Back to Journals » International Journal of Nanomedicine » Volume 19
Prosthetic Metals: Release, Metabolism and Toxicity
Authors Zhong Q, Pan X, Chen Y, Lian Q, Gao J, Xu Y, Wang J, Shi Z, Cheng H
Received 12 January 2024
Accepted for publication 13 May 2024
Published 5 June 2024 Volume 2024:19 Pages 5245—5267
DOI https://doi.org/10.2147/IJN.S459255
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Xing Zhang
Qiang Zhong,* Xin Pan,* Yuhang Chen, Qiang Lian, Jian Gao, Yixin Xu, Jian Wang, Zhanjun Shi, Hao Cheng
Department of Orthopedics, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhanjun Shi; Hao Cheng, Nanfang Hospital, Southern Medical University, 1838# Guangzhou North Avenue, Guangzhou, People’s Republic of China, Tel +86 2062787924, Email [email protected]; [email protected]
Abstract: The development of metallic joint prostheses has been ongoing for more than a century alongside advancements in hip and knee arthroplasty. Among the materials utilized, the Cobalt-Chromium-Molybdenum (Co-Cr-Mo) and Titanium-Aluminum-Vanadium (Ti-Al-V) alloys are predominant in joint prosthesis construction, predominantly due to their commendable biocompatibility, mechanical strength, and corrosion resistance. Nonetheless, over time, the physical wear, electrochemical corrosion, and inflammation induced by these alloys that occur post-implantation can cause the release of various metallic components. The released metals can then flow and metabolize in vivo, subsequently causing potential local or systemic harm. This review first details joint prosthesis development and acknowledges the release of prosthetic metals. Second, we outline the metallic concentration, biodistribution, and elimination pathways of the released prosthetic metals. Lastly, we discuss the possible organ, cellular, critical biomolecules, and significant signaling pathway toxicities and adverse effects that arise from exposure to these metals.
Keywords: metallic joint prostheses, prosthetic metal release, potential toxicity and adverse effects
Graphical Abstract:
History and Characteristics of the Metallic Prosthesis
History of the Hip and Knee Prosthesis
The development of hip and knee replacement has continued for over a century as orthopedic surgeons and researchers strive to identify suitable materials to replace diseased joints (Figure 1). John Murray Carnochan attempted to complete the first mandibular arthroplasty by inserting an oak chip in 1840,1 marking the introduction of prosthesis implantation. However, the implant failed immediately after, resulting in the loosening of the chip. In the 1860s, Verneuil introduced knee arthroplasty to treat diseased knee rigidity by establishing a septum in the joint space using surrounding soft tissue or fascia.2 Gluck performed a total hip arthroplasty (THA) in 1891 using a femoral head and acetabular cup constructed from ivory, subsequently fixed with Nickel plated screws.3 The significance of the stiffness and durability of implantations in bearing joints emerged over time, leading to the utilization of metallic insertion. Robert Jones designed a golden cover four years later to resurface a diseased femoral head.4 Despite the subsequent utilization of prostheses made from rubber,5 glass,3 and stainless steel,5 long-term outcomes indicated dissatisfaction with the implantations. Attempts to use materials like nylon and glass in diseased knees likewise failed.2 Smith Peterson debuted the first acetabular cup in 1938 constructed using Cobalt-Chromium-Molybdenum alloy (Co-Cr-Mo), inspired by dental materials.6 However, a single metallic cup worsened friction between itself and the femoral head, resulting in bone necrosis and pain. To address this, the Judet brothers designed an artificial head with a short stem or a long stem by Austin Moore.6 Different types of metallic molds were also utilized as femoral or tibial hemiarthroplasties in knee replacements until the mid-twentieth century, inspired by the application of Co-Cr-Mo alloy in diseased hip joints.2 After 1950, knee prosthesis development focused more on biomechanics than on materials. The transition from fully restrictive hinged prostheses to semi-restrictive and non-restrictive total condylar prostheses for total knee arthroplasty (TKA) today was a result of such focus.7 On the other hand, John Charnley, regarded as the founder of modern hip replacement, used high molecular polyethylene and acrylic cement to anchor the artificial femoral head in 1958,3 a milestone in the low friction arthroplasty theory.3 Since then, arthroplasty has been widely accepted and recognized as a standard treatment for adult joint diseases.8 Nowadays, press-fit or anatomical prostheses have been developed, with some being modified further by hydroxyapatite coating to enhance stability or durability.3
 |
Figure 1 History of joint replacement. |
 |
Figure 2 Biodistribution and excretion of prosthetic metals. |
 |
Figure 3 Macrophage, osteoblasts and osteoclasts influenced by prosthetic metals. |
Along with the continuous development of joint prostheses, arthroplasty is now being hailed as the greatest surgery of the 21st century and has become the premier solution for end-stage diseases such as osteoarthritis and osteonecrosis of the femoral head. According to the 2022 American Joint Replacement Registry (AJRR) annual report, over 2.8 million hip and knee procedures was registered in the whole United States, representing a cumulative registered procedural volume growth of 14% compared to the previous year.9 Undoubtedly, the solid development of joint prostheses has guaranteed total joint replacement as the most successful option for the treatment of end-stage joint diseases.
Major Characteristics of the Modern Metallic Prosthesis
The current mainstream in clinical applications for joint replacements has been shaped by advancements in alloy technology. Specifically, the Titanium-Aluminium-Vanadium (Ti-Al-V) and Co-Cr-Mo metallic prostheses are predominately utilized.10 Ti-Al-V elements often feature as the tibial plateau in artificial knee systems or the femoral stem and acetabular cup in artificial hip systems.11 Co-Cr-Mo, on the other hand, mainly consists of the bearing femoral head in an artificial hip or the femoral condyle in an artificial knee.12,13 The exceptional biocompatibility, mechanical strength, and corrosion resistance ability of these alloys account for their broad applications.11
Biocompatibility
Biosafety concerns associated with Ti-Al-V and Co-Cr-Mo alloys are rare due to their biological inertia. Co, Cr, Mo, and V essentially participate in the metabolism of vitamins, glucose, or carbohydrate by contributing to the formation of several critical enzymes in vivo.14–22 Although Ti, Ta, and Al are not considered essential for normal bio-functioning detected in the human body, they are detected in vivo, possibly through daily intake of food and water.23,24 Generally, it is suggested that the aforementioned metals remaining at a physiologically acceptable level result in few adverse events. Additionally, the metals could be excreted through multiple organs. Table 1 summarizes the physiological function of these metals.25–35
 |
Table 1 Physiological Function Affected by Prosthetic Metals |
Mechanical Strength
When treating weight-bearing joints, such as the hips and knees, appropriate mechanical strength is a key advantage of Ti-Al-V and Co-Cr-Mo alloys compared to other materials with lower elastic moduli. The elastic moduli of the Ti-Al-V alloy are approximately four to six times greater than those of cortical bone, allowing for stress-sharing with the periprosthetic bone and bearing the body weight as the femoral stem or tibial plateau.36 The Co-Cr-Mo alloy, which has an elastic modulus approximately twice that of the Ti-Al-V alloy, is commonly used in grinding components such as the artificial femoral head and condyle.36 The mechanical properties of mainstream medical metals was summarized in Table 2.37 However, implants with extremely high elastic moduli can hinder osteointegration and result in an unstable state due to excessive stress sharing (known as the stress shielding effect), preventing adequate bone growth.11
 |
Table 2 Elastic Modulus of Prosthetic Metals and Natural Bone Tissues |
Corrosion Resistance
The alloys possess excellent corrosion resistance characteristics, which are highly desired in ensuring long-term stability. Upon being implanted in vivo, gentle redox reactions start on the prosthetic surface, and a thin protective oxide membrane is known as a passivation film gradually forms.38 This passivation film provides a clear antioxidant effect and shields the alloys from excessive redox reactions. Specifically, Ti-Al-V generates a stable Ti oxide film on their surface attributed to their strong affinity with oxygen, while Co-Cr-Mo produces a thin but denser protective oxide layer to avoid electrochemical corrosion.39,40 These membranes can rapidly recover and resupply, even if the film is destroyed by fretting or wearing.38
Potential Mechanism of Metals Released from the Prosthesis
Both the hip and knee joints are load-bearing joints subjected to complex forces. In load-bearing conditions, the prosthesis must withstand the comprehensive effects of tension, compression, torsion, interface shearing, and repeated fatigue over many years. Despite the excellent corrosion resistance of Ti-Al-V and Co-Cr-Mo, metals in different forms (ions, particles, and debris) were detected in vivo under the combined effects mentioned above. The metals are released from prostheses as a result of diverse factors involving mechanical wearing, electrochemical corrosion, and inflammation.41
Mechanical wearing, a tribological effect of two surfaces in contact, generally includes fretting and impinging. Factors associated with fretting include the number of cycles, load to the interface, motion amplitude, frequency, and temperature. Such actions can cause abrasion of metallic particle fragments from the materials. These metallic microparticles have an irregular shape and a diameter of less than 400 μm.42
Electrochemical corrosion may occur on the partial or entire surface of the prosthesis, depending on the electric potential energy of the metals.41 When active metals come into contact with other cations, the difference in electric potential energy causes electron flow, indicating the onset of corrosion. This phenomenon is more noticeable when different metals are used in alloy components, such as a Co-Cr-Mo femoral and a Ti-Al-V stem.43 Although the passivation film mentioned earlier exists, the protective effect of the oxide membrane is cracked by the simultaneous action of electrochemical corrosion and mechanical wear. This cracking causes the continuous release of metallic debris or ions.41
The metallic debris and ions generated by wear and corrosion can lead to a series of cascading effects on multiple cells, including inflammation, necrosis, fibrosis, and osteolysis.42 Macrophages play a central role in the cascade, phagocytosing the metals and releasing inflammatory mediators such as Interleukin (IL) and Tumour Necrosis Factor (TNF).43 A specific phenotype, distinct from traditional M1 and M2 types of macrophages, has even been proposed to describe this situation.43 Larger but non-degradable debris can be released after cell death and subsequently re-phagocytosed, triggering a negative feedback loop.44 Furthermore, macrophages can attach to and affect the oxide surface of alloys, compromising the passivation films and accelerating corrosion.45 Other cells, including osteoclasts and osteoblasts, are also influenced by the released metals and inflammatory mediators, amplifying the adverse events.46,47
Metabolism Profiles of the Prosthetic Metals
Elevation of Metals in vivo
The blood of the ordinary population contains very low levels of metals as a result of frequent exposure to metal products in their environment,14,15,48 as indicated in Table 1. Therefore, a baseline metallic concentration was established in whole blood or serum using references from the Laboratory Test Information Guide of the London Health Sciences Centre and Mayo Clinic Laboratory data.49–60 These measurements follow the guidelines from the Centre for Disease Control and Prevention of America (Table 3).61
 |
Table 3 Reference Range and Postoperative Concentration of Prosthetic Metals in the Blood |
Metallic prostheses can cause an increase in metal concentration in patients’ blood that exceeds the baseline level. As indicated in a comprehensive systematic review of over 400 cases of Co-Cr hip prosthesis implantation, the mean Co concentrations were found to be 0.70 ~ 3.40 μg/L and 0.30 ~ 7.50 μg/L in the whole blood and serum, respectively.80 Similarly, the average Cr concentrations were 0.50 ~ 2.50 μg/L and 0.80 ~ 5.10 μg/L, respectively, in the whole blood and serum, as shown by the study.80
Table 3 62–79 presents evidence of increased levels of Co and Cr in the blood of patients with Co-Cr-Mo prostheses. The increased concentration persisted for 72 months, and the concentration of both Co and Cr exhibited two peaks, as observed at 24 months (6.20 μg/L of Co, 4.70 μg/L of Cr) and 60 months (8.42 μg/L of Co, 4.58 μg/L of Cr) following surgery.81 The second peak may be attributed to secondary wearing and corrosion due to prosthetic dysfunction or patient factors, such as renal dysfunction.82,83 However, others have suggested that after implantation of Co-Cr-Mo prostheses, the concentration of Co and Cr may initially soar before stabilizing or gradually decreasing over time.84 The postoperative in-vivo fluctuations in Mo levels are still debatable Some studies have reported that Mo concentration peaked at 60 months following THA, reaching levels over 6 ng/mL.81 However, other studies have shown that there was no notable rise in Mo levels detected in the blood.85,86
Individuals with Ti-Al-V prostheses exhibit obvious Ti accumulation (12 times higher than baseline) in their blood, peaking at 13.6 μg/L after 3 months post-surgery before declining. Ti concentration in a case with Ti-Al-V prostheses may even reach 50 times the baseline.87 Al tends to bind to transferrin, albumin, and some low molecular weight compounds, primarily citrate,88 in the blood, facilitating its penetration through biological barriers and subsequent clearance from the kidneys,89 thereby producing a gentler rise compared to Ti. V primarily occurs as Vanadate or Vanadyl in the blood, binding also to transferrin, and it has a similar duration of peaking (3~6 months post-surgery) as Al.90,91
The release of metals into the body can be influenced by various factors that include prosthetic factors such as interfaces, designs, and manufacturing processes; patient-specific factors such as weight and physical activity; and operational factors such as instability and mismatched components. Additionally, the methods used to sample and detect metal ions in the body and the timing of these evaluations can also have an impact on the results. Specifically, the use of a Metal-on-Metal bearing interface tends to result in higher concentrations of metals in the body compared to the Metal-on-Polyethylene or the Metal-on-Ceramics bearings.63–68,74,75,92 Moreover, higher levels of metals are commonly detected when the prosthesis is not functioning optimally.72,93 Elevation in metallic concentration is more likely to occur when bilateral implantation is performed.62,71,94 Other factors that can affect metal release include the surface coating,77,78 the size of the femoral head,66,73 and the design and manufacture of the prosthesis.76,79
Thus, long-term monitoring of the state of the prosthesis after implantation is essential. Prostheses in an abnormal state are more prone to severe wear and may result in a secondary surge in metal concentration following surgery. Continuous monitoring of metal concentration fluctuations, for example, at 3- or 6-month intervals following implantation, can aid in evaluating the state of the implanted prosthesis and predicting its lifespan. Therefore, postoperative metal concentration can be an essential indicator of the state of the implanted prosthesis. However, some aspects, including sensitivity, specificity, and the time lag between increased metal concentration and the occurrence of an unstable prosthesis, require further confirmation. The findings presented in this study may provide some insights; however, further detailed investigations are necessary.
