Back to Journals » International Journal of Nanomedicine » Volume 19
Nano-Proteolysis Targeting Chimeras (Nano-PROTACs) in Cancer Therapy
Authors Song Y, Dong QQ, Ni YK, Xu XL , Chen CX, Chen W
Received 1 December 2023
Accepted for publication 30 May 2024
Published 12 June 2024 Volume 2024:19 Pages 5739—5761
DOI https://doi.org/10.2147/IJN.S448684
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Yue Song,1,* Qing-Qing Dong,2,* Yi-Ke Ni,3 Xiao-Ling Xu,3 Chao-Xiang Chen,3 Wei Chen2
1Stomatology Hospital, School of Stomatology, Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, 310003, People’s Republic of China; 2ICU, Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, 200032, People’s Republic of China; 3Shulan International Medical College, Zhejiang Shuren University, Hangzhou, Zhejiang Province, 310015, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiao-Ling Xu; Chao-Xiang Chen, Shulan International Medical College, Zhejiang Shuren University, 8 Shuren Street, Hangzhou, 310015, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Proteolysis-targeting chimeras (PROTACs) are heterobifunctional molecules that have the capability to induce specific protein degradation. While playing a revolutionary role in effectively degrading the protein of interest (POI), PROTACs encounter certain limitations that impede their clinical translation. These limitations encompass off-target effects, inadequate cell membrane permeability, and the hook effect. The advent of nanotechnology presents a promising avenue to surmount the challenges associated with conventional PROTACs. The utilization of nano-proteolysis targeting chimeras (nano-PROTACs) holds the potential to enhance specific tissue accumulation, augment membrane permeability, and enable controlled release. Consequently, this approach has the capacity to significantly enhance the controllable degradation of target proteins. Additionally, they enable a synergistic effect by combining with other therapeutic strategies. This review comprehensively summarizes the structural basis, advantages, and limitations of PROTACs. Furthermore, it highlights the latest advancements in nanosystems engineered for delivering PROTACs, as well as the development of nano-sized PROTACs employing nanocarriers as linkers. Moreover, it delves into the underlying principles of nanotechnology tailored specifically for PROTACs, alongside the current prospects of clinical research. In conclusion, the integration of nanotechnology into PROTACs harbors vast potential in enhancing the anti-tumor treatment response and expediting clinical translation.
Keywords: Proteolysis-targeting chimeras, nanotechnology, delivery, linker, designing principles
Graphical Abstract:
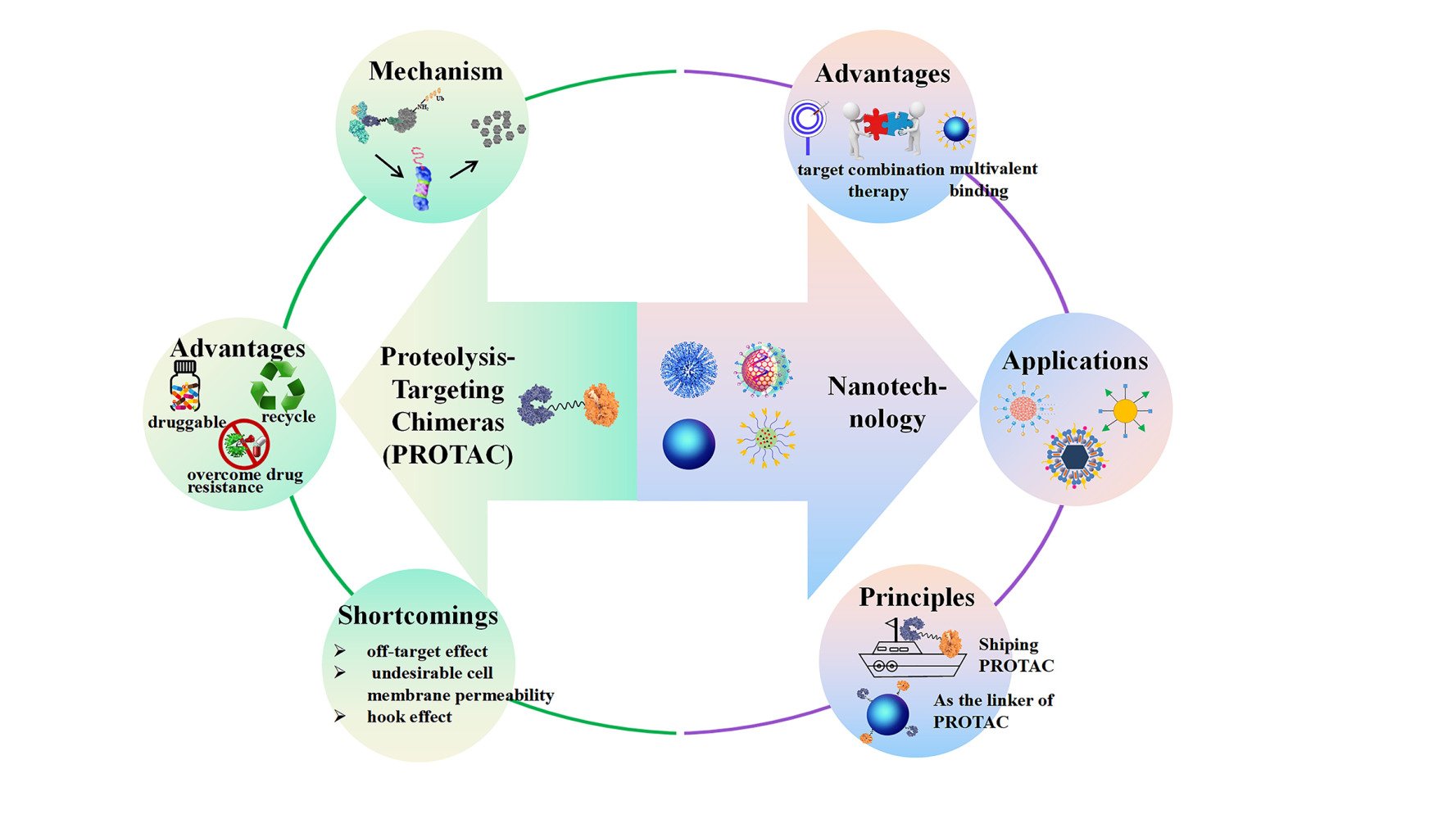
Introduction
Proteolysis-Targeting Chimeras (PROTAC)
Proteolysis-targeting chimeras (PROTAC) are a class of small molecule drugs designed to target and degrade intracellular proteins.1 PROTAC consists of three parts; E3 ubiquitin ligase ligand, target protein ligand, and linker for conjugation. Due to the proper hydrophile-lipophile balance, PROTAC may be rapidly internalized into the cytoplasm, facilitating effective degradation of the protein of interest (POI). The target protein ligand and E3 ubiquitin ligase ligand guide PROTAC to recognize the target protein and E3 ubiquitin ligase, respectively, resulting in the formation of a ternary complex. As E3 ubiquitin ligase is an enzyme that can transfer ubiquitin molecules to a lysine of the target protein, the target protein turns to proteins marked with ubiquitin. The ubiquitin-proteasome systems rapidly and irreversibly initiate the degradation process, contributing to reduced levels of target proteins.2–5 The schematic route of PROTAC is illustrated in Figure 1.
 |
Figure 1 The mechanism of PROTACs. Figure generated using BioRender, Agreement number: KE26PEXHAO. |
PROTAC offers several advantages over traditional small molecule inhibitors (SMIs), as described below, and puts it ahead of alternative drugs: 1) Changing the target from “undruggable” to “druggable”.6–10 “Undruggable” proteins refer to therapeutic targets with clinical significance that are characterized by the lack of definite ligand binding pockets, non-catalytic interaction regions, and unresolved crystal structures, accounting for 80% of proteins in human cells. Antibodies with high molecular weights cannot enter because the majority are located in cells or nuclei. Moreover, the protein surface is relatively smooth without evident “pockets”, thus, SMIs cannot be firmly grasped. Hence, these target proteins (including C-Myc, NF-κB, and brachyury (TBXTA)) are difficult or impossible to make into drugs via traditional methods. Conversely, PROTACs only require weak binding with the target protein to specifically “label” it regardless of catalytic ability or binding pockets of an enzyme. Currently, approximately 80% of the “undruggable” proteins may be solved by PROTAC technology.11,12 2) Overcoming drug resistance caused by mutation/overexpression of target proteins.13–18 Over the course of the clinical use of SMIs or antagonists, acquired drug resistance will inevitably occur. For example, epidermal growth factor receptor (EGFR)-T790M19 and C797S20,21 are resistant. Even if the problem of drug resistance can be solved by creating new inhibitor generations, like the third and fourth generation of EGFR, using these generations of medications will lead to new drug resistance. The use of PROTAC technology has been beneficial in combating medication resistance. Recently, a C4 therapeutics company announced its EGFR degrading agent CFT8919, making the fourth-generation inhibitor currently under development inferior. CFT8919 exhibits strong degradation activity to a variety of EGFR mutants, including the C797S drug-resistant mutation that is currently unavailable in clinics, and can avoid the degradation of the EGFR wild-type. The transplanted tumor model in vivo further verified that the degradation agent exhibited good tumor inhibitory activity and had little effect on body weight; indicating its safety.22 Although the specific structure of CFT8919 has not been disclosed, through the analysis of relevant patents we can determine that the connected ligand binding to EGFR is based on allosteric inhibitors and the connected ligand of E3 ligase is based on CRBN. 3) Recycling mode.23 After one round of degradation, PROTAC will be released, which enables the PROTAC to be reused. Therefore, theoretically, the dosage of PROTAC can be efficiently reduced while achieving the equivalent therapeutic outcomes. 4) Tissue-and/or tumor-specific E3 ligase ligands can be used to achieve tumor selectivity.24 Selective degradation of tumor-associated proteins of interest (POIs) can be achieved using PROTACs through various strategies. When the POI is specific to the tumor, tumor-specific PROTACs can be generated by directing the POI towards any available E3 ligase present in the tumor tissue. For instance, Crews et al utilized von-Hippel Lindau (VHL) ligands to create PROTACs that target the oncogenic fusion protein BCR-ABL1 in chronic myeloid leukemia (CML) cells, which are exclusively expressed in CML cells.25 Additionally, tumor-selective PROTACs can be generated by directing the POI towards any available E3 ligase in tumor-derived tissue, even if the POIs are not specific to the tumor but functionally unnecessary in normal tissue. Several PROTACs have been developed to target BTK, a protein crucial for normal B cell development and B cell lymphoma progression, showing promise in treating ibrutinib-resistant non-Hodgkin lymphoma.26 In cases where the POI is not specific to a particular tissue or tumor, targeting tissue- or tumor-specific E3 ligases can generate tumor-selective PROTACs, thereby reducing the toxicity of the POI. An example of this strategy is DT2216, a BCL-XL PROTAC that directs BCL-XL toward the VHL E3 ligase for degradation.27 Platelets rely on BCL-XL for survival, and the use of dual BCL-XL/BCL-2 inhibitors like ABT263 or selective BCL-XL inhibitors (A1155463 or A1331852) can lead to severe platelet toxicity, limiting their potential as safer anticancer therapies. Researchers have employed PROTAC technology to selectively target anti-apoptotic BCL-XL proteins in tumor cells by utilizing the least expressed VHL or CRBN E3 ligases in platelets, thereby reducing platelet toxicity.
