Back to Journals » International Journal of Nanomedicine » Volume 19
Nanoparticles in Periodontitis Therapy: A Review of the Current Situation
Authors Wang D, Li Q , Xiao C , Wang H, Dong S
Received 20 February 2024
Accepted for publication 10 June 2024
Published 9 July 2024 Volume 2024:19 Pages 6857—6893
DOI https://doi.org/10.2147/IJN.S465089
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Di Wang,1,2 Qiqi Li,1,2 Chunsheng Xiao,2 Hao Wang,2 Shujun Dong1
1The First Outpatient Department, Jilin Provincial Key Laboratory of Tooth Development and Bone Remodeling, Hospital of Stomatology, Jilin University, Changchun, 130021, People’s Republic of China; 2Key Laboratory of Polymer Ecomaterials, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022, People’s Republic of China
Correspondence: Hao Wang, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, 5625 Renmin Street, Chaoyang District, Changchun, 130022, People’s Republic of China, Tel +86-431-85262110, Email [email protected] Shujun Dong, The First Outpatient Department, Hospital of Stomatology, Jilin University, Room 101, Building G5, Phase 3, China Resources Ziyun Mansion, the Intersection of Jinhu Road and Nanhu Middle Street, Nanguan District, Changchun, 130021, People’s Republic of China, Tel +86-431-80621155, Email [email protected]
Abstract: Periodontitis is a disease of inflammation that affects the tissues supporting the periodontium. It is triggered by an immunological reaction of the gums to plaque, which leads to the destruction of periodontal attachment structures. Periodontitis is one of the most commonly recognized dental disorders in the world and a major factor in the loss of adult teeth. Scaling and root planing remain crucial for managing patients with persistent periodontitis. Nevertheless, exclusive reliance on mechanical interventions like periodontal surgery, extractions, and root planning is insufficient to halt the progression of periodontitis. In response to the problem of bacterial resistance, some researchers are committed to finding alternative therapies to antibiotics. In addition, some scholars focus on finding new materials to provide a powerful microenvironment for periodontal tissue regeneration and promote osteogenic repair. Nanoparticles possess distinct therapeutic qualities, including exceptional antibacterial, anti-inflammatory, and antioxidant properties, immunomodulatory capacities, and the promotion of bone regeneration ability, which made them can be used for the treatment of periodontitis. However, there are many problems that limit the clinical translation of nanoparticles, such as toxic accumulation in cells, poor correlation between in vitro and in vivo, and poor animal-to-human transmissibility. In this paper, we review the present researches on nanoparticles in periodontitis treatment from the perspective of three main categories: inorganic nanoparticles, organic nanoparticles, and nanocomposites (including nanofibers, hydrogels, and membranes). The aim of this review is to provide a comprehensive and recent update on nanoparticles-based therapies for periodontitis. The conclusion section summarizes the opportunities and challenges in the design and clinical translation of nanoparticles for the treatment of periodontitis.
Keywords: anti-inflammatory, antibacterial, guided tissue regeneration, nanofibers, hydrogel, films
Graphical Abstract:
- Discussing the pathogenic factors and clinical treatment of periodontitis.
- Emphasizing the role played by nanoparticles in periodontitis therapy: antibacterial, anti-inflammatory, or periodontal regeneration.
- Reviewing the present and future research on the application of nanoparticles in periodontitis treatment from the perspective of three main categories: inorganic nanoparticles, organic nanoparticles, and nanocomposites.
- Presenting the challenges and prospects of nanoparticles in periodontitis therapy.
Introduction
Periodontitis is a complex inflammatory disease that occurs in periodontal supporting tissue.1 In a statistical analysis investigating the occurrence of periodontitis in 17 nations over the course of the last decade, it was found that the prevalence of periodontitis was approximately 60%. Furthermore, the proportion of severe periodontitis reached a significant 24%.2
Periodontitis is often referred to as a “silent” disease because it is seldom associated with obvious signs and symptoms unless the disease has progressed to an advanced stage.3,4 Untreated periodontitis may end up in the destruction of the supporting tissue of the teeth, potentially causing tooth loss and diminished quality of life, particularly in severe instances.5 However, periodontitis is not only a local phenomenon, periodontal pathogens and their metabolites and inflammatory mediators can enter the bloodstream, thus causing the development of systemic diseases.6,7 Such as, there is an established link between periodontitis with diabetes, metabolic syndrome, cardiovascular disease, and chronic kidney disease.8–10 In addition, associations to be confirmed exist between periodontitis and several other diseases, such as adverse pregnancy outcomes,11 Alzheimer’s disease,12 and cancer.13
Scaling and root planing (SRP) is the prevailing treatment for periodontitis,14 with the goal of eliminating both supragingival and subgingival plaque and tartar. For the clinician, proper execution of SRP is an effective but challenging process that requires a rigorous, meticulous approach.15,16 Mechanical treatment alone cannot stop progressive periodontitis because of periodontitis is driven by bacteria that cannot be removed thoroughly by mechanical instruments. This requires some complementary treatment to improve, such as mouthwash, antibiotics, probiotics, and so forth.17–19 However, in recent years, periodontal pathogens have also developed antibiotic resistance, which has affected the success of periodontitis treatment. Hence, alternative antibacterial approaches are required to effectively manage and cure periodontal disease.20 In addition, guided tissue regeneration/guided bone regeneration (GTR/GBR) seems to be a favorable alternative for some patients with severe periodontitis who continue to have unsatisfactory results after the completion of basic periodontal treatment.21 However, no GTR/GBR membrane meets all the requirements of good mechanical strength, suitable degradation rate, satisfactory osteogenesis, and clinical operability at the same time.22 Therefore, it is crucial to pursue more efficient therapies to tackle these issues.
Nanomaterials have been widely noticed in many biomedical fields, particularly in the realm of cancer research.23–28 In the past few years, therapeutic nanoparticles (NPs), as a developing engineering solution for treating periodontitis, have received extensive attention.29–32 NPs possess distinct therapeutic qualities, including exceptional antibacterial, anti-inflammatory, and antioxidant properties, immunomodulatory capacities, and the promotion of bone regeneration ability. These attributes are a result of the material’s inherent characteristics and its combination with various medications. Silver NPs have excellent antimicrobial activity against periodontitis pathogens.33 The combination of silver nanoparticles with the anti-inflammatory drug ebselen improves biosafety and provides synergistic anti-inflammatory and antibacterial effects.34 Bai et al delivered minocycline hydrochloride (MH) using polydopamine (PDA)-functionalized mesoporous silica. The remodeling of the periodontitis microenvironment by the synergistic action of PDA and MH resulted in a decrease in ROS levels and the conversion of macrophages to an anti-inflammatory phenotype.35 According to the latest research, we are of the opinion that NPs have the potential to be crucial in the future management of periodontitis.
