Back to Journals » International Journal of Nanomedicine » Volume 19
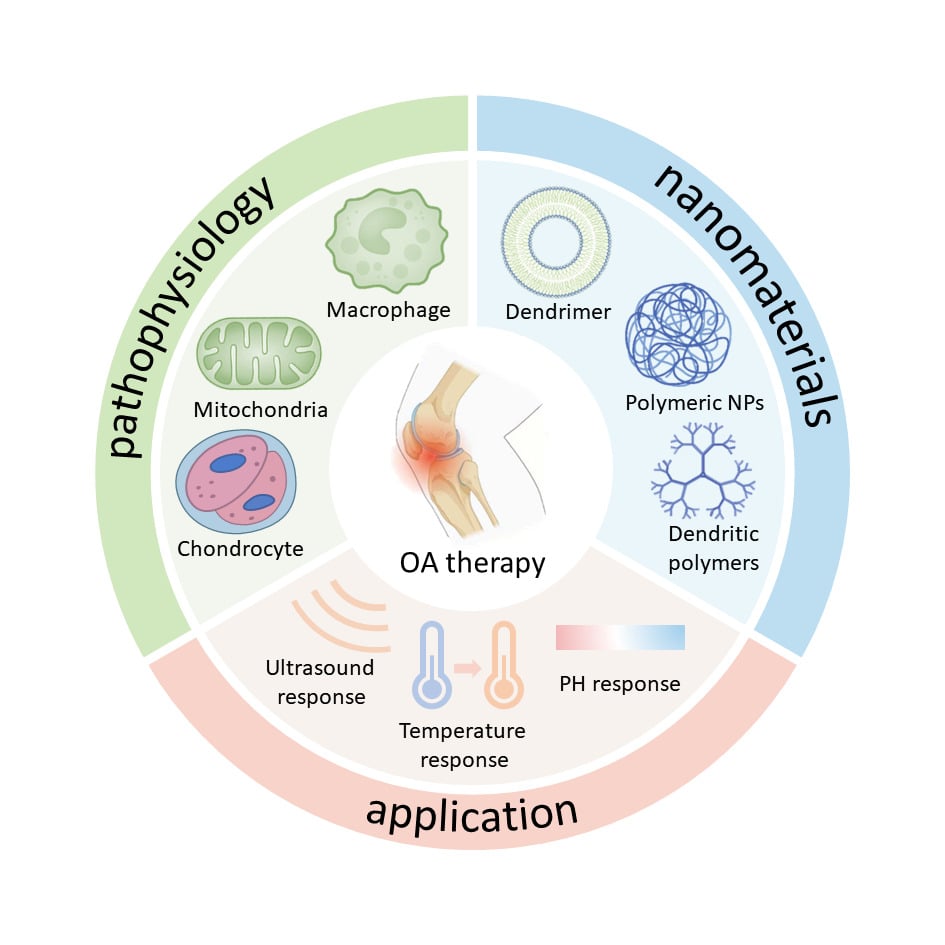
Functional Nanomaterials for the Treatment of Osteoarthritis
Authors Yi X , Leng P, Wang S, Liu L, Xie B
Received 21 February 2024
Accepted for publication 15 June 2024
Published 4 July 2024 Volume 2024:19 Pages 6731—6756
DOI https://doi.org/10.2147/IJN.S465243
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. RDK Misra
Xinyue Yi,1,2,* Pengyuan Leng,1,* Supeng Wang,2 Liangle Liu,1 Bingju Xie1
1The Third Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang Province, People’s Republic of China; 2Clinical Medical College, Ningxia Medical University, Yinchuan, Ningxia Hui Autonomous Region, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liangle Liu; Bingju Xie, Email [email protected]; [email protected]
Abstract: Osteoarthritis (OA) is the most common degenerative joint disease, affecting more than 595 million people worldwide. Nanomaterials possess superior physicochemical properties and can influence pathological processes due to their unique structural features, such as size, surface interface, and photoelectromagnetic thermal effects. Unlike traditional OA treatments, which suffer from short half-life, low stability, poor bioavailability, and high systemic toxicity, nanotherapeutic strategies for OA offer longer half-life, enhanced targeting, improved bioavailability, and reduced systemic toxicity. These advantages effectively address the limitations of traditional therapies. This review aims to inspire researchers to develop more multifunctional nanomaterials and promote their practical application in OA treatment.
Keywords: nanostructures, osteoarthritis, drug delivery systems, nanomedicine, precision medicine
Graphical Abstract:
Introduction
Osteoarthritis (OA), a chronic degenerative joint disease, involves the progressive degeneration of joint cartilage and the sclerotic hyperplasia of subchondral bone.1,2 A new study published in The Lancet Rheumatology reports that OA has been increasing globally since 1990 and will likely continue to rise, with 595 million people affected in 2020, or 7.6% of the global population. This marks a 132% increase in cases since 1990. By 2050, the number of OA cases is projected to grow by 48.6% to 95.1%. The global age-standardized disability rate from OA rose by 9.5% in 2020 compared to 1990, making OA the seventh leading cause of motor disability among those aged 70 and older. Notably, a high body mass index contributes to approximately 20.4% of OA cases, with the knees being the most commonly affected joints.3 The pathophysiology of osteoarthritis (OA) is multifaceted and involves complex interactions between several factors, including cartilage degradation, chronic inflammatory responses, changes in subchondral bone structure, and atypical joint mechanics.4 The key events in joint pathology during the progression of OA are shown in Figure 1A. In the initial phase, the joint space narrows, bony encumbrances appear, and chondrocytes enlarge and progressively break down, eventually leading to friction between bone ends. Thereafter, synovial fibrosis and inflammation increase, along with cartilage calcification, eventually leading to loss of joint function (Figure 1A).5 Notably, articular cartilage, being avascular with weak regenerative capacity, is virtually irreparable once damaged, inevitably leading to degeneration. Currently nonsteroidal anti-inflammatory drugs (NSAIDs) and analgesics are the mainstay of treatment for OA, but they only provide temporary pain relief and do not completely alter the progression of the disease.6,7 Few options exist for subsequent treatment after NSAIDs fail. Moreover, traditional drug delivery methods, including oral administration, present issues such as decreased bioavailability, gastrointestinal complications, increased cardiovascular risk, osteoporosis.8 Local intra-articular (IA) administration of drugs directly into the joints offers benefits such as improved local bioavailability and fewer side effects compared to systemic administration.9,10 However, because synovial capillaries and lymphatic vessels in the bone and joint rapidly remove drugs (especially small molecules) from the joint and because the extracellular matrix (ECM) has a dense natural cartilage barrier, patients require frequent injections of medications to maintain efficacy. Inevitably, there is an increased risk of further joint damage.10 In more severe cases, surgical interventions, such as joint replacement, may seem preferable; however, issues such as allogeneic donor rejection and inherent surgical risks limit their suitability for treating OA.11 Moreover, these therapeutic strategies can only ameliorate symptoms without stopping disease progression;12 this represents a significant challenge in current clinical practice.
 |
Figure 1 Pathology of Joint OA. (A) OA is a progressive disease that gradually transitions from a normal phenotype (left image) to an early OA phenotype (center image). In the initial stages of the lesion (center panel), the joint space becomes narrower, bony growths develop, chondrocytes multiply, and cartilage starts to deteriorate until the ends of the bones rub against each other. As OA advances (right panel), there is noticeable synovial fibrosis and inflammation, along with cartilage calcification and decreased joint function. Adapted reprinted with permission from Paesa M, Alejo T, Garcia-Alvarez F, Arruebo M, Mendoza G. New insights in osteoarthritis diagnosis and treatment: nano-strategies for an improved disease management. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023;15(2):e1844. Copyright © 2022, John Wiley and Sons.5 (B) Chronic synovial hypertrophy and synovial hypoinflammation are well-known characteristics of OA. Furthermore, inflammatory mediators and receptors can be expressed by chondrocytes and osteoblasts in cartilage and subchondral bone. The presence of localized inflammation in all joint tissues contributes to a complicated pathological process, leading to increased pain and tissue damage. Reprinted from Osteoarthritis and Cartilage, 31/10, De Roover A, Escribano-Núñez A, Monteagudo S, Lories R. Fundamentals of osteoarthritis: inflammatory mediators in osteoarthritis, 1303–1311. Copyright 2023 with permission from Elsevier.13 |
Over the past decade, nanomedicine has emerged in research for the treatment of a variety of diseases such as cancer, infectious diseases and cardiovascular diseases. Several therapeutic nanoplatforms have been approved for cancer treatment, including (Liposomes, albumin nanoparticles (NPs), and polymer vesicles, with more therapeutic modalities undergoing clinical trials).14 Emerging research has demonstrated a growing need for precisely targeted therapies for the treatment of OA, with nanomaterial-based precision medicine representing a promising strategy.15 Biomaterials currently used in arthritis treatment mainly include: hyaluronic acid, stem cell therapy, bioactive factors, liposomal drug delivery systems, and biodegradable materials. These biomaterials play an important role in arthritis treatment and can help reduce pain, improve joint function, and promote cartilage repair and regeneration. Although other materials have been developed in the field of osteoarthritis treatment, the size of the nanomaterial creates her unique properties.16 Our literature review indicates that nanomaterials are primarily used in OA therapy for drug delivery. Nanocarrier particles, compared to drug molecules, have a large specific surface area, small size, and the potential for surface modification. These features improve drug-loading, extend in vivo circulation time, increase bioavailability, target specific organs or tissues, and enhance drug stability. Consequently, they improve therapeutic efficacy while reducing systemic toxicity, side effects, and treatment costs. In addition, functionalized nanomaterials can be used as carriers to achieve targeted transport and controlled release of drugs to achieve better drug efficacy. Moreover, surface-modified nanomaterials loaded with photosensitizers, photothermal agents, chemotherapeutic agents, magnetic materials, and genes can be used in various therapies—such as photodynamic, photothermal, chemotherapy, magnetic, antibacterial, and gene therapies—by responding to internal or external stimuli.17,18
Here, we review advanced approaches to nanomaterial-based OA treatment. First, we provide an Introduction to the pathophysiology of OA. Secondly, we explored the various types of nanomaterials used in OA therapy, including nanogels (NGs), liposomes, NPs, extracellular vesicles, nanozymes, dendritic polymers, and micellar polymers. Thirdly, we describe various nanomaterial-based strategies for OA therapy, highlighting their advantages. The modification and design of nanomaterials are subsequently described. Finally, we conclude by discussing the current challenges and opportunities for the use of nanomaterials in the treatment of OA.
Pathophysiology of OA
OA, a degenerative and progressive inflammatory condition, affects articular cartilage, synovium, and accessory tissues. The onset of OA has been linked to several variables, including genes, signaling pathways, matrix metalloproteinases (MMPs), and cytokines. In addition, a number of other important factors come into play, including hormone levels, sex, age, obesity, endocrine disorders, and aberrant mechanical stresses within the joints. The interplay of these factors establishes a complex pathogenesis for OA,19 with cartilage destruction being its main characteristic.20 Cartilage, a non-vascularized covering on the surface of joints, reduces the wear and tear of bone tissue by maintaining minimal surface friction during joint movement. During the initial phases of OA, the breakdown of articular cartilage occurs because of excessive mechanical stress, leading to the release of acute damage-associated pattern molecules. These molecules then trigger the activation of immune cells within the synovium, resulting in the release of pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, initiating an inflammatory cascade. Chondrocytes respond by secreting catabolic enzymes, such as matrix metalloproteinase 3, matrix metalloproteinase 13, and platelet-responsive proteolytic integrin metallopeptidases, causing cartilage matrix degradation. Moreover, cartilage releases additional damage-associated pattern molecules, initiating a positive feedback loop that creates an inflammatory cascade in the synovium and maintains a chronic inflammatory (Figure 1B).13 Furthermore, cartilage tissue is devoid of nerves and blood vessels, consisting only of chondrocytes and ECM. Thus, cartilage tissue exhibits extremely low metabolic activity. While chondrocytes can heal minor joint injuries, severely injured joints have limited regenerative capacity OA advances when the breakdown of the cartilage’s ECM surpasses its ability to repair itself. This leads to the deterioration of cartilage, remodeling of the bone beneath it, the generation of bony outgrowths (osteophytes), inflammation of the synovial membrane (synovitis), degeneration of ligaments, damage to the meniscus, and enlargement of the joint capsule. In addition, during the development of OA, the levels of reactive oxygen species (ROS) within the cartilage as well as chondrocytes of patients with OA are significantly increased.21,22 Moreover, when chondrocytes are exposed to pro-oxidants, such as H2O2, menaquinone, 3-morpholinoaniline (SIN1), and tert-butyl hydroperoxide (TBHP), which can generate excessive free radicals and ROS, the frequency of inflammatory reactions and apoptosis is greatly increased. This suggests that ROS can induce inflammatory reactions in chondrocytes, further damaging cartilage tissues and accelerating the onset and development of OA.2 Studies suggest a strong link between ROS production and cartilage matrix metabolism, with ROS accelerating collagen degradation and inhibiting proteoglycan synthesis.23,24 Excessive ROS levels can cause severe cartilage damage, and without treatment, OA has a slim chance of self-recovery.25 In addition, mitochondrial dysfunction is related to apoptosis, aging, and a variety of pathological states. Mitochondrial dysfunction affects the respiratory chain activity of chondrocytes, increases inflammation, and promotes apoptosis, making it one of the primary factors leading to OA.26
While OA has traditionally been recognized as a disease caused by the progressive degeneration of cartilage, recent studies have highlighted the function of persistent synovial inflammation in the progression of OA.13,27 The synovial surface, characterized by a discontinuous layer of synovial cells, lack of a basement membrane, and a capillary network, serves as a key entry and exit point for molecules in and out of joints. This specific structure increases the likelihood and severity of synovial membrane injury.13 Histological features of synovitis in patients with OA include hyperplasia of the inner synovial layer, macrophage and lymphocyte infiltration, angiogenesis, and fibrosis.28 Current clinical treatments involve IA injections of corticosteroids or pro-inflammatory cytokine antagonists. However, their efficacy is limited, mainly because the drugs are cleared rapidly in the articular cavity and are therefore unable to maintain long-term effective therapeutic concentrations to markedly ameliorate the inflammatory processes within the whole joint. Therefore, the development of effective drug delivery vehicles has become a popular research avenue.29 With the gradual deepening of multidisciplinary integration, many researchers have found that nanomaterials exhibit excellent physicochemical properties. Their popularity as drug delivery carriers is expected to rise, achieving on-demand, controllable drug accumulation at lesion sites over an extended period. Moreover, researchers are exploring nanomaterials with ROS scavenging abilities and antioxidant effects, and some progress has been made with regard to developing nanomaterials for treating OA.30
Nanomaterials
Nanomaterials, defined as materials with at least one dimension ranging from 1–100 nm, exhibit significant variations in quality and origin. Choosing nanomaterials from appropriate sources is crucial for OA treatment, and their morphology influences drug delivery efficiency and release rate. This section introduces dominant nanomaterials and their properties in current OA treatment (Table 1).
