Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Correlation Between Growth Differentiation Factor-15 and Peripheral Neuropathy in Patients with Type 2 Diabetes Mellitus
Authors Li Y , Wang Y, Cao Y, Zhang X, Dai W, Zhao Y, Zhang L, Han X
Received 18 January 2024
Accepted for publication 18 July 2024
Published 14 August 2024 Volume 2024:17 Pages 3019—3028
DOI https://doi.org/10.2147/DMSO.S454531
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Yue Li,1,2,* Yuhui Wang,2,3,* Yonghong Cao,1,2 Xinxiu Zhang,1,2 Wu Dai,1,2 Yiran Zhao,1,2 Lei Zhang,1,2 Xiaofang Han1,2
1Department of Endocrinology, Hefei Hospital Affiliated to Anhui Medical University, Hefei, People’s Republic of China; 2The Fifth Clinical College of Anhui Medical University, Hefei, People’s Republic of China; 3Department of Cardiology, Hefei Hospital Affiliated to Anhui Medical University, Hefei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaofang Han, Email [email protected]
Purpose: To inquire into the relationship between diabetic peripheral neuropathy (DPN) and serum levels of growth differentiation factor-15 (GDF-15) in individuals with type 2 diabetes mellitus (T2DM).
Patients and Methods: Out of 162 T2DM patients classified according to the diagnostic criteria of DPN, 75 were allocated to the non-DPN group and 87 to the DPN group. In turn, based on serum GDF-15 quartiles, all patients were additionally divided (GDF-15 low to high) into group A (40 cases), group B (41 cases), group C (41 cases), and group D (40 cases). General data and laboratory indexes of patients were collected, and enzyme-linked immunosorbent assay (ELISA) was used to determine serum GDF-15 levels.
Results: Compared to the non-DPN group, in the DPN group GDF-15 levels were noticeably greater (P < 0.001). Using serum GDF-15 as a grouping variable, DPN prevalence and body mass index were gradually increased, motor and sensory nerve latencies were gradually lengthened, and amplitude (Amp) and nerve conduction velocity (NCV) were gradually decreased with increasing GDF-15 levels (P < 0.05). Linear regression modeling revealed that GDF-15 levels correlated positively with the latencies of sensory and motor nerves, and negatively with their corresponding NCV (P < 0.05). Binary logistic regression results indicated GDF-15 as an independent predictor for DPN (P < 0.05), whereas restricted cubic spline analysis indicated a dose-response, nonlinear relationship between GDF-15 and DPN.
Conclusion: Serum GDF-15 level strongly correlates with DPN, and may represent an independent predictor and a biological marker for the disease.
Keywords: growth differentiation factor 15, diabetic neuropathy, nerve conduction study, type 2 diabetes mellitus
Introduction
The most common chronic complication of diabetes mellitus (DM) is diabetic peripheral neuropathy (DPN).1 The prevalence of DPN in adults with diabetes in the United States is estimated to be 28%, and a meta-analysis indicated that DPN occurs in up to 90% of patients with diabetes of >20 years’ duration.2,3 DPN imposes a substantial economic burden and diminishes quality of life for individuals with diabetes. In the United States, the projected expenses associated with DPN ranged from $3000 to $4000 annually per patient.4 It is estimated that 43% of DPN patients experience anxiety, depression, and sleep disorders,5 and previous studies have reported an average loss of 5.5 working days per month due to pain.6 Additionally, DPN-induced loss of protective sensation increases the risk of foot ulcers and lower limb amputations. Approximately 25% of DM patients suffer from a foot ulcer throughout their lifespan,7 whereas approximately 14%–24% will require limb amputation.8 Therefore, early detection and identification of potential predictors for DPN is necessary.
A constituent of the transforming growth factor-β (TGF-β) family, growth differentiation factor-15 (GDF-15) is extensively expressed throughout the peripheral and central nervous systems.9 It was found that damage to Schwann cells can directly cause upregulation of GDF-15 expression.9 As a marker of oxidative stress, endothelial dysfunction, atherosclerosis, and inflammation, GDF-15 participates actively in type 2 diabetes mellitus (T2DM) and its complications.10,11 Peripheral nerve injury caused by multiple pathways, including metabolic disorders and inflammation, is the primary mechanism of DPN development.12 Accordingly, we hypothesize that elevated GDF-15 may be related to the pathogenesis of DPN.
