Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Evidence Construction of Chuankezhi Injection Against Chronic Obstructive Pulmonary Disease: A Systematic Review and Network Pharmacology
Authors Wei X , Zhong Y, Yi X, Li T, Ling Z, Ming M, Zhang S, He Z
Received 2 February 2024
Accepted for publication 20 May 2024
Published 27 May 2024 Volume 2024:19 Pages 1177—1196
DOI https://doi.org/10.2147/COPD.S442281
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Xuan Wei,1,2,* Yu Zhong,3,* Xiaofei Yi,1 Tingting Li,1 Zhougui Ling,2 Moyu Ming,2 Shuang Zhang,1 Zhiyi He1
1Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, The Fourth Affiliated Hospital of Guangxi Medical University, Liuzhou, People’s Republic of China; 3Department of Emergency Medicine, The Fourth Affiliated Hospital of Guangxi Medical University, Liuzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhiyi He, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Guangxi Medical University, NO. 6 Shuangyong Road, Qingxiu District, Nanning City, Guangxi Zhuang Autonomous Region, People’s Republic of China, 530000, Tel +86 187 7801 7698, Email [email protected]
Objective: Chronic obstructive pulmonary disease (COPD) is a chronic respiratory disease with high prevalence, morbidity, and mortality. Chuankezhi (CKZ) injection, a Chinese patent medicine, has been commonly used for treating COPD. This study evaluated the clinical efficacy of CKZ injections in COPD patients and explored potential underlying mechanisms by integrating meta-analysis and network pharmacology.
Research Methods: Randomized controlled trials (RCTs) were search in database by Web of Science, Cochrane Library and PubMed as of November 2022 for literature collection, and the Review Manager 5.4 was used to analyze the data. Through the network pharmacology method, the chemical components and their targets, as well as the disease targets were further analyzed.
Results: A total of 15 RCTs including 1212 patients were included. The results of meta-analysis showed that CKZ injection can significantly improve the clinical effective rate (RR = 1.25, 95% CI: 1.14 to 1.36), and the clinical advantage was that it can significantly reduced acute exacerbation rate (RR = 0.29, 95% CI: 0.12 to 0.70) and COPD assessment test (CAT) scores (MD =− 4.62, 95% CI:-8.966 to-0.28). A total of 31 chemical compounds and 178 potential targets for CKZ injection were obtained from the online databases. Molecular docking revealed that most key components and targets could form stable structure.
Conclusion: This systematic review with meta-analysis and network pharmacology demonstrates that CKZ could effectively improve the clinical efficacy and safety in the treatment of COPD. Such efficacy may be related to an anti-inflammatory effect and immunoregulation of CKZ via multiple components, multiple targets and multiple pathways.
Keywords: meta-analysis, network pharmacology, chronic obstructive pulmonary disease, Chuankezhi injection
Graphical Abstract:
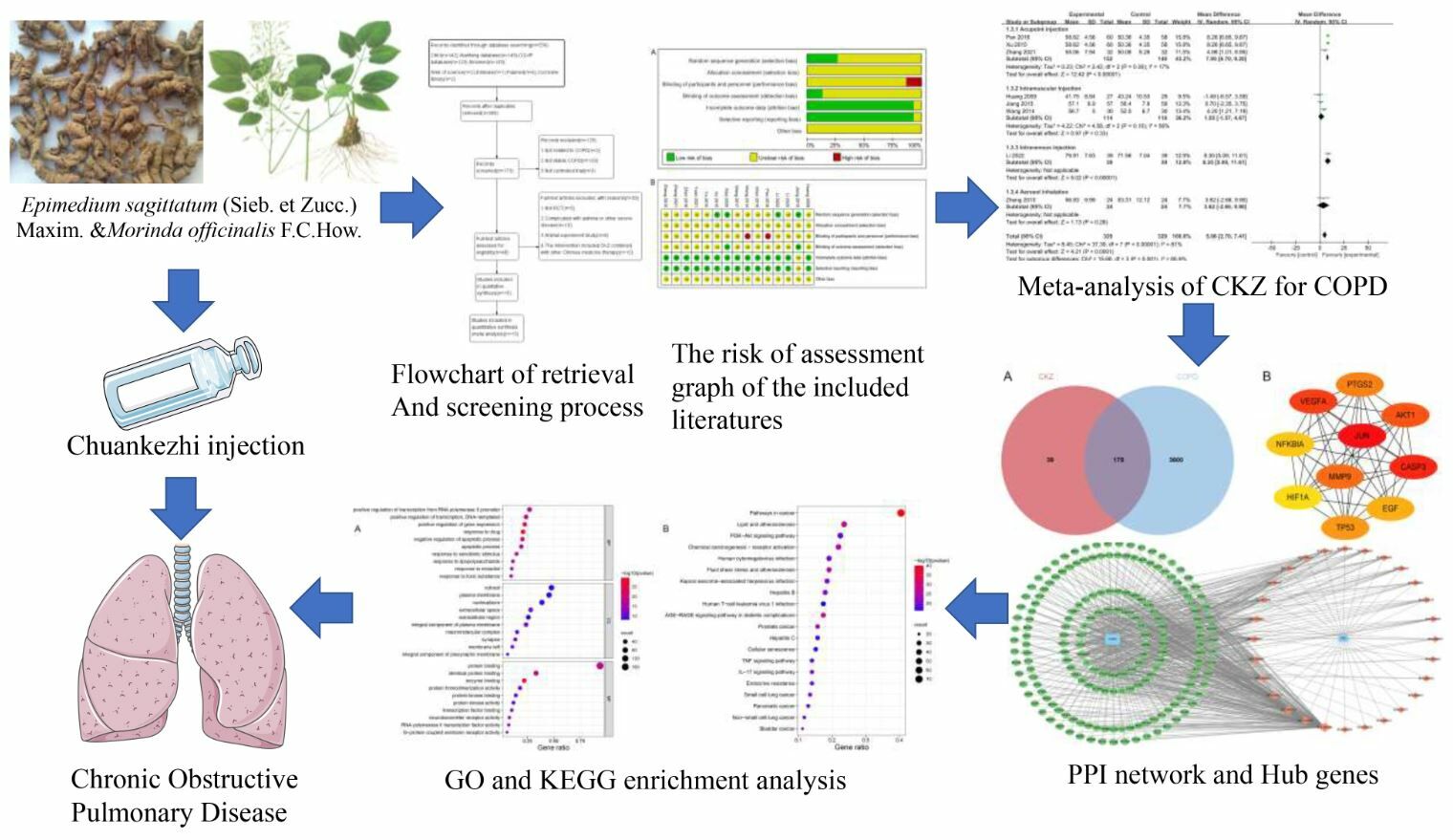
Introduction
Chronic obstructive pulmonary disease (COPD) is a common obstructive pulmonary disease, which can be prevented and treated.1 It is characterized by persistent respiratory symptoms and airway obstruction induced by abnormalities in the airways alveoli as a result of prolonged exposure to noxious substances, particularly tobacco smoke and pollutants.2 More than 300 million individuals worldwide suffer from COPD, which is now a significant global burden and is responsible for over 3.2 million annual deaths.3 This disease will become the third biggest cause of mortality worldwide by 2030, according to the World Health Organization (WHO).4 Importantly, reduplicative acute exacerbations can lead to a loss of pulmonary function and quality of life, culminating in death.5 Thus, it is necessary to manage and prevent the progression of COPD6 since inhaled corticosteroids and bronchodilators, which are the front-line therapies, are oftentimes ineffective.7
Due to its relative safety and distinct superiority, Traditional Chinese medicine (TCM) has begun to gain international recognition during the past few years.8 TCM is widely used in China to treat COPD patients and is gradually being accepted by the public due to its clinical efficacy and low incidence of side effects.9 The Chuankezhi injection (CKZ) is made up of two traditional Chinese herbal medicines, Epimedium sagittatum (Sieb. et Zucc.) Maxim. and Morinda officinalis F.C.How.10 These medicines are originated from the Shen Nong Ben Cao Jing, in which Epimedium sagittatum (Sieb. et Zucc.) Maxim.is thought to “warm the kidneys” and “help yang to stop coughing”.11 It is said in “Ben Cao Jing Shu” that Morinda officinalis F.C.How. can subsidize Yuan Yang, while “Ben Cao Qi Yuan” states that it can resolve phlegm and stop coughing and wheezing.12 According to a previous study, Epimedium sagittatum (Sieb. et Zucc.) Maxim. was shown to alleviate lipopolysaccharide (LPS)-induced inflammatory response by inhibiting the NF-κB signaling pathway.13 Meanwhile, Epimedium sagittatum (Sieb. et Zucc.) Maxim. can attenuate oxidative stress in human lung epithelial cells via quenching reactive oxygen species (ROS), up-regulating GSH and modulating PI3K/Akt/Nrf2 signaling.14
