrated that T-B@NPs + laser had the strongest antitumor effect (84.2%), which was greater than that of T@NPs + laser (77.6%), B@NPs (65.2%), free mTHPC + laser (40.0%) and bufalin alone (45.8%), by relieving the oxygen deficiency and inducing antiangiogenic effects. Moreover, T-B@NPs + laser greatly prolonged the survival rate of the mice, and no obvious organ damage was observed during treatment, which was attributed to RGD-mediated active targeting and the antitumor ability in combination with PDT.139
Moreover, erythrocyte‑cancer hybrid membrane-coated cinobufagin and Chlorin e6 (Ce6)-coloaded polyacrylic acid-modified cerium nanoparticles [CeO2@PAA@CS-1/Ce6@M (CPCCM NPs)] were developed for synergistic chemo/PDT against TNBC. The hybrid erythrocyte/MDA-MB-231 cell membrane endows the nanomedicine with homologous targeting ability and long circulation times in the blood. The PAA modification could control the release of drugs. Approximately 22.31% of the cinobufagin was released from the CPCCM NPs after 5 h of incubation at pH 7.4, while the release rate increased to 39.78% at pH 6.8, and laser irradiation further accelerated drug release (72.84% within 90 min), which effectively prevented the side effects of free cinobufagin/Ce6 and enhanced the therapeutic effect. Interestingly, CPCCM NPs can also autonomously generate O2 by decomposing excess H2O2 within tumor tissue and reprogramming the hypoxic tumor microenvironment, which accordingly enhances the effectiveness of cinobufagin and PDT. In vivo studies also revealed that cinobufagin + PDT had a high tumor growth inhibition rate (85.5%) and markedly prevented lung and liver metastases by inhibiting HIF-1α and MMP-9 expression. In addition, CPCCM NPs + laser could reduce the inflammatory response (white blood cells) or reverse the side effects (ALT and AST) caused by free cinobufagin or PDT, and H&E-stained pathological sections also revealed no obvious damage to the main organs, suggesting that the CPCCM NPs have obvious biosafety in vivo (Figure 6).140
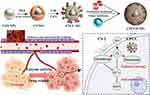 |
Figure 6 Schematic diagram of the designed strategy for the combined therapy of CPCCM NPs. Reproduced with permission from Zeng Z, Wang Z, Chen S, et al. Bio-nanocomplexes with autonomous O(2) generation efficiently inhibit triple negative breast cancer through enhanced chemo-PDT. J Nanobiotechnol. 2022;20(1):500. Copyright 2022, BioMed Central.140 Abbreviations: CS-1, Cinobufagin; Ce6, Chlorin e6; PAA, Polyacrylic acid; MMP9, Matrix metalloproteinase 9; HIF 1α, Hypoxia-inducible factor-1alpha; CPCC, Ce6 and CS-1 loaded cerium nanoparticles. |
Codelivery of Active Ingredients of Toad Skin/Toad Venom and Photothermal Therapy Drugs
PTT is an optical treatment strategy that can convert light energy into heat energy for tumor treatment and has the advantages of minimal invasiveness, few adverse reactions, high specificity, and repeatable treatment. Some studies have shown that bufalin, cinobufagin and gamabufotalin combined with PTT can achieve synergistic chemo/photothermal therapeutic effects.
Hybrid erythrocyte/SW480 cell membranes (M) camouflaged with cinobufagin-loaded Prussian blue nanoparticles (PC@M NPs) were prepared for combined chemo/PTT against colorectal cancer. The camouflaged hybrid membrane exhibited enhanced cellular internalization and a favorable ability to escape the endolysosomal system, along with a strong homologous targeting capacity to tumor cells. In vitro release studies have shown that low pH (24 h, pH = 5.2/7.4, 59.2% vs 42.4%) and near-infrared (NIR) light stimulation (24 h, pH = 5.2, + laser irradiation reached 74.8%) can increase the release of drugs. Moreover, by increasing ROS levels and the Bax/Bcl-2 ratio and decreasing vimentin, MMP-9, and HIF-1a expression, PC@M NPs have the potential to both efficiently trigger apoptosis and suppress metastasis in colorectal cancer cells. In vivo studies also revealed that PC@M NPs + laser markedly inhibited tumor growth (84.57%) by activating mitochondria-driven apoptosis signaling and markedly prevented lung metastasis and recurrence by reducing the MMP-9 level and blocking the EMT process. In addition, PC@M NPs + laser effectively reduced the cardiotoxicity caused by cinobufagin alone, reduced the number of white blood cells due to its strong anti-inflammatory effect, and caused no obvious damage to the liver and kidney indices or major organs, which may be attributed to the excellent homologous targeting ability of the cell membrane, pH-responsive behavior and PTT ability. Notably, PC@M NPs + laser reversed the proliferation of detrimental bacteria and the reduction in the number of good bacteria in the intestinal tract of tumor-bearing mice (Figure 7).141
 |
Figure 7 Schematic depiction of preparation (A) and in vivo application (B) of PC@M NPs. Reproduced with permission from Luo M, Tan C, Cao R, et al. Hybrid membrane camouflaged prussian blue nanoparticles with cinobufagin loading for chemo/photothermal therapy of colorectal cancer. Mater Design. 2023;232:112088. Copyright 2023, Elsevier Ltd.141 Abbreviations: CS-1, Cinobufagin; PTT, Photothermal therapy; ROS, Reactive oxygen species; Bax, Bcl-2-like protein 4; bcl-2, B-cell lymphoma; EMT, Epithelial-mesenchymal transition; MMP-9, Matrix metalloproteinase 9. |
Red blood cell (RBC) membrane-camouflaged hyaluronic acid (HA)-modified PB NPs loaded with gamabutolin, referred to as HA@RBC@PB@CS-6 NPs (HRPCs), were developed for synergistic chemo/PTT against breast cancer. The erythrocyte membrane and HA on the nanoparticles increased the blood circulation time by 10 h, increased immune evasion by more than 60%, and increased drug accumulation at the tumor site by binding specifically to the CD44 receptor. Moreover, the release behavior of HRPC is pH- and temperature dependent. The cumulative rate of gamabutolin release at pH 5.0 was 33.40% after 4 cycles of laser on/off treatment, whereas it was only 19.34% at pH 7.4, which is favorable for increasing the release of drugs in the tumor region and reducing the adverse effects on other normal organs. Notably, the excellent antitumor activity of the gamabutolin-containing nanomedicine is partly due to its ability to suppress heat shock protein 70 (HSP70), which accordingly enhances the effectiveness of PTT. Moreover, in vivo studies also revealed that HRPCs + laser had the strongest antitumor effect (93.4%), which was superior to that of HRPCs (23.1%), RPCs + laser (45.4%) and free gamabutolin (58.8%) in an MDA-MB-231 tumor-bearing model. In addition, HRPCs + laser significantly reversed gamabutolin-induced hepatorenal toxicity (AST, ALT and creatinine levels returned to normal), and no damage to major organs (heart, liver, spleen or kidney) was detected.142
FA-modified cinobufagin-loaded polydopamine (PDA) nanoparticles were constructed for synergistic chemo/PTT against lung cancer. PDA nanoparticles can achieve active targeting of tumor cells via specific binding of FA and FA receptors. The PDA nanomedicine subsequently achieved pH-responsive and NIR irradiation-triggered drug release (12 h, pH = 7.4/5.0 released 24% vs 53%, pH = 7.4/5.0 + laser released 33%/74%). In vivo experiments on an LLC tumor-bearing model revealed that the PDA nanomedicine + NIR laser had excellent multimodal therapeutic effects (tumor inhibition rate: 67%). In addition, compared with free cinobufagin, the PDA nanodrug could decrease hepatorenal toxicity (ALT, AST, and creatinine), which may be attributed to FA-mediated active targeting and pH- and light-triggered drug release.143
An Ln229 glioma cell membrane camouflaged with cinobufotalin-loaded Cu2-xSe nanoparticles (Cu2-xSe-CB@MEM, CCM) was designed to enhance orthotopic glioblastoma therapy through synergistic chemo/photothermal therapeutic effects. The coated Ln229 cell membrane allowed the nanoparticles to penetrate blood‒brain barrier, and the nanoparticles exhibited a homologous tumor-targeting capacity. In vitro release studies revealed that CCM could achieve pH-responsive drug release (120 min, pH = 7.4/5.0, 39.6% vs 54.1% release, respectively). Cinobufotalin + Cu2-xSe-mediated PTT exhibited remarkable glioma cell inhibitory efficacy through the induction of G2/M phase arrest and apoptosis. Moreover, CCM + laser significantly suppressed tumor growth and extended the survival time in an Ln229-luc glioblastoma orthotopic nude mouse model. In addition, CCM + laser reversed the negative effects of cinobufotalin on the liver (ALT and AST) and caused no obvious damage to the main organs (heart, liver, spleen, lung, and kidney).144
Moreover, black phosphorus nanosheets (BPNSs) and bufalin coloaded with a temperature-sensitive supramolecular polypeptide hydrogel (BP-bufalin@SH) were successfully developed for synergistic chemo/PTT. Upon NIR irradiation (808 nm, 1 W/cm2), the temperature of the BP-bufalin@SH mixture rapidly increased and promoted the smart, photocontrolled release of bufalin, which significantly reduced the adverse effects of bufalin. This hydrogel was able to increase cell apoptosis by disrupting the ΔΨm, achieve a remarkable tumor growth inhibition effect and prolong the survival time in a HepG2 tumor-bearing mouse model. In addition, an injection of BP-bufalin@SH did not cause infection or inflammation in mice, and no significant hepatorenal toxicity or organ damage was observed, indicating its excellent biosafety in vivo.145
