Back to Journals » International Journal of Nanomedicine » Volume 19
Emerging Nanotechnology in Preclinical Pancreatic Cancer Immunotherapy: Driving Towards Clinical Applications
Authors Pan X, Han T, Zhao Z , Wang X, Fang X
Received 29 February 2024
Accepted for publication 16 June 2024
Published 2 July 2024 Volume 2024:19 Pages 6619—6641
DOI https://doi.org/10.2147/IJN.S466459
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dongwoo Khang
Xuan Pan,1,* Ting Han,2,* Zixuan Zhao,3,4 Xiaoming Wang,1 Xiaosan Fang1
1Department of Hepato-Biliary-Pancreatic Surgery, The First Affiliated Hospital of Wannan Medical College (Yijishan Hospital of Wannan Medical College), Wuhu, 241000, People’s Republic of China; 2Department of Gastroenterology, The First Affiliated Hospital of Wannan Medical College (Yijishan Hospital of Wannan Medical College), Wuhu, 241000, People’s Republic of China; 3The Translational Research Institute for Neurological Disorders of Department of Neurosurgery, The First Affiliated Hospital of Wannan Medical College (Yijishan Hospital of Wannan Medical College), Wuhu, 241000, People’s Republic of China; 4The Institute of Brain Science, Wannan Medical College, Wuhu, 241000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaosan Fang; Xiaoming Wang, Department of Hepato-Biliary-Pancreatic Surgery, The First Affiliated Hospital of Wannan Medical College (Yijishan Hospital of Wannan Medical College), 2 West Zheshan Road, Wuhu, 241002, People’s Republic of China, Tel +86-0553-5739219, Email [email protected]; [email protected]
Abstract: The high malignant degree and poor prognosis of pancreatic cancer (PC) pose severe challenges to the basic research and clinical translation of next-generation therapies. The rise of immunotherapy has improved the treatment of a variety of solid tumors, while the application in PC is highly restricted by the challenge of immunosuppressive tumor microenvironment. The latest progress of nanotechnology as drug delivery platform and immune adjuvant has improved drug delivery in a variety of disease backgrounds and enhanced tumor therapy based on immunotherapy. Based on the immune loop of PC and the status quo of clinical immunotherapy of tumors, this article discussed and critically analyzed the key transformation difficulties of immunotherapy adaptation to the treatment of PC, and then proposed the rational design strategies of new nanocarriers for drug delivery and immune regulation, especially the design of combined immunotherapy. This review also put forward prospective views on future research directions, so as to provide information for the new means of clinical treatment of PC combined with the next generation of nanotechnology and immunotherapy.
Keywords: pancreatic cancer, immunotherapy, nanomedicine, immunosuppressive tumor microenvironment regulation, combined drug delivery strategy
Graphical Abstract:
Introduction
Pancreatic cancer (PC) is a highly lethal malignant tumor with the characteristics of insidious onset, rapid progression, and poor prognosis. According to the World Health Organization (WHO) classification, PC has been divided into epithelial and non-epithelial origins based on tissue origin, with ductal adenocarcinoma originating from adenocarcinoma accounting for 80–90% of all PC types. Statistics show that PC mortality still ranks fourth among tumor-related causes of death, while the overall five-year survival rate is only about 7% and the mortality-to-incidence ratio (M/I) is approximately 0.94, ranking first among all common tumors.1 The extremely malignant nature and poor prognosis reflected thus pose a more severe challenge to the basic transformation and clinical research of PC. Surgery is the only potential cure for PC but over 80% of patients are already in locally advanced or metastatic PC at the time of diagnosis, missing the opportunity for surgical resection and cure. Therefore, the treatment of advanced PC mainly relies on drug therapy. For a long time, the drug options for first-line treatment of PC have been very limited, with gemcitabine-based treatment regimens and the FOLFIRINOX (a four-drug combination chemotherapy regimen of fluorouracil, calcium folinate, irinotecan and oxaliplatin) regimen and its modified regimens remaining the main treatment options.2–4 However, with the advancement of treatment, increasing drug resistance and cumulative toxicity, patients will soon face a dilemma where there are no drugs available.5
The development of immunotherapies to activate the immune system to effectively eradicate tumor cells has already changed the outlook for patients with advanced solid tumors. Mainstream of tumor immunotherapy includes immune checkpoint blockade (ICB) represented by PD-1/PD-L1 inhibitors, tumor vaccines that induce immune responses by presenting specific antigens, and CAR-T therapy that modifies T cells for specific recognition and killing.6–8 However, only 1% of PC patients with microsatellite instability may benefit from a single dose of immunotherapy because of the unique resistance of PC to immunotherapy. The failure is mainly blamed on the immunosuppressed tumor microenvironment (TME) of PC, which characterized by significant infiltration of tumor-promoting immune cells (such as myeloid-derived suppressor cells (MDSCs), which mainly composed of immature neutrophils and monocytes) and often lacks cytotoxic CD8+ T cells.9 Furthermore, another significant challenge faced by current immunotherapies is their limited ability to specifically target tumors. Therapeutic drugs often have difficulty accumulating effectively within tumor tissues, resulting in suboptimal treatment outcomes. Therefore, there is a constant need to explore innovative treatment methods.
In recent years, the development of nanotechnology has played an important role in improving the efficacy of precise cancer treatment based on immunotherapy.10 Due to the unique size effect, nanomedicine delivery platforms enable target and release drugs to enrich TME cells and reprogram the elusive immunosuppressive TME. At the same time, the controllable pharmacokinetics behavior and modifiable functional domains of the nanomedicine delivery platform can enhance the release and presentation of tumor antigens to antigen-presenting cells (APCs) and enhance their uptake by dendritic cells (DCs), strengthening the treatment of low immunogenic cancers.11,12 Finally, the development of nanocarriers provides a possibility for the efficient and simultaneous delivery of various immunotherapeutic drugs, with the potential to enhance immune activation through synergistic effects, which may overcome the obstacles of effective immune therapy for PC. Currently, new immunotherapy delivery platforms are being researched and developed, such as liposomes, polymer micelle, scaffolds, and nanogels, to protect therapeutic agents, target delivery, and intelligently control release, promoting safer and more effective cancer immunotherapy.13,14
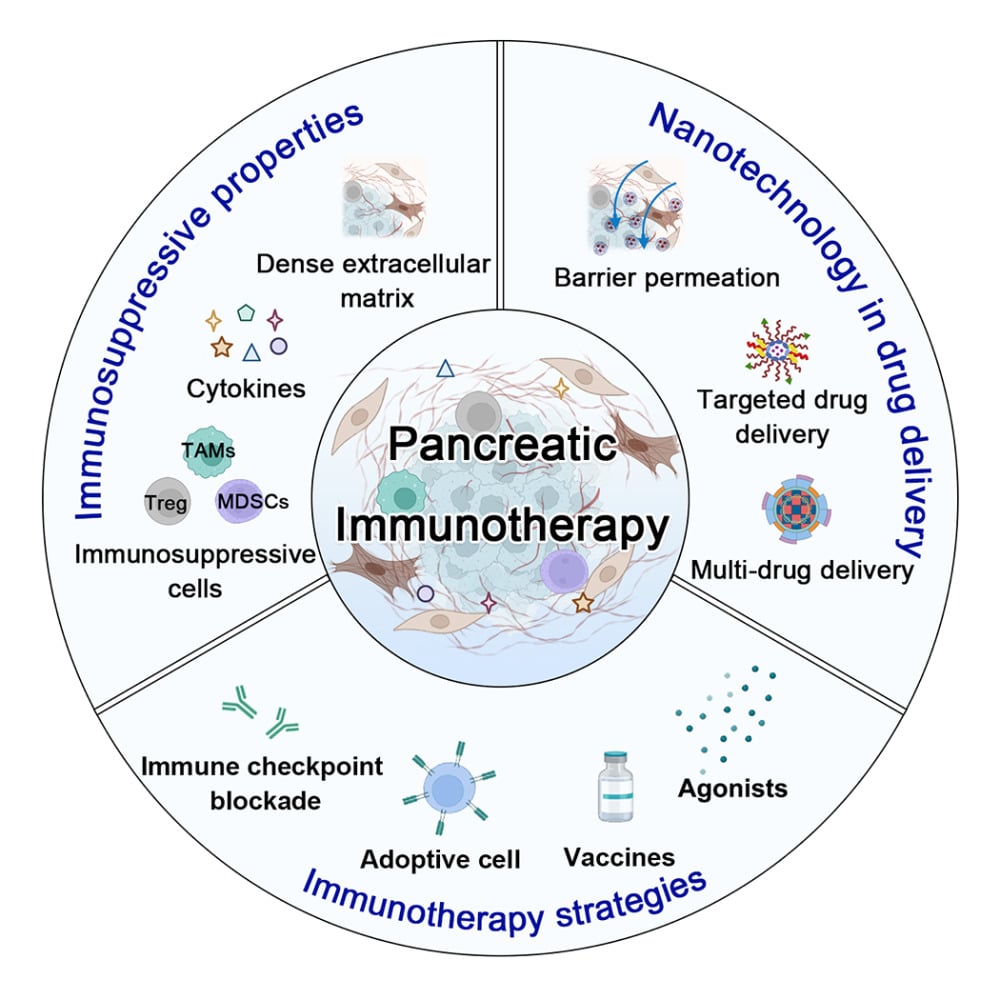
As the advanced comprehension of the intrinsic characteristics of PC and its microenvironment, researchers have engineered a variety of PC-targeting nanomedicine employing diverse targeting strategies. Despite these innovations, the dense tumor stroma present within PC tissues continues to impede the effective penetration, thereby limiting their anticancer efficacy in scenarios characterized by significant tissue fibrosis.15 Harnessing strategies that target both the PC stroma and cancer-associated cells present an opportunity to modulate the TME, thereby potentially augmenting the therapeutic efficacy of chemotherapeutic agents against PC.16 Concurrently, the complexity of the immunological microenvironment poses a challenge to monotherapy approaches, which often fall short in their ability to effect a comprehensive reversal of immunosuppressive conditions. Given the unique features of the TME, a synergistic combination of multiple immunotherapeutic strategies may be essential to counteract the suppressive effects inherent in the pancreatic cancer immunological landscape.17 These judicious methodologies in nanomedicine design and therapeutic combination necessitate an in-depth comprehension of the intricate cross-talk within the PC immune microenvironment, coupled with an appraisal of the developmental effectiveness of these approaches from the vantage point of clinical immunotherapy. Therefore, this article will focus on their characteristics and their impact on cancer immunotherapy, and based on the current status of PC immunotherapy methods, discuss how to better utilize nanotechnology for targeted delivery (Figure 1).
