Back to Journals » Clinical Ophthalmology » Volume 18
Morphological and Clinical Characterization of Foveal Bulge Sign Three Years After Retinal Detachment Repair: A Longitudinal Prospective Evaluation
Authors Arend N , Vounotrypidis E , Schumann RG, Kampik A, Lob F, Priglinger S, Wolf A
Received 5 February 2024
Accepted for publication 17 June 2024
Published 13 August 2024 Volume 2024:18 Pages 2261—2270
DOI https://doi.org/10.2147/OPTH.S463004
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr John B Miller
Nicole Arend,1,* Efstathios Vounotrypidis,2,* Ricarda G Schumann,3 Anselm Kampik,3 Felice Lob,4 Siegfried Priglinger,5 Armin Wolf2
1Department of Ophthalmology, Eye Center Olching, Olching, Germany; 2Department of Ophthalmology, Ulm University, Ulm, Germany; 3Munich Eye Center “Augenzentrum im Brienner Hof”, Munich, Germany; 4Department of Ophthalmology, Realeyes Eye clinic, Munich, Germany; 5Department of Ophthalmology, Ludwig Maximilian University, Munich, Germany
*These authors contributed equally to this work
Correspondence: Armin Wolf, Department of Ophthalmology Ulm University, Ulm, Germany, Email [email protected]
Purpose: To evaluate optical-coherence-tomography (OCT)-morphological and clinical parameters three years after primary rhegmatogenous retinal detachment (RRD) repair surgery and the role of postoperative foveal bulge sign.
Methods: Of the 32 initially enrolled patients with primary RRD 20 (14 fovea-on, 6 fovea-off) completed this prospective study. Preoperatively and 3 years after surgery best corrected visual acuity (BCVA) and OCT including macula status, central retinal thickness (CRT), central choroidal thickness (CCT), thickness of each single retinal layer, integrity of cone-interdigitation-zone (CIZ) and ellipsoid zone (EZ), and presence of foveal bulge were evaluated.
Results: Preoperatively fovea-off RRD patients show significantly thinner CCT, inner nuclear layer (INL) and inner plexiform layer (IPL) compared to fovea-on RRD patients, whereas only IPL and INL were significantly thicker compared to the fellow eye. Three years after surgery this thickening recovered. Final BCVA did not differ statistically significantly between fovea-off and fovea-on patients, no difference in CIZ-integrity or presence of foveal bulge was observed. Presence of foveal bulge at 3 years showed significantly better final BCVA and was associated with intact preoperative CIZ-integrity and postoperative EZ- and CIZ-integrity. The preoperative fovea status showed no correlation to the postoperative presence of foveal bulge.
Conclusion: Three years after RRD repair retinal layers show similar thickness. The presence of foveal bulge is associated with better final BCVA. Sufficient pre- and postoperative CIZ-integrity as well as postoperative CIZ-integrity seem to be strongly associated with the restoration of foveal bulge. No correlation was found with the preoperative macular status or BCVA.
Plain Language Summary: We analyzed long-term optical-coherence-tomography changes after rhegmatogenous retinal detachment repair. Three years after rhegmatogenous retinal detachment repair retinal layers show similar thickness and visual acuity did not differ between fovea off and fovea on patients. The restoration of foveal bulge sign was the most important factor for good postoperative visual acuity. Integrity of specific retinal layers, such as the ellipsoid zone and cone-interdigitation-zone, seems to be strongly associated with the restoration of the foveal bulge.
Keywords: retinal detachment, visual acuity, optic coherence tomography, foveal bulge, retinal layers, retinal thickness, visual outcome, OCT
Introduction
Rhegmatogenous retinal detachment (RRD) occurs at an incidence of almost 1 to 10.000 persons annually,1 with myopic or pseudophakic patients being at higher risk for developing this disease.2,3 In up to 50% of cases, the macula is involved,4 which may result in a lack of visual recovery despite anatomically successful surgery.5 Clinical studies showed that time of intervention impacts final visual acuity6 in macula-off eyes.
