Back to Journals » Cancer Management and Research » Volume 16
Focusing on the Immune Cells: Recent Advances in Immunotherapy for Biliary Tract Cancer
Authors Ni L, Xu J, Li Q, Ge X , Wang F, Deng X, Miao L
Received 29 April 2024
Accepted for publication 17 July 2024
Published 29 July 2024 Volume 2024:16 Pages 941—963
DOI https://doi.org/10.2147/CMAR.S474348
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Luohang Ni,* Jianing Xu,* Quanpeng Li, Xianxiu Ge, Fei Wang, Xueting Deng, Lin Miao
Medical Center for Digestive Diseases, Second Affiliated Hospital, Nanjing Medical University, Nanjing, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xueting Deng; Lin Miao, Email [email protected]; [email protected]
Abstract: Biliary tract cancer (BTC) represents a challenging malignancy characterized by aggressive behavior, high relapse rates, and poor prognosis. In recent years, immunotherapy has revolutionized the treatment landscape for various cancers, but its efficacy in BTC remains limited. This article provides a comprehensive overview of the advances in preclinical and clinical studies of immunotherapy for BTC. We explore the potential of immune checkpoint inhibitors in reshaping the management of BTC. Despite disappointing results thus far, ongoing clinical trials are investigating the combination of immunotherapy with other treatment modalities. Furthermore, research on the tumor microenvironment has unveiled novel targets for immunotherapeutic interventions. By understanding the current state of immunotherapy in BTC and highlighting future directions, this article aims to fuel further exploration and ultimately improve patient outcomes in this challenging disease.
Keywords: biliary tract cancer, cancer immunotherapy, tumor microenvironment, immune checkpoint inhibitors, clinical trials
Introduction
Biliary tract cancers (BTCs) encompass a diverse group of adenocarcinomas characterized by genetic variability and a grim prognosis. They are categorized based on their anatomical origin into intrahepatic cholangiocarcinoma (iCCA), extrahepatic cholangiocarcinoma (eCCA, including perihilar CCA (pCCA) and distal CCA), and gallbladder cancer (GBC).1 The global incidence of BTC varies, with higher rates observed in Asian countries like Korea, Thailand, and China.2 This disparity may arise from multifaceted risk factors such as liver disease, lifestyle choices, age, gender, and hereditary factors. Additionally, although BTC was previously considered a rare tumor, accounting for approximately 3% of all gastrointestinal tumors,3 these years have witnessed a rising trend of its incidence,4 especially among younger adults.5,6
Surgical resection remains the sole potentially curative treatment option for BTC.7 Unfortunately, due to its asymptomatic clinical course, most patients are diagnosed only after the disease has metastasized extensively.8 Consequently, merely 20–30% of patients qualify for surgical intervention.9,10 Moreover, despite initial eligibility for surgery, 40–85% of patients experience disease recurrence either during or after radical resection,11,12 resulting in a high case–fatality ratio and a median overall survival (OS) of less than 12 months.13 Limited data exist regarding the impact of adjuvant therapy on biliary tract cancer (BTC); however, certain clinical trials have demonstrated the potential of this treatment to offer long-term benefits to BTC patients.14 Despite a recent meta-analysis of the French PRODIGE-12 and Japanese BCAT Phase III studies showing no significant enhancement in relapse-free survival (RFS) or overall survival (OS) with adjuvant therapy,15–17 the UK BILCAP phase III trial, which randomly assigns patients with BTC after resection to receive oral capecitabine or observation, reports a clinical meaningful prolonged median OS for capecitabine group.18 In a French single-center study, patients with locally advanced iCCA who receive surgery following adjuvant chemotherapy have similar short- and long-term results to patients with initially resectable iCCA treated by surgery alone, indicating that this therapeutic option may facilitate patients with initially unresectable disease.19 Of note, recent Phase II trials also indicate promise for Teysuno in resected BTC patients.20,21
For advanced BTC, the cisplatin-gemcitabine (GemCis) combination stands as the internationally accepted first-line treatment regimen, endorsed by studies like UK ABC-02 and Japanese BT22.22,23 Further refinement includes the GCS triplet (cisplatin, gemcitabine, and S1) tested in the KHBO1401 trial.24 However, the addition of albumin-bound paclitaxel to GemCis in the SWOG 1815 trial did not significantly enhance median OS.25
Advancements in understanding BTC’s genetic diversity have identified potential therapeutic targets. For instance, ivosidenib targeting Isocitrate dehydrogenase 1 (IDH1) and pemigatinib against the Fibroblast Growth Factor Receptor (FGFR) pathway represent notable breakthroughs.26–28 HER2 overexpression, amplification, or mutation can occur in GBC (19%), eCCA (17.4%), and is rarer in iCCA (4.8%).29 The HERIZON-BTC-1 phase II trial investigates the application of zanidatamab, a bispecific antibody targeting two distinct HER2 epitopes, in patients with HER2-amplified BTC. The trial reports an impressive objective response rate (ORR) of 41.3% and observes grade 3 treatment-related adverse events in 16% of patients.30 These findings support the potential of zanidatamab as a future treatment option for HER2-positive biliary tract cancer.
In addition to the emerging therapeutic options mentioned above, immunotherapy, which includes immune checkpoint inhibitors (ICI), adoptive cell therapy (ACT), cancer vaccines, and chimeric antigen receptor T cell (CAR-T cell) therapy, has revolutionized cancer treatment by enhancing the immune system’s ability to recognize and attack cancer cells.31 The TOPAZ-1 trial, which randomly assigns advanced BTC patients to receive durvalumab or placebo in combination with GemCis, followed by durvalumab or placebo monotherapy, demonstrates superior OS in favor of the durvalumab and GemCis triplet,32 This combination has thus become a first-line treatment option for advanced BTC. Despite these advancements, only a minority of BTC patients currently benefit from immunotherapy.33,34 In conclusion, while significant progress has been made in understanding and treating BTC, challenges remain in improving early detection, expanding treatment options, and personalizing therapies based on molecular profiles. Ongoing research and clinical trials continue to explore new avenues, offering hope for further advancements in BTC management. Herein, we will describe recent results and future directions of immunotherapy in BTC.
Immune Cells in Tumor Microenvironment (TME) of BTC
BTCs are characterized by desmoplastic tumors with a dense TME. This TME comprises various components, including nonmalignant cells, lymphoid tissue, blood vessels, nerves, intercellular components, and metabolites. It develops within the tumor lesion and the internal environment of tumor cells, influenced by the recruitment and adaptation of normal cells surrounding the mutated ones. Cancer cells actively shape the TME to promote tumor development, contributing to its dynamic heterogeneity.35,36 Within the TME, immune cells play a crucial dual role in tumor biology, affecting initiation, progression, and metastasis. For instance, CD4+ and CD8+ T cells exhibit potent antitumor activity through distinct mechanisms, such as direct cytotoxicity and cytokine release. Conversely, immunosuppressive cells like regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) are also present, dampening immune responses and facilitating tumor immune evasion.37 The intricate interplay between these immune cell subsets within the TME presents both challenges and opportunities for immunotherapy. Immune cells respond dynamically to intrinsic and extrinsic stimuli, influencing tumor behavior and response to treatment. Harnessing these interactions holds promise for developing novel immunotherapeutic strategies tailored to combat BTC effectively.
Adaptive Immune Cells
CD8+ T Cell
CD8+ T cells, originating in the red bone marrow, possess a remarkable ability to selectively recognize tumor antigens and induce apoptosis in target cells by releasing cytotoxic substances like perforin and granzyme. This mechanism is pivotal in antitumor responses and forms the cornerstone of immunotherapy.
When CD8+ T cells become overactivated, several immune-checkpoint molecules including programmed cell death protein 1 (PD-1), cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), and T cell immunoglobulin and ITIM domain (TIGIT) can dampen immune responses. These mechanisms can also contribute to creating an immunosuppressive TME.38 Research by Qiao et al demonstrate that downregulating PD-1, TIGIT, T cell immunoglobulin mucin domain-containing protein 3 (Tim-3), and certain immunosuppressive cytokine receptors within CAR-T cells significantly enhances their efficacy in suppressing BTC.39 Additionally, the PD-1-CD28 switch receptor (SR) has shown the ability to convert the immunosuppressive signals from PD-1/PD-L1 interactions into stimulatory signals via CD28. CAR-T cells engineered to include SR exhibit enhanced cytotoxicity against targeted tumor cells.40 The mechanism of CAR-T cells with reduced immune-checkpoints and immunosuppressive cytokine receptors is shown in Figure 1.39
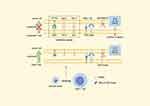 |
Figure 1 Lentiviral vectors carrying EGFR or B7H3 specific CAR moiety, together with shRNAs targeting immune checkpoints and cytokine receptors, are designed to transduce primary human CD8+ T cells into CAR-T cells with decreased expression of PD-1, Tim-3, TIGIT and cytokine receptors. These CAR-T cells revealed an enhanced cytotoxicity.39 Abbreviations: EGFR, Epidermal growth factor receptor; B7H3, B7 homolog 3 protein; shRNAs, Small hairpin RNAs; CAR T cell, Chimeric Antigen Receptor T Cell; TIGIT, T cell immunoglobulin and ITIM domain; Tim-3, T cell immunoglobulin and mucin domain-containing protein 3; PD-1, programmed cell death protein; TGF-β, transforming growth factor-β; Gal-9, galectin-9; TCR, T cell receptor; MHC-1, major histocompatibility complex-1. Note: By Figdraw. |
Several gene mutations that influence the activity and recruitment of CD8+ T cells are highlighted as promising therapeutic targets for treating BTC. For instance, IDH1 mutations, which generate (R)-2-hydroxyglutarate [(R)-2HG], can hinder the accumulation of CD8+ T cells.41 (R)-2HG also restricts glycolysis and mitochondrial function in CD8+ T cells, thereby impairing cytokine production in cholangiocarcinoma (CCA). IDH1 inhibitors can counteract this mechanism and promote the recruitment and activation of CD8+ T cells. However, these inhibitors may also enhance the recruitment of regulatory T cells (Tregs) and the expression of CTLA-4. Therefore, combination therapy involving IDH1 and CTLA-4 inhibitors is utilized to achieve nearly complete tumor regression and sustained immunological memory.42 Furthermore, the endoplasmic reticulum stress (ER) response gene, X-box binding protein 1 (XBP1), is found to be upregulated in CD8+ T cells within the TME of BTC, particularly in those with low levels of PD-1 expression. In contrast, the rest of the CD8+ T cells exhibit the opposite pattern. Via using the specific inhibitors of XBP1 (4μ8C), researchers report a downregulation of TIGIT in tumor tissue, suggesting the potential benefits 4μ8C have on BTC patients who are refractory to PD-1 blockers.43 In addition, Liu et al report a negative correlation between the expression of B-cell-specific Moloney murine leukemia virus integration site 1 (BMI1) and CD8 in CCA samples. Subsequent investigations validate the effectiveness of BMI1 inhibitors in inducing the transcription of CD8+ T cell-recruiting chemokines such as C-C motif ligand 5 (CCL5) and C-X-C motif ligand 9 (CXCL9).44 Through investigating tumor tissues from patients treated by Gemcitabine, Oxaliplatin, Lenvatinib, and anti-PD1 antibody (GOLP) therapy, Lu et al report that GZMB+ CD8+ T cell, GZMK+ CD8+ T cell and proliferating CD8+ T cell are the three most active CD8+ subtypes in response to GOLP treatment. Intriguingly, these three clusters share high clonal similarities, suggesting common origins. Further cell trajectory analysis reveals that proliferating CD8+ T cell is at a naive stage with a relatively high exhausted score and low cytotoxic score, while GZMK+ CD8+ T cell is terminally differentiated with the highest cytotoxic score and lowest exhausted score. GZMB+ CD8+ T cell fall in an intermediate stage. CD5L+ macrophage can promote macrophage M2 polarization.45 The interaction of CD5L+ macrophages with GZMB+ CD8+ T cells may contribute to their exhausted status, potentially leading to resistance to GOLP therapy in iCCA patients.46 According to the study carried out by Li et al, about 45.6% of their iCCA patients have been infected by hepatitis b virus (HBV). They report an upregulation of the expression of CD3, CD4, and CD8 in HBV infected iCCA patients, showing that their immune system is activated. Meanwhile, TNFSF9, which suppress the expression of IL-12 and subsequently attenuates the cytotoxicity of CD8+ T cells,47 is higher in HBV-negative iCCA patients. After inhibiting the expression of TNFSF9, there is a significant decrease in cell viability in HBV-negative iCCA organoids. The altered TME observed in HBV-positive iCCA patients may explain their better overall survival compared to HBV-negative patients.48
It is well established that CD8+ T cells confer significant prognostic value in BTC. A high density of CD8+ T cells, especially those in the tumor margin,49 correlates with improved OS in BTC patients.50 Moreover, the infiltration of PD-1−eomesodermin (EOMES)−CD8+ and Granzyme-B+CD8+ T cells is associated with a favorable clinical outcome in BTC patients. Notably, the prognostic impact of PD-1-EOMES-CD8+ T cells is predominantly observed within tumor areas rather than peripheries.51 As a marker of activated CD8+ T cells in ovarian cancer,52 a high level of CXCL13 expression are found to be an indicator of “hot” TME, thus leading to a better prognosis in GBC patients receiving immunotherapy.53 Tissue-resident CD103+CD8+ T cells exhibit persistent antitumor effects within tumor tissues and may serve as prognostic predictors. Patients with higher infiltration of these T cells tend to benefit more from ICI therapies. This unique activity may be attributed to interactions between E-cadherin produced by cancer cells and CD103.54 C-reactive protein (CRP), produced by the liver in response to inflammation, negatively correlates with the prognosis of iCCA patients.55 The lymphocyte-to-CRP ratio (LCR), which combines lymphocyte count and CRP level, is validated as a prognostic marker across various cancers.56–59 Similarly, iCCA patients with unresectable tumors often exhibit lower LCR values along with reduced CD8+ T cell densities.60
