Back to Journals » International Journal of Nanomedicine » Volume 19
Nanomaterials Enhance Pyroptosis-Based Tumor Immunotherapy
Authors Ji F, Shi C, Shu Z, Li Z
Received 15 January 2024
Accepted for publication 22 May 2024
Published 10 June 2024 Volume 2024:19 Pages 5545—5579
DOI https://doi.org/10.2147/IJN.S457309
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Mian Wang
Fujian Ji, Chunyu Shi, Zhenbo Shu, Zhongmin Li
Department of Gastrointestinal and Colorectal Surgery, China-Japan Union Hospital of Jilin University, Changchun, 130033, People’s Republic of China
Correspondence: Zhongmin Li, Email [email protected]
Abstract: Pyroptosis, a pro-inflammatory and lytic programmed cell death pathway, possesses great potential for antitumor immunotherapy. By releasing cellular contents and a large number of pro-inflammatory factors, tumor cell pyroptosis can promote dendritic cell maturation, increase the intratumoral infiltration of cytotoxic T cells and natural killer cells, and reduce the number of immunosuppressive cells within the tumor. However, the efficient induction of pyroptosis and prevention of damage to normal tissues or cells is an urgent concern to be addressed. Recently, a wide variety of nanoplatforms have been designed to precisely trigger pyroptosis and activate the antitumor immune responses. This review provides an update on the progress in nanotechnology for enhancing pyroptosis-based tumor immunotherapy. Nanomaterials have shown great advantages in triggering pyroptosis by delivering pyroptosis initiators to tumors, increasing oxidative stress in tumor cells, and inducing intracellular osmotic pressure changes or ion imbalances. In addition, the challenges and future perspectives in this field are proposed to advance the clinical translation of pyroptosis-inducing nanomedicines.
Keywords: pyroptosis, programmed cell death, nanomaterial, immunotherapy
Graphical Abstract:
Introduction
Pyroptosis, a novel type of programmed cell death (PCD), has gained increasing attention in recent years. The morphological features of pyroptosis include chromatin condensation, DNA breakage into fragments, cell swelling with large bubbles, cell membrane breakage, and the release of various cellular contents. The gasdermin (GSDM) family of proteins plays an important role in pyroptosis by inducing the formation of cell membrane pores. Gasdermin D (GSDMD) was discovered in 2015 as an executor of pyroptosis,1 and pyroptosis was classified into caspase-1-dependent canonical pathway and caspase-4/5/11-dependent noncanonical pathway. In the canonical pathway, pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) are recognized by pattern recognition receptors, leading to the recruitment and assembly of inflammasomes composed of sensor proteins, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and pro-caspase-1. Subsequently, activated caspase-1 promotes the maturation of interleukin-1β (IL-1β) and interleukin-18 (IL-18) and cleaves GSDMD into N-terminal fragment (GSDMD-N) and C-terminal fragment (GSDMD-C). GSDMD-N forms pores in the plasma membrane and triggers pyroptosis. In the noncanonical pathway, caspase-4/5 (human) and caspase-11 (mouse) are activated by lipopolysaccharide (LPS) and then they cleave GSDMD. Potassium efflux induced by cell membrane pores activates inflammasomes and caspase-1, which promote the maturation of IL-1β and IL-18.2,3 Recent studies have shown that pyroptosis can also be initiated through other pathways. Some chemotherapeutic agents cause pyroptosis in gasdermin E (GSDME)-positive cells via the caspase-3/GSDME pathway.4 Under hypoxic conditions, since programmed cell death-ligand 1 (PD-L1) up-regulates the expression of gasdermin C (GSDMC) in tumor cells, macrophage-derived tumor necrosis factor-α (TNF-α) induces caspase-8/GSDMC pathway-mediated pyroptosis.5 Furthermore, granzymes produced by activated natural killer (NK) cells or cytotoxic T cells (CTLs) initiate caspase-independent pyroptosis in gasdermin B (GSDMB)- or GSDME-positive cancer cells.6,7 With the deeper study of pyroptosis, more pathways may be discovered in the future.
Increasing evidence suggests that pyroptosis is strongly associated with tumor immunotherapy.8 Pyroptosis of tumor cells releases tumor antigens, DAMPs, and a large number of pro-inflammatory factors such as IL-1β and IL-18, thus triggering immune responses. As representatives of DAMPs, high mobility group box 1 (HMGB1) and adenosine triphosphate (ATP) enhance antigen presentation by promoting the infiltration and maturation of dendritic cells (DCs). IL-1β promotes DC maturation and facilitates intratumoral infiltration of immune cells. IL-18 promotes the proliferation, activation, and tumor-killing effects of NK cells, and modulates the polarization of type 1 T helper cells. Lieberman and co-workers demonstrated that increased GSDME expression in tumors effectively inhibited tumor growth by converting apoptosis into pyroptosis. This has been attributed to the increased number and enhanced antitumor activity of tumor-infiltrating CTLs and NK cells.9 In another study, Lin et al confirmed that oncolytic parapoxvirus ovis triggered tumor cell pyroptosis via GSDME, thus reversing the immunosuppressive tumor microenvironment (TME) and sensitizing nonresponsive tumors to immune checkpoint blockade (ICB) therapy.10 The dysfunction of pyroptosis in tumor tissues may be related to immune escape of the tumor. Kumar et al found that LncRNA Malat1 inhibited GSDMD-mediated pyroptosis, thereby preventing T cells from recognizing and killing incipient metastatic cells.11 Therefore, pyroptosis has great potential for enhancing tumor immunotherapy.
Although tumor cell pyroptosis activates antitumor immunity, pyroptosis in normal cells usually causes adverse effects, such as cardiovascular diseases (atherosclerosis, myocardial infarction, hypertension, and cardiac hypertrophy), neurological diseases (Alzheimer’s disease, Parkinson’s disease, stroke, and amyotrophic lateral sclerosis), and kidney diseases (diabetic kidney disease).12,13 Moreover, hepatocyte pyroptosis causes non-alcoholic steatohepatitis14 and GSDME-mediated pyroptosis of intestinal epithelial cells increases susceptibility to Crohn’s disease.15 Therefore, it is critical to trigger pyroptosis of tumor cells while avoiding damage to normal tissues or cells. However, current initiators of pyroptosis, including chemotherapeutic drugs, small-molecule inhibitors or agonists, and specific metal ions, often face the problems of rapid in vivo clearance and insufficient accumulation in tumor. Although pyroptosis may activate the antitumor immune response by promoting antigen presentation, increasing the infiltration of immune cells within the tumor, and modulating the TME, it often needs to be combined with other therapeutic strategies to amplify the antitumor effect, which may inevitably increase the side effects. An increasing number of studies indicate that pyroptosis plays a dual role in inhibiting tumor growth and promoting tumor progression.16 The selective induction of pyroptosis to enhance antitumor efficacy requires further research.
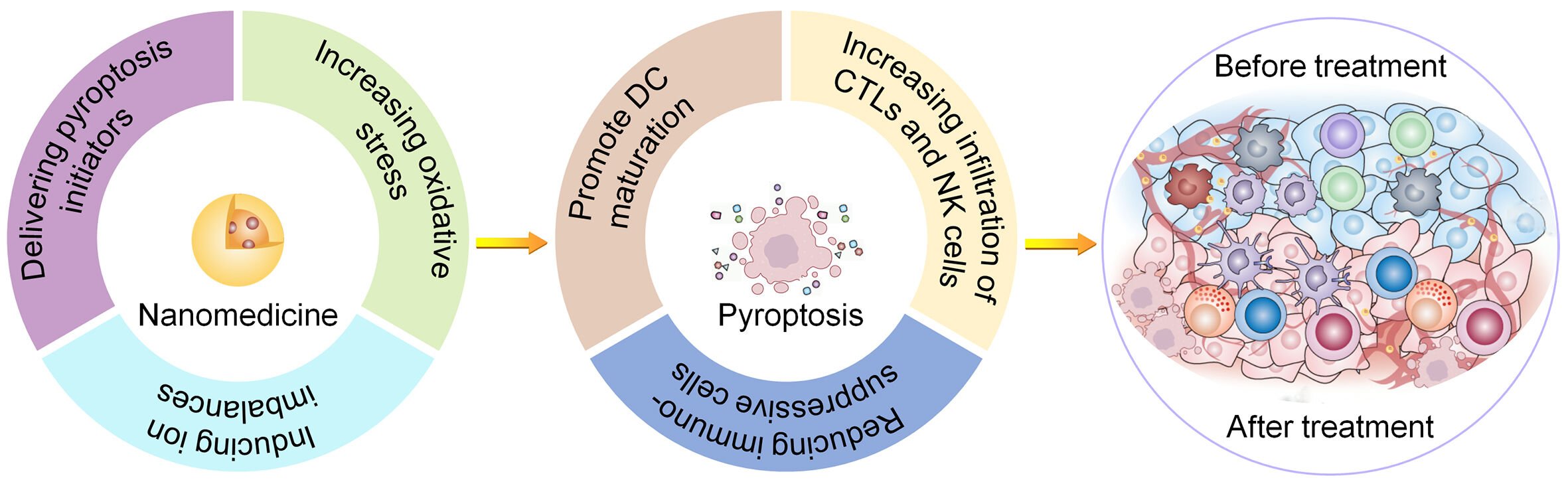
Currently, nanotechnology has undergone rapid development and has been widely used in biomedical fields such as disease diagnosis, cancer therapy, and drug delivery. Different forms of drug carriers such as inorganic nanoparticles, nanofibers, polymer micelles, liposomes, hydrogels, and scaffolds have been developed to improve the efficacy of antitumor therapy.17,18 Nanotechnology provides an effective platform for pyroptosis-based tumor immunotherapy (Scheme 1). First, nanomaterials increase the stability and biocompatibility of proteins, bacteria, bacterial exotoxins, and viruses that cause pyroptosis and reduce their immunogenicity. Second, nanomaterials specifically deliver pyroptosis initiators to tumor tissues via enhanced permeability retention (EPR) effects or active targeting surface modifications, thereby increasing their blood circulation time and intratumoral accumulation. Third, some nanomaterials containing specific metal ions directly induce tumor cell pyroptosis by triggering osmotic pressure changes, ionic imbalances, and oxidative stress without loading other drugs.19,20 Multifunctional nanoplatforms with multi-model imaging and therapeutic capabilities play an increasing role in cancer therapy.21 Nanomaterials with imaging functions provide multi-model imaging of tumors, including photoacoustic imaging, fluorescence imaging, computed tomography imaging, and magnetic resonance imaging, to achieve more accurate drug delivery and treatment efficacy monitoring.22 Finally, stimulus-responsive nanomaterials loaded with multiple drugs, including pyroptosis initiators, mediate tumor combination therapy by spatiotemporally releasing drugs in response to intrinsic or extrinsic stimuli, thereby alleviating systemic side effects by reducing the nonspecific distribution and release of drugs in normal tissues and organs.
 |
Scheme 1 Schematic illustration of the mechanism of nanomaterials for enhancing pyroptosis-based tumor immunotherapy. |
Immunotherapy has become an essential part of current antitumor treatment strategies, and nanotechnology has shown broad application prospects in enhancing immunotherapy.23 Nanomaterial-induced pyroptosis contributes to improving the response rate of immunotherapy and reducing adverse effects. Herein, we highlight the advantages of nanomaterials in enhancing pyroptosis-based tumor immunotherapy according to their mechanisms of inducing pyroptosis, including delivering pyroptosis initiators, increasing intracellular oxidative stress, and causing changes in osmotic pressure or inducing ion imbalance. In addition, we discuss prospects and challenges in this field based on the latest research.
Nanomaterials for Enhancing Pyroptosis-Based Immunotherapy
Nanomaterials for Delivering Pyroptosis Initiators
As mentioned above, chemotherapeutic drugs, epigenetic drugs, molecular targeted drugs, specific proteins or nucleic acids, and bacteria or viruses have been reported to induce pyroptosis.24 To increase the accumulation of these pyroptosis initiators in tumors, prolong their blood circulation time, and improve their in vivo stability, biocompatibility, and biosafety, various nanomaterials have been designed to deliver them to tumor tissues. We will introduce these nano-drug delivery platforms based on the characteristics of different pyroptosis initiators. The unique properties of nanomaterials such as stimulus responsiveness, tumor targeting, deep tumor penetration, and biocompatibility will also be highlighted. The syntheses and applications of these nanomaterials are described in detail.
Chemotherapeutic Drugs
As the traditional modality of cancer treatment, chemotherapy is still the main treatment strategy for most malignancies. However, the use of chemotherapeutic drugs is usually limited because of chemotherapeutic resistance and systemic side effects. It has been confirmed that some chemotherapeutic drugs such as doxorubicin (DOX), cisplatin (DDP), oxaliplatin (OXA), and paclitaxel (PTX) not only induce tumor cell apoptosis but also trigger pyroptosis. Chemotherapeutic drugs activate caspase-3 to cleave GSDME to form the N-terminal domain (GSDME-N), which promotes the formation of cell membrane pores and ultimately results in pyroptosis.25 Su et al demonstrated that inhibition of Src reactivated pyroptosis and reversed chemoresistance in pancreatic and lung cancer cells.26 Therefore, switching from chemotherapeutic drug-induced apoptosis to pyroptosis may help overcome chemoresistance. Furthermore, pyroptosis modulates TME to facilitate immune activation, providing new opportunities for the application of chemotherapeutic drugs. Owing to the continuous progress and improvement of nanotechnology, a series of nanomaterials have been used to deliver chemotherapeutic drugs to tumor tissues to induce pyroptosis and enhance immunotherapy, independently or in combination with other therapeutic strategies.
Lack of targeting to tumors and uncontrolled drug release are the main factors limiting the use of chemotherapeutic agents as pyroptosis initiators. Wan et al prepared a diselenide nanoprodrug (DSe@POC) that was covalently self-assembled using a PTX-OXA prodrug, chlorin e6 (Ce6), and a diselenide-containing cross-linker.27 In vitro experiments showed that DSe@POC was efficiently taken up by tumor cells and released payloads in response to high levels of glutathione (GSH) in the TME and reactive oxygen species (ROS) produced by photodynamic therapy (PDT). DSe@POC + laser triggered the most significant tumor pyroptosis compared with the other groups. In vivo drug distribution experiments showed that DSe@POC specifically accumulated in the tumor and maintained a high concentration for at least 72 h, confirming the excellent tumor-targeting capability of DSe@POC. In vivo antitumor experiments showed that DSe@POC + laser significantly inhibited 4T1 tumor growth and completely eradicated tumors in some mice. For the DSe@POC + laser treatment group, flow cytometry (FCM) showed that the percentage of matured DCs was up-regulated in the tumor-draining lymph nodes (TDLNs) and the number of CD8+ T cells was increased in the spleens. Moreover, reduced levels of regulatory T cells (Tregs) in the TDLNs and myeloid-derived suppressor cells (MDSCs) in the spleens were observed. Finally, DSe@POC + laser + anti-programmed death 1 (PD-1) obviously suppressed primary and distant tumor growth and inhibited metastasis, confirming that DSe@POC successfully activated the immune response and enhanced ICB.
In another study, Xiao et al prepared a ROS/GSH dual-responsive nanoprodrug (MCCP) containing a PTX-SS-PTX dimer linked by disulfate, purpurin 18 (P18) photosensitizer, and methoxypolyethylene glycols-4-cyano-4-(phenylcarbonothioylthio)pentanoic acid-block-P(M4) copolymer (Figures 1A and B).28 MCCP + laser induced pyroptosis of CT26 cells, and the pyroptosis index was as high as 32.1 at 48 h (Figure 1C). Increased release of lactate dehydrogenase (LDH), calreticulin (CRT), and HMGB1 was observed in the MCCP + laser treatment group. This excellent pyroptosis-inducing ability may be attributed to the synergistic effect of chemotherapy and PDT. After intravenous injection, MCCP selectively accumulated in the tumors of CT26 tumor-bearing mice, and the fluorescence intensity of MCCP in the tumors was significantly higher than that of free P18 at 96 h. In CT26 tumor-bearing mice, the MCCP + laser treatment group exhibited the smallest tumor volume. FCM showed increased CD103 expression on DCs and up-regulated levels of CD3+ T cells in the TDLNs of the MCCP + laser treatment group, suggesting that MCCP promoted DC activation and T cell expansion. MCCP + laser + anti-PD-1 treatment significantly arrested tumor growth and prolonged the survival time of mice. The combination treatment also protected mice from tumor re-challenge, indicating the generation of an immunological memory.
 |
Figure 1 TME-responsive nano-prodrug (MCPP) enhanced cancer immunotherapy by inducing pyroptosis. (A) Schematic illustration of the synthesis of MCPP. (B) Schematic illustration of the mechanism of MCPP for activating the antitumor immune response. (C) Representative image of CT26 cells after different treatments observed by microscopy; the pyroptotic cells were marked by arrows. Note: Reprinted with permission from Xiao Y, Zhang T, Ma XB et al. Microenvironment-responsive prodrug-induced pyroptosis boosts cancer immunotherapy. Adv Sci. 2021;8(24):2,101,840. doi: 10.1002/advs.202101840.28. |
Src is abnormally activated in head and neck squamous cell carcinoma and is closely related to chemoresistance.29 To overcome chemoresistance and potentiate antitumor immunity, Zhu et al synthesized a prodrug DAS-OXA by covalently binding an Src inhibitor (dasatinib, DAS) to OXA and further encapsulated it in poly(ethylene glycol)-b-poly(2-(diisopropylamino) ethyl methacrylate) (iPDPA).30 The prepared prodrug nanomicelles (PDO NPs) accumulated at the tumor site and released DAS-OXA in response to the tumor acidity. Subsequently, OXA and DAS were released from the prodrug in the reductive TME. In vitro assays showed that PDO NPs significantly inhibited Src phosphorylation and up-regulated the levels of cleaved caspase-3 and GSDME-N in SCC7 and 4MOSC2 cells, indicating that PDO NPs successfully prevented Src activation and induced pyroptosis. Compared to the control and free drugs, PDO NPs exhibited the most significant tumor inhibition effect in 4MOSC2 tumor-bearing mice. Immunohistochemical staining showed increased levels of CD8 and granzyme B and decreased levels of arginase 1 in tumors treated with PDO NPs. FCM analysis showed that the proportion of CD8+ T cells was increased and the proportion of monocytic MDSCs (M-MDSCs) was decreased in the spleens of the PDO NPs treatment group. These results demonstrate that PDO NPs have excellent synergistic antitumor effects and significantly activate the immune response.