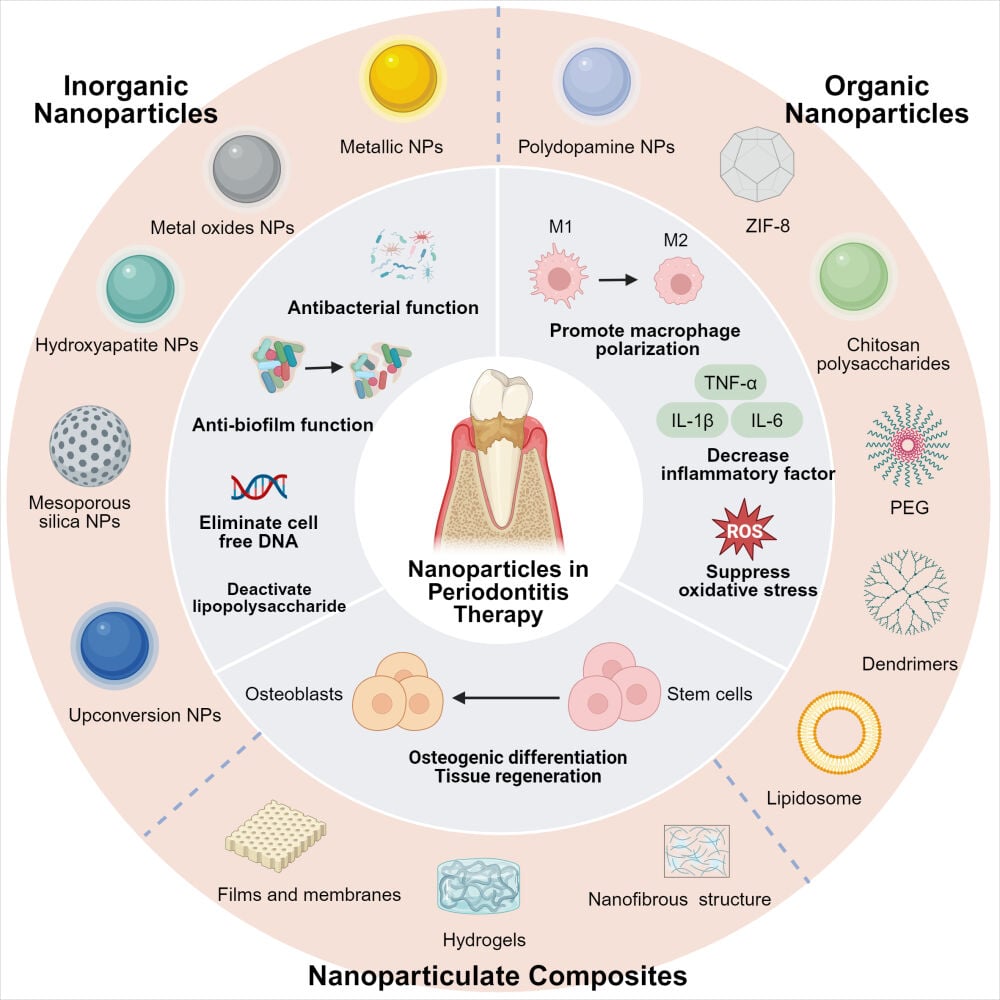
There are few reviews about nanoparticles in the treatment of periodontitis. Previously, the role of some single classes of nanoparticles in periodontitis treatment has been summarized in the literature, such as metal nanoparticles.36 Several studies have also investigated nanoparticles for use in periodontal delivery systems.37–40 Shawky et al presented the application of periodontal membranes loaded with antibiotics, metal nanoparticles, and metal oxides for topical periodontal drug delivery.41 In conclusion, the current study lacks a systematic introduction of nanoparticles for the treatment of periodontitis. This article examines the NPs currently employed in the treatment of periodontitis. These NPs vary in size from 1 to 1000 nm36,42 and are categorized into three groups: inorganic NPs, organic NPs, and nanocomposites (including nanofibers, hydrogels, and membranes) (Figure 1). The objective of this review is to offer a more thorough analysis of the present research progress on the use of NPs in managing periodontitis. This review summarizes the challenges and future directions of NPs in the treatment of periodontitis.
Nanomaterials, A New Strategy Treating Periodontitis
Pathologic Mechanisms and Treatment of Periodontitis
Periodontal disease is an infectious disease involving multiple pathogenic factors and risk promoters. Conventional wisdom suggests that penetration of periodontal tissues by specific bacteria and/or their metabolites is a key step in the pathogenesis of periodontitis.43 For instance, Porphyromonas gingivalis (P. gingivalis) has been recognized as such a key pathogen.44 However, monocausal ideas lack support from in vivo or situ evidences. Instead, more evidence suggests that periodontitis is associated with ecological disorders.45 Ecological dysbiosis refers to changes in the quantity or influence of specific species within a diverse microbial community, which disrupts the homeostasis of the host microorganisms and leads to immune-related destructive inflammation.46 Periodontal health requires a controlled state of immune inflammation capable of maintaining a state of host-microbe homeostasis within the periodontium.47 Plaque biofilms, believed to be the causative agent of periodontitis, are organized aggregates of microorganisms that exist within a complex intercellular matrix.48 Once the host-microbe homeostasis is disrupted, periodontal inflammation (unattached loss and resorption of alveolar bone) occurs. When ecologically dysfunctional microbiota act on the periodontal tissue of a susceptible host. Plaque microorganisms are pathogenic either directly by damaging periodontal tissues through their metabolites or indirectly by causing an immune response in the host.49
Host susceptibility is also essential in the progression of periodontitis. In individuals who are susceptible, the host response is dysregulated and destructive. Although bacteria are necessary for disease pathogenesis, it is primarily an excessive inflammatory response of the host to this microbial challenge that ultimately leads to periodontal tissue damage. In turn, the tissue breakdown products caused by inflammation can serve as nutrients for bacteria. Thus, inflammation and ecological disorders are mutually reinforcing.49–51 Neutrophils are the first line of defense against pathogens and employ many defense mechanisms such as degranulation, chemotaxis, phagocytosis, neutrophil extracellular traps, and the generation of reactive oxygen species (ROS).52 Normally, ROS are produced primarily to act as antimicrobials, but excessive ROS can lead to an increased oxidative load. When the balance between antioxidants and ROS is disrupted, oxidative stress is generated within the affected tissues, resulting in pathological change that ultimately leads to the destruction of host tissues.
The primary objective of periodontal therapy is to remove inflammation while promoting the restoration and regeneration of periodontal tissues, thereby restoring the physiology and function of periodontal tissues. Current clinical treatments focus on eliminating bacteria, but regeneration of periodontal tissues remains a challenge. Periodontal tissues have limited regenerative capacity and do not restore their function on their own after disease.53,54 Scholars have been trying to explore more effective therapeutic strategies to restore periodontal tissue function to address the shortcomings and deficiencies of existing treatments. The progress in nanotechnology has enabled the creation of nanomaterials with diverse functional characteristics for the supplementary treatment of periodontitis and the regeneration of periodontal tissues.35,53,55,56 Nevertheless, these endeavors are currently in the exploratory phase. It will be a great breakthrough in periodontitis treatment if solving the problems of tissue regeneration after periodontitis.
Adjunctive pharmacotherapy aims to inhibit “periodontal pathogens” and the pathological process of periodontitis, including inhibition of reactive oxygen species production, excessive inflammatory reaction, and apoptotic cell death. Improvement of the microenvironment in periodontitis protects both the undamaged tissues from damage and some of the already damaged tissues from further deterioration. In addition, the combination of nanoparticles and scaffolds has the characteristics of both. The integration of nanoparticles’ functional properties with the scaffold material’s exceptional mechanical characteristics and biocompatibility holds promise for addressing the limitations in bioactivities and physicochemical functions observed in current tissue-engineered membranes, which will offer a fresh approach to therapy for periodontal tissue regeneration.
Nanoparticles Improve the Periodontal Microenvironment
Based on the above characteristics, functional nanomaterials ameliorate these pathological processes of periodontitis. More precisely, these methods can be classified into the subsequent three domains. The first method is directly impeding the formation and maturation of periodontally relevant pathogenic bacteria and plaque biofilms. For instance, silver NPs57 and iron oxide NPs58 with magnetic fields can kill bacteria, platinum NPs are both antibacterial and biofilm-eliminating,59 and carbon quantum dots can penetrate biofilms.60,61 Due to its different antibacterial mechanism in comparison to antibiotics, the main advantage of NPs is their ability to slow down the emergence of antimicrobial resistance.
