Back to Journals » Drug Design, Development and Therapy » Volume 18
Development of Intratumoral Drug Delivery Based Strategies for Antitumor Therapy
Received 26 March 2024
Accepted for publication 23 May 2024
Published 11 June 2024 Volume 2024:18 Pages 2189—2202
DOI https://doi.org/10.2147/DDDT.S467835
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Zhimei Jiang,1– 3 Yuzhi Fu,1– 3 Hongxin Shen1– 3
1Department of Pharmacy, West China Second University Hospital of Sichuan University, Chengdu, People’s Republic of China; 2Evidence-Based Pharmacy Center, West China Second University Hospital of Sichuan University, Chengdu, People’s Republic of China; 3Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, People’s Republic of China
Correspondence: Hongxin Shen, West China Second University Hospital, Sichuan University, No. 20, Third Section, Renmin South Road, Chengdu, Sichuan, 610041, People’s Republic of China, Email [email protected]
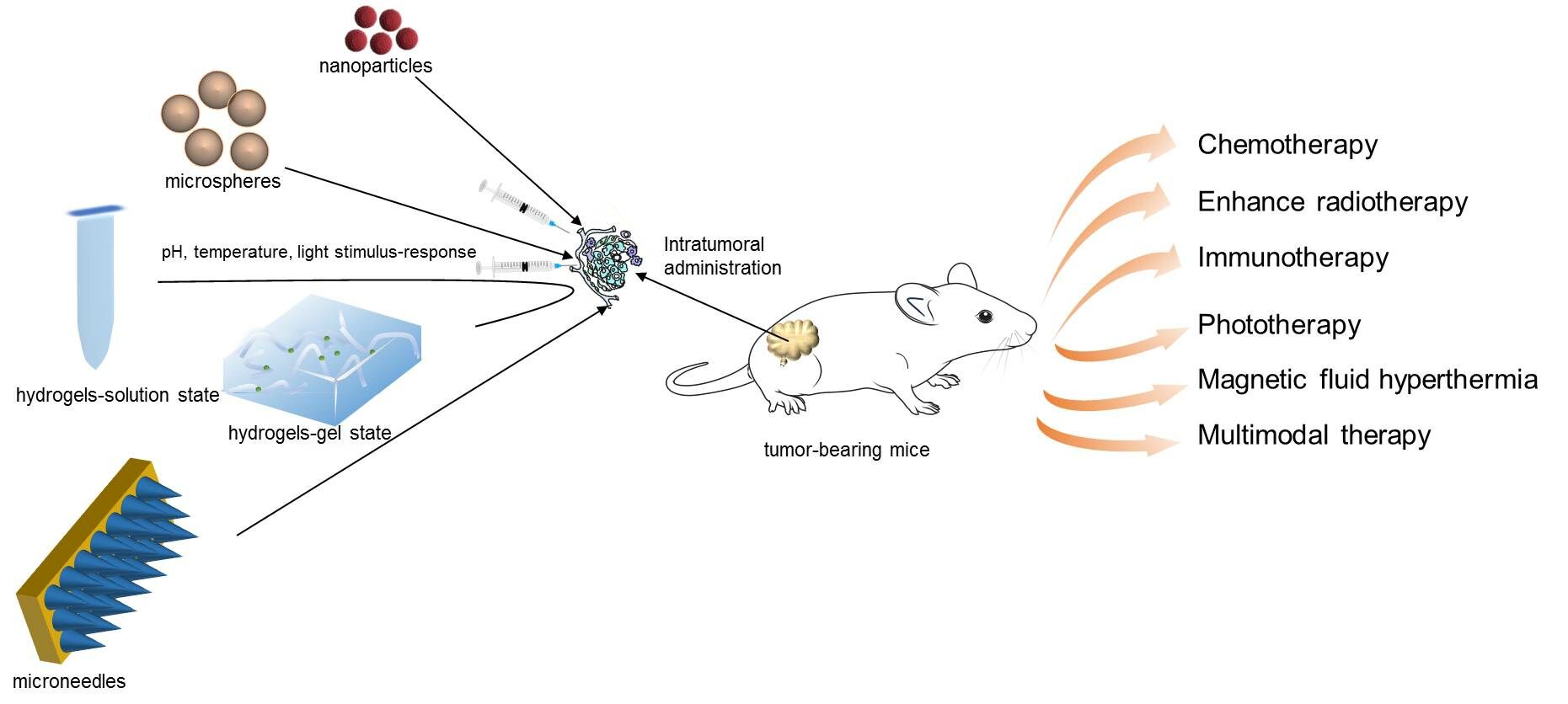
Abstract: Research for tumor treatment with significant therapy effects and minimal side-effects has been widely carried over the past few decades. Different drug forms have received a lot of attention. However, systemic biodistribution induces efficacy and safety issues. Intratumoral delivery of agents might overcome these problems because of its abundant tumor accumulation and retention, thereby reducing side effects. Delivering hydrogels, nanoparticles, microneedles, and microspheres drug carriers directly to tumors can realize not only targeted tumor therapy but also low side-effects. Furthermore, intratumoral administration has been integrated with treatment strategies such as chemotherapy, enhancing radiotherapy, immunotherapy, phototherapy, magnetic fluid hyperthermia, and multimodal therapy. Some of these strategies are ongoing clinical trials or applied clinically. However, many barriers hinder it from being an ideal and widely used option, such as decreased drug penetration impeded by collagen fibers of a tumor, drug squeezed out by high density and high pressure, mature intratumoral injection technique. In this review, we systematically discuss intratumoral delivery of different drug carriers and current development of intratumoral therapy strategies.
Keywords: intratumoral drug delivery, drug carrier, treatment strategy, antitumor therapy
Graphical Abstract:
Introduction
At present, many kinds of strategies have been developed for the therapy of various tumors, such as surgery, chemotherapy, radiotherapy, immunotherapy, and photothermal therapy. Some early solid tumors are preferentially treated by surgical resection; however, advanced carcinomas at stage III–IV, inoperable regions, or elderly patients unable to undergo surgical treatment require improved systemic treatment strategies.1 Currently, there are still many challenges in cancer treatment. Traditional treatment methods such as radiation therapy and chemotherapy can inhibit tumor growth to a certain extent but are unlikely to completely kill tumor cells, resulting in significant side-effects, tumor recurrence, and metastasis. Therapeutic drug delivery systems have been widely investigated because of their anticancer potential to target tumors, enhance radiotherapy, or immunotherapy. However, challenges and limitations also urgently need to be solved. Drugs show the characteristics of non-specific delivery, causing various adverse reactions and inefficient treatment. Hopefully, the intratumoral delivery system which refers to drugs directly injected into tumors may provide a meaningful response. Its unique anticancer efficacy was associated with increased tumor distribution, prolonged retention of therapeutic drugs within the tumors, and decreased normal tissue uptake.2,3
Intratumoral therapy is different from systemic administration, because it directly delivers therapeutic substances into the tumor sites, increases the concentration and station of antitumor drugs, thereby enhancing the antitumor effect and reducing systemic toxicity.4 Recently, intratumoral delivery systems have been extensively explored. This mainly focused on nanoparticles,5 hydrogels,6 microneedles,7 microspheres,8 etc. For example, Chattopadhyay et al3,9 employed the intratumoral injection of trastuzumab-gold nanoparticles to maximize tumor localization for X-ray radio sensitization and reduce uptake by the liver and spleen. It caused 25-fold higher tumor uptake, 10-fold lower spleen uptake of trastuzumab-gold nanoparticles than that post-intravenous injection at 48 hours. Its liver uptake was also modestly decreased. In addition, the pharmacodynamics revealed that tumor size was decreased by 2-fold in a trastuzumab-gold nanoparticles and X-radiation group that in an X-radiation alone group.
