Back to Journals » Drug Design, Development and Therapy » Volume 18
Low-Dose Alfentanil Inhibits Sufentanil-Induced Cough During Anesthesia Induction: A Prospective, Randomized, Double-Blind Study
Authors Xu Q , Zou X, Wu J, Duan G , Lan H, Wang L
Received 20 February 2024
Accepted for publication 11 May 2024
Published 17 May 2024 Volume 2024:18 Pages 1603—1612
DOI https://doi.org/10.2147/DDDT.S464823
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Tin Wui Wong
Qiaomin Xu,1 Xintong Zou,2 Jimin Wu,1 Gongchen Duan,1 Haiyan Lan,1 Liangrong Wang2
1Department of Anesthesiology, Lishui People’s Hospital, Lishui, 323000, People’s Republic of China; 2Department of Anesthesiology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325000, People’s Republic of China
Correspondence: Liangrong Wang, The First Affiliated Hospital of Wenzhou Medical University, No. 2, Fuxue Road, Lucheng District, Wenzhou City, Zhejiang Province, 325000, People’s Republic of China, Tel +86 13587884540, Email [email protected]
Background: Cough is one of the most common complications following intravenous administration of sufentanil during anesthesia induction. The study aimed to investigate the protective effect of alfentanil, afentanyl derivative with short onset time and short duration, in reducing sufentanil-induced cough.
Patients and methods: Eighty patients that scheduled for thyroid surgery under general anesthesia were randomly divided into the alfentanil group and normal saline group, with 40 cases per group. Patients in the alfentanil group received intravenous administration of 2 μg/kg alfentanil prior to sufentanil injection during general anesthesia induction, while the same dose of normal saline was administered in the normal saline group. The outcomes measures included the incidence and severity of cough and common side effects of opioids following the administration of sufentanil during the induction of general anesthesia, intraoperative hemodynamics parameters and major adverse events during anesthesia recovery period.
Results: The incidence of cough within one minute after the injection of sufentanil during anesthesia induction was 40% in the normal saline group, and the pretreatment of alfentanil significantly reduced the incidence of sufentanil-induced cough to 5% (p < 0.05). Correspondingly, the patients in the alfentanil group had decreased severity of sufentanil-induced cough compared with the normal saline group (p < 0.05). No significant differences in the incidences of common side effects of opioids (dizziness, nausea and vomiting, chest tightness and respiratory depression) within one minute after sufentanil injection were found (p > 0.05). Furthermore, there were no significant differences between the two groups in intraoperative hemodynamic parameters, extubation time, or the incidences of emergence agitation, respiratory depression, delayed recovery from anesthesia and postoperative nausea and vomiting during Postanesthesia Care Unit stay (p > 0.05).
Conclusion: Pretreatment with low-dose alfentanil (2 μg/kg) effectively and safely reduced both the incidence and severity of sufentanil-induced cough during anesthesia induction.
Clinical Trial Registration Number: Chinese Clinical Trial Registry (identifier: ChiCTR2300069286).
Keywords: alfentanil, sufentanil, cough, anesthesia induction
Introduction
Sufentanil, a synthesized opioid agonist, has been widely used for the induction of general anesthesia in clinical practice due to its reliable analgesic efficacy, lack of histamine release and little effect on hemodynamics. Whereas, the use of intravenous sufentanil can be associated with a number of undesirable effects, such as nausea, vomiting, cough, chest tightness, dizziness and even respiratory depression, depending on the dose, injection speed and individual variation. Sufentanil-induced cough, with an incidence of up to 31.9%,1 is usually transient and explosive, and in severe cases it can increase intracranial, intraocular and intraabdominal pressures, which may even cause a range of adverse effects in patients with high-risk comorbidities.2 Several medicines, such as lidocaine, propofol, fentanyl, remifentanil, dezocine, dexmedetomidine, tramadol or magnesium sulfate, have been shown to be effective in reducing the incidence and / or severity of opioid-induced cough.1,3–9 However, there are potential risks associated with these pharmacological interventions and it is therefore necessary to identify an alternative pretreatment agent with minimal complications to reduce sufentanil-induced cough.