Erythrocyte‒gastric cancer hybrid cell membrane (HM)-wrapped indocyanine green (ICG) and gamabufotalin coloaded GOQD nanoparticles (GIC@HM NPs) were also successfully designed and established for combined photothermal‒chemotherapy against gastric cancer. The camouflaged hybrid membrane not only improved the nanoparticles’ biocompatibility and in vivo circulation time but also increased drug accumulation at the tumor site. Moreover, the release of gamabufotalin from the GIC@HM NPs could be accelerated by the acidic microenvironment and laser (72 h, pH = 7.4/5.4, 40% vs 60% released, pH = 5.4 + laser 80% release), which effectively reduced the adverse reactions of free drugs and improved the therapeutic effect. In vitro and in vivo experiments on BGC-823 cells and a BGC-823 tumor-bearing mouse model proved that GIC@HM NPs + laser had excellent synergistic antitumor effects. In addition, GIC@HM NPs + laser reversed the negative effects of the free drugs (AST, ALT, uric acid, creatinine and blood urea nitrogen levels returned to normal), and all the indices of the blood samples were within the normal range. No damage to major organs (heart, liver, spleen or kidney) was detected, indicating the excellent biosafety of GIC@HM NPs + laser in vivo.146
Furthermore, human serum albumin (HSA)-modified IR780 or bufalin-loaded trimethyl chitosan (TMC) nanoparticles (TIH or TBH) were prepared for synergistic chemo/PTT against metastatic breast cancer. Notably, TIH-mediated PTT was able to disturb the tumor microenvironment, allowing deeper drug penetration and accordingly leading to increased accumulation of a second TBH dose in the tumor tissue. Moreover, TBH could responsively release bufalin in the acidic tumor environment, which could further enhance the therapeutic effect. Upon NIR irradiation (808 nm, 3 W/cm2, 3 min), the TIH + laser and second TBH injection (TIH+L/TBH) resulted in a high tumor growth inhibition rate of 93% and a remarkable lung metastasis inhibition rate of 98.46%. In addition, good compatibility was revealed by the histological examination and weight changes, which may be related to the ability of HSA modification to improve drug targeting.147
Using superparamagnetic Fe3O4 nanoparticles as photothermal agents and magnetic targeting agents, a photomagnetic dual-sensitive liposome coloaded with Fe3O4 nanoparticles and bufalin (BF-Fe3O4-L) was prepared. Under the action of a magnetic field, BF-Fe3O4-L targeted sentinel lymph nodes located near the tumor and increased the drug uptake rate by 1.6 times. When irradiated by an 808 nm NIR laser (2 W/cm2, 5 min), Fe3O4 nanoparticles can effectively convert light energy into thermal energy (46 °C) and bufalin can be released from liposomes to achieve combined photothermal therapy and chemotherapy for sentinel lymph node metastatic breast cancer. Compared with the PBS group, the BF-Fe3O4-L group presented a 91.0% inhibition of sentinel lymph node metastasis and an 81.5% reduction in distal lung metastasis. In addition, treatment with BF-Fe3O4-L did not result in sudden weight loss in mice, indicating good biosafety in vivo.148
Perspectives and Conclusions
As valuable traditional Chinese medicines, toad skin and toad venom have been shown to exert significant antitumor effects in clinical practice for more than one thousand years. This article reviews three aspects of combined antitumor therapeutic strategies based on toad skin- and toad venom-related preparations and active monomer components: clinical efficacy and safety, antitumor effects and mechanisms in vitro and in vivo, and nanodelivery systems. Clinical studies have shown that chemotherapy drugs, small-molecule targeted drugs or TACE combined with toad skin- and toad venom-related preparations can effectively improve the objective response rate of cancer patients, prolong the survival time of patients, improve the QOL of patients, improve the immune function of patients and reduce the incidence of adverse reactions. However, the current research has focused mainly on combinations with chemotherapy drugs, and no reports on combinations with tumor immunotherapy are available. In recent years, tumor immunotherapy involving PD-1/PD-L1 antibodies has greatly influenced the pattern of tumor therapy and has shown great application potential in tumor therapy. TCM preparations such as toad skin and toad venom also have unique advantages in improving the immune function of the body. Therefore, clinical trials of the combination of toad skin- and toad venom-related preparations with tumor immunotherapy are necessary in the future to better exploit the advantages of TCM in “strengthening the body’s resistance to eliminate pathogenic factors”. In addition, studies of the combined antitumor effect of toad skin- and toad venom-related preparations with additional targeted drugs, such as regorafenib, bevacizumab, and cetuximab, as well as the combined antitumor effect with new anticancer therapies, such as PDT, antibody–drug conjugates, and oncolytic virus therapy, are necessary to provide cancer patients with more options for personalized treatment plans. In addition, evidence-based medical studies have shown that Huachansu, a water-soluble extract of toad skin, has another unique role in the clinical treatment of cancer pain. Huachansu combined with the three-step analgesic method is superior to the three-step analgesic method alone in the treatment of cancer pain, improving QOL and reducing the occurrence of side effects, and has the advantages of shortening the onset time and prolonging the duration of pain relief. Moreover, it can reduce the average daily dosage of opioids and greatly improve the quality of life of patients with middle-stage and advanced cancer. However, some problems exist in the clinical studies reported thus far, such as a small number of case samples, insufficient rigorous study design, and inconsistent clinical efficacy evaluation indicators. In the future, randomized controlled trials with a reasonable design, rigorous implementation, standardized reporting, large sample sizes, multiple centers, and sufficient follow-up time should be conducted to verify the effectiveness and safety of toad skin- and toad venom-related preparations in the treatment of cancer and cancer pain.
Mechanistically, active ingredients in toad skin and toad venom (gamabufotalin, arenobufagin, bufalin, cinobufagin, and cinobufotalin) can enhance the efficacy of chemotherapy, targeted therapy, radiotherapy, hyperthermia, signaling pathway inhibitors, traditional Chinese medicine therapy, PDT, mRNA therapy, siRNA therapy and other therapeutic methods by inducing the apoptosis of tumor cells; arresting the cell cycle; inhibiting proliferation, migration and invasion; and inhibiting tumor angiogenesis in in vitro cell and in vivo animal models. In addition, the active ingredients in toad skin and toad venom, especially bufalin, can effectively reverse resistance to chemotherapeutic drugs and targeted drugs by inducing the apoptosis of tumor cells, regulating cancer cell stemness, reducing M2 macrophage polarization, decreasing drug efflux pump activity, inhibiting the epithelial‒mesenchymal transition, and other processes. In general, the antitumor mechanisms of toad skin and toad venom have not been studied in sufficient depth. How these preparations regulate the tumor microenvironment to exert antitumor immune effects needs to be elucidated. In addition, whether they can enhance antitumor effects through ferroptosis, pyroptosis, cell senescence, glycolysis and other pathways also needs to be further studied. In addition, TCMs, such as toad skin and toad venom, have multiple components and multiple targets, and the study of a single pathway or single active ingredient cannot easily interpret the therapeutic idea of the “holistic concept” of TCM. Network pharmacology combined with multiomics technology can explain the molecular mechanism of TCM in the treatment of diseases from multiple angles and aspects, which is consistent with the research idea of TCM. Therefore, in the future, multiomics technologies such as transcriptomics, proteomics, metabolomics and network pharmacology research methods can be applied to study toad skin and toad venom and reveal their antitumor mechanisms more comprehensively. In addition, Huachansu can significantly relieve a variety of types of cancer pain in the clinic and may be related to inhibiting inflammation, regulating opioid receptor pathways, affecting the function of Ca2+ and K+ channels, alleviating nerve compression by tumor tissue, and regulating the function of the 5-hydroxytryptamine system.151 Unfortunately, the understanding of the analgesic mechanism of Huachansu combined with the three-step analgesic method is insufficient at present. How to systematically study its mechanism of inhibiting cancer pain at multiple levels and in multiple disciplines needs to be further explored by researchers.