However, for intratumoral drug delivery systems, the complex and chaotic vasculatures formed in and around the tumor, and the properties of their abnormal mesenchyme,10 hindered effective permeation of therapeutic drugs to tumor cells which reduced their anticancer activity.11 Moreover, uniform distribution within the tumor mass was another challenge which might determine the therapeutic efficacy.12 Promisingly, the smart formulations have higher potential, owing to their superior advantages, such as target delivery, easy tumor penetration, stimulus-responsive property, sustained drug release, as well as minimal systemic side-effects.13,14 The smart intratumoral delivery systems may be suitable to utilize drug carriers overcoming short retention times in tumors and the penetration barriers.15 In addition, further ligand modifications enable tumor targeted delivery to be possible. Stimulus-responsive natures such as pH, reactive oxygen species, light, or heat make drugs a good choice for local tumor-specific targeting. Currently, in order to achieve the desired antitumor effects with high safety, the intratumoral delivery systems have been intensively developed in the past decades. Fortunately, recent progress has revealed that intratumoral delivery systems enable tumor targeted delivery of not only chemotherapeutic drugs and immunotherapeutic agents, but also radiosensitizers, photothermal agents, or other sensitive substances to enhance its effects and reduce side-effects.15–17
Intratumoral administration can be realized through direct injection or image-guided injection, which enables not only high concentrations of drugs in tumor tissue, but also reduces systemic side-effects. A suitable drug carrier may contribute to reasonable drug loading and release. Intratumoral administrations have been integrated with treatment strategies. Some of these strategies are ongoing clinical trials or applied clinically. This review focuses on the intratumoral delivery of well-studied drug carriers and current development of intratumoral therapy strategies.
Application of Intratumoral Delivery System with Different Drug Carriers for Antitumor Therapy
Hydrogels for Intratumoral Administration
Hydrogels are prepared by biocompatible materials and form a three-dimensional network structure, which is suitable for loading various antitumor drugs. Currently, in-situ forming hydrogels have attracted increasing attention because of their many advantages. Local delivery of therapeutic agents through hydrogels can directly provide continuous release and a high dose of therapeutic drugs in tumor tissues,18 which may prevent prolonged blood circulation and reduce side-effects among normal tissues. Stimulus responsive hydrogels are solutions which can easily flow during intratumoral administration, but rapidly change to gel phase once injected into tumor sites.19 Hence, the application of intratumoral injectable in-situ forming hydrogel is a promising route to local drug delivery.
Hydrogels can be prepared through in-situ cross-linking, of which chemical and physical based cross-linking are most commonly used. A mixture of polymer, cross-linker, and antitumor drugs will form in-situ hydrogels through chemical reaction. In addition, physical hydrogels are prepared by electrostatic interaction, hydrogen bonds, and entanglement of chains.20 Recent studies have revealed that smart hydrogels can also emerge intelligent sol-gel changes according to environmental stimuli of tumors such as light, temperature, pH, etc. Zhang et al21 explored an in-situ hydrogel system triggered by near-infrared (NIR) laser. It showed multi-mechanism antitumor therapy. A solution containing poly (ethylene glycol) double acrylates (PEGDA), Doxorubicin (DOX), and TiO2 introduced by multi-walled carbon nanotubes (MWCNTs) substrate was injected into the tumor of mice, and then quickly gelled through photo-crosslinking triggered by a NIR laser. It allowed for convenient injection in vitro and sustained release in vivo. Similarly, the temperature sensitivity is another intelligent approach to form in-situ hydrogels. Wan et al22 synthesized D-PNA100 nanomedicines via acid-base neutralization of DOX and pAA100-bpNIPAM200-b-pAA100 (PNA100). Within a specific concentration range (5.0–10.0% of D-PNA100), the phase behavior transition of temperature sensitive sol-gel occurred. Temperature sensitive hydrogels were in a liquid state with good fluidity at room temperature and became in a gel state at 37°C with good retention. An in vitro release experiment of D-PNA100 hydrogels revealed a sustained DOX-release over ten days. Due to its excellent retention, intratumoral administration of temperature-sensitive D-PNA100 hydrogels achieved the highest antitumor activity among all treatment groups. Furthermore, Raza et al23 prepared a pH responsive hydrogel for the intratumoral delivery of paclitaxel to enhance the efficacy of antitumor chemotherapy. Since the acidic pH of tumor cell microenvironment is relatively lower than that of normal cell, the smart hydrogel is designed to be acid-sensitive. The pH responsive FER-8 peptide hydrogel is stable and rarely degradative at neutral pH 7.4, which can be explained by the uncharged basic amino acids, resulting in no repulsion and completely compound solubilized. However, a higher decomposition rate was observed in the acidic environment because of electrostatic repulsion among glutamic acid residues which facilitated drug release from hydrogels in a tumor microenvironment. It is indicated that environmental stimuli is promising to be explored as the nature of novel in-situ forming hydrogels for intratumoral administration against tumors.
Nanoparticles for Intratumoral Administration
A lot of nanoparticles have been developed for antitumor therapy, but it is still difficult to achieve tumor high targeting or distribution of drugs though systemic administration.24 The rapid liver and spleen uptake upon i.v. administration led to reduced uptake of target tisssues.25 It may be attributed to the basis of enhanced permeability and retention (EPR) effect which is seriously doubted in human bodies.26 Furthermore, the survival percentages of marketed nanoscale anticancer drugs (such as Doxil®, Abraxane®) are not higher than that of free drug solutions.27 Therefore, compared to the intratumoral therapy with free drugs, intratumoral delivery of drugs through nanoparticle formulations provides opportunities to realize higher encapsulate rates, lower systemic toxicity, target property, and sustained release.
As an alternative strategy for cancer treatment, intratumoral injecting nanoparticles directly into tumors to deliver therapeutic drugs to the tumor site has been widely developed. Reversed lipid-based nanoparticles (RLBN) have been explored as carriers for hydrophilic drugs which spread rapidly from injection sites to systemic circulation.28 RLBN had a polar core and a reversed lipophilic periphery, which made it soluble and stable in hydrophobic liquids and entrap hydrophilic drugs. In addition, DOX entrapped by RLBN after a single intratumoral injection in HepG2 tumor-bearing mice showed much more remarkable antitumor activity than that of free DOX. Furthermore, nanoparticles as drug carriers have also been used to improve short effective diffusion distance such as nitric oxide (NO) which possessed powerful cytotoxicity and lack of off-target side-effects. Lee et al29 prepared poly(lactic-co-glycolic acid) (PLGA)-conjugated linear polyethylenimine diazeniumdiolate (LP/NO)-modified with albumin nanoparticles. It offered sufficient NO-loading as well as sustained NO-release. Moreover, surface coating with albumin changed the surface charge of nanoparticles, which enhanced tumor penetration by decreasing dense extracellular matrix the (ECM) interaction. Enhanced antitumor activity was demonstrated by in vivo experiment in B16F10-tumor-bearing mice which indicated improved spatial distribution and matrix penetration in tumor tissue. Taken together, nanoparticles as carriers of intratumoral delivery drugs represent a promising modality for tumor treatment.
Even though a lot of effort has been made to increase total tumor accumulation of systemic administration of nanoparticles such as modified with monoclonal antibodies or targeting ligands, these approaches have been proved to be moderately successful.9 Studies revealed that tumor-targeting nanoparticles through intravenous administration did not improve accumulation in solid tumors, but improved internalization within tumor cells compared with non-targeting nanoparticles.30–32 This may be attributed to a large number of proteins in blood which can tightly bind to the surface of nanoparticles, thereby changing their physical and chemical characteristic and stability and hindering the specific binding of targeted molecules to the receptor.33,34 Furthermore, the uptake and clearance by liver and spleen hinder further delivery to tumor tissue and lead to toxicity of healthy tissues.35 High heterogeneity of enhanced permeability and retention effect in different tumor types and different regions of the same tumor is another challenge.36 However, intratumoral injection of targeted nanoparticles may be a feasible treatment option for cancer therapy because of blood sequestration, high tumor retention and tumor internalization, as well as reduced exposure of the liver and spleen.9
Microneedles for Intratumoral Administration
Microneedles (MNs) are needle arrays of micrometer length, which establish hundreds of microchannels in the skin through almost painlessly direct piercing into the cuticula in a minimally invasive pattern and increased efficacy of transdermal administration.37 Recently, MNs have displayed great potential to facilitate intratumoral delivery of therapy drugs within the lesion site of tumors.2,38 MN patches can offer uniform drug delivery throughout the tumor tissue, which is critical for in-situ chemotherapy and immunotherapy. After administration, needle tips are dissolved by the environment fluid of the tumor tissue and gradually release encapsulated drugs or nanoparticles. Loading therapeutic agents into nanoparticles further leads to sustained release. In addition, the side-effects of normal tissues related to system circulation are remarkably reduced.39
MNs can integrate with nanoparticles concentrated at needle tips, demonstrating superior synergistic antitumor effects compared with intravenous and intratumoral injection of nanoparticles.38 Furthermore, functionalized MNs with thermal-sensitive, pH-sensitive properties were prepared to achieve specific controlled release of the drug. Wu et al explored metal-organic frameworks (MOFs) constructed using MIL-88 as an internal core which is composed of iron ions and 2-aminoterephthalic acid and ZIF-8 as an external shell which consists of zinc ions and 2-methyl imidazole.38 Indocyanine green (ICG) was loaded into the internal core and DOX was encapsulated into the external shell, which formed MIL-88-ICG@ZIF-8-DOX nanoparticles. The above nanoparticles were further loaded into dissolving microneedles. It was thermal-sensitive attributed to IGG converting light energy into heat and pH-sensitive because of ZIF-8 degradation triggered by tumor acid microenvironment. MNs could also be designed to delivery drugs deeper into the tumor tissues. This feature was realized though active MNs loaded with magnesium (Mg) microparticles, which exhibited improved overall survival as well as tumor growth inhibition compared to injection and passive MNs.40 Due to its ability to generate hydrogen gas bubbles, a Mg micromotor exerts a thrust on drug payloads penetrating deep into tumor tissues. Therefore, well-designed MNs are suitable for intratumoral delivery to enhance anti-tumor efficacy.