Alfentanil is a synthetic, short-acting opioid analgesic classified as a small molecule derivative of fentanyl. Alfentanil exhibits the less potency, the faster onset and shorter duration of action compared to analogous anesthetics such as fentanyl and sufentanil, making it an ideal analgesic supplement during various surgical procedures. In addition, alfentanil injection could prevent bronchial spasms and reduce airway secretions,10 and its single bolus administration has been reported to be useful in reducing cough during emergence from desflurane or isoflurane anesthesia.11,12 The intravenous bolus of alfentanil is associated with a significantly lower incidence of cough than the equipotent dose of remifentanil,13 so alfentanil would theoretically be superior to remifentanil as a pretreatment for reducing sufentanil-induced cough, but there are no studies investigating the protective effect of alfentanil in preventing opioid-induced cough.
Herein, the present study was designed to evaluate the efficacy and safety of low-dose alfentanil pretreatment against sufentanil-induced cough. Outcomes measures included the incidence and severity of cough and common side effects of opioids after the administration of sufentanil during the induction of general anesthesia, intraoperative hemodynamics parameters and major adverse events during anesthesia recovery.
Methods
This study was approved by the Medical Ethics Committee of The Lishui People’s Hospital in Lishui, China (LLW-FO-403), and was registered with the Chinese Clinical Trial Registry (ChiCTR2300069286, 12 March 2023). Written informed consent was obtained from all the enrolled patients. The study was conducted at Lishui People’s Hospital from March 2023 to June 2023 in accordance with the criteria of the Declaration of Helsinki.
Patient Enrollment
Patients who were scheduled for elective thyroid surgery under general anesthesia were screened. Inclusion criteria included American Society of Anesthesiologists (ASA) physical status I or II, New York Heart Association (NYHA) functional class I or II, age between 18–65 years old, body mass index (BMI) between 18–30 kg/m2 and procedure time between 1–6 hours. Exclusion criteria were current or recent (within 2 weeks) upper respiratory tract infection; history of smoking or chronic respiratory disease (eg, asthma, chronic bronchitis, chronic obstructive pulmonary disease); prevalent gastroesophageal reflux disease; the presence of signs indicating elevated intracranial pressure, intraabdominal pressure or intraocular pressure; prevalent severe cardiac, cerebral and renal diseases (eg, uncontrolled hypertension, diabetes mellitus, heart disease); preoperative use of cough-inducing or suppressing medications; long-term opioid use; psychiatric disorders; Modified Mallampati Score of IV or predicted difficult airway; known allergies or contraindications to the agents. Patients with intraoperative blood loss greater than 600 mL or unplanned transfer to Intensive Care Unit after the surgery were also excluded.
Randomization and Group Allocation
All the patients were visited and assessed the day before surgery. After screening, eighty patients were recruited and randomized by computer-generated codes into 2 groups, designated as the alfentanil group (group A) and the normal saline group (group C), with 40 patients in each group. Patients in the group A received intravenous administration of 2 μg/kg afentanil (diluted with normal saline to a final volume of 5 mL) 1 minute prior to the sufentanil injection, and the same volume of normal saline was administered in the group C in the same manner. The group allocation was sealed in the closed envelopes by investigator Haiyan Lan, and only opened before induction of general anesthesia by the investigator Qiaomin Xu who was not involved in data collection. Both the patients and the investigators who performed anesthesia and assessment were blinded to the group allocation.
Anaesthetic Management
Patients received no premedication before the surgery. Continuous monitoring including electrocardiography, non-invasive blood pressure and pulse oximetry saturation (SpO2), was initiated after the patients were admitted to the operating room. Venous access was established on the dorsal vein using an 18-G intravenous cannula, which was then connected to a T-connector for the administration of the agents (Figure 1). Patients were preoxygenated with 100% oxygen 6L/min via a tight-fitting mask for 3 minutes before induction of anesthesia. Intravenous pretreatment of 2 μg/kg alfentanil that diluted to 5 mL with normal saline or an equal volume of normal saline was completed within 5 seconds.8 One minute after the pretreatment, sufentanil was administered at a dose of 0.5 μg/kg, which was completed within 5 seconds. One minute after sufentanil bolus, an intravenous injection of 1.5 mg/kg propofol and 0.1 mg/kg vecuronium bromide was consequently initiated for anesthesia induction, followed by endotracheal intubation and mechanical ventilation. A tidal volume of 6–8 mL/kg, a respiratory rate of 12–14 breaths/minute, a positive end-expiratory pressure of 5 cmH2O and an I:E ratio of 1:2 were set and adjusted to keep end-tidal carbon dioxide between 35 and 45 mm Hg. Maintenance of anaesthesia was achieved with 1% to 3% sevoflurane, and the inhalational concentration was adjusted according to the bispectral index (BIS) value, which was expected to remain between 40 and 60. Intermittent boluses of 0.1μg/kg sufentanil and 0.02 mg/kg vecuronium bromide were injected as required. At the end of the procedure, the administration of sevoflurane was terminated, the patient was then transferred to the Post-Anesthesia Care Unit (PACU), and the tracheal tube was removed when regular breathing with adequate tidal volume (> 6 mL/kg) and recovery of consciousness were confirmed.
