Back to Journals » Journal of Inflammation Research » Volume 17
Effect of Preoperative Serum Lactate Dehydrogenase-to-Albumin Ratio on the Survival of Oral Cancer: A Retrospective Study
Received 3 April 2024
Accepted for publication 16 July 2024
Published 31 July 2024 Volume 2024:17 Pages 5129—5138
DOI https://doi.org/10.2147/JIR.S472041
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Xuming Wang, Xiaoli Ji
Department of Stomatology, The Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University, Huaian, Jiangsu, People’s Republic of China
Correspondence: Xiaoli Ji, Email [email protected]
Background: Several studies have investigated the relationship between serum lactate dehydrogenase-to-albumin ratio (LAR) and the prognosis of cancers. However, no studies have explored the association between serum LAR and the survival of oral cancer (OC). This study was aimed to determine the association of serum LAR with the overall survival (OS) of OC.
Methods: One hundred and ninety patients with OC were included in this study between January 2018 and December 2019. Log rank test and Kaplan–Meier method were used to compare the survival rate of OC between the low LAR group and the high LAR group. The association between serum LAR and the survival of OC patients was determined via univariate and multivariate Cox regression analyses.
Results: Kaplan-Meier analysis and Log rank test indicated that the OS rate in low LAR group was significantly higher than that in high LAR group (P < 0.05). Univariate cox analysis showed that TNM III-IV stage, serum LDH > 162 U/L, and serum LAR > 3.79 were significantly associated with the OS of OC patients. Multivariate Cox analysis suggested that the TNM III-IV stage (HR, 2.317; 95% CI, 1.423– 3.774, P = 0.001) and serum LAR > 3.79 (HR, 5.138; 95% CI, 2.245– 11.756, P = 0.000) were independently related with poor OS of OC patients.
Conclusion: High serum LAR (> 3.79) is an independent predictor of adverse prognosis in OC patients. LAR could be used as a promising marker for predicting the OS of OC patients.
Keywords: oral cancer, lactate dehydrogenase to albumin ratio, prognosis, marker
Introduction
Oral cancer (OC) occurs commonly in middle-aged and elder people; however, it is also being reported in younger individuals in recent years.1,2 The OC ranks as the 6th most common malignancy in the world,3 which is an issue of global health burden. There are approximately 354,864 cases of oral cavity and lip cancer worldwide, with about 177,384 deaths every year.4 Betel quid, diet and nutrition, mouthwash, alcohol, tobacco, occupational risks, and genetic factors were associated with the risk of OC.5 Despite advances in therapeutic methods,6 the five-year survival rate of OC is about 50%, which was not improved remarkably in recent years.7 The poor prognosis of OC is mainly attributed to delayed diagnosis and treatment. Therefore, early-stage diagnosis of OC is a crucial step in reducing its mortality rate.8 It is important to identify effective prognostic factors for OC patients to select suitable treatment regimens.
Lactate dehydrogenase (LDH), a key enzyme in the glycolytic pathway, could convert pyruvate to lactate. A meta-analysis showed that high LDH was related to the adverse prognosis of many solid tumors.9 Mafessoni et al found that salivary LDH could help with the early diagnosis of OC in individuals with Fanconi anemia.10 In addition, the serum albumin (ALB) level could reflect the individual’s the nutritional status. Previous studies indicated that low serum ALB level was related with poor prognosis of different types of cancers,11–13 including OC.14,15 Thus, high LDH and low ALB levels may be good predictors for the poor survival of OC patients. The serum LDH-to-ALB ratio (LAR), consisting of LDH and ALB, may be more effective in predicting the survival of OC than each alone. Several studies showed that serum LAR correlated with adverse survival in colorectal cancer (CRC),16–19 bladder cancer,20 breast cancer,21 and non-small cell lung cancer (NSCLC).22 However, the relationship between serum LAR and the survival of OC has not been investigated before. In this study, we determined to probe into the prognostic value of serum LAR in OC patients.