Microspheres for Intratumoral Administration
Microspheres (MS) have been increasingly developed as drug delivery systems for anticancer therapy. MS can serve as drug reservoirs, slowly release drugs by multiple mechanisms such as hydrolysis, self-diffusion, transport, and erosion.41,42 The sustained release characteristics are profit to increasing drug retention at the tumor site. Furthermore, MS can protect drugs from chemical degradation and transformation, thereby improving the drug stability.43,44 Especially the biocompatible and biodegradable poly(lactic-co-glycolic-acid) (PLGA) material has been widely used. Nowadays, intratumoral delivery of MS results in high drug concentration in tumors, minor leakage in plasma, and good therapeutic effect.45
Despite its efficient cancer therapy, the core-shell MS was prepared to obtain high drug loading capacity. Ni et al46 explored PLGA microspheres, in which the polydopamine (PDA) nanoparticles as photothermal therapeutic agent and DOX as chemotherapy agent were loaded in the interior hollow space with the PLGA as the outer shell layer. NIR irradiation at the tumor site triggered a hyperthermia effect through PDA nanoparticles, while releasing DOX and diffusing into tumor cells. Noteworthy, drug accumulation in the tumor was shown to be 6-fold higher in intratumoral delivery of the microspheres group than that in intravenous injection of the DOX group. The above description further demonstrates the advantages of intratumoral administration compared with systemic delivery.
Additionally, the morphology of MS plays an important role in solubility, encapsulation efficiency, and drug release behavior, which are affected by oil phase viscosity, stirring rate, and organic solvent evaporation.47,48 The above characteristics further affect antitumor activity of MS preparations. Zhang et al49 prepared smooth paclitaxel (PTX) PLGA-MS by a characteristic of internal sporadic porosity and rough PTX-PLGA-MS with porous internal structures and microporous surface. The above different morphological types were explored to study the difference in pharmacodynamics. Finally, the rough MS group after a single injection achieved higher antitumor activity (tumor inhibition rate ¼ 58.33%) than that in the free PTX and smooth MS group. It might be attributed to sustained drug release and adhering throughout tumor tissues of rough MS. Decreased drug administration frequency and reduced drug distribution in normal tissues alleviated the adverse reactions. Microspheres with the characteristic of biodegradability, biocompatibility, and minimal systemic toxicity are an important strategy for the delivery of short serum half-life, dose limiting side-effect drugs. As drug carriers, they serve as drug reservoirs allowing sustained drug release which improves therapeutic efficiency.50
Strategies Based on Intratumoral Drug Delivery System for Antitumor Therapy
Design Intratumoral Delivery System for Chemotherapy
Chemotherapy is one of the most classic antitumor treatments and widely applied in clinical practice. An intratumoral delivery system has been demonstrated to be effective in delivering chemotherapeutic agents to tumors. Merdan et al prepared curcumin-loaded nanoparticles with particle size about 169 ± 4.8 nm which significantly decreased the tumor size (from 66.6 to 34.9 mm2) after intratumoral injection compared with intravenous administration on glioblastoma. It was demonstrated that the intratumoral administration of nanoparticles with chemotherapeutics was a preferred method in brain tumors by offering target drug accumulation at the tumor site. Furthermore, drug carriers could be functionalized by surface modification to enhance target activity. Farokhzad et al51 reported docetaxel-PLGA-b-PEG nanoparticles conjugated with A10 2’-fluoropyrimidine RNA aptamers which recognized prostate-specific membrane antigen (PSMA). PSMA was usually expressed on the surface of prostate cancer cells which enabled increasing cell absorption leading to significantly enhanced cellular toxicity compared with nontargeted nanoparticles. Consistently, complete tumor reduction in five of seven and 100% of animals surviving for 109-days were shown in targeted nanoparticles, whereas complete tumor reduction in two of seven and 57% of survivability were shown for nontargeted nanoparticles.
Traditional chemotherapy against malignant tumors was usually accompanied with metastasis. Therefore, it is meaningful to enhance antitumor and anti-metastasis activity simultaneously. Yang et al52 explored an intratumoral injectable phospholipids-based phase separation gel encapsulated with 5-fluotouracil and magnesium oxide (5FU + MgO-PPSG). It exhibited sustained release 5-fluotouracil to achieve anti-tumor efficacy and alkaline substance neutralizing tumor acidic microenvironment as well as magnesium oxide to reach anti-meta stasis activity. Systemic toxicity is another important consideration factor for chemotherapy. DOX-loaded polymer-lipid hybrid nanoparticles were prepared for intratumoral delivery.53 It was demonstrated that the unwanted normal tissue toxicity was very low for intratumoral treatment. In conclusion, intratumoral delivery systems have great potential to improve chemotherapy efficacy.
Design Intratumoral Delivery System to Enhance Radiotherapy
Radiotherapy (RT) is the mainstay of cancer therapy. Unfortunately, it generally results in significant side-effects to normal tissues and organs due to the lack of selectivity and the following high dose.54 Therefore, research has been conducted to achieve safe and effective radiation therapy. An active area focused on developing radiosensitizers which made tumor cells easier to kill for radiotherapy.17 Gold nanoparticles could improve the biological effective dose of radiation.55 Targeted gold nanoparticles by attaching trastuzumab was prepared to target human epidermal growth factor receptor-2 (HER-2) positive tumors.9 This acted as a radiosensitizer and has demonstrated that systemically administration of targeted nanoparticles had a fast clearance from blood, while intratumoral injection led to high tumor retention and low systemic exposure. Intratumoral delivery of radio sensitizers represents an attractive delivery strategy.
To extend the short half-life and increase the antitumor effect of radiosensitizers, their delivery modes and cancer cell targeting features could be incorporated. Tang et al explored cytarabine hyaluronic acid-tyramine (Are-HA-Tyr) hydrogel to sensitize tumor cells to RT. The combination of RT and Are-HA-Tyr hydrogel increased survival and prolonged the tumor growth delay compared to either monotherapy in a Lewis lung cancer xenograft model. Furthermore, the combination therapy could significantly induce cell cycle arrest in the G2/M phase, decrease the proliferation index, and increase apoptosis. In addition, lower toxicity was also observed in intratumoral delivery of Ara-HA-Tyr hydrogel and RT group which was consistent with improved targeting and lower toxicity.56
The radiosensitizing effect of different doses was reasonably evaluated to suggest the maximally effective dose. Liu et al investigated the radiosensitizing effect of silver nanoparticles (AgNPs) with 10/20 μg/10 μL on glioma cells. Most importantly, the combination of AgNPs and radiation significantly enhanced survival time compared with irradiation alone (P<0.001). However, the average survival time and cure rate of glioma-bearing rats observed in 10 and 20 μg of AgNPs and 6-MV X–irradiation group were equal, indicating that 10 μg of AgNPs represents the maximum effective dose. The negligible accumulation in normal tissues around the tumor was quantitatively determined, which further indicated the clinical potential of AgNPs combined with radiotherapy.
Intratumoral administration of radioisotopes was investigated to overcome the lack of selectivity by external therapy which led to a high systemic radiation dose and damage of normal tissues. Furthermore, a carrier system for radioisotope was applied to remain long enough at the tumor site enhancing the treatment effect and reducing side-effects to normal tissues. Several studies have already reported a prolonged retention time in tumor site and low radioactive concentration in blood for 8 days.57,58 Therefore, radiotherapy using a carrier with radioisotopes when injected directly into the tumor could provide a promising modality for antitumor treatment.