Helper T Cell (Th Cell)
The interaction between MHC-antigen-peptide complex and T cell receptor (TCR), alongside co-stimulatory signals and specific cytokine milieu, drives the differentiation of naive CD4+ T cells into distinct functional subsets through activation of multiple signaling pathways. Once activated, Th1 cells exert cytotoxic effects on CD8+ T cells and directly target tumor cells by secreting interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and interleukin (IL)-2. In line with their antitumor activity, low expression of CD4 in both the tumor center and the margin is correlated with reduced OS.61,62 Various strategies aimed at enhancing the expression of costimulatory molecules or suppressing immune checkpoints on CD4+ T cells are proven effective as supplements to traditional ICIs. Listeria monocytogenes (Lm) serves as an antigen provider for MHC class I or II-dependent presentation, contingent on whether Lm undergoes lysosomal degradation. This process stimulates cytotoxic lymphocytes and promotes cell-mediated immunity.63 By deleting virulence factors internalin B and actA, the double-deficient strain L. monocytogenes ∆actA/∆inlB (LmAI) exhibits reduced off-target toxicity while maintaining comparable antitumor responses to wild-type Lm.64 Building upon this research, LmAIO, a derivative of LmAI delivering the model antigen Ovalbumin (Ova), is shown to induce significant Th1 responses, reduce the expression of immune checkpoint molecules on CD4+ and CD8+ T cells, and prolong survival in mice with CCA and Hepatocellular carcinoma (HCC) expressing Ova.65 Additionally, the CD40 agonist and mitogen-activated protein kinase/ERK kinase (MEK) inhibitor can also enhance the activation of CD4+ and CD8+ T cells via various mechanisms.66,67
Regulatory T Cell (Treg)
The Forkhead box protein P3 (FoxP3) expressing Tregs dampen overactivated immune responses, while the crosstalk between Tregs and several other cells in TME can impede antitumor immune responses and further induce the accumulation of Tregs. Recombinant Human Signal-Regulatory Protein gamma (SIRPG) and CD200, known suppressors of immune responses,68,69 are upregulated in the intratumoral Tregs.70 Meanwhile, the interaction between Inducible T cell CO-Stimulator (ICOS) and ICOSL, pivotal in Treg accumulation,71 is notably strengthened in tumoral tissues.70 Several other interplay between Tregs and TME is shown in Figure 2.70,72,73
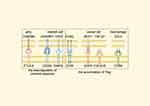 |
Figure 2 The crosstalk between Tregs and other components of TME which is found enhanced in biliary tract cancer. The interaction between CD80/86 and CTLA-4 is important for the Treg-mediated inhibition of immune responses.70 Through combining to their ligands, MUC1, TGF-β1 (secreted by cancer cell) and CCL4 (secreted by macrophage) can lead to the accumulation of Tregs.72,73 Abbreviations: Treg, regulatory T cell; TME, tumor microenvironment; APC, Antigen-presenting cells; CCR8, Chemokine receptor 8; EGFR, Epidermal growth factor receptor; TGF-β1, Transforming growth factor-β1; ICOS, Inducible T cell CO-Stimulator; MUC1, Mucin1; CTLA-4, Cytotoxic T lymphocyte-associated antigen-4; SIRPG, Recombinant Human Signal-Regulatory Protein gamma. Note: By Figdraw. |
Upon infiltration into the TME of BTC, Tregs demonstrate heightened expression of several transcription factors (TFs). Forkhead box M1 (FoxM1), a member of the Forkhead box O (FOXO) family, binds to the FoxP3 promoter, thereby enhancing its transcription in CCA.74 Similarly, IKZF2 increases the expression of FoxP3 and effector cytokines by upregulating the IL-2Rα-STAT5B pathway.75 NVP-DKY709, a selective molecular glue degrader of IKZF2, diminishes Treg immunosuppressive activity and restores cytokine production in exhausted T-effector cells.76 Interferon regulatory factor 4 (IRF4) modulates genes critical for Treg differentiation and chemokine receptors expression and is more abundant in patients with advanced cancer.77 Signal transducers and activators of transcription 5A (STAT5A), activated by IL-2, binds to promoter and enhancer elements to induce FoxP3 expression.78 SMAD-1, a component of the TGF-β1 signaling pathway, facilitates Treg induction.79 Enhancer of zeste homolog2 (EZH2) activity, inhibitable to disrupt Treg transcriptome, is also elevated in intratumoral Tregs.80 Meanwhile, mesenchyme homeobox 1 (MEOX1) transduction leads to increased accessibility of multiple genes involved in immunosuppression in Tregs. The upregulation of the aforementioned TFs provides insights into the transcriptional and epigenetic landscape of tumor-infiltrating Tregs.70 The TFs upregulated in the intra-tumoral Tregs and the pathways in which they are involved are shown in Figure 3.74,75,78,79
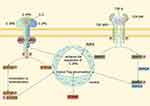 |
Figure 3 TFs upregulated in the intra-tumoral Tregs and the pathways in which they are involved. Upon binding of IL-2 and IL-2R, Jak1 coupled to the IL-2Rβ and Jak3 on the IL-2Rγ will phosphorylate and activate each other. The activated Jak1 and Jak3 can in turn phosphorylate the tyrosine on the IL-2Rβ, introducing a binding site for the STAT5A protein. The bound STAT5A protein is activated, generating dimers or tetramers and transferring to the nucleus, increasing FoxP3 transcription.78 IKZF2 can induce the expression of IL-2Rα and enhance the activity of IL-2Rα–STAT5B pathway.75 TGF-β dimers can interact with TGF-βRI and TGF-βRII, inducing TGF-βRII to phosphorylate TGF-βRI and activating it. The activated TGF-βRI is able to phosphorylate SMAD1, which has a strong affinity for SMAD4. The phosphorylated SMAD1/SMAD4 complex enters the nucleus and binds to transcription promoters to induce DNA transcription, leading to the Treg accumulation.79 In addition, FOXM1 can play a similar role to STAT5A and SMAD1.74 Abbreviations: TF, Transcription factor; Jak1, Janus kinase 1; STAT5A, Signal transducers and activators of transcription 5A; FoxM1, Forkhead box M1; TGF-β, Transforming growth factor-β; FOXM1, forkhead box protein M1. Note: By Figdraw. |
Intra-tumoral tertiary lymphoid structures (TLSs) serve as specialized sites for T cell activation and antigen presentation, correlating with improved prognosis in various solid tumors.81 Conversely, peri-tumoral TLSs negatively impact overall survival in iCCA patients, potentially due to higher CD4+Bcl6+ follicular helper T cell (Tfh) and CD4+Foxp3+ Treg cell densities. Intriguingly, Treg cell frequencies within intra-tumoral TLSs escalate with increased peri-tumoral TLS density, suggesting a mechanism for inducing an immunosuppressive intratumoral environment.82 Deemed as a blocker of leukocyte associated immunoglobulin like receptor 1 (LAIR-1),83 LAIR-2 demonstrates efficacy in restoring exhausted CD8+ T cells in lung cancer.84 Nevertheless, LAIR-2 is predominantly expressed by Tregs and CD8+GZMB+ T cells in CCA, highly associated with various immune inhibitory and stimulatory markers, thereby serving as a marker for T cell exhaustion.85 Although Tregs accumulating at the tumor center typically signal poor prognosis in BTC patients,86 TregIII, one of the three Treg subtypes,87 exhibits a significant negative correlation with iCCA recurrence post-surgery. Higher TregIII infiltration is also linked to reduced perineural invasion.88
B Cell
B cells, activated by the stimulatory signals from antigens and co-stimulatory signals from the interaction between CD40 and CD40L, play a crucial role in augmenting innate and adaptive immune responses through antibody production, antigen delivery, and the release of cytokines and cytotoxic effector molecules. Early B cell Factor 1 (EBF1), involved in B cell differentiation, has emerged as a potential tumor suppressor in various cancers,89–92 including CCA. Oxidative stress is reported to decrease the expression of EBF1, possibly facilitating CCA development.93 This downregulation is likely mediated by DNA hypermethylation in the EBF1 promoter, with high methylation rates correlating with poorer prognosis.94 Elevated infiltration levels of B cells in CCA are associated with improved prognosis, and the expression of propronociceptin (PNOC) by B cells holds similar prognostic significance.85 Unlike T cells, the specific pathogenic role of B cells in BTC remains unclear. Further exploration of B cell functions and their interactions with other immune cells within the BTC tumor TME holds promise for extending the efficacy and durability of immunotherapy strategies.
Innate Immune Cells
Dendritic Cell (DC)
Dendritic cells (DCs), functioning as antigen-presenting cells capable of producing various cytokines and fostering different T cell responses against diverse pathogens, wield significant influence over both anti- and pro-tumor activities, profoundly impacting clinical outcomes. DCs incubated with lysates of cancer cells are capable of generating a broad spectrum of tumor-associated antigens (TAAs), thereby enhancing their cytotoxic potential.95 Investigations into this mechanism reveal promising therapeutic avenue. Honokiol, a pleiotropic compound isolated from magnolia species known for inducing apoptosis in cancer cells, shows potential in this context.96 DCs loaded with tumor cell lysates from honokiol-treated CCA cells exhibit enhanced IL-12 secretion, facilitating heightened activation of CD8+ T cells and Th cells.97 Furthermore, DCs pulsed with tumor lysates and transduced with adenoviruses encoding human CD40L (Ad-hCD40L) exhibit elevated expression of both soluble (s)CD40L and membrane-bound (m)CD40L. These engineered DCs also show increased levels of costimulatory markers and cytokines. Co-culturing these DCs with cytokine-induced killer (CIK) cells results in augmented proliferation and cytotoxicity. Interestingly, soluble CD40L appears to induce predominantly Th2 cytokines, whereas membrane-bound CD40L predominantly induces Th1 cytokines.98 Neoantigens derived from common mutations like TP53, KRAS, and RNF43 in CCA can also stimulate DCs during differentiation, enhancing their ability to selectively target cancer cells and activate T cells.99 Conversely, interactions between tumor associated macrophages (TAMs), CCA cells, and DCs via IL-10 and Transforming Growth Factor Beta receptor (TGF-βR) signaling contribute to reduced expression of MHC class II molecules, chemokine receptors, co-stimulatory molecules, and cytokines on DCs.100 Subsequent studies employing lentiviral short-hairpin RNAs to knock down TGF-βRII and IL-10RA mRNA in DCs have demonstrated improved enhancement of effector T cell cytotoxicity.101
Consistent with its activity in inducing an immunosuppressive TME in various tumors,102 the accumulation of peritumoral Plasmacytoid Dendritic Cells (pDCs) is reported as a predictor of poor prognosis in iCCA patients and are positively correlated with peritumoral Treg abundance.103 Moreover, the high expression of four m6A-related mRNAs (AIP, CEBPB, SDC1, and VPS25), associated with adverse outcomes in CCA patients, correlates with increased infiltration of resting DCs, suggesting a possible contribution to poor prognosis in the high m6A-related mRNA risk group.104 In addition, CD1a, a transmembrane glycoprotein expressed by immature DCs,105 has been linked to improved survival in GBC patients following surgery.106
Natural Killer (NK) Cell
NK cells are cytotoxic lymphocytes of the innate immune system with potent activity against virally infected and/or transformed cells via direct cell killing and production of pro-inflammatory cytokines.107 A plethora of methods to augment the cytotoxicity and longevity of NK cells are currently under clinical investigation. For instance, natural compounds are capable of sensitizing cancer cells to be susceptible to NK cells and display less side effects compared to traditional chemotherapy. Cordycepin, a bioactive compound from Cordyceps militaris extract, possesses anti-tumor properties. Suthida et al demonstrated that cordycepin upregulates mRNA expression of DR4 and DR5 on intrahepatic cholangiocarcinoma (iCCA) cells, enhancing the antitumor immune response and inducing NK cell cytotoxicity.108,109 Genistein, an isoflavone derived from soybean products, is well documented for its anti-tumor effect, similarly upregulating DR4 and DR5 expression on iCCA cells.110
NK cell activity can also be modulated through alternative mechanisms. MHC class I polypeptide-related sequence A (MICA) and MHC class I polypeptide-related sequence B (MICB) are expressed in iCCA tissues under cellular stress and serve as ligands for NK group 2 member D (NKG2D), pivotal for NK cell activation and subsequent cancer cell elimination. However, endoplasmic reticulum protein 5 (ERp5) can bind to the MICA/B α-3 domain and facilitate their cleavage. The monoclonal antibody 7C6 competes for binding to the MICA/B α-3 domain, thereby preventing the loss of cell surface MICA/B and enhancing NK cell cytotoxicity in peripheral and tumor-infiltrating NK cells from iCCA patients.111,112 Globo H ceramide (GHCer), a tumor‐associated carbohydrate antigen, promotes tumor angiogenesis and immune evasion.113 Existing research demonstrate that high Globo H expression is correlated with poor clinical outcomes in rats bearing iCCA. After the administration of anti-Globo H VK9, boosted cytotoxicity of NK cells and suppressed tumor growth are witnessed.114 High mesenchymal–epithelial transition (MET) expression is discovered in 53.1% (135 of 254) of resected samples from CCA patients in a Chinese cohort study. High MET expression is also found to be correlated with short OS in CCA patients.115 Therefore, CAR-NK cells containing humanized single-chain antibody fragment (scFv) targeting MET are designed and has revealed its potent activity to kill specific cancer cells.116 In addition, Fukuda et al’s immunohistochemical analysis reveals that high endogenous expression of CXCL9, a chemoattractant for activated NK cells, is associated with favorable postoperative survival in iCCA patients.117
Neutrophil