 |
Table 1 Classification of Advantages and Disadvantages of Nanomaterials |
NGs
NGs are hydrophilic or amphiphilic polymer hydrogel networks created by physical or chemical cross-linking. These structures exhibit water-absorbing and swelling properties, boasting a robust water retention capacity.31,32 Compared with other forms of nanomaterials in the biomedical industry, NGs combine the advantages of hydrogels and NPs. NGs offer high drug-loading capacity, slow release, improved permeability due to their small particle size, and a large surface area for specific modifications. They also have excellent biocompatibility and biodegradability.32 These properties make it easier for NGs to reach the damaged site and prolong the residence time of the drug in the joint. Additionally, NGs provide better stability, particle size management, and resistance to enzyme stimulation, as well as pH, temperature, light, and redox conductivity. NGs achieve sustained release of encapsulated drugs by undergoing structural changes in response to stimuli such as temperature, pH, glucose, enzymes and redox potential.33,34
Improvements in NGs synthesis enable the fine control of size and shape, influencing biological distribution. Advanced characterization techniques can also be used to anticipate the actions of NGs when they are utilized in vivo.48 Nedunchezian et al used HA methacryloyl chloride/gelatin methacryloyl chloride hydrogels coupled with acrylate-functionalized silica NPs to fabricate novel photo-crosslinked hybrid hydrogels for loading human fat-derived stems cells and promoting their differentiation into cartilage in OA treatment.49 Li developed NGs containing glycol chitosan and fucoidan, loaded with the anti-inflammatory peptide KAFAK (referred to as GC/Fu@KAFAK NGs), using a combination of electrostatic interactions and dye-wood symbiotic cross-linking. In an experimental rat model of OA, the GC/Fu@KAFAK NGs exhibited significant therapeutic effects. These NGs not only suppressed the manifestation of inflammatory mediators, specifically interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), leading to reduced glycosaminoglycan depletion and reduced release of inflammatory cytokines, but also demonstrated the capacity to enhance the production of type II collagen and aggregated glycans, as well as the chondrogenic marker Sox9 (Figure 2).50 Additionally, Yang et al designed a versatile anti-inflammatory drug using a peptide-dendrimer nanohydrogel, encapsulating the carbon monoxide-releasing molecule with folic acid-modified HA. This nano-complex hydrogel effectively targeted synovial macrophages, inhibiting cell proliferation, reducing reactive oxygen components in osteoarticular joints, and significantly inhibiting the release of IL-6, TNF-α, and IL-1β. It also stimulated the activity of hemoglobin oxygenase, decreased the activity of P38 mitogen-activated protein kinase, and increased the production of κ-light chain and Toll-like receptor 2 in nuclear factor-activated B cells, leading to beneficial therapeutic outcomes.51 All three examples involve the use of nanogels in combination with various functional ingredients to achieve therapeutic effects for OA by reducing inflammation, or promoting cartilage regeneration, or improving joint function. Each of these approaches represents a current cutting-edge research direction in the field of arthritis treatment and demonstrates the potential of hydrogels for medical applications. Despite their promising potential, no nanogel-based drugs are currently available for clinical applications, and extensive research and technology are required to ensure the efficacy and safety of NGs-based clinical treatments.52
 |
Figure 2 (A) Protocols for NGs preparation and intra-articular injection; (B) NGs IA injection decreases the levels of inflammatory factors in a rat model of OA induced by ACLT. Immunohistochemical examination of (i) IL-6 and (ii) TNF-α in sections of the knee tibial plateau. All images are reprinted from International Journal of Biological Macromolecules, 170, Li T, Yang J, Weng C, et al. Intra-articular injection of anti-inflammatory peptide-loaded glycol chitosan/fucoidan nanogels to inhibit inflammation and attenuate osteoarthritis progression, 469–478. Copyright 2021 with permission from Elsevier.50 |
Liposomes
Liposomes are water-nucleated globular shaped vesicles surrounded by a phospholipid bilayer that encapsulate drugs, exhibiting high toxicity, poor stability, and rapid degradation. Because of their unique structural properties, liposomes are highly suitable for the delivery of fat-soluble drugs. Upon fusion with the cell membrane, liposomes can concentrate drugs at the lesion site, improving drug efficacy, reducing toxicity, and enhancing drug stability. In addition, the lubricating ability of liposomes can reduce bone wear in OA joints, making them a preferred drug carrier.35 Depending on the buffering agent and lipid structure, liposome sizes can range from 50–5000 nm, generating miniature unilamellar (~100 nm), large unilamellar (200–800 nm), and multi-lamellar (500–5000 nm) vesicles. Enhanced mobility of immunoliposomes can be achieved by encapsulating polymers or antibodies or by modifying the surface chemistry. Dravid et al designed a nanoliposomal formulation containing Resolvin D1 (RvD1), referred to as Lipo-RvD1. This formulation demonstrated controlled release for up to 11 days in vitro, targeted and inhibited osteochondritis dissecans formation, and showed analgesic effects by inhibiting synovial macrophage pro-inflammatory activity and recruiting more M2 macrophages to the inflammation site.36 OA associated with obesity is characterized by enhanced macrophage infiltration of synovial tissue.53–56 Moreover, clodronate liposomes induce the reduction of macrophages levels. Bader et al recently demonstrated that the administration of IA injections containing clodronate liposomes decreased synovitis and cartilage degradation in a mouse model of obesity-related OA by depleting macrophages and reducing collagen X levels. Liposomal formulations provide numerous benefits, including superb biocompatibility, minimal toxicity, and the capacity to encapsulate both lipophilic and hydrophilic medicines. The above studies demonstrated about the application of nanoliposomal formulations in dealing with the treatment of joint diseases, proving the potential and importance of nanoliposomes in the field of OA therapy. Despite these benefits, liposomes have some drawbacks. Firstly, they exhibit high sensitivity to external reactions, making them vulnerable to changes in shear pressure, temperature, diluent, pH, and diluent concentration. Secondly, liposomal systems possess a degree of toxicity, potentially inducing acute hypersensitivity syndromes. Challenges remain with cytotoxicity, leakage rates, synthetic reproducibility, and effective sterilization techniques. Despite these issues, liposomal NPs have been highly successful in clinical applications. They are used in over 20 small molecule drug formulations and play a crucial role in developing RNA vaccines, highlighting their significant potential in drug delivery.57
NPs
Inorganic NPs
Inorganic NPs, made from nanoscale inorganic materials (with at least one dimension under 100 nm), feature large specific surface areas, controllable structures, diverse surface chemistry, and unique optical-physical properties, making them advantageous for biomedical applications. Researchers have shown that inorganic NPs and their released metal ions can act as therapeutic agents in target tissues or treat various diseases without causing acute toxicity.37 Moreover, the use of metal NPs in drug delivery systems (DDSs) offers significant advantages such as increased stability and half-life of drug carriers in circulation, favorable biodistribution, and passive or active targeting of desired target sites.58 Gold NPs (GNPs) possess anti-arthritic effects due to their antioxidative as well as anti-inflammatory characteristics.59 Sarkar et al labeled GNPs with fish oil proteins (FPs) possessing anti-inflammatory properties. Subsequently, they encapsulated these FP-GNPs in dipalmitoyl phosphatidylcholine (DPPC) liposomes, which were subsequently administered to a rat model of OA via IA injection. They observed a constant release of FP-GNPs into the synovial fluid, accompanied by a decrease in apoptotic markers including Bax, cysteine 3, and p53, as well as a reduction in cytokines that promote inflammation such as TNF-a, IL-6, and NF-kB.59 Copper is an important trace metal with multiple uses.60 Notably, Cu-based antibacterial and anticancer NPs have been developed that convert ROS into harmless hydroxyl radicals (-OH).61 Among such NPs, CuS NPs have demonstrated great versatility and adaptability for a wide range of biomedical uses.60 It is important to mention that the malfunctioning of mitochondria, which is a characteristic feature of aged and damaged cartilage cells in OA, triggers the generation of ROS within the cells, resulting in oxidative stress.62 Excess hydrogen peroxide in the cartilage of patients with OA can be problematic,63 Copper-based NPs with enzyme-like properties have shown efficacy in eliminating senescent chondrocytes. Furthermore, prior research has demonstrated that Cu-based materials facilitate the chondrogenic process and enhance the regeneration of cartilage through mesenchymal stem cells (MSCs).64 Therefore, Cu-based materials enhance the microenvironment of cartilage in the joints following the elimination of aged chondrocytes.
Polymer NPs
Polymer NPs (PNPs) are firm particles in the size range of 10–1000 nm, prepared from natural or synthetic polymers.35 They are capable of being engineered into smart NPs with various structures and properties, serving as biocompatible and biodegradable vehicles for drug delivery.38 Their adaptability allows for precise control over nanomaterial characteristics, including surface modifications for delivering drugs, proteins, and genetic materials to target tissues. PNPs are versatile for drug delivery, as drugs can be encapsulated within nanomaterials and polymer molecules, chemically bound to polymers, or bound to the surface of nanomaterials.65 Thus, compounds, both hydrophobic and hydrophilic in nature, together with drugs of varying molecular weights, can be delivered via them.66 Synthetic polymer NPs offer advantages such as good biocompatibility, controlled release, and high drug-carrying capacity, but they also face challenges with degradation rate, stability, and safety. Therefore, when selecting synthetic polymer NPs for drug delivery, factors such as drug properties, in vivo environmental condition, and potential side effects need to be considered. Among natural polymers, chitosan, alginate, gelatin, collagen, dextran, heparin, as well as albumin are favored for drug delivery. Chitosan-based hydrogels with good biocompatibility, biodegradability and antimicrobial properties are ideal candidates for drug delivery and tissue engineering. However, the application of chitosan scaffolds in tissue regeneration is limited due to insufficient mechanical strength and degradation rate, so composite scaffolds prepared by mixing chitosan with other functional substances tend to exhibit enhanced tissue regeneration capabilities.67 NPs prepared from natural polysaccharides such as alginate and gelatin exhibit good biocompatibility and biodegradability, enabling slow and targeted drug release, improving efficacy, and reducing side effects. Moreover, they are easy to prepare and, inexpensive making them suitable for mass production. However, they exhibit poor stability easily affected by pH and other environmental factor.
Plant Exocysts
Extracorporeal-like nanovesicles (ELNVs) are biological nanostructures with diameters between 40–150 nm that are secreted in most cells and play an imperative role in transmitting signals among cells and organisms across all three living worlds. Plant ELNV (PELNV) is not only naturally enriched with a variety of pharmacologically effective monomers, like bioactive lipids, proteins, RNA. It also serves as a natural nano-vehicle due to its peculiar morphologic and constitutive features.68 In contrast to animal-derived vesicles, which are morphologically and functionally identical to their mammalian analogs, PELNVs express a number of lipid as well as cell attachment factors, like phosphatidic acid, enabling targeted delivery through specific receptor cell binding. Specifically, binding of phosphatidic acid to Raf-1 can localize Raf-1 to specific cell membranes or membrane regions.40 PELNV-derived nanocarriers also offer customization of bioactive molecule surfaces, enhancing application and precision of targeted transport. Most importantly, the primary explanation for the immune system’s failure to adjudicate PELNV as a xenobiotic for removal is its natural provenance and ingredients, resulting in longer circulation periods and higher bioavailability.41 In addition, they do not carry any zoonotic or human pathogens. Thus, PELNV surpass mammalian cell-derived ELNVs as drug delivery vehicles and are efficiently absorbed at the target site for efficient drug delivery. These compelling physicochemical characteristics underpin the regulatory role of PELNVs in physiological processes, showcasing their potential as next-generation biotherapeutic and drug delivery nanoplatforms.42,43 However, OA research is currently focused on animal models. They are difficult, time-consuming and expensive, which is a major obstacle to their use. Therefore, more clinical trials will be needed in the future to validate these findings.68
Nanozymes
OA-induced inflammation alters the peripheral microenvironment (eg, pH, temperature, and protease levels), leading to the loss of activity of natural enzymes. With the gradual advancement of research, researchers have discovered a class of bifunctional nanomaterials, such as cerium oxide, manganese dioxide (MnO2), and platinum (Pt), which possess both physical properties (eg, magnetic and photovoltaic properties) and catalytic activity.44,45 Termed Nanozymes, these materials exhibit light, sound, and electricity properties while regulating enzyme activity. For example, researchers have found that CeO2 and Pt NPs mimic SOD, catalase, and peroxidase activities, whereas MnO2 NPs mimic SOD and catalase-like activities. Such nanozymes also serve as effective antioxidants for cellular protection.46,47 Although, many forms of nanozymes show cytoprotective effects, conventional synthesis methods still pose environmental and human health risks. Therefore, the environmentally sustainable synthesis of nanozymes, utilizing microbial and plant extracts, is gaining interest. Green synthesis represents a novel approach for the synthesis of NPs from microbial as well as botanical derivatives. To enhance toxicological studies in the context of OA treatments, reliable experimental data is crucial for elucidating the toxicity associated with inorganic NPs.39
Dendritic Polymers
Dendritic polymers are repeating chain macromolecules exhibiting a topologic tree-like nanostructure, consisting of a core, branches, and a shell. The shell forms the outer surface, enabling the attachment of cargo or targeting ligands. As a drug delivery technology, dendritic polymers offer several advantages, including a precisely defined number of surface functional groups, manageable dimensions, uniformity, and high cargo-carrying efficiency. The unique composition and characteristics of dendrimers have garnered significant interest across various fields.69
Dendritic polymers serve as drug carriers through various routes of administration, including dermal, intravenous, and oral routes. Catabolism degrades OA cartilage, surpassing the effects of anabolic processes in cartilage cells. Insulin-like growth factor 1 (IGF-1) is an anti-inflammatory factor that stimulates the biogenesis of cartilage matrix. Geiger et al developed cationic polyethylene glycolated Polyamide-Amino (PAMAM) that binds to IGF-1, thereby targeting anionic cartilage tissue for the treatment of OA.70 In vivo studies using a surgically induced rat model of OA have demonstrated that polyethylene glycol dendritic polymer-IGF-1 efficiently penetrates the entire layer of articular cartilage in rats through electrostatic interactions and rescues cartilage from degeneration. These findings support clinical trials of new OA treatments. Similarly, partially polyethylene-glycolated PAMAM dendrimers have been used as carriers for anti-OA drugs. To create the PEG-PAMAM-KGN (PPK) and KGN-PEG-PAMAM (KPP) couplings, Hu et al conjugated KGN to the polyethylene glycol (PEG) terminal groups and the PAMAM surface, respectively. PPK and KPP, differently affected MSCs’ chondrogenic differentiation in vitro, with KPP more effectively promoting chondrogenic development. PEG-PAMAM could thus serve as an effective nanodrug carrier system for OA treatment. Glycerol and sulfate groups combine to form the polymer known as dendritic polyglycerol sulfate (dPGS). This polymer demonstrates anti-inflammatory action by lowering leukocyte extravasation and complement C3 and C5 levels, However, its use also presents challenges associated with the inability to encapsulate hydrophilic drugs and the cytotoxicity of micelles.