Current Status of Clinical Immunotherapy for Pancreatic Cancer
Immunosuppressive Properties of Pancreatic Cancer
Intrinsic Immune Escape Properties
Pancreatic ductal adenocarcinoma (PDAC), which accounts for 95% of PC, is characterized by distinctive genomic mutations, with a staggering 92% of cases manifesting mutations in the KRAS gene.18 This genetic alteration endows PDAC cells with the capability to elude immune surveillance through the activation of diverse signaling pathways (Figure 2A). Specifically, the KRAS mutation (mKRAS) disrupts tumor-specific antigens presentation pathways while concurrently suppressing T cell infiltration and activity, posing a formidable challenge for the immune system to discern and kill PDAC cells.19 In PDAC cells, the mKRAS mutation causes major histocompatibility complex class I (MHC-I) to be selectively targeted for lysosomal degradation through an autophagy-dependent mechanism. This leads to the downregulation of MHC-I expression on the surface of tumor cells, impairing the antigen recognition process, and ultimately contributing to immune evasion.20,21 Ongoing clinical endeavors suggest that pharmacological inhibition of autophagy, exemplified by hydroxychloroquine administration, may amplify the anti-tumor immune response.22 Moreover, the overexpression of CD47 by PDAC cells further impedes the phagocytic and antigen-presenting functions of macrophages and DCs, achieved through binding with signal regulatory protein alpha (SIRPα).23 Furthermore, these malignant cells contribute to immune evasion by secreting Indoleamine 2.3-dioxygenase (IDO), which catalyzes the degradation of tryptophan, a pivotal component for the survival of cytotoxic lymphocytes (CTLs), consequently inducing T cell inactivation.24 And approximately 12.5% of patients with PDAC showcase heightened expression of programmed death-ligand 1 (PD-L1) on tumor cells.25 This elevated PD-L1 interacts with the abundant programmed cell death protein 1 (PD-1) on the surface of T cells, effectively inhibiting their cytotoxic capabilities. Furthermore, PDAC cells, activated through the WNT/β-catenin pathway, directly secrete immunoregulatory cytokines such as interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β).26 These cytokines impede the maturation and differentiation of DCs, and promote the accumulation of regulatory T cells (Tregs).27 The heightened expression of CCL5 plays a pivotal role in attracting Tregs to the tumor microenvironment.28 Additionally, PC orchestrates the recruitment of tumor-associated macrophages and MDSCs through the hyperactivation of the CCL/CCR2 axis.29 A comprehensive understanding of these aberrant physiological mechanisms not only elucidates the intricacies of immune evasion in PC but also provides indispensable insights for the development of efficacious treatment modalities. Isolated strategies to overcome specific immune targets have also had limited success, possibly due to the presence of multiple immunomodulatory pathways in the pancreatic ductal adenocarcinoma microenvironment.
 |
Figure 2 (A) The PDAC-intrinsic immunosuppressive properties and immunosuppressive tumor microenvironment. (B) Clinical immunotherapy strategies for PDAC. |
Immunosuppressive Tumor Microenvironment
PDAC cells, beyond manifesting intrinsic immune evasion characteristics, intricately engage with the fibroinflammatory microenvironment. Pancreatic stellate cells (PSCs) recognized as the predominant precursors of cancer-associated fibroblasts (CAFs), which exert pivotal roles in developmental processes, tissue homeostasis, and the progression of tumors.30 CAFs exhibit prolific secretion of proteins, including collagen isoforms (eg, type I and type III collagen), fibronectin, and hyaluronic acid, thereby augmenting the density of the extracellular matrix (ECM).31,32 This augmentation not only impedes the penetration of therapeutic agents and the infiltration of immune cells but also induces heightened interstitial fluid pressure and fosters a hypoxic microenvironment.33,34 The dense ECM further contributes to a malnourished milieu for PDAC, inherently restraining tumor cell proliferation. More importantly, CAFs release various metabolites that serve as an energy source for cancer cells, fostering their proliferation within a nutritionally deprived environment.
CAFs also participate in orchestrating immune responses in PDAC. They attenuate the activity of T cells and natural killer cells through the secretion of immunosuppressive factors, such as IL-6, IL-10, VEGF and TGF-β, thereby facilitating immune evasion of the tumor.35 And CAFs curtail the infiltration of CTLs into the tumor by hyperactivating Focal Adhesion Kinase (FAK) and generating excessive levels of CXCL12.36 Importantly, the proximity of CAFs to PDAC cells delineates their diverse functionalities, underscoring spatial heterogeneity.37 For instance, CAFs positioned in close proximity to cancer cells adopt a classical activated myofibroblast-like phenotype, while those distributed in the surrounding stromal tissue exhibit a partially inflammatory phenotype characterized by cytokine expression.38 This intricate interplay among tumor cells, CAFs, T cells, and myeloid cells underscores the interwoven mechanisms propelling tumor growth within the TME. In conclusion, an exhaustive comprehension of the intricacies of immune suppression in PDAC, coupled with concerted strategies targeting both the stromal components and the immune system, holds promise for delineating more efficacious targets and strategies in the treatment paradigm for PDAC.
Clinical Immunotherapy Strategies
Dendritic Cells Activation
To reverse the immunosuppressive TME, various immunotherapy strategies have been clinically tested (Figure 2B). In the case of PDAC, some vaccine trials aim to stimulate the immune system to generate a strong immune response against tumor antigens (Table 1).39 Multiple trials in PDAC patients have investigated different types of vaccines, such as whole-cell vaccines (eg, GVAX), MUC1 peptide vaccines, Wilm’s tumor 1 (WT-1) vaccines, algenpantucel-L, mutant KRAS peptide vaccines, and Listeria monocytogenes-expressing mesothelin (CRS-207) vaccines.40 Clinical trial reports assessing autologous whole-cell GVAX and CRS-207 vaccines in combination with ICB have shown promising results, highlighting improvements in anti-tumor immune infiltration and suggesting this therapeutic approach for maintenance treatment in metastatic PDAC patients.41 While still in the early stages of research, cancer vaccines offer a promising avenue for eliciting a stronger immune response against PC.
 |
Table 1 Select Immunotherapy-Based Clinical Trials |
Additionally, DCs express co-stimulatory receptors that, when activated, enhance the antigen presentation process in an immunosuppressive environment, thereby stimulating T cells. Among these receptors, CD40 agonists show great potential in the treatment of PDAC.42 The anti-tumor effect of CD40 is achieved by binding to T cell surfaces, promoting the production of cytokines and chemokines, inducing the expression of co-stimulatory molecules, and enhancing antigen presentation. In a Phase I clinical trial involving a potent monoclonal antibody targeting CD40, the immunoactivation effects, including CD8+ T cell enrichment, increased mature DCs, and decreased tumor-associated macrophages (TAMs) were confirmed.43 Moreover, an enhanced DCs response to CD40 agonists was found to be correlated with increased PD-L1 expression in the PDAC TME.41 This suggests that combining ICBs with CD40 agonists could be a valuable treatment strategy.
T Cell Activation
In addition to targeting DCs, common therapeutic strategies in clinical practice include enhancing the tumoricidal activity of the adaptive immune system. Treatment modalities encompass CAR-T therapy and immune checkpoint inhibitors. The currently intensively studied CAR-T cell therapy has been attempted in PDAC patients but has not yielded satisfactory results. CAR-T therapy involves genetically engineering the patient’s own T cells to express a CAR capable of recognizing and attacking tumor cells.44
In clinical research, T lymphocytes activated with MUC-1 peptide have been used for unresectable or recurrent PDAC patients, achieving complete remission in patients with multiple lung metastases and 25% exhibiting stable disease (SD).45 However, subsequent studies have not demonstrated significant efficacy, possibly due to the high heterogeneity and variability of tumor cells, rendering T cells activated against limited tumor-associated antigens ineffective in producing a sustained and potent cytotoxic effect. Additionally, the upregulation of various inhibitory receptors, such as the common PD-1, on the surface of CAR-T cells over time may contribute to this limitation.46 Therefore, combining adoptive cell therapy with immune checkpoint blockade may play a role in the future.
Myeloid Cells Regulation
The immune cell population in pancreatic tumors is primarily composed of medullary cells, sparking interest in studying the role of various medullary populations in PDAC biology. PDAC cells activate GM-CSF production through oncogenic KRAS signaling, leading to the recruitment of MDSCs. Novel agonists targeting CD11b/CD18 receptors in a specific myeloid subpopulation within PDAC tumors have shown promising preclinical results and are currently being evaluated in clinical trials.47 The CCL2-CCR2 chemokine pathway plays a crucial role in attracting TAM to the TME, garnering attention from researchers. In a Phase I trial involving advanced PDAC, the oral CCR2 inhibitor PF-04136309 combined with FOLFIRINOX achieved a 49% ORR and 97% DCR. CSF1-R inhibition, like CCR2 inhibition, disrupts TAMs recruitment and enhances T cell activation.46,48 However, the combination of cabiralizumab (anti-CSF-1R) with anti-PD-1 therapy did not meet the primary endpoint of increasing PFS in advanced PDAC.49 Ongoing research is investigating the use of other CSF1-R inhibitors in combination with chemotherapy and immunotherapy to enhance treatment effectiveness. Targeting MDSCs is a promising aspect of immunotherapy strategies aimed at overcoming the immunosuppressive TME.
Targeting Stromal Cells
Therapies targeting the stromal components of PDAC aim to weaken their protective role against tumors, enhance drug penetration, and improve immune cell infiltration. Various research studies have been conducted clinically to explore this treatment strategy, yielding diverse outcomes. One of the primary targets within the PDAC stroma is HA, a key component of the ECM.50 Reports indicate that an enzyme degrading HA, PEGPH20, can enhance the efficacy of chemotherapy drugs in KPC mice.34,51 In a Phase II clinical study, its combination with albumin-bound paclitaxel/gemcitabine extended the progression-free survival (PFS) of PDAC patients with high HA levels.52 However, the disappointment arose when the Phase III clinical trial did not demonstrate any improvement in overall survival (OS), ultimately leading to its termination.50 This failure prompted researchers to redirect their focus towards rational combinations of hyaluronic acid (HA) with other immunotherapies, including the combination of PEGPH20 with immune checkpoint blockade (ICB). Furthermore, deactivating pancreatic stellate cells (PSCs), a primary source of CAFs, is crucial in disrupting the tumor stroma. The TGF-β signaling pathway is crucial in activating PSCs, and efforts to block this pathway’s transmission have progressed to clinical trial phases, with drugs like Galunisertib (a TGFβRI kinase inhibitor) and Ceritinib (a TGF-β receptor ALK inhibitor) under investigation.53 On the other hand, CAFs promote the proliferation and survival of other immunosuppressive cells by secreting various chemokines and cytokines. By disrupting the interaction pathways between CAFs and other cells, their connections can be severed. Several therapeutic molecules aimed at this approach have advanced to different clinical stages, including tocilizumab (an anti-IL6R antibody), anakinra (an IL-1R antagonist), and MSC-1 (an anti-LIF antibody).
In general, PC displays robust immune evasion capabilities, which pose challenges for current immunotherapy treatments. However, numerous clinical trials are underway to investigate combined immunotherapies and to gain insight into immune evasion mechanisms in hopes of identifying more effective treatment approaches in future studies. The use of nanotechnology in the treatment of PC is gaining momentum, with research focusing on enhancing drug permeability and targeting, boosting immune cell activity, and improving the tumor microenvironment to enhance the impact of immunotherapy on PC.