Patients and Methods
Patients and Data Collection
A total of 232 patients with OC were initially screened from January 2018 to December 2019. Finally, 190 cases were included in this study. The inclusion criteria of OC cases were as follows: (1) diagnosis of OC was pathologically confirmed; (2) the follow-up data and clinicopathologic characteristics were complete; (3) OC patients received surgical treatment. The exclusion criteria were: (1) case had incomplete data; (2) cases refused to be included; (3) the pathological results were contradictory to the clinical diagnosis of OC; (4) cases had other cancers. Figure 1 shows the flow chart of OC patient selection. The study was approved by the Ethics Committee of the Affiliated Huai’an No.1 People’s Hospital of Nanjing Medical University, which was consistent with the Helsinki Declaration. All patients provided informed consent.
 |
Figure 1 Flow chart of patient selection. |
The demographic and clinical characteristics of OC patients were recorded. Age, sex, drinking, body mass index (BMI), hypertension, smoking, tumor node metastasis (TNM) stage, diabetes mellitus, lymphatic and vascular invasion (LVI), perineural invasion and anemia (PNI), serum ALB, and serum LDH were collected. The LAR was calculated as follows: LAR = LDH (U/L) / ALB (g/L). All OC patients had their LAR calculated at the time of OC diagnosis in this study. All relevant data were measured before treatments. All information was independently checked by two experienced clinicians.
Follow-Up
The follow-up of patients with OC was as follows: OC cases were recorded monthly in the first half of the year after treatment, and then twice a year thereafter. Different ways of follow-up (telephone, medical records, and outpatient/inpatient) were used. The deadline time of follow-up was December 31, 2022. Overall survival (OS) was defined from the time of diagnosis to the time of death due to any cause.
Statistical Analysis
Categorical data were shown as number (percentage), whereas continuous data were presented as median (range) or means (± standard deviations). Chi-square test or Fisher’s exact test was used for analyzing categorical variables, while the Student’s t-test or Mann–Whitney U-test was used for calculating continuous variables. The Kaplan–Meier method and Log rank test were used to analyze survival rates among different groups. The risk factors associated with the survival of OC patients were identified via univariate and multivariate Cox regression analyses. The variables with P value <0.05 in univariate Cox analysis were selected into multivariate Cox analysis. Hazard ratios (HRs) and 95% confidence interval (CI) were calculated. P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 21.0, MedCalc 20, and GraphPad Prism version 8.0.
Results
Characteristics of OC Patients
Demographic and clinical characteristics of OC patients are presented in Table 1. Totally, 190 patients with OC were included, among which 108 males (56.8%) and 82 females (43.2%) were analyzed. There were 122 (64.2%) cases aged >60 years, while 68 (35.8%) cases aged ≤60 years. The percentages of smokers and drinkers among the OC patients were 7.4% and 8.9%, respectively. The percentages of OC patients with hypertension and diabetes mellitus were 18.4% and 23.7%, respectively. The median values of ALB, LDH, and LAR were 42.6g/L, 162 U/L, and 3.79, which were used for the grouping criteria. Other parameters regarding OC patients were shown in Table 1.
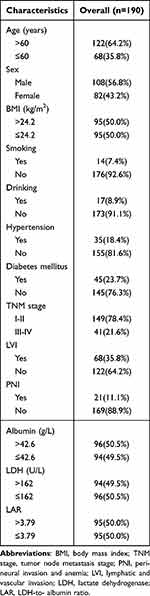 |
Table 1 Demographic and Clinical Characteristics of Oral Cancer Patients |
Relationship Between LAR and Clinicopathological Characteristics of OC
The median value of LAR was 3.79, which was used for dividing OC patients into two groups. The high LAR group was >3.79, and low LAR group was ≤3.79. The associations between serum LAR and clinicopathological characteristics of OC were shown in Table 2. This study indicated that the percentage of OC patients aged >60 years in the high LAR group was significantly higher than that in the low LAR group. The percentage of OC patients with higher serum LDH levels (>162 U/L) in the high LAR group was remarkedly higher than that in the low LAR group. Regarding sex, BMI, drinking, smoking, diabetes mellitus, hypertension, TNM stage, LVI, PNI, and serum ALB, no significant association was obtained between high LAR group and low LAR group.