Biodistribution and Deposition
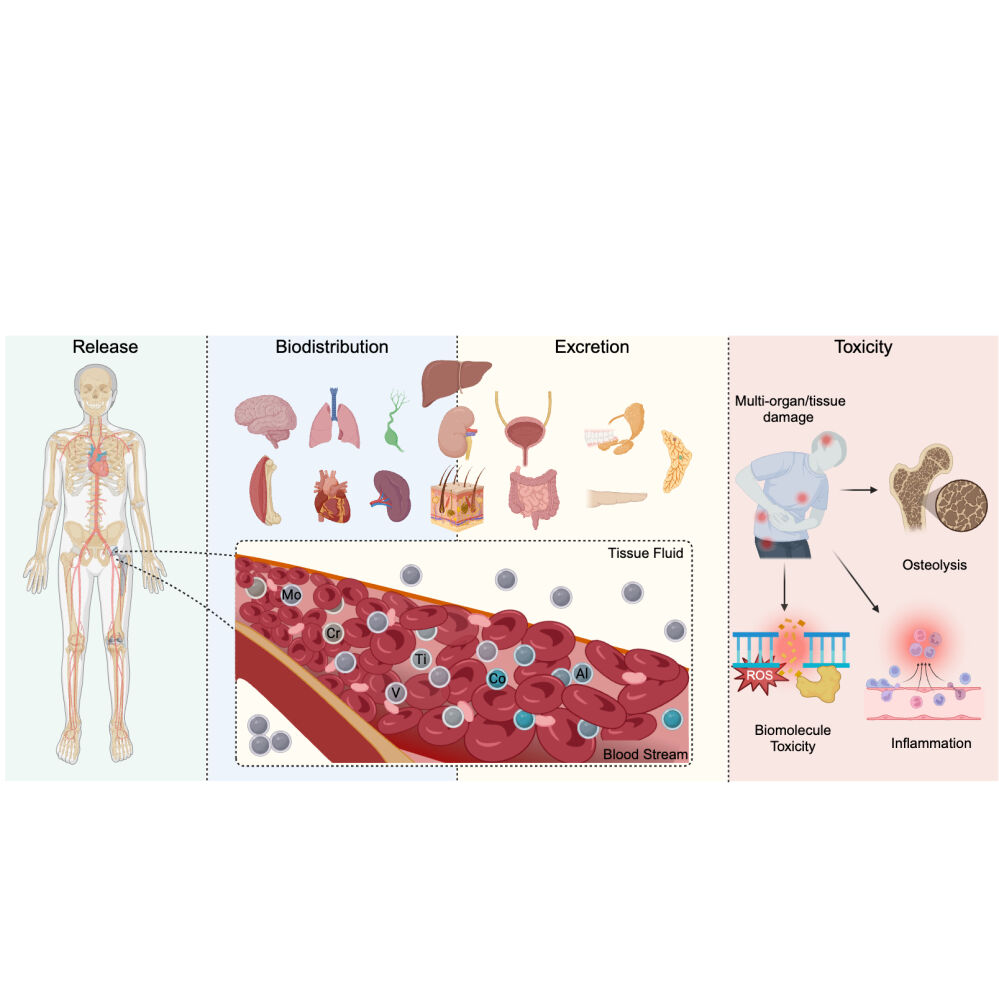
Metals released from implanted prostheses eventually deposit in various organs and tissues through transportation via blood or interstitial fluid. Although direct detection of metal accumulation in tissues of THA or TKA patients remains lacking, the biodistribution or deposition of blood metals has been confirmed via experiments conducted in human or animal models, as well as biokinetic models. Figure 2 summarizes the biodistribution of these metals.
Bone and Muscle
Concentration of Co up to 380 ppm was detected in the mineralized periprosthetic bone two years after implantation of the prosthesis.95 In an animal trial, Co distribution in muscle was also observed.96 Although the Cr level in the mineralized periprosthetic bone exhibited no significant change,95 a biokinetic investigation provided evidence of Cr distribution in bone.97 Biopsy results from THA or TKA patients or animal models failed to present any relevant evidence of Mo accumulation.
While Ti deposition from prostheses was not detected in bone and muscle, Ti from dental implants was found in the jawbone and surrounding soft tissue.95 Evidence of Ti retention in bone is also found in an animal trial.98 Bone and muscle serve as the primary accumulation sites for Al, with 60% and 10%, respectively.99,100 V is detectable in human muscle tissue, with elevated levels also found in rat bone after a long-term high V diet or intravenous injection.101
Kidney and Liver
The kidney and liver are organs that filter and purify blood flow. Biopsies from patients with bilateral THA have shown concentrations of Co, Cr, and Mo nearly fifty times higher than the standard value,102 similar to findings from studies in rats with metallic implants.103,104
Elevated levels of Ti concentration in the liver of Metal-on-Metal bearing THA patients are confirmed 4 to 10 years post-implantation.105 Both laboratory rats with hamster dose injections and New Zealand rabbits with proximal tibial screw implants have experienced Ti accumulation in the liver and kidney.24,105 Approximately 3% of the total body burden of Al is found in the liver,99 whereas evidence of Al kidney retention is only present in rats in intravenous injection tests.100 Limited evidence of V accumulation has been found in the kidney or liver of THA or TKA patients; however, biokinetic calculation suggests V tends to first accumulate in the kidneys, then the liver of rats treated with oral V administration.106
Brain
The ability of metals to permeate the brain depends on their ability to cross the blood-brain barrier (BBB). Ti dioxide nanoparticles have been reported to cause damage to the BBB ultrastructure and increase BBB permeability.107 Elevated levels of Ti in brain tissue were found in laboratory rats after intravenous injection of Ti nanoparticles,108 with the metal mainly concentrating in the hippocampus.109 It is estimated that with the assistance of transferrin and citrate, approximately 1% of the total Al burden and 5% of the total V burden are deposited in the brain.90,99 V dose-dependently accumulates primarily in the olfactory bulb, brain stem, and cerebellum,110,111 while experimental studies show Al retention in cerebral tissue detected in rats treated with a dose of intravenous Al.100 However, no significant increase in Ti, Al, and V has been reported in patients with metallic prostheses.
Co and Cr may enter the brains of THA patients through cerebrospinal fluid circulation,112 with animal trials showing accumulation in cerebral tissue.113,114 Evidence for Mo accumulation in brain tissue is limited.
Lung
Although few reports indicate the metal deposition of Co-Cr-Mo or Ti-Al-V in human lungs, elevated concentrations of Ti, Al, and V were found in the lungs of laboratory baboons with Ti-Al-V alloy implantation.115 Furthermore, increased Co, Cr, and Mo levels were found in lung tissue after Co-Cr-Mo debris was implanted under rat skin, with peak concentrations recorded at 48 hours.116
Spleen
Studies have reported Co, Cr, and Mo deposition in the spleen of THA and TKA patients, with concentrations exceeding baseline values.102,117,118 In an animal trial, Ti retention in the spleen of rats was detected after intraperitoneal or intravenous injection of Ti dioxide.109 Biokinetic modeling based on multiple human and animal studies confirms Al accumulation in the spleen.119 Accumulation of V in humans was confirmed in the spleen, a fact also evidenced in rats.106
Other Organs and Tissues
In addition to the previously mentioned organs, traces of these metals have been found in other tissues in the human body. Elevated levels of Co, Cr, Mo, Ti, and Al have been detected in the hair of patients that have prostheses.120,121 A high level of Co was found in the heart of a patient after they experienced fatal cardiomyopathy due to severe wear of the Co-Cr-Mo alloy.122 Additionally, an investigation showed higher levels of Co in the seminal plasma of implanted patients (2.89 μg/L) compared to control patients (1.12 μg/L).123 An implantation model of metallic debris also suggested elevated levels of Co in the heart and testis.116 In addition, deposition of Al in body fat and accumulation of Mo in lymph nodes were also confirmed in patients with THA.124
Excretion and Elimination
The kidneys were the primary excretion site for all the metals mentioned.88,96,106,109,125–127 TKA or THA patients had high metal concentrations in their urine.128–130 Strong evidence indicated high average Co concentrations in the urine of patients who had undergone THA.81,92,131–139 An experiment administering Co intravenously in humans demonstrated that 40% was eliminated within the first 24 hours, 70% within a week, 80% within a month, and 90% within a year.140 The geometric mean Mo level in the blood was 15.36 nmol/L, while the highest level in urine was 58.41 μmol/L.141 Furthermore, a volunteer experiment demonstrated rapid Mo excretion,142 which could explain the low Mo level in the blood and the low risk of adverse effects from Mo. Al and V are rapidly excreted by converting to hydrophilic forms (Vanadyl or Vanadate)106 or combining with hydrophilic substances (citric acid, transferrin, or albumin).89 These processes enhance penetration and improve kidney clearance, but only 10% can pass through the glomerular membrane as free ions.89 Nevertheless, due to the continuous metallic release in THA patients, the excretion of both metals gradually slows down after the initial rapid phase and persists for several years.90,91,128
In addition to urination, faecal excretion is an important elimination pathway for metals. Metals, including Cr, Al, V, and Ti, in the gastrointestinal tract primarily come from saliva, bile, and gastrointestinal secretions,88,90,106,109,143,144 and due to their poor absorption rate, they are eliminated with feces.90,125,145–148 Moreover, Cr and Ti can be eliminated through the shedding of nails, hair, sweat, and milk.109,143 Ti particles can also be phagocytosed by cells and coughed up as sputum from the respiratory tract (Figure 2).109
Toxicity Induced by the Prosthetic Metals
Toxicity to Organs and Tissues
Continuously high concentrations of these metals can cause damage to multiple organs, with diverse clinical manifestations. Reports show that elevated Co in THA or TKA patients can cause various organ damages, including neurotoxicity (lethargy, hearing loss, numbness, paresthesia, tinnitus, visual and auditory abnormalities, and peripheral neuropathy), cardiomyopathy, pericardial effusion, hypothyroidism, hepatotoxicity, allergic dermatitis (rash) and polycythemia.149–153 High levels of Co can also cause systemic toxicity that can lead to hard metal asthma, hard metal disease (pulmonary fibrosis) and myocardial toxicity better known as “Beer Drinkers’ Cardiomyopathy”.25,154–157 Excess Cr can lead to similar systemic toxicity in the nervous and circulatory systems.152
Chronic accumulation of Al in the brain can cause neurological damage, including Alzheimer’s and Parkinson’s diseases, with symptoms such as brain degeneration, disorientation, memory impairment, dementia, and changes in personality and intelligence.100,158 Al accumulation in the kidneys can cause fibrosis in glomeruli or Bowman’s capsule,100 leading to renal dysfunction and subsequent microcytic anemia.159 Al systemic toxicity can also manifest as bone diseases such as osteomalacia due to interference with parathyroid hormone and bone calcium metabolism.100 Excessive V deposition can cause systemic toxicity, including peripheral neuropathy, skin allergies, diarrhea, kidney damage, and reproductive system damage, as reported in several studies.160–166 Systematic pathological changes from high Ti, and Mo levels after THA or TKA are uncommonly reported.167–172
Aside from causing systemic changes, accumulated metals in situ can also trigger adverse local tissue reactions (ALTRs) in the periprosthetic tissues, such as inflammatory pseudotumor, osteolysis, and tissue necrosis.173–176 Excessive Ti, in the form of metallic oxide nanoparticles, can often cause periprosthetic osteolysis and inflammation.24,109,146,177,178 Another possible localized adverse reaction known as aseptic lymphocyte-dominated vascular-associated lesions (ALVAL), may occur in patients with especially a Metal-on-Metal bearing prosthesis. ALVAL is resulted by activated cytotoxic T lymphocytes and macrophages induced via the T lymphocyte-mediated type IV hypersensitivity reactions, for the metal debris or ions released from the prosthesis diffuse into the surrounding tissue, complexing with natural proteins to form hemi-antigens and leading to the allergic reaction.179 The histopathological manifestation of ALVAL is characterized as an aseptic and chronic perivascular inflammatory response dominated by lymphocytic infiltration.179 ALVAL has been shown to have a positive correlation with elevated Co and Cr concentrations, but no evidence with Ti, Al, V, and Mo has been found.180,181 The reported cases also focused only on the abnormally elevated levels of Co and Cr in the patients’ blood.181 Most patients with ALVAL manifest as persistent periarticular pain, especially persistent groin pain after THA, and further examination also reveals pathologic changes such as joint effusion and osteolysis.180
Metals are also known to pose considerable risks of both carcinogenicity and mutagenicity. Experimental evidence has confirmed the carcinogenic and mutagenic potential of Co, Cr, and both ionic and oxide forms of Ti,177,182–196 and they are more likely to pose risks that are time-dependent rather than concentration-dependent.197 The carcinogenicity of Al is yet to be confirmed, although a correlation has been observed between Al and breast cancer.100 It is still unclear whether the metals act as a single causative factor or as a co-inducing factor with other substances during tumor development.90
Toxicity to Cells and Biomolecules
To gain a deeper understanding of metal toxicity like inflammation, osteolysis, and mutagenicity, researchers have studied their toxic effects on the cell and biomolecule aspects. Therefore, we will further discuss the potential mechanisms of how the release of metals correlates with the regulation of the inflammatory cascade, the inhibition of osteoblasts, the activation of macrophages and osteoclasts, and the damage to the biomolecules and organelles.
