Back to Journals » Journal of Pain Research » Volume 17
Identification of Presurgical Risk Factors for the Development of Chronic Postsurgical Pain in Adults: A Comprehensive Umbrella Review
Authors Sydora BC , Whelan LJ, Abelseth B, Brar G, Idris S, Zhao R , Leonard AJ , Rosenbloom BN, Clarke H , Katz J , Beesoon S, Rasic N
Received 1 March 2024
Accepted for publication 15 July 2024
Published 30 July 2024 Volume 2024:17 Pages 2511—2530
DOI https://doi.org/10.2147/JPR.S466731
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jinlei Li
Beate C Sydora,1 Lindsay Jane Whelan,1,2 Benjamin Abelseth,2 Gurpreet Brar,3 Sumera Idris,3 Rachel Zhao,4 Ashley Jane Leonard,4 Brittany N Rosenbloom,5 Hance Clarke,6 Joel Katz,6,7 Sanjay Beesoon,1 Nivez Rasic2,8
1Department of Surgery Strategic Clinical Network, Alberta Health Services, Edmonton, AB, Canada; 2Cumming School of Medicine, University of Calgary, Calgary, AB, Canada; 3Health Systems Knowledge and Evaluation, Alberta Health Services, Edmonton, AB, Canada; 4Knowledge Resource Service, Alberta Health Services, Edmonton, AB, Canada; 5Toronto Academic Pain Medicine Institute, Toronto, ON, Canada; 6Department of Anesthesia and Pain Management, Toronto General Hospital, UHN, Toronto, ON, Canada; 7Department of Psychology, York University, Toronto, ON, Canada; 8Department of Anesthesiology, Perioperative & Pain Medicine, University of Calgary, Calgary, AB, Canada
Correspondence: Nivez Rasic, Department of Anesthesiology, Perioperative and Pain Medicine, University of Calgary, Medical Lead, Vi Riddell Pain & Rehabilitation Program, Acute Pain Lead, Alberta Pain Strategy, Alberta Children’s Hospital, 28 Oki Drive NW, Calgary, AB, T3B 6A8, Canada, Tel +403-955-7810, Email [email protected] Sanjay Beesoon, Assistant Scientific Director, Surgery Strategic Clinical Network, Alberta Health Services, 02-048 South Tower, Seventh Street Plaza, 10030 107 St NW, Edmonton, AB, T5J 3E4, Canada, Tel +780-735-1682 ; +780-218-4786, Email [email protected]
Purpose: Risk factors for the development of chronic postsurgical pain (CPSP) have been reported in primary studies and an increasing number of reviews. The objective of this umbrella review was to compile and understand the published presurgical risk factors associated with the development of CPSP for various surgery types.
Methods: Six databases were searched from January 2000 to June 2023 to identify meta-analyses, scoping studies, and systematic reviews investigating presurgical CPSP predictors in adult patients. Articles were screened by title/abstract and subsequently by full text by two independent reviewers. The selected papers were appraised for their scientific quality and validity. Data were extracted and descriptively analyzed.
Results: Of the 2344 retrieved articles, 36 reviews were selected for in-depth scrutiny. The number of primary studies in these reviews ranged from 4 to 317. The surgery types assessed were arthroplasty (n = 13), spine surgery (n = 8), breast surgery (n = 4), shoulder surgery (n = 2), thoracic surgery (n = 2), and carpal tunnel syndrome (n = 1). One review included a range of orthopedic surgeries; six reviews included a variety of surgeries. A total of 39 presurgical risk factors were identified, some of which shared the same defining tool. Risk factors were themed into six broad categories: psychological, pain-related, health-related, social/lifestyle-related, demographic, and genetic. The strength of evidence for risk factors was inconsistent across different reviews and, in some cases, conflicting. A consistently high level of evidence was found for preoperative pain, depression, anxiety, and pain catastrophizing.
Conclusion: This umbrella review identified a large number of presurgical risk factors which have been suggested to be associated with the development of CPSP after various surgeries. The identification of presurgical risk factors is crucial for the development of screening tools to predict CPSP. Our findings will aid in designing screening tools to better identify patients at risk of developing CPSP and inform strategies for prevention and treatment.
Plain Language Summary: Chronic postsurgical pain (CPSP) is pain experienced predominantly at the surgical site for longer than 3 months after a surgical procedure. Depending on surgery type, it can affect between 10 and 80% of people undergoing major surgeries, which may have negative effects such as a lower quality of life, disability, and persistent opioid use. Targeted identification and management of at risk patients in the presurgical phase may decrease the risk of CPSP. This umbrella review generated a list of potential risk factors for CPSP from evidence-based reviews of the current literature.
Thirty-nine presurgical risk factors were identified in this review. Risk factors are divided into six broad categories: psychological, pain-related, health-related, demographic, genetic, and social/lifestyle-related. Although the strength of evidence for individual risk factors varied across reviews, risk factors in the psychological category consistently showed a strong impact on the development of CPSP.
It is vital to understand which individuals are vulnerable and at risk for CPSP. The findings of this umbrella review will aid in designing screening tools to identify surgical candidates at risk. Some risk factors, such as genetics, cannot be altered. However, many identified risk factors are modifiable and may inform strategies for the prevention and treatment of CPSP using screening tools. Our findings may guide future research to consider an in-depth analysis of risk factor characterization to group modifiable presurgical risk factors. At risk patients will be offered psychological, physical, and pharmacological treatments accordingly to mitigate their risk of developing CPSP and ultimately improve patient outcomes in surgery.
Keywords: chronic postsurgical pain, risk factors, predictors, umbrella review, surgery
Introduction
In 2019, the International Classification of Diseases (ICD-11) published an updated definition of chronic postsurgical pain (CPSP).1 Previously described as a collection of symptoms, the ICD definition now defines CPSP as:
Chronic pain developing or increasing in intensity after a surgical procedure and persisting beyond the healing process, ie, at least 3 months after surgery. The pain is either localised to the surgical field, projected to the innervation territory of a nerve situated in this area, or referred to a dermatome (after surgery/injury to deep somatic or visceral tissues). Other causes of pain, such as preexisting pain conditions, infections, or malignancies, need to be excluded. Depending on the type of surgery, CPSP may be neuropathic pain. (ICD-11)
The proposed biochemical mechanisms by which chronic pain develops have been described at the molecular level,2 and the new definition of CPSP provides better clarity around the pathology itself. Nonetheless, a significant gap remains in the understanding of individual risk factors for the development of CPSP in patients presenting for surgery. Numerous reviews have been performed investigating risk factors for CPSP; commonly identified risk factors being medical history (pain, trauma, substance use), psychological factors (depression, anxiety, pain catastrophizing), sex (female) and younger age.3–7 However, most of these reviews have focused on a single surgery type or have investigated a specific risk factor. The importance in identifying those at risk of CPSP should not be understated, as the incidence of CPSP has been reported to be up to 85% for certain surgical procedures such as amputations.8 Beyond the obvious adverse clinical effects of pain itself, CPSP may result in impaired functional recovery, deleterious mental health effects, an increased risk of developing opioid use disorders (OUD) and medicolegal implications.9,10 Furthermore, CPSP has been found to have major downstream economic impacts, including direct health-related costs such as expenditures for medications, health care provider fees and indirect costs associated with absence from work, caregiver time for the patients, disability allowance and unemployment benefits.11 For example, a recent Canadian study on CPSP after cardiac surgery found that the average monthly cost of CPSP to the system at six months after surgery is CAN$ 989.52 per patient, driven mostly by excess health care utilization.12
With an increase in systematic reviews, scoping reviews and meta-analyses, the objective of our study was to conduct an umbrella review (a review of reviews) to collate the full range of published presurgical risk factors for the development of CPSP for various surgery types. Our primary goal was to establish a catalogue of a variety of presurgical risk factors with demonstrated association with CPSP. Secondary goals were to correlate the risk factors with type of surgery and to evaluate their quality, strength, and reliability, as described in the literature. Our study is the first umbrella review to summarize the assessment of a variety of risk factors that may result in the development of CPSP for various surgery types. Our findings may aid in implementing approaches to better identify patients at risk of developing CPSP and in initiating strategic methods for the prevention and early treatment of CPSP.
