Back to Journals » International Journal of Nanomedicine » Volume 19
Delivery of miRNAs Using Nanoparticles for the Treatment of Osteosarcoma
Authors Wang C, Zhang Y , Kong W, Rong X, Zhong Z, Jiang L, Chen S, Li C , Zhang F, Jiang J
Received 2 April 2024
Accepted for publication 31 July 2024
Published 22 August 2024 Volume 2024:19 Pages 8641—8660
DOI https://doi.org/10.2147/IJN.S471900
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kamakhya Misra
Chengran Wang,1,* Yihong Zhang,1,* Weihui Kong,2 Xin’ao Rong,1 Ziming Zhong,1 Lei Jiang,3 Shuhan Chen,1 Chuang Li,1 Fuqiang Zhang,1 Jinlan Jiang1
1Department of Scientific Research Center, China–Japan Union Hospital of Jilin University, Changchun, Jilin Province, People’s Republic of China; 2Department of Stomatology, the First Hospital of Jilin University, Changchun, Jilin Province, People’s Republic of China; 3Department of Geriatric Medicine, Changchun Central Hospital, Changchun, Jilin Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fuqiang Zhang; Jinlan Jiang, Department of Scientific Research Center, China–Japan Union Hospital of Jilin University, Changchun, Jilin Province, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Osteosarcoma is the predominant primary malignant bone tumor that poses a significant global health challenge. MicroRNAs (miRNAs) that regulate gene expression are associated with osteosarcoma pathogenesis. Thus, miRNAs are potential therapeutic targets for osteosarcoma. Nanoparticles, widely used for targeted drug delivery, facilitate miRNA-based osteosarcoma treatment. Numerous studies have focused on miRNA delivery using nanoparticles to inhibit the progress of osteosarcoma. Polymer-based, lipid-based, inorganic-based nanoparticles and extracellular vesicles were used to deliver miRNAs for the treatment of osteosarcoma. They can be modified to enhance drug loading and delivery capabilities. Also, miRNA delivery was combined with traditional therapies, for example chemotherapy, to treat osteosarcoma. Consequently, miRNA delivery offers promising therapeutic avenues for osteosarcoma, providing renewed hope for patients. This review emphasizes the studies utilizing nanoparticles for miRNA delivery in osteosarcoma treatment, then introduced and summarized the nanoparticles in detail. And it also discusses the prospects for clinical applications.
Keywords: osteosarcoma, MicroRNAs, nanoparticle delivery, molecular targeted therapy
Graphical Abstract:
Introduction
Osteosarcoma originates from primitive mesenchymal cells and is the predominant malignant primary bone tumor in children and adolescents aged 0–24 years.1 Most osteosarcoma cases manifest in the lower long bones.2 Osteosarcoma can be classified into the osteoblastic, chondroblastic, and fibroblastic subtypes.3 A defining feature of osteosarcoma is the osteoid extracellular matrix, which is predominantly composed of collagen I.4 Osteosarcoma is characterized by notable inter- and intra-tumoral heterogeneity.5 The development of osteosarcoma is linked to intricate genetic mutations, notably chromothripsis, chromoplexies, and kataegis mutations.6 The most frequently mutated genes involved in osteosarcoma pathogenesis are tumor suppressor p53 (TP53) and the retinoblastoma susceptibility gene (RB1). Additionally, approximately 90% of osteosarcomas exhibit mutations in breast cancer (BRCA)-related genes.6 The recurrence and metastasis of osteosarcoma significantly affect patients’ survival.7 Consequently, novel osteosarcoma treatments with substantial research potential are urgently needed to target mutated genes.
Currently, the standard treatment for osteosarcoma is neoadjuvant chemotherapy supplemented by a combination of surgical and adjuvant therapies.8 Osteosarcoma metastasis, influenced by the bone microenvironment, poses considerable challenges for both surgical and chemotherapeutic interventions.9 Immunotherapy and targeted chemotherapy are emerging strategies for osteosarcoma treatment, encompassing the innovation of novel drug delivery systems.10
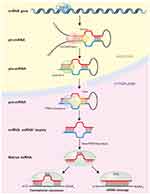
MiRNAs are single-stranded endogenous RNA, approximately 22 nucleotides in length.11 Lee et al12 first identified miRNAs in Caenorhabditis elegans in 1993. Within the nucleus, RNA polymerase II transcribes miRNA genes into pri-miRNAs, which are subsequently cleaved into pre-miRNAs by Drosha and its cofactor DGCR8/Pasha.13 Subsequently, Exportin 5 transports pre-miRNAs to the cytoplasm, where Dicer cleaves them into small double-stranded RNAs termed miRNA duplexes.14 The miRNA duplex is incorporated into the guide strand channel of an argonaute protein, resulting in the formation of an RNA-induced silencing complex (RISC).15 The RISC complex then facilitates the recognition of the targeted mRNA, leading to either mRNA destabilization or translational repression (Figure 1).16
MiRNAs, functioning in association with argonaute and the 182 kDa glycine-tryptophan protein, engage with the mRNA 3′-untranslated region (UTR). This interaction leads to translational repression, deadenylation, and degradation, culminating in specific biological effects.17 MiRNAs play pivotal roles in modulating diverse physiological processes, including proliferation, differentiation, and immunity.18 Concurrently, miRNAs participate in the orchestration of cancer-associated pathological processes, including cell cycle regulation, proliferation, apoptosis, invasion, migration, and angiogenesis.19,20 Therapies based on the gene-regulating functions of miRNAs have been used for tumor treatment.21
MiRNA delivery involves safeguarding and stabilizing endogenous or exogenous miRNA structures via diverse techniques. This process ensures targeted delivery to disease sites, facilitates gene regulation, and aids in disease treatment.22 These miRNAs include tumor suppressors (TS miRNAs), oncogenes (oncomiRs), miRNA mimics, and molecules specifically targeting miRNAs (anti-miRs).23 TS miRNAs, which are typically downregulated in cancer, and oncomiRs, which are often overexpressed in cancer, are endogenous.24 Utilizing exogenous miRNAs to modulate the expression of endogenous miRNAs offers a therapeutic approach for cancer.
Encapsulation of miRNAs into nanocarriers can improve delivery efficiency and become a promising strategy for targeting therapy of cancer.25 Currently, many types of vectors are used for delivery of miRNAs.26 Polymer-based carriers are capable of adsorbing or encapsulating miRNAs and can also be modified with groups or molecules to increase performance.27,28 Liposomes are highly biocompatible and have been widely used to deliver miRNAs.29 Exosomes, secreted by cells, are capable of carrying various secretions and have homing properties to target lesions.30 Inorganic nanoparticle can be used in conjunction with a variety of methods, such as magnetic fields and photothermal therapy, to enhance the effect of miRNA delivery.31
Strategies centered on miRNA-targeted delivery for tumor treatment have demonstrated efficacy and have been transitioned into clinical trials, revealing significant application potential.32 This advancement also kindles optimism for miRNA-based gene-targeted therapies for osteosarcoma. In this context, our study delves into the recent discoveries related to osteosarcoma treatment via miRNA delivery, summarizes the vectors used and explores the potentials for clinical applications.
Roles of miRNAs in Tumors
In 2002, Carlin et al33 discovered that both miR-15 and miR-16 were either deleted or downregulated in 68% of patients with chronic lymphocytic leukemia, marking the inaugural identification of the association between miRNAs and tumors. Subsequently, researchers have progressively delved deeper into the relationship between miRNAs and tumors.34,35 The dysregulation of expression of miRNAs in cancer is intricately linked to tumor pathogenesis.36 Therefore, targeting and modulating miRNA expression may be a novel therapeutic approach for cancer treatment.37 Deregulated miRNAs in pathological conditions can be addressed through miRNA replacement therapies using miRNA mimics or by inhibiting the function of miRNAs using anti-miRs, both of which hold therapeutic promise.23 In 2013, MRX34, a synthetic double-stranded miR-34a mimic, was introduced clinically for the first time in tumor treatment.38 A deeper exploration of the role of miRNAs in tumorigenic mechanisms could expedite their therapeutic application in tumor treatments.39
Multiple miRNAs play pivotal roles in the evolution and progression of breast and prostate cancers.40 Xu et al41 identified that miRNA-135 curbed the onset of epithelial-mesenchymal transition (EMT) in breast cancer. This was achieved by targeting and downregulating zinc finger protein 217 (ZNF217) and subsequently preventing Nanog homeobox (NANOG) upregulation by reducing N6-methyladenosine levels via methyltransferase-like 13 (METTL13). Gan et al42 observed that elevated levels of miR-375 in patients with castration-resistant PCa could expedite prostate cancer progression and resistance to enzalutamide. This was achieved by disrupting the expression of phosphatase non-receptor type 4, which subsequently stabilized phosphorylated signal transducer and activator of transcription 3 (STAT3). Khan et al43 determined that miR-1 directly targeted the 3’-UTR of CXC chemokine receptor type 4 (CXCR4), which hindered Forkhead box M1 (FOXM1) from binding to the Ribonucleotide reductase M2 (RRM2) promoter, thereby inhibiting the growth and metastasis of small cell lung cancer. Additionally, researches indicated that tumor-derived exosomes significantly influenced cancer development by promoting cancer proliferation, invasion, metastasis, EMT, and immune evasion.44–46 Qiu et al47 discovered that exo-miR-519a-3p derived from gastric cancer triggered M2 polarization in intrahepatic macrophages, leading to the establishment of premetastatic niches rich in angiogenesis. Zeng et al48 discovered that exosomal miR-25-3p, originating from colorectal cancer cells, elevated the expression of vascular endothelial growth factor receptor-2 (VEGFR2), zonula occludens-1 (ZO-1), occludin, and Claudin5 in endothelial cells by targeting Krüppel-like factor 2 (KLF2) and Krüppel-like factor 4 (KLF4). This action enhanced vascular permeability and angiogenesis, thereby promoting colorectal cancer metastasis. Consequently, miRNAs are intricately linked to the biological behaviors of tumors and are promising targets for tumor therapy.49
Effects of miRNAs in the Occurrence and Development of Osteosarcoma
Numerous studies have explored the role of miRNAs in promoting osteosarcoma development, highlighting their potential therapeutic targets (Table 1).
 |
Table 1 MiRNAs in Osteosarcoma |
Cell Proliferation
Cell proliferation is a defining characteristic of the cancer cells. Many studies explored the mechanisms by which miRNAs promote the proliferation of osteosarcoma cells.69 The phosphatase and tensin homolog (PTEN) can curtail osteosarcoma proliferation by negatively modulating the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway.70 A research indicated that miR-93 could target PTEN, leading to the inhibition of osteosarcoma proliferation.50 In osteosarcoma cells, while miR-let-7a expression diminished, its target gene E2F Transcription Factor 2 (E2F2) was upregulated. Overexpression of let-7a in these cells markedly reduced tumor growth in vivo.51 Additionally, Zhang et al53 discovered that overexpression of miR-221 in osteosarcoma cells bolstered osteosarcoma proliferation and induced apoptosis. This effect was attributed to the downregulation of F-box and WD repeat domain containing 11 (FBXW11), which amplified Wnt signaling activity.