Since reliable biomarkers and targets for predicting DPN are currently insufficient, we conducted this study to investigate the relationship between GDF-15 and DPN.
Material and Methods
Study Participants
We enrolled 162 consecutive T2DM patients who were admitted to the Second People’s Hospital of Hefei City from August 2022 to June 2023 and met the inclusion and exclusion criteria. According to the diagnostic criteria of DPN, two groups of patients, ie the DPN group (n = 87) and the non-DPN group (n = 75), were established.
Inclusion criteria: (1) diagnosis based on the diagnostic criteria established by the World Health Organization (WHO) in 1999; (2) completion of electromyography (EMG). Exclusion criteria: (1) acute complications of diabetes; (2) gestational diabetes; (3) severe diseases and conditions, such as tumors and infections (C-reactive protein > 10 mg/L), heart failure (NYHA classification > II), liver failure (Prothrombin activity < 40%, Total bilirubin > 170 μmol/L), renal failure (estimated glomerular filtration rate < 30 mL/min per 1.73 m2 by the CKD-EPI formula), etc.; (4) diagnosed and/or treated mental illness; (5) peripheral neurological disorders caused by other conditions, such as Parkinson’s disease, herniated lumbar discs, Guillain-Barré syndrome, etc.
Data Collection
The patients’ baseline data and laboratory findings were registered: (1) The demographics, medical history, and body measurement data, including age (years), sex, height (m), weight (kg), duration of DM (from now on referred to as duration, years), metformin medication, and hypertension status were collected through interviews. Body mass index (BMI, kg/m2) was recorded through measurements by trained clinicians. (2) Collection and determination of peripheral blood specimens was performed after an 8-hour fasting period, from a sample of venous blood obtained in the early morning. After blood centrifugation, an immunoturbidimetric assay (ADVIA2400, Siemens, Germany) was used to determine triglyceride (TG, mmol/L), total cholesterol (TC, mmol/L), high-density lipoprotein (HDL, mmol/L), low-density lipoprotein (LDL, mmol/L), serum uric acid (UA, µmol/L), serum creatinine (Scr, µmol/L), and fasting blood glucose (FBG, mmol/L). Fasting insulin (INS, pmol/L) was measured with an automated chemiluminescence immunoassay (IMMULITE 2000, Siemens, Germany). Glycosylated hemoglobin A1c (HbA1c) was measured using a Trinity Biotech Premier Hb9210 kit (Trinity Biotech Plc., Ireland). Urine albumin creatine ratio (ACR) was measured using a urine-formed elements analyzer (AVE-764, AVE, China). (3) For GDF-15 determination, all blood samples were stored at −80°C before measurements. An enzyme-linked immunosorbent assay kit (Cusabio Biotechnology, China) was used to measure GDF-15 (pg/mL).
Electrophysiologic Testing
All enrolled subjects were tested using an electromyographic evoked potential apparatus (Neptune NDI-092; Haishen Suzhou Medical Instruments Co., Ltd., China). Motor nerves were selected from the median, ulnar, common peroneal, and tibial nerves; sensory nerves were chosen from the median, ulnar, and sural nerves. The latencies, amplitude (Amp), and nerve conduction velocity (NCV) of the motor and sensory nerves were recorded bilaterally in the study subjects. Given the complexity and specialized nature of this procedure, the assessment of electrophysiological parameters was assisted by two experienced electrophysiologists (Supplementary Appendix S1).
Definitions
(1) T2DM was defined based on the 1999 WHO diagnostic criteria (Supplementary Appendix S2).13
(2) DPN was determined using a tiered structure consisting of three levels of confirmed, clinical, or subclinical diagnostic criteria. The evaluation was primarily by clinical observations and nerve conduction test results (Supplementary Appendix S3).14
(3) Homeostasis model assessment of insulin resistance (HOMA-IR) index = FPG (mmol/L) × fasting insulin (μU/mL)/22.5 (Supplementary Appendix S4).