Nanomedicines in Immunotherapy Agent Delivery
Immunotherapy has been developed against highly aggressive malignant PC.54 However, its efficacy in clinical PC patients is not satisfying, which is due to various reasons. Firstly, the unique composition of pancreatic tumor contributed to an immune-excluded TME.55 And the fibrotic stroma composed of cancer-associated fibroblasts (CAFs) encapsulates tumor cells and prevents it from being infiltrated by systemic drugs and immune cells, which will even accelerate tumor progression, metastasis, and trigger the apoptosis of T cells. In addition to the low infiltration performance of anti-tumor agents, the inefficiency of immunotherapy drugs and even uncontrollable adverse reactions have also hindered the progress of immunotherapy drugs in clinical PC.56 The only FDA-approved immune checkpoint inhibitor Pembrolizumab against PC has been exactly plagued by repair-deficient mismatch with great individual different frequencies and consequent drug resistance.57,58 Therefore, new strategies are yelled to be developed to enhance the tumor specificity and immunogenicity of immunotherapy drugs.
Nanomaterials have participated in the forefront in drug delivery area to address some tricky but critical obstacle in immunotherapy.59 Not only small-molecule drugs but also complex macromolecules such as protein drugs and nucleic acid drugs can be successfully delivered via nano-carrier.60,61 For example, nab-paclitaxel, a nanomedicine approved by the FDA for PC treatment, showing significant advantages over conventional paclitaxel formulation, including longer circulation times, better systemic distribution, and lower toxicity.62 Liposomal irinotecan, in combination with 5-fluorouracil (5-FU) and leucovorin, has been approved by the FDA as a therapeutic option for PDAC patients bearing resistance to gemcitabine.63 In contrast to free irinotecan, liposomal nanomedicine demonstrate both lower toxicity and higher efficacy. All of these clinical cases demonstrate the benefits of nanomedicine strategies, such as enhanced drug penetration into tumors, remodeling of the immunosuppressive TME, and a stronger safety profile.10 Herein, different nanotechnologies would be introduced according to their therapeutic mechanism.
Nanomedicines to Enhance Immunogenic Cell Death
When tumor cells are attacked by external stimuli, they would release damage-associated molecular patterns (DAMPs) and activate sequent tumor-specific immune responses. This process is known as immunogenic cell death (ICD).64 Continued ICD contributes to the recruitment and activation of cytotoxic T cell lymphocytes. Interestingly, some chemotherapy drugs and photodynamic therapy can evoke the occurrence of ICD.65 Therefore, researchers designed nanomedicines in the hope of enhancing ICD-induced immunity.
The most common used ICD-inducible chemotherapeutic agent is oxaliplatin. In order to reduce its intrinsic dose-limiting toxicity, Liu et al loaded it into mesoporous silica nanoparticles (MSNP).66 This system realized a high drug loading efficiency and in vivo stability of oxaliplatin (Figure 3A). After intravenous injection, orthotopic Kras PDAC model mice showed stronger tumor killing response induced by ICD, and longer survival with decreased bone marrow toxicity. Nanocarriers are also capable of inducing ICD, thus overcoming the immunosuppressive microenvironment by themselves. A sequential receptor–mediated mixed-charge targeted delivery system was designed to realize ICD effects.67 In addition, this nano-carrier also owned lysosomal escape capacity and mitochondrial targeting ability. After helping ingenol-3-mebutate (I3A), a tumor-suppressing botanical ingredient, transport into tumor, stronger efficacy than first-line clinical drugs in Panc02 model mice was exhibited.
 |
Figure 3 Nanomedicines in immunotherapy agent delivery. (A) Design, synthesis, and characterization of MSNPs that contain the activated Pt drug. And approximately 5.6× fold improvement in drug loading was also achieved for DAPt. Reprinted from Liu X, Jiang J, Chang CH, et al. Development of facile and versatile platinum drug delivering silicasome nanocarriers for efficient pancreatic cancer chemo-immunotherapy. Small. 2021;17:e2005993. © 2021 Wiley-VCH GmbH.66 (B) Schematic illustration of FAP-α and ROS dual-activatable nanoregulator for tumor-specific delivery of CAFi and IDO1i. (C) Masson staining and IHC examination validated decreased collagen and FAP-α expression in the PMFPL group. And nanoparticle treatment increased the rate of DC maturation, TIL and CTL. Reprinted from Pan J, Lai Y, Zhang S, et al. Self-adaptive nanoregulator to mitigate dynamic immune evasion of pancreatic cancer. Adv Mater. 2023;35:e2305798. © 2023 Wiley-VCH GmbH.68 (D) Schematic and TEM pictures to show the structure of OX-laden MSN. (E) Immuno-PET imaging to demonstrate the induction of the systemic immune response by OX/IND-MSNP administration to animals carrying orthotopic KRAS-mediated pancreatic carcinoma tumors. Reprinted from Lu J, Liu X, Liao Y-P, et al. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat Commun. 2017;8:1811. Creative Commons.69 (F) Schematic of antitumor immune response induced by pyroptosis pathway activation and COX-2 inhibition. (G) Pt-In NP sensitize platinum drugs by inhibiting COX-2 to enhance cellular pyroptosis and produce anticancer activity in vitro. Reprinted from Yu B, Wang Y, Bing T, et al. Platinum prodrug nanoparticles with COX-2 inhibition amplify pyroptosis for enhanced chemotherapy and immune activation of pancreatic cancer. Adv Mater. 2023;36:e2310456. © 2023 Wiley-VCH GmbH.70 *p < 0.05, **p < 0.01 and ***p < 0.001. |
In summary, nanomaterials have achieved great improvements in both safety and immunotherapy efficiency with ICD involvement.
Nanomedicines to Modulate Tumor Microenvironment
As previously mentioned, a “physical barrier” formed by dense stroma exists in pancreatic tumor environment. This stoma is also capable of inducing immunosuppressive lymphocytes and secreting related cytokines, thus creating an immunosuppressive TME.71 Consequently, PC patients derive limited benefits from current immunotherapies. To achieve efficient therapy, modulating the TME, especially the dense stroma, is considered to be one of the most potential strategies for PC treatment.72 Small-molecule drugs and proteins have been utilized in stroma regulation. For instance an adaptive nanomodulator engineered a peptide-drug conjugate (PDC) as an anchor sequence has been created.68 This nanoparticle is modified with a CAF homing peptide and PEG chain to prolong circulation time, loaded with the photosensitizer pyropheophorbide and CAF inhibitor (Figure 3B). It enabled the retention of nanoparticles in tumor, ultimately alleviating CAF-mediated immune resistance (Figure 3C). Moreover, indoleamine 2.3-dioxygenase 1 inhibitor (IDO1i) was added to the nanoparticles to further address CD8+ T cell exhaustion and adaptive immune evasion.
In addition, hyaluronic acid (HA) is also one of the important components of the dense stromal matrix protecting cancer cells. It is responsible for increasing the pressure of interstitial fluid, which compresses the blood vessels.73 Recently, human hyaluronidase PH20 (PEGPH20) has been utilized in combination with gemcitabine in clinical trials to degrade HA, resulting in prolonged survival for patients. However, side effects such as HA depletion in other organs have been observed because of the systemic administration with non-targeting ability, leading to dosage limitations. To address this issue, various attenuated ST strains with high tumor specificity have been developed and screened. Studies have shown that bH-ST can target orthotopic PDAC tumors after systemic delivery and significantly degrade HA in TME, thereby further enhancing the intratumoral spread of ST. However, HA depletion alone cannot achieve the desired results, as studies have combined it with other treatments. Zhang et al developed a nanoparticle of human serum albumin coloaded with autophagy inhibitor HCQ and ICD initiator DOX. Meanwhile, HAase and hypoxia-targeted probiotic EcN were decorated on the surface obtain HD@HH/EcN.74 Once the HAase breaks the dense fence wall, nanoparticles could effectively kill the tumor with the help of subsequent autophagy inhibition and immune cell attack. A growing number of combination therapies based on matrix disruption are also being studied.
Nanomedicines in Combination with Chemotherapy
Though chemotherapy is the most common clinical treatment for PC, it shows dismal prognosis.75 It may be attributed to the fact that the chemotherapy drugs themselves show side effects in both safety and efficiency. Even combinations with other immunomodulators have emerged, the clinical result is still disappointing due to the intrinsic immunosuppressive TME of PC and the dense matrix around tumor. Effort has been made to enhance the antitumor effects of chemoimmunotherapy. Nanoparticle-based chemoimmunotherapy is one of the most attractive methods to deliver the drug deeper in tumor.
Currently, the FDA has approved the application of albumin nanocarriers to deliver gemcitabine and irinotecan, resulting in improved pharmacokinetic performance of both drugs. For further improvement, Liu et al utilized mesoporous silica nanoparticles (MSNP) as carriers for the first time, incorporating synergy therapy to enhance antitumor immune response.69 MSNP co-delivered oxaliplatin and IDO inhibitors. IDO inhibitor could reverse immunosuppressive TME through kynurenine pathway (Figure 3D and E). Following the induction of ICD by oxaliplatin, the synergistic treatment composed of oxaliplatin and IDO inhibitor enhances anti-tumor immunotherapy in combination with IDO inhibitors.
In addition, self-assemble to form nanoparticles by altering the structure of the chemotherapy drugs themselves to avoid the side effects from carrier materials is another way. Platinum chemotherapy drugs can induce pyroptosis, which is a newfound programmed mechanism of cell death, accompanying by subsequent anti-tumor immune response and undesirable cyclooxygenase-2 (COX-2) expression increase. To effectively inhibit COX-2 expression while promoting pyroptosis, an amphiphilic polymer composing of indomethacin and platinum prodrug was designed.70 It allowed for self-assembling into nanoparticles (Pt-In NP) and disintegrating in response to glutathione at the tumor site to release two different drugs (Figure 3F and G). After administration, Pt-In-NP could induce pyroptosis in mice bearing PC, thereby prolonging the duration of the immune response.
In summary, the nano-delivery platform provides greater efficiency in the delivery of chemotherapy drugs as well as the potential of combination therapy with other treatment options. This brings more possibilities for the future preclinical researches and clinical practice of chemoimmunotherapy.
Nanomedicines with Other Therapeutic Mechanism
Photodynamic therapy (PDT) is a non-invasive and highly effective treatment that can activate photosensitizers (PSs) via light, thereby producing toxic reactive oxygen species (ROS) to kill tumor cells. Moreover, PDT can induce the development of ICD effect against cancer.76 However, traditional PSs have the disadvantage of insufficient targeting. And the dense extracellular matrix in the TME obstructs the permeability and retention of PSs, limiting the efficacy of PDT. Considering challenge in PDT therapy, Qu et al designed a new light-activated nanoparticle decorated with a tumor-targeting factor MDK.12 After reaching the tumor site, nanoparticles could generate a significant amount of ROS when activated by light to induce cell death. At the same time, ICD was initiated to facilitate TME remodeling and promotion of immune cell infiltration. Furthermore, upon the addition of PD-1 antibody, the anti-tumor efficacy of nanoparticles was further enhanced. Similarly, photothermal therapy (PTT), which converts light energy into heat energy, can also trigger ICD and boost immune responses.77 Researchers have also designed size-adjustable nanoparticles by combining immune checkpoint blockers and PTT thermosensitizers.78 The nanoparticles are able to be widely distributed within tumors and reverse TME.
In addition, Sonodynamic therapy (SDT) and chemodynamic therapy (CDT) which could transform ROS by the Fenton reaction are also gradually being combined with nanotechnology.79,80 Among them, nanocarrier help them achieve deeper penetration, or co-load other drugs to promote the anti-tumor ability of therapeutics.