Cell Apoptosis
Apoptosis, a structured and coordinated cell death process, is prevalent in both physiological and pathological states but is notably reduced in cancer.71 Endoplasmic reticulum stress (ERS) arises from the accumulation of misfolded proteins within the ER, culminating in apoptosis of osteosarcoma cells.72 Jiang et al54 observed that during ERS, p53 upregulates miR-1281, which subsequently targeted and suppressed ubiquitin-specific protease 39 (USP39), thereby inducing apoptosis in osteosarcoma cells. Zhang et al55 identified that in osteosarcoma cells subjected to tunicamycin or thapsigargin-induced ERS, miR-663a directly bound to ZBTB7A 3′-UTR, reducing its expression. This action negated ZBTB7A transcriptional repression of lncRNA GAS5, amplifying ERS-induced apoptosis.
Cell Metastasis
Metastasis involves the proliferation of cancer cells at sites distant from their origin and encompasses processes such as dissemination, dormancy, and colonization.73 MiRNAs interact with multiple signaling pathways that regulate osteosarcoma metastasis, presenting potential targets for metastasis inhibition and osteosarcoma treatment.74 Osteosarcoma cells can lose polarity and initiate invasion and migration via EMT, a process modulated by miRNAs.75 Xu et al56 observed the downregulation of miR-382 in osteosarcoma cells. However, elevating miR-382 levels curtailed EMT and osteosarcoma cell metastasis by targeting Y box-binding protein 1. Zhang et al57 observed that miR-101 expression was markedly reduced in metastatic osteosarcoma cells compared with non-metastatic cells. However, amplifying miR-101 expression hindered osteosarcoma cell invasion and migration by suppressing the expression of B-cell lymphoma 6 (BCL6), an osteosarcoma tumor suppressor. Cheng et al58 discovered that elevated miR-487b-3p levels suppressed osteosarcoma cell migration by targeting aldehyde dehydrogenase family 1 member 3 (ALDH1A3).
Angiogenesis
Angiogenesis is the process through which new capillaries emerge from an existing vascular network.76 The vascular endothelial growth factor (VEGF) plays a pivotal role in regulating angiogenesis.77 VEGF facilitates the detachment of pericytes from the basement membrane, weakens the extracellular matrix via proteolytic degradation, and subsequently drives the migration and proliferation of the endothelial cells lining the inner walls of blood vessels.78 VEGF promotes the proliferation and survival of endothelial cells and contributes to tumor angiogenesis.79 Thus, targeting VEGF could be a strategy for curtailing tumor growth.
Emerging evidence indicates that miRNAs play a pivotal role in regulating tumor angiogenesis.80 Numerous studies have demonstrated that miRNAs modulate osteosarcoma angiogenesis by targeting VEGF-A. Zhang et al59 discovered that exosomal miR-199a-5p derived from osteosarcoma cells translocated to human umbilical vein endothelial cells (HUVECs), targeting and suppressing VEGFA expression, thereby inhibiting osteosarcoma growth and angiogenesis. Zhang et al60 observed that miR-134 expression was reduced in osteosarcoma cells. However, when overexpressed, miR-134 targeted and suppressed VEGFA and VEGFR1, thereby inhibiting osteosarcoma angiogenesis and proliferation. Tsai et al61 discovered that Wnt-induced signaling protein 1 (WISP-1) enhanced VEGFA expression and angiogenesis by diminishing miR-381 expression, thereby advancing osteosarcoma progression. Raimondi et al62 identified that miR-CT3 curtailed tumor angiogenesis by targeting VEGFA. Moreover, anti-angiogenic medications can suppress VEGFA synthesis through miRNAs, consequently inhibiting angiogenesis in osteosarcoma. Vimalraj et al63 revealed that melatonin elevated miR-424-5p expression in osteosarcoma, leading to VEGFA inhibition.
Immune Regulation
The tumor microenvironment (TME) comprises a network of immune cells that secrete numerous cytokines, regulating immune responses and influencing tumor behavior.81 Macrophages are pivotal components of the TME. Nirala et al64 determined that hyperactivation of myelocytomatosis oncogene (MYC) resulted in macrophage colony-stimulating factor 1 (CSF1) downregulation due to elevated miR-17/20a expression, leading to reduced macrophage presence in the osteosarcoma TME. Yan et al52 observed the upregulation of let-7a levels in exosomes derived from tumor-associated macrophages (TAMs). This upregulation targeted the 3’-UTR of C15orf41, leading to increased invasion and migration in osteosarcoma.
Drug Resistance
Chemotherapy resistance poses a significant challenge to the treatment of osteosarcoma. However, strategies targeting the genes responsible for this resistance are promising.82 MiRNAs can target signaling pathways associated with chemoresistance genes in osteosarcoma, thereby enhancing the sensitivity of cells to chemotherapy.83 Wang et al65 discovered that transforming growth factor-β (TGFβ)-induced EMT reduced miR-499a expression via Snail1/Zeb1 binding directly to the miR-499a promoter. This subsequently elevated SH3KBP1-binding protein 1 (SHKBP1) expression, a target of miR-499a, thereby increasing erlotinib resistance in osteosarcoma cells. Di Fiore et al66 observed the downregulation of miR-29b-1 in human osteosarcoma cells. However, miR-29b-1 upregulation suppressed stemness and increased chemosensitivity in the 3AB-osteosarcoma cancer stem cell (CSC) human osteosarcoma cell line. Song et al67 revealed that miR-140 enhanced chemoresistance in osteosarcoma cells by targeting histone deacetylase 4. Song et al84 determined that miR-215 induced G2 phase blockade by targeting and suppressing denticleless E3 ubiquitin protein ligase homolog (DTL) expression, thereby reducing cell proliferation. This resulted in the increased resistance of osteosarcoma cells to methotrexate and Tomudex.
MiRNA Delivery
MiRNAs that are intricately linked to the signaling pathways involved in the onset and progression of osteosarcoma can be modulated to hinder osteosarcoma progression.68 While miRNA-based therapies hold significant promise for tumor treatment, the direct introduction of miRNAs as drugs into the body can substantially reduce their efficacy.85 The direct introduction of exogenous miRNA mimics and anti-miRs into the body poses challenges, including degradation by RNases, potential absorption by tissues and organs, and possible harm to the organism.86 Consequently, drug delivery systems that safeguard miRNAs, ensure targeted delivery, enhance their efficacy, and accelerate their clinical application, are needed.
Currently, vectors for miRNA delivery for tumor treatment are categorized into viral and nonviral types.87,88 While viral vectors have a high delivery efficiency, they pose significant safety concerns. In contrast, nonviral vectors, despite their lower delivery efficiency, offer greater safety and hold substantial promise for clinical use.89 Consequently, recent clinical studies on miRNA delivery vectors have predominantly focused on nonviral vectors.90
Treatment of Osteosarcoma by miRNA Delivery
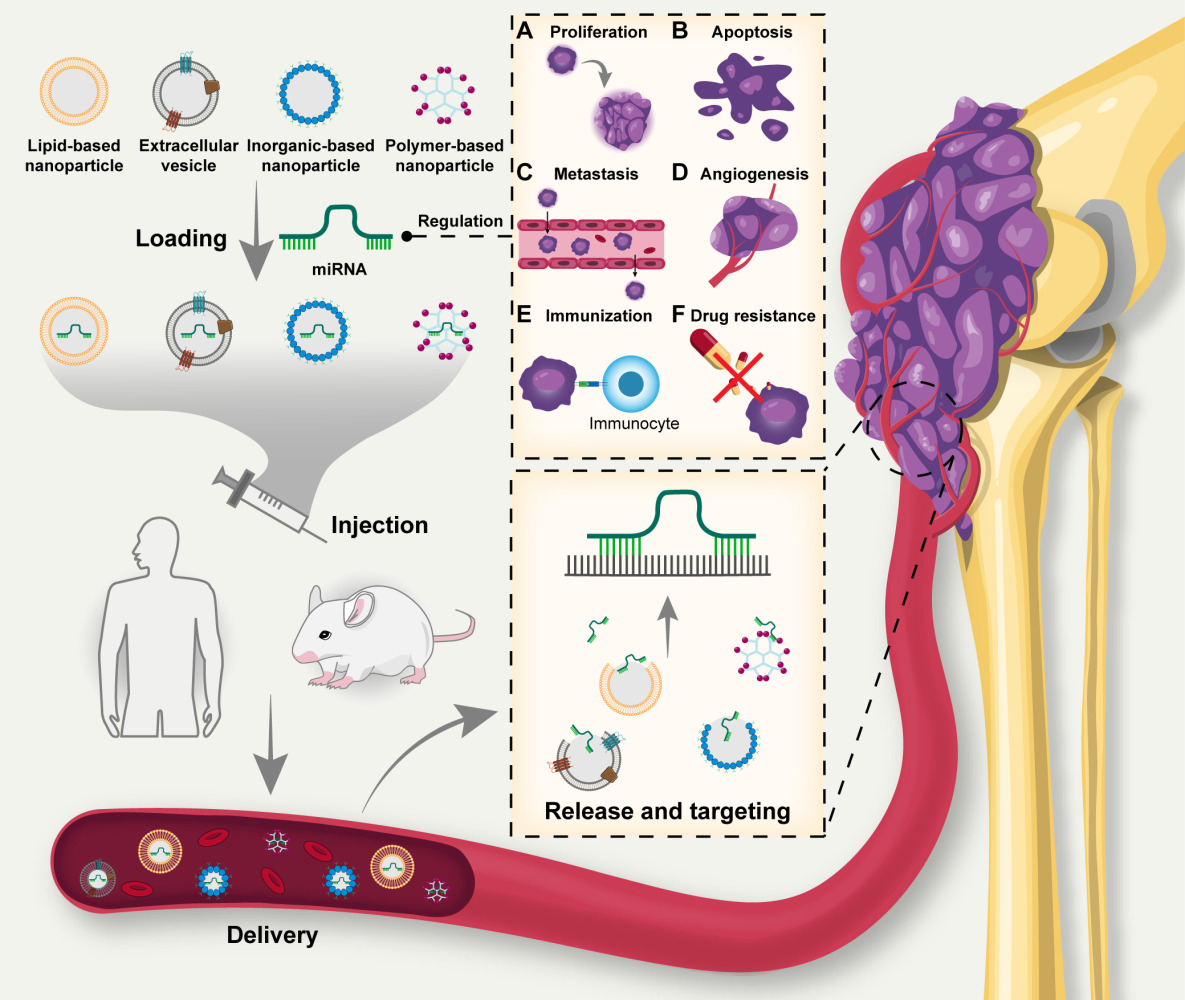
Using nanoparticles to deliver miRNAs for cancer treatment has proven to be feasible.91 Moreover, miRNA delivery can be used to treat osteosarcomas. In this section, we discuss studies on the use of nanomaterials for miRNA delivery in osteosarcoma treatment (Figure 2 and Table 2).
 |
Table 2 Nanoparticles for miRNA Delivery |
MiRNA Delivery via Polymer-Based Nanoparticles
Dextran is a water-soluble, biocompatible, and biodegradable substance that is non-toxic and non-immunogenic. Its properties can be enhanced by structural modifications, making it a popular drug delivery system.106 Zhang et al92 used lipid-modified dextran-based polymeric nanoparticles to introduce miR-199a-3p and let-7a into osteosarcoma cells, inhibiting proliferation and growth of osteosarcoma cells. The lipid-modified dextran is amphiphilic dextran copolymers, which can self-assemble in an aqueous environment by hydrophobic interactions, forming spherical nanoparticles known as micelles.107 The drug can then be encapsulated in a hydrophobic environment inside the carrier, increasing stability of the drug.108 Also, the polyethylene glycol (PEG) on its surface reduces the clearance of the carrier by the reticuloendothelial system, thereby increasing the retention time of the drug in the circulation.109 Zhang et al was the first to use polymers to encapsulate miRNAs to inhibit osteosarcoma. The effectivity and targetability of lipid-modified dextran-based polymeric nanoparticles to the osteosarcoma within organisms are uncertain because Zhang et al did not conduct vivo experiments in osteosarcoma. In addition, the safety of the lipid-modified dextran-based polymeric nanoparticles on organisms is still needed to verify by more experiments.
