Back to Journals » Open Access Journal of Sports Medicine » Volume 15
Do Combined Oculomotor and Bimanual Coordination Exercises Instantly Stabilize Balance in Athletes?
Authors Matsuura Y , Sakairi Y, Sato H, Takiura K
Received 23 May 2024
Accepted for publication 9 July 2024
Published 17 July 2024 Volume 2024:15 Pages 77—89
DOI https://doi.org/10.2147/OAJSM.S472125
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Andreas Imhoff
Yuki Matsuura,1 Yosuke Sakairi,2 Haruki Sato,1 Koki Takiura3
1Cooperative Faculty of Education, Utsunomiya University, Utsunomiya, Japan; 2Faculty of Health and Sport Sciences, University of Tsukuba, Tsukuba, Japan; 3Institute for Social Innovation and Cooperation, Utsunomiya University, Utsunomiya, Japan
Correspondence: Yuki Matsuura, Cooperative Faculty of Education, Utsunomiya University, 350 Mine, Utsunomiya-City, Tochigi, 321-8505, Japan, Tel +81-28-649-5378, Email [email protected]
Purpose: This study examined the immediate effects of oculomotor and bimanual coordination exercises, as well as a combination of the two, on stability of balance in athletes.
Patients and Methods: Changes in center-of-gravity sway were measured in 30 college student athletes before and after the following three exercise conditions: 1) oculomotor exercises, 2) bimanual coordination exercises, and 3) a combination of oculomotor and bimanual coordination exercises (1+2). The order of these exercises was counterbalanced.
Results: The combination of exercises (condition 3) reduced large swaying during balancing and immediately increased postural stability. Moreover, the oculomotor and bimanual coordination exercises (conditions 1 and 2) immediately reduced large sway during balancing when performed independently. Thus, the present study revealed that the combination of oculomotor and bimanual coordination exercises immediately reduced accidental swaying during balancing and also improved postural stability.
Conclusion: This combination could be effective as an immediate balance adjustment method for athletes.
Keywords: postural stability, warming up, balance training, oculomotor exercise, bimanual coordination exercise
Introduction
Balance training in athletes has been shown to reduce the risk of sports-related injuries and improve functional performance.1–3 It has also been reported to improve posture and neuromuscular control in athletes,4,5 and to induce, promote, and assist in the recovery of neuromuscular function and rehabilitation after an injury.6 A meta-analysis of the effects of balance training in youth reported moderate to large effects on static and dynamic balance,7 highlighting the importance of balance training in young athlete’s training programs.8
According to a meta-analysis by Lesinski et al9 balance training comprises static/dynamic steady-state, proactive, and reactive balance exercises. The training includes standing with one or both legs on stable or unstable surfaces (eg, BOSU ball, DynaDisc, balance platform, etc.) with one’s eyes open or closed. In addition, most balance training studies with athletes have examined the effects of balance training over several weeks in combination with strength training, as in a review by Granacher and Behm,8 which recommends a combination of balance and strength training. On the other hand, it has been suggested that balance control may also be affected by warming up, which aims to improve functional performance and reduce the risk of sports-related injuries.10
Warming up is essential for achieving optimal performance11 and increasing the efficiency of the somatosensory system12 and neuromuscular responses.13 Athletes warm up immediately before a game to achieve better performance, and warm-up exercises that immediately improve balance are essential in such situations.14 However, the aforementioned balance training as a warm-up does not necessarily lead to the improvement of balance stability. For instance, training on balance control as a warm-up exercise is not effective in improving balance immediately, indicating the need for a recovery time of about 20 minutes after training.15,16 Furthermore, research has supported the notion that proprioceptive training using an unstable platform has a short-term negative effect on balance stability in athletes.17
Therefore, we focused on an approach that restores or improves balance by directly targeting the vestibular system, which is directly involved in maintaining posture and balance. The approaches used in this study are oculomotor and gaze stabilization exercises. The effectiveness of such exercises in improving balance has been reported mainly in the field of rehabilitation for stroke and other disorders.18–20 These exercises also help improve balance in healthy young adults without vestibular or ocular problems.21
In addition to the vestibular system, the cerebellum is involved in maintaining posture and balance.22 Specifically, through its connections with the vestibular nuclei and the vestibular apparatus, the cerebellum plays an important role in maintaining balance and coordinating head and eye movements.23,24 It has also been suggested that cerebellar activation improves balance.25 We focused on bimanual coordinated movements, which are closely related to cerebellar control and activation.26–28 Although the cerebellar activation during bimanual coordination exercises may stabilize balance, no study has confirmed the effect of bimanual coordination exercises on balance. Therefore, we hypothesized that a combination of oculomotor exercises, which are beneficial to the vestibular system, and bimanual coordination exercises, which activate the cerebellum, would have a more beneficial effect on balance. In summary, this study aimed to examine whether oculomotor exercises, bimanual coordination exercises, and a combination of the two are beneficial for immediate balance stability in athletes.