Clinical and preclinical evidence underscores the considerable potential of immunotherapy in the management of PC. The advent of nanotechnology has introduced a paradigm of enhanced efficacy and safety in the delivery of immunotherapeutic agents. The nanoscale dimensions of these particles enable them to surmount the dense fibrotic stroma, facilitating profound tumor penetration. The combination of chemical modifications and advanced technologies (such as PTT, PDT) endow nanoparticles capabilities such as precise drug release, targeted delivery, and prolonged circulation, thereby amplifying the therapeutic effect in pharmaceutical applications. Nonetheless, it is imperative to address the inherent challenges accompanying nanomedicine, which encompass considerations of nanocarrier biocompatibility and toxicity, individual patient differences, and the transition from preclinical research to clinical validation.
Nanomedicines for Pancreatic Cancer Immunotherapy
Introduction of Immunogenic Cell Death (ICD)
The low immunogenicity of PC underscores the importance of tumor-associated antigens (TAAs) to activate the immune system. ICD is a specific type of cell death that involves releasing damage-associated molecular patterns (DAMPs), triggering antigen-specific immune responses against tumors.81 Certain chemotherapy drugs (eg, DOX and oxaliplatin) have shown the ability to continually generate TAAs and create in situ whole-cell vaccines with multiple specific epitopes by inducing ICD. Therefore, delivering these ICD inducers is a promising strategy for immune activation in PC. Importantly, the immune-boosting effects of ICD inducers depend on the function of DCs and CTLs, and anti-tumor immunity might be compromised if these immune cells are inhibited. Therefore, it is essential to enhance the selectivity of ICD inducers to prevent adverse effects on immune cells.
In addition to chemotherapeutic agents, photothermal therapy (PTT), photodynamic therapy (PDT), and sonodynamic therapy (SDT) can also stimulate an antitumor immune response by inducing ICD in tumor cells. Combining PTT/PDT/SDT with immunotherapy holds great potential as a treatment for PC. However, challenges remain in this synergistic immunotherapy approach. For example, PDT induces ICD but also leads to oxygen consumption and microvascular damage, worsening hypoxia, increasing glycolysis, and intensifying immunosuppression in TME.82,83 To tackle this issue, Sun et al developed a hyaluronic acid (HA)-based supramolecular prodrug nanoplatform for co-delivery of a photosensitizer and a prodrug of bromodomain-containing protein 4 inhibitor (BRD4i).84 In this nanoplatform, PDT boosted immunogenicity and promoted CTL infiltration, while BRD4i counteracted PDT-induced immune evasion by antagonizing the oncogene c-Myc and activating CTLs. Another study utilized tumor-derived re-assembled exosomes as carriers for delivering the photosensitizer chlorin e6 to produce reactive oxygen species (ROS) and as antigens captured by innate immune cells to enhance immune responses, offering a novel approach for immunotherapy combined with PDT.85 SDT utilizes low-intensity ultrasound to activate sonosensitizers, generating ROS to eliminate tumor cells and induce ICD. It is particularly effective for deep tumors like PC due to its excellent penetration capability. However, the short half-life and limited diffusion distance of ROS impede the ICD effect, particularly in pancreatic tumors with dense stroma and poor blood perfusion. To address this, Chen et al developed cavitation-assisted endoplasmic reticulum (ER) targeted sonodynamic droplets that achieved deep penetration and accumulated in the ER of the PC cells.86 Upon ultrasound irradiation, the modified sonosensitizer generated a significant amount of ROS in the ER, amplifying ICD and enhancing the effectiveness of anti-PD-L1 immunotherapy in both orthotopic and distant PDAC models. These studies demonstrate that combined strategies offer promising benefits in PC immunotherapy and represent a crucial research direction for PC.
Promotion of DCs to Antigen Presentation
The dendritic cells (DCs) serve as antigen-presenting cells (APCs) in both innate and adaptive immunity, playing a role in initiating and regulating immune responses. And the effective operation of DC is tied to functional antitumor T cell responses.87 However, in PC patients, the down-regulation of chemokine (C-X-C motif) ligand 17 (CXCL17) and intercellular adhesion molecule 2 (ICAM2) accelerate the process of change from immune surveillance to immune tolerance.88 These two factors are responsible for promoting the infiltration and accumulation of immature myeloid DCs in tumor epithelium, which is a prerequisite for the subsequent cellular immune response. In addition, the decreased number of DCs has been considered as a character in this highly aggressive pancreatic cancer.22 Considering the functional plasticity of DC, regulating the state of DCs is a potential strategy to break the cellular immune dilemma of PC.
Anti-tumor DC vaccines have been developed specifically to reactivate DCs to reverse immunosuppression, and ultimately delaying the progression of PC. During the course of action, dendritic cells (DCs) are supposed to efficiently phagocytose and present tumor antigens to naive T cells, which induces their differentiation into effector cells, thus exerting tumor killing effect.89 However, clinical DC-based vaccines face low antigen internalization efficiency and invalid tumor antigen. To conquer these limitations, nano-adjuvants owning advantages of promoting antigen stability and absorption were designed and widely used. Polyethyleneimine (PEI) modified aluminum hydroxide nanoparticle (LV@HPA/PEI) was designed by Heng Dong et al to serve as a novel adjuvant of DCs in immunotherapy.90 The core LV@HPA showed many superiorities like high antigen loading efficiency and safety. The researchers combined DRibbles, a kind of tumor-derived autophagosomes which contain a large number of tumor-associated antigens (TAAs), with nanocore LV@HPA. Results demonstrated that the immune infiltration and anti-tumor potency of three kinds of DCs (murine mutu DCs, murine bone marrow-derived DCs, and human DCs) were improved. Moreover, the LV@HPA/PEI-DRibbles-DCs activated the tumor-specific T cell, thus inhibiting the pancreatic tumor growth and increasing lifespan of tumor-bearing mice. A wide range of other antigens has been also utilized to obtain antigen-specific immune responses.
Though immunotherapy has been commonly used in clinical practice, recent researches found its ineffectiveness in most PC. Therefore, focus was shifted to the combination therapy of clinical surgery and immunotherapy. Irreversible electroporation (IRE) nonthermal ablation with minimal invade is emerging as a strong candidate for clinical surgery for unresectable PC. However, high tumor recurrence after surgery still bothers the survival of patients. An injectable hydrogel microsphere vaccine loaded with FLT3L and CD40L was developed to mobilize tumor-residue DCs.91 In the mouse model of orthotopic PC surgery, this “immune amplifier” following with IRE effectively activate DCs and enhanced antigen cross-presentation and CD8 T cell activation, transforming the immuno-cold tumor microenvironment into a hot one and eliminating the possibility of postoperative tumor recurrence.
Activation of CTLs and NKs to Kill Tumor Cells
CTLS and NKs play crucial roles in eliminating cancer cells through mechanisms such as granule exocytosis and the death ligand/death receptor system.92 However, PC cells evade immune detection via immune checkpoint pathways (eg, the PD-1/PD-L1 axis), hindering the immune cells’ ability to eradicate cancer cells. Therefore, targeting immune checkpoints is a promising strategy to enhance immune cells’ tumor-killing capacity. Using immune checkpoint inhibitors (eg, monoclonal antibodies (mAbs) targeting PD-1 or PD-L1) has emerged as a leading approach for PC immunotherapy. In addition to antibodies or small-molecule inhibitors, small interfering RNA (siRNA) can directly silence disease-causing genes (eg, PD-L1), providing high specificity and requiring lower effective doses.93,94 Polymer-based nanomaterials are being designed to enhance the stability and tumor-targeting capabilities of siRNA therapeutics for PC immunotherapy.95 For example, poly(lactic-co-glycolic acid)-based nanoparticles loaded with PD-L1 siRNA (siPD-L1) effectively suppressed PD-L1 expression in tumor cells and improved the function of NK cells and CTLs in a humanized mouse model of PC, resulting in reduced pancreatic tumor growth.96 Further, certain signaling pathways (eg, TGFβ signaling) can dampen the tumor response to PD-L1 blockade by T cell exclusion.97 Therefore, the co-delivery of siPD-L1 and inhibitors blocking these signaling pathways is actively explored and has shown anti-tumor efficacy in PC models.98
Inhibition of Immunosuppressive Cells
In the microenvironment of pancreatic tumors, an abundance of immunosuppressive cells such as Tregs, TAMs, and MDSCs inhibit the activity of CTLs and NKs, resulting in a diminished immune response against PC. Current research primarily targets reprogramming TAMs and decreasing Tregs and MDSCs to establish a more responsive immune microenvironment.
Reprogramming of TAMs
In the pancreatic TME, TAMs play a crucial role in treatment resistance, metastasis, and poor survival outcomes. TAMs can transition from an M1 (anti-tumor) to an M2 (pro-tumor) phenotype as the tumor progresses.99 M1-TAMs produce high levels of IL-12 to hinder tumor growth, whereas M2-TAMs secrete immunosuppressive cytokines (eg, IL-10 and TGFβ), along with enzymes that degrade extracellular matrix to support immunosuppression and tumor spread.100 Therefore, reprogramming M2-TAMs to M1-TAMs is a promising strategy to combat immunosuppression in PC and enhance the efficacy of immunotherapy. Since M2-TAM polarization is regulated by various signaling pathways (eg, PI3K-γ and CSF-1/CSF-1RPI3Kγ pathways), Li et al devised a nanomicelle modified with an M2-TAM-targeted peptide for co-delivery of the PI3K-γ inhibitor BEZ 235 and a CSF-1R siRNA to shift TAMs towards an anti-tumor phenotype (Figure 4A).101,102 With enhanced drug accumulation in M2-TAMs, these nanomicelles significantly reduced M2-TAM numbers while increasing M1 TAMs in PDAC (Figure 4B and C). This dual-pathway inhibition strategy focusing on repolarization of M2-TAMs presents a fresh approach for immunotherapy in PC. Furthermore, disrupting tumor lipid metabolism by inhibiting monoacylglycerol lipase (MGLL) activity has shown promise in eliminating tumor cells by disrupting nutrient supply.103 However, inhibiting MGLL has been found to upregulate endocannabinoid receptor-2 (CB-2) on TAMs, potentially shifting them towards a M2 phenotype.104 To address this, Cao et al developed a poly(disulfide amide)-based nanoplatform for co-delivery of MGLL siRNA and CB-2 siRNA, resulting in synergistic antitumor effects through nutrient supply inhibition and repolarization of TAMs to an M1 phenotype, along with increased secretion of tumoricidal cytokines (eg, TNF-α and IL-12).105 Additionally, TAMs in the pancreatic TME are influenced by various physical and chemical factors (eg, prostaglandin E2 (PGE2) and tumor necrosis factor (TNF)) in the pancreatic TME.106 Further exploration of the key pathways involved in TAM regulation will facilitate the development of targeted drugs to manipulate TAMs and advance PC immunotherapy.