Statistical Analysis
SPSS, version 26.0 (IBM Company, Chicago, IL, USA), and R Studio, version 4.2.1 (R Foundation for Statistical Computing) were used to conduct the statistical analysis. Shapiro–Wilk test was used to test the normality of a variable. Normally distributed measures were expressed as mean ± standard deviation (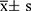 ), Student’s t-tests were used to compare two groups, and one-way analysis of variance (ANOVA) was used to compare several groups. Non-normally distributed measures were expressed as median and interquartile spacing [M (P25, P75)], and the Kruskal–Wallis H-test or the Mann–Whitney U-test was used to compare intergroup differences. Count data were presented as cases (percentage) [n (%)], and chi-squared tests were used to compare groups. We explored the correlation between GDF-15 and nerve conduction using linear regression modeling. The restricted cubic spline (RCS) method was performed to test for a nonlinear relationship between GDF-15 and DPN. Predictors for DPN were analyzed using multivariable binary logistic regression. To maintain the odds ratio (OR) values, beta values, and their 95% confidence intervals (CI) for GDF-15 at an appropriate scale, we reduced the value of GDF-15 to one percent of its original value when constructing linear regression and logistic regression models. Therefore, the results should be interpreted per 100 unit change. P < 0.05 indicated a statistically significant difference.
), Student’s t-tests were used to compare two groups, and one-way analysis of variance (ANOVA) was used to compare several groups. Non-normally distributed measures were expressed as median and interquartile spacing [M (P25, P75)], and the Kruskal–Wallis H-test or the Mann–Whitney U-test was used to compare intergroup differences. Count data were presented as cases (percentage) [n (%)], and chi-squared tests were used to compare groups. We explored the correlation between GDF-15 and nerve conduction using linear regression modeling. The restricted cubic spline (RCS) method was performed to test for a nonlinear relationship between GDF-15 and DPN. Predictors for DPN were analyzed using multivariable binary logistic regression. To maintain the odds ratio (OR) values, beta values, and their 95% confidence intervals (CI) for GDF-15 at an appropriate scale, we reduced the value of GDF-15 to one percent of its original value when constructing linear regression and logistic regression models. Therefore, the results should be interpreted per 100 unit change. P < 0.05 indicated a statistically significant difference.
Results
Population Characteristics
On comparison of clinical data among patient groups, patients with DPN were significantly older and had higher duration, BMI, HbA1c, UA, ACR, and GDF-15 than those in the non-DPN group (Table 1). We compared also the clinical data based on serum GDF-15 as a grouping variable (Table 2). The results showed that there were remarkable differences in sex, prevalence of DPN, BMI, UA, Scr, and ACR between the four different groups. Overall, as the level of GDF-15 increased, the prevalence of DPN and BMI increased gradually (Table 2).
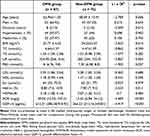 |
Table 1 Characteristics of the Study Population |
 |
Table 2 Characteristics of the Study Population Based on GDF-15 Levels |
Correlation Analysis of Nerve Conduction and GDF-15
We next compared electromyography data from T2DM patients with different GDF-15 levels. The results showed differences in motor and sensory nerves between groups with different GDF-15 levels (P < 0.05). We noted that electromyography activity varied with GDF-15 levels, with increasing GDF-15 levels correlating with prolonged motor and sensory nerve latencies and decreased Amp and NCV (Table 3).
 |
Table 3 Characterization of Nerve Conduction in Patients with Different GDF-15 Levels |
Multivariable Linear Regression
A multiple linear regression analysis, which included variables with P < 0.05 in the single-factor logistic regression, and variance inflation factor (VIF) <5 was next conducted. The results demonstrated that GDF-15 correlated positively with the latency of sensory and motor nerves, and negatively with their NCV (Table 4 and Supplementary Tables 1–6).
 |
Table 4 Multivariable Linear Regression Analysis of Nerve Conduction and GDF-15 |
Multivariable Logistic Regression
A multivariate binary logistic regression analysis, which included variables with P < 0.05 in the single-factor logistic regression, and VIF <5 was next conducted. The results indicated a strong correlation between GDF-15 and an elevated risk of DPN. Specifically, the likelihood of developing DPN rose by ~24% with each 100 pg/mL increment in GDF-15 levels (OR = 1.24, 95% confidence interval (CI): 1.09–1.43, P = 0.002) (Table 5).