Neutrophils, as versatile phagocytes involved in both tumor-promoting and tumor-suppressing roles, play complex and evolving roles in cancer biology. Zhou et al utilize whole-exome sequencing to identify SLIT2 mutations specifically in relapsed intrahepatic iCCA, where SLIT2 inactivation enhances neutrophil chemotaxis and contributes to metastasis.118 In early GBC, upregulation of proteins associated with neutrophil degranulation (MPO, PRTN3, S100A8) is observed, while proteins involved in extracellular matrix (ECM) organization (COL14A1, COL1A2, COL6A1) are downregulated. This suggests that neutrophil infiltration is promoted in early GBC stages, with neutrophil degranulation facilitating ECM degradation and cancer cell invasion.119 Interaction between GBC cells and neutrophils increases the expression of OLR1, a gene mediating oxidized low-density lipoprotein (oxLDL) uptake in neutrophils. This uptake leads to lipid peroxidation and ferroptosis in neutrophils, enhancing their pro-metastatic effects.120,121 Multiplex immunofluorescent staining of iCCA tissues reveals co-distribution and strong correlation in local densities between tumor-associated neutrophils (TANs) and TAMs. Co-cultured TANs and TAMs secrete Oncostatin M (OSM) and IL-11, which promote iCCA tumor growth and metastasis. TANs express TAM chemoattractants (CCL2, CCL5, CSF1), while TAMs express chemokines (CXCL8, CSF3) that induce TAN infiltration, suggesting reciprocal crosstalk between these cell types.122
Sciellin (SCEL), a precursor of the cornified envelope, is demonstrated to be overexpressed in GBC tissues and to enhance carcinogenesis both in vitro and in vivo by stabilizing EGFR expression. Improved EGFR expression also induce the generation of IL-8, leading to the release of chromatin and granular proteins from polymorphonuclear neutrophils (PMN) and the formation of neutrophil extracellular traps (NETs), an extracellular fibrillar matrix that promotes the motility and migration ability of GBC cells.123,124 Meanwhile, it is found that the binding of platelets to cancer cells can also increase the NET formation in iCCA,125 which may consequently lead to adverse prognostic effects in CCA patients.126 Through its interplay with αV integrin, NET can increase the activity of NFκB, and consequently enhance the expression of vascular endothelial growth factor (VEGF), inducing the proliferation, angiogenesis, and metastasis in CCA. Interestingly, It is NET-DNA, rather than NET protein, that can induce tumor growth.127
Neutrophil infiltration levels in tumor regions negatively correlate with OS in patients with GBC post-cholecystectomy.128 The neutrophil-to-lymphocyte ratio (NLR), reflecting circulating blood neutrophil/lymphocyte counts, emerges as a promising prognostic factor for tumor metastasis and surgical infectious complications in BTC patients.129–132 Low albumin levels are also associated with significantly shortened survival, suggesting albumin as a potential prognostic biomarker for BTC patients.133 To further optimize NLR’s efficiency as a biomarker, the cachexia index (CXI), incorporating skeletal muscle index (SMI), albumin levels, and NLR, has been developed and shown to independently predict OS.134 Meanwhile, multiple NLR-based models have been devised to predict long-term outcomes in BTC patients.135–138
Macrophage
Macrophages, as pivotal phagocytic innate immune cells, exhibit distinct polarization states into pro-inflammatory M1 macrophages or anti-inflammatory M2 macrophages. During cancer progression, M1 TAMs initially dominate but are gradually supplanted by M2 TAMs.139 Therapeutic strategies aimed at reducing M2 TAM polarization have thus emerged as promising anti-cancer approaches. Increased expression of secreted midkine (MDK) in GBC with epidermal growth factor receptor (ErbB) mutations promotes M2 TAM polarization via crosstalk with Low-Density Lipoprotein Receptor-Related Protein 1 (LRP1), highlighting MDK as a potential immunotherapy target.140 Yes-associated protein (YAP) is a transcriptional regulator which can activate various oncogenic pathways/target genes.141 Targeting at YAP, verteporfin is identified to induce a higher percentage of M1 TAMs.142 Meanwhile, IGF2BP3 and leptin, involved in the GBC cells-derived exosomes, are also found to increase the proportion of M2-polarized TAMs.143,144 Furthermore, the TME of KRAS-mutated BTC is characterized by a higher M1 macrophage activation and interferon-γ expression.145 The metabolic pathways of M1 and M2 macrophages are different. M1 macrophages prefer glycolytic metabolism, while M2 macrophages are in fond of mitochondrial respiration.146 Immune responsive gene 1 (IRG1), encoding itaconate, can break the TCA cycle and restrain M2 macrophage polarization.147
Interactions between TAMs and the TME critically influence tumor progression. Yang et al demonstrate that TGFβ1 derived from M2 macrophages upregulates the expression of aPKCι, which consequently activates NF-κB signaling pathway, leading to epithelial–mesenchymal transition (EMT) and immunosuppression in CCA. Reciprocally, macrophages are recruited by CCA cells via the secretion of CCL5.148 It is revealed that the Macrophage migration inhibitory factor (MIF) can promote cancer metastasis and progression.149 After the application of ICIs, MIF-CD74 signaling between cancer cells and TAMs is found to be enhanced. In addition, M1 gene signatures are found to be enriched in ICIs treatment-naïve TAMs, whereas M2 gene signatures and SPP1+ TAMs, which is reported to be correlated with poor prognosis, are enriched in TAMs after ICIs treatment.150,151 These findings may help explain the limited efficacy of immune checkpoint inhibitors (ICIs) in iCCA. High-throughput RNA-sequencing by Luo et al identifies elevated miR-183-5p in exosomes from iCCA cells, inhibiting phosphatase and tensin homolog (PTEN) to increase PD-L1 expression on macrophages.152 Involved in the exosomes from TAMs, Circ_0020256 is also reported to promote the proliferation, migration and invasion of cancer cells by interacting with its intra-cellular microRNA target, miR-432-5p.153
As a main cause of malignant biliary diseases, Pancreaticobiliary reflux (PBR) is featured by a high-amylase bile.154 To figure out how high-amylase bile promotes CCA progression, Wu et al measured the concentration of inflammatory factor in the tumor tissue after treatment of high-amylase bile and report an upregulation of IL-8 derived from macrophage.155 IL-8 has been demonstrated to induce metastasis and tumor growth in iCCA via activating PI3K/AKT signaling pathway.156 From chronic cholecystitis to GBC, the expression of Olfactomedin 4 (OLFM4) is gradually upregulated in the tumor tissue and is negatively correlated with survival in GBC patients. OLFM4 depletion can sensitize GBC cells to cis in vivo.157 Via ligand-receptor pairs such as ICAM1-integrin, CCL15-CCR1 and AREG-MMP9, OLFM4+epithelium can interact with TAM intensively. Furthermore, OLFM4 is demonstrated to induce the expression of PD-L1, thus impairing the function of T cells.158 Dermatopontin (DPT) is an extracellular matrix (ECM) protein rich in tyrosine. It is established that the expression of DPT is positively associated with the immune infiltration and the survival of CCA patients. The further study reveals DPT’s activity to stimulate macrophages to release CCL19, which may increase the immune infiltration in CCA TME.159
Myeloid-Derived Suppressor Cell (MDSC)
As stated by their name, MDSCs are immature myeloid cells with immunosuppressive activities. Guided by cytokines such as IL-6 and GM-CSF, MDSCs presence in resectable tumor regions is correlated with the aggressiveness of BTCs. It is reported that Anti-CSF-1R, a TAM blockade, fails to delay CCA progression, likely due to the compensatory upregulation of the expression of granulocytic MDSC (G-MDSC) chemoattractant CXCL2 by cancer associated fibroblasts (CAFs). Combined inhibition of MDSCs and TAMs has proven significantly more effective than monotherapy in subsequent studies.160 Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), expressed predominantly on immune cells, can induce cell death by correlating with its receptor such as DR4 and DR5.108 It is reported that TRAIL reduces the abundance of MDSCs by promoting MDSCs apoptosis in breast cancer.161 However, MDSCs in CCA are resistant to TRAIL-mediated apoptosis due to increased expression of cellular FLICE inhibitory protein, an inhibitor of proapoptotic TRAIL signaling. Moreover, TRAIL can enhance the activation of NF-κB, thus promoting proliferation of MDSCs in CCA.162 MDSC recruitment by CCL2 from CAFs is another mechanism identified. Both the knockdown of fibroblast activation protein (FAP) and high tumor clostridia abundance are able to impair this mechanism.163,164 Additionally, CAFs are demonstrated to promote the activity of MDSCs through the secretion of IL-6 and IL-33. Hyperactivated 5-lipoxygenase (5-LO) metabolism is mediated by these two cytokines in MDSCs. 5-LO consequently induces the synthesis of leukotriene B4 (LTB4), which interacts with leukotriene B4 receptor type 2 (BLT2) to facilitate the iCCA tumor progression, emphasizing this pathway as a potential target for immunotherapy.165
Cancer Associated Fibroblast (CAF)
Mainly originating from hepatic stellate cells (HSCs) and portal fibroblasts (PFs) in BTC,166,167 cancer associated fibroblasts (CAFs) are a very heterogeneous group of cells. The myofibroblastic CAFs (myCAFs) and the inflammatory CAFs (iCAFs), the two most abundant CAF subtypes in the CCA TME, are demonstrated to promote the progression of iCCA through distinct mediator. An upregulation of hyaluronan synthase 2 (Has2) are witnessed in myCAFs, while iCAFs can modulate the growth of carcinoma via the interplay between hepatocyte growth factor (HGF) and mesenchymal to epithelial transition factor.168 Intriguingly, according to the transcriptional similarity between myCAFs and iCAFs, iCAFs might represent an inflamed immunologically activated population of myCAFs rather than an independent subtype.169
As a major cellular component of desmoplastic stroma of BTC, CAFs are activated myofibroblasts that express a wide range of factors, thus playing a pivotal role in BTC growth and progression.170 Alpha smooth muscle actin (α-SMA) is one of the specific markers of CAFs. Accordingly, patients with high α-SMA expression in the tumor stroma have shorten survival.171 Due to strong cell–cell interaction between CAFs and other cell types in TME, the number of CAFs and other cells can also help predict the prognosis of BTC patients.169 Expressed by CAFs, transgelin-2 (TAGLN2) is witnessed to be upregulated in the serum of CCA patients and can be used as a diagnostic marker for BTC. The silenced TAGLN2 expression sensitizes BTC cells to gemcitabine and other chemotherapeutic drugs.172 Furthermore, high expression of IL-33 in CAFs is correlated with better two-year survival of patients with BTC,173 while the matricellular protein periostin is a poor prognostic factor in post-resected BTC.174
Yan et al find that CAF-derived Platelet-derived growth factor (PDGF)-BB is a regulator of lymph node metastasis in CCA. Via correlating with its receptor PDGFR-β on lymphatic endothelial cells (LECs), PDGF-BB can upregulate lymphangiogenesis. Anti-PDGF-BB antibodies and PDGFR inhibitor STI571 significantly inhibit the activation of GSK3β and P65, leading to the suppression of tumor growth, revealing PDGF-BB/PDGFR-β-GSK3β/P65 axis as a potential therapeutic target in CCA.175 CXCR4 is increased tremendously in CAFs, and this overexpression is reported to contribute to the resistance to bortezomib of CCA tissue. Moreover, the combination of CXCR4 and bortezomib sensitizes CCA to anti-PD-1 treatment in the further study.176 Polo-like kinase 1 (PLK1)’s ability to phosphorylate glucose-6-phosphate dehydrogenase (G6PD) and to transform hepatic stellate cell into CAFs phenotype allow it plays a dual role in the regulation of both tumor cells and CAFs. The sigma receptor, which is increased in tumor cells and CAFs, has high affinity with anisamide-derived ligands, making it an ideal target for targeted drug delivery system loaded with PLK1 inhibitor.177,178 Thrombospondin 4 (TSP-4) is highly expressed by CAFs in GBC. TSP-4 can bind to its receptor on cancer cells to induce the phosphorylation of heat shock factor 1 (HSF1), which in turn upregulate the expression of TGF-β1 to promote the transdifferentiation of Peritumoral fibroblasts (PTFs) into CAFs, thus forming a positive feedback loop. This discovery provides a potential therapeutic target for GBC patients.179
Via single-cell transcriptomes analysis, Lewinska et al discovered that iCAF are responsible for upregulated Lysyl oxidase (LOX) expression in CCA. LOX is copper-dependent ECM protein which promote collagen and elastin crosslinking, leading to increased ECM stiffness.180 CCA cells exposed to LOX show a significantly decreased expression of apoptotic genes and an increased cell migration. The interaction between LOX and mitochondrial transcription factor TFAM contributes to the increase in mitochondrial fitness, which in turn promote the metastasis of CCA. Accordingly, LOX is correlated with poor survival in CCA patients. Moreover, LOX overexpression is also found in CCA early lesions and livers of patients with primary sclerosis cholangitis (PSC), indicating that LOX is involved in CCA initiation.181 Although both cancer-promoting CAFs (pCAFs) and cancer-restraining CAFs (rCAFs) have been reported in several cancers so far,182 only pCAFs have been found in CCA. Hu et al measure the CAFs’ effects on the tumor progression by transwell assays and demonstrate the existence of both subtypes of CAFs in CCA. Intriguingly, pCAFs and rCAFs regulate the protein stability of polycomb group ring finger 4 (PCGF4) in a reversed way to affect the migration of the CCA. pCAFs activate the IL-6/phosphorylated STAT3 pathway to increase the stability of PCGF4, while rCAFs trigger the proteasome-dependent degradation of PCGF4.183
ICI Combination Trials
In the Phase II, multi-cohort, non-randomized KEYNOTE-158 study, the efficacy of pembrolizumab, a PD-1 inhibitor, is assessed in 104 patients with previously treated BTC. The mOS is 7.4 months, and the mPFS is 2 months, with a poor objective response rate (ORR) of 5.8%. This study reported a patient with grade 5 renal failure and no patient with grade 4 treatment-related adverse events (TRAE).184 The similar outcomes are shown in several other studies applying ICI monotherapy.34,185 Moreover, ICI monotherapy and immune-based combinations may lead to increased risk of all-grade and grade 3–4 hypertransaminasemia.186 These disappointing results prompt researchers to explore combination therapies involving ICIs and other drugs with lower TRAE incidence. Trials of ICI use in treatment to biliary tract cancers are shown in Table 1.