Despite these distinct advantages, the development of PROTACs is still limited in terms of clinical translation. 1) Off-target effect.28–30 A lack of tissue-targeting ability when PROTACs are administrated orally or intravenously may produce off-target effects on extra organs, causing adverse effects. 2) Cell membrane permeability.31 PROTACs need cell membrane penetration to be effective, as reflected by an appropriate hydrophile-lipophile balance in the components. Moreover, a linker of appropriate length is required, which does not bind to two target proteins due to too the close distance or ubiquitinate target proteins due to the far distance. Therefore, constant optimization of the chemical structure of PROTACs has been performed, which is time-consuming and causes a high workload and economic burden. 3) Hook effect.32 Even though the obtained PROTACs can pass through cell membranes, the amount of target proteins and PROTACs are still not equivalent. This may be attributable to the inappropriate concentration and affinity between supply and demand. Thus, the development of PROTACs is still a long way from completion. Additional methods should be initiated to improve therapeutic response and advance clinical translations of PROTACs.
Discovery of Nano-PROTACs
The emergence of nanotechnology forms the foundation for the clinical translation of PROTACs due to its intrinsic physicochemical properties. Nanotechnology commonly refers to nanoscale structures up to 200 nm in size. Nanoplatforms designed for drug delivery include liposomes,33–37 solid nanoparticles,38–40 nanoparticles,41–43 polymeric micelles,44,45 nanogels,46–48 and extracellular vesicles.49–51 Agents can be loaded into the core or on the surface of nanosystems via chemical conjugation, physical encapsulation, or electrostatic adsorption. This nanosized process will endow drugs with an extended blood circulation time,52 enhanced bioavailability,53 improved therapeutic response,54 and reduced side effects.55 Over the last few decades, the advantages of nano drug delivery systems (NDDS) have been powerfully explored for a variety of treatments. To date, over 30 nanosystems types have entered clinical trials.56 A randomized study concerning liposomal vitamin C in healthy, adult, human subjects revealed that, compared with non-liposomal encapsulated vitamin C,57 liposomal preparations represented enhanced bioavailability accompanied by uniform particle size. In parallel, a study on amikacin liposome inhalation suspension demonstrated that this type of nanoscale particle exerted a potential benefit in patients with Mycobacterium avium complex lung disease.58–60 Nanotechnology offers great advantages for tumor treatments. Pegylated liposomal doxorubicin, at a tolerated dose of 40 mg/m2 every four weeks without serious adverse effects, is effective as a first-line treatment for platinum-resistant or -refractory recurrent ovarian cancer, according to a meta-analysis.61 Patients who had severe tumors in solid tissue had another liposomal mimic of microRNA-34a (miR-34a) identified and assessed.62 Findings showed modest therapeutic effectiveness and a reasonable toxicity profile in the majority of patients, demonstrating the viability of miRNA-based cancer therapy.
In general, nanodevices manifested three key advantages that may be beneficial for PROTAC delivery.
- Improvement in drug concentrations at a lesion. It was reported fenestrations occurred in the tumor vascular wall which were not closely arranged and permeable. Further, the internal lymphatic drainage of the tumor was insufficient. When nanoparticles penetrate the tumor’s interstitium, they stay there. This phenomenon refers to the enhanced permeability and retention (EPR) effect. Moreover, the possibility of nanoparticles entering the tumor site can be further improved by modification (ligands,63 oligonucleotides,64 antibodies, and cell membranes) on the surface of nanoparticles. Thus, the application of nanotechnology may contribute to improved drug concentration in tumor tissues.
- High specific surface area and multivalent binding possibility. There are many modification sites on the nanosized surface of NDDS which may realize multivalent binding and increase the probability of binding target proteins.
- Combination therapy in one location. Nano-architecture materials provided sufficient space to carry a variety of drugs to achieve synergistic therapy in one system.
- Spatiotemporally-controlled drug release. To avoid off-target effects, PROTACs need to be released in the specific target cells to ensure proper function. With appropriate design, some NDDSs could function in response to temperature/light/magnetic fields locally, contributing to precisely controlled drug release based on requirements.
In this review, we introduce the current applications of nanotechnology for PROTACs, including nano-sized PROTACs for single or combination therapy for solid tumors, and NDDS designed for delivering PROTACs to tumor tissues (Table 1). Finally, we provide a future outlook on PROTAC-based nanoplatforms for tumor therapy.
 |
Table 1 Application of Nanotechnology for Proteolysis-Targeting Chimeras |
Nanotechnology for PROTAC
Delivering Existing PROTACs
Single Tumor Treatment
Murine double minute X (MDMX) can induce p53 binding to non-specific chromatin regions while preventing p53 binding to specific target promoters, hence severely inhibiting the tumor suppressor gene p53. Therefore, lowering the amount of MDMX in cancer cells may improve the result of p53-mediated anti-tumor activity.75 Thus, a novel concept for MDMX inhibition will be offered by peptide-derived protein degradation. Based on this, Yan et al66 created a peptide-based PROTAC for MDMX degradation that includes the target recruitment component in the Cul2-Rbx1-EloB/C-VHL E3 ubiquitin ligase complex, the MDMX binding motif, and a flexible tripolymer glycol linker (Figure 2). Due to the tumor expansion, insufficient cell permeability, and proteolytic resistance, a macromolecule called polyacryl sulfydryl imidazole (PSI) and nanoengineered gold were introduced, both of which have pH-triggered charge reversal. As anticipated, Nano-MP@PSI was able to considerably increase tumor-specific accumulation and cellular absorption by shifting the Zeta potential from 28 mV at pH 7.4 to 50 mV at pH 6.0. Furthermore, the increased glutathione content in cancer cells would cause Au(I)-alkanethiol links to break, allowing for the regulated release of MDMX-based PROTACs. As a result, the MDMX expression was significantly down-regulated in Nano-MP@PSI-treated WERI-Rb-1 cells compared with Nano-MMP@PSI, Nano-PEG@PSI, or Nano-DPMI@PSI, leading to stabilized p53, p73, and p21 (Figure 3). Accordingly, Nano-MP@PSI displayed a remarkable therapeutic response both in retinoblastoma-bearing mice and a patient-derived xenograft model, offering the possibility for a more precise delivery of PROTACs.
 |
Figure 2 The MDMX degrader (MG) developed from peptides and its nanoscale approach. Notes: Reprinted from Yan S, Yan J, Liu D, et al. A nano-predator of pathological MDMX construct by clearable supramolecular gold(I)-thiol-peptide complexes achieves safe and potent anti-tumor activity. Theranostics. 2021;11(14):6833–6846.66 Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). |
 |
Figure 3 In WERI-Rb-1 cells, nano-MP@PSI down-regulated MDMX expression and then stabilized the expressions of p53, p73, and p21. Notes: (A) Western blotting revealed the MDMX, p53, p73, and p21 protein expressions. Various preparations were added to cells and incubated for 6 hours at a dose of 100 nM. (B) The quantitative analysis of the Western blot (inserted photos) shows the dose-dependent degradation of MDMX. (C) Hierarchical clustering of genes differentially expressed in cells during a 12-hour incubation period at a dose of 1 µM with Nano-MP@PSI and no treatment. The p53 signaling pathway includes gene set enrichment analysis (GSEA, D) and hierarchical gene clustering (E). GSEA demonstrates the differential expression of the p53 (F) and p73 (G) pathways in response to Nano-MP@PSI. KEGG, Kyoto Encyclopedia of Genes and Genomes; PID, Pathway Interaction Database; NES, normalized enrichment score. Reprinted from Yan S, Yan J, Liu D, et al. A nano-predator of pathological MDMX construct by clearable supramolecular gold(I)-thiol-peptide complexes achieves safe and potent anti-tumor activity. Theranostics. 2021;11(14):6833–6846.66 Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/). |
A frequent mutation in Kirsten RAS (KRAS) is detected in 95% of cases of pancreatic cancer (PC). However, because the KRAS has a roughly spherical form and no appropriate catalytic pockets, directly addressing it remains an extremely difficult process. The first-in-class PROTAC-induced PDEδ degrader (PIPD), created by Fan et al,72 was able to effectively induce PDEδ degradation and show markedly enhanced anti-tumor activity against malignancies with KRAS mutations. However, the penetration efficiency at the PROTAC target cell membrane is poor, and untargeted delivery induces undesired “off-target” side effects. In order to solve this challenge, scientists were able to remove the PATU-8988 cells’ cytomembrane, wrap PIPD molecules with ultrasonography, and create an intelligent nano-drug delivery system (CM8988-PIPD) for the targeted distribution of PIPD. Excellent serum stability, a perfectly regulated drug release profile, immunocompatibility, and biocompatibility were all present in CM8988-PIPD. Cell membrane coverage could effectively promote the endocytosis biomimetic nanosized CM8988-PIPD by homologous targeting. CM8988-PIPD had the highest apoptosis rate in all groups (53.7% in PATU-8988 cells and 54.3% in PL-45 cells). Additionally, it can downregulate p-AKT and p-Erk, effectively promote PDEδ breakdown in both concentration and time dependency, and decrease proliferation by blocking RAS signaling.
Glutathione peroxidase 4 (GPX4) is one kind of selenoprotein that could specifically catalyze lipid peroxides into lipoids, playing an important role in regulating ferroptosis. We hypothesize that inhibiting GPX4 overexpression or degradation may impair lipid peroxide elimination, which might result in cell ferroptosis.76 To confirm this possibility, Luo et al74 created a PROTAC degrader that targets GPX4, and dGPX4. They achieved this by covalently coupling pomalidomide, which attracts cereblon (CRBN) E3 ubiquitin ligase, with ML162, an inhibitor of GPX4. The enhancement of intracellular ROS levels and lipid peroxidation after dGPX 4 treatment suggested that dGPX4 degradation induces ferroptosis in cancer cells. Furthermore, dGPX4 treatment caused ferroptosis in cancer cells and showed generality in various cancer cells (e g A375 melanoma cells, HeLa cervical cancer cells, 4T1 breast cancer cells, B16-F10 murine melanoma cells and SH-SY5Y human neuroblastoma cells). Because of its low biostability and poor water solubility, PROTACs limited its clinical transformation. Reactive oxygen species (ROS)-degradable lipid 401-TK-12 is used to encapsulate dGPX4 in lipid nanoparticles to get around these restrictions. Lipid nanoparticle-based dGPX4 formulation has the potential to inhibit tumor development while simultaneously improving GPX4 breakdown in tumors, all without causing noticeable adverse effects. The selective release of dGPX4 into cancer cells for the breakdown of GPX4 was caused by the upregulated intracellular ROS level, which also prompted the degradation of 401-TK-12. After 12 hours of treatment, more than 60% of the dGPX4 was thus released from the dGPX4@401-TK-12 nanoparticles, with the least amount of dGPX4 released under the same incubation conditions without H2O2.