Most dendrimers are non-degradable and inevitably carry the risk of accumulating in the organism, analogous to traditional methods of administering medication. In addition, lots of dendritic polymers, such as PAMAM and PPI, are densely packed with numerous terminal amino groups on the surface of their three-dimensional spheres. As the number of branching generations increases, the cationicity strengthens, leading to potential issues such as cell membrane rupture and apoptosis, contributing to the cytotoxicity of dendrimers. Current development strategies for dendrimers involve modifications such as glycosylation, acetylation, PEG, or peptide modification to neutralize cations, reduce cytotoxicity, or facilitate dendrimer degradation.
Nanotherapeutic Strategies for OA
DDSs
DDSs are complex mechanisms designed to precisely control the spatial, temporal and dosage distribution of drugs within an organism. Their aim is to improve the efficiency of drug use, therapeutic efficacy, and cost effectiveness while reducing potentially harmful side effects, by delivering the required dose of drug precisely to the target site at the right time. Common drug delivery methods for OA treatment include direct IA injection and oral drug delivery, employed in clinical treatment.71,72 IA effectively avoids the systemic side effects caused by oral administration, allowing direct drug delivery to the joints and improving drug bioavailability while reducing treatment costs.73 Nevertheless, the high efficiency of IA lymphatic drainage results in short drug retention times, necessitating repeated injections, potentially increasing the risk of joint infections.74 Nanomaterial-based DDSs offer advantages such as reduced injection frequency, prolonged drug residence in joints, and improved patient compliance, ultimately enhancing treatment efficacy.72 Despite the progress in nanotechnology-based orthopedic DDS, concerns persist regarding long-term safety and metabolic pathways, emphasizing the need for further research.75
Traditional pain relief strategies for OA involve the use of NSAIDs. Lornoxicam (Lnx) is a newly developed NSAID capable of reducing inflammation, relieving pain, and promoting the repair of joints.76 Unfortunately, its poor water solubility and upper gastrointestinal absorption result in side effects, such as gastrointestinal reactions when administered orally or via simple IA injections.77 Zhang et al administered Lnx-encapsulated PLGA microspheres (Lnx-MS) via IA injection into the rats’ knee joints of rats. They found that IA injection of Lnx-MS reduced the plasma drug concentration and increased drug retention in rat joints, demonstrating good targeting efficiency and reduced systemic toxicity.78 Meloxicam (Mlx) is an NSAID that is both hydrophobic and lipophilic.79 Unlike other NSAIDs, Mlx is a selective inhibitor of COX2,80 which leads to mild gastrointestinal symptoms.81 Additionally, its low solubility in water hinders its absorption and decreases its bioavailability. Shohreh et al conducted a comparison between two Mlx delivery systems and observed that chondrocytes demonstrated enhanced proliferation and adherence in the NPs system. Furthermore, their behavior was impacted by the density of NPs and the concentration of the Mlx solution. The carboxymethyl chitosan-methylcellulose-polycellulose hydrogel (CMC-MC-P hydrogel) combined with Mlx NPs demonstrated improved biocompatibility, bioadhesion, and cell growth expansion compared with hydrogels containing Mlx solution.82 However, Mlx liposomes have drawbacks such as low drug-loading capacity, poor storage stability, and potential burst release risks with passive loading. To overcome these problems, Zhong et al proposed a new method to fabricate meloxicam liposomes using the active loading method (Figure 3A).83 By adding glucosamine and divalent metal solution, they successfully improved the water solubility and encapsulation efficiency of meloxicam, effectively solving the main problems of poor water solubility and lipophilicity of the drug (Figure 3B).83 It is expected that MLX-Ca(AC)2Lipo not only effectively reduces chondrocyte apoptosis and extracellular matrix degeneration by decreasing COX-2 expression, but also increases hydration and lubrication of the joints and inhibits the degeneration of cartilage (Figure 3C).83 Etoricoxib is an NSAID that selectively inhibits the COX-2 enzyme. It received its initial approval for use in clinical practice in 2002. Due to cardiovascular risks from systemic administration, local methods such as direct intra-arterial injection are recommended for etoricoxib.84 Rapamycin (Rap) known to inhibit OA progression, faces challenges due to its hydrophobicity, which causes instability after IA administration. Large doses and repeated injections are often needed to clear lymph from the joint effectively.74 To address this issue, Ma et al incorporated iRap poly (lactic acid-ethanolic acid copolymer) NPs (RNPs). These NPs had high drug encapsulation capacity and provided gradual release. RNPs reduced the need for multiple injections, promoted chondrogenic cell differentiation in ATDC5 cells, and prevented oxidative stress-induced senescence in articular chondrocytes.65 Sohn et al also showed that RAP lipid NPs induce immunomodulation of type II collagen in joints and alleviate OA by suppressing inflammation.85
 |
Figure 3 (A) Diagram illustrating the active loading of meloxicam liposomes (MLX-Ca(AC) 2Lipo) and its key benefits over passive loading methods, including efficient drug encapsulation, secure drug storage, and controlled drug release. (B) In this diagram, we illustrate the administration of MLX-Ca (AC) 2Lipo via local intra-articular injection for treating osteoarthritis in the temporomandibular joint of rats, as well as the meloxicam release profile from the system. (C) Demonstration of MLX-Ca(AC)2 injected into the joint of rats and its potential mechanism for producing anti-inflammatory effects. Figure parts reprinted with permission from Zhong Y, Zhou Y, Ding R, et al. Intra-articular treatment of temporomandibular joint osteoarthritis by injecting actively-loaded meloxicam liposomes with dual-functions of anti-inflammation and lubrication. Mater Today Bio. 2023;19:100573.https://creativecommons.org/licenses/by/4.0/.83 |
Oxidative stress is the primary contributor to osteoarthritic chronic inflammation, while glutathione (GSH) attenuates oxidative stress.86 GSH is a tri-peptide composed of glutamic acid, cyclamic acid and glycine containing a γ-amide bond and mercapto group. It is present in almost every cell in the body and exhibits antioxidant properties.87 However, GSH is only synthesized in the cytoplasm, and its quantity may be defective under redox imbalance. Loading GHS in CNPs and transporting it to chondrocytes addresses these defects, allowing cells to regulate oxidative state reduction and reduce oxidative stress-associated damage in OA.87,88 HA is usually administered via IA injection for viscosupplementation in OA; however, it has notable post-treatment limitations. For example, it is rapidly removed from the joint after injection, and any biological advantage is lost within hours. Mota et al constructed a replacement DDs on the basis of HA hydrogels combined with oleic acid-containing poly (lactic acid) (PLGA) particles. The superiority of the combination of HA and PLGA particles lies in the improved steadiness of the HA and a marked reduction in the incidence of in situ pneumonia.89 Colchicine reduces the release of inflammatory mediators associated with OA.90 However, orally administered colchicine has a number of limitations, including widespread first-pass effects, low bioavailability, as well as serious gastrointestinal adverse outcomes. Percutaneous administration can bypass these issues, but colchicine’s high aqueous solubility and poor dermal permeability pose challenges. Mohamed et al developed mesoporous silica NPs as colchicine encapsulants within self-healing hydrogels. The resulting patches show significant potential for efficient, safe, and patient-friendly formulations for OA management.91 PNPs offer advantages such as binding both hydrophilic and hydrophobic substances, controllable drug release, and robustness. However, they face challenges like poor drug-loading capacity and toxicity, requiring modifications and further toxicological studies. Jiang et al demonstrated that engineered macrophage membrane vesicles loaded with antioxidant NPs effectively scavenged mitochondrial ROS, reprogrammed pro-inflammatory macrophages, and suppressed synovial inflammation and early OA.92 The CBFβ-RUNX1 pathway activator katoglobulin (KGN) is a prospective active minor pharmaceutical ingredient (API) to regenerate and preserve arthrocartilage in OA.93,94 Maudens et al encapsulated high-payload KGN nanocrystals into polymer particles of poly(DL-propylene lactone) (PLA). These polymer particles that are embedded with 320 nm KGN nanocrystals show a remarkably high drug-carrying capacity of 31.5% (w/w), with a 62% longer drug release time at 3 months, achieving more effective joint protection compared to KGN solutions.95 The examples mentioned above all demonstrate that the use of nanomaterials for drug delivery in the treatment of OA ensures that effective therapeutic concentrations of drugs are maintained at the site of injury for a long period of time, reduces the systemic side effects of large doses of drugs, and solves the inconvenience of repeated injections in the daily life of patients.
Gene Therapy
Gene therapy is a cutting-edge medical breakthrough that involves the introduction of exogenous, targeted genes into reactive target cells via vectors. This technique aims to correct overactivation or compensate for defective genes, marking a significant advance in medicine by controlling the disease at its root, rather than merely alleviating the symptoms of the disease, distinguishing it from other therapeutic strategies. MicroRNAs (miRNAs) are key elements in gene delivery.96 It has recently been demonstrated that miRNAs play a role in the maturation of osteoblasts and the osteogenic differentiation of cells that precede them such as MSCs.97 The miR-200 gene family, specifically miR-200c-3p,98 has garnered interest because of its low expression levels observed in OA tissue samples. Despite this, the specific function of miR-200c-3p in OA has not yet fully elucidated.99,100 Investigations into its role have identified potential targets involved in OA’s pathological processes his suggests that miRNAs hold promise for addressing orthopedic illnesses by controlling gene expression related to epigenetics, transcription, and various activities such as cellular self-renewal, proliferation, differentiation, and apoptosis. Despite their promise, miRNAs face limitations in clinical applications because of their negative charge, limited cell membrane permeation, and stability.101 Moreover, chondrocytes possess a compact ECM and proteoglycans that are strongly negatively charged, posing additional challenges for OA-related drug penetration.102 To Address these issues, researchers have focused on enhancing gene drug concentration, transfection efficiency, and gene vector permeability in miRNA gene therapy for OA.59 Nevertheless, the potential of miRNAs is hindered by their limited effectiveness in specifically targeting desired cells, their brief duration in circulation, and their tendency to affect unintended targets. Consequently, miRNA-loaded NPs have emerged as a solution, providing protection against inactivation or degradation, increasing circulation time, and promoting target accumulation.103 Utilizing miRNAs for therapeutic applications presents distinct benefits compared to conventional gene therapy methods that rely on delivering larger molecules like mRNA or DNA. Since a single miRNA can regulate multiple mRNAs concurrently, they have a broader range of therapeutic targets. Additionally, unlike siRNAs and mRNAs, which are limited to repressing or overexpressing a single gene, miRNAs, in the form of miRNA inhibitors and miRNA mimics, can regulate the expression and repression of numerous genes. Finally, due to their small size, miRNAs can be efficiently delivered to cells via NPs and operate at the cytoplasmic level.104
Despite the ongoing challenges in delivering miRNAs using nanocarriers, such as low encapsulation efficiency and the necessity for cellular targeting, progress is being made.105 When nanoprecipitation or emulsion-based preparation methods are utilized, the rapid diffusion of miRNAs into the aqueous phase due to their high affinity for water results in low encapsulation efficiency.105 While NPs can enhance the tissue distribution and site-specific localization of miRNAs, the degree of improvement is generally inadequate. As a result, many studies have focused on modifying the NP surface with ligands that target specific receptors on the cells of interest, thereby facilitating receptor-mediated endocytosis for NP uptake and reducing the required treatment dose and side effects. It is crucial to recognize that the disease environment plays a critical role in determining the physical and biological barriers that NPs must overcome, as well as the underlying obstacles that hinder miRNA delivery.106 Consequently, various strategies have been developed to prepare NPs capable of efficiently delivering miRNAs to target cells, with a particular emphasis on ensuring the colloidal stability of NPs in complex physiological media for cell-targeted delivery of miRNAs.107 Upon administration, NPs should ideally remain in circulation until they reach the intended location and achieve endosomal escape, facilitating effective interactions between miRNAs and their intracellular targets (eg, leveraging the proton sponge effect). Nevertheless, the duration of cycling is contingent upon the interaction between NPs and the biological microenvironment, potentially resulting in their prompt elimination. Upon exposure to bodily fluids, the surface of NPs becomes coated with plasma proteins.108 This leads to the concealment, indiscriminate uptake, and decrease in the stability, of surface ligands. The NP cycle half-life, MPS separation and biodistribution are affected by various variables, such as size, surface charge, shape, and hydrophobicity.103 Neutral particles exhibit lower susceptibility to conditioning compared to strongly charged particles, especially those with a positive charge (ie, cationic particles).109 Water repellency can lead to faster clearance from the body, but this can be countered by surface modifications such as PEGylation, which improves particle circulation time in the bloodstream.110 Lipids are a major component of cell membranes, enabling lipid-based NPs (LNPs) to interact with membranes and facilitate uptake of their contents by cells. LNPs are structured with lipid bilayers that enclose miRNAs within aqueous cores, or, in the case of multilamellar liposomes, between the lipid bilayers. These LNPs typically comprise cationic lipids, neutral lipids, and PEGylation.111 The natural electrostatic attraction between cationic lipids and negatively charged miRNAs facilitates the effective condensation of miRNAs, offering protection against enzymatic degradation. In addition, positive charges contribute to interactions with oppositely charged cell membranes.111 Nevertheless, the utilization of cationic lipids is commonly linked to cytotoxicity due to their ability to disturb the integrity of cell membranes, stimulate the formation of cytoplasmic vacuoles, and decrease cellular activity.112 Furthermore, cationic lipids have the ability to interact with serum proteins that have a negative charge, leading to the creation of aggregates that are later cleared by the liver and spleen. Proposed methods to decrease the positive charge, such as incorporation with neutral lipids like cholesterol (Chol), dioleoylphosphatidylethanolamine (DOPE), and phosphatidylcholine (PC) (co-lipids), have been suggested to improve stability and minimize toxicity.113 Furthermore, PEGylation has been employed to enhance the half-life of LNPs, as evidenced by the application of polyethylene glycolated cationic/neutral LNPs for delivering the liver-specific tumor suppressor miR-122 in the treatment of hepatocellular carcinoma (HCC). Leading to a reduction in tumor growth of approximately 50% within a 30-day period.114 Multiple studies have shown the increasing benefits of miRNAs in treating OA. Thus, the integration of innovative nanotechnology with miRNAs exhibits potential for attaining optimal therapeutic outcomes in the imminent future.