Methods
Study Design
An umbrella review was conducted to capture the breadth of outcomes from systematic assessments of risk factors for CPSP and to evaluate their quality, strength, and reliability. This umbrella review follows the PRIOR (Preferred Reporting Items for Overviews of Reviews) guidelines.13 An unpublished protocol had been drafted which is available on request by the author. The umbrella review is registered with Prospero under ID: CRD42023474748.
Search Strategy
Six databases (Wiley Cochrane Database of Systematic reviews, EBSCOhost CINAHL Complete, APA PsycInfo, Ovid EMBASE, Ovid MEDLINE(R) ALL, and Web of Science Core Collection) were searched systematically using keywords related to surgery, risk factors, and chronic pain. The keywords and search strategy were developed in collaboration with two research librarians (RZ, AJL). The search was restricted to articles published in English from 1 January 2000 to 2 August 2022. The search was updated on 27 June 2023. All meta-analyses, systematic reviews, and scoping reviews that were identified using a predetermined keyword search were included. We did not restrict the publication dates of the primary papers, eg, the papers or studies that are subject of the selected reviews. The detailed search strategy is available in the appendix (Supplementary Table 1, Database search strategy). Retrieved articles were imported into Covidence, a software for managing and streamlining systematic reviews; exact duplicates were automatically removed.14
Study Selection
Articles were first reviewed by title and abstract, each by two of three independent researchers (LW, BA, BCS) according to predetermined inclusion and exclusion criteria following the Covidence screening design; a third reviewer (SI or GB) was consulted in case of discrepancies. The second round of screening involved the review of full text articles by two reviewers. Four reviewers (LW, BA, SI, GB) shared this task. Disagreements were resolved by another researcher (BCS) and/or discussed in reviewer team meetings until a consensus was reached.
Inclusion Criteria
- Scoping review, systematic review and/or meta-analysis
- Description of risk factors/predictors associated with CPSP (non-validated tools were accepted if the risk factor was well described)
- Outcome assessment of the risk factors regarding pain at >3 months post-surgery
- All surgery types
- No restriction on the number of primary studies included in the reviews
- Adult patients (18 years and older)
Exclusion Criteria
- Not a scoping review, systematic review and/or meta-analysis
- No clear description/definition of the risk factors for CPSP
- Description of acute but not long-term pain (between 3 and 12 months post-hospital discharge)
- Pediatric patients only (under 18 years)
- Diagnostic procedures (CT/MRI, endoscopy)
- Minimally invasive procedures such as percutaneous, dental and lumbar puncture.
Data Extraction
A standardized data collection form was developed specifically for this study in a Microsoft Excel spreadsheet. The extracted data included author, year, type of review (systematic review, scoping review, meta-analysis), reporting guidelines used, protocol registration if applicable, databases searched for individual reviews, number, study design and quality assessment of primary papers included in the reviews, rating/assessment of bias and strength of primary studies, information on study participants in primary studies, surgery type studied, tools/descriptors used to define risk factors, strength and effect of risk factors, and pain intensity measures or tools used in studies. Data were extracted from each selected paper by one of five researchers (BCS, LW, BA, SI, GB) and verified by a second and occasionally a third researcher, depending on the complexity of the data. We collected data only from the text, tables/figures, and supplementary material of the included reviews and meta-analyses but did not examine the primary papers to extract data. Furthermore, we did not attempt to contact the authors of studies with missing data to gather additional unpublished information, as this was not feasible.
Quality Appraisal of Selected Reviews
We employed the Centre for Evidence-Based Medicine (CEBM) University of Oxford appraisal tool for systematic reviews to appraise the validity and reliability of included papers.15 Although this appraisal tool was developed for systematic reviews, it has been used to rate the validity of other reviews such as umbrella reviews as well.16 This tool consists of five questions addressing the following: (i) a clearly stated objective, (ii) a comprehensive search strategy, (iii) the appropriateness of selection criteria, (iv) the quality of individual studies, and (v) the homogeneity versus heterogeneity of study results. The appraisal requires a yes/no rating for each question. For our assessment, we determined that the objective was clearly stated if the review clearly outlined the inclusion and exclusion criteria for the selection of primary papers on the type of surgery and risk factors studied. To determine whether a search strategy was comprehensive, three or more of the following criteria had to be met:
- Searched at least three electronic databases using a variety of keywords and MeSH (Medical Subject Headings) words.
- Searched reference lists of relevant studies to find articles not uploaded to electronic databases.
- Search not limited to the English language.
- Search included grey literature.
The appropriateness of the selection criteria was determined by the explicit statement of similar selection criteria of the primary studies. We determined the quality of the included reviews to be sufficient if at least 50% of the primary studies were rated good quality or had a low-to-moderate risk of bias. We considered the heterogeneity adequately low, when statistics of the heterogeneity tests were deemed non-significant or when the forest plots showed homogenous results. Our review authors defined results as “unclear” if assessment could not be determined; for example, if the author of the selected reviews had neither applied a statistical test nor compared the results of primary status descriptively. According to the positive scoring of the five item rating scale, 5 points indicated high validity, 4 indicated good validity, 3 indicated fair validity, 2 indicated poor validity, and 1 indicated low validity. Studies were appraised independently by two reviewers. Discordance in appraisal outcomes was discussed in team meetings among five researchers (LW, BA, SI, GB, SB) until a consensus was reached.
Risk of Bias and Strength of Evidence of the Primary Studies
Risk of bias of the primary studies was collected from text and tables of the selected reviews. Additionally, we collected primary studies’ appraisal tools used to assess risk of bias and/or strength of evidence. Depending on the tool used and authors’ interpretations, risk of bias and strength of evidence could be rated as low, moderate, or high, or rated as good, fair, or poor. We did not attempt to consult the primary studies in cases where risk of bias and/or strength of evidence was unclear or not effectively reported in the reviews.
Data Synthesis and Analysis
Data was tabulated in Excel, and descriptive statistics were applied. Data not available from the selected reviews or appendices therein were labeled as not recorded or not specified. The number of participants combined from the primary papers was recorded as stated in the reviews; if the combined number was not stated in the text or a table, the number was summed from the primary study numbers provided in the tables in the reviews. Data was synthesized to document the effects of risk factors on CPSP. In the analysis, we focused on the risk factors with a demonstrated effect on developing CPSP. Risk factors described in individual reviews with only an effect on postsurgical disability, function, patient satisfaction, or quality of life (QOL) but not CPSP, were not included in our analysis. The risk factors were grouped into categories, and the findings of their assessment and association with CPSP were listed according to surgery type. The total number a risk factor was assessed, and the number this risk factor was positively associated with CPSP was taken from the tables or supplementary materials of the reviews. If factors or characteristics, such as age, sex, or BMI, were only used as effect modifiers but not investigated as independent predictors, they were not included in the count. If a potential risk factor was investigated at several follow-up times beyond three months, we only reported its effect on CPSP once and used the first follow-up date after a period of three months or longer.