Numerous studies have demonstrated that CKZ is able to cure cough and asthma through its anti-allergy, anti-inflammatory, stress response, and immunoregulatory functions.15,16 CKZ has also been used in the adjuvant treatment of COPD patients with its with its remarkable effects being demonstrated in previous studies in China.17,18 Nevertheless, systematical assessment of efficacy, safety and underlying mechanism of CKZ against COPD is also necessary to be further processed. Combination of evidenced-based medicine study and network pharmacology have been regarded as an effective approach to better clarify above issues. Hence, we conducted a meta-analysis to estimate the efficacy and safety of CKZ against COPD. Furthermore, network pharmacology and molecular docking were utilized to explore potential pharmacological mechanisms of CKZ against COPD.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist was used to guide this study.19 The systematic review protocol was registered at PROSPERO(CRD:42022370971).
A literature search was conducted by screening four English databases (PubMed, Cochrane Library, Web of science and Embase) and four Chinese databases (China National Knowledge Infrastructure (CNKI), the Wan fang database, the China Biology Medicine disc (SinoMed) database and CQVIP Database (VIP) from their inceptions to November 2022. Additionally, the Chinese clinical trial registration website (http://www.chictr) and the international clinical trial register website (http://clinicaltrials.gov/) were used as resources. We also looked through the publication of meeting minutes, grey literature, and reference lists. English and Chinese were considered the primary search languages. The terms were entered alone or in different combinations and included: “Chronic Obstructive Pulmonary Disease”, “Chronic Obstructive Lung Disease”, “Chronic Obstructive Airway Disease”, “Chuankezhi” and “Chuankezhi Injection”. Details on search terms and search strategy are listed in Supplementary Table 1.
Inclusion Criteria
We incorporated randomized controlled trials (RCTs) that examined the effectiveness and safety of CKZ in patients with stable COPD. Adult patients (>18 years) were diagnosed according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD)4 with a postbronchodilator fixed ratio of forced expiratory volume in first second (FEV1)/forced vital capacity (FVC) < 0.70 measured by spirometry without restrictions in terms of gender, race, educational attainment, or socioeconomic situation. Stable COPD is defined as stable or mild symptoms of cough, expectoration, and dyspnea, with no acute exacerbation within 4 weeks.2
Conventional treatments of control groups included: oxygen inhalation therapy, anti-inflammatories, cough alleviation drugs, sputum reduction drugs, and inhaled bronchodilators. The intervention of the experimental group was a combination of CKZ injection and conventional treatments.
The following outcomes were collected: clinical total effective rate, COPD assessment test (CAT) score, pulmonary function index (FEV1%pred, FEV1/FVC), St George’s Respiratory Questionnaire (SGRQ) score, six-minute walk distance (6MWD), Quality of Life Instruments for Chronic Diseases-COPD (QLICD-COPD), modified Medical Research Council (mMRC) dyspnea score, TCM symptom score and adverse events.
Exclusion Criteria
The exclusion criteria included: (1) non-RCTs or uncontrolled clinical trials; (2) studies that used CKZ in conjunction other TCM therapies such as acupuncture, herbal paste applied to acupoints and decoction therapy; (3) patients with major disorders such as diffuse bronchiolitis, tuberculosis, congestive heart failure, asthma, bronchiectasis, or congestive heart failure; (4) the trial’s specifics were unclear or the data it produced were insufficient.
Study Selection
Two review authors (Xuan Wei and Yu Zhong) identified possibly suitable studies after individually examining titles and abstracts that were returned by the literature search. The same review writers then obtained the full text of each study and independently evaluated them in accordance with the inclusion and exclusion criteria. In cases of disagreement, a third reviewer (Xiaofei Yi) served as the arbitrator.
Data Extraction
Two review authors (Xuan Wei and Xiaofei Yi) extracted data and evaluated the literature quality for each qualifying study. Then, the above two reviewers checked the data extracted by the other party to ensure accuracy. A third researcher (Yu Zhong) was invited to make a final decision in cases of disagreement.
The following information and data were extracted according to a standard data extraction form that was created prior to analyzing the studies: title, first author name, year of publication, country, study design, participant characteristics, treatment duration, outcomes, and adverse events. To retrieve relevant information that could not be found in the published report, we e-mailed the corresponding author.
Risk of Bias Assessment
The Cochrane Risk of Bias Assessment Tool, Version 5.1.0 was adopted by two reviewers to assess the methodological quality of included studies.20 The items used in this analysis were as follows: (1) random sequence generation (selection bias); (2) allocation concealment (selection bias); (3) blinding of participants and personnel (performance bias); (4) blinding of outcome assessment (detection bias); (5) incomplete outcome data (attrition bias); (6) selective reporting (reporting bias), and other biases. Each type of bias was ranked as: high risk, unclear risk or low risk. Reviewers settled disagreements by dialogue and, when that was impracticable, through adjudication by a third party.