Macrophage and Osteoclasts Activation
After the uptake of metals by macrophages and osteoclasts through phagocytosis, these cells get activated to release pro-inflammatory mediators, such as IL-1β, IL-6, TNF-α, and Prostaglandin E2 (PGE2), which participate in the receptor activator of nuclear factor-kappa B/ligand (RANK/RANKL) signaling to provoke osteolysis and inflammation.198–202 The larger non-degradable debris is re-phagocytosed after cell death, creating a closed circuit that promotes inflammation continuously.44 Moreover, mature osteoclasts also produce acid and enzymes that erode the prosthetic surface and enable the uptake of dissolved metals, thereby compounding the damage.203 The effects of metals on osteoclasts and macrophages vary according to the concentration.200 Extremely high metal concentrations are believed to have a direct harmful effect on cell viability.200
Osteoblasts and Mesenchymal Cells Inhibition
Apart from macrophages and osteoclasts activation, osteoblasts and mesenchymal cells also have an essential role in the emergence of ALTR, given the imbalanced homeostasis of bone resorption and formation.204 After internalizing prosthetic metals, osteoblasts are impacted, and their viability and proliferation are affected, which can cause cell death.205–207 On the other hand, osteoblasts exhibit downregulation of the type-I collagen gene expression, while cytokines like IL-6 and TNF-α occur synchronously.205,206 Factors derived from osteoblasts promote osteoclastogenesis by facilitating its progression.207 Also, exposure to prosthetic wear products in vivo and in vitro reduced mesenchymal cells’ alkaline phosphatase activity and matrix mineralization for osteogenesis.208
Inflammatory Cascade Regulation
Cytokines that metallic-activated cells release act as messengers that disperse the functions of different cells throughout the regulatory network of local osteolysis and inflammation. For instance, PGE2, and TNF-α are essential activators of the RANK/RANKL pathway and suppressors of type-I collagen generation.207,209–211 TNF-α production activates the osteoclast precursor through calcineurin/nuclear factor of activated T cells 1 (CaN/NFATc1) signaling212–214 and upregulates the RANKL expression in osteoblasts.213,215–219 Additionally, chemokines like granulocyte-macrophage colony stimulating factor (GM-CSF) and macrophage colony stimulating factor (M-CSF) enhance and accumulate inflammatory cells. Furthermore, the chemokines like monocyte chemotactic protein-1 (MCP-1) and macrophage inflammatory protein-1α (MIP-1α) are responsible for osteoclasts migrating to periprosthetic tissues.198,220–225
The regulation of most cytokines is thought to occur following a metallic concentration and time-dependent pattern. Elevated levels of Co and Cr can lead to increased TNF-α upregulation.226 Furthermore, Co promotes other cytokines and chemokines, such as IL-1β, IL-6, IL-8, PGE2, INF-γ, MCP-1, MIP-1α and vascular endothelial growth factor-a (VEGF-A),227–229 while Cr is believed to promote IL-1β, MCP-1, and MIP-1α.228,230 Promotion of cytokines by Mo includes IL-1β, IL-6, and TNF-α.231 Ti causes upregulation of cytokines, including IL-1β, IL-6, IL-8, TNF-α, and PGE2.232–234 Moreover, M-CSF and GM-CSF are promoted by Ti to induce the maturation and gathering of inflammatory cells.235 V upregulates TNF-α, IL-6, and IL-8 expression, while suppressing IFN-γ and IL-10 expression.236–239 Besides, common inflammatory cytokines such as IL-1β, IL-8, and TNF-α, Al promotes cytokines including MIP-1α.240,241 Figure 3 and Table 4 summarize the cytokine profiles that were upregulated. Contrarily, metals such as Co, Cr, and Ti are known to downregulate transforming growth factor-β (TGF-β), the factor that promotes anti-inflammation and type-I collagen synthesis.242,243
 |
Table 4 Inflammatory Cytokines, Chemokines and Signaling Related to Prosthetic Metals |
Toxicity to Biomolecules
Studies have shown that excess metals can cause molecular-level hazards such as structural damage to DNA and chromosome abnormalities (including aberrations, translocations, and aneuploidy), which can increase the risk of genotoxicity and carcinogenicity.268,269 In summary, the study of toxicology can be classified into three basic categories: i. metals binding directly to molecules and influencing their functions or structure; ii. metals generating free radicals and oxidative species that lead to significant damage; iii. metals interfering with signaling molecules in specific pathways.
The damage induced by direct combination mostly emerges in the ionic metals through the direct combination with negatively charged components due to ionic interactions. The toxicity is strengthened with the higher charges of the cations.270 Toxicity of bivalent Co (Co2+) is attributed to their notable capacity to interact with Zinc finger motifs, which mediates the interactions between proteins themselves and nucleic acids,271 thus resulting in DNA single-strand break, DNA repairing interruption, DNA-protein cross-linkage and sister chromatid exchange.272–276 The decreased function of the osteogenic-related alkaline phosphatase due to the direct substitution of Zn2+ by Co2+ has also been reported.277 Furthermore, the high affinity to sulfur atoms enables Co2+ to suppress the antioxidation of lipoic acid in mitochondria, subsequently blocking the citric acid cycle, the core of adenosine triphosphate (ATP) production,275 which has been evidenced by the Co-dose-dependent ATP depletion and the consequently decreased mitochondrial membrane potential.258 Trivalent Cr (Cr3+) can be directly combined with DNA to form a stable Cr3+-DNA admixture and DNA-DNA cross-linkage. Hence, the single or double-strand structure of nucleic acid is destroyed.278,279 As the Cr3+ concentration significantly increases, enzyme activity is directly inhibited, where the active center is restricted by Cr3+, leading to the more pronounced toxicity.280 The primary toxicology of ionic Ti is its direct combination with cellular structures, including phosphorylated proteins, phospholipids, and nucleotides,203 consequently hindering cellular functions. The combination of Trivalent Al (Al3+) with phospholipids, the chief component of the membrane of a cell or organelle, can render the peroxidation and alter membrane fluid dynamics.159 Once the mitochondrial membrane is affected, cytochrome c can be released, initiating cell apoptosis.159 V appears more as Vanadate and Vanadyl, rather than in the ionic form. The metallic salt is proven to be able to inactivate a series of bioligands, including Glutathione, ATP, adenosine diphosphate (ADP), nicotinamide adenine dinucleotide (NAD), amino acids and some enzymes (phosphatases and dynein ATPase), after the direct combination.239,281
Reactive oxygen species (ROS) is a group of oxygen-free radicals typically generated by neutrophils and macrophages during inflammation or by metal-catalyzed reactions.282 ROS can be hazardous if they overwhelm the body’s antioxidant protection, leading to interference with DNA bases, enhanced lipid peroxidation, and changes in calcium and sulfhydryl homeostasis.282 The generated hydroxyl radicals play a major role in later intensive oxidative damage to biomolecules (DNA, chromosomes, proteins, and polyunsaturated fatty acids) and cell organelles (such as mitochondria and lysosomes) due to the concentration-dependent result of Co2+,275 Cr3+,278,279,283 and Mo283 overload. Detectable elevation of superoxide dismutase (SOD)283 and catalase activity280 is further evidence of metal-induced oxidative stress caused by ROS. The hexavalent (Cr6+) form is often used for prosthetic fabrication.284 However, Cr6+ has a transient biological half-life due to its high potential energy, which tends to switch to the more stable Cr3+ form - The most common oxidation form found in humans. This means that the hexavalent form is likely to be reduced to trivalent swiftly by redox reactions in cells, potentially leading to intensive oxidation damage to biomolecules and organelles.285
For Ti-Al-V alloys, ROS is generated by Ti dioxide (TiO2) nanoparticles.286 The following oxidative stressing leads to repeated cell damage and proliferation, indirect DNA damage, and finally, abnormal cell growth behavior.287 Trivalent Al (Al3+)-associated with oxidative damage affects the mitochondria functioning,288 inducing p53 elevation, chromosome translocation and DNA fragmentation, followed by the activation of caspase-3 and caspase-12.159 This implies genomic instability, the hallmark of tumorigenesis and a prerequisite condition for malignant transformation.289 A series of oxide compounds of V (V2O5, V2O3, V2O4) and Vanadyl and Vanadate is the source of ROS, which is dose- or time-dependently.239,290–293 The consequences are upregulation of cyclooxygenase-2,294 activation of p21 and p53,295 and inhibition of a cyclin named Cdc25C,296 finally leading to abnormal anti-apoptotic effect, arrested cell cycle and improper cell growth. Additionally, increased DNA strands are broken and chromosomal damages involving aberration, sister chromatid exchange and aneuploidy also happen because of V oxide compounds.297–299
Metals, in addition to causing structural damage and inactivating functional biomolecules, can inhibit signaling networks. For instance, the elevation of Co can create a hypoxic environment, which can result in the regulation of the PI3K/AKT/mTOR pathway, leading to cell autophagy or proliferation.244,245 Moreover, Co-generated ROS can decrease macrophage motility by downregulating the RhoA signaling pathway.246 Cr can cause endoplasmic reticulum stress, mitochondrial dysfunction, and carcinogenicity via Ca2+/CaMK, SRC/Ras, and AMPK/PGC-1α.247–249 Mo can trigger DNA damage, the release of IL-6, and cell death through JNK, ERK, and AMPK signaling.250,251
Ti is responsible for PI3K/AKT/p38MAPK and NF-κB signaling, leading to cell autophagy or apoptosis.252,253 NF-κB controlled by PKCε and ERK1/2 stimulated by Ti has a pro-inflammatory effect.254 On the other hand, Ti can inhibit osteogenesis via several signaling, including GSK-3β/β-catenin, MAPK/JNK, and HIPPO/YAP.255–257 Abnormal activation of JNK and Ca2+/CaMK pathway in osteoblasts in the process of Al-associated cell apoptosis,259,260 plus the inhibition of osteogenesis via Al interfering with Wnt/β-catenin pathway,261 both enhance osteolysis. Furthermore, Al-associated dysfunctional mitochondria affect Ca2+-ATPase, hindering the Ca2+ influx.258,262 The obstacle on the Ca2+ channel triggers the altered Ca2+ signaling, leading to the generation of pro-apoptotic factors and cell death.263 Besides, Al-associated ROS also affects the PI3K pathways, with downstream effects on cell growth and proliferation and a close relationship to tumor development.264 In nerve tissue, Al induces inflammation and synaptic damage through DDX3X/N2RP3 and PHF8/H3K9me2/BDNF pathways.265,266 V can promote inflammation by elevating arachidonic acid via calcium-dependent PLA2,267 and on the other hand, by facilitating COX-2 synthesis through SRC or EGFR and the downstream PKC and MAPK.267 Besides, ROS generated by V also influences NF-κB.267
Conclusion and Perspective
Metallic prostheses have been developed and perfected over nearly a century, and are now used in joint replacements. However, the release of metals from Co-Cr-Mo and Ti-Al-V prostheses cannot be overlooked. Several studies have found a significant link between these metals and long-term adverse consequences of the implanted prosthesis. Metals released from implants can distribute in multiple organs and accumulate in surrounding tissues. While blood metals can be presented in various ways, significantly elevated blood metals have been detected in patients undergoing TKA and THA. High levels of metals can have considerable toxic and destructive effects on distributed organs and surrounding tissues. This adverse effect is linked to the disturbance of the inflammatory cascade and the dysfunction of critical cells and biomolecules.
Factors such as unstable implantation or a mismatch of prosthetic components can lead to an increase in metal concentration. The clinical application of digital technology may help solve problems and improve the curative effect. Robot technology is currently the most advanced representative of digital technologies. Although the current use of robot-assisted artificial joint replacements has some limitations, it represents the future development trend in joint surgery and is an inevitable result of industry advancement. Current hip and knee replacement surgery robots provide doctors with visual 3D preoperative planning, which allows for more precise operations. Robotic assistance can reduce human error and help ensure the accurate placement of the prosthesis during the operation, which can reduce the risk of postoperative complications. The use of this technology can also help achieve better soft tissue balance in knee joint replacement. Joint replacement prostheses may become highly personalized in the future, with support from imaging technology, 3D printing, computer technology, and relevant laws and regulations.
Improved prosthetic implant materials are a promising direction of research. Four main design factors have been identified: reducing the elastic modulus to better match that of human bone (~30 GPa), improving the biocompatibility and corrosion resistance of metal alloys, using non-toxic alloy elements (avoiding toxic Al and V), and enhancing the tensile and fatigue strength of titanium alloys. Personalized design, particularly for children and young adults, can prevent damage during implant removal by designing new titanium alloys that do not grow well into bone. In the future, joint replacement prostheses may be highly personalized, but this will require support from imaging technology, 3D printing, computer technology, relevant laws, and regulations.
Acknowledgments
This study was funded by the National Science Foundation of China (82272463), and Clinical Research Specialty Foundation of Nanfang Hospital, Southern Medical University (2018CR032).
Disclosure
The authors declare no competing interests in this work.
References
1. Mercuri LG. Alloplastic temporomandibular joint reconstruction. Oral Surg, Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):631–637. doi:10.1016/S1079-2104(98)90028-2
2. Makridis KG, Karachalios T. A Brief History of Total Knee Arthroplasty. J Med Case Rep. 2015.
3. Learmonth ID, Young C, Rorabeck C. The operation of the century: total Hip replacement. Lancet. 2007;370(9597):1508–1519. doi:10.1016/S0140-6736(07)60457-7
4. Markatos K, Savvidou OD, Foteinou A, et al. Hallmarks in the History and Development of Total Hip Arthroplasty. Surg Innov. 2020;27(6):691–694. doi:10.1177/1553350620947209
5. Mellon SJ, Liddle AD, Pandit H. Hip replacement: landmark surgery in modern medical history. Maturitas. 2013;75(3):221–226. doi:10.1016/j.maturitas.2013.04.011
6. Kovochich M, Finley BL, Novick R, et al. Understanding outcomes and toxicological aspects of second generation metal-on-metal Hip implants: a state-of-The-art review. Crit. Rev. Toxicol. 2018;48(10):853–901. doi:10.1080/10408444.2018.1563048
7. Ranawat CS. History of total knee replacement. J South Orthop Assoc. 2002;11(4):218–226.
8. Rizzo TD. Chapter 61 - Total Hip Replacement**Based on a chapter in the third edition written by Juan A. Cabrera, MD and Alison L. Cabrera, MD. In: Frontera WR, Silver JK, Rizzo TD, editors. Essentials of Physical Medicine and Rehabilitation (Fourth Edition). Philadelphia: Elsevier; 2020:337–345.
9. Hegde V, Stambough JB, Levine BR, Springer BD. Highlights of the 2022 American Joint Replacement Registry Annual Report. Arthroplast Today. 2023;21:101137. doi:10.1016/j.artd.2023.101137
10. Head WC, Bauk DJ, Emerson RH. Titanium as the material of choice for cementless femoral components in total Hip arthroplasty. Clin Orthop Relat Res. 1995;311:85–90.
11. Kaur M, Singh K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater Sci Eng C Mater Biol Appl. 2019;102:844–862.
