Back to Journals » International Journal of Nanomedicine » Volume 19
Heterologous Prime-Boost Immunization Strategies Using Varicella-Zoster Virus gE mRNA Vaccine and Adjuvanted Protein Subunit Vaccine Triggered Superior Cell Immune Response in Middle-Aged Mice
Authors Li D, Bian L , Cui L , Zhou J, Li G, Zhao X , Xing L , Cui J , Sun B, Jiang C, Kong W, Zhang Y , Chen Y
Received 19 February 2024
Accepted for publication 25 July 2024
Published 6 August 2024 Volume 2024:19 Pages 8029—8042
DOI https://doi.org/10.2147/IJN.S464720
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Dongdong Li,1 Lijun Bian,1 Lili Cui,2 Jingying Zhou,1 Gaotian Li,1 Xiaoyan Zhao,1 Liao Xing,1 Jiaxing Cui,1 Bo Sun,1 Chunlai Jiang,1,3,4 Wei Kong,1,3,4 Yong Zhang,1,3,4 Yan Chen1,3,4
1National Engineering Laboratory for AIDS Vaccine, School of Life Sciences, Jilin University, Changchun, People’s Republic of China; 2Beijing Institute of Drug Metabolism, Beijing, People’s Republic of China; 3Key Laboratory for Molecular Enzymology and Engineering of Ministry of Education, School of Life Sciences, Jilin University, Changchun, People’s Republic of China; 4NMPA Key Laboratory of Humanized Animal Models for Evaluation of Vaccines and Cell Therapy Products, Jilin University, Changchun, People’s Republic of China
Correspondence: Yan Chen; Yong Zhang, National Engineering Laboratory for AIDS Vaccine, School of Life Sciences, Jilin University, Changchun, 130012, People’s Republic of China, Tel +86-431-85167751, Fax +86-431-85167674, Email [email protected]; [email protected]
Purpose: Heterologous immunization using different vaccine platforms has been demonstrated as an efficient strategy to enhance antigen-specific immune responses. In this study, we performed a head-to-head comparison of both humoral and cellular immune response induced by different prime-boost immunization regimens of mRNA vaccine and adjuvanted protein subunit vaccine against varicella-zoster virus (VZV) in middle-aged mice, aiming to get a better understanding of the influence of vaccination schedule on immune response.
Methods: VZV glycoprotein (gE) mRNA was synthesized and encapsulated into SM-102-based lipid nanoparticles (LNPs). VZV-primed middle-aged C57BL/6 mice were then subjected to homologous and heterologous prime-boost immunization strategies using VZV gE mRNA vaccine (RNA-gE) and protein subunit vaccine (PS-gE). The antigen-specific antibodies were evaluated using enzyme-linked immunosorbent assay (ELISA) analysis. Additionally, cell-mediated immunity (CMI) was detected using ELISPOT assay and flow cytometry. Besides, in vivo safety profiles were also evaluated and compared.
Results: The mRNA-loaded lipid nanoparticles had a hydrodynamic diameter of approximately 130 nm and a polydispersity index of 0.156. Total IgG antibody levels exhibited no significant differences among different immunization strategies. However, mice received 2×RNA-gE or RNA-gE>PS-gE showed a lower IgG1/IgG2c ratio than those received 2×PS-gE and PS-gE> RNA-gE. The CMI response induced by 2×RNA-gE or RNA-gE>PS-gE was significantly stronger than that induced by 2×PS-gE and PS-gE> RNA-gE. The safety evaluation indicated that both mRNA vaccine and protein vaccine induced a transient body weight loss in mice. Furthermore, the protein vaccine produced a notable inflammatory response at the injection sites, while the mRNA vaccine showed no observable inflammation.
Conclusion: The heterologous prime-boost strategy has demonstrated that an mRNA-primed immunization regimen can induce a better cell-mediated immune response than a protein subunit-primed regimen in middle-aged mice. These findings provide valuable insights into the design and optimization of VZV vaccines with the potentials to broaden varicella vaccination strategies in the future.
Keywords: varicella-zoster Virus, mRNA vaccine, glycoprotein E, lipid nanoparticles, heterologous prime-boost, cell-mediated immunity
Introduction
Varicella-zoster virus (VZV), a member of the herpesvirus family, is a neurotropic and lymphotropic virus with double-stranded DNA.1 The VZV virus can cause two types of diseases.1 The first is chickenpox, which primarily results from primary infection and typically occurs mostly in childhood. VZV is transmitted through respiratory droplets and skin-to-skin contact. Due to its high contagiousness, almost all adults have been exposed to VZV and become virus carriers since childhood.2,3 Although patients recover from chickenpox, the VZV virus, which cannot be completely eradicated from the body, may reside in trigeminal nerve and other dorsal root ganglia. When the body’s immune system weakens or declines, VZV virus can be reactivated, causing herpes zoster (HZ).4,5 HZ leads to multiple complications, such as postherpetic neuralgia (PHN), a painful condition that may last for more than 3 months and cannot be effectively alleviated by available antiviral therapies.6 Therefore, it is necessary to develop a safe and effective vaccine to manage clinical discomfort caused by HZ.
Vaccines against varicella and HZ are different in their needs to induce an immune response. Varicella vaccines are primarily required to induce a humoral immune response to prevent VZV infection. Whereas, HZ vaccines are required to induce a cell-mediated immunity (CMI) to control or eliminate reactivated VZV from infected cells.7–10 The leading HZ vaccine currently in the market is Shingrix™ (Glaxo Smith Kline, Rockville, MD, USA), a VZV glycoprotein (gE)-based HZ subunit vaccine with AS01B as adjuvant. This vaccine is administered by intramuscular injection and is available since 2017. Compared with the live attenuated HZ vaccine Zostavax® (Merck & Co., Inc., Kenilworth, NJ, USA), Shingrix™ provides higher immunological efficiency.11–16 Clinical trials have shown that Shingrix™ provides >90% protection efficiencies in people aged 50–59 years and older than 70 years and reduces the risk of postherpetic neuralgia.13,16 Unlike Zostavax® which is challenging to manufacture and requires maintenance of high titers of live-attenuated virus, Shingrix™ is relatively straightforward to produce and store. Despite of obvious advantages of VZV subunit vaccines, QS21, a key component of adjuvant AS01B, has obvious limitations in production and availability and cannot be synthesized in a cost-effective and large-scale way.17,18 This is partially why subunit vaccines are very expensive and cannot be sufficiently produced. In contrast, mRNA vaccines have emerged as a promising alternative, demonstrated by the success of COVID-19 mRNA vaccines Comirnaty (BNT162b2) and Spikevax (mRNA-1273). To be specific, mRNA vaccines can be rapidly synthesized and self-assembled in vitro, with no need to purify virus and express the protein.19,20 Moreover, mRNA vaccines can effectively induce humoral and cellular immune responses without the addition of adjuvants.21,22 Therefore, the development of an mRNA vaccine for HZ is a promising strategy for the production challenges associated with subunit vaccines. Besides, strong cellular immune response elicited by mRNA vaccine may better meet the requirements for the prevention of vesicular diseases.