Nanomolecular Drugs
Agents used for nanomedicine are mainly categorized into nanomolecular drugs and nanocarrier drugs. Nanomolecular drugs directly processing raw materials into NPs, such as aprepitant capsules used for anti-nausea treatment.115 Dai et al utilized the autofluorescence of curcumin (Cur) to design a novel carrier-free self-assembled nanodrug comprising Cur and icariin (ICA) NPs. These Cur/ICA NPs exhibited low cytotoxicity, superior cellular absorption, and sustained drug release. They effectively inhibited inflammatory cytokine secretion and reduced chondrogenic deterioration. Furthermore, in vitro and in vivo trials demonstrated that NPs exhibited improved synergistic anti-inflammatory as well as chondroprotective effects compared with those exerted by Cur or ICA individually.116 Polydopamine (PDA), a naturally occurring biopolymer with active surface functionality, was utilized by Wu et al to synthesize polydopamine NPs (PDA-PEG NPs) from methoxy polyethylene glycol amine (mPEG-NH2). These NPs slowed down the degradation of cartilage in OA mice by inhibiting subchondral bone resorption and angiogenesis, showcasing their potential for treating OA.117 Overall, nanomolecular drugs show great promise in OA therapy.
Modification of Nanomaterials
Effective loading of therapeutic OA biomolecules can be achieved by selecting different forms of nanomaterials, which, to some extent, fulfills the functional requirements for carriers designed to restore and rejuvenate cartilage and control inflammatory responses. Numerous investigations have demonstrated that “the structure of nanomaterials, including factors such as size and apparent surface charge significantly influences the loading efficiency of drugs”. Recently, there has been increased attention on activating nanomaterials by incorporating corresponding stimulating components, leading to targeted delivery and controlled drug release, thereby enhancing therapeutic effects.118 In the subsequent sections, we delve into specific aspects of nanomaterial design to offer fresh insights to researchers.
Size of Nanomaterials
Particle size emerges as a critical factor determining the depth of tissue penetration for nanocarriers. Interaction between injected NPs and synovial fluid is influenced by NP size. For instance, PLGA NPs undergo dimensional and surface charge changes upon interaction with synovial fluid, affecting cellular phagocytosis. Protein crowns formed by synovial fluid on the particle surface can obscure surface modifications or targeting ligands, impacting their interaction with cells or tissues.119 Singh et al prepared polymer NPs that were 500–900 nm in size utilizing a polyhydroxymethacrylate backbone as well as hydrophobic pyridine side chains. They revealed that the 900-nm NPs have a half-life of 2.5 d, whereas that of the 500-nm NPs was only 1.9 d.120 In patients with OA, damaged areas are primarily concentrated in the cartilage and synovium, each requiring nanocarriers of different sizes. NPs 55–60-nm can penetrate entire cartilage, even though the cartilage matrix has an average pore diameter of approximately 6 nm. even in healthy individuals.121,122 However, as OA progresses, cartilage tissue damage leads to fewer chondrocytes, decreased proteoglycan content, and increased pore size,123 facilitating easier penetration of drug nanocarriers Simultaneously, a dilemma arises as composite nanocarriers become more difficult to retain in the ECM, resulting in an impossibility to sustain an efficacious drug concentration in the damaged cartilage for a long period of time. In an ex vivo experiment, researchers observed that NPs with a particle size of 138 nm failed to penetrate the injured cartilage, indicating that the size threshold of the NPs for penetrating OA cartilage was between 55 and 140 nm.124 The synovium is another major site of damage in patients with OA. The surface of the synovium consists of a discrete layer of synoviocytes with cellular gaps ranging from 0.1 to 5.5 μm and without a basement membrane. In addition, because of the abundant network of perforated or continuous capillaries on the superficial surface, the synovium is facilitates the entry and exit of various particles from the joint. The synovium is composed of two main types of cells.125 Encompassing type A synoviocytes or macrophages, which proliferate rapidly in an inflammatory environment as well as generate numerous pro-inflammatory factors; and type B synoviocytes, which are similar to fibroblasts and comprise the majority of synoviocytes (approximately 70%). These cells are capable of synthesizing HA, which is the main lubricant in synovial fluid. The histological features of synovitis in patients with OA include synovial lining proliferation, macrophage and lymphocyte infiltration, angiogenesis, and fibrosis.126 Although IA injections of corticosteroids or pro-inflammatory cytokine antagonists are being used to treat OA, their rapid removal from the joints and inability to markedly ameliorate the inflammatory milieu throughout the joints is a major challenge associated with their clinical application.127 Hence, the development of more effective drug delivery vehicles for synovitis is urgently needed. Lipid-soluble molecules diffuse through the synovial tissue and into synovial cells more readily than hydrophilic molecules. In isolated porcine synovium, researchers found that only 5 nm gold NPs could penetrate the entire synovial tissue,128 while NPs with a particle diameter over 250 nm did not readily leak from synovial cells to synovial cells.125 Consequently, most large particles tended to accumulate in synovial membranes, leading to passive accumulation and prolonged retention time of nanomedicine in the synovium, maintaining effective concentration levels to some extent. In addition, macrophages in the synovium are targeted for OA treatment due to inherent phagocytosis and their crucial role in the disease Champion et al found that medium-sized particles (2–3 μm) were most readily phagocytosed and adhered to by macrophages,129 whereas particles with a size of 26.5 μm were not phagocytosed by macrophages. In contrast, submicron-sized NPs not only translocate into deep synovial tissue but are also phagocytosed by macrophages.
In conclusion, the size of a nanocarrier affects its penetration ability. Researchers have long noted that cartilage degeneration occurs earliest in the superficial region.130 Consequently, for patients with early stage of OA, it is possible to consider increasing the carrier particle size at the expense of the depth of tissue penetration to increase drug-loading for improved therapeutic results. However, this proposition needs to be confirmed through further in-depth studies. Additionally, small animal models are now the dominant choice for animal investigations, neglecting differences in the thicknesses of cartilage and joint spaces among different species. For example, the thickness of cartilage is about 50.0 μm in mice, 604.0 μm in mini-pigs, 1.0 mm in crab-eating monkeys, 1.5–2.0 mm in human beings, and 1.1 mm in sheep.131–133 Therefore, it is likely that NPs that penetrate the cartilage in mice are rapidly removed from human joints. With the goal of accurately modeling the potential effectiveness that NPs may have in human joints and fostering clinical translation, larger animals must be selected for further in vivo experiments. Another important factor to consider when developing IA nanotherapies to treat arthritis is the potential impact of nanotherapies on the subchondral bone. The majority of current in vivo investigations have concentrated on the effects that particles have on synovial and cartilaginous tissues, ignoring the fact that supracartilaginous bone is similarly susceptible to articular disorders and therapies. Furthermore, limited information regarding this aspect is currently available, emphasizing the need for further studies.
Surface Charge of Nanomaterials
Healthy adult cartilage matrix is characterized by sulfated glycosaminoglycans, carrying numerous anions and creating a negatively charged environment ranging from −182 to −158 mm.134 A large body of literature suggests that the surface charge of nanomaterials strongly influences drug delivery efficiency. Zeta potential, as a passive targeting strategy, plays a crucial role in tissues like cartilage and synovium through electrostatic interactions. Simultaneously, weak and reversible non-specific electrostatic interactions enhance drug penetration and residence in cartilage. Therefore, the effect of the surface charge should be emphasized when designing nanomaterials for drug delivery. Researchers have synthesized NPs with different surface charges using ammonium (cationic), phosphonate (neutral), and carboxylate (anionic) as terminal functional group modifications. Notably, ammonium-modified NPs at +7.6 mV could effectively enter the cartilage of mice, while phosphonate and carboxylate NPs accumulated solely in the superficial region of the cartilage, exhibiting slower absorption.135 This suggests that cations are suitable as composite nanocarriers. Both the tiny dimension of manganese dioxide NPs (less than 20 nm in size) and their cationic nature are structural features that facilitate their access to cartilage, demonstrating a chondroprotective effect. Their retention and biodistribution properties post IA injection suggest a promising approach to protecting arthritic chondrocytes.136 Furthermore, research indicates that using positively charged NPs in damaged joints of patients with OA accelerates drug transport, shortens the time to reach therapeutic concentrations, as well as successfully prolongs the half-life of drug within the body.137 In vitro experiments, avidin, a positively charged drug delivery particle with a diameter of 10 nm, penetrated the entire thickness of a cartilage explant and remained in the cartilage for more than 15 d.138 Moreover, multivalent cationic nanostructured avidin showed favorable results in rapidly penetrating cartilage throughout its full thickness in elevated concentrations and extended IA preservation time.139 When PLGA NPs were surface-modified using quaternary ammonium-cationized dodecylammonium bromide, the particles could be passively localized to cartilage. In in vitro experiments, the retention rate of modified PLGA NPs was discovered to be four times greater than that of negatively charged NPs.119
Yao et al used mercapto polyhedral oligosilsesquioxane as a nanoconstructive platform,140 connecting PEG, 2-([1,1-biphenyl]-4-yl carbamoyl), benzoic acid, hydrogenated soybean phosphatidylcholine, and fluorescein to construct cartilage repair nanomaterials with fluorescence visualization. They then used microfluidic technology to prepare microfluidic HA methacrylate microspheres loaded with these nanomaterials. The resultant nanocomposite hydrogel was then injected in situ into the joint cavity, creating a cushioning lubrication layer in the joint space, reducing friction between the articular cartilage. Simultaneously, it released positively charged mercapto polyhedral oligosilsesquioxane/polyethylene glycol/benzoic acid/hydrogenated soya phosphatidylcholine NPs into the deep cartilage through electromagnetic force. This facilitated the promotion of bone marrow mesenchymal stem cell differentiation into chondrocytes. Fluorescence visualization aided in determining the drug’s location and understanding the progress of cartilage repair. However, as OA progresses and sulfated proteoglycans are lost, the negative surface charge of the cartilage decreases, which is not conducive to a targeting strategy that relies solely on electrostatic action. In addition, nanomedicines may adsorb anions in the synovial fluid, thereby altering or even reversing the cationic charge.119 In conclusion, the benefit of zeta potential varies in various phases of OA, and requires careful consideration when designing nanomedicine carriers.
Composite Nanomaterials
Several studies have reported the efficacy of delivering chondroprotectants with NPs and microparticles.141 In vivo experiments showed statistically indistinguishable histological scores for the two drugs, emphasizing the importance of considering the mechanism and site of drug action when designing carriers. Chondrocytes, the sole resident cells in cartilage organization, oversee the maintenance and production of cartilage ECM and serve as delivery targets for enhanced drug efficacy. Numerous drugs have been developed for chondrocytes, including nucleic acids, phages, growth factors, chondroprotectors, and matrix metalloproteinase inhibitors.75,142 For these therapeutic strategies to be effective, the drug must reach the chondrocytes and remain there for a certain period of time at a sufficient concentration. Nanomaterials targeting cartilage facilitate the clinical translation of these emerging therapies. The uptake of nanomedicines by chondrocytes can be enhanced using cell-penetrating peptide surface modifications.124 The binding of cell-penetrating peptide-modified nanocarriers to chondrocytes is significantly higher than that of unmodified nanocarriers. Furthermore, HA modification can improve the affinity of nanocarriers for chondrocytes.143 Cell uptake experiments have shown that this affinity is mainly derived from the fact that HA can bind to the CD44 receptor on the surface of chondrocytes and participate in the NP endocytosis process. Research on cartilage-targeted vectors is in its infancy, and the specific efficacy of this strategy lacks definition due to limited large animal samples or clinical studies, necessitating further exploratory studies.