12. Chen Q, Thouas GA. Metallic implant biomaterials. Mater Sci Eng. 2015;87:1–57.
13. Patel NR, Gohil PP. A review on biomaterials: scope, applications & human anatomy significance. Int J Emerging Technol Adv Eng. 2012;2(4):91–101.
14. Osamu W. What are Trace Elements? J Med Case Rep. 2018. doi:10.1186/s13256-018-1703-2
15. Berdanier CD, Dwyer JT, Feldman EB. Handbook of Nutrition and Food. CRC press; 2007.
16. Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Acad Nutr Diet. 2001;101(3):294.
17. Eastley R, Wilcock GK, Bucks RS. Vitamin B12 deficiency in dementia and cognitive impairment: the effects of treatment on neuropsychological function. Int j Geriatric Psychiatry. 2000;15(3):226–233. doi:10.1002/(SICI)1099-1166(200003)15:3<226::AID-GPS98>3.0.CO;2-K
18. Badmaev V, Prakash S, Majeed M. Vanadium: a review of its potential role in the fight against diabetes. J Altern Complementary Med. 1999;5(3):273–291. doi:10.1089/acm.1999.5.273
19. Beliaeva N, Gorodetskiĭ V, Tochilkin A, Golubev M, Semenova N, Kovel’man I. Vanadium compounds--a new class of therapeutic agents for the treatment of diabetes mellitus. Voprosy meditsinskoi khimii. 2000;46(4):344–360.
20. Anderson RA. Nutritional factors influencing the glucose/insulin system: chromium. J Am Coll Nutr. 1997;16(5):404–410. doi:10.1080/07315724.1997.10718705
21. Kimura K. Role of essential trace elements in the disturbance of carbohydrate metabolism. Nihon rinsho Japan j clin med. 1996;54(1):79–84.
22. Cefalu WT, Hu FB. Role of chromium in human health and in diabetes. Diabetes Care. 2004;27(11):2741–2751. doi:10.2337/diacare.27.11.2741
23. Chao E, Frassica F, Pritchard D, Moyer T. Metal ion release in patients with porous coated megaprostheses. Trans Orthop Res Soc. 1995;20:731.
24. Buettner KM, Valentine AM. Bioinorganic chemistry of titanium. Chem. Rev. 2012;112(3):1863–1881. doi:10.1021/cr1002886
25. Berk L, Burchenal JH, Castle WB. Erythropoietic Effect of Cobalt in Patients with or without Anemia. N Engl J Med. 1949;240(19):754–761. doi:10.1056/NEJM194905122401903
26. Simonsen LO, Brown AM, Harbak H, Kristensen BI, Bennekou P. Cobalt uptake and binding in human red blood cells. Blood Cells Mol. Dis. 2011;46(4):266–276. doi:10.1016/j.bcmd.2011.02.009
27. Schwarz G, Mendel RR, Ribbe MW. Molybdenum cofactors, enzymes and pathways. Nature. 2009;460(7257):839–847. doi:10.1038/nature08302
28. Schwarz G. Molybdenum cofactor and human disease. Curr. Opin. Chem. Biol. 2016;31:179–187. doi:10.1016/j.cbpa.2016.03.016
29. Vincent JB. Chromium: biological Relevance. Encyclopedia Inorganic Bioinorganic Chemistry. 2011.
30. Sahin K, Onderci M, Tuzcu M, et al. Effect of chromium on carbohydrate and lipid metabolism in a rat model of type 2 diabetes mellitus: the fat-fed, streptozotocin-treated rat. Metabolism. 2007;56(9):1233–1240. doi:10.1016/j.metabol.2007.04.021
31. Agency EP. Toxicological review of trivalent chromium. J Med. 1998.
32. Ortega R, Bresson C, Fraysse A, et al. Cobalt distribution in keratinocyte cells indicates nuclear and perinuclear accumulation and interaction with magnesium and zinc homeostasis. Toxicol Lett. 2009;188(1):26–32.
33. Czarnek K, Terpiłowska S, Siwicki AK. Selected aspects of the action of cobalt ions in the human body. Central-Eur J Immunol. 2015;40(2):236–242.
34. Springer. Vanadium. Netherlands: Dordrecht: Springer; 2012.
35. Malay Chatterjee SD, Chatterjeeand Kaushik Roy M. Vanadium in Biological Systems. Encyclopedia Metalloproteins. 2013.
36. Niinomi M. Recent metallic materials for biomedical applications. Metall Mater Trans A. 2002;33(3):477–486. doi:10.1007/s11661-002-0109-2
37. Shanmuganantha L, Baharudin A, Sulong AB, Shamsudin R, Ng MH. Prospect of Metal Ceramic (Titanium-Wollastonite) Composite as Permanent Bone Implants: a Narrative Review. Materials (Basel). 2021;14(2):2. doi:10.3390/ma14020277
38. Oldani C, Dominguez AJRAIA. Titanium as a Biomaterial for Implants. Phys Chem Chem Phys. 2012;218:149–162.
39. Metikos-Hukovic M, Kwokal A, Piljac J. The influence of niobium and vanadium on passivity of titanium-based implants in physiological solution. Biomaterials. 2003;24(21):3765–3775. doi:10.1016/S0142-9612(03)00252-7
40. Liao Y, Hoffman E, Wimmer M, Fischer A, Jacobs J, Marks L. CoCrMo metal-on-metal Hip replacements. Phys Chem Chem Phys. 2013;15(3):746–756. doi:10.1039/C2CP42968C
41. Virtanen S. Metal release mechanisms in Hip replacement. Acta orthopaedica. 2006;77(5):695–696. doi:10.1080/17453670610012854
42. Huber M, Reinisch G, Trettenhahn G, Zweymuller K, Lintner F. Presence of corrosion products and hypersensitivity-associated reactions in periprosthetic tissue after aseptic loosening of total Hip replacements with metal bearing surfaces. Acta Biomater. 2009;5(1):172–180. doi:10.1016/j.actbio.2008.07.032
43. Bijukumar DR, Salunkhe S, Zheng G, et al. Wear particles induce a new macrophage phenotype with the potential to accelerate material corrosion within total Hip replacement interfaces. Acta Biomater. 2020;101:586–597. doi:10.1016/j.actbio.2019.10.039
44. Gill HS, Grammatopoulos G, Adshead S, Tsialogiannis E, Tsiridis E. Molecular and immune toxicity of CoCr nanoparticles in MoM Hip arthroplasty. Trends Mol Med. 2012;18(3):145–155. doi:10.1016/j.molmed.2011.12.002
45. Heise G, Black CM, Smith R, Morrow BR, Mihalko WM. In vitro effects of macrophages on orthopaedic implant alloys and local release of metallic alloy components. Bone Joint j. 2020;102-B(7_Supple_B):116–121. doi:10.1302/0301-620X.102B7.BJJ-2019-1556.R1
46. Gallo J, Goodman SB, Konttinen YT, Wimmer MA, Holinka M. Osteolysis around total knee arthroplasty: a review of pathogenetic mechanisms. Acta Biomater. 2013;9(9):8046–8058. doi:10.1016/j.actbio.2013.05.005
47. Hallab NJ, Jacobs JJ. Chemokines Associated with Pathologic Responses to Orthopedic Implant Debris. Front Endocrinol (Lausanne). 2017;8:5. doi:10.3389/fendo.2017.00005
48. Merritt K, Brown SA. Biological Effects of Corrosion Products from Metals. Corrosion and degradation of implant materials: second symposium, ASTM International; 1985.
49. ALS - Clinical: aluminum, Serum. Available from: https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/8373.
50. TIS - Clinical: titanium, Serum. Available from: https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/89367.
51. CRS - Clinical: chromium, Serum. Available from: https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/8638.
52. COS - Clinical: cobalt, Serum. Available from: https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/80084.
53. MOWB - Clinical: molybdenum, Blood. Available from: https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/64272.
54. CRWB - Clinical: chromium, Blood. Available from: https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/65601.
55. COWB - Clinical: cobalt, Blood. Available from: https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/60355.
56. MOS - Clinical: molybdenum, Serum. Available from: https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/89270.
57. VS - Clinical: vanadium, Serum. Available from: https://ltig.lhsc.on.ca/?action=view_rec&test=Vanadium%2C%20Plasma.
58. VWB - Clinical: vanadium, Blood. Available from: https://ltig.lhsc.on.ca/?action=view_rec&test=Vanadium%2CWhole%20Blood.
59. ALWB - Clinical: aluminum, Blood. Available from: https://ltig.lhsc.on.ca/?action=view_rec&test=Aluminum%2C%20Whole%20Blood.
60. TIWB - Clinical: titanium, Blood. Available from: https://ltig.lhsc.on.ca/?action=view_rec&test=Titanium%2C%20Whole%20Blood.
61. Control, C. f. D. Prevention, Guidelines for Collecting and Handling Blood Lead Samples [Video]; 2004.
62. Smolders JM, Hol A, Rijnberg WJ, van Susante JL. Metal ion levels and functional results after either resurfacing Hip arthroplasty or conventional metal-on-metal Hip arthroplasty. Acta orthopaedica. 2011;82(5):559–566. doi:10.3109/17453674.2011.625533
63. Moroni A, Nocco E, Hoque M, et al. Cushion bearings versus large diameter head metal-on-metal bearings in total Hip arthroplasty: a short-term metal ion study. Arch Orthopaedic Trauma Surgery. 2012;132(1):123–129. doi:10.1007/s00402-011-1364-8
64. Schouten R, Malone AA, Frampton CM, Tiffen C, Hooper G. Five-year follow-up of a prospective randomised trial comparing ceramic-on-metal and metal-on-metal bearing surfaces in total Hip arthroplasty. Bone Joint j. 2017;99-b(10):1298–1303. doi:10.1302/0301-620X.99B10.BJJ-2016-0905.R1
65. Cadossi M, Mazzotti A, Baldini N, Giannini S, Savarino L. New couplings, old problems: is there a role for ceramic-on-metal Hip arthroplasty? J Biomed Mater Res Part B. 2016;104(1):204–209. doi:10.1002/jbm.b.33383
66. Engh CA, MacDonald SJ, Sritulanondha S, Korczak A, Naudie D, Engh C. Metal ion levels after metal-on-metal total Hip arthroplasty: a five-year, prospective randomized trial. J Bone Joint Surg Am Vol. 2014;96(6):448–455. doi:10.2106/JBJS.M.00164
67. Hailer NP, Blaheta RA, Dahlstrand H, Stark A. Elevation of circulating HLA DR(+) CD8(+) T-cells and correlation with chromium and cobalt concentrations 6 years after metal-on-metal Hip arthroplasty. Acta orthopaedica. 2011;82(1):6–12. doi:10.3109/17453674.2010.548028
68. Zijlstra WP, van der Veen HC, van den Akker-Scheek I, Zee MJ, Bulstra SK, van Raay JJ. Acetabular bone density and metal ions after metal-on-metal versus metal-on-polyethylene total Hip arthroplasty; short-term results. Hip International. 2014;24(2):136–143. doi:10.5301/hipint.5000087
69. Vendittoli PA, Rivière C, Roy AG, Barry J, Lusignan D, Lavigne M. Metal-on-metal Hip resurfacing compared with 28-mm diameter metal-on-metal total Hip replacement: a randomised study with six to nine years’ follow-up. Bone Joint j. 2013;95-b(11):1464–1473. doi:10.1302/0301-620X.95B11.31604
70. Smolders JM, Bisseling P, Hol A, Van Der Straeten C, Schreurs BW, van Susante JL. Metal ion interpretation in resurfacing versus conventional Hip arthroplasty and in whole blood versus serum. How should we interpret metal ion data. Hip International. 2011;21(5):587–595. doi:10.5301/HIP.2011.8643
71. Bisseling P, Smolders JM, Hol A, van Susante JL. Metal ion levels and functional results following resurfacing Hip arthroplasty versus conventional small-diameter metal-on-metal total Hip arthroplasty; a 3 to 5 year follow-up of a randomized controlled trial. J Arthroplasty. 2015;30(1):61–67. doi:10.1016/j.arth.2014.07.036
72. Smolders JM, Hol A, van Susante JL. Metal ion trend may be more predictive for malfunctioning metal-on-metal implants than a single measurement. Hip International. 2013;23(5):434–440. doi:10.5301/hipint.5000066
73. Ando W, Yasui H, Yamamoto K, et al. A comparison of the effect of large and small metal-on-metal bearings in total Hip arthroplasty on metal ion levels and the incidence of pseudotumour: a five-year follow-up of a previous report. Bone Joint j. 2018;100-b(8):1018–1024. doi:10.1302/0301-620X.100B8.BJJ-2018-0414.R1
74. Briggs TW, Hanna SA, Kayani B, et al. Metal-on-polyethylene versus metal-on-metal bearing surfaces in total Hip arthroplasty: a prospective randomised study investigating metal ion levels and chromosomal aberrations in peripheral lymphocytes. Bone Joint j. 2015;97-b(9):1183–1191. doi:10.1302/0301-620X.97B9.34824
75. Malviya A, Ramaskandhan JR, Bowman R, et al. What advantage is there to be gained using large modular metal-on-metal bearings in routine primary Hip replacement? A preliminary report of a prospective randomised controlled trial. J Bone Joint Surg Br Vol. 2011;93(12):1602–1609. doi:10.1302/0301-620X.93B12.27533
76. Hutt J, Lavigne M, Lungu E, Belzile E, Morin F, Vendittoli PA. Comparison of Whole-Blood Metal Ion Levels Among Four Types of Large-Head, Metal-on-Metal Total Hip Arthroplasty Implants: a Concise Follow-up, at Five Years, of a Previous Report. J Bone Joint Surg Am Vol. 2016;98(4):257–266. doi:10.2106/JBJS.O.00201
77. Lützner J, Hartmann A, Dinnebier G, Spornraft-Ragaller P, Hamann C, Kirschner S. Metal hypersensitivity and metal ion levels in patients with coated or uncoated total knee arthroplasty: a randomised controlled study. International Orthopaedics. 2013;37(10):1925–1931. doi:10.1007/s00264-013-2010-6
78. Postler A, Beyer F, Lützner C, Tille E, Lützner J. Similar outcome during short-term follow-up after coated and uncoated total knee arthroplasty: a randomized controlled study. Knee Surg Sports Traumatol Arthrosc. 2018;26(11):3459–3467. doi:10.1007/s00167-018-4928-0
79. Bistolfi A, Cimino A, Lee GC, et al. Does metal porosity affect metal ion release in blood and urine following total Hip arthroplasty? A short term study. Hip International. 2018;28(5):522–530. doi:10.1177/1120700018762167
80. Jantzen C, Jorgensen HL, Duus BR, Sporring SL, Lauritzen JB. Chromium and cobalt ion concentrations in blood and serum following various types of metal-on-metal Hip arthroplasties: a literature overview. Acta orthopaedica. 2013;84(3):229–236. doi:10.3109/17453674.2013.792034
81. Cui P, Jiang W, Fan M, Wan Y. Detection and influence factor of serum metal ions concentration level after resurfacing arthroplasty of the Hip. Chin J Reparative Reconst Surg. 2017;31(4):404–409. doi:10.7507/1002-1892.201608018
82. Lainiala O, Reito A, Jamsa P, Eskelinen A. Mild or moderate renal insufficiency does not increase circulating levels of cobalt and chromium in patients with metal-on-metal Hip arthroplasty. Bone Joint j. 2017;99-B(9):1147–1152. doi:10.1302/0301-620X.99B9.BJJ-2016-0773.R2
83. Manninen E, Lainiala O, Karsikas M, Reito A, Jamsa P, Eskelinen A. Do cobalt or chromium accumulate in metal-on-metal Hip arthroplasty patients who have mild, moderate, or severe renal insufficiency? Bone Joint j. 2021;103-B(7):1231–1237. doi:10.1302/0301-620X.103B7.BJJ-2020-0836.R2
84. Hartmann A, Hannemann F, Lutzner J, et al. Metal ion concentrations in body fluids after implantation of Hip replacements with metal-on-metal bearing--systematic review of clinical and epidemiological studies. PLoS One. 2013;8(8):e70359. doi:10.1371/journal.pone.0070359
85. Friesenbichler J, Maurer-Ertl W, Sadoghi P, Lovse T, Windhager R, Leithner A. Serum metal ion levels after rotating-hinge knee arthroplasty: comparison between a standard device and a megaprosthesis. Int Orthop. 2012;36(3):539–544. doi:10.1007/s00264-011-1317-4
86. Reiner T, Sorbi R, Muller M, et al. Blood Metal Ion Release After Primary Total Knee Arthroplasty: a Prospective Study. Orthop Surg. 2020;12(2):396–403. doi:10.1111/os.12591
87. Nuevo-Ordonez Y, Montes-Bayon M, Blanco-Gonzalez E, et al. Titanium release in serum of patients with different bone fixation implants and its interaction with serum biomolecules at physiological levels. Anal Bioanal Chem. 2011;401(9):2747–2754. doi:10.1007/s00216-011-5232-8