Dendritic polyglycerolamine (dPG-NH2) is a nanocarrier derived from polyglycerol. dPG-NH2 possesses 175 amines per mole of polymer, enabling it to bind negatively charged miRNAs.93 Tiram et al93 successfully delivered miR-93,50 miR-200c,110 and miR-34a111 into human osteosarcoma cells both in vitro and in vivo by dPG-NH2. These miRNAs induced tumor dormancy which extending time window of treatment.93 Therefore, miRNA delivery not only inhibits osteosarcoma growth, but also brings hopes for the applications of other treatments of osteosarcoma. And it inspires the idea that we could combine other treatments, such as chemotherapy and surgery, with miRNA delivery to increase the therapeutic effect. Additionally, the enhanced permeability and retention effect allows nanoparticles to penetrate and accumulate more effectively in tumor cells, thus enhancing the therapeutic impact of drugs encapsulated within the nanoparticles.112,113 Tiram et al93 demonstrated the accumulation of miRNAs in tumor cells through in vivo experiments, which suggested that dPG-NH2 successfully delivered miRNAs into the tumor tissue. But we do not know the distribution of miRNAs in other tissues and organs in the body compared to tumor tissues, which is related to the vector’s tropism to tumors. One study highlighted that cationic nanoparticles disrupted cell membrane integrity more significantly than anionic nanoparticles, posing a potential biological risk.114 Hence, experiments are also needed to explore the biotoxicity of dPG-NH2.
Poly-beta-amino esters (pBAE) are cationic polymers synthesized through the Michael addition of acrylates to amines.115 pBAE has been extensively used in drug delivery research because of its biocompatibility, biodegradability.116 Freeman et al94 used pBAE combined with hyaluronic acid (HA) to deliver miR-29b for osteosarcoma treatment. As the HA underwent swelling and degradation, it gradually released pBAE to deliver miRNAs progressively,94 which contributed to reduce the number of administrations and increase patient compliance in clinical therapy. In addition, the receptor for HA, cluster of differentiation-44 (CD44), which is upregulated in OS cell lines,117 is overexpressed in various cancers and is used for CD44-mediated tumor targeting.118 Therefore, the aggregation of nanoparticles and miRNAs within osteosarcoma cells under HA targeted delivery in vivo can be further investigated in the future. Additionally, Freeman et al94 combined systemic doxorubicin (Dox) administration with the targeted delivery of miR-29b. They demonstrated that miR-29b offered benefits in terms of reduced osteolysis which resulted from Dox.94 Thus, delivery of miRNAs not only increases the efficacy of chemotherapeutic drugs, but also alleviates the suffering of body caused by chemotherapy. Furthermore, the above studies demonstrated that miRNA-targeted delivery combined with chemotherapeutic agents was effective and feasible.
Magalhães et al95 employed a micellar nanosystem composed of the amphiphilic copolymer Pluronic® L64 and the cationic polymer polyethyleneimine (PEI) for the delivery of therapeutic miRNA-145 to osteosarcoma cells. PEI is a cationic polymer with several positive charges.119 Conversely, RNA is a biomolecule with a prominent negative charge.120 It can bind to PEI, facilitating its passage through negatively charged cell membranes.121 Pluronic® L64, a neutral amphiphilic triblock copolymer, has a hydrophobic core and a hydrophilic shell, which can interact with phospholipid pairs in cell membranes and alter the membrane structure and function.122 Chen et al122 discovered that in a cellular internalization assay of PEI/pDNA, L64 enhanced the permeability of endosomal/lysosomal membranes, facilitating the escape of PEI/pDNA from endosomal/lysosomal catabolism. So Pluronic® L64 serves as a guardian, increasing the retention time of miRNAs in the circulation to serve as an effective vector for miRNA delivery. miR-145, which is diminished in osteosarcoma tissues and cell lines, inhibits osteosarcoma cell proliferation and invasion by targeting and silencing Rho-associated protein kinase 1.123 It also targets VEGF to inhibit osteosarcoma cell invasion and angiogenesis.124 Additionally, miR-145 suppresses EMT in osteosarcoma by targeting Snail, a potent repressor of E-cadherin transcription.125 In an in vitro study using the osteosarcoma cell line MG-63, Magalhães et al95 demonstrated that the L64-PEI/miR-145 drug formulation effectively transported and released miR-145 into the cell cytoplasm which led to apoptosis and hindered cell migration. But PEI, due to its numerous amine groups, has evident cytotoxicity to cells.126,127 However, as mentioned earlier, PEI readily binds to RNA and serves to act as a transporter or bridge to other substances and it is an excellent carrier for RNA delivery. Therefore, we can modify PEI to reduce its toxicity.128,129 Pluronic® L64 has been studied for the use of intramuscular delivery of genes.130,131 But the study on Pluronic® L64 in tumor therapy is not sufficient. Thus, its toxicity in living organisms still needs to be further investigated.
Chen et al96 employed TGIC-CA (TC), a hydroxyl-rich, reduction-responsive cationic polymeric nanoparticle. It was synthesized via a one-step epoxy ring-opening reaction between 1,3,5-triglycidyl isocyanurate (TGIC) and cystamine (CA) to deliver miRNA-22 for osteosarcoma treatment.96 Owing to its abundant hydroxyl groups, this polyhydroxy cationic polymer, synthesized using reagents with multiple amino or epoxy groups through a direct ring-opening reaction, exhibited higher transfection efficiency and lower cytotoxicity than PEI.126 Additionally, Volasertib, a potent cell cycle kinase inhibitor, was delivered using TC along with miR-22.96 Research has indicated that both miR-22 and Volasertib inhibit the PI3K/Akt pathway.132,133 The combined therapeutic effect of TC/miR-22 and Volasertib was proved to be better than that of TC/miR-22 alone.96 Hence, combining miRNA-targeted delivery of polymer with chemotherapeutic agents holds significant promise for osteosarcoma treatment.
Ethanolamine-modified poly (glycidyl methacrylate) (PGEA) is a cation carrier enriched with hydroxyl groups. It has low toxicity and high transfection efficiency, making it a popular choice in recent gene therapy studies.134,135 Research has indicated that miR-223 plays a role in hindering the progression of osteosarcoma.136,137 Chen et al97 used PGEA to deliver miR-223 in vivo, which effectively inhibited the proliferation, invasion, and migration of osteosarcoma cells without causing notable toxicity. Additionally, the adjacent nonionic hydrophilic hydroxyl groups boost the affinity of the cationic polymer for the anionic gene, enhancing transfection efficiency and also shielding the potentially harmful cationic charge of the carrier.138,139 The presence of hydroxyl groups diminishes the interaction between PGEA and serum proteins, thereby promoting its stability in the bloodstream.140–143 Hence, the introduction of hydroxyl group modifications significantly enhances cationic polymer carriers’ affinity for miRNAs and reduces the probability of being disturbed by substances in the blood circulation.
The in vivo metabolism, accumulation, and side effects of polymer vectors also need to be considered if polymer vectors are to be used in the clinic for delivery of miRNAs for the treatment of osteosarcoma.144–146
MiRNA Delivery via Lipid-Based Nanoparticles
Liposomes have been widely studied for in vivo drug delivery.147 PEGylation shields nanocapsules from plasma protein adsorption and mononuclear phagocyte system (MPS) detection, thereby facilitating their circulation in the bloodstream and subsequent drug release.148 Liposomes with a particle size of 20–100 nm were reported to exhibit uniform drug encapsulation, stable drug release, and longer circulation times.149 And the particle size of PEGylated Staramine was about 80–100 nm according to a previous research.150 Yang et al98 used PEGylated Staramine to deliver anti-miR-20a oligonucleotides targeting Fas to suppress lung metastatic osteosarcoma by intravenous injection. Therefore, to address the early metastatic nature of osteosarcoma, systemic administration, for example, intravenous injection, may plays a more effective role in inhibiting potential osteosarcoma metastases.151 However, research has indicated that PEGylated lipid nanocarriers possess immunogenic properties, potentially diminishing carrier absorption and posing safety concerns.152 However, subsequent research showed that polyethylene glycosylation did not increase cell death rates.153 And perhaps the formulation of different kinds of lipids affects the cytotoxicity of lipid nanoparticles.153,154 In addition, application of liposomes with excessive diameter should be avoided as it may lead to fat embolization.155,156 And whether the immune evasion of liposomes for a long period of time will have adverse effects on the organism still needs to be further investigated.
MiRNA Delivery via Extracellular Vesicles
Mesenchymal stem cells (MSCs) are pluripotent cells with the ability to differentiate into diverse cell types and exhibit immunomodulatory and tumor-homing properties.157 Given their low immunogenicity and tumor-homing capabilities, MSCs can be engineered to produce antitumorigenic miRNAs.158 Exosomes are extracellular vesicles (EVs) measuring 40–160 nm in diameter that carry substances from their originating cells. They are secreted and play a role in intercellular signaling.159 Mesenchymal stem cell-extracellular vesicles (MSC-EVs) exhibit low immunogenicity and possess tumor-targeting capabilities, making them suitable for the delivery of miRNAs in tumor treatment.160 Numerous studies have explored miRNA delivery using MSC-EVs.161
MSC-EVs significantly influence the onset, progression, and treatment of osteosarcoma.162 However, exosomes from original MSCs may facilitate progression of cancer.163 That is because primitive MSCs are able to secrete exosomes carrying miRNAs which can promote proliferation of osteosarcoma.164–166 Thus, engineered exosomes from MSCs are ideal miRNA carriers for targeted tumor therapy.167 Given that certain MSC-EVs can enhance tumor growth, employing exosomes from MSCs with tumor-suppressive traits can be an effective strategy for cancer therapy.168
Numerous studies have focused on engineered MSC exosomes carrying miRNAs targeting osteosarcoma and their specific mechanism. Zhang et al99 introduced miR-206 mimics into human bone marrow-derived mesenchymal stem cell (hBMSC). This miR-206 was then transferred to the human osteosarcoma cell line 143 B via hBMSC-derived exosomes, which subsequently inhibited osteosarcoma growth and spread by targeting transformer 2 protein homolog beta (TRA2B). Zhang et al57 employed a lentivirus to make adipose-derived mesenchymal stem cell (AD-MSC) express miR-101, using exosomes for targeted delivery, which inhibited the lung metastasis of osteosarcoma. MSC-EVs loaded with miR-22 also inhibited osteosarcoma by targeting the Twist1/CADM1 axis.100 Thus, engineering MSC-EVs can turn them into excellent carriers for miRNA delivery for the treatment of osteosarcoma.169,170 However, it remains to be explored whether the other contents of the exosomes may cause a negative effect on the body, and whether miRNAs within exosomes will pair spontaneously reducing efficacy.171,172 Preparation procedures of exosomes are cumbersome and can only be stored at low temperatures, resulting in high costs, which may limit their widespread use.173,174 Therefore, more safety validation and clinical trials are needed in order to use engineered MSC exosomes for the clinical treatment of osteosarcoma.175,176
Exosomes derived from tumor cells preferentially target tumors because of their homotypic characteristics.177 In addition, exosomes derived from osteosarcoma cells can serve as miRNA delivery vectors. Shimbo et al101 introduced miR-143 into human osteosarcoma cell line 143 B. The resulting 143B-derived exosomes then conveyed miR-143 back to 143 B cells, inhibiting their migration. Recently, induction of ferroptosis in tumor cells has become a new direction in tumor therapy.178,179 Engineering exosomes to induce ferroptosis in tumor cells is a promising therapy strategy of tumor.180 Jiang et al102 enhanced the expression of miR-144-3p in the human osteosarcoma cell line, 143 B. miR-144-3p then promoted ferroptosis, curbing osteosarcoma growth and spread by modulating ZEB1 expression. However, exosomes originating from tumors contain elements that can promote cancer progression.44 Drug resistance of osteosarcoma cells can be transmitted via miRNAs.181 Therefore, the use of tumor-derived exosomes as miRNA carriers in cancer therapy poses potential risks. Future research should explore the viability of tumor-derived exosomes as carriers for cancer treatment.