Active targeting of the synovial membrane is mainly directed at macrophages. The utilization of macrophage surface receptors can enhance the uptake of drugs by macrophages, thereby improving the efficacy of nanomedicines modified by targeting ligands. For instance, prophagocytic peptides can promote macrophage phagocytosis by binding to Fc receptors or neurofibrillary protein 1 receptors on macrophages. Jain et al encapsulated DNA from plasmids encoding the inflammation-fighting cytokine IL-10 into alginate NPs and achieved the targeting of active macrophages through pro-phagocytin peptide surface modification.144 This showed that pro-phagocytin-modified NPs successfully increased the percentage of M2 type macrophages in OA from 46% to 66% compared to untargeted NPs, this led to a significant decrease in pro-inflammatory cytokine expression (IL-1β, IL-6, and TNF-α) both systemically and in the joints. Moreover, Li et al produced a macrophage-derived microvesicle (MMV) inspired by the ability of macrophages to target inflammatory infiltrates to treat rheumatoid arthritis.145 Proteomic analyses indicated that MMV could be targeting intercellular adhesion molecule 1 or P-selectin. PLGA NPs loaded with tacrolimus, an immunomodulatory agent that inhibits T cell activation, were encapsulated with MMVs. Results of in vivo experiments performed using mice showed that MMV-targeted NPs significantly alleviated paw swelling and subchondral bone erosion compared with non-targeted controls. In conclusion, many studies designing actively targeted nanocarriers have demonstrate their enhanced efficacy compared to non-targeted nanocarriers, indicating promising developments in this field.
Design of Stimulus-Responsive Nanomaterials
The treatment of OA presents a multifaceted and dynamically evolving process. While nanomaterials are generally perceived as inert because of their unalterable structural characteristics, such as their size, the dynamic demands of OA treatment have led researchers to propose the integration of stimulus-responsive components into nanomaterials, creating responsive nanocarriers. These nanocarriers, categorized as being internal stimulus-responsive (pH-responsive, ROS-responsive, etc) or external stimulus-responsive (infrared light-responsive, magnetic-responsive, and ultrasound-responsive) based on the type of stimulation, hold great promise for the treatment of OA.146 The microenvironment of OA joint sites exhibits pathological alterations related to pH and ROS, prompting the development of current DDSs responsive to endogenous stimuli to achieve optimum and targeted drug delivery in the joint. The acidic microenvironment of osteoarthritic joints, attributed to increased metabolic activity and inadequate vascular supply, supports the use of pH-responsive drug release nanomaterials. Zhou et al devised a system wherein MnO2-encapsulated hydrogel NPs, crosslinked by the Schiff base reaction using bovine serum albumin (BSA)-MnO dispersed in a HA/platelet-rich plasma gel network, were developed. This system demonstrated responsiveness to the acidic microenvironment, enabling the delivery of BM NPS and growth factors.147 Wang et al also engineered a pH-responsive nanofibrous membrane composed of polycaprolactone and poly (ethylene glycol)-naringenin (PCL/PEG-Nar), that sustains the release of the anti-inflammatory agent Nar in weakly acidic OA microenvironments.148 Qin et al designed inflammatory microenvironmentally dual-responsive deformable NPs (DKPNPs) consisting of the amyloid β-derived peptide KLVFF and polysialic acid (PSA) coupled to the anti-inflammatory drug dexamethasone (Dex). In normal physiological environments, these NPs remain stable, with the PSA shell conferring long-term circulation and inflammatory macrophage-targeting abilities to the DKPNPs. Upon reaching inflammatory articulation, acidic pH-triggered Dex dissociation or inflammatory macrophage-induced PSA ligand-receptor-specific binding induces the conversion of DKPNPs from NPs to nanofibers, which inhibits the clearance of the drug from the lymphatic vessels, ultimately enhancing the effectiveness of medications.149
Because more severe ROS are present in the pathologic microscopic environment associated with OA than in healthy joints,150 a number of ROS-responsive drug delivery platforms for OA therapeutics have been developed. For instance, a nanoprobe with reduced toxicity and ROS-responsive properties was successfully developed by Wu et al. The nanoprobe was prepared from ROS-sensitive thione linker (TK) and cartilage-targeting peptide (TKCP)-modified PEG micelles. It is able to specifically target articular cartilage via CAP and respond to high levels of ROS (Figure 4A).151 In inflamed tissues, the thioaldehyde bond is cleaved as a result of abundant ROS, leading to progressive degradation of the polymer and release of Cy5.5 and drug (Figure 4B).151 Wu et al also prepared an ROS scavenging and drug release platform by encapsulating ROS-erasable poly(ethylene glycol)-b-poly(thioacetal)-b-poly(ethylene glycol) (PEG-PTK-PEG) micelles (PDM) loaded with dexamethasone acetate into an injectable hydrogel. The multifunctional injectable hydrogel effectively reduced joint inflammation via the increased consumption of ROS, suppression of the production of inflammatory cytokines, and promotion of macrophage polarization from the M1 to the M2 state.152
 |
Figure 4 (A) Illustration of the self-assembly process of targeted NPs for treating osteoarthritis. The findings indicate that the nanoprobes can be effectively activated in the presence of high levels of ROS. (B) TKCP@DEX is engineered to target cartilage directly, responding effectively to ROS and gradually releasing DEX. This advanced nanoprobe, activated by the body’s own processes, shows potential for both treating and diagnosing osteoarthritis. Figure parts reprinted with permission from Shen C, Gao M, Chen H, et al. Reactive oxygen species (ROS)-responsive nanoprobe for bioimaging and targeting therapy of osteoarthritis. J Nanobiotechnol. 2021;19:1–6. https://creativecommons.org/licenses/by/4.0/.151 |
In contrast to endogenous stimulus-responsive drug delivery platforms, exogenous stimulus-responsive stimulation platforms allow external stimuli application, enabling precise control over the amount and rate of drug delivery by adjusting exogenous signal power and stimulation time. This offers a promising strategy for controlled drug delivery in OA therapy. Among the light-responsive drug delivery protocols at different wavelengths, near-infrared (NIR) light-responsive drug release platforms are receiving growing attention. Xu et al developed NIR-sensitive multifunctional nanozymes (E@Au-Ag) with the potential to restore mitochondrial dysfunction, preserve cartilage, and rejuvenate cartilage. Serving as a versatile nanoplatform for OA treatment, E@Au-Ag exerts a cooperative effect in repairing mitochondrial injury, promoting cartilage migration, and reducing chondrocyte apoptosis during OA treatment. Moreover, it exhibits intrinsic antioxidative stress properties and can reduce chondrocyte apoptosis by 83.3% in vitro. In rats, the IA injection of E@Au-Ag resulted in the heating of the joint cavity to 46.6 °C under near-infrared conditions, facilitating the additional release of EGCG and stimulating cartilage regeneration. Moreover, this treatment significantly improved cartilage wear and tear in arthritic rats, leading to a considerable thickening of the cartilage layer. Moreover, the excellent antimicrobial ability of E@Au-Ag effectively prevented infections caused by IA injections.153 Additionally, owing to the advantages of magnetic materials, numerous magnetically induced drug delivery nanoplatforms have been successfully designed for OA therapy. For example, Chen et al synthesized a hyaluronic acid-polyacrylic acid (HA-pAA) hydrogel using polyacrylic acid-ferrocene (pAA-Fc) and hyaluronic acid-cyclodextrin (HA-CD) and designed porous polylactic acid-hydroxyglycolic acid (PLGA) magnetic microcapsules (PPMMs) containing glutathione (GSH) and iron oxide NPs. This approach demonstrated potential in enhancing the regeneration of degenerated cartilage by enhancing chondrocyte transportation to the injured region through CD44 receptors on the HA polymer chains of layer-by-layer (LbL)-PPMMs via endomagnetism.154 Su et al labeled chondrocytes with magnetic NPs which were cultured on type II collagen chitosan/polylactoglycolic acid scaffolds. These magnetic NPs had no significant effect on the chondrocyte phenotype. After culturing on scaffolds, better chondrogenic and osteogenic characteristics were observed in labeled cells.155 Orthopedic issues such as fracture healing and cartilage lesion detection have been resolved using pressure wave ultrasound at 20 kHz or higher.156 Ultrasound can also be used to design therapeutic DDSs for osteoarthritis, such as ultrasound-responsive microbubbles (MBs) that can remotely activate and modulate drug release as well as increase cell permeability.17 For instance, Yu et al developed KGN loaded with poly (propyleneglycolide-hydroxyglycolic acid) MBs (MBs@KGN), which were then embedded in carboxymethyl chitosan-oxidized chondroitin sulfate (CMC-OCS) hydrogel to produce ultrasound-responsive scaffolds (CMC-OCS/MBs@KGN). The results indicated that the ultrasound-responsive scaffold CMC-OCS/MBs@KGN sustained the release of KGN and enhanced the expression and differentiation of rabbit bone marrow mesenchymal stem cells.157 Specifically, ultrasound is used as a stimulus for the release of calcium ions from liposomes, which activates the enzymatic activity of transglutaminase (Figure 5A).158 The activated enzyme promotes the formation of fibrinogen hydrogels by catalyzing covalent intermolecular cross-linking (Figure 5B).158
 |
Figure 5 Ultrasound-responsive hydrogels work through the following mechanisms: (A) Ultrasound activates enzyme-catalyzed protein binding; (B) Ultrasound initiates fibrinogen cross-linking. Figure parts reprinted with permission from Nele V, Schutt CE, Wojciechowski JP, et al. Ultrasound-triggered enzymatic gelation. Adv Mater. 2020;32(7):e1905914. WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim. https://creativecommons.org/licenses/by/4.0/.158 |
Regarding the choice of stimulus response, it has been proposed that exogenous stimulus-responsive nanomedicine release platforms offer advantages such as the possibility for precise remote regulation and multifunctional integration compared to endogenous stimulus-responsive platforms.17 This is because stimuli can be externally applied rather than being confined to pre-existing pathological factors at the focal site. Many endogenous triggers are not specific to OA, which can lead to unintended release of the drug at non-focal sites. Therefore, exogenous stimulus responses are superior to endogenous stimuli, allowing precise control of on/off switching and drug release rates by controlling the on/off switching and power of the external stimulus source. However, some scholars argue that while exogenous stimulus-responsive nanocarriers can enable precise control of drug release behavior, this ability tends to be limited by the depth of tissue penetration of the stimulus source,17 whereas endogenous stimulus-responsive platforms are not similarly affected.
As research progresses, dual- or multiple-stimulus-responsive/functional controlled drug release platforms have become viable options to further enhance therapeutic efficacy. Jiang et al prepared dual-responsive nanomedicines with active targeting functions by loading glucocorticoids with pH/ROS dual-responsive carriers, modifying and the surface of the NPs with PEG as well as RGD peptides. In vitro cellular experiments suggested that the nanomedicines were readily phagocytosed by macrophages and synoviocytes and significantly promoted the conversion of M1-type macrophages to the M2 phenotype. In vivo imaging results demonstrated that the nanomedicines significantly aggregated at RA inflammation sites compared to unencapsulated drugs. Results of in vivo animal trials revealed that the nanomedicine reduced the expression of inflammatory factors, such as TNF-α, IL-1β, and IL-6, and blocked the NF-KB modeling channel. This, in turn, markedly alleviated the inflammatory swelling response and joint destruction in mice. By addressing challenges such as the low effective concentration of glucocorticoid drugs in the inflammatory area and high incidence of systemic side effects, this nanomedicine offers a novel targeted therapy for rheumatoid arthritis.159 Additionally, Xue et al reported a novel nanomaterial platform RB@MPMW. This nano-sized sphere, consisting of a metal-organic framework shell layer and a mesoporous polydopamine (MPDA) core layer, with the shell layer loaded with bilirubin (Br) and the core layer loaded with rapamycin (Rap), was surface-modified with a type II collagen-targeting peptide (WYRGRL). This structure guided accurate drug delivery to the articular cartilage and facilitated Br and Rap release in response to photothermal and pH stimulation. Br demonstrated significant antioxidative effects by neutralizing oxygen free radicals (ROS) and converting them to bilirubin, while Rap, a potent autophagy activator, enhanced autophagy activation and chondrocyte protection. In an anterior cruciate ligament transection-induced rat OA model, this nanomaterial effectively delayed disease progression and promoted cartilage repair through these dual mechanisms, achieving positive therapeutic results.160
Challenges Associated with, and Prospects for, the Use of Nanomaterials in OA Treatment
Nanomaterials demonstrate a wide range of potential applications and research value in OA applications.161 The main applications include the following. 1. Nanometer drug-carrying system: These systems can increase the concentration of drugs in OA lesion tissues by controlling the release rate and targeting of drugs, thus effectively reducing pain, alleviating inflammatory response, and promoting tissue repair. This customized treatment plan improves therapeutic efficacy and reduces side effects.162 2. Nanometer immunotherapy: Nanomaterials can also be used for immunotherapy, by regulating immune cell activity through suitable nanocarriers, reducing inflammatory response and pain.3. Nanomaterials delivery of genes. Nanomaterials effectively encapsulate and protect gene drugs, enabling targeted delivery through surface modification or functionalization to address underlying factors of OA. Several nanomaterials have successfully entered clinical trials for the treatment of OA (Table 2). For example, Leite et al conducted a randomized controlled trial of patients with knee cartilage using a nanoemulsion containing glucosamine and chondroitin sulfate (NANO-CG). They observed pain relief and full articular cartilage recovery in some patients treated with NANO-CG;163 Additionally, Hashemzadeh et al conducted a subgroup trial of two groups of patients taking placebo and nanocurcumin and found that nanocurcumin significantly improved the symptoms of osteoarthritis patients with OA;164 Furthermore, Shakouri et al used leech saliva extract (LSE) in liposomal gel as a complementary treatment for the relief of signs and symptoms of OA. They found that after 1 month of a LSE liposomal gel administration, patients experienced pain relief and enhanced quality of life.165 Although significant progress has been made in nanotechnology, its development and translation face a number of challenges and limitations that need to be addressed to achieve successful clinical translation.166,167 Firstly, there are significant differences in cartilage composition and thickness between animals and humans. For example, mouse cartilage, is 30–40 times thinner than human cartilage, and nanomaterials may be rapidly removed before they reach the human joint cavity, resulting in lower-than-expected efficacy.168 Current research on nanomaterials relies on animal models, which poses a great challenge for further clinical applications of nanomaterials. Therefore, establishing natural models for IA administration in OA is crucial to improve clinical relevance.169 Secondly, the safety and biocompatibility of NPs are important factors for clinical translation.170 The long-term effects of many materials in vivo are not fully understood; therefore, it is critical that the safety of nanomaterials be thoroughly evaluated before they are used in the clinical treatment of OA. In addition to safety issues, complex joint structures and dynamic joint environments complicate the clinical application of nanomaterials in the treatment of OA.171 Therefore, to maximize the therapeutic potential of nanomaterials, researchers must carefully and comprehensively consider the structural and environmental characteristics of the joints when designing nanomaterials for the treatment of OA.69
 |
Table 2 Nanomaterials Entering Clinical Trials for OA |
Despite these challenges, the potential of nanomaterials in OA treatment remains promising several future research directions, including advanced DDSs, individualized nanomedicine, clinical trials, translational research, biomimetic nanomaterials, and combination therapies, could further enhance the application of nanotechnology in the management of OA. Monitoring these prospective research avenues could lead to the development of nanotechnology-enhanced biomaterials for OA therapy, potentially revolutionizing treatments and offering effective and safe approaches for managing OA.30
Abbreviations
OA, Osteoarthritis; NSAIDs, Nonsteroid anti-inflammatory drugs; IA, intra-articular; ECM, extracellular matrix; HA, hyaluronic acid; MMPs, matrix metalloproteinases; ROS, reactive oxygen species; SIN1, 3-morpholinoaniline; TBHP, tert-butyl hydroperoxide; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; RvD1, Resolvin D1; GNPs, Gold NPs; FPs, fish oil proteins; DPPC, dipalmitoyl phosphatidyl choline; GSH, glutathione reductase; SOD, superoxide dismutase; SPIONs, superparamagnetic iron oxide NPs; MRI, magnetic resonance imaging; MSCs, mesenchymal stem cells; PNPs, Polymer NPs; PGA, polyglycolic acid; PLA, polylactic acid; PLGA, polylactic acid-glycolic acid; PCL, poly-ε-caprolactone; PMMA, polymethyl methacrylate; ELNVs, Extracorporeal-like nanovesicles; PELNVs, Plant exosome-like nanovesicles; SOD, superoxide dismutase; IGF-1, Insulin-like growth factor 1; PPK, PEG-PAMAM-KGN; KPP, KGN-PEG-PAMAM; PEG, polyethylene glycol; DDS, Drug delivery systems; Lnx-MS, Lnx-encapsulated PLGA microspheres; GSH, glutathione; KGN, katoglobulin; API, active minor pharmaceutical ingredient; miRNAs, MicroRNAs; NPs, nanoparticles; Chol, cholesterol; DOPE, dioleoyl phosphatidylethanolamine; PC, phosphatidylcholine; LNPs, lipid NPs; HCC, hepatocellular carcinoma; Cur, curcumin; ICA, Icariin; PDA, Polydopamine; MMV, macrophage derived microvesicle; PSA, Polysialic acid; DKPNPs, dual-responsive deformable NPs; Dex, dexamethasone; NIR, near-infrared; PPMMs, porous polylactic magnetic microcapsules; LBL, layer-by-layer; MB, microbubbles; MPDA, mesoporous polydopamine; Br, bilirubin; Rap, rapamycin; WYRGRL, type II collagen-targeting peptide; NANO-CG, nanoemulsions combining glucosamine and chondroitin sulfate; LSE, Leech saliva extract.