Design Intratumoral Delivery System for Immunotherapy
Cancer immunotherapy is gaining increasing attention due to its ability to stimulate immune cells to recognize and attack tumor cells with high specificity, effectiveness, and persistence. Currently, its main design is to deliverantigen vaccines, immune checkpoint inhibitors, genetic vaccines, antibodies, or cytokines to treat tumors.59 Additionally, the route of administration affects behavior and therapeutic efficacy. Most current applications are based on systemic administration and corresponding immune activation.60 A growing amount of evidence suggests that intratumoral delivery of oncolytic circus or innate immune stimulators exhibits therapeutic potential against tumors.61,62 The in-situ vaccination was able to alter the tumor microenvironment to anti-tumor state.63 Furthermore, it was discovered that intratumoral injection of nanovaccine achieved a more significant antitumor effect than subcutaneous delivery with the same dose.64 Mohsen et al65 explored intratumoral injections of microcrystalline tyrosine decorated with cucumber mosaic virus-like particles to activate tumor antigen-specific T cells. This “immune-enhancer” turned immunologically cold tumors into hot tumors, and inhibited tumor growth locally and systemically.
Cytokines as the important immune-therapeutics fail to achieve adequate concentrations in tumors via systemic administration due to dose-limiting toxicity.66 However, intratumoral delivery may improve the therapeutic effect, because it can deliver cytokines to tumors as immunostimulants. Liu et al67 used novel lipid nanoparticles for efficient intratumoral delivery of cytokine mRNAs. Dual IL-12 and IL-27 delivery showed the most potent inhibition of tumor growth due to enhanced influence of immune effectors on the tumor vascular system and activating the secretion of downstream anti-tumor signaling molecules by effector cells. In addition, sustained tumor inhibition after treatment stops might suggest that immune memory responses had been established among the treated mice. Severe adverse side-effects for cytokine-based cancer therapy were not induced, which was speculatively attributed to the intratumoral injection approach with low systemic release of cytokine and rapid cytokine utilized by nearby immune cells. Therefore, the intratumoral delivery of appropriate cytokines loaded by nanoparticles might be an appropriate modality for current immunotherapy.
Furthermore, the intratumoral delivery system could be explored as the administration of immunotherapeutic agents, with encouraging clinical data to improve their degradation and specificity, such as synthetic non-methylated cytosine-phosphate-guanine (CpG).68 CpG has been extensively studied for its efficacy in tumor prevention and regression.69 It was susceptible to nuclease-mediated degradation and lacked specificity to target tumor cells after systemic administration. Zhang et al70 designed 3-aminopropyltriethoxysilane (APTES)-modified Fe3O4 nanoparticles loaded with CpG (FeNP/CpG), and the particle size was approximately 50 nm. Compared with free CpG, FeNP/CpG showed improved cellular uptake of CpG in vitro in bone marrow-derived dendritic cells and enhanced antitumor efficacy through stimulating better humoral as well as cellular immune responses in vivo.
Design Intratumoral Delivery System for Phototherapy
Phototherapy is one of the most promising cancer therapy methods, as it has many advantages such as negligible drug resistance, noninvasive features, high spatial selectivity, low side-effects, and manual control through light radiation.71,72 It mainly includes photodynamic therapy (PDT) and photothermal therapy (PTT). PDT is a cancer treatment strategy that uses light and a photosensitizer (PS) to produce cytotoxic singlet oxygen (1O2) as well as reactive oxygen species (ROS) to kill surrounding cancer cells.73 PTT is based on a photo absorbing agent, which converts absorbed light energy into heat to destroy cancer cells.74,75 However, limited success has been achieved in vivo because of the major uptake by the reticuloendothelial system after systemic injection, especially the liver, kidney, and spleen.76 Therefore, high tumor distribution or targeting may be difficult to realize. Currently, a lot of research has focused on intratumoral injection, which could be able to increase accumulation of drugs for PDT and PTT.24,77 This may be a potential method of drug administration for phototherapy.
Due to the light absorption and scattering by tissues, the limited penetration depth of most traditional photosensitizers results in poor therapeutic efficacy on large or internal tumors. Therefore, the promising carrier of photosensitizer should enhance penetration depth such as upconversion nanoparticles (UCNPs). Due to the ideal choice of near-infrared light as optical tissue penetration for phototherapy, UCNPs could convert NIR light to visible photons for activating a photosensitizer to generate ROS.78 Moreover, environmentally controlled PDT could be realized through pH-responsive UCNPs, which are negatively charged in alkaline and neutral surroundings and switched to positively charged under slightly acidic environments. The smart feature facilitates their interaction with cell membranes to improve cellular uptake. Wang et al obtained charge-reversible UCNPs through coating with dimethyl maleic acid groups and polyethylene glycol chains, which remarkably improved intracellular uptake and increased tumor retention. In addition, to prevent local pain and postoperative complications caused by frequent intratumoral injections, hydrogels provided a novel means of PDT strategies to promote multiple rounds of therapy after a single injection.79
In PTT, efficient delivery of a photo absorbing agent to the whole tumor is crucial for minimizing tumor recurrence by achieving a cytotoxic temperature anywhere within the tumor.80 Cell-mediated delivery systems were widely applied because they could cross the almost impermeable biological barriers.81,82 Li et al83 explored bovine serum albumin (BSA)-coated Au nanorods (7 nm)-laden-macrophages. They showed significant improvement in photothermal conversion nearly anywhere in the tumor. After intratumoral injection, this resulted in minimized tumor recurrence rates compared with non-macrophages laden preparations in vivo. Hence, it is highly desirable to enhance tumor coverage in PTT. Besides, multi-modal imaging-guided PTT has attracted increasing attention recently as it can offer comprehensive information for intensive therapy. Sun et al84 successfully prepared melanin-manganese nanoparticles for magnetic resonance/photoacoustic dual-modal imaging guided PTT. In vivo results revealed that nanoparticles after intratumoral injection began to diffuse and spread throughout the entire tumor sites at 3 hours, indicating the optimal treatment time. Melanin-manganese nanoparticles for PTT in vivo showed efficient tumor ablation, no recurrence, and negligible side-effects.
Design Intratumoral Delivery System for Magnetic Fluid Hyperthermia
Magnetic fluid hyperthermia (MFH) is the process of introducing magnetic fluid into the tumor area through a certain method and placing it in an alternating magnetic field. Magnetic particles are heated up under the action of the alternating magnetic field by relaxing the rearrangement of the magnetic vector in the magnetic field, thereby achieving the temperature required to kill tumor cells. However, it has high targeting and specificity due to the non-distribution of magnetic fluids and unremarkable heating in normal tissues around tumors.85 It renders MFH as an attractive new approach for deep tumors hyperthermia. Therefore, intratumoral delivery of magnetic fluids could realize tumor deposition, allowing for a series of MFH therapies without repeated administration and tumor inhibition.
Johannsen et al86 treated an orthotopic prostate tumor model of rat with two MFH therapies following a single intratumoral injection of magnetic fluid. The mean iron content in the prostate of rats was 82.5% of the injected dose, while the iron content in the liver, spleen, and lungs was 5.3%, 0.5%, and 1.0%, respectively. Furthermore, compared to the control group, the stable tumor deposition resulted in 44–51% tumor inhibition. Kettering et al87 demonstrated that magnetic nanoparticles accumulation was not affected by magnetic heating and remained within the intratumoral injection side for 7 days, thereby not affecting healthy tissue. Chauhan et al88 explored chitosan-coated Fe3O4 nanoparticles to evaluate the antitumor response in an ectopic tumor model of Ce glioblastoma for MHT. The magnetic nanoparticles showed high heating efficiency, rapidly inhibiting 69.4% of tumors within 8 days, and completely inhibiting within 32 days after intratumoral injection on the first and seventh day. What is more, no recurrence was observed over a 5-month follow-up. Studies were also conducted on magnetic nanoparticles mediated hyperthermia in animal models of melanoma89,90 and breast tumors.91 Furthermore, the first clinical experiences with hyperthermia using magnetic nanoparticles in prostate carcinoma had been published.92
Design Intratumoral Delivery System for Multimodal Therapy
Development of combination therapy plays a crucial role in enhancing antitumor activity and inhibiting tumor recurrence. Multifunctional formulations have been widely studied for cancer therapy, such as nanomaterials. Nanocarriers have been prepared as chemo-photo-thermal therapy, chemo-immunotherapy, chemo-radiotherapy, radio-immunotherapy, thermo-radiotherapy agents, and even two or more synergistic therapies. Hashimoto et al93 combined DOX-loaded functional dendrimers and gold nanorods to achieve chemo-photothermal therapy. Under laser irradiation, the formulation showed almost complete tumor growth inhibition, especially through intratumoral injection. Kossatz et al94 explored superparamagnetic iron oxide nanoparticles functionalized with DOX and Nucant multivalent pseudo peptide (MF66-N6LDOX) for magnetic hyperthermia and chemotherapy. MF66-N6LDOX showed increased nanoparticle uptake in tumor cells and greater cytotoxicity for breast cancer cells than both individual free ligands. Furthermore, it exhibited significant tumor growth suppression after intratumoral injection in vivo, which took advantage of the direct deposition at the target site for magnetic nanoparticles. Wang et al95 developed an ultrasound based drug delivery strategy for the treatment of prostate cancer which had poor response to routine immunotherapy because of the tumor immunosuppressive microenvironment. To generate effective antitumor immunity, exosomes were encapsulated with immune adjuvant R848 and sonosensitizer Chlorin e6 and accumulated at the tumor site through intratumoral injection. Ultrasonic irradiation and engineered exosomes synergistically improved R848-mediated maturation of dendritic cells and reprocessed the phenotype of macrophages from immunosuppressive M2-like to antitumor M1-like, further reverting the immunosuppressive microenvironment. Therefore, the ultrasound-based immunotherapy showed obvious tumor growth and stable body weights of mice. More types of intratumoral delivery systems for multimodal cancer therapy are summarized in Table 1.