Methods
Participants
Participants were students who were performing the athletic club activites at the author’s institution. They played baseball, soccer, basketball, volleyball, track and field, tennis, handball, badminton, swimming, and kendo. Healthy student athletes were notified of the study and those who agreed to cooperate were eligible to participate. The exclusion criteria were: injury, physical disability, any disease, poor physical condition on the day of the experiment, alcohol consumption within 24 hours of the experiment, or less than 5 hours of sleep the day before the experiment. In total, 30 Japanese university student athletes (23 men and 7 women; mean age 21.20 years, SD=0.98) who met the criteria were included in the study. All participants performed at the regional competition level.
Participants were informed of the nature of the study, the safety of the activities, and that their privacy would be protected. Only those who signed the informed consent sheet were included. The study was approved by the Ethics Committee on Research Involving Human Subjects of Utsunomiya University (H23-0118) and complied with the ethical principles of the Declaration of Helsinki.
Exercise Conditions
The exercise program was conducted under the following three conditions: 1) oculomotor exercises, 2) bimanual coordination exercises, and 3) a combination of the two (1+2). The oculomotor program comprised six exercises—two smooth pursuit exercises and four adaptation exercises—based on a study by Morimoto et al.21 Bimanual coordinated movements comprised three bimanual in-phase movements and three bimanual anti-phase movements, as both movements can be performed without extensive practice.29 The combination condition comprised six exercises: one smooth pursuit exercise, two adaptation exercises, two bimanual in-phase movements, and one bimanual anti-phase movement. The order in which the oculomotor and bimanual coordination exercises were performed was counterbalanced. The duration of each program was approximately 5 minutes. The exercises performed in each program are shown in Table 1 and Figure 1.
 |
Table 1 Details of the Exercise Conducted |
 |
Figure 1 Methods of performing oculomotor and bimanual coordination exercises. The oculomotor exercises were created based on the movements in Morimoto et al21 (A) Oculomotor exercise 1 is one of the pursuit exercises. This exercise involves moving the thumbs horizontally and tracking them with the eyes while keeping the head still. (B) Oculomotor exercise 2 is one of the pursuit exercises. This exercise involves moving the thumbs vertically and tracking them with your eyes while keeping your head still. (C) Oculomotor exercise 3 is one of the adaptation exercises. This exercise involves moving the head horizontally while keeping your gaze on a stationary thumb. (D) Oculomotor exercise 4 is one of the adaptation exercises. This exercise involves moving the head vertically while keeping your gaze on a stationary thumb. (E) Oculomotor exercise 5 is one of the adaptation exercises. This exercise involves moving the head and thumbs in opposite directions horizontally while tracking the thumb with your eyes. (F) Oculomotor exercise 6 is one of the adaptation exercises. This exercise involves moving the head and thumbs in opposite directions vertically while tracking the thumb with your eyes. (G) Bimanual-coordination exercise 1 is one of the in-phase bimanual-coordination exercises. This exercise involves extending both arms to a position horizontal to the floor, dorsiflexing your wrists to 90 degrees, and performing a bimanual coordinated movement in-phase. (H) Bimanual-coordination exercise 2 is one of the anti-phase bimanual-coordination exercises. This exercise involves extending both arms to a position horizontal to the floor, dorsiflexing your wrists to 90 degrees, and performing a bimanual coordinated movement in anti-phase. |
Experimental Equipment and Measurement
Experimental Equipment
The Wii Balance Board (WBB, Nintendo, Japan) was used to measure center-of-gravity sway. In addition, stabometry software, namely FitTri ver. 2, developed by Yoshimura,30 was employed to continuously acquire the center of pressure (COP) position. The accuracy of the WBB has been confirmed in previous studies.31 To ensure that participant’s toes did not stick out of the WBB when measuring the tandem stance, a wooden board that was 450 mm long, 600 mm wide, and 9 mm thick was placed on the WBB for measurement.
