Back to Journals » Breast Cancer: Targets and Therapy » Volume 16
Hypofractionated versus Conventional Postmastectomy Irradiation for Breast Cancer: Comparison of Acute Skin Toxicity
Authors Wu Z, Hou L, Li C, Li X, Li Y
Received 2 April 2024
Accepted for publication 24 July 2024
Published 30 July 2024 Volume 2024:16 Pages 423—432
DOI https://doi.org/10.2147/BCTT.S471901
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Pooja Advani
Zhiyuan Wu,1,* Lili Hou,2,* Cheng Li,1,* Xiaohua Li,2 Ying Li1
1Department of Radio-Oncology, Suzhou Municipal Hospital, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou, People’s Republic of China; 2Department of Breast Surgery, Suzhou Wuzhong People’s Hospital, Suzhou, People’s Republic of China
*These authors contributed equally to this works
Correspondence: Ying Li; Xiaohua Li, Email [email protected]; [email protected]
Purpose: Breast cancer is the leading cause of cancer mortality among women. Radiotherapy can reduce recurrence and prolong survival of patients accepting breast-conserving surgery (BCS). This study aims to compare acute skin reactions in patients receiving hypofractionated versus conventional radiotherapy at a single institution and to summarize the relevant influencing factors.
Methods: This study analyzed 152 patients who underwent either hypofractionated or conventional whole-breast irradiation (WBI) after BCS. Acute skin toxicity was assessed according to the Radiation Therapy Oncology Group (RTOG) criteria. Predictive factors for acute skin toxicity were identified using multivariate analysis and visualized using a forest spot.
Results: Grade 0 reactions occurred in 75.34% vs 70.89%, grade 1 in 16.44% vs 15.19%, grade 2 in 8.22% vs 12.66%, and grade 3 in 0% vs 1.27% of patients receiving hypofractionated and conventional WBI, respectively. There was no statistically significant difference in acute skin reaction in patients treated with hypofractionated radiation compared with conventional radiation (P = 0.62). Multivariate analysis revealed that metastatic lymph nodes (P = 0.021), whole-breast planning target volume (PTV-WB) (P < 0.001), and tumor bed planning target volume (PTV-TB) (P = 0.002) were significantly correlated with higher rates of acute skin toxicity.
Conclusion: Hypofractionated WBI demonstrated similar acute skin adverse reactions compared to conventional WBI. These findings indicate that hypofractionated radiotherapy offers comparable tolerance, equivalent curative effect, convenience, and economic benefits, supporting its clinical promotion.
Keywords: breast cancer, hypofractionated radiation, conventional radiation, acute skin toxicity
Introduction
Breast cancer is the leading cause of cancer mortality among women worldwide, with an estimated 2,100,000 newly diagnosed cases and 627,000 death cases in 2018.1 As the largest developing country, China has a relatively low level of morbidity of breast cancer globally. However, the incidence of breast cancer has increased rapidly since the 1990s.2 Recent statistics indicate that breast cancer ranks fifth in cancer mortality in China, accounting for 30% of new cancer cases and 15% of cancer-related death cases among women.3 Breast cancer places a significant financial and health burden on women in rural China because of increasing high-risk factors and the rapid expansion of the economy, population growth, and aging. Nowadays, the main treatments for breast cancer include surgery, chemotherapy, radiotherapy, endocrinotherapy, targeted therapy, and immunotherapy. Patients undergoing breast-conserving surgery (BCS) benefit substantially from radiotherapy (RT), which can reduce local and distant recurrence, as well as prolong survival in patients with early-stage breast cancer. In the last 25 years, the standard of adjuvant whole-breast irradiation (WBI) has been a conventional fraction with a total dose of 50Gy in 25 fractions over a period of 5–6 weeks after BCS. Thus, the conventional fraction imposes a substantial time and economic burden on healthcare systems and patients. However, with more than 10 years of follow-up, several large randomized controlled trials have demonstrated that hypofractionated WBI (40–42Gy in 15–16 fractions), with better acute tolerance and equal efficacy, is an equivalent or even superior WBI regimen compared with conventional radiation.4–8
Hypofractionated radiation (HypoRT) has been identified as an alternative regimen in breast cancer because of its better acute tolerance, equal efficacy, late toxicity, equivalent outcomes, and compelling data. The present study evaluated acute toxicity in patients undergoing conventional WBI therapy with sequential boost (SEB) to the tumor bed and hypofractionated WBI with simultaneous integrated boost (SIB) to the tumor bed.