MiRNA Delivery via Inorganic-Based Nanoparticles
Magnetofection is an delivery strategy that combines magnetic drug targeting and gene delivery.182 The kinds of magnetic nanoparticles (MNPs), coating molecules on the surface that can affect their size and charge, external magnetic field, method of administration will influence their biodistribution.183,184 Surface functionalization of MNPs enhances their ability to adsorb miRNAs and prevents biodegradation.185 The use of ammonium terminal groups on the nanoparticle surface allows the electrostatic interactions between nanoparticles and miRNAs.186 So enhancing magnetic nanoparticles with specific substances, such as the cationic polymer PEI, can boost their transfection efficiencies.183 While iron oxide nanoparticles show significant promise in biomedicine, they have a drawback in terms of their hydrophobic surfaces.187 The modification of iron oxide with dextran can counteract this limitation.106 Gong et al103 used a magnetic gene carrier composed of PEI, dextran, and iron oxide nanoparticles (PDIs) to deliver miR-302b, targeting YOD1 deubiquitinase (YOD1) and suppressing osteosarcoma. In their cytotoxicity tests, the toxicity of PDI rose with increasing concentrations of low molecular weight (≤2000) PEI. However, at low concentrations, its toxicity was minimal, especially when compared to that of PEI with a molecular weight of 25 kDa.103 Lin et al188 created a chitosan-PEI crosslinked polymer (Chi-xPEI) by crosslinking low-molecular-weight PEI with chitosan. This significantly reduced the cytotoxicity of PEI. Additionally, NP-Chi-xPEI, produced by combining iron oxide nanoparticles with Chi-xPEI, demonstrated superior drug-loading capabilities that were reliant on PEI.188 Thus, modifying PEI cuts down requirement for miRNAs, offers lower cytotoxicity, and enhances the drug-loading efficiency of the inorganic nanoparticles. This approach capitalizes on strengths while mitigating weaknesses. Gong et al103 highlighted the limitations in drug release and intratumoral distribution of PDI. Studies have suggested that surface functionalization can address these issues.189,190
Photothermal therapy (PTT) involves irradiation of a photosensitizer with a near-infrared laser to produce high temperatures that kill cancer cells. This method has been extensively studied for the treatment of bone cancer.191 Li et al104 combined miR-520a-3p with polydopamine (PDA)-coated, folate-modified iron oxide (Fe2O3@PDA-FA) for osteosarcoma PTT, which effectively inhibited its growth. PDA modification enhances the biocompatibility and hydrophilicity of iron oxide and offers robust photothermal conversion capabilities suitable for PTT.192 The folate receptor is overexpressed in numerous tumor cells, allowing folate-modified nanocarriers to specifically target them.193–195 Similarly, Luo et al196 employed a multifunctional nanoplatform crafted from FePS3 and poly-L-lysine-PEG-folic acid (PPF) (FePS@PPF), to convey anti-miR-19a, suppressing osteosarcoma growth. Li et al104 utilized an 808 nm near-infrared (NIR) laser for irradiation, whereas Luo et al196 employed a 1064 nm NIR-II laser. The chosen irradiation wavelength depends on the photothermal conversion efficiency and the depth of lesion.197 And for osteosarcomas that are deep in the tissue, long wavelength, such as the NIR-II window (1000–1700 nm), is required for effective treatment.105 Moreover, the control of the duration, temperature level and uniformity of photothermal therapy at the lesion site also affects its clinical translatability.198 Except from cancer, Fe2O3@PDA-FA and FePS@PPF mainly aggregated in the spleen, and their metabolic patterns and effects on the body still needed to be further clarified.104,196 In conclusion, merging the photothermal therapy with miRNA genes targeting, especially when utilizing inorganic nanocarriers, holds great promise.
Directions and Challenges in the Future
MiRNAs, which are key players in epigenetic regulation, have demonstrated potent regulatory and therapeutic effects on tumors with aberrant gene expression. MiRNAs are promising diagnostic biomarkers of osteosarcoma.199 And then numerous miRNAs have been linked to the onset and progression of osteosarcoma, positioning them as potential therapeutic targets.200 By analyzing expression profiles of miRNAs across various osteosarcoma types, specific miRNAs can be identified for targeted treatment.
MiRNAs are susceptible to be cleared by RNase in vivo, so they need to be protected and delivered by vectors.32,201 Targeted miRNA delivery for tumor therapy is a burgeoning research area with immense potential. Safety remains paramount when selecting delivery vectors. While viral vectors offer robust transfection capabilities, they pose safety concerns.202 In contrast, nonviral vectors may have limited drug-loading capacity but are generally safer. Current researches on miRNA delivery for osteosarcoma treatment employed nanocarriers, such as polymers, liposomes, exosomes, and inorganic particles. Cationic polymers can be cytotoxic because of their abundant amino groups.203 Intravenous injection of large liposomes has a risk of causing embolism.155,156 Exosomes excel in drug loading and biocompatibility; however, some studies have suggested that they may inadvertently promote cancer growth.164–166 The exact metabolism of inorganic-based nanoparticles in the body still needs to be explored.104,196 Fortunately, with proper engineering, these challenges associated with nanocarriers can be addressed.
Modifications are crucial for enhancing the nanocarrier performance. While abundant surface amino groups of PEI contribute to its cytotoxicity, modifications, such as ethylenediamine, can mitigate this effect.139 Using low molecular weight (≤2000) PEI to modify nanocarriers can reduce positive charge cytotoxicity while retaining its advantages.103 Additionally, suitable modifications can enhance the properties of polymeric supports. For instance, HA and folate modifications can enhance the tumor-targeting ability of nanocarriers.117,118 PEGylation extends the blood retention time of nanocarriers, thereby facilitating drug release.109 Choosing engineered exosomes from MSCs to inhibit osteosarcoma may address the issue of safety.167 These solutions have significant potential for safety.
Given that osteosarcoma tends to metastasize early, the mode of administration is worth being considered. In the review, miRNA delivery modalities used to treat osteosarcoma are categorized into local and systemic applications. Chen et al96 successfully inhibited osteosarcoma metastasis by local application of miRNAs. However, osteosarcoma tends to metastasize early and often has been metastatic by the time it is diagnosed.204,205 Zhang et al57 shrank pulmonary metastasis of osteosarcoma by tail vein injection of miR-101 encapsulated in exosomes from adipose-derived mesenchymal stem cells. Therefore, systemic delivery might be suitable for osteosarcoma, especially for metastasis. More experiments are needed to explore the difference in efficacy between systemic and topical drug administration.
Combining miRNA delivery with other therapies offers promising potential for osteosarcoma treatment. When combined with chemotherapeutic drugs, miRNAs enhance the chemosensitivity of osteosarcoma, significantly improving the efficacy of chemotherapy.94 The integration of PTT with miRNA delivery holds considerable promise.104,196
Several miRNA-based therapies have been tested in clinical trials.22,206,207 However, no miRNA delivery therapy for osteosarcoma has entered clinical trials. Osteosarcoma development is characterized by intricate molecular mechanisms and alterations in the expression of several miRNAs.68 Gaining insight into the regulatory networks and associated signaling pathways of miRNAs in osteosarcoma could pave the way for initiating clinical trials of miRNA delivery.
Recently, plant-derived nanoparticles have become a hot research topic.208 Plant-derived exosome-like nanoparticles are widely available which relatively saves the cost, and are capable of delivering miRNAs.209 Thus, the feasibility of using plant-derived exosome-like nanoparticles to deliver miRNAs for the treatment of osteosarcoma could be explored in the future.
In summary, when modified with specific substances, the capabilities of nanocarriers can be optimized, making them highly effective for miRNA delivery in osteosarcoma treatment. Compared to exosomes whose contents are uncertain, polymers, liposomes, and inorganic-based nanoparticles have relatively more defined influence on the organism and thus have advantages in clinical applications. Furthermore, integrating miRNA delivery with therapies such as chemotherapy and PTT presents vast potentials, warranting additional researches. In conclusion, multiple kinds of carriers and miRNAs can be combined into a lot of delivery systems, which generates various research orientations in the future.
Conclusion
Overall, miRNA delivery holds significant promise for osteosarcoma treatment, particularly when combined with other therapies. However, the application of nanocarriers for miRNA delivery in osteosarcoma treatment remains in the early stages of development. The in vivo metabolism mechanism, toxicity, mode of administration, and dosage of miRNA delivery vectors still need to be clarified through further studies to accelerate clinical translation. In conclusion, however, miRNA delivery holds great promise for the treatment of osteosarcoma.
Ethics Approval and Consent to Participate
Not applicable. Ethical approval was not required as this umbrella review is a synthesis and analysis of existing studies.
Consent for Publication
All authors declare full consent for publication.
Acknowledgments
The authors thank all researchers who contributed to the advancement of science.
Funding
This work was supported by Science and Technology Development Plan Project of Changchun (21ZGY29); Science and Technology Development Plan Project of Jilin Province (20220505032ZP); College Students’ Innovative Entrepreneurial Training Plan Program (202310183310).