Data Sharing Statement
No data was used for the research describe.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting or writing, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for the contents of this article.
Funding
This study was supported by the medical and health research project of Zhejiang Province (No. 2022KY354).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105(1):185–199. doi:10.1093/bmb/lds038
2. Collins JA, Wood ST, Nelson KJ, et al. Oxidative stress promotes peroxiredoxin hyperoxidation and attenuates pro-survival signaling in aging chondrocytes. J Biol Chem. 2016;291(13):6641–6654. doi:10.1074/jbc.M115.693523
3. Steinmetz JD, Culbreth GT, Haile LM, et al. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023;5(9):e508–e522. doi:10.1016/S2665-9913(23)00163-7
4. Liu L, Tang H, Wang Y. Nanotechnology-boosted biomaterials for osteoarthritis treatment: current status and future perspectives. Int J Nanomed. 2023;18:4969–4983. doi:10.2147/ijn.S423737
5. Paesa M, Alejo T, Garcia-Alvarez F, Arruebo M, Mendoza G. New insights in osteoarthritis diagnosis and treatment: nano-strategies for an improved disease management. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023;15(2):e1844. doi:10.1002/wnan.1844
6. Pelletier JP, Martel-Pelletier J, Rannou F, Cooper C. Efficacy and safety of oral NSAIDs and analgesics in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45(4 Suppl):S22–S27. doi:10.1016/j.semarthrit.2015.11.009
7. Alcaraz MJ, Guillén MI, Ferrándiz ML. Emerging therapeutic agents in osteoarthritis. Biochem Pharmacol. 2019;165:4–16. doi:10.1016/j.bcp.2019.02.034
8. Nelson AE. Osteoarthritis year in review 2017: clinical. Osteoarthritis Cartilage. 2018;26(3):319–325. doi:10.1016/j.joca.2017.11.014
9. Liang Y, Xu X, Xu L, et al. Non-surgical osteoarthritis therapy, intra-articular drug delivery towards clinical applications. J Drug Target. 2021;29(6):609–616. doi:10.1080/1061186x.2020.1870231
10. Jones IA, Togashi R, Wilson ML, Heckmann N, Vangsness CT. Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol. 2019;15(2):77–90. doi:10.1038/s41584-018-0123-4
11. Liukkonen R, Honkanen M, Skyttä E, Eskelinen A, Karppelin M, Reito A. Trends in revision knee arthroplasty for prosthetic joint infection: a single-center study of 384 knees at a high-volume center between 2008 and 2021. J Arthroplasty. 2023;38(11):2447–2454. doi:10.1016/j.arth.2023.05.033
12. Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376–387. doi:10.1016/s0140-6736(14)60802-3
13. De Roover A, Escribano-Núñez A, Monteagudo S, Lories R. Fundamentals of osteoarthritis: inflammatory mediators in osteoarthritis. Osteoarthritis Cartilage. 2023;31(10):1303–1311. doi:10.1016/j.joca.2023.06.005
14. Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37. doi:10.1038/nrc.2016.108
15. Zhang X, Chen X, Song J, Zhang J, Ren X, Zhao Y. Size-transformable nanostructures: from design to biomedical applications. Adv Mater. 2020;32(48):2003752. doi:10.1002/adma.202003752
16. Zhang X, Chen X, Zhao Y. Nanozymes: versatile platforms for cancer diagnosis and therapy. Nanomicro Lett. 2022;14(1):95. doi:10.1007/s40820-022-00828-2
17. Jiang Q, Zhang S. Stimulus-responsive drug delivery nanoplatforms for osteoarthritis therapy. Small. 2023;19(23):2206929. doi:10.1002/smll.202206929
18. Chen G, Roy I, Yang C, Prasad PN. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem Rev. 2016;116(5):2826–2885. doi:10.1021/acs.chemrev.5b00148
19. Conaghan PG, Cook AD, Hamilton JA, Tak PP. Therapeutic options for targeting inflammatory osteoarthritis pain. Nat Rev Rheumatol. 2019;15(6):355–363. doi:10.1038/s41584-019-0221-y
20. Ng L, Grodzinsky AJ, Patwari P, Sandy J, Plaas A, Ortiz C. Individual cartilage aggrecan macromolecules and their constituent glycosaminoglycans visualized via atomic force microscopy. J Struct Biol. 2003;143(3):242–257. doi:10.1016/j.jsb.2003.08.006
21. Ansari MY, Khan NM, Ahmad I, Haqqi TM. Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthritis Cartilage. 2018;26(8):1087–1097. doi:10.1016/j.joca.2017.07.020
22. Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(7):412–420. doi:10.1038/nrrheum.2016.65
23. Altindag O, Erel O, Aksoy N, Selek S, Celik H, Karaoglanoglu M. Increased oxidative stress and its relation with collagen metabolism in knee osteoarthritis. Rheumatol Int. 2007;27(4):339–344. doi:10.1007/s00296-006-0247-8
24. Bolduc JA, Collins JA, Loeser RF. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med. 2019;132:73–82. doi:10.1016/j.freeradbiomed.2018.08.038
25. Thoene M, Bejer-Olenska E, Wojtkiewicz J. The current state of osteoarthritis treatment options using stem cells for regenerative therapy: a Review. Int J Mol Sci. 2023;24(10). doi:10.3390/ijms24108925
26. Zhou S, Si H, Peng L, Shen B. 软骨细胞线粒体生物发生在骨关节炎发病机制中的作用 [The role of chondrocyte mitochondrial biogenesis in the pathogenesis of osteoarthritis]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2022;36(2):242–248. Chinese. doi:10.7507/1002-1892.202109091
27. Zhao K, Ruan J, Nie L, Ye X, Li J. Effects of synovial macrophages in osteoarthritis. Front Immunol. 2023;14:1164137. doi:10.3389/fimmu.2023.1164137
28. Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6(11):625–635. doi:10.1038/nrrheum.2010.159
29. Martel-Pelletier J, Barr AJ, Cicuttini FM, et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. doi:10.1038/nrdp.2016.72
30. Zhang S, Wang L, Kang Y, Wu J, Zhang Z. Nanomaterial-based reactive oxygen species scavengers for osteoarthritis therapy. Acta Biomater. 2023;162:1–19. doi:10.1016/j.actbio.2023.03.030
31. Qureshi MA, Khatoon F. Different types of smart nanogel for targeted delivery. J Sci. 2019;4(2):201–212. doi:10.1016/j.jsamd.2019.04.004
32. Neamtu I, Rusu AG, Diaconu A, Nita LE, Chiriac AP. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017;24(1):539–557. doi:10.1080/10717544.2016.1276232
33. Li D, van Nostrum CF, Mastrobattista E, Vermonden T, Hennink WE. Nanogels for intracellular delivery of biotherapeutics. J Control Release. 2017;259:16–28. doi:10.1016/j.jconrel.2016.12.020
34. Soni KS, Desale SS, Bronich TK. Nanogels: an overview of properties, biomedical applications and obstacles to clinical translation. J Control Release. 2016;240:109–126. doi:10.1016/j.jconrel.2015.11.009
35. Hans ML, Lowman AM. Biodegradable NPs for drug delivery and targeting. Curr Opin Solid State Mater Sci. 2002;6(4):319–327. doi:10.1016/S1359-0286(02)00117-1
36. Dravid AA, Md K, Agarwal S, Agarwal R. Resolvin D1-loaded nanoliposomes promote M2 macrophage polarization and are effective in the treatment of osteoarthritis. Bioeng Transl Med. 2022;7(2):e10281. doi:10.1002/btm2.10281
37. Liu Q, Kim Y-J, G-b I, et al. Inorganic NPs applied as functional therapeutics. Adv Funct Mater. 2021;31(12):2008171. doi:10.1002/adfm.202008171
38. Xiao R, Zhou G, Wen Y, Ye J, Li X, Wang X. Recent advances on stimuli-responsive biopolymer-based nanocomposites for drug delivery. Compos Part B Eng. 2023;266:111018. doi:10.1016/j.compositesb.2023.111018
39. Zandieh M, Liu J. Nanozymes: definition, activity, and mechanisms. Adv Mater. 2024;36(10):2211041. doi:10.1002/adma.202211041
40. Wang X, Devaiah SP, Zhang W, Welti R. Signaling functions of phosphatidic acid. Prog Lipid Res. 2006;45(3):250–278. doi:10.1016/j.plipres.2006.01.005
41. Liu C, Su C. Design strategies and application progress of therapeutic exosomes. Theranostics. 2019;9(4):1015–1028. doi:10.7150/thno.30853
42. An Q, van Bel AJ, Hückelhoven R. Do plant cells secrete exosomes derived from multivesicular bodies? Plant Signal Behav. 2007;2(1):4–7. doi:10.4161/psb.2.1.3596
43. Zhuang X, Teng Y, Samykutty A, et al. Grapefruit-derived nanovectors delivering therapeutic miR17 through an intranasal route inhibit brain tumor progression. Mol Ther. 2016;24(1):96–105. doi:10.1038/mt.2015.188
44. Hou W, Ye C, Chen M, et al. Excavating bioactivities of nanozyme to remodel microenvironment for protecting chondrocytes and delaying osteoarthritis. Bioact Mater. 2021;6(8):2439–2451. doi:10.1016/j.bioactmat.2021.01.016
45. Huang Y, Ren J, Qu X. Nanozymes: classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem Rev. 2019;119(6):4357–4412. doi:10.1021/acs.chemrev.8b00672
46. Jiang X, Gray P, Patel M, Zheng J, Yin JJ. Crossover between anti- and pro-oxidant activities of different manganese oxide NPs and their biological implications. J Mater Chem B. 2020;8(6):1191–1201. doi:10.1039/c9tb02524c
47. Zhang Y, Gao W, Ma Y, et al. Integrating Pt NPs with carbon nanodots to achieve robust cascade superoxide dismutase-catalase nanozyme for antioxidant therapy. Nano Today. 2023;49:101768. doi:10.1016/j.nantod.2023.101768
48. Altuntaş E, Özkan B, Güngör S, Özsoy Y. Biopolymer-Based Nanogel Approach in Drug Delivery: basic Concept and Current Developments. Pharmaceutics. 2023;15(6). doi:10.3390/pharmaceutics15061644
49. Nedunchezian S, Wu CW, Wu SC, Chen CH, Chang JK, Wang CK. Characteristic and chondrogenic differentiation analysis of hybrid hydrogels comprised of Hyaluronic Acid Methacryloyl (HAMA), Gelatin Methacryloyl (GelMA), and the acrylate-functionalized nano-silica crosslinker. Polymers. 2022;14(10). doi:10.3390/polym14102003
50. Li T, Yang J, Weng C, et al. Intra-articular injection of anti-inflammatory peptide-loaded glycol chitosan/fucoidan nanogels to inhibit inflammation and attenuate osteoarthritis progression. Int J Biol Macromol. 2021;170:469–478. doi:10.1016/j.ijbiomac.2020.12.158
51. Yang G, Fan M, Zhu J, et al. A multifunctional anti-inflammatory drug that can specifically target activated macrophages, massively deplete intracellular H(2)O(2), and produce large amounts CO for a highly efficient treatment of osteoarthritis. Biomaterials. 2020;255:120155. doi:10.1016/j.biomaterials.2020.120155
52. Duan Q-Y, Zhu Y-X, Jia H-R, Wang S-H, Wu F-G. Nanogels: synthesis, properties, and recent biomedical applications. Pro Mater Sci. 2023;139:101167. doi:10.1016/j.pmatsci.2023.101167
53. Nedunchezhiyan U, Varughese I, Sun AR, Wu X, Crawford R, Prasadam I. Obesity, Inflammation, and Immune System in Osteoarthritis. Front Immunol. 2022;13:907750. doi:10.3389/fimmu.2022.907750
54. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi:10.1172/jci19246
55. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. doi:10.1172/jci29881
56. Yao Z, Qi W, Zhang H, et al. Down-regulated GAS6 impairs synovial macrophage efferocytosis and promotes obesity-associated osteoarthritis. Elife. 2023;12. doi:10.7554/eLife.83069
57. Thi TTH, Suys EJA, Lee JS, Nguyen DH, Park KD, Truong NP. Lipid-based NPs in the clinic and clinical trials: from cancer nanomedicine to COVID-19 vaccines. Vaccines. 2021;9(4). doi:10.3390/vaccines9040359
58. Chandrakala V, Aruna V, Angajala G. Review on metal NPs as nanocarriers: current challenges and perspectives in drug delivery systems. Emergent Mater. 2022;5(6):1593–1615. doi:10.1007/s42247-021-00335-x
59. Abdel-Aziz MA, Ahmed HMS, El-Nekeety AA, Sharaf HA, Abdel-Aziem SH, Abdel-Wahhab MA. Biosynthesis of gold NPs for the treatment of osteoarthritis alone or in combination with Diacerein(®) in a rat model. Inflammopharmacology. 2021;29(3):705–719. doi:10.1007/s10787-021-00833-8