Materials and Methods
Participants
The present study was conducted as a retrospective investigation of breast cancer that had been referred to the Affiliated Suzhou Hospital of Nanjing Medical University (Jiangsu, China) between September 2018 and June 2023. The study analyzed 152 patients who underwent RT after BCS. The study was approved by the Medical Ethics Committees of the Affiliated Suzhou Hospital of Nanjing Medical University. All patients have signed informed consent before RT, and their ages ranged from 28 to 78 years. The staging of cancer was made according to tumor-nodulus-metastases (TNM) classification and classified through the American Joint Committee on Cancer (AJCC) recommendations. Among 152 patients, 108 patients had stage I disease, 39 had stage II disease, and 5 patients had stage III disease. Among 152 patients, 126 were diagnosed with invasive ductal carcinoma, 21 with ductal carcinoma in situ, 3 with mucinous carcinoma, 1 with adenoid cystic carcinoma, and 1 with metaplastic carcinoma.
Radiotherapy Planning and Delivery
All participants underwent computed tomography (CT)-imaging while in the supine position with their arms above the head for radiotherapy planning and scanned in free breathing. Patients underwent virtual simulation CT using a GE workstation (GE Medical Systems Ltd., Pollards Wood, United Kingdom) with 5-mm-thick slices obtained at 5-mm intervals. Dose optimization and calculations were performed with Eclipse Version 11.0 (Varian Medical Systems, Palo Alto, CA, USA). The superior margin of scanning was the sternoclavicular joint, and the inferior margin was 2 cm below the inferior margin of the breast. For patients requiring irradiation in the supraclavicular lymph node region, the superior margin was increased to 2 cm above the cricothyroid membrane.
Target Delineation
All contouring was performed by an attending physician experienced in breast radiotherapy and then confirmed by a chief physician. Target delineation was referred to guidelines of the National Health Commission (Version 2018) and American Society for Radiation Oncology (ASTRO) guidelines (Version 2018). Whole-breast planning target volume (PTV-WB) was defined as the entire ipsilateral breast parenchyma. The setup margin: the superior margin did not exceed the sternoclavicular joint; the inferior margin was the point where breast prominence diminishes; the anterior margin was 0.5 cm under the skin; the posterior margin was in front of pectoralis major muscle fascia, ribs, and intercostal muscles; the external margin did not exceed midaxillary line or lateral thoracic artery; the internal margin did not exceed sternocostal joint or internal mammary artery. Tumor bed planning target volume (PTV-TB) was defined by surgical clips placed in the lumpectomy cavity during surgery, with an additional margin of 1.5 cm. PTV-TB did not exceed PTV-WB. The patients with metastasis in 1–2 sentinel lymph nodes were treated with high tangent field (HTF) radiation. The setup margin of HTF was 2 cm below the humeral head. Four patients with over four lymph node metastases were treated with conventional fraction and additional irradiation to the internal mammary and supraclavicular lymph node region. The internal mammary region was defined as the medial intercostal region of the first to the third rib. Supraclavicular lymph node region: the superior margin was the inferior margin of cricoid cartilage; the inferior margin was the confluence of brachiocephalic vein and axillary vein; the anterior margin was sternocleidomastoid muscle; the posterior margin was the anterior margin of scalene muscle. Organs at risk (OARs) defined were contralateral breast, ipsilateral, and contralateral lung, heart, and spinal cord.
Radiation Protocol
A total of 73 patients received hypofractionated WBI at 4005cGY/15F with SIB of 800cGY/15F to the tumor bed, whereas 79 patients were treated with conventional fractionated WBI at 5000cGY/25F with SEB of 1000cGY/5F to the tumor bed. Regional lymph node irradiation (RNI), including the supraclavicular region and the internal mammary region at 5000cGY/25F, was given to four patients with more than four axillary lymph node metastasis. The criteria for target coverage were as follows: D100% for both PTV-WB and PTV-TB was >90%, and D95% for both PTV-WB and PTV-TB was 99%. D110% for both PTV-WB and PTV-TB was <1%. Dmax of each scan slice was contained in PTV. The criteria for OARs were as follows: the Dmax of the spine cord was <35Gy; the Dmax of the contralateral breast was <5Gy; the Dmax of the heart was <8Gy; the ipsilateral lung was V20 <25% and V5 <40%.