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the surveillance, epidemiology, and end results program. Cancer. 2009;115(7):1531–1543. doi:10.1002/cncr.24121
2. Cole S, Gianferante DM, Zhu B, Mirabello L. Osteosarcoma: a Surveillance, Epidemiology, and End Results program-based analysis from 1975 to 2017. Cancer. 2022;128(11):2107–2118. doi:10.1002/cncr.34163
3. Choi JH, Ro JY. The 2020 WHO classification of tumors of bone: An updated review. Adv Anat Pathol. 2021;28(3):119–138. doi:10.1097/PAP.0000000000000293
4. Rozeman LB, Cleton-Jansen AM, Hogendoorn PCW. Pathology of primary malignant bone and cartilage tumours. Int Orthop. 2006;30(6):437–444. doi:10.1007/s00264-006-0212-x
5. Whelan JS, Osteosarcoma DLE. Chondrosarcoma, and Chordoma. J Clin Oncol off J Am Soc Clin Oncol. 2018;36(2):188–193. doi:10.1200/JCO.2017.75.1743
6. B D. Pathogenesis and genetics of osteosarcoma: Current concepts and developments. Pathol. 2018;39(2). doi:10.1007/s00292-017-0365-y
7. Meltzer PS, Helman LJ. New horizons in the treatment of osteosarcoma. N Engl J Med. 2021;385(22):2066–2076. doi:10.1056/NEJMra2103423
8. Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: Current treatment and a collaborative pathway to success. J Clin Oncol off J Am Soc Clin Oncol. 2015;33(27):3029–3035. doi:10.1200/JCO.2014.59.4895
9. Yang C, Tian Y, Zhao F, et al. Bone microenvironment and osteosarcoma metastasis. Int J Mol Sci. 2020;21(19):6985. doi:10.3390/ijms21196985
10. Misaghi A, Goldin A, Awad M, Kulidjian AA. Osteosarcoma: A comprehensive review. SICOT-J. 2018;4:12. doi:10.1051/sicotj/2017028
11. Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6(5):376–385. doi:10.1038/nrm1644
12. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi:10.1016/0092-8674(93)90529-y
13. Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15(8):509–524. doi:10.1038/nrm3838
14. Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234(5):5451–5465. doi:10.1002/jcp.27486
15. Bartel DP. Metazoan MicroRNAs. Cell. 2018;173(1):20–51. doi:10.1016/j.cell.2018.03.006
16. Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat Rev Mol Cell Biol. 2019;20(1):5–20. doi:10.1038/s41580-018-0059-1
17. Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610. doi:10.1038/nrg2843
18. Dong H, Lei J, Ding L, Wen Y, Ju H, Zhang X. MicroRNA: function, detection, and bioanalysis. Chem Rev. 2013;113(8):6207–6233. doi:10.1021/cr300362f
19. Jansson MD, Lund AH. MicroRNA and cancer. Mol Oncol. 2012;6(6):590–610. doi:10.1016/j.molonc.2012.09.006
20. Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199–227. doi:10.1146/annurev.pathol.4.110807.092222
21. Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med. 2012;4(3):143–159. doi:10.1002/emmm.201100209
22. Gupta A, Andresen JL, Manan RS, Langer R. Nucleic acid delivery for therapeutic applications. Adv Drug Deliv Rev. 2021;178:113834. doi:10.1016/j.addr.2021.113834
23. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–222. doi:10.1038/nrd.2016.246
24. Otmani K, Rouas R, Lewalle P. OncomiRs as noncoding RNAs having functions in cancer: Their role in immune suppression and clinical implications. Front Immunol. 2022;13:913951. doi:10.3389/fimmu.2022.913951
25. Zare M, Pemmada R, Madhavan M, et al. Encapsulation of miRNA and siRNA into nanomaterials for cancer therapeutics. Pharmaceutics. 2022;14(8):1620. doi:10.3390/pharmaceutics14081620
26. Yadav K, Sahu KK, Null S, et al. Biomedical applications of nanomaterials in the advancement of nucleic acid therapy: Mechanistic challenges, delivery strategies, and therapeutic applications. Int J Biol Macromol. 2023;241:124582. doi:10.1016/j.ijbiomac.2023.124582
27. Höbel S, Aigner A. Polyethylenimines for siRNA and miRNA delivery in vivo. Wiley Interdiscip Rev Nanomed Nanobiotech. 2013;5(5):484–501. doi:10.1002/wnan.1228
28. Bober Z, Bartusik-Aebisher D, Aebisher D. Application of dendrimers in anticancer diagnostics and therapy. Mol. 2022;27(10):3237. doi:10.3390/molecules27103237
29. Zhang X, Hai L, Gao Y, Yu G, Sun Y. Lipid nanomaterials-based RNA therapy and cancer treatment. Acta Pharm Sin B. 2023;13(3):903–915. doi:10.1016/j.apsb.2022.10.004
30. Muskan M, Abeysinghe P, Cecchin R, Branscome H, Morris KV, Kashanchi F. Therapeutic potential of RNA-enriched extracellular vesicles: the next generation in RNA delivery via biogenic nanoparticles. Mol Ther J Am Soc Gene Ther. 2024;S1525-0016(24). doi:10.1016/j.ymthe.2024.02.025
31. Amaldoss MJN, Yang JL, Koshy P, Unnikrishnan A, Sorrell CC. Inorganic nanoparticle-based advanced cancer therapies: promising combination strategies. Drug Discov Today. 2022;27(12):103386. doi:10.1016/j.drudis.2022.103386
32. Kara G, Calin GA, Ozpolat B. RNAi-based therapeutics and tumor targeted delivery in cancer. Adv Drug Deliv Rev. 2022;182:114113. doi:10.1016/j.addr.2022.114113
33. Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99(24):15524–15529. doi:10.1073/pnas.242606799
34. Zhang Z, Huang Q, Yu L, et al. The role of miRNA in tumor immune escape and miRNA-based therapeutic strategies. Front Immunol. 2021;12:807895. doi:10.3389/fimmu.2021.807895
35. Kousar K, Ahmad T, Abduh MS, et al. miRNAs in regulation of tumor microenvironment, chemotherapy resistance, immunotherapy modulation and miRNA therapeutics in cancer. Int J Mol Sci. 2022;23(22):13822. doi:10.3390/ijms232213822
36. Hill M, Tran N. miRNA interplay: mechanisms and consequences in cancer. Dis Model Mech. 2021;14(4):dmm047662. doi:10.1242/dmm.047662
37. Ali Syeda Z, Langden SSS, Munkhzul C, Lee M, Song SJ. Regulatory mechanism of MicroRNA expression in cancer. Int J Mol Sci. 2020;21(5):1723. doi:10.3390/ijms21051723
38. Bouchie A. First microRNA mimic enters clinic. Nat Biotechnol. 2013;31(7):577. doi:10.1038/nbt0713-577
39. Mishra S, Yadav T, Rani V. Exploring miRNA based approaches in cancer diagnostics and therapeutics. Crit Rev Oncol Hematol. 2016;98:12–23. doi:10.1016/j.critrevonc.2015.10.003
40. Sidorova EA, Zhernov YV, Antsupova MA, et al. The role of different types of microRNA in the pathogenesis of breast and prostate cancer. Int J Mol Sci. 2023;24(3):1980. doi:10.3390/ijms24031980
41. Xu LM, Zhang J, Ma Y, et al. MicroRNA-135 inhibits initiation of epithelial-mesenchymal transition in breast cancer by targeting ZNF217 and promoting m6A modification of NANOG. Oncogene. 2022;41(12):1742–1751. doi:10.1038/s41388-022-02211-2
42. Gan J, Liu S, Zhang Y, et al. MicroRNA-375 is a therapeutic target for castration-resistant prostate cancer through the PTPN4/STAT3 axis. Exp Mol Med. 2022;54(8):1290–1305. doi:10.1038/s12276-022-00837-6
43. Khan P, Siddiqui JA, Kshirsagar PG, et al. MicroRNA-1 attenuates the growth and metastasis of small cell lung cancer through CXCR4/FOXM1/RRM2 axis. Mol Cancer. 2023;22(1):1. doi:10.1186/s12943-022-01695-6
44. Kok VC, Yu CC. Cancer-Derived Exosomes: Their role in cancer biology and biomarker development. Int J Nanomed. 2020;15:8019–8036. doi:10.2147/IJN.S272378
45. Dai J, Su Y, Zhong S, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5(1):145. doi:10.1038/s41392-020-00261-0
46. Yi M, Xu L, Jiao Y, Luo S, Li A, Wu K. The role of cancer-derived microRNAs in cancer immune escape. J Hematol Oncol. 2020;13(1):25. doi:10.1186/s13045-020-00848-8
47. Qiu S, Xie L, Lu C, et al. Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J Exp Clin Cancer Res CR. 2022;41(1):296. doi:10.1186/s13046-022-02499-8
48. Zeng Z, Li Y, Pan Y, et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9(1):5395. doi:10.1038/s41467-018-07810-w
49. Kim T, Croce CM. MicroRNA: trends in clinical trials of cancer diagnosis and therapy strategies. Exp Mol Med. 2023;55(7):1314–1321. doi:10.1038/s12276-023-01050-9
50. Kawano M, Tanaka K, Itonaga I, Ikeda S, Iwasaki T, Tsumura H. microRNA-93 promotes cell proliferation via targeting of PTEN in Osteosarcoma cells. J Exp Clin Cancer Res CR. 2015;34(1):76. doi:10.1186/s13046-015-0192-z
51. Iwasaki T, Tanaka K, Kawano M, Itonaga I, Tsumura H. Tumor-suppressive microRNA-let-7a inhibits cell proliferation via targeting of E2F2 in osteosarcoma cells. Int J Oncol. 2015;46(4):1543–1550. doi:10.3892/ijo.2015.2867
52. Yan CF, Xia J, Qun WS, et al. Tumor-associated macrophages-derived exo-let-7a promotes osteosarcoma metastasis via targeting C15orf41 in osteosarcoma. Environ Toxicol. 2023;38(6):1318–1331. doi:10.1002/tox.23766
53. Zhang Q, Yin X, Zhang Y. MicroRNA-221 promotes cell proliferation and inhibits apoptosis in osteosarcoma cells by directly tARGeting FBXW11 and regulating wnt signaling. Arch Med Res. 2021;52(2):191–199. doi:10.1016/j.arcmed.2020.10.017
54. Jiang J, Ma B, Li X, et al. MiR-1281, a p53-responsive microRNA, impairs the survival of human osteosarcoma cells upon ER stress via targeting USP39. Am J Cancer Res. 2018;8(9):1764–1774.