The second approach involves mitigating tissue damage by improving the periodontitis microenvironment and utilizing the tissue’s own reparative and regenerative capacity. For example, cerium oxide NPs62 and polydopamine NPs63 have antioxidant and ROS scavenging effects. In addition, some nanomaterials are used for drug transportation. Including inorganic NPs such as hydroxyapatite NPs (deliver tetracycline and ibuprofen),64 silver NPs (deliver chlorhexidine or metronidazole),65 and mesoporous silica NPs (deliver chlorhexidine and silver ions);66 organic NPs such as chitosan (deliver minocycline),67 poly lactic-co-glycolic acid NPs (deliver metformin).68
In addition, a third approach is to promote periodontal tissue regeneration, using NPs by themselves or in combination with other active ingredients as scaffolds to treat periodontitis and achieve substantial functional recovery. For example, AuNPs can promote osteogenic differentiation by activating autophagy and other pathways.69 Incorporation of MgONPs into scaffold materials enhances membrane tensile strength and modulates degradation rate, while small-dose release of magnesium ions promotes osteogenic differentiation.70
Inorganic NPs
Inorganic nanoparticles can be readily constructed, engineered, and fabricated into a variety of sizes, structures, and geometries, such as nanospheres, nanorods, and nanostars.71–74 The nanomaterials synthesized typically exhibit consistent dimensions, primarily in the range of tens of nanometers, and possess excellent dispersibility. The inherent characteristics of certain inorganic elements, such as the osteogenic differentiation-promoting effect of Au, may help to treat periodontitis.75,76 Appropriate modifications can make them easier to internalize and improve bioavailability.77,78 It is widely acknowledged, therefore, that studies are necessary to ensure the safety of inorganic nanoparticles for in vivo applications.
Metallic NPs
Silver NPs
Silver NPs (AgNPs) with a size of less than 100 nm and a uniform spherical shape have high and broad-spectrum antimicrobial activity.57 Antibacterial mechanisms of AgNPs include but are not limited to, the released silver ions can disrupt the cell walls and cell membranes, denature ribosomes, interrupt adenosine triphosphate production, and interfere with DNA replication.79 Beyond the common roles of antibacterial, antifungal, and drug carriers, AgNPs have been observed to enhance human periodontal ligament fibroblasts (HPDLFs) osteogenic differentiation in a dose-responsive way at the concentration range of 25–100 μM.80
The biosynthetic approach offers significant advantages over physical and chemical synthesis methods as it represents a non-toxic and more sustainable approach to the synthesis of NPs. The green chemistry approach focuses on environmental concerns, efficiency, and economy.81 Prapaipittayakhun et al biosynthesized AgNPs using the stem bark extract of Oroxylum indicum (L.) Kurz (OI) as a reducer. They then investigated the biological properties of OI/Ag NPs on human periodontal ligament stem cells (hPDLSCs). Acquired materials enhanced the proliferation of H2O2-treated hPDLSCs and reduced the production of interleukin 1 beta (IL-1β) in lipopolysaccharide (LPS)-treated hPDLSCs. LPS, also known as endotoxin, which is a crucial constituent of the outermost membrane of gram-negative bacteria, might activate the immune system and cause severe infectious diseases.82 OI/Ag NPs improved the alkaline phosphatase (ALP) activity and calcium concentration of hPDLSCs, which was beneficial for osteoblast differentiation.83
In another study, Steckiewicz et al created AgNPs that were combined with chlorhexidine (AgNPs-CHL) or metronidazole (AgNPs-PEG-MET). Compared to AgNPs-PEG-MET, AgNPs-CHL had greater antibacterial efficacy despite having higher cytotoxicity. Anyway, both NPs showed beneficial properties at non-toxic concentrations. They both decreased the levels of the pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, and TNF-α and the metalloproteinases MMP3 and MMP8 which suggests that they hinder tissue breakdown.65
AuNPs
The first biomedical applications of AuNPs occurred in the 1970s.84 Since then, different modified AuNPs have been employed to make progress in the management of periodontitis. It reported that 45 nm AuNPs could induce human periodontal ligament cells (hPDLCs) differentiation and regulate the periodontitis microenvironment by modulating the phenotype of macrophages. In vivo, treatment with 45 nm AuNPs showed a significant increase in newly developed periodontal tissues.85 Their capacity to stimulate differentiation may rely on the initiation of the autophagic pathway by upregulating the expression of microtubule-associated protein light chain 3 (LC3) and downregulating the expression of sequestosome 1/p62.69 In another study, Zhang et al effectively synthesized L/D-cysteine-anchored AuNPs (L/D-Cys-AuNPs). In vitro experiments showed that more L-type NPs tended to be internalized in hPDLCs than D-type NPs and had a more noticeable effect on the osteogenesis of hPDLCs. Moreover, animal experiments indicated that L-type NPs could activate the autophagy of cells, leading to the greatest effect on cell differentiation and periodontal regeneration.75
The researchers found that treatment with AuNPs repaired the autophagic lysosomal system damaged by inflammation. As a result, the ability of bone formation in periodontal ligament stem cells (PDLSCs) nurtured under inflammatory circumstances was restored.86 Transcriptional factor EB (TFEB), a key controller of autophagy and the creation of lysosomes, is commonly recognized as a component that promotes cell survival.87 The collapse of TFEB in PDLSC rendered AuNPs ineffective. Meanwhile, human β-defensin 3 (hBD3), a peptide with wide-ranging antibacterial properties, has been demonstrated to possess diverse biological activities in periodontitis.88 The combination of hBD3 and AuNPs has a notable effect on enhancing the process of osteogenic differentiation in hPDLCs inside inflammatory microenvironments by stimulating the Wnt/β-catenin signaling pathway.76 Additionally, AuNPs can enhance the process of bone-forming differentiation in hBD3 gene-modified hPDLCs and contribute to periodontal regeneration through the activation of the p38 MAPK pathway.89
In addition to the above-mentioned applications of AuNPs in promoting osteogenic differentiation, AuNPs have also been employed as photothermal therapeutic agents in the treatment of periodontitis. Using near-infrared (NIR) light as an excitation source is preferred because red light can penetrate deeper compared to other lasers.90 AuNPs have favorable photothermal and antibacterial characteristics when exposed to NIR radiation.91 Among AuNPs, metal-phenolic networks (MPNs) are versatile hybrid nanomaterials created through the binding of metal ions with polyphenols. These nanoplatforms have a diverse array of potential uses in the field of pharmaceuticals.92 Wang et al prepared a novel photothermal nanocomposite by encapsulating MPNs onto the surface of branched AuAg NPs. The photothermal characteristics of AuAg NPs (AuAg@PC-Fe) were enhanced by this method, along with a decrease in oxidative stress and inflammation. AuAg@PC-Fe demonstrated the ability to efficiently eradicate periodontal bacteria when exposed to 808 nm near-infrared light. In addition, AuAg@PC-Fe inhibited the nuclear factor kappa-B signaling pathway to treat periodontitis.93 Dong et al incorporated epigallocatechin gallate (EGCG) into a hydrogel modified with gold nanoparticles (E-Au@H) to enable NIR photosensitization, antibacterial activity, and regeneration of periodontal tissue (Figure 2A). The E-Au@H was quickly heated to a temperature of 50.7°C in under 5 minutes when exposed to NIR light, demonstrating a remarkable photothermal effect (Figure 2B and C). Moreover, the release of EGCG was effectively controlled by NIR irradiation, and the released EGCG could enhance the antibacterial impact, stimulate angiogenesis, and improve the process of osseous regeneration. In a rat periodontitis model, after a 4-week treatment, the E-Au@H+NIR group inhibited 87% of plaque biofilms and reduced the distance between the cementoenamel junction and the alveolar bone crest (CEJ-ABC distance) by approximately 38% compared to the periodontitis group (Figure 2D and E). Based on the experimental findings, this hydrogel including a NIR responsive composite nano platform presents novel prospects for the management of periodontal disease.94