Spectral-domain optical-coherence-tomography (OCT) gives structural insight into the different retinal layers and provides with additional retinal imaging details in many retinal diseases.7,8
Studies regarding OCT changes after retinal detachment repair confirmed the correlation between the inner segment–outer segment-junction (IS/OS) status, presence of epiretinal membranes (ERM) or macular edema (ME) and reduced visual acuity after retinal detachment.9–13 Kim et al examined structural changes at the extrafoveal border of detached and undetached retina, and detected that, after RRD repair, the thickness of the outer nuclear layer (ONL) was significantly thinner in the previously detached retina than in the previously undetached retina.14 Delolme et al11 and Gharbiya et al10 investigated the impact of changes in the subfoveal ONL after retinal detachment surgery on postoperative best corrected visual acuity (BCVA), however without any comparison to preoperative OCT parameters. After defining new anatomical landmarks in the OCT during the international OCT consensus development conference,15 recent studies showed varying results regarding the changes of the ONL in retinal detachment.16,17 However, most of them enhanced research on the cone-interdigitation-zone (CIZ), and the thickness of the inner and outer segments of the photoreceptor. Significant changes within these structures, especially after fovea-off retinal detachment, have been detected,16,17 and the possibly relevant role of the foveal bulge sign for the restoration of the outer retinal layers and visual acuity recovery was recognized.17 However, no previous studies have been published yet reporting on changes of inner retinal layers, the comparison to preoperative findings and their long-term course. Therefore, the present study evaluates the long-term OCT findings of all retinal layers and their correlation with the visual outcome after macula-on and macula-off RRD.
Methods
Patients that presented with primary RRD in the Department of Ophthalmology at the Ludwig-Maximilians-University Munich, Germany from April to September 2013 were screened for the present study. All practice and procedures followed strictly the declaration of Helsinki. Institutional review board approval was obtained for this non-interventional observational study and ethic committee approval was waived. Inclusion criteria were primary RRD with SD-OCT defined fovea-on or fovea-off status, age >18 years and informed consent to the study plan. Exclusion criteria were previous vitreoretinal surgery, preoperative ME, status post retinal vein or artery occlusion, diabetic retinopathy, presence of ERM, or any known macular abnormalities and highly bullous retinal detachment making a valid OCT image impossible. Preoperative data assessment included best corrected distance visual acuity (BCVA), age, sex, previous eye disease history, slit lamp examination, intraocular pressure (IOP) and preoperative macula and fovea status captured by OCT image. The macula was defined as the retinal area between the temporal vessel arches centered at the fovea with an approximately 6mm diameter. The fovea was defined according to Cho et al as the central retinal depression approximately 4mm temporal and 0.8mm inferior of the optic disc.9 OCT was obtained in the morning before surgery with Heidelberg Spectral Domain OCT (HRA-Spectralis OCT, version 5.6.1.0) and evaluated with Heidelberg eye explorer (Version 1.7.1.0, Heidelberg Engineering, Heidelberg, Germany). All scans were conducted by the same examiner (NA). The posterior pole was scanned with the 30-degree program with 61 consecutive high-density scans with a space of 120µm covering a 30 x 25-degree field in standard and EDI (enhanced depth imaging) mode with 12 frames art mode. Additionally, a single line scan of the fovea was added in standard and EDI mode with art mode 100 frames. The layers of the retina were defined according to the international consensus on OCT imaging15 as nerve fiber layer (NFL), ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), ONL, external limiting membrane (ELM), ellipsoid zone (EZ), CIZ and retinal pigment epithelium (RPE). EDI-OCT offers also imaging of the choroid. In the preoperative OCT scans, macula and fovea status, central retinal thickness (CRT) in the foveal center as reported by the retinal thickness map of the Heidelberg eye explorer, as well as the thickness of all retinal layers and the choroid measured in the central 100 frames art mode scan subfoveal by the caliper tool of Heidelberg eye explorer, were assessed. By evaluating each of the 61 scans, the integrity of the IS/OS junction, CIZ and ELM and the presence of foveal depression, foveal bulge, ERM or ME were assessed and quantified as absent (score 0) or present in two different graduations (score 0.5 or 1). Images were independently evaluated by 2 examiners (NA, FL). Three years postoperatively the OCT scans were repeated using the follow-up eye tracker mode and the same OCT evaluations were performed on both eyes. Additionally, BCVA, IOP and a complete clinical examination were performed at the 3 years postoperative visit. For each patient the type of vitreoretinal surgery was documented as vitrectomy (with gas tamponade) or scleral buckle. Surgical procedures were performed by two different surgeons (AW, AK). Each OCT and clinical evaluation were performed by two independent examiners (NA, FL), except for BCVA, which was conducted by a person masked for the study.