Center-of-Gravity Test
In the center-of-gravity test, unit time trajectory length and sway area were calculated to examine changes in center-of-gravity stability before and after each exercise. The characteristics of each index, based on Yamanaka,32 are shown below. Unit time trajectory length is the value obtained by dividing the moving distance of the COP due to body sway (total trajectory length) by the recording time. The higher the value, the more unstable the postural control. The sway area is calculated by multiplying the maximum moving distances in the left–right and front–back directions. The higher the value, the greater the effect of the unintentional sway, indicating that unintentional large sway has occurred. The average of three pre-measurements was used as the reference value for each participant, and the amount of change obtained by subtracting this reference value from the measured value of each trial was used as the index for the study.
The center-of-gravity sway was measured in a closed-eye tandem standing posture with arms crossed over the chest (Figure 2). This stance was used for the following reason: the participants are athletes and have better balance than non-athletes, and the body movements during the tandem standing posture emphasize differences that are not seen in the normal standing posture; therefore, they are suitable for understanding the neural mechanisms that control stability.33,34 In the tandem standing posture, the participants were placed in a toe and heel position with the toes and heels at the center of the WBB. In the tandem standing posture, the dominant foot was the rear limb. On the first day of measurement, participants were instructed on the balance posture (Figure 2) and confirmed that they were able to maintain this posture before the measurement began. The measurement period was 60 seconds after the participant stepped onto the WBB and the examiner confirmed that the initial transient body movement had subsided. The measurement time was determined based on the work of Carpenter et al.35 Measurements were usually taken while participants were barefoot, although light socks were allowed.
 |
Figure 2 The closed-eye tandem standing posture with arms crossed over the chest. |
Two-Dimensional Mood Scale
The Two-Dimensional Mood Scale (TDMS)36 was used to determine whether each exercise condition caused participants to experience unpleasant mood states. This scale comprises eight words selected based on pleasure and arousal and is capable of measuring momentary mood states.
Procedure
The study was conducted as a quasi-randomized controlled trial, with participants assigned as shown in Table 2. The order in which the exercise programs were implemented was counterbalanced. One exercise program was performed for only one condition per day, and another was performed 3–7 days later. Data were measured by two collaborators between 9:00 a.m. and 3:00 p.m. All measurements were performed in the same laboratory.
 |
Table 2 Counterbalance of the Exercise Programs and Participants Assigned |
Participants were measured on the first day of the experiment in the resting condition of sitting in a chair for 5 minutes, followed by one of the exercise conditions. Participants performed one exercise condition in a standing position, after which they rested in a chair for 2 minutes. They also reported changes in mood using the TDMS before and after each exercise condition, and changes in subjective stability after the post-center-of-gravity test. The study protocol is illustrated in Figure 3.