 |
Table 1 Summary of Different Types of Intratumoral Drug Delivery Based Multimodal Therapy |
Summary and Perspective
To realize further improvements in quality-of-life and survival, numerous efforts have been made in the fight against cancer, starting from understanding the mechanism of cancer to patient treatment. Various treatment strategies for cancer treatment are being developed. Intratumoral injection may provide a meaningful response due to directly delivering therapeutic substances into tumor tissues.1 This still needs to go through a series of complex screenings to become a suitable candidate anticancer drug, such as cytotoxic screening through cell lines or animal models to evaluate the effect of drugs on cell viability, cell proliferation, and analysis applied to evaluate the apoptosis inducing effect of drugs, the cell cycle analysis, animal models to evaluate the effects of tumor growth and migration in vivo, pharmacokinetic and toxicological evaluations, and clinical trials. The safety of intratumoral injection has been proved by numerous researchers, and this technique has been used in clinics. Intratumoral injection of E1B gene-deleted adenovirus combined with cisplatin-based chemotherapy has been conducted for Phase III randomized clinical trials.106–109 Furthermore, if the tumor is not visible on the skin, drugs can be administered by image or endoscope guided intervention. Endobronchial ultrasound-guided intratumoral administration of chemotherapeutic agents was clinically applied in lung cancer treatment.110,111
Intratumoral administration is a preferred method integrated with other modalities to minimize systemic side-effects such as the targeted delivery of cytokines IL-2 and IFN for cancer immunotherapy. Delivery of oncolytic virus through intratumoral injection has been clinically approved such as talimogene laherparepvec for intratumoral injection of melanoma.112 Furthermore, intratumoral delivery systems have been extensively studied based on different formulations, involving hydrogels, nanoparticles, microneedles, microspheres, etc. These drug carriers enhance tumor targeting and retention, thereby improving treatment efficacy and reducing distribution of other organs to cope with mild systemic adverse reactions. Furthermore, physical methods were also applied to enhance the effect of intratumoral drug delivery such as cryotherapy and electroporation. Cryotherapy induced tissue necrosis at low temperature to achieve tumor lysis which was demonstrated to further stimulate local immune response. Electroporation was high voltage electrical pulses transmitted to the tumor through electrodes causing membrane rupture which could promote the entry of anticancer drugs into cancer cells.113
Despite its enormous potential, there are still challenges for intratumoral delivery systems in successful tumor therapy. For example, thermo-sensitive hydrogel has been widely investigated to overcome drug squeezing out of pinprick by high pressure difference.4 Furthermore, the high pressure of dense tumor tissues may result in poor penetration of the formulation. Multipoint injection and combined local treatment with active substances were studied in an effort to improve uniform drug distribution within tumors. Losartan potassium as an anti-fibrotic agent was able to improve intratumoral penetration by reducing collagens in tumors.114,115 Combined drug release delivery systems, such as microsphere-based hydrogel and nanoparticles contained hydrogel, have been evaluated for improved drug delivery and drug loading property as well as controlled drug release to prolong antitumor efficiency.116 Novel preparations in-depth research for optimization of intratumoral therapy must be conducted to further select injection form, location, number, and amount, and accelerate successful translation for clinical use. Especially, the efficacy of intratumoral administration might be operator-dependent because of the quality of in-situ delivery. A serious concern for clinically applied intratumoral therapies was the technical aspect, especially when considering multi-center phase III registration trials.117 Therefore, it is essential to develop mate intratumoral puncture techniques.
In summary, the combination therapy with enhanced antitumor efficiency and reduced systemic adverse reactions represents a high-potential cancer treatment strategy, of which an intratumoral delivery system will play an integral role.
Disclosure
The authors report no conflicts of interest in this work.
References
1. chu X-Y, Huang W, Wang Y-L. Improving antitumor outcomes for palliative intratumoral injection therapy through lecithin–chitosan nanoparticles loading paclitaxel–cholesterol complex. Int J Nanomed. 2019;14:689–705.
2. Wang C, Ye Y, Hochu GM, Sadeghifar H, Gu Z. Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of Anti-PD1 Antibody. Nano Lett. 2016;16:2334–2340.
3. Chattopadhyay N, Cai Z, Kwon YL, Lechtman E, Pignol JP, Reilly RM. Molecularly targeted gold nanoparticles enhance the radiation response of breast cancer cells and tumor xenografts to X-radiation. Breast Cancer Res Treat. 2013;137(1):81–91. doi:10.1007/s10549-012-2338-4
4. Al-Abd AM, Hong KY, Song SC, Kuh HJ. Pharmacokinetics of doxorubicin after intratumoral injection using a thermosensitive hydrogel in tumor-bearing mice. J Control Release. 2010;142(1):101–107. doi:10.1016/j.jconrel.2009.10.003
5. Ho-Chun S, Kun N, Park K-H. Intratumoral administration of rhenium-188-labeled pullulan acetate nanoparticles (PAN) in mice bearing CT-26 cancer cells for suppression of tumor growth. J Microbiol Biotechnol. 2006;16(10):1491–1498.
6. Brachi G, Ruiz-Ramírez J, Dogra P. Intratumoral injection of hydrogel-embedded nanoparticles enhances retention in glioblastoma. Nanoscale. 2020;12(46):23838–23850. doi:10.1039/D0NR05053A
7. Boone CE, Wang C, Lopez-Ramirez MA. Active Microneedle Administration of Plant Virus Nanoparticles for Cancer In Situ Vaccination Improves Immunotherapeutic Efficacy. ACS Appl. Nano Mater. 2023;3(8):8037–8051.
8. Fan L, Duan M, Sun X. Injectable Liquid Metal- and Methotrexate-Loaded Microsphere for Cancer Chemophotothermal Synergistic Therapy. ACS Appl. Bio Mater. 2020;3(6):3553–3559. doi:10.1021/acsabm.0c00171
9. Chattopadhyay N, Fonge H, Cai Z, et al. Role of antibody-mediated tumor targeting and route of administration in nanoparticle tumor accumulation in vivo. Mol Pharm. 2012;9(8):2168–2179.
10. Paszek MJ, Zahir N, Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi:10.1016/j.ccr.2005.08.010
11. Tredan O, Galmarini CM, Patel K, Tannock IF, Natl J. Drug resistance and the solid tumor microenvironment. Cancer Inst. 2007;99(19):1441–1454. doi:10.1093/jnci/djm135
12. Shido Y, Nishida Y, Suzuki Y, Kobayashi T, Ishiguro N. Targeted hyperthermia using magnetite cationic liposomes and an alternating magnetic field in a mouse osteosarcoma model. J Bone Joint Surg Br. 2010;92(4):580–585. doi:10.1302/0301-620X.92B4.22814
13. Xu X, Huang Z, Huang Z, et al. Injectable, NIR/pH-Responsive Nanocomposite Hydrogel as Long-Acting Implant for Chemophotothermal Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces. 2017;9(24):20361–20375. doi:10.1021/acsami.7b02307
14. Qu Y, Chu BY, Peng JR, et al. A biodegradable thermo-responsive hybrid hydrogel: therapeutic applications in preventing the post-operative recurrence of breast cancer. NPG Asia Materials. 2015;7(8):e207. doi:10.1038/am.2015.83
15. Zhu Y, Yang Z, Dong Z, et al. CaCO3‑Assisted Preparation of pH‑Responsive Immune‑Modulating Nanoparticles for Augmented Chemo‑Immunotherapy. Nano-Micro Lett. 2021;13(1):29. doi:10.1007/s40820-020-00549-4
16. Jianbo G, Xue L, Hongdan Y, et al. The Anti-Melanoma Efficiency of the Intratumoral Injection of Cucurbitacin-Loaded Sustained-Release Carriers: a PLGA Particle. System. 2013;102:2550–2563.