Large-sized nanoparticles (approximately 100 nm) tend to accumulate at the tumor site via the EPR effect; however, disorganized tumor vasculature, high interstitial fluid pressure, and dense extracellular matrix (ECM) in solid tumors limit their further penetration into the tumor. In contrast, small-sized nanoparticles (approximately 30 nm) are able to penetrate deep into tumors but suffer from a short blood circulation time.31 To overcome biological barriers and achieve efficient drug delivery, Liang et al fabricated a cascaded pH-activated supramolecular nanoprodrug (PDNP) that can gradually shrink in size to induce pyroptosis and enhance antitumor immunity.32 PDNPs were self-assembled by PEGylated DOX blocks, which consisted of acid-responsive benzoic imine and hydrazone bonds. At physiological pH (pH 7.4), PDNPs (129.7 nm) remained intact and accumulated within the tumor owing to the protection of PEG. Subsequently, PDNPs broke down into small particles (37.9 nm) in the acidic TME (pH 6.5) because of the breaking of benzoimine bonds and detachment of PEG to achieve deep penetration. Finally, with the breaking of the hydrazone bonds, the PDNPs completely fragmented to 8.1 nm in the endolysosome (pH 5.0) and released DOX into the nucleus. In vitro studies showed that PDNPs successfully escaped from the lysosomes and triggered pyroptosis in CT26 cells. RNA sequencing revealed significantly increased levels of gene expression associated with the inflammatory response, antigen presentation, and T cell function in the cells treated with PDNPs. Compared with free DiR, DiR-loaded PDNPs exhibited longer blood circulation and deeper tumor penetration in vivo. In CT26 and 4T1 tumor-bearing mice, the tumor inhibition rates of PDNPs were as high as 74% and 85%, respectively, which were markedly higher than those of the other treatments. FCM revealed increased proportion of mature DCs and infiltrating T cells in the PDNP-treated tumor tissues. Furthermore, the number of M-MDSCs in the spleens was significantly lower in the PDNP treatment group.
Epigenetic Drugs
In recent years, epigenetic drugs, including DNA methyltransferase inhibitors (DNMTIs) and histone deacetylase inhibitors (HDACIs), have achieved outstanding efficacy in some hematogenous and solid tumors.33 Epigenetic drugs have been shown to increase tumor sensitivity to conventional treatments such as chemotherapy, radiotherapy, and molecular targeted therapy.34 GSDME is a key protein in chemotherapy-induced pyroptosis, but it is not expressed or is expressed at low levels in most tumor cells.9 GSDME is silenced in various tumor cells because of the high methylation of the promoter region.35 Therefore, inhibition of GSDME gene methylation by epigenetic drugs can up-regulate GSDME expression in tumor cells, thus contributing to the initiation of pyroptosis. Studies have shown that DNMTIs alone or in combination with chemotherapeutic drugs is sufficient to trigger pyroptosis.36 Fan et al proposed a therapeutic strategy that combines decitabine (DAC) and chemotherapeutic nanodrugs to induce pyroptosis and amplify the antitumor immune effect.37 DAC acts as a preconditioning agent that increases GSDME levels via demethylation. The results showed that DAC combined with DDP effectively induced pyroptosis in 4T1 cells. Furthermore, DDP was loaded into liposomes (LipoDDP) to avoid non-specific accumulation outside the tumor. In 4T1 tumor-bearing mice, DAC + LipoDDP effectively suppressed tumor volume to less than 200 mm3, which was noticeably smaller than that in the control group (1200 mm3). FCM showed that DAC + LipoDDP obviously up-regulated the proportion of CTLs in the tumors and increased the proportion of mature DCs in the TDLNs from 22.5% to 63.1%, confirming that the combination therapy activated the immune response. Xie et al encapsulated DAC and DOX in porous poly (lactic-co-glycolic acid) (PLGA) microspheres to synthesize CO-MPs.38 CO-MPs successfully reversed GSDME silencing and triggered pyroptosis in tumor cells. In lung tumor-bearing mice, CO-MPs effectively suppressed lung tumors and metastatic lung nodules. Elevated levels of CD8+ T cells in tumor tissues and mature DCs in TDLNs were observed in the CO-MPs treatment group.
In addition to inducing pyroptosis, some epigenetic drugs have also been shown to modulate the immunosuppressive TME to enhance immunotherapy.39 Zhou et al encapsulated mitoxantrone (MIT) and hydralazine (HYD) in a pH-responsive zeolitic imidazolate framework-8 (ZIF-8) and further coated them with hyaluronic acid (HA) to prepare (M+H)@ZIF/HA nanoparticles.40 HYD, a DNA demethylating agent, not only up-regulates the expression of GSDME and triggers pyroptosis in combination with MIT but also alleviates T cell paralysis by blocking methylglyoxal formation in MDSCs.41 The results showed that (M+H)@ZIF/HA effectively triggered pyroptosis in 4T1 cells and significantly decreased the methylglyoxal levels in MDSCs. In 4T1 tumor-bearing mice, (M+H)@ZIF/HA exhibited the most significant tumor-suppressive effect without causing serious systemic toxicity. FCM revealed that (M+H)@ZIF/HA increased the number of mature DCs from 18.8% to 36.6%. The proportions of intratumoral CLTs and helper T cells in the (M+H)@ZIF/HA treatment group were significantly higher than those in the other groups. Immunofluorescence assays demonstrated that (M+H)@ZIF/HA reduced the infiltration of MDSCs and Tregs into the tumors. Further studies showed that (M+H)@ZIF/HA inhibited lung metastasis and increased the percentage of CD8+ central memory T cells (TCMs) by 1.82-fold compared with the control group. Chen et al fabricated a GSH-responsive prodrug-based nanogel containing LAQ824 (a typical HDACI) and DOX.42 The prepared LAQ824-Dox nanoparticles (LD NPs) showed an excellent pyroptosis-inducing ability and activated the immune response by promoting DC maturation, increasing CD8+ T cell infiltration, and decreasing the number of MDSCs and Tregs. LD NPs combined with anti-PD-1 extended the survival of 4T1 tumor-bearing mice from 25.375 to 50.125 days. These results indicate that epigenetic drugs have huge potential for application in pyroptosis-based immunotherapy and deserve further study.
Similar to chemotherapy, PDT and hyperthermia therapy rely on GSDME to initiate pyroptosis. The combination of epigenetic drugs with these therapeutic strategies promotes the transformation of apoptosis to pyroptosis. To enhance antitumor photoimmunotherapy, Zheng et al fabricated a nanoagonist (R@IrP) by loading RG108 (a DNMTi) with an amphiphilic iridium-based photosensitizer (IrP).43 RG108 up-regulates GSDME expression in tumor cells and synergistically induces pyroptosis with IrP-mediated PDT, thus reversing immunosuppressive TME and sensitizing anti-PD-1 therapy (Figure 2A). The results showed that R@IrP was effectively internalized by A375 cells and elevated the intracellular ROS levels under light irradiation. RG108 increased the expression of GSDME in a concentration-dependent manner, and the overexpression of GSDME-N fragments was observed in cells treated with R@IrP + light. R@IrP + light significantly inhibited tumor growth in A375 tumor-bearing nude mice. Additionally, R@IrP + light + anti-PD-1 showed better tumor growth control than anti-PD-1 alone in B16 tumor-bearing mice. FCM showed that R@IrP + light increased CD4+ and CD8+ T cell levels in the spleens (Figures 2B and C) and lymph nodes (Figures 2D and E) of C57BL/6 mice. An increased percentage of effector T cells and decreased levels of Tregs and granulocytic MDSCs were observed in the R@IrP + light + anti-PD-1 treatment group. These results suggest that pyroptosis-based photoimmunotherapy effectively reshapes the TME and activates the immune system to kill tumors.
 |
Figure 2 Nanoagonist-mediated pyroptosis remodeled the inflammatory TME for synergistic photoimmunotherapy. (A) Schematic illustration of R@IrP nanoparticles triggering pyroptosis under light irradiation to enhance anti-PD-L1 immunotherapy by reversing immunosuppressive TME. Representative images (B) and quantification (C) of CD8+ T cells and CD4+ T cells gated on CD3+ cells in the spleens of B16 tumor-bearing C57BL/6 mice in different treatment groups detected by FCM. Representative images (D) and quantification (E) of CD8+ T cells and CD4+ T cells gated on CD3+ cells in the lymph nodes of B16 tumor-bearing C57BL/6 mice in different treatment groups detected by FCM. *P < 0.05. Note: Reprinted from Zheng LL, Fan Y, Wang X et al. Nanoagonist-mediated GSDME-dependent pyroptosis remodels the inflammatory microenvironment for tumor photoimmunotherapy. Adv Funct Mater. 2023;33(6):2,200,811. © 2022 Wiley-VCH GmbH.43. |
Radiofrequency ablation is a hyperthermia therapy commonly used in clinical practice. However, invasive procedures, insufficient tumor ablation, and thermal damage to normal tissues limit its application.44 To address these challenges, Zhang et al synthesized a radiofrequency-responsive bivalent gold nanocluster (biGC@PNA) via sequential redox reactions of lipoic acid and NaBH4 and further modification with temperature-sensitive poly(N-isopropylacrylamide-b-acrylic acid) (PNA).45 Under radiofrequency irradiation, biGC@PNA combined with DAC significantly induced pyroptosis in 4T1 cells and promoted HMGB1 and ATP release. In 4T1 tumor-bearing mice, biGC@PNA + DAC + radiofrequency reduced the average tumor volume to 27.21 ± 22.1 mm3 at day 14, and the tumors were radically ablated in two mice. The highest expression of GSDME-N was detected in mice treated with biGC@PNA + DAC + radiofrequency. Systemic immune activation was detected in bilateral 4T1 tumor-bearing mice. FCM showed that biGC@PNA + DAC + radiofrequency promoted DC maturation and increased the levels of CTLs and effector memory T cells (TEMs) in distant tumors, indicating that the combination therapy effectively provoked an antitumor immune response. This study demonstrates that epigenetic drugs can amplify radiofrequency-activated pyroptosis and is a promising strategy for enhancing immunotherapy.
Molecular Targeted Drugs
Molecular targeted drugs inhibit tumor growth and metastasis by specifically targeting well-defined oncogenic targets at the cellular and molecular levels, and have become the standard treatment for many advanced malignant tumors.46 However, drug resistance, low response rates, and systemic adverse effects limit their clinical use.47 Recent studies have found that some molecular targeted drugs enhance antitumor immunotherapy by promoting DC maturation, elevating T cell infiltration in tumors, and remodeling the TME; however, the specific mechanism is unclear.48 With the discovery of pyroptosis and the continuous exploration of its mechanism, some molecular targeted drugs have been confirmed to induce pyroptosis. For example, Erkes et al found that BRAF inhibitor combined with MEK inhibitor caused pyroptosis via the caspase-3/GSMDE pathway in melanoma and that the antitumor effects were immune-mediated.49 In another study, BI2536, a PLK1 kinase inhibitor, increased the chemosensitivity of the tumor to DDP by promoting the conversion of apoptosis to pyroptosis in esophageal squamous cell carcinoma.50 Therefore, it is necessary to further explore the use of molecular targeted drugs for pyroptosis-based tumor therapy, and nanomaterials can further improve the therapeutic effect.
The phosphatidylinositol 3-kinase (PI3K)–Akt–mammalian target of rapamycin (mTOR) and cyclin-dependent kinase (CDK) signaling pathways are two oncogenic pathways closely related to tumorigenesis, metastasis, and immune evasion.51,52 The abnormal activation of the CDK pathway often hinders the therapeutic effects of PI3K inhibitors.53 Sun and co-workers developed a TME-activatable prodrug nanomicelle (PNM) by crosslinking PF-04691502 (PF, a PI3K/mTOR inhibitor) and flavopiridol (Flav, a CDK inhibitor) using a GSH-responsive crosslinker (DBHD).54 The diameter of the PNM was 126.7 nm as detected by dynamic light scattering, and the loading efficiencies of PF and Flav were 28.92% and 29.33%, respectively. When incubated with 10 mM GSH for 24 h, the amount of PF released from the PNM reached 80%. In vitro assay showed that PNM was effectively internalized by 4T1 cells and escaped from the lysosomes. Moreover, up-regulated levels of cleaved caspase-3 and GSDME-N, and increased secretion of ATP, HMGB1, and CRT were detected in the cells treated with PNM, suggesting that PNM successfully induced pyroptosis. In 4T1 tumor-bearing mice, the PNM-treated group exhibited the smallest tumor volume. The infiltration ratio of CD8+ T cells in the PNM treatment group was 50.15%, which was significantly higher than that of the other groups. Compared with the control group, PNM significantly increased the proportion of mature DCs in the TDLNs and decreased the proportion of Tregs and polymorphonuclear MDSCs in the tumors. Further studies showed that PNM + anti-PD-1 extended the survival time of mice from 36 to 50 days. These results suggest that PNM-induced pyroptosis effectively activates antitumor immunity and improves the response rate of ICB. Furthermore, they fabricated GSH-responsive nanogels (CDNPs) by crosslinking dabrafenib (a BRAF inhibitor) and celecoxib (a COX2 inhibitor) using DBHD.55 They demonstrated that CDNPs induced pyroptosis through the caspase-3/GSDME pathway and activated a robust antitumor immune response.
The ATP-adenosine (ADO) axis plays a key role in modulating innate and adaptive immunity in the TME.56 Extracellular ATP (eATP) produced by stressed and dying cells releases inflammatory signals that activate antitumor immunity.57 However, eATP is hydrolyzed into the immunosuppressive ADO by a cascade of two ectonucleotidases: CD39 and CD73. CD39 is responsible for degrading ATP and adenosine diphosphate to adenosine monophosphate (AMP), whereas CD73 ultimately catalyzes the conversion of AMP to ADO.58 ADO promotes immunosuppression by inhibiting the activation and function of T cells and NK cells, while enhancing the function of immunosuppressive cells. Reducing ADO production by inhibiting CD39 or CD73 is a promising therapeutic strategy to reinvigorate antitumor immunity. Wu et al synthesized C-PMet nanovesicles by encapsulating the CD39 antagonist POM1 and the AMP-activated protein kinase agonist metformin in cancer cell-derived exosomes to activate the immunity system.59 Metformin promotes the generation of ATP, and POM1 inhibits the decomposition of eATP, thus synergistically elevating eATP levels in the TME. In vitro experiments revealed that C-PMet promoted the polarization of M1-type macrophages, advanced the maturation of DCs, and enhanced the proliferation and function of CD8+ T cells. In B16F10 tumor-bearing mice, C-PMet markedly suppressed tumor growth and extended the survival of mice without causing serious systemic side effects. Compared with the PBS group, C-PMet increased the concentration of ATP in the tumor from 20.3 μM to 89.2 μM and decreased the concentration of ADO from 54.6 μM to 5.6 μM. Notably, C-PMet decreased the number of tumor-infiltrating macrophages and increased IL-18 levels, indicating that increased eATP levels induced by C-PMet triggered pyroptosis in macrophages. Moreover, C-PMet increased the proportions of mature DCs, NK cells, and CD4+ and CD8+ T cells and decreased the percentages of Tregs and MDSCs in the tumor tissues. These results demonstrate that C-PMet successfully activates innate and adaptive immune responses and reverses immunosuppression. Further studies showed that C-PMet + anti-PD-1 therapy effectively inhibited distant metastasis and tumor recurrence.
In order to overcome tumor resistance to ICB, Sun and co-workers prepared GSH-responsive prodrug nanomicelles (AOZNs) by crosslinking epigenetic modulator γ-oryzanol (Orz) and CD73 inhibitor α, β-methylene adenosine 5′ diphosphate (AMPCP) through DBHD.60 In vivo distribution experiments showed that AOZNs preferentially accumulated in tumors. At the end of the treatment, the average tumor volume of the AOZN-treated group was approximately 3.5-fold lower than that of the control group in B16F10 tumor-bearing mice. Increased expression of caspase-1, GSDMD, the N-terminal of GSDMD, and HMGB1 was detected in the AOZNs treatment group, indicating that AOZNs caused pyroptosis via the caspase-1/GSDMD pathway. AOZNs effectively inhibited the activity of DNA methyltransferase and decreased the level of ADO in tumors. FCM showed that AOZNs significantly increased T cell infiltration into the tumor, promoted DC maturation, reduced MDSC accumulation, and inhibited M2-type macrophage polarization. Further studies showed that AOZNs + anti-PD-L1 therapy resulted in better tumor control and longer survival than monotherapy. Therefore, AOZNs represent a promising platform for potentiating tumor immunotherapy.
Specific Proteins or Nucleic Acids
GSDM is a key protein that triggers pyroptosis; therefore, the delivery of GSDM proteins or nucleic acids into tumor cells contributes to the initiation of pyroptosis. However, in vivo delivery of proteins or nucleic acids faces a series of problems, including poor in vivo stability, high immunogenicity, premature degradation, difficulty in penetrating biological barriers, and lack of tumor targeting.61 Nanotechnology helps overcome these obstacles, and a wide range of nanoplatforms have been developed and show broad application potential.62,63 Shao and co-workers designed a bioorthogonal system based on phenylalanine trifluoroborate (Phe-BF3) desilylation to deliver gasdermin A3 (GSDMA3) and induce pyroptosis.64 In detail, NP–GSDMA3 was prepared by conjugating GSDMA3 to 60 nm diameter nanoparticles via a triethylsilyl ether linker. When incubation with Phe-BF3, GSDMA3 dissociated from NP–GSDMA3 via desilylation. Pyroptosis was detected in 40%, 35%, and 20% of the NP-GSDMA3- and Phe-BF3-treated HeLa, EMT6, and 4T1 cells, respectively. In 4T1 and EMT6-tumor bearing mice, NP-GSDMA3 + Phe-BF3 caused the most significant tumor shrinkage. Notably, less than 15% of the tumor cells undergoing pyroptosis was able to completely eliminate the 4T1 tumor. In contrast, the antitumor effect of NP-GSDMA3 + Phe-BF3 disappeared in athymic Nu/Nu mice, indicating that tumor regression was mediated by T cells. Elevated proportions of CD4+ and CD8+ T cells and reduced levels of CD4+FOXP3+ Tregs were detected in the GSDMA3 + Phe-BF3-treated tumors. This study provides a new strategy for exploring immune activation during pyroptosis.