88. Alfrey AC. Aluminum metabolism. Kidney Int Suppl. 1986;18:S8–11.
89. Riihimaki V, Aitio A. Occupational exposure to aluminum and its biomonitoring in perspective. Crit. Rev. Toxicol. 2012;42(10):827–853. doi:10.3109/10408444.2012.725027
90. Barceloux DG. Vanadium. J Toxicol. 1999;37(2):265–278. doi:10.1081/clt-100102425
91. Rehder D. Vanadium. Its role for humans. Metal Ions Life Sci. 2013;13:139–169.
92. Dahlstrand H, Stark A, Wick MC, Anissian L, Hailer NP, Weiss RJ. Comparison of metal ion concentrations and implant survival after total Hip arthroplasty with metal-on-metal versus metal-on-polyethylene articulations. Acta orthopaedica. 2017;88(5):490–495. doi:10.1080/17453674.2017.1350370
93. Bosker BH, Ettema HB, Boomsma MF, Kollen BJ, Maas M, Verheyen CC. High incidence of pseudotumour formation after large-diameter metal-on-metal total Hip replacement: a prospective cohort study. J Bone Joint Surg Br Vol. 2012;94(6):755–761. doi:10.1302/0301-620X.94B6.28373
94. Omlor GW, Kretzer JP, Reinders J, et al. In vivo serum titanium ion levels following modular neck total Hip arthroplasty--10 year results in 67 patients. Acta Biomater. 2013;9(4):6278–6282. doi:10.1016/j.actbio.2012.12.001
95. Hahn M, Busse B, Procop M, Zustin J, Amling M, Katzer A. Cobalt deposition in mineralized bone tissue after metal-on-metal Hip resurfacing: quantitative mu-X-ray-fluorescence analysis of implant material incorporation in periprosthetic tissue. J Biomed Mater Res Part B. 2017;105(7):1855–1862. doi:10.1002/jbm.b.33667
96. Simonsen LO, Harbak H, Bennekou P. Cobalt metabolism and toxicology--a brief update. Sci Total Environ. 2012;432:210–215. doi:10.1016/j.scitotenv.2012.06.009
97. Katz SA, Salem H. The toxicology of chromium with respect to its chemical speciation: a review. J Appl Toxicol. 1993;13(3):217–224. doi:10.1002/jat.2550130314
98. Merritt K, Brown SA. Distribution of titanium and vanadium following repeated injection of high-dose salts. J Biomed Mater Res. 1995;29(10):1175–1178. doi:10.1002/jbm.820291003
99. Krewski D, Yokel RA, Nieboer E, et al. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J Toxicol Environ Health B Crit Rev. 2007;10 Suppl 1:1–269. doi:10.1080/10937400701597766
100. Peto MV. Aluminium and iron in humans: bioaccumulation, pathology, and removal. Rejuvenation Res. 2010;13(5):589–598. doi:10.1089/rej.2009.0995
101. Trevino S, Diaz A, Sanchez-Lara E, Sanchez-Gaytan BL, Perez-Aguilar JM, Gonzalez-Vergara E. Vanadium in Biological Action: chemical, Pharmacological Aspects, and Metabolic Implications in Diabetes Mellitus. Biol. Trace Elem. Res. 2019;188(1):68–98. doi:10.1007/s12011-018-1540-6
102. Dobbs HS, Minski MJ. Metal ion release after total Hip replacement. Biomaterials. 1980;1(4):193–198. doi:10.1016/0142-9612(80)90016-2
103. Jakobsen SS, Danscher G, Stoltenberg M, et al. Cobalt-chromium-molybdenum alloy causes metal accumulation and metallothionein up-regulation in rat liver and kidney. Basic Clin Pharmacol Toxicol. 2007;101(6):441–446. doi:10.1111/j.1742-7843.2007.00137.x
104. Rubio JC, Garcia-Alonso MC, Alonso C, et al. Determination of metallic traces in kidneys, livers, lungs and spleens of rats with metallic implants after a long implantation time. J Mater Sci Mater Med. 2008;19(1):369–375. doi:10.1007/s10856-007-3002-0
105. Golasik M, Herman M, Piekoszewski W. Toxicological aspects of soluble titanium - a review of in vitro and in vivo studies. Metallomics. 2016;8(12):1227–1242. doi:10.1039/C6MT00110F
106. Mukherjee B, Patra B, Mahapatra S, Banerjee P, Tiwari A, Chatterjee M. Vanadium--an element of atypical biological significance. Toxicol Lett. 2004;150(2):135–143. doi:10.1016/j.toxlet.2004.01.009
107. Hong F, Mu X, Ze Y, Li W, Zhou Y, Ji J. Damage to the Blood Brain Barrier Structure and Function from Nano Titanium Dioxide Exposure Involves the Destruction of Key Tight Junction Proteins in the Mouse Brain. J biomedical nanotechnol. 2021;17(6):1068–1078. doi:10.1166/jbn.2021.3083
108. Shelly S, Liraz Zaltsman S, Ben-Gal O, et al. Potential neurotoxicity of titanium implants: prospective, in-vivo and in-vitro study. Biomaterials. 2021;276:121039. doi:10.1016/j.biomaterials.2021.121039
109. Shi H, Magaye R, Castranova V, Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 2013;10(1):15. doi:10.1186/1743-8977-10-15
110. Sun L, Wang K, Li Y, Fan Q, Zheng W, Li H. Vanadium exposure-induced striatal learning and memory alterations in rats. Neurotoxicology. 2017;62:124–129. doi:10.1016/j.neuro.2017.06.008
111. Fatola OI, Olaolorun FA, Olopade FE, Olopade JO. Trends in vanadium neurotoxicity. Brain Res Bull. 2019;145:75–80. doi:10.1016/j.brainresbull.2018.03.010
112. Harrison-Brown M, Scholes C, Field C, et al. Limited penetration of cobalt and chromium ions into the cerebrospinal fluid following metal on metal arthroplasty: a cross-sectional analysis. Clin toxicol. 2020;58(4):233–240. doi:10.1080/15563650.2019.1636993
113. Gomez-Arnaiz S, Tate RJ, Grant MH. Cobalt Neurotoxicity: transcriptional Effect of Elevated Cobalt Blood Levels in the Rodent Brain. Toxics. 2022;10(2):2. doi:10.3390/toxics10020059
114. Salama A, Hegazy R, Hassan A. Intranasal Chromium Induces Acute Brain and Lung Injuries in Rats: assessment of Different Potential Hazardous Effects of Environmental and Occupational Exposure to Chromium and Introduction of a Novel Pharmacological and Toxicological Animal Model. PLoS One. 2016;11(12):e0168688. doi:10.1371/journal.pone.0168688
115. Woodman JL, Jacobs JJ, Galante JO, Urban RM. Metal ion release from titanium-based prosthetic segmental replacements of long bones in baboons: a long-term study. J Orthop Res. 1984;1(4):421–430. doi:10.1002/jor.1100010411
116. Afolaranmi GA, Akbar M, Brewer J, Grant MH. Distribution of metal released from cobalt-chromium alloy orthopaedic wear particles implanted into air pouches in mice. J Biomed Mater Res Part A. 2012;100(6):1529–1538. doi:10.1002/jbm.a.34091
117. Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc’h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with Hip or knee replacement. J Bone Joint Surg Am Vol. 2000;82(4):457–476. doi:10.2106/00004623-200004000-00002
118. Urban RM, Tomlinson MJ, Hall DJ, Jacobs JJ. Accumulation in liver and spleen of metal particles generated at nonbearing surfaces in Hip arthroplasty. J Arthroplasty. 2004;19(8 Suppl 3):94–101. doi:10.1016/j.arth.2004.09.013
119. Hethey C, Hartung N, Wangorsch G, Weisser K, Huisinga W. Physiology-based toxicokinetic modelling of aluminium in rat and man. Arch Toxicol. 2021;95(9):2977–3000. doi:10.1007/s00204-021-03107-y
120. Hernandez-Vaquero D, Rodriguez de la Flor M, Fernandez-Carreira JM, Sariego-Muniz C. Detection of metal ions in hair after metal-metal Hip arthroplasty. Revista espanola de cirugia ortopedica y traumatologia. 2014;58(5):267–273. doi:10.1016/j.recot.2014.01.008
121. Rodriguez de la Flor M, Hernandez-Vaquero D, Fernandez-Carreira JM. Metal presence in hair after metal-on-metal resurfacing arthroplasty. J Orthop Res. 2013;31(12):2025–2031. doi:10.1002/jor.22450
122. Zywiel MG, Brandt JM, Overgaard CB, Cheung AC, Turgeon TR, Syed KA. Fatal cardiomyopathy after revision total Hip replacement for fracture of a ceramic liner. Bone Joint j. 2013;95-B(1):31–37. doi:10.1302/0301-620X.95B1.30060
123. Matusiewicz H. Potential release of in vivo trace metals from metallic medical implants in the human body: from ions to nanoparticles--a systematic analytical review. Acta Biomater. 2014;10(6):2379–2403. doi:10.1016/j.actbio.2014.02.027
124. Zeiner M, Zenz P, Lintner F, Schuster E, Schwagerl W, Steffan I. Influence on elemental status by Hip-endoprostheses. Microchem J. 2007;85(1):145–148. doi:10.1016/j.microc.2006.04.019
125. Mertz W. Chromium occurrence and function in biological systems. Physiol Rev. 1969;49(2):163–239. doi:10.1152/physrev.1969.49.2.163
126. Turnlund JR, Keyes WR, Peiffer GL, Chiang G. Molybdenum absorption, excretion, and retention studied with stable isotopes in young men during depletion and repletion. The American Journal of Clinical Nutrition. 1995;61(5):1102–1109. doi:10.1093/ajcn/61.5.1102
127. Rodushkin I, Engstrom E, Stenberg A, Baxter DC. Determination of low-abundance elements at ultra-trace levels in urine and serum by inductively coupled plasma-sector field mass spectrometry. Anal Bioanal Chem. 2004;380(2):247–257. doi:10.1007/s00216-004-2742-7
128. Catalani S, Stea S, Beraudi A, et al. Vanadium release in whole blood, serum and urine of patients implanted with a titanium alloy Hip prosthesis. Clin toxicol. 2013;51(7):550–556. doi:10.3109/15563650.2013.818682
129. Sarmiento-González A, Marchante-Gayón JM, Tejerina-Lobo JM. High-resolution ICP-MS determination of Ti, V, Cr, Co, Ni, and Mo in human blood and urine of patients implanted with a Hip or knee prosthesis. Anal Bioanal Chem. 2008;391(7):2583–2589. doi:10.1007/s00216-008-2188-4
130. Takai S, Yoshino N, Kusaka Y, Watanabe Y, Hirasawa Y. Dissemination of metals from a failed patellar component made of titanium-base alloy. J Arthroplasty. 2003;18(7):931–935. doi:10.1016/S0883-5403(03)00277-8
131. La Barbera L, D’Apolito R, Peretti GM, Piergiovanni M, Banfi G, Zagra L; Xix Congresso Nazionale S.I.C.O.O.P. Societa’ Italiana Chirurghi Ortopedici Dell’Ospedalita’ Privata, A. Modular implant design affects metal ion release following metal-on-metal Hip arthroplasty: a retrospective study on 75 cases. J Biol Regul Homeost Agents. 2019;33(2 Suppl. 1):79–88.