Treatment Verification
Treatment planning was performed using Eclipse 11.0 (Varian, Palo Alto, CA, USA). All patients were treated with intensity-modulated radiotherapy (IMRT). Target region and OARs were estimated using a dose-volume histogram (DVH). The target volumes and OARs were contoured by a radiotherapist and later confirmed by the chief radiotherapist and radiation oncologist. All treatment plans were approved by a chief radiation oncologist prior to treatment. All patients were treated with the Varian TrueBeam STx (Palo Alto, California, USA) linear accelerator in a supine position by two radiotherapists and underwent weekly cone-beam computed tomography (CBCT) for validation.
Patient Evaluation
The assessment of acute skin toxicity in four weeks after RT was performed by physician-assessed subjective dermatitis scoring by visual inspections or photographs according to the Radiation Therapy Oncology Group (RTOG) criteria. RTOG criteria: grade 0: no change; grade 1: Follicular, faint or dull erythema/epilation/dry desquamation/decreased sweating; grade 2: Tender or bright erythema, patchy moist desquamation/moderate edema; grade 3: Confluent, moist desquamation other than skin folds, pitting edema; grade 4: Ulceration, hemorrhage, and necrosis.
Criteria for the Assessment of Acute Skin Toxicity
Cosmetic outcomes were evaluated using quantitative measurements by Limbergen at 1- and 6-month post-treatment.9 The assessment of acute toxicity, including skin edema, radiodermatitis, radiation pneumonitis, radiation-induced heart injury, late toxicity, and local recurrence, was solely performed according to the RTOG criteria.10 Local recurrence of breast and distant metastasis was monitored through physical examination, ultrasound scan, and CT scanning. All patients were followed until December 2023.
Statistical Analysis
Statistical analyses were performed with SPSS Statistics Software Version 19.0 (IBM Corporation, Chicago, IL). Continuous variables that complied with normal distribution are expressed in means ± standard deviation (SD), and subgroups were compared using a t-test. Continuous variables that did not comply with the normal distribution are expressed in median (Quartile), and subgroups were compared using a nonparametric test. Categorical variables were evaluated by number of cases and percentage. The χ2 test and Fisher exact tests were used to compare the categorical variables. Factors predicting acute skin toxicity were identified using univariate and multivariate Cox regression analysis models and then visualized using Forest spots. All values of P < 0.05 were considered statistically significant.
Results
Clinical Effect
The follow-up period was 6–61 months, with a median follow-up time of (32.5 ± 2.5) months. No instances of recurrence were observed during the entire follow-up duration. The local control rate achieved 100% in both cohorts until December 2023.
Clinical Characteristics of Patients
The present study enrolled 79 patients who underwent conventional WBI with SEB and 73 patients of hypofractionated WBI with SIB after BCS between 2018 and 2023. Patient characteristics are shown in Table 1. No statistically significant difference was observed between hypofractionated and conventional WBI. Acute skin toxicity was found to be associated with body mass index (BMI), tumor size, metastatic lymph nodes, TNM stage, V20 of the affected lung, lung volume on the affected side, breast volume on the healthy side, PTV-WB volume, and PTV-TB volume. Patients suffering from acute skin toxicity had significantly higher BMI, V20, breast volume, PTV-WB volume, PTV-TB volume, larger tumor size, and positive lymph nodes compared with those without acute skin toxicity. The proportion of stage IIB and above in patients who suffered from acute skin toxicity was significantly higher than those without acute skin toxicity. The lung volume of patients suffering from acute skin toxicity was significantly lower than those without acute skin toxicity.