 |
Table 5 Logistic Regression Analysis of DPN Predictors |
Nonlinear Relationship Between GDF-15 and DPN
The RCS suggested that when GDF-15 is below 914.28 pg/mL, the occurrence of DPN increases remarkably as the level of GDF-15 increases (OR = 1.34, 95% 1.12~1.64, P = 0.002). In turn, a saturation effect is seen when GDF-15 exceeds 914.28 pg/mL, determining a nonlinear correlation between GDF-15 and DPN in our study subjects (Figure 1 and Supplementary Table 7).
 |
Figure 1 Correlation between GDF-15 and DPN. Odds ratios (OR) are shown as solid lines, and 95% confidence intervals (CI) are shown as purple areas. The dashed line corresponds to the turning point. |
Discussion
Approximately 50% of individuals with diabetes are projected to acquire diabetic peripheral neuropathy (DPN) over time, making the identification of biomarkers for early-stage DPN crucial.15 The purpose of this study was to examine the link between GDF-15 and DPN by revealing the correlation between GDF-15 levels and nerve conduction parameters and DPN risk.
GDF-15 is a stress-responsive chemokine member of the transforming growth factor-β (TGF-β) superfamily. Elevated GDF-15 expression is associated with metabolic diseases, inflammation, cancer, and cardiovascular disease. Numerous studies have confirmed that GDF-15 is significantly correlated with the incidence of diabetes and is a high-risk biomarker for this condition.16,17 In addition, research has showed that GDF-15 levels have a tendency to rise when microvascular injury worsens, and are positively linked to complications of macrovascular disease.18,19 In our study, significant differences in BMI, UA, Scr, and ACR were found among patients with different GDF-15 levels. More importantly, a high level of GDF-15 was a predictor for DPN independent of traditional predictors such as age, BMI, duration, UA, HbA1c, and ACR (OR: 1.24, 95% CI: 1.09~1.43, P = 0.002).
The present study revealed a significantly higher BMI in the highest GDF-15 group (group D) compared to the lowest GDF-15 group (group A) (r = 0.197, P = 0.017), a finding consistent with Lee et al.20 However, contradictory results emerged in previous research, which found that elevated levels of GDF-15 caused macrophage M2 polarization and promoted an improved inflammatory state and weight loss.21 Notably, studies by Vila and Ho et al found no correlation between GDF-15 and BMI.22,23 In turn, a controlled study concluded that T2DM patients taking metformin had 40% higher GDF-15 levels than those who did not take this medication.24 In contrast, in the present study no relationship was found between oral metformin and GDF-15 level. This discrepancy may be related to patient selection, study design, and/or drug use. Another study reported that metformin prevented weight gain in wild-type mice fed a high fat diet. The authors hypothesized that metformin induces intestinal secretion of GDF-15 into the bloodstream, which activates the hindbrain and the nucleus accumbens via the GFRAL pathway, thereby affecting feeding behavior and reducing body weight.25
The complex pathophysiological mechanisms of DPN are thought to arise in the context of diabetes. As a common vascular factor, high glucose levels are a significant predictor for DM and its complications.26 Indicators of dysglycemia, including chronic hyperglycemia (HbA1c) and time in range, have been shown in this and other studies to be predictors for DPN.27 Additionally, the progression of DPN is linked to oxidative stress, mitochondrial dysfunction, inflammatory response, and lipid metabolism disorders.28–31 Serum GDF-15 has been shown to predict tibial motor neuropathy and ulnar and median sensory neuropathy in patients with DM, suggesting that there is a significant correlation between elevated levels of GDF-15 and DPN.32 Consistent with this, in this work we discovered that GDF-15 levels were markedly elevated in patients with T2DM without comorbid DPN, whereas the occurrence of DPN gradually increased with GDF-15. In this respect, a dose-response correlation between GDF-15 and DPN was found by RCS analysis. When GDF-15 was below 914.28 pg/mL, the risk of DPN prevalence increased with increasing GDF-15 levels (OR = 1.34, 95% 1.12~1.64, P = 0.002). We also found that GDF-15 levels showed a positive linear correlation with the latency of sensory and motor nerves and a negative linear correlation with NCV. This was consistent with the study of Weng et al,32 suggesting that DPN-related nerve damage was aggravated by increased GDF-15 levels. Therefore, elevated circulating GDF-15 levels may be a potential predictor for DPN.