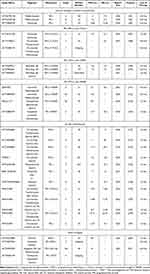 |
Table 1 Trials of Immune Checkpoint Inhibitor (ICI) Use in Treatment to Biliary Tract Cancers |
Combination of PD-1/PD-L1 and CTLA-4 Blockade
Due to the joint effects PD-1/PD-L1 and CTLA have on Effector T cells, Tregs, NK cells and macrophages, combination of inhibitors of these two immune checkpoints is expected to improve the poor prognosis of BTC patients. The phase II study (NCT03101566) of nivolumab and ipilimumab combination demonstrates improved mOS and mPFS in patients with advanced BTC. Of interest, the likelihood of survival at 2 years in this trial is significantly higher than in patients receiving chemotherapy alone. Treatment is feasible with no grade 3 or higher hematologic TRAE reported and the most common nonhematologic TRAE are Elevated aspartate aminotransferase and alanine aminotransferase.187 The low TRAE incidence of this combination is also confirmed in the Phase I trial (NCT01938612).188 Recently, a phase II study investigating the combination of Tremelimumab plus Durvalumab with Radiation (NCT03482102) is ongoing.
Combination of ICI and Transforming Growth Factor Beta Receptor (TGFβR)
Bintrafusp alfa, the first bifunctional fusion protein comprising the extracellular domain of TGF-βRII and a PD-L1 blocker, is expected to revolutionize BTC treatment. A phase I study (NCT02699514) reports its promising antitumor activity in 30 previously treated patients. Importantly, five patients have responses lasting over 12.5 months, and responses are observed even in patients with immune-desert and immune-excluded TME, highlighting the significant value of bintrafusp alfa.189 These results are further verified In the following phase II study (NCT03833661) including 159 patients.190 Unfortunately, the phase II/III study (NCT04066491) evaluating bintrafusp alfa in combination with GemCis as a first-line treatment is terminated.
Combination of ICI and Vascular endothelial growth factor receptor (VEGFR) Blockade
The combination of ICI and VEGFR inhibitors is highly anticipated in the treatment of advanced BTC due to their potential to reprogram the immunosuppressive TME into an immunostimulatory one.202 In the ongoing phase II LEAP-005 study, the durable efficacy of lenvatinib plus pembrolizumab is observed in 31 patients previously treated for BTC. Encouraged by the promising results, the study enrollment is expanded to include 100 patients.191 Patients receiving the same therapy report a much higher ORR in another phase IIb study (NCT03895970), probably due to a high percentage of patients who receive two or more anticancer treatments previously in this study.192 To investigate the combination of PD-L1 and VEGF inhibition with chemotherapy as a first-line treatment for BTC, the phase II double-blind, placebo-controlled IMbrave-151 trial randomly assigns 162 participants to receive bevacizumab plus atezolizumab and GemCis or placebo plus atezolizumab and GemCis. The bevacizumab group shows a superior mPFS.193 The feasibility of combining toripalimab and lenvatinib with radiotherapy (RT) is evaluated in the NCT03892577 clinical trials. This study randomizes BTC patients into the RT group or non-RT (NRT) group, demonstrating significant benefits for patients receiving RT. However, patients treated with RT have a higher incidence of Grade ≥3 treatment-related adverse events (TRAEs).194
Combination of ICI and Chemotherapy
Through immunogenic cell death (ICD) and directly reduction of immunosuppressive cells,203,204 chemotherapy is proven to be able to reach a better result in treatment to many malignancies, including lung cancer,205 breast cancer,206 nasopharyngeal cancer,207 gastric cancer208 and urothelial cancer.209 Via the application of GemCis combined with anti-PD-1 and anti-CTLA-4 in orthotopic murine models of iCCA, Chen et al discover that GemCis induce effector CD8+ T cell recruitment by the normalization of iCCA vasculature, which is caused by proangiogenic factors from cancer cells killed by GemCis, thus enhancing the antitumor effect of ICI.210
In a phase II study conducted in South Korea, 121 advanced BTC patients are randomly divided into three cohorts: in the biomarker cohort (BMC) to receive GemCis, followed by GemCis, durvalumab and tremelimumab; in the 3 combo cohort (3C) to receive GemCis and durvalumab; in the 4 combo cohort (4C) to receive GemCis, durvalumab, and tremelimumab. Amazing benefits are observed in all three cohorts. The most common Grade ≥3 TRAEs are neutropenia and anemia.195 This study lays the groundwork for subsequent phase III trials.
KEYNOTE-966, the second phase III study investigating ICI plus chemotherapy treatment for BTC following TOPAZ-1, enrolls 1069 patients at 175 medical centers worldwide. Among all the participants, 533 receive pembrolizumab plus GemCis and 536 receive placebo plus GemCis between 2019 and 2021. The efficacy boundary for a statistically significant overall survival benefit for the pembrolizumab group is met in this study. The most common TRAEs are decreased neutrophil count and anemia. Of note, an absence of the relationship between PD-L1 expression and outcomes is observed in both KEYNOTE-966 and TOPAZ-1,196 which is also reported in other studies.195,211
The safety and efficacy of toripalimab in combination with gemcitabine plus S-1 are assessed in the single-arm, phase II JS001-ZS-BC001 study in patients with advanced BTCs. Of the 48 treatment naive patients with BTCs, ORR is 27.1%, the mPFS is 7.0 months and the mOS is 16.0 months. The activation of PI3K pathway may associate with shorter PFS in this research.197
In a single-arm, phase II trial (ChiCTR2000036652), 30 BTC patients receive sintilimab plus GemCis as a frontline treatment. The presence of gene alteration in homologous recombination repair (HRR) pathway might bring additional benefits to patients in this study, compared to the wild-type group. Differential expression gene enrichment between responders and non-responders is mainly concentrated in cytokine–cytokine receptor interaction and chemokine signaling pathways. High levels of TAMs are associated with significantly shorter survival outcomes,198 consistent with other studies.212
To determine the efficacy of the combination of durvalumab (D) and tremelimumab (T) in addition with Gem or GemCis, the IMMUCHEC study, which includes five arms, is conducted. The trial interventions: arm A (n=22): D, T and Gem; Arm B (n=22): D, T and GemCis (n=35); Arm C (n=35): GemCis; Arm D (n=30): D, T and GemCis; Arm E (n=29): D and GemCis. The omission of Cis results in inferior outcomes: the ORR is only 4.6% in arm A, while it is 18.2% and 26.7% in arm B and D. Regimen B in arm D seems to have some benefits to patients, with higher mPFS and mOS compared with arm B. The incidence of Grade ≥3 TRAE in both arms is almost unanimous.199
Other Strategies
Dickkopf-1 (DKK1), a secreted WNT signaling modulator, can promote tumor growth by disabling antitumor cells. It is found that DKN-01, an inhibitor of DKK1, reduces tumor growth in mice with CCA.213 The feasibility and efficacy of DKN-01 is also demonstrated in several other tumors.214,215 In a phase I study, 47 BTC patients receive DKN-01 plus GemCis. This therapy is well tolerated by participants and is consistent with the toxicity profile of GemCis. It is noteworthy that improved outcomes are associated with biomarkers of angiogenesis inhibition and reduced inflammation. However, this combination fails to show an additional activity beyond historically reported efficacy of GemCis.200 To further explore the antiangiogenic and immunomodulatory activity of DKN-01, the phase II study (NCT04057365) investigating Nivolumab plus DKN-01 is currently ongoing.
Based on the previous finding that allogeneic NK cells (SMT-NK) show cytolytic activity against CCA in mice216 and clinical trial investigating SMT-NK in patients with lung cancer,217 The phase I and IIa study (NCT03937895) is carried out. In this study, SMT-NK plus pembrolizumab are given to 40 patients. Data from the 23 patients who can be obtained efficacy evaluation for highlight its therapeutic potential.201 Meanwhile, ociperlimab, a TIGIT inhibitor, in combination with tislelizumab and GemCis, are being tested in an ongoing phase II trial (NCT05023109).
Biomarkers for ICI Therapy
Based on the KEYNOTE-028 clinical trial, patients with high tumor mutation burden (TMB) and high PD-L1 expression may benefit more from ICI therapy.218 The first study exploring the correlation between genomic characteristics and immunotherapy response in BTC is carried out by Li et al. In their study, genetic mutations of LRP1B show higher TMB and predict for better immunotherapy response, which is in line with another multicenter analysis involving multiple-tumor types.219 Better PFS is also discovered in patients with ERBB2 and PKHD1 alterations. However, the functional significance of ERBB2 missense mutations remained unclear, and ERBB2 mutations are higher in patients with worse survival in another study.220 Furthermore, Chromosome arm‐level somatic copy-number alterations (SCNAs) can also affect the response to immunotherapy. 19q amplification and 9p deletion are associated with poor prognosis in this research.221 KRAS, correlated with poor prognosis in patients treated with ICI, is one of the most common mutations in BTC.220 It is also found that patients with both KRAS mutation and PD-L1 positivity have longer PFS than patients with KRAS mutation and PD-L1 negativity. Interestingly, this finding is not discovered in patients without KRAS mutation.222 Fatty acid-binding protein 1 (FABP1) is reported to induce the uptake of fatty acids to mediate lipid metabolism of cancer cells during metastasis.223 The expression of FABP1 is significantly higher in direct liver invasion tumor than in primary tumor of GBC and is associated with lymph node metastasis and OS.224 The stem cell marker octamer-binding transcription factor 4 (OCT4) maintains the pluripotent properties of embryonic stem cells and is demonstrated to induce chemoresistance in CCA.225 Compared with patients with no circulating tumor cell (CTC) or patients with OCT4-CTC, patients with OCT4+CTC have a higher chance of developing lymph node metastases and a worse survival.226 Moreover, CD8+ T cell infiltration, NLR, cutaneous TRAEs, expression of immune checkpoint and DPT can also be used to predict the efficacy of ICI in BTC.159,220,227–230
Future Directions and Conclusion
BTCs are rare and devastating solid tumors characterized by a highly heterogeneous TME comprising adaptive and innate immune cells. Due to the typically asymptomatic nature of BTC, there is significant variability in patient responses to immunotherapy, highlighting the challenge of developing personalized treatment strategies. Researchers can use molecular biology, genetics, bioinformatics, and other technologies to explore tailored treatment approaches based on specific gene expressions or mutations. Although the roles of immune cells in tumor progression were previously believed to have fixed positive or negative impacts, it is increasingly evident that their exact functions within the tumor microenvironment are influenced by various factors, such as TME crosstalk and tumor stage. It is feasible to employ single-cell sequencing to explore the phenotype and functional characteristics of different immune cell types within BTC tissues. We can also collect and analyze clinical samples from BTC patients, such as tissue biopsies or blood samples. Analyzing immune cell types, functional states, and levels of immune suppression factors in these samples provides insights into the immune status of BTC patients and its relationship with disease progression and treatment responses. While BTC often exhibit resistance to immune ICI monotherapy, combination therapies, which combine immunotherapy with emerging treatment modalities such as targeted therapy or gene therapy to form comprehensive treatment strategies, could offer BTC patients more treatment options and opportunities. To find the optimal combination, we should conduct preclinical studies to elucidate tumor microenvironment changes and potential resistance mechanisms when combining therapies. Clinical trials are needed to help determine treatment sequences, dosing schedules, and potential synergistic effects in the following research. Furthermore, identifying biomarkers for prognosis and treatment response in BTC patients is a critical research direction. Biomarker discovery can help identify patient populations best suited for immunotherapy, thereby advancing personalized treatment strategies. In order to find powerful biomarkers, it is crucial to collect and analyze biological samples from clinical patients, such as tissue biopsies or serum samples. By comparing immune therapy treatment responses and biomarker expressions in different patient populations, potential biomarkers associated with treatment response can be identified. Meanwhile, combining big data and bioinformatics analysis will help us integrate information from different sources, discover new biomarker candidates, and validate their potential value in immunotherapy. However, Immunotherapy can induce immune-related adverse reactions such as immune-related hepatitis. As a result, focusing on optimizing treatment protocols to minimize adverse effects and identifying more effective management strategies is important. Currently, immunotherapy for BTC remains in the exploratory stage. Over the next five years, more clinical trials animal model studies will be necessary to verify its effectiveness and safety across different patient groups, particularly exploring its potential in advanced-stage BTC.
Overall, the future development of immunotherapy for BTC will depend on deeper understanding of immune mechanisms, which may inspire researchers to invent new combination therapies with minimized adverse effects. Patients can be treated by personalized treatment strategies targeting at specific gene expressions or mutations. Their response to immunotherapy will be predicted by measuring the expression of biomarkers. With advancements in technology and theory, we anticipate immunotherapy to play an increasingly important role in the treatment of the exquisitely heterogeneous BTC, providing new hope and choices for patients.