 |
Figure 1 T-connector. |
Outcome Measurements
The primary outcome was the incidence of cough within one minute after the injection of sufentanil during anesthesia induction.
The secondary outcomes were measured as followings:
- The severity of cough within one minute after the injection of sufentanil during anesthesia induction: the severity of cough was scaled based on its frequency, with “mild” indicating 1–2 coughs, “moderate” indicating 2–4 coughs and “severe” indicating 5 or more coughs.13,14
- Common side effects of opioids: the occurrence of dizziness, nausea and vomiting, chest tightness and respiratory depression within one minute after the injection of sufentanil during anesthesia induction were recorded, where respiratory depression was defined as a persistent decrease in respiratory rate (< 10 breaths/minute) and / or SpO2 value (<90%). When respiratory depression occurred, manual ventilation was provided to improve the patient’s oxygenation until SpO2 ≥ 98%.
- Hemodynamic parameters and pulse oximetry: mean arterial pressure (MAP), heart rate (HR) and SpO2 were recorded immediately before (T0) and 2 minutes after (T1) the initiation of pretreatment, immediately before (T2) and after (T3) intubation, and 2 minutes after intubation (T4). Hypotension, defined by a systolic blood pressure < 80 mmHg or a fall in blood pressure of more than 20% from baseline, was treated with intravenous administration of 5 mg ephedrine, and hypertension was defined as a blood pressure > 160 / 95 mmHg or an increase of more than 20% from baseline, and appropriate antihypertensive medication was given. Tachycardia (HR greater than 100 beats per minute) and bradycardia (HR less than 50 beats per minute) were treated with intravenous administration of esmolol and 0.5 mg atropine, respectively.
- Extubation time: extubation time was defined as the time (min) from discontinuation of sevoflurane to extubation.
- Adverse events during PACU stay: emergence agitation, respiratory depression, delayed recovery from anesthesia and postoperative nausea and vomiting (PONV) were documented during PACU stay. Emergence agitation was defined as a Riker sedation-agitation score ≥ 515 and treated appropriately according to the cause. Delayed recovery from anesthesia was defined as the failure to regain consciousness 90 minutes after the termination of general anesthetic. One or more episodes of nausea or vomiting were considered PONV, where nausea was defined as the subjectively unpleasant feeling associated with the perception of the urge to vomit, and vomiting was defined as the forceful expulsion of gastric contents from the mouth. When PONV occurred, the serotonin type 3 (5-HT3) receptor antagonists were used.
Sample Size Calculation
The sample size was calculated using G*power 3.1 software based on the incidence of cough within one minute after the injection of sufentanil during anesthesia induction as the primary outcome. In our pilot study with 20 patients in each group, we found that the incidence of cough after the injection of sufentanil was 45%, and the incidence of sufentanil-induced cough decreased to 10% when the patients were pretreated with 2 μg/kg alfentanil. For a two-tailed significance level of 5% alpha error level and 80% power, 32 cases in each group was needed. To allow for a 20% dropout rate, a sample size of 40 patients per group (80 in total) was required.
Statistical Analysis
All statistical analyses were performed using SPSS statistical software (version 26.0, IBM Corp). Continuous variables were tested for normal distribution using the Shapiro–Wilk test. Continuous data are presented as mean ± standard deviation (SD) and were compared between the two groups using the independent t-test. Categorical data are presented as frequencies or percentages and were compared between the two groups using the chi-square test or Fisher’s exact test according to their expected counts. The data of hemodynamic parameters and pulse oximetry were analyzed by two-way repeated measures analysis of variance (ANOVA) followed by Bonferroni multiple comparison correction. A P < 0.05 was considered statistically significant, except for the repeated measured variables.