Although toad venom and toad skin exhibit excellent antitumor activity, they also possess certain toxicity. Toad venom is a toxic TCM according to the 2020 edition of the Chinese Pharmacopoeia.12 At present, with the widespread clinical use of toad venom- and toad skin-related preparations such as Huachansu injection and Toad venom injection, the frequent occurrence of adverse reactions has garnered widespread attention. Adverse reactions involve multiple organ systems, including the nervous, circulatory, and digestive systems, among which adverse reactions of the cardiovascular system should receive increased attention because of their serious consequences.152,153 Clinical reports have shown that adverse reactions such as arrhythmia and shortness of breath may occur within 30 minutes after the administration of Huachansu injection.154 Additionally, adverse reactions such as atrioventricular block, myocardial damage, and sinus arrhythmia can also occur after the administration of preparations containing toad venom.155–157 Furthermore, abnormal changes in cardiac troponin I (cTn-I) levels were observed after intravenous injection of Huachansu injection (5 g/kg and 10 g/kg) in rats, confirming its cardiotoxicity.158 In beagles, intravenous injection of 3 g/kg Huachansu injection immediately caused shortness of breath, with arrhythmia detected at 10 minutes posttreatment. Additionally, 3 g/kg Huachansu injection resulted in significant increases in cTn-I, CK-MB, and AST levels at 1.5 h and 3 h postinjection.159 Moreover, the administration of a large dose of a 75% ethanol extract of toad venom resulted in gradually reduced activity, slow and deep respiration, a weakened or absent righting reflex before death, as well as slow and weak breathing in mice, with an LD50 of 0.6006 g/kg.160 In addition, a study reported that IL-6 can exacerbate the inhibitory effect of Toad venom injection on the Ca2+ current, significantly increasing the risk of atrioventricular block.161 Another study has shown that toad venom can impair normal myocardial function by affecting ion homeostasis, actin construction and cell apoptosis.162 Numerous studies have confirmed that the main active ingredients of toad venom and toad skin are bufadienolides, which have similar structures to those of cardiac glycosides, and thus they can produce side effects such as cardiotonic effects in cancer treatment,163 causing long-term Na+-K+-ATP blockage and resulting in cardiac arrest.164 Currently, the cardiotoxicity of bufadienolides has been widely reported. For example, symptoms of cardiac glycoside toxicity, such as palpitations, tachypnea and convulsions, were observed in mice intraperitoneally injected with 1 mg/kg, 2 mg/kg and 10 mg/kg bufalin. Mice intraperitoneally injected with 1 mg/kg bufalin showed inverted T waves on the electrocardiogram, and mice injected with 2 mg/kg and 10 mg/kg bufalin showed 80% and 100% lethality, respectively,165 with instant death observed after the injection of 30 mg/kg.166 Moreover, studies have shown that inhibition of the Nav1.5 channel, enhancement of the late sodium channel current and enhancement of the sodium‒calcium exchange current are among the main mechanisms of the cardiotoxicity induced by toad venom.167 In addition, an intraperitoneal injection of 10 mg/kg/day bufalin or cinobufagin induced 100% death at 1 and 2 days postadministration, respectively, with death occurring within 10 minutes after the intravenous injection of 1 mg/kg cinobufagin.168 The LD50 of arenobufagin administered via intraperitoneal injection in mice was 16.2 mg/kg.169 Furthermore, some studies have reported that the LD50 values of various components of toad venom in mice are as follows: 41.0 mg/kg (intravenous), 96.6 mg/kg (subcutaneous), and 36.24 mg/kg (intraperitoneal) for toad venom; 2.2 mg/kg (intraperitoneal) for bufalin; 4.38 mg/kg (intraperitoneal) for cinobufagin; and 4.25 mg/kg (intravenous), 15 mg/kg (slow intravenous), 14 mg/kg (intraperitoneal), 124.5 mg/kg (subcutaneous), and 64 mg/kg (oral) for resibufogenin. In dogs, reported LD50 values for bufotalin are approximately 0.36 mg/kg (intravenous) and 0.98 mg/kg (oral minimal lethal dose).170 In summary, the toxicity of toad venom and toad skin should not be underestimated, and ensuring their safe use is a key issue in their clinical application. At present, toad venom- and toad skin-related preparations are mainly used as clinical antitumor adjuvant drugs to enhance the effects of chemotherapy, radiotherapy, targeted therapy, and other treatments. However, most of the current studies on the cardiotoxicity of toad venom and toad skin are limited to single drugs, and an exploration of the toxicity and mechanism of clinical combination drugs is lacking. Since toxic reactions are often closely related to the dose, mode and course of administration and more factors need to be considered in combination administration than in single administration, the toxicity and mechanism of combined drug administration based on toad skin and toad venom should be further studied to better guide rational drug use in the clinic. In addition, some studies have shown that taurine,171 phenytoin sodium, lidocaine and propranolol172 can alleviate bufadienolide-induced cardiotoxicity or arrhythmia, suggesting that we can use these drugs in combination to alleviate toad venom- and toad skin-induced cardiotoxicity.
Compared with the conventional multidrug combination strategy, the multidrug combination strategy based on a nanodelivery system can achieve the accurate loading of combined drugs and carry the drug to overcome physiological and pathological barriers, complete the efficient enrichment in the tumor tissue, and achieve targeted delivery to tumor cells and controlled drug release, resulting in enhanced antitumor efficacy and reduced toxic effects. Although preclinical studies have shown that the codelivery strategy of the active ingredients of toad skin and toad venom (bufalin, gamabufotalin, and cinobufagin) combined with chemotherapy drugs, small molecule-targeted drugs, monoclonal antibodies, active ingredients of TCM, drugs for PDT and PTT can reduce the side effects of drugs on normal tissues, especially cardiac toxicity, can reverse the MDR of tumor cells to a certain extent, and achieve the synergistic therapeutic effect of “1+1>2”, many problems remain to be solved to achieve the clinical transformation of nanomedicines. The optimal ratio of drugs is the key to realizing the synergistic effect of drugs, and how to screen the optimal compatibility ratio of combined drugs needs to be studied. How to select drug delivery carriers to achieve high drug loading rates, high encapsulation rates, and stable production technology and storage; how to achieve the optimal ratio of combined drugs unchanged and stable delivery into tumor tissues during the preparation of coloaded drug nanodelivery systems; and how to control the simultaneous release or sequential release of combined drugs need to be further studied. In addition, the toxicity of nanomaterials and the safety of the long-term application of nanomedicines also need to be studied. Liposomes are spherical vesicles with special bilayers composed of lipid components such as phospholipids and cholesterol. Due to their high biocompatibility, biodegradability, low toxicity, and wide range of drug delivery, liposomes have become some of the most widely studied nanocarriers in the current nanodrug delivery system. At present, a variety of drugs, such as doxorubicin hydrochloride, paclitaxel, irinotecan hydrochloride, daunorubicin, mifamurtide, vincristine and cytarabine, have been successfully encapsulated in liposomes for clinical use.173 Notably, in August 2017, the US FDA officially approved the market of the world’s first compound liposome (Vyxeos). Vyxeos is a liposomal compound preparation composed of cytarabine and daunorubicin at a molar ratio of 5:1 for the treatment of therapy-related acute myeloid leukemia (t-AML) and acute myeloid leukemia with myelodysplastic-related changes (AML-MRC). In addition, the irinotecan hydrochloride/floxuridine compound liposome (LY01616) is also undergoing clinical trials.23 Therefore, liposomes can be used as the starting point for the development of nanomedicines for the active ingredients of toad skin and toad venom.
In conclusion, combined antitumor strategies based on related preparations and active ingredients of toad skin and toad venom, along with drug delivery systems, can improve cancer therapy through multiple pathways and targets, reduce adverse events, and provide better treatment options for patients with tumors. However, many areas for improvement in current clinical combination treatment strategies still remain. The mechanistic basis is the rational foundation of antineoplastic drug combination therapy. In the future, researchers should continue to conduct in-depth mechanistic studies of combination therapy. Concurrently, adequate single-agent clinical pharmacological and safety data of the proposed combination drugs should be obtained, including human pharmacokinetic parameters, safe dose ranges, and dose‒exposure‒effect relationships, to fully evaluate the possible drug interactions and the superimposed risk of toxicity in vital organs. These findings provide a basis for the scientific and reasonable selection of combined drug dosages, formulation of dosage sequences, and design of safety and risk control measures. This approach could also provide a foundation for developing low-toxicity, high-efficiency, stable, and quality-controlled codelivery systems. Additionally, continuously enriching and improving the clinical trial technical guidelines for antitumor drug combination therapy is necessary to promote the scientific and orderly development of the combination of toad skin and toad venom, thus providing patients with combination therapy solutions with real clinical value. With further research, the combination of toad skin and toad venom is anticipated to have wider, safer, and more rational clinical applications, leading to more effective therapeutic outcomes.