A heterologous prime-boost immunization strategy involves sequential immunization with vaccines from different antigen types, such as DNA, mRNA and protein-subunit vaccines.23 Previous studies have demonstrated that heterologous prime-boost immunization is an effective strategy to increase antigen immunogenicity and provide better protection efficiencies, including vaccines against COVID-19, HIV influenza, and hepatitis viruses.24–28 For example, various heterologous immunization combinations, such as mRNA and protein subunit vaccines for COVID-19 vaccines, mRNA and inactivated virus vaccines, mRNA and adenovirus-vectored (Adv) vaccines, inactivated virus and recombinant protein vaccines, have been applied in pre-clinical and clinical studies.26,29–33 These combinations have been demonstrated to provide a superior or equivalent immunogenicity and protection efficiency than homologous vaccine immunization. Zhang et al performed a comparative study, in which BALB/c mice were immunized with COVID-19 mRNA and protein subunit vaccines at a homologous, heterologous, or mixed immunization strategy.21 Their results demonstrated significant differences in humoral and cellular immune responses induced by different immunization combinations. Currently, no studies have investigated heterologous immunization strategies for VZV, probably due to limited choices in vaccine options. Here, we immunized VZV-primed middle-aged C57BL/6 mice with a VZV protein-subunit vaccine formulated with a MPL- and QS-21-loaded liposomal adjuvant and mRNA vaccine by a heterologous or homologous strategy. The effect of different immunization regimens on immune responses, particularly CMI, which is generally required for protection against herpes zoster, was evaluated and compared.
Materials and Methods
Materials
The recombinant pET-28a (+) plasmid harboring the sequence of interest downstream of a T7 promoter was synthesized by Nanjing GenScript Biotechnology Co., Ltd. The restriction endonuclease HindIII was purchased by TransGen Biotech (Cat. No. JH101-01, Beijing, China). The TIANquick Midi Purification Kit (Cat. No. DP204-02) was purchased from TIANGEN BIOTECH, Beijing, China). The T7-FlashScribe™ Transcription Kit (Cat. No. C-ASF3507) and ScriptCap™ Cap 1 Capping System (Cat. No. C-SCCS1710) were purchased from Cellscript (USA). N1-methylpseudouridine-5’-triphosphate (Cat. No. N-1081-5) was purchased from Trilink (San Diego, CA). The MEGAclear transcription clean-up kit (Cat. No. AM1908), Lipofectamine™ 2000 (Cat. No. 11668030) and Quant-iTTM RiboGreenR RNA Reagent Kit (Cat. No. R11490) were purchased from Invitrogen (Carlsbad, CA, USA). RIPA buffer (Cat. No. P0013B) was purchased from Beyotime Biotechnology (Shanghai, China), which was added with 1% phosphatase-inhibitor mixture (Cat. No. P1048, Beyotime). The anti-VZV gE antibody (Cat. No. ab272686, 1:1000) was purchased from Abcam (Cambridge, UK). The lipid components 1.2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), monophosphoryl lipid A (PHAD), 1.2-distearoyl-sn-glycero-3-phosphocholine (DSPC) (Cat. No. 850365C) and DMG-PEG2000 (Cat. No. 880150P) were purchased from Avanti Polar Lipids (Alabaster, AL). Cholesterol (Cat. No. C3045) was purchased from Sigma (USA). SM-102 was purchased from AVT Pharmaceutical Technology Co., Ltd. (Shanghai, China). QS-21 was purchased from Desert King International (San Diego, CA, USA). The gE proteins and live herpes zoster vaccine Ganwei® were kindly provided by Changchun BCHT Biotechnology Co., Ltd. (Changchun, China). The ELISpot assay kit (Cat. No. 3321–4HPW-10) was purchased from Mabtech (Cincinnati, OH, USA). The Protein transport inhibitor cocktail (500X, Cat. No. 00–4980-93), anti-CD3-eFluor™ 506 (69–0032-82), anti-CD4-APC-eFluor™780 (47–0041-82), anti-CD8-FITC (11–0081-82), anti-TNF alpha-eFluor 450 (48–7321-82), anti-IL-2-PE (12–7021-82) and anti-IFN gamma-APC (17–7311-82) were purchased from Thermo Fisher (Eugene, OR, USA). The anti-CD3-Percp 5.5 (Cat. No. 100218), anti-CD4-FITC (Cat. No. 100405), anti-CD8-APC (Cat. No. 100712), anti-CD44-PE (Cat. No. 103008) and anti-CD62L-Brilliant Violet 421 (Cat. No. 104436) were purchased from Biolegend (San Diego, CA, USA).
mRNA Synthesis and Expression
The VZV glycoprotein (gE) mRNA sequence, which included 5’ untranslated region (UTR), open reading frame (ORF), 3’UTR and an 80-nt poly(A) tail, was referenced from previous study.34 For mRNA synthesis, the pET-28a (+) plasmid harboring full length of gE sequence was first linearized by HindIII and purified by a TIANquick Midi Purification Kit. Following linearization, gE mRNA sequence was synthesized with a T7 polymerase transcription reaction using a T7-FlashScribe™ Transcription Kit. In the present study, N1-methylpseudouridine-5’-triphosphate was used to replace UTP (uridine 5’-triphosphate) to generate a modified mRNA. gE mRNA was purified with a MEGAclear transcription clean-up kit from Invitrogen. Synthesized mRNA was further capped using a ScriptCap™ Cap 1 Capping System and purified again. The length of gE mRNA was analyzed using a denaturing agarose gel electrophoresis. The human embryonic kidney cell-line HEK293T was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). For mRNA expression, HEK293T cells were seeded into a 6-well plate and grown to 50%–70% confluence. 2μg of gE mRNA was then transfected into HEK293T cells using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA). After transfection for 48 h, the cells were lysed by a RIPA buffer with the addition of phosphatase-inhibitor mixture. Afterward, the total proteins were separated by 10% SDS-PAGE gels and transferred to Western-Blot. The interest protein was detected using an anti-VZV gE antibody (Cat. No. ab272686, 1:1000, Abcam, Cambridge, UK).