60. Ramadan S, Guo L, Li Y, Yan B, Lu W. Hollow copper sulfide nanoparticle-mediated transdermal drug delivery. Small. 2012;8(20):3143–3150. doi:10.1002/smll.201200783
61. Wang X, Cai Y, Wu C, et al. Conversion of senescent cartilage into a pro-chondrogenic microenvironment with antibody-functionalized copper sulfate NPs for efficient osteoarthritis therapy. J Nanobiotechnology. 2023;21(1):258. doi:10.1186/s12951-023-02036-5
62. Kan S, Duan M, Liu Y, Wang C, Xie J. Role of mitochondria in physiology of chondrocytes and diseases of osteoarthritis and rheumatoid arthritis. Cartilage. 2021;13(2_suppl):1102s–1121s. doi:10.1177/19476035211063858
63. Yudoh K, Nguyen VT, Nakamura H, Hongo-Masuko K, Kato T, Nishioka K. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res Therapy. 2005;7(2):R380–R391. doi:10.1186/ar1499
64. Xu C, Chen J, Li L, et al. Promotion of chondrogenic differentiation of mesenchymal stem cells by copper: implications for new cartilage repair biomaterials. Mater Sci Eng C Mater Biol Appl. 2018;93:106–114. doi:10.1016/j.msec.2018.07.074
65. Ma JC, Luo T, Feng B, et al. Exploring the translational potential of PLGA NPs for intra-articular rapamycin delivery in osteoarthritis therapy. J Nanobiotechnology. 2023;21(1):361. doi:10.1186/s12951-023-02118-4
66. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision NPs for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124. doi:10.1038/s41573-020-0090-8
67. Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric NPs based drug delivery systems. Colloids Surf B Biointerf. 2010;75(1):1–18. doi:10.1016/j.colsurfb.2009.09.001
68. Yin H, Li M, Tian G, et al. The role of extracellular vesicles in osteoarthritis treatment via microenvironment regulation. Biomater Res. 2022;26(1):52. doi:10.1186/s40824-022-00300-7
69. Zhou D, Zhou F, Sheng S, Wei Y, Chen X, Su J. Intra-articular nanodrug delivery strategies for treating osteoarthritis. Drug Discov Today. 2023;28(3):103482. doi:10.1016/j.drudis.2022.103482
70. Geiger BC, Wang S, Padera RF, Grodzinsky AJ, Hammond PT. Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci Trans Med. 2018;10(469):eaat8800. doi:10.1126/scitranslmed.aat8800
71. Kou L, Xiao S, Sun R, Bao S, Yao Q, Chen R. Biomaterial-engineered intra-articular drug delivery systems for osteoarthritis therapy. Drug Delivery. 2019;26(1):870–885. doi:10.1080/10717544.2019.1660434
72. Huang H, Lou Z, Zheng S, et al. Intra-articular drug delivery systems for osteoarthritis therapy: shifting from sustained release to enhancing penetration into cartilage. Drug Deliv. 2022;29(1):767–791. doi:10.1080/10717544.2022.2048130
73. Ma L, Zheng X, Lin R, et al. Knee osteoarthritis therapy: recent advances in intra-articular drug delivery systems. Drug Des Devel Ther. 2022;16:1311–1347. doi:10.2147/dddt.S357386
74. Evans CH, Kraus VB, Setton LA. Progress in intra-articular therapy. Nat Rev Rheumatol. 2014;10(1):11–22. doi:10.1038/nrrheum.2013.159
75. Brown S, Kumar S, Sharma B. Intra-articular targeting of nanomaterials for the treatment of osteoarthritis. Acta Biomater. 2019;93:239–257. doi:10.1016/j.actbio.2019.03.010
76. Berry H, Bird HA, Black C, et al. A double blind, multicentre, placebo controlled trial of lornoxicam in patients with osteoarthritis of the Hip and knee. Ann Rheum Dis. 1992;51(2):238–242. doi:10.1136/ard.51.2.238
77. Hamza Yel S, Aburahma MH. Design and in vitro evaluation of novel sustained-release double-layer tablets of lornoxicam: utility of cyclodextrin and xanthan gum combination. AAPS Pharm Sci Tech. 2009;10(4):1357–1367. doi:10.1208/s12249-009-9336-9
78. Zhang Z, Huang G. Intra-articular lornoxicam loaded PLGA microspheres: enhanced therapeutic efficiency and decreased systemic toxicity in the treatment of osteoarthritis. Drug Delivery. 2012;19(5):255–263. doi:10.3109/10717544.2012.700962
79. Blair HA. Bupivacaine/meloxicam prolonged release: a review in postoperative pain. Drugs. 2021;81(10):1203–1211. doi:10.1007/s40265-021-01551-9
80. Ziesenitz VC, Welzel T, van Dyk M, Saur P, Gorenflo M, van den Anker JN. Efficacy and safety of NSAIDs in infants: a comprehensive review of the literature of the past 20 years. Paediatr Drugs. 2022;24(6):603–655. doi:10.1007/s40272-022-00514-1
81. Yu Y, Tian Y, Zhang H, et al. The evaluation of meloxicam nanocrystals by oral administration with different particle sizes. Molecules. 2022;27(2). doi:10.3390/molecules27020421
82. Fattahpour S, Shamanian M, Tavakoli N, et al. An injectable carboxymethyl chitosan-methylcellulose-pluronic hydrogel for the encapsulation of meloxicam loaded NPs. Int J Biol Macromol. 2020;151:220–229. doi:10.1016/j.ijbiomac.2020.02.002
83. Zhong Y, Zhou Y, Ding R, et al. Intra-articular treatment of temporomandibular joint osteoarthritis by injecting actively-loaded meloxicam liposomes with dual-functions of anti-inflammation and lubrication. Mater Today Bio. 2023;19:100573. doi:10.1016/j.mtbio.2023.100573
84. Mao L, Wu W, Wang M, et al. Targeted treatment for osteoarthritis: drugs and delivery system. Drug Deliv. 2021;28(1):1861–1876. doi:10.1080/10717544.2021.1971798
85. Sohn HS, Choi JW, Jhun J, et al. Tolerogenic NPs induce type II collagen-specific regulatory T cells and ameliorate osteoarthritis. Sci Adv. 2022;8(47):eabo5284. doi:10.1126/sciadv.abo5284
86. Ansari MY, Ahmad N, Haqqi TM. Oxidative stress and inflammation in osteoarthritis pathogenesis: role of polyphenols. Biomed Pharmacother. 2020;129:110452. doi:10.1016/j.biopha.2020.110452
87. Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009;30(1–2):1–12. doi:10.1016/j.mam.2008.08.006
88. Ramírez-Noguera P, Zetina Marín I, Gómez Chavarin BM, Valderrama ME, López-Barrera LD, Díaz-Torres R. Study of the early effects of chitosan NPs with glutathione in rats with osteoarthrosis. Pharmaceutics. 2023;15(8). doi: 10.3390/pharmaceutics15082172
89. Mota AH, Direito R, Carrasco MP, et al. Combination of hyaluronic acid and PLGA particles as hybrid systems for viscosupplementation in osteoarthritis. Int J Pharm. 2019;559:13–22. doi:10.1016/j.ijpharm.2019.01.017
90. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. doi:10.1038/nature04516
91. Mohamed AL, Elmotasem H, Salama AAA. Colchicine mesoporous silica NPs/hydrogel composite loaded cotton patches as a new encapsulator system for transdermal osteoarthritis management. Int J Biol Macromol. 2020;164:1149–1163. doi:10.1016/j.ijbiomac.2020.07.133
92. Zhang L, Chen X, Cai P, et al. Reprogramming mitochondrial metabolism in synovial macrophages of early osteoarthritis by a camouflaged meta-defensome. Adv Mater. 2022;34(30):e2202715. doi:10.1002/adma.202202715
93. Johnson K, Zhu S, Tremblay MS, et al. A stem cell-based approach to cartilage repair. Science. 2012;336(6082):717–721. doi:10.1126/science.1215157
94. Marini JC, Forlino A. Replenishing cartilage from endogenous stem cells. N Engl J Med. 2012;366(26):2522–2524. doi:10.1056/NEJMcibr1204283
95. Maudens P, Seemayer CA, Thauvin C, Gabay C, Jordan O, Allémann E. Nanocrystal-polymer particles: extended delivery carriers for osteoarthritis treatment. Small. 2018;14(8). doi:10.1002/smll.201703108
96. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi:10.1016/S0092-8674(04)00045-5
97. Lian JB, Stein GS, van Wijnen AJ, et al. MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol. 2012;8(4):212–227. doi:10.1038/nrendo.2011.234
98. Klicka K, Grzywa TM, Mielniczuk A, Klinke A, Włodarski PK. The role of miR-200 family in the regulation of hallmarks of cancer. Front Oncol. 2022;12:965231. doi:10.3389/fonc.2022.965231
99. Proctor CJ, Smith GR, van Wijnen A. Computer simulation models as a tool to investigate the role of microRNAs in osteoarthritis. PLoS One. 2017;12(11):e0187568. doi:10.1371/journal.pone.0187568
100. Davies SR, Sakano S, Zhu Y, Sandell LJ. Distribution of the transcription factors Sox9, AP-2, and [delta]EF1 in adult murine articular and meniscal cartilage and growth plate. J Histochem Cytochem. 2002;50(8):1059–1065. doi:10.1177/002215540205000808
101. Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13(4):423–433. doi:10.1038/ncb2210
102. Barry F, Chai F, Chijcheapaza-Flores H, Garcia-Fernandez MJ, Blanchemain N, Nicot R. Systematic review of studies on drug-delivery systems for management of temporomandibular-joint osteoarthritis. J Stomatol Oral Maxillofac Surg. 2022;123(5):e336–e341. doi:10.1016/j.jormas.2021.08.003
103. Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–951. doi:10.1038/nbt.3330
104. Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18(5):504–511. doi:10.1101/gad.1184404
105. Cun D, Jensen DK, Maltesen MJ, et al. High loading efficiency and sustained release of siRNA encapsulated in PLGA NPs: quality by design optimization and characterization. Eur J Pharm Biopharm. 2011;77(1):26–35. doi:10.1016/j.ejpb.2010.11.008
106. Chen Y, Gao DY, Huang L. In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev. 2015;81:128–141. doi:10.1016/j.addr.2014.05.009
107. Caracciolo G, Caputo D, Pozzi D, Colapicchioni V, Coppola R. Size and charge of NPs following incubation with human plasma of healthy and pancreatic cancer patients. Colloids Surf B Biointerfaces. 2014;123:673–678. doi:10.1016/j.colsurfb.2014.10.008
108. Nierenberg D, Khaled AR, Flores O. Formation of a protein Corona influences the biological identity of nanomaterials. Rep Pract Oncol Radiother. 2018;23(4):300–308. doi:10.1016/j.rpor.2018.05.005
109. He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric NPs. Biomaterials. 2010;31(13):3657–3666. doi:10.1016/j.biomaterials.2010.01.065
110. Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric NPs as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108(27):10980–10985. doi:10.1073/pnas.1106634108
111. Zhao Y, Huang L. Lipid NPs for gene delivery. Adv Genet. 2014;88:13–36. doi:10.1016/b978-0-12-800148-6.00002-x
112. Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114(1):100–109. doi:10.1016/j.jconrel.2006.04.014
113. Cheng X, Lee RJ. The role of helper lipids in lipid NPs (LNPs) designed for oligonucleotide delivery. Adv Drug Deliv Rev. 2016;99(Pt A):129–137. doi:10.1016/j.addr.2016.01.022
114. Hsu SH, Yu B, Wang X, et al. Cationic lipid NPs for therapeutic delivery of siRNA and miRNA to murine liver tumor. Nanomedicine. 2013;9(8):1169–1180. doi:10.1016/j.nano.2013.05.007
115. Yongvongsoontorn N, Chung JE, Gao SJ, et al. Carrier-enhanced anticancer efficacy of sunitinib-loaded green tea-based micellar nanocomplex beyond tumor-targeted delivery. ACS Nano. 2019;13(7):7591–7602. doi:10.1021/acsnano.9b00467
116. Dai W, Jin P, Li X, et al. A carrier-free nano-drug assembled via π-π stacking interaction for the treatment of osteoarthritis. Biomed Pharmacother. 2023;164:114881. doi:10.1016/j.biopha.2023.114881
117. Wu Z, Yuan K, Zhang Q, Guo JJ, Yang H, Zhou F. Antioxidant PDA-PEG NPs alleviate early osteoarthritis by inhibiting osteoclastogenesis and angiogenesis in subchondral bone. J Nanobiotechnology. 2022;20(1):479. doi:10.1186/s12951-022-01697-y
118. Zhang M, Hu W, Cai C, Wu Y, Li J, Dong S. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Mater Today Bio. 2022;14:100223. doi:10.1016/j.mtbio.2022.100223