 |
Figure 1 Classification and structural description of nanoparticles for the treatment of periodontitis. Created with BioRender.com. |
 |
Figure 2 (A) Schematic of near-infrared-triggered tea polyphenol-modified gold nanoparticle-loaded hydrogel for the treatment of periodontitis. The E-Au@H was rapidly heated under NIR irradiation to release EGCG, which sterilizes the bacteria, enhances vascular development, and accelerates the regeneration of periodontal bone tissue. (B) Thermal image of the sample captured after 5 minutes of exposure to infrared radiation. (C) The temperature variation profiles of various materials following 5 minutes of 808 nm NIR irradiation. (D) Quantification of CEJ-ABC distance in maxillary molars. (E) Quantitative results of the bacterial standard plate counting method. **P < 0.01, and ***P < 0.001. Reprinted from Materials & Design, 225, Dong Z, Lin Y, Xu S, et al. NIR-triggered tea polyphenol-modified gold nanoparticles-loaded hydrogel treats periodontitis by inhibiting bacteria and inducing bone regeneration, 111487. Copyright 2023 with permission from Elsevier.94 Abbreviations: NaBH4, sodium borohydride; EGCG, epigallocatechin gallate; Alg, sodium alginate; PVA, polyvinyl alcohol; NIR, near-infrared; E-Au@H, EGCG was loaded into gold nanoparticle-modified hydrogels; PTT, photothermal therapy; BMP2, bone morphogenetic protein2; Runx2, runt-related transcription factor 2; OCN, osteocalcin; OPN, osteopontin; ALP, alkaline phosphatase; CEJ-ABC, from the cementoenamel junction to the alveolar bone crest; CFU, colony-forming unit; HL (Red), healthy group; Control (Yellow), periodontitis untreated group; E-Au@H (Blue), periodontitis treated with E-Au@H; E-Au@H+NIR (Green), periodontitis treated with E-Au@H+NIR. |
Platinum NPs
Platinum NPs (PtNPs) are agglomerations of platinum atoms that measure between 1 to 100 nanometers in size. PtNPs exhibit exceptional biocompatibility, remarkable durability, and possess surface chemistry, and are widely applied in the biomedical domain, particularly in the areas of cancer treatment and photothermal therapy. However, relatively few studies have been conducted on the application of PtNPs in periodontal treatment.
Itohiya et al examined the antimicrobial properties and potential for decomposition of organic matter of PtNPs made by infrared pulsed laser irradiation methods.59 Colony formation of P. gingivalis was completely inhibited at concentrations of PtNPs greater than 5 ppm. Results proved that PtNPs could deactivate LPS by decomposing it. In short, PtNPs are promising for use in periodontal therapy.
Wu et al prepared an injectable ointment using Pt nanoclusters (PtNC) modified graphitic carbon nitride (CN) and PEG400/PEG4000. The ointment (named CN-PtNCs) showed oxidase-like or peroxidase-like abilities and could produce ROS to kill pathogens. Experiments showed that CN-PtNCs exhibited strong biofilm elimination against Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli). The results showed that CN-PtNC groups reduced the CEJ-ABC distance and probing depth (distance from the gingival margin to the bottom of the periodontal pocket) (P < 0.05), which means that periodontal inflammation was relieved and the results were better than the clinical standard drug. Finally, the CN-PtNCs, characterized by good biocompatibility, high bioavailability, and anti-inflammatory and analgesic properties, demonstrated good therapeutic effects on periodontitis in rats.95
Metal Oxides NPs
With the development of nanomaterials, some nanomaterials, such as metal oxides, have been found to have efficient catalytic activity similar to that of natural enzymes. Nanomaterials with enzyme-like properties are called “nanoenzymes”.96,97 Since the first report of Fe3O4 nanoenzymes in 2007, many kinds of nanoenzymes have been developed and have shown great potential for applications in biochemical testing, environmental management, and disease diagnosis. Metal oxide nanoenzymes, which avoid the instability and low yield of natural enzymes, also demonstrate significant promise for utilization in the field of periodontitis treatment.
Cerium oxide NPs (CeO2NPs) are typical and widely explored nanoenzymes with multiple enzymatic activities, including superoxide dismutase (SOD) and catalase (CAT)-like activities, oxidase mimetic activity, and hydroxyl radicals (·OH) scavenging capacity. Yu et al investigated the impact of CeO2NPs on ameliorating the microenvironment of periodontitis, and the mechanism by which it exerts anti-inflammatory effects. The findings from both in vitro and in vivo provided evidence of the ability of CeO2NPs to scavenge various types of ROS and suppress inflammatory responses induced by ROS in the presence of LPS. Furthermore, CeO2NPs could hinder the MAPK-NF-κB signaling pathway, so effectively suppressing pro-inflammatory factors’ production. CeO2NPs have been found to effectively reduce alveolar bone resorption, osteoclast activity, and inflammation in a rat model of periodontitis.62 Ren et al synthesized a multifunctional nanocomposite (MSN@Ce@PEG NPs) by loading CeO2 onto the surface of mesoporous silica (MSN) and then being modified with PEG (Figure 3A). The authors explored the anti-oxidative stress damage and in vivo therapeutic effects of the obtained NPs in a rat model, as well as potential biotoxicity. The results of the experiment showed that the obtained NPs exhibited good biocompatibility. The materials were able to prevent the inflammatory destruction of periodontal tissues in addition to effectively regulating intracellular ROS and enhancing the differentiation of hPDLSCs. The levels of bone loss and BV/TV ratios indicated that injection of MSN@Ce@PEG inhibited bone resorption (Figure 3B-D).98
 |
Figure 3 (A) Synthesis and application of MSN@Ce@PEG NPs in the treatment of periodontitis. (B-D) Quantitative measurements of alveolar bone loss of both buccal and palatal areas. *P < 0.05, **P < 0.01, ***P < 0.001, and N.S. means P > 0.05. Reprinted from Chemical Engineering Journal, 423, Ren S, Zhou Y, Fan R, et al. Constructing biocompatible MSN@Ce@PEG nanoplatform for enhancing regenerative capability of stem cell via ROS-scavenging in periodontitis, 130207. Copyright 2021 with permission from Elsevier.98 Abbreviations: MSN, mesoporous silica nanoparticles; CeO2, cerium oxide; MSN@Ce, CeO2 onto the surface of MSN; MSN@Ce@PEG, MSN@Ce modified with PEG; ROS, reactive oxygen species; BV/TV, bone volume/tissue volume; Control, no periodontitis group; P+Saline, periodontitis with saline; P+MSN, periodontitis with MSNs; P+MSN@Ce@PEG, periodontitis with MSN@Ce@PEG NPs. |
Engineered magnetic nanoparticles (MNP) have a unique design and precision for a range of medical uses in the environmental and biomedical fields.99,100 Sun et al co-loaded Ce6 and Coumarin 6 (C6) into Fe3O4-silane core-shell structures to form multifunctional NPs (Fe3O4-silane@Ce6/C6 MNPs). The multifunctional NPs are non-cytotoxic and have a range of characteristics, such as chemical stability, water solubility, and superparamagnetic qualities. Compared to the control, Fe3O4-silane@Ce6/C6-mediated antimicrobial PDT demonstrated a significantly higher reduction in biofilm. The reduction of biofilm colony-forming units by Fe3O4-silane@Ce6/C6-mediated antimicrobial PDT was about 4–5 orders of magnitude. The co-loading of Ce6 and C6 allowed real-time monitoring of antimicrobial PDT by ratiometric emission at the same wavelength. Fe3O4 with the magnetic field was able to kill bacteria by the magnetic field and thus target the site of infection. All things considered, Fe3O4-silane@Ce6/C6 MNPs showed strong anti-biofilm action against pathogens linked to periodontitis while maintaining good biocompatibility, time-monitoring, and magnetic localization capability. The use of multifunctional nanoparticles in antimicrobial applications to stop the progression of periodontitis shows considerable promise.58