Acknowledgments
The authors are grateful to all who assisted in the preparation of this manuscript. All individuals included in this section have consented to the acknowledgement.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Valle JW, Kelley RK, Nervi B, Oh D-Y, Zhu AX. Biliary tract cancer. Lancet. 2021;397(10272):428–444. doi:10.1016/S0140-6736(21)00153-7
2. Florio AA, Ferlay J, Znaor A, et al. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer. 2020;126:2666–2678. doi:10.1002/cncr.32803
3. Rizzo A, Brandi G. Pitfalls, challenges, and updates in adjuvant systemic treatment for resected biliary tract cancer. Expert Rev Gastroenterol Hepatol. 2021;15:547–554. doi:10.1080/17474124.2021.1890031
4. Koshiol J, Yu B, Kabadi SM, Baria K, Shroff RT. Epidemiologic patterns of biliary tract cancer in the United States: 2001–2015. BMC Cancer. 2022;22:1178. doi:10.1186/s12885-022-10286-z
5. Rahman R, Ludvigsson JF, von Seth E, Lagergren J, Bergquist A, Radkiewicz C. Age trends in biliary tract cancer incidence by anatomical subtype: a Swedish cohort study. Eur J Cancer. 2022;175:291–298. doi:10.1016/j.ejca.2022.08.032
6. Van Dyke AL, Shiels MS, Jones GS, et al. Biliary tract cancer incidence and trends in the United States by Demographic Group, 1999–2013. Cancer. 2019;125:1489–1498. doi:10.1002/cncr.31942
7. Gunasekaran G, Bekki Y, Lourdusamy V, Schwartz M. Surgical treatments of hepatobiliary cancers. Hepatology. 2021;73(1):128–136. doi:10.1002/hep.31325
8. Zhang W, Zhou H, Wang Y, et al. Systemic treatment of advanced or recurrent biliary tract cancer. Biosci Trends. 2020;14:328–341. doi:10.5582/bst.2020.03240
9. Rizzo A, Ricci AD, Brandi G. Recent advances of immunotherapy for biliary tract cancer. Expert Rev Gastroenterol Hepatol. 2021;15:527–536. doi:10.1080/17474124.2021.1853527
10. Moris D, Palta M, Kim C, Allen PJ, Morse MA, Lidsky ME. Advances in the treatment of intrahepatic cholangiocarcinoma: an overview of the current and future therapeutic landscape for clinicians. CA Cancer J Clin. 2023;73(2):198–222. doi:10.3322/caac.21759
11. Schweitzer N, Fischer M, Kirstein MM, et al. Risk estimation for biliary tract cancer: development and validation of a prognostic score. Liver Int. 2017;37:1852–1860. doi:10.1111/liv.13517
12. Vogel A, Wege H, Caca K, Nashan B, Neumann U. The diagnosis and treatment of cholangiocarcinoma. Dtsch Arztebl Int. 2014;111:748–754. doi:10.3238/arztebl.2014.0748
13. Mirallas O, López-Valbuena D, García-Illescas D, et al. Advances in the Systemic Treatment of Therapeutic Approaches in Biliary Tract Cancer. ESMO Open. 2022;7:100503. doi:10.1016/j.esmoop.2022.100503
14. Rizzo A, Brandi G. Neoadjuvant therapy for cholangiocarcinoma: a comprehensive literature review. Can Treat Res Communicat. 2021;27:100354. doi:10.1016/j.ctarc.2021.100354
15. Edeline J, Hirano S, Bertaut A, et al. Individual patient data meta-analysis of adjuvant gemcitabine-based chemotherapy for biliary tract cancer: combined analysis of the BCAT and PRODIGE-12 studies. Eur J Cancer. 2022;164:80–87. doi:10.1016/j.ejca.2022.01.009
16. Ebata T, Hirano S, Konishi M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg. 2018;105:192–202. doi:10.1002/bjs.10776
17. Edeline J, Benabdelghani M, Bertaut A, et al. Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): a randomized Phase III study. J Clin Oncol. 2019;37:658–667. doi:10.1200/JCO.18.00050
18. Bridgewater J, Fletcher P, Palmer DH, et al. Long-term outcomes and exploratory analyses of the randomized Phase III BILCAP study. J Clin Oncol. 2022;40:2048–2057. doi:10.1200/JCO.21.02568
19. Le Roy B, Gelli M, Pittau G, et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg. 2018;105:839–847. doi:10.1002/bjs.10641
20. Seita K, Ebata T, Mizuno T, et al. Phase 2 trial of adjuvant chemotherapy with S - 1 for Node-Positive Biliary Tract Cancer (N-SOG 09). Ann Surg Oncol. 2020;27:2348–2356. doi:10.1245/s10434-020-08355-3
21. Kobayashi S, Nagano H, Tomokuni A, et al. A prospective, randomized Phase II Study of adjuvant gemcitabine versus S-1 after major hepatectomy for biliary tract cancer (KHBO 1208): Kansai Hepato-Biliary Oncology Group. Ann Surg. 2019;270:230–237. doi:10.1097/SLA.0000000000002865
22. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi:10.1056/NEJMoa0908721
23. Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103:469–474. doi:10.1038/sj.bjc.6605779
24. Ioka T, Kanai M, Kobayashi S, et al. Randomized Phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401- MITSUBA). J Hepatobiliary Pancreat Sci. 2023;30:102–110. doi:10.1002/jhbp.1219
25. Shroff RT, Guthrie KA, Scott AJ, et al. SWOG 1815: a Phase III randomized trial of gemcitabine, cisplatin, and Nab-Paclitaxel versus gemcitabine and cisplatin in newly diagnosed, advanced biliary tract cancers. JCO. 2023;41:LBA490. doi:10.1200/JCO.2023.41.4_suppl.LBA490
26. Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, Chemotherapy-Refractory Cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, Phase 3 study. Lancet Oncol. 2020;21:796–807. doi:10.1016/S1470-2045(20)30157-1
27. Lee S, Shroff RT, Makawita S, et al. Phase II study of ramucirumab in advanced biliary tract cancer previously treated by gemcitabine-based chemotherapy. Clin Cancer Res. 2022;28:2229–2236. doi:10.1158/1078-0432.CCR-21-3548
28. Kim RD, Sanoff HK, Poklepovic AS, et al. A multi-institutional Phase 2 trial of regorafenib in refractory advanced biliary tract cancer. Cancer. 2020;126:3464–3470. doi:10.1002/cncr.32964
29. Valle JW, Lamarca A, Goyal L, Barriuso J, Zhu AX. New horizons for precision medicine in biliary tract cancers. Cancer Discov. 2017;7:943–962. doi:10.1158/2159-8290.CD-17-0245
30. Harding JJ, Fan J, Oh D-Y, et al. Zanidatamab for HER2-Amplified, Unresectable, Locally Advanced or Metastatic Biliary Tract Cancer (HERIZON-BTC-01): a multicentre, single-arm, Phase 2b study. Lancet Oncol. 2023;24:772–782. doi:10.1016/S1470-2045(23)00242-5
31. Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17:807–821. doi:10.1038/s41423-020-0488-6
32. Oh D-Y, He AR, Bouattour M, et al. Durvalumab or Placebo plus Gemcitabine and Cisplatin in Participants with Advanced Biliary Tract Cancer (TOPAZ-1): updated overall survival from a randomised Phase 3 study. Lancet Gastroenterol Hepatol. 2024. doi:10.1016/S2468-1253(24)00095-5
33. Piha-Paul SA, Oh D-Y, Ueno M, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: results from the KEYNOTE-158 and KEYNOTE-028 Studies. Int, J, Cancer. 2020;147:2190–2198. doi:10.1002/ijc.33013
34. Kim RD, Chung V, Alese OB, et al. A Phase 2 multi-institutional study of nivolumab for patients with advanced refractory biliary tract cancer. JAMA Oncol. 2020;6:888–894. doi:10.1001/jamaoncol.2020.0930
35. Cao H, Huang T, Dai M, et al. Tumor microenvironment and its implications for antitumor immunity in cholangiocarcinoma: future perspectives for novel therapies. Int J Biol Sci. 2022;18:5369–5390. doi:10.7150/ijbs.73949
36. Jin M-Z, Jin W-L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 2020;5:166. doi:10.1038/s41392-020-00280-x
37. Leone RD, Powell JD. Metabolism of immune cells in cancer. Nat Rev Cancer. 2020;20:516–531. doi:10.1038/s41568-020-0273-y
38. Shevchenko I, Bazhin AV. Metabolic checkpoints: novel avenues for immunotherapy of cancer. Front Immunol. 2018;9:1816. doi:10.3389/fimmu.2018.01816
39. Qiao Y, Chen J, Wang X, et al. Enhancement of CAR-T cell activity against cholangiocarcinoma by simultaneous knockdown of six inhibitory membrane proteins. Cancer Commun. 2023;43:788–807. doi:10.1002/cac2.12452
40. Supimon K, Sangsuwannukul T, Sujjitjoon J, Chieochansin T, Junking M, Yenchitsomanus P-T. Cytotoxic activity of anti-mucin 1 chimeric antigen receptor T cells expressing PD-1-CD28 switch receptor against cholangiocarcinoma cells. Cytotherapy. 2023;25:148–161. doi:10.1016/j.jcyt.2022.10.006
41. Kohanbash G, Carrera DA, Shrivastav S, et al. Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas. J Clin Invest. 2017;127:1425–1437. doi:10.1172/JCI90644
42. Wu M-J, Shi L, Dubrot J, et al. Mutant IDH inhibits IFNγ-TET2 signaling to promote immunoevasion and tumor maintenance in cholangiocarcinoma. Cancer Discov. 2022;12:812–835. doi:10.1158/2159-8290.CD-21-1077
43. Shi X, Li Z, Yao R, et al. Single-cell atlas of diverse immune populations in the advanced biliary tract cancer microenvironment. NPJ Precis Oncol. 2022;6:58. doi:10.1038/s41698-022-00300-9
44. Liu Z, Hu C, Zheng L, et al. BMI1 promotes cholangiocarcinoma progression and correlates with antitumor immunity in an exosome-dependent manner. Cell Mol Life Sci. 2022;79:469. doi:10.1007/s00018-022-04500-1
45. Sanjurjo L, Aran G, Téllez É, et al. CD5L promotes M2 macrophage polarization through autophagy-mediated upregulation of ID3. Front Immunol. 2018;9:480. doi:10.3389/fimmu.2018.00480
46. Lu J-C, Wu -L-L, Sun Y-N, et al. Macro CD5L+ deteriorates CD8+T cells exhaustion and impairs combination of gemcitabine-oxaliplatin-lenvatinib-anti-PD1 therapy in intrahepatic cholangiocarcinoma. Nat Commun. 2024;15:621. doi:10.1038/s41467-024-44795-1
47. Kang SW, Lee SC, Park SH, et al. Anti-CD137 suppresses tumor growth by blocking reverse signaling by CD137 ligand. Cancer Res. 2017;77:5989–6000. doi:10.1158/0008-5472.CAN-17-0610
48. Li Z, Gao Q, Wu Y, et al. HBV infection effects prognosis and activates the immune response in intrahepatic cholangiocarcinoma. Hepatol Commun. 2024;8:e0360. doi:10.1097/HC9.0000000000000360
49. Liu D, Heij LR, Czigany Z, et al. The role of tumor-infiltrating lymphocytes in cholangiocarcinoma. J Exp Clin Cancer Res. 2022;41:127. doi:10.1186/s13046-022-02340-2
50. Lan C, Kitano Y, Yamashita Y-I, et al. Cancer-associated fibroblast senescence and its relation with tumour-infiltrating lymphocytes and PD-L1 expressions in intrahepatic cholangiocarcinoma. Br J Cancer. 2022;126:219–227. doi:10.1038/s41416-021-01569-6
51. Xia T, Li K, Niu N, et al. Immune cell atlas of cholangiocarcinomas reveals distinct tumor microenvironments and associated prognoses. J Hematol Oncol. 2022;15:37. doi:10.1186/s13045-022-01253-z
52. Yang M, Lu J, Zhang G, et al. CXCL13 shapes immunoactive tumor microenvironment and enhances the efficacy of PD-1 checkpoint blockade in high-grade serous ovarian cancer. J Immunother Cancer. 2021;9. doi:10.1136/jitc-2020-001136
53. Xie L, Ning Z, Hua Y, Wang P, Meng Z. Single-cell transcriptome analysis revealed the immune profile of PD-1 blockade in gallbladder carcinoma liver metastasis. Hepatol Commun. 2023;7:e0131. doi:10.1097/HC9.0000000000000131
54. Chen L, Huang H, Huang Z, et al. Prognostic values of tissue-resident CD8+T cells in human hepatocellular carcinoma and intrahepatic cholangiocarcinoma. World J Surg Oncol. 2023;21:124. doi:10.1186/s12957-023-03009-6
55. Gerhardt T, Milz S, Schepke M, et al. C-reactive protein is a prognostic indicator in patients with perihilar cholangiocarcinoma. World J Gastroenterol. 2006;12:5495–5500. doi:10.3748/wjg.v12.i34.5495
56. Okugawa Y, Toiyama Y, Yamamoto A, et al. Lymphocyte-to-C-reactive protein ratio and score are clinically feasible nutrition-inflammation markers of outcome in patients with gastric cancer. Clin Nutr. 2020;39:1209–1217. doi:10.1016/j.clnu.2019.05.009
57. Yamamoto T, Kawada K, Obama K. Inflammation-related biomarkers for the prediction of prognosis in colorectal cancer patients. Int J Mol Sci. 2021;22(8002). doi:10.3390/ijms22158002
58. Sata A, Fukui R, Miyagawa Y, et al. C-reactive protein and absolute lymphocyte count can predict overall survival of patients treated with eribulin. Anticancer Res. 2020;40:4147–4156. doi:10.21873/anticanres.14414
59. Fan Z, Luo G, Gong Y, et al. Prognostic value of the C-reactive protein/lymphocyte ratio in pancreatic cancer. Ann Surg Oncol. 2020;27:4017–4025. doi:10.1245/s10434-020-08301-3
60. Miyazaki K, Morine Y, Imura S, et al. Preoperative Lymphocyte/C-reactive protein ratio and its correlation with CD8+ tumor-infiltrating lymphocytes as a predictor of prognosis after resection of intrahepatic cholangiocarcinoma. Surg Today. 2021;51:1985–1995. doi:10.1007/s00595-021-02295-5