Statistical Analysis
The meta-analysis was conducted by the Review Manager (RevMan) 5.4 software. To appraise dichotomous and continuous variables outcomes, Relative risk (RR) and its 95% confidence intervals (CIs), mean difference (MD) and its 95% CIs were respectively used. Data that could not be included in a meta-analysis are demonstrated in the final table I2 was used to assess statistical heterogeneity among included studies. A fixed-effect model was chosen for when I2<50%; otherwise, a random-effect model was applied. We also performed a subgroup analysis to identify potential sources of heterogeneity. In order to perform the subgroup analysis, characteristics and differences between included studies were examined including intervention and follow-up times, including the treatment philosophies associated with TCM syndromes, disease severity, location and age of participants.
This study used secondary data from published RCTs, in which there was no personal information disclaimed. Hence, it was not necessary to obtain ethical approval and consent from participants of included studies.
CKZ Active Ingredients and Targets
The Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP; https://old.tcmsp-e.com/index.php) was used to identify active components of CKZ. The Canonical Simplified molecular input line entry system (Canonical SMILES) of each ingredient was searched at PubChem (https://pubchem.ncbi.nlm.nih.gov/). After that, the Swiss Target Prediction database (http://swisstargetprediction.ch/) was applied to forecast the therapeutic targets of CKZ and the UniProt database (https://www.uniprot.org/) was utilized to acquire canonical gene names of each target. CKZ-related targets were obtained after removing duplicates.
Screening for COPD-Related Targets
GeneCards (https://www.genecards.org/), Online Mendelian Inheritance in Man (OMIM; https://omim.org/), Drugbank (https://www.drugbank.com/), DisGeNET (https://www.disgenet.org/), and Target Database (TTD; http://db.idrblab.net/ttd/) were used to search COPD-related targets with “Chronic Obstructive Pulmonary Disease” as the keyword.
Network Topology Analysis of Protein-Protein Interaction
Potential targets of CKZ relevant to COPD were defined as the common intersection of drug and disease targets. Owing to the multi-component and multi-target characteristic of traditional Chinese herbal prescription, a protein-protein interaction (PPI) network was constructed to demonstrate the complex therapeutic mechanisms of CKZ. First, intersection target data were imported into the STRING database (https://string-db.org/) to build the PPI network.21 The conditions were set as “Homo sapiens” and the confidence score was defined as >0.4. Then, the PPI network was visualized in Cytoscape 3.9.0. The CytoHubba plugin was used to obtain hub genes. A node’s size corresponds to its degree value in the network, and there is a positive correlation between the two.
Target Function and Pathway Enrichment Analysis
The Database for Annotation, Visualization, and Integrated Discovery (DAVID) 6.8 (https://david.ncifcrf.gov/) was used to analyze the enrichment of GO and KEGG pathways to investigate the biological pathways of key genes. An adjusted P value<0.05, was deemed statistically significant.
Molecular Docking
To confirm the binding force of hub genes and key ingredients from the PPI network, we performed molecular docking. Specifically, we used CB-Dock, which is an online website providing an online protein-ligand docking approach to ascertain binding sites, evaluate center and size, and demonstrate molecular docking.
The Protein Data Bank database (PDB; https://www.rcsb.org) was utilized to obtain the 3D structure of target proteins, while the chemical structures of active components were acquired from PubChem (https://pubchem.ncbi.nlm.nih.gov). The Vina score represents the binding energy and the stability of the interconnection module has a positive correlation with the absolute Vina score value. Finally, PyMOL software was used to visually represent molecular docking results.
Results
Study Characteristics
A total of 15 RCTs published in Chinese were included. The study flowchart is outlined in Figure 1. These 15 trials included a total of 1, 212 participants and were published between 2009 and 2022. Further details are presented in Table 1.
 |
Table 1 Characteristics of Included Studies |
 |
Figure 1 Flow chart of meta-analysis. |
Bias Assessment
The Cochrane Risk of Bias Assessment Tool was used to estimate the quality of included studies. Four studies reported the use of randomization (eg using a random number table). Hence, these RCTs were judged as having a “low risk” for selection bias. The remainder were unclear in regard to their randomization techniques and were therefore classified as having an “unclear risk” for selection bias. None of the studies mentioned missing values as a result of withdrawals or dropouts. In all studies, there was a low risk of bias in selective result reporting. None of the research made reference to study design, sample size estimation, or randomization scheme concealment. Figure 2 depicts that it was unclear whether there were other biases in the RCTs herein analyzed.
 |
Figure 2 Risk bias of included studies. Notes: (A) Overall risk; (B) Detailed risk. |
Efficacy Assessment
Clinical Effective Rate
This meta-analysis included six studies that provided categorical data for clinical effective rates. According to the data analysis, the heterogeneity was not statistically significant (x2 = 3.92, P = 0.56; I2 = 0%); hence, a fixed-effect model was chosen. The analysis results revealed that the clinical effective rate of the experimental group was higher than that of the control group (RR = 1.25; 95% CI: 1.14 to 1.36; Z = 4.91, P<0.00001) (Figure 3).
 |
Figure 3 Forest plot of clinical effective rate. |
Acute Exacerbation Rate
Two trials that provided categorical data for the acute exacerbation rate were included. Since there was no heterogeneity (x2 =0.00, P = 1.00; I2 = 0%), a fixed-effect model was used. The data analysis demonstrated that the experimental group had a significantly reduced acute exacerbation rate compared with the control group (RR = 0.29; 95% CI: 0.12 to 0.70; Z = 2.77, P = 0.006) (Figure 4).
 |
Figure 4 Forest plot of acute exacerbation rate. |
Pulmonary Function
Eight studies reported FEV1%pred as a measure of pulmonary function. Again, there was no heterogeneity (x2 = 8.34, P = 0.30; I2 = 16%), hence, a fixed-effect model was chosen. The combined data demonstrated that the improvement of FEV1%pre in the experiment group was superior to the improvement in the control group (MD = 7.35;95% CI:5.87 to 8.83; Z = 9.72, P<0.00001) (Figure 5). Meanwhile, eight studies provided numerical data for FEV1/FVC and a random effects model was utilized due to considerable heterogeneity. In a subgroup analysis investigating different administration routes, one study found no statistically significant change in FEV1/FVC between groups that used aerosol inhalation (MD = 3.62; 95% CI: −2.66 to 9.90; Z = 1.13, P = 0.96) (Figure 6), while the outcomes for studies using intramuscular injections of CKZ combined with conventional treatment were comparable in the subgroup analysis (MD = 1.55; 95% CI:-1.57 to 4.67; Z = 0.97, P = 0.33) (Figure 6). One study showed that a combination of intravenous injection of CKZ and conventional treatment resulted in better FEV1/FVC values than the control group (MD = 8.35; 95% CI: 5.09 to 11.61; Z = 5.02, P<0.00001) (Figure 6), similar to when CKZ was administered through acupoint injection (MD = 7.95; 95% CI: 6.70 to 9.20; Z = 12.42, P<0.00001) (Figure 6).