To assess the use of screening tools for individual CPSP risk factors, the number of times a tool was used was extracted from the text or table of reviews, whenever available. For the rating of the strength of evidence for risk factors, we used the evidence for a given risk factor’s strength from narrative summaries and tables in the reviews. This allowed for a rating independent of the heterogeneous metrics used in primary papers and the lack of explicit reporting on primary papers’ statistical results in some of the reviews. According to the authors’ description, for best evidence synthesis we distinguished weak (limited evidence of association, high risk of bias, low quality studies), moderate or conflicting (multiple studies with inconsistent results and/or mostly high risk and low quality studies), and strong (studies with generally consistent findings of CPSP association and low risk of bias) evidence; in cases where the strength of evidence was not rated, we indicated this with “not rated”. Evidence maps were created and stratified according to the surgery type and risk factors.
Results
Selection of Included Reviews
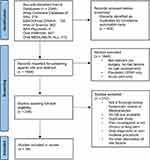
A total of 2344 articles were retrieved from the six databases and exported into Covidence, where duplicates (n = 450) were removed. The first selection step was title and abstract screening, which eliminated all irrelevant articles (n = 1646). A subsequent selection step involved reading full text articles and removing those not meeting our selection criteria (n = 212) which resulted in the final inclusion of 36 studies (Figure 1).17–52 A detailed list of all included reviews can be found in Supplementary Table 2 (Supplementary Table 2: List of 36 included reviews).
Characteristics of Included Reviews and Study Participants
All, but one of the 36 articles included in our umbrella review, were systematic reviews of which 18 included a meta-analysis (Table 1). The only non-systematic review was a scoping review on pain associated with breast cancer surgery.46 In order to include a large number of potential risk factors assessed in a wide selection of surgeries, we did not restrict the study design of the primary papers in the selected reviews. Most reviews (97%) were based on primary observational studies, which included a variety of study types, such as prospective or retrospective cohort and case–control studies, analysis of registries or databases, and cross-sectional or longitudinal studies. Twelve reviews (33%) also included interventional studies with randomised or nonrandomised trials or before-and-after designs (Supplementary Table 2). One review (Hernandez et al 2015)32 included four systematic reviews within their selection of 37 primary papers; findings from these four systematic reviews were excluded from our analysis because they did not meet our inclusion criteria. Seventeen systematic reviews (47%) had a protocol registered with PROSPERO, and the scoping review was listed with an identifier under the Open Science Framework (Supplementary Table 2).
 |
Table 1 Characteristics of Selected Reviews Included in the Umbrella Review |
While we used six databases for our search, the total number of different databases covered by the 36 reviews was 22, including searches for grey literature. The number of databases per review ranged from 2 to 8. The full list of databases used in the 36 reviews is available in Supplementary Table 3 (Supplementary Table 3: List of databases). The databases most frequently used in the reviews corresponded to the databases we employed for the search in our umbrella review (Table 1). The number of primary studies included in each of the 36 reviews ranged from 4 to 317, with an approximate range of the total number of participants in each review from 662 to over 1 million. Based on our goal of exploring CPSP risk factors in the adult population, 31 of the included 36 reviews (86%) involved primary studies investigating adult participants; however, two articles included surgery patients aged 15 years and older25,27 and one article included those aged 17 years and older;52 two studies did not specify the age group.21,26 The inclusion of these five reviews in our umbrella review was deemed appropriate and unanimously approved by the research team. Most reviews (75%) investigated primary studies with both male and female participants; only five (14%) reviews reported on studies with only female participants, including three studies on breast surgery,38,41,46 one on spine surgery for lumbar degenerative disease,52 and one on total hip and knee arthroplasty.28
Appraisal of Included Reviews
The quality appraisal of the selected reviews is presented in Table 2. According to the CEBM appraisal tool, if all five criteria are answered with a “yes”, this garners the highest points and suggests a high validity and reliability of the review. In our selection, there were only two reviews with the highest score of five points, indicating high validity. Most reviews (72%) scored either three (n = 13) or four (n = 13) points, representing fair and good validity, respectively; six (17%) had two points, and two had one point for low validity. The majority of the selected reviews had a clearly stated question for their study (94%) with appropriate selection criteria (92%); however, a homogeneity of included primary papers was only reported for 7 (19%) reviews.
 |
Table 2 Appraisal of Included Reviews: the Centre for Evidence-Based Medicine (CEBM) University of Oxford Appraisal Tool for Systematic Reviews was used |
Risk of Bias and Strength of Evidence of Primary Studies
Due to the various tools used to assess risk of bias and reporting quality of evidence in the primary studies as indicated in the 36 selected reviews, it was not feasible to create a consensus on the rating of quality and risk of bias for the primary studies. The tools used included Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) (n = 15), Quality in Prognosis Studies (QUIPS) (n = 12), National Heart, Lung, and Blood Institute (NHLBI) quality assessment tools (n = 3), the Joanna Briggs Institute (JBI) critical appraisal tools (n = 2), the Oxford Center for Evidence-Based Medicine criteria (OCEBM) (n = 2), the Newcastle–Ottawa assessment scale (NOS) (n = 2), the Downs & Black checklist (DB) (n = 1), STrengthening the REporting of Genetic Association studies (STREGA) (n = 1), Evidence-Based Recommendation Development (EBRO) (n = 1), and a variety of modified appraisal or appraisal tools of unknown sources. Three reviews did not report on any rating tools for primary studies (Supplementary Table 2). A high variability in risk of bias rating was also evident when the same tool was used. For example, the QUIPS tool53 and a modification of it were used in 12 reviews (33%) to assess risk of bias. Primary studies in reviews using this tool had a range of reported low risk of bias from 2 to 86.7%, moderate risk from 20.4 to 77.8%, and high risk from 5.5 to 87.5%. A similar variability was found in the assessment of the strength of evidence in the primary studies.
Risk Factor Definition and Tools
Thirty-nine risk factors with a demonstrable effect on CPSP were identified from the 36 selected reviews. Several of these risk factors were defined by the same outcome tool, for example the Hospital Anxiety and Depression Scale (HADS), or subsections of the same tool, such as the HADS depression subscale (HADS-D) or HADS anxiety subscale (HADS-A). Table 3 lists the most frequently used Patient Reported Outcome Measures (PROMs) and questionnaires of a total of 106 PROMs and questionnaires that were recorded in the 36 reviews to be used as an assessment tool for potential pain-related, physical, and psychological predictors for CPSP.
 |
Table 3 PROMs and Questionnaires Use to Define the Risk Factors |
Mental health constructs, such as depression, anxiety, and pain catastrophizing, were often studied separately and with specifically defined tools; however, in some reviews, mental health constructs were less distinguished and were just combined under the term “mental health”. The foremost tools used for mental risk factors were the Beck’s Depression Inventory (BDI), various forms of the Hospital Anxiety and Depression Scale (HADS), the Pain Catastrophizing Scale (PCS), and the State Trait Anxiety Inventory (STAI). The impact of preoperative pain-related risk factors was predominantly assessed with the Visual Analogue Scale (VAS) prior to surgery. Comorbidity was mostly established based on the number, type, and severity of the comorbid illnesses. A large variety of risk factor-specific PROMs and questionnaires were used for individual risk factors, such as the Coping Strategies Questionnaire (CSQ), the Perceived Stress Scale (PSS), the Tampa Kinesiophobia Scale (TKS), the Pain Self-Efficacy Questionnaire (PSEQ), and others (Table 3).