The epigenetic reader bromodomain-containing protein 4 (BRD4) is a member of the BET bromodomain family, with two conserved bromodomains and the extra-terminal (ET) domain. Since BRD4 is often shown to be overexpressed in lung tumors, targeting BRD4 has gained popularity as a treatment strategy for the disease. To accurately destroy BRD4, Zhang et al68 created a unique type of adaptable nano-PROTAC called CREATE. The pH/GSH-responsive polymer (DS-PLGA) loaded BRD4-targeted PROTAC (dBET6) was used to form the CREATE (CRV-LLC membrane /DS-PLGA/dBET6), which was subsequently concealed with CRV-engineered Lewis lung carcinoma (LLC) cell membranes (CRV-LLCM). The rapid breakdown of CREATE caused by the combination of low pH and high GSH concentration enhanced the therapeutic efficacy of dBET6. In contrast to the mixture containing pH-responsive but GSH-insensitive components, CREATE resulted in a notably elevated rate of apoptosis in both LLC cells and M2-like macrophages. The first TAMs-lung cancer cell spheroids (M2/LLC spheroids) were created by co-cultivating LLC cells with M2-like macrophages to assess the tumor suppressive impact in a three-dimensional tumor spheroid test. As anticipated, in comparison to DS‐PLGA/DiD or LLCM/DS‐PLGA/DiD, CRV‐LLCM/DS‐PLGA/DiD caused a much larger infiltration in the 3D M2/LLC spheroids and resulted in considerable cell death. CREATE demonstrates the capacity to target lung cancer cells and TAMs simultaneously with the use of CRV-overexpressing LLCM for camouflage. These results offer a new approach to lung cancer recurrence.
Combination Tumor Therapy
Currently, PROTAC-based immunotherapy predicts responses to a variety of malignant tumors,70 providing a new framework for long-lasting tumor responses and augmented tumor therapeutic effects. Nevertheless, due to the inability to target particular tissues and the ensuing off-target impact, there are still a lot of obstacles and restrictions concerning low patient response rates and immune-related side effects. To enable enhanced response to immunotherapy, combination therapy can be introduced, for example between chemotherapy,77–79 photodynamic therapy,80 photothermal therapy,81 and immunotherapy.82 However, PROTACs-based off-target problems induced by nonspecific distribution remain an obstacle. To overcome this, Zhang et al29 synthesized a semiconducting polymer nano-PROTAC (SPNpro) for photo-switchable combination therapy of immunotherapy and photodynamic therapy (Figure 4). The obtained nanodevices were composed of a semiconducting polymer backbone, a cathepsin B-cleavable peptide, and an indoleamine 2,3-dioxygenase (IDO)-targeting PROTAC. After intravenous injection, SPNpro was passively distributed into the lesion via the EPR effect. The overexpressed cathepsin B in the tumor microenvironment activated SPNpro by breaking down the cathepsin B-cleavable peptide, which facilitated the release of IDO-targeting PROTAC. PROTACs recruited E3 ubiquitin ligase and IDO, respectively, contributing to an 89.3% decreased expression of IDO, therefore altering tumor metabolism and ameliorating the tumor immunosuppressive microenvironment. The cathepsin B-switchable behavior of SPNpro rendered the local activation of PROTACs, avoiding serious off-target effects. Meanwhile, NIR irradiation activated SPNpro to generate a large quantity of 1O2, exerting photodynamic therapy, followed by the release of tumor-associated antigens and immunogenic cell death. As the microenvironment was reprogrammed, more cytotoxic T lymphocytes could filtrate into the tumor tissue and boost immunotherapy, realizing effective tumor growth inhibition both in primary and distant tumors without any signs of adverse effects.
 |
Figure 4 The antitumor mechanism of the semiconducting polymer nano-PROTAC (SPNpro). Notes: (a) The structure and activation mechanism of SPNpro that is unique to cathepsin B (CatB). (b) Activatable photo-immunometabolic treatment mediated by SPNpro using two mechanisms. Reprinted from Zhang C, Zeng Z, Cui D, et al. Semiconducting polymer nano-PROTACs for activatable photo-immunometabolic cancer therapy. Nat Commun. 2021;12(1):2934.29 Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). |
Based on the same method, Zhang et al70 synthesized another semiconducting polymer, onto which COX-1/2-targeting PROTACs were connected using a Cathepsin B-cleavable peptide. This resulted in the development of smart nano-PROTACs (SPNCOX), which are intended for photo-metabolic cancer immunotherapy. The diameter of this prepared nanosystem was measured at 30 nm. Under laser irradiation, SPNCOX indicated the potential to generate a large quantity of 1O2 in a time-dependent manner. After incubation with Cathepsin B, the FK peptide sequence containing SPNCOX was cleaved, resulting in the rapid release of COX-1/2-targeting PROTACs followed by persistent degradation on COX-1/2. As a result, SPNCOX demonstrated an accelerated ability to remodel the immunosuppressive microenvironment and reinvigorate immunotherapy, thereby causing significant inhibition of tumor growths without originating tumor recurrence (Figure 5). This combination therapy of PROTACs-mediated immunotherapy and photodynamic therapy overcomes the off-target obstacles and expands our understanding of antitumor synergistic therapy.
 |
Figure 5 In vivo anti-tumor and anti-metastatic effect of SPNCOX. Notes: (a) Schedule for 4T1 tumor implantation and treatment. (b) Biodistribution of various preparations in 4T1 tumor-bearing mice at various intervals following the intravenous injection. (c) A quantitative examination of Figure 4b’s principal organs. (d) Confocal pictures of tumors treated with various preparations, either with or without 6 minutes of NIR photoirradiation (0.3 W cm−2 at 808 nm). Reactive oxygen species concentration was detected by the green fluorescent signal produced by the SOSG probe. (e) Tumor volume in 5 4T1 tumor-bearing mice following various treatments throughout 14 days of observation. (f) Lung tissues in 4T1 tumor-bearing mice stained with hematoxylin and eosin following various treatments. The metastatic nodes were shown with black arrows. (g) Quantitative evaluation of caspase-3 expression in tumors following various therapeutic approaches (n = 5). *** p<0.001 and **** p<0.0001. Reprinted with permission from Zhang C, He S, Zeng Z, Cheng P, Pu K. Smart Nano-PROTACs Reprogram Tumor Microenvironment for Activatable Photo-metabolic Cancer Immunotherapy. Angew Chem Int Ed Engl. 2022;61(8):e202114957.70 Copyright 2022 Wiley, under the license number 5770710257931. |
As a new type of antitumor therapy, immune checkpoint blockade is a milestone innovation in the history of malignancy therapy. Despite these advances, it also has some problems such as inefficiency and off-target adverse effects. It is very necessary to develop an immunological checkpoint PROTAC to support the immune response against tumors. In light of this, Zhang et al69 described a checkpoint PROTAC method that enhances the anticancer immune response by focusing on the Src homology 2 domain-containing phosphatase 2 (SHP2). This technique works in concert with immunogenic phototherapy. Photosensitizer (protoporphyrin IX, PpIX), caspase 3 lysable peptide substrate (DEVD), UBR E3 ligase targeting fragment (RLAA), and the SHP2-targeted peptide (SP, SLNpYIDLDLVK) were all part of the checkpoint nano-PROTAC (NPRO). Caspases 3 and associated with apoptosis during photodynamic therapy-induced 4T1 cell death may activate NPRO and then be transported to T cells or macrophages to cause SHP2 degradation. Because of NPRO-mediated therapy’s phototherapeutic potential and strong T-cell activation through SHP2 degradation, tumor immunogenicity was increased. The immune-suppressive checkpoint signaling pathways, including PD-1/PD-L1 and CD47/SIRPα (“Don’t eat me”), were suppressed by the degradation of SHP2. Following this, effector T cells (Teffs) mediated antitumor immunity and M1-type macrophages (M1Macs) mediated phagocytosis effect were triggered. Therefore, NPRO-mediated therapy’s superior biosafety and biocompatibility might efficiently reverse immunosuppression to activate checkpoint signaling and strengthen immune responses against tumors.
It is well recognized that a variety of tumors and malignancies are encouraged to form, progress, and spread when the polycomb ring finger oncogene BMI1 is present. Additionally, it has been demonstrated that an unusually high BMI1 suppresses the innate immune response of tumor cells and increases resistance, especially to immunotherapy. Consequently, Wu et al65 devised a plan to influence TME immunosuppression and concurrently lower BMI1 to enhance the clinical prognosis of patients with head and neck squamous cell cancer (HNSCC). Two types of nanoparticles were grafted onto an injectable thermosensitive nanocomposite hydrogel that they designed. The first type was coated mesoporous silica nanoparticles (MSNs) with the cancer cell membrane (CCM) and paclitaxel (Pac) in their pores, connected to a peptide-based BMI1 PROTAC drug via disulfide bonds (PepM@PacC); the second type were R837-loaded CaCO3(“RC”) nanoparticles. In short, thermosensitive nanocomposite hydrogel injected intratumorally was able to gel in situ but eventually broke down in TME. Following the release of PepM@PacC from the deteriorating hydrogel, cleavage of the BMI1-targeting PROTAC from the MSNs and the release of paclitaxel are facilitated by the CCM-mediated homologous targeting of cancer cells and GSH-dependent drug release. Then, in the acidic TME, the RC nanoparticles quickly dissolved and released the pre-loaded R837, which encouraged DC maturation and increased the T cell-mediated immune response even more. The PP-RC-H treatment dramatically increased the therapeutic efficacy of the tumor by reducing the tumor cells’ ability to evade the immune system and inducing BMI1 degradation in addition to chemotherapy.