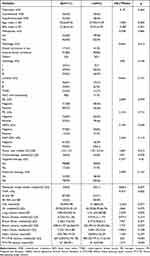 |
Table 1 Association of Clinical Factors with Acute Skin Toxicity |
Physician-Assessed Toxic Effects
The following reactions were observed in patients treated with conventional radiation: 56 grade 0 reaction (70.89%), 12 grade 1 reaction (15.19%), 10 grade 2 reaction (12.66%), and 1 grade 3 reaction (1.27%), whereas those treated with HypoRT exhibited the following reactions: 55 grade 0 reaction (75.34%), 12 grade 1 reaction (16.44%), and 6 grade 2 reaction (8.22%). Overall, no grade 4 reaction was observed in the present study, and only one patient developed a grade 3 reaction treated with conventional radiation. Grade 0 reaction occurred in 75.34% vs 70.89%, grade 1 in 16.44% vs 15.19%, grade 2 in 8.22% vs 12.66%, and grade 3 in 0% vs 1.27% of patients accepting hypofractionated and conventional WBI, respectively (Figure 1).
 |
Figure 1 Comparison of histograms for acute skin adverse reactions. |
Quantitative Data Synthesis
Univariate analyses indicated that age (Odds ratio [OR]=1.036; 95% confidence interval [CI] 1.002–1.071; P = 0.037), BMI (OR = 1.162; 95% CI: 1.062–1.272; P = 0.001), tumor size (OR 2.219; 95% CI: 1.295–3.805; P = 0.004), lymph nodes (OR = 1.576; 95% CI: 1.042–2.383; P = 0.031), TNM stage (OR = 3.452; 95% CI: 1.614–7.386; P = 0.001), V20 (OR = 1.07; 95% CI: 1.007–1.137; P = 0.028), lung volume (HR 3.452; 95% CI: 1.614–7.386; P = 0.001), breast volume (OR = 0.998; 95% CI: 0.996–1; P = 0.003), PTV-WB volume (OR = 1.007; 95% CI: 1.005–1.009; P<0.001), and PTV TB volume (OR = 1.034; 95% CI: 1.018–1.051; P<0.001) might be correlated with acute radiation-induced skin toxicity (Figure 2).
 |
Figure 2 Forest plot comparing acute skin toxicity of CF with that of HypoRT in breast cancer (Univariate Cox regression analysis). |
In the multivariate analysis, lymph nodes (OR = 2.004; 95% CI: 1.158–4.055; P = 0.021), PTV-WB volume (OR = 1.009; 95% CI: 1.005–1.014; P<0.001), and PTV-TB volume (OR = 1.039; 95% CI: 1.016–1.066; P = 0.002) had a significant effect on increased risk of acute radiation-induced skin toxicity. The risk of reaction increased by 100.4% for each increase in lymph node metastasis. The risk of reaction increased by 0.9% for every 1 cm3 increase in PTV-WB volume. The risk of reaction increased by 3.9% for every 1 cm3 increase in PTV-TB volume. Moreover, the TNM stage showed marginal significance, suggesting that it might be a potential risk factor for acute radiation-induced skin toxicity. Patients with stage IIB and above had a 5.339 times higher risk of acute radiation-induced skin toxicity than those with stage IIA and below (OR = 5.339) (Figure 3).
 |
Figure 3 Forest plot comparing acute skin toxicity of CF with that of HypoRT in breast cancer (Multivariate Cox regression analysis). |
Discussion
Based on a substantial number of pathological specimens and clinical research, most recurrence in the ipsilateral breast developed at or near the original tumor site.11 Previous randomized clinical studies have reported that a radiation boost in the tumor bed after WBI could improve local control and reduce the risk of recurrence by half, especially in young patients.12 Conventional fraction has been recognized as standard adjuvant radiotherapy after BCS. Typically, a conventional regimen is delivered in 25 fractions of 2Gy with a boost of 10Gy/5F to the tumor bed of 5–7 weeks, which impacts the patients and the healthcare system. Because of the rapid development of radiation treatment, studies have exerted direct effects on the reduction of radiation toxicity, treatment period, and healthcare spending. A hypofractionated regimen with fewer, larger fractions was thus born. The theoretical basis of a hypofractionated regimen was based on the radiobiological characteristics of tumor tissue. A low α/β value of 2–4Gy for breast cancer,13 which was similar to late response tissue, suggests that an increased single dose will not aggravate the toxicity, indicating equivalent efficacy to a conventional regimen. Multiple prospective studies have demonstrated that HypoRT with SEB to the tumor bed shows equivalent efficacy and would not increase toxicity compared with conventional fractions.5–7 The IMPOWER HIGH trial also reported in 2023 that HypoRT with SIB to the tumor bed does not result in a higher local recurrence rate or an increased incidence of adverse reactions in 5 years.8 Therefore, as the hypofractionated regimen had substantially shortened the treatment period without an associated increase in risk and reduced the healthcare and time cost of patients and the healthcare system, it deserves full-scale promotion in clinical practice.