Different subgroups were compared to each other regarding preoperative foveal status and postoperative BCVA >0.3 logMAR (20/40 Snellen). The level of 0.3 logMAR was chosen because it is the level required for reading standard newspaper letters.18 Additionally, pre- to postoperative changes were evaluated and compared with contralateral eye.
Statistical analyses were conducted using SPSS Version 23 (SPSS Statistics, IBM). Normal distribution was tested with the Shapiro–Wilk test. Metrical data was expressed as mean and standard deviation, scores as median. Differences between the examined groups were analyzed using parametrical and non-parametrical tests regarding normal distribution. Multiple linear regression analysis was conducted for evaluation of parameters affecting final BCVA. For associations between categorical variables Cramer’s V test was used. Results were considered statistically significant when p<0.05. Post-hoc analysis with G*power software was performed.19 Based on the squared multiple correlation r2 from our model we calculated post hoc the effect size f2, which was 2.125. Given an α-error of 0.05, the post hoc calculated effect size of 2.125 and a sample size of 20 eyes the calculated achieved power in our study was 0.85.
Results
Thirty-two eyes of 32 patients were primary included in the study and measured preoperatively, 20 with fovea attached (on) on, 12 with fovea detached (off). Unfortunately, 12 patients were lost to follow-up and after 3 years, only 20 patients (3 female, 17 male) with a mean age of 58±8.5 years completed the study with postoperative measurements. Clinical characteristics of these patients are summarized in Table 1. Fourteen patients (10 pseudophakic, 4 phakic) underwent primary pars plana vitrectomy (ppV), two phakic (1 fovea-on, 1 fovea-off) underwent combined phacovitrectomy and four phakic patients underwent scleral buckling. In all cases the retina was successfully reattached. Regarding the clinical parameters, no significant difference was observed between the fovea-on and the fovea-off groups (Table 1).
 |
Table 1 Baseline Parameters Regarding Preoperative Macular Status |
Comparison with the Fellow Eye and Time-Related Changes
Table 2 summarizes relevant parameters such as thickness of retinal layers, integrity of CIZ, presence of foveal bulge sign and BCVA of the affected eye preoperatively and 3 years postoperatively. Figures 1 and 2 demonstrate thickness of each retinal layer and the choroid in fovea on, fovea off and in fellow eyes. Briefly, CRT, central choroidal thickness (CCT), INL and IPL show higher values in fovea-off eyes preoperatively in comparison to fovea-on or fellow eyes (CRT: p = 0.068 and p = 0.009, respectively; CCT: p = 0.020 and p = 0.177, respectively; INL p = 0.004 and p = 0.004, respectively; IPL (p = 0.001 and p<0.001, respectively). Three years postoperatively all retinal layers except of GCL showed a thickness reduction in fovea-off eyes compared with preoperative measurements. Moreover, all retinal layers showed similar thickness except of OPL of fovea-off eyes, which was statistically significantly thinner compared with fovea-on eyes (p = 0.018). The postoperative integrity of CIZ (fovea on score: 1; fovea off score: 0.5) and the formation of the postoperative foveal bulge (fovea-on score: 1; fovea-off score: 1) did not differ statistically significantly between fovea-on and fovea-off patients.
 |
Table 2 Mean and Median Values of Numeric and Ordinal Variables with Regard to Preoperative Fovea Status |
Preoperative BCVA was significantly worse in fovea-off eyes compared to fovea-on (p = 0.006) or fellow eyes (p<0.001). However, 3 years postoperatively BCVA was comparable with fovea-on or fellow eyes (p = 0.779 and p = 0.566 respectively). Visually relevant cataract was preoperatively observed in two patients that underwent combined phacovitrectomy and were equally distributed to the fovea-on and fovea-off group, avoiding any additional bias.