Grouping of Relevant Risk Factors and Their Implication in Surgery Type
Several surgery types were included in the 36 selected reviews: 13 studied risk factors in arthroplasty surgery (knee only n = 8, hip only n = 2, and both knee and hip n = 3), eight in spine, four in breast, two in shoulder and two in thoracic surgery. One review investigated risk factors separately in both breast and thoracic surgeries, and another explored risk factors in an assortment of orthopedic surgeries including knee, hip, shoulder, elbow, and spine. In addition, one review specifically studied only Carpal Tunnel Syndrome. Six reviews included combinations of different surgeries (Table 4).
 |
Table 4 Risk Factor Assessment in Primary Studies Stratified by Body Part |
Risk factors were grouped into six broad categories with the following number of risk factors per group: psychological (n = 14), pain-related (n = 5), health condition-related (n = 7), social/lifestyle-related (n = 9), demographic (n = 3), and genetic (n = 1). Psychological risk factors were the most studied risk factors and were the subject of 24 reviews (67%). While many reviews (47%) investigated risk factors included in several categories, some reviews only considered one individual predictor; for example, fibromyalgia was the only risk factor assessed in two reviews: one review focused on shoulder surgeries,24 another studied a variety of orthopedic surgeries.26 Similarly, the effect of preoperative opioid use was studied in only two reviews.23,30 Variations in the potassium voltage-gated channel KCNS1 gene as a possible genetic predictor for CPSP were examined in only one review.22 The effect of obesity, while considered in several studies in addition to other risk factors, was the only risk factor investigated for an effect on CPSP following arthroplasty surgery in a review.42 Table 5 provides the frequency of risk factors found to be associated with CPSP in relation to the total number of risk factors assessed, stratified by surgery type (Table 5 and Supplementary Table 2).
 |
Table 5 Risk Factor Grouping and Frequency of CPSP Association According to Surgery Type |
Potential Strength of Risk Factors in the Development of CPSP
The heterogeneous and subjective nature of the data collected in the reviews on the strength of evidence for the studied risk factors from primary papers made an attempt to quantify the effects of individual risk factors challenging. Therefore, data for the strength of evidence of the association of a given risk factor with CPSP was obtained from tables or narrative summaries within the text of the reviews and converted into a three-scale rating system of strong, moderate/conflicting, and weak, as outlined in the methodology. In addition, we computed the frequency that a risk factor was found to be associated with CPSP in primary studies, independent of the imputed strength of evidence for this association. Our analysis showed that depression, anxiety, pain catastrophizing, and high pain level in the surgical area were the most often studied risk factors, with a reported association with CPSP between 55 and 65%; evidence for these risk factors was mostly described as strong (Table 6).
 |
Table 6 Risk Factor Frequency and Strength of Evidence |
Discussion
This study aimed to generate an evidence-based pool of preoperative risk factors most likely to predict the development of CPSP in adult patients. Using the methodology of an umbrella review enables the integration of information from multiple systematic reviews to provide an organized, comprehensive analysis of the most up-to-date published medical literature on CPSP. Included were all papers that reviewed the assessment of CPSP predictors, some of which were only characterized for specific surgery types. Thirty-six reviews were included in our analysis, providing an overview of evidence in the current literature up to June 2023. These reviews identified 39 presurgical risk factors for CPSP among a large variety of invasive surgical procedures, including orthopedic, spinal, thoracic, and breast surgeries. The risk factors were defined by 106 different tools and questionnaires, in addition to demographic or lifestyle descriptors such as age, sex, smoking, and others, including those concerning work satisfaction and life events.
Psychological Risk Factors
Based on our results, the most consistent predictors of CPSP in the current literature are mental health symptoms of emotional distress (eg, symptoms of anxiety, depression, somatization, pain catastrophizing) or beliefs and behaviours regarding pain (eg, lower self-efficacy, fearful and avoidant behaviours). Emotional distress or symptoms of mental health disorders as risk factors for CPSP were present across all surgery types, with symptoms of anxiety and depression having the highest strength of evidence compared with other psychological risk factors. Symptoms of anxiety and depression increase pain perception and impede the body’s inherent pain management mechanisms. Additionally, individuals with catastrophic thinking or pessimistic beliefs about pain are more likely to develop CPSP. Fear avoidance, which involves abstaining from physical activity due to apprehension of pain or re-injury, can intensify the severity of pain symptoms and exacerbate the condition. Several models have proposed that certain vulnerability factors increase the risk of pain54 with some noting the importance of the interaction between multiple genes with each other and with a person’s environment.55,56
The practicality of using mental health factors as a risk factor tool does become complicated when applied in a pre-operative setting. As shown by this umbrella review, multiple screening instruments have been used to assess any given risk factor, with some studies showing an association with one instrument and not with another. It is not possible at this stage to determine why this is the case. It may be due to methodological issues, such as a small number of studies, characteristics of the sample under study, or it may be measure specific.
With many of the selected reviews, it was not possible to determine which screening tools were applied to demonstrate the strength of evidence for associated risk factors. Unless a candidate for surgery has a previous mental health diagnosis from a psychodiagnostics assessment, the validity of an assessment in the preoperative period is unknown.
Preoperative Pain-Related Risk Factors
Pain-related risk factors were found to be highly predictive for CPSP in our umbrella review, which corroborates previous evidence in the literature that the most robust predictor of CPSP in adults is prior pain.5,8,9,57 Specifically, higher pain at the surgical site has been reported as a risk factor across most surgical types. Interestingly, one review found that in lumbar spine surgery, preoperative leg pain had a reverse predictive effect, in that patients with more pain at a separate site had a lower risk of CPSP.17 In this case, leg pain may be considered as a referred pain, and spinal fusion might be the optimal surgery for this type of pain, which could explain the negative association between pain at other sites as a risk factor and CPSP. It is also possible that patients starting with a higher symptom burden had overall more relief from surgery or, alternatively, that these patients had been conditioned to tolerate higher levels of pain, so their development of CPSP might have been lower. Although the pre-existing literature and our current review support the idea that more intense preoperative pain is associated with higher risk of CPSP, it may not be as simple a correlation of more pain preoperatively equals more pain postoperatively.