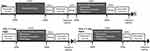 |
Figure 3 Protocol. |
Analysis
A one-way within-subjects analysis of variance (ANOVA) was performed for each measurement item related to the center-of-gravity test. When a significant main effect was observed, Dunnett’s multiple comparisons were performed. To examine changes in mood before and after exercise, a two-way within-subjects (pre-post x 3 conditions) ANOVA was performed to assess the TDMS comfort scores. Bonferroni was employed to adjust for multiple comparisons, with the significance level set at less than 5%, and less than 10% was considered a significant trend. Regarding effect sizes, generalized eta-squared (ηG2) was calculated for one-way and two-way ANOVAs, and Cohen’s d37 was calculated for multiple comparisons. IBM SPSS Statistics 28 was used as the statistical software. No statistical sample size calculations were performed. For power, based on the sample size of this study (n = 30), a post hoc analysis was conducted using G*power 3.1 for one-way and two-way within-subjects ANOVA with a medium effect size (ηG2 = 0.25) and a significance level of p < 0.05. The post hoc powers were 0.840 and 0.751, respectively.
Results
The Shapiro–Wilk test indicated that all the data were normally distributed, and the Levene test indicated that equality of error variances was assumed at the 5% level.
Center-of-Gravity Test
Effect of Measurement Period
Unit Time Trajectory Length
There was no significant difference in unit time trajectory length between the pre-measurement (F (2, 58) = 0.24, p = 0.791, ηG2 = 0.02) and post-measurement (F (2, 58) = 0.20, p = 0.819, ηG2 = 0.02) periods (Table 3).
 |
Table 3 Comparison of Center-of-Gravity Sway by Measurement Period |
Sway Area
There was no significant difference in sway area between the pre-measurement (F (2, 58) = 0.02, p = 0.981, ηG2 = 0.00) and post-measurement (F (2, 58) = 0.21, p = 0.812, ηG2 = 0.01) periods (Table 3).
Effects of Exercise Conditions
Unit Time Trajectory Length
Significant differences in unit time trajectory length were found between exercise conditions (F (3, 87) = 3.09, p = 0.031, ηG2 = 0.25). Multiple comparisons confirmed a significant decrease in unit time trajectory length for the combination condition compared with rest (p = 0.017, d = 0.67). There was no significant difference in the degree of reduction in unit time trajectory length between rest and the oculomotor condition (p = 0.718, d = 0.21) or rest and the bimanual coordination condition (p = 0.961, d = 0.09) (Table 4, Figure 4A).
 |
Table 4 Comparison of Center-of-Gravity Sway by Exercises Condition |
Sway Area
A significant difference in sway area was observed depending on exercise condition (F (3, 87) = 6.57, p <0.001, ηG2 = 0.33). Multiple comparisons confirmed that the sway area was significantly reduced in all conditions, compared with rest: oculomotor (p = 0.003, d = 0.65), bimanual coordination (p = 0.006, d = 0.59), and combination (p <0.001, d = 0.77; Table 4, Figure 4B).
TDMS
A significant interaction was observed for mood comfort scores (F (2, 58) = 3.54, p = 0.035, ηG2 = 0.05). Multiple comparisons revealed a significant increase in comfort scores for the bimanual coordination condition (p = 0.003, d = 0.45) with respect to changes in the measurement period. No significant changes were observed for the oculomotor (p = 0.870, d = 0.02) or combination (p = 0.278, d = 0.16) conditions (Table 5).
 |
Table 5 Change in the Mood Pleasure Before and After Exercises |
Discussion
Unit time trajectory length is an index of standing postural instability and indicates fine control of posture. This study confirmed that only the combination exercise significantly reduced unit time trajectory length compared with rest. Sway area represents the degree of unintentional large sway and the extent of back-and-forth and left-and-right movements. In this study, sway area was significantly reduced compared with rest, in all three conditions. These results confirmed that the oculomotor, bimanual coordination, and combination conditions were all effective in reducing unintentional large sway. They also confirmed that the combination condition resulted in less sway and better stability. In this study, balance was measured repeatedly on the same participants. Therefore, we showed that the effect of measurement period showed no significant difference in any of the indicators. Such results suggest that the learning effect of repeated measurements can be excluded. Regarding the mood state, no change was observed for the oculomotor or combination conditions, whereas the bimanual coordination condition was found to increase pleasure. Although threatening and anxious emotions affect center-of-gravity sway,38 these uncomfortable moods and emotions were not observed in this study. However, the effect size for the change in mood was small, indicating insufficient detection power, suggesting the need for further study.