Subgroup Analysis
In the subgroup analysis 14 patients had BCVA<0.3 logMAR (group A) and 6 patients >0.3 logMAR (group B) 3 years postoperatively. Patients of group A had a significantly better postoperative integrity of CIZ (score 1 vs. score 0; p = 0.009) and EZ (score 1 vs. score 0; p = 0.028). Moreover, the postoperative foveal bulge sign was observed significantly more often than in group B (score 1 vs. score 0; p = 0.031). There was no statistically significant difference in preoperative BCVA (0.3 vs. 0.4 logMAR) or preoperative thickness of retinal layers between patients of groups A and B (Table 3).
 |
Table 3 Different Parameters with Regard to Final BCVA at 3 Years |
Figures 3 and 4 show an exemplary OCT course of two fovea-off eyes, with good BCVA, recovery of CIZ and foveal bulge sign 3 years postoperatively.
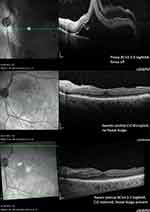 |
Figure 3 OCT example of a fovea-off patient with a 3-year postoperative foveal bulge sign, intact CIZ and a final BCVA of 0.2 logMAR. |
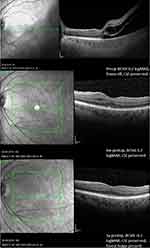 |
Figure 4 OCT example of a fovea-off patient with a 3-year postoperative foveal bulge sign, intact CIZ and a final BCVA of −0.1 logMAR. |
Further analysis was performed regarding BCVA and the presence of foveal bulge sign at 3 years postoperatively. Postoperative presence of foveal bulge sign was associated with better final BCVA (Multiple linear regression analysis; r2= 0.653; p = 0.002). Twelve patients showed a foveal bulge, whereas 8 did not. In patients with foveal bulge at 3 years BCVA was better (0.1 logMAR vs. 0.4 logMAR). Presence of foveal bulge was correlated significantly with preoperative CIZ-Integrity (Cramer’s V: 0.596, p = 0.029), postoperative CIZ-Integrity (Cramer’s V: 0.612, p = 0.024) and postoperative EZ-Integrity (Cramer’s V: 0.612, p = 0.006), but not with preoperative foveal status (Cramer’s V: 0.363, p = 0.267).
Discussion
Reduced visual acuity (VA) after retinal detachment (RD) surgery may occur due to formation of membranes or cataract. In RD cases with macular involvement VA remains often poor and it seems that the time of intervention plays a significant role on the postoperative BCVA as well as the preservation of the foveal depression of the detached retina.6,20 Earlier studies tried to enlighten this field by analyzing postoperative OCT scans within the first year after retinal detachment repair surgery. Delolme et al showed that in patients with macula-off retinal detachments, the postoperative integrity of the IS/OS junction, the ELM and the photoreceptors were significantly correlated with the postoperative BCVA in both “macula-on” and “macula-off” cases.10,11 In particular, the photoreceptor layer thickness has been recognized for its role in the recovery of VA and its increase within the first year.16,17 Additionally, in these studies the postoperative foveal bulge sign has been identified as a relevant factor in the restoration of the BCVA.16,17 Other studies have investigated further preoperative risk factors that may affect the final functional outcome, such as the extent of retinal detachment, the volume of the submacular fluid, as well as the integrity of the EZ and the preoperative continuity of the ELM. 21–24 With an increase of the photoreceptor layer within the first year, our study aimed at looking at OCT changes over a longer period after successful RD repair in a prospective manner. Additionally, our study assessed preoperative OCT findings which have not been previously evaluated in detail.
When comparing the affected eye with the healthy fellow eye, fovea-on patients did not show any changes pre- to postoperatively. IPL-thickening, which primarily contains fibers of Muller cells and dendrites of retinal ganglion cells, may be explained by focal contraction due to peripheral fixation loss at the detachment.25 The choroidal thickness can be influenced, among other factors, by age and axial length.26 Preoperatively, in fovea-off patients, each retinal layer (except of GCL) and the choroid of the affected eye showed relevant thickening in comparison to the fellow eye. This may be explained by intraretinal fluid accumulating during retinal detachment.27 Over the period of three years, this thickening resolved until there was no longer any statistically significant difference to the layer thickness of the fellow eye. While other groups have shown a postoperative thinning of outer retinal layers after detachment,16,17 followed by a gradually re-thickening within the first postoperative year, we were not able to confirm these findings at the third postoperative year. This may be explained by different timepoints of measurements. While differences in the groups of “fovea-on” and “fovea-off” RRD or affected eye and fellow eye during the follow up were observed, there seems to be no difference in layer thicknesses at 3 years postoperatively anymore. To our understanding this confirms the previously mentioned plasticity of retinal layers over a period of more than 3 years.