 |
Figure 4 Strategies for inhibiting immunosuppressive cells. (A) Schematic diagram of effects of a M2-TAM targeting nanomicelle for TIM reprogramming. (B) Co-localization of M2-TAMs and nanomicelles. (C) Percentage of M1-TAMs (left) and M2-TAMs (right) in tumors after treatment. Reprinted from J Control Release, 321, Li M, Li M, Yang Y, et al. Remodeling tumor immune microenvironment via targeted blockade of PI3K-γ and CSF-1/CSF-1R pathways in tumor associated macrophages for pancreatic cancer therapy. 23–35, copyright 2020, with permission from Elsevier.102 (D and E) The capability of CLANsiIDO1 to knockdown IDO1, leading to increased tumor-infiltrating CTLs and decreased Tregs. (F) Treatment with CLANsiIDO1 significantly decreased the sizes of visible tumor nodules. Reprinted from Huang H, Jiang CT, Shen S, et al. Nanoenabled reversal of IDO1-mediated immunosuppression synergizes with immunogenic chemotherapy for improved cancer therapy. Nano Lett. 2019;19:5356–5365. Copyright 2019, American Chemical Society.107 (G and H) Treatment with PLT/PTX effectively reduced the recruitment of MDSCs by inhibiting adhesion of MDSCs on the blood vessel endothelium. Reprinted from Eur J Pharm Biopharm, 165, Lu Z, Long Y, Wang Y, et al. Phenylboronic acid modified nanoparticles simultaneously target pancreatic cancer and its metastasis and alleviate immunosuppression. 164–173, Copyright 2021, with permission from Elsevier. 108*p < 0.05 and **p < 0.01 and ***p < 0.001. |
Depletion of Tregs
The high frequency of Treg infiltration in pancreatic tumors is strongly linked to a poor prognosis due to the immunosuppressive effects of Tregs. These effects include inhibiting DC antigen presentation, suppressing T cell proliferation and NK cell cytotoxicity, and secreting inhibitory cytokines.109,110 Indoleamine 2.3-dioxygenase-1 (IDO1), an enzyme overexpressed in PC, promotes Treg proliferation by limiting tryptophan and increasing kynurenine catabolites.111 Inhibiting IDO shows potential in reversing Treg-mediated immunosuppression to boost tumor immunotherapy. Cationic lipid-assisted nanoparticles (CLANsiIDO1) were employed to deliver IDO1-targeting siRNA (siDO1) into pancreatic tumors.107 This treatment reduced IDO1 expression, decreased Treg infiltration, and increased antigen-specific T cells, which promoted anti-tumor effects in mice with Panc02 orthotopic pancreatic tumors (Figure 4D–F). Another study found that tumor cell CD73 boosts Treg recruitment by upregulating CCL5 transcription.112 Targeting the CD73/CCL5 axis may thus hold promise for removing tumor-infiltrating Tregs without triggering autoimmunity risk.
Inhibition of MDSCs
MDSCs exert immune-suppressive effects by impairing APC activity, inhibiting T cell activation, and inducing T cell exhaustion.113 Consequently, MDSCs have been recognized as a promising target for immunotherapy in PC. However, therapeutic depletion of MDSCs often results in severe adverse effects and compensatory recruitment of alternative cell populations, leading to treatment failure.114,115 Alternatively, inhibiting the proliferation and tumor trafficking of MDSCs could be an effective and safe approach to mitigate their immunosuppressive effects. A recent study found that low-molecular-weight heparin (LMWH) competitively inhibited the binding between P-selectin and PSGL-1, thereby preventing adhesion between vascular endothelial cells and MDSCs and inhibiting MDSC infiltration into pancreatic tumor tissues.108 Building on this discovery, Lu et al developed PTX-loaded nanoparticles (PLT/PTX) using a self-assembly method with LMWH-based conjugates. Treatment with PLT/PTX successfully reduced MDSC recruitment to tumor tissues, relieved immunosuppression, and inhibited tumor metastasis (Figure 4G and H). Moreover, considering the influence of various cytokines (eg, GM-CSF and CXCL12) on MDSC formation and movement, blocking cytokine–receptor interactions could be a potential immunotherapeutic strategy for PC.116 The development of related nanomedicine in this direction remains to be explored in the future.
Modulation of Pancreatic Stroma Cells
The dense stroma in PC, composed of extracellular matrix and stromal cells (eg, pancreatic stellate cells (PSCs) and CAFs), presents a major obstacle to successful immunotherapy. Stromal cells produce collagen and hyaluronic acid, leading to the formation of dense stroma, which hinders the delivery of drugs and the infiltration of CTLs. Furthermore, drugs that reach the pancreas are mainly absorbed by stromal cells, resulting in off-target effects and impacting the anti-tumor response. Therefore, various strategies have been developed to enhance the effectiveness of PC immunotherapy by targeting the ECM and overactive stromal cells. For example, Huang et al developed a nano-sapper loaded with phosphates-modified α-mangostin (an antifibrotic agent) and plasmid encoding LIGHT (an immune-enhanced cytokine).117 In PDAC model mice, the nano-sapper reshaped the tumor microenvironment and improved the efficacy of checkpoint inhibitors by reducing collagen deposition, normalizing intratumoral vasculature, and promoting the lymphocyte recruiting (Figure 5A–D). Although matrix depletion strategies have shown promise in preclinical studies, they have failed to display positive outcomes in clinical trials and even led to an increased incidence of adverse events.52,118 This may be due to stromal depletion allowing for the emergence of a more aggressive tumor phenotype and the infiltration of regulatory T cells. Therefore, the focus should shift towards selectively modifying the matrix instead of broadly depleting it to enhance the effectiveness of immunotherapy for PC. Chen et al designed a nanoparticle (T-RKP) carrying both PTX and phosphorylated gemcitabine.119 The T-RKP disrupted the central stroma to enhance the anti-tumor effects of PTX and promote infiltration of CTLs (Figure 5E and F). Simultaneously, they preserved the external stroma to maintain the neighboring suppression effect and prevent tumor metastasis (Figure 5G). Recognizing the significant role of tumor–matrix interactions in PC progression, Xie et al further proposed a triple combined matrix remodeling approach. They designed cholesterol-modified polymeric CXCR4 antagonist nanoparticles to co-deliver anti-miR-210 and siKRASG12D, in which CXCR4 antagonist blocked cancer–stroma interactions, anti-miR-210 deactivated PSCs, and siKRASG12D targeted and killed PC cells (Figure 5H).120 The triple-action nanoparticles, administered via intraperitoneal (IP) delivery, exhibited enhanced therapeutic efficacy by reducing immunosuppression, inhibiting metastasis, and prolonging survival. Importantly, due to differences in the surface mesothelium between pancreatic tumor and normal organs, IP delivery serves as a feasible alternative to maximize local efficacy while minimizing systemic side effects in PC treatment.121
 |
Figure 5 Strategies for modulation of pancreatic stroma cells. (A) Schematic diagram of effects of Nano-sapper synergized with immune-checkpoint inhibitor. (B) All the phosphates-modified α-mangostin contained treatments downregulated collagen content. (C) Nano-sapper treatment normalized tumor vessels. (D) Nano-sapper treatment enhanced CTLs infiltration. Reprinted from Huang Y, Chen Y, Zhou S, et al. Dual-mechanism based CTLs infiltration enhancement initiated by Nano-sapper potentiates immunotherapy against immune-excluded tumors. Nat Commun. 2020;11:622. Creative Commons.117 (E) Representative α-SMA and TUNEL-positive images. White dots indicate the disruptive (up) and apoptosis (down) area of pancreatic stroma, respectively. (F) T-RKP treatment increased the infiltration of CTLs. (G) T-RKP treatment reduced metastatic foci in the livers. Reprinted from Chen X, Zhou W, Liang C, et al. Codelivery nanosystem targeting the deep microenvironment of pancreatic cancer. Nano Lett. 2019;19:3527–3534. Copyright 2019, American Chemical Society.119 (H) Schematic diagram of triple combined matrix remodeling strategy. Reprinted from Xie Y, Hang Y, Wang Y, et al. Stromal modulation and treatment of metastatic pancreatic cancer with local intraperitoneal triple miRNA/siRNA nanotherapy. ACS Nano. 2020;14(1):255–271. Copyright 2020, American Chemical Society.120 **p < 0.01. |
In addition to producing matrix components, CAFs also secret immunosuppressive cytokines (eg, TGF-β and CXCL12) to inhibit CTL infiltration and attract immunosuppressive cells (eg, Treg and MDSCs), creating an immunosuppressive environment in PC.122 However, excessive depletion of CAFs can lead to an increase in Tregs and contribute to a more aggressive tumor phenotype, underscoring the importance of appropriately deactivating CAFs.123 Sonic hedgehog (SHH) overexpression, which activates CAFs, has been identified as a crucial target for remodeling the pancreatic stroma.123 To address this, Zhao et al constructed a polymeric micelle-based nanoformulation (M-CPA/PTX) for co-delivering paclitaxel (PTX) and cyclopamine (CPA), an SHH inhibitor that can normalize intratumoral vasculature.15 This strategy increased intratumoral vasculature density without depleting CAFs or collagen in the stroma. As a result, it enhanced infiltration of cytotoxic lymphocytes and prolonged survival in various mouse models of PDAC when combined with PD-1 checkpoint blockade. Additionally, a lipid-protamine-DNA nanoparticle loaded with trap genes was developed to block the immunosuppressive effects of CAF-derived cytokines such as CXCL12.124 These trap genes encoded proteins that bond specifically to and trap IL-10 and CXCL12 within tumors. In PC model mice, treatment with CXCL12-trap showed improved efficacy in reducing tumor growth and prolonging host survival, highlighting the promising potential of blocking or clearing CAF-derived immunosuppressive factors for PC treatment.
Conclusion
Pancreatic cancer (PC) is an almost universally fatal malignancy characterized by extensive infiltration of immunosuppressive myeloid cells, including tumor-associated macrophages and myeloid-derived suppressor cells. Immunotherapy has been shown to be effective in a variety of solid tumors. However, for PC, known as the “king of cancers”, immunotherapy regimen has been ineffective, in large part because of its strong immunosuppression of TME. Therefore, the preclinical research of immunotherapy focuses on reversing the immunosuppressive mechanism and promoting precise targeted drug delivery to cells in PC.
In recent years, with the discovery of more regulatory pathways in tumor immune circulation, the birth of corresponding immunologically active carrier treatment strategies is particularly promising. At the same time, the higher levels of genomic, transcriptomic, and new antigen analysis related to mutation load provide more convenience for identifying meaningful new therapeutic targets and the nature of new antigens.124,125 With the advancement of complex nanomedicine system production, these methods are expected to completely change the success rates of PC clinical presentation and the high-impact care of PC patients. Compared to single immunotherapy, the introduction of nanomedicine can effectively target drug delivery, response release functions, and even activate innate immune regulation in tumors in situ through the rational design of functional carriers. This strategy is expected to provide disruptive therapeutic benefits for the challenging immunotherapy of PC. However, so far, only a few anti-cancer nanomedicines, including drug-antibody conjugates, have entered clinical trials, indicating that there are still some challenges for nanotechnology to improve the effectiveness of immunotherapy for PC.