132. Reiner T, Bader N, Panzram B, et al. In vivo blood metal ion levels in patients after total shoulder arthroplasty. J Shoulder Elbow Surgery. 2019;28(3):539–546. doi:10.1016/j.jse.2018.08.027
133. Pernaa K, Saltychev M, Makela K. Relationship between Pelvic Incidence Angle and Blood Concentration of Chromium and Cobalt Ions after Metal-on-Metal Hip Replacement: a Brief Report. Scand J Surg. 2018;107(1):91–94. doi:10.1177/1457496917731182
134. Kasparek MF, Renner L, Faschingbauer M, Waldstein W, Weber M, Boettner F. Predictive factors for metal ion levels in metal-on-metal total Hip arthroplasty. Arch Orthopaedic Trauma Surgery. 2018;138(2):281–286. doi:10.1007/s00402-017-2856-y
135. Wyles CC, Wright TC, Bois MC, et al. Myocardial Cobalt Levels Are Elevated in the Setting of Total Hip Arthroplasty. J Bone Joint Surg Am Vol. 2017;99(22):e118. doi:10.2106/JBJS.17.00159
136. Ohtsuru T, Morita Y, Murata Y, Shimamoto S, Munakata Y, Kato Y. Blood metal ion concentrations in metal-on-metal total Hip arthroplasty. Eurn J of Orthop Surg and Traumatol. 2017;27(4):527–532. doi:10.1007/s00590-017-1931-y
137. Nam D, Salih R, Brown KM, Nunley RM, Barrack RL. Metal Ion Levels in Young, Active Patients Receiving a Modular, Dual Mobility Total Hip Arthroplasty. J Arthroplasty. 2017;32(5):1581–1585. doi:10.1016/j.arth.2016.12.012
138. Milosev I, Levasic V, Vidmar J, Kovac S, Trebse R. pH and metal concentration of synovial fluid of osteoarthritic joints and joints with metal replacements. J Biomed Mater Res Part B. 2017;105(8):2507–2515. doi:10.1002/jbm.b.33793
139. Lehtovirta L, Reito A, Parkkinen J, et al. Analysis of bearing wear, whole blood and synovial fluid metal ion concentrations and histopathological findings in patients with failed ASR Hip resurfacings. BMC Musculoskeletal Disorders. 2017;18(1):523. doi:10.1186/s12891-017-1894-5
140. Smith T, Edmonds CJ, Barnaby CF. Absorption and retention of cobalt in man by whole-body counting. Health Phys. 1972;22(4):359–367. doi:10.1097/00004032-197204000-00007
141. Clark NA, Teschke K, Rideout K, Copes R. Trace element levels in adults from the west coast of Canada and associations with age, gender, diet, activities, and levels of other trace elements. Chemosphere. 2007;70(1):155–164. doi:10.1016/j.chemosphere.2007.06.038
142. Barceloux DG. Molybdenum. J Toxicol. 1999;37(2):231–237. doi:10.1081/clt-100102422
143. Barceloux DG. Chromium. J Toxicol. 1999;37(2):173–194. doi:10.1081/clt-100102418
144. Tripathi D, Mani V, Pal RP. Vanadium in Biosphere and Its Role in Biological Processes. Biol. Trace Elem. Res. 2018;186(1):52–67. doi:10.1007/s12011-018-1289-y
145. Leggett RW. The biokinetics of inorganic cobalt in the human body. Sci Total Environ. 2008;389(2–3):259–269. doi:10.1016/j.scitotenv.2007.08.054
146. Hong F, Yu X, Wu N, Zhang YQ. Progress of in vivo studies on the systemic toxicities induced by titanium dioxide nanoparticles. Toxicol Res (Camb). 2017;6(2):115–133. doi:10.1039/C6TX00338A
147. Aguilar F, Autrup H, Barlow S, et al. Safety of aluminium from dietary intake scientific opinion of the panel on food additives, flavourings, processing aids and food contact materials (AFC). J Med. 2008;754:1–34.
148. Fleshman DG, Silva AJ, Shore B. The metabolism of tantalum in the rat. Health Phys. 1971;21(3):385–392. doi:10.1097/00004032-197109000-00004
149. Ho JH, Leikin JB, Dargan PI, Archer JRH, Wood DM, Brent J. Metal-on-Metal Hip Joint Prostheses: a Retrospective Case Series Investigating the Association of Systemic Toxicity with Serum Cobalt and Chromium Concentrations. J Med Toxicol. 2017;13(4):321–328. doi:10.1007/s13181-017-0629-1
150. Ikeda T, Takahashi K, Kabata T, Sakagoshi D, Tomita K, Yamada M. Polyneuropathy caused by cobalt-chromium metallosis after total Hip replacement. Muscle and Nerve. 2010;42(1):140–143. doi:10.1002/mus.21638
151. Gilbert CJ, Cheung A, Butany J, et al. Hip pain and heart failure: the missing link. Can J Cardiol. 2013;29(5):639 e1–2. doi:10.1016/j.cjca.2012.10.015
152. Bradberry SM, Wilkinson JM, Ferner RE. Systemic toxicity related to metal Hip prostheses. Clin toxicol. 2014;52(8):837–847. doi:10.3109/15563650.2014.944977
153. Kavanagh KT, Kraman SS, Kavanagh SP. An Analysis of the FDA MAUDE Database and the Search for Cobalt Toxicity in Class 3 Johnson & Johnson/DePuy Metal-on-Metal Hip Implants. J Patient Saf. 2018;14(4):e89–e96. doi:10.1097/PTS.0000000000000534
154. Peters K, Unger RE, Barth S, Gerdes T, Kirkpatrick CJ. Induction of apoptosis in human microvascular endothelial cells by divalent cobalt ions. Evidence for integrin-mediated signaling via the cytoskeleton. J Mater Sci Mater Med. 2001;12(10–12):955–958. doi:10.1023/A:1012852814570
155. Prescott E, Netterstrom B, Faber J, Hegedus L, Suadicani P, Christensen JM. Effect of occupational exposure to cobalt blue dyes on the thyroid volume and function of female plate painters. Scand J Work Environ Health. 1992;18(2):101–104. doi:10.5271/sjweh.1605
156. Holly RG. Studies on iron and cobalt metabolism. Journal of the American Medical Association. 1955;158(15):1349–1352. doi:10.1001/jama.1955.02960150019005
157. Barceloux DG. Cobalt. J Toxicol. 1999;37(2):201–206. doi:10.1081/clt-100102420
158. Parkinson I, Ward M, Kerr D. Dialysis encephalopathy, bone disease and anaemia: the aluminum intoxication syndrome during regular haemodialysis. J Clin Pathol. 1981;34(11):1285. doi:10.1136/jcp.34.11.1285
159. Willhite CC, Karyakina NA, Yokel RA, et al. Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts. Crit. Rev. Toxicol. 2014;44 Suppl 4(Suppl 4):1–80. doi:10.3109/10408444.2014.934439
160. Domingo JL. Vanadium: a review of the reproductive and developmental toxicity. Reprod Toxicol. 1996;10(3):175–182. doi:10.1016/0890-6238(96)00019-6
161. Peat F, Coomber R, Rana A, Vince A. Vanadium allergy following total knee arthroplasty. BMJ Case Rep. 2018;2018:548.
162. Granchi D, Cenni E, Tigani D, Trisolino G, Baldini N, Giunti A. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials. 2008;29(10):1494–1500. doi:10.1016/j.biomaterials.2007.11.038
163. Moretti B, Pesce V, Maccagnano G, et al. Peripheral neuropathy after Hip replacement failure: is vanadium the culprit? Lancet. 2012;379(9826):1676. doi:10.1016/S0140-6736(12)60273-6
164. Cancilleri F, De Giorgis P, Verdoia C, Parrini L, Lodi A, Crosti C. Allergy to components of total Hip arthroplasty before and after surgery. Ital J Orthop Traumatol. 1992;18(3):407–410.
165. Smith D, Pickering R. A systematic review of vanadium oral supplements for glycaemic control in type 2 diabetes mellitus. QJM. 2008;101(5):351–358. doi:10.1093/qjmed/hcn003
166. Barceloux DG, Barceloux D. Vanadium. J Toxicol. 1999;37(2):265–278.
167. Vyskočil A, Viau C. Assessment of molybdenum toxicity in humans. J Appl Toxicol. 1999;19(3):185–192. doi:10.1002/(SICI)1099-1263(199905/06)19:3<185::AID-JAT555>3.0.CO;2-Z
168. Lewis RC, Johns LE, Meeker JD. Exploratory analysis of the potential relationship between urinary molybdenum and bone mineral density among adult men and women from NHANES 2007–2010. Chemosphere. 2016;164:677–682. doi:10.1016/j.chemosphere.2016.08.142
169. Parry NM, Phillippo M, Reid MD, McGaw BA, Flint DJ, Loveridge N. Molybdenum-induced changes in the epiphyseal growth plate. Calcified Tissue Int. 1993;53(3):180–186. doi:10.1007/BF01321835
170. Meeker JD, Rossano MG, Protas B, et al. Cadmium, lead, and other metals in relation to semen quality: human evidence for molybdenum as a male reproductive toxicant. Environ. Health Perspect. 2008;116(11):1473–1479. doi:10.1289/ehp.11490
171. Meeker JD, Rossano MG, Protas B, et al. Environmental exposure to metals and male reproductive hormones: circulating testosterone is inversely associated with blood molybdenum. Fertil Sterility. 2010;93(1):130–140. doi:10.1016/j.fertnstert.2008.09.044
172. Ribeiro AM, Flores-Sahagun THS, Paredes RC. A perspective on molybdenum biocompatibility and antimicrobial activity for applications in implants. J Mater Sci. 2015;51(6):2806–2816. doi:10.1007/s10853-015-9664-y
173. Thomas WC, Prieto HA. Total Hip replacement failure due to adverse local tissue reaction from both ceramic abrasive wear and trunnion corrosion. Arthroplast Today. 2019;5(4):384–388.
174. Cadossi M, Chiarello E, Savarino L, et al. Fast growing pseudotumour in a hairdresser after metal-on-metal Hip resurfacing: a case report. Eur. Rev. Med. Pharmacol. Sci. 2014;18(1 Suppl):29–33.
175. Grote CW, Cowan PC, Anderson DW, Templeton KJ. Pseudotumor from Metal-on-Metal Total Hip Arthroplasty Causing Unilateral Leg Edema: case Presentation and Literature Review. Biores Open Access. 2018;7(1):33–38. doi:10.1089/biores.2017.0035
176. Lainiala O, Eskelinen A, Elo P, Puolakka T, Korhonen J, Moilanen T. Adverse reaction to metal debris is more common in patients following MoM total Hip replacement with a 36 mm femoral head than previously thought: results from a modern MoM follow-up programme. Bone Joint j. 2014;96-B(12):1610–1617. doi:10.1302/0301-620X.96B12.33742
177. Czajka M, Sawicki K, Sikorska K, Popek S, Kruszewski M, Kapka-Skrzypczak L. Toxicity of titanium dioxide nanoparticles in central nervous system. Toxicol in vitro. 2015;29(5):1042–1052. doi:10.1016/j.tiv.2015.04.004
178. Hou J, Wang L, Wang C, et al. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J Environ Sci (China). 2019;75:40–53. doi:10.1016/j.jes.2018.06.010
179. Miller RA, Ro JY, Schwartz MR. Adverse tissue reactions after total Hip arthroplasty. Ann Diagn Pathol. 2017;27:83–87. doi:10.1016/j.anndiagpath.2016.07.006
180. Wu D, Bhalekar RM, Marsh JS, Langton DJ, Stewart AJ. Periarticular metal hypersensitivity complications of Hip bearings containing cobalt-chromium. EFORT Open Rev. 2022;7(11):758–771. doi:10.1530/EOR-22-0036
181. Rajamaki A, Lehtovirta L, Niemelainen M, et al. Mild aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL)-type reactions also present in patients with failed knee prostheses. Bone Joint Res. 2024;13(4):149–156. doi:10.1302/2046-3758.134.BJR-2023-0255.R1
182. Cohen MD, Kargacin B, Klein CB, Costa M. Mechanisms of chromium carcinogenicity and toxicity. Crit. Rev. Toxicol. 1993;23(3):255–281. doi:10.3109/10408449309105012
183. Gad SC. Acute and chronic systemic chromium toxicity. Sci Total Environ. 1989;86(1–2):149–157. doi:10.1016/0048-9697(89)90201-5
184. Pavesi T, Moreira JC. Mechanisms and individuality in chromium toxicity in humans. J Appl Toxicol. 2020;40(9):1183–1197. doi:10.1002/jat.3965
185. Wang Y, Su H, Gu Y, Song X, Zhao J. Carcinogenicity of chromium and chemoprevention: a brief update. Onco Targets Ther. 2017;10:4065–4079. doi:10.2147/OTT.S139262
186. Chen QY, DesMarais T, Costa M. Metals and Mechanisms of Carcinogenesis. Annu Rev Pharmacol Toxicol. 2019;59(1):537–554. doi:10.1146/annurev-pharmtox-010818-021031
187. Gomes CC, Moreira LM, Santos VJ, et al. Assessment of the genetic risks of a metallic alloy used in medical implants. Genetics Mol Biol. 2011;34(1):116–121. doi:10.1590/S1415-47572010005000118
188. Huang C, Sun M, Yang Y, et al. Titanium dioxide nanoparticles prime a specific activation state of macrophages. Nanotoxicology. 2017;11(6):737–750. doi:10.1080/17435390.2017.1349202
189. Santos Filho RD, Vicari T, Santos SA, et al. Genotoxicity of titanium dioxide nanoparticles and triggering of defense mechanisms in Allium cepa. Genet Mol Biol. 2019;42(2):425–435. doi:10.1590/1678-4685-gmb-2018-0205
190. Wakeman TP, Yang A, Dalal NS, et al. DNA mismatch repair protein Mlh1 is required for tetravalent chromium intermediate-induced DNA damage. Oncotarget. 2017;8(48):83975–83985. doi:10.18632/oncotarget.20150
191. Papis E, Gornati R, Prati M, Ponti J, Sabbioni E, Bernardini G. Gene expression in nanotoxicology research: analysis by differential display in BALB3T3 fibroblasts exposed to cobalt particles and ions. Toxicol Lett. 2007;170(3):185–192. doi:10.1016/j.toxlet.2007.03.005