Based on these results, it can be assumed that the changes in center-of-gravity sway were not influenced by mood state but were due to the effects of the individual exercises. The effect of oculomotor exercises on reducing sway during balancing is similar to that reported in previous studies;21 however, the present study confirms the effect of eye movements on reducing immediate and unintentional large sway in athletes. Gaze stabilization exercises have been reported to immediately improve the vestibular function required for postural stability, mediated by an improvement in the vestibulospinal reflex.39 In the closed-eye tandem standing balance test in the present study, the effect might have been achieved by the same mechanism as that used in previous studies.
The effect of the reduction in sway area by the bimanual coordination exercise may be due to the activation of the cerebellum. A previous study that examined the structural relationship between human postural balance and the brain reported that almost all regions of the brain are involved in balance, with the greatest number of reports on the gray and white matter of the cerebellum; the cerebellum plays an important role in the acquisition and ability to balance.40 In particular, individuals with balancing skill, such as athletes, have increased gray matter volume in the cerebellum compared with individuals with average balance.41 Furthermore, it has been reported that cerebellar transcranial direct current stimulation (ctDCS) reduces balance in healthy adults when cathodal ctDCS is applied to the cerebellum.42 This suggests that the degree of cerebellar activity affects balance.
It has also been hypothesized that simple, repetitive, and symmetrical bimanual coordination relies on complex patterns of interaction within spatially distributed neural networks that are closely related to functional connections between the left and right hemispheres of the cerebellum and information transfer between cortical and subcortical structures;43 thus, balance involves nearly every region of the brain.40 Moreover, anodal transcranial direct current stimulation (atDCS) over the cerebellum improves motor performance and motor transmission in bimanual coordination tasks.44 Accordingly, these findings suggest that the reason for the greatest effect on increased balance stability in the combination exercise condition is the synergistic effect produced by the improvement of the vestibular system via eye movements and the activation of the neural network centered on the cerebellum via the bimanual coordination exercise.
Notably, the in-phase and anti-phase motions performed in the bimanual coordinated movements in this study were reported to be different control processes.45 Specifically, the dominant hemisphere controls the movement in the in-phase motion, whereas both hemispheres are controlled more independently during the anti-phase movement. Although there is no difference between in-phase and anti-phase cerebellar activation, the cerebellum has been reported to be more strongly connected to the cingulate gyrus motor area (CMA) during anti-phase movements than during in-phase movements.46 The reason for the greater activation of the CMA during anti-phase movements is thought to be the suppression of contrastive movements47 along the intrinsically preferred midline.48 As both in-phase and anti-phase exercises were performed in this study, it is unclear whether the effect is due to the combination of the exercises or due to one or the other. This effect should be investigated further.
Several participants reported headache and dizziness immediately after the eye movement, although these symptoms were transient and resolved after 2 minutes of rest. The participant’s mood did not change to an unpleasant state in any of the conditions, indicating that the exercise was not distressing for them.
The limitations of this study include the fact that only immediate effects were investigated; therefore, it remains unclear how long the effects of improved balance will be maintained, and to what extent they will be achieved in the actual performance situations of each athlete. In addition, the participants in this study were engaged in various sports. The effects on postural control are considered to vary depending on the sport; hence, it is necessary to examine the effects of each sport in future studies.
However, as the present study confirmed that each of the training exercises performed had an immediate effect on balance and that there was no mental strain on the participant, it is possible to conclude that the training can be used, for example, as a warm-up immediately prior to an athlete’s performance demonstration situation. Future studies should implement the exercises performed in this study for a long period, such as 3 months, as a warm-up exercise and examine the effects on the actual performance demonstration situation of each athlete. The exercises performed in this study, if added to conventional balance training, may also have the potential to be used as a new type of training to further improve balance.