Several possible mechanisms may explain the relationship between GDF-15 and DPN. Insulin resistance is considered a common factor in the pathogenesis of T2DM and DPN. Studies have shown that GDF-15 is a reliable indicator of insulin resistance, regardless of other factors.33 On the one hand, increasing GDF-15 levels during stress improves insulin resistance through the PPAR β/δ- Adenosine 5- monophosphate (AMP)- activated protein kinase (AMPK) pathway.34, On the other hand, overexpression of GDF-15 increases glucose uptake and promotes glucose-induced insulin secretion by upregulating the major glucose transporter and downregulating metallothionein-1 (MT-1) expression.35 In addition, GDF-15 protects pancreatic islet β cells function by activating the PI3K-Akt-eNOS signaling pathway and attenuating the expression of NF-κB, JNK, and cysteinyl aspartate-specific proteinase-3 (Caspase-3), and by exerting anti-apoptotic properties.36–38
Studies have confirmed that DPN is characterized by low-grade chronic subclinical inflammation.31 High glucose levels trigger increased release of pro-inflammatory factors and active production of reactive oxygen species, which induce apoptosis of neurons and Schwann cells in peripheral nerves, resulting in neurological and vascular changes.30 Data suggest that Schwann cells directly release GDF-15 upon cell injury.39 GDF-15, being an anti-inflammatory protein, hinders the activation of macrophages and has a beneficial impact on chronic inflammatory conditions like T2DM.10
Study Limitations
There are certain constraints in the current investigation. First, this is a cross-sectional study, which cannot confirm a causal relationship between GDF-15 and the development of DPN. Hence, sampling size should be further enlarged to conduct a prospective study in the future. Second, the specific mechanism(s) underlying the association between GDF-15 and DPN need to be identified. Third, the degree of abnormality of the nerves examined was not accounted for in this study, so it was not possible to explore lesion differences between sensory and motor or upper and lower limb nerves. Fourth, this study included a relatively limited number of participants and did not include a control group with normal blood glucose. The bias brought by the small-sample study was unavoidable, and there were some markers or indicators (such as FBG, HbA1c, HOMA-IR, etc). whose serum levels were theoretically associated with GDF-15 but did not show significance.
Conclusion
In summary, high levels of GDF-15 are an independent predictor for DPN. As the GDF-15 level increased, the latency period of sensory and motor nerves of DM patients was prolonged. The concomitant decrease in NCV indicates a close correlation between GDF-15 expression and diabetic peripheral nerve injury. Since this correlation is highly significant for assessing the pathophysiology of DPN and the severity of the disease, future studies should focus on the mechanisms and pathways involved, to aid convenient screening for DPN in primary hospitals. Furthermore, because of its role in enhancing insulin sensitivity and reducing body weight, GDF-15 offers possible avenues for developing insulin sensitizers and weight loss drugs.
Abbreviations
ANOVA, Analysis of variance; BMI, Body mass index; CI, Confidence intervals; DM, Diabetes mellitus; DPN, Diabetic peripheral neuropathy; FBG, Fasting blood glucose; HDL, High-density lipoprotein; LDL, Low-density lipoprotein; NCV, Nerve conduction velocity; OR, Odds ratios; RCS, Restricted cubic spline; ROS, Reactive oxygen species; TC, Total cholesterol; TIR, Time in range; UA, Uric acid; WHO, World Health Organization; AMPK, Adenosine 5‘- monophosphate (AMP)- activated protein kinase.
Ethics Approval and Informed Consent
The study conforms to the principles of the Helsinki Declaration, and it was approved by the hospital’s Medical Ethics Committee (No. 2023-keyan-108). Each patient signed an informed permission form. Blood samples were obtained from the remaining samples of routine admission tests, ensuring that there was no additional risk to the patients.
Acknowledgments
Gratitude is extended to the individuals who participated in the research and to the personnel from the Endocrinology and Metabolism Department at Hefei Hospital, affiliated with Anhui Medical University, for their contributions to this endeavor.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Disclosure
All authors have declared no conflicts of interest and have consented to the publication of the data described in this manuscript.