Non-Surgical Health-Related Risk Factors
Comorbidities such as diabetes, hypertension, dyslipidemia, and cardiovascular diseases were identified as risk factors for CPSP in arthroplasty, spinal, and thoracic surgeries. Multiple reviews examined comorbid conditions as a single discrete variable being either present or absent, rather than describing singular diagnoses and associated risks.28,31,32,37 In two reviews, diabetes itself was demonstrated to be an independent comorbidity that increased risk of CPSP.19,40 Meert40 described a possible pathophysiological explanation of how increased insulin resistance and inflammatory markers could impact the postoperative recovery period. The study concluded that diabetes is a highly influential risk factor significantly impacting the recovery process after surgery. For all comorbidities described in the reviews, further questions regarding whether the severity of disease affects the risk of CPSP should be considered. Comorbidities could be regarded as potential confounding factors rather than independent primary risk factors. Nevertheless, it is necessary to take appropriate measures to manage comorbid conditions preoperatively and to mitigate their potential impact on long-term postoperative pain.
Social and Lifestyle-Related Risk Factors
Social and lifestyle-related risk factors were not extensively studied in many reviews. However, when investigated, socioeconomic status, low income, and poor job satisfaction were deemed strong predictors of CPSP.25,32 The effect of lower education levels was studied in four reviews, three of which reported strong evidence of association of lower education as a risk factor for CPSP. The likelihood of low socioeconomic status and lower levels of education as risk factors for CPSP is supported by evidence showing that these social conditions are closely associated with the development of chronic pain.58,59
Our umbrella review found little information on substance use as a potential risk factor. Smoking was studied as a CPSP predictor in two selected reviews, one studying spine surgery,27 the other both breast and thoracic surgery;36 nevertheless, there was little evidence of an association between smoking and CPSP. Assessment of preoperative opioid use, including medically prescribed opioids, was the main risk factor assessed for arthroplasty surgery in one review.30 Although an association was reported, no discernible strength of evidence was provided. Another review investigating thoracic surgery did not find an association between presurgical opioid use and CPSP.23 The reason for the low number of studies assessing presurgical opioid use may be because most studies assessing presurgical opioid use might have long-term opioid addiction as their main outcome rather than CPSP. Because of the outcome measure, we did not include reviews studying opioid addiction in our selection; yet, an association might exist, and additional studies may be required. The use of opioids can be an effective treatment for acute pain before and after surgery; however, prolonged opioid treatment is generally not indicated for CPSP because it can lead to deleterious side effects and opioid use disorders. Opioid use prior to surgery has been previously recognized as a risk factor for CPSP but was not borne out in this umbrella review.60,61
Demographic Risk Factors
Age and female sex were identified as risk factors in several reviews, albeit at various levels of strength of evidence. For age, this could include both older age21,27,31,32,37 and younger age.18,19,28,36,46 Older age was generally more dominant as a risk factor in spine and arthroplasty surgeries for both sexes, whereas younger age as a risk factor was primarily predictive of CPSP in female breast cancer patients.
Sex and gender have a complex relationship with pain and treatment of pain. Five reviews assessed CPSP predictors restricted to a female population, including three breast surgeries,38,41,46 one spine surgery52 and one arthroplasty surgery.28 None of the reviews reported male sex as a predictor for CPSP; however, seven reviews reported female sex as a predictor.18,19,21,23,28,32,36 For some studies, the type of surgery influenced the sex difference for the development of CPSP (ie, breast surgery); nevertheless, female sex as a risk factor was found for spine, thoracic, and arthroplasty surgeries. There is a range of explanations for the higher prevalence of chronic pain among females than males and for other pain experiences (eg, intensity and interference). The reasons are manyfold and include variations in clinical vulnerability, differences in pain reporting, and a variety of intersecting biological and social psychological factors.62–64
African American race was identified as a risk factor for CPSP in two reviews, albeit with contrasting strengths of evidence.19,32 Both reviews studied risk factors in arthroplasty surgeries. An association with CPSP for non-white race was found in spinal surgery. A recent literature review found that patients of African American and Hispanic race reported higher postoperative pain than non-Hispanic white patients.65 It is also not clear whether the reported higher pain intensity translates to a longer experience of CPSP. Nevertheless, the unique sociocultural background should be considered when exploring CPSP management.
Genetic Risk Factors
Genetic variants may play a role in identifying surgical candidates at risk of developing CPSP;66 however, extensive research of genetic risk factors for CPSP is lacking to date. Genetic risk factors were only investigated in one of the selected reviews, and only one genetic marker was found to be marginally associated with CPSP.22 This genetic marker included variations in the KCNS1 gene, which codes for a subunit of the potassium voltage-gated channel protein involved in sensory transport and implicated in higher sensitivity to pain.67 Genes that have been studied as possible candidates for a genetic link to CPSP included genes involved in immunological responses, neurotransmission, and neuroendocrine receptor interactions and signalling.22,68,69 In addition, gene modifications such as single nucleotide polymorphisms and epigenetics have been considered.68,69 While most of the studied genes did not show an effective association with CPSP, a recent review discovered another genetic variant (catecholamine metabolism enzyme variant (COMT V158M) involved in the metabolism of neurotransmitters) with moderate implications for the development of CPSP.70 Knowledge of genetic risk factors could provide the basis for pharmaceutical treatment options for CPSP; however, the complex interaction of confounding risk factors from the categories discussed above poses a challenge for the clear identification of genetic risk factors.
Quality of the Selected Reviews and Risk of Bias
Assessing the strength of evidence from the included reviews is challenging, mainly because of the heterogeneity in methodology, sample size, duration of follow-up, and population characteristics across the reviews. The association of identified risk factors with CPSP can vary considerably depending on a variety of factors such as surgery type, outcome measures and tools employed, and patient characteristics. Many of the reviews have methodological shortcomings and limitations, making it difficult to compare results across the various reviews. Most of the reviews stated their own limitations in reporting conclusive evidence for certain risk factors. There is inconsistency between primary studies in outcome reporting, including the lack of reporting on confounding factors.17,36,42 Reviews reported an overall low study quality, partly due to the inclusion of primary studies with poor study designs, leading to insufficient results rather than robust studies with strong statistical methodologies that enable comprehensive and effective data analysis and interpretation.31,36 There were significant differences in sample sizes among primary studies, with a small sample size leading to a potential overestimation of predictors.18,25,46 In addition, results might be distorted due to the considerable heterogeneity in study characteristics, methodology and statistical analysis, in the tools used to quantify CPSP, and in the length of time after surgery to assess outcomes which can range from three months to several years.20,22,29,34,37,47,51
Risk of bias was not always clearly described or transparent in the reviews. Owing to the limited information provided in some reviews and the heterogeneity in bias reporting, it was not feasible to devise an independent rating system. Therefore, we relied on the authors’ assessment of bias regarding the primary studies (Supplementary Table 2). Frequencies of high, medium, and low risk of bias in primary studies were reported in 15 of the selected reviews, with a mean frequency of 44.2% for low, 21.9% for medium, and 36.6% for high risk of bias. About half of these reviews (n = 7) rated risk of bias as high (frequency >50%) for most primary studies, while four reviews rated risk of bias for primary studies mainly as low. A narrative report on risk of bias for primary studies was available for five reviews, of which one reported an overall high risk, two reported a moderate risk, one reported a medium to low risk, and one reported an overall low risk for the primary papers. Sixteen reviews did not assess risk of bias; however, nine of those reviews reported on the quality and certainty of evidence of the primary papers, which were mostly found to be in the range of moderate to good.