ARV-825 (ARV) is a form of BRD4-targeting PROTACs that holds promising effects for the treatment of vemurafenib-resistant melanoma. However, its therapeutic effect required improvements in hindering the dense extracellular matrix in tumor tissue and extremely poor aqueous solubility. Liposomes are large unilamellar vesicles consisting of phospholipids and cholesterol, which were confirmed to be available for co-delivery of both hydrophilic and hydrophobic drugs, long-circulating time83 and enhanced therapeutic outcomes.84 Considering the potential of liposomes, Fu et al71 loaded BRD4-targeting PROTACs (ARV) and an anti-fibrotic agent (nintedanib) into the lipid bilayer of PEGylated liposomes for combination therapy (Figure 6). Critic acid was added during the drug loading procedure to increase the entrapment efficacy from 21.67% to over 90%. The particle size of the nano-liposome (ARNIPIL) was evaluated at 111.1 nm with a uniform size distribution. Moreover, the preparations remain stable in one month storage. An in vitro release investigation showed that ARNIPIL released the medication continuously, without experiencing any burst release. Consequently, ARNIPL significantly altered the number of colonies, vasculogenic mimicry and BRD4 expression when compared to ARV or nintedanib alone. In three-dimensional (3D) multicellular tumor spheroids, ARNIPL reduced 71.43% of tumor volume on day six. In contrast, the reduction of tumor volume with an individual drug was less than 58%. The augmented efficacy may be attributable to the enhanced penetration of liposomes. Hence, liposome-based co-delivery nanovesicles present a promising combination approach for vemurafenib-resistant melanoma therapy.
 |
Figure 6 The preparation route and anti-tumor response of BRD4-targeting PROTACs (ARV) and an anti-fibrotic agent (nintedanib) co-loaded liposome. Notes: Reprinted from Fu Y, Saraswat A, Wei Z, et al. Development of Dual ARV-825 and Nintedanib-Loaded PEGylated Nano-Liposomes for Synergistic Efficacy in Vemurafenib-Resistant Melanoma. Pharmaceutics. 2021;13(7):1005.71 Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/). |
Monoclinic 1t-phase TaTe2 has remarkably paramagnetic, charge transfer, and biocompatible properties. TaTe2 can exhibit photothermal and photodynamic qualities due to the surface’s charge transfer characteristics. This suggests that TaTe2 might be used as a nanotherapeutic. A nanotherapy platform (T-ARV) based on TaTe2 nanosheets loaded with ARV-825 was created by Liu et al.73 T-ARV outperformed the conventional TMDs in terms of photostability, exhibiting a photothermal conversion of up to 62.3% and high absorption in the near-infrared spectrum. For ten minutes at 62°C, T-ARV dispersions were exposed to an NIR laser, producing singlet oxygen and superoxide anions. ARV-825 bound to a nearby E3 ligase upon coming into contact with BRD4 and T-ARVs, which started the process of BRD4 ubiquitination and proteasomal destruction. TaTe2 nanosheets’ large surface area allowed ARV-825 to attach to target proteins with efficiency, which in turn aided the proteasomal breakdown process. For a brief time, T-ARV briefly and at low concentrations broke down BRD4 and the downstream protein C-MYC. Under near-infrared light irradiation, T-ARV efficiently sensitized the photothermal and photodynamic properties of tumor tissue. Additionally, T-ARV was developed as a contrast agent for photothermal optical coherence tomography imaging (PT-OCT) used in tumor imaging. It can direct oral squamous cell carcinoma multimodal therapy.
Nano-Sized PROTAC (Nanocarriers as the Linker)
Lymphoma kinase receptor anaplastic lymphoma kinase (ALK) contributes to the growth and endurance of lung cancer epithelial cells. Thus, degradation of ALK appears to be crucial for enhanced anti-tumor effects. Several ALK-targeting PROTACs have been well explored,72,73 showing notable advantages in specificity and efficacy. However, challenges remain in clinical applications. The chemical structure of PROTACs needs to be optimized to meet the requirements of cell penetrability and appropriate binding space. Even though optimization is finished, the prepared PROTACs still lack tissue targeting ability, which is an empirically important cause of off-target effects. Inspired by two-headed PROTACs, gold nanoparticles (GNPs) with large surface areas and versatile functionalization were excavated for the construction of multi-headed PROTAC nanoplatforms. ALK binding moiety (ceritinib) and E3 ligase ligand (pomalidomide) were grafted onto spherical GNPs (30 nm) coated with PEG2000 as a linker by Wang et al,67 creating a nanoscale PROTAC (Cer/Pom-PEG@GNP) (Figure 7). Cer/Pom-PEG@GNP never lost its stability in the presence of serum, tumor tissue homogenate, or cell culture media, however, the particle size was slightly altered. After cellular internalization, it co-localized in the cytoplasm, providing the possibility for targeting ALK proteins. Once incubated for 12 h, 76.21% of ALK fusion proteins were degraded. As ALK protein is involved in cell proliferation, a cell viability assay was conducted. Accordingly, the Cer/Pom-PEG@GNP nanoplatforms decreased the percentage of living cells with an IC50 of 4.8 μM, implying the potential anti-tumor response. This nanodevice presents a novel strategy to design a nano-PROTAC using nanocarriers as the linker, thereby creating a multivalent combination with target proteins and more efficient degradation.
 |
Figure 7 The schematic illustration of the nanosized PROTAC (Cer/Pom-PEG@GNP). Notes: (A) The preparation process and anti-tumor mechanism of Cer/Pom-PEG@GNP. (B) The EML4-ALK expression was measured by Western blot under different conditions. Reprinted from Colloids and Surfaces B: Biointerfaces, volume 188, Wang Y, Han L, Liu F, et al. Targeted degradation of anaplastic lymphoma kinase by gold nanoparticle-based multi-headed proteolysis targeting chimeras. 110795, with permission from Elsevier.67 |
Discussion
Despite significant achievements and advancements made using nanotechnology, nano-PROTACs still require further design. Some principles of nano-sized PROTACs or NDDS designed for PROTACs are summarized as follows:
For Delivering Existing PROTACs
Improvement in Tumor Distribution and Cellular Uptake
The particle size, morphology, and Zeta potential of nanoparticles determined their biological distribution in different organs, including the lung, liver, spleen, and kidney. Therefore, these parameters should be managed to obtain ideal NDDS delivering existing PROTACs, thereby improving tumor accumulation and cellular uptake.
Nanoparticles with a diameter larger than 2000 nm were essential to accumulate in the capillaries of the spleen, liver, and lung. Particle sizes in the range of 100–200 nm can escape the filtration of the liver and spleen and potentially accumulate in the tumor site through the EPR effect. As the size increased to more than 200 nm, more and more nanoparticles were distributed in the liver and spleen. In contrast, small-sized nanoparticles (<5 nm) were filtered out by the kidney.
Nanoparticles with different shapes, including spherical, rod, cylindrical, and disk, can be prepared by adjusting the synthesis conditions. Nanoparticles with different shapes have varying flow characteristics, cycle life, cell membrane interaction, and macrophage uptake, which in turn affect their pharmacokinetics and biodistribution among different organs.85–87 Of all the particles under investigation, spherical forms with smaller surface areas often showed the greatest tumor-to-blood ratios and accumulation.88 Furthermore, the in vivo dispersion of nanoparticles with varied nanostructures varies for different cells. For breast cancer cells, rods offer the best specificity and absorption efficiency.89 In contrast, spheres performed the highest uptake in macrophages.90
Nanoparticles with different surface charges affect the opsonization, cycle time, and interaction with resident macrophages contained in reticuloendothelial systems.91 Macrophages in the lung, liver, and spleen are more prone to seize positively charged particles. Neutral and slightly negatively charged nanoparticles have reduced accumulation in the reticuloendothelial system, thus, prolonging the cycle life.
Furthermore, proper surface modification of nanoparticles with targeting ligands,92,93 antibodies,94 or oligonucleotides95 can potentially improve tissue accumulation. It is worth noting that membranes derived from target cells can be coated on the surface of biomaterials, providing further improvements in delivery efficiency. Similarly, extracellular vesicles were also regarded as nano-swimmers for efficient drug delivery, displaying precise tissue traffic and a homing effect.
Tumor Microenvironment-Responsive Drug Release
To avoid off-target effects, tissue-specific PROTAC release should be considered. Tumor microenvironment-responsive NDDS can serve as intelligent nanoplatforms for controlled drug release by endogenous or exogenous stimuli. Endogenous factors contain differences in tumor microenvironments from healthy tissue, including pH, enzymes, glutathione, reactive oxygen hypoxia,96 adenosine-triphosphate,97 and inflammatory factors.98 PROTACs can be conjugated onto the surface of nanoplatforms with tumor microenvironment-cleavable linkers. Once they enter into tumor tissues, PROTACs-based nanodevices can be triggered to release and realize tissue-specific effects.
Unlike endogenous stimuli-responsive nanoplatforms, exogenous stimuli-triggered NDDS have the potential benefit of overcoming individual pathological differences. These technologies allow for the exact regulation of medication release with the application of external variables locally.99 Exogenous agents refer to photo/light,100 magnetic field,101 ultrasound,102 microwave,103 mechanical forces,104 and electric field.105
Various wavelengths of light, including ultraviolet, near-infrared, and visible light, have been reported and discussed. Due to their limited capacity to permeate tissues, visible and ultraviolet light are not appropriate for use in in vivo treatments. On the other hand, near-infrared spectroscopy is thought to be the best light source for controlled medication release. Near-infrared light can transform photons into heat through photothermal substances, producing a photothermal effect and splitting temperature-sensitive linkers to spatiotemporally control drug release.106 It can also convert light into reactive oxygen species through photosensitizers to produce photodynamic effects, thereby breaking down the reactive oxygen species sensitive bonds and performing spatiotemporally controlled drug release. Furthermore, it can upconvert nanoparticles from near-infrared light to ultraviolet or visible light, therefore releasing medicines from the backbone of UV-triggered drug release from near-infrared light.-responsive, highly monodispersity diblock copolymer-coated up-conversion nanoparticles.107
This is convenient to externally applied magnetic fields because magnetic field-activated materials are often used as drug release platforms.108,109 The mechanism of controlled release mainly focuses on the abundant energy generated by the application of magnetic fields locally, which increases local temperature and breaks down the temperature-sensitive bonds.110,111
Similarly, ultrasound, microwave, and electric fields primarily induce local high temperatures to break the temperature-sensitive bond to produce a controlled release of drugs and reduce toxic and side effects.112,113 In addition, ultrasound can also act on ultrasound contrast agents to create openings on the surface of NDDS to release drugs.114 Electrochemical reactions can also alter the local pH and break the pH-sensitive bond of nanoparticles to control drug release.
Combination Therapy
Extremely aggressive tumors require combination therapy to kill them via multiple pathways. AS NDDS possess large cores with a specific surface area, they can interact with various drugs to deliver multiple drugs and realize combination therapy. Furthermore, some nanocarriers have intrinsic functions, such as photothermal conversion,115 reactive oxygen-generating ability,116 and neutralizing acid microenvironments,117,118 thus, achieving synergistic effects.