Research on magnesium oxide nanomaterials in periodontitis treatment has focused on periodontal tissue regeneration. The findings demonstrated that adding magnesium oxide NPs (MgONPs) to poly (L-lactic acid) (PLA)/gelatin considerably enhanced the membranes’ overall performance. This included boosting the membranes’ tensile strength to preserve structural stability and modifying their rate of degradation to account for periodontal tissue. In vitro, studies have shown that the multifunctional membranes have considerable antimicrobial and osteogenic properties and are of great value for the regeneration of tissues. In a model of periodontal defects in rats, the MgO-embedded membranes effectively guided the regeneration of periodontal tissues.70 Peng et al prepared polycaprolactone (PCL)/gelatin core-shell nanocellulose membranes doped with MgONPs, called Coaxial-MgO, using the coaxial electrostatic spinning technique.101 The obtained films have better hydrophilicity and can consistently release Mg2+ at a concentration of about 5 mM. It has been indicated that 5–10 mM Mg2+ has a mild toxic effect on cells and can enhance the development of bone cells.102 Coaxial-MgO membranes showed an improved proliferation rate of hPDLSCs in comparison to Blending-MgO and Blending-Blank and significantly enhanced the adhesion of hPDLCs. In vitro, Coaxial-MgO membranes enhanced ALP activity, mineralized nodule formation, osteogenesis-related genes [runt-related transcription factor 2(Runx2), type I collagen (Col I), ALP], and high antimicrobial effects against E. coli and S. aureus in comparison to controls.
Hydroxyapatite NPs
Hydroxyapatite, calcium-deficient hydroxyapatite (CDHA), and β-tricalcium phosphate (β-TCP) are often utilized in calcium phosphate cements (CPCs) in the field of biological applications.103 HA is highly regarded as a ceramic biomaterial for the replacement and regeneration of bone and hard tissues. This is because it possesses exceptional biocompatibility, osteoconductivity, and a composition that closely resembles that of bone.104 Madhumathi et al developed a dual topical drug delivery system consisting using calcium phosphate bioceramic nanocarriers. CDHA and β-TCP were synthesized using a microwave-accelerated wet chemical method. The drug transporter CDHA was selected for tetracycline, meanwhile, TCP was chosen as the carrier for ibuprofen. The CDHA/TCP (CTP) system exhibited a better regulated drug release profile in comparison to the HA/TCP (BCP) biphasic system. The in vitro biological investigations demonstrated that the CTP system exhibits biocompatibility and possesses notable antimicrobial and anti-inflammatory properties. In a rat model of cranial defects, the drug-loaded CTP system demonstrated superior bone repair and fresh bone production when compared with the control group (without carrier) after 12 weeks.64
Xiang et al exploited a bioactive dual-functionalized apatite nanocrystal. In short, a biomimetic nanohydroxyapatite material was created by employing a polydopamine structure as a template, which was termed tHA. This material was then changed on its surface with bone-forming peptide-1 (BFP-1) and vascular endothelial growth factor-mimicking peptide (QK) using a single step of catechol chemistry. In vitro experiments showed that the synthesized apatite nanocomposites, particularly tHA-BFP/QK, enhanced the cytocompatibility of the original apatite. The nanocomposites have unaffected BFP-1 and QK activities, which can accelerate the biological activity proliferation and development of PDLSCs. The tHA-BFP/QK NPs group significantly enhanced periodontal bone formation in animal experiments.105
Excessive activation of toll-like receptor 9 (TLR9), a component of the immune system, plays a key role in the progress of numerous inflammatory disorders.106 Elevated levels of TLR9 are also linked to the destruction of the tissues of periodontal disease. Studies on animals have demonstrated that immunological responses mediated by TLR9 result in the development of inflammatory periodontal bone loss. Additionally, mice lacking TLR9 have been found to be resistant to periodontitis.107,108 Cell-free DNA (cfDNA), a ligand for TLR9, refers to the release of nuclear and mitochondrial DNA from injured host cells, and it also includes the presence of bacterial or viral DNA.109 Previous studies have demonstrated that positively charged nanomaterials can effectively treat several inflammatory illnesses in animal models by removing negatively charged cfDNA.110 Huang et al explored the correlation between cfDNA levels and the intensity of periodontitis. They utilized selenium-doped hydroxyapatite NPs (SeHAN) with the cationic polyamidoamine dendrimers (PAMAM-G3) to create cationic NPs cfDNA scavengers (G3@SeHANs). Figure 4A illustrates the process by which cfDNA contributes to bone loss. Figure 4B-D show the size, SEM and Zeta potential changes of SeHANs before and after coating with PAMAM-G3. The researchers then explored the therapeutic potential of this nanoparticle in periodontitis treatment. The results indicated that both G3@SeHANs and PAMAM-G3 effectively suppressed the proinflammatory response associated with periodontitis in laboratory experiments by eliminating cfDNA. Additionally, both substances were able to reduce the extent of bone loss caused by inflammation in a mouse model of periodontitis generated by ligation. G3@SeHANs also modulated macrophage polarization and promoted M2 over M1 macrophage phenotype. Furthermore, G3@SeHANs exhibited superior therapeutic efficacy compared to PAMAM-G3 in vivo, specifically in terms of decreasing pro-inflammatory responses and alveolar bone loss. The expression of TLR9 in periodontal tissues epithelium, as well as the levels of pro-inflammatory factors, were reduced after treatment with nanoscavengers (Figure 4E-G).111
 |
Figure 4 (A) The diagram illustrates the process by which cfDNA contributes to bone loss. The cfDNA-scavenging nanoparticles can prevent bone loss and reduce the symptoms of periodontitis. (B) Size measurement of nanoparticles. (C) Scanning electron microscopy imaging of SeHANs before and after coating with PAMAM-G3. (D) Zeta potential of nanoparticles. PBS, PAMAM-G3, or G3@SeHANs were injected on both sides of mice into six sites around the ligature on days 0, 3, 6, 9, and 12; samples were collected on day 15. (E) Expression of TLR9 in periodontal tissues epithelium. (F) TNF-α and (G) IL-6 levels in periodontal tissues. *P < 0.05, **P < 0.01, and ****P < 0.0001. Reproduced from Huang H, Pan W, Wang Y, et al. Nanoparticulate cell-free DNA scavenger for treating inflammatory bone loss in periodontitis. Nat Commun. 2022;13(1):5925. Published 2022 Springer Nature. Available under a CC-BY license.111 Abbreviations: TLR9, toll-like receptor 9; cfDNA, Cell-free DNA; SeHANs, selenium-doped hydroxyapatite nanoparticles; G3@SeHANs, SeHANs with the cationic dendrimer PAMAM-G3.; Normal, healthy group; Untreated, periodontitis treated with phosphate-buffered saline; PAMAM-G3, periodontitis treated with PAMAM-G3; G3@SeHANs, periodontitis treated with G3@SeHANs. |
Mesoporous Silica NPs
Mesoporous silica NPs (MSNs) are commonly used as nanocarriers. It has unique physicochemical properties such as high porosity, large surface area, and pore volume, functionalization, tunable pore, and particle size, biocompatibility, and high-loading cavity.112
Silver ion has been used in antibacterial experimental studies to address the problem of bacterial resistance arising from the application of conventional antibiotics. However, it has also been reported that silver ions can induce resistance in bacteria.113 Meanwhile, the development of nanoparticles containing antimicrobial compounds with mutually enhancing properties can prevent this resistance.114 Lu et al utilized mesoporous silica nanoparticles as carriers of chlorhexidine (CHX) and silver ions to exert synergistic antimicrobial activity while preventing the development of drug resistance. The effective antimicrobial concentration of Ag-MSNs@CHX was less cytotoxic to normal cells. Due to their synergistic bactericidal properties and favorable biocompatibility, these nanoparticles have the potential for widespread and effective use in treating bacterial infections in clinical settings. In another research, the authors synthesized redox/pH dual-responsive MSNs (Ag-MSNs@CHX) to inhibit microbial film formation by controlling the release of CHX and Ag+.115 The results indicated that the Ag-MSNs@CHX remarkably inhibited the growth of Streptococcus pyogenes and their biofilms. Importantly, Ag-MSNs@CHX was more effective than equivalent amounts of free CHX in limiting Streptococcus pyogenes biofilm formation via causing the demise of bacterial cells, especially in the long run. In addition, Ag-MSNs significantly reduced the toxicity of CHX to mouse oral epithelial cells. The authors have developed a safe and highly effective method of combating biofilms, which shows promise for use as an oral biofilm therapy.66