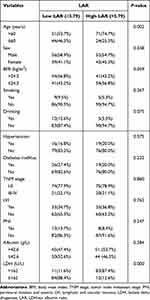 |
Table 2 Correlation Between LAR and Clinicopathological Features in Oral Cancer Patients |
Prognostic Significance of Serum LAR on OC Patients
The OC cases were divided into a low LAR group and a high LAR group according to the median LAR value. The Kaplan-Meier method and Log rank test showed that the high LAR group showed poorer OS compared with the low LAR group (Figure 2, P < 0.0001). We also compared the OS rates between two groups, and found that the high LDH group presented a shorter OS than the low LDH group (Supplementary Figure 1A). In addition, this study indicated that the high ALB group did not show a higher OS than the low ALB group (Supplementary Figure 1B, P > 0.05).
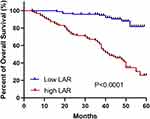 |
Figure 2 Comparison of overall survival rate between high LAR group and low LAR group among OC patients. |
Univariate and multivariate analyses were used to identify the risk factors of survival for OC patients. Univariate Cox analysis indicated that TNM III-IV stage, serum LDH >162 U/L, and LAR > 3.79 were significantly associated with poor OS of OC patients (Table 3). Further multivariate Cox analysis suggested that the TNM III-IV stage (HR, 2.317; 95% CI, 1.423–3.774, P = 0.001) and serum LAR > 3.79 (HR, 5.138; 95% CI, 2.245–11.756, P = 0.000) were independent risk factors for OS among OC patients (Table 3); however, serum LDH > 162 U/L was not associated with the OS of OC patients.
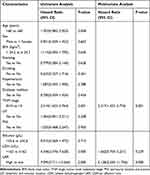 |
Table 3 Univariate and Multivariate COX Regression Analysis for Overall Survival in Oral Cancer |
Discussion
Herein, we introduced a novel biomarker, LAR, for predicting the survival of OC patients among a Chinese Han population. We found that a high serum LAR (>3.79) was an independent predictor of OS for OC patients.
LDH is a glycolytic enzyme, which could convert pyruvate to lactate and lead to a hypoxic environment. Increased serum levels of LDH were considered as a marker of poor prognosis for cancers, which was attributed to elevated cancer metabolism and tumor burden.23–25 A host of studies found that elevated LDH level was associated with poor survival in various cancers.26–29 In this study, univariate Cox regression analysis showed that higher serum LDH was a risk factor for the OS of OC patients; however, multivariate Cox regression analysis did not uncover that LDH was associated with the prognosis of OC. Limited sample size, different treatment strategies, and clinical heterogeneity may contribute to these inconsistent findings. In addition, ALB is an important protein for cancers, which could reflect the nutritional status of cancer patients. Several studies have demonstrated that low serum ALB levels could predict poor prognosis of cancers.30–33 In this study, we did not find that serum ALB was related to the survival of OC, which was partly consistent with the findings by Cui et al.34
LAR, combing ALB and LDH, reflected both tumor burden and nutritional status. Therefore, LAR may be more effective in predicting the cancer survival than a single marker, such as LDH or ALB. LAR is cost-effective and easily available, and it could be used for cancer patients at high risk of recurrence, progression, and death. A host of studies have explored the relationship between the survival of cancers and LAR values. We summarized the findings in Table 4. Gan et al found that LAR was an independent prognostic predictor in cases with hepatocellular carcinoma undergoing curative resection.35 They showed that LAR was a significant prognostic marker for both recurrence-free survival and OS.35 Feng et al suggested that LAR was an independent risk factor of cancer-specific survival in patients with resectable esophageal squamous cell carcinoma.36 Gao et al uncovered that high LAR was a prognostic factor for pancreatic cancer.37 For Gastrointestinal tumors, four Chinese studies17–19,38 and two Turkish studies16,39 have addressed this issue before. Aday et al indicated that LAR was not a significant predictor for patients with gastric cancer.39 In their further study, they found that LAR ≥ 52.7 was significantly related to worse disease-free survival and OS in CRC patients.16 Hu et al17 and Shu et al18 demonstrated that high LAR was associated with poor OS and disease-free survival among CRC patients. Xie et al suggested that high LAR was associated with progression-free survival and OS in cases with colon cancer.38 They did not include patients with rectal cancer.38 Wu et al found that LAR was a significant predictor of OS among patients with CRC.19 For nasopharyngeal carcinoma (NPC), two Chinese studies showed a significant association with the survival of NPC.40,41 In addition, two studies indicated that LAR was significantly associated with the survival of breast cancer21 and bladder cancer.20 A Turkish study by Menekse et al showed that LAR was an independent predictor of nivolumab in cases with NSCLC.22 In line with abovementioned studies, this study showed that high LAR was an independent prognostic marker of OS among OC patients. To the best of our knowledge, this is the first study to uncover an association between the LAR value and survival of OC patients.