Nano-Sized PROTACs
Nanocarriers can also be used as a part of PROTACs to become nano-sized PROTACs. Generally, nanocarriers are regarded as the platform to load the E3 ubiquitin ligase ligand and target protein ligand. It should be noted that the particle size of nanocarriers applied should be controlled to fulfill the formation of the ternary complex. If the size is too large, it may affect the binding efficiency and subsequent ubiquitination of the target protein and E3 enzyme due to insufficient space. Similarly, if the size is too small, it may be rapidly eliminated in the tumor site, reducing the accumulation efficiency.
Concurrently, most nanoparticles are internalized into cells via clathrin-mediated endocytosis, resulting in degradation in lysosomes rather than perforation in cytoplasm. Hence, if internalized, nanoparticles should escape from degradative endo-lysosomal compartments. Cationic nanocarriers were reported to possess the ability to escape from lysosomes. However, a positive charge that is too strong may easily form a “protein crown” during circulation at physiologic conditions, which severely affects the targeting ability. This implies that the net charge and surface charge of nanocarriers indicated a dominant position in the design of nano-sized PROTACs.
Perspect in Clinical Translation
Since its inception, PROTAC technology has garnered significant attention due to its potential to overcome the limitations associated with current cancer treatments by facilitating the degradation of target proteins.
In terms of clinical applications, Arvinas stands as the pioneering biotechnology company exclusively dedicated to the development of PROTACs.119 Following the announcement of Phase 1 clinical trials for two PROTAC candidate drugs in 2019, numerous pharmaceutical companies have invested in the advancement of PROTACs as therapeutic agents. Arvinas LLC has successfully developed two PROTAC probes, namely ARV-110 and ARV-471, which specifically target the androgen receptor (AR) and ERα for the treatment of metastatic castration-resistant prostate cancer and metastatic ER+/HER2− breast cancer, respectively.120,121 Numerous protein degraders, targeting a wide range of solid tumors and hematologic malignancies, have been evaluated in Phase I–III clinical trials.122,123
The structural elucidation of eight compounds has been completed, with seven derivatives modeled on various CRBN E3 ligase ligands the eighth being VHL PROTAC DT-2216.124,125 In clinical evaluations, each of the seven CRBN PROTACs was administered orally, and oral bioavailability in murine models was reported for four, all exceeding 30%. In stark contrast, DT-2216 exhibited markedly low bioavailability (<0.03% in mice) and required intravenous infusion. This discrepancy in bioavailability corresponds well with predictions based on Lipinski’s Rule of 5126 and Veber’s rules.127 Generally, PROTACs are characterized by high efficacy and a catalytic mechanism of action, potentially allowing effective oral administration at low oral bioavailability or lower dosages.128 However, this theory is contradicted by the actual dosing regimens of three CRBN PROTACs currently in Phase II or III trials, which involve substantial daily oral doses: ARV-766 is administered in Phase II trials at doses of 100 mg and 300 mg,129 ARV-110 in Phase II at a recommended dose of 420 mg,130 and ARV-471 in Phase III at a recommended dose of 200 mg.131 Notably, both ARV-110 and ARV-471 demonstrate bioavailabilities exceeding 30%, although data for ARV-766 are not yet available.
Despite the significant advantages associated with PROTAC technology, it is not without its challenges, including issues related to drug permeability, diminished bioavailability, and unintended off-target effects. Nonetheless, these technologies open expansive new avenues for biomedical research. In the realm of cancer therapy, nanomedicines have demonstrated considerable promise.132 Nano-PROTACs, in particular, offer distinct benefits over traditional PROTAC methodologies.133 The primary mechanism by which nanoparticles exert their effects involves augmenting intracellular permeability through pathways such as endocytosis and endosomal transport. Furthermore, leveraging the EPR effect or employing active targeting strategies to guide nanoparticles can significantly mitigate the exposure of non-target tissues to high concentrations of PROTACs. It is noteworthy that most nanomedicines, both in clinical and preclinical phases, exhibit reduced deleterious impacts on healthy tissues. Over the last decade, advances in nanomedicine have led to the development of several efficacious oral delivery systems that have improved the bioavailability of therapeutic agents, thus paving the way for the formulation of effective nano-PROTACs for cancer treatment.134 For instance, Eudragit®, a polymer coating specifically engineered to stabilize oral drug formulations, has been shown to bolster the stability of liposomal systems for oral administration.135 The functionalization of PLGA nanoparticles enables targeted interaction with intestinal transport proteins, enhancing drug delivery efficiency.136 Additionally, the incorporation of polycaprolactone in the production of polymeric nanoparticles has been found to improve the oral bioavailability of ellagic acid, a potent anticancer compound with otherwise limited gastrointestinal uptake.137 Furthermore, the clinical application of docetaxel encapsulated within the polymeric matrix BIND-014® has demonstrated profound therapeutic outcomes in treating advanced metastatic cancers and non-small cell lung cancer.138
An additional impediment in the realm of PROTAC design is the constrained availability of efficacious ligands for ubiquitin ligases. Although the human proteome encompasses over 600 E3 ligases,139 the repertoire of these enzymes that are actively employed within PROTAC frameworks is limited to fewer than ten. Among these, small molecule ligands targeting VHL represent some of the earliest examples employed in PROTAC development. Conversely, CRBN molecular glue has emerged as a preferred ligand in numerous PROTAC configurations, owing to its robust degradative efficacy across a variety of substrates, its diminutive molecular size, and its relative safety profile. As delineated previously, the emergence of nanotechnology presents an innovative approach to the deployment of E3 ligases.
In summation, the application of nanotechnology to enhance the delivery and the action mechanism of PROTACs offers a viable and promising avenue for advancement.
Conclusion
The PROTAC-based targeted degradation strategy has shown significant promise in cancer treatment and is positioned to be a groundbreaking therapeutic approach. Despite its revolutionary potential in achieving precise and effective degradation of the protein of interest (POI) within the drug discovery paradigm, PROTAC still faces limitations that hinder its clinical application. These limitations include off-target effects, poor cell membrane permeability, and the hook effect. The emergence of nanotechnology provides a promising opportunity to address these challenges associated with traditional PROTACs. Previous studies have discussed successful implementations of nanotechnology in this area.29,72,74 Notably, Zhang et al140 reported intracellular fabricated nano-PROTACs that employ self-assembling peptides as carriers for linking the POI and E3 ligase. Through the formation of polyvalent POI cellular nanofibrils, effective dose-dependent protein degradation is achieved in vivo, leading to the inhibition of tumor growth. This innovative approach successfully overcomes the off-target side effects and “hook effect” associated with traditional PROTACs.
The application of nanoplatforms for the effective delivery of PROTACs may yield improvements in biodistribution, membrane permeability, and controlled PROTAC release, thereby fundamentally enhancing the precise degradation of target proteins and enabling more effective treatment. Furthermore, the utilization of intelligent drug delivery systems can mitigate off-target toxicity risks by improving drug solubility, membrane permeability, and cell targeting. While current research efforts have primarily concentrated on evaluating the anti-tumor efficacy of nano-PROTACs, it is important to recognize that addressing potential safety risks through appropriate preclinical models is essential for the successful clinical translation of any promising new drug modality. Therefore, conducting a comprehensive safety assessment and thorough research on nano-PROTACs is crucial.
In Conclusion, the emerging nano-PROTAC drug modality holds great promise for tumor therapy and potentially other diseases. However, careful attention must be given to key safety risks and issues throughout the clinical translation.
Funding
This study was supported by the Introduction plan of high-end foreign experts (G2022016010L).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sakamoto KM, Kim KB, Kumagai A, Mercurio F, Crews CM, Deshaies RJ. Protacs: chimeric molecules that target proteins to the Skp1–Cullin–F box complex for ubiquitination and degradation. Proc Natl Acad Sci U S A. 2001;98(15):8554–8559. doi:10.1073/pnas.141230798
2. Abbas R, Larisch S. Killing by degradation: regulation of apoptosis by the ubiquitin-proteasome-system. Cells. 2021;10(12):3465. doi:10.3390/cells10123465
3. Su T, Yang M, Wang P, Zhao Y, Ma C. Interplay between the ubiquitin proteasome system and ubiquitin-mediated autophagy in plants. Cells. 2020;9(10):2219. doi:10.3390/cells9102219
4. Fhu CW, Ali A. Dysregulation of the ubiquitin proteasome system in human malignancies: a window for therapeutic intervention. Cancers. 2021;13(7):1513. doi:10.3390/cancers13071513
5. LaPlante G, Zhang W. Targeting the ubiquitin-proteasome system for cancer therapeutics by small-molecule inhibitors. Cancers. 2021;13(12):3079. doi:10.3390/cancers13123079
6. Si J, Shi X, Sun S, et al. Hematopoietic Progenitor Kinase1 (HPK1) mediates T cell dysfunction and is a druggable target for T cell-based immunotherapies. Cancer Cell. 2020;38(4):551–566.e11. doi:10.1016/j.ccell.2020.08.001
7. Rothweiler EM, Brennan PE, Huber KVM. Covalent fragment-based ligand screening approaches for identification of novel ubiquitin proteasome system modulators. Biol Chem. 2022;403(4):391–402. doi:10.1515/hsz-2021-0396
8. Schneider M, Radoux CJ, Hercules A, et al. The PROTACtable genome. Nat Rev Drug Discov. 2021;20(10):789–797. doi:10.1038/s41573-021-00245-x
9. Ding Y, Fei Y, Lu B. Emerging new concepts of degrader technologies. Trends Pharmacol Sci. 2020;41(7):464–474. doi:10.1016/j.tips.2020.04.005
10. Alabi SB, Crews CM. Major advances in targeted protein degradation: pROTACs, LYTACs, and MADTACs. J Biol Chem. 2021;296:100647. doi:10.1016/j.jbc.2021.100647
11. Békés M, Langley DR, Crews CM. PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov. 2022;21(3):181–200. doi:10.1038/s41573-021-00371-6
12. Guenette RG, Yang SW, Min J, Pei B, Potts PR. Target and tissue selectivity of PROTAC degraders. Chem Soc Rev. 2022;51(14):5740–5756. doi:10.1039/d2cs00200k
13. Zeng S, Huang W, Zheng X, et al. Proteolysis targeting chimera (PROTAC) in drug discovery paradigm: recent progress and future challenges. Eur J Med Chem. 2021;210:112981. doi:10.1016/j.ejmech.2020.112981
14. Burke MR, Smith AR, Zheng G. Overcoming cancer drug resistance utilizing PROTAC technology. Front Cell Dev Biol. 2022;10:872729. doi:10.3389/fcell.2022.872729
15. Giardina SF, Valdambrini E, Warren JD, Barany F. PROTACs: promising approaches for epigenetic strategies to overcome drug resistance. Curr Cancer Drug Targets. 2021;21(4):306–325. doi:10.2174/1568009621666210203110857
16. Ma D, Zou Y, Chu Y, et al. A cell-permeable peptide-based PROTAC against the oncoprotein CREPT proficiently inhibits pancreatic cancer. Theranostics. 2020;10(8):3708–3721. doi:10.7150/thno.41677
17. Wang K, Zhou H. Proteolysis targeting chimera (PROTAC) for epidermal growth factor receptor enhances anti-tumor immunity in non-small cell lung cancer. Drug Dev Res. 2021;82(3):422–429. doi:10.1002/ddr.21765
18. Fischer PD, Papadopoulos E, Dempersmier JM, et al. A biphenyl inhibitor of eIF4E targeting an internal binding site enables the design of cell-permeable PROTAC-degraders. Eur J Med Chem. 2021;219:113435. doi:10.1016/j.ejmech.2021.113435
19. Dhillon S. Lazertinib: first approval [published correction appears in Drugs. 2021 Jun 9;:]. Drugs. 2021;81(9):1107–1113. doi:10.1007/s40265-021-01533-x
20. Duggirala KB, Lee Y, Lee K. Chronicles of EGFR tyrosine kinase inhibitors: targeting EGFR C797S containing triple mutations. Biomol Ther. 2022;30(1):19–27. doi:10.4062/biomolther.2021.047
21. Dong RF, Zhu ML, Liu MM, et al. EGFR mutation mediates resistance to EGFR tyrosine kinase inhibitors in NSCLC: from molecular mechanisms to clinical research. Pharmacol Res. 2021;167:105583. doi:10.1016/j.phrs.2021.105583
22. Discovery of CFT8919 as an oral, CNS-active, mutant-selective allosteric degrader of EGFR L858R for the treatment of EGFR inhibitor resistant non-small cell lung cancer; 2021. Available from: https://ir.c4therapeutics.com/static-files/7fe6aac1-0797-4f1e-b576-60bdb86339a7.