Apart from the application of MSNs for the delivery of antimicrobial materials and drugs, a series of studies have been carried out by authors using them as delivery systems in the promotion of osteogenic differentiation. Li et al successfully synthesized quaternary ammonium salts-modified core-shell mesoporous silica containing AgNPs (Ag@QHMS). Ag@QHMS demonstrated more effective and consistent concentration-dependent antimicrobial effects compared to QHMS’s single-contact antimicrobial activity. The lowest inhibitory concentration was within 100 μg/mL. In addition, AG@QHMS could promote the production of osteogenic-related factors such ALP, bone sialoprotein (BSP), Col I, osteocalcin (OCN), Runx2, and osteopontin (OPN), apoptosis does not occur. Within safe concentrations of Ag@QHMS stimulate the deposition of cellular alkaline phosphatase and stromal calcium salts.116 In another study, Casarrubios et al demonstrated that hollow mesoporous nanospheres in SiO2-CaO (nanoMBGs) systems loaded with isoflavones (IPs) enter preosteoblasts mainly through clathrin-dependent mechanisms. The present study demonstrates the positive binding of MC3T3-E1 osteoprogenitor cells to nanoMBG-IPs, stimulating their differentiation into a mature osteoblast phenotype and increasing alkaline phosphatase activity.117
Mitochondrial dysfunction in mesenchymal stem cells (MSCs) of chronic inflammatory bone disease origin is due to excessive intra-mitochondrial calcium and accumulation of damaged mitochondria due to inhibition of mitosis.118,119 Mitochondrial dysfunction is involved in the pathogenesis and progression of periodontitis by influencing oxidative stress and modulating inflammatory responses.120,121 Based on this finding, Zhai et al designed novel precision-engineered NPs. Figure 5 illustrates the composition of the NPs. They consist of a core made of mesoporous silica NPs (TMAMSN) with a positive charge. These nanoparticles act as carriers for loading siRNA and releasing it in a targeted manner in MSCs. The NPs also have a shell made of a composite of ethylene glycol tetraacetic acid (EGTA), which is a calcium chelator, and triphenylphosphine (TPP), which targets mitochondria. This shell is responsible for trapping calcium ions associated with mitochondria. Additionally, the NPs have a PEG corona, which is attached to EGTA fragments through ester bonds. The METP NPs are composed of core-shell structured corona NPs, specifically TMA-MSN-EGTA/TPP-PEG. The obtained METP NPs possess mitochondrial targeting and intracellular microenvironment (esterase and low pH) responsiveness to regulate mitochondrial calcium flux in MSCs by trapping calcium ions around mitochondria in MSCs with mitochondrial dysfunction and transporting small interfering RNA to tissue-specific MSCs to inhibit the Wnt/β-catenin pathway, regulate mitosis and remove damaged mitochondria. These bifunctional METP NPs are anticipated to serve as effective treatments for various chronic inflammation-related bone disorders, such as periodontitis and osteoarthritis.122 In a separate study, calcium, magnesium, and strontium co-doped MSNs were prepared using the sol-gel method by Pouroutzidou et al Upon immersion in simulated body fluids, all co-doped MSNs exhibited the development of hydroxycarbonate apatite on their surface. This, in turn, enhanced the activity of mitochondria and the proliferation of cells.123
 |
Figure 5 Diagram illustrates the structure and function of METP NPs, along with the process by which siβ-catenin loaded METP NPs (METP/siβ-catenin) remove malfunctioning mitochondria and restore the functionality of both mitochondria and MSCs. Reprinted from Zhai Q, Chen X, Fei D, et al. Nanorepairers Rescue Inflammation‐Induced Mitochondrial Dysfunction in Mesenchymal Stem Cells. Adv Sci (Weinh). 2021;9(4):e2103839. Published 2021 Wiley-VCH GmbH. Available under http://creativecommons.org/licenses/by/4.0/.122 Abbreviations: MSCs, mesenchymal stem cells; ER, endoplasmic reticulum; EGTA, Ethylenebis(oxyethylenenitrilo)tetraacetic acid; METP/siβ-catenin, siβ-catenin loaded METP NPs; siRNA, siβ-catenin. |
Upconversion NPs
Upconversion NPs (UCNPs) are inorganic crystalline nanomaterials that possess several notable benefits. These include a prolonged fluorescence lifetime, minimal potential for toxicity to living organisms, the ability to penetrate deeply into biological tissues, and the absence of background light interference. Recently, they have been widely used in the fields of PDT, bioimaging, and bio-detection. They are capable of producing visible or NIR emissions when continuously stimulated by NIR.124,125 The large depth of light penetration of UCNPs can be used to address the problem of depth of tissue penetration that exists in conventional photodynamic therapy. Zhang et al used the hydrophobic chains of silane to attach to the hydrophobic groups of UCNPs via hydrophobic-hydrophobic interactions, then loaded Ce6 molecules within this hydrophobic layer. Given that the Ce6 molecule is excited in the red area, the addition of Mn ions (doping content was 30%) was implemented to amplify the red light, hence enhancing the function of PDT. The final composite (NaYF4@Ce6@silane NPs) with extremely effective red upconversion luminescence, demonstrated notable therapeutic properties against P. gingivalis, Prevotella intermedia, and Fusobacterium nucleatum (F. nucleatum) and their respective biofilms when exposed to 980 nm irradiation, with superior biocompatibility and low cytotoxicity (90% cell viability at the concentration of 200 μg/mL). Moreover, NaYF4-Mn@Ce6@silane NPs showed more than 2 log reduction in the number of colony-forming units (CFU) for all three bacterial 4-day biofilms and significantly decreased the production of polysaccharides generated by live bacteria.126
Qi et al obtained a NIR-triggered core-shell nanostructure (UCNPs@TiO2) of which the β-NaYF4:Yb3+, Tm3+ core was synthesized by thermal decomposition and the TiO2 shell further modified by hydrothermal method. UCNPs@TiO2 was hexagonal with an average diameter of 39.7 nm and had a positive surface charge (+12.4 mV). The UCNPs@TiO2 were biocompatible and non-cytotoxic. NIR light (980 nm) can excite the core NaYF4:Yb3+, Tm3+ UCNPs to emit intense ultraviolet (UV) light, which further triggers aPDT function of the shell TiO2 through energy transfer, thus achieving significant antibacterial effects. NIR-triggered aPDT exhibited strong inhibition of Streptococcus sanguinis, P. gingivalis, and F. nucleatum, resulting in 3–4 orders of magnitude reduction in biofilm CFU that was remarkably greater than that of control groups.127
In another study, Hu et al developed a NIR light-responsive nanodelivery system based on carvacrol (CA). The system consists of UCNPs that upconvert 808 nm NIR light to blue light (BL), mesoporous silica (mSiO2, carrier channel), and hydrophobic CA (BL responsive properties). Under NIR light (808 nm), UCNPs@mSiO2-CA (UCMCs) exhibited antimicrobial, anti-inflammatory, and immunomodulatory properties due to the synergistic effect of CA and upconverted BL. Significantly, the results of high-throughput sequencing showed that several well-known signaling pathways associated with inflammation and the immune response, such as the MAPK signaling pathway, TNF signaling pathway, and IL-17 signaling pathway, were found to be more prevalent in the altered immunological microenvironment. In a rat periodontitis model induced by ligation, the group of UCMCs irradiated by NIR 808 nm exhibited significant antibacterial and reduced inflammatory factor production. In addition, The 3D reconstructed images show that the UCMCs group irradiated by NIR exhibited better periodontal restorations, which was also demonstrated by the quantitative results of CEJ-ABC distance and BV/TV.128
Although studies have shown that surface-modified UCNPs are biocompatible and not biotoxic within a certain dosage range, the potential toxicity of UCNPs over a long period has not been monitored. A lot of systematic investigations need to be done before they can be applied to the clinic.