192. Lison D, De Boeck M, Verougstraete V, Kirsch-Volders M. Update on the genotoxicity and carcinogenicity of cobalt compounds. Occup Environ Med. 2001;58(10):619–625. doi:10.1136/oem.58.10.619
193. De Boeck M, Kirsch-Volders M, Lison D. Cobalt and antimony: genotoxicity and carcinogenicity. Mutat Res. 2003;533(1–2):135–152. doi:10.1016/j.mrfmmm.2003.07.012
194. Magaye R, Zhao J, Bowman L, Ding M. Genotoxicity and carcinogenicity of cobalt-, nickel- and copper-based nanoparticles. Exp Ther Med. 2012;4(4):551–561. doi:10.3892/etm.2012.656
195. Behl M, Stout MD, Herbert RA, et al. Comparative toxicity and carcinogenicity of soluble and insoluble cobalt compounds. Toxicology. 2015;333:195–205. doi:10.1016/j.tox.2015.04.008
196. Savarino L, Fotia C, Roncuzzi L, et al. Does chronic raise of metal ion levels induce oxidative DNA damage and hypoxia-like response in patients with metal-on-metal Hip resurfacing? J Biomed Mater Res Part B. 2017;105(2):460–466. doi:10.1002/jbm.b.33555
197. Christian WV, Oliver LD, Paustenbach DJ, Kreider ML, Finley BL. Toxicology-based cancer causation analysis of CoCr-containing Hip implants: a quantitative assessment of genotoxicity and tumorigenicity studies. J Appl Toxicol. 2014;34(9):939–967. doi:10.1002/jat.3039
198. Ollivere B, Wimhurst JA, Clark IM, Donell ST. Current concepts in osteolysis. J Bone Joint Surg Br Vol. 2012;94(1):10–15. doi:10.1302/0301-620X.94B1.28047
199. Purdue PE, Koulouvaris P, Nestor BJ, Sculco TP. The central role of wear debris in periprosthetic osteolysis. HSS J. 2006;2(2):102–113. doi:10.1007/s11420-006-9003-6
200. Sansone V, Pagani D, Melato M. The effects on bone cells of metal ions released from orthopaedic implants. A review. Clin Cases Miner Bone Metab. 2013;10(1):34–40. doi:10.11138/ccmbm/2013.10.1.034
201. Kudo O, Sabokbar A, Pocock A, Itonaga I, Fujikawa Y, Athanasou NA. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone. 2003;32(1):1–7. doi:10.1016/S8756-3282(02)00915-8
202. Niki Y, Matsumoto H, Suda Y, et al. Metal ions induce bone-resorbing cytokine production through the redox pathway in synoviocytes and bone marrow macrophages. Biomaterials. 2003;24(8):1447–1457. doi:10.1016/S0142-9612(02)00531-8
203. Cadosch D, Al-Mushaiqri MS, Gautschi OP, Meagher J, Simmen HP, Filgueira L. Biocorrosion and uptake of titanium by human osteoclasts. J Biomed Mater Res Part A. 2010;95(4):1004–1010. doi:10.1002/jbm.a.32914
204. Kim BJ, Koh JM. Coupling factors involved in preserving bone balance. Cell Mol Life Sci. 2019;76(7):1243–1253. doi:10.1007/s00018-018-2981-y
205. Vermes C, Glant TT, Hallab NJ, Fritz EA, Roebuck KA, Jacobs JJ. The potential role of the osteoblast in the development of periprosthetic osteolysis: review of in vitro osteoblast responses to wear debris, corrosion products, and cytokines and growth factors. J Arthroplasty. 2001;16(8 Suppl 1):95–100. doi:10.1054/arth.2001.28719
206. Hallab NJ. Concentration- and composition-dependent effects of metal ions on human MG-63 osteoblasts. J Biomed Mater Res. 2002;60(3):420–433. doi:10.1002/jbm.10106
207. Vermes C, Chandrasekaran R, Jacobs JJ, Galante JO, Roebuck KA, Glant TT. The effects of particulate wear debris, cytokines, and growth factors on the functions of MG-63 osteoblasts. J Bone Joint Surg Am Vol. 2001;83(2):201–211. doi:10.2106/00004623-200102000-00007
208. Rakow A, Schoon J, Dienelt A, et al. Influence of particulate and dissociated metal-on-metal Hip endoprosthesis wear on mesenchymal stromal cells in vivo and in vitro. Biomaterials. 2016;98:31–40. doi:10.1016/j.biomaterials.2016.04.023
209. Roebuck KA, Vermes C, Carpenter LR, Fritz EA, Narayanan R, Glant TT. Down-regulation of procollagen alpha1[I]] messenger RNA by titanium particles correlates with nuclear factor kappaB (NF-kappaB) activation and increased rel A and NF-kappaB1 binding to the collagen promoter. J Bone Miner Res. 2001;16(3):501–510. doi:10.1359/jbmr.2001.16.3.501
210. Vermes C, Roebuck KA, Chandrasekaran R, Dobai JG, Jacobs JJ, Glant TT. Particulate wear debris activates protein tyrosine kinases and nuclear factor kappaB, which down-regulates type I collagen synthesis in human osteoblasts. J Bone Miner Res. 2000;15(9):1756–1765. doi:10.1359/jbmr.2000.15.9.1756
211. Ormsby RT, Solomon LB, Yang D, et al. Osteocytes respond to particles of clinically-relevant conventional and cross-linked polyethylene and metal alloys by up-regulation of resorptive and inflammatory pathways. Acta Biomater. 2019;87:296–306. doi:10.1016/j.actbio.2019.01.047
212. Minematsu H, Shin MJ, Celil AA, et al. Orthopedic implant particle-induced tumor necrosis factor-alpha production in macrophage-monocyte lineage cells is mediated by nuclear factor of activated T cells. Ann N Y Acad Sci. 2007;1117:143–150. doi:10.1196/annals.1402.026
213. Orhue V, Kanaji A, Caicedo MS, et al. Calcineurin/nuclear factor of activated T cells (NFAT) signaling in cobalt-chromium-molybdenum (CoCrMo) particles-induced tumor necrosis factor-alpha (TNFalpha) secretion in MLO-Y4 osteocytes. J Orthop Res. 2011;29(12):1867–1873. doi:10.1002/jor.21458
214. Kanaji A, Caicedo MS, Virdi AS, Sumner DR, Hallab NJ, Sena K. Co-Cr-Mo alloy particles induce tumor necrosis factor alpha production in MLO-Y4 osteocytes: a role for osteocytes in particle-induced inflammation. Bone. 2009;45(3):528–533. doi:10.1016/j.bone.2009.05.020
215. Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26(11):1271–1286. doi:10.1016/j.biomaterials.2004.04.035
216. Sun L, Blair HC, Peng Y, et al. Calcineurin regulates bone formation by the osteoblast. Proc Natl Acad Sci USA. 2005;102(47):17130–17135. doi:10.1073/pnas.0508480102
217. Winslow MM, Pan M, Starbuck M, et al. Calcineurin/NFAT signaling in osteoblasts regulates bone mass. Dev Cell. 2006;10(6):771–782. doi:10.1016/j.devcel.2006.04.006
218. Yamanaka Y, Abu-Amer W, Foglia D, Otero J, Clohisy JC, Abu-Amer Y. NFAT2 is an essential mediator of orthopedic particle-induced osteoclastogenesis. J Orthop Res. 2008;26(12):1577–1584. doi:10.1002/jor.20714
219. Celil AA, Minematsu H, Gardner TR, Kim KO, Ahn JM, Lee FY. Nuclear factor of activated T cells mediates fluid shear stress- and tensile strain-induced Cox2 in human and murine bone cells. Bone. 2010;46(1):167–175. doi:10.1016/j.bone.2009.08.061
220. Magone K, Luckenbill D, Goswami T. Metal ions as inflammatory initiators of osteolysis. Arch Orthopaedic Trauma Surgery. 2015;135(5):683–695. doi:10.1007/s00402-015-2196-8
221. Gallo J, Slouf M, Goodman SB. The relationship of polyethylene wear to particle size, distribution, and number: a possible factor explaining the risk of osteolysis after Hip arthroplasty. J Biomed Mater Res Part B. 2010;94(1):171–177. doi:10.1002/jbm.b.31638
222. Gallo J, Raska M, Mrazek F, Petrek M. Bone remodeling, particle disease and individual susceptibility to periprosthetic osteolysis. Physiol Res. 2008;57(3):339–349. doi:10.33549/physiolres.931140
223. Goodman SB, Ma T. Cellular chemotaxis induced by wear particles from joint replacements. Biomaterials. 2010;31(19):5045–5050. doi:10.1016/j.biomaterials.2010.03.046
224. Gallo J, Kaminek P, Ticha V, Rihakova P, Ditmar R. Particle disease. A comprehensive theory of periprosthetic osteolysis: a review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2002;146(2):21–28. doi:10.5507/bp.2002.004
225. Merkel KD, Erdmann JM, McHugh KP, Abu-Amer Y, Ross FP, Teitelbaum SL. Tumor necrosis factor-alpha mediates orthopedic implant osteolysis. Am J Pathol. 1999;154(1):203–210. doi:10.1016/S0002-9440(10)65266-2
226. Catelas I, Petit A, Zukor DJ, Antoniou J, Huk OL. TNF-alpha secretion and macrophage mortality induced by cobalt and chromium ions in vitro-qualitative analysis of apoptosis. Biomaterials. 2003;24(3):383–391. doi:10.1016/S0142-9612(02)00351-4
227. Queally JM, Devitt BM, Butler JS, et al. Cobalt ions induce chemokine secretion in primary human osteoblasts. J Orthop Res. 2009;27(7):855–864. doi:10.1002/jor.20837
228. Eltit F, Noble J, Sharma M, et al. Cobalt ions induce metabolic stress in synovial fibroblasts and secretion of cytokines/chemokines that may be diagnostic markers for adverse local tissue reactions to Hip implants. Acta Biomater. 2021;131:581–594. doi:10.1016/j.actbio.2021.06.039
229. Liu G, Wang X, Zhou X, et al. Modulating the cobalt dose range to manipulate multisystem cooperation in bone environment: a strategy to resolve the controversies about cobalt use for orthopedic applications. Theranostics. 2020;10(3):1074–1089. doi:10.7150/thno.37931
230. Baskey SJ, Lehoux EA, Catelas I. Effects of cobalt and chromium ions on lymphocyte migration. J Orthop Res. 2017;35(4):916–924. doi:10.1002/jor.23336
231. Caicedo MS, Pennekamp PH, McAllister K, Jacobs JJ, Hallab NJ. Soluble ions more than particulate cobalt-alloy implant debris induce monocyte costimulatory molecule expression and release of proinflammatory cytokines critical to metal-induced lymphocyte reactivity. J Biomed Mater Res Part A. 2010;93(4):1312–1321. doi:10.1002/jbm.a.32627
232. Schwab LP, Marlar J, Hasty KA, Smith RA. Macrophage response to high number of titanium particles is cytotoxic and COX-2 mediated and it is not affected by the particle’s endotoxin content or the cleaning treatment. J Biomed Mater Res Part A. 2011;99(4):630–637. doi:10.1002/jbm.a.33222
233. Hotchkiss KM, Reddy GB, Hyzy SL, Schwartz Z, Boyan BD, Olivares-Navarrete R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016;31:425–434. doi:10.1016/j.actbio.2015.12.003
234. Rader CP, Sterner T, Jakob F, Schutze N, Eulert J. Cytokine response of human macrophage-like cells after contact with polyethylene and pure titanium particles. J Arthroplasty. 1999;14(7):840–848. doi:10.1016/S0883-5403(99)90035-9
235. Valles G, Gil-Garay E, Munuera L, Vilaboa N. Modulation of the cross-talk between macrophages and osteoblasts by titanium-based particles. Biomaterials. 2008;29(15):2326–2335. doi:10.1016/j.biomaterials.2008.02.011
236. Carter JD, Ghio AJ, Samet JM, Devlin RB. Cytokine production by human airway epithelial cells after exposure to an air pollution particle is metal-dependent. Toxicol Appl Pharmacol. 1997;146(2):180–188. doi:10.1006/taap.1997.8254
237. Ye J, Ding M, Zhang X, et al. Induction of TNFalpha in macrophages by vanadate is dependent on activation of transcription factor NF-kappaB and free radical reactions. Mol Cell Biochem. 1999;198(1–2):193–200. doi:10.1023/A:1006969008056
238. Tsave O, Petanidis S, Kioseoglou E, et al. Role of Vanadium in Cellular and Molecular Immunology: association with Immune-Related Inflammation and Pharmacotoxicology Mechanisms. Oxid Med Cell Longev. 2016;2016:4013639. doi:10.1155/2016/4013639
239. Zwolak I. Vanadium carcinogenic, immunotoxic and neurotoxic effects: a review of in vitro studies. Toxicol Mech Methods. 2014;24(1):1–12. doi:10.3109/15376516.2013.843110
240. Pineton de Chambrun G, Body-Malapel M, Frey-Wagner I, et al. Aluminum enhances inflammation and decreases mucosal healing in experimental colitis in mice. Mucosal Immunol. 2014;7(3):589–601. doi:10.1038/mi.2013.78
241. Johnson VJ, Sharma RP. Aluminum disrupts the pro-inflammatory cytokine/neurotrophin balance in primary brain rotation-mediated aggregate cultures: possible role in neurodegeneration. Neurotoxicology. 2003;24(2):261–268. doi:10.1016/S0161-813X(02)00194-8
242. Drynda A, Drynda S, Kekow J, Lohmann CH, Bertrand J. Differential Effect of Cobalt and Chromium Ions as Well as CoCr Particles on the Expression of Osteogenic Markers and Osteoblast Function. Int J Mol Sci. 2018;19(10):10. doi:10.3390/ijms19103034
243. Wang JY, Wicklund BH, Gustilo RB, Tsukayama DT. Titanium, chromium and cobalt ions modulate the release of bone-associated cytokines by human monocytes/macrophages in vitro. Biomaterials. 1996;17(23):2233–2240. doi:10.1016/0142-9612(96)00072-5