Preparation of Vaccines
gE mRNA was encapsulated into lipid nanoparticles (LNPs) using a modified procedure described in a previous study.35,36 In brief, the gE mRNA was dissolved into 100 mM citrate buffer (pH 4.0) to a final concentration of 0.17 mg/mL. Lipid components, including SM-102, DSPC, cholesterol and DMG-PEG2000, were dissolved in ethanol at a molar ratio of 50:10:38.5:1.5. The lipid mixture was combined with mRNA solution at a volume ratio of 1:3 using a microfluidic mixer (Precision Nanosystems, Vancouver, BC, Canada). The synthesized LNPs/gE mRNA were then dialyzed against PBS, concentrated using Amicon ultra centrifugal filters (EMD Millipore, Billerica, MA, USA) and stored at 4°C until used. Hydrodynamic particle size and polydispersity index of LNPs were detected using Zetasizer Nano ZS particle size analyzer (Malvern Panalytical, Malvern, UK). The encapsulated mRNA was analyzed using a Quant-iTTM RiboGreenR RNA Reagent Kit. The morphological characters of LNPs were analyzed using a Talos transmission electron microscope (TEM, FEI Company, Hillsboro, OR, USA).
MPL- and QS-21-loaded liposomal adjuvant was prepared using a thin-film method reported in a previous study.37 Liposome consists of 1.2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and cholesterol, and the DOPC/cholesterol weight ratio is 4:1. The gE protein antigen was obtained from Changchun BCHT Biotechnology Co., Ltd. The hydrodynamic particle size and polydispersity index of the resultant liposomal adjuvant were detected using the Zetasizer Nano ZS particle size analyzer (Malvern Panalytical, Malvern, UK). Antigens and adjuvants were mixed immediately before use.
Animal Studies
Twenty-five specific pathogen-free (SPF) C57BL/6 mice (6–8 weeks old, 15–18 g, female) were purchased from Liaoning Changsheng Biotechnology Co. Ltd (Liaoning, China) and maintained in the SPF conditions with free access to food and water. Routine laboratory tests and regular examinations by veterinarians during a quarantine period were used to ensure that these experimental animals were pathogen-free and in good physical condition. To simulate the real-world scenario where most people have already been infected by VZV in childhood before inoculate VZV vaccine, we used a VZV-primed mouse model.38 Briefly, C57BL/6 mice were primed with VZV via subcutaneous injection of 1/5 human dose of attenuated live herpes zoster vaccine Ganwei®. Eleven months later, the mice were randomly divided into five groups (n = 5) and immunized as follows. It is worth noting that, in this case, mice with 12.5–13 months old are equivalent to human adults of 42–45 years old at which shingles disease begins to occur.39–41
RNA-gE used in this study was an mRNA vaccine that encoded VZV gE antigen. PS-gE was a VZV gE protein subunit vaccine formulated with an AS01B-like liposomal adjuvant containing 5 μg MPL, 5 μg QS-21 and 5 μg gE. The mice were divided into five groups: 1) PBS; 2) 15 μg of RNA-gE for two doses (2×RNA-gE); 3) Primed with 15 μg of RNA-gE and boosted with 5 μg of PS-gE (RNA-gE>PS-gE); 4) Primed with 5 μg of PS-gE and boosted with 15 μg of RNA-gE (PS-gE>RNA-gE); 5) 5 μg of PS-gE for two doses (2×PS-gE). All immunizations were performed twice during a 2 week interval. Blood was collected from the cheek (submandibular) at week 2 after first immunization and weeks 2, 3 after boosted immunization. The volume of collected blood was approximately 100 μL for each mouse. Animal studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council), and all animal procedures were reviewed and approved by the Animal Welfare and Research Ethics Committee at Jilin University (Ethical approval number 2022YNPZSY1007). At the end of the experiment, mice were sacrificed by CO2 asphyxiation.
Enzyme-Linked Immunosorbent Assay (ELISA) of Antibody Titers
The gE-specific antibodies were detected by ELISA assay. The gE protein antigen was dissolved into a sodium carbonate buffer (5 µg/mL) and added into a 96-well ELISA plate at a volume of 100 μL/well. After incubation at 4°C overnight, the plates were washed with PBST buffer (phosphate-buffered saline containing 0.05% Tween-20) and blocked with PBS containing 3% bovine serum albumin (BSA) for 1 h. After washed with PBST buffer for three times, the plates were added with 3.5-fold diluted serum samples and incubated for 1 h at 37°C. After incubation, the plates were washed with a PBST buffer for three times and then incubated with HRP-labeled goat anti-mouse IgG, IgG1 and IgG2c for 1 h at 37°C, followed by development with TMB substrate (Solarbio, Beijing, China). The OD values at 450 nm were measured by an ELISA plate reader (Gene Company, Beijing, China). The dilution factor and OD values were subjected to a linear regression analysis. The antibody titer of each sample was defined as log10 of the highest serum dilutions yielding 2.1-fold OD value of negative controls.
Enzyme-Linked Immunospot Assay (ELISpot) of Splenocytes
At 3 weeks after boost immunization, mice were executed and spleens were excised. The monodispersed spleen cells were acquired using a 70 µm cell strainer (BD, USA). Red blood cells were then lysed by ammonium–chloride–potassium (ACK) lysis buffer at room temperature for 5 minutes. The remaining cells were resuspended in a Roswell Park Memorial Institute (RPMI) 1640 medium with 10% v/v fetal bovine serum (Thermo Fisher) and 1% penicillin–streptomycin (Thermo Fisher). After cell counting, the splenocytes density was adjusted to 1×107 cells/mL. For ELISpot assay, gE proteins containing extracellular domain were added into 96-well plates of ELISpot assay kit at a concentration of 10 μg/mL. 100 μL of splenocytes solution was then added to each well of the 96-well plates and incubated for 24 h. The IFNγ secreting cell spots were detected on an ELISPOT reader system (Autoimmun Diagnostika GmbH, Germany).