Conclusion
The combined oculomotor and bimanual coordination exercise examined in the present study was found to reduce unintentional large sway during balancing and immediately increase postural stability. Independent oculomotor and bimanual coordinated movements also showed an immediate reduction in unintentional large sway during balancing. These findings suggest that the exercises investigated in this study should be included as part of athlete’s warm-up immediately prior to their performance, to enable them to achieve greater feats.
Acknowledgments
We would like to express our great appreciation to H. Matsuoka (University of Tsukuba, Japan) for assisting with the statistical analysis.
Funding
This work was supported by JSPS KAKENHI Grant Number JP21K11543, JP23H03294.
Disclosure
The authors have no conflicts of interest directly relevant to the content of this article.
References
1. Emery CA, Cassidy JD, Klassen TP, Rosychuk RJ, Rowe BH. Effectiveness of a home-based balance-training program in reducing sports-related injuries among healthy adolescents: a cluster randomized controlled trial. Can Med Assoc J. 2005;172(6):749–754. doi:10.1503/cmaj.1040805
2. McGuine TA, Keene JS. The effect of a balance training program on the risk of ankle sprains in high school athletes. Am J Sports Med. 2006;34(7):1103–1111. doi:10.1177/0363546505284191
3. Gebel A, Prieske O, Behm DG, Granacher U. Effects of balance training on physical fitness in youth and young athletes: a narrative review. Strength Conditioning J. 2020;42(6):35–44. doi:10.1519/SSC.0000000000000548
4. Brachman A, Kamieniarz A, Michalska J, Pawłowski M, Słomka KJ, Juras G. Balance training programs in athletes – a systematic review. J HumKinet. 2017;58(1):45–64.
5. DiStefano LJ, Clark MA, Padua DA. Evidence supporting balance training in healthy individuals: a systemic review. J Strength Cond Res. 2009;23(9):2718–2731. doi:10.1519/JSC.0b013e3181c1f7c5
6. Zech A, Huebscher M, Vogt L, Banzer W, Hänsel F, Pfeifer K. Neuromuscular training for rehabilitation of sports injuries: a systematic review. Med Sci Sports Exercise. 2009;41(10):1831–1841. doi:10.1249/MSS.0b013e3181a3cf0d
7. Gebel A, Lesinski M, Behm DG, Granacher U. Effects and dose–response relationship of balance training on balance performance in youth: a systematic review and meta-analysis. Sports Med. 2018;48(9):2067–2089. doi:10.1007/s40279-018-0926-0
8. Granacher U, Behm DG. Relevance and effectiveness of combined resistance and balance training to improve balance and muscular fitness in healthy youth and youth athletes: a scoping review. Sports Med. 2023;53(2):349–370. doi:10.1007/s40279-022-01789-7
9. Lesinski M, Hortobágyi T, Muehlbauer T, Gollhofer A, Granacher U. Dose-response relationships of balance training in healthy young adults: a systematic review and meta-analysis. Sports Med. 2015;45(4):557–576. doi:10.1007/s40279-014-0284-5
10. Chatzopoulos D, Galazoulas C, Patikas D, Kotzamanidis C. Acute effects of static and dynamic stretching on balance, agility, reaction time and movement time. J Sports Sci Med. 2014;13(2):403–409.
11. Pasanen K, Parkkari J, Pasanen M, Kannus P. Effect of a neuromuscular warm-up programme on muscle power, balance, speed and agility - A randomised controlled study. Br J Sports Med. 2009;43(13):1073–1078. doi:10.1136/bjsm.2009.061747
12. Subasi SS, Gelecek N, Aksakoglu G. Effects of different warm-up periods on knee proprioception and balance in healthy young individuals. J Sport Rehabil. 2008;17(2):186–205. doi:10.1123/jsr.17.2.186
13. Painter R, Pearce A, Rostami M, Frazer A, Kidgell D. Determining the corticospinal and neuromuscular responses following a warm-up protocol. J Sci Med Sport. 2020;2(2):1–12.