Because of the favorable prognosis and extended survival time associated with early-stage breast cancer, the detrimental effects of acute and late radiation therapy in breast cancer are increasingly acknowledged for their effects on patients, particularly among younger patients. Adverse reactions of radiotherapy include skin toxicity, radiation-induced lung injury, radiation-induced heart disease, upper-limb lymphedema, and radiation-induced brachial plexus neuropathy. Common acute adverse reactions in breast cancer include radiation dermatitis, desquamation, edema, and pain, whereas late adverse events include breast shrinkage, distortion, induration, breast tenderness on palpation, breast discomfort, and telangiectasia. Studies have reported that the conventional regimen induced significantly more acute skin toxicity than the hypofractionated regimen.14,15 For instance, DBCG HYPO reported that low rate of breast pain, edema, telangiectasia, dyspigmentation, and scar appearance were observed in HypoRT (40Gy/15F) cohort, and there was either no significant difference or better cosmetic outcomes in patients underwent HypoRT cohort compared with standard fractionated radiotherapy (50Gy/25F). Moreover, 9-year overall survival showed no difference between HypoRT cohort and conventional fraction cohort.16 The FAST trial reported no significant difference in normal tissue effects between the hypofractionated (28.5Gy/5F) and conventional (50Gy/25F) regimens.17 The RTOG1005 trial also reported no statistically significant difference between C-WBI (50/25 F or 42.7Gy/16 F with SEB of 12Gy/6 F or 14Gy/7F) and H-WBI (40Gy/15 F with SIB of 8Gy/15 F). Moreover, there were no discernible variations in adverse reactions observed between the two treatment cohorts, as evidenced by comparable rates of grade ≥3 treatment-related adverse events at 3.3% in Group I and 3.5% in Group II (P = 0 0.79).18 The findings of this present study are consistent with those of previous clinical trials: HypoRT with SIB does not increase the incidence of acute radioactive skin adverse reactions.
In the present study, univariate Cox regression analysis revealed that BMI, tumor size, metastatic lymph nodes, TNM stage, V20 of the affected lung, lung volume on the affected side, breast volume on the healthy side, PTV-WB volume, and PTV-TB volume might have correlations with acute skin toxicity. Obesity has been widely recognized as a risk factor for the development and recurrence of breast cancer.19 Arti Parekh et al demonstrated that higher BMI was correlated with an increased risk of moist desquamation.20 Additionally, in another study by Celine et al, grade ≥2 acute skin toxicity events were significantly correlated with higher BMI (≥25).21 Previous studies have highlighted that large-breasted patients faced more challenges in the delivery of radiotherapy. In the study of Corbin et al, the mean breast volume of patients who suffered dry and/or focal moist desquamation was significantly higher compared with those without dry and/or moist desquamation. Multivariate analysis demonstrated that breast volume was significantly correlated with moist desquamation.22 Moreover, patients treated with conventional radiation suffered a higher rate of focal moist desquamation than those treated with HypoRT.22 Consistent with the present study, Celine et al demonstrated that grade ≥2 acute skin toxicity events were significantly associated with large breast volume.21 In the present study, the mean breast volume of patients without skin adverse reactions was significantly lower than those suffered from grade ≥1 acute skin toxicity (P = 0.037). Moreover, Celine et al also reported that grade ≥2 acute skin toxicity was significantly correlated with larger breast clinical tumor volume (CTV) 1 (>500 cm3) and CTV2 (>25 cm3).21 Furthermore, in a recent study by Montero et al, a statistically significant correlation was found between the median PTV-WB and skin toxicity.23 Fodor et al reported that PTV-WB volume was significantly associated with grade ≥2 edema and hyperpigmentation.24 In the present study, multivariate Cox regression analysis suggested that PTV-WB volume and PTV-TB volume had a significant correlation with increased risk of acute skin toxicity.