As mentioned above, the application of pyroptosis is limited by the lack of GSDME expression in tumor cells due to DNA methylation. Additionally, an active cell membrane repair system is involved in the regulation of pyroptosis. Once the formation of membrane pores caused by GSDMD occurs, calcium influx through membrane pores activates the endosomal sorting complexes required for transport (ESCRT) to initiate membrane repair, thus preventing pyroptosis and inhibiting IL-1β release.65 Therefore, the inhibition of ESCRT-dependent repair of cell membrane damage is conducive to enhancing pyroptosis. Li et al designed a hydrogel-based drug delivery platform integrating GSDMD delivery and ESCRT inhibition to efficiently trigger pyroptosis and enhance immunotherapy (Figures 3A and B).66 GSDMD proteins were assembled into nanocages using GSH-responsive linkers and subsequently conjugated to Salmonella typhimurium (VNP) bacteria to form VNP-GD. An ESCRT inhibitor was loaded into biodegradable dextran nanoparticle to prepare EI-NP. VNP-GD and EI-NP were encapsulated in injectable hydrogels (VNP-GD/EI-NP@Gel) and cell patches (VNP-GD/EI-NP@Patch) for the treatment of primary and metastatic tumors and unresectable tumors, respectively. Because of the good tumor-targeting ability and biosafety of VNP, VNP-GD successfully delivered GSDMD nanocages into tumor cells, and GSDMD was released under the stimulation of high intracellular GSH. After incubation with PBS, VNP-GD, and VNP-GD + EI-NP for 24 h, the cellular uptake of SYTOX green was 3.8%, 30%, and 52%, respectively, indicating that VNP-GD successfully triggered pyroptosis and that EI-NP further promoted pyroptosis by preventing cell membrane damage repair. In 4T1 tumor-bearing mice, VNP-GD/EI-NP@Gel combined with anti-PD-1 obviously suppressed tumor growth, extended the survival of mice, and reduced lung metastatic nodules (Figure 3C). The excellent antitumor effects of VNP-GD/EI-NP@Gel were also verified in a B16F10 melanoma tumor model. Further studies showed that VNP-GD/EI-NP@Gel elevated the percentage of mature DCs from 12.9% (PBS control) to 49.4% (Figure 3D) and VNP-GD/EI-NP@Gel + anti-PD-1 elevated the levels of tumor-infiltrating CD8+ T cells from 76 per mg tumor (PBS control) to 493 per mg tumor (Figure 3E). Compared with the control group, VNP-GD/EI-NP@Gel clearly up-regulated the levels of HMGB1, TNF-α, and interferon-γ (IFN-γ) in the tumor tissues. Additionally, VNP-GD/EI-NP@Gel or VNP-GD/EI-NP@Patch combined with anti-PD-1 showed enhanced tumor control in unresectable ovarian tumor-bearing mice. Thus, blocking ESCRT-mediated cell membrane damage enhances pyroptosis and acts synergistically with ICB to suppress tumor growth.
 |
Figure 3 GSDM protein combined with ESRCT inhibitor triggered tumor cell pyroptosis to activate the antitumor immunity. (A) Schematic illustration of the fabrication process and administration of the hydrogel-based bacteria protein cage delivery system. (B) Schematic illustration of the underlying mechanism of VNP-GD and EI-NP. (C) Tumor volumes of 4T1 tumor-bearing mice in different treatment groups. (D) Proportion of mature DCs in the lymph nodes of 4T1 tumor-bearing mice receiving different treatments. (E) The number of CD8+ T cells per mg tumor tissue in 4T1 tumor-bearing mice receiving different treatments. ns: no significance, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns = not significant. Note: Reprinted from Li ZT, Mo FY, Wang YX et al. Enhancing gasdermin-induced tumor pyroptosis through preventing ESCRT-dependent cell membrane repair augments antitumor immune response. Nat Commun. 2022;13(1):6321.66. |
The delivery of GSDM nucleic acids into tumor cells is an alternative pathway for triggering pyroptosis. As a medium for intercellular communication, small extracellular vesicles (EVs) with a size of 30–200 nm have become new drug delivery vectors.67 Xing et al designed a GSDMD-N mRNA delivery platform (EVTx) by encapsulating GSDMD-N mRNA in EVs, incorporating Ce6 into the membrane of EVs, and displaying HER2 antibody on the surface of EVs.68 To ensure cell viability and facilitate mRNA encapsulation in EVs, optimized puromycin concentration was used to repress the translation of GSDMD-N mRNA in EV-donor cells. Once EVTx is internalized by HER2+ breast cancer cells, GSDMD-N translation is initiated when puromycin is diluted and inactivated by Ce6-mediated sonodynamic treatment (SDT). In SKBR3 tumor xenograft model, EVTx markedly suppressed tumor growth, improved the survival of mice, and promoted the secretion of IL-1β and IL-18. Moreover, EVTx + anti-PD-1 induced the most obvious tumor regression and suppressed lung metastasis in a 4T1-HER2 syngeneic mouse model. FCM analysis of tumor tissues showed that EVTx increased the proportion of mature DCs and CTLs and reduced the level of Tregs. To realize the self-supply of GSDME, Wang et al fabricated a nano-CRISPR scaffold (Nano-CD) to deliver CRISPR/dCas9 and DDP.69 Amino acid-modified polyetherimide (PEI) and CRISPR/dCas9 plasmids formed the CRISPR/dCas9 polyplex as the core through electrostatic adsorption, and DDP and TAT (a cell membrane penetrating peptide) were covalently linked to PEGylated polyacrylic acid to form the shell. Nano-CD showed enhanced cellular uptake and up-regulated the expression of GSDME RNA by 7.5 times. After incubation with Nano-CD, B16F10 cells exhibited typical cell membrane swelling with large bubbles and increased release of ATP, LDH, and IL-1β, suggesting that GSDME and DDP synergistically triggered pyroptosis. In B16F10 tumor-bearing mice, Nano-CD + anti-PD-1 effectively inhibited primary tumor growth, tumor recurrence, and distant metastasis. Nano-CD + anti-PD-1 dramatically elevated the levels of tumor-infiltrating CD4+ and CD8+ T cells and reduced the levels of intratumoral M2-type macrophages and Tregs. Therefore, the delivery of GSDM nucleic acids into tumor cells using nanoplatforms to induce pyroptosis is a feasible and efficient strategy.
ASC is another key protein involved in pyroptosis as it activates caspase-1 to initiate pyroptosis.70 Therefore, the delivery of ACS to tumor cells is also conducive to inducing pyroptosis. Liu et al designed a smart drug delivery system that integrated pyroptosis, starvation therapy, and chemotherapy to treat breast cancer.71 Glucose oxidase (GOx) was chemically attached to iron oxide nanoparticles, further encapsulated with the ASC plasmid and PTX in PLGA-chitosan nanoparticles, and finally modified with the EpCAM aptamer to form PTX-ASC-GO@MPCSApt NPs. The results showed that PTX-ASC-GO@MPCSApt NPs obviously promoted the production of IL-1β and LDH in 4T1 cells, indicating that PTX-ASC-GO@MPCSApt NPs successfully triggered pyroptosis of tumor cells. In 4T1 tumor-bearing mice, PTX-ASC-GO@MPCSApt NPs induced the most significant tumor shrinkage, with a tumor inhibition rate of 95%, which was much higher than that in the other groups. Further analysis revealed that PTX-ASC-GO@MPCSApt NPs increased the proportions of activated T helper cells and activated cytotoxic T cells from 8.51% and 16.2% (control group) to 22.3% and 29.4%, respectively.
Bacteria or Viruses
DAMPs such as bacteria and viruses successively activate inflammasome and caspase-1 to induce pyroptosis through the canonical pathway. Studies have shown that various bacteria, such as Shigella flexneri, Salmonella, Listeria monocytogenes (Lmo), and Legionella pneumophila can induce pyroptosis.72 Lipopolysaccharides in gram-negative bacteria activate caspase-4/5/11 to trigger pyroptosis through a noncanonical pathway. Some anaerobic bacteria have a natural tumor-targeting ability; they can penetrate and preferentially colonize the core area of the tumor through peripheral blood circulation.73 Therefore, bacteria, viruses, and their components or products have great potential to induce pyroptosis. However, the potential pathogenicity, immunogenicity, rapid clearance in vivo, and unavoidable side effects limit the application of bacteria-based therapeutic strategies. Nanotechnology provides an opportunity for the clinical transformation of bacteria-mediated pyroptosis.74
Studies have shown that Lmo can directly kill tumor cells and activate antitumor immune response.75 To preserve the immunogenicity of Lmo and attenuate its pathogenicity, Liu et al developed a living bacterial delivery platform (Lmo@RBC) by encapsulating Lmo into natural red blood cell (RBC) membranes (Figure 4A).76 The RBC membrane coating significantly improved the biosafety of Lmo, prolonged the in vivo circulation time, and increased its accumulation within the tumor. In CT26 tumor-bearing mice, Lmo@RBC showed the best tumor inhibition effect. Transcriptome analysis of the tumor tissue revealed that Lmo@RBC up-regulated genes associated with immune activation. Further studies showed that Lmo@RBC caused pyroptosis in CT26 cells through the caspase-8/GSDMC pathway (Figure 4B). FCM showed that Lmo@RBC increased the percentage of mature DCs and promoted the infiltration of CD8+ T cells within the tumor. Lmo@RBC combined with BMS-1 (a small-molecule inhibitor of the PD-1/PD-L1 interaction) synergistically inhibited tumor growth and prevented tumor reattack (Figure 4C).
 |
Figure 4 Red blood cell membrane-coated Listeria monocytogenes induced tumor pyroptosis to motivate antitumor immune response. (A) Schematic illustration of the preparation of Lmo@RBC and the underlying mechanism by which Lmo@RBC activates the antitumor immunity. (B) Immunofluorescence staining of the expressions of DAPI (blue), GSDMC (green), and NLRP3 (red) in tumor tissues from CT26 tumor-bearing mice receiving different treatments, scale bar = 50 μm. (C) Distant tumor volumes of CT26 tumor-bearing mice in different treatment groups. *P < 0.05. Note: Reprinted with permission from Liu Y, Lu YP, Ning B et al. Intravenous delivery of living listeria monocytogenes elicits gasdmermin-dependent tumor pyroptosis and motivates anti-tumor immune response. ACS Nano. 2022;16(3):4102–4115. Copyright 2022 American Chemical Society.76. |
Bacterial outer membrane vesicles (OMVs) are spherical nanovesicles with diameters of 20–300 nm derived from gram-negative bacteria.77 OMVs are rich in PAMPs and can be efficiently internalized by antigen-presenting cells, thereby strongly stimulating the innate immune system. Therefore, OMVs are ideal delivery platforms for vaccines and immunotherapy drugs.78 Li et al prepared an OMV-based therapeutic platform (DOX/Ce6-OMVs@M) by encapsulating Ce6- and DOX-loaded OMVs in macrophages.79 OMVs were derived from Escherichia coli. Ce6/DOX-OMVs@M exhibited an enhanced and controlled drug release under laser irradiation. In 4T1 tumor-bearing mice, Ce6/DOX-OMVs@M effectively suppressed tumor growth and eliminated most tumors by the end of the treatment. Moreover, Ce6/DOX-OMVs@M completely prevented lung metastasis. FCM analysis revealed that Ce6/DOX-OMVs@M elevated the percentage of M1-type macrophages and the ratio of M1/M2 macrophages in the tumors. Subsequent studies showed that treatment with OMVs induced pyroptosis in 4T1 cells. Therefore, the excellent antitumor effect of OMVs may be attributed to the modulation of macrophages and the initiation of pyroptosis. This study provides a promising strategy for OMV-mediated combined photodynamic, chemo-, and immunotherapy. OMV is an ideal vector for delivering LPS to tumor cells to induce pyroptosis through the noncanonical pathway. Chen et al designed molecularly engineered OMVs (Apt-OMVs) by coating DNA aptamers (AS1411) on the surface of OMVs.80 AS1411 modification helps mask the immunogenicity of OMVs and enhances their targeting to tumor cells. After intravenous administration, Apt-OMVs specifically accumulated in tumor tissues and successfully delivered LPS into the cytosol of tumor cells, thereby effectively triggering pyroptosis and activating the immune response.
In addition to OMVs, some bacterial exotoxins also have been shown to trigger pyroptosis.81 Pseudomonas aeruginosa exotoxin A (PE24) and Corynebacterium diphtheriae exotoxin (DITOX) are two representative exotoxins that cause pyroptosis and have been used to prepare nanotoxins for tumor therapy.82–84 Zhao et al synthesized a T22-PE24 nanotoxin by the self-assembly of PE24 and the CXCR4 antagonist T22 peptide.84 The results showed that T22-PE24 effectively induced pyroptosis in A2058CXCR4 cells via the caspase-3/GSDME pathway. T22-PE24 effectively inhibited tumor growth in A2058CXCR4 tumor-bearing mice. Moreover, T22-PE24 combined with anti-PD-1 achieved 60% tumor elimination in B16-luci-bearing C57 mice. Immunofluorescence analysis of tumor tissues showed that the combination therapy markedly elevated the levels of CD4+ and CD8+ T cells in the tumors.
Oncolytic viruses (OVs) are natural or artificial viruses that preferentially infect and kill tumor cells while sparing healthy ones. OVs not only directly eliminate tumor cells but also evoke antitumor immune effects, making them a promising strategy for cancer treatment.85 Current research has demonstrated that OVs can induce pyroptosis in tumor cells, thus facilitating tumor immunotherapy.10 Sun and co-workers developed a novel tumor immunotherapy strategy by combining signal transducer and activator of transcription 3 (STAT3) inhibitor nanoprodrugs (MPNPs) and oncolytic herpes simplex virus type 1 (oHSV) (Figure 5A).86 MPNPs were prepared by loading niclosamide (a STAT3 inhibitor) into the ROS/pH dual-responsive mPEG-b-P(2-(methylthio)ethyl methacrylate-co-2-(diisopropylamino) ethyl methacrylate) (mPEG-b-P(MTE-co-PDA)). The results showed that oHSV + MPNPs induced rapid and durable GSDME-mediated pyroptosis. MPNPs specifically accumulated in the tumor via the EPR effect, and oHSV promoted the tumor penetration of MPNPs (Figure 5B). In 4T1 and 4MOSC2 tumor-bearing mice, oHSV + MPNPs showed the most prominent tumor inhibition effect. Histological analysis showed that oHSV + MPNPs induced pyroptosis in tumor tissues and decreased the stemness of tumor cells. FCM analysis showed that oHSV + MPNPs elevated the proportion of CD8+ T cells and mature DCs in 4T1 tumors from 22.16% and 19.31% (control group) to 31.96% and 33.95%, respectively (Figure 5C). Additionally, oHSV + MPNPs significantly decreased the intratumoral infiltration of MDSCs and Tregs, thereby overcoming the immunosuppression of the TME (Figure 5D). Further studies showed that oHSV + MPNPs improved the response rate of anti-PD-1 therapy and effectively inhibited tumor recurrence and metastasis. Therefore, OV-mediated pyroptosis represents a viable tactic for improving antitumor immunotherapy.
 |
Figure 5 ROS/pH dual-responsive STAT3 inhibitor-loaded prodrug nanoparticles (MPNPs) in combination with oncolytic virus synergically inhibited tumor growth by triggering pyroptosis. (A) Schematic illustration of the synthesis of MPNPs and the mechanism by which MPNPs and oncolytic virus elicit antitumor immune response. (B) In vivo distribution of MPNPs@DiR in 4T1-tumor-bearing mice. The tumor is marked by a red circle. (C) Proportion of CD8+ T cells in the tumor of 4T1 tumor-bearing mice receiving different treatments. (D) Proportion of MDSCs in the tumor of 4T1 tumor-bearing mice receiving different treatments. *P < 0.05, **P < 0.01, ***P < 0.001. Note: Reprinted with permission from Su W, Qiu W, Li SJ et al. A dual-responsive STAT3 inhibitor nanoprodrug combined with oncolytic virus elicits synergistic antitumor immune responses by igniting pyroptosis. Adv Mater. 2023;35(11): e2209379. © 2023 Wiley-VCH GmbH.86 |
Nanomaterials for Increasing Intracellular Oxidative Stress
Oxidative stress greatly affects the proliferation, differentiation, and death of tumor cells. Oxidative stress is involved in various forms of PCD including apoptosis, autophagy, pyroptosis, and ferroptosis.87 Studies have shown that increased ROS levels in tumor cells induce pyroptosis of tumor cells through various signaling pathways. First, high levels of ROS promote the dissociation of thioredoxin-interacting protein from thioredoxin and its binding to NOD-like receptor thermal protein domain associated protein 3 (NLRP3), which subsequently activates the inflammasome and triggers pyroptosis via the caspase-1/GSDMD pathway.88 Nicotine,89 saikosaponin-D,90 polyphyllin VI,91 and cadmium92 have been reported to cause ROS-NLNP3-mediated pyroptosis. Second, some substances, such as tetraarsenic hexoxide,93 miltirone,94 CBL0137,95 and apoptin96 elevate intracellular ROS levels and promote the secretion of cytochrome C, thereby triggering caspase-3/GSDME-dependent pyroptosis. In addition, metabolite α-ketoglutarate, a common ROS inducer, initiates pyroptosis through caspase-8/GSDMC pathway.97 However, the relationship between the intracellular ROS levels and pyroptosis requires further investigation.
Tumor treatment strategies such as radiotherapy, PDT, sonodynamic therapy (SDT), and chemodynamic therapy (CDT) can elevate ROS levels in tumor cells to induce pyroptosis, which provides an opportunity for their combination with immunotherapy. However, inherent limitations of these treatments often hinder their ability to induce pyroptosis. Nanomaterials help overcome these defects and enhance the antitumor effects.98 Moreover, some nanodelivery systems deliver ROS inducers into tumors to induce pyroptosis more efficiently.