Abbreviations
PDT, photodynamic therapy; TCM, traditional Chinese medicine; MDR, multidrug resistance; TACE, transarterial chemoembolization; QOL, quality of life; PFS, progression-free survival; OS, overall survival; HCC, hepatocellular carcinoma; DCR, disease control rate; ORR, objective response rate; NSCLC, non-small-cell lung cancer; PSA, prostate specific antigen; f-PSA, free PSA; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DOX, doxorubicin; ASK1, apoptosis signal-regulating kinase 1; JNK, c-Jun N-terminal kinase; FAK, focal adhesion kinase; Bax, Bcl-2-like protein 4; PDCD4, programmed cell death 4; GSK‑3β, Glycogen synthase kinase 3beta; Bcl‑xl, B-cell lymphoma-extra large; p-Akt, phosphorylated protein kinase B; mTOR, mechanistic target of rapamycin; MTX, Methotrexate; DHFR, dihydrofolate reductase; 5-FU, 5-Fluorouracil; MRP1, multidrug resistance-associated protein 1; VCR, Vincristine; CDC25, cell division cycle 25; CDK1, cyclin-dependent kinase 1; Bcl-2, B-cell lymphoma; ΔΨm, mitochondrial membrane potential; ROS, reactive oxygen species; L-OHP, Oxaliplatin; ADR, Adriamycin; P-gp, P-glycoprotein; DCT, Docetaxel; ABCB1, ATP-binding cassette subfamily B member 1; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase-1; IRE1a, inositol-requiring enzyme 1a; TRAF2, tumor necrosis factor receptor associated factor 2; ER, endoplasmic reticulum; NF-κB, nuclear factor-kappaB; MDR1, multidrug resistance gene 1; OCT4, octamer-binding transcription factor 4; SOX2, sex-determining region Y-box 2; ABCG2, ATP-binding cassette transporter G2; MIF, macrophage migration inhibitory factor; SRC-3, steroid receptor coactivator 3, PI3K, phosphoinositide 3-kinase; MYH9, myosin heavy chain 9; USP7, ubiquitin-specific protease 7; EMT, epithelial-mesenchymal transition; S6K, S6 kinase; 4EBP1, eIF4E-binding protein 1; FOXO1, forkhead box O1; CIPNP, chemotherapy-induced peripheral neuropathic pain; PIPNP, Paclitaxel-induced peripheral neuropathic pain; TNF-α, tumor necrosis factor-alpha; IL-1β, interleukin-1beta; TRPV1, transient receptor potential vanilloid 1; ERK, extracellular signal-regulated kinase; PCNA, Proliferating cell nuclear antigen; CDK2¸ cyclin-dependent kinase 2; HUVECs, human umbilical vein endothelial cells; PDGF-BB, platelet-derived growth factor-BB; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; EGFR, epidermal growth factor receptor; HGF, hepatocyte growth factor; APAF-1, apoptotic protease activating factor-1; SOD, superoxide dismutase; Bad, BCL2-associated agonist of cell death; MAPK, mitogen-activated protein kinase; SOS-1, son of sevenless homolog 1; MMP-2, matrix metalloproteinase-2; RhoA, Ras homolog gene family, member A; uPA, urokinase-type plasminogen activator; TIMP1, tissue inhibitor of matrix metalloprotease 1; MCL-1, myeloid cell leukemia-1; HR, homologous recombination; γ-H2AX, phosphorylated histone H2AX; MM, multiple myeloma; BMSC, bone marrow stromal cells; IL-6, Interleukin-6; LC3B, light chain 3B; Atg, autophagy-related gene; Ptch1, patched homolog 1; Gli1, GLI family zinc finger 1; Gli3, GLI family zinc finger 3; GSH, glutathione; LC-MS, liquid chromatography-mass spectrometry; MALDI-MSI, matrix-assisted laser desorption ionization-mass spectrometry imaging; PC, phosphatidylcholines; PG, phosphatidylglycerol; PS, phosphatidylserine; PE, phosphatidylethanolamine; HIFU, high-intensity focused ultrasound; HIF-1a, hypoxia-inducible factor-1alpha; SDF-1, stromal cell-derived factor 1; CXCR4, C-X-C chemokine receptor type 4; MiR-497, MicroRNA-497; TRAIL, TNF‑related apoptosis‑inducing ligand; DR5, death receptor 5; cFLIP, cellular Fas‑associated death domain‑like interleukin‑1β‑converting enzyme inhibitory protein; XIAP, X‑linked inhibitor of apoptosis protein; PTT, photothermal therapy; CA, Cholic acid; TPGS, Tocopherol polyethylene glycol 1000 succinate; NTCP, Na+-taurocholate co-transporting polypeptide; GOQDs, graphene oxide quantum dots; MMP-9, matrix metalloproteinase 9; COX-2, chemotherapy-activated cyclooxygenase-2; VES-CSO, vitamin-E-succinate grafted chitosan oligosaccharide; EPR, enhanced permeability and retention; FGFR, fibroblast growth factor receptor; PDGFR, platelet-derived growth factor receptor; α-SMA, alpha-smooth muscle actin; AUC, area under the curve; MRT, mean residence time; STAT3, signal transducer and activator of transcription 3; PD-L1, programmed death ligand-1; FA, folic acid; mSiO2, monodisperse mesoporous silica nanoparticle; CCA, cholangiocarcinoma; INF-γ, interferon-gamma; NR, nano-realgar; TSE, Toad Skin extracts; BJO, Brucea javanica oil; mTHPC, meta-tetrahydroxyphenylchlorin; Ce6, Chlorin e6; RBC, red blood cell; HA, hyaluronic acid; HSP70, heat shock protein 70; PDA, polydopamine; NIR, near-infrared; BPNSs, black phosphorus nanosheets; HM, hybrid cell membrane; ICG, indocyanine green; HSA, human serum albumin; TMC, trimethyl chitosan; cTn-I, cardiac troponin I; t-AML, therapy-related acute myeloid leukaemia; AML-MRC, acute myeloid leukaemia with myelodysplastic-related changes.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by grants from National Natural Science Foundation of China (grant No. 82204935), Open Project of Shaanxi Key Laboratory of Chinese Medicine Fundamentals and New Drugs Research (grant No. KF2201).
Disclosure
The authors declare no competing interests.
References
1. Jassim A, Rahrmann EP, Simons BD, Gilbertson RJ. Cancers make their own luck: theories of cancer origins. Nat Rev Cancer. 2023;23(10):710–724. doi:10.1038/s41568-023-00602-5
2. Zhou H, Zhang M, Cao H, et al. Research progress on the synergistic anti-tumor effect of natural anti-tumor components of Chinese herbal medicine combined with chemotherapy drugs. Pharmaceuticals-Base. 2023;16(12):1734. doi:10.3390/ph16121734
3. Silva J, Pinto B, Monteiro L, Silva P, Bousbaa H. Combination therapy as a promising way to fight oral cancer. Pharmaceutics. 2023;15(6):1653. doi:10.3390/pharmaceutics15061653
4. Wang Y, Minden A. Current molecular combination therapies used for the treatment of breast cancer. Int J Mol Sci. 2022;23(19):11046. doi:10.3390/ijms231911046
5. Songca SP. Combinations of photodynamic therapy with other minimally invasive therapeutic technologies against cancer and microbial infections. Int J Mol Sci. 2023;24(13):10875. doi:10.3390/ijms241310875
6. Wang H, Xu Y, Zuo F, Liu J, Yang J. Immune-based combination therapy for esophageal cancer. Front Immunol. 2022;13:1020290. doi:10.3389/fimmu.2022.1020290
7. Jin H, Wang L, Bernards R. Rational combinations of targeted cancer therapies: background, advances and challenges. Nat Rev Drug Discov. 2023;22(3):213–234. doi:10.1038/s41573-022-00615-z
8. Zhang Y, Lou Y, Wang J, Yu C, Shen W. Research status and molecular mechanism of the traditional Chinese medicine and antitumor therapy combined strategy based on tumor microenvironment. Front Immunol. 2020;11:609705. doi:10.3389/fimmu.2020.609705
9. Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens J. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol. 2019;30(2):219–235. doi:10.1093/annonc/mdy551
10. Wang X, Li J, Chen R, Li T, Chen M. Active ingredients from Chinese medicine for combination cancer therapy. Int J Biol Sci. 2023;19(11):3499–3525. doi:10.7150/ijbs.77720
11. Nanjing University of Chinese Medicine. Dictionary of Traditional Chinese Medicine. Shanghai: Shanghai Scientific and Technical Publisher; 2006.
12. National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China (Part I). Beijing: China Medical Science and Technology Publisher; 2020.
13. Wu Q, Wang SP, Sun XX, et al. HuaChanSu suppresses tumor growth and interferes with glucose metabolism in hepatocellular carcinoma cells by restraining Hexokinase-2. Int J Biochem Cell B. 2022;142:106123. doi:10.1016/j.biocel.2021.106123
14. Chen C, Wu H, Fu X, et al. A UPLC-QTOF/MS-based hepatic tissue metabolomics approach deciphers the mechanism of Huachansu tablets-based intervention against hepatocellular carcinoma. J Pharmaceut Biomed. 2023;239:115875. doi:10.1016/j.jpba.2023.115875
15. Wang M, Li Y, Li S, et al. Cinobufacini injection delays hepatocellular carcinoma progression by regulating lipid metabolism via SREBP1 signaling pathway and affecting macrophage polarization. J Ethnopharmacol. 2024;321:117472. doi:10.1016/j.jep.2023.117472
16. Xu Y, Peng W, Han D, et al. Material basis and mechanism of chansu injection for COVID-19 treatment based on network pharmacology and molecular docking technology. Evid-Based Complementary Altern Med. 2021;2021:7697785. doi:10.1155/2021/7697785
17. Asrorov AM, Kayumov M, Mukhamedov N, et al. Toad venom bufadienolides and bufotoxins: an updated review. Drug Dev Res. 2023;84(5):815–838. doi:10.1002/ddr.22072
18. Jia J, Li J, Zheng Q, Li D. A research update on the antitumor effects of active components of Chinese medicine ChanSu. Front Oncol. 2022;12:1014637. doi:10.3389/fonc.2022.1014637
19. Cao Y, Wu J, Pan H, Wang L. Chemical profile and multicomponent quantitative analysis for the quality evaluation of toad venom from different origins. Molecules. 2019;24(19):3595. doi:10.3390/molecules24193595
20. Huang J, Chen F, Zhong Z, et al. Interpreting the pharmacological mechanisms of Huachansu Capsules on hepatocellular carcinoma through combining network pharmacology and experimental evaluation. Front Pharmacol. 2020;11:414. doi:10.3389/fphar.2020.00414
21. Deng LJ, Li Y, Qi M, et al. Molecular mechanisms of bufadienolides and their novel strategies for cancer treatment. Eur J Pharmacol. 2020;887:173379. doi:10.1016/j.ejphar.2020.173379
22. Shao H, Li B, Li H, et al. Novel strategies for solubility and bioavailability enhancement of bufadienolides. Molecules. 2021;27(1):51. doi:10.3390/molecules27010051
23. Tian H, Zhao F, Qi QR, Yue BS, Zhai BT. Targeted drug delivery systems for elemene in cancer therapy: the story thus far. Biomed Pharmacother. 2023;166:115331. doi:10.1016/j.biopha.2023.115331
24. Zhai BT, Sun J, Shi YJ, et al. Review targeted drug delivery systems for norcantharidin in cancer therapy. J Nanobiotechnol. 2022;20(1):509. doi:10.1186/s12951-022-01703-3
25. Gao H, He J, Cheng CS, Zhuang L, Chen H, Meng Z. Unresectable hepatocellular carcinoma: transarterial chemoembolisation plus Huachansu - a single-center randomised controlled trial. BMJ Support Palliat. 2023;2022–3870. doi:10.1136/spcare-2022-003870
26. Wang T, Zhang L, Han L, et al. Clinical effect of intravenous infusion of zoledronic acid combined with oral medication of cinobufagin in the treatment of metastatic bone tumors. Pak J Pharm Sci. 2018;31(4):1609–1612.