61. Kim H-D, Kim JH, Ryu Y-M, et al. Spatial distribution and prognostic implications of tumor-infiltrating FoxP3- CD4+ T cells in biliary tract cancer. Cancer Res Treat. 2021;53:162–171. doi:10.4143/crt.2020.704
62. Carapeto F, Bozorgui B, Shroff RT, et al. The immunogenomic landscape of resected intrahepatic cholangiocarcinoma. Hepatology. 2022;75:297–308. doi:10.1002/hep.32150
63. Wood LM, Paterson Y. Attenuated listeria monocytogenes: a powerful and versatile vector for the future of tumor immunotherapy. Front Cell Infect Microbiol. 2014;4:51. doi:10.3389/fcimb.2014.00051
64. Flickinger JC, Rodeck U, Snook AE. Listeria monocytogenes as a vector for cancer immunotherapy: current understanding and progress. Vaccines. 2018;6:48. doi:10.3390/vaccines6030048
65. Hochnadel I, Hoenicke L, Petriv N, et al. Safety and efficacy of prophylactic and therapeutic vaccine based on live-attenuated listeria monocytogenes in hepatobiliary cancers. Oncogene. 2022;41:2039–2053. doi:10.1038/s41388-022-02222-z
66. Wabitsch S, Tandon M, Ruf B, et al. Anti-PD-1 in combination with trametinib suppresses tumor growth and improves survival of intrahepatic cholangiocarcinoma in mice. Cell Mol Gastroenterol Hepatol. 2021;12:1166–1178. doi:10.1016/j.jcmgh.2021.05.011
67. Diggs LP, Ruf B, Ma C, et al. CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma. J Hepatol. 2021;74:1145–1154. doi:10.1016/j.jhep.2020.11.037
68. Caserta S, Nausch N, Sawtell A, et al. Chronic infection drives expression of the inhibitory receptor CD200R, and its ligand CD200, by Mouse and human CD4 T cells. PLoS One. 2012;7. doi:10.1371/journal.pone.0035466
69. Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47–SIRPα signalling pathway. Trends Cell Biol. 2009;19:72–80. doi:10.1016/j.tcb.2008.12.001
70. Alvisi G, Termanini A, Soldani C, et al. Multimodal single-cell profiling of intrahepatic cholangiocarcinoma defines hyperactivated tregs as a potential therapeutic target. J Hepatol. 2022;77:1359–1372. doi:10.1016/j.jhep.2022.05.043
71. Kornete M, Sgouroudis E, Piccirillo CA. ICOS-dependent homeostasis and function of Foxp3+ regulatory T cells in islets of nonobese diabetic mice. J Immunol. 2012;188:1064–1074. doi:10.4049/jimmunol.1101303
72. Xu L, Lu Y, Deng Z, et al. Single-cell landscape of immunocytes in patients with extrahepatic cholangiocarcinoma. J Transl Med. 2022;20:210. doi:10.1186/s12967-022-03424-5
73. Deng X, Jiang P, Chen J, et al. GATA6 promotes epithelial-mesenchymal transition and metastasis through MUC1/β-catenin pathway in cholangiocarcinoma. Cell Death Dis. 2020;11:860. doi:10.1038/s41419-020-03070-z
74. Ma K, Sun Z, Li X, Guo J, Wang Q, Teng M. Forkhead Box M1 recruits FoxP3+ treg cells to induce immune escape in hilar cholangiocarcinoma. Immun Inflamm Dis. 2022;10:e727. doi:10.1002/iid3.727
75. Kim H-J, Barnitz RA, Kreslavsky T, et al. Stable inhibitory activity of regulatory T cells requires the transcription factor helios. Science. 2015;350:334–339. doi:10.1126/science.aad0616
76. Bonazzi S, d’Hennezel E, Beckwith REJ, et al. Discovery and characterization of a selective IKZF2 glue degrader for cancer immunotherapy. Cell Chem Biol. 2023;30:235–247.e12. doi:10.1016/j.chembiol.2023.02.005
77. Alvisi G, Brummelman J, Puccio S, et al. IRF4 instructs effector treg differentiation and immune suppression in human cancer. J Clin Invest. 2020;130:3137–3150. doi:10.1172/JCI130426
78. Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for Interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi:10.1038/ni1263
79. Gandhi R, Kumar D, Burns EJ, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell–like and Foxp3+ regulatory T cells. Nat Immunol. 2010;11:846–853. doi:10.1038/ni.1915
80. DuPage M, Chopra G, Quiros J, et al. The chromatin-modifying enzyme Ezh2 is critical for the maintenance of regulatory T cell identity after activation. Immunity. 2015;42:227–238. doi:10.1016/j.immuni.2015.01.007
81. Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary Lymphoid Structures in the Era of Cancer Immunotherapy. Nat Rev Cancer. 2019;19:307–325. doi:10.1038/s41568-019-0144-6
82. Ding G-Y, Ma J-Q, Yun J-P, et al. Distribution and density of tertiary lymphoid structures predict clinical outcome in intrahepatic cholangiocarcinoma. J Hepatol. 2022;76:608–618. doi:10.1016/j.jhep.2021.10.030
83. Verbrugge A, Ruiter TD, Clevers H, Meyaard L. Differential contribution of the immunoreceptor tyrosine-based inhibitory motifs of human leukocyte-associated ig-like receptor-1 to inhibitory function and phosphatase recruitment. Int Immunol. 2003;15:1349–1358. doi:10.1093/intimm/dxg134
84. Peng DH, Rodriguez BL, Diao L, et al. Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8+ T Cell exhaustion. Nat Commun. 2020;11:4520. doi:10.1038/s41467-020-18298-8
85. Chen Z, Yu M, Yan J, et al. PNOC expressed by B cells in cholangiocarcinoma was survival related and LAIR2 could be a T cell exhaustion biomarker in tumor microenvironment: characterization of immune microenvironment combining single-cell and bulk sequencing technology. Front Immunol. 2021;12:647209. doi:10.3389/fimmu.2021.647209
86. Kinoshita M, Kobayashi S, Gotoh K, et al. Heterogeneity of Treg/Th17 according to cancer progression and modification in biliary tract cancers via self-producing cytokines. Dig Dis Sci. 2020;65:2937–2948. doi:10.1007/s10620-019-06011-9
87. Wang L, Simons DL, Lu X, et al. Connecting blood and intratumoral treg cell activity in predicting future relapse in breast cancer. Nat Immunol. 2019;20:1220–1230. doi:10.1038/s41590-019-0429-7
88. Zheng Y, Huang N, Kuang S, et al. The clinicopathological significance and relapse predictive role of tumor microenvironment of intrahepatic cholangiocarcinoma after radical surgery. Cancer. 2023;129:393–404. doi:10.1002/cncr.34552
89. Shen A, Chen Y, Liu L, et al. EBF1-mediated upregulation of ribosome assembly factor PNO1 contributes to cancer progression by negatively regulating the P53 signaling pathway. Cancer Res. 2019;79:2257–2270. doi:10.1158/0008-5472.CAN-18-3238
90. Mullighan CG, Goorha S, Radtke I, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–764. doi:10.1038/nature05690
91. Cheng Y, Ni Y-J, Tang L-M. ZNF521/EBF1 axis regulates AKR1B1 to promote the proliferation, migration, and invasion of gastric cancer cells. Kaohsiung J Med Sci. 2023;39:244–253. doi:10.1002/kjm2.12624
92. Neve RM, Chin K, Fridlyand J, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi:10.1016/j.ccr.2006.10.008
93. Armartmuntree N, Murata M, Techasen A, et al. Prolonged oxidative stress down-regulates early B cell factor 1 with inhibition of its tumor suppressive function against cholangiocarcinoma genesis. Redox Biol. 2018;14:637–644. doi:10.1016/j.redox.2017.11.011
94. Armartmuntree N, Jusakul A, Sakonsinsiri C, et al. Promoter hypermethylation of early B cell factor 1 (EBF1) is associated with cholangiocarcinoma progression. J Cancer. 2021;12:2673–2686. doi:10.7150/jca.52378
95. Junking M, Grainok J, Thepmalee C, Wongkham S, Yenchitsomanus P-T. Enhanced cytotoxic activity of effector T-cells against cholangiocarcinoma by dendritic cells pulsed with pooled mRNA. Tumour Biol. 2017;39:1010428317733367. doi:10.1177/1010428317733367
96. Rauf A, Olatunde A, Imran M, et al. Honokiol: a review of its pharmacological potential and therapeutic insights. Phytomedicine. 2021;90:153647. doi:10.1016/j.phymed.2021.153647
97. Jiraviriyakul A, Songjang W, Kaewthet P, Tanawatkitichai P, Bayan P, Pongcharoen S. Honokiol-enhanced cytotoxic T lymphocyte activity against cholangiocarcinoma cells mediated by dendritic cells pulsed with damage-associated molecular patterns. World J Gastroenterol. 2019;25:3941–3955. doi:10.3748/wjg.v25.i29.3941
98. Sadeghlar F, Vogt A, Mohr RU, et al. Induction of cytotoxic effector cells towards cholangiocellular, pancreatic, and colorectal tumor cells by activation of the immune checkpoint CD40/CD40L on dendritic cells. Cancer Immunol Immunother. 2021;70:1451–1464. doi:10.1007/s00262-020-02746-x
99. Panya A, Thepmalee C, Sawasdee N, Saengmuang S, Luangwattananun P, Yenchitsomanus P-T. Enhancing cholangiocarcinoma immunotherapy with adoptive T cells targeting HLA-restricted neoantigen peptides derived from driver gene mutations. Biomed Pharmacother. 2023;168:115827. doi:10.1016/j.biopha.2023.115827
100. Thepmalee C, Panya A, Junking M, Chieochansin T, Yenchitsomanus P-T. Inhibition of IL-10 and TGF-β receptors on dendritic cells enhances activation of effector T-cells to kill cholangiocarcinoma cells. Hum Vaccin Immunother. 2018;14(6):1423–1431. doi:10.1080/21645515.2018.1431598
101. Thepmalee C, Panya A, Sujjitjoon J, et al. Suppression of TGF-β and IL-10 receptors on self-differentiated dendritic cells by short-hairpin RNAs enhanced activation of effector T-cells against cholangiocarcinoma cells. Hum Vaccin Immunother. 2020;16:2318–2327. doi:10.1080/21645515.2019.1701913
102. Vermi W, Soncini M, Melocchi L, Sozzani S, Facchetti F. Plasmacytoid dendritic cells and cancer. J Leukoc Biol. 2011;90:681–690. doi:10.1189/jlb.0411190
103. Hu Z-Q, Zhou Z-J, Luo C-B, et al. Peritumoral plasmacytoid dendritic cells predict a poor prognosis for intrahepatic cholangiocarcinoma after curative resection. Cancer Cell Int. 2020;20:582. doi:10.1186/s12935-020-01676-z
104. Zhu H, Zhao H, Wang J, et al. Potential prognosis index for m6A-related mRNA in cholangiocarcinoma. BMC Cancer. 2022;22:620. doi:10.1186/s12885-022-09665-3
105. Moody DB, Suliman S. CD1: from Molecules to diseases. F1000Res. 2017;6:1909. doi:10.12688/f1000research.12178.1
106. Kai K, Tanaka T, Ide T, Kawaguchi A, Noshiro H, Aishima S. Immunohistochemical analysis of the aggregation of CD1a-positive dendritic cells in resected specimens and its association with surgical outcomes for patients with gallbladder cancer. Transl Oncol. 2021;14:100923. doi:10.1016/j.tranon.2020.100923
107. Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18:85–100. doi:10.1038/s41571-020-0426-7
108. Sag D, Ayyildiz ZO, Gunalp S, Wingender G. The role of TRAIL/DRs in the modulation of immune cells and responses. Cancers. 2019;11:1469. doi:10.3390/cancers11101469
109. Panwong S, Wathikthinnakon M, Kaewkod T, et al. Cordycepin sensitizes cholangiocarcinoma cells to be killed by natural killer-92 (NK-92) cells. Molecules. 2021;26:5973. doi:10.3390/molecules26195973
110. Chiawpanit C, Panwong S, Sawasdee N, Yenchitsomanus P-T, Panya A. Genistein sensitizes human cholangiocarcinoma cell lines to be susceptible to natural killer cells. Biology. 2022;11:1098. doi:10.3390/biology11081098
111. Ferrari de Andrade L, Tay RE, Pan D, et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science. 2018;359:1537–1542. doi:10.1126/science.aao0505
112. Oliviero B, Varchetta S, Mele D, et al. MICA/B-targeted antibody promotes NK cell-driven tumor immunity in patients with intrahepatic cholangiocarcinoma. Oncoimmunology. 2022;11:2035919. doi:10.1080/2162402X.2022.2035919
113. Cheng J-Y, Wang S-H, Lin J, et al. Globo-H ceramide shed from cancer cells triggers translin-associated factor X-dependent angiogenesis. Cancer Res. 2014;74:6856–6866. doi:10.1158/0008-5472.CAN-14-1651
114. Hung T-H, Hung J-T, Wu C-E, et al. Globo H is a promising theranostic marker for intrahepatic cholangiocarcinoma. Hepatol Commun. 2022;6:194–208. doi:10.1002/hep4.1800
115. Mao Z-Y, Zhu G-Q, Ren L, Guo X-C, Su D, Bai L. Prognostic value of C-met expression in cholangiocarcinoma. Technol Cancer Res Treat. 2016;15:227–233. doi:10.1177/1533034615578959
116. Chiawpanit C, Wathikthinnakorn M, Sawasdee N, et al. Precision immunotherapy for cholangiocarcinoma: pioneering the use of human-derived anti-cMET single chain variable fragment in anti-cMET Chimeric Antigen Receptor (CAR) NK cells. Int Immunopharmacol. 2024;136:112273. doi:10.1016/j.intimp.2024.112273
117. Fukuda Y, Asaoka T, Eguchi H, et al. Endogenous CXCL9 affects prognosis by regulating tumor-infiltrating natural killer cells in intrahepatic cholangiocarcinoma. Cancer Sci. 2020;111:323–333. doi:10.1111/cas.14267
118. Zhou S-L, Luo C-B, Song C-L, et al. Genomic evolution and the impact of SLIT2 mutation in relapsed intrahepatic cholangiocarcinoma. Hepatology. 2022;75:831–846. doi:10.1002/hep.32164