 |
Figure 5 Forest plot of FEV1% pre. |
 |
Figure 6 Forest plot of FEV1/FVC. |
CAT Score
Three of the fifteen included studies reported variations in CAT scores. The pooled analysis revealed that the experimental group displayed a considerable elevation in their CAT score (MD =−4.62; 95% CI:-8.966 to-0.28; Z = 2.08, P = 0.04) compared to control patients. However, there was significant heterogeneity between the groups (x2 = 22.36, P<0.0001; I2 = 91%). To pinpoint the source of variability, a subgroup analysis of different CKZ administration routes was carried out. The results demonstrated that CAT scores decreased in both studies that used acupoint injections (MD =−5.34; 95% CI:-10.73 to 0.05; Z = 1.94, P<0.0001) and intramuscular injections of CKZ (MD =−2.74; 95% CI:-6.98 to 1.50; Z = 1.27, P = 0.20). However, heterogeneity was still present in this subgroup analysis (Figure 7A).
 |
Figure 7 Forest plot of quality of life score. (A) CAT score. (B) QLICD-COPD score. (C) SGRQ score. |
SGRQ Score
With 118 participants, two of the fifteen included studies mentioned changes in the SGRQ score. Indeed, CKZ combined with conventional treatment improved the SGRQ score of COPD patients to a greater extent than what was observed with conventional treatment alone (MD =−6.42; 95% CI:-10.71 to-2.13; Z = 2.93, P = 0.003). There was no statistical heterogeneity between studies (x2 = 0.00, P = 1.00; I2 = 0%) (Figure 7B).
QLICD-COPD
The QLICD-COPD score was reported in 2 of 15 studies, comprising 232 participants. The pooled analysis demonstrated that when CKZ was added to conventional treatment, the QLICD-COPD score significantly improved compared to conventional treatment alone (MD = 6.07; 95% CI: 3.79 to 8.35; Z = 5.22, P<0.0001). In addition, there was no statistical heterogeneity among groups (x2 = 0.00, P = 1.00; I2 = 0%) (Figure 7C).
6MWD
Four trials with 420 participants reported 6MWD data. Data analysis indicated that there was significant heterogeneity (x2 = 13.21, P = 0.004; I2 = 77%); hence, a random-effect model was adopted. The results revealed a significant improvement in 6MWD for patients in the experimental group compared to that of the control group (MD = 32.17; 95% CI: 17.76 to 46.95; Z = 4.37, P < 0.00001) (Figure 8).
 |
Figure 8 Forest plot of 6MWD. |
TCM Syndrome Score
Five trials with a total of 390 participants reported data for the TCM Syndrome Score. We identified significant heterogeneity (x2 = 26.90, P < 0.0001; I2 = 85%); hence, a random-effect model was chosen. The results demonstrated that the TCM syndrome score of the experimental group was much lower than that of the control group, suggesting a lower occurrence of severe clinical symptoms in patients treated with CKZ (MD =−3.39; 95% CI:-5.06 to-1.72; Z = 3.98, P < 0.0001) (Figure 9).
 |
Figure 9 Forest plot of TCM Syndrome Score. |
Other Outcomes
In addition, two studies compared immunoglobulin M, immunoglobulin A and immunoglobulin G levels and one study compared BODE scores between groups (Table 2). It was clear that CKZ treatment could improve circulating levels of immunoglobulins and reduce the BODE score compared with outcomes of the control group.
 |
Table 2 Other Outcomes |
Adverse Reactions
Four studies reported adverse reactions, such as palpitation, cough, hoarseness, and dizziness, among others. All adverse reactions were temporary and had no long-term negative repercussions. There were no adverse reactions or incidents reported by other studies.
Active Ingredients and Targets Screening of CKZ
According to pharmacokinetic parameters (drug-likeness (DL) ≥0.18, drug half-life (HL) ≥4, and relative molecular weight (MW)≤500), 31 active ingredients of CKZ were identified at the TCMSP database (Table 3). Through PubChem and Swiss Target Prediction databases, related targets of the 31 active ingredients were also identified. A total of 217 CKZ-related targets were obtained after eliminating redundant ones and standardizing them through UniProt.
 |
Table 3 List of CKZ Ingredients |
COPD-Related Target Gene Collection
COPD-related genes were collected from GeneCards, OMIM, DisGeNET, DrugBank and TTD databases. The score in the Genecards database has a positive correlation with how prevalent the condition is. The maximum score of the target obtained by GeneCards was 142.24, while the minimum score was 0.28, and the median was 8.11. Hence, the target with a score higher than the median was classified as a target associated with COPD. Overall, 3215 targets were identified from GeneCards, 544 targets from OMIM, 73 targets from TTD, 83 targets from DrugBank and 180 targets from DisGeNET. Finally, a total of 3778 targets associated with COPD were collected after eliminating duplicate genes.
Screening of Hub Genes and PPI Network Construction
We discovered that COPD and CKZ shared 178 targets by comparing the two sets of genes (Figure 10A). The common targets were imported into STRING online database to establish the PPI network. Hub genes were chosen using the CytoHubba plugin and the Maximum Cross-Correlation (MCC) computation method. The top 10 hub genes include: JUN, Casp3, VEGFA, AKT1, MMP9, PTGS2, TP53, EGF, NF-κB1A and HIF1A (Figure 10B). An active ingredient-target network was created in Cytoscape, in which green represents the targets, and orange represents the active ingredients of CKZ (Figure 11). The importance of each protein and component in the network was examined in accordance with the node degree, and a network diagram was created for visualization. The top five most significant elements in CKZ, as determined by the Analyze Network plugin and based on node degree, were: quercetin, luteolin, kaempferol, Anhydroicaritin and β-sitosterol.
 |
Figure 10 Common targets and PPI network of hub genes. Notes: (A) Common targets between CKZ and COPD; (B)PPI network of 10 hub genes. |
 |
Figure 11 Active ingredient-target network. Notes: Green oval represent targets, orange rhombus represent active ingredients of CKZ. |
GO Enrichment Analysis
To systematically identify the treatment pathways of CKZ in COPD, a GO enrichment analysis was carried out. We identified 110 items for cellular components (CC), 172 items for molecular functions (MF), and 836 items for biological processes (BP). Separately, we chose the top 10 BP, CC, and MF data to depict in a bubble diagram (Figure 12A).
 |
Figure 12 GO and KEGG enrichment bubble diagram. Notes: (A) GO enrichment bubble diagram; (B) KEGG enrichment bubble diagram. |
KEGG Pathway Enrichment Analysis
The top 20 pathways were chosen for presentation and visualization out of a total of 181 pathway items that were enriched in the KEGG enrichment analysis. These items primarily included the AGE-RAGE signaling pathway, Cellular senescence, and PI3K/Akt signaling pathway. Based on the results of the KEGG enrichment, the first 20 associated enrichment results were visualized via a bubble diagram (Figure 12B).