27. Liu Q, Zhang H, Feng X, Wang S. Huachansu injection combined with cemt regimen on patients with relapsed or refractory multiple myeloma. Blood. 2013;122(21):5384. doi:10.1182/blood.V122.21.5384.5384
28. Tian Y, Yang P, Tan B, et al. Efficacy of toad venom injection combined with apatinib in patients with advanced gastric cancer. J Pract Med. 2020;36(18):2583–2586.
29. Wang Y, Wu J. Clinical study on Kang’aiping Pills combined with capecitabine in treatment of rectal cancer. Drugs Clin. 2019;34(7):2142–2146.
30. Zhang Y, Lu P. Clinical study of xianchan tablets combined with chemotherapy in the treatment of elderly patients with esophageal cancer. J Clin Med Pract. 2020;24(3):75–77.
31. Dong J, Lu N, Shi G, Shi H, Ye X. Combination of“Delisheng Injection”and chemotherapy for the treatment of liver metastases of colon cancer. SH J TCM. 2014;48(11):27–29.
32. Xu J, Wang Q. Clinical study on hechan tablets combined with DP chemotherapy in treatment of non-small cell lung cancer. Drugs Clin. 2018;33(11):2963–2968.
33. Liu C, Li F, Wang L, et al. Short-term efficacy of tianfoshen oral liquid combined with gemcitabine and cisplatin in treatment of elderly patients with advanced non-small cell lung cancer. Oncol Prog. 2017;15(3):294–296.
34. Zhang J, Bai H. Clinical study on the treatment of platinum sensitive recurrent epithelial ovarian cancer by toad venom injection combined with TC regimen. Pract Clin J Integr Tradit West Med. 2020;20(8):46–47.
35. Zhang J. Clinical study of toad venom injection in treatment of advanced ovarian cancer. Henan Tradit Chin Med. 2017;37(5):869–871.
36. Chen F, Luo J, Du M, Zhang X. Effect of anti-cancer pill combined with trastuzumab and chemotherapy on short term efficacy and long-term efficacy of HER2 positive patients with advanced gastric cancer. Pract J Cancer. 2019;34(2):264–266.
37. Xie J, Zou Y. The clinical effects of xianchanpian and docetaxel in the treatment of advanced non-small cell lung cancer and its influence on the immune function, life quality and long-term effects in patients. Pract J Cancer. 2019;34(5):766–769.
38. Yang R, Bai J, Yang B. Clinical study on Xianchan Tablets combined with trastuzumab and mFOLFOX6 regimen in treatment of elderly with advanced gastric cancer of HER2 positive. Drugs Clin. 2018;33(7):1736–1741.
39. Kang T, Liu M, Bao H, Duan W, Sun X. Clinical study on Xianchan Tablets combined with GP chemotherapy in treatment of elderly advanced non-small cell lung cancer. Drugs Clin. 2017;32(10):1980–1984.
40. Li DH, Su YF, Fan HF, Guo N, Sun CX. Acupuncture combined with three-step analgesic drug therapy for treatment of cancer pain: a systematic review and meta-analysis of randomised clinical trials. Evid-Based Complementary Altern Med. 2021;2021:5558590. doi:10.1155/2021/5558590
41. Chen J. Analysis of curative effect of oxycontin combined with huachansu capsule in the treatment of advanced cancer pain. China Prac Med. 2020;15(34):123–125.
42. Nan X. The effect of OxyContin combined with Huachansu in the treatment of moderate to severe cancer pain. World J Complex Med. 2019;5(11):181–183.
43. Yang S, Zhao H, Wang Z, et al. Efficacy of cinobufacin capsule combined with fentanyl transdermal patch in the treatment of moderate and severe cancer pain with bone metastasis. Chin Gen Pract. 2019;22(32):3993–3996.
44. Zhou Y, Wu D, Liu D, Wang J, Tang Q, Yu J. Study on huachansu capsule combined with zoledronic acid for osseous metastasis pain relief in prostatic cancer patients. World Clin Drug. 2017;38(11):781–784.
45. Sun L. Effect of Huachansu Capsule in the Treatment of Cancer Pain. Beijing: Beijing University of Chinese Medicine; 2020.
46. Bao W. Clinical Study of Cinobufotalin Combined with Opioids in the Treatment of Cancer Pain in Patients with Opioid Tolerance. Chengdu: Chengdu University of Chinese Medicine; 2019.
47. Wen Y, Bai D, Wu Y, Ma Q, Feng L. Observation on the efficacy of huachan element injection combined acetaminophen oxycodone in remission of moderately severe pain caused by cancer. J Clin Exp Med. 2018;17(7):731–733.
48. Wu S, Chen W, Lin H. Discussion on the efficacy of morphine hydrochloride sustained-release tablets combined with Huachansu capsule in the treatment of cancer pain. China Prac Med. 2021;16(12):136–138.
49. Wang Y, Li Y, Zhang Y, Pan Y, Dong X, Mai X. The clinical observation of sodium ibandronate injection combined with cinobufacini injection on the treatment of bone metastases with cancer pain. Internal Med. 2018;13(2):170–172.
50. Wang Y, Dong M. Clinical efficacy of tianchan capsules combined with oxycodone hydrochloride sustained release tablets in the treatment of cancer pain of advanced gastric cancer and its effect on quality of life. Eval Anal Drug-Use Hosp China. 2022;22(2):172–175.
51. Gao X, Mei J, Li H. Clinical observation of Tianchan Capsules combined with oxycodone in treatment of moderate cancer pain. Drugs Clin. 2020;35(11):2201–2205.
52. Li Y, Jin H, Wang H, Ji L, Sheng S. Clinical observation of acupoint sticking with chan wu gel in releasing cancer pain. Shanghai J Acupunct Moxibustion. 2017;36(4):397–400.
53. Wang X, Chen L, He Y, Liu D. Treatment effect of Cinobufacini capsule for digestive cancer pain. J North Sichuan Med Coll. 2017;32(1):71–74.
54. Zhang Y, Li X, Wan L. Therapeutic effect of Huachansu capsule combined with oxycodone hydrochloride sustained release tablets in the treatment of cancer pain. Forum Tradit Chin Med. 2021;36(3):45–47.
55. Yu B, Yu H, Su Z, Yuan B, Yuan Y. Observe on the clinical curative effect of cinobufotalin tablets in the standardized treatment of cancer pain. Mod J Integr Tradit Chin West Med. 2015;24(36):3997–3999.
56. Zhang X, Zhang J, Qi H, Zhao X. Effects of Huachansu tablets combined with oxycodone hydrochloride sustained release tablets on cancer pain and quality of life in elderly patients with advanced liver cancer. Electron J Pract Clin Nurs Sci. 2019;4(37):38–39.
57. Dong X, Li Y, Zhang Y, Zhao Y, Wu S, Xiao Y. Efficacy of morphine sulfate sustained-release tablets combined with cinobufotalin capsule in the treatment of cancer pain. Chin Community Doctors. 2018;34(16):84–85.
58. Chen Z, Li K. Effect analysis of oxycodone hydrochloride sustained-release tablets combined with Huachansu in the treatment of cancer pain. Chin Community Doctors. 2019;35(8):130–131.
59. Li LL, Su YX, Mao Y, et al. The effectiveness and safety of cinobufotalin injection as an adjunctive treatment for lung cancer: a meta-analysis of randomized controlled trials. Evid-Based Complementary Altern Med. 2021;2021:8852261. doi:10.1155/2021/8852261
60. Peng W, Xu Y, Feng F, et al. Meta-analysis of therapy of cinobufacini capsule adjunct with first-line platinum based chemotherapy for the treatment of advanced NSCLC. Evid-Based Complementary Altern Med. 2021;2021:5596415. doi:10.1155/2021/5596415
61. Wu J, Zhang D, Ni M, et al. Effectiveness of huachansu injection combined with chemotherapy for treatment of gastric cancer in China: a systematic review and meta-analysis. J Tradit Chin Med. 2020;40(5):749–757. doi:10.19852/j.cnki.jtcm.2020.05.004
62. Guo N, Miao Y, Sun M. Transcatheter hepatic arterial chemoembolization plus cinobufotalin injection adjuvant therapy for advanced hepatocellular carcinoma: a meta-analysis of 27 trials involving 2079 patients. Oncol Targets Ther. 2018;11:8835–8853. doi:10.2147/OTT.S182840
63. Zhang Z, Yang Y. Effectiveness and safety of bufalin praeparatum in the adjuvant therapy of tumor: a systematic review and meta-analysis. Acad J Shanghai Univ Tradit Chin Med. 2013;27(5):35–40.