Results
One hundred patients scheduled for elective thyroid surgery under general anesthesia were screened, and eighty of them who met the inclusion and exclusion criteria were randomly assigned in a 1:1 ratio to either the group A or group C, with 40 cases in each group. All the patients completed the study and were included in the final analysis. The Consolidated Standards of Reporting Trials (CONSORT) flowchart diagram of the study was demonstrated in Figure 2. The demographics and clinical data were comparable between the two groups, as shown in Table 1 (p > 0.05).
 |
Table 1 Demographic and Clinical Data of the Patients |
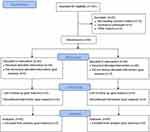 |
Figure 2 The Consolidated Standards of Reporting Trials (CONSORT) flowchart diagram of the study. |
The Incidence and Severity of Sufentanil-Induced Cough
During anesthesia induction, 40% of the patients in the group C and 5% in the group A developed cough within one minute after sufentanil injection, respectively (p < 0.05). The group A had a lower proportion of mild cough compared to the group C among those who developed cough (p < 0.05). However, there were no significant differences in the proportion of moderate and severe cough between the two groups (p > 0.05, see Table 2).
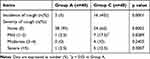 |
Table 2 The Incidence and Severity of Sufentanil-Induced Cough |
Common Side Effects of Opioids During Anesthesia Induction
There were no significant differences between the two groups in the incidence of dizziness, nausea and vomiting, chest tightness and respiratory depression within one minute after sufentanil injection during the induction of anesthesia (p > 0.05, see Table 3).
 |
Table 3 The Common Side Effects of Opioids During Anesthesia Induction |
Hemodynamic Data and SpO2 Values
There were no statistical differences in the data of MAP, HR and SpO2 between two groups at five corresponding time points (p > 0.05, see Table 4).
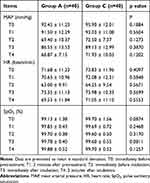 |
Table 4 Hemodynamic Data and SpO2 Values |
Extubation Time and Adverse Events During PACU Stay
As demonstrated in Table 5, extubation time was comparable between the two groups, and there were no significant differences in incidence of emergence agitation, respiratory depression, delayed recovery from anesthesia and PONV during PACU stay (p > 0.05).
 |
Table 5 Extubation Time and Adverse Events During PACU Stay |
Discussion
The main findings of our present study were that 1) the pretreatment with low-dose alfentanil (2 μg/kg) significantly reduced both the incidence and severity of sufentanil-induced cough during the induction of anesthesia; and 2) pretreatment with low-dose alfentanil did not increase the common side effects of opioids or had little effect on intraoperative hemodynamic stability and postoperative emergence from anesthesia. To sum up, our study provided a safe and effective alternative method to prevent sufentanil-induced cough during the induction of anesthesia.
Cough following opioid administration during the induction of anesthesia is not uncommon, and independent risk factors, such as age, cigarette smoking, previous epidural injection of lidocaine or a priming dose of muscle relaxant, may contribute to the variable incidence and severity of cough following opioid administration, but gender, the presence of either bronchial asthma and chronic obstructive pulmonary disease and the use of atropine have limited effects on the occurrence of opioid-induced cough.16 Additionally, variations in dose, concentration, route of administration and injection speed of opioids also affect the incidence of cough.17 To date, the mechanisms involved in sufentanil-induced cough are still undetermined and several possibilities have been proposed to explain it. The relative vagal predominance secondary to central sympathetic outflow inhibition by sufentanil administration would facilitate cough and reflex bronchoconstriction.14,18 As the pulmonary chemoreflex, mediated by either fast adapting receptors (irritant receptors) or vagal C-fibre receptors, is located close to the pulmonary vessels, stimulation or enhancement of irritant receptor excitability by opioid drugs could induce tracheal smooth muscle constriction.19,20 By acting on prejunctional μ-receptors, opioids cause an increased release of histamine and neuropeptides21,22 and decreased compliance of the chest wall, which would lead to sudden vocal cord closure or supraglottic obstruction by soft tissue, causing tussive effects. In addition, several studies have shown that opioid receptor dualism may be an important factor contributing to opioid-induced cough.1,20