17. Sung Duk J, Lee J, Kyung Joo M, et al. PEG−PLA-Coated and Uncoated Radio-Luminescent CaWO4 Micro- and Nanoparticles for Concomitant Radiation and UV-A/RadioEnhancement Cancer Treatments. ACS Biomater Sci Eng. 2018;4:1445–1462. doi:10.1021/acsbiomaterials.8b00119
18. Yu L, Ding J. Injectable hydrogels as unique biomedical materials. Chem Soc Rev. 2008;37(8):1473–1481. doi:10.1039/b713009k
19. Mathew AP, Uthaman S, Cho KH, Cho CS, Park IK. Injectable hydrogels for delivering biotherapeutic molecules. Int JBiol Macromol. 2018;110:17–29. doi:10.1016/j.ijbiomac.2017.11.113
20. Ru Shin G, Eun Kim H, Kim JH, et al. Advances in Injectable In Situ-Forming Hydrogels for Intratumoral Treatment. Pharmaceutics. 2021;13(11):1953. doi:10.3390/pharmaceutics13111953
21. Zhang H, Zhu X, Yandan J, et al. Near-Infrared-Triggered In-situ Hybrid Hydrogel System for Synergistic Cancer Therapy. J Mater Chem B. 2015;3(30):6310–6326. doi:10.1039/C5TB00904A
22. Wan J, Geng S, Zhao H, et al. Doxorubicin-induced co-assembling nanomedicines with temperature-sensitive acidic polymer and their in-situ-forming hydrogels for intratumoral administration. J Control Release. 2016;235:328–336. doi:10.1016/j.jconrel.2016.06.009
23. Raza F, Zhu Y, Chen L, et al. Paclitaxel-loaded pH Responsive Hydrogel Based on Self-assembly Peptides for Tumor Targeting. Biomater Sci. 2019;7(5):2023–2036. doi:10.1039/C9BM00139E
24. Sun X, Zhuang B, Zhang M, et al. Intratumorally Injected Photothermal Agent-Loaded Photodynamic Nanocarriers for Ablation of Orthotopic Melanoma and Breast Cancer. ACS Biomater Sci Eng. 2019;5(2):724–739. doi:10.1021/acsbiomaterials.8b01111
25. Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2004;56:1649–1659. doi:10.1016/j.addr.2004.02.014
26. Danhier F. To exploit the tumor microenvironment: since the EPR effect fails in the clinic, what is the future of nanomedicine? J Control Release. 2016;244(Pt A):108–121. doi:10.1016/j.jconrel.2016.11.015
27. Dawidczyk CM, Kim C, Park JH, et al. State-of-the-art in design rules for drug delivery platforms: lessons learned from FDA-approved nanomedicines. J Control Release. 2014;187:133–144. doi:10.1016/j.jconrel.2014.05.036
28. Shen L, Zhang Z, Wang T, et al. Reversed lipid-based nanoparticles dispersed in oil for malignant tumor treatment via intratumoral injection. Drug Deliv. 2017;24(1):857–866. doi:10.1080/10717544.2017.1330373
29. Lee J, Phyu Hlaing S, Hasan N, et al. Tumor-Penetrable Nitric Oxide-Releasing Nanoparticles Potentiate Local Antimelanoma Therapy. ACS Appl Mater Interfaces. 2021;13(26):30383–30396. doi:10.1021/acsami.1c07407
30. Pirollo KF, Chang EH. Does a targeting ligand influence nanoparticle tumor localization or uptake? Trends Biotechnol. 2008;26:552–558. doi:10.1016/j.tibtech.2008.06.007
31. Huang X, Peng X, Wang Y, et al. A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano. 2010;4(10):5887–5896. doi:10.1021/nn102055s
32. Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci USA. 2007;104(39):15549–15554. doi:10.1073/pnas.0707461104
33. Mahmoudi M, Bertrand N, Zope H, et al. Emerging understanding of the protein Corona at the nano-bio interfaces. Nano Today. 2016;11(6):817–832. doi:10.1016/j.nantod.2016.10.005
34. Chen FF, Wang GK, Griffin JI, et al. Complement proteins bind to nanoparticle protein Corona and undergo dynamic exchange in vivo. Nat Nanotechnol. 2017;12(4):387–393. doi:10.1038/nnano.2016.269
35. Hayashi Y, Takamiya M, Jensen PB, et al. Differential Nanoparticle Sequestration by Macrophages and Scavenger Endothelial Cells Visualized in Vivo in Real-Time and at Ultrastructural Resolution. ACS Nano. 2020;14(2):1665–1681. doi:10.1021/acsnano.9b07233
36. Hansen AE, Petersen AL, Henriksen JR, et al. Positron emission tomography based elucidation of the enhanced permeability and retention effect in dogs with cancer using copper-64 liposomes. ACS Nano. 2015;9(7):6985–6995. doi:10.1021/acsnano.5b01324
37. Hou A, Quan G, Yang B, et al. Rational Design of Rapidly Separating Dissolving Microneedles for Precise Drug Delivery by Balancing the Mechanical Performance and Disintegration Rate. Adv Healthc Mater. 2019;8(21):e1900898. doi:10.1002/adhm.201900898
38. Biyuan W, Jintao F, Zhou Y, et al. Tailored core‒shell dual metaleorganic frameworks as a versatile nanomotor for effective synergistic antitumor therapy. Acta Pharm Sin B. 2020;10(11):2198–2211. doi:10.1016/j.apsb.2020.07.025
39. Qin W, Quan G, Sun Y, et al. Dissolving Microneedles with Spatiotemporally controlled pulsatile release Nanosystem for Synergistic Chemo-photothermal Therapy of Melanoma. Theranostics. 2020;10(18):8179–8196. doi:10.7150/thno.44194
40. Boone CE, Wang C, Angel Lopez-Ramirez M, et al. Active Microneedle Administration of Plant Virus Nanoparticles for Cancer In Situ Vaccination Improves Immunotherapeutic Efficacy. ACS Appl Nano Mater. 2020;3(8):8037–8051. doi:10.1021/acsanm.0c01506
41. Tian L, Gao J, Yang Z, Huang G. Tamibarotene-loaded PLGA microspheres for intratumoral injection administration: preparation and evaluation. AAPS Pharm Sci Tech. 2018;19(1):275–283. doi:10.1208/s12249-017-0827-9
42. Doty AC, Weinstein DG, Hirota K. Mechanisms of in vivo release of triamcinolone acetonide from PLGA microspheres. J Control Release. 2017;256:19–25. doi:10.1016/j.jconrel.2017.03.031
43. Doty AC, Zhang Y, Weinstein DG. Mechanistic analysis of triamcinolone acetonide release from PLGA microspheres as a function of varying in vitro release conditions. Eur J Pharm Biopharm. 2017;113:24–33. doi:10.1016/j.ejpb.2016.11.008
44. Guo WJ, Quan P, Fang L, Cun DM, Yang MS. Sustained release donepezil loaded PLGA microspheres for injection: preparation, in vitro and in vivo study. Asian J Pharm Sci. 2015;10(5):405–414. doi:10.1016/j.ajps.2015.06.001
45. Benny O, Kim SK, Gvili K. In vivo fate and therapeutic efcacy of PF-4/CTF microspheres in an orthotopic human glioblastoma model. FASEB J. 2008;22(2):488–499. doi:10.1096/fj.07-8801com
46. Guoli N, Yang G, Yang H, et al. Uniformly sized hollow microspheres loaded with polydopamine nanoparticles and doxorubicin for local chemo-photothermal combination therapy. Chem Eng J. 2020;379:122317. doi:10.1016/j.cej.2019.122317
47. Zhou YL, Wang HT, Du QG. Effect of initiation site location on morphology of polymer microspheres via pickering polymerization. J Polym Sci. 2012;50:3537–3545. doi:10.1002/pola.26138
48. Wang SY, Shi XD, Gan ZH, Wang F. Preparation of PLGA Microspheres with Different Porous Morphologies Chin. J Polym Sci. 2015;33(1):128–136.