Nanomaterials for PDT
PDT is a noninvasive cancer treatment technique that mainly activates photosensitizers in tumor tissues to produce ROS to kill tumors through laser irradiation. Studies have shown that PDT can trigger pyroptosis by increasing ROS levels in tumor cells, thus activating antitumor immune effects.99 Photosensitizers are key factors influencing the efficacy of PDT; however, poor water solubility, lack of stability, and the inability to effectively aggregate in tumors limit their clinical application. Li et al developed a carrier-free chemo-photodynamic nanosystem (A-C/NPs) via the self-assembly of cytarabine (Ara-C) and Ce6 for the treatment of breast cancer.100 Ara-C and Ce6 self-assemble into a single nanoplatform through π-π stacking and hydrophobic interaction, which not only increases the stability of Ara-C but also improves the water solubility of Ce6. Under near-infrared (NIR) laser irradiation, A-C/NPs obviously elevated ROS levels in tumor cells and effectively induced pyroptosis. Compared with free drugs, A-C/NPs exhibited increased intratumoral accumulation and prolonged blood circulation time. In bilateral subcutaneous 4T1 tumor-bearing mice, A-C/NPs + laser achieved the best inhibitory effect on distant tumors. FCM revealed that A-C/NPs + laser remarkably increased the percentage of CD4+ and CD8+ T cells in the spleens and distant tumors. Further studies showed that A-C/NPs + laser effectively inhibited tumor recurrence and increased the levels of TCMs and TEMs in the spleens.
To achieve aggregation and specific release of photosensitizers within the tumor, various stimulus-responsive nanoparticles have been designed to enhance PDT-induced pyroptosis. Ma et al synthesized an endogenous/exogenous stimulus-activatable unimolecular polyprodrug (CCNP) containing camptothecin (CPT) and Ce6 as precise nanoinducers of pyroptosis.101 CPT polyprodrug (CDCPT) was prepared from a GSH/ROS dual-responsive CPT monomer and poly (ethylene glycol) methyl ether methacrylate via one-step atom transfer radical polymerization. Subsequently, CCNP was obtained by self-assembly of CDCPT and Ce6 through π-π stacking and hydrophobic interaction. The CCNP showed excellent GSH/ROS-responsive drug release and was effectively internalized by 4T1 cells. Further studies showed that CCNP + laser increased ROS levels in tumor cells by 8.29-fold and successfully caused pyroptosis via the caspase-3/GSDME pathway. RNA sequencing confirmed that CCNP + laser significantly up-regulated the expression of immune-related genes. In 4T1 tumor-bearing mice, CCNP + laser nearly completely eliminated the tumors. FCM analysis revealed that CCNP + laser markedly elevated the levels of mature DCs in the TDLNs and elevated the percentage of CD4+ and CD8+ T cells and M1-type tumor-associated macrophages (TAMs) in the tumors. Moreover, the proportions of M2-type TAMs and MDSCs in the tumors were reduced in the CCNP + laser treatment group. Finally, CCNP + laser + anti-PD-1 effectively inhibited secondary tumor growth, suggesting that CCNP-induced pyroptosis provoked a systemic immune response. Furthermore, Qiu et al prepared acidic TME-evoked MRC nanoparticles to deliver Ce6 and an immune agonist, RGX-104;102 Zhou et al fabricated a GSH-responsive prodrug consisting of Ce6 and a heat shock protein 90 inhibitor, tanespimycin.103 The results demonstrated that these stimuli-responsive nanoparticles enhanced PDT-mediated pyroptosis and activated potent antitumor immune effects.
Oxygen is an essential element in PDT; however, tumor hypoxia greatly impedes PDT efficacy. Tumor hypoxia is a typical characteristic of solid tumors and is mainly due to an imbalance between the inadequate oxygen flow resulting from aberrant vascularization within the tumor and the high oxygen consumption of tumor cells.104 Oxygen consumption and microvascular damage during PDT further exacerbate tumor hypoxia. Therefore, overcoming tumor hypoxia and enhancing the effects of PDT are urgent challenges to be addressed.105 Su et al developed a carbonic anhydrase IX (CAIX)-anchored rhenium photosensitizer (CA-Re) to induce pyroptosis under hypoxia.106 The results showed that CA-Re not only produced singlet oxygen in an oxygen-dependent manner but also produced hydroxyl radicals in an oxygen-independent manner. In vitro experiments confirmed that CA-Re effectively anchored to the cell membrane and down-regulated the expression of CAIX and hypoxia inducible factor-1α (HIF-1α) in hypoxic MDA-MB-231 cells. CA-Re + light effectively caused tumor cell lysis and triggered GSDMD-mediated pyroptosis. In bilateral 4T1 tumor bearing mice, CA-Re + light inhibited primary and distant tumor growth by 78% and 73%, respectively. Compared to that in the control group, CA-Re + light increased the proportion of mature DCs in the TDLNs from 17.3% to 28.4%. The percentage of tumor-infiltrating CD4+ and CD8+ T cells was also significantly increased in the group treated with CA-Re + light. In another study, TiO2 nanoparticles were modified by ruthenium (Ru) complex and further loaded with siRNA of HIF-1α to prepare TiO2@Ru@siRNA.107 TiO2@Ru@siRNA effectively increased intracellular ROS levels in both oxygen-dependent and oxygen-independent manners under 525 nm light irradiation, thus efficiently causing lysosomal damage and inducing pyroptosis. At the same time, siRNA was released to inhibit HIF-1α expression. The results showed that TiO2@Ru@siRNA-mediated PDT dramatically slowed the growth of oral squamous cell carcinoma, which was attributed to the successful overcoming of tumor hypoxia and the reversal of the immunosuppressive TME.
Organelles play important roles in signal transmission, energy conversion, metabolic processes, and protein synthesis in tumor cells. Studies have shown that targeting specific organelles is expected to further improve the therapeutic efficacy of antitumor strategies.108 Since ROS generated by PDT are rapidly eliminated in vivo, and various subcellular organelles play different roles in pyroptosis, precise delivery of photosensitizers to subcellular organelles helps to maximize the induction of pyroptosis. Zeng et al synthesized photosensitizers targeting the mitochondria, lysosomes, and endoplasmic reticulum (Mito-ZS, Lyso-ZS, and ER-ZS) to evaluate the potential for precisely elevating ROS in different subcellular organelles to trigger pyroptosis (Figure 6A).109 Photosensitizers are composed of parental cyanine chromophores, heavy atoms, and targeting moieties for subcellular organelles. The results showed that the subcellular organelle-targeting photosensitizers accumulated in the mitochondria, lysosomes, and endoplasmic reticulum with colocalization coefficients of 0.96, 0.84, and 0.87, respectively. Under irradiation with 520 nm light, subcellular organelle-targeting photosensitizers significantly increased ROS levels in 4T1 cells and successfully triggered pyroptosis via the caspase-1/GSDMD pathway. Notably, Mito-ZS and ER-ZS induced more significant pyroptosis than Lyso-ZS. Transcriptomic analysis showed that PDT activated the pyroptotic pathway while inhibiting the apoptotic and necrotic pathways (Figure 6B). In bilateral 4T1 tumor-bearing mice, subcellular organelle-targeting photosensitizer-induced PDT almost completely suppressed primary tumor growth. However, only Mito-ZS + light and ER-ZS + light achieved better inhibition of distant tumors, which may be attributed to the triggering of a higher grade of pyroptosis. Further studies confirmed that Mito-ZS + light and ER-ZS + light significantly elevated the proportion of mature DCs and CD4+ and CD8+ T cells and reduced the level of Tregs in the tumors. Moreover, Mito-ZS + light and ER-ZS + light increased the percentage of TEMs (Figures 6C and E) and TCMs (Figures 6D and F) in the spleens and effectively inhibited lung metastasis. This study provides a basis for PDT that precisely targets subcellular organelles to maximize pyroptosis.
 |
Figure 6 Organelle-targeting PDT induced pyroptosis to boost antitumor immunotherapy. (A) Schematic illustration of the underlying mechanism of Mito-ZS, Lyso-ZS, and ER-ZS for arousing antitumor immune response. (B) Transcriptomic analysis of pyroptosis pathway in 4T1 cells treated with PDT. Proportion of CD4+ TEMs (C) and TCMs (D) in the spleens of 4T1 tumor-bearing mice receiving different treatments. Proportion of CD8+ TEMs (E) and TCMs (F) in the spleens of 4T1 tumor-bearing mice receiving different treatments. **P < 0.01, ***P < 0.001. Note: Reprinted with permission from Zeng S, Chen C, Zhang LW et al. Activation of pyroptosis by specific organelle-targeting photodynamic therapy to amplify immunogenic cell death for anti-tumor immunotherapy. Bioact Mater. 2023;25:580–593. https://creativecommons.org/licenses/by-nc-nd/4.0/.109 |
After endocytosis, the nanomedicine enters the lysosome at different stages of endosome maturation. Endosomes and lysosomes play important roles in nanomedicine-induced PCD. To explore the effects of targeting different phases of endosome maturation on pyroptosis, Chen et al designed an acid-activatable nanophotosensitizer (ANPS) library as a pyroptosis nanotuner.110 ANPS was prepared by encapsulating Ce6 and a fluorescence quencher (QSY21) in the amphiphilic copolymers mPEG-b-P(R1-r-R2) with a pH transition (pHt) from 5.2 to 6.8. The results show that ANPS with different pHt (from 5.3 to 6.9) precisely targeted different stage of endosome maturation and thus induced endocytic organelle-targeted PDT. ANPS was divided into early endosome (EE)-targeted ANPS (pHt = 6.3–6.9) and late endosome and lysosome-targeted ANPS (pHt = 5.3–6.1) according to the photocytotoxicity efficacy. EE-targeted ANPS-mediated PDT effectively induced the pyroptosis of GSDME-positive tumor cells, but the pyroptosis-inducing activity was reduced when ANPS were transported into late endosomes/lysosomes. In vivo experiments revealed that EE-targeted ANPS-mediated PDT achieved better tumor control and longer survival than late endosome- and lysosome-targeted ANPS-mediated PDT. In 4T1 tumor-bearing mice, ANPS-mediated PDT significantly elevated the number and antitumor capacity of tumor-infiltrating CD8+ T cells.
Nanomaterials for CDT
CDT is an emerging treatment modality that utilizes endogenous hydrogen peroxide (H2O2) in tumors to produce toxic hydroxyl radicals via a Fenton or Fenton-like reaction, catalyzed by metal ions, to kill tumor cells.111 Compared to other ROS-elevating therapies, CDT presents the advantages of higher ROS catalytic performance, deep tissue penetration, less dependence on external stimuli, and less susceptibility to treatment resistance.112 However, insufficient H2O2 in tumors and the complex reducing microenvironment severely restrict the efficacy of CDT. Numerous nanomaterials have been created to increase the efficacy of CDT and induce pyroptosis.
Ling and co-workers fabricated a virus-spike TME-responsive pyroptotic agent (VTPA) composed of an organosilica-coated iron oxide nanoparticle core and spiky manganese dioxide protrusions.113 The virus-spike structure contributes to intracellular lysosomal rupture. Stimulated by high concentrations of intracellular GSH, VTPA degraded and released Mn ions and iron oxide nanoparticles, which generated robust ROS through the Fenton reaction, activating inflammasome and inducing pyroptosis. In another study, Xu and co-workers encapsulated oligomycin A (OA), an inhibitor of mitochondrial respiratory, in a cyclic arginine–glycine–aspartic (c(RGDyC))-modified iron constructed metal-organic framework (FeMOF) to form RGD-OA@FeMOF nanoparticles (FeOA).114 FeOA nanoparticles exhibited GSH-responsive drug release, and the released iron generated large amounts of oxidative hydroxyl radicals (•OH) via the Fenton reaction. After co-incubation with tumor cells, FeOA nanoparticles effectively triggered pyroptosis, mainly due to the elevated oxidative stress caused by OA-induced mitochondrial dysfunction and the FeMOF-mediated Fenton reaction. In B16-xenograft bearing nude mice, FeOA nanoparticles achieved optimal tumor growth control with a tumor suppression rate as high as 65.13%. The combination of FeOA nanoparticles and anti-PD-L1 synergistically slowed tumor growth and prevented lung metastasis in B16 tumor-bearing mice. Further studies showed that FeOA nanoparticles + anti-PD-L1 significantly elevated the proportion of mature DCs in the lymph nodes and the level of CD8+ T cells in the tumors, indicating that FeOA nanoparticle-induced pyroptosis sensitized melanoma immunotherapy.
However, the therapeutic efficacy of single treatment modality is limited. To induce pyroptosis more effectively, CDT is often combined with other therapeutic strategies to inhibit tumor growth, and nanomaterials provide a platform for combination therapy.115 Xu et al prepared polyvinyl pyrrolidone (PVP)-modified NiS2/FeS2 hybrid nanoparticles (PVP-NiS2/FeS2 NPs) for synergistic cancer therapy.116 Because of the abundance of Fe2+/Fe3+ and Ni2+/Ni3+ redox couples, PVP-NiS2/FeS2 NPs showed GSH peroxidase-like reactivity and produced a large quantity of •OH through the Fenton reaction, thus inducing pyroptosis and ferroptosis. Moreover, PVP-NiS2/FeS2 NPs mediated the combined effects of CDT/PDT/photothermal therapy (PTT) under NIR irradiation and enabled photoacoustic and magnetic resonance imaging. PVP-NiS2/FeS2 NPs + NIR irradiation dramatically suppressed 4T1 tumor growth and prevented lung metastasis. In the bilateral tumor model, the expression of immune activation-related genes was elevated, and the expression of immunosuppression-related molecules was greatly reduced in the distant tumors of mice receiving PVP-NiS2/FeS2 NPs + NIR treatment, indicating the activation of systemic antitumor immune effects.
Nanomaterials for SDT
SDT is a noninvasive therapeutic modality that uses ultrasound (US) to activate sonosensitizers to generate ROS to kill tumor cells. Due to the high tissue penetration, non-ionizing radiation, and high controllability of US, SDT has the advantages of high antitumor efficacy and few side effects.117 SDT not only kills tumors directly through ROS, but also activates the immune response by triggering immunogenic cell death. Various multifunctional nanoplatforms have been established to enhance SDT-based immunotherapy.118 As a therapeutic strategy for increasing ROS levels, SDT can also induce pyroptosis. Chen and co-workers encapsulated Ce6 and hydrophilic tirapazamine (TPZ) in zeolitic imidazole frameworks-8 (ZIF-8) to obtain ZIF-8@TPZ/Ce6 nanoparticles and further modified them with the cytomembrane of gastric cancer cells.119 The prepared ZTC@M nanoparticles exhibited enhanced cellular uptake and significantly elevated intracellular ROS levels under US irradiation, which aggravated tumor hypoxia and activated TPZ. In AGS tumor-bearing mice, ZTC@M combined with US inhibited tumor growth, and the tumor inhibition rate was 87%. Further studies confirmed that ZTC@M + US triggered pyroptosis in tumor cells through the caspase-1/GSDMD pathway. This study provides a promising for treating gastric cancer by combining SDT and chemotherapy.
Although pyroptosis of tumor cells activates antitumor immune effects, the dense ECM impedes immune cell infiltration into the tumor, thereby promoting tumor immune escape. In order to remodel the TME for enhancing tumor immunotherapy, Chen et al loaded LY364947, a specific inhibitor of transforming growth factor-β type I receptor (TGF-βR1), in porous coordination network (PCN-224) nanoparticles and further decorated them with RBC membranes to prepare LY364947@PCN-224@membrane (LPM) nanoparticles.120 Under US irradiation, LPM significantly increased the ROS levels in 4T1 cells and triggered pyroptosis in tumor cells pretreated with DAC through the caspase-3/GSDME pathway. In vivo experiments showed that LPM accumulated specifically within tumors, removed 48% of the collagen, and increased the number of tumor-infiltrating lymphocytes by 1.9-fold. In 4T1 tumor-bearing mice, LPM + DAC + US almost completely eliminated tumors. FCM analysis showed that LPM + DAC + US significantly increased the number of mature DCs in the TDLNs and increased the proportion of tumor-infiltrating CD8+ T cells. Notably, the proportion of tumor-infiltrating CD8+ T cells in the LPM + DAC + US treatment group was 1.3 times higher than that in the PM (PCN@RBC) + DAC + US treatment group, indicating that collagen depletion amplified pyroptosis-induced immune activation. LPM + DAC + US also effectively prevented tumor re-challenge and elevated the levels of TCMs and TEMs in the spleens.
Nanomaterials for Cancer Starvation Therapy
Abnormal cellular metabolism and increased nutrient intake are the primary physiological characteristics of cancer cells. Cancer starvation therapy is an attractive tumor treatment strategy aimed at killing tumor cells by blocking the nutrient supply. Inhibition of tumor angiogenesis, direct blocking of tumor blood vessels, inhibition of glycolysis, and intervention in amino acid metabolism are commonly used strategies in cancer starvation therapy.121 The use of GOx and other enzymes to rapidly consume glucose and thus block the energy supply of tumors is an effective way to inhibit tumor growth.122 Compared with normal cells, tumor cells consume more glucose to maintain their growth. As a natural oxidoreductase, GOx catalyzes the oxidation of glucose to produce gluconic acid and H2O2, thereby increasing oxidative stress in the TME. Studies have shown that a GOx-based ROS elevation strategy can induce pyroptosis and reverse tumor resistance to apoptosis.123 However, the application of GOx faces challenges, such as poor stability in vivo, short blood circulation time, and off-target side effects. A series of nanodelivery platforms have been developed to address these issues.
In order to maintain the catalytic activity of GOx and improve its catalytic efficiency, Li et al designed a ROS-responsive nanoreactor (GOx@PICsomes) by loading GOx in polyion complex vesicles (PICsomes) made of poly([2-[[1-[(2-aminoethyl)thio]-1methylethyl]thio]ethyl]-α,β-aspartamide) (PATK) and PEG-b-poly(α,β-aspartic acid) (PEG-b-PAsp).124 Upon H2O2 exposure, the volume and surface area of the PICsomes expanded by 256% and 187%, respectively, as the ROS-responsive linkers in PATK were cleaved, while the structure of the vesicles remained intact. Size expansion of PICsomes increases membrane permeability and allows more glucose to cross the membrane to be catalyzed by GOx. At the same time, PICsomes can effectively protect GOx from degradation by proteases. Subsequent studies confirmed that GOx@PICsomes exhibited enhanced catalytic efficiency, longer catalytic activity, and higher cytotoxicity. When tumor cells were incubated with GOx@PICsomes, typical morphological changes associated with pyroptosis were observed. The secretion of pro-inflammatory cytokines and immune activators, including IL-1β, CRT, and HMGB1, were significantly increased, indicating that GOx@PICsomes-mediated pyroptosis has great potential to boost antitumor immunotherapy.