55. Zhang L, Wang Y, Zhang L, et al. ZBTB7A, a miR-663a target gene, protects osteosarcoma from endoplasmic reticulum stress-induced apoptosis by suppressing LncRNA GAS5 expression. Cancer Lett. 2019;448:105–116. doi:10.1016/j.canlet.2019.01.046
56. Xu M, Jin H, Xu CX, et al. miR-382 inhibits osteosarcoma metastasis and relapse by targeting Y box-binding protein 1. Mol Ther J Am Soc Gene Ther. 2015;23(1):89–98. doi:10.1038/mt.2014.197
57. Zhang K, Dong C, Chen M, et al. Extracellular vesicle-mediated delivery of miR-101 inhibits lung metastasis in osteosarcoma. Theranostics. 2020;10(1):411–425. doi:10.7150/thno.33482
58. Cheng M, Duan PG, Gao ZZ, Dai M. MicroRNA‑487b‑3p inhibits osteosarcoma chemoresistance and metastasis by targeting ALDH1A3. Oncol Rep. 2020;44(6):2691–2700. doi:10.3892/or.2020.7814
59. Zhang L, Cao H, Gu G, et al. Exosomal MiR-199a-5p Inhibits Tumorigenesis and Angiogenesis by Targeting VEGFA in Osteosarcoma. Front Oncol. 2022;12:884559. doi:10.3389/fonc.2022.884559
60. Zhang L, Lv Z, Xu J, et al. MicroRNA-134 inhibits osteosarcoma angiogenesis and proliferation by targeting the VEGFA/VEGFR1 pathway. FEBS J. 2018;285(7):1359–1371. doi:10.1111/febs.14416
61. Tsai HC, Tzeng HE, Huang CY, et al. WISP-1 positively regulates angiogenesis by controlling VEGF-A expression in human osteosarcoma. Cell Death Dis. 2017;8(4):e2750. doi:10.1038/cddis.2016.421
62. Raimondi L, Gallo A, Cuscino N, et al. Potential anti-metastatic role of the novel miR-CT3 in tumor angiogenesis and osteosarcoma invasion. Int J Mol Sci. 2022;23(2):705. doi:10.3390/ijms23020705
63. Vimalraj S, Saravanan S, Raghunandhakumar S, Anuradha D. Melatonin regulates tumor angiogenesis via miR-424-5p/VEGFA signaling pathway in osteosarcoma. Life Sci. 2020;256:118011. doi:10.1016/j.lfs.2020.118011
64. Nirala BK, Patel TD, Kurenbekova L, et al. MYC regulates CSF1 expression via microRNA 17/20a to modulate tumor-associated macrophages in osteosarcoma. JCI Insight. 2023;8(13):e164947. doi:10.1172/jci.insight.164947
65. Wang T, Wang D, Zhang L, et al. The TGFβ-miR-499a-SHKBP1 pathway induces resistance to EGFR inhibitors in osteosarcoma cancer stem cell-like cells. J Exp Clin Cancer Res CR. 2019;38(1):226. doi:10.1186/s13046-019-1195-y
66. Di Fiore R, Drago-Ferrante R, Pentimalli F, et al. MicroRNA-29b-1 impairs in vitro cell proliferation, self‑renewal and chemoresistance of human osteosarcoma 3AB-OS cancer stem cells. Int J Oncol. 2014;45(5):2013–2023. doi:10.3892/ijo.2014.2618
67. Song B, Wang Y, Xi Y, et al. Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells. Oncogene. 2009;28(46):4065–4074. doi:10.1038/onc.2009.274
68. Todosenko N, Khlusov I, Yurova K, Khaziakhmatova O, Litvinova L. Signal Pathways and microRNAs in Osteosarcoma Growth and the Dual Role of Mesenchymal Stem Cells in Oncogenesis. Int J Mol Sci. 2023;24(10):8993. doi:10.3390/ijms24108993
69. Scuderi SA, Calabrese G, Paterniti I, et al. The biological function of MicroRNAs in bone tumors. Int J Mol Sci. 2022;23(4):2348. doi:10.3390/ijms23042348
70. Zheng C, Tang F, Min L, Hornicek F, Duan Z, Tu C. PTEN in osteosarcoma: recent advances and the therapeutic potential. Biochim Biophys Acta Rev Cancer. 2020;1874(2):188405. doi:10.1016/j.bbcan.2020.188405
71. Wong RSY. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res CR. 2011;30(1):87. doi:10.1186/1756-9966-30-87
72. Zhu P, Li T, Li Q, et al. Mechanism and Role of Endoplasmic Reticulum Stress in Osteosarcoma. Biomolecules. 2022;12(12):1882. doi:10.3390/biom12121882
73. Gerstberger S, Jiang Q, Ganesh K. Metastasis. Cell. 2023;186(8):1564–1579. doi:10.1016/j.cell.2023.03.003
74. Soghli N, Ferns GA, Sadeghsoltani F, Qujeq D, Yousefi T, Vaghari-Tabari M. MicroRNAs and osteosarcoma: potential targets for inhibiting metastasis and increasing chemosensitivity. Biochem Pharmacol. 2022;201:115094. doi:10.1016/j.bcp.2022.115094
75. Chong ZX, Yeap SK, Ho WY. Unraveling the roles of miRNAs in regulating epithelial-to-mesenchymal transition (EMT) in osteosarcoma. Pharmacol Res. 2021;172:105818. doi:10.1016/j.phrs.2021.105818
76. Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8(6):464–478. doi:10.1038/nrm2183
77. Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15(6):385–403. doi:10.1038/nrd.2015.17
78. Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis. 2017;20(4):409–426. doi:10.1007/s10456-017-9562-9
79. Apte RS, Chen DS, Ferrara N. VEGF in Signaling and Disease: beyond Discovery and Development. Cell. 2019;176(6):1248–1264. doi:10.1016/j.cell.2019.01.021
80. Wang Y, Wang L, Chen C, Chu X. New insights into the regulatory role of microRNA in tumor angiogenesis and clinical implications. Mol Cancer. 2018;17(1):22. doi:10.1186/s12943-018-0766-4
81. Chen C, Xie L, Ren T, Huang Y, Xu J, Guo W. Immunotherapy for osteosarcoma: fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. 2021;500:1–10. doi:10.1016/j.canlet.2020.12.024
82. Lilienthal I, Herold N. Targeting Molecular Mechanisms Underlying Treatment Efficacy and Resistance in Osteosarcoma: a Review of Current and Future Strategies. Int J Mol Sci. 2020;21(18):6885. doi:10.3390/ijms21186885
83. Lin Z, Xie X, Lu S, Liu T. Noncoding RNAs in osteosarcoma: implications for drug resistance. Cancer Lett. 2021;504:91–103. doi:10.1016/j.canlet.2021.02.007
84. Song B, Wang Y, Titmus MA, et al. Molecular mechanism of chemoresistance by miR-215 in osteosarcoma and colon cancer cells. Mol Cancer. 2010;9:96. doi:10.1186/1476-4598-9-96
85. Sayed N, Allawadhi P, Khurana A, et al. Gene therapy: comprehensive overview and therapeutic applications. Life Sci. 2022;294:120375. doi:10.1016/j.lfs.2022.120375
86. PTB H, Clark IM, LTT L. MicroRNA-Based Diagnosis and Therapy. Int J Mol Sci. 2022;23(13):7167. doi:10.3390/ijms23137167
87. Santana-Armas ML, Tros de Ilarduya C. Strategies for cancer gene-delivery improvement by non-viral vectors. Int J Pharm. 2021;596:120291. doi:10.1016/j.ijpharm.2021.120291
88. Li X, Le Y, Zhang Z, Nian X, Liu B, Yang X. Viral Vector-Based Gene Therapy. Int J Mol Sci. 2023;24(9):7736. doi:10.3390/ijms24097736
89. Holjencin C, Jakymiw A. MicroRNAs and Their Big Therapeutic Impacts: delivery Strategies for Cancer Intervention. Cells. 2022;11(15):2332. doi:10.3390/cells11152332
90. Menon A, Abd-Aziz N, Khalid K, Poh CL, Naidu R. miRNA: a Promising Therapeutic Target in Cancer. Int J Mol Sci. 2022;23(19):11502. doi:10.3390/ijms231911502
91. Ferdows BE, Patel DN, Chen W, Huang X, Kong N, Tao W. RNA cancer nanomedicine: nanotechnology-mediated RNA therapy. Nanoscale. 2022;14(12):4448–4455. doi:10.1039/d1nr06991h
92. Zhang L, Lyer AK, Yang X, et al. Polymeric nanoparticle-based delivery of microRNA-199a-3p inhibits proliferation and growth of osteosarcoma cells. Int J Nanomed. 2015;10:2913–2924. doi:10.2147/IJN.S79143
93. Tiram G, Segal E, Krivitsky A, et al. Identification of Dormancy-Associated MicroRNAs for the Design of Osteosarcoma-Targeted Dendritic Polyglycerol Nanopolyplexes. ACS Nano. 2016;10(2):2028–2045. doi:10.1021/acsnano.5b06189
94. Freeman FE, Dosta P, Shanley LC, et al. Localized Nanoparticle-Mediated Delivery of miR-29b Normalizes the Dysregulation of Bone Homeostasis Caused by Osteosarcoma whilst Simultaneously Inhibiting Tumor Growth. Adv Mater Deerfield Beach Fla. 2023;35(23):e2207877. doi:10.1002/adma.202207877
95. Magalhães M, Almeida M, Tavares-da-silva E, et al. miR-145-loaded micelleplexes as a novel therapeutic strategy to inhibit proliferation and migration of osteosarcoma cells. Eur J Pharm Sci off J Eur Fed Pharm Sci. 2018;123:28–42. doi:10.1016/j.ejps.2018.07.021
96. Chen D, Lei C, Liu W, et al. Reduction-responsive nucleic acid nanocarrier-mediated miR-22 inhibition of PI3K/AKT pathway for the treatment of patient-derived tumor xenograft osteosarcoma. Bioact Mater. 2023;28:376–385. doi:10.1016/j.bioactmat.2023.05.012
97. Chen DF, Zhang BW, Cao J, et al. Preparation of polycation with hydroxyls for enhanced delivery of miRNA in osteosarcoma therapy. Biomater Sci. 2022;10(11):2844–2856. doi:10.1039/d2bm00253a
98. Yang Y, Huang G, Zhou Z, Fewell JG, Kleinerman ES. miR-20a Regulates FAS Expression in Osteosarcoma Cells by Modulating FAS Promoter Activity and Can be Therapeutically Targeted to Inhibit Lung Metastases. Mol Cancer Ther. 2018;17(1):130–139. doi:10.1158/1535-7163.MCT-17-0042
99. Zhang H, Wang J, Ren T, et al. Bone marrow mesenchymal stem cell-derived exosomal miR-206 inhibits osteosarcoma progression by targeting TRA2B. Cancer Lett. 2020;490:54–65. doi:10.1016/j.canlet.2020.07.008
100. Ruan Q, Wang C, Wu Y, Zhu Q. Exosome microRNA-22 inhibiting proliferation, migration and invasion through regulating Twist1/CADM1 axis in osteosarcoma. Sci Rep. 2024;14(1):761. doi:10.1038/s41598-023-50612-4
101. Shimbo K, Miyaki S, Ishitobi H, et al. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem Biophys Res Commun. 2014;445(2):381–387. doi:10.1016/j.bbrc.2014.02.007
102. Jiang M, Jike Y, Liu K, et al. Exosome-mediated miR-144-3p promotes ferroptosis to inhibit osteosarcoma proliferation, migration, and invasion through regulating ZEB1. Mol Cancer. 2023;22(1):113. doi:10.1186/s12943-023-01804-z
103. Gong M, Liu H, Sun N, Xie Y, Yan F, Cai L. Polyethylenimine-dextran-coated magnetic nanoparticles loaded with miR-302b suppress osteosarcoma in vitro and in vivo. Nanomed. 2020;15(7):711–723. doi:10.2217/nnm-2019-0218
104. Li X, Wang S, Gao Q, et al. MiRNA-520a-3p combined with folic acid conjugated Fe2O3@PDA multifunctional nanoagents for MR imagine and antitumor gene-photothermal therapy. Nanotechnology. 2023;34(37). doi:10.1088/1361-6528/acd5d9
105. Xie M, Gong T, Wang Y, et al. Advancements in Photothermal Therapy Using Near-Infrared Light for Bone Tumors. Int J Mol Sci. 2024;25(8):4139. doi:10.3390/ijms25084139
106. Huang G, Huang H. Application of dextran as nanoscale drug carriers. Nanomed. 2018;13(24):3149–3158. doi:10.2217/nnm-2018-0331