In this review, we initially commence from the successes and shortcomings of clinical PC immunotherapies, elucidating the efficacy of immunotherapy against PC. More importantly, based on clinical treatment experiences, we propose the limitations imposed by the TME on PC immunotherapy and, drawing from clinical trials, we discuss the regulatory strategies and outcomes related to TME, highlighting the necessity and rational approaches for a delivery platform in PC immunotherapy. This concept, which is oriented by clinical experiences and needs in PC immunotherapy design, supports the development of therapies with greater potential for clinical translation. Then, the limitations of nanoparticles in cancer immunotherapy are largely attributed to the limited knowledge of the immune network during tumorigenesis. The innate and adaptive immunity constitutes a complex network, where the inhibition of one or a few components might be compensated by upregulating other pathways. Therefore, we emphasize the biochemical and physical structural characteristics of the PC immune microenvironment, especially analyzing the design requirements for combined therapies in PC immunotherapy from experiences in clinical PC therapies, and then systematically discuss the design of combined therapy strategies for PC adaptability, providing directional guidance for the development of drug combination for PC therapies. Concurrently, due to the heterogeneity of different tumor structures, the response to the same immunotherapy varies significantly among different tumors, primarily due to the differences in the tumor vasculature. Therefore, building upon various clinical immunotherapies applied to other solid tumors, we explore their application value and therapeutic shortcomings in PC, and then, in light of the characteristics of the PC microenvironment, discuss the design methods for a pancreatic adaptive drug delivery system.
In order to further advance its clinical translation, particular attention should be paid to the following points in future research. Firstly, the safety and efficacy of immunologically active nanocarriers need further evaluation, especially regarding whether the introduction of existing biological materials will affect the complex immune microenvironment balance in the human body, even though preliminary assessments have been conducted at the animal level.125 Nanomedicines have the intrinsic potential to act as antigens, and the elicitation of an immune response against the nanomedicine can expedite their systemic clearance, ultimately compromising their therapeutic effectiveness. Recent studies have indicated that the size and surface property of nanomedicine can significantly affect macrophage migration and uptake, for instance, smaller nanovesicles can substantially decelerate the movement of macrophages but are more readily ingested. Thus, balancing drug loading, cellular uptake, and migration through the optimization of nanomedicine configuration represents a highly promising direction for future research.126 Additionally, due to the “cold tumor” characteristics of PC, the decisive factor for the effectiveness of immunotherapy is not the quantity of drug delivery, but the quality. Therefore, the combined use of multiple approaches, such as radiotherapy, photodynamic therapy, photothermal therapy, etc., to enhance the immunogenicity of “cold tumors” and integrate deeply with immunotherapy, is expected to provide more possibilities for PC immunotherapy.127 Moreover, results from immunotherapeutic efficacy and safety studies conducted in animal models may not necessarily reflect equivalent outcomes in humans, as the in vivo fate of tested nanomedicine and its interactions with the immune microenvironment can be highly variable. This encourages researchers to actively develop highly consistent platforms for evaluating efficacy, such as ex vivo immune microenvironments simulant based on induced pluripotent stem cells (iPSCs).128 Finally, it is noteworthy that the cells targeted by existing immunotherapies are predominantly located in secondary lymphoid organs and regional lymph nodes. Although tumor-draining lymph nodes are considered crucial sites for immune activation as they collect tumor antigens and antigen-specific T cells, they also accumulate immunosuppressive cytokines, Tregs, and metastatic cancer cells, which may promote an environment of immune tolerance rather than immune defense. Therefore, the delivery of immune stimuli to local lymph nodes represents an important field in immunotherapy. For rational nanomedicines design, it is important to consider that the physicochemical properties of nanomedicines that facilitate their accumulation at the primary tumor site do not necessarily favor accumulation in lymph nodes. This implies that in the design of nanomedicines, particular attention must be given to characteristics that can promote the delivery of therapeutics to lymph nodes, thereby achieving more effective immune stimulation and treatment.129
In general, in the future through more precise targeting, more effective drug delivery and more in-depth immunotherapy combination, nanomedicine is expected to become an important exploration direction for PC treatment. At the same time, further research and exploration are needed to continuously improve the design and application of nanomedicine to better serve clinical treatment and achieve personalized and accurate immunotherapy strategies.
Acknowledgments
This work was supported by grants from the Natural Science Research Project of Higher Education Institutions of Anhui Provincial Department of Education (2023AH040254), the Funding of “Peak” Training Program for Scientific Research of Yijishan Hospital, Wannan Medical College (KGF2019T02), the Funding of Climbing Peak Training Program for Innovative Technology team of Yijishan Hospital, Wannan Medical College (KPF2019011), the Special Project for Clinical Medicine Research and Transformation in Anhui Province (202204295107020059), and the Health Research Program of Anhui Province (AHWJ2023A10151).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Zhao Y, Tang J, Jiang K, et al. Liquid biopsy in pancreatic cancer - Current perspective and future outlook. Biochim Biophys Acta Rev Cancer. 2023;1878:188868. doi:10.1016/j.bbcan.2023.188868
2. Hu ZI, O’Reilly EM. Therapeutic developments in pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2024;21:7–24. doi:10.1038/s41575-023-00840-w
3. Labori KJ, Bratlie SO, Andersson B, et al. Neoadjuvant FOLFIRINOX versus upfront surgery for resectable pancreatic head cancer (NORPACT-1): a multicentre, randomised, Phase 2 trial. Lancet Gastroenterol Hepatol. 2024;9:205–217. doi:10.1016/S2468-1253(23)00405-3
4. Conroy T, Castan F, Lopez A, et al. Five-year outcomes of FOLFIRINOX vs gemcitabine as adjuvant therapy for pancreatic cancer: a randomized clinical trial. JAMA Oncol. 2022;8:1571–1578. doi:10.1001/jamaoncol.2022.3829
5. Bergonzini C, Gregori A, Hagens TMS, et al. ABCB1 overexpression through locus amplification represents an actionable target to combat paclitaxel resistance in pancreatic cancer cells. J Exp Clin Cancer Res. 2024;43:4. doi:10.1186/s13046-023-02879-8
6. Tang HY, Cao YZ, Zhou YW, et al. The power and the promise of CAR-mediated cell immunotherapy for clinical application in pancreatic cancer. J Adv Res. 2024. doi:10.1016/j.jare.2024.01.014
7. Kiaie SH, Sanaei MJ, Heshmati M, et al. Immune checkpoints in targeted-immunotherapy of pancreatic cancer: new hope for clinical development. Acta Pharm Sin B. 2021;11:1083–1097. doi:10.1016/j.apsb.2020.12.011
8. Pant S, Wainberg ZA, Weekes CD, et al. Lymph-node-targeted, mKRAS-specific amphiphile vaccine in pancreatic and colorectal cancer: the Phase 1 AMPLIFY-201 trial. Nat Med. 2024;30:531–542. doi:10.1038/s41591-023-02760-3
9. Liu X, Tang R, Xu J, et al. CRIP1 fosters MDSC trafficking and resets tumour microenvironment via facilitating NF-κB/p65 nuclear translocation in pancreatic ductal adenocarcinoma. Gut. 2023;72:2329–2343. doi:10.1136/gutjnl-2022-329349
10. Liu L, Kshirsagar PG, Gautam SK, et al. Nanocarriers for pancreatic cancer imaging, treatments, and immunotherapies. Theranostics. 2022;12:1030–1060. doi:10.7150/thno.64805
11. Chen L, Xue W, Cao J, et al. TiSe(2)-mediated sonodynamic and checkpoint blockade combined immunotherapy in hypoxic pancreatic cancer. J Nanobiotechnology. 2022;20:453. doi:10.1186/s12951-022-01659-4
12. Qu C, Yuan H, Tian M, et al. Precise photodynamic therapy by midkine nanobody-engineered nanoparticles remodels the microenvironment of pancreatic ductal adenocarcinoma and potentiates the immunotherapy. ACS Nano. 2024;18:4019–4037. doi:10.1021/acsnano.3c07002
13. Zhao R, Xiao Q, Wu Y, et al. Dual-crosslinking immunostimulatory hydrogel synchronously suppresses pancreatic fistula and pancreatic cancer relapse post-resection. Biomaterials. 2024;305:122453. doi:10.1016/j.biomaterials.2023.122453
14. Bhandari C, Moffat A, Shah N, et al. PD-L1 immune checkpoint targeted photoactivable liposomes (iTPALs) prime the stroma of pancreatic tumors and promote self-delivery. Adv Healthc Mater;2024. e2304340. doi:10.1002/adhm.202304340
15. Zhao J, Xiao Z, Li T, et al. Stromal modulation reverses primary resistance to immune checkpoint blockade in pancreatic cancer. ACS Nano. 2018;12:9881–9893. doi:10.1021/acsnano.8b02481
16. Zinger A, Koren L, Adir O, et al. Collagenase nanoparticles enhance the penetration of drugs into pancreatic tumors. ACS Nano. 2019;13:11008–11021. doi:10.1021/acsnano.9b02395
17. Wan Z, Huang H, West RE, et al. Overcoming pancreatic cancer immune resistance by codelivery of CCR2 antagonist using a STING-activating gemcitabine-based nanocarrier. Mater Today. 2023;62:33–50. doi:10.1016/j.mattod.2022.11.008
18. Christenson ES, Jaffee E, Azad NS. Current and emerging therapies for patients with advanced pancreatic ductal adenocarcinoma: a bright future. Lancet Oncol. 2020;21:e135–e145. doi:10.1016/S1470-2045(19)30795-8
19. Qian ZR, Rubinson DA, Nowak JA, et al. Association of alterations in main driver genes with outcomes of patients with resected pancreatic ductal adenocarcinoma. JAMA Oncol. 2018;4:e173420.