The retinal recovery may further be demonstrated by the fact that not only layer thickness, but also the layer integrity (CIZ, ELM and EZ), was affected after fovea-off retinal detachment during early follow-up, which recovered almost completely at 3 years. At this timepoint, there was no difference regarding the CIZ integrity in correlation to the foveal preoperative status. This process seems to take place within the first months after retinal reattachment repair, as previously shown by Dell´Omo and Kobayashi et al.16,17
Interestingly, BCVA, which was significantly reduced preoperatively in fovea-off patients, had recovered after 3 years to the same mean BCVA as in fovea-on patients. This must be interpreted carefully due to the limited number of study participants, the different lens status and the possible development of an ERM, as 2 fovea-off and 1 fovea-on patients developed an extrafoveal ERM, which did not affect BCVA. Despite that we believe that this is an important finding, as there was no impact of cataract formation or ERM during the follow-up period as explained above. Furthermore, in our study except of 3 patients having symptoms for a period longer than 1 week, all other patients had a duration of symptoms ranging between 1 and 3 days. As all patients were treated in our clinic within 24 hours after presentation, we assume that the on-time treatment within the 3 days timespan is another major reason for the good BCVA.6
The postoperative presence of the postoperative foveal bulge sign seems to be the most important predictive factor for BCVA at 3 years, which was already indicated by studies with a shorter observation time.16,17 Additionally, an association was indicated between postoperative formation of foveal bulge sign and pre- and postoperative CIZ-integrity, as well as postoperative EZ-integrity. However, preoperative CIZ-integrity did not show any high correlation with good postoperative BCVA at 3 years (p = 0.571, Chi-square). Several previous studies indicated the importance of the foveal bulge for visual acuity in different retinal diseases.16,17,28–30 Another study showed a correlation of the postoperative presence of the foveal bulge and the preoperative fovea status in RRD.28 Despite the long follow-up of our study, we were not able to confirm this correlation in our cohort. We assume that the major reason for this is the low number of study participants. Nevertheless, we observed that the pre- and post-operative CIZ-integrity as well as EZ-Integrity seem to be associated with formation of the foveal bulge, whereas previous studies indicated only a correlation to the postoperative CIZ-integrity.17 A recent study showed in eyes with ERM showed a better BCVA when foveal bulge was present, indicating its significance regarding BCVA.31 Another study in healthy subjects revealed the presence of the foveal bulge in only 60% of the subjects and showed an age-dependent decrease of the foveal bulge height.32 In our cohort, 6 of the 20 healthy fellow eyes had no foveal bulge. Fortunately, the age distribution in our cohort is constant and with a small standard deviation, while others had a deviation of 20 years. Thus, we believe this effect could be either age-dependent or an individual predisposition.
This study has certain advantages and limitations. The major drawback is the rather small number of patients that completed the long-term follow-up and the different number of macula-on and macula-off RRD participants. Therefore, we do not feel confident to draw generalized conclusions rather than discuss the observed trends regarding the examined parameters. On the other hand, the prospective nature of the present study and the long follow-up period strengthen our findings. Furthermore, we tried to reduce bias due to cataract formation by excluding such patients. Furthermore, ERM formation was low and did not affect BCVA especially in fovea-off eyes. Nevertheless, this study provides long-term insights into the changes of all retinal layers and foveal bulge sign after successful RRD repair surgery.
In summary, we demonstrate that previous structural OCT-changes of retinal layers diminish in the long-term after successful RRD repair surgery. The foveal bulge sign seems to be the most accurate tool to predict a good BCVA. However, it seems that its restoration is affected by multiple factors and prediction of BCVA remains difficult. Given the fact, that VA deterioration can occur even after 3 days of macular off RD it would be interesting to investigate any correlations between foveal bulge sign and time from detachment to intervention to enlighten further any correlations between these factors and VA. Further studies with larger cohorts, automated OCT segmentation, inclusion of microperimetry findings and age-matched groups are warranted in the need of further investigation of structural OCT changes after RRD repair surgery and the role of the foveal bulge and its influence on final visual outcome.