49. Zhang Z, Wang X, Binbin L, et al. Paclitaxel-loaded PLGA microspheres with a novel morphology to facilitate drug delivery and antitumor efficiency. RSC Adv. 2018;8(6):3274–3285. doi:10.1039/C7RA12683B
50. Zou Y, Song Y, Yang WJ, et al. Galactose-installed photo-crosslinked pH-sensitive degradable micelles for active targeting chemotherapy of hepatocellular carcinoma in mice. J Control Release. 2014;193:154–161. doi:10.1016/j.jconrel.2014.05.016
51. Omid C, Jianjun C, Benjamin AT, et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A. 2006;103(16):6315–6320. doi:10.1073/pnas.0601755103
52. Yang L, Song X, Gong T, et al. Enhanced anti-tumor and anti-metastasis efficacy against breast cancer with an intratumoral injectable phospholipids-based phase separation gel co-loaded with 5-fluotouracil and magnesium oxide by neutralizing acidic microenvironment. Int J Pharm. 2018;547(1–2):181–189. doi:10.1016/j.ijpharm.2018.05.072
53. Lun Wong H, Mike Rauth A, Bendayan R, Wu XY. In vivo evaluation of a new polymer-lipid hybrid nanoparticle (PLN) formulation of doxorubicin in a murine solid tumor model. Eur J Pharm Biopharm. 2007;65(3):300–308. doi:10.1016/j.ejpb.2006.10.022
54. Kyoo Seong S, Man Ryu J, Hyuk Shin D, et al. Biodistribution and excretion of radioactivity after the administration of 166 Ho-chitosan complex (DW-166HC) into the prostate of rat. Eur J Nucl Med Mol Imaging. 2005;32(8):910–917. doi:10.1007/s00259-005-1792-1
55. Geng F, Song K, Xing JZ, et al. Thio-glucose bound gold nanoparticles enhance radio-cytotoxic targeting of ovarian cancer. Nanotechnology. 2011;22(28):285101. doi:10.1088/0957-4484/22/28/285101
56. Yoon HY, Koo H, Choi KY, et al. Tumor-targeting hyaluronic acid nanoparticles for photodynamic imaging and therapy. Biomaterials. 2012;3315:3980–3989. doi:10.1016/j.biomaterials.2012.02.016
57. Nakajo M, Kobayashi H, Shimabukuro K, et al. Biodistribution and in vivo kinetics of iodine-131 lipiodol infused via the hepatic artery of patients with hepatic cancer. J Nucl Med. 1988;29(6):1066–1077.
58. Suzuki YS, Momose Y, Higashi N, et al. Biodistribution and kinetics of holmium-166-chitosan complex (DW-166HC) in rats and mice. J Nucl Med. 1998;39(12):2161–2166.
59. Li D, Hu D, Xu H, et al. Progress and perspective of microneedle system for anti-cancer drug delivery. Biomaterials. 2021;264:120410. doi:10.1016/j.biomaterials.2020.120410
60. Marabelle A, Kohrt H, Caux C, et al. Intratumoral immunization: a new paradigm for cancer therapy. Clin Cancer Res. 2014;20(7):1747–1756. doi:10.1158/1078-0432.CCR-13-2116
61. Corrales L, Glickman LH, McWhirter SM, et al. Direct activation of sting in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 2015;11(7):1018–1030. doi:10.1016/j.celrep.2015.04.031
62. Macedo N, Miller DM, Haq R, et al. Clinical landscape of oncolytic virus research in 2020. J Immunother Cancer. 2020;8(2):e001486. doi:10.1136/jitc-2020-001486
63. Lee KL, Murray AA, Le DHT, et al. Combination of Plant Virus Nanoparticle-Based in Situ Vaccination with Chemotherapy Potentiates Antitumor Response. Nano Lett. 2017;17(7):4019–4028. doi:10.1021/acs.nanolett.7b00107
64. Jiang X, Wang J, Zheng X, et al. Intratumoral administration of STINGactivating nanovaccine enhances T cell immunotherapy. J Immunother Cancer. 2022;10(5):e003960. doi:10.1136/jitc-2021-003960
65. Mohsen MO, Heath M, Kramer MF, et al. In situ delivery of nanoparticles formulated with micron-sized crystals protects from murine melanoma. J Immunother Cancer. 2022;10(9):e004643. doi:10.1136/jitc-2022-004643
66. Liu JQ, Zhu J, Hu A. Is AAV-delivered IL-27 a potential immunotherapeutic for cancer? Am J Cancer Res. 2020;10(11):3565–3574.
67. Liu J-Q, Zhang C, Zhang X, et al. Intratumoral delivery of IL-12 and IL-27 mRNA using lipid nanoparticles for cancer immunotherapy. J Control Release. 2022;345:306–313. doi:10.1016/j.jconrel.2022.03.021
68. Link BK, Ballas ZK, Weisdorf D, et al. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J Immunother. 2006;29(5):558–568. doi:10.1097/01.cji.0000211304.60126.8f
69. Maurer T. Immunostimulatory CpG-DNA and PSApeptide vaccination elicits profound cytotoxic T cell responses. Urol Oncol. 2013;31(7):1395–1401. doi:10.1016/j.urolonc.2011.09.002
70. Zhang X, Fengbo W, Men K, et al. Modified Fe3O4 Magnetic Nanoparticle Delivery of CpG Inhibits Tumor Growth and Spontaneous Pulmonary Metastases to Enhance Immunotherapy. Nanoscale Res Lett. 2018;13(1):240. doi:10.1186/s11671-018-2661-8
71. Celli JP, Spring BQ, Rizvi I, et al. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev. 2010;110(5):2795–2838. doi:10.1021/cr900300p
72. Chen Q, Wen J, Li H, Xu Y, Liu F, Sun S. Recent advances in different modal imaging-guided photothermal therapy. Biomaterials. 2016;106:144–166. doi:10.1016/j.biomaterials.2016.08.022
73. Robertson CA, Hawkins Evans D, Abrahamse H. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J Photochem Photobiol B. 2009;96(1):1–8. doi:10.1016/j.jphotobiol.2009.04.001
74. Dickerson EB, Dreaden EC, Huang X, et al. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008;269(1):57–66. doi:10.1016/j.canlet.2008.04.026
75. Niidome T, Yamagata M, Okamoto Y, et al. PEG-modified gold nanorods with a stealth character for in vivo applications. J Control Release. 2006;114(3):343–347. doi:10.1016/j.jconrel.2006.06.017
76. De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJ, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29(12):1912–1919. doi:10.1016/j.biomaterials.2007.12.037
77. Green HN, Crockett SD, Martyshkin DV, et al. A histological evaluation and in vivo assessment of intratumoral near infrared photothermal nanotherapy-induced tumor regression. Int J Nanomed. 2014;9:5093–5102. doi:10.2147/IJN.S60648
78. Wang C, Tao H, Cheng L, Liu Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials. 2011;32(26):6145–6154. doi:10.1016/j.biomaterials.2011.05.007
79. Zhue D, Zhengb Z, Luo G, et al. Single injection and multiple treatments: an injectable nanozyme hydrogel as AIEgen reservoir and release controller for efficient tumor therapy. Nano Today. 2021;37:101091. doi:10.1016/j.nantod.2021.101091
80. Von Maltzahn G, Park JH, Agrawal A, et al. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009;69(9):3892–3900. doi:10.1158/0008-5472.CAN-08-4242
81. Choi J, Kim HY, Ju EJ, et al. Use of macrophages to deliver therapeutic and imaging contrast agents to tumors. Biomaterials. 2012;33(16):4195–4203. doi:10.1016/j.biomaterials.2012.02.022
82. Mooney R, Roma L, Zhao D, et al. Neural stem cell-mediated delivery of gold nanorods improves photothermal therapy. ACS Nano. 2014;8(12):12450–12460. doi:10.1021/nn505147w
83. Zhibin L, Huang H, Tang S, et al. Small Gold Nanorods Laden Macrophages for Enhanced Tumor Coverage in Photothermal Therapy. Biomaterials. 2016;74:144–154. doi:10.1016/j.biomaterials.2015.09.038
84. Sun J, Wen X, Liping L, et al. Ultrasmall Endogenous Biopolymer Nanoparticles for Magnetic Resonance /Photoacoustic Dual-Modal Imaging-Guided Photothermal Therapy. Nanoscale. 2018;10(22):10584–10595. doi:10.1039/C8NR01215F
85. Kobayashi T. Cancer hyperthermia using magnetic nanoparticles. Biotechnol J. 2011;6(11):1342–1347. doi:10.1002/biot.201100045