20. Zhang Z, Song B, Wei H, et al. NDRG1 overcomes resistance to immunotherapy of pancreatic ductal adenocarcinoma through inhibiting ATG9A-dependent degradation of MHC-1. Drug Resist Updat. 2024;73:101040. doi:10.1016/j.drup.2023.101040
21. Yamamoto K, Venida A, Yano J, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581:100–105. doi:10.1038/s41586-020-2229-5
22. Bellone G, Carbone A, Smirne C, et al. Cooperative induction of a tolerogenic dendritic cell phenotype by cytokines secreted by pancreatic carcinoma cells. J Immunol. 2006;177:3448–3460. doi:10.4049/jimmunol.177.5.3448
23. Pan Y, Lu F, Fei Q, et al. Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer. J Hematol Oncol. 2019;12:124. doi:10.1186/s13045-019-0822-6
24. Mahalingam D, Chen S, Xie P, et al. Combination of pembrolizumab and pelareorep promotes anti-tumour immunity in advanced pancreatic adenocarcinoma (PDAC). Br J Cancer. 2023;129:782–790. doi:10.1038/s41416-023-02344-5
25. Coelho MA, de Carné Trécesson S, Rana S, et al. Oncogenic RAS signaling promotes tumor immunoresistance by stabilizing PD-L1 mRNA. Immunity. 2017;47:1083–1099.e6. doi:10.1016/j.immuni.2017.11.016
26. Hecht JR, Lonardi S, Bendell J, et al. Randomized phase III study of FOLFOX alone or with pegilodecakin as second-line therapy in patients with metastatic pancreatic cancer that progressed after gemcitabine (SEQUOIA). J Clin Oncol. 2021;39:1108–1118. doi:10.1200/JCO.20.02232
27. Clark CE, Hingorani SR, Mick R, et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–9527. doi:10.1158/0008-5472.CAN-07-0175
28. Chen K, Wang Y, Hou Y, et al. Single cell RNA-seq reveals the CCL5/SDC1 receptor-ligand interaction between T cells and tumor cells in pancreatic cancer. Cancer Lett. 2022;545:215834. doi:10.1016/j.canlet.2022.215834
29. Fan J-Q, Wang M-F, Chen H-L, et al. Current advances and outlooks in immunotherapy for pancreatic ductal adenocarcinoma. Mol Cancer. 2020;19:32. doi:10.1186/s12943-020-01151-3
30. Wang LM, Silva MA, D’Costa Z, et al. The prognostic role of desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget. 2016;7:4183–4194. doi:10.18632/oncotarget.6770
31. Tian C, Clauser KR, Öhlund D, et al. Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. Proc Natl Acad Sci U S A. 2019;116:19609–19618. doi:10.1073/pnas.1908626116
32. Whatcott CJ, Diep CH, Jiang P, et al. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin Cancer Res. 2015;21:3561–3568. doi:10.1158/1078-0432.CCR-14-1051
33. Jacobetz MA, Chan DS, Neesse A, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi:10.1136/gutjnl-2012-302529
34. Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi:10.1016/j.ccr.2012.01.007
35. Jiang H, Hegde S, Knolhoff BL, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nature Med. 2016;22:851–860. doi:10.1038/nm.4123
36. Ene-Obong A, Clear AJ, Watt J, et al. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology. 2013;145:1121–1132. doi:10.1053/j.gastro.2013.07.025
37. Öhlund D, Handly-Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214:579–596. doi:10.1084/jem.20162024
38. Elyada E, Bolisetty M, Laise P, et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 2019;9:1102–1123. doi:10.1158/2159-8290.CD-19-0094
39. Lutz ER, Wu AA, Bigelow E, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2:616–631. doi:10.1158/2326-6066.CIR-14-0027
40. Le DT, Wang-Gillam A, Picozzi V, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33:1325–1333. doi:10.1200/JCO.2014.57.4244
41. Reiss KA, Mick R, O’Hara MH, et al. Phase II study of maintenance rucaparib in patients with platinum-sensitive advanced pancreatic cancer and a pathogenic germline or somatic variant in BRCA1, BRCA2, or PALB2. J Clin Oncol. 2021;39:2497–2505. doi:10.1200/JCO.21.00003
42. Beatty GL, Li Y, Long KB. Cancer immunotherapy: activating innate and adaptive immunity through CD40 agonists. Expert Rev Anticancer Ther. 2017;17:175–186. doi:10.1080/14737140.2017.1270208
43. Zippelius A, Schreiner J, Herzig P, et al. Induced PD-L1 expression mediates acquired resistance to agonistic anti-CD40 treatment. Cancer Immunol Res. 2015;3:236–244. doi:10.1158/2326-6066.CIR-14-0226
44. June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–1476. doi:10.1172/JCI32446
45. Kondo H, Hazama S, Kawaoka T, et al. Adoptive immunotherapy for pancreatic cancer using MUC1 peptide-pulsed dendritic cells and activated T lymphocytes. Anticancer Res. 2008;28:379–387.
46. Stromnes IM, Schmitt TM, Hulbert A, et al. T cells engineered against a native antigen can surmount immunologic and physical barriers to treat pancreatic ductal adenocarcinoma. Cancer Cell. 2015;28:638–652. doi:10.1016/j.ccell.2015.09.022
47. Panni RZ, Herndon JM, Zuo C, et al. Agonism of CD11b reprograms innate immunity to sensitize pancreatic cancer to immunotherapies. Sci Transl Med. 2019;11. doi:10.1126/scitranslmed.aau9240
48. Nywening TM, Wang-Gillam A, Sanford DE, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016;17:651–662. doi:10.1016/S1470-2045(16)00078-4
49. Zhu Y, Knolhoff BL, Meyer MA, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–5069. doi:10.1158/0008-5472.CAN-13-3723
50. Chen IM, Willumsen N, Dehlendorff C, et al. Clinical value of serum hyaluronan and propeptide of type III collagen in patients with pancreatic cancer. Int, J, Cancer. 2020;146:2913–2922. doi:10.1002/ijc.32751
51. Kim PK, Halbrook CJ, Kerk SA, et al. Hyaluronic acid fuels pancreatic cancer cell growth. Elife. 2021;10. doi:10.7554/eLife.62645
52. Van Cutsem E, Tempero MA, Sigal D, et al. Randomized phase III trial of pegvorhyaluronidase alfa with nab-paclitaxel plus gemcitabine for patients with hyaluronan-high metastatic pancreatic adenocarcinoma. J Clin Oncol. 2020;38:3185–3194. doi:10.1200/JCO.20.00590
53. Biffi G, Oni TE, Spielman B, et al. IL1-Induced JAK/STAT signaling is antagonized by TGFβ to Shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 2019;9:282–301. doi:10.1158/2159-8290.CD-18-0710
54. Elzoghby AO, Ferrone CR, Ferrone S, et al. Engineered nanomedicines to overcome resistance of pancreatic cancer to immunotherapy. Drug Discov Today. 2023;28:103434. doi:10.1016/j.drudis.2022.103434
55. Hosein AN, Brekken RA, Maitra A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat Rev Gastroenterol Hepatol. 2020;17:487–505. doi:10.1038/s41575-020-0300-1
56. Mizrahi JD, Surana R, Valle JW, et al. Pancreatic cancer. Lancet. 2020;395:2008–2020. doi:10.1016/S0140-6736(20)30974-0
57. Marcus L, Lemery SJ, Keegan P, et al. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25:3753–3758. doi:10.1158/1078-0432.CCR-18-4070
58. Cox RE, Mahipal A, Chakrabarti S. A patient with locally advanced mismatch-repair-deficient pancreatic ductal adenocarcinoma successfully treated with neoadjuvant immunotherapy. Cureus. 2021;13:e14640.
59. Tarannum M, Vivero-Escoto JL. Nanoparticle-based therapeutic strategies targeting major clinical challenges in pancreatic cancer treatment. Adv. Drug Delivery Rev. 2022;187:114357. doi:10.1016/j.addr.2022.114357
60. Krenz B, Gebhardt-Wolf A, Ade CP, et al. MYC- and MIZ1-dependent vesicular transport of double-Strand RNA controls immune evasion in pancreatic ductal adenocarcinoma. Cancer Res. 2021;81:4242–4256. doi:10.1158/0008-5472.CAN-21-1677
61. Zhou W, Zhou Y, Chen X, et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. 2021;268:120546. doi:10.1016/j.biomaterials.2020.120546
62. Carrato A, Pazo-Cid R, Macarulla T, et al. Nab-Paclitaxel plus gemcitabine and FOLFOX in metastatic pancreatic cancer. NEJM Evid. 2024;3:EVIDoa2300144.
63. Frampton JE. Liposomal irinotecan: a review in metastatic pancreatic adenocarcinoma. Drugs. 2020;80:1007–1018. doi:10.1007/s40265-020-01336-6
64. Ren E, Wang Y, Liang T, et al. Local drug delivery techniques for triggering immunogenic cell death. Small Methods. 2023;7:e2300347.
65. Ahmed A, Tait SWG. Targeting immunogenic cell death in cancer. Mol Oncol. 2020;14:2994–3006. doi:10.1002/1878-0261.12851
66. Liu X, Jiang J, Chang CH, et al. Development of facile and versatile platinum drug delivering silicasome nanocarriers for efficient pancreatic cancer chemo-immunotherapy. Small. 2021;17:e2005993.
67. Shen J, Sun C, Wang Z, et al. Sequential receptor-mediated mixed-charge nanomedicine to target pancreatic cancer, inducing immunogenic cell death and reshaping the tumor microenvironment. Int J Pharm. 2021;601:120553. doi:10.1016/j.ijpharm.2021.120553
68. Pan J, Lai Y, Zhang S, et al. Self-adaptive nanoregulator to mitigate dynamic immune evasion of pancreatic cancer. Adv Mater. 2023;35:e2305798.
69. Lu J, Liu X, Liao Y-P, et al. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat Commun. 2017;8:1811. doi:10.1038/s41467-017-01651-9
70. Yu B, Wang Y, Bing T, et al. Platinum prodrug nanoparticles with COX-2 inhibition amplify pyroptosis for enhanced chemotherapy and immune activation of pancreatic cancer. Adv Mater. 2023;36:e2310456. doi:10.1002/adma.202310456
71. Zhou J, Wang G, Chen Y, et al. Immunogenic cell death in cancer therapy: present and emerging inducers. J Cell Mol Med. 2019;23:4854–4865. doi:10.1111/jcmm.14356
72. Jia M, Zhang D, Zhang C, et al. Nanoparticle-based delivery systems modulate the tumor microenvironment in pancreatic cancer for enhanced therapy. J Nanobiotechnol. 2021;19:384. doi:10.1186/s12951-021-01134-6
73. Ebelt ND, Zuniga E, Passi KB, et al. Hyaluronidase-expressing salmonella effectively targets tumor-associated hyaluronic acid in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2020;19:706–716. doi:10.1158/1535-7163.MCT-19-0556
74. Zhang H, Wang Y, Zhu L, et al. A “bulldozer” driven by anoxic bacteria for pancreatic cancer chemo-immunotherapy. J Contr Rel. 2023;360:660–671. doi:10.1016/j.jconrel.2023.07.014
75. Aglietta M, Barone C, Sawyer MB, et al. A phase I dose escalation trial of tremelimumab (CP-675, 206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol. 2014;25:1750–1755. doi:10.1093/annonc/mdu205
76. Chibaya L, Murphy KC, DeMarco KD, et al. EZH2 inhibition remodels the inflammatory senescence-associated secretory phenotype to potentiate pancreatic cancer immune surveillance. Nat Cancer. 2023;4:872–892. doi:10.1038/s43018-023-00553-8
77. Huang X, Lu Y, Guo M, et al. Recent strategies for nano-based PTT combined with immunotherapy: from a biomaterial point of view. Theranostics. 2021;11:7546–7569. doi:10.7150/thno.56482
78. Yu Q, Tang X, Zhao W, et al. Mild hyperthermia promotes immune checkpoint blockade-based immunotherapy against metastatic pancreatic cancer using size-adjustable nanoparticles. Acta Biomater. 2021;133:244–256. doi:10.1016/j.actbio.2021.05.002
79. Li M, Liu Y, Zhang Y, et al. Sono-activatable semiconducting polymer nanoreshapers multiply remodel tumor microenvironment for potent immunotherapy of orthotopic pancreatic cancer. Advan Sci. 2023;10:e2305150.