Radiation pneumonitis is a potentially fatal complication of radiation therapy, with a 1–3% incidence rate among patients undergoing whole-breast irradiation. However, most patients experience symptom relief after hormone therapy. The DBCG HYPO study demonstrated that both hypofractionated and conventional fractionated radiotherapy resulted in extremely rare cases of radiation-related lung injury, with no statistically significant difference between the two treatment approaches.16 A recent study by Indian researchers reported that comparable rates of radiation pneumonitis were caused by hypofractionated radiotherapy (40Gy/15f) and conventional fractionated radiotherapy (50Gy/25f).25 Previous large-scale studies on conventional fractionated radiotherapy have confirmed the unavoidable occurrence of cardiac complications following chest wall irradiation in breast cancer patients undergoing radiation therapy. However, there are limited prospective studies investigating the effect of hypofractionated radiotherapy on cardiac health. Khan et al quantified the effects of hypofractionated radiotherapy on the heart and found no significant acute or late cardiac adverse events in patients followed up for 2 years after receiving hypofractionated radiotherapy (42.5Gy/16f).26 Consistent with findings from the DBCG HYPO study, both hypofractionated and conventional fractionated radiotherapy were associated with extremely rare cases of radiation-related cardiopulmonary injury without any statistical difference between them.16 Although the present study did not include data on the occurrence of radiation pneumonitis or radiation-induced cardiac damage, future research should explore additional predictive indicators and assessments related to these conditions.
In summary, the present study demonstrated that acute skin toxicity in patients accepting HypoRT was similar to conventional radiation. Multivariate analysis suggested that lymph nodes, PTV-WB volume, and PTV-TB volume had a significant effect on increased risk of acute radiation-induced skin toxicity. With the advantage of better tolerance, equal efficacy, and equivalent outcomes, HypoRT has been considered an alternative regimen for breast cancer. However, the present study has some limitations. First, it is a retrospective study with a relatively small sample size. Second, only acute skin toxicity was investigated in the present study. Third, longer follow-up is required to evaluate late adverse events. Many efforts are required to grasp a more conclusive understanding of the feasibility of HypoRT. Future studies should probe into radiation-induced cardiac and lung toxicity of HypoRT.
Data Sharing Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
Given that this study was conducted relying on retrospective data collected as part of routine clinical practice, ethical committee waived the consent. In this retrospective study, no patient identifiers were used, and data were anonymized. This study followed the Declaration of Helsinki (2013 revision).
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Disclosure
Zhiyuan Wu, Lili Hou, and Cheng Li are co-first authors for this study. The authors have no relevant financial or non-financial interests to disclose for this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Lei S, Zheng R, Zhang S, et al. Breast cancer incidence and mortality in women in China: temporal trends and projections to 2030. Cancer Biol Med. 2021;18(3):900–909. doi:10.20892/j.issn.2095-3941.2020.0523
3. DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–451. doi:10.3322/caac.21583
4. Owen JR, Ashton A, Bliss JM, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol. 2006;7(6):467–471. doi:10.1016/S1470-2045(06)70699-4
5. Group ST, Bentzen SM, Agrawal RK, et al. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9(4):331–341. doi:10.1016/S1470-2045(08)70077-9
6. Group ST, Bentzen SM, Agrawal RK, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371(9618):1098–1107. doi:10.1016/S0140-6736(08)60348-7
7. Krug D, Vonthein R, Schreiber A, et al. Impact of guideline changes on adoption of hypofractionation and breast cancer patient characteristics in the randomized controlled HYPOSIB trial. Strahlenther Onkol. 2021;197(9):802–811. doi:10.1007/s00066-020-01730-9