Oxygen is necessary for GOx to catalyze glucose decomposition; however, a hypoxic TME often limits the catalytic efficiency of GOx. Zhang et al fabricated HA-modified GOx-Mn nanoparticles (GOx-Mn/HA) with two enzymatic activities using a biomineralization-like method to regulate tumor glycometabolism and induce pyroptosis of tumor cells (Figure 7A).125 Mn-containing nanozymes catalyze H2O2 within the tumor to produce oxygen, which can be used by GOx to degrade glucose to generate H2O2, thus forming a catalytic circulation system that consumes glucose. In vitro experiments showed that GOx-Mn/HA induced pyroptosis of 4T1 cells through the caspase-1/GSDMD pathway and increased PD-L1 expression on tumor cells (Figures 7B and C). In 4T1 tumor-bearing mice, GOx-Mn/HA + anti-PD-L1 demonstrated the best antitumor efficacy with a tumor inhibition rate of 92.9%. FCM analysis showed that GOx-Mn/HA + anti-PD-L1 significantly increased the proportion of mature DCs in the TDLNs and the percentages of CD3+, CD4+, and CD8+ T cells in the tumors (Figures 7D and E). Furthermore, combination therapy downregulated the levels of immunosuppressive cells, including M2-type macrophages, MDSCs, and Tregs. Real-time polymerase chain reaction revealed that GOx-Mn/HA + anti-PD-L1 up-regulated genes related to immune activation in tumors and inhibited the expression of immunosuppressive genes (Figure 7F). Finally, the combination therapy effectively prevented tumor recurrence and distant metastasis, indicating that the immune memory effect was activated. In another study, Ding et al designed pyroptosis adjuvants by loading GOx into urchin-like manganese oxide and demonstrated the excellent antitumor efficacy and potent immune activation of pyroptosis adjuvants.126 These results provide a basis for activating antitumor immune effects by inhibiting tumor glycometabolism.
 |
Figure 7 Biomineralized two-enzyme nanoparticles triggered tumor cell pyroptosis by regulating tumor glycometabolism to boost antitumor immunotherapy. (A) Schematic illustration of the synthesis and mechanism of biomineralized two-enzyme nanoparticles. (B) Representative image of 4T1 cells after different treatments observed by microscopy; the pyroptotic cells were marked by arrows, scale bar = 50 μm. (C) The expression of PD-L1 on 4T1 tumor cells receiving different treatments. Proportion of CD4+ T cells (D) and CD8+ T cells (E) in the tumor of 4T1 tumor-bearing mice receiving different treatments. (F) The expression of multiple genes in 4T1 tumors detected by RT-PCR. (I) PBS, II: Mn-NP/HA, III: GOx-Mn, IV: GOx-Mn/HA, (V) GOx-Mn/HA + anti-PD-L1. *P < 0.05, **P < 0.01, ***P < 0.001. Note: Reprinted with permission from Zhang SJ, Zhang Y, Feng YJ et al. Biomineralized two-enzyme nanoparticles regulate tumor glycometabolism inducing tumor cell pyroptosis and robust antitumor immunotherapy. Adv Mater. 2022;34(50):e2206851. © 2022 Wiley-VCH GmbH.125 |
The tricarboxylic acid cycle not only provides energy to tumor cells, but also produces intermediate metabolites that are strongly associated with the occurrence and metastasis of tumor.127 Pyruvate dehydrogenase kinase 1 (PDHK1), a mitochondrial protein, inhibits pyruvate dehydrogenase (PDH) activity through phosphorylation, thereby limiting the inflow of the tricarboxylic acid cycle. PDHK1 is closely associated with metabolic remodeling, growth, and metastasis of tumors.128 Jin et al developed an amphiphilic block polymer (OPDEA-b-PDCA) that targets the mitochondria through the self-assembly of tertiary amine-oxide-based zwitterionic poly[2-(N-oxide-N,N-diethylamino)ethyl methacrylate] (OPDEA) and dichloroacetate (DCA).129 DCA, a selective inhibitor of PDHK, has been reported to increase mitochondrial oxidative stress and inhibit tumor growth.130 The results revealed that OPDEA-b-PDCA precisely colocalized with mitochondria and significantly increased ROS levels in the mitochondria after internalization by tumor cells. Further analysis showed that OPDEA-b-PDCA significantly reduced intracellular lactate levels, suggesting that OPDEA-b-PDCA increased the inflow of pyruvate into mitochondria and promoted oxidative phosphorylation. OPDEA-b-PDCA effectively triggered pyroptosis in tumor cells by releasing cytochrome C and cleaving GSDMD. Additionally, OPDEA-b-PDCA-induced pyroptosis up-regulated extracellular PD-L1 expression. In K7M2 tumor-bearing mice, OPDEA-b-PDCA + anti-PD-L1 slowed the tumor growth and extended the survival of mice. FCM analysis showed that OPDEA-b-PDCA not only increased the percentage of CD8+ T cells in the tumors, but also markedly increased the proportion of exhausted (PD-1+Tim-3+CD8+) T cells. In contrast, OPDEA-b-PDCA + anti-PD-L1 significantly reduced the number of exhausted T cells, suggesting that the combination therapy is necessary and effective.
Nanomaterials for Delivering ROS Inducers
Apart from the above strategies that can increase ROS, some drugs or compounds can directly or indirectly increase ROS levels in tumors, thereby inducing pyroptosis. Nanomaterials help deliver ROS inducers specifically to tumor tissues and improve their stability and biocompatibility. Wang et al confirmed that CyNH2 (an amino-containing hemicyanine fluorophore) causes mitochondrial membrane damage through aggregation in mitochondria, further increasing intracellular ROS levels, promoting the cytochrome C release, and finally inducing pyroptosis of tumor cells through the caspase-3/GSDME pathway.131 However, its high toxicity and inability to target tumor cells limit its application in antitumor therapies. As NAD(P)H: quinone oxidoreductase isozyme 1 (NQO1) is highly expressed in a variety of tumor cells, they prepared an NQO1-responsive CyNH2 theranostic (NCyNH2) and further encapsulated it in poly(ethylene glycol)-block-poly(lactic-co-glycolic acid) (PEG-b-PLGA) to form NCyNP. The results showed that NCyNP selectively aggregated in tumor tissues with high expression of NQO1. In 4T1 tumor-bearing mice, NCyNP + anti-PD-1 achieved the highest tumor suppression rate (71.5%) without causing significant weight loss or organ damage. Further studies showed that the levels of mature DCs in the TDLNs and the proportion of CTLs in the tumors were significantly increased in the NCyNP + anti-PD-1 treatment group. Moreover, the production of IFN-γ and TNF-α was much higher in the combination treatment group than in the control group. These results demonstrate that NCyNP-triggered pyroptosis boosts the antitumor effects of anti-PD-1.
The delivery of H2O2 into tumor tissues is an effective way to increase oxidative stress in the TME. Zheng et al fabricated ZnO2 nanoparticles by mixing Zn(CH3COO)2 and H2O2 in a polyethylenimine solution for the specific delivery of H2O2 to tumors.132 The ZnO2 nanoparticles remained stable at pH 7.4, but degraded and released H2O2 and Zn2+ under acidic conditions. After co-incubation with 4T1 cells, high concentration of ZnO2 nanoparticles (HZnO2) triggered pyroptosis, while low concentration of ZnO2 nanoparticles (LZnO2) induced apoptosis, which may be attributed to the different amounts of H2O2 produced. The immune activation assay revealed that the proportions of mature DCs and CD8+ T cells in the HZnO2 group were 44.1% and 33.7%, respectively, which were obviously higher than those in the LZnO2 group (20.2% and 11.7%, respectively), indicating that HZnO2-induced pyroptosis effectively activated the immune response. In 4T1 tumor-bearing mice, HZnO2 significantly inhibited tumor growth and lung metastasis. The depletion of GSH within the TME is another means of disrupting redox equilibria. Zhen et al prepared (-)-epigallocatechin-3-o-gallate-molybdenum ion coordination nanoparticles (BEM NPs) to consume intracellular GSH via the Michael addition reaction.133 The results showed that BEM NPs successfully induced pyroptosis and provoked a strong antitumor immune effect.
Nanomaterials for Causing Changes in Osmotic Pressure or Inducing Ion Imbalance
Ions are essential for maintaining the normal activities of living organisms and are widely involved in a variety of complex physiological and biochemical processes, such as material transport, energy conversion, signal transduction, and metabolic regulation. The type and distribution of ions are closely related to the physicochemical properties and biological effects of cells. An imbalance in ion homeostasis seriously affects the normal physiological functions of cells and even leads to cell death.134 Ion interference therapy is a novel tumor treatment strategy that alters the physicochemical properties and biological functions of cancer cells by regulating intracellular ion distribution, ultimately killing tumor cells.135 However, tumor cells have a strong self-regulatory ability, and when the ion distribution is abnormal, they initiate a self-regulatory mechanism to restore ion homeostasis. Preventing tumor cells from initiating the self-regulation of ionic imbalance, and thus achieving a reverse distribution of concentration gradients of specific ions in tumor cells, is a challenge that needs to be addressed for ion interference therapy. Bioactive nanomaterials can release excess ions that exceed the self-regulation threshold of cells in a very short time, causing sudden changes in ion distribution, osmotic pressure, pH, or redox imbalances, and functional protein inactivation, thereby inducing cell death.136 Research has shown that the use of nanomaterials to deliver sodium, potassium, or calcium ions to tumor cells to cause sudden changes in their concentrations can lead to pyroptosis, which is a potential new strategy for treating tumors.
Nanomaterials for Causing Sodium Ion Imbalance
Sodium ions are the major cations in the extracellular fluid of the human body and play a key role in the physiological activities of the body, mainly in the maintenance of cellular osmolality, acid-base balance, neuromuscular excitability, cell membrane permeability, and the normal morphology of cells. Studies have shown that elevated intracellular sodium and chloride concentrations induce cell death by elevating intracellular ROS levels, promoting cytochrome C release from the mitochondria, and activating caspases.137 However, it is challenging to enable the specific accumulation of sodium and chloride ions in tumor cells and avoid the ionic self-regulatory mechanisms of tumor cells. To address this challenge, Jiang et al prepared NaCl nanoparticles (SCNPs) via a microemulsion reaction and modified them with PEGylated phospholipids to prepare phospholipid-coated SCNPs (PSCNPs).138 After being taken up by tumor cells through endocytosis, PSCNPs broke down and released sodium and chloride ions, significantly increasing their concentrations. After incubation with PSCNPs for 6 h, the ATP levels in PC-3 cells decreased by 36.0%, whereas the ROS levels increased by 22.3%. Morphological observation showed that PSCNPs induced pyroptosis of tumor cells, which was further confirmed by increased caspase-1, GSDMN, LDH, and IL-1β levels. In vivo antitumor experiments showed that PSCNPs obviously inhibited tumor growth and that their antitumor effect in syngeneic tumor models was much better than that in xenograft tumor models. In bilateral SCC VII tumor-bearing mice, PSCNPs markedly suppressed the growth of secondary tumors, with a tumor inhibition rate of 53% on day 12. FCM analysis showed that PSCNPs significantly increased the percentage of CD8+ T cells in the spleens and the levels of effector T cells (CD8+IFN-γ+) and activated DCs in the primary tumors, indicating that PSCNPs activated the antitumor immunity.
However, the ability of a single factor to induce pyroptosis remains limited. Combining multiple triggers helps induce more potent pyroptosis. Liu et al fabricated phospholipid-coated Na2S2O8 nanoparticles (PNSO NPs) to increase ROS levels in tumor cells and induce a sudden increase in sodium ion concentration to trigger pyroptosis.139 PNSO NPs entered tumor cells through endocytosis and generated Na+ and S2O82− via gradual degradation. Subsequently, S2O82− transformed into toxic •SO4− and •OH to increase oxidative stress within the cell. The large amount of sodium ions released from the PNSO NPs obviously increased the intracellular osmolarity, leading to cell rupture and lysis. In vitro experiments confirmed that PNSO NPs triggered pyroptosis of tumor cells and promoted DC maturation. In bilateral 4T1 tumor-bearing mice, PNSO NPs markedly slowed the growth of both primary and distant tumors, and almost completely eliminated tumors when combined with anti-CTLA4. Compared with the control group, the proportion of mature DCs in the TDLNs and the levels of CD8+ and CD4+ T cells in the distant tumors of the PNSO NPs group were obviously increased. Moreover, the concentrations of perforin and granzyme B in the PNSO NPs group were much higher than those in the control group.
Tumor vaccines have gained increasing attention in recent years, which control or remove tumors by transferring tumor antigens in different forms into the patient’s body to activate the immune system and evoke cellular and humoral immune responses.140 Because pyroptosis helps to recruit and activate immune cells, pyroptosis-based tumor vaccines have attractive application prospects. Tang et al prepared Na-IVAl-DMSN by modifying dendritic mesoporous silica nanoparticles with sodium-stabilized four-coordinate aluminum species to induce DC pyroptosis and activate immune response.141 Na-IVAl-DMSN showed outstanding antigen-loading capacity and sustained antigen-release properties. After internalization by DCs, Na-IVAl-DMSN significantly increased the concentration of sodium ions and promoted the efflux of potassium through pH-responsive intracellular ion exchange, thus triggering pyroptosis in DCs through the caspase-1/GSDMD pathway. The release of pro-inflammatory cytokines and cell contents from pyroptotic DCs further promoted the hyperactivation of DCs. In vivo experiments showed that CT26 cell lysate-loaded Na-IVAl-DMSN obviously inhibited the growth of CT26 tumors. FCM analysis showed that the percentage of activated DCs and the number of activated memory T cells in the spleens of the Na-IVAl-DMSN group were markedly higher than those of the control group. Furthermore, Na-IVAl-DMSN significantly elevated the proportion of IFN-γ+CD8+ T cells, IFN-γ+CD4+ T cells, and IFN-γ+ NK cells in the spleens and tumors. This research provides insights for the rational design of pyroptosis-based tumor vaccines.
Nanomaterials for Causing Potassium Ion Imbalance
Potassium ions are one of the main cations in intracellular fluids and are mainly involved in osmotic pressure, acid-base balance, energy metabolism, and the maintenance of normal neuromuscular function. They are closely associated with the proliferation, apoptosis, and immune escape of tumor cells.142 Studies have shown that intracellular potassium imbalances such as potassium efflux activate the NLRP3 inflammasome, which in turn initiates caspase-1-dependent pyroptosis.143 Nigericin (Nig), a polyether antibiotic derived from Streptomyces hygroscopicus, acts as a potassium ionophore that promotes K+/H+ exchange through the lipid bilayer. Nig has been reported to decrease the intracellular potassium ion concentration, thereby activating NLRP3 inflammasome-mediated pyroptosis.144 Niu et al encapsulated Nig and DAC in hexahistidine (His6)-metal assembly particles ((Nig+DAC)@HmA) to induce pyroptosis and boost antitumor immunity.145 The prepared (Nig+DAC)@HmA nanoparticles were efficiently taken up by tumor cells and successfully escaped from the lysosomes. After incubation with (Nig+DAC)@HmA, MB49 cells showed increased expression of NLRP3 and GSDMD, as well as typical morphological features of pyroptosis. (Nig+DAC)@HmA-induced pyroptosis promoted macrophage polarization into M1-type macrophages. In MB49 tumor-bearing mice, (Nig+DAC)@HmA showed the most significant tumor suppressive effects. Increased levels of mature DCs, M1-type macrophages, CD3+, CD4+, and CD8+ T cells were observed in the (Nig+DAC)@HmA treatment group, indicating that an antitumor immune response was activated.
Rare earth-doped upconversion nanoparticles (UCNPs) have been extensively employed in tumor therapy owing to their unique physicochemical properties.146 UCNPs are promising vehicles for ion delivery, owing to their richness in a wide range of ions. Ding et al fabricated biodegradable K3ZrF7:Yb/Er upconversion nanoparticles (ZrNPs) using a thermal decomposition method to induce pyroptosis by disrupting ion and osmotic pressure homeostasis in tumor cells.147 ZrNPs were internalized by tumor cells through endocytosis and released large amounts of K+ and [ZrF7]3− ions, resulting in sudden changes in intracellular osmolarity and ion imbalance. Subsequent experiments revealed that ZrNPs successfully triggered pyroptosis in 4T1 cells via the caspase-1/GSDMD pathway. Subsequent assessment showed that ZrNPs increased the percentages of CD3+ T cells, CD8+ T cells, TEMs, and DCs in the TDLNs and spleens. In 4T1 tumor-bearing mice, ZrNPs obviously inhibited tumor growth and distant metastasis.
Nanomaterials for Causing Calcium Ion Imbalance
As second messengers in intracellular signaling, calcium ions play a crucial role in maintaining the normal physiological functions of cells and subcellular organelles. Studies have shown that a sudden increase in the intracellular calcium ion concentration can disable the function of calcium ion channels in tumor cells, resulting in calcium overload and promoting tumor cell death. The induction of intracellular calcium overload is considered a promising method to defeat tumors with efficient tumor inhibition and less susceptibility to drug resistance.148 Various nanomaterials have been developed to trigger calcium overload in tumor cells. Zheng et al fabricated biodegradable Ca2+ nanomodulators (CaNMs) composed of CaCO3 and curcumin (CUR) to trigger pyroptosis by promoting mitochondrial calcium overload (Figure 8A).149 CaCO3 releases Ca2+ into the acidic TME, whereas CUR promotes the release of Ca2+ from the endoplasmic reticulum and inhibits the efflux of Ca2+, which synergistically triggers calcium overload. At pH 5.5, CaNMs dissociated and released Ca2+ and CUR. With the concentration of 200 μg mL−1, CaNMs effectively induced pyroptosis of 4T1 cells. Increased level of Ca2+ and up-regulated expression of cytochrome C and GSDME-N were detected in CaNM-treated tumor cells. In vivo immune activation assays revealed that the percentage of mature DCs and the proportion of CD8+ T cells in the CaNMs group were 2.5-fold and 3.8-fold higher than those in the control group, respectively (Figures 8B-E). In 4T1 tumor-bearing mice, CaNMs clearly inhibited tumor growth and lung metastasis.
 |
Figure 8 Biodegradable Ca2+ nanomodulators triggered tumor cell pyroptosis through mitochondrial Ca2+ overload to enhance immunotherapy. (A) Schematic illustration of the fabrication and mechanism of Ca2+ nanomodulators for activating the antitumor immune response. Representative images (B) and quantification (C) of mature DCs in the spleens of 4T1 tumor-bearing mice in different treatment groups detected by FCM. Representative images (D) and quantification (E) of CD8+ T cells in the spleens of 4T1 tumor-bearing mice in different treatment groups detected by FCM. ***P < 0.001. Note: Reprinted with permission from Zheng P, Ding BB, Zhu GQ, Li CX, Lin J. Biodegradable Ca2+ nanomodulators activate pyroptosis through mitochondrial Ca2+ overload for cancer immunotherapy. Angew Chem, Int Ed. 2022;61(36):e202204904. © 2022 Wiley-VCH GmbH.149 |
Studies have shown that the photothermal effect of NIR light irradiation promotes the opening of specific calcium ion channels, thereby increasing the concentration of calcium ions in cell.150 Zhao et al synthesized biomimetic nanoparticles (BNP) by encapsulating indocyanine green (ICG) and DAC in breast cancer membrane-coated PLGA to induce pyroptosis in cancer cells.151 Under NIR irradiation, BNP degraded due to hyperthermia and released the loaded drug. BNP showed 77% higher cellular uptake by 4T1 cells than free ICG due to modification of the tumor cell membrane. Under low-dose NIR irradiation for 2 minutes, the intracellular concentration of Ca2+ in 4T1 cells treated with BNP was 4.1-fold higher than that in 4T1 cells treated with free ICG. Subsequent studies showed that BNP-triggered Ca2+ influx promoted cytochrome C release and caspase-3 activation. Simultaneously, because DAC increased GSDME expression in tumor cells, BNP successfully triggered pyroptosis in tumor cells. In bilateral 4T1 tumor-bearing mice, BNP combined with photoactivation markedly suppressed the growth of primary and distant tumors and extended the survival of the mice. Further studies showed that BNP combined with photoactivation enhanced the infiltration of DCs in the primary tumors and significantly elevated the level of mature DCs in the tumors and TDLNs. Moreover, increased infiltration of CD4+ and CD8+ T cells was detected in the distant tumors and spleens. Immunofluorescence staining revealed reduced Treg levels in the distant tumors in the combination treatment group. These results suggest that the Ca2+ influx induced by the photothermal effect is an alternative way to trigger pyroptosis.