Flow Cytometry
1×106 splenocytes were incubated with 10 µg/mL extracellular domain of gE for 2 h at 37°C. Protein transport inhibitor cocktail (500X, Cat. No. 00–4980-93, Thermo Fisher) was added and incubated for 6 h to block cytokine release. After incubation with Fc receptor blocking antibodies for 10 min at 4°C, splenocytes were stained with anti-CD3-eFluor™ 506, anti-CD4-APC-eFluor™780 and anti-CD8-FITC for 30 min at 4°C, followed by cell fixation and permeabilization. Cells were then stained with eFluor 450-TNF, PE-IL-2 and APC-IFN-gamma for 30 min at room temperature. After being washed with permeabilization wash buffer, the polyfunctional T cells were analyzed using flow cytometry on a CytoFLEX flow cytometer (Beckman, Indianapolis, IN, USA). For the evaluation of memory T cells, splenocytes were incubated with Fc receptor blocking antibodies for 10 min at 4°C and then stained with anti-CD3-Percp 5.5, anti-CD4-FITC, anti-CD8-APC, anti-CD44-PE and anti-CD62L-Brilliant Violet 421 for 30 min at 4°C. After being washed by a staining buffer, cells were analyzed by flow cytometry.
Histological Staining
The mice were immunized with PBS, RNA-gE (15 μg) or PS-gE (5 μg) by intramuscular injection. The muscle tissues at the injection sites were collected at Day 1, Day 3 and Day 7. The heart, liver, spleen, lung and kidney were collected at Day 7. The tissues samples were fixed with paraformaldehyde for 24 h dehydrated in an alcohol gradient and placed on a paraffin slicer for sectioning. After stained with Hematoxylin and Eosin (HE, Sigma-Aldrich), tissues sections were observed on an Olympus IX51 microscope with a Qlmaging digital camera (Qicam Fast, Qimaging, Surrey, Canada).
Statistical Analysis
Data were shown as mean and standard deviation (SD). Differences among groups were analyzed with one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons test. GraphPad Prism 8.0 (GraphPad Software Inc., La Jolla, CA. USA) was used for statistical analyses.
Results
VZV gE mRNA Was in vitro Transcribed from DNA Vector and Encapsulated into LNP with a Uniform Particle Size
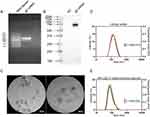
In order to prepare a VZV mRNA vaccine, gE full-length sequence, 3’ UTR and 5’ UTR were inserted into the DNA vector and then transcribed into gE mRNA in vitro using RNA polymerase. As shown in Figure 1A, the synthesized gE mRNA showed good integrity in denatured agarose gel as bands of approximately 2000 bases. To verify antigen expression, gE mRNA was transfected into HEK193T cells and cell lysate was subjected to Western blot. As shown in Figure 1B, the gE mRNA group exhibited a clear protein band at 75–100 KD, indicating an effective protein expression. The gE mRNA was then encapsulated into the LNP. TEM images indicated that the size of LNP/mRNA was about 100 nm (Figure 1C). The hydrodynamic diameter of LNP/mRNA was 133.2 ± 0.608 nm (Figure 1D). The zeta potential of LNP/mRNA was 5.47 ± 0.205 mV. The polydisperse index (PDI) of the LNP/mRNA was 0.156 ± 0.016 indicating a good uniformity of the particle sizes. The larger diameter of gE mRNA-LNPs detected by dynamic light scattering technique than that by TEM is as expected as the former includes the thickness of the hydrated water layers around LNP/mRNA nanoparticles. The hydrodynamic diameter of the liposomal adjuvant used in the protein subunit vaccine was 105.3 ± 0.872 nm (Figure 1E). The zeta potential of the liposomal adjuvant was −23.47 ± 1.65 mV. The polydisperse index (PDI) was 0.116 ± 0.024, suggesting a monodisperse system.
The Safety Profiles of VZV mRNA Vaccine and Protein Subunit Vaccine
The experimental procedure for animal study is shown in Figure 2A. The mice were divided into five groups: PBS; Two doses of VZV gE mRNA vaccine (2×RNA-gE); RNA-gE boosted with PS-gE (RNA-gE>PS-gE); PS-gE boosted with RNA-gE (PS-gE>RNA-gE); Two doses of protein subunit vaccine (2×PS-gE). In order to evaluate the safety profiles of mRNA and protein vaccine in vivo, C57BL/6 mice were injected with 15 μg of RNA-gE or 5 μg of PS-gE and mice body weight, temperature and possible tissue inflammations were monitored. As shown in Figure 2B, both RNA-gE and PS-gE groups experienced a transient weight loss on Day 1 after immunization (P < 0.05). Then the mice rapidly regained weight. The body temperature exhibited no significant change in both groups during 5 h after immunization (Figure 2C). HE staining results are shown in Figure 2D and E. The PBS and RNA-gE groups exhibited no inflammation at injection sites. However, PS-gE group displayed an obvious inflammatory cell infiltration on Day 3 as evidenced by the increasing amount of blue (nuclear) staining. The inflammatory state in PS-gE group was rapidly alleviated at Day 7. The HE staining of visceral organs at Day 7 after immunization had no significant pathological condition in all groups.
Immune Response Types Triggered by Homologous and Heterologous Immunization with RNA-gE and PS-gE
In order to determine a superior immunization strategy for mRNA vaccine and protein subunit vaccine, our synthesized gE mRNA vaccine (RNA-gE) and a gE protein subunit vaccine (PS-gE) harboring N-terminal 546 amino acids were used for homologous and heterologous prime-boost immunization. At week 2 after first immunization, mice produced a high titer of IgG antibodies (~106), with no significant differences between different immunization strategies (P > 0.05, Figure 3A). After a homologous or a heterologous booster immunization, the total IgG levels in all groups still remained at comparable levels (Figure 3B–D). The above results suggest that homologous and heterologous prime-boost regimens have no remarkable influence on the immunogenicity of vaccines in middle-aged mice. Further study is needed to illustrate the possible effect of inoculation doses on vaccine immunogenicity.