Organic NPs
Organic NPs can be constructed with diverse shapes to maximize drug loading, reduce adverse effects, or slow down the pace of drug release. They are mostly made of polymers with a variety of components and functionalities. Organic NPs can also be equipped with active and targeted delivery capabilities by connecting particular functional groups. The biosafety is significantly higher, the raw ingredients are less expensive, and the physicochemical properties are easier to manage when compared to inorganic NPs. Certain organic polymers, such as ZIF-8 for its antibacterial and osteogenic properties129 and polydopamine for its antioxidant properties,63 can also be therapeutically effective against periodontitis in and of themselves.
Polydopamine NPs
Polydopamine (PDA) and its derivatives are extensively utilized in biomedical applications as they possess exceptional NIR absorbance, strong chelating ability for metal ions, and high biocompatibility. These properties make them valuable for uses such as photoacoustic contrast agents, photothermolysis, and chelating agents.130–133 To address the low operational stability and difficult reusability of natural enzymes and antioxidants, this work employed PDA NPs as scavengers of ROS to scavenge multiple ROS and inhibit ROS-induced inflammatory responses.63 Figure 6A shows the general synthesis process of PDA NPs and a schematic diagram of its application as an effective scavenger of ROS for periodontitis treatment. Figure 6B presents an SEM image of the PDA NPs. PDA NPs effectively reduced the expression of inflammatory mediators in LPS-treated HGE cells (Figure 6C and D) and led to a significant reduction in ROS levels (Figure 6E). In a mouse periodontitis model, PDA NPs were used as powerful antioxidants to scavenge ROS and reduce periodontitis without causing any adverse effects (Figure 6F). Doping polymeric PolymP-n Active NPs with Ag, and doxycycline (Dox) demonstrated strong antimicrobial effects in in vitro subgingival biofilm model tests.134
 |
Figure 6 (A) Synthesis process of PDA NPs and its application as an effective scavenger of ROS in periodontitis treatment. (B) Scanning electron microscopy image. (C and D) Expression of inflammatory mediators in LPS-treated HGE cells in the absence or presence of PDA NPs (***P < 0.001). (E) Fluorescence images of HGE cells upon various treatments. (Scale bar = 100 μm.) (F) The results of in vivo fluorescence imaging and relative quantification showed that the fluorescence signal gradually diminished when PDA NPs were injected in situ in the LPS-treated group (***P < 0.001). Adapted with permission from Bao X, Zhao J, Sun J, Hu M, Yang X. Polydopamine Nanoparticles as Efficient Scavengers for Reactive Oxygen Species in Periodontal Disease. ACS Nano. 2018;12(9):8882–8892. Copyright 2018 American Chemical Society.63 Abbreviations: LPS, lipopolysaccharides; PDA, polydopamine; ROS reactive oxygen species; NPs, nanoparticles; HGE, human gingival epithelia. |
ZIF-8
Zinc ions (Zn2+) have a connection by imidazolate (Im) ligands to form the metal-organic framework which is also called zeolitic imidazole framework-8 (ZIF-8). ZIF-8 is promising for use in a variety of biological fields because of its prolonged release of Zn2+, which is essential for osteogenic and antibacterial activities. Antimicrobials encapsulating ZIF-8 exhibit higher biological activity compared to free antimicrobials.129 Li et al doped cerium (Ce) into ZIF-8 to produce a novel nanoparticle (ZIF-8:Ce) with antibacterial and anti-inflammatory properties. The findings showed that the regular and homogenous structure of ZIF-8 was unaffected by the addition of 1% to 10% of Ce. At concentrations lower than 30 μg/mL, ZIF-8:Ce demonstrated strong catalase and superoxide dismutase activity, as well as prolonged release of Zn2+ and Ce3+/Ce4+. When it came to periodontitis pathogens, ZIF-8 had higher anti-biofilm activity. Although the antibacterial effect of ZIF-8 decreased slightly after the incorporation of Ce at 10%, the reduction of CFU remained at about 2 orders of magnitude. More crucially, the anti-inflammatory properties of the novel nanoparticles became stronger with the increase of Ce doping. Furthermore, ZIF-8:Ce10% demonstrated superior anti-inflammatory actions by inhibiting the expression of pro-inflammatory factors by suppressing the translocation of the NF-κB/p65 subunit (p < 0.05). In the meantime, ZIF-8:Ce10% increased macrophage M2 phenotypic polarization and stimulated the release of anti-inflammatory cytokines.135
Gelatin methacrylate (GelMA) is a gelatin derivative, first introduced by Van den Bulcke and colleagues in 2000.136 Liu et al loaded ZIF-8 NPs into GelMA to obtain an injectable photopolymerizable composite hydrogel (GelMA-Z). The gel exhibits a liquid form when exposed to body temperature and can transition into a gel state upon exposure to UV irradiation (Figure 7A). GelMA-Z exhibits sustained release of Zn2+ ions for a duration exceeding 7 days, while also demonstrating favorable compatibility with living cells. In vitro experiments indicated that GelMA-Z up-regulated the expression of osteogenic-related genes and proteins, increased ALP activity, and promoted extracellular matrix mineralization. Moreover, GelMA-Z exhibited notable antibacterial efficacy against P. gingivalis. The hydrogel containing 0.2% w/v ZIF-8 NPs was named GelMA-ZH. The reduction of CEJ-ABC distance in the GelMA-ZH group was seen on both 3D reconstructed images and 2D images of the rat maxilla (Figure 7B and C), which was also demonstrated by the quantification results in CEJ-ABC distance and BV/TV index (Figure 7D and E). Overall, GelMA-Z was shown to promote alveolar bone regeneration in the rat model.137
 |
Figure 7 (A) Synthesis and characterization of composite hydrogels. (B) 3D reconstruction images of maxillary alveolar bone. (M1: the first maxillary molar, M2: the second maxillary molar). (C) 2D images of maxillary alveolar bone. (D and E) Quantification of CEJ-ABC distance and BV/TV index after treatment. *P < 0.05, and **P < 0.01. Reprinted from Acta Biomaterialia, 146, Liu Y, Li T, Sun M, et al. ZIF-8 modified multifunctional injectable photopolymerizable GelMA hydrogel for the treatment of periodontitis, 37-48. Copyright 2022 with permission from Elsevier.137 Abbreviations: ZIF-8, zeolitic imidazolate framework-8; GelMA, gelatin methacryloyl; GelMA-Z, ZIF-8/GelMA composite hydrogel; GelMA-ZH, the hydrogel contains 0.2% w/v ZIF-8 nanoparticles; CEJ-ABC, from the cementoenamel junction to the alveolar bone crest; BV/TV, bone volume/tissue volume. |