 |
Table 4 The Baseline Characteristics of the Studies Regarding the Association Between LAR and Cancer Survival |
In addition, univariate and multivariate Cox regression analyses showed that TNM III-IV stage was a significant prognostic factor of OS among OC patients in this study, which was shown in other cancers.18 It is of note that the cutoff values of the LAR level differed among studies, which may exert effects on the final results of LAR. Various definitions of LAR may explain this discrepancy. The cutoff value of the LAR level in the study by Aday et al was quite doubtful,16 which was 52.7. Notably, the cutoff values of the LAR in most of abovementioned studies were lower than 10.0.
Several limitations were shown in this study. First, this single-center study was retrospective. Multi-center prospective studies are urgently needed in the future. Second, the sample size was not large enough. Third, the optimal threshold value of LAR in this study was different from other studies. Fourth, confounding factors affecting the findings were not included in this study. Fifth, the dynamic monitoring of LAR value was not shown. Sixth, all studies investigating the relationship between LAR and the survival of OC patients were from China and Turkey; studies from other countries are needed. Last, other combined indexes including LDH or ALB may also be useful for evaluating the survival of OC patients.
Conclusions
Totally, this study finds that serum LAR could be served as a marker for the survival of patients with OC. High serum LAR is associated with poor OS of OC patients. LAR can help physicians make suitable treatment decisions and more effective management for OC patients. However, these results should be interpreted as hypothesis generated rather than practice changing due to multiple study limitations (small sample size, retrospective analysis, single-institution experience, heterogeneous patient population, etc).
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sarode G, Maniyar N, Sarode SC, Jafer M, Patil S, Awan KH. Epidemiologic aspects of oral cancer. Dis Mon. 2020;66(12):100988. doi:10.1016/j.disamonth.2020.100988
2. Wong T, Wiesenfeld D. Oral cancer. Aust Dent J. 2018;63(Suppl 1):S91–S99. doi:10.1111/adj.12594
3. Warnakulasuriya S. Living with oral cancer: epidemiology with particular reference to prevalence and life-style changes that influence survival. Oral Oncol. 2010;46(6):407–410. doi:10.1016/j.oraloncology.2010.02.015
4. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
5. Kumar M, Nanavati R, Modi TG, Dobariya C. Oral cancer: etiology and risk factors: a review. J Cancer Res Ther. 2016;12(2):458–463. doi:10.4103/0973-1482.186696
6. Baskar G, Palaniyandi T, Viswanathan S, et al. Recent and advanced therapy for oral cancer. Biotechnol Bioeng. 2023;120(11):3105–3115. doi:10.1002/bit.28452
7. Slieker FJB, de Bree R, Van Cann EM. Oral squamous cell carcinoma involving the maxillae: factors affecting local recurrence and the value of salvage treatment for overall survival. Head Neck. 2020;42(8):1821–1828. doi:10.1002/hed.26108
8. Kaur J, Srivastava R, Borse V. Recent advances in point-of-care diagnostics for oral cancer. Biosens Bioelectron. 2021;178:112995. doi:10.1016/j.bios.2021.112995
9. Zhang J, Yao YH, Li BG, Yang Q, Zhang PY, Wang HT. Prognostic value of pretreatment serum lactate dehydrogenase level in patients with solid tumors: a systematic review and meta-analysis. Sci Rep. 2015;5(1):9800. doi:10.1038/srep09800