Several agents, including lidocaine, tramadol, dezocine, dexmedetomidine and magnesium sulfate, have been reported to be effective in reducing opioid-induced cough.1,3,5,9,23 The additional pretreatments are likely to increase the drug-related side effects and potentially compromise patient safety. For instance, an injection with dexmedetomidine is liable to cause bradycardia and hypotension, and the 10-minute onset of action may be inappropriate for emergency use. In addition, magnesium sulfate may cause obvious burning and significantly increased plasma magnesium levels. Intravenous lidocaine increases the risk of systemic toxicity of the local anesthetic, while a mixed agonist-antagonist opioid, dezocine, is not suitable for the anesthesia for long surgical procedures. Tramadol-induced dizziness, nausea and vomiting may also be a problem, affecting the patient experience. What’s more, although long-acting opioids such as priming fentanyl or intramuscular morphine are effective in reducing opioid-induced cough, they also have major drawbacks, including a long onset time or prolonged duration of action.4,20 To avoid the side effects of the additional agents, opioids with short onset time and short duration of action will be favoured to provide protection against sufentanil-induced cough during anesthesia induction, and remifentanil and alfentanil are two promising candidates. Recently, low-dose (0.3 μg/kg) pretreatment with remifentanil has been shown to effectively and safely reduce the incidence and severity of sufentanil-induced cough during induction of anesthesia.8 But, rapid administration of remifentanil may cause muscle rigidity, hypotension and significant bradycardia.24 Apart from the almost 20-fold greater potency of remifentanil, the two opioid drugs are pharmacodynamically similar.25 In a study comparing the effects of a bolus injection of remifentanil and alfentanil on reducing the incidence of cough on emergence from isoflurane anaesthesia, alfentanil at 15 µg/kg significantly reduced the incidence of cough from 82% to 6%,12 whereas 1 µg/kg of remifentanil at the end of surgery was found to be ineffective, while 1 µg/kg remifentanil at the end of surgery was proved invalid.26 Collectively, alfentanil therefore appears to be favourable alternative to remifentanil in suppressing sufentanil-induced cough.
Indeed, as shown in our study, the pretreatment of low-dose alfentanil significantly decreased both the incidence and severity of sufentanil-induced cough during the induction of anesthesia. We hypothesized that opioid receptor dualism may be a possible mechanism to partially explain this phenomenon. Alfentanil at a low and subanalgesic dose may inhibit the cough reflex by acting directly on the cough centre in the medulla, thus exerting a central anti-tussive effect against cough induced by a subsequent large dose of sufentanil.1 Furthermore, pretreatment with alfentanil at a dose of 2 μg/kg was not associated with increased opioid-related side effects during induction or impaired recovery quality after surgery. Alfentanil has a different pharmacokinetic profile from fentanyl. Theoretically, alfentanil, with a lower blood / brain solubility, is able to easily penetrate through the blood-brain barrier and the equilibrium between plasma and central nervous system can be rapidly achieved (1.5 minutes), ensuring a very rapid onset of action when compared to fentanyl or sufentanil.27,28 In addition, compared with an equipotent dose of fentanyl, alfentanil has a more rapid redistribution and a sharp decrease in concentration below the therapeutic threshold, also the small volume of distribution indicates a short terminal elimination half-time.29 These factors result in a short duration of action of alfentanil. We assumed that the low dose of alfentanil and its less potency and relatively short duration of action would help to explain these findings.
It was noticed that although there was no significant difference in the incidence of dizziness between the two groups, approximately 25% of cases in each group complained of this side effect with 1 minute after intravenous sufentanil injection, which was similar to the previous report by Chen et al.30 In this study, the authors found that the incidence of dizziness after the intravenous administration of sufentanil did not differ within the dose range of 0.1–1.0 μg/kg. In addition, bolus alfentanil administration also shows specific advantage in clinical anesthesia because of its relatively fewer adverse effects. Previous studies have shown hemodynamic fluctuation in patients during remifentanil anesthesia, whereas alfentanil is associated with a significantly lower incidence of respiratory depression and improved hemodynamic stability when compared with remifentanil,31,32 and our present study showed that pretreatment with low-dose alfentanil did not cause intraoperative hemodynamic instability, suggesting the safety of this regimen.
Our study has several limitations. Firstly, the current study did not evaluate the effects of different doses of alfentanil pretreatment in reducing the incidence and severity of sufentanil-induced cough during the induction of anaesthesia, therefore, the dose-response effect of alfentanil pretreatment was not studied and the optimal dose of alfentanil pretreatment was not investigated. Secondly, the clinical efficacy of low-dose alfentanil pretreatment in reducing sufentanil-induced cough during the induction of anaesthesia has been demonstrated, but the underlying physiological or molecular mechanisms have not yet been determined. Finally, the efficacy and safety of alfentanil pretreatment in preventing sufentanil-induced cough was not evaluated in specific patient populations, such as obese and pediatric patients, which may limit the generality of the conclusion.