14. Patti A, Giustino V, Cataldi SEA. Effects of 5-week of FIFA 11+ warm-up program on explosive strength, speed, and perception of physical exertion in elite female futsal athletes. Sports. 2022;10(7):100. doi:10.3390/sports10070100
15. Brighenti A, Noé F, Stella F, Schena F, Mourot L. Warm-up improves balance control differently in the dominant and non-dominant leg in young sportsmen according to their experience in asymmetric or symmetric sports. Int J Environ Res Public Health. 2022;19(8):4562. doi:10.3390/ijerph19084562
16. Paillard T, Kadri MA, Nouar MB, Noé F. Warm-up optimizes postural control but requires some minutes of recovery. J Strength Cond Res. 2018;32(10):2725–2729. doi:10.1519/JSC.0000000000002592
17. Romero-Franco N, Martínez-López EJ, Lomas-Vega R, Hita-Contreras F, Osuna-Pérez MC, Martínez-Amat A. Short-term effects of proprioceptive training with unstable platform on athlete’s stabilometry. J Strength Cond Res. 2013;27(8):2189–2197. doi:10.1519/JSC.0b013e31827de04c
18. Pimenta C, Correia A, Alves M, Virella D. Effects of oculomotor and gaze stability exercises on balance after stroke: clinical trial protocol. Porto Biomed J. 2017;2(3):76–80. doi:10.1016/j.pbj.2017.01.003
19. Correia A, Pimenta C, Alves M, Virella D. Better balance: a randomised controlled trial of oculomotor and gaze stability exercises to reduce risk of falling after stroke. Clin Rehabil. 2021;35(2):213–221. doi:10.1177/0269215520956338
20. Do Amaral CMS, de Almeida SB, de Almeida RP, Do Nascimento SL, Ribeiro RM, Braga-Neto P. Effectiveness of vestibular rehabilitation on postural balance in Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. BMC Neurol. 2024;24(1):161. doi:10.1186/s12883-024-03649-5
21. Morimoto H, Asai Y, Johnson EG, et al. Effect of oculo-motor and gaze stability exercises on postural stability and dynamic visual acuity in healthy young adults. Gait Posture. 2011;33(4):600–603. doi:10.1016/j.gaitpost.2011.01.016
22. Ango F, Dos Reis R. Sensing how to balance. Elife. 2019;8:e46973. doi:10.7554/eLife.46973
23. Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78(3–5):272–303. doi:10.1016/j.pneurobio.2006.02.006
24. Barlow JS. The Cerebellum and Adaptive Control. Cambridge: Cambridge University Press; 2002.
25. Nissim M, Livny A, Barmatz CEA. Effects of Ai-Chi practice on balance and left cerebellar activation during high working memory load task in older people: a controlled pilot trial. Int J Environ Res Public Health. 2021;18(23):12756. doi:10.3390/ijerph182312756
26. Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Cerebellar and premotor function in bimanual coordination: parametric neural responses to spatiotemporal complexity and cycling frequency. Neuroimage. 2004;21(4):1416–1427. doi:10.1016/j.neuroimage.2003.12.011
27. Pollok B, Butz M, Gross J, Schnitzler A. Intercerebellar coupling contributes to bimanual coordination. J Cognit Neurosci. 2007;19(4):704–719. doi:10.1162/jocn.2007.19.4.704
28. van Dun K, Brinkmann P, Depestele S, Verstraelen S, Meesen R. Cerebellar activation during simple and complex bimanual coordination: an activation likelihood estimation (ALE) meta-analysis. Cerebellum. 2021;21(6):987–1013. doi:10.1007/s12311-021-01261-8
29. Kelso JA. Phase transitions and critical behavior in human bimanual coordination. Am J Physiol Regul Intgr Comp Physiol. 1984;246(6):R1000–R1004. doi:10.1152/ajpregu.1984.246.6.R1000
30. Yoshimura M A study group of rehabilitation tool. 2014; [online] [retrieved on 2014-12-25]. Retrieved from the Internet: http://www.eonet.ne.jp/~rpt/.