To investigate the effect of homologous and heterologous prime-boost schedules on immune response types, the levels of VZV gE protein-specific IgG1 and IgG2c levels were detected at 2 weeks after boost immunization. In C57BL/6 mice, serum IgG2c is widely recognized as a marker of Th1 cellular immune response and IgG1 indicates a humoral immune response.42 As shown in Figure 4A, both 2×PS-gE and PS-gE>RNA-gE induced the highest levels of IgG1, while that in 2×RNA-gE group were the lowest. The IgG1 levels of RNA-gE>PS-gE and PS-gE>RNA-gE groups had no significant differences (P > 0.05). 2×RNA-gE, RNA-gE>PS-gE and PS-gE>RNA-gE induced a similar level of IgG2c, all of which were higher than that induced by PS-gE (Figure 4B). The IgG1/IgG2c ratio was similar between 2×RNA-gE and RNA-gE>PS-gE groups, both were lower than that of PS-gE>RNA-gE and 2×PS-gE (Figure 4C). The above results suggested that 2×RNA-gE and RNA-gE>PS-gE tended to induce a Th1-biased immune response, while PS-gE>RNA-gE and 2×PS-gE tended to induce a Th2-biased immune response in middle-aged mice.
Two doses of VZV mRNA vaccine or mRNA vaccine boosted with protein subunit vaccine produced superior CMI in middle-aged mice
CMI induced by homologous and heterologous prime-boost immunization was evaluated using ELISPOT assay. As shown in Figure 5, after gE protein stimulation, 2×RNA-gE and RNA-gE>PS-gE produced the most IFN-γ-producing splenocytes (127 spots per 5×105 splenocytes for 2×RNA-gE and 146 spots for RNA-gE>PS-gE), followed by PS-gE>RNA-gE and 2×PS-gE groups (31 spots per 5×105 splenocytes for PS-gE>RNA-gE and 13 spots for 2×PS-gE).
 |
Figure 5 Analysis of IFNγ-secreting T cells induced by homologous and heterologous prime-boost immunization. Evaluation of cell-mediated immune response using ELISPOT assay after gE protein stimulation. n=5 for each group. (A) The images of IFNγ-producing splenocytes splenocytes. (B) The numbers of IFNγ-producing splenocytes were calculated from the ELISPOT assay. *P<0.05, **P<0.01 and ***P<0.001.The Figure 5 legend was as above. Besides, please change the + sign to superscript for CD4+ and CD8+ in all figure legends. |
To further investigate the effect of different vaccination regimens on the frequency of antigen-specific multifunctional T cells, the whole spleen flow cytometry assay was performed. Splenocytes from immunized mice were collected and stimulated with gE protein. The proportions of CD4+/CD8+ T cells expressing IFNγ, IL-2, and/or TNFα were determined. As shown in Figure 6A and B, individual cytokine analysis shows that 2×RNA-gE and RNA-gE>PS-gE had the highest levels of IFN-γ and TNFα in both CD4+ and CD8+ T cells, with similar IL-2 expression among all groups. In general, there were no significant differences between 2×RNA-gE and RNA-gE>PS-gE, as well as PS-gE>RNA-gE and 2×PS-gE. The proportions of polyfunctional T cells were also analyzed. Polyfunctional T cells represent a category of T cells that can simultaneously express two or more functional proteins, including cytokines, on a per cell basis. Previous studies have demonstrated that polyfunctional T cells play a crucial role in vaccine-induced protective immunity across multiple virus infections, such as HIV and VZV.35,43 As shown in Figure 6C and D, 2×RNA-gE and RNA-gE>PS-gE exhibited the higher levels of CD4+/CD8+ T cells expressing two or three kinds of cytokines than PS-gE>RNA-gE and 2×PS-gE groups. In summary, the mRNA vaccine-primed immunization strategy induced a superior cell-mediated immune response compared to the protein subunit vaccine-primed regimen in middle-aged mice.
Different Immunization Strategies Induced Similar T Cell Memory
Central memory T (TCM) cells, highly expressing CD44 and CD62L, have strong abilities to proliferate and differentiate into effector T cells upon repeated infection with identical or similar viruses.44,45 TCM cells are located in peripheral lymphatic organs, such as spleen. Here, splenocytes were collected from immunized mice and TCM cell proportions were determined using flow cytometry 3 weeks after boost. As shown in Figure 7, there were no significant differences in the proportions of CD4+CD44+CD62L+ and CD8+ CD44+CD62L+ among all groups, which implies that middle-aged mice received different immunization strategies have similar capacities to induce T-cell memory. Further investigation is needed to illustrate the kinetics of memory T cells in the spleens of middle-aged mice.
 |
Figure 7 Flow cytometry analysis of memory T cell response. (A) Proportions of memory CD4+ T cells; (B) Proportions of memory CD8+ T cells. n=5 for each group. |
Discussion
A variety of vaccine platforms have been developed to date, including live attenuated and inactivated vaccines, protein-subunit vaccines, nucleic acid vaccines (DNA-based or mRNA-based), and viral vector vaccines. These platforms differ significantly in the strength and type of immune responses they induce. With the advent of COVID-19 epidemic and the widespread use of Comirnaty (BNT162b2) and Spikevax (mRNA-1273) vaccines, mRNA vaccines have emerged as a promising strategy to prevent infectious diseases in recent years. These vaccine delivery antigen-encoding mRNAs to host cells using lipid nanoparticles, where they are translated into viral antigens. In contrast, traditional vaccine platforms, such as live attenuated and inactivated vaccines and protein subunit vaccines, involve the direct uptake and processing of engineered antigens by antigen-presenting cells, which are then presented to the adaptive immune system to elicit an immune response. mRNA vaccines have demonstrated the ability to induce strong cellular and humoral immune responses, while subunit vaccines tend to favor humoral responses unless paired with adjuvants that promote Th1-biased immunity. Therefore, combining mRNA vaccines with other platforms may be a reasonable attempt to induce stronger and longer-lasting immune responses. For VZV, several mRNA-based vaccines have shown strong immune responses in mice and nonhuman primates.35,36,46 In this study, a VZV mRNA vaccine encoding gE antigen named RNA-gE and a protein subunit vaccine named PS-gE were evaluated to determine optimal immunization strategy in middle-aged C57BL/6 mice. Middle-aged mice were used as they are equivalent to human adults of 42–45 years old at which shingles typically begin to occur. This model may better reflect the real-world clinical situation. In addition, a deep understanding of heterologous prime–boost regimens with VZV mRNA vaccine and adjuvanted protein subunit vaccine may provide valuable information to broaden varicella vaccination strategies in the future.