244. Lee YW, Cherng YG, Yang ST, Liu SH, Chen TL, Chen RM. Hypoxia Induced by Cobalt Chloride Triggers Autophagic Apoptosis of Human and Mouse Drug-Resistant Glioblastoma Cells through Targeting the PI3K-AKT-mTOR Signaling Pathway. Oxid Med Cell Longev. 2021;2021:5558618. doi:10.1155/2021/5558618
245. Zhang C, Chen M, Tao Q, Chi Z. Cobalt chloride-stimulated hypoxia promotes the proliferation of cholesteatoma keratinocytes via the PI3K/Akt signaling pathway. Int J Med Sci. 2021;18(15):3403–3411. doi:10.7150/ijms.60617
246. Xu J, Yang J, Nyga A, et al. Cobalt (II) ions and nanoparticles induce macrophage retention by ROS-mediated down-regulation of RhoA expression. Acta Biomater. 2018;72:434–446. doi:10.1016/j.actbio.2018.03.054
247. Zhang X, Wang Y, Chen M, Zeng M. Hexavalent chromium-induced apoptosis in Hep3B cells is accompanied by calcium overload, mitochondrial damage, and AIF translocation. Ecotoxicol Environ Saf. 2021;208:111391. doi:10.1016/j.ecoenv.2020.111391
248. Ganapathy S, Li P, Lafontant J, et al. Chromium IV exposure, via Src/Ras signaling, promotes cell transformation. Mol, Carcinog. 2017;56(7):1808–1815. doi:10.1002/mc.22639
249. Yang Q, Han B, Xue J, et al. Hexavalent chromium induces mitochondrial dynamics disorder in rat liver by inhibiting AMPK/PGC-1alpha signaling pathway. Environ Pollut. 2020;265(Pt A):114855. doi:10.1016/j.envpol.2020.114855
250. Bozinovic K, Nestic D, Centa UG, et al. In-vitro toxicity of molybdenum trioxide nanoparticles on human keratinocytes. Toxicology. 2020;444:152564. doi:10.1016/j.tox.2020.152564
251. Yang TY, Yen CC, Lee KI, et al. Molybdenum induces pancreatic beta-cell dysfunction and apoptosis via interdependent of JNK and AMPK activation-regulated mitochondria-dependent and ER stress-triggered pathways. Toxicol Appl Pharmacol. 2016;294:54–64. doi:10.1016/j.taap.2016.01.013
252. Gholinejad Z, Khadem Ansari MH, Rasmi Y. Titanium dioxide nanoparticles induce endothelial cell apoptosis via cell membrane oxidative damage and p38, PI3K/Akt, NF-kappaB signaling pathways modulation. J Trace Elem Med Biol. 2019;54:27–35. doi:10.1016/j.jtemb.2019.03.008
253. Xian G, Chen W, Gu M, et al. Titanium particles induce apoptosis by promoting autophagy in macrophages via the PI3K/Akt signaling pathway. J Biomed Mater Res Part A. 2020;108(9):1792–1805. doi:10.1002/jbm.a.36938
254. Yu X, Hong F, Zhang YQ. Cardiac inflammation involving in PKCepsilon or ERK1/2-activated NF-kappaB signalling pathway in mice following exposure to titanium dioxide nanoparticles. J Hazard Mater. 2016;313:68–77. doi:10.1016/j.jhazmat.2016.03.088
255. Gu Y, Wang Z, Shi J, et al. Titanium particle-induced osteogenic inhibition and bone destruction are mediated by the GSK-3beta/beta-catenin signal pathway. Cell Death Dis. 2017;8(6):e2878. doi:10.1038/cddis.2017.275
256. Zhu WQ, Ming PP, Zhang SM, Qiu J. Role of MAPK/JNK signaling pathway on the regulation of biological behaviors of MC3T3E1 osteoblasts under titanium ion exposure. Mol Med Rep. 2020;22(6):4792–4800. doi:10.3892/mmr.2020.11575
257. Zhu WQ, Ming PP, Qiu J, et al. Effect of titanium ions on the Hippo/YAP signaling pathway in regulating biological behaviors of MC3T3-E1 osteoblasts. J Appl Toxicol. 2018;38(6):824–833. doi:10.1002/jat.3590
258. Diaz D, Bartolo R, Delgadillo DM, Higueldo F, Gomora JC. Contrasting effects of Cd2+ and Co2+ on the blocking/unblocking of human Cav3 channels. J Membr Biol. 2005;207(2):91–105. doi:10.1007/s00232-005-0804-1
259. Li X, Han Y, Guan Y, Zhang L, Bai C, Li Y. Aluminum induces osteoblast apoptosis through the oxidative stress-mediated JNK signaling pathway. Biol. Trace Elem. Res. 2012;150(1–3):502–508. doi:10.1007/s12011-012-9523-5
260. Cao Z, Liu D, Zhang Q, Sun X, Li Y. Aluminum Chloride Induces Osteoblasts Apoptosis via Disrupting Calcium Homeostasis and Activating Ca(2+)/CaMKII Signal Pathway. Biol. Trace Elem. Res. 2016;169(2):247–253. doi:10.1007/s12011-015-0417-1
261. Sun X, Cao Z, Zhang Q, et al. Aluminum trichloride impairs bone and downregulates Wnt/beta-catenin signaling pathway in young growing rats. Food Chem Toxicol. 2015;86:154–162. doi:10.1016/j.fct.2015.10.005
262. Persson B, Carlenor E, Clyne N, et al. Binding of dietary cobalt to sarcoplasmic reticulum proteins. Scand J Clin Lab Invest. 1992;52(2):137–140. doi:10.3109/00365519209088777
263. Karovic O, Tonazzini I, Rebola N, et al. Toxic effects of cobalt in primary cultures of mouse astrocytes. Similarities with hypoxia and role of HIF-1alpha. Biochem Pharmacol. 2007;73(5):694–708. doi:10.1016/j.bcp.2006.11.008
264. Assem FL, Levy LS. A review of current toxicological concerns on vanadium pentoxide and other vanadium compounds: gaps in knowledge and directions for future research. J Toxicol Environ Health B Crit Rev. 2009;12(4):289–306. doi:10.1080/10937400903094166
265. Hao W, Hao C, Wu C, et al. Aluminum impairs cognitive function by activating DDX3X-NLRP3-mediated pyroptosis signaling pathway. Food Chem Toxicol. 2021;157:112591. doi:10.1016/j.fct.2021.112591
266. Li H, Xue X, Li Z, Pan B, Hao Y, Niu Q. Aluminium-induced synaptic plasticity injury via the PHF8-H3K9me2-BDNF signalling pathway. Chemosphere. 2020;244:125445. doi:10.1016/j.chemosphere.2019.125445
267. Korbecki J, Baranowska-Bosiacka I, Gutowska I, Chlubek D. Vanadium Compounds as Pro-Inflammatory Agents: effects on Cyclooxygenases. Int J Mol Sci. 2015;16(6):12648–12668. doi:10.3390/ijms160612648
268. Gajski G, Jelcic Z, Orescanin V, Geric M, Kollar R, Garaj-Vrhovac V. Physico-chemical characterization and the in vitro genotoxicity of medical implants metal alloy (TiAlV and CoCrMo) and polyethylene particles in human lymphocytes. Biochim Biophys Acta. 2014;1840(1):565–576. doi:10.1016/j.bbagen.2013.10.015
269. Chen F, Shi X. Intracellular signal transduction of cells in response to carcinogenic metals. Crit Rev Oncol Hematol. 2002;42(1):105–121. doi:10.1016/S1040-8428(01)00211-6
270. Gulati K, Scimeca JC, Ivanovski S, Verron E. Double-edged sword: therapeutic efficacy versus toxicity evaluations of doped titanium implants. Drug Discov Today. 2021;26(11):2734–2742. doi:10.1016/j.drudis.2021.07.004
271. Hartwig A. Zinc finger proteins as potential targets for toxic metal ions: differential effects on structure and function. Antioxid Redox Signal. 2001;3(4):625–634. doi:10.1089/15230860152542970
272. Baldwin EL, Byl JA, Osheroff N. Cobalt enhances DNA cleavage mediated by human topoisomerase II alpha in vitro and in cultured cells. Biochemistry. 2004;43(3):728–735. doi:10.1021/bi035472f
273. Lloyd DR, Carmichael PL, Phillips DH. Comparison of the formation of 8-hydroxy-2’-deoxyguanosine and single- and double-strand breaks in DNA mediated by Fenton reactions. Chem Res Toxicol. 1998;11(5):420–427. doi:10.1021/tx970156l
274. Beyersmann D, Hartwig A. The genetic toxicology of cobalt. Toxicol Appl Pharmacol. 1992;115(1):137–145. doi:10.1016/0041-008X(92)90377-5
275. Paustenbach DJ, Tvermoes BE, Unice KM, Finley BL, Kerger BD. A review of the health hazards posed by cobalt. Crit. Rev. Toxicol. 2013;43(4):316–362. doi:10.3109/10408444.2013.779633
276. Lison D, van den Brule S, Van Maele-Fabry G. Cobalt and its compounds: update on genotoxic and carcinogenic activities. Crit. Rev. Toxicol. 2018;48(7):522–539. doi:10.1080/10408444.2018.1491023
277. Wang J, Stieglitz KA, Kantrowitz ER. Metal specificity is correlated with two crucial active site residues in Escherichia coli alkaline phosphatase. Biochemistry. 2005;44(23):8378–8386. doi:10.1021/bi050155p
278. Snow ET. Effects of chromium on DNA replication in vitro. Environ Health Perspect. 1994;102(Suppl 3):41–44. doi:10.1289/ehp.94102s341
279. Bijukumar DR, Segu A, Souza J, et al. Systemic and local toxicity of metal debris released from Hip prostheses: a review of experimental approaches. Nanomedicine. 2018;14(3):951–963. doi:10.1016/j.nano.2018.01.001
280. Chen L, Zhang J, Zhu Y, Zhang Y. Interaction of chromium(III) or chromium(VI) with catalase and its effect on the structure and function of catalase: an in vitro study. Food Chem. 2018;244:378–385. doi:10.1016/j.foodchem.2017.10.062
281. Aureliano M. Recent perspectives into biochemistry of decavanadate. World J Biol Chem. 2011;2(10):215–225. doi:10.4331/wjbc.v2.i10.215
282. Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160(1):1–40. doi:10.1016/j.cbi.2005.12.009
283. Terpilowska S, Siwicki AK. Interactions between chromium(III) and iron(III), molybdenum(III) or nickel(II): cytotoxicity, genotoxicity and mutagenicity studies. Chemosphere. 2018;201:780–789. doi:10.1016/j.chemosphere.2018.03.062
284. Ducros V. Chromium metabolism. A literature review. Biol. Trace Elem. Res. 1992;32(1–3):65–77. doi:10.1007/BF02784589
285. Merritt K, Brown SA. Release of hexavalent chromium from corrosion of stainless steel and cobalt-chromium alloys. J Biomed Mater Res. 1995;29(5):627–633. doi:10.1002/jbm.820290510
286. Braakhuis HM, Gosens I, Heringa MB, et al. Mechanism of Action of TiO 2: recommendations to Reduce Uncertainties Related to Carcinogenic Potential. Annu Rev Pharmacol Toxicol. 2021;61(1):203–223. doi:10.1146/annurev-pharmtox-101419-100049
287. Shukla RK, Sharma V, Pandey AK, Singh S, Sultana S, Dhawan A. ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol In Vitro. 2011;25(1):231–241. doi:10.1016/j.tiv.2010.11.008
288. Liu M, Wu X, Cui Y, et al. Mitophagy and apoptosis mediated by ROS participate in AlCl3-induced MC3T3-E1 cell dysfunction. Food Chem Toxicol. 2021;155:112388. doi:10.1016/j.fct.2021.112388
289. Mandriota SJ, Tenan M, Nicolle A, et al. Genomic Instability Is an Early Event in Aluminium-Induced Tumorigenesis. Int J Mol Sci. 2020;21:23. doi:10.3390/ijms21239332
290. Rojas E, Valverde M, Herrera LA, Altamirano-Lozano M, Ostrosky-Wegman P. Genotoxicity of vanadium pentoxide evaluate by the single cell gel electrophoresis assay in human lymphocytes. Mutat Res. 1996;359(2):77–84. doi:10.1016/S0165-1161(96)90254-X
291. Ivancsits S, Pilger A, Diem E, Schaffer A, Rudiger HW. Vanadate induces DNA strand breaks in cultured human fibroblasts at doses relevant to occupational exposure. Mutat Res. 2002;519(1–2):25–35. doi:10.1016/S1383-5718(02)00138-9
292. Rodriguez-Mercado JJ, Mateos-Nava RA, Altamirano-Lozano MA. DNA damage induction in human cells exposed to vanadium oxides in vitro. Toxicol in vitro. 2011;25(8):1996–2002. doi:10.1016/j.tiv.2011.07.009
293. Shi X, Jiang H, Mao Y, Ye J, Saffiotti U. Vanadium(IV)-mediated free radical generation and related 2’-deoxyguanosine hydroxylation and DNA damage. Toxicology. 1996;106(1–3):27–38. doi:10.1016/0300-483X(95)03151-5
294. Tang H, Sun Y, Xiu Q, Lu H, Han H. Cyclooxygenase-2 induction requires activation of nuclear factor of activated T-cells in Beas-2B cells after vanadium exposure and plays an anti-apoptotic role. Arch Biochem Biophys. 2007;468(1):92–99. doi:10.1016/j.abb.2007.09.016
295. Zhang Z, Huang C, Li J, Shi X. Vanadate-induced cell growth arrest is p53-dependent through activation of p21 in C141 cells. J Inorg Biochem. 2002;89(1–2):142–148. doi:10.1016/S0162-0134(01)00409-3
296. Zhang Z, Huang C, Li J, et al. Vanadate-induced cell growth regulation and the role of reactive oxygen species. Arch Biochem Biophys. 2001;392(2):311–320. doi:10.1006/abbi.2001.2464
297. Rodriguez-Mercado JJ, Roldan-Reyes E, Altamirano-Lozano M. Genotoxic effects of vanadium(IV) in human peripheral blood cells. Toxicol Lett. 2003;144(3):359–369. doi:10.1016/S0378-4274(03)00255-8
298. Geyikoglu F, Turkez H. Boron compounds reduce vanadium tetraoxide genotoxicity in human lymphocytes. Environ Toxicol Pharmacol. 2008;26(3):342–347. doi:10.1016/j.etap.2008.07.002
299. Ramirez P, Eastmond DA, Laclette JP, Ostrosky-Wegman P. Disruption of microtubule assembly and spindle formation as a mechanism for the induction of aneuploid cells by sodium arsenite and vanadium pentoxide. Mutat Res. 1997;386(3):291–298. doi:10.1016/S1383-5742(97)00018-5
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