References
1. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–154. doi:10.2337/dc16-2042
2. Gregg EW, Gu Q, Williams D, et al. Prevalence of lower extremity diseases associated with normal glucose levels, impaired fasting glucose, and diabetes among U.S. adults aged 40 or older. Diabet Res Clin Pract. 2007;77(3):485–488. doi:10.1016/j.diabres.2007.01.005
3. Zheng C, Ou W, Shen H, et al. Combined therapy of diabetic peripheral neuropathy with breviscapine and mecobalamin: a systematic review and a meta-analysis of Chinese studies. Biomed Res Int. 2015;2015:680756. doi:10.1155/2015/680756
4. Wu N, Chen S, Boulanger L, et al. Duloxetine compliance and its association with healthcare costs among patients with diabetic peripheral neuropathic pain. J Med Econ. 2009;12(3):192–202. doi:10.3111/13696990903240559
5. Tölle T, Xu X, Sadosky AB. Painful diabetic neuropathy: a cross-sectional survey of health state impairment and treatment patterns. J Diabetes Its Compl. 2006;20(1):26–33. doi:10.1016/j.jdiacomp.2005.09.007
6. McDermott AM, Toelle TR, Rowbotham DJ, et al. The burden of neuropathic pain: results from a cross-sectional survey. Eur J Pain. 2006;10(2):127–135. doi:10.1016/j.ejpain.2005.01.014
7. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–228. doi:10.1001/jama.293.2.217
8. Consensus Development Conference on Diabetic Foot Wound Care. Boston, Massachusetts. Am Diab Assoc Diab Ca. 1999;22(8):1354–1360. doi:10.2337/diacare.22.8.1354
9. Mensching L, Börger A-K, Wang X, et al. Local substitution of GDF-15 improves axonal and sensory recovery after peripheral nerve injury. Cell Tissue Res. 2012;350(2):225–238. doi:10.1007/s00441-012-1493-6
10. Lu J, Zhang Y, Dong X, et al. Association between MIC-1 and type 2 diabetes: a combined Analysis. Dis Markers. 2019;2019:7284691. doi:10.1155/2019/7284691
11. Schernthaner-Reiter MH, Kasses D, Tugendsam C, et al. Growth differentiation factor 15 increases following oral glucose ingestion: effect of meal composition and obesity. Eur J Endocrinol. 2016;175(6):623–631. doi:10.1530/EJE-16-0550
12. Shakher J, Stevens MJ. Update on the management of diabetic polyneuropathies. Diabetes Metab Syndr Obes. 2011;4:289–305. doi:10.2147/DMSO.S11324
13. Consultation W. Definition, Diagnosis and classification of diabetes mellitus and its complications; 1999.
14. Martin CL, Albers JW, Pop-Busui R, et al. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):31–38. doi:10.2337/dc13-2114
15. Partanen J, Niskanen L, Lehtinen J, et al. Natural history of peripheral neuropathy in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333(2):89–94. doi:10.1056/NEJM199507133330203
16. Xiao QA, He Q, Zeng J, et al. GDF-15, a future therapeutic target of glucolipid metabolic disorders and cardiovascular disease. Biomed Pharmacother. 2022;146:112582. doi:10.1016/j.biopha.2021.112582
17. Bao X, Borné Y, Muhammad IF, et al. Growth differentiation factor 15 is positively associated with incidence of diabetes mellitus: the Malmö Diet and Cancer-Cardiovascular Cohort. Diabetologia. 2019;62(1):78–86. doi:10.1007/s00125-018-4751-7
18. Carlsson AC, Nowak C, Lind L, et al. Growth differentiation factor 15 (GDF-15) is a potential biomarker of diabetic kidney disease and future cardiovascular events in cohorts of individuals with type 2 diabetes: a proteomics approach. Ups J Med Sci. 2020;125(1):37–43. doi:10.1080/03009734.2019.1696430
19. Bonfiglio V, Platania CBM, Lazzara F, et al. TGF-β serum levels in diabetic retinopathy patients and the role of anti-VEGF therapy. Int J Mol Sci. 2020;21(24):9558. doi:10.3390/ijms21249558
20. Lee SH, Lee JY, Lim K-H, et al. Associations between plasma growth and differentiation Factor-15 with aging phenotypes in muscle, adipose tissue, and bone. Calcif Tissue Int. 2022;110(2):236–243. doi:10.1007/s00223-021-00912-6