Ethical Statement
All practice and procedures of this study followed strictly the declaration of Helsinki and the guidelines of the LMU ethical commission. Institutional review board approval was given for the present study. Informed consent was obtained by the study participants prior to study commencement.
Acknowledgment
The authors thank Ebru Memis and Jakob Piskorowski for their dedicated assistance.
Disclosure
Dr Efstathios Vounotrypidis reports personal fees from Bayer, personal fees from Novartis, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Wilkes SR, Beard CM, Kurland LT, et al. The incidence of retinal detachment in Rochester, Minnesota, 1970-1978. Am J Ophthalmol. 1982;94(5):670–673. doi:10.1016/0002-9394(82)90013-7
2. Sheu SJ, Ger LP, Chen JF. Axial myopia is an extremely significant risk factor for young-aged pseudophakic retinal detachment in Taiwan. Retina. 2006;26(3):322–327. doi:10.1097/00006982-200603000-00011
3. Ripandelli G, Scassa C, Parisi V, et al. Cataract surgery as a risk factor for retinal detachment in very highly myopic eyes. Ophthalmology. 2003;110(12):2355–2361. doi:10.1016/S0161-6420(03)00819-4
4. Thelen U, Amler S, Osada N, et al. Outcome of surgery after macula-off retinal detachment - results from MUSTARD, one of the largest databases on buckling surgery in Europe. Acta Ophthalmol. 2012;90(5):481–486. doi:10.1111/j.1755-3768.2010.01939.x
5. van de Put MA, Croonen D, Nolte IM, et al. Postoperative recovery of visual function after macula-off rhegmatogenous retinal detachment. PLoS One. 2014;9(6):e99787. doi:10.1371/journal.pone.0099787
6. Greven MA, Leng T, Silva RA, et al. Reductions in final visual acuity occur even within the first 3 days after a macula-off retinal detachment. Br J Ophthalmol. 2019;103(10):1503–1506. doi:10.1136/bjophthalmol-2018-313191
7. Sander B. Optical coherence tomography in ophthalmology: an overview. Acta Ophthalmol. 2009;87(3):245–246. doi:10.1111/j.1755-3768.2008.01386.x
8. Rodrigues EB, Medeiros F, Mennel S, et al. Optical coherence tomography in ophthalmology. J Ophthalmol. 2012;2012:134569. doi:10.1155/2012/134569
9. Cho M, Witmer MT, Favarone G, Chan RP, D’Amico DJ, Kiss S. Optical coherence tomography predicts visual outcome in macula-involving rhegmatogenous retinal detachment. Clin Ophthalmol. 2012; 6:91–96.
10. Gharbiya M, Grandinetti F, Scavella V, et al. Correlation between spectral-domain optical coherence tomography findings and visual outcome after primary rhegmatogenous retinal detachment repair. Retina. 2012;32(1):43–53. doi:10.1097/IAE.0b013e3182180114
11. Delolme MP, Dugas B, Nicot F, et al. Anatomical and functional macular changes after rhegmatogenous retinal detachment with macula off. Am J Ophthalmol. 2012;153(1):128–136. doi:10.1016/j.ajo.2011.06.010
12. Lai WW, Leung GYO, Chan CWS, et al. Simultaneous spectral domain OCT and fundus autofluorescence imaging of the macula and microperimetric correspondence after successful repair of rhegmatogenous retinal detachment. Br J Ophthalmol. 2010;94(3):311–318. doi:10.1136/bjo.2009.163584
13. Dell’Omo R, Mura M, Lesnik Oberstein SY, et al. Early simultaneous fundus autofluorescence and optical coherence tomography features after pars plana vitrectomy for primary rhegmatogenous retinal detachment. Retina. 2012;32(4):719–728. doi:10.1097/IAE.0b013e31822c293e
14. Kim JH, Park DY, Ha HS, et al. Topographic changes of retinal layers after resolution of acute retinal detachment. Invest Ophthalmol Vis Sci. 2012;53(11):7316–7321. doi:10.1167/iovs.12-10155
15. Staurenghi G, Sadda S, Chakravarthy U, et al. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN*OCT consensus. Ophthalmology. 2014;121(8):1572–1578. doi:10.1016/j.ophtha.2014.02.023