10. Mafessoni TP, Mazur CE, Amenabar JM. Salivary lactate dehydrogenase (LDH) as a tool for early diagnosis of oral cancer in individuals with Fanconi anemia. Med Hypotheses. 2018;119:29–31. doi:10.1016/j.mehy.2018.07.021
11. Duzkopru Y, Kocanoglu A, Dogan O, Sahinli H, Cilbir E, Altinbas M. Hemoglobin, albumin, lymphocyte, and platelet score as a predictor of prognosis in metastatic gastric cancer. World J Gastrointest Oncol. 2023;15(9):1626–1635. doi:10.4251/wjgo.v15.i9.1626
12. Li Q, Kong F, Ma J, et al. Nomograms based on fibrinogen, albumin, neutrophil-lymphocyte ratio, and carbohydrate antigen 125 for predicting endometrial cancer prognosis. Cancers. 2022;14(22):5632. doi:10.3390/cancers14225632
13. Wang X, Xu J, Zhang H, Qu P. The effect of albumin and hemoglobin levels on the prognosis of early-stage cervical cancer: a prospective, single-center-based cohort study. BMC Women's Health. 2023;23(1):553. doi:10.1186/s12905-023-02713-5
14. Bao X, Liu F, Lin J, et al. Nutritional assessment and prognosis of oral cancer patients: a large-scale prospective study. BMC Cancer. 2020;20(1):146. doi:10.1186/s12885-020-6604-2
15. Hiraoka SI, Shimada Y, Kawasaki Y, Akutagawa M, Tanaka S. Preoperative nutritional evaluation, surgical site infection, and prognosis in patients with oral cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;134(2):168–175. doi:10.1016/j.oooo.2022.01.009
16. Aday U, Boyuk A, Akkoc H. The prognostic significance of serum lactate dehydrogenase-to-albumin ratio in colorectal cancer. Ann Surg Treat Res. 2020;99(3):161–170. doi:10.4174/astr.2020.99.3.161
17. Hu Y, Zhou Y, Cao Y, et al. Nomograms based on lactate dehydrogenase to albumin ratio for predicting survival in colorectal cancer. Int J Med Sci. 2022;19(6):1003–1012. doi:10.7150/ijms.71971
18. Shu XP, Xiang YC, Liu F, Cheng Y, Zhang W, Peng D. Effect of serum lactate dehydrogenase-to-albumin ratio (LAR) on the short-term outcomes and long-term prognosis of colorectal cancer after radical surgery. BMC Cancer. 2023;23(1):915. doi:10.1186/s12885-023-11446-5
19. Wu J, Wu A, Wang S, et al. The value of lactate dehydrogenase to albumin ratio and immune inflammation biomarkers in colorectal cancer. Front Surg. 2023;10:1118403. doi:10.3389/fsurg.2023.1118403
20. Xu H, Lin T, Ai J, et al. Utilizing the lactate dehydrogenase-to-albumin ratio for survival prediction in patients with bladder cancer after radical cystectomy. J Inflamm Res. 2023;16:1733–1744. doi:10.2147/JIR.S384338
21. He J, Tong L, Wu P, Wu Y, Shi W, Chen L. Prognostic significance of preoperative lactate dehydrogenase to albumin ratio in breast cancer: a retrospective study. Int J Gen Med. 2023;16:507–514. doi:10.2147/IJGM.S396871
22. Menekse S, Kut E, Almuradova E. Elevated serum lactate dehydrogenase to albumin ratio is a useful poor prognostic predictor of nivolumab in patients with non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2023;27(5 Suppl):86–94. doi:10.26355/eurrev_202310_34076
23. Claps G, Faouzi S, Quidville V, et al. The multiple roles of LDH in cancer. Nat Rev Clin Oncol. 2022;19(12):749–762. doi:10.1038/s41571-022-00686-2
24. Miholjcic TBS, Halse H, Bonvalet M, et al. Rationale for LDH-targeted cancer immunotherapy. Eur J Cancer. 2023;181:166–178. doi:10.1016/j.ejca.2022.11.032
25. Sharma D, Singh M, Rani R. Role of LDH in tumor glycolysis: regulation of LDHA by small molecules for cancer therapeutics. Semin Cancer Biol. 2022;87:184–195. doi:10.1016/j.semcancer.2022.11.007
26. Anami S, Doi H, Nakamatsu K, et al. Serum lactate dehydrogenase predicts survival in small-cell lung cancer patients with brain metastases that were treated with whole-brain radiotherapy. J Radiat Res. 2019;60(2):257–263. doi:10.1093/jrr/rry107