Conclusions and Perspectives
PCD is a genetically controlled, autonomous, and orderly cell death process that involves the activation, expression, and regulation of a series of genes, and plays a critical role in maintaining the stability of the body’s internal environment. In addition to apoptosis, an increasing number of forms of PCD have been identified in recent years, including necroptosis, autophagy, pyroptosis, ferroptosis, and cuproptosis.152 As a proinflammatory PCD, the mechanism and function of pyroptosis have gradually been discovered in recent years. Growing evidence suggests that pyroptosis has enormous potential for enhancing antitumor immunity.153 By releasing tumor antigens and a large number of pro-inflammatory factors, pyroptosis of tumor cells contributes to promoting the maturation of DCs, increasing the intratumoral infiltration of CTLs and NK cells, and reducing the accumulation of immunosuppressive cells. Some existing tumor therapeutic strategies, such as chemotherapy, radiotherapy, PDT, PTT, CDT, and SDT, have been proven to be capable of inducing pyroptosis in tumor cells, providing a basis for the development of pyroptosis-based immunotherapy. For example, Cao et al demonstrated that ionizing radiation induced pyroptosis in GSDME-overexpressing 4T1 tumors via the caspase-9/caspase-3/GSDME pathway, which significantly increased the levels of CTLs and related cytokines within the tumors.154 However, pyroptosis of healthy tissues or normal cells is associated with increased side effects. Therefore, precisely triggering pyroptosis in tumor cells and avoiding damage to normal tissues are challenges that need to be urgently addressed.
Nanotechnology provides opportunities for the application and development of pyroptosis in immunotherapy. Currently, a variety of nanomaterials have been developed to trigger pyroptosis and activate antitumor immune responses. (Table 1). First, they enhance the in vivo stability, biocompatibility, and biodegradability of pyroptosis initiators, particularly proteins, nucleic acids, bacteria, and viruses. LPS is a representative pyroptosis initiator, but high immunogenicity and systemic toxicity limit its in vivo application. Chen et al utilized DNA aptamer-equipped OMVs to deliver LPS into tumor cells, effectively masking the immunogenicity of LPS and improving its biocompatibility and biosafety.80 A variety of functionalized polymers have been developed for intracellular delivery of proteins to improve their stability, avoid immune clearance, enhance their cellular uptake, and promote lysosomal escape.155 Second, nanomaterials precisely deliver pyroptosis initiators to tumor tissues, cells, or target organelles through the EPR effect or active targeting, thereby efficiently triggering pyroptosis in tumor cells and reducing side effects on normal cells. For example, cell membrane-coated nanoparticles not only inherit some of the functions from the source cell but also acquire better biocompatibility, weaker immunogenicity, long blood circulation, and excellent tumor-targeting ability; therefore, they are gradually being used in various therapeutic strategies.156 Third, compared with the environment of normal tissues, the TME possesses many abnormal physiological features, including weak acidity, hypoxia, imbalance of redox homeostasis, and high expression of some specific enzymes. Stimuli-responsive nanomaterials release drugs responsively in the tumor tissue or tumor cells, thus enabling their precise spatiotemporal release and on-demand release.157,158 Nanomaterials also provide multi-mode imaging techniques to detect tumors at an early stage and monitor treatment response. Therefore, nanomaterials offer a promising approach for precise and personalized cancer therapy with improved efficacy and reduced side effects. Fourth, nanomaterials achieve deep tumor penetration by promoting tumor vascular normalization, degrading the extracellular matrix, regulating the particle size or charge, and utilizing active transcellular transport.159,160 Furthermore, some metal nanomaterials such as MOF nanoparticles directly induce pyroptosis by increasing oxidative stress, inducing ion imbalance, and causing sudden changes in osmotic pressure. Ploetz et al prepared lipid-coated iron-based MOF nanoparticles (Lip-MOFs) and showed that the delivery of large amounts of iron ions into tumor cells by Lip-MOFs induced pyroptosis rather than ferroptosis.161 Finally, the antitumor effects of a single therapeutic strategy are usually limited, and nanomaterials provide a platform for multimodal combination therapies based on pyroptosis.162
 |
Table 1 Summary of Representative Nanomaterials for Pyroptosis-Based Tumor Immunotherapy |
Although nanomaterials have led to tremendous developments in pyroptosis-based immunotherapy in recent years, some challenges remain unaddressed. First, the antitumor mechanism of pyroptosis and the related signaling pathways need to be further evaluated and studied. Most current studies suggest that pyroptosis plays a double-edged role in tumors. Pyroptosis of tumor cells helps to overcome the resistance of tumor cells to apoptosis, recruit immune cells, and reverse the immunosuppressive TME, so as to effectively suppress tumor growth and metastasis. In contrast, the chronic inflammatory microenvironment created by pyroptosis promotes the malignant transformation of normal cells, thereby promoting tumorigenesis and progression. Pyroptosis may play different roles in normal and tumor tissues and at different stages of tumor development. In addition to tumor cells, studies have shown that pyroptosis of DCs and macrophages activates antitumor immune responses, but the mechanisms and effects of immune regulation may differ.141,163 Therefore, further research is needed to explore the mechanism of pyroptosis to maximize its antitumor effects.
Second, the efficient induction of pyroptosis or conversion of apoptosis into pyroptosis is another concern that requires careful consideration. Most compounds or therapeutic strategies trigger pyroptosis via the caspase-3/GSDME pathway. However, GSDME is underexpressed in most tumors because of GSDME gene hypermethylation, which greatly limits the occurrence of pyroptosis. The delivery of DNA methylase inhibitors, GSDME proteins, or genes into tumor cells through nanomaterials is an effective measure for elevating GSDME expression. In addition to GSDMD and GSDME, the roles of other members of the GSDM protein family in pyroptosis have not been fully elucidated. Recent studies have shown that GSDMB splice variants play different roles in the induction of pyroptosis. The generation of cytotoxic GSDMB isoforms by selective mRNA splicing may be a potential strategy for triggering pyroptosis and enhancing antitumor immunity.164,165 Exploring GSDME pathway-independent pyroptosis initiators may provide new directions for antitumor therapy.
Third, the TME, which is composed of the ECM, immunosuppressive cells, and immunosuppressive cytokines, severely limits the efficacy of pyroptosis-based immunotherapy. Although pyroptosis recruits CTLs and NK cells, dense ECM often limits their infiltration into tumors. Nanomaterials that degrade or remodel the ECM are beneficial for promoting immune cell infiltration.166 Immunosuppressive cells, such as tumor-associated macrophages, Tregs, and MDSCs, greatly limit the immune activation effect of pyroptosis, and targeted elimination of these cells may further enhance antitumor immunotherapy.40 Tumor hypoxia is an important cause of immune escape in solid tumors and inhibits the function of T cells and increases the number of immunosuppressive cells.167 Multifunctional nanomaterials alleviate tumor hypoxia by delivering oxygen directly into the tumor, generating oxygen in situ from H2O2 in the tumor tissue, and promoting tumor vascular normalization, thus reversing immunosuppression.168 Improving tumor hypoxia is a potential strategy for enhancing pyroptosis-based tumor therapy.
In addition, nanomaterials not only serve as delivery vehicles for pyroptosis initiators but also as immune adjuvants to help activate immune responses. Recent studies have found that some nanomaterials such as PEI,169 pH-sensitive polymer bearing a seven-membered ring with a tertiary amine (PC7A),170 poly(D-lactic acid) nanoparticles,171 and chiral polypeptide nanoparticles172 evoke cellular or humoral immune responses. The underlying mechanism of nanoadjuvants may be related to the activation of toll-like receptors or stimulator of interferon genes pathways. Xu et al prepared achiral and left- and right-handed gold biomimetic nanoparticles and evaluated their immune-activating effects.173 The results showed that chiral nanoparticles activated the immune response by binding to cluster of differentiation 97 and epidermal growth factor-like-module receptor 1 on the surface of APCs, and left-handed nanoparticles exhibited the best immune activation. This study opens a new path for the application of chiral nanomaterials in immunology and therapeutic vaccines. The combination of nanoadjuvants and pyroptosis initiators is expected to improve the immunotherapy response rate.
Finally, although various nanomaterials have been developed to induce pyroptosis, the clinical translation of nanomaterials remains a major obstacle to be overcome. The clinical translation of nanomaterials faces challenges including safety concerns due to potential toxicity, biocompatibility issues, targeting and delivery efficiency, scale-up and manufacturing difficulties, cost-effectiveness, and a lack of robust clinical evidence.174 The biosafety and long-term toxicity of nanomaterials require further evaluation. Most preclinical studies have assessed the biosafety of nanomaterials through weight changes in tumor-bearing mice, histopathological examination of vital organs, and key serological indicators; however, long-term safety monitoring of nanomaterials is lacking. Various inorganic nanomaterials have been used to induce pyroptosis, especially metal-based nanomaterials, which face problems such as the inability to degrade, in vivo accumulation, and toxicity to organs. For example, mesoporous silica nanoparticles175 and CdSe/ZnS quantum dots176 cause liver injury, and zinc oxide nanoparticles177 combined with UVB can cause skin toxicity by triggering pyroptosis via NLRP3 inflammasome activation. Improving the targeting of nanomaterials to tumor cells or target organelles to achieve precise tumor therapy is an effective measure for reducing their toxic side effects. Polymer nanocarriers often suffer from limited drug loading capacity, complex preparation process, difficulty in ensuring uniformity of preparation, and susceptibility to clearance by the immune system. Liposomes have the limitations of poor in vivo stability, susceptibility to drug leakage, short circulation time, and poor tumor penetration capacity.178 More preclinical and clinical trials are required to assess the therapeutic efficacy, long-term safety, and in vivo toxicity of pyroptosis-inducing nanomaterials. Large-scale fabrication of nanomaterials with consistent quality is a major challenge, as is ensuring their biocompatibility and minimizing immune responses. Additionally, the high costs associated with developing and producing nanomaterials limit their accessibility and widespread adoption in the clinic. More robust clinical studies and data are needed to demonstrate the efficacy and safety of nanomaterial-based therapies for a variety of diseases. In recent years, organoid technology has provided new opportunities for tumor therapy, and the combination of nanotechnology and patient-derived organoids may accelerate the development and clinical translation of nanomedicine.179
In conclusion, the relationship between pyroptosis and the immune response, as well as the mechanisms involved, are gradually being uncovered. Nanomaterials have shown significant advantages in amplifying the immunomodulatory effects of pyroptosis. With the rapid development of nanotechnology and precision tumor therapy, clinical application of pyroptosis-based tumor immunotherapy is expected to be realized in the near future.
Funding
This work was financially supported by the Science and Technology Development Program of Jilin Province (Grant No. YDZJ202301ZYTS103, YDZJ202201ZYTS002, YDZJ202201ZYTS011, and 20240601025RC) and Norman Bethune Program of Jilin University (Grant No. 2022B15).
Disclosure
The authors declared that they have no conflicts of interest in this work.
References
1. Shi JJ, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi:10.1038/nature15514
2. Baker PJ, Boucher D, Bierschenk D, et al. NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur J Immunol. 2015;45(10):2918–2926. doi:10.1002/eji.201545655
3. Rühl S, Broz P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K+ efflux. Eur J Immunol. 2015;45(10):2927–2936. doi:10.1002/eji.201545772
4. Wang YP, Gao WQ, Shi XY, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99–103. doi:10.1038/nature22393
5. Hou JW, Zhao RC, Xia WY, et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat Cell Biol. 2020;22(10):1264–1275. doi:10.1038/s41556-020-0575-z
6. Zhou ZW, He HB, Wang K, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020;368(6494):eaaz7548. doi:10.1126/science.aaz7548
7. Liu YY, Fang YL, Chen XF, et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci Immunol. 2020;5(43):eaax7969. doi:10.1126/sciimmunol.aax7969
8. Lu L, Zhang Y, Tan X, et al. Emerging mechanisms of pyroptosis and its therapeutic strategy in cancer. Cell Death Discov. 2022;8(1):338. doi:10.1038/s41420-022-01101-6
9. Zhang ZB, Zhang Y, Xia SY, et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579(7799):415–420. doi:10.1038/s41586-020-2071-9
10. Lin J, Sun S, Zhao K, et al. Oncolytic parapoxvirus induces gasdermin E-mediated pyroptosis and activates antitumor immunity. Nat Commun. 2023;14(1):224. doi:10.1038/s41467-023-35917-2
11. Kumar D, Gurrapu S, Wang Y, et al. LncRNA Malat1 suppresses pyroptosis and T cell-mediated killing of incipient metastatic cells. Nat Cancer. 2024;5:262–282. doi:10.1038/s43018-023-00695-9
12. Jin XY, Ma YC, Liu DD, Huang Y. Role of pyroptosis in the pathogenesis and treatment of diseases. Medcomm. 2023;4(3):e249. doi:10.1002/mco2.249
13. Pan YH, Cai WJ, Huang J, et al. Pyroptosis in development, inflammation and disease. Front Immunol. 2022;13:991044. doi:10.3389/fimmu.2022.991044
14. Koh EH, Yoon JE, Ko MS, et al. Sphingomyelin synthase 1 mediates hepatocyte pyroptosis to trigger non-alcoholic steatohepatitis. Gut. 2021;70(10):1954–1964. doi:10.1136/gutjnl-2020-322509
15. Tan G, Huang CY, Chen JY, Chen BX, Zhi FC. Gasdermin-E-mediated pyroptosis participates in the pathogenesis of crohn’s disease by promoting intestinal inflammation. Cell Rep. 2021;35(11):109265. doi:10.1016/j.celrep.2021.109265
16. Wei X, Xie F, Zhou XX, et al. Role of pyroptosis in inflammation and cancer. Cell Mol Immunol. 2022;19(9):971–992. doi:10.1038/s41423-022-00905-x
17. Chen L, Sun XQ, Cheng K, et al. Temperature-regulating phase change fiber scaffold toward mild photothermal-chemotherapy. Adv Fiber Mater. 2022;4(6):1669–1684. doi:10.1007/s42765-022-00199-8
18. Yang J, Xu L, Ding YN, et al. NIR-II-triggered composite nanofibers to simultaneously achieve intracranial hemostasis, killing superbug and residual cancer cells in brain tumor resection surgery. Adv Fiber Mater. 2023;5(1):209–222. doi:10.1007/s42765-022-00210-2
19. Shi JY, Tian HL, Peng LY, et al. A nanoplatform reshaping intracellular osmolarity and redox homeostasis against colorectal cancer. J Control Release. 2022;352:766–775. doi:10.1016/j.jconrel.2022.11.003