First, we evaluated the immunogenicity of VZV mRNA vaccine and protein subunit vaccine. The results showed that a 15 μg dose of RNA-gE elicits a total IgG titer (~106) comparable to a 5 μg of PS-gE. Similar results were observed in the RNA-gE>PS-gE and PS-gE>RNA-gE groups. However, antibody isotyping results revealed that RNA-gE induced a much lower IgG1/IgG2c ratio than PS-gE, indicating a bias of cellular immune response for RNA-gE vaccine and a bias of humoral immune response for PS-gE vaccine. For heterologous prime-boost regimens, IgG2c was the dominant IgG subtype in the groups given RNA-gE as prime immunization, while the PS-gE>RNA-gE regimen produced a higher IgG1 proportion in total IgG. It revealed that RNA-gE as the prime immunization tended to exhibit cellular immune response while PS-gE>RNA-gE had a bias of humoral immune response. During humoral immune response, activated B cells will produce different Ig isotypes under the influence of various factors, including cytokines exposure and T-dependent (TD) or T-independent (TI) activation mode. In C57BL/6 mice, when B cells are stimulated with IFNγ, they will switch to IgG2c.47 Compared with IgG1, IgG2c strongly activates receptor FcγRIV, which mediates antibody-dependent cellular cytotoxicity (ADCC) of NK cells.48 The previous study has demonstrated a correlation between ADCC-associated antibodies and vaccine efficiency, as well as herpes zoster (HZ) episodes. Furthermore, IgG1/IgG2c ratio can be used to indicate Th1/Th2 immune bias to some extent.49
The effect of VZV vaccine on preventing the onset of HZ and reducing the severity of HZ-related pain in patients is mainly dependent on VZV-specific cellular immune response rather than humoral immune response.7 Here, we demonstrated that mRNA vaccine primed regimens induced a stronger gE-specific cell immune response in middle-aged mice compared to protein subunit vaccine primed immunization regimens, consistent with the relatively lower level of IgG1/IgG2a in these groups. In previous studies on the heterologous prime-boost immunization of COVID-19 vaccine, researchers demonstrated that administration of RBD encoded mRNA vaccine boosted with protein subunit vaccine produced a higher IgG2a/IgG1 ratio and IFNγ secreting T lymphocytes than protein subunit vaccine primed regimens in mice.21 Protein subunit vaccine primed regimens exhibited a strong capability in inducing neutralizing antibodies against COVID-19 virus.21 However, studies on the adenovirus vectored (Adv) vaccine (another type of nucleic acid vaccine) suggest that the Adv vaccine administered as a boost to immunization after a recombinant protein vaccine or an inactivated viral vaccine would produce a stronger CMI, but a down-regulated neutralizing antibody level than Adv-primed regimens.50,51 The differential results between mRNA vaccine and Adv vaccine might be due to the underlying differences between mRNA and DNA vaccines in antigen expression, presentation, and immune activation. As reported in previous reviews, the immune efficacies of heterogeneous prime-boost immunization strategies often involve various factors, such as immunodominant epitopes of the antigen, anti-vector immunity, the relative strengths of cellular and humoral immunity et al52,53 mRNA vaccines could imitate the process of natural viral infections, displaying more conformational epitopes than recombinant proteins. Therefore, co-delivery of mRNA vaccine and protein subunit vaccine can induce antibodies with broader neutralizing epitopes.21 Additionally, the lipid components and mRNA in mRNA vaccines can provide extra-adjuvant effects for protein vaccines.21 In another study, researchers proposed a mechanistic model for sequential immunization with an inactivated virus vaccine and a protein vaccine.54 In this model, the prime immunization with a protein vaccine generates antigen-specific CD8+ T cells, which significantly enhances the proliferation of antigen-specific CD8+ T cells upon the subsequent administration of the inactivated virus vaccine. In contrast, the inactivated virus vaccine-prime regimen often leads to the generation of CD8+ T cells that targeted non-specific virus epitopes, which could weaken the cellular immune response of the booster protein vaccine. As mRNA vaccine mainly induced a Th1-biased immunity, it results in a higher proportion of Th1-CD4+ T cells and CD8+ T cells, as well as corresponding memory cells. Consequently, the boosted protein vaccine will lead to a stronger activation and proliferation of these cell-mediated immunity-related T cells, while the activation of humoral immune response will be relatively weaker. Furthermore, the broad-spectrum CD8+ T cell epitopes provided by mRNA prime immunization may also be a crucial influence factor in mRNA prime-protein boost strategy. In addition, age is an important risk factor for HZ onset. Along with age, the incidences of HZ and associated PHN increase significantly after the age of 40–50 years, largely due to the age-related decline of CMI response.55,56 Furthermore, aging also leads to a weakened response to vaccines in vaccinated individuals, diminishing the protective efficiency of VZV vaccine over time.13,16 Therefore, the age of mice used in this study may influence the observed immune responses, warranting further investigation.