8. Coles CE, Haviland JS, Kirby AM, et al. Dose-escalated simultaneous integrated boost radiotherapy in early breast cancer (IMPORT HIGH): a multicentre, Phase 3, non-inferiority, open-label, randomised controlled trial. Lancet. 2023;401(10394):2124–2137. doi:10.1016/S0140-6736(23)00619-0
9. Van Limbergen E, van der Schueren E, Van Tongelen K. Cosmetic evaluation of breast conserving treatment for mammary cancer. 1. Proposal of a quantitative scoring system. Radiother Oncol. 1989;16(3):159–167. doi:10.1016/0167-8140(89)90016-9
10. Brunt AM, Wheatley D, Yarnold J, et al. Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3-week regimen delivered in the UK FAST-Forward Trial. Radiother Oncol. 2016;120(1):114–118. doi:10.1016/j.radonc.2016.02.027
11. Faverly DR, Hendriks JH, Holland R. Breast carcinomas of limited extent: frequency, radiologic-pathologic characteristics, and surgical margin requirements. Cancer. 2001;91(4):647–659. doi:10.1002/1097-0142(20010215)91:4<647::aid-cncr1053>3.0.co;2-z
12. Bartelink H, Maingon P, Poortmans P, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015;16(1):47–56. doi:10.1016/S1470-2045(14)71156-8
13. Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086–1094. doi:10.1016/S1470-2045(13)70386-3
14. Tortorelli G, Di Murro L, Barbarino R, et al. Standard or hypofractionated radiotherapy in the postoperative treatment of breast cancer: a retrospective analysis of acute skin toxicity and dose inhomogeneities. BMC Cancer. 2013;13:230. doi:10.1186/1471-2407-13-230
15. De Langhe S, Mulliez T, Veldeman L, et al. Factors modifying the risk for developing acute skin toxicity after whole-breast intensity modulated radiotherapy. BMC Cancer. 2014;14:711. doi:10.1186/1471-2407-14-711
16. Offersen BV, Alsner J, Nielsen HM, et al. Hypofractionated versus standard fractionated radiotherapy in patients with early breast cancer or ductal carcinoma in situ in a randomized phase iii trial: the DBCG HYPO trial. J Clin Oncol. 2020;38(31):3615–3625. doi:10.1200/JCO.20.01363
17. Brunt AM, Haviland JS, Sydenham M, et al. Ten-year results of FAST: a randomized controlled trial of 5-fraction whole-breast radiotherapy for early breast cancer. J Clin Oncol. 2020;38(28):3261–3272. doi:10.1200/JCO.19.02750
18. Vicini FA, Winter K, Freedman GM, et al. NRG RTOG 1005: a phase III trial of hypofractionated whole breast irradiation with concurrent boost vs. conventional whole breast irradiation plus sequential boost following lumpectomy for high-risk early-stage breast cancer. IJROGP. 2022:114. doi:10.1016/j.ijrobp.2022.07.2320
19. van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152(6):514–527. doi:10.1093/aje/152.6.514
20. Parekh A, Dholakia AD, Zabranksy DJ, et al. Predictors of radiation-induced acute skin toxicity in breast cancer at a single institution: role of fractionation and treatment volume. Adv Radiat Oncol. 2018;3(1):8–15. doi:10.1016/j.adro.2017.10.007
21. Bourgier C, Cowen D, Castan F, et al. Quality assurance program and early toxicities in the phase III BONBIS randomized trial evaluating the role of a localized radiation boost in ductal carcinoma in situ. Radiother Oncol. 2021;164:57–65. doi:10.1016/j.radonc.2021.09.014
22. Corbin KS, Dorn PL, Jain SK, Al-Hallaq HA, Hasan Y, Chmura SJ. Hypofractionated radiotherapy does not increase acute toxicity in large-breasted women: results from a prospectively collected series. Am J Clin Oncol. 2014;37(4):322–326. doi:10.1097/COC.0b013e31827b45b7
23. Montero A, Ciervide R, Canadillas C, et al. Acute skin toxicity of ultra-hypofractionated whole breast radiotherapy with simultaneous integrated boost for early breast cancer. Clin Transl Radiat Oncol. 2023;41:100651. doi:10.1016/j.ctro.2023.100651
24. Fodor A, Brombin C, Mangili P, et al. Toxicity of hypofractionated whole breast radiotherapy without boost and timescale of late skin responses in a large cohort of early-stage breast cancer patients. Clin Breast Cancer. 2022;22(4):e480–e487. doi:10.1016/j.clbc.2021.11.008
25. Malik D, Singh A, Birajdar MM, Vyas VJ. Feasibility, tolerance, and quality of life for hypofractionation versus conventional fractionation for post-mastectomy radiotherapy in Indian patients. Cureus. 2022;14(3):e23497. doi:10.7759/cureus.23497
26. Khan M, Gupta M, Seam R. Analysis of cardiac adverse events following postmastectomy hypofractionated radiotherapy. Chin Clin Oncol. 2014;3(4):47. doi:10.3978/j.issn.2304-3865.2014.06.06
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