20. Li Y, Lin JY, Wang PY, et al. Tumor microenvironment-responsive yolk-shell NaCl@virus-inspired tetrasulfide- organosilica for ion-interference therapy via osmolarity surge and oxidative stress amplification. ACS Nano. 2022;16(5):7380–7397. doi:10.1021/acsnano.1c09496
21. Wen M, Liu XH, Yu N, et al. Multifunctional hemoporfin-Cu9S8-MnO2 for magnetic resonance imaging-guided catalytically-assisted photothermal-sonodynamic therapies. J Colloid Interface Sci. 2022;626:77–88. doi:10.1016/j.jcis.2022.06.116
22. Wen M, Wang S, Jiang RQ, et al. Tuning the NIR photoabsorption of CuWO4−x nanodots with oxygen vacancies for CT imaging guided photothermal therapy of tumors. Biomater Sci UK. 2019;7(11):4651–4660. doi:10.1039/c9bm00995g
23. Yang YQ, Zhao TJ, Chen QH, et al. Nanomedicine strategies for heating “cold” ovarian cancer (OC): next evolution in immunotherapy of OC. Adv Sci. 2022;9(28):2202797. doi:10.1002/advs.202202797
24. Wang WY, Zhang L, Sun ZJ. Eliciting pyroptosis to fuel cancer immunotherapy: mechanisms and strategies. Cancer Biol Med. 2022;19(7):948–964. doi:10.20892/j.issn.2095-3941.2022.0049
25. Li LJ, Wang SM, Zhou WH. Balance cell apoptosis and pyroptosis of caspase-3-activating chemotherapy for better antitumor therapy. Cancers (Basel). 2023;15(1):26. doi:10.3390/cancers15010026
26. Su LP, Chen YT, Huang C, et al. Targeting Src reactivates pyroptosis to reverse chemoresistance in lung and pancreatic cancer models. Sci Transl Med. 2023;15(678):eabl7895. doi:10.1126/scitranslmed.abl7895
27. Wan S-C, M-J Y, Yang Q-C, et al. Diselenide-based dual-responsive prodrug as pyroptosis inducer potentiates cancer immunotherapy. Adv Healthc Mater. 2022;12(7):e2202135. doi:10.1002/adhm.202202135
28. Xiao Y, Zhang T, Ma XB, et al. Microenvironment-responsive prodrug-induced pyroptosis boosts cancer immunotherapy. Adv Sci. 2021;8(24):2101840. doi:10.1002/advs.202101840
29. Wang XT, Wang B, Li F, et al. The c-Src/LIST positive feedback loop sustains tumor progression and chemoresistance. Adv Sci. 2023;10(20):e2300115. doi:10.1002/advs.202300115
30. Zhu S-W, Ye M, Ma X, et al. pH-responsive nanoprodrugs combining a Src inhibitor and chemotherapy to potentiate antitumor immunity via pyroptosis in head and neck cancer. Acta Biomater. 2022;154:497–509. doi:10.1016/j.actbio.2022.10.051
31. Tan J, Li H, Hu XX, et al. Size-tunable assemblies based on ferrocene-containing DNA polymers for spatially uniform penetration. Chem. 2019;5(7):1775–1792. doi:10.1016/j.chempr.2019.05.024
32. Liang MY, Zhang MJ, Qiu W, et al. Stepwise size shrinkage cascade-activated supramolecular prodrug boosts antitumor immunity by eliciting pyroptosis. Adv Sci. 2022;9(26):2203353. doi:10.1002/advs.202203353
33. Hogg SJ, Beavis PA, Dawson MA, Johnstone RW. Targeting the epigenetic regulation of antitumour immunity. Nat Rev Drug Discov. 2020;19(11):776–800. doi:10.1038/s41573-020-0077-5
34. Morel D, Jeffery D, Aspeslagh S, Almouzni G, Postel-Vinay S. Combining epigenetic drugs with other therapies for solid tumours - past lessons and future promise. Nat Rev Clin Oncol. 2020;17(2):91–107. doi:10.1038/s41571-019-0267-4
35. Kim MS, Chang X, Yamashita K, et al. Aberrant promoter methylation and tumor suppressive activity of the DFNA5 gene in colorectal carcinoma. Oncogene. 2008;27(25):3624–3634. doi:10.1038/sj.onc.1211021
36. Mei Z, Chen XY, Chen Y, Su XD, Lv SX, Wei SC. Improved antitumor immunity of chemotherapy in OSCC treatment by gasdermin-E mediated pyroptosis. Apoptosis. 2023;28(3–4):348–361. doi:10.1007/s10495-022-01792-3
37. Fan JX, Deng RH, Wang H, et al. Epigenetics-based tumor cells pyroptosis for enhancing the immunological effect of chemotherapeutic nanocarriers. Nano Lett. 2019;19(11):8049–8058. doi:10.1021/acs.nanolett.9b03245
38. Xie BB, Liu TT, Chen S, et al. Combination of DNA demethylation and chemotherapy to trigger cell pyroptosis for inhalation treatment of lung cancer. Nanoscale. 2021;13(44):18608–18615. doi:10.1039/d1nr05001j
39. Dai EY, Zhu Z, Wahed S, Qu ZX, Storkus WJ, Guo ZS. Epigenetic modulation of antitumor immunity for improved cancer immunotherapy. Mol Cancer. 2021;20(1):171. doi:10.1186/s12943-021-01464-x
40. Zhou SY, Shang Q, Ji JB, Luan YX. A nanoplatform to amplify apoptosis-to-pyroptosis immunotherapy via immunomodulation of myeloid-derived suppressor cells. ACS Appl Mater Interfaces. 2021;13(40):47407–47417. doi:10.1021/acsami.1c16154
41. Baumann T, Dunkel A, Schmid C, et al. Regulatory myeloid cells paralyze T cells through cell-cell transfer of the metabolite methylglyoxal. Nat Immunol. 2020;21(5):555–566. doi:10.1038/s41590-020-0666-9
42. Chen DR, Gao Y, Xiao Y, et al. Engineered nanogels simultaneously implement HDAC inhibition and chemotherapy to boost antitumor immunity via pyroptosis. Appl Mater Today. 2022;26:101363. doi:10.1016/j.apmt.2022.101363
43. Zheng LL, Fan Y, Wang X, et al. Nanoagonist-mediated GSDME-dependent pyroptosis remodels the inflammatory microenvironment for tumor photoimmunotherapy. Adv Funct Mater. 2023;33(6):2200811. doi:10.1002/adfm.202200811
44. Guo HL, Huang JS, Tan Y, et al. Nanodrug shows spatiotemporally controlled release of anti-PD-L1 antibody and STING agonist to effectively inhibit tumor progression after radiofrequency ablation. Nano Today. 2022;43:101425. doi:10.1016/j.nantod.2022.101425
45. Zhang QQ, Shi DW, Guo MQ, Zhao H, Zhao YB, Yang XL. Radiofrequency-activated pyroptosis of Bi-valent gold nanocluster for cancer immunotherapy. ACS Nano. 2023;17(1):515–529. doi:10.1021/acsnano.2c09242
46. Lopez JS, Banerji U. Combine and conquer: challenges for targeted therapy combinations in early phase trials. Nat Rev Clin Oncol. 2017;14(1):57–66. doi:10.1038/nrclinonc.2016.96
47. Schram AM, Chang MT, Jonsson P, Drilon A. Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nat Rev Clin Oncol. 2017;14(12):735–748. doi:10.1038/nrclinonc.2017.127
48. Li Z, Liu Y, Fang X, Shu Z. Nanomaterials enhance the immunomodulatory effect of molecular targeted therapy. Int J Nanomed. 2021;16:1631–1661. doi:10.2147/IJN.S290346
49. Erkes DA, Cai W, Sanchez IM, et al. Mutant BRAF and MEK inhibitors regulate the tumor immune microenvironment via pyroptosis. Cancer Discov. 2020;10(2):254–269. doi:10.1158/2159-8290.Cd-19-0672
50. Wu MJ, Wang Y, Yang D, et al. A PLK1 kinase inhibitor enhances the chemosensitivity of cisplatin by inducing pyroptosis in oesophageal squamous cell carcinoma. Ebiomedicine. 2019;41:244–255. doi:10.1016/j.ebiom.2019.02.012
51. He Y, Sun MM, Zhang GG, et al. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Ther. 2021;6(1):425. doi:10.1038/s41392-021-00828-5
52. Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17(2):93–115. doi:10.1038/nrc.2016.138
53. Shi RS, Li M, Raghavan V, et al. Targeting the CDK4/6-Rb pathway enhances response to PI3K inhibition in PIK3CA-mutant lung squamous cell carcinoma. Clin Cancer Res. 2018;24(23):5990–6000. doi:10.1158/1078-0432.Ccr-18-0717
54. Yang QC, Ma XB, Xiao Y, et al. Engineering prodrug nanomicelles as pyroptosis inducer for codelivery of PI3K/mTOR and CDK inhibitors to enhance antitumor immunity. Acta Pharm Sin B. 2022;12(7):3139–3155. doi:10.1016/j.apsb.2022.02.024
55. Zhang MJ, Liang MY, Yang SC, et al. Bioengineering of BRAF and COX2 inhibitor nanogels to boost the immunotherapy of melanoma via pyroptosis. Chem Commun. 2023;59(7):932–935. doi:10.1039/d2cc05498a
56. Di Virgilio F, Sarti AC, Falzoni S, De Marchi E, Adinolfi E. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat Rev Cancer. 2018;18(10):601–618. doi:10.1038/s41568-018-0037-0
57. Vultaggio-Poma V, Sarti AC, Di Virgilio F. Extracellular ATP: a feasible target for cancer therapy. Cells-Basel. 2020;9(11):2496. doi:10.3390/cells9112496
58. Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol Rev. 2017;276(1):121–144. doi:10.1111/imr.12528
59. Wu L, Xie W, Li Y, et al. Biomimetic nanocarriers guide extracellular ATP homeostasis to remodel energy metabolism for activating innate and adaptive immunity system. Adv Sci. 2022;9(17):2105376. doi:10.1002/advs.202105376
60. Xiong HG, Ma XB, Wang XL, et al. Inspired epigenetic modulation synergy with adenosine inhibition elicits pyroptosis and potentiates cancer immunotherapy. Adv Funct Mater. 2021;31(20):2100007. doi:10.1002/adfm.202100007
61. Vargason AM, Anselmo AC, Mitragotri S. The evolution of commercial drug delivery technologies. Nat Biomed Eng. 2021;5(9):951–967. doi:10.1038/s41551-021-00698-w
62. Qin XF, Yu CM, Wei J, et al. Rational design of nanocarriers for intracellular protein delivery. Adv Mater. 2019;31(46):1902791. doi:10.1002/adma.201902791
63. Mendes BB, Conniot J, Avital A, et al. Nanodelivery of nucleic acids. Nat Rev Meth Primers. 2022;2(1):24. doi:10.1038/s43586-022-00104-y
64. Wang QY, Wang YP, Ding JJ, et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature. 2020;579(7799):421–426. doi:10.1038/s41586-020-2079-1
65. Ruhl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science. 2018;362(6417):956–960. doi:10.1126/science.aar7607
66. Li ZT, Mo FY, Wang YX, et al. Enhancing gasdermin-induced tumor pyroptosis through preventing ESCRT-dependent cell membrane repair augments antitumor immune response. Nat Commun. 2022;13(1):6321. doi:10.1038/s41467-022-34036-8
67. Zhang X, Zhang HB, Gu JM, et al. Engineered extracellular vesicles for cancer therapy. Adv Mater. 2021;33(14):2005709. doi:10.1002/adma.202005709
68. Xing YQ, Zhang FY, Ji PP, et al. Efficient delivery of GSDMD-N mRNA by engineered extracellular vesicles induces pyroptosis for enhanced immunotherapy. Small. 2023;19(20):e2204031. doi:10.1002/smll.202204031
69. Wang N, Liu C, Li Y, et al. A cooperative nano- CRISPR scaffold potentiates immunotherapy via activation of tumour-intrinsic pyroptosis. Nat Commun. 2023;14(1):779. doi:10.1038/s41467-023-36550-9
70. de Souza JG, Starobinas N, Ibanez OCM. Unknown/enigmatic functions of extracellular ASC. Immunology. 2021;163(4):377–388. doi:10.1111/imm.13375
71. Liu YX, Guo K, Ding M, et al. Engineered magnetic polymer nanoparticles can ameliorate breast cancer treatment inducing pyroptosis-starvation along with chemotherapy. ACS Appl Mater Interfaces. 2022;14(37):42541–42557. doi:10.1021/acsami.2c13011
72. Xia XJ, Wang X, Zheng Y, Jiang JQ, Hu JH. What role does pyroptosis play in microbial infection? J Cell Physiol. 2019;234(6):7885–7892. doi:10.1002/jcp.27909
73. Huang XH, Pan JM, Xu FN, et al. Bacteria-based cancer immunotherapy. Adv Sci. 2021;8(7):2003572. doi:10.1002/advs.202003572
74. Li ZT, Wang YX, Liu J, et al. Chemically and biologically engineered bacteria-based delivery systems for emerging diagnosis and advanced therapy. Adv Mater. 2021;33(38):2102580. doi:10.1002/adma.202102580
75. Howell LM, Forbes NS. Bacteria-based immune therapies for cancer treatment. Semin Cancer Biol. 2022;86:1163–1178. doi:10.1016/j.semcancer.2021.09.006
76. Liu Y, Lu YP, Ning B, et al. Intravenous delivery of living listeria monocytogenes elicits gasdmermin-dependent tumor pyroptosis and motivates anti-tumor immune response. ACS Nano. 2022;16(3):4102–4115. doi:10.1021/acsnano.1c09818
77. Dhital S, Deo P, Stuart I, Naderer T. Bacterial outer membrane vesicles and host cell death signaling. Trends Microbiol. 2021;29(12):1106–1116. doi:10.1016/j.tim.2021.04.003
78. Wang SM, Guo JY, Bai Y, et al. Bacterial outer membrane vesicles as a candidate tumor vaccine platform. Front Immunol. 2022;13:987419. doi:10.3389/fimmu.2022.987419
79. Li YJ, Wu JY, Qiu XH, et al. Bacterial outer membrane vesicles-based therapeutic platform eradicates triple-negative breast tumor by combinational photodynamic/chemo-/immunotherapy. Bioact Mater. 2023;20:548–560. doi:10.1016/j.bioactmat.2022.05.037
80. Chen LL, Ma X, Liu WJ, Hu QQ, Yang HH. Targeting pyroptosis through lipopolysaccharide-triggered noncanonical pathway for safe and efficient cancer immunotherapy. Nano Lett. 2023;23(18):8725–8733. doi:10.1021/acs.nanolett.3c02728
81. Deng WY, Bai Y, Deng F, et al. Streptococcal pyrogenic exotoxin B cleaves GSDMA and triggers pyroptosis. Nature. 2022;602(7897):496–502. doi:10.1038/s41586-021-04384-4
82. Sala R, Rioja-Blanco E, Serna N, et al. GSDMD-dependent pyroptotic induction by a multivalent CXCR4-targeted nanotoxin blocks colorectal cancer metastases. Drug Deliv. 2022;29(1):1384–1397. doi:10.1080/10717544.2022.2069302
83. Rioja-Blanco E, Arroyo-Solera I, Alamo P, et al. CXCR4-targeted nanotoxins induce GSDME-dependent pyroptosis in head and neck squamous cell carcinoma. J Exp Clin Cancer Res. 2022;41(1):49. doi:10.1186/s13046-022-02267-8
84. Zhao Z, Huang YB, Wang J, et al. A self-assembling CXCR4-targeted pyroptosis nanotoxin for melanoma therapy. Biomater Sci UK. 2023;11(6):2200–2210. doi:10.1039/d2bm02026b
85. Rahman MM, McFadden G. Oncolytic viruses: newest frontier for cancer immunotherapy. Cancers (Basel). 2021;13(21):5452. doi:10.3390/cancers13215452
86. Su W, Qiu W, Li SJ, et al. A dual-responsive STAT3 inhibitor nanoprodrug combined with oncolytic virus elicits synergistic antitumor immune responses by igniting pyroptosis. Adv Mater. 2023;35(11):e2209379. doi:10.1002/adma.202209379
87. Gao WT, Wang XY, Zhou Y, Wang XQ, Yu Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther. 2022;7(1):196. doi:10.1038/s41392-022-01046-3
88. Zhou RB, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–140. doi:10.1038/ni.1831
89. Wu XX, Zhang HY, Qi W, et al. Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death Dis. 2018;9:171. doi:10.1038/s41419-017-0257-3
90. Chen MQ, Hu CY, Yang L, Guo QX, Liang YL, Wang WJ. Saikosaponin-D induces the pyroptosis of lung cancer by increasing ROS and activating the NF-κB/NLRP3/caspase-1/GSDMD pathway. J Biochem Mol Toxicol. 2023;37(8):e23444. doi:10.1002/jbt.23444
91. Teng JF, Mei QB, Zhou XG, et al. Polyphyllin VI induces caspase-1-mediated pyroptosis via the induction of ROS/NF-κB/NLRP3/GSDMD signal axis in non-small cell lung cancer. Cancers (Basel). 2020;12(1):193. doi:10.3390/cancers12010193
92. Zhou JZ, Zeng L, Zhang YW, et al. Cadmium exposure induces pyroptosis in testicular tissue by increasing oxidative stress and activating the AIM2 inflammasome pathway. Sci Total Environ. 2022;847:157500. doi:10.1016/j.scitotenv.2022.157500
93. An H, Heo JS, Kim P, et al. Tetraarsenic hexoxide enhances generation of mitochondrial ROS to promote pyroptosis by inducing the activation of caspase-3/GSDME in triple-negative breast cancer cells. Cell Death Dis. 2021;12(2):159. doi:10.1038/s41419-021-03454-9
94. Zhang XW, Zhang P, An L, et al. Miltirone induces cell death in hepatocellular carcinoma cell through GSDME-dependent pyroptosis. Acta Pharm Sin B. 2020;10(8):1397–1413. doi:10.1016/j.apsb.2020.06.015
95. Yang C, Wang ZQ, Zhang ZC, Lou G, Jin WL. CBL0137 activates ROS/BAX signaling to promote caspase-3/GSDME-dependent pyroptosis in ovarian cancer cells. Biomed Pharmacother. 2023;161:114529. doi:10.1016/j.biopha.2023.114529
96. Liu ZR, Li YQ, Zhu YL, et al. Apoptin induces pyroptosis of colorectal cancer cells via the GSDME-dependent pathway. Int J Biol Sci. 2022;18(2):717–730. doi:10.7150/ijbs.64350
97. Zhang JY, Zhou B, Sun RY, et al. The metabolite α-KG induces GSDMC-dependent pyroptosis through death receptor 6-activated caspase-8. Cell Res. 2021;31(9):980–997. doi:10.1038/s41422-021-00506-9
98. Ding Y, Ye BL, Sun ZQ, Mao ZW, Wang WL. Reactive oxygen species-mediated pyroptosis with the help of nanotechnology: prospects for cancer therapy. Adv Nanobiomed Res. 2022;3(1):2200077. doi:10.1002/anbr.202200077
99. Wu M, Liu XG, Chen H, et al. Activation of pyroptosis by membrane-anchoring AIE photosensitizer design: new prospect for photodynamic cancer cell ablation. Angew Chem Int Ed. 2021;60:9093–9098. doi:10.1002/anie.202016399
100. Li L, Tian HL, Zhang Z, et al. Carrier-free nanoplatform via evoking pyroptosis and immune response against breast cancer. ACS Appl Mater Interfaces. 2022;15(1):452–468. doi:10.1021/acsami.2c17579