Conclusion
In conclusion, pretreatment with low-dose alfentanil effectively reduced both the incidence and severity of sufentanil-induced cough during the induction of anesthesia without increasing adverse events and complications.
Data Sharing Statement
The data that support the findings of this study are available on reasonable request from the corresponding author, LW.
Ethics Approval and Consent to Participate
This study was approved the Medical Ethics Committee of Lishui People’s Hospital in Lishui, China (LLW-FO-403). Written informed consent was obtained from all patients.
Funding
We received no funding source.
Disclosure
The authors declare no competing interests in this work.
References
1. Liu XS, Xu G-H, Shen Q-Y, et al. Dezocine prevents sufentanil-induced cough during general anesthesia induction: a randomized controlled trial. Pharmacol Rep. 2015;67(1):52–55. doi:10.1016/j.pharep.2014.08.004
2. Schug SA, Zech D, Grond S. Adverse effects of systemic opioid analgesics. Drug Saf. 1992;7(3):200–213. doi:10.2165/00002018-199207030-00005
3. An LJ, Gui B, Su Z, et al. Magnesium sulfate inhibits sufentanil-induced cough during anesthetic induction. Int J Clin Exp Med. 2015;8(8):13864–13868.
4. Hung KC, Chen C-W, Lin VC-H, et al. The effect of pre-emptive use of minimal dose fentanyl on fentanyl-induced coughing. Anaesthesia. 2010;65(1):4–7. doi:10.1111/j.1365-2044.2009.06109.x
5. He L, Xu JM, Dai RP. Dexmedetomidine reduces the incidence of fentanyl-induced cough: a double-blind, randomized, and placebo-controlled study. Ups J Med Sci. 2012;117(1):18–21. doi:10.3109/03009734.2011.629749
6. Kim JY, Park KS, Kim JS, et al. The effect of lidocaine on remifentanil-induced cough. Anaesthesia. 2008;63(5):495–498. doi:10.1111/j.1365-2044.2007.05414.x
7. Tang Q, Qian Y, Zhang Q, et al. Effects of different priming doses of propofol on fentanyl-induced cough during anesthesia induction: a preliminary randomized controlled study. Ups J Med Sci. 2010;115(2):121–124. doi:10.3109/03009730903291034
8. Lin W, Sun J, Fu S. A small dose of remifentanil pretreatment suppresses sufentanil-induced cough during general anesthesia induction: a randomized, double-blind, placebo-controlled trial. BMC Anesthesiol. 2019;19(1):164. doi:10.1186/s12871-019-0836-1
9. Zou Y, Ling Y, Kong G, et al. Effect of tramadol pretreatment on sufentanil-induced cough. J Perianesth Nurs. 2019;34(6):1181–1186. doi:10.1016/j.jopan.2019.01.013
10. Sridharan K, Sivaramakrishnan G. Comparison of fentanyl, remifentanil, sufentanil and alfentanil in combination with propofol for general anesthesia: a systematic review and meta-analysis of randomized controlled trials. Curr Clin Pharmacol. 2019;14(2):116–124. doi:10.2174/1567201816666190313160438
11. Lee MG, Chang YJ, Park JM, et al. The clinical effective dose of alfentanil for suppressing cough during emergence from desflurane anesthesia. Korean J Anesthesiol. 2011;61(4):292–296. doi:10.4097/kjae.2011.61.4.292
12. Mendel P, Fredman B, White PF. Alfentanil suppresses coughing and agitation during emergence from isoflurane anesthesia. J Clin Anesth. 1995;7(2):114–118. doi:10.1016/0952-8180(94)00024-X
13. Agarwal A, Gautam S, Nath SS, et al. Comparison of the incidence and severity of cough induced by sufentanil and fentanyl: a prospective, randomised, double-blind study. Anaesthesia. 2007;62(12):1230–1232. doi:10.1111/j.1365-2044.2007.05249.x
14. Agarwal A, Azim A, Ambesh S, et al. Salbutamol, beclomethasone or sodium chromoglycate suppress coughing induced by iv fentanyl. Can J Anaesth. 2003;50(3):297–300. doi:10.1007/BF03017801