Molecular Docking
According to the ingredient-target network diagram, the top 6 core ingredients were selected for molecular docking with their respective hub genes relevant to COPD. The core target protein ID codes were obtained through the PDB database for JUN (6Y3V), Casp3 (1RHJ), VEGFA (5DN2), AKT1 (4EJN), MMP9 (5I12), and PTGS2 (5F19). The results of docking scores of quercetin (MOL000098), luteolin (MOL000006), kaempferol (MOL000422), Anhydroicaritin (MOL004373) and β-sitosterol (MOL000358) with key targets are listed in Figure 13A. The binding energies of all ingredients were all lower than −5 kcal/mol, indicating a stable interaction. The top 5 interconnection modules with the lowest vina score were chosen for visual demonstration (Figure 13B).
 |
Figure 13 Molecular docking results. Notes: (A) Heat map of molecule docking affinity; (B) Molecular docking visualization results. |
Discussion
COPD is a complex pulmonary inflammatory disease, with airway and parenchymal lung damage, which is now the third leading cause of death in the world and is estimated to cause the greatest economic burden of disease in China.35,36 It is generally considered that the intake of pollutants, particularly cigarette smoke, is the primary cause of COPD.37
Reiterative airway inflammation and airflow limitation can trigger an imbalance of immune function, which is strongly associated with the continuous development of COPD.38 The TCM theory states that COPD falls within the categories of “cough”, “phlegm” and “lung distension”.39 Lung-kidney Qi deficiency is the most common syndrome in stable COPD.40 Hence, the treatment of COPD with TCM focuses on nourishing the kidney and warming yang.41 The two herbs in CKZ exert these properties. Therefore, this research first evaluated the efficacy and safety of CKZ for COPD using an evidence-based analytical approach. Our systematic review included 15 RCTs with 1212 individuals and suggested that CKZ was beneficial to COPD patients since it alleviated the risk of acute exacerbation, improved quality of life, pulmonary function, clinical effectiveness and activity endurance.
Exacerbation is a recurring issue in the management of COPD since it might impair pulmonary function if it occurs frequently.36 Clinical effective rate is widely considered to be a subjective outcome measure in TCM treatment, which reflects symptom change.42 The SGRQ is recommended as the golden standard to evaluate the symptoms of COPD patients, given that it assesses the psychological state of COPD patients.43 It also has an intimate correlation with FEV1%pre, FEV1/FVC, and clinical symptoms.44 Meanwhile, the CAT score is a multi-dimensional assessment of a patient’s health, based on a comprehensive symptom score, and the mMRC is mainly used to evaluate the degree of dyspnea in COPD patients.45 The QLICD-COPD, which includes modules on social, psychological, and physical functions, was created to assess the health condition of individuals with COPD.46 Another significant feature of COPD is lower exercise tolerance, which may be caused by muscle atrophy and impaired cardiovascular function. Hence, the 6MWD test measures this decline in activity tolerance.47 Based on subgroup analyses, we suggest that CKZ combined with conventional treatment improves the: SGRQ score, CAT score, 6MWD, QLICD-COPD score and clinical effective rate when compared to participants using conventional treatment alone. Both FEV1/FVC and FEV1%pre play a significant role in diagnostic standards as well as in evaluating disease progression.48 Furthermore, our analyses suggest that acupoint injection of CKZ leads to a significant elevation in FEV1/FVC compared to other administration routes. Therefore, the effectiveness of CKZ may depend on the route of administration.
Only four of the included research documented precise randomization methods, such as random number tables, while the remaining studies just referenced randomization without further details. Double-blinding was not stated in any of the included RCTs, and its absence may result in measurement bias. Only four of the 15 studies that were included reported adverse events. Even though the adverse events were tolerated by the patients, did not interfere with the course of the treatment, or disappeared following drug withdrawal, a personalized treatment plan must be created to account for individual variances. Due to the incompleteness of raw and comprehensive data, the baseline characteristics of different study participants (such as disease severity and progression) may also vary. This could be one of the reasons for the high heterogeneity of some analyses. While conducting the meta-analysis, we attempted to avert and prevent compromises due to high heterogeneity. Nevertheless, a high-quality RCT with large sample size is necessary to corroborate our findings, owing to the small sample size, multiple confounding variables, and poor quality of studies herein analyzed. Based on the aforementioned research findings, it is clear that CKZ can benefit COPD patients by improving clinical efficacy. In addition, the pooled analysis indicated that serum IgM, IgA and IgG were increased in the experimental group, suggesting that the therapeutic effectiveness of CKZ for COPD might be related to an enhancement in host immunity. These findings lay the groundwork for additional research into the pharmacological mechanisms of action of CKZ.
Therefore, we explored the pharmacological mechanisms of CKZ in COPD by network pharmacology and molecule docking. A total of 217 targets related to CKZ and 31 active components were found and PPI networks were established by integrating 178 intersecting targets of CKZ and COPD. The top 6 ingredients of the drug-active ingredient-target network were: quercetin, luteolin, kaempferol, Anhydroicaritin and β-sitosterol. It was found that JUN, Casp3, VEGFA, AKT1, MMP9 and PTGS2 were the most critical potential targets of CKZ relevant to COPD.