27. Liu J, Wu D, Shen B, et al. Serum lactate dehydrogenase predicts brain metastasis and survival in limited-stage small cell lung cancer patients treated with thoracic radiotherapy and prophylactic cranial irradiation. Strahlenther Onkol. 2022;198(12):1094–1104. doi:10.1007/s00066-022-01977-4
28. Ma L, Qiu J, Zhang Y, et al. Prognostic factors for operable biliary tract cancer: serum levels of lactate dehydrogenase, a strong association with survival. Onco Targets Ther. 2018;11:2533–2543. doi:10.2147/OTT.S150502
29. Pelizzari G, Basile D, Zago S, et al. Lactate dehydrogenase (LDH) response to first-line treatment predicts survival in metastatic breast cancer: first clues for a cost-effective and dynamic biomarker. Cancers. 2019;11(9):1243. doi:10.3390/cancers11091243
30. Chen CC, Tang WH, Wu CC, et al. Pretreatment circulating albumin, platelet, and rdw-sd associated with worse disease-free survival in patients with breast cancer. Breast Cancer. 2024;16:23–39. doi:10.2147/BCTT.S443292
31. Dai D, Balega J, Sundar S, et al. Serum albumin as a predictor of survival after interval debulking surgery for advanced ovarian cancer (aoc): a retrospective study. J Invest Surg. 2022;35(2):426–431. doi:10.1080/08941939.2020.1827314
32. Liu C, Li X. Stage-dependent changes in albumin, nlr, plr, and afr are correlated with shorter survival in patients with gastric cancer. Clin Lab. 2019;65(9). doi:10.7754/Clin.Lab.2019.190132
33. Tang Q, Li X, Sun CR. Predictive value of serum albumin levels on cancer survival: a prospective cohort study. Front Oncol. 2024;14:1323192. doi:10.3389/fonc.2024.1323192
34. Cui L, Yu H, Sun Q, Miao Y, Jiang K, Fang X. Effects of body mass index and serum albumin on overall survival in patients with cancer undergoing pancreaticoduodenectomy: a single-center retrospective cohort study. World J Surg Oncol. 2022;20(1):221. doi:10.1186/s12957-022-02678-z
35. Gan W, Zhang MX, Wang JX, et al. Prognostic impact of lactic dehydrogenase to albumin ratio in hepatocellular carcinoma patients with child-Pugh I who underwent curative resection: a prognostic nomogram study. Cancer Manag Res. 2018;10:5383–5394. doi:10.2147/CMAR.S176317
36. Feng JF, Wang L, Yang X, Jiang YH. Prognostic value of lactate dehydrogenase to albumin ratio (LAR) in patients with resectable esophageal squamous cell carcinoma. Cancer Manag Res. 2019;11:7243–7251. doi:10.2147/CMAR.S208320
37. Gao S, Wu M, Chen Y, et al. Lactic dehydrogenase to albumin ratio in prediction of unresectable pancreatic cancer with intervention chemotherapy. Future Oncol. 2018;14(14):1377–1386. doi:10.2217/fon-2017-0556
38. Xie Z, Zhou H, Wang L, Wu Y. The significance of the preoperative lactate dehydrogenase/albumin ratio in the prognosis of colon cancer: a retrospective study. PeerJ. 2022;10:e13091. doi:10.7717/peerj.13091
39. Aday U, Tatli F, Akpulat FV, et al. Prognostic significance of pretreatment serum lactate dehydrogenase-to-albumin ratio in gastric cancer. Contemp Oncol. 2020;24(3):145–149. doi:10.5114/wo.2020.100219
40. Peng RR, Liang ZG, Chen KH, Li L, Qu S, Zhu XD. Nomogram based on lactate dehydrogenase-to-albumin ratio (LAR) and platelet-to-lymphocyte ratio (plr) for predicting survival in nasopharyngeal carcinoma. J Inflamm Res. 2021;14:4019–4033. doi:10.2147/JIR.S322475
41. Zhao R, Liang Z, Chen K, Zhu X. Nomogram based on inflammatory biomarkers and nutritional indicators for predicting overall survival in locoregionally advanced nasopharyngeal carcinoma. J Inflamm Res. 2022;15:2971–2981. doi:10.2147/JIR.S366299
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