Limitations of the Umbrella Review
Although we included a sufficiently large number of databases in our search, we cannot exclude the possibility that relevant reviews may have been missed. As our goal was to collect a comprehensive catalogue of potential risk factors for CPSP, we did not limit the number of primary papers in the reviews selected for inclusion in our umbrella review. Consequently, the number of primary papers in some of the included reviews is large, making it unfeasible to establish a potential overlap of primary studies within the reviews. Although the overlap might have led to an overestimation of the importance for predictors, this effect would be small in the current umbrella review as an inspection of primary paper overlap within our largest group of arthroplasty surgeries showed only minimal overlap with a percent overlap of 14.2 and a calculated CCA (corrected cover area) of 0.014.71 Our analysis is only based on data available in the selected reviews; we did not verify any information by consulting the primary papers as this was not feasible given the number of papers included. The large heterogeneity among the selected reviews with regard to analytical methods, differences in follow-up times, and inclusion of potentially low-quality studies makes it difficult to draw explicit conclusions on the strength of evidence. Variations in the length of follow-up may affect pain intensity scores. Furthermore, little consideration has been given to confounders and effect modifiers. There may be several unmeasured confounders and effect modifiers, such as comorbidities, illnesses, and other patient characteristics in individual studies possibly influencing the results and strength of evidence for the assessment of a given risk factor. Consequently, while this review found a large number of risk factors for CPSP with a comprehensive search, it is possible that not all risk factors were identified, or their strengths of evidence adequately assessed.
Strength of the Umbrella Review
Our umbrella review was able to capture a large number of potential risk factors for a variety of surgery types. To our knowledge, this is the first review of reviews systematically accumulating and summarizing data from presurgical risk factors and assessing their association with the development of CPSP. To accurately assess the risk of CPSP in the preoperative setting, it is useful to have data on specific risk factors and surgeries, as demonstrated by the articles included in this review, and a collective assessment across multiple surgical types, as presented in our umbrella review. With our comprehensive approach, we can uncover trends and patterns that may not be apparent when focusing on specific surgeries.
An understanding of the underlying individual experiences with pain and the reasons for developing CPSP are central to targeted personalized treatments and pain management. While some risk factors, such as age, sex, and genetic factors, may not be modifiable, they may have intersecting properties and may be instrumental for the development of risk factor identification tools. Other risk factors, for instance psychological risk factors, have the potential to be modified using a variety of treatment approaches, including Cognitive Behavioral Therapy (CBT), Acceptance and Commitment Therapy (ACT),72 and mindfulness-based interventions.73 Future research should consider targeting the treatment of these factors to prevent and mitigate the development of CPSP.74
A recent umbrella review on the prevalence of CPSP and postoperative opioid use (PPOU) reports on an estimated prevalence of CPSP ranging from 4.7 to 58% depending on the type of surgery and follow-up time.75 The authors highlight the importance of a comprehensive analysis of risk factors for the development of screening tools to determine the presurgical risk factors most likely to be involved in CPSP.75 It is critical to understand which individuals are vulnerable and at risk for developing CPSP. Recognizing risk factors may aid in the early identification of at risk patients and prevention or management of CPSP. Our findings will assist in the creation of predictive models to identify patients at risk for developing CPSP. Ultimately, this will enable us to make more informed decisions for surgical patients and to improve patient outcomes.
Conclusions
Our review provides a comprehensive analysis of the various risk factors for CPSP in adult surgical patients, with psychological factors being the most notable. Identification of presurgical risk factors is crucial for the development of predictive screening tools to manage and prevent CPSP. Our findings may guide future research to consider an in-depth analysis of risk factor characterization to group modifiable presurgical risk factors. By having a comprehensive understanding of modifiable risk factors, we may be able to mitigate the development of CPSP in at risk patients.
Abbreviations
CEBM, Center for Evidence-Based Medicine; CPSP, Chronic postsurgical pain; ICD, International Classification of Diseases; OUD, Opioid use disorder; PROM, Patient Reported Outcome Measure.
Acknowledgments
Dr. Katz is supported by a Canadian Institutes of Health Research Canada Research Chair in Health Psychology at York University. Dr. Rosenbloom was funded by a CIHR Banting Postdoctoral Fellowship for part of this work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The production of this document has been made possible through a financial contribution from Health Canada. The views expressed herein do not necessarily represent the views of Health Canada.
Disclosure
Dr. Clarke is supported by a merit award from the University of Toronto, Department of Anesthesia and Pain Medicine. The authors report no other conflicts of interest in this work.
References
1. International classification of diseases, eleventh revision (ICD-11), World Health Organization (WHO) 2019/2021. Available from: https://icd.who.int/browse11.
2. Richebé P, Capdevilla X, Rivat C. Persistent Postsurgical Pain: pathophysiology and Preventative Pharmacologic Considerations. Anesthesiology. 2018;129:590–607. doi:10.1097/ALN.0000000000002238
3. Schug SA, Bruce J. Risk stratification for the development of chronic postsurgical pain. Pain Rep. 2017;2(6):e627. doi:10.1097/PR9.0000000000000627
4. VanDenKerkhof EG, Peters ML, Bruce J. Chronic pain after surgery: time for standardization? A framework to establish core risk factor and outcome domains for epidemiological studies. Clin J Pain. 2013;29(1):2–8.
5. Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother. 2009;9(5):723–744. PMID: 19402781 Review. doi:10.1586/ern.09.20
6. Lopes A, Seligman Menezes M, Moreira de Barros GA. Chronic postoperative pain: ubiquitous and scarcely appraised: narrative review. Braz J Anesthesiol. 2021;71(6):649–655. ISSN 0104-0014. doi:10.1016/j.bjane.2020.10.014
7. Bruce J, Quinlan J. Chronic Post Surgical Pain. Rev Pain. 2011;5(3):23–29. PMID: 26526062; PMCID: PMC4590073. doi:10.1177/204946371100500306
8. Rosenberger DC, Pogatzki-Zahn EM. Chronic post-surgical pain – update on incidence, risk factors and preventive treatment options. British J Anesth. 2022;22(5):190–196. doi:10.1016/j.bjae.2021.11.008
9. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. The Lancet Series PostoperPain Manag Opioids. 2019;393(10180):1537–1546. doi:10.1016/S0140-6736(19)30352-6
10. Hanley C, Ladha SK, Clarke HA, Cuthbertson BC, Wijeysundera DN. Association of postoperative complications with persistent post-surgical pain: a multicentre prospective cohort study. Br J Anaesth. 2022;128(2):311–320. doi:10.1016/j.bja.2021.10.027
11. Raftery MN. Chronic noncancer pain major economic burden in Ireland. Pharmacoecon Outcomes News. 2012;647:5. doi:10.2165/00151234-201206470-00011
12. Guertin JR, Pagé MG, Tarride J, Talbot D, Watt-T-Watson J, Choinière M. Just how much does it cost? A cost study of chronic pain following cardiac surgery. J Pain Res. 2018;11:2741–2759.
13. Gates M, Gates A, Pieper D, et al. Reporting guideline for overviews of reviews of healthcare interventions: development of the PRIOR statement. BMJ. 2022;378:e070849. doi:10.1136/bmj-2022-070849
14. Covidence systematic review software, Veritas Health Innovation. Melbourne, Australia. Available from: www.covidence.org.
15. University of Oxford. Centre for Evidence-Based Medicine, Systematic Review Critical Appraisal Sheet. Available from: https://www.cebm.ox.ac.uk/resources/ebm-tools/critical-appraisal-tools.