16. dell’Omo R, Viggiano D, Giorgio D, et al. Restoration of foveal thickness and architecture after macula-off retinal detachment repair. Invest Ophthalmol Vis Sci. 2015;56(2):1040–1050. doi:10.1167/iovs.14-15633
17. Kobayashi M, Iwase T, Yamamoto K, et al. Association between photoreceptor regeneration and visual acuity following surgery for rhegmatogenous retinal detachment. Invest Ophthalmol Vis Sci. 2016;57(3):889–898. doi:10.1167/iovs.15-18403
18. Zhang J, Liu J, Jasti S, et al. Visual demand and acuity reserve of Chinese versus English newspapers. Optom Vis Sci. 2020;97(10):865–870. doi:10.1097/OPX.0000000000001585
19. Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. doi:10.3758/BRM.41.4.1149
20. Konstantinidis L, Stappler T, Potic J, et al. Characteristics of patients with complete visual acuity recovery after vitrectomy for macula-off retinal detachment. Eye. 2021;35(10):2834–2839. doi:10.1038/s41433-020-01322-y
21. Ng H, Vermeer KA, van Meurs JC, et al. Visual acuity inadequately reflects vision-related quality of life in patients after macula-off retinal detachment surgery. Invest Ophthalmol Vis Sci. 2020;61(10):34. doi:10.1167/iovs.61.10.34
22. Noda H, Kimura S, Morizane Y, et al. RELATIONSHIP BETWEEN PREOPERATIVE FOVEAL MICROSTRUCTURE AND VISUAL ACUITY IN MACULA-OFF RHEGMATOGENOUS RETINAL DETACHMENT: imaging analysis by swept source optical coherence tomography. Retina. 2020;40(10):1873–1880. doi:10.1097/IAE.0000000000002687
23. Angermann R, Mosböck S, Palme C, et al. Impact of submacular fluid volume on visual outcome in macula-off rhegmatogenous retinal detachment using automated optical coherence tomography volumetric quantification. Clin Exp Ophthalmol. 2021;49(5):439–447. doi:10.1111/ceo.13929
24. Yeo YD, Kim YC. Significance of outer retinal undulation on preoperative optical coherence tomography in rhegmatogenous retinal detachment. Sci Rep. 2020;10(1):15747. doi:10.1038/s41598-020-72907-6
25. Szigeti A, Tátrai E, Varga BE, et al. the effect of axial length on the thickness of intraretinal layers of the macula. PLoS One. 2015;10(11):e0142383. doi:10.1371/journal.pone.0142383
26. Ho M, Liu DTL, Chan VCK, et al. Choroidal thickness measurement in myopic eyes by enhanced depth optical coherence tomography. Ophthalmology. 2013;120(9):1909–1914. doi:10.1016/j.ophtha.2013.02.005
27. Choudhry N, Golding J, Manry MW, et al. Ultra-widefield steering-based spectral-domain optical coherence tomography imaging of the retinal periphery. Ophthalmology. 2016;123(6):1368–1374. doi:10.1016/j.ophtha.2016.01.045
28. Hasegawa T, Ueda T, Okamoto M, et al. Relationship between presence of foveal bulge in optical coherence tomographic images and visual acuity after rhegmatogenous retinal detachment repair. Retina. 2014;34(9):1848–1853. doi:10.1097/IAE.0000000000000160
29. Wu Q, Hu Y, Liu B, et al. Factors associated with the presence of foveal bulge in eyes with resolved diabetic macular edema. Front Med. 2021;8:755609. doi:10.3389/fmed.2021.755609
30. Hasegawa T, Ueda T, Okamoto M, et al. Presence of foveal bulge in optical coherence tomographic images in eyes with macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2014;157(2):390–396e1. doi:10.1016/j.ajo.2013.10.007
31. Icoz M, Toklu Y, Arikan Yorgun M. Evaluation of the optical coherence tomography findings of patients with idiopathic epiretinal membrane. Photodiagnosis Photodyn Ther. 2023;41:103286. doi:10.1016/j.pdpdt.2023.103286
32. Saurabh K, Roy R, Sharma P, et al. Age-related changes in the foveal bulge in healthy eyes. Middle East Afr J Ophthalmol. 2017;24(1):48–50. doi:10.4103/meajo.MEAJO_347_16
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