Quercetin is a natural compound found in a variety of plants that exerts anti-inflammatory, antioxidant, anti-cancer effects.49 It was found that quercetin can reduce inflammatory storms and oxidative stress induced by LPS.50 Besides, quercetin could inhibit oxidative stress, reduce inflammatory factors and the expression of MMP9, MMP12 and MUC5AC to decelerate emphysema in elastase-induced mice.51 Luteolin is found in a variety of plants and it is a flavonoid with anti-inflammatory, antibacterial, antioxidant, and anti-cancer properties.52 Indeed, luteolin considerably reduces the production of superoxide anion, and Raf1 activity to alleviate inflammation storms and oxidative stress induced by neutrophils in inflammatory arthritis.53 Interestingly, luteolin can regulate the levels of Treg and Tregs-derived IL-10, and alleviate caspase-11-dependent pyroptosis to inhibit inflammation responses in the CLP-induced acute lung injury mouse model.54 Kaempferol is an antimicrobial, anti-inflammatory, antioxidant, and antitumor flavonoid aglycone widely distributed in numerous plants.55 Kaempferol was shown to mitigate allergic response and airway inflammation induced by OVA via the NF-κB signal pathway and by inhibiting the expression of otaxin-1 in eosinophils.55 Anhydroicaritin, a natural component originated from Epimedii Herba, was shown to inhibit cancer cell multiplication, cell differentiation, invasion, and metastasis.56 In addition, Anhydroicaritin can regulate the LKB1/AMPK/mTOR pathway to ameliorate diet-induced obesity and alleviate insulin resistance.57 β-sitosterol is a primary component from common plants and vegetables that exerts anti-hyperlipidemia, anti-inflammation and anti-tumor effects.58
Analysis of the disease target-ingredient network and PPI network suggested that JUN, Casp3, VEGFA, AKT1, MMP9, PTGS2, TP53 and EGF may be the primary targets of CKZ in COPD. The GO BP functional enrichment analysis revealed that the co-targets of CKZ and COPD were mainly enriched in the positive regulation of transcription from RNA polymerase II promoter, positive regulation of transcription, positive regulation of gene expression, response to drug, and other functions. The GO CC functional analysis mostly focused on the extracellular space, cytosol, nucleoplasm, plasma membrane, and other processes, while the GO MF functional analysis was primarily enriched in protein binding, enzyme binding, protein homodimerization activity, protein kinase binding, and other functions. The primary KEGG pathway included Pathways in cancer, lipid and atherosclerosis, AGE-RAGE signaling pathway in diabetic complications, PI3K/Akt signaling pathway, chemical carcinogenesis-receptor activation, human cytomegalovirus infection, IL-17 signaling pathway, and fluid shear stress and atherosclerosis. These functions and pathways are associated with immunity and inflammatory response during the onset and progression of COPD. Hence, these data are indicative that CKZ mediates airway and pulmonary inflammation via regulating inflammatory factors, which is crucial for the treatment of COPD. Finally, molecular docking was conducted to further explore the mechanism of CKZ in COPD. Our data demonstrate that most ingredients could stably bind to their target proteins. PTGS2, a cyclooxygenase, inhibits cellular and humoral immune responses in endometriosis via participating in the angiogenesis and regulation of T cell differentiation.59 It has been be proved that Luteolin could suppress protein expression such as VEGF, MMP-2 and MMP-9, which are related to cell migration and invasion, and regulate PI3K/Akt and MAPK signaling pathways to prevent tumorigenesis of non-small cell lung cancer.60 Quercetin was shown to accelerate the release of CASP8 and decrease the expression of EGFR, VEGFA, CCND1, and ERBB2 in cervical cancer cells, which improved the anticancer effects of cisplatin.61 Thus, the above molecular docking results reiterate that the active ingredients of CKZ are likely to bind to proteins implicated in COPD.
This study integrated meta-analysis and network pharmacology to evaluate the clinical efficacy of CKZ in COPD and explore potential mechanisms. Here we show how TCM can be used to treat COPD. Nevertheless, there were still some limitations. First, since CKZ is a TCM, all research included were published in Chinese and had limited sample sizes. More research should be conducted outside China to validate our findings. Second, a large-scale, multicenter, randomized controlled trials are needed to validate our data. Finally, since this study is primarily based on literature and database research, experiments are required to verify identified mechanisms.
Conclusion
In summary, based on meta-analysis, network pharmacology, and molecular docking, this study revealed that CKZ is an effective adjuvant therapy for COPD, and its mechanisms may be related to reducing inflammatory and responses.
Abbreviations
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; HE, hematoxylin and eosin; CAT, COPD Assessment Test; mMRC, modified Medical Research Council scale; SGRQ, St George’s Respiratory Questionnaire; 6MWD, six-minute walk distance; QLICD-COPD, Quality of Life Instruments for Chronic Diseases-COPD; BC, Betweenness centrality; CC, Closeness centrality; DL, drug-like; DAVID, Database for Annotation, Visualization, and Integrated Discovery; TCM, traditional Chinese medicine; TCMSP, Traditional Chinese Medicine Systems Pharmacology; OB, oral bioavailability; TTD, Therapeutic Target Database; OMIM, Online Mendelian Inheritance in Man database; PPI, protein‒protein interaction; DC, Degree centrality; SD, standard deviation; BP, biological processes; MF, molecular functions; CC, cellular components; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; PDB, Protein Data Bank.
Ethics Approval and Consent to Participate
Ethical approval was not necessary for the current study because our study gathered data only from Embase, PubMed, the Cochrane Library, the China National Knowledge Infrastructure Database (CNKI), the Wanfang Database, the CQVIP Database (VIP), the China Biology Medicine disc (SinoMed) database, the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP), PubChem, UniProt database, Therapeutic Target Database (TTD), Online Mendelian Inheritance in Man (OMIM), DisGeNET, and STRING database, and this procedure did not address any patient’s personal data or harm any patient.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81860010) and Guangxi Medical High-level Backbone Personnel Training “139” Plan Training Project (Grant No. G201903008, G202002002).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gloeckl R, Schneeberger T, Jarosch I, Kenn K. Pulmonary rehabilitation and exercise training in chronic obstructive pulmonary disease. Dtsch Arztebl Int. 2018;115(8):117–123. doi:10.3238/arztebl.2018.0117
2. Christenson SA, Smith BM, Bafadhel M, Putcha N. Chronic obstructive pulmonary disease. Lancet. 2022;399(10342):2227–2242. doi:10.1016/S0140-6736(22)00470-6
3. Iyer AS, Sullivan DR, Lindell KO, Reinke LF. The role of palliative care in COPD. Chest. 2022;161(5):1250–1262. doi:10.1016/j.chest.2021.10.032
4. Halpin DMG, Criner GJ, Papi A, et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203(1):24–36. doi:10.1164/rccm.202009-3533SO
5. MacLeod M, Papi A, Contoli M, et al. Chronic obstructive pulmonary disease exacerbation fundamentals: diagnosis, treatment, prevention and disease impact. Respirology. 2021;26(6):532–551. doi:10.1111/resp.14041
6. Li L, Zhong X, Zheng A, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in Kashi region, northwestern China. Int J Chron Obstruct Pulmon Dis. 2021;16:655–663. doi:10.2147/COPD.S289620
7. Bourbeau J, Bafadhel M, Barnes NC, et al. Benefit/risk profile of single-inhaler triple therapy in COPD. Int J Chron Obstruct Pulmon Dis. 2021;16:499–517. doi:10.2147/COPD.S291967
8. Huang K, Zhang P, Zhang Z, et al. Traditional Chinese Medicine (TCM) in the treatment of COVID-19 and other viral infections: efficacies and mechanisms. Pharmacol Ther. 2021;225:107843. doi:10.1016/j.pharmthera.2021.107843
9. Zhang F, Chen X, Wu X, et al. TCM nonpharmacological interventions for chronic obstructive pulmonary disease (COPD): protocol for a systematic review and network meta-analysis. Medicine. 2019;98(23):e15979. doi:10.1097/MD.0000000000015979
10. Xu SJ, Zhu YL, Yu JJ, Sun S, Xu YJ, Yang L. 喘可治注射液中朝藿定A,朝藿定B,朝藿定C和淫羊藿苷在大鼠体内的药物动力学研究 [Pharmacokinetics of epimedin A, B, C and icariin of Chuankezhi injection in rat]. Zhongguo Zhong Yao Za Zhi. 2016;41(1):129–133. Chinese. doi:10.4268/cjcmm20160125
11. Chen XJ, Tang ZH, Li XW, Xie CX, Lu JJ, Wang YT. Chemical constituents, quality control, and bioactivity of epimedii folium (Yinyanghuo). Am J Chin Med. 2015;43(5):783–834. doi:10.1142/S0192415X15500494
12. Zhao JQ, Shao J, Zhong WW. Clinical study on effect of chuankezhi injection in treating children with bronchial asthma. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24(6):511–513.