23. Maneiro MA, Forte N, Shchepinova MM, et al. Antibody-PROTAC conjugates enable HER2-dependent targeted protein degradation of BRD4. ACS Chem Biol. 2020;15(6):1306–1312. doi:10.1021/acschembio.0c00285
24. Khan S, He Y, Zhang X, et al. PROteolysis TArgeting Chimeras (PROTACs) as emerging anticancer therapeutics. Oncogene. 2020;39(26):4909–4924. doi:10.1038/s41388-020-1336-y
25. Burslem GM, Schultz AR, Bondeson DP, et al. Targeting BCR-ABL1 in Chronic Myeloid Leukemia by PROTAC-Mediated Targeted Protein Degradation. Cancer Res. 2019;79(18):4744–4753. doi:10.1158/0008-5472.CAN-19-1236
26. Sun Y, Ding N, Song Y, et al. Degradation of Bruton’s tyrosine kinase mutants by PROTACs for potential treatment of ibrutinib-resistant non-Hodgkin lymphomas. Leukemia. 2019;33(8):2105–2110. doi:10.1038/s41375-019-0440-x
27. Khan S, Zhang X, Lv D, et al. A selective BCL-XL PROTAC degrader achieves safe and potent antitumor activity. Nat Med. 2019;25(12):1938–1947. doi:10.1038/s41591-019-0668-z
28. Liu X, Zhang Y, Ward LD, et al. A proteomic platform to identify off-target proteins associated with therapeutic modalities that induce protein degradation or gene silencing. Sci Rep. 2021;11(1):15856. doi:10.1038/s41598-021-95354-3
29. Zhang C, Zeng Z, Cui D, et al. Semiconducting polymer nano-PROTACs for activatable photo-immunometabolic cancer therapy. Nat Commun. 2021;12(1):2934. doi:10.1038/s41467-021-23194-w
30. Chen Y, Tandon I, Heelan W, Wang Y, Tang W, Hu Q. Proteolysis-targeting chimera (PROTAC) delivery system: advancing protein degraders towards clinical translation. Chem Soc Rev. 2022;51(13):5330–5350. doi:10.1039/d1cs00762a
31. He S, Gao F, Ma J, Ma H, Dong G, Sheng C. Aptamer-PROTAC Conjugates (APCs) for tumor-specific targeting in breast cancer. Angew Chem Int Ed Engl. 2021;60(43):23299–23305. doi:10.1002/anie.202107347
32. Klein VG, Bond AG, Craigon C, Lokey RS, Ciulli A. Amide-to-ester substitution as a strategy for optimizing PROTAC permeability and cellular activity. J Med Chem. 2021;64(24):18082–18101. doi:10.1021/acs.jmedchem.1c01496
33. Wu K, Yu B, Li D, Tian Y, Liu Y, Jiang J. Recent Advances in Nanoplatforms for the Treatment of Osteosarcoma. Front Oncol. 2022;12:805978. doi:10.3389/fonc.2022.805978
34. Yu B, Wu K, Xu X, Liu Y, Jiang J. Recent advances in nanoplatforms for the treatment of neuropathic pain. Spinal Cord. 2022;60(7):594–603. doi:10.1038/s41393-021-00746-x
35. Zhang Y, Fowlkes B. Liposomes-based nanoplatform enlarges ultrasound-related diagnostic and therapeutic precision. Curr Med Chem. 2022;29(8):1331–1341. doi:10.2174/0929867328666210804092624
36. Cao J, Huang D, Peppas NA. Advanced engineered nanoparticulate platforms to address key biological barriers for delivering chemotherapeutic agents to target sites. Adv Drug Deliv Rev. 2020;167:170–188. doi:10.1016/j.addr.2020.06.030
37. Rajpoot K. Lipid-based Nanoplatforms in Cancer Therapy: recent Advances and Applications. Curr Cancer Drug Targets. 2020;20(4):271–287. doi:10.2174/1568009620666200115160805
38. Zhao Z, Li D, Wu Z, Wang Q, Ma Z, Zhang C. Research progress and prospect of nanoplatforms for treatment of oral cancer. Front Pharmacol. 2020;11:616101. doi:10.3389/fphar.2020.616101
39. Mauro N, Utzeri MA, Varvarà P, Cavallaro G. Functionalization of metal and carbon nanoparticles with potential in cancer theranostics. Molecules. 2021;26(11):3085. doi:10.3390/molecules26113085
40. Zhu M, Shi Y, Shan Y, et al. Recent developments in mesoporous polydopamine-derived nanoplatforms for cancer theranostics. J Nanobiotechnology. 2021;19(1):387. doi:10.1186/s12951-021-01131-9
41. Giannousi K, Koutroumpis E, Georgiadou V, Karagkounis V, Dendrinou-Samara C. Nanoplatforms of manganese ferrite nanoparticles functionalized with anti-inflammatory drugs. Eur J Inorg Chem. 2019;1895–1903. doi:10.1002/ejic.201801539
42. Jiao W, Zhang T, Peng M, Yi J, He Y, Fan H. Design of magnetic nanoplatforms for cancer theranostics. Biosensors. 2022;12(1):38. doi:10.3390/bios12010038
43. Chen YC, Huang Y, Jin Q. Polymeric nanoplatforms for the delivery of antibacterial agents. Macromol Chem Phys. 2022;223(5). doi:10.1002/macp.202100440
44. Suliman K, Vahdani Y, Hussain A, et al. Polymeric micelles functionalized with cell penetrating peptides as potential pH-sensitive platforms in drug delivery for cancer therapy: a review. Arabian J Chem. 2021;14(8). doi:10.1016/j.arabjc.2021.103264
45. Tian Y, Zhou S. Advances in cell penetrating peptides and their functionalization of polymeric nanoplatforms for drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13(2):e1668. doi:10.1002/wnan.1668
46. Maddiboyina B, Desu PK, Vasam M, et al. An insight of nanogels as novel drug delivery system with potential hybrid nanogel applications. J Biomater Sci Polym Ed. 2022;33(2):262–278. doi:10.1080/09205063.2021.1982643
47. Sabir F, Asad MI, Qindeel M, et al. Polymeric nanogels as versatile nanoplatforms for biomedical applications. J Nanomater. 2019;2019. doi:10.1155/2019/1526186
48. Li X, Sun H, Li H, et al. Multi-responsive biodegradable cationic nanogels for highly efficient treatment of tumors. Adv Funct Mater. 2021;31:26. doi:10.1002/adfm.202100227
49. Xing Y, Cheng Z, Wang R, Lv C, James TD, Yu F. Analysis of extracellular vesicles as emerging theranostic nanoplatforms. Coord Chem Rev. 2020;424. doi:10.1016/j.ccr.2020.213506
50. Li YJ, Wu JY, Wang JM, Hu XB, Xiang DX. Emerging strategies for labeling and tracking of extracellular vesicles. J Control Release. 2020;328:141–159. doi:10.1016/j.jconrel.2020.08.056
51. Ma Y, Dong S, Li X, Kim BYS, Yang Z, Jiang W. Extracellular vesicles: an emerging nanoplatform for cancer therapy. Front Oncol. 2021;10:606906. doi:10.3389/fonc.2020.606906
52. Yang J, Yang Y, Kawazoe N, Chen G. Encapsulation of individual living cells with enzyme responsive polymer nanoshell. Biomaterials. 2019;197:317–326. doi:10.1016/j.biomaterials.2019.01.029
53. Gazaille C, Sicot M, Akiki M, et al. Characterization of biological material adsorption to the surface of nanoparticles without a prior separation step: a case study of glioblastoma-targeting peptide and lipid nanocapsules. Pharm Res. 2021;38(4):681–691. doi:10.1007/s11095-021-03034-8
54. Lu L, Duong VT, Shalash AO, Skwarczynski M, Toth I. Chemical conjugation strategies for the development of protein-based subunit nanovaccines. Vaccines. 2021;9(6):563. doi:10.3390/vaccines9060563
55. Teran-Saavedra NG, Sarabia-Sainz JA, Velázquez-Contreras EF, Ramos-Clamont Montfort G, Pedroza-Montero M, Vazquez-Moreno L. Albumin-Albumin/Lactosylated core-shell nanoparticles: therapy to treat hepatocellular carcinoma for controlled delivery of doxorubicin. Molecules. 2020;25(22):5432. doi:10.3390/molecules25225432
56. Andra VVSNL, Pammi SVN, Bhatraju LVKP, Ruddaraju LK. A comprehensive review on novel liposomal methodologies, commercial formulations, clinical trials and patents. Bionanoscience. 2022;12(1):274–291. doi:10.1007/s12668-022-00941-x
57. Gopi S, Balakrishnan P. Evaluation and clinical comparison studies on liposomal and non-liposomal ascorbic acid (vitamin C) and their enhanced bioavailability. J Liposome Res. 2021;31(4):356–364. doi:10.1080/08982104.2020.1820521
58. Li Z, Perkins W, Cipolla D. Robustness of aerosol delivery of amikacin liposome inhalation suspension using the eFlow® Technology. Eur J Pharm Biopharm. 2021;166:10–18. doi:10.1016/j.ejpb.2021.05.021
59. Bilton D, Pressler T, Fajac I, et al. Amikacin liposome inhalation suspension for chronic Pseudomonas aeruginosa infection in cystic fibrosis. J Cyst Fibros. 2020;19(2):284–291. doi:10.1016/j.jcf.2019.08.001
60. Winthrop KL, Flume PA, Thomson R, et al. Amikacin liposome inhalation suspension for Mycobacterium avium complex lung disease: a 12-month open-label extension clinical trial. Ann Am Thorac Soc. 2021;18(7):1147–1157. doi:10.1513/AnnalsATS.202008-925OC
61. Li XR, Zhu Y, Zhang GN, Huang JM, Pei LX. The impact of Pegylated liposomal doxorubicin in recurrent ovarian cancer: an updated meta-analysis of randomized clinical trials. J Ovarian Res. 2021;14(1):42. doi:10.1186/s13048-021-00790-4