119. Akhtar J, Jain V, Kansal R, et al. Quantitative tissue proteome profile reveals neutrophil degranulation and remodeling of extracellular matrix proteins in early stage gallbladder cancer. Front Oncol. 2022;12:1046974. doi:10.3389/fonc.2022.1046974
120. Kim R, Hashimoto A, Markosyan N, et al. Ferroptosis of tumor neutrophils causes immune suppression in cancer. Nature. 2022;612:338–346. doi:10.1038/s41586-022-05443-0
121. Rao D, Li J, Zhang M, et al. Multi-model analysis of gallbladder cancer reveals the role of OxLDL-absorbing neutrophils in promoting liver invasion. Exp Hematol Oncol. 2024;13:58. doi:10.1186/s40164-024-00521-7
122. Zhou Z, Wang P, Sun R, et al. Tumor-associated neutrophils and macrophages interaction contributes to intrahepatic cholangiocarcinoma progression by activating STAT3. J Immunother Cancer. 2021;9:e001946. doi:10.1136/jitc-2020-001946
123. Li Y, Yuan R, Ren T, et al. Role of sciellin in gallbladder cancer proliferation and formation of neutrophil extracellular traps. Cell Death Dis. 2021;12:30. doi:10.1038/s41419-020-03286-z
124. Herre M, Cedervall J, Mackman N, Olsson A-K. Neutrophil extracellular traps in the pathology of cancer and other inflammatory diseases. Physiol Rev. 2023;103:277–312. doi:10.1152/physrev.00062.2021
125. Yoshimoto M, Kagawa S, Kajioka H, et al. Dual antiplatelet therapy inhibits neutrophil extracellular traps to reduce liver micrometastases of intrahepatic cholangiocarcinoma. Cancer Lett. 2023;567:216260. doi:10.1016/j.canlet.2023.216260
126. Yamamoto H, Nakanishi Y, Mitsuhashi T, et al. Impact of neutrophil extracellular traps identified by citrullinated histone H3 immunohistochemistry for postoperative prognosis in patients with extrahepatic cholangiocarcinomas. Ann Surg Oncol. 2024;31:2090–2100. doi:10.1245/s10434-023-14638-2
127. Zhang C, Wu D, Dong B, et al. The scaffold of neutrophil extracellular traps promotes CCA progression and modulates angiogenesis via ITGAV/NFκB. Cell Commun Signal. 2024;22:103. doi:10.1186/s12964-024-01500-5
128. Bosch DE, Salipante SJ, Schmidt RA, et al. Neutrophilic inflammation in gallbladder carcinoma correlates with patient survival: a case-control study. Ann Diagn Pathol. 2022;56:151845. doi:10.1016/j.anndiagpath.2021.151845
129. Ogul A, Kidi MM, Buyuksimsek M. Advanced biliary tract cancer treated with Gemcitabine plus Cisplatin (GEMCIS) and novel inflammatory markers. J Gastrointest Cancer. 2021;52:294–299. doi:10.1007/s12029-020-00428-6
130. Hashimoto Y, Ajiki T, Yanagimoto H, et al. Risk factors for occult metastasis detected by inflammation-based prognostic scores and tumor markers in biliary tract cancer. World J Clin Cases. 2021;9:9770–9782. doi:10.12998/wjcc.v9.i32.9770
131. Ruzzenente A, Alaimo L, Caputo M, et al. Infectious complications after surgery for perihilar cholangiocarcinoma: a single western center experience. Surgery. 2022;172:813–820. doi:10.1016/j.surg.2022.04.028
132. Fu S, Li J, Fan H, et al. NLCECA score: a serum inflammatory-tumor biomarker score to predict survival of advanced perihilar cholangiocarcinoma after hepatic arterial infusion chemotherapy. Sci Rep. 2024;14:4466. doi:10.1038/s41598-024-53883-7
133. Guven DC, Sahin TK, Erul E, et al. The association between albumin levels and survival in patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Mol Biosci. 2022;9:1039121. doi:10.3389/fmolb.2022.1039121
134. Hamura R, Haruki K, Shirai Y, et al. Preoperative Cachexia Index Can predict the prognosis of extrahepatic biliary tract cancer after resection. Surg Oncol. 2022;44:101825. doi:10.1016/j.suronc.2022.101825
135. Zhang B, Yao W. Prognostic role of the Systemic Immune-Inflammation Index in biliary tract cancers: a meta-analysis of 3515 patients. World J Surg Oncol. 2022;20:320. doi:10.1186/s12957-022-02783-z
136. Filippi R, Montagnani F, Lombardi P, et al. A prognostic model in patients with advanced biliary tract cancer receiving first-line chemotherapy. Acta Oncol. 2021;60:1317–1324. doi:10.1080/0284186X.2021.1953704
137. Du F, Qiu Z, Ai W, et al. Blood tests predict the therapeutic prognosis of anti-PD-1 in advanced biliary tract cancer. J Leukoc Biol. 2021;110:327–334. doi:10.1002/JLB.5MA1220-631R
138. Liu F, Wang J-K, Ma W-J, Hu H-J, Jin Y-W, Li F-Y. Prognostic value of combined preoperative inflammatory marker neutrophil-lymphocyte ratio and platelet distribution width in patients with gallbladder carcinoma. Langenbecks Arch Surg. 2024;409:51. doi:10.1007/s00423-024-03247-6
139. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi:10.1038/nri2448
140. Zhang Y, Zuo C, Liu L, et al. Single-cell RNA-sequencing atlas reveals an MDK-dependent immunosuppressive environment in ErbB pathway-mutated gallbladder cancer. J Hepatol. 2021;75:1128–1141. doi:10.1016/j.jhep.2021.06.023
141. Shen Y, Wang X, Liu Y, et al. STAT3-YAP/TAZ signaling in endothelial cells promotes tumor angiogenesis. Sci Signal. 2021;14:eabj8393. doi:10.1126/scisignal.abj8393
142. Golino JL, Wang X, Bian J, et al. Anti-cancer activity of verteporfin in cholangiocarcinoma. Cancers. 2023;15:2454. doi:10.3390/cancers15092454
143. Qin Y, Zhang M, Lei H, et al. Knockdown of IGF2BP3 inhibits the tumorigenesis of gallbladder cancer and modifies tumor microenvironment. Drug Dev Res. 2022;83:1831–1844. doi:10.1002/ddr.22000
144. Liu L, Liu Y, Zhu Y, et al. Exosomal leptin accelerates gallbladder carcinoma by promoting M2-subtype macrophage polarization. Minerva Med. 2024;115:253–254. doi:10.23736/S0026-4806.23.08612-3
145. Moffat GT, Hu ZI, Meric-Bernstam F, et al. KRAS allelic variants in biliary tract cancers. JAMA Network Open. 2024;7:e249840. doi:10.1001/jamanetworkopen.2024.9840
146. Koo S, Garg NJ. Metabolic programming of macrophage functions and pathogens control. Redox Biol. 2019;24:101198. doi:10.1016/j.redox.2019.101198
147. Zhou M, Yu H, Bai M, et al. IRG1 restrains M2 macrophage polarization and suppresses intrahepatic cholangiocarcinoma progression via the CCL18/STAT3 pathway. Cancer Sci. 2024;115:777–790. doi:10.1111/cas.16068
148. Yang T, Deng Z, Xu L, et al. Macrophages-aPKCɩ-CCL5 feedback loop modulates the progression and chemoresistance in cholangiocarcinoma. J Exp Clin Cancer Res. 2022;41:23. doi:10.1186/s13046-021-02235-8
149. Sumaiya K, Langford D, Natarajaseenivasan K, Shanmughapriya S. Macrophage Migration Inhibitory Factor (MIF): a multifaceted cytokine regulated by genetic and physiological strategies. Pharmacol Ther. 2022;233:108024. doi:10.1016/j.pharmthera.2021.108024
150. Bill R, Wirapati P, Messemaker M, et al. CXCL9: SPP1 macrophage polarity identifies a network of cellular programs that control human cancers. Science. 2023;381:515–524. doi:10.1126/science.ade2292
151. Sun B-Y, Zhou C, Guan R-Y, et al. Dissecting intra-tumoral changes following immune checkpoint blockades in intrahepatic cholangiocarcinoma via single-cell analysis. Front Immunol. 2022;13:871769. doi:10.3389/fimmu.2022.871769
152. Luo C, Xin H, Zhou Z, et al. Tumor-derived exosomes induce immunosuppressive macrophages to foster intrahepatic cholangiocarcinoma progression. Hepatology. 2022;76:982–999. doi:10.1002/hep.32387
153. Chen S, Chen Z, Li Z, et al. Tumor-associated macrophages promote cholangiocarcinoma progression via exosomal Circ_0020256. Cell Death Dis. 2022;13:94. doi:10.1038/s41419-022-04534-0
154. Wang L, Zhang Z-W, Guo T, Xie P, Huang X-R, Yu Y-H. Occult pancreaticobiliary reflux is a pathogenic factor of some benign biliary diseases and gallbladder cancer. Hepatobiliary Pancreat Dis Int. 2023;22:288–293. doi:10.1016/j.hbpd.2022.08.010
155. Wu T, Gao R, Wang X, et al. Pancreatobiliary reflux increases macrophage-secreted IL-8 and activates the PI3K/NFκB pathway to promote cholangiocarcinoma progression. Transl Oncol. 2024;45:101967. doi:10.1016/j.tranon.2024.101967
156. Meng Z-W, Zhang L, Cai X-R, Wang X, She -F-F, Chen Y-L. IL-8 is a novel prometastatic chemokine in intrahepatic cholangiocarcinoma that induces CXCR2-PI3K/AKT signaling upon CD97 activation. Sci Rep. 2023;13:18711. doi:10.1038/s41598-023-45496-3
157. Lin Z, Yang S, Zhou Y, et al. OLFM4 depletion sensitizes gallbladder cancer cells to cisplatin through the ARL6IP1/Caspase-3 axis. Transl Oncol. 2022;16:101331. doi:10.1016/j.tranon.2021.101331
158. He H, Chen S, Yu Y, et al. Comprehensive single-cell analysis deciphered microenvironmental dynamics and immune regulator olfactomedin 4 in pathogenesis of gallbladder cancer. Gut. 2024. doi:10.1136/gutjnl-2023-331773
159. Xu P, Li S, Liu K, et al. Downregulation of dermatopontin in cholangiocarcinoma cells suppresses CCL19 secretion of macrophages and immune infiltration. J Cancer Res Clin Oncol. 2024;150:66. doi:10.1007/s00432-023-05532-1
160. Loeuillard E, Yang J, Buckarma E, et al. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J Clin Invest. 2020;130:5380–5396. doi:10.1172/JCI137110
161. Nalawade SA, Shafer P, Bajgain P, et al. Selectively targeting myeloid-derived suppressor cells through TRAIL receptor 2 to enhance the efficacy of CAR T cell therapy for treatment of breast cancer. J Immunother Cancer. 2021;9:e003237. doi:10.1136/jitc-2021-003237
162. Loeuillard EJ, Li B, Stumpf HE, et al. Noncanonical TRAIL signaling promotes myeloid-derived suppressor cell abundance and tumor growth in cholangiocarcinoma. Cell Mol Gastroenterol Hepatol. 2024;17:853–876. doi:10.1016/j.jcmgh.2024.01.006
163. Ma W-J, Li Z-H, Wu Z-R, et al. PI3K-CCL2-CCR2-MDSCs axis: a potential pathway for tumor clostridia-promoted CD 8+ T lymphocyte infiltration in bile tract cancers. Neoplasia. 2023;43:100920. doi:10.1016/j.neo.2023.100920
164. Lin Y, Li B, Yang X, et al. Fibroblastic FAP promotes intrahepatic cholangiocarcinoma growth via MDSCs recruitment. Neoplasia. 2019;21:1133–1142. doi:10.1016/j.neo.2019.10.005
165. Lin Y, Cai Q, Chen Y, et al. CAFs shape myeloid-derived suppressor cells to promote stemness of intrahepatic cholangiocarcinoma through 5-lipoxygenase. Hepatology. 2022;75:28–42. doi:10.1002/hep.32099
166. Dranoff JA, Wells RG. Portal fibroblasts: underappreciated mediators of biliary fibrosis. Hepatology. 2010;51:1438–1444. doi:10.1002/hep.23405
167. Okabe H, Beppu T, Hayashi H, et al. Hepatic stellate cells may relate to progression of intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16:2555–2564. doi:10.1245/s10434-009-0568-4
168. Affo S, Nair A, Brundu F, et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell. 2021;39:866–882.e11. doi:10.1016/j.ccell.2021.03.012
169. Martin-Serrano MA, Kepecs B, Torres-Martin M, et al. Novel microenvironment-based classification of intrahepatic cholangiocarcinoma with therapeutic implications. Gut. 2023;72:736. doi:10.1136/gutjnl-2021-326514
170. Biffi G, Tuveson DA. Diversity and biology of cancer-associated fibroblasts. Physiol Rev. 2021;101:147–176. doi:10.1152/physrev.00048.2019
171. Itou RA, Uyama N, Hirota S, et al. Immunohistochemical characterization of cancer-associated fibroblasts at the primary sites and in the metastatic lymph nodes of human intrahepatic cholangiocarcinoma. Hum Pathol. 2019;83:77–89. doi:10.1016/j.humpath.2018.08.016
172. Jo JH, Park SB, Chung J, et al. Transgelin-2, a novel cancer stem cell-related biomarker, is a diagnostic and therapeutic target for biliary tract cancer. BMC Cancer. 2024;24:357. doi:10.1186/s12885-024-12082-3
173. Yangngam S, Thongchot S, Pongpaibul A, et al. High level of interleukin-33 in cancer cells and cancer-associated fibroblasts correlates with good prognosis and suppressed migration in cholangiocarcinoma. J Cancer. 2020;11:6571–6581. doi:10.7150/jca.48327
174. Okamoto K, Tajima H, Nakanuma S, et al. Angiotensin II enhances epithelial-to-mesenchymal transition through the interaction between activated hepatic stellate cells and the stromal cell-derived factor-1/CXCR4 axis in intrahepatic cholangiocarcinoma. Int J Oncol. 2012;41:573–582. doi:10.3892/ijo.2012.1499
175. Yan J, Xiao G, Yang C, et al. Cancer-associated fibroblasts promote lymphatic metastasis in cholangiocarcinoma via the PDGF-BB/PDGFR-β mediated paracrine signaling network. Aging Dis. 2024;15:369–389. doi:10.14336/AD.2023.0420