119. Brown S, Pistiner J, Adjei IM, Sharma B. Nanoparticle properties for delivery to cartilage: the implications of disease state, synovial fluid, and off-target uptake. Mol Pharm. 2019;16(2):469–479. doi:10.1021/acs.molpharmaceut.7b00484
120. Singh A, Agarwal R, Diaz-Ruiz CA, et al. Nanoengineered particles for enhanced intra-articular retention and delivery of proteins. Adv Healthcare Mater. 2014;3(10):1525, 1562–1567. doi:10.1002/adhm.201400051
121. DiDomenico CD, Lintz M, Bonassar LJ. Molecular transport in articular cartilage - what have we learned from the past 50 years? Nat Rev Rheumatol. 2018;14(7):393–403. doi:10.1038/s41584-018-0033-5
122. Yan H, Duan X, Pan H, et al. Suppression of NF-κB activity via nanoparticle-based siRNA delivery alters early cartilage responses to injury. Proc Natl Acad Sci USA. 2016;113(41):E6199–E6208. doi:10.1073/pnas.1608245113
123. Torzilli PA, Arduino JM, Gregory JD, Bansal M. Effect of proteoglycan removal on solute mobility in articular cartilage. J biomech. 1997;30(9):895–902. doi:10.1016/s0021-9290(97)00059-6
124. Elsaid KA, Ferreira L, Truong T, Liang A, Machan J, D’Souza GG. Pharmaceutical nanocarrier association with chondrocytes and cartilage explants: influence of surface modification and extracellular matrix depletion. Osteoarthritis Cartilage. 2013;21(2):377–384. doi:10.1016/j.joca.2012.11.011
125. Edwards SH. Intra-articular drug delivery: the challenge to extend drug residence time within the joint. Vet j. 2011;190(1):15–21. doi:10.1016/j.tvjl.2010.09.019
126. Prieto-Potin I, Largo R, Roman-Blas JA, Herrero-Beaumont G, Walsh DA. Characterization of multinucleated giant cells in synovium and subchondral bone in knee osteoarthritis and rheumatoid arthritis. BMC Musculoskeletal Disord. 2015;16:226. doi:10.1186/s12891-015-0664-5
127. Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021;325(6):568–578. doi:10.1001/jama.2020.22171
128. Labens R, Lascelles BD, Charlton AN, et al. Ex vivo effect of gold NPs on porcine synovial membrane. Tissue Barriers. 2013;1(2):e24314. doi:10.4161/tisb.24314
129. Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008;25(8):1815–1821. doi:10.1007/s11095-008-9562-y
130. Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11(1):21–34. doi:10.1038/nrrheum.2014.157
131. Dufour A, Lafont JE, Buffier M, et al. Repair of full-thickness articular cartilage defects using IEIK13 self-assembling peptide hydrogel in a non-human primate model. Sci Rep. 2021;11(1):4560. doi:10.1038/s41598-021-83208-x
132. Malda J, de Grauw JC, Benders KE, et al. Of mice, men and elephants: the relation between articular cartilage thickness and body mass. PLoS One. 2013;8(2):e57683. doi:10.1371/journal.pone.0057683
133. Ruediger T, Horbert V, Reuther A, et al. Thickness of the stifle joint articular cartilage in different large animal models of cartilage repair and regeneration. Cartilage. 2021;13(2_suppl):438s–452s. doi:10.1177/1947603520976763
134. Shapiro EM, Borthakur A, Gougoutas A, Reddy R. 23Na MRI accurately measures fixed charge density in articular cartilage. Magnet Resonan Med. 2002;47(2):284–291. doi:10.1002/mrm.10054
135. Freedman JD, Lusic H, Snyder BD, Grinstaff MW. Tantalum oxide NPs for the imaging of articular cartilage using X-ray computed tomography: visualization of ex vivo/in vivo murine tibia and ex vivo human index finger cartilage. Angew Chem. 2014;53(32):8406–8410. doi:10.1002/anie.201404519
136. Kumar S, Adjei IM, Brown SB, Liseth O, Sharma B. Manganese dioxide NPs protect cartilage from inflammation-induced oxidative stress. Biomaterials. 2019;224:119467. doi:10.1016/j.biomaterials.2019.119467
137. Morgen M, Tung D, Boras B, Miller W, Malfait AM, Tortorella M. NPs for improved local retention after intra-articular injection into the knee joint. Pharm Res. 2013;30(1):257–268. doi:10.1007/s11095-012-0870-x
138. Bajpayee AG, Wong CR, Bawendi MG, Frank EH, Grodzinsky AJ. Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis. Biomaterials. 2014;35(1):538–549. doi:10.1016/j.biomaterials.2013.09.091
139. He T, Zhang C, Vedadghavami A, et al. Multi-arm Avidin nano-construct for intra-cartilage delivery of small molecule drugs. J Control Release. 2020;318:109–123. doi:10.1016/j.jconrel.2019.12.020
140. Yao Y, Wei G, Deng L, Cui W. Visualizable and lubricating hydrogel microspheres via NanoPOSS for cartilage regeneration. Adv Sci. 2023;10(15):e2207438. doi:10.1002/advs.202207438
141. Kang ML, Ko JY, Kim JE, Im GI. Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials. 2014;35(37):9984–9994. doi:10.1016/j.biomaterials.2014.08.042
142. Chongchai A, Waramit S, Wongwichai T, et al. Targeting human osteoarthritic chondrocytes with ligand directed bacteriophage-based particles. Viruses. 2021;13(12). doi:10.3390/v13122343
143. Chen Z, Chen J, Wu L, et al. Hyaluronic acid-coated bovine serum albumin NPs loaded with brucine as selective nanovectors for intra-articular injection. Int j Nanomed. 2013;8:3843–3853. doi:10.2147/ijn.S50721
144. Jain S, Tran TH, Amiji M. Macrophage repolarization with targeted alginate NPs containing IL-10 plasmid DNA for the treatment of experimental arthritis. Biomaterials. 2015;61:162–177. doi:10.1016/j.biomaterials.2015.05.028
145. Li R, He Y, Zhu Y, et al. Route to Rheumatoid Arthritis by Macrophage-Derived Microvesicle-Coated NPs. Nano Lett. 2019;19(1):124–134. doi:10.1021/acs.nanolett.8b03439
146. Shi G, Jiang H, Yang F, et al. NIR-responsive molybdenum (Mo)-based nanoclusters enhance ROS scavenging for osteoarthritis therapy. Pharmacol Res. 2023;192:106768. doi:10.1016/j.phrs.2023.106768
147. Zhou T, Ran J, Xu P, et al. A hyaluronic acid/platelet-rich plasma hydrogel containing MnO(2) nanozymes efficiently alleviates osteoarthritis in vivo. Carbohydr Polym. 2022;292:119667. doi:10.1016/j.carbpol.2022.119667
148. Wang Z, Zhong Y, He S, et al. Application of the pH-responsive PCL/PEG-Nar nanofiber membrane in the treatment of osteoarthritis. Front Bioeng Biotechnol. 2022;10:859442. doi:10.3389/fbioe.2022.859442
149. Qin X, Pan L, Chen T, et al. Inflammation-responsive NPs suppress lymphatic clearance for prolonged arthritis therapy. J Control Release. 2022;352:700–711. doi:10.1016/j.jconrel.2022.11.005
150. Riegger J, Schoppa A, Ruths L, Haffner-Luntzer M, Ignatius A. Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: a narrative review. Cell Mol Biol Lett. 2023;28(1):76. doi:10.1186/s11658-023-00489-y
151. Shen C, Gao M, Chen H, et al. Reactive oxygen species (ROS)-responsive nanoprobe for bioimaging and targeting therapy of osteoarthritis. J Nanobiotechnol. 2021;19:1–6.
152. Zhou T, Xiong H, Wang SQ, et al. An injectable hydrogel dotted with dexamethasone acetate-encapsulated reactive oxygen species-scavenging micelles for combinatorial therapy of osteoarthritis. Mat Today Nano. 2022;17:100164. doi:10.1016/j.mtnano.2021.100164
153. Xu S, Chang L, Zhao X, et al. Preparation of epigallocatechin gallate decorated Au-Ag nano-heterostructures as NIR-sensitive nano-enzymes for the treatment of osteoarthritis through mitochondrial repair and cartilage protection. Acta Biomater. 2022;144:168–182. doi:10.1016/j.actbio.2022.03.038
154. Chiang MY, Cheng IY, Chou SH, et al. A smart injectable composite hydrogel with magnetic navigation and controlled glutathione release for promoting in situ chondrocyte array and self-healing in damaged cartilage tissue. J Mater Chem B. 2021;9(45):9370–9382. doi:10.1039/d1tb02030g
155. Su JY, Chen SH, Chen YP, Chen WC. Evaluation of magnetic nanoparticle-labeled chondrocytes cultivated on a type II Collagen-Chitosan/Poly(Lactic-co-Glycolic) Acid Biphasic Scaffold. Int J Mol Sci. 2017;18(1). doi:10.3390/ijms18010087
156. Angle SR, Sena K, Sumner DR, Virdi AS. Osteogenic differentiation of rat bone marrow stromal cells by various intensities of low-intensity pulsed ultrasound. Ultrasonics. 2011;51(3):281–288. doi:10.1016/j.ultras.2010.09.004
157. Yuan FZ, Wang HF, Guan J, et al. Fabrication of injectable chitosan-chondroitin sulfate hydrogel embedding kartogenin-loaded microspheres as an ultrasound-triggered drug delivery system for cartilage tissue engineering. Pharmaceutics. 2021;13(9). doi:10.3390/pharmaceutics13091487
158. Nele V, Schutt CE, Wojciechowski JP, et al. Ultrasound-triggered enzymatic gelation. Adv Mater. 2020;32(7):e1905914. doi:10.1002/adma.201905914
159. Lu Y, Zhou J, Wang Q, et al. Glucocorticoid-loaded pH/ROS dual-responsive NPs alleviate joint destruction by downregulating the NF-κB signaling pathway. Acta Biomater. 2023;164:458–473. doi:10.1016/j.actbio.2023.04.012
160. Xue S, Zhou X, Sang W, et al. Cartilage-targeting peptide-modified dual-drug delivery nanoplatform with NIR laser response for osteoarthritis therapy. Bioact Mater. 2021;6(8):2372–2389. doi:10.1016/j.bioactmat.2021.01.017
161. Jin GZ. Current nanoparticle-based technologies for osteoarthritis therapy. Nanomaterials. 2020;10(12). doi:10.3390/nano10122368
162. Guo X, Lou J, Wang F, Fan D, Qin Z. Recent advances in nano-therapeutic strategies for osteoarthritis. Front Pharmacol. 2022;13:924387. doi:10.3389/fphar.2022.924387
163. Leite CBS, Coelho JM, Ferreira-Nunes R, et al. Phonophoretic application of a glucosamine and chondroitin nanoemulsion for treatment of knee chondropathies. Nanomedicine. 2020;15(7):647–659. doi:10.2217/nnm-2019-0317
164. Hashemzadeh K, Davoudian N, Jaafari MR, Mirfeizi Z. The effect of nanocurcumin in improvement of knee osteoarthritis: a randomized clinical trial. Curr Rheumatol Rev. 2020;16(2):158–164. doi:10.2174/1874471013666191223152658
165. Shakouri A, Adljouy N, Balkani S, et al. Effectiveness of topical gel of medical leech (Hirudo medicinalis) saliva extract on patients with knee osteoarthritis: a randomized clinical trial. Complement Therap Clin Pract. 2018;31:352–359. doi:10.1016/j.ctcp.2017.12.001
166. Amirsaadat S, Amirazad H, Hashemihesar R, Zarghami N. An update on the effect of intra-articular intervention strategies using nanomaterials in osteoarthritis: possible clinical application. Front Bioeng Biotechnol. 2023;11:1128856. doi:10.3389/fbioe.2023.1128856
167. Delbaldo C, Tschon M, Martini L, Fini M, Codispoti G. Benefits of applying nanotechnologies to hydrogels in efficacy tests in osteoarthritis models-a systematic review of preclinical studies. Int J Mol Sci. 2022;23(15). doi:10.3390/ijms23158236
168. Rahimi M, Charmi G, Matyjaszewski K, Banquy X, Pietrasik J. Recent developments in natural and synthetic polymeric drug delivery systems used for the treatment of osteoarthritis. Acta Biomater. 2021;123:31–50. doi:10.1016/j.actbio.2021.01.003
169. Tryfonidou MA, de Vries G, Hennink WE, Creemers LB. ”Old Drugs, New Tricks” - Local controlled drug release systems for treatment of degenerative joint disease. Adv Drug Deliv Rev. 2020;160:170–185. doi:10.1016/j.addr.2020.10.012
170. Feng X, Chen A, Zhang Y, Wang J, Shao L, Wei L. Central nervous system toxicity of metallic NPs. Int J Nanomed. 2015;10:4321–4340. doi:10.2147/ijn.S78308
171. Mwangi TK, Berke IM, Nieves EH, Bell RD, Adams SB, Setton LA. Intra-articular clearance of labeled dextrans from naive and arthritic rat knee joints. J Control Release. 2018;283:76–83. doi:10.1016/j.jconrel.2018.05.029
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