62. Hong DS, Kang YK, Borad M, et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br J Cancer. 2020;122(11):1630–1637. doi:10.1038/s41416-020-0802-1
63. Du Y, Liu D, Sun M, et al. Multifunctional Gd-CuS loaded UCST polymeric micelles for MR/PA imaging-guided chemo-photothermal tumor treatment. Nano Res. 2022;15(3):2288–2299. doi:10.1007/s12274-021-3812-2
64. Wang J, Qi J, Jin F, et al. Spatiotemporally light controlled ”drug-free” macromolecules via upconversion-nanoparticle for precise tumor therapy. Nano Today. 2022;42:101360. doi:10.1016/j.nantod.2021.101360
65. Wu Y, Chang X, Yang G, et al. A physiologically responsive nanocomposite hydrogel for treatment of head and neck squamous cell carcinoma via proteolysis-targeting chimeras enhanced immunotherapy. Adv Mater. 2023;35(12):e2210787. doi:10.1002/adma.202210787
66. Yan S, Yan J, Liu D, et al. A nano-predator of pathological MDMX construct by clearable supramolecular gold(I)-thiol-peptide complexes achieves safe and potent anti-tumor activity. Theranostics. 2021;11(14):6833–6846. doi:10.7150/thno.59020
67. Wang Y, Han L, Liu F, et al. Targeted degradation of anaplastic lymphoma kinase by gold nanoparticle-based multi-headed proteolysis targeting chimeras. Colloids Surf B Biointerfaces. 2020;188:110795. doi:10.1016/j.colsurfb.2020.110795
68. Zhang HT, Peng R, Chen S, et al. Versatile nano-PROTAC-induced epigenetic reader degradation for efficient lung cancer therapy. Adv Sci. 2022;9(29):e2202039. doi:10.1002/advs.202202039
69. Zhang C, Xu M, He S, Huang J, Xu C, Pu K. Checkpoint nano-PROTACs for activatable cancer photo-immunotherapy. Adv Mater. 2023;35(6):e2208553. doi:10.1002/adma.202208553
70. Zhang C, He S, Zeng Z, Cheng P, Pu K. Smart nano-PROTACs reprogram tumor microenvironment for activatable photo-metabolic cancer immunotherapy. Angew Chem Int Ed Engl. 2022;61(8):e202114957. doi:10.1002/anie.202114957
71. Fu Y, Saraswat A, Wei Z, et al. Development of Dual ARV-825 and Nintedanib-Loaded PEGylated nano-liposomes for synergistic efficacy in vemurafnib-resistant melanoma. Pharmaceutics. 2021;13(7):1005. doi:10.3390/pharmaceutics13071005
72. Fan R, He S, Wang Y, et al. Targeted delivery of a PROTAC induced PDEδ degrader by a biomimetic drug delivery system for enhanced cytotoxicity against pancreatic cancer cells. Am J Cancer Res. 2022;12(3):1027–1041. PMCID: PMC8984894.
73. Liu H, Chen C, Chen HL, et al. 2D-PROTACs with augmented protein degradation for super-resolution photothermal optical coherence tomography guided momentary multimodal therapy. Chem Eng J. 2022;446(1):137039. doi:10.1016/j.cej.2022.137039
74. Luo T, Zheng Q, Shao L, Ma T, Mao L, Wang M. Intracellular delivery of glutathione peroxidase degrader induces ferroptosis in vivo. Angew Chem Int Ed Engl. 2022;61(39):e202206277. doi:10.1002/anie.202206277
75. Marine JC, Dyer MA, Jochemsen AG. MDMX: from bench to bedside. J Cell Sci. 2007;120(Pt 3):371–378. doi:10.1242/jcs.03362
76. Yang WS, SriRamaratnam R, Welsch ME, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1–2):317–331. doi:10.1016/j.cell.2013.12.010
77. Huang Y, Yan X, Ren T, Yi F, Li Q, Zhang C. The safety and efficacy of chemotherapy combined with immunotherapy for pancreatic cancer: a meta-analysis. Medicine. 2021;100(29):e26673. doi:10.1097/MD.0000000000026673
78. Davern M, Lysaght J. Cooperation between chemotherapy and immunotherapy in gastroesophageal cancers. Cancer Lett. 2020;495:89–99. doi:10.1016/j.canlet.2020.09.014
79. Chan HY, Choi J, Jackson C, Lim M. Combination immunotherapy strategies for glioblastoma. J Neurooncol. 2021;151(3):375–391. doi:10.1007/s11060-020-03481-0
80. Meng X, Wang K, Lv L, et al. Photothermal/photodynamic therapy with immune-adjuvant liposomal complexes for effective gastric cancer therapy. Part Part Syst Charact. 2019;36(6):1900015. doi:10.1002/ppsc.201900015
81. Chen Z, Zhang Q, Huang Q, et al. Photothermal MnO2 nanoparticles boost chemo-photothermal therapy-induced immunogenic cell death in tumor immunotherapy. Int J Pharm. 2022;617:121578. doi:10.1016/j.ijpharm.2022.121578
82. Liu ZB, Zhang L, Bian J, Jian J. Combination strategies of checkpoint immunotherapy in metastatic breast cancer. Onco Targets Ther. 2020;13:2657–2666. doi:10.2147/OTT.S240655
83. Cheraga N, Ouahab A, Shen Y, Huang NP. Characterization and pharmacokinetic evaluation of oxaliplatin long-circulating liposomes. Biomed Res Int. 2021;2021:5949804. doi:10.1155/2021/5949804
84. He H, Lu Y, Qi J, Zhu Q, Chen Z, Wu W. Adapting liposomes for oral drug delivery. Acta Pharm Sin B. 2019;9(1):36–48. doi:10.1016/j.apsb.2018.06.005
85. Henna TK, Raphey VR, Sankar R, Ameena Shirin VK, Gangadharappa HV, Pramod K. Carbon nanostructures: the drug and the delivery system for brain disorders. Int J Pharm. 2020;587:119701. doi:10.1016/j.ijpharm.2020.119701
86. Ion R, Necula MG, Mazare A, et al. Drug delivery systems based on titania nanotubes and active agents for enhanced osseointegration of bone implants. Curr Med Chem. 2020;27(6):854–902. doi:10.2174/0929867326666190726123229
87. Guan C, Zhu X, Feng C. DNA nanodevice-based drug delivery systems. Biomolecules. 2021;11(12):1855. doi:10.3390/biom11121855
88. Alaarg A, Pérez-Medina C, Metselaar JM, et al. Applying nanomedicine in maladaptive inflammation and angiogenesis. Adv Drug Deliv Rev. 2017;119:143–158. doi:10.1016/j.addr.2017.05.009
89. Yang Y, Nie D, Liu Y, Yu M, Gan Y. Advances in particle shape engineering for improved drug delivery. Drug Discov Today. 2019;24(2):575–583. doi:10.1016/j.drudis.2018.10.006
90. Li Z, Sun L, Zhang Y, Dove AP, O’Reilly RK, Chen G. Shape effect of glyco-nanoparticles on macrophage cellular uptake and immune response. ACS Macro Lett. 2016;5(9):1059–1064. doi:10.1021/acsmacrolett.6b00419
91. Di J, Gao X, Du Y, Zhang H, Gao J, Zheng A. Size, shape, charge and “stealthy” surface: carrier properties affect the drug circulation time in vivo. Asian J Pharm Sci. 2021;16(4):444–458. doi:10.1016/j.ajps.2020.07.005
92. Zununi Vahed S, Fathi N, Samiei M, Maleki Dizaj S, Sharifi S. Targeted cancer drug delivery with aptamer-functionalized polymeric nanoparticles. J Drug Target. 2019;27(3):292–299. doi:10.1080/1061186X.2018.1491978
93. Zia A, Wu Y, Nguyen T, Wang X, Peter K, Ta HT. The choice of targets and ligands for site-specific delivery of nanomedicine to atherosclerosis. Cardiovasc Res. 2020;116(13):2055–2068. doi:10.1093/cvr/cvaa047
94. Cao Y, Wu J, Zheng X, et al. Assessing the activity of antibodies conjugated to upconversion nanoparticles for immunolabeling. Anal Chim Acta. 2022;1209:339863. doi:10.1016/j.aca.2022.339863
95. Qi J, Li W, Xu X, et al. Cyto-friendly polymerization at cell surfaces modulates cell fate by clustering cell-surface receptors. Chem Sci. 2020;11(16):4221–4225. doi:10.1039/c9sc06385d
96. Zou T, Lu W, Mezhuev Y, et al. A review of nanoparticle drug delivery systems responsive to endogenous breast cancer microenvironment. Eur J Pharm Biopharm. 2021;166:30–43. doi:10.1016/j.ejpb.2021.05.029
97. Wang J, Xu L, Liu X, Yang R, Wang D. A facile adenosine triphosphate-responsive nanoplatform for efficacious therapy of esophageal cancer. Oncol Lett. 2020;20(4):108. doi:10.3892/ol.2020.11969
98. Huang C, You Q, Xu J, et al. An mTOR siRNA-loaded spermidine/DNA tetrahedron nanoplatform with a synergistic anti-inflammatory effect on acute lung injury. Adv Healthc Mater. 2022;11(11):e2200008. doi:10.1002/adhm.202200008
99. Raza A, Rasheed T, Nabeel F, Hayat U, Bilal M, Iqbal HMN. Endogenous and exogenous stimuli-responsive drug delivery systems for programmed site-specific release. Molecules. 2019;24(6):1117. doi:10.3390/molecules24061117
100. Lv Z, He S, Wang Y, Zhu X. Noble metal nanomaterials for NIR-triggered photothermal therapy in cancer. Adv Healthc Mater. 2021;10(6):e2001806. doi:10.1002/adhm.202001806
101. Liang J, Yang B, Zhou X, Han Q, Zou J, Cheng L. Stimuli-responsive drug delivery systems for head and neck cancer therapy. Drug Deliv. 2021;28(1):272–284. doi:10.1080/10717544.2021.1876182
102. Zhang N, Wang J, Foiret J, Dai Z, Ferrara KW. Synergies between therapeutic ultrasound, gene therapy and immunotherapy in cancer treatment. Adv Drug Deliv Rev. 2021;178:113906. doi:10.1016/j.addr.2021.113906