Conclusion
To summarize, we investigated the optimal immune strategy of VZV mRNA vaccine and protein-subunit vaccine in VZV-primed C57BL/6 mice, which are equivalent to human adults of 42–45 years old at which shingles disease begins to occur, and found that the cell immune response elicited by heterologous prime-boost regimen of “RNA-gE prime and PS-gE boost” was equal with mRNA vaccine, but significantly stronger than protein-subunit vaccine primed regimens. These findings provide valuable information to the design and optimization of VZV vaccines, which helps broaden varicella vaccination strategies in the future. The complexity of the immune response resulting from various vaccination combinations will require further investigation into the underlying mechanism in order to apply them in real world situation.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zerboni L, Sen N, Oliver SL, Arvin AM. Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev Microbiol. 2014;12(3):197–210. doi:10.1038/nrmicro3215
2. Arruti M, Piñeiro LD, Salicio Y, Cilla G, Goenaga MA, López de Munain A. Incidence of varicella zoster virus infections of the central nervous system in the elderly: a large tertiary hospital-based series. J Neurovirol. 2017;23(3):451–459. doi:10.1007/s13365-017-0519-y
3. Breuer J. Molecular Genetic Insights Into Varicella Zoster Virus (VZV), the vOka vaccine strain, and the pathogenesis of latency and reactivation. J Infect Dis. 2018;218(suppl_2):S75–s80. doi:10.1093/infdis/jiy279
4. Gilden DH, Vafai A, Shtram Y, Becker Y, Devlin M, Wellish M. Varicella-zoster virus DNA in human sensory ganglia. Nature. 1983;306(5942):478–480. doi:10.1038/306478a0
5. Weller TH. Varicella and herpes zoster. changing concepts of the natural history, control, and importance of a not-so-benign virus. N Engl J Med. 1983;309(23):1434–1440. doi:10.1056/NEJM198312083092306
6. Gershon AA, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nature Reviews Disease Primers. 2015;1(1):15016. doi:10.1038/nrdp.2015.16
7. Asada H. VZV-specific cell-mediated immunity, but not humoral immunity, correlates inversely with the incidence of herpes zoster and the severity of skin symptoms and zoster-associated pain: the SHEZ study. Vaccine. 2019;37(44):6776–6781. doi:10.1016/j.vaccine.2019.09.031
8. Steain M, Sutherland JP, Rodriguez M, Cunningham AL, Slobedman B, Abendroth A. Analysis of T cell responses during active varicella-zoster virus reactivation in human ganglia. J Virol. 2014;88(5):2704–2716. doi:10.1128/JVI.03445-13
9. Haberthur K, Engelmann F, Park B, et al. CD4 T cell immunity is critical for the control of simian varicella virus infection in a nonhuman primate model of VZV infection. PLoS Pathog. 2011;7(11):e1002367. doi:10.1371/journal.ppat.1002367
10. Gilbert PB, Gabriel EE, Miao XP, et al. Fold rise in antibody titers by measured by glycoprotein-based enzyme-linked immunosorbent assay is an excellent correlate of protection for a herpes zoster vaccine, demonstrated via the vaccine efficacy curve. J Infect Dis. 2014;210(10):1573–1581. doi:10.1093/infdis/jiu279
11. Cunningham AL, Heineman TC, Lal H, et al. Immune responses to a recombinant glycoprotein e herpes zoster vaccine in adults aged 50 years or older. J Infect Dis. 2018;217(11):1750–1760. doi:10.1093/infdis/jiy095
12. Cohen JI. A New Vaccine to Prevent Herpes Zoster. N Engl J Med. 2015;372(22):2149–2150. doi:10.1056/NEJMe1505050
13. Chlibek R, Bayas JM, Collins H, et al. Safety and Immunogenicity of an AS01-adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults ≥50 years of age. J Infect Dis. 2013;208(12):1953–1961. doi:10.1093/infdis/jit365
14. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–2284. doi:10.1056/NEJMoa051016
15. Gilderman LI, Lawless JF, Nolen TM, et al. A double-blind, randomized, controlled, multicenter safety and immunogenicity study of a refrigerator-stable formulation of Zostavax. Clin Vaccine Immunol. 2008;15(2):314–319. doi:10.1128/CVI.00310-07
16. Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–1032. doi:10.1056/NEJMoa1603800
17. Wang PF. Natural and synthetic saponins as vaccine adjuvants. Vaccines. 2021;9(3):1.
18. Upadhyay S, Jeena GS, Shikha RK, Shukla RK. Recent advances in steroidal saponins biosynthesis and in vitro production. Planta. 2018;248(3):519–544. doi:10.1007/s00425-018-2911-0
19. Vitiello A, Ferrara F. Brief review of the mRNA vaccines COVID-19. Inflammopharmacology. 2021;29(3):645–649. doi:10.1007/s10787-021-00811-0
20. Li M, Ren J, Si X, et al. The global mRNA vaccine patent landscape. Hum Vaccin Immunother. 2022;18(6). doi:10.1080/21645515.2022.2095837.
21. Zhang J, He Q, Yan X, et al. Mixed formulation of mRNA and protein-based COVID-19 vaccines triggered superior neutralizing antibody responses. MedComm. 2022;3(4):e188. doi:10.1002/mco2.188
22. Li D, Zhao H, Liao Y, et al. Long-term cross immune response in mice following heterologous prime-boost COVID-19 vaccination with full-length spike mRNA and recombinant S1 Protein. Vaccines. 2023;11(5):1.
23. Lu S. Heterologous prime-boost vaccination. Curr Opin Immunol. 2009;21(3):346–351. doi:10.1016/j.coi.2009.05.016
24. Burm R, Maravelia P, Ahlen G, et al. Novel prime-boost immune-based therapy inhibiting both hepatitis B and D virus infections. Gut. 2023;72(6):1186–1195. doi:10.1136/gutjnl-2022-327216
25. Wang SX, Arthos J, Lawrence JA, et al. Enhanced immunogenicity of gp120 protein when combined with recombinant DNA priming to generate antibodies that neutralize the JR-FL primary isolate of human immunodeficiency virus type 1. J Virol. 2005;79(12):7933–7937. doi:10.1128/JVI.79.12.7933-7937.2005
26. Liu X, Shaw RH, Stuart ASV, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398(10303):856–869. doi:10.1016/S0140-6736(21)01694-9
27. Excler J-L, Kim JH. Novel prime-boost vaccine strategies against HIV-1. Expert Rev Vacci. 2019;18(8):765–779. doi:10.1080/14760584.2019.1640117
28. Aleshin SE, Timofeev AV, Khoretonenko MV, et al. Combined prime-boost vaccination against tick-borne encephalitis (TBE) using a recombinant vaccinia virus and a bacterial plasmid both expressing TBE virus non-structural NSI protein. BMC Microbiol. 2005;5:5. doi:10.1186/1471-2180-5-5
29. Kaku CI, Champney ER, Normark J, et al. Broad anti-SARS-CoV-2 antibody immunity induced by heterologous ChAdOx1/mRNA-1273 vaccination. Science. 2022;375(6584):1041. doi:10.1126/science.abn2688
30. Barros-Martins J, Hammerschmidt SI, Cossmann A, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021;27(9):1525–+. doi:10.1038/s41591-021-01449-9
31. Cohen G, Jungsomsri P, Sangwongwanich J, et al. Immunogenicity and reactogenicity after heterologous prime-boost vaccination with CoronaVac and ChAdox1 nCov-19 (AZD1222) vaccines. Hum Vaccin Immunother. 2022;18(5). doi:10.1080/21645515.2022.2052525.