86. Johannsen M, Thiesen B, Jordan A, et al. Magnetic Fluid Hyperthermia (MFH) Reduces Prostate Cancer Growth in the Orthotopic Dunning R3327 Rat Model. Prostate. 2005;64(3):283–292. doi:10.1002/pros.20213
87. Kettering M, Richter H, Wiekhorst F, et al. Minimal-invasive magnetic heating of tumors does not alter intra-tumoral nanoparticle accumulation, allowing for repeated therapy sessions: an in vivo study in mice. Nanotechnology. 2011;22(50):505102. doi:10.1088/0957-4484/22/50/505102
88. Chauhan A, Midha S, Kumar R, et al. Rapid tumor inhibition via magnetic hyperthermia regulated by caspase 3 with time-dependent clearance of iron oxide nanoparticles. Biomater Sci. 2021;9(8):2972–2990. doi:10.1039/D0BM01705A
89. Ito A, Tanaka K, Kondo K, et al. Tumor regression by combined immunotherapy and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Sci. 2003;94(3):308–313. doi:10.1111/j.1349-7006.2003.tb01438.x
90. Tanaka K, Ito A, Kobayashi T, et al. Intratumoral injection of immature dendritic cells enhances antitumor effect of hyperthermia using magnetic nanoparticles. Int, J, Cancer. 2005;116(4):624–633. doi:10.1002/ijc.21061
91. Hilger I, Hiergeist R, Hergt R, et al. Thermal ablation of tumors using magnetic nanoparticles: an in vivo feasibility study. Invest Radiol. 2002;37(10):580–586. doi:10.1097/00004424-200210000-00008
92. Johannsen M, Gneveckow U, Eckelt L, et al. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: presentation of a new interstitial technique. Int J Hyperther. 2005;21(7):637–647. doi:10.1080/02656730500158360
93. Hashimoto T, Yuba E, Harada A, Kono K. Preparation of photothermal-chemotherapy nanohybrids by complexation of gold nanorods with polyamidoamine dendrimers having poly(ethylene glycol) and hydrophobic chains. J Mater Chem B. 2020;8(14):2826–2833. doi:10.1039/C9TB02163A
94. Kossatz S, Grandke J, Couleaud P, et al. Efficient treatment of breast cancer xenografts with multifunctionalized iron oxide nanoparticles combining magnetic hyperthermia and anti-cancer drug delivery. Breast Cancer Res. 2015;17(1):66. doi:10.1186/s13058-015-0576-1
95. Wang D, Wan Z, Yang Q, et al. Sonodynamical reversion of immunosuppressive microenvironment in prostate cancer via engineered exosomes. Drug Deliv. 2022;29(1):702–713. doi:10.1080/10717544.2022.2044937
96. Tingting L, Zhang M, Wang J, et al. Thermosensitive Hydrogel Co-loaded with Gold Nanoparticles and Doxorubicin for Effective Chemoradiotherapy. AAPS J. 2016;18(1):146–155. doi:10.1208/s12248-015-9828-3
97. Wang Z, Liu B, Jingyao T, et al. PLGA Nanoparticles Loaded with Sorafenib Combined with Thermosensitive Hydrogel System and Microwave Hyperthermia for Multiple Sensitized Radiotherapy. Pharmaceutics. 2023;15(2):487. doi:10.3390/pharmaceutics15020487
98. Zeng W, Liu C, Wang S, Wang Z, Huang Q. SnFe2O4 Nanozyme Based TME Improvement System for Anti-Cancer Combination Thermoradiotherapy. Front Oncol. 2021;11:768829. doi:10.3389/fonc.2021.768829
99. Gogoi M, Jaiswal MK, Dev Sarma H, Bahadur D, Banerjee R. Biocompatibility and therapeutic evaluation of magnetic liposomes designed for self-controlled cancer hyperthermia and chemotherapy. Integr Biol (Camb). 2017;9(6):555–565. doi:10.1039/C6IB00234J
100. Manfred J, Burghard T, Uwe G, et al. Thermotherapy Using Magnetic Nanoparticles Combined With External Radiation in an Orthotopic Rat Model of Prostate Cancer. Prostate. 2006;66(1):97–104. doi:10.1002/pros.20324
101. Janic B, Brown SL, Neff R, et al. Gold Nanoparticle (AuNP) as a Therapeutic Enhancer for Radio – and Immunotherapy Therapy Combination in Triple Negative Breast Cancer. Int J Radiat Oncol Biol Phys. 2022;114(3):e522–e522. doi:10.1016/j.ijrobp.2022.07.2114
102. Banstola A, Pandit M, Duwa R, et al. Reactive oxygen species-responsive dual-targeted nanosystem promoted immunogenic cell death against breast cancer. Bioeng Transl Med. 2022;8(5):e10379. doi:10.1002/btm2.10379
103. Gorgizadeh M, Behzadpour N, Salehi F, et al. A MnFe2O4/C nanocomposite as a novel theranostic agent in MRI, sonodynamic therapy and photothermal therapy of a melanoma cancer model. Journal of Alloys and Compounds. 2020;816.
104. Yata T, Takahashi Y, Tan M, et al. DNA nanotechnology-based composite-type gold nanoparticle-immunostimulatory DNA hydrogel for tumor photothermal immunotherapy. Biomaterials. 2017;146:136–145. doi:10.1016/j.biomaterials.2017.09.014
105. Yang Z, Tao D, Zhong W, et al. Perfluorocarbon loaded fluorinated covalent organic polymers with effective sonosensitization and tumor hypoxia relief enable synergistic sonodynamic-immunotherapy. Biomaterials. 2022;280:121250. doi:10.1016/j.biomaterials.2021.121250
106. Xia ZJ, Chang JH, Zhang L, et al. Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus. Chin J Cancer. 2004;23(12):1666e70.
107. Zhu HL, Xie P. The safety and short-term clinical observation of endoscopic intratumoral injection of recombinant human p53 adenovirus on advanced esophageal cancer. Pract J Clin Med. 2012;9(4):79e80.
108. Jenks N, Myers R, Greiner SM, et al. Safety studies on intrahepatic or intratumoral injection of oncolytic vesicular stomatitis virus expressing interferon-b in rodents and nonhuman primates. Hum Gene Ther. 2010;21:451e62. doi:10.1089/hum.2009.111
109. Mazzolini G, Alfaro C, Sangro B, et al. Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J Clin Oncol. 2005;23(5):999e1010. doi:10.1200/JCO.2005.00.463
110. Mehta HJ, Begnaud A, Penley AM, et al. Treatment of isolated mediastinal and hilar recurrence of lung cancer with bronchoscopic endobronchial ultrasound guided intratumoral injection of chemotherapy with cisplatin. Lung Cancer. 2015;90(3):542–547. doi:10.1016/j.lungcan.2015.10.009
111. Mehta HJ, Jantz MA. Endobronchial ultrasound-guided intratumoral injection of cisplatin for the treatment of isolated mediastinal recurrence of lung cancer. J Vis Exp. 2017;120:54855.
112. Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–2788. doi:10.1200/JCO.2014.58.3377
113. Kepp O, Marabelle A, Zitvogel L. Guido KroemerOncolysis without viruses - inducing systemic anticancer immune responses with local therapies. Nat Rev Clin Oncol. 2020;17(1):49–64. doi:10.1038/s41571-019-0272-7
114. Qutachi O, Wright EJ, Bray G, et al. Improved delivery of PLGA microparticles and microparticle-cell scaffolds in clinical needle gauges using modified viscosity formulations. Int J Pharm. 2018;546(1–2):272–278. doi:10.1016/j.ijpharm.2018.05.025
115. Nasiri R, Almaki JH, Idris A, et al. Targeted delivery of bromelain using dual mode nanoparticles: synthesis, physicochemical characterization, in vitro and in vivo evaluation. RSC Adv. 2017;7(64):40074–40094. doi:10.1039/C7RA06389J
116. Meiling Y, Zhang C, Tang Z, Tang X, Hui X. Intratumoral injection of gels containing losartan microspheres and (PLG-g-mPEG)-cisplatin nanoparticles improves drug penetration, retention and anti-tumor activity. Cancer Lett. 2019;442:396–408. doi:10.1016/j.canlet.2018.11.011
117. Melero I, Castanon E, Alvarez M, Champiat S, Marabelle A. Intratumoural administration and tumour tissue targeting of cancer immunotherapies. Nat Rev Clin Oncol. 2021;18(9):558–576. doi:10.1038/s41571-021-00507-y
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