101. Ma XB, Su W, Ye MJ, et al. Endogenous/exogenous stimulies inspired polyprodrug nano-inducer switches pyroptosis path for promoting antitumor immunity. Nano Today. 2023;48:101727. doi:10.1016/j.nantod.2022.101727
102. Qiu W, Su W, Xu JM, et al. Immunomodulatory-photodynamic nanostimulators for invoking pyroptosis to augment tumor immunotherapy. Adv Healthc Mater. 2022;11(21):2201233. doi:10.1002/adhm.202201233
103. Zhou JJ, Ma XB, Li H, et al. Inspired heat shock protein alleviating prodrug enforces immunogenic photodynamic therapy by eliciting pyroptosis. Nano Res. 2022;15(4):3398–3408. doi:10.1007/s12274-021-3946-2
104. Singleton DC, Macann A, Wilson WR. Therapeutic targeting of the hypoxic tumour microenvironment. Nat Rev Clin Oncol. 2021;18(12):751–772. doi:10.1038/s41571-021-00539-4
105. Wan YL, Fu LH, Li CY, Lin J, Huang P. Conquering the hypoxia limitation for photodynamic therapy. Adv Mater. 2021;33(48):2103978. doi:10.1002/adma.202103978
106. Su XX, Wang WJ, Cao Q, et al. A carbonic anhydrase IX (CAIX)-anchored Rhenium(I) photosensitizer evokes pyroptosis for enhanced anti-tumor immunity. Angew Chem Int Ed. 2022;61(8):e202115800. doi:10.1002/anie.202115800
107. Zhou JY, Wang WJ, Zhang CY, et al. Ru(II)-modified TiO2 nanoparticles for hypoxia-adaptive photo-immunotherapy of oral squamous cell carcinoma. Biomaterials. 2022;289:121757. doi:10.1016/j.biomaterials.2022.121757
108. Zhen WY, An SJ, Wang SQ, et al. Precise subcellular organelle targeting for boosting endogenous-stimuli-mediated tumor therapy. Adv Mater. 2021;33(51):2101572. doi:10.1002/adma.202101572
109. Zeng S, Chen C, Zhang LW, et al. Activation of pyroptosis by specific organelle-targeting photodynamic therapy to amplify immunogenic cell death for anti-tumor immunotherapy. Bioact Mater. 2023;25:580–593. doi:10.1016/j.bioactmat.2022.07.016
110. Chen BL, Yan Y, Yang Y, et al. A pyroptosis nanotuner for cancer therapy. Nat Nanotechnol. 2022;17(7):788–798. doi:10.1038/s41565-022-01125-0
111. Tang ZM, Liu YY, He MY, Bu WB. Chemodynamic therapy: tumour microenvironment-mediated Fenton and Fenton-like reactions. Angew Chem Int Ed. 2019;58(4):946–956. doi:10.1002/anie.201805664
112. Tian QW, Xue FF, Wang YR, et al. Recent advances in enhanced chemodynamic therapy strategies. Nano Today. 2021;39:101162. doi:10.1016/j.nantod.2021.101162
113. Nadeem S, Yang C, Du Y, et al. A virus-spike tumor-activatable pyroptotic agent. Small. 2021;17(8):2006599. doi:10.1002/smll.202006599
114. Wang LY, Lu D, Huo MF, Xu HX. Oligomycin A induces apoptosis-to-pyroptosis switch against melanoma with sensitized immunotherapy. Adv Funct Mater. 2022;32(4):2106332. doi:10.1002/adfm.202106332
115. Deng HZ, Zhang J, Yang YF, et al. Chemodynamic and photothermal combination therapy based on dual-modified metal-organic framework for inducing tumor ferroptosis/pyroptosis. ACS Appl Mater Interfaces. 2022;14(21):24089–24101. doi:10.1021/acsami.2c00574
116. Xu SX, Zhou SY, Xie LYJ, et al. A versatile NiS2/FeS2 hybrid nanocrystal for synergistic cancer therapy by inducing ferroptosis and pyroptosis. Chem Eng J. 2023;460:141639. doi:10.1016/j.cej.2023.141639
117. Liang S, Yao JJ, Liu D, Rao L, Chen XY, Wang ZH. Harnessing nanomaterials for cancer sonodynamic immunotherapy. Adv Mater. 2023;35(33):e2211130. doi:10.1002/adma.202211130
118. Yang YR, Huang J, Liu M, et al. Emerging sonodynamic therapy-based nanomedicines for cancer immunotherapy. Adv Sci. 2023;10(2):2204365. doi:10.1002/advs.202204365
119. Yu Z, Cao WL, Han CY, et al. Biomimetic metal-organic framework nanoparticles for synergistic combining of SDT-chemotherapy induce pyroptosis in gastric cancer. Front Bioeng Biotechnol. 2022;10:796820. doi:10.3389/fbioe.2022.796820
120. Chen Z, Liu W, Yang Z, et al. Sonodynamic-immunomodulatory nanostimulators activate pyroptosis and remodel tumor microenvironment for enhanced tumor immunotherapy. Theranostics. 2023;13(5):1571–1583. doi:10.7150/thno.79945
121. Yu SJ, Chen ZW, Zeng X, Chen XS, Gu Z. Advances in nanomedicine for cancer starvation therapy. Theranostics. 2019;9(26):8026–8047. doi:10.7150/thno.38261
122. Fu LH, Qi C, Hu YR, Lin J, Huang P. Glucose oxidase-instructed multimodal synergistic cancer therapy. Adv Mater. 2019;31(21):1808325. doi:10.1002/adma.201808325
123. Chang MQ, Wang ZY, Dong CH, et al. Ultrasound-amplified enzyodynamic tumor therapy by perovskite nanoenzyme-enabled cell pyroptosis and cascade catalysis. Adv Mater. 2023;35(7):e2208817. doi:10.1002/adma.202208817
124. Li JJ, Anraku Y, Kataoka K. Self-boosting catalytic nanoreactors integrated with triggerable crosslinking membrane networks for initiation of immunogenic cell death by pyroptosis. Angew Chem Int Ed. 2020;59(32):13526–13530. doi:10.1002/anie.202004180
125. Zhang SJ, Zhang Y, Feng YJ, et al. Biomineralized two-enzyme nanoparticles regulate tumor glycometabolism inducing tumor cell pyroptosis and robust antitumor immunotherapy. Adv Mater. 2022;34(50):e2206851. doi:10.1002/adma.202206851
126. Ding BB, Zheng P, Tan J, et al. Pyroptosis adjuvants: gram-scale production, cascade catalysis, and in situ antitumor immunity activation. Chem Mater. 2022;34(4):1800–1808. doi:10.1021/acs.chemmater.1c04048
127. Cai Z, Li CF, Han F, et al. Phosphorylation of PDHA by AMPK drives TCA cycle to promote cancer metastasis. Mol Cell. 2020;80(2):263–278. doi:10.1016/j.molcel.2020.09.018
128. Xie DB, Xu YT, Zhang YM, Cai WP, Lan X, Yan H. Pyruvate dehydrogenase kinase 1-dependent metabolic reprogramming: a promising target for postmenopausal osteoporosis treatment. Biomed Pharmacother. 2023;160:114411. doi:10.1016/j.biopha.2023.114411
129. Jin JK, Yuan PC, Yu W, et al. Mitochondria-targeting polymer micelle of dichloroacetate induced pyroptosis to enhance osteosarcoma immunotherapy. ACS Nano. 2022;16(7):10327–10340. doi:10.1021/acsnano.2c00192
130. Kolb D, Kolishetti N, Surnar B, et al. Metabolic modulation of the tumor microenvironment leads to multiple checkpoint inhibition and immune cell infiltration. ACS Nano. 2020;14(9):11055–11066. doi:10.1021/acsnano.9b10037
131. Wang KW, Xiao X, Jiang ML, Li JS, Zhou JL, Yuan YY. An NIR-fluorophore-based theranostic for selective initiation of tumor pyroptosis-induced immunotherapy. Small. 2021;17(36):2102610. doi:10.1002/smll.202102610
132. Zheng P, Ding BB, Zhu GQ, Wang M, Li CX, Lin J. Biodegradable hydrogen peroxide nanogenerator for controllable cancer immunotherapy via modulating cell death pathway from apoptosis to pyroptosis. Chem Eng J. 2022;450:137967. doi:10.1016/j.cej.2022.137967
133. Zhen WY, Liu Y, An SJ, Jiang XE. Glutathione-induced in situ Michael addition between nanoparticles for pyroptosis and immunotherapy. Angew Chem Int Ed. 2023;62(20):e202301866. doi:10.1002/anie.202301866
134. Lee D, Ha J, Kang M, Yang ZG, Jiang W, Kim BYS. Strategies of perturbing ion homeostasis for cancer therapy. Adv Ther. 2022;5(2):2100189. doi:10.1002/adtp.202100189
135. Chi YJ, Sun P, Gao Y, Zhang J, Wang LY. Ion interference therapy of tumors based on inorganic nanoparticles. Biosensors-Basel. 2022;12(2):100. doi:10.3390/bios12020100
136. Liu YY, Zhang M, Bu WB. Bioactive nanomaterials for ion-interference therapy. View. 2020;1(2):e18. doi:10.1002/viw2.18
137. Ko SK, Kim SK, Share A, et al. Synthetic ion transporters can induce apoptosis by facilitating chloride anion transport into cells. Nat Chem. 2014;6(10):885–892. doi:10.1038/nchem.2021
138. Jiang W, Yin L, Chen HM, et al. NaCl nanoparticles as a cancer therapeutic. Adv Mater. 2019;31(46):e1904058. doi:10.1002/adma.201904058
139. Liu Y, Zhen WY, Wang YH, Song SY, Zhang HJ. Na2S2O8 nanoparticles trigger antitumor immunotherapy through reactive oxygen species storm and surge of tumor osmolarity. J Am Chem Soc. 2020;142(52):21751–21757. doi:10.1021/jacs.0c09482
140. Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21(6):360–378. doi:10.1038/s41568-021-00346-0
141. Tang J, Yang Y, Qu JJ, et al. Mesoporous sodium four-coordinate aluminosilicate nanoparticles modulate dendritic cell pyroptosis and activate innate and adaptive immunity. Chem Sci. 2022;13(29):8507–8517. doi:10.1039/d1sc05319a
142. Eil R, Vodnala SK, Clever D, et al. Ionic immune suppression within the tumour microenvironment limits t cell effector function. Nature. 2016;537(7621):539–543. doi:10.1038/nature19364
143. Rivers-Auty J, Brough D. Potassium efflux fires the canon: potassium efflux as a common trigger for canonical and noncanonical NLRP3 pathways. Eur J Immunol. 2015;45(10):2758–2761. doi:10.1002/eji.201545958
144. Wu LS, Bai SM, Huang J, et al. Nigericin boosts anti-tumor immune response via inducing pyroptosis in triple-negative breast cancer. Cancers (Basel). 2023;15(12):3221. doi:10.3390/cancers15123221
145. Niu Q, Liu Y, Zheng Y, et al. Co-delivery of nigericin and decitabine using hexahistidine-metal nanocarriers for pyroptosis-induced immunotherapeutics. Acta Pharm Sin B. 2022;12(12):4458–4471. doi:10.1016/j.apsb.2022.11.002
146. Wang C, Cheng L, Liu Z. Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics. Theranostics. 2013;3(5):317–330. doi:10.7150/thno.5284
147. Ding BB, Sheng JY, Zheng P, et al. Biodegradable upconversion nanoparticles induce pyroptosis for cancer immunotherapy. Nano Lett. 2021;21(19):8281–8289. doi:10.1021/acs.nanolett.1c02790
148. Zhang M, Song RX, Liu YY, et al. Calcium-overload-mediated tumor therapy by calcium peroxide nanoparticles. Chem. 2019;5(8):2171–2182. doi:10.1016/j.chempr.2019.06.003
149. Zheng P, Ding BB, Zhu GQ, Li CX, Lin J. Biodegradable Ca2+ nanomodulators activate pyroptosis through mitochondrial Ca2+ overload for cancer immunotherapy. Angew Chem Int Ed. 2022;61(36):e202204904. doi:10.1002/anie.202204904
150. Ma ZY, Zhang J, Zhang WY, et al. Intracellular Ca2+ cascade guided by NIR-II photothermal switch for specific tumor therapy. Iscience. 2020;23(5):101049. doi:10.1016/j.isci.2020.101049
151. Zhao PF, Wang M, Chen M, et al. Programming cell pyroptosis with biomimetic nanoparticles for solid tumor immunotherapy. Biomaterials. 2020;254:120142. doi:10.1016/j.biomaterials.2020.120142
152. Moujalled D, Strasser A, Liddell JR. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021;28(7):2029–2044. doi:10.1038/s41418-021-00814-y
153. Huang H, Weng Y, Tian W, Lin X, Chen J, Luo L. Molecular mechanisms of pyroptosis and its role in anti-tumor immunity. Int J Biol Sci. 2023;19(13):4166–4180. doi:10.7150/ijbs.86855
154. Cao W, Chen GD, Wu LJ, et al. Ionizing radiation triggers the antitumor immunity by inducing gasdermin E-mediated pyroptosis in tumor cells. Int J Radiat Oncol Biol Phys. 2023;115(2):440–452. doi:10.1016/j.ijrobp.2022.07.1841
155. Zhang YH, Shi JH, Ma B, et al. Functionalization of polymers for intracellular protein delivery. Prog Polym Sci. 2023;146:101751. doi:10.1016/j.progpolymsci.2023.101751
156. Yu XY, Xing GZ, Sheng SP, et al. Neutrophil camouflaged stealth nanovehicle for photothermal-induced tumor immunotherapy by triggering pyroptosis. Adv Sci. 2023;10(5):e2207456. doi:10.1002/advs.202207456
157. Wen M, Yu N, Wu SW, et al. On-demand assembly of polymeric nanoparticles for longer-blood-circulation and disassembly in tumor for boosting sonodynamic therapy. Bioact Mater. 2022;18:242–253. doi:10.1016/j.bioactmat.2022.03.009
158. Wen M, Yu N, Yi ZG, et al. On-demand phototoxicity inhibition of sensitizers and H2S-triggered in situ activation for precise therapy of colon cancer. Nano Today. 2023;50:101863. doi:10.1016/j.nantod.2023.101863
159. Ding JX, Chen JJ, Gao LQ, et al. Engineered nanomedicines with enhanced tumor penetration. Nano Today. 2019;29:100800. doi:10.1016/j.nantod.2019.100800
160. Zhou Q, Dong CY, Fan WF, et al. Tumor extravasation and infiltration as barriers of nanomedicine for high efficacy: the current status and transcytosis strategy. Biomaterials. 2020;240:119902. doi:10.1016/j.biomaterials.2020.119902
161. Ploetz E, Zimpel A, Cauda V, et al. Metal-organic framework nanoparticles induce pyroptosis in cells controlled by the extracellular pH. Adv Mater. 2020;32(19):1907267. doi:10.1002/adma.201907267
162. Wang H, Gao ZY, Jiao D, et al. A microenvironment dual-responsive nano-drug equipped with PD-L1 blocking peptide triggers immunogenic pyroptosis for prostate cancer self-synergistic immunotherapy. Adv Funct Mater. 2023;33(16):2214499. doi:10.1002/adfm.202214499
163. Hage C, Hoves S, Strauss L, et al. Sorafenib induces pyroptosis in macrophages and triggers natural killer cell-mediated cytotoxicity against hepatocellular carcinoma. Hepatology. 2019;70(4):1280–1297. doi:10.1002/hep.30666
164. Kong Q, Xia SY, Pan XX, et al. Alternative splicing of GSDMB modulates killer lymphocyte-triggered pyroptosis. Sci Immunol. 2023;8(82):eadg3196. doi:10.1126/sciimmunol.adg3196
165. Zhong X, Zeng H, Zhou ZW, et al. Structural mechanisms for regulation of GSDMB pore-forming activity. Nature. 2023;616(7957):598–605. doi:10.1038/s41586-023-05872-5
166. Zhang C, Xu MK, Zeng ZL, et al. A polymeric extracellular matrix nanoremodeler for activatable cancer photo-immunotherapy. Angew Chem Int Ed. 2023;62(12):e202217339. doi:10.1002/anie.202217339
167. Vignali PDA, DePeaux K, Watson MJ, et al. Hypoxia drives CD39-dependent suppressor function in exhausted T cells to limit antitumor immunity. Nat Immunol. 2023;24(2):267–279. doi:10.1038/s41590-022-01379-9
168. Pan Y, Liu L, Mou X, Cai Y. Nanomedicine strategies in conquering and utilizing the cancer hypoxia environment. ACS Nano. 2023;17(21):20875–20924. doi:10.1021/acsnano.3c07763
169. Li AW, Sobral MC, Badrinath S, et al. A facile approach to enhance antigen response for personalized cancer vaccination. Nat Mater. 2018;17(6):528–534. doi:10.1038/s41563-018-0028-2
170. Li SX, Luo M, Wang ZH, et al. Prolonged activation of innate immune pathways by a polyvalent STING agonist. Nat Biomed Eng. 2021;5(5):455–466. doi:10.1038/s41551-020-00675-9
171. Xu WG, Su YZ, Ma Y, et al. Immunologically effective poly(D-lactic acid) nanoparticle enhances anticancer immune response. Sci China Chem. 2023;66(4):1150–1160. doi:10.1007/s11426-022-1441-7
172. Su YZ, Xu WG, Wei Q, Ma Y, Ding JX, Chen XS. Chiral polypeptide nanoparticles as nanoadjuvants of nanovaccines for efficient cancer prevention and therapy. Sci Bull. 2023;68(3):284–294. doi:10.1016/j.scib.2023.01.024
173. Xu LG, Wang XX, Wang WW, et al. Enantiomer-dependent immunological response to chiral nanoparticles. Nature. 2022;601(7893):366–373. doi:10.1038/s41586-021-04243-2
174. Zhang PF, Xiao YF, Sun X, et al. Cancer nanomedicine toward clinical translation: obstacles, opportunities, and future prospects. Med. 2023;4(3):147–167. doi:10.1016/j.medj.2022.12.001
175. Zhang XY, Luan JY, Chen W, et al. Mesoporous silica nanoparticles induced hepatotoxicity via NLRP3 inflammasome activation and caspase-1-dependent pyroptosis. Nanoscale. 2018;10(19):9141–9152. doi:10.1039/c8nr00554k
176. Lu YH, Xu SC, Chen HY, et al. CdSe/ZnS quantum dots induce hepatocyte pyroptosis and liver inflammation via NLRP3 inflammasome activation. Biomaterials. 2016;90:27–39. doi:10.1016/j.biomaterials.2016.03.003
177. Chen YY, Lee YH, Wang B, Chen RJ, Wang YJ. Skin damage induced by zinc oxide nanoparticles combined with UVB is mediated by activating cell pyroptosis via the NLRP3 inflammasome-autophagy-exosomal pathway. Part Fibre Toxicol. 2022;19(1):2. doi:10.1186/s12989-021-00443-w
178. Tan C, Wang J, Sun BG. Biopolymer-liposome hybrid systems for controlled delivery of bioactive compounds: recent advances. Biotechnol Adv. 2021;48:107727. doi:10.1016/j.biotechadv.2021.107727
179. Zhao DK, Liang J, Huang XY, Shen S, Wang J. Organoids technology for advancing the clinical translation of cancer nanomedicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023;15(5):e1892. doi:10.1002/wnan.1892
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