13. Yan N, Wen DS, Zhao YR, Xu SJ. Epimedium sagittatum inhibits TLR4/MD-2 mediated NF-kappaB signaling pathway with anti-inflammatory activity. BMC Complement Altern Med. 2018;18(1):303. doi:10.1186/s12906-018-2363-x
14. Wu J, Xu H, Wong PF, Xia S, Xu J, Dong J. Icaritin attenuates cigarette smoke-mediated oxidative stress in human lung epithelial cells via activation of PI3K-AKT and Nrf2 signaling. Food Chem Toxicol. 2014;64:307–313. doi:10.1016/j.fct.2013.12.006
15. Li M, Zheng W, Zhang C, et al. Chuankezhi injection for asthma: protocol of a systematic review and meta-analysis. Medicine. 2019;98(33):e16630. doi:10.1097/MD.0000000000016630
16. Zhao JJ, Pan K, Wang QJ, et al. Effect of anti-asthma Chinese medicine Chuankezhi on the anti-tumor activity of cytokine-induced killer cells. Chin J Cancer. 2013;32(10):553–560. doi:10.5732/cjc.012.10249
17. Pan J, Chen Y. Clinical study on Chuankezhi injection in treatment of Chronic Obstructive Pulmonary Diseases of spleen-kidney deficiency syndrome in stable phase. Chinese Archives Traditional Chin Med. 2016;34(01):57–59. doi:10.13193/j.issn.1673-7717.2016.01.015
18. Shan Y, Guo J, Li G. Clinical effects of Zusanli acupoint injection of Chuankezhi injection combined with routine treatment on chronic obstructive pulmonary disease patients due to lung and Kidney Qi Deficiency. Chinese Traditional Patent Med. 2019;41(04):799–803.
19. Cohen JF, Deeks JJ, Hooft L, et al. Preferred reporting items for journal and conference abstracts of systematic reviews and meta-analyses of diagnostic test accuracy studies (PRISMA-DTA for Abstracts): checklist, explanation, and elaboration. BMJ. 2021;372:n265. doi:10.1136/bmj.n265
20. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi:10.1002/14651858.ED000142
21. Szklarczyk D, Gable AL, Nastou KC, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49(D1):D605–D612. doi:10.1093/nar/gkaa1074
22. Huang X. The effects of Chuankezhi injection in patients with chronic obstructive pulmonary disease in stable phase. [Master’s Thesis]; 2009.
23. Jiang J, Zhou X. Curative effect of Chuankezhi injection combined with budesonide and formoterol fumarate powder for inhalation in the treatment of COPD. China Pharm. 2015;18(11):1938–1940.
24. Li S. Effects of Chuankezhi injection on pulmonary function and PaO2 and PaCO2 levels in COPD patients. Mod Med Health Res Elect J. 2020;4(04):36–38.
25. Li Z. Clinical value of Chuankezhi injection combined with budesonide in chronic obstructive pulmonary disease. Cont Med Educ. 2022;36(06):129–132.
26. Xiao Y, Liao W. The efficacy of Chuankezhi combined with Budesonide in the treatment of stable COPD. Mod Diagnos Treat. 2020;31(04):529–530.
27. Wang K, Dong C. Clinical efficacy of Seretide combined with Chuankezhi injection in the treatment of stabilized stage of COPD. Health Vocat Educ. 2014;32(08):141–143.
28. Wang X. Analysis on the curative effect of Chuankezhi in the treatment of chronic obstructive pulmonary disease. Chinese Community Doctors. 2017;33:1.
29. Xu X, Pan J, Chen Y. An investigation on clinical therapeutic effect of acupoint injection combined with health education for treatment of spleen kidney yang deficiency syndrome in patients with chronic obstructive pulmonary disease at stable stage. Chin J Integrat Trad West Med Inten Crit Care. 2015;(6):565–568. doi:10.3969/j.issn.1008-9691.2015.06.002
30. Yu Z, Li J, Zhu X. The clinical effects of chuankezhi injection on stable-stage patients with chronic obstructive pulmonary disease. J Clin Pulm Med. 2010;15(09):1280–1281.
31. Yuan H, Wang X. Influence of Chuankezhi injection combined with tiotropium Bromide on lung function and inflammatory factor levels in patients with stable COPD. Clin Med Enginee. 2021;28(03):327–328.
32. Zhan S, Dong H, Li B. Method of tonifying kidney on the immune function of patients with chronic obstructive pulmonary disease stabilization and the influence of the quality of life. J Liaoning Univ Traditional Chin Med. 2016;18(01):135–138. doi:10.13194/j.issn.1673-842x.2016.01.044
33. Zhang L. The clinical effects of acupoint injection of Chuankezhi injection on COPD in stable phase based on tonifying kidney. [Master’s Thesis]; 2021.
34. Zheng L, Zhuo M. Study on the effect of Chuankezhi nebulization on pulmonary function in patients with chronic obstructive pulmonary disease in stable stage. China Health Care & Nutr. 2010;2010:1.