31. Clark RA, Mentiplay BF, Pua YH, Bower KJ. Reliability and validity of the Wii Balance Board for assessment of standing balance: a systematic review. Gait Posture. 2018;61:40–54. doi:10.1016/j.gaitpost.2017.12.022
32. Yamanaka T. Clinicalposturography / stabilometry. Equilib Res. 2022;81(1):1–15. doi:10.3757/jser.81.1
33. Sozzi S, Monti A, De Nunzio AM, Do MC, Schieppati M. Sensori-motor integration during stance: time adaptation of control mechanisms on adding or removing vision. Hum Mov Sci. 2011;30(2):172–189. doi:10.1016/j.humov.2010.06.002
34. O’Connor SM, Kuo AD. Direction-dependent control of balance during walking and standing. J Neurophysiol. 2009;102(3):1411–1419. doi:10.1152/jn.00131.2009
35. Carpenter MG, Frank JS, Winter DA, Peysar GW. Sampling duration effects on centre of pressure summary measures. Gait Posture. 2001;13(1):35–40. doi:10.1016/S0966-6362(00)00093-X
36. Sakairi Y, Nakatsuka K, Shimizu T. Development of the two - dimensional mood scale for self-monitoring and self-regulation of momentary mood states. Jpn Psychol Res. 2013;55(4):338–349. doi:10.1111/jpr.12021
37. Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi:10.1037/0033-2909.112.1.155
38. Adkin AL, Carpenter MG. New insights on emotional contributions to human postural control. Front Neurol. 2018;9:789. doi:10.3389/fneur.2018.00789
39. Matsugi A, Ueta Y, Oku K, et al. Effect of gaze-stabilization exercises on vestibular function during postural control. Neuroreport. 2017;28(8):439–443. doi:10.1097/WNR.0000000000000776
40. Surgent OJ, Dadalko OI, Pickett KA, Travers BG. Balance and the brain: a review of structural brain correlates of postural balance and balance training in humans. Gait Posture. 2019;71:245–252. doi:10.1016/j.gaitpost.2019.05.011
41. Hüfner K, Binetti C, Hamilton DA, et al. Structural and functional plasticity of the hippocampal formation in professional dancers and slackliners. Hippocampus. 2011;21(8):855–865. doi:10.1002/hipo.20801
42. Foerster Á, Melo L, Mello MEA. Cerebellar transcranial direct current stimulation (ctDCS) impairs balance control in healthy individuals. Cerebellum. 2017;16(4):872–875. doi:10.1007/s12311-017-0863-8
43. Pollok B, Südmeyer M, Gross J, Schnitzler A. The oscillatory network of simple repetitive bimanual movements. Cogn Brain Res. 2005;25(1):300–311. doi:10.1016/j.cogbrainres.2005.06.004
44. Azarpaikan A, Torbati HRT, Sohrabi M, Boostani R, Ghoshuni M. The effect of parietal and cerebellar transcranial direct current stimulation on bimanual coordinated adaptive motor learning. J Psychophysiol. 2019;35(1). doi:10.1027/0269-8803/a000254
45. Shih PC, Steele CJ, Nikulin V, Villringer A, Sehm B. Kinematic profiles suggest differential control processes involved in bilateral in-phase and anti-phase movements. Sci Rep. 2019;9(1):3273. doi:10.1038/s41598-019-40295-1
46. Lin Q, Li H, Mao Y, et al. The difference of neural networks between bimanual antiphase and in-phase upper limb movements: a preliminary functional magnetic resonance imaging study. Behav Neurol. 2017;2017:8041962. doi:10.1155/2017/8041962
47. Mechsner F, Knoblich G. Do muscles matter for coordinated action? J Exp Psychol Hum Percept Perform. 2004;30(3):490–503. doi:10.1037/0096-1523.30.3.490
48. Wenderoth N, Debaere F, Sunaert S, Swinnen SP. The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. Eur J Neurosci. 2005;22(1):235–246. doi:10.1111/j.1460-9568.2005.04176.x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