21. Chang JY, Hong HJ, Kang SG, et al. The role of growth differentiation Factor 15 in energy metabolism. Diabetes Metab J. 2020;44(3):363–371. doi:10.4093/dmj.2020.0087
22. Vila G, Riedl M, Anderwald C, et al. The relationship between insulin resistance and the cardiovascular biomarker growth differentiation factor-15 in obese patients. Clin Chem. 2011;57(2):309–316. doi:10.1373/clinchem.2010.153726
23. Ho JE, Mahajan A, Chen M-H, et al. Clinical and genetic correlates of growth differentiation factor 15 in the community. Clin Chem. 2012;58(11):1582–1591. doi:10.1373/clinchem.2012.190322
24. Natali A, Nesti L, Venturi E, et al. Metformin is the key factor in elevated plasma growth differentiation factor-15 levels in type 2 diabetes: a nested, case-control study. Diabetes Obes Metab. 2019;21(2):412–416. doi:10.1111/dom.13519
25. Coll AP, Chen M, Taskar P, et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature. 2020;578(7795):444–448. doi:10.1038/s41586-019-1911-y
26. Clair C, Cohen MJ, Eichler F, et al. The effect of cigarette smoking on diabetic peripheral neuropathy: a systematic review and meta-analysis. J Gen Intern Med. 2015;30(8):1193–1203. doi:10.1007/s11606-015-3354-y
27. Yu C, Zhuang L, Xu F, et al. Increased levels of serum adenosine deaminase and increased risk of diabetic peripheral neuropathy in type 2 diabetes. Front Endocrinol. 2022;13:997672. doi:10.1080/03009734.2019.1696430
28. Gonçalves NP, Vægter CB, Andersen H, et al. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat Rev Neurol. 2017;13(3):135–147. doi:10.1038/nrneurol
29. Marshall A, Alam U, Themistocleous A, et al. Novel and emerging electrophysiological biomarkers of diabetic neuropathy and painful diabetic neuropathy. Clin Ther. 2021;43(9):1441–1456. doi:10.1016/j.clinthera.2021.03.020
30. De Gregorio C, Contador D, Díaz D, et al. Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res Ther. 2020;11(1):168. doi:10.1186/s13287-020-01680-0
31. Vincent AM, Callaghan BC, Smith AL, et al. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011;7(10):573–583. doi:10.1038/nrneurol.2011.137
32. Weng S-W, Chen W-C, Shen F-C, et al. Circulating growth differentiation Factor 15 is associated with diabetic neuropathy. J Clin Med. 2022;11(11):3033. doi:10.3390/jcm11113033
33. Kempf T, Guba-Quint A, Torgerson J, et al. Growth differentiation factor 15 predicts future insulin resistance and impaired glucose control in obese nondiabetic individuals: results from the XENDOS trial. Eur J Endocrinol. 2012;167(5):671–678. doi:10.1530/EJE-12-0466
34. Kobayashi M, Zochodne DW. Diabetic neuropathy and the sensory neuron: new aspects of pathogenesis and their treatment implications. J Diabetes Investig. 2018;9(6):1239–1254. doi:10.1111/jdi.12833
35. Mohammad MG, Saeed R, Mohammed AK, et al. GDF15 plays a critical role in insulin secretion in INS-1 cells and human pancreatic islets. Exp Biol Med. 2023;248(4):339–349. doi:10.1177/15353702221146552
36. Aguilar-Recarte D, Barroso E, Gumà A, et al. GDF15 mediates the metabolic effects of PPARβ/δ by activating AMPK. Cell Rep. 2021;36(6):109501. doi:10.1016/j.celrep.2021.109501
37. Eltokhy AK, Khattab HA, Rabah HM. The impact of Cichorium intybus L. On GDF-15 level in obese diabetic albino mice as compared with metformin effect. J Diabetes Metab Disord. 2021;20(2):1119–1128. doi:10.1007/s40200-021-00828-w
38. Sarkar S, Syed F, Webb-Robertson B-J, et al. Protection of β cells against pro-inflammatory cytokine stress by the GDF15-ERBB2 signaling. medRxiv. 2023. doi:10.1101/2023.11.27.23298904
39. Strelau J, Strzelczyk A, Rusu P, et al. Progressive postnatal motoneuron loss in mice lacking GDF-15. J Neurosci. 2009;29(43):13640–13648. doi:10.1523/JNEUROSCI.1133-09.2009
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