35. Alcazar-Navarrete B, Jamart L, Sanchez-Covisa J, Juarez M, Graefenhain R, Sicras-Mainar A. Clinical characteristics, treatment persistence, and outcomes among patients with COPD treated with single- or multiple-inhaler triple therapy: a retrospective analysis in Spain. Chest. 2022;162(5):1017–1029. doi:10.1016/j.chest.2022.06.033
36. Vogelmeier CF, Roman-Rodriguez M, Singh D, Han MK, Rodriguez-Roisin R, Ferguson GT. Goals of COPD treatment: focus on symptoms and exacerbations. Respir Med. 2020;166:105938. doi:10.1016/j.rmed.2020.105938
37. Radicioni G, Ceppe A, Ford AA, et al. Airway mucin MUC5AC and MUC5B concentrations and the initiation and progression of chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2021;9(11):1241–1254. doi:10.1016/S2213-2600(21)00079-5
38. Gu XY, Chu X, Zeng XL, Bao HR, Liu XJ. Effects of PM2.5 exposure on the Notch signaling pathway and immune imbalance in chronic obstructive pulmonary disease. Environ Pollut. 2017;226:163–173. doi:10.1016/j.envpol.2017.03.070
39. Wang J, Wu Q, Ding L, et al. Therapeutic effects and molecular mechanisms of bioactive compounds against respiratory diseases: traditional Chinese medicine theory and high-frequency use. Front Pharmacol. 2021;12:734450. doi:10.3389/fphar.2021.734450
40. Li Q, Wang G, Xiong SH, et al. Bu-Shen-Fang-Chuan formula attenuates cigarette smoke-induced inflammation by modulating the PI3K/Akt-Nrf2 and NF-kappaB signalling pathways. J Ethnopharmacol. 2020;261:113095. doi:10.1016/j.jep.2020.113095
41. Rahman MM, Bibi S, Rahaman MS, et al. Natural therapeutics and nutraceuticals for lung diseases: traditional significance, phytochemistry, and pharmacology. Biomed Pharmacother. 2022;150:113041. doi:10.1016/j.biopha.2022.113041
42. Zhang HY, Huang H, Pang LJ, Lv XD, Zheng WD. Effectiveness and safety of acupoint application for chronic obstructive pulmonary disease: a protocol for updated systematic review and meta-analysis. Medicine. 2021;100(18):e25802. doi:10.1097/MD.0000000000025802
43. Choi JY, Yoon HK, Shin KC, et al. CAT Score and SGRQ definitions of chronic bronchitis as an alternative to the classical definition. Int J Chron Obstruct Pulmon Dis. 2019;14:3043–3052. doi:10.2147/COPD.S228307
44. Stamenova V, Liang K, Yang R, et al. Technology-enabled self-management of chronic obstructive pulmonary disease with or without asynchronous remote monitoring: randomized controlled trial. J Med Internet Res. 2020;22(7):e18598. doi:10.2196/18598
45. von Siemens S M, Alter P, Lutter JI, et al. CAT score single item analysis in patients with COPD: results from COSYCONET. Respir Med. 2019;159:105810. doi:10.1016/j.rmed.2019.105810
46. Liu Y, Ruan J, Wan C, Tan J, Wu B, Zhao Z. Canonical correlation analysis of factors that influence quality of life among patients with chronic obstructive pulmonary disease based on QLICD-COPD (V2.0). BMJ Open Respir Res. 2022;9(1):1.
47. Diaz AA, Pinto-Plata V, Hernandez C, et al. Emphysema and DLCO predict a clinically important difference for 6MWD decline in COPD. Respir Med. 2015;109(7):882–889. doi:10.1016/j.rmed.2015.04.009
48. Mansour KMK, Goulart CDL, Carvalho-Junior LCS, Trimer R, Borghi-Silva A, Silva A. [Pulmonary function and functional capacity cut-off point to establish sarcopenia and dynapenia in patients with COPD]. Pontos de corte da funcao pulmonar e capacidade funcional determinantes para sarcopenia e dinapenia em pacientes com DPOC. J Bras Pneumol. 2019;45(6):e20180252. Portuguese. doi:10.1590/1806-3713/e20180252
49. Li Y, Yao J, Han C, et al. Quercetin, Inflammation and Immunity. Nutrients. 2016;8(3):167. doi:10.3390/nu8030167
50. Tang J, Diao P, Shu X, Li L, Xiong L. Quercetin and quercitrin attenuates the inflammatory response and oxidative stress in LPS-Induced RAW264.7 cells: in vitro assessment and a theoretical model. Biomed Res Int. 2019;2019:7039802. doi:10.1155/2019/7039802
51. Ganesan S, Faris AN, Comstock AT, et al. Quercetin prevents progression of disease in elastase/LPS-exposed mice by negatively regulating MMP expression. Respir Res. 2010;11:131. doi:10.1186/1465-9921-11-131
52. Aziz N, Kim MY, Cho JY. Anti-inflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J Ethnopharmacol. 2018;225:342–358. doi:10.1016/j.jep.2018.05.019
53. Yang SC, Chen PJ, Chang SH, et al. Luteolin attenuates neutrophilic oxidative stress and inflammatory arthritis by inhibiting Raf1 activity. Biochem Pharmacol. 2018;154:384–396. doi:10.1016/j.bcp.2018.06.003
54. Zhang ZT, Zhang DY, Xie K, Wang CJ, Xu F. Luteolin activates Tregs to promote IL-10 expression and alleviating caspase-11-dependent pyroptosis in sepsis-induced lung injury. Int Immunopharmacol. 2021;99:107914. doi:10.1016/j.intimp.2021.107914
55. Imran M, Salehi B, Sharifi-Rad J, et al. Kaempferol: a key emphasis to its anticancer potential. Molecules. 2019;24(12):1.
56. Shi Y, Wu Y, Li F, et al. Identifying the anti-metastasis effect of Anhydroicaritin on breast cancer: coupling network pharmacology with experimental validation. J Ethnopharmacol. 2022;293:115326. doi:10.1016/j.jep.2022.115326
57. Zheng ZG, Zhou YP, Zhang X, et al. Anhydroicaritin improves diet-induced obesity and hyperlipidemia and alleviates insulin resistance by suppressing SREBPs activation. Biochem Pharmacol. 2016;122:42–61. doi:10.1016/j.bcp.2016.10.016
58. Liu R, Hao D, Xu W, et al. beta-Sitosterol modulates macrophage polarization and attenuates rheumatoid inflammation in mice. Pharm Biol. 2019;57(1):161–168. doi:10.1080/13880209.2019.1577461
59. Smolarz B, Szyllo K, Romanowicz H. Endometriosis: epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature). Int J Mol Sci. 2021;22(19):10554. doi:10.3390/ijms221910554
60. Pan J, Cai X, Zheng X, Zhu X, Feng J, Wang X. Luteolin inhibits viability, migration, angiogenesis and invasion of non-small cell lung cancer vascular endothelial cells via miR-133a-3p/purine rich element binding protein B-mediated MAPK and PI3K/Akt signaling pathways. Tissue Cell. 2022;75:101740. doi:10.1016/j.tice.2022.101740
61. Ji H, Li K, Xu W, Li R, Xie S, Zhu X. Prediction of the mechanisms by which quercetin enhances cisplatin action in cervical cancer: a network pharmacology study and experimental validation. Front Oncol. 2021;11:780387. doi:10.3389/fonc.2021.780387
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