107. Abeylath SC, Amiji M. “Click” Synthesis of Dextran Macrostructures for Combinatorial-Designed Self-Assembled Nanoparticles Encapsulating Diverse Anticancer Therapeutics. Bioorg Med Chem. 2011;19(21):6167–6173. doi:10.1016/j.bmc.2011.09.024
108. Hu Q, Lu Y, Luo Y. Recent advances in dextran-based drug delivery systems: from fabrication strategies to applications. Carbohydr Polym. 2021;264:117999. doi:10.1016/j.carbpol.2021.117999
109. Lukyanov AN, Torchilin VP. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv Drug Deliv Rev. 2004;56(9):1273–1289. doi:10.1016/j.addr.2003.12.004
110. Liu Y, Zhu ST, Wang X, et al. MiR-200c regulates tumor growth and chemosensitivity to cisplatin in osteosarcoma by targeting AKT2. Sci Rep. 2017;7(1):13598. doi:10.1038/s41598-017-14088-3
111. Yang Z, Liu T, Ren X, Yang M, Tu C, Li Z. Mir-34a: a regulatory hub with versatile functions that controls osteosarcoma networks. Cell Cycle Georget Tex. 2022;21(20):2121–2131. doi:10.1080/15384101.2022.2087755
112. Shi Y, van der Meel R, Chen X, Lammers T. The EPR effect and beyond: strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics. 2020;10(17):7921–7924. doi:10.7150/thno.49577
113. Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev. 2015;91:3–6. doi:10.1016/j.addr.2015.01.002
114. Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomed. 2012;7:5577–5591. doi:10.2147/IJN.S36111
115. Iqbal S, Zhao Z. Poly (β amino esters) copolymers: novel potential vectors for delivery of genes and related therapeutics. Int J Pharm. 2022;611:121289. doi:10.1016/j.ijpharm.2021.121289
116. Wei J, Zhu L, Lu Q, et al. Recent progress and applications of poly(beta amino esters)-based biomaterials. J Control Release off J Control Release Soc. 2023;354:337–353. doi:10.1016/j.jconrel.2023.01.002
117. Kong L, Ji H, Gan X, Cao S, Li Z, Jin Y. Knockdown of CD44 inhibits proliferation, migration and invasion of osteosarcoma cells accompanied by downregulation of cathepsin S. J Orthop Surg. 2022;17(1):154. doi:10.1186/s13018-022-03048-x
118. Luo Z, Dai Y, Gao H. Development and application of hyaluronic acid in tumor targeting drug delivery. Acta Pharm Sin B. 2019;9(6):1099–1112. doi:10.1016/j.apsb.2019.06.004
119. Chen Z, Lv Z, Sun Y, Chi Z, Qing G. Recent advancements in polyethyleneimine-based materials and their biomedical, biotechnology, and biomaterial applications. J Mater Chem B. 2020;8(15):2951–2973. doi:10.1039/c9tb02271f
120. Li X, Bhullar AS, Binzel DW, Guo P. The dynamic, motile and deformative properties of RNA nanoparticles facilitate the third milestone of drug development. Adv Drug Deliv Rev. 2022;186:114316. doi:10.1016/j.addr.2022.114316
121. Wang C, Pan C, Yong H, et al. Emerging non-viral vectors for gene delivery. J Nanobiotech. 2023;21(1):272. doi:10.1186/s12951-023-02044-5
122. Chen J, Luo J, Zhao Y, et al. Increase in transgene expression by pluronic L64-mediated endosomal/lysosomal escape through its membrane-disturbing action. ACS Appl Mater Interfaces. 2015;7(13):7282–7293. doi:10.1021/acsami.5b00486
123. Li E, Zhang J, Yuan T, Ma B. MiR-145 inhibits osteosarcoma cells proliferation and invasion by targeting ROCK1. Tumour Biol J Int Soc Oncodevelopl Biol Med. 2014;35(8):7645–7650. doi:10.1007/s13277-014-2031-9
124. Fan L, Wu Q, Xing X, Wei Y, Shao Z. MicroRNA-145 targets vascular endothelial growth factor and inhibits invasion and metastasis of osteosarcoma cells. Acta Biochim Biophys Sin. 2012;44(5):407–414. doi:10.1093/abbs/gms019
125. Zhang Z, Zhang M, Chen Q, Zhang Q. Downregulation of microRNA-145 promotes epithelial-mesenchymal transition via regulating Snail in osteosarcoma. Cancer Gene Ther. 2017;24(2):83–88. doi:10.1038/cgt.2017.1
126. Duan S, Yu B, Gao C, Yuan W, Ma J, Xu FJ. A Facile Strategy to Prepare Hyperbranched Hydroxyl-Rich Polycations for Effective Gene Therapy. ACS Appl Mater Interfaces. 2016;8(43):29334–29342. doi:10.1021/acsami.6b11029
127. Casper J, Schenk SH, Parhizkar E, Detampel P, Dehshari A, Huwyler J. Polyethylenimine (PEI) in gene therapy: current status and clinical applications. J Control Release off J Control Release Soc. 2023;362(23):667–691. doi:10.1016/j.jconrel.2023.09.001
128. Xue L, Yan Y, Kos P, Chen X, Siegwart DJ. PEI fluorination reduces toxicity and promotes liver-targeted siRNA delivery. Drug Deliv Transl Res. 2021;11(1):255–260. doi:10.1007/s13346-020-00790-9
129. Xin J, Lu X, Cao J, et al. Fluorinated Organic Polymers for Cancer Drug Delivery. Adv Mater Deerfield Beach Fla. 2024. doi:10.1002/adma.202404645
130. Song H, Liu S, Li C, Geng Y, Wang G, Gu Z. Pluronic L64-mediated stable HIF-1α expression in muscle for therapeutic angiogenesis in mouse hindlimb ischemia. Int J Nanomed. 2014;9:3439–3452. doi:10.2147/IJN.S65353
131. He Y, Liu Y, Sun Z, et al. The proper strategy to compress and protect plasmid DNA in the Pluronic L64-electropulse system for enhanced intramuscular gene delivery. Regen Biomater. 2019;6(5):289–298. doi:10.1093/rb/rby028
132. Meng CY, Zhao ZQ, Bai R, et al. MicroRNA-22 mediates the cisplatin resistance of osteosarcoma cells by inhibiting autophagy via the PI3K/Akt/mTOR pathway. Oncol Rep. 2020;43(4):1169–1186. doi:10.3892/or.2020.7492
133. Choi EJ, Koo BK, Hur EH, et al. Inhibition of DNMT3B and PI3K/AKT/mTOR and ERK Pathways as a Novel Mechanism of Volasertib on Hypomethylating Agent-Resistant Cells. Biomol Ther. 2023;31(3):319–329. doi:10.4062/biomolther.2022.117
134. Li Y, Nie JJ, Yang Y, et al. Redox-Unlockable Nanoparticle-Based MST1 Delivery System to Attenuate Hepatic Steatosis via the AMPK/SREBP-1c Signaling Axis. ACS Appl Mater Interfaces. 2022;14(30):34328–34341. doi:10.1021/acsami.2c05889
135. Nie JJ, Liu Y, Qi Y, et al. Charge-reversal nanocomolexes-based CRISPR/Cas9 delivery system for loss-of-function oncogene editing in hepatocellular carcinoma. J Control Release off J Control Release Soc. 2021;333:362–373. doi:10.1016/j.jconrel.2021.03.030
136. Xu J, Yao Q, Hou Y, et al. MiR-223/Ect2/p21 signaling regulates osteosarcoma cell cycle progression and proliferation. Biomed Pharm Biomed Pharm. 2013;67(5):381–386. doi:10.1016/j.biopha.2013.03.013
137. Tang Q, Yuan Q, Li H, et al. miR-223/Hsp70/JNK/JUN/miR-223 feedback loop modulates the chemoresistance of osteosarcoma to cisplatin. Biochem Biophys Res Commun. 2018;497(3):827–834. doi:10.1016/j.bbrc.2018.02.091
138. MHJr A, Green MD, Getaneh HK, Miller KM, Long TE. Tailoring Charge Density and Hydrogen Bonding of Imidazolium Copolymers for Efficient Gene Delivery. Biomacromolecules. 2011;12(6):2243–2250. doi:10.1021/bm2003303
139. Xu FJ, Zhu Y, Chai MY, Liu FS. Comparison of ethanolamine/ethylenediamine-functionalized poly(glycidyl methacrylate) for efficient gene delivery. Acta Biomater. 2011;7(8):3131–3140. doi:10.1016/j.actbio.2011.04.023
140. Xu FJ, Chai MY, Li WB, et al. Well-Defined Poly(2-hydroxyl-3-(2-hydroxyethylamino)propyl methacrylate) Vectors with Low Toxicity and High Gene Transfection Efficiency. Biomacromolecules. 2010;11(6):1437–1442. doi:10.1021/bm100309y
141. hua LX, wei HF, yong QS, et al. A strategy to improve serum-tolerant transfection activity of polycation vectors by surface hydroxylation. Biomaterials. 2011;32(36):9925–9939. doi:10.1016/j.biomaterials.2011.09.011
142. Li RQ, Wu Y, Zhi Y, et al. PGMA-Based Star-Like Polycations with Plentiful Hydroxyl Groups Act as Highly Efficient miRNA Delivery Nanovectors for Effective Applications in Heart Diseases. Adv Mater. 2016;28(33):7204–7212. doi:10.1002/adma.201602319
143. Li L, Tian H, He J, Zhang M, Li Z, Ni P. Fabrication of aminated poly(glycidyl methacrylate)-based polymers for co-delivery of anticancer drugs and the p53 gene. J Mater Chem B. 2020;8(41):9555–9565. doi:10.1039/d0tb01811b
144. Sadaquat H, Akhtar M, Nazir M, Ahmad R, Alvi Z, Akhtar N. Biodegradable and biocompatible polymeric nanoparticles for enhanced solubility and safe oral delivery of docetaxel: in vivo toxicity evaluation. Int J Pharm. 2021;598:120363. doi:10.1016/j.ijpharm.2021.120363
145. Asl FD, Mousazadeh M, Taji S, et al. Nano drug-delivery systems for management of AIDS: liposomes, dendrimers, gold and silver nanoparticles. Nanomed. 2023;18(3):279–302. doi:10.2217/nnm-2022-0248
146. Wang X, Zhang M, Li Y, Cong H, Yu B, Shen Y. Research status of dendrimer micelles in tumor therapy for drug delivery. Small Weinh Bergstr Ger. 2023;19(50):e2304006. doi:10.1002/smll.202304006
147. Liu Y, Castro Bravo KM, Liu J. Targeted liposomal drug delivery: a nanoscience and biophysical perspective. Nanoscale Horiz. 2021;6(2):78–94. doi:10.1039/d0nh00605j
148. Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99(Pt A):28–51. doi:10.1016/j.addr.2015.09.012
149. Large DE, Abdelmessih RG, Fink EA, Auguste DT. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv Drug Deliv Rev. 2021;176:113851. doi:10.1016/j.addr.2021.113851
150. Sparks J, Slobodkin G, Matar M, et al. Versatile cationic lipids for siRNA delivery. J Control Release off J Control Release Soc. 2012;158(2):269–276. doi:10.1016/j.jconrel.2011.11.006
151. Beird HC, Bielack SS, Flanagan AM, et al. Osteosarcoma. Nat Rev Dis Primer. 2022;8(1):77. doi:10.1038/s41572-022-00409-y
152. Tenchov R, Sasso JM, Zhou QA. PEGylated Lipid Nanoparticle Formulations: immunological Safety and Efficiency Perspective. Bioconjug Chem. 2023;34(6):941–960. doi:10.1021/acs.bioconjchem.3c00174
153. Barzegari Firouzabadi F, Oryan SH, Sheikhha MH, Kalantar SM, Javed A. Preparation and evaluation of a novel liposomal nano-formulation in metastatic cancer treatment studies. Cell J. 2019;21(2):135–142. doi:10.22074/cellj.2019.6008
154. Soliman B, Wen MM, Kandil E, El-Agamy B, Gamal-Eldeen AM, ElHefnawi M. Preparation and Optimization of MiR-375 nano-vector using two novel chitosan-coated nano-structured lipid carriers as gene therapy for hepatocellular carcinoma. Pharmaceutics. 2024;16(4):494. doi:10.3390/pharmaceutics16040494
155. Godoy DA, Di Napoli M, Rabinstein AA. Cerebral Fat Embolism: recognition, Complications, and Prognosis. Neurocrit Care. 2018;29(3):358–365. doi:10.1007/s12028-017-0463-y