176. Li L, Zhou Y, Zhang Y, Hu H, Mao H-Q, Selaru FM. A combination therapy of bortezomib, CXCR4 Inhibitor, and checkpoint inhibitor is effective in cholangiocarcinoma in vivo. iScience. 2023;26:106095. doi:10.1016/j.isci.2023.106095
177. Ma X, Wang L, Huang D, et al. Polo-like kinase 1 coordinates biosynthesis during cell cycle progression by directly activating pentose phosphate pathway. Nat Commun. 2017;8:1506. doi:10.1038/s41467-017-01647-5
178. Zhou Y, Xu L, Wang Z, et al. Sequentially targeting and intervening mutual polo-like kinase 1 on CAFs and tumor cells by dual targeting nano-platform for cholangiocarcinoma treatment. Theranostics. 2022;12:3911–3927. doi:10.7150/thno.70557
179. Shi Y, Sun L, Zhang R, et al. Thrombospondin 4/integrin Α2/HSF1 axis promotes proliferation and cancer stem-like traits of gallbladder cancer by enhancing reciprocal crosstalk between cancer-associated fibroblasts and tumor cells. J Exp Clin Cancer Res. 2021;40:14. doi:10.1186/s13046-020-01812-7
180. Herchenhan A, Uhlenbrock F, Eliasson P, et al. Lysyl oxidase activity is required for ordered collagen fibrillogenesis by tendon cells. J Biol Chem. 2015;290:16440–16450. doi:10.1074/jbc.M115.641670
181. Lewinska M, Zhuravleva E, Satriano L, et al. Fibroblast-derived lysyl oxidase increases oxidative phosphorylation and stemness in cholangiocarcinoma. Gastroenterology. 2024;166. doi:10.1053/j.gastro.2023.11.302
182. Miyai Y, Esaki N, Takahashi M, Enomoto A. Cancer-associated fibroblasts that restrain cancer progression: hypotheses and perspectives. Can Sci. 2020;111:1047–1057. doi:10.1111/cas.14346
183. Hu J, Xu H, Ma X, et al. Modulating PCGF4/BMI1 stability is an efficient metastasis-regulatory strategy used by distinct subtypes of cancer-associated fibroblasts in intrahepatic cholangiocarcinoma. Am J Pathol. 2024;194. doi:10.1016/j.ajpath.2024.03.012
184. Marabelle A, Fakih M, Lopez J, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, Phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–1365. doi:10.1016/S1470-2045(20)30445-9
185. Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. 2019;38:255. doi:10.1186/s13046-019-1259-z
186. Rizzo A, Mollica V, Tateo V, et al. Hypertransaminasemia in cancer patients receiving immunotherapy and immune-based combinations: the MOUSEION-05 study. Cancer Immunol Immunother. 2023;72:1381–1394. doi:10.1007/s00262-023-03366-x
187. Sahai V, Griffith KA, Beg MS, et al. A randomized Phase 2 trial of nivolumab, gemcitabine, and cisplatin or nivolumab and ipilimumab in previously untreated advanced biliary cancer: bilT-01. Cancer. 2022;128:3523–3530. doi:10.1002/cncr.34394
188. Doki Y, Ueno M, Hsu C-H, et al. Tolerability and efficacy of durvalumab, either as monotherapy or in combination with tremelimumab, in patients from Asia with advanced biliary tract, esophageal, or head-and-neck cancer. Cancer Med. 2022;11:2550–2560. doi:10.1002/cam4.4593
189. Yoo C, Oh D-Y, Choi HJ, et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. J Immunother Cancer. 2020;8:e000564. doi:10.1136/jitc-2020-000564
190. Yoo C, Javle MM, Verdaguer Mata H, et al. Phase 2 trial of bintrafusp alfa as second-line therapy for patients with locally advanced/metastatic biliary tract cancers. Hepatology. 2023;78:758–770. doi:10.1097/HEP.0000000000000365
191. Villanueva L, Lwin Z, Chung HC, et al. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort Phase II LEAP-005 study. JCO. 2021;39:321. doi:10.1200/JCO.2021.39.3_suppl.321
192. Lin J, Yang X, Long J, et al. Pembrolizumab combined with lenvatinib as non-first-line therapy in patients with refractory biliary tract carcinoma. Hepatobiliary Surg Nutr. 2020;9:414–424. doi:10.21037/hbsn-20-338
193. Shemesh CS, Chan P, Marchand M, et al. Early decision making in a randomized Phase II trial of atezolizumab in biliary tract cancer using a tumor growth inhibition-survival modeling framework. Clin Pharmacol Ther. 2023;114:644–651. doi:10.1002/cpt.2953
194. Wang Y, Zhang N, Xue J, et al. Safety and feasibility of toripalimab plus lenvatinib with or without radiotherapy in advanced BTC. Front Immunol. 2023;14:1084843. doi:10.3389/fimmu.2023.1084843
195. Oh D-Y, Lee K-H, Lee D-W, et al. Phase II study assessing tolerability, efficacy, and biomarkers for Durvalumab (D) ± Tremelimumab (T) and Gemcitabine/Cisplatin (GemCis) in Chemo-Naïve Advanced Biliary Tract Cancer (aBTC). JCO. 2020;38:4520. doi:10.1200/JCO.2020.38.15_suppl.4520
196. Kelley RK, Ueno M, Yoo C, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet. 2023;401:1853–1865. doi:10.1016/S0140-6736(23)00727-4
197. Li W, Yu Y, Xu X, et al. Toripalimab with Chemotherapy as first-line treatment for advanced biliary tract tumors: update analytic results of an open-label Phase II clinical study (JS001-ZS-BC001). JCO. 2021;39:e16170. doi:10.1200/JCO.2021.39.15_suppl.e16170
198. Zeng T-M, Yang G, Lou C, et al. Clinical and biomarker analyses of sintilimab plus gemcitabine and cisplatin as first-line treatment for patients with advanced biliary tract cancer. Nat Commun. 2023;14:1340. doi:10.1038/s41467-023-37030-w
199. Vogel A, Boeck S, Waidmann O, et al. 52MO A randomized Phase II trial of durvalumab and tremelimumab with gemcitabine or gemcitabine and cisplatin compared to gemcitabine and cisplatin in treatment-naïve patients with CHolangio- and gallbladdEr Carcinoma (IMMUCHEC). Ann Oncol. 2022;33:S563. doi:10.1016/j.annonc.2022.07.080
200. Goyal L, Sirard C, Schrag M, et al. Phase I and biomarker study of the wnt pathway modulator DKN-01 in combination with gemcitabine/cisplatin in advanced biliary tract cancer. Clin Cancer Res. 2020;26:6158–6167. doi:10.1158/1078-0432.CCR-20-1310
201. Leem G, Jang S-I, Cho J-H, et al. Safety and efficacy of allogeneic natural killer cells in combination with pembrolizumab in patients with chemotherapy-refractory biliary tract cancer: a multicenter open-label Phase 1/2a trial. Cancers. 2022;14:4229. doi:10.3390/cancers14174229
202. Kudo M. Scientific rationale for combined immunotherapy with PD-1/PD-L1 antibodies and VEGF inhibitors in advanced hepatocellular carcinoma. Cancers. 2020;12:1089. doi:10.3390/cancers12051089
203. Vincent J, Mignot G, Chalmin F, et al. 5-fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi:10.1158/0008-5472.CAN-09-3690
204. Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic Cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. doi:10.1038/nri.2016.107
205. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:1497–1508. doi:10.1016/S1470-2045(16)30498-3
206. Zhang J-Y, Yan -Y-Y, Li -J-J, Adhikari R, Fu L-W. PD-1/PD-L1 based combinational cancer therapy: icing on the cake. Front Pharmacol. 2020;11:722. doi:10.3389/fphar.2020.00722
207. Mai H-Q, Chen Q-Y, Chen D, et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized Phase 3 trial. Nat Med. 2021;27:1536–1543. doi:10.1038/s41591-021-01444-0
208. Guan W-L, He Y, Xu R-H. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol. 2023;16:57. doi:10.1186/s13045-023-01451-3
209. Lopez-Beltran A, Cimadamore A, Blanca A, et al. Immune Checkpoint Inhibitors for the Treatment of Bladder Cancer. Cancers. 2021;13:131. doi:10.3390/cancers13010131
210. Chen J, Amoozgar Z, Liu X, et al. Reprogramming the intrahepatic cholangiocarcinoma immune microenvironment by chemotherapy and CTLA-4 blockade enhances anti-PD-1 therapy. Cancer Immunol Res. 2024;12:400–412. doi:10.1158/2326-6066.CIR-23-0486
211. Garassino MC, Gadgeel S, Speranza G, et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non-small-cell lung cancer: 5-year outcomes from the Phase 3 KEYNOTE-189 study. J Clin Oncol. 2023;41:1992–1998. doi:10.1200/JCO.22.01989
212. Reddy SM, Reuben A, Barua S, et al. Poor response to neoadjuvant chemotherapy correlates with mast cell infiltration in inflammatory breast cancer. Cancer Immunol Res. 2019;7:1025–1035. doi:10.1158/2326-6066.CIR-18-0619
213. Jarman EJ, Horcas-Lopez M, Waddell SH, et al. DKK1 drives immune suppressive phenotypes in intrahepatic cholangiocarcinoma and can be targeted with anti-DKK1 therapeutic DKN-01. Liver Int. 2023;43:208–220. doi:10.1111/liv.15383
214. Arend R, Dholakia J, Castro C, et al. DKK1 is a predictive biomarker for response to DKN-01: results of a Phase 2 basket study in women with recurrent endometrial carcinoma. Gynecol Oncol. 2023;172:82–91. doi:10.1016/j.ygyno.2023.03.013
215. Klempner SJ, Bendell JC, Villaflor VM, et al. Safety, efficacy, and biomarker results from a Phase Ib study of the anti-DKK1 antibody DKN-01 in combination with pembrolizumab in advanced esophagogastric cancers. Mol Cancer Ther. 2021;20:2240–2249. doi:10.1158/1535-7163.MCT-21-0273
216. Jung IH, Kim DH, Yoo DK, et al. In vivo study of Natural Killer (NK) cell cytotoxicity against cholangiocarcinoma in a nude mouse model. In Vivo. 2018;32:771–781. doi:10.21873/invivo.11307
217. Lin M, Luo H, Liang S, et al. Pembrolizumab plus allogeneic NK cells in advanced non-small cell lung cancer patients. J Clin Invest. 2020;130:2560–2569. doi:10.1172/JCI132712
218. Ott PA, Bang Y-J, Piha-Paul SA, et al. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol. 2019;37:318–327. doi:10.1200/JCO.2018.78.2276
219. Brown LC, Sedhom R, Schwartz EB, et al. Association of LRP1B pathogenic genomic alterations with favorable outcomes with immune checkpoint inhibitors across multiple tumor types. J Clin Oncol. 2020. doi:10.1200/JCO.2020.38.15_suppl.3007
220. Guo J, Zhou Q, Zhou M, et al. Survival benefit and biomarker of PD-1 inhibitor combination therapy in first-line of advanced biliary tract cancer: a retrospective study. Cancer Med. 2023;12:20699–20711. doi:10.1002/cam4.6628
221. Li J, Wei Q, Wu X, et al. Integrative clinical and molecular analysis of advanced biliary tract cancers on immune checkpoint blockade reveals potential markers of response. Clin Translat Med. 2020;10. doi:10.1002/ctm2.118
222. Jeong SY, Hong JY, Park JO, et al. The efficacy of immune checkpoint inhibitors in biliary tract cancer with KRAS mutation. Therap Adv Gastroenterol. 2023;16:17562848231170484. doi:10.1177/17562848231170484
223. Bergers G, Fendt S-M. The metabolism of cancer cells during metastasis. Nat Rev Cancer. 2021;21:162–180. doi:10.1038/s41568-020-00320-2
224. Qiu S, Liu Z, Hu J, et al. Fatty acid binding protein 1 is an independent prognostic biomarker for gallbladder cancer with direct hepatic invasion. Int J Med Sci. 2024;21:862–873. doi:10.7150/ijms.93413
225. Choodetwattana P, Proungvitaya S, Jearanaikoon P, Limpaiboon T. The upregulation of OCT4 in acidic extracellular pH is associated with gemcitabine resistance in cholangiocarcinoma cell lines. Asian Pac J Cancer Prev. 2019;20:2745–2748. doi:10.31557/APJCP.2019.20.9.2745
226. Pei F, Tao Z, Lu Q, Fang T, Peng S. Octamer-binding transcription factor 4-positive circulating tumor cell predicts worse treatment response and survival in advanced cholangiocarcinoma patients who receive immune checkpoint inhibitors treatment. World J Surg Oncol. 2024;22:110. doi:10.1186/s12957-024-03369-7
227. Cheng Z, Yang C, Zhao Q, et al. Efficacy and predictors of immune checkpoint inhibitors in patients with gallbladder cancer. Cancer Sci. 2024;115:1979–1988. doi:10.1111/cas.16142
228. Huang Y, Du Z, Kan A, et al. Clinical and biomarker analyses of hepatic arterial infusion chemotherapy plus lenvatinib and PD-1 inhibitor for patients with advanced intrahepatic cholangiocarcinoma. Front Immunol. 2024;15:1260191. doi:10.3389/fimmu.2024.1260191
229. Rimini M, Masi G, Lonardi S, et al. Durvalumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin in biliary tract cancer: a real-world retrospective, multicenter study. Target Oncol. 2024;19:359–370. doi:10.1007/s11523-024-01060-1
230. Ma Y, Pan Y, Li Y, Guan H, Dai G. Prognosis of patients with advanced bile tract carcinoma: assessment using the Modified-Gustave Roussy Immune Score (mGRIm-s) as a clinico-immunological tool. J Cancer Res Clin Oncol. 2024;150:247. doi:10.1007/s00432-024-05771-w
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