16. Biondi-Zoccai G. Umbrella Reviews: evidence Synthesis with Overviews of Reviews and Meta-Epidemiologic Studies. Springer Int Publish. 2016. ISBN 978-3-319-25653-5. doi:10.1007/978-3-319-25655-9
17. Achttien RJ, Powell A, Zoulas K, et al. Prognostic factors for outcome following lumbar spine fusion surgery: a systematic review and narrative synthesis. Eur Spine J. 2022;31:623–668.
18. Andreoletti H, Dereu D, Combescure C, Rehberg B. A systematic review and meta-analysis of three risk factors for chronic postsurgical pain: age, sex and preoperative pain. Minerva Anestesiol. 2022;88:827–841.
19. Ashoorion V, Sadeghirad B, Wang L, et al. Predictors of Persistent Post-Surgical Pain Following Total Knee Arthroplasty: a Systematic Review and Meta-Analysis of Observational Studies. Pain Med. 2023;24:369–381.
20. Burns LC, Ritvo SE, Ferguson MK, et al. Pain catastrophizing as a risk factor for chronic pain after total knee arthroplasty: a systematic review. J Pain Res. 2015;8:21–32.
21. Celestin J, Edwards RR, Jamison RN. Pretreatment psychosocial variables as predictors of outcomes following lumbar surgery and spinal cord stimulation: a systematic review and literature synthesis. Pain Med. 2009;10:639–653.
22. Chidambaran V, Gang Y, Pilipenko V, et al. Systematic Review and Meta-Analysis of Genetic Risk of Developing Chronic Postsurgical Pain. J Pain. 2020;21:2–24.
23. Clephas PRD, Hoeks SE, Singh PM, et al. Prognostic factors for chronic post-surgical pain after lung and pleural surgery: a systematic review with meta-analysis, meta-regression and trial sequential analysis. Anaesthesia. 2023;78:1005–1019.
24. Compagnoni R, Gualtierotti R, Luceri F, et al. Fibromyalgia and Shoulder Surgery: a Systematic Review and a Critical Appraisal of the Literature. J Clin Med. 2019;8(10):1518. doi:10.3390/jcm8101518
25. den Boer JJ, Oostendorp RA, Beems T, et al. A systematic review of bio-psychosocial risk factors for an unfavourable outcome after lumbar disc surgery. Eur Spine J. 2006;15:527–536.
26. D’Onghia M, Ciaffi J, McVeigh JG, et al. Fibromyalgia syndrome - a risk factor for poor outcomes following orthopaedic surgery: a systematic review. Semin Arthritis Rheum. 2021;51:793–803.
27. Dorow M, Lobner M, Stein J, et al. Risk Factors for Postoperative Pain Intensity in Patients Undergoing Lumbar Disc Surgery: a Systematic Review. PLoS One. 2017;12:e0170303.
28. Ghoshal A, Bhanvadia S, Singh S, et al. Factors associated with persistent postsurgical pain after total knee or Hip joint replacement: a systematic review and meta-analysis. Pain Rep. 2023;8:e1052.
29. Giusti EM, Lacerenza M, Manzoni GM, Castelnuovo G. Psychological and psychosocial predictors of chronic postsurgical pain: a systematic review and meta-analysis. Pain. 2021;162:10–30.
30. Goplen CM, Verbeek W, Kang SH, et al. Preoperative opioid use is associated with worse patient outcomes after Total joint arthroplasty: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2019;20:234.
31. Halicka M, Duarte R, Catherall S, et al. Predictors of Pain and Disability Outcomes Following Spinal Surgery for Chronic Low Back and Radicular Pain: a Systematic Review. Clin J Pain. 2022;38(5):368–380. PMID: 35413024. doi:10.1097/AJP.0000000000001033
32. Hernández C, Díaz-Heredia J, Berraquero ML, Crespo P, Loza E, Ruiz Ibán MÁ. Pre-operative Predictive Factors of Post-operative Pain in Patients with Hip or Knee Arthroplasty: a Systematic Review. Reumatol Clin. 2015. 11(6):361–380. English, Spanish. PMID: 25840826. doi:10.1016/j.reuma.2014.12.008
33. Hinrichs-Rocker A, Schulz K, Jarvinen I, et al. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP) - a systematic review. Eur J Pain. 2009;13:719–730.
34. Innocenti T, Ristori D, Galantini P, et al. The influence of central pain modulation on postoperative outcomes after shoulder surgery: a systematic review. Acta Orthop Traumatol Turc. 2021;55:227–234.
35. Lewis GN, Rice DA, McNair PJ, Kluger M. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. Br J Anaesth. 2015;114:551–561.
36. Lim J, Chen D, McNicol E, et al. Risk factors for persistent pain after breast and thoracic surgeries: a systematic literature review and meta-analysis. Pain. 2022;163:3–20.
37. Lungu E, Maftoon S, Vendittoli PA, Desmeules F. A systematic review of preoperative determinants of patient-reported pain and physical function up to 2 years following primary unilateral total Hip arthroplasty. Orthop Traumatol Surg Res. 2016;102:397–403.
38. McCowat M, Fleming L, Vibholm J, Dixon D. The Psychological Predictors of Acute and Chronic Pain in Women Following Breast Cancer Surgery: a Systematic Review. Clin J Pain. 2019;35(3):261–271. PMID: 30531400. doi:10.1097/AJP.0000000000000672
39. McKillop AB, Carroll LJ, Battie MC. Depression as a prognostic factor of lumbar spinal stenosis: a systematic review. Spine J. 2014;14:837–846.
40. Meert L, Mertens MG, Meeus M, et al. Identification of Metabolic Factors and Inflammatory Markers Predictive of Outcome after Total Knee Arthroplasty in Patients with Knee Osteoarthritis: a Systematic Review. Int J Environ Res Public Health. 2023;20(10). doi:10.3390/ijerph20105796
41. Moloney NA, Pocovi NC, Dylke ES, Graham PL, De Groef A. Psychological Factors Are Associated with Pain at All Time Frames After Breast Cancer Surgery: a Systematic Review with Meta-Analyses. Pain Med. 2021;22(4):915–947. PMID: 33547465. doi:10.1093/pm/pnaa363
42. Hjh N, Loke WJ, James WLH. The Influence of Obesity on Unicompartmental Knee Arthroplasty Outcomes: a Systematic Review and Meta-Analysis. Arch Bone Jt Surg. 2021;9:618–632.
43. Nunez-Cortes R, Cruz-Montecinos C, Torres-Castro R, et al. Effects of Cognitive and Mental Health Factors on the Outcomes Following Carpal Tunnel Release: a Systematic Review and Meta-analysis. Arch Phys Med Rehabil. 2022;103:1615–1627.
44. O’Connor JP, Holden P, Gagnier JJ. Systematic review: preoperative psychological factors and total Hip arthroplasty outcomes. J Orthop Surg Res. 2022;17:457.
45. Olsen U, Lindberg MF, Rose C, et al. Factors correlated with pain after total knee arthroplasty: a systematic review and meta-analysis. PLoS One. 2023;18:e0283446.
46. Rogowsky LC, Illmann CF, Isaac KV. Chronic pain in breast cancer patients post mastectomy with alloplastic reconstruction: a scoping review. Eur J Cancer Care. 2022;31:e13631.