156. Guimarães D, Cavaco-Paulo A, Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Int J Pharm. 2021;601:120571. doi:10.1016/j.ijpharm.2021.120571
157. Lan T, Luo M, Wei X. Mesenchymal stem/stromal cells in cancer therapy. J Hematol Oncol. 2021;14(1):195. doi:10.1186/s13045-021-01208-w
158. Harrell CR, Volarevic A, Djonov VG, Jovicic N, Volarevic V. Mesenchymal stem cell: A friend or foe in anti-tumor immunity. Int J Mol Sci. 2021;22(22):12429. doi:10.3390/ijms222212429
159. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. doi:10.1126/science.aau6977
160. Weng Z, Zhang B, Wu C, et al. Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J Hematol Oncol. 2021;14(1):136. doi:10.1186/s13045-021-01141-y
161. Liu H, Deng S, Han L, et al. Mesenchymal stem cells, exosomes and exosome-mimics as smart drug carriers for targeted cancer therapy. Colloids Surf B Biointerfaces. 2022;209(Pt 1):112163. doi:10.1016/j.colsurfb.2021.112163
162. Sarhadi VK, Daddali R, Seppänen-Kaijansinkko R. Mesenchymal Stem Cells and Extracellular Vesicles in Osteosarcoma Pathogenesis and Therapy. Int J Mol Sci. 2021;22(20):11035. doi:10.3390/ijms222011035
163. Vakhshiteh F, Atyabi F, Ostad SN. Mesenchymal stem cell exosomes: a two-edged sword in cancer therapy. Int J Nanomed. 2019;14:2847–2859. doi:10.2147/IJN.S200036
164. Qin F, Tang H, Zhang Y, Zhang Z, Huang P, Zhu J. Bone marrow-derived mesenchymal stem cell-derived exosomal microRNA-208a promotes osteosarcoma cell proliferation, migration, and invasion. J Cell Physiol. 2020;235(5):4734–4745. doi:10.1002/jcp.29351
165. Liu W, Wang B, Duan A, et al. Exosomal transfer of miR-769-5p promotes osteosarcoma proliferation and metastasis by targeting DUSP16. Cancer Cell Int. 2021;21(1):541. doi:10.1186/s12935-021-02257-4
166. Qi J, Zhang R, Wang Y. Exosomal miR-21-5p derived from bone marrow mesenchymal stem cells promote osteosarcoma cell proliferation and invasion by targeting PIK3R1. J Cell Mol Med. 2021;25(23):11016–11030. doi:10.1111/jcmm.17024
167. Zhang M, Hu S, Liu L, et al. Engineered exosomes from different sources for cancer-targeted therapy. Signal Transduct Target Ther. 2023;8(1):124. doi:10.1038/s41392-023-01382-y
168. Zhang F, Guo J, Zhang Z, et al. Mesenchymal stem cell-derived exosome: a tumor regulator and carrier for targeted tumor therapy. Cancer Lett. 2022;526:29–40. doi:10.1016/j.canlet.2021.11.015
169. Shams F, Pourjabbar B, Hashemi N, et al. Current progress in engineered and nano-engineered mesenchymal stem cells for cancer: from mechanisms to therapy. Biomed Pharm Biomed Pharm. 2023;167:115505. doi:10.1016/j.biopha.2023.115505
170. Deng M, Wu S, Huang P, Liu Y, Li C, Zheng J. Engineered exosomes-based theranostic strategy for tumor metastasis and recurrence. Asian J Pharm Sci. 2023;18(6):100870. doi:10.1016/j.ajps.2023.100870
171. Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi:10.1146/annurev-biochem-060308-103103
172. Jeppesen DK, Fenix AM, Franklin JL, et al. Reassessment of Exosome Composition. Cell. 2019;177(2):428–445.e18. doi:10.1016/j.cell.2019.02.029
173. Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int J Nanomed. 2020;15:6917–6934. doi:10.2147/IJN.S264498
174. Tian J, Han Z, Song D, et al. Engineered exosome for drug delivery: recent development and clinical applications. Int J Nanomed. 2023;18:7923–7940. doi:10.2147/IJN.S444582
175. Zhang M, Wan L, Li R, Li X, Zhu T, Lu H. Engineered exosomes for tissue regeneration: from biouptake, functionalization and biosafety to applications. Biomater Sci. 2023;11(22):7247–7267. doi:10.1039/d3bm01169k
176. Al-Madhagi H. The landscape of exosomes biogenesis to clinical applications. Int J Nanomed. 2024;19:3657–3675. doi:10.2147/IJN.S463296
177. Shao J, Zaro J, Shen Y. Advances in exosome-based drug delivery and tumor targeting: from tissue distribution to intracellular Fate. Int J Nanomed. 2020;15:9355–9371. doi:10.2147/IJN.S281890
178. Tang R, Xu J, Zhang B, et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol. 2020;13(1):110. doi:10.1186/s13045-020-00946-7
179. Gao W, Wang X, Zhou Y, Wang X, Yu Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther. 2022;7(1):196. doi:10.1038/s41392-022-01046-3
180. Chen A, Zhang W, Jiang C, Jiang Z, Tang D. The engineered exosomes targeting ferroptosis: a novel approach to reverse immune checkpoint inhibitors resistance. Int J Cancer. 2024;155(1):7–18. doi:10.1002/ijc.34934
181. Meng C, Yang Y, Feng W, Ma P, Bai R. Exosomal miR-331-3p derived from chemoresistant osteosarcoma cells induces chemoresistance through autophagy. J Orthop Surg. 2023;18(1):892. doi:10.1186/s13018-023-04338-8
182. Sizikov AA, Kharlamova MV, Nikitin MP, Nikitin PI, Kolychev EL. Nonviral Locally Injected Magnetic Vectors for In Vivo Gene Delivery: a Review of Studies on Magnetofection. Nanomater Basel Switz. 2021;11(5):1078. doi:10.3390/nano11051078
183. Sizikov AA, Nikitin PI, Nikitin MP. Magnetofection in vivo by nanomagnetic carriers systemically administered into the bloodstream. Pharmaceutics. 2021;13(11):1927. doi:10.3390/pharmaceutics13111927
184. Nowak-Jary J, Machnicka B. In vivo biodistribution and clearance of magnetic iron oxide nanoparticles for medical applications. Int J Nanomed. 2023;18:4067–4100. doi:10.2147/IJN.S415063
185. Rezaei B, Yari P, Sanders SM, et al. Magnetic nanoparticles: A review on synthesis, characterization, functionalization, and biomedical applications. Small Weinh Bergstr Ger. 2024;20(5):e2304848. doi:10.1002/smll.202304848
186. Hoang J, Patil SL, Srinoi P, et al. Transfection of Unmodified MicroRNA Using Monolayer-Coated Au Nanoparticles as Gene-Delivery Vehicles. ACS Appl Bio Mater. 2024;7(1):230–237. doi:10.1021/acsabm.3c00837
187. Ali A, Zafar H, Zia M, et al. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol Sci Appl. 2016;9:49–67. doi:10.2147/NSA.S99986
188. Lin G, Huang J, Zhang M, Chen S, Zhang M. Chitosan-Crosslinked Low Molecular Weight PEI-Conjugated Iron Oxide Nanoparticle for Safe and Effective DNA Delivery to Breast Cancer Cells. Nanomater Basel Switz. 2022;12(4):584. doi:10.3390/nano12040584
189. Vangijzegem T, Stanicki D, Laurent S. Magnetic iron oxide nanoparticles for drug delivery: applications and characteristics. Expert Opin Drug Deliv. 2019;16(1):69–78. doi:10.1080/17425247.2019.1554647
190. Stanicki D, Vangijzegem T, Ternad I, Laurent S. An update on the applications and characteristics of magnetic iron oxide nanoparticles for drug delivery. Expert Opin Drug Deliv. 2022;19(3):321–335. doi:10.1080/17425247.2022.2047020
191. Sun J, Xing F, Braun J, et al. Progress of phototherapy applications in the treatment of bone cancer. Int J Mol Sci. 2021;22(21):11354. doi:10.3390/ijms222111354
192. Jin A, Wang Y, Lin K, Jiang L. Nanoparticles modified by polydopamine: working as “drug” carriers. Bioact Mater. 2020;5(3):522–541. doi:10.1016/j.bioactmat.2020.04.003
193. Mi X, Hu M, Dong M, et al. Folic acid decorated zeolitic imidazolate framework (ZIF-8) Loaded with Baicalin as a Nano-Drug Delivery System for Breast Cancer Therapy. Int J Nanomed. 2021;16:8337–8352. doi:10.2147/IJN.S340764
194. Chen C, Du S, Zhong W, et al. Accurate delivery of pristimerin and paclitaxel by folic acid-linked nano-micelles for enhancing chemosensitivity in cancer therapy. Nano Converg. 2022;9(1):52. doi:10.1186/s40580-022-00343-5
195. Parvathaneni V, Shukla SK, Gupta V. Development and Characterization of Folic Acid-Conjugated Amodiaquine-Loaded Nanoparticles-Efficacy in Cancer Treatment. Pharmaceutics. 2023;15(3):1001. doi:10.3390/pharmaceutics15031001
196. Luo T, Jiang M, Cheng Z, et al. Biodegradable FePS3 nanoplatform for efficient treatment of osteosarcoma by combination of gene and NIR-II photothermal therapy. J Nanobiotechnology. 2023;21(1):224. doi:10.1186/s12951-023-01961-9
197. Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17(11):657–674. doi:10.1038/s41571-020-0410-2
198. Bravo M, Fortuni B, Mulvaney P, et al. Nanoparticle-mediated thermal Cancer therapies: Strategies to improve clinical translatability. J Control Release off J Control Release Soc. 2024;372:751–777. doi:10.1016/j.jconrel.2024.06.055
199. Huber J, Longaker MT, Quarto N. Circulating and extracellular vesicle-derived microRNAs as biomarkers in bone-related diseases. Front Endocrinol. 2023;14:1168898. doi:10.3389/fendo.2023.1168898
200. Celik B, Cicek K, Leal AF, Tomatsu S. Regulation of molecular targets in osteosarcoma treatment. Int J Mol Sci. 2022;23(20):12583. doi:10.3390/ijms232012583
201. Soutschek J, Akinc A, Bramlage B, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432(7014):173–178. doi:10.1038/nature03121
202. Bulcha JT, Wang Y, Ma H, Tai PWL, Gao G. Viral vector platforms within the gene therapy landscape. Signal Transduct Target Ther. 2021;6(1):53. doi:10.1038/s41392-021-00487-6
203. Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release off J Control Release Soc. 2006;114(1):100–109. doi:10.1016/j.jconrel.2006.04.014
204. Harris MA, Hawkins CJ. Recent and ongoing research into metastatic osteosarcoma treatments. Int J Mol Sci. 2022;23(7):3817. doi:10.3390/ijms23073817
205. Jin J, Cong J, Lei S, et al. Cracking the code: deciphering the role of the tumor microenvironment in osteosarcoma metastasis. Int Immunopharmacol. 2023;121:110422. doi:10.1016/j.intimp.2023.110422
206. Raue R, Frank AC, Syed SN, Brüne B. Therapeutic Targeting of MicroRNAs in the Tumor Microenvironment. Int J Mol Sci. 2021;22(4):2210. doi:10.3390/ijms22042210
207. Llobat L, Gourbault O. Role of MicroRNAs in human osteosarcoma: future perspectives. Biomedicines. 2021;9(5):463. doi:10.3390/biomedicines9050463
208. Bravo-Vázquez LA, Méndez-García A, Rodríguez AL, et al. Applications of nanotechnologies for miRNA-based cancer therapeutics: current advances and future perspectives. Front Bioeng Biotechnol. 2023;11:1208547. doi:10.3389/fbioe.2023.1208547
209. Yi C, Lu L, Li Z, et al. Plant-derived exosome-like nanoparticles for microRNA delivery in cancer treatment. Drug Deliv Transl Res. 2024. doi:10.1007/s13346-024-01621-x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.