80. Zhang R, Liu T, Li W, et al. Tumor microenvironment-responsive BSA nanocarriers for combined chemo/chemodynamic cancer therapy. J Nanobiotechnol. 2022;20:223. doi:10.1186/s12951-022-01442-5
81. Li Z, Lai X, Fu S, et al. Immunogenic cell death activates the tumor immune microenvironment to boost the immunotherapy efficiency. Adv Sci. 2022;9:e2201734. doi:10.1002/advs.202201734
82. Brown TP, Bhattacharjee P, Ramachandran S, et al. The lactate receptor GPR81 promotes breast cancer growth via a paracrine mechanism involving antigen-presenting cells in the tumor microenvironment. Oncogene. 2020;39:3292–3304. doi:10.1038/s41388-020-1216-5
83. Brand A, Singer K, Koehl GE, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016;24:657–671. doi:10.1016/j.cmet.2016.08.011
84. Sun F, Zhu Q, Li T, et al. Regulating glucose metabolism with prodrug nanoparticles for promoting photoimmunotherapy of pancreatic cancer. Adv Sci. 2021;8:2002746. doi:10.1002/advs.202002746
85. Jang Y, Kim H, Yoon S, et al. Exosome-based photoacoustic imaging guided photodynamic and immunotherapy for the treatment of pancreatic cancer. J Control Release. 2021;330:293–304. doi:10.1016/j.jconrel.2020.12.039
86. Chen J, Feng L, Jin P, et al. Cavitation assisted endoplasmic reticulum targeted sonodynamic droplets to enhanced anti-PD-L1 immunotherapy in pancreatic cancer. J Nanobiotechnology. 2022;20:283. doi:10.1186/s12951-022-01459-w
87. Boltjes A, van Wijk F. Human dendritic cell functional specialization in steady-state and inflammation. Front Immunol. 2014;5:131. doi:10.3389/fimmu.2014.00131
88. Hiraoka N, Yamazaki-Itoh R, Ino Y, et al. CXCL17 and ICAM2 are associated with a potential anti-tumor immune response in early intraepithelial stages of human pancreatic carcinogenesis. Gastroenterology. 2011;140:310–321. doi:10.1053/j.gastro.2010.10.009
89. Han J-A, Kang YJ, Shin C, et al. Ferritin protein cage nanoparticles as versatile antigen delivery nanoplatforms for dendritic cell (DC)-based vaccine development. Nanomedicine. 2014;10:561–569. doi:10.1016/j.nano.2013.11.003
90. Dong H, Wen Z-F, Chen L, et al. Polyethyleneimine modification of aluminum hydroxide nanoparticle enhances antigen transportation and cross-presentation of dendritic cells. Int J Nanomed. 2018;13:3353–3365. doi:10.2147/IJN.S164097
91. Liu X, Zhuang Y, Huang W, et al. Interventional hydrogel microsphere vaccine as an immune amplifier for activated antitumour immunity after ablation therapy. Nat Commun. 2023;14:4106. doi:10.1038/s41467-023-39759-w
92. Martínez-Lostao L, Anel A, Pardo J. How do cytotoxic lymphocytes kill cancer cells? Clin Cancer Res. 2015;21:5047–5056. doi:10.1158/1078-0432.CCR-15-0685
93. Wu Y, Chen W, Xu ZP, et al. PD-L1 distribution and perspective for cancer immunotherapy-blockade, knockdown, or inhibition. Front Immunol. 2019;10:2022. doi:10.3389/fimmu.2019.02022
94. Yoo B, Jordan VC, Sheedy P, et al. RNAi-mediated PD-L1 inhibition for pancreatic cancer immunotherapy. Sci Rep. 2019;9:4712. doi:10.1038/s41598-019-41251-9
95. Wang C, Shi X, Song H, et al. Polymer-lipid hybrid nanovesicle-enabled combination of immunogenic chemotherapy and RNAi-mediated PD-L1 knockdown elicits antitumor immunity against melanoma. Biomaterials. 2021;268:120579. doi:10.1016/j.biomaterials.2020.120579
96. Jung JY, Ryu HJ, Lee SH, et al. siRNA nanoparticle targeting PD-L1 activates tumor immunity and abrogates pancreatic cancer growth in humanized preclinical model. Cells. 2021;10:2734. doi:10.3390/cells10102734
97. Mariathasan S, Turley SJ, Nickles D, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi:10.1038/nature25501
98. Wang Y, Gao Z, Du X, et al. Co-inhibition of the TGF-β pathway and the PD-L1 checkpoint by pH-responsive clustered nanoparticles for pancreatic cancer microenvironment regulation and anti-tumor immunotherapy. Biomater Sci. 2020;8:5121–5132. doi:10.1039/D0BM00916D
99. Cullis J, Siolas D, Avanzi A, et al. Macropinocytosis of Nab-paclitaxel drives macrophage activation in pancreatic cancer. Cancer Immunol Res. 2017;5:182–190. doi:10.1158/2326-6066.CIR-16-0125
100. Ernsting MJ, Hoang B, Lohse I, et al. Targeting of metastasis-promoting tumor-associated fibroblasts and modulation of pancreatic tumor-associated stroma with a carboxymethylcellulose-docetaxel nanoparticle. J Control Release. 2015;206:122–130. doi:10.1016/j.jconrel.2015.03.023
101. Wang N, Wang S, Wang X, et al. Research trends in pharmacological modulation of tumor-associated macrophages. Clin Transl Med. 2021;11:e288.
102. Li M, Li M, Yang Y, et al. Remodeling tumor immune microenvironment via targeted blockade of PI3K-γ and CSF-1/CSF-1R pathways in tumor associated macrophages for pancreatic cancer therapy. J Control Release. 2020;321:23–35. doi:10.1016/j.jconrel.2020.02.011
103. Currie E, Schulze A, Zechner R, et al. Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18:153–161. doi:10.1016/j.cmet.2013.05.017
104. Xiang W, Shi R, Kang X, et al. Monoacylglycerol lipase regulates cannabinoid receptor 2-dependent macrophage activation and cancer progression. Nat Commun. 2018;9:2574. doi:10.1038/s41467-018-04999-8
105. Cao S, Saw PE, Shen Q, et al. Reduction-responsive RNAi nanoplatform to reprogram tumor lipid metabolism and repolarize macrophage for combination pancreatic cancer therapy. Biomaterials. 2022;280:121264. doi:10.1016/j.biomaterials.2021.121264
106. Caronni N, La Terza F, Vittoria FM, et al. IL-1β(+) macrophages fuel pathogenic inflammation in pancreatic cancer. Nature. 2023;623:415–422. doi:10.1038/s41586-023-06685-2
107. Huang H, Jiang CT, Shen S, et al. Nanoenabled reversal of IDO1-mediated immunosuppression synergizes with immunogenic chemotherapy for improved cancer therapy. Nano Lett. 2019;19:5356–5365. doi:10.1021/acs.nanolett.9b01807
108. Lu Z, Long Y, Wang Y, et al. Phenylboronic acid modified nanoparticles simultaneously target pancreatic cancer and its metastasis and alleviate immunosuppression. Eur J Pharm Biopharm. 2021;165:164–173. doi:10.1016/j.ejpb.2021.05.014
109. Wing JB, Tanaka A, Sakaguchi S. Human FOXP3(+) regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity. 2019;50:302–316. doi:10.1016/j.immuni.2019.01.020
110. De Simone M, Arrigoni A, Rossetti G, et al. Transcriptional landscape of human tissue lymphocytes unveils uniqueness of tumor-infiltrating T regulatory cells. Immunity. 2016;45:1135–1147. doi:10.1016/j.immuni.2016.10.021
111. Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37:193–207. doi:10.1016/j.it.2016.01.002
112. Tang T, Huang X, Lu M, et al. Transcriptional control of pancreatic cancer immunosuppression by metabolic enzyme CD73 in a tumor-autonomous and -autocrine manner. Nat Commun. 2023;14:3364. doi:10.1038/s41467-023-38578-3
113. Greten TF. Myeloid-derived suppressor cells in pancreatic cancer: more than a hidden barrier for antitumor immunity? Gut. 2014;63:1690–1691. doi:10.1136/gutjnl-2014-306790
114. Fleming V, Hu X, Weber R, et al. Targeting myeloid-derived suppressor cells to bypass tumor-induced immunosuppression. Front Immunol. 2018;9:398. doi:10.3389/fimmu.2018.00398
115. Stromnes IM, Brockenbrough JS, Izeradjene K, et al. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut. 2014;63:1769–1781. doi:10.1136/gutjnl-2013-306271
116. Gargett T, Christo SN, Hercus TR, et al. GM-CSF signalling blockade and chemotherapeutic agents act in concert to inhibit the function of myeloid-derived suppressor cells in vitro. Clin Transl Immunology. 2016;5:e119. doi:10.1038/cti.2016.80
117. Huang Y, Chen Y, Zhou S, et al. Dual-mechanism based CTLs infiltration enhancement initiated by Nano-sapper potentiates immunotherapy against immune-excluded tumors. Nat Commun. 2020;11:622. doi:10.1038/s41467-020-14425-7
118. Ramanathan RK, McDonough SL, Philip PA, et al. Phase IB/II randomized study of FOLFIRINOX plus pegylated recombinant human hyaluronidase versus FOLFIRINOX alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. J Clin Oncol. 2019;37:1062–1069. doi:10.1200/JCO.18.01295
119. Chen X, Zhou W, Liang C, et al. Codelivery nanosystem targeting the deep microenvironment of pancreatic cancer. Nano Lett. 2019;19:3527–3534. doi:10.1021/acs.nanolett.9b00374
120. Xie Y, Hang Y, Wang Y, et al. Stromal modulation and treatment of metastatic pancreatic cancer with local intraperitoneal triple miRNA/siRNA nanotherapy. ACS Nano. 2020;14(1):255–271. doi:10.1021/acsnano.9b03978
121. Dakwar GR, Shariati M, Willaert W, et al. Nanomedicine-based intraperitoneal therapy for the treatment of peritoneal carcinomatosis - Mission possible? Adv Drug Deliv Rev. 2017;108:13–24. doi:10.1016/j.addr.2016.07.001
122. Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol. 2020;17:527–540. doi:10.1038/s41571-020-0363-5
123. Steele NG, Biffi G, Kemp SB, et al. Inhibition of hedgehog signaling alters fibroblast composition in pancreatic cancer. Clin Cancer Res. 2021;27:2023–2037. doi:10.1158/1078-0432.CCR-20-3715
124. Shen L, Li J, Liu Q, et al. Local blockade of interleukin 10 and C-X-C motif chemokine ligand 12 with nano-delivery promotes antitumor response in murine cancers. ACS Nano. 2018;12:9830–9841. doi:10.1021/acsnano.8b00967
125. Yan S, Yan J, Liu D, et al. A nano-predator of pathological MDMX construct by clearable supramolecular gold(I)-thiol-peptide complexes achieves safe and potent anti-tumor activity. Theranostics. 2021;11:6833–6846. doi:10.7150/thno.59020
126. Sakamoto Y, Fujii S, Takano S, et al. Manipulation of macrophage uptake by controlling the aspect ratio of graft copolymer micelles. Nano Lett. 2024;24:5838–5846. doi:10.1021/acs.nanolett.4c01054
127. Wang J, Gai J, Zhang T, et al. Neoadjuvant radioimmunotherapy in pancreatic cancer enhances effector T cell infiltration and shortens their distances to tumor cells. Sci Adv. 2024;10:eadk1827.
128. Bi J, Huang C, Jin X, et al. TIPE2 deletion improves the therapeutic potential of adoptively transferred NK cells. J Immunother Cancer. 2023;11:e006002. doi:10.1136/jitc-2022-006002
129. Schudel A, Francis DM, Thomas SN. Material design for lymph node drug delivery. Nat Rev Mater. 2019;4:415–428. doi:10.1038/s41578-019-0110-7
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