103. Rammohan A, Reddy GM, Garcia JR, et al. Microwave-assisted synthesis of N-substituted maleimide derivatives as exogenous antioxidant agents. J Heterocycl Chem. 2019;56(2):470–476. doi:10.1002/jhet.3421
104. Thomas RG, Jonnalagadda US, Kwan JJ. Biomedical applications for gas-stabilizing solid cavitation agents. Langmuir. 2019;35(31):10106–10115. doi:10.1021/acs.langmuir.9b00795
105. Mohapatra A, Wells C, Jennings A, Ghimire M, Mishra SR, Morshed BI. Electric stimulus-responsive chitosan/MNP composite microbeads for a drug delivery system. IEEE Trans Biomed Eng. 2020;67(1):226–233. doi:10.1109/TBME.2019.2911579
106. Zhang F, Wu Q, Liu H. NIR light-triggered nanomaterials-based prodrug activation towards cancer therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12(6):e1643. doi:10.1002/wnan.1643
107. Xiang J, Tong X, Shi F, Yan Q, Yu B, Zhao Y. Near-infrared light-triggered drug release from UV-responsive diblock copolymer-coated upconversion nanoparticles with high monodispersity. J Mater Chem B. 2018;6(21):3531–3540. doi:10.1039/c8tb00651b
108. Wang Y, Li B, Xu F, et al. Tough magnetic chitosan hydrogel nanocomposites for remotely stimulated drug release. Biomacromolecules. 2018;19(8):3351–3360. doi:10.1021/acs.biomac.8b00636
109. Li Z, Li Y, Chen C, Cheng Y. Magnetic-responsive hydrogels: from strategic design to biomedical applications. J Control Release. 2021;335:541–556. doi:10.1016/j.jconrel.2021.06.003
110. Thirunavukkarasu GK, Cherukula K, Lee H, Jeong YY, Park IK, Lee JY. Magnetic field-inducible drug-eluting nanoparticles for image-guided thermo-chemotherapy. Biomaterials. 2018;180:240–252. doi:10.1016/j.biomaterials.2018.07.028
111. Wang D, Xie W, Gao Q, et al. Non-magnetic injectable implant for magnetic field-driven thermochemotherapy and dual stimuli-responsive drug delivery: transformable liquid metal hybrid platform for cancer theranostics. Small. 2019;15(16):e1900511. doi:10.1002/smll.201900511
112. Paris JL, Manzano M, Cabañas MV, Vallet-Regí M. Mesoporous silica nanoparticles engineered for ultrasound-induced uptake by cancer cells. Nanoscale. 2018;10(14):6402–6408. doi:10.1039/C8NR00693H
113. Shen Q, Tang T, Hu Q, et al. Microwave hyperthermia-responsible flexible liposomal gel as a novel transdermal delivery of methotrexate for enhanced rheumatoid arthritis therapy. Biomater Sci. 2021;9(24):8386–8395. doi:10.1039/d1bm01438b
114. Liu X, Shi D, Guo L, et al. Echogenic, ultrasound-sensitive chitosan nanodroplets for spatiotemporally controlled DKK-2 gene delivery to prostate cancer cells. Int J Nanomed. 2021;16:421–432. doi:10.2147/IJN.S286474
115. Liang P, Mao L, Dong Y, et al. Design and application of near-infrared nanomaterial-liposome hybrid nanocarriers for cancer photothermal therapy. Pharmaceutics. 2021;13(12):2070. doi:10.3390/pharmaceutics13122070
116. Li X, Wu Y, Zhang R, Bai W, Ye T, Wang S. Oxygen-based nanocarriers to modulate tumor hypoxia for ameliorated anti-tumor therapy: fabrications, properties, and future directions. Front Mol Biosci. 2021;8:683519. doi:10.3389/fmolb.2021.683519
117. Chen M, Xu X, Shu G, et al. Multifunctional microspheres dual-loaded with doxorubicin and sodium bicarbonate nanoparticles to introduce synergistic trimodal interventional therapy. ACS Appl Bio Mater. 2021;4(4):3476–3489. doi:10.1021/acsabm.1c00033
118. Honda R, Gyobu T, Shimahara H, Miura Y, Hoshino Y. Electrostatic Interactions between acid-/base-containing polymer nanoparticles and proteins: impact of polymerization pH. ACS Appl Bio Mater. 2020;3(6):3827–3834. doi:10.1021/acsabm.0c00390
119. Scudellari M. Protein-slaying drugs could be the next blockbuster therapies. Nature. 2019;567(7748):298–300. doi:10.1038/d41586-019-00879-3
120. Neklesa T, Snyder LB, Willard RR, et al. Abstract 5236: ARV-110: an androgen receptor PROTAC degrader for prostate cancer. Cancer Res. 2018;78(13 Supplement):5236. doi:10.1158/1538-7445.AM2018-5236
121. Flanagan J, Qian Y, Gough S, et al. Abstract P5-04-18: ARV-471, an oral estrogen receptor PROTAC degrader for breast cancer. Cancer Res. 2019;79:P5-04–18. doi:10.1158/1538-7445.SABCS18-P5-04-18
122. Chirnomas D, Hornberger KR, Crews CM. Protein degraders enter the clinic - a new approach to cancer therapy. Nat Rev Clin Oncol. 2023;20(4):265–278. doi:10.1038/s41571-023-00736-3
123. Zeng S, Ye Y, Xia H, et al. Current advances and development strategies of orally bioavailable PROTACs. Eur J Med Chem. 2023;261:115793. doi:10.1016/j.ejmech.2023.115793
124. Apprato G, Poongavanam V, Garcia Jimenez D, et al. Exploring the chemical space of orally bioavailable PROTACs. Drug Discov Today. 2024;29(4):103917. doi:10.1016/j.drudis.2024.103917
125. Liu X, Ciulli A. Proximity-based modalities for biology and medicine. ACS Cent Sci. 2023;9(7):1269–1284. doi:10.1021/acscentsci.3c00395
126. Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1–3):3–26. doi:10.1016/s0169-409x(00)00129-0
127. Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45(12):2615–2623. doi:10.1021/jm020017n
128. Bondeson DP, Mares A, Smith IE, et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat Chem Biol. 2015;11(8):611–617. doi:10.1038/nchembio.1858
129. Petrylak DP, Stewart TF, Gao X, et al. A Phase 2 expansion study of ARV-766, a PROTAC androgen receptor (AR) degrader, in metastatic castration-resistant prostate cancer (mCRPC). Clin Oncol. 2023;41(6 Supplement):TPS290. doi:10.1200/JCO.2023.41.6_suppl.TPS2
130. Gao X, Burris HA, Vuky J, et al. Phase 1/2 study of ARV-110, an androgen receptor (AR) PROTAC degrader, in metastatic castration-resistant prostate cancer (mCRPC). Clin Oncol. 2022;40(6 Supplement):017. doi:10.1200/JCO.2022.40.6_suppl.017
131. Campone M, Ma CX, De Laurentiis M, et al. VERITAC-2: a global, randomized Phase 3 study of ARV-471, a proteolysis targeting chimera (PROTAC) estrogen receptor (ER) degrader, vs fulvestrant in ER+/human epidermal growth factor receptor 2 (HER2)- advanced breast cancer. Clin Oncol. 2023;41(16 Supplement):TPS1122. doi:10.1200/JCO.2023.41.16_suppl.TPS112
132. Gonzalez-Valdivieso J, Girotti A, Schneider J, Arias FJ. Advanced nanomedicine and cancer: challenges and opportunities in clinical translation. Int J Pharm. 2021;599:120438. doi:10.1016/j.ijpharm.2021.120438
133. Juan A, Del Mar Noblejas-López M, Arenas-Moreira M, Alonso-Moreno C, Ocaña A. Options to improve the action of PROTACs in Cancer: development of controlled delivery nanoparticles. Front Cell Dev Biol. 2022;9:805336. doi:10.3389/fcell.2021.805336
134. Parodi A, Buzaeva P, Nigovora D, et al. Nanomedicine for increasing the oral bioavailability of cancer treatments. J Nanobiotechnology. 2021;19(1):354. doi:10.1186/s12951-021-01100-2
135. Hua S. Orally administered liposomal formulations for colon targeted drug delivery. Front Pharmacol. 2014;5:138. doi:10.3389/fphar.2014.00138
136. Kou L, Yao Q, Sun M, et al. Cotransporting ion is a trigger for cellular endocytosis of transporter-targeting nanoparticles: a case study of high-efficiency SLC22A5 (OCTN2)-mediated carnitine-conjugated nanoparticles for oral delivery of therapeutic drugs. Adv Healthc Mater. 2017;6(17). doi:10.1002/adhm.201700165
137. Mady FM, Shaker MA. Enhanced anticancer activity and oral bioavailability of ellagic acid through encapsulation in biodegradable polymeric nanoparticles. Int J Nanomed. 2017;12:7405–7417. doi:10.2147/IJN.S147740
138. Jain MM, Gupte SU, Patil SG, et al. Paclitaxel injection concentrate for nanodispersion versus nab-paclitaxel in women with metastatic breast cancer: a multicenter, randomized, comparative phase II/III study. Breast Cancer Res Treat. 2016;156(1):125–134. doi:10.1007/s10549-016-3736-9
139. Li W, Bengtson MH, Ulbrich A, et al. Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the organelle’s dynamics and signaling. PLoS One. 2008;3(1):e1487. doi:10.1371/journal.pone.0001487
140. Zhang NY, Hou DY, Hu XJ, et al. Nano Proteolysis Targeting Chimeras (PROTACs) with anti-hook effect for tumor therapy. Angew Chem Int Ed Engl. 2023;62(37):e202308049. doi:10.1002/anie.202308049
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