64. Xu J, Qin S, Chen Y, Li D, Yan Q. Systematic review and meta-analysis of efficacy and safety of huachansu in treating cancer-related pain. China J Chin Mater Med. 2019;44(12):2627–2636.
65. Yuan J, Guo Y. Meta-analysis of efficacy and safety of Huachansu combined with three-step analgesic therapy in the treatment of cancer pain. Zhejiang J Integr Tradit Chin West Med. 2023;33(2):169–177.
66. Tan X, Liang X, Xi J, et al. Clinical efficacy and safety of Huachansu injection combination with platinum-based chemotherapy for advanced non-small cell lung cancer: a systematic review and meta-analysis of randomized controlled trials. Medicine. 2021;100(36):e27161. doi:10.1097/MD.0000000000027161
67. Xu Y, Han D, Feng F, et al. Meta-analysis of cinobufacini injection combined with platinum-contained first-line chemotherapy in treatment of non-small cell lung cancer. China J Chin Mater Med. 2019;44(21):4728–4737.
68. Zhang F, Yin Y, Xu T. Cinobufotalin injection combined with chemotherapy for the treatment of advanced NSCLC in China: a PRISMA-compliant meta-analysis of 29 randomized controlled trials. Medicine. 2019;98(35):e16969. doi:10.1097/MD.0000000000016969
69. Zhang X, Yuan Y, Xi Y, et al. Cinobufacini injection improves the efficacy of chemotherapy on advanced stage gastric cancer: a systemic review and meta-analysis. Evid-Based Complementary Altern Med. 2018;2018:7362340. doi:10.1155/2018/7362340
70. Zhou B, Wu F, Yuan L, Miao Z, Zhu S. Is huachansu beneficial in treating advanced non-small-cell lung cancer? Evidence from a meta-analysis of its efficacy combined with chemotherapy. Evid-Based Complementary Altern Med. 2015;2015:408145. doi:10.1155/2015/408145
71. Wu T, Sun R, Wang Z, Yang W, Shen S, Zhao Z. A meta-analysis of Cinobufacini combined with transcatheterarterial chemoembolization in the treatment of advanced hepatocellular carcinoma. J Cancer Res Ther. 2014;10 Suppl 1:60–64. doi:10.4103/0973-1482.139763
72. Xie X, Huang X, Li J, et al. Efficacy and safety of Huachansu combined with chemotherapy in advanced gastric cancer: a meta-analysis. Med Hypotheses. 2013;81(2):243–250. doi:10.1016/j.mehy.2013.04.038
73. Huang T, Gong WH, Li XC, et al. Efficient killing effect of osteosarcoma cells by cinobufacini and cisplatin in combination. Asian Pac J Cancer Prev. 2012;13(6):2847–2851. doi:10.7314/apjcp.2012.13.6.2847
74. Xia J, Inagaki Y, Gao J, et al. Combination of cinobufacini and doxorubicin increases apoptosis of hepatocellular carcinoma cells through the fas- and mitochondria-mediated pathways. Am J Chinese Med. 2017;45(7):1537–1556. doi:10.1142/S0192415X17500835
75. Chen Y, Guo Q, Zhang B, Kang M, Xie Q, Wu Y. Bufalin enhances the antitumor effect of gemcitabine in pancreatic cancer. Oncol Lett. 2012;4(4):792–798. doi:10.3892/ol.2012.783
76. Liu F, Tong D, Li H, et al. Bufalin enhances antitumor effect of paclitaxel on cervical tumorigenesis via inhibiting the integrin alpha2/beta5/FAK signaling pathway. Oncotarget. 2016;7(8):8896–8907. doi:10.18632/oncotarget.6840
77. Liu J, Zhang Y, Sun S, et al. Bufalin induces apoptosis and improves the sensitivity of human glioma stem-like cells to temozolamide. Oncol Res. 2019;27(4):475–486. doi:10.3727/096504018X15270916676926
78. Gu R, Zhang Q. Effects of low-dose bufalin combined with hydroxycamptothecin on human castration-resistant prostate cancer xenografts in nude mice. Exp Ther Med. 2021;22(3):1015. doi:10.3892/etm.2021.10447
79. Zhao H, Zhao D, Jin H, et al. Bufalin reverses intrinsic and acquired drug resistance to cisplatin through the AKT signaling pathway in gastric cancer cells. Mol Med Rep. 2016;14(2):1817–1822. doi:10.3892/mmr.2016.5426
80. Dai G, Yu L, Yang J, et al. The synergistic antitumor effect of cinobufagin and cisplatin in human osteosarcoma cell line in vitro and in vivo. Oncotarget. 2017;8(49):85150–85168. doi:10.18632/oncotarget.19554
81. Yuan B, Xu K, Shimada R, et al. Cytotoxic effects of arsenite in combination with gamabufotalin against human glioblastoma cell lines. Front Oncol. 2021;11:628914. doi:10.3389/fonc.2021.628914
82. Yuan B, Li J, Miyashita SI, et al. Enhanced cytotoxic effects of arenite in combination with active bufadienolide compounds against human glioblastoma cell line U-87. Molecules. 2022;27(19). doi:10.3390/molecules27196577
83. Yin JQ, Shen JN, Su WW, et al. Bufalin induces apoptosis in human osteosarcoma U-2OS and U-2OS methotrexate300-resistant cell lines. Acta Pharmacol Sin. 2007;28(5):712–720. doi:10.1111/j.1745-7254.2007.00559.x
84. Gu W, Liu L, Fang FF, Huang F, Cheng BB, Li B. Reversal effect of bufalin on multidrug resistance in human hepatocellular carcinoma BEL-7402/5-FU cells. Oncol Rep. 2014;31(1):216–222. doi:10.3892/or.2013.2817
85. Zhai X, Lu J, Wang Y, Fang F, Li B, Gu W. Reversal effect of bufalin on multidrug resistance in K562/VCR vincristine-resistant leukemia cell line. J Tradit Chin Med. 2014;34(6):678–683. doi:10.1016/s0254-6272(15)30082-0
86. Zhang DM, Liu JS, Tang MK, et al. Bufotalin from venenum bufonis inhibits growth of multidrug resistant hepG2 cells through G2/M cell cycle arrest and apoptosis. Eur J Pharmacol. 2012;692(1–3):19–28. doi:10.1016/j.ejphar.2012.06.045
87. Yuan Z, Shi X, Qiu Y, et al. Reversal of P-gp-mediated multidrug resistance in colon cancer by cinobufagin. Oncol Rep. 2017;37(3):1815–1825. doi:10.3892/or.2017.5410
88. Zhang D, Jia T, Chen X, et al. Bufalin reverses ABCB1-mediated resistance to docetaxel in breast cancer. Heliyon. 2023;9(3):e13840. doi:10.1016/j.heliyon.2023.e13840
89. Xie Y, Yan X, Sun L. The mechanism of bufalin-induced apoptosis of K562/A02. Med Sci Monit. 2019;25:2542–2552. doi:10.12659/MSM.915802
90. Yuan ZT, Shi XJ, Yuan YX, et al. Bufalin reverses ABCB1-mediated drug resistance in colorectal cancer. Oncotarget. 2017;8(29):48012–48026. doi:10.18632/oncotarget.18225
91. Zhan Y, Qiu Y, Wang H, et al. Bufalin reverses multidrug resistance by regulating stemness through the CD133/nuclear factor-kappaB/MDR1 pathway in colorectal cancer. Cancer Sci. 2020;111(5):1619–1630. doi:10.1111/cas.14345
92. Sun J, Xu K, Qiu Y, et al. Bufalin reverses acquired drug resistance by inhibiting stemness in colorectal cancer cells. Oncol Rep. 2017;38(3):1420–1430. doi:10.3892/or.2017.5826
93. Chen J, Wang H, Jia L, et al. Bufalin targets the SRC-3/MIF pathway in chemoresistant cells to regulate M2 macrophage polarization in colorectal cancer. Cancer Lett. 2021;513:63–74. doi:10.1016/j.canlet.2021.05.008
94. Liu JH, Yang HL, Deng ST, et al. The small molecule chemical compound cinobufotalin attenuates resistance to DDP by inducing ENKUR expression to suppress MYH9-mediated c-Myc deubiquitination in lung adenocarcinoma. Acta Pharmacol Sin. 2022;43(10):2687–2695. doi:10.1038/s41401-022-00890-x
95. Ma X, Suo Z, Ma X, Zhan C, Luo G, Song J. Cinobufagin inhibits tumor progression and reduces doxorubicin resistance by enhancing FOXO1-mediated transcription of FCGBP in osteosarcoma. J Ethnopharmacol. 2022;296:115433. doi:10.1016/j.jep.2022.115433
96. Sisignano M, Baron R, Scholich K, Geisslinger G. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat Rev Neurol. 2014;10(12):694–707. doi:10.1038/nrneurol.2014.211
97. Ba X, Wang J, Zhou S, et al. Cinobufacini protects against paclitaxel-induced peripheral neuropathic pain and suppresses TRPV1 up-regulation and spinal astrocyte activation in rats. Biomed Pharmacother. 2018;108:76–84. doi:10.1016/j.biopha.2018.09.018
98. Hao Y, Luo X, Ba X, et al. Huachansu suppresses TRPV1 up-regulation and spinal astrocyte activation to prevent oxaliplatin-induced peripheral neuropathic pain in rats. Gene. 2019;680:43–50. doi:10.1016/j.gene.2018.09.035
99. Gao Y, Li HX, Xu LT, et al. Bufalin enhances the anti-proliferative effect of sorafenib on human hepatocellular carcinoma cells through downregulation of ERK. Mol Biol Rep. 2012;39(2):1683–1689. doi:10.1007/s11033-011-0908-x
100. Wang H, Zhang C, Chi H, Meng Z. Synergistic anticancer effects of bufalin and sorafenib by regulating apoptosis associated proteins. Mol Med Rep. 2018;17(6):8101–8110. doi:10.3892/mmr.2018.8927
101. Wang Z, Xu Z, Wang J, Dai E, Xu R. Synergy with the active ingredients of toad venom and sorafenib suppresses hepatocellular carcinoma HepG2 cells proliferation through down-regulating Akt/NF-κB signaling pathways. Chin Pharmacol Bull. 2017;33(11):1510–1516.