32. Campos GRF, Almeida NBF, Filgueiras PS, et al. Booster dose of BNT162b2 after two doses of CoronaVac improves neutralization of SARS-CoV-2 Omicron variant. Communicat Med. 2022;2(1). doi:10.1038/s43856-022-00141-4.
33. Xu X, Liao Y, Jiang G, et al. Immunological evaluation of an mRNA vaccine booster in individuals fully immunized with an inactivated SARS-CoV-2 vaccine. Clini Trans Med. 2022;12(6). doi:10.1002/ctm2.875.
34. Zhang H, Zhang L, Lin A, et al. Algorithm for optimized mRNA design improves stability and immunogenicity. Nature. 2023;621(7978):396–+.
35. Monslow MA, Elbashir S, Sullivan NL, et al. Immunogenicity generated by mRNA vaccine encoding VZV gE antigen is comparable to adjuvanted subunit vaccine and better than live attenuated vaccine in nonhuman primates. Vaccine. 2020;38(36):5793–5802. doi:10.1016/j.vaccine.2020.06.062
36. Cao H, Wang Y, Luan N, Lin K, Liu C. Effects of varicella-zoster virus glycoprotein e carboxyl-terminal mutation on mRNA vaccine efficacy. Vaccines. 2021;9(12):1.
37. He L, Sun B, Guo Y, et al. Immune response of C57BL/6J mice to herpes zoster subunit vaccines formulated with nanoemulsion-based and liposome-based adjuvants. Int Immunopharmacol. 2021;1:101.
38. Dendouga N, Fochesato M, Lockman L, Mossman S, Giannini SL. Cell-mediated immune responses to a varicella-zoster virus glycoprotein E vaccine using both a TLR agonist and QS21 in mice. Vaccine. 2012;30(20):3126–3135. doi:10.1016/j.vaccine.2012.01.088
39. Rivers JR, Ashton JC. Age matching animal models to humans--theoretical considerations. Curre Drug Disco Tech. 2013;10(3):177–181. doi:10.2174/1570163811310030001
40. Hope-Simpson RE. THE NATURE OF HERPES ZOSTER: a LONG-TERM STUDY AND A NEW HYPOTHESIS. Proc R Soc Med. 1965;58(1):9–20.
41. Patil A, Goldust M, Wollina U. Herpes zoster: a review of clinical manifestations and management. Viruses-Basel. 2022;14(2):192. doi:10.3390/v14020192
42. Singh M, O’Hagan D. Advances in vaccine adjuvants. Nat Biotechnol. 1999;17(11):1075–1081. doi:10.1038/15058
43. Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8<SUP>+</SUP> T cells. Blood. 2006;107(12):4781–4789. doi:10.1182/blood-2005-12-4818
44. Harari A, Enders FB, Cellerai C, Bart P-A, Pantaleo G. Distinct profiles of cytotoxic granules in memory CD8 T cells correlate with function, differentiation stage, and antigen exposure. J Virol. 2009;83(7):2862–2871. doi:10.1128/JVI.02528-08
45. Hansen SG, Vieville C, Whizin N, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2011;17(12):1692. doi:10.1038/nm1211-1692
46. Wang Y, Cao H, Lin K, Hu J, Luan N, Liu C. Evaluation of the immunological efficacy of an LNP-mRNA vaccine prepared from varicella zoster virus glycoprotein gE with a double-mutated carboxyl terminus in different untranslated regions in mice. Vaccines. 2023;11(9):1475. doi:10.3390/vaccines11091475
47. Deenick EK, Hasbold J, Hodgkin PD. Decision criteria for resolving isotype switching conflicts by B cells. Eur J Immunol. 2005;35(10):2949–2955. doi:10.1002/eji.200425719
48. Kaugars K, Dardick J, de Oliveira AP, et al. A recombinant herpes virus expressing influenza hemagglutinin confers protection and induces antibody-dependent cellular cytotoxicity. Proc Natl Acad Sci U S A. 2021;118(34). doi:10.1073/pnas.2110714118.
49. Kanai N, Min W-P, Ichim TE, Wang H, Zhong R. Th1/Th2 xenogenic antibody responses are associated with recipient dendritic cells. Microsurgery. 2007;27(4):234–239. doi:10.1002/micr.20342
50. He Q, Mao Q, An C, et al. Heterologous prime-boost: breaking the protective immune response bottleneck of COVID-19 vaccine candidates. Emerging Microbes Infect. 2021;10(1):629–637. doi:10.1080/22221751.2021.1902245
51. He Q, Mao Q, Zhang J, et al. Heterologous immunization with adenovirus vectored and inactivated vaccines effectively protects against SARS-CoV-2 variants in mice and macaques. Front Immunol. 2022;1:13.
52. Pan Y, Jia R, Li J, et al. Heterologous prime-boost: an important candidate immunization strategy against Tembusu virus. Virol J. 2020;17(1). doi:10.1186/s12985-020-01334-w.
53. Kardani K, Bolhassani A, Shahbazi S. Prime-boost vaccine strategy against viral infections: mechanisms and benefits. Vaccine. 2016;34(4):413–423. doi:10.1016/j.vaccine.2015.11.062
54. Schneider J, Gilbert SC, Hannan CM, et al. Induction of CD8 + T cells using heterologous prime-boost immunisation strategies. Immunol Rev. 1999;170(1):29–38. doi:10.1111/j.1600-065X.1999.tb01326.x
55. Harpaz R, Ortega-Sanchez IR, Seward JF. Advisory committee on immunization practices centers for disease C, prevention. prevention of herpes zoster: recommendations of the advisory committee on immunization practices (ACIP). MMWR Reco Rep. 2008;57(RR–5):1.
56. Yawn BP, Gilden D. THE GLOBAL EPIDEMIOLOGY OF HERPES ZOSTER. Neurology. 2013;81(10):928–930. doi:10.1212/WNL.0b013e3182a3516e
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.