15. Riker RR, Picard JT, Fraser GL. Prospective evaluation of the sedation-agitation scale for adult critically ill patients. Crit Care Med. 1999;27(7):1325–1329. doi:10.1097/00003246-199907000-00022
16. Oshima T, Kasuya Y, Okumura Y, et al. Identification of independent risk factors for fentanyl-induced cough. Can J Anaesth. 2006;53(8):753–758. doi:10.1007/BF03022790
17. Shuying L, Ping L, Juan N, et al. Different interventions in preventing opioid-induced cough: a meta-analysis. J Clin Anesth. 2016;34:440–447. doi:10.1016/j.jclinane.2016.05.034
18. Canning BJ, Mori N, Mazzone SB. Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol. 2006;152(3):223–242. doi:10.1016/j.resp.2006.03.001
19. Yasuda I, Hirano T, Yusa T, et al. Tracheal constriction by morphine and by fentanyl in man. Anesthesiology. 1978;49(2):117–119. doi:10.1097/00000542-197808000-00012
20. Böhrer H, Fleischer F, Werning P. Tussive effect of a fentanyl bolus administered through a central venous catheter. Anaesthesia. 1990;45(1):18–21. doi:10.1111/j.1365-2044.1990.tb14496.x
21. Kamei J, Nakanishi Y, Asato M, et al. Fentanyl enhances the excitability of rapidly adapting receptors to cause cough via the enhancement of histamine release in the airways. Cough. 2013;9(1):3. doi:10.1186/1745-9974-9-3
22. Karlsson JA, Sant’Ambrogio G, Widdicombe J. Afferent neural pathways in cough and reflex bronchoconstriction. J Appl Physiol. 1988;65(3):1007–1023. doi:10.1152/jappl.1988.65.3.1007
23. Pandey CK, Raza M, Ranjan R, et al. Intravenous lidocaine 0.5 mg.kg-1 effectively suppresses fentanyl-induced cough. Can J Anaesth. 2005;52(2):172–175. doi:10.1007/BF03027724
24. Shen JC, Xu J-G, Zhou Z-Q, et al. Effect of equivalent doses of fentanyl, sufentanil, and remifentanil on the incidence and severity of cough in patients undergoing abdominal surgery: a prospective, randomized, double-blind study. Curr Ther Res Clin Exp. 2008;69(6):480–487. doi:10.1016/j.curtheres.2008.12.002
25. Egan TD, Minto CF, Hermann DJ, et al. Remifentanil versus alfentanil: comparative pharmacokinetics and pharmacodynamics in healthy adult male volunteers. Anesthesiology. 1996;84(4):821–833. doi:10.1097/00000542-199604000-00009
26. Shajar MA, Thompson JP, Hall AP, et al. Effect of a remifentanil bolus dose on the cardiovascular response to emergence from anaesthesia and tracheal extubation. Br J Anaesth. 1999;83(4):654–656. doi:10.1093/bja/83.4.654
27. Stanski DR. Narcotic pharmacokinetics and dynamics: the basis of infusion applications. Anaesth Intensive Care. 1987;15(1):23–26. doi:10.1177/0310057X8701500105
28. Scott JC, Ponganis KV, Stanski DR. EEG quantitation of narcotic effect: the comparative pharmacodynamics of fentanyl and alfentanil. Anesthesiology. 1985;62(3):234–241. doi:10.1097/00000542-198503000-00005
29. Stanski DR, Hug CC. Alfentanil--A kinetically predictable narcotic analgesic. Anesthesiology. 1982;57(6):435–438. doi:10.1097/00000542-198212000-00001
30. Chen P, Zeng P, Gong Y, et al. Recommended dose of sufentanil during induction of general anesthesia to avoid coughing and drastic hemodynamic fluctuations in patients undergoing surgery. J Int Med Res. 2021;49(3):300060521996143. doi:10.1177/0300060521996143
31. Brosnan RJ, Pypendop BH, Stanley SD. Phenylpiperidine opioid effects on isoflurane minimum alveolar concentration in cats. J Vet Pharmacol Ther. 2020;43(6):533–537. doi:10.1111/jvp.12886
32. Li H, Zhang H, Cheng Z, et al. Effects of alfentanil hydrochloride on cough and hemodynamics during induction of general anesthesia in daytime surgery. Minerva Surg. 2023;78(6):730–732. doi:10.23736/S2724-5691.21.09341-2
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