102. Wang Z. Synergy with the Active Ingredients of Toad Venom and Sorafenib Suppresses Hepatocellular Carcinoma HepG2 Cells Proliferation Through Down-Regulating Akt/NF-Κb Signaling Pathways. Jinzhou: Jinzhou Medical University; 2018.
103. Wang H, Zhang C, Ning Z, Xu L, Zhu X, Meng Z. Bufalin enhances anti-angiogenic effect of sorafenib via AKT/VEGF signaling. Int J Oncol. 2016;48(3):1229–1241. doi:10.3892/ijo.2016.3326
104. Wang H, Zhang C, Chi H, Meng Z. Synergistic anti-hepatoma effect of bufalin combined with sorafenib via mediating the tumor vascular microenvironment by targeting mTOR/VEGF signaling. Int J Oncol. 2018;52(6):2051–2060. doi:10.3892/ijo.2018.4351
105. Kang X, Gong Y, Wang L, et al. Effect of bufalin combined gefitinib on lung cancer H1975 cells and its mechanisms research. Chin J Integr Tradit West Med. 2013;33(8):1081–1085.
106. Han Y, Ma R, Cao G, et al. Combined treatment of cinobufotalin and gefitinib exhibits potent efficacy against lung cancer. Evid-Based Complementary Altern Med. 2021;2021:6612365. doi:10.1155/2021/6612365
107. Kuo JY, Liao CL, Ma YS, et al. Combination treatment of sorafenib and bufalin induces apoptosis in NCI-H292 human lung cancer cells in vitro. Vivo. 2022;36(2):582–595. doi:10.21873/invivo.12741
108. Kang X, Lu P, Cui Y, et al. Bufalin reverses hepatocyte growth factor-induced resistance to Afatinib in H1975 lung cancer cells. Chin J Oncol. 2015;37(7):490–496.
109. Zhai B, Hu F, Yan H, et al. Bufalin reverses resistance to sorafenib by inhibiting akt activation in hepatocellular carcinoma: the role of endoplasmic reticulum stress. PLoS One. 2015;10(9):e138485. doi:10.1371/journal.pone.0138485
110. Huang AC, Yang MD, Hsiao YT, et al. Bufalin inhibits gefitinib resistant NCI-H460 human lung cancer cell migration and invasion in vitro. J Ethnopharmacol. 2016;194:1043–1050. doi:10.1016/j.jep.2016.11.004
111. Cao F, Gong YB, Kang XH, et al. Degradation of MCL-1 by bufalin reverses acquired resistance to osimertinib in EGFR mutant lung cancer. Toxicol Appl Pharm. 2019;379:114662. doi:10.1016/j.taap.2019.114662
112. Zhang X, Huang Q, Wang X, et al. Bufalin enhances radiosensitivity of glioblastoma by suppressing mitochondrial function and DNA damage repair. Biomed Pharmacother. 2017;94:627–635. doi:10.1016/j.biopha.2017.07.136
113. Wang L, Raju U, Milas L, et al. Huachansu, containing cardiac glycosides, enhances radiosensitivity of human lung cancer cells. Anticancer Res. 2011;31(6):2141–2148.
114. Emam H, Refaat A, Jawaid P, et al. Hyperthermia and radiation reduce the toxic side-effects of bufadienolides for cancer therapy. Oncol Lett. 2017;14(1):1035–1040. doi:10.3892/ol.2017.6256
115. Wang Z, Liu F, Huang C, Zhang J, Wu J. Bufalin inhibits epithelial-mesenchymal transition and increases radiosensitivity of non-small cell lung cancer via inhibition of the Src signaling. J Thorac Dis. 2023;15(1):123–134. doi:10.21037/jtd-22-1859
116. Huang H, Lin XJ, Lin Y, Yao RX, He MQ. Bufalin enhances the cytotoxity of human multiple myeloma cells H929 to AKT INhibitor MK2206: the role of protein AKT phosphorylation. Indian J Hematol Blo. 2018;34(2):268–272. doi:10.1007/s12288-017-0883-z
117. Xiang RF, Wang Y, Zhang N, et al. MK2206 enhances the cytocidal effects of bufalin in multiple myeloma by inhibiting the AKT/mTOR pathway. Cell Death Dis. 2017;8(5):e2776. doi:10.1038/cddis.2017.188
118. Xu Z, Bao J, Jin X, et al. The effects of cinobufagin on hepatocellular carcinoma cells enhanced by MRT68921, an autophagy inhibitor. Am J Chinese Med. 2023;51(6):1595–1611. doi:10.1142/S0192415X23500726
119. Sheng X, Sun X, Sun K, Sui H, Qin J, Li Q. Inhibitory effect of bufalin combined with Hedgehog signaling pathway inhibitors on proliferation and invasion and metastasis of liver cancer cells. Int J Oncol. 2016;49(4):1513–1524. doi:10.3892/ijo.2016.3667
120. Zhang J, Hong Y, Jiang L, et al. Global metabolomic and lipidomic analysis reveal the synergistic effect of bufalin in combination with cinobufagin against HepG2 cells. J Proteome Res. 2020;19(2):873–883. doi:10.1021/acs.jproteome.9b00681
121. Zhang J, Hong Y, Xie P, et al. Spatial lipidomics reveals anticancer mechanisms of bufalin in combination with cinobufagin in tumor-bearing mice. Front Pharmacol. 2020;11:593815. doi:10.3389/fphar.2020.593815
122. Yin Z. The Antitumor Effect and Its Mechanism by Photoexcited TiO2 Combined with Bufalin on Human Melanoma A375 Cells. Shanghai: The Second Military Medical University; 2012.
123. Ning Z, Zhu Z, Wang H, et al. High-intensity focused ultrasound enhances the effect of bufalin by inducing apoptosis in pancreatic cancer cells. Oncol Targets Ther. 2019;12:1161–1170. doi:10.2147/OTT.S185953
124. Liu X, Zhou Y, Peng J, Xie B, Shou Q, Wang J. Silencing c-Myc enhances the antitumor activity of bufalin by suppressing the HIF-1alpha/SDF-1/CXCR4 pathway in pancreatic cancer cells. Front Pharmacol. 2020;11:495. doi:10.3389/fphar.2020.00495
125. Qiu YY, Hu Q, Tang QF, et al. MicroRNA-497 and bufalin act synergistically to inhibit colorectal cancer metastasis. Tumour Biol. 2014;35(3):2599–2606. doi:10.1007/s13277-013-1342-6
126. Kang KH, Han MH, Jeong JW, et al. Bufalin sensitizes human bladder carcinoma cells to TRAIL-mediated apoptosis. Oncol Lett. 2017;14(1):853–859. doi:10.3892/ol.2017.6223
127. Liu Y, Lu X, Zhang Z, Jiang S, Lv H. mPEG-Cholic acid/TPGS mixed micelles for combined delivery of paclitaxel and bufalin to treat hepatocellular carcinoma. Pharm Dev Technol. 2022;27(2):215–227. doi:10.1080/10837450.2022.2037140
128. Fan J, Liu B, Long Y, et al. Sequentially-targeted biomimetic nano drug system for triple-negative breast cancer ablation and lung metastasis inhibition. Acta Biomater. 2020;113:554–569. doi:10.1016/j.actbio.2020.06.025
129. Yuan Z, Yuan Y, Han L, et al. Bufalin-loaded vitamin E succinate-grafted-chitosan oligosaccharide/RGD conjugated TPGS mixed micelles demonstrated improved antitumor activity against drug-resistant colon cancer. Int J Nanomed. 2018;13:7533–7548. doi:10.2147/IJN.S170692
130. Xu Y, Liu Y, Liu Q, et al. Co-delivery of bufalin and nintedanib via albumin sub-microspheres for synergistic cancer therapy. J Control Release. 2021;338:705–718. doi:10.1016/j.jconrel.2021.08.049
131. Chen P, Lu S, Pan B, Xu Y. Development, optimization, and pharmacokinetics study of bufalin/nintedanib co-loaded modified albumin sub-microparticles fabricated by coaxial electrostatic spray technology. AAPS Pharm Sci Tech. 2021;23(1):13. doi:10.1208/s12249-021-02163-y
132. Long Y, Wang Z, Fan J, et al. A hybrid membrane coating nanodrug system against gastric cancer via the VEGFR2/ STAT3 signaling pathway. J Mater Chem B. 2021;9(18):3838–3855. doi:10.1039/d1tb00029b
133. Ning Z, Zhao Y, Yan X, Hua Y, Meng Z. Flower-like composite material delivery of co-packaged lenvatinib and bufalin prevents the migration and invasion of cholangiocarcinoma. Nanomaterials-Basel. 2022;12(12):2048. doi:10.3390/nano12122048
134. Li Y, Yuan J, Yang Q, et al. Immunoliposome co-delivery of bufalin and anti-CD40 antibody adjuvant induces synergetic therapeutic efficacy against melanoma. Int J Nanomed. 2014;9:5683–5700. doi:10.2147/IJN.S73651
135. Zhang S, Zhang Y, Wang Z, et al. Temperature-sensitive gel-loaded composite nanomedicines for the treatment of cervical cancer by vaginal delivery. Int J Pharmaceut. 2020;586:119616. doi:10.1016/j.ijpharm.2020.119616
136. Zuo W, Li N, Zhao Y, et al. Synchronized release of bufadienolides in a stable Lutrol F127 based solid dispersion prepared with spray congealing. Drug Dev Ind Pharm. 2018;44(11):1817–1825. doi:10.1080/03639045.2018.1503290
137. Huang N. Construction of Compound Immunoliposomes Loaded with Melittin and Bufalin and Its Synergistic Mechanism in Inhibiting Sorafenib Resistance in Hepatocellular Carcinoma. Shanghai: Naval Medical University; 2019.