47. Terradas-Monllor M, Beltran-Alacreu H, Tabuenca JV, et al. Are Psychosocial Factors Predictors of Pain and Functional Outcomes After Knee Arthroplasty at 6 and 12 Months After Surgery? A Systematic Review. Top Geriatric Rehabil. 2021;37:244–251.
48. Theunissen M, Peters ML, Bruce J, et al. Preoperative anxiety and catastrophizing: a systematic review and meta-analysis of the association with chronic postsurgical pain. Clin J Pain. 2012;28:819–841.
49. Van Bogaert W, Tegner H, Coppieters I, et al. The Predictive Value of Fear Avoidance Beliefs for Outcomes Following Surgery for Lumbar Degenerative Disease: a Systematic Review and Best Evidence Synthesis. Pain Physician. 2022;25(6):441–457. PMID: 36122254.
50. Varallo G, Giusti EM, Manna C, et al. Sleep disturbances and sleep disorders as risk factors for chronic postsurgical pain: a systematic review and meta-analysis. Sleep Med Rev. 2022;63:101630. doi:10.1016/j.smrv.2022.101630
51. Wluka AE, Yan MK, Lim KY, et al. Does preoperative neuropathic-like pain and central sensitisation affect the post-operative outcome of knee joint replacement for osteoarthritis? A systematic review and meta-analysis. Osteoarthritis Cartilage. 2020;28:1403–1411.
52. Zhao Z, Li J, Zhang R, et al. The prognostic value of fear-avoidance beliefs on postoperative pain and dysfunction for lumbar degenerative disk disease: a meta-analysis. Int J Rehabil Res. 2023;46:3–13.
53. Totten AM, Cheney TP, O’Neil ME, et al. Physiologic Predictors of Severe Injury: systematic Review [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018. (Comparative Effectiveness Review, No. 205.) Table E1, Quality in Prognostic Studies (QUIPS) tool, modified. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537447/table/appe.tab1/.
54. Turk DC. A diathesis-stress model of chronic pain and disability following traumatic injury. Pain Res Manag. 2002;7(1):9–19. PMID: 16231063. doi:10.1155/2002/252904
55. Lethem J, Slade PD, Troup JD, Bentley G. Outline of a fear-avoidance model of exaggerated pain perception: i. Behav Res Ther. 1983;21(4):401–408. doi:10.1016/0005-7967(83)90009-8
56. Carson SH. Creativity and Psychopathology: a Shared Vulnerability Model. Can J Psychiatry. 2011;56(3):144–153.
57. Katz J. One man’s risk factor is another man’s outcome: difference in risk factor profiles for chronic postsurgical pain maintenance vs transition. PAIN. 2012;153(3):505–506. doi:10.1016/j.pain.2011.10.044
58. Teasell RW, Bombardier C. Employment-related factors in chronic pain and chronic pain disability. Clin J Pain. 2001;17:S39–S45.
59. Poleshuck EL, Green CR. Socioeconomic disadvantage and pain. Pain. 2008;136(3):235–238. PMID: 18440703; PMCID: PMC2488390. doi:10.1016/j.pain.2008.04.003
60. VanDenKerkhof EG, Hopman WM, Goldstein DH, et al. Impact of perioperative pain intensity, pain qualities, and opioid use on chronic pain after surgery: a prospective cohort study. Reg Anesth Pain Med. 2012;37(1):19–27. doi:10.1097/AAP.0b013e318237516e
61. Katz J, Weinrib AZ, Clarke H. Chronic postsurgical pain: from risk factor identification to multidisciplinary management at the Toronto General Hospital Transitional Pain Service. Can J Pain. 2019;3(2):49–58. doi:10.1080/24740527.2019.1574537
62. Morin C, Lund JP, Villarroel T, Clokie CM, Feine JS. Differences between the sexes in post-surgical pain. Pain. 2000;85(1–2):79–85. PMID: 10692605. doi:10.1016/s0304-3959(99)00248-1
63. Keogh E. Sex and gender differences in pain: past, present, and future. Pain. 2022;163(Suppl 1):S108–S116. PMID: 36099334. doi:10.1097/j.pain.0000000000002738
64. Mogil JS. Sources of Individual Differences in Pain. Annu Rev Neurosci. 2021;44:1–25. PMID: 34236890. doi:10.1146/annurev-neuro-092820-105941
65. Perry M, Baumbauer K, Young EE, Dorsey SG, Taylor JY, Starkweather AR. The Influence of Race, Ethnicity and Genetic Variants on Postoperative Pain Intensity: an Integrative Literature Review. Pain Manag Nurs. 2019;20(3):198–206. PMID: 31080143; PMCID: PMC7841600. doi:10.1016/j.pmn.2018.11.002
66. Clarke H, Katz J, Flor H, Rietschel M, Diehl SR, Seltzer Z. Genetics of chronic post-surgical pain: a crucial step toward personal pain medicine. Can J Anaesth. 2015;62(3):294–303. PMID: 25471684. doi:10.1007/s12630-014-0287-6
67. Costigan M, Belfer I, Griffin RS, et al. Multiple chronic pain states are associated with a common amino acid–changing allele in KCNS1. Brain. 2010;133(9):2519–2527. doi:10.1093/brain/awq195
68. Hoofwijk DMN, van Reij RRI, Rutten BP, Kenis G, Buhre WF, Joosten EA. Genetic polymorphisms and their association with the prevalence and severity of chronic postsurgical pain: a systematic review. Br J Anaesth. 2016;117(6):708–719.
69. James SK. Chronic postsurgical pain: is there a possible genetic link? Br J Pain. 2017;11(4):178–185. PMID: 29123662; PMCID: PMC5661692. doi:10.1177/2049463717723222
70. Frangakis SG, MacEachern M, Akbar TA, et al. Association of Genetic Variants with Postsurgical Pain: a Systematic Review and Meta-analyses. Anesthesiology. 2023;139:827–839. doi:10.1097/ALN.0000000000004677
71. Pieper D, Antoine SL, Mathes T, Neugebauer EA, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. 2014;67(4):368–375. PMID: 24581293. doi:10.1016/j.jclinepi.2013.11.007
72. Weinrib AZ, Azam MA, Birnie KA, Burns LC, Clarke H, Katz J. The psychology of chronic post-surgical pain: new frontiers in risk factor identification, prevention and management. Br J Pain. 2017;11(4):169–177. PMID: 29123661; PMCID: PMC5661689. doi:10.1177/2049463717720636
73. Nicholls JL, Azam MA, Burns LC, et al. Psychological treatments for the management of postsurgical pain: a systematic review of randomized controlled trials. Patient Relat Outcome Meas. 2018;9:49–64. PMID: 29403322; PMCID: PMC5783145. doi:10.2147/PROM.S121251
74. Clarke H, Azargive S, Montbriand J, et al. Opioid weaning and pain management in postsurgical patients at the Toronto General Hospital Transitional Pain Service. Can J Pain. 2018;2(1):236–247. PMID: 35005382; PMCID: PMC8730554. doi:10.1080/24740527.2018.1501669
75. Bansal N, Ang S, Chen LC. Prevalence and determinants of chronic pain and persistent opioid use after surgery: a review of systematic reviews. Br J Pain. 2024;18(1):95–103. PMID: 38344265; PMCID: PMC10851888. doi:10.1177/20494637231204549
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.