138. Li Q, Chen X, Lin W, Guo X, Ma Y. Application of a novel multicomponent nanoemulsion to tumor therapy based on the theory of ”unification of drugs and excipients”. Pharm Dev Technol. 2023;28(3–4):351–362. doi:10.1080/10837450.2023.2196330
139. Yuan Z, Liu C, Sun Y, et al. Bufalin exacerbates photodynamic therapy of colorectal cancer by targeting SRC-3/HIF-1alpha pathway. Int J Pharmaceut. 2022;624:122018. doi:10.1016/j.ijpharm.2022.122018
140. Zeng Z, Wang Z, Chen S, et al. Bio-nanocomplexes with autonomous O(2) generation efficiently inhibit triple negative breast cancer through enhanced chemo-PDT. J Nanobiotechnol. 2022;20(1):500. doi:10.1186/s12951-022-01706-0
141. Luo M, Tan C, Cao R, et al. Hybrid membrane camouflaged prussian blue nanoparticles with cinobufagin loading for chemo/photothermal therapy of colorectal cancer. Mater Design. 2023;232:112088. doi:10.1016/j.matdes.2023.112088
142. Liu B, Wang W, Fan J, et al. RBC membrane camouflaged prussian blue nanoparticles for gamabutolin loading and combined chemo/photothermal therapy of breast cancer. Biomaterials. 2019;217:119301. doi:10.1016/j.biomaterials.2019.119301
143. Li J, Zhang Z, Deng H, Zheng Z. Cinobufagin-loaded and folic acid-modified polydopamine nanomedicine combined with photothermal therapy for the treatment of lung cancer. Front Chem. 2021;9:637754. doi:10.3389/fchem.2021.637754
144. Song Z, Zhao L, Fang W, et al. Glioma cell membrane camouflaged cinobufotalin delivery system for combinatorial orthotopic glioblastoma therapy. Nano Res. 2023;16(8):11164–11175. doi:10.1007/s12274-023-5807-7
145. He J, Chen G, Zhao P, Ou C. Near-infrared light-controllable bufalin delivery from a black phosphorus-hybrid supramolecular hydrogel for synergistic photothermal-chemo tumor therapy. Nano Res. 2021;14(11):3988–3998. doi:10.1007/s12274-021-3325-z
146. Lei Z, Fan J, Li X, et al. Biomimetic graphene oxide quantum dots nanoparticles targeted photothermal-chemotherapy for gastric cancer. J Drug Target. 2023;31(3):320–333. doi:10.1080/1061186X.2022.2162060
147. Hu H, Qi Q, Dong Z, et al. Albumin coated trimethyl chitosan-based targeting delivery platform for photothermal/chemo-synergistic cancer therapy. Carbohyd Polym. 2020;241:116335. doi:10.1016/j.carbpol.2020.116335
148. Hu W. Preliminary Study of Photomagnetic Dual-Sensitive Bufalin Liposome Against Breast Cancer Metastasis. Beijing: Beijing University of Chinese Medicine; 2022.
149. Nezhadi S, Dorkoosh FA. Co-delivery systems: hope for clinical application? Drug Deliv Transl Re. 2022;12(6):1339–1354. doi:10.1007/s13346-021-01041-1
150. Al BR, Abuwatfa WH, Husseini GA. Recent advances in nanoparticle-based co-delivery systems for cancer therapy. Nanomaterials-Basel. 2022;12(15):2672. doi:10.3390/nano12152672
151. Ba X, Zhou S, Luo X, Jiang C, Xiao L, Hao Y. Research progress on pharmacological action and mechanism of Huachansu in anticancer pain. Chin J Pain Med. 2019;25(9):695–698.
152. Liu B. Adverse drug reactions induced by cinobufotalin injection:literature analysis of 252 cases. China Pharm. 2011;22(12):1096–1098.
153. Li M, Li H, Wang X, Ma J. Progress of experimental research on cardiotoxicity of Chansu. Chin J Pharmacol Toxicol. 2016;30(5):605–610.
154. Cheng M. Analysis on 272 cases of adverse reactions/incidents induced by cinobufacini injection. China Pharm. 2013;22(16):71–72.
155. Sun X. A case of neonatal toxic myocarditis of toad venom. J Neonatology. 1996;11(4):179.
156. Zhao W. A case report of acute poisoning with Liushen Pill. Clin J Tradit Chin Med. 2009;21(4):327.
157. Zhou C, Liu X. Two cases of tachyarrhythmia caused by toad venom poisoning. Chin Community Doctors. 2004;1(22):66.
158. Li M, Huang L, Shao S, et al. Cardiotoxicity assessment of cinobufotalin injection. Chin J New Drugs. 2020;29(13):1485–1494.
159. Li M, Wang X, Qiu Y, et al. Safety reassessment of cinobufotalin injection: new findings into cardiotoxicity. Toxicol Res-UK. 2020;9(4):390–398. doi:10.1093/toxres/tfaa035
160. Li X, Lei L, Hu J, Liu J, Deng W. Study on acute toxicity of toad venom and danling xinshu capsule. Pharmacol Clin Chin Mater Med. 2012;28(6):127–129.
161. Gao Q, Xiao Y, Wang Y, et al. IL-6 exacerbates potential arrhythmia of chan su intravenous injection. Chin Pharmacol Bull. 2022;38(5):712–718.
162. Yang A, Fan X, Li X, Liang Q, Wang Y, Luo G. Acute toxicity of venenum bufonis and compatibility of heart musk protecting pills by microarray expression analysis. Chem J Chin Univ. 2011;32(5):1058–1064.
163. Cheng CS, Wang J, Chen J, et al. New therapeutic aspects of steroidal cardiac glycosides: the anticancer properties of Huachansu and its main active constituent Bufalin. Cancer Cell Int. 2019;19:92. doi:10.1186/s12935-019-0806-1
164. Zuo Q, Xu DQ, Yue SJ, Fu RJ, Tang YP. Chemical composition, pharmacological effects and clinical applications of cinobufacini. Chin J Integr Med. 2024;30(4):366–378. doi:10.1007/s11655-024-3708-6
165. Zhang W, Fan Y, Zhang J, et al. Cell membrane-camouflaged bufalin targets NOD2 and overcomes multidrug resistance in pancreatic cancer. Drug Resist Update. 2023;71:101005. doi:10.1016/j.drup.2023.101005
166. Yang L, Zhou F, Zhuang Y, et al. Acetyl-bufalin shows potent efficacy against non-small-cell lung cancer by targeting the CDK9/STAT3 signalling pathway. Br J Cancer. 2021;124(3):645–657. doi:10.1038/s41416-020-01135-6
167. Li M, Wang XJ, Zhao Q, et al. Bufalin-induced cardiotoxicity: new findings into mechanisms. Chin J Nat Medicines. 2020;18(7):550–560. doi:10.1016/S1875-5364(20)30065-0
168. Jin YH, Jeon S, Lee J, et al. Broad spectrum antiviral properties of cardiotonic steroids used as potential therapeutics for emerging coronavirus infections. Pharmaceutics. 2021;13(11):1839. doi:10.3390/pharmaceutics13111839
169. Han L. Chemical Analysis, Metabolism, Activity and Toxicity Research of Bufadeinoliedes in Huachansu. Beijing: China Academy of Chinese Medical Sciences; 2018.
170. Editorial Board of Chinese Materia Medica. State Administration of Traditional Chinese Medicine. Chinese Materia Medica. Shanghai: Shanghai Scientific and Technical Publisher; 1999.
171. Ma H, Jiang J, Zhang J, et al. Protective effect of taurine on cardiotoxicity of the bufadienolides derived from toad (Bufo bufo gargarizans Canto) venom in guinea-pigs in vivo and in vitro. Toxicol Mech Method. 2012;22(1):1–8. doi:10.3109/15376516.2011.583295
172. Lu W. Research on the Cardiac Toxicity and Attenuated Drug Screening of Toad Venoms. Nanjing: Nanjing University of Chinese Medicine; 2012.
173. Liu P, Chen G, Zhang J. A review of liposomes as a drug delivery system: current status of approved products, regulatory environments, and future perspectives. Molecules. 2022;27(4):1372. doi:10.3390/molecules27041372
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.