Chitosan Polysaccharides
Chitosan is a polysaccharide biopolymer with diverse functional properties, including biocompatibility, biodegradability, antibacterial and antioxidant effects.138 The degradation by-products of chitosan are non-cytotoxic. Chitosan and its derivatives have extensive applications in biomedical applications.139 Carboxymethyl cellulose (CMC), the same as chitosan (Chi), is an adhesive biopolymer that has become an important biomaterial for oral mucosal drug delivery because of its ability to interact well with living organisms and lacks harmful effects. Alvarez Echazú et al combined the two with silica to develop a pH-responsive biopolymer-silica composite (Chi-SiO2, Chi-CMC-SiO2). In addition, a plant extract with antioxidant properties, Larrea divaricata Cav. aqueous extract (Ld), was added to the composite. The Chi-CMC-SiO2 composites indicated the maximum binding rate and reached 100% extract release in nearly 4 days while they maintained their properties that prevent or inhibit oxidation. The results of cytotoxicity tests on 3T3 fibroblasts showed the addition of Ld to the composites increased the proliferation of fibroblasts. Only Chi-SiO2 composites in simulated bodily fluids facilitate potential biomineralization, but the inclusion of CMC in the composites hinders calcium buildup.140
In addition to utilizing chitosan’s properties, some studies have also used it as a carrier for some antibiotics in research on periodontitis treatment to improve biocompatibility and drug utilization. For example, Xu et al prepared polyelectrolyte complex NPs (CS/CMCS-NPs) using chitosan (CS) and carboxymethyl chitosan (CMCS) by ionic gelation, and then used them as Dox carriers (Dox: CS/CMCS-NPs). The results showed that the synthesized complex nanoparticles had an ordered morphology and good cytocompatibility. The obtained NPs showed strong inhibition of P. gingivalis in comparison to the control group. Furthermore, the NPs effectively reduced the expression of NLRP3 inflammasome and IL-1β at both the gene and protein levels in human gingival fibroblasts (HGFs).141 In another study, Martin et al developed chitosan nanoparticles that contained minocycline (MH-NPs) to be administered directly into periodontal pockets (Figure 8A). The findings indicated that MH-NPs were cytocompatible enough for HGFs to be internalized by cells through macrophagocytosis and clathrin-mediated endocytosis (Figure 8B and C). P. gingivalis LPS-stimulated fibroblasts cultured with MH-NPs showed dramatically decreased expression of inflammation-related markers such as TNF-α, IL-1β, NFκB1, and CXCL-8 (Figure 8D). In conclusion, MH-NPs possess the capability to specifically deliver drugs to the interior of cells and exhibit notable anti-inflammatory properties.67
 |
Figure 8 (A) Chitosan NPs, as a carrier for intracellular delivery of minocycline, were successfully internalized by HGFs through megaloblast drinking effect or lattice protein-based endocytosis and regulated autophagy via endosomal/lysosomal pathway within the cell. (B) NPs uptake by HGFs. Red circles delimitate inset areas of analysis. In insets, white arrows indicate NPs aggregates, red arrows indicate membrane ruffles, white circles delimitate clathrin-coated pits and blue circles delimitate clathrin-coated vesicles. (C) The intracellular trafficking of b-NPs and MH-NPs in HGFs. Open red arrow indicates the formation of early endosomes, colored red arrows indicate the late endosomes, and colored black arrows indicate the lysosomes. (D) The levels of inflammation-related markers were significantly reduced in the presence of MH-NPs. *P < 0.05, significantly different from LPS-stimulated condition; ** P < 0.05, significantly different from b-NPs and MH. Reprinted from International Journal of Pharmaceutics, 572, Martin V, Ribeiro IAC, Alves MM, et al. Understanding intracellular trafficking and anti-inflammatory effects of minocycline chitosan-nanoparticles in human gingival fibroblasts for periodontal disease treatment, 118821. Copyright 2019 with permission from Elsevier.67 Abbreviations: HGFs, human gingival fibroblasts; P. gingivalis, Porphyromonas gingivalis; LPS, lipopolysaccharide; MH, minocycline hydrochloride; b-NPs, Chitosan blank nanoparticles; MH-NPs, chitosan-nanoparticles loaded with minocycline; NFκB1, Nuclear factor kappa-B p105 subunit; IL-1β, Interleukin 1 beta; IL-6, Interleukin 6; CXCL-8, Interleukin 8. |
Poly Lactic-Co-Glycolic Acid (PLGA)
Nanomedicine has extensively investigated the use of polymers. Among these options, PLGA stands out as a synthetic polymer that is both biocompatible and biodegradable. It has been approved by the United States Food and Drug Administration (USFDA). PLGA may be fabricated into many forms and dimensions, exhibits excellent water solubility, and allows for adjustable drug release.142,143
BAR (SspB Adherence Region) is a region of streptococcal antigens that suppresses P. gingivalis or Streptococcus gordonii (S. gordonii) interaction and biofilm formation.144 BAR-encapsulated PLGA and methoxy-polyethylene glycol PLGA (mPEG-PLGA) NPs not only effectively inhibited biofilm formation (IC50 = 0.7 μM) but also presented a dose-dependent disruption of pre-existing biofilms (IC50 = 1.3 μM). Moreover, the nanoparticles were effective in inhibiting biofilm formation by releasing BAR within the first 2 h of administration, whereas it took 3 h to disrupt pre-existing biofilms. These NPs provide a platform for encapsulating BAR to ensure high local concentrations of BAR and prolonged exposure to exert inhibitory and disruptive effects on biofilms.145 On this basis, Mohamed et al modified the surfaces of PLGA NPs with BAR, and results showed that the BAR-modified NPs (BNPs) were more effective in disrupting P. gingivalis/S. gordonii biofilms than free peptides. BNPs-treatment could inhibit the alveolar bone loss and IL-17 expression. The vitality of telomerase immortalized gingival keratin-forming cells (TIGKs) treated with BNPs or free BAR was above 90%, and no significant signs of lysis or apoptosis were observed. Both BNP and free BAR did not exhibit hemolytic activity, indicating good biocompatibility.146
Cohesion factor A (CafA) is a fibronectin expressed by early colonizer actinomycetes.147 Desai et al modified BAR-encapsulated NPs with CafA, which was able to increase the adhesion of nanoparticles to S. gordonii, thus prolonging the retention time of nanoparticles in the oral cavity. The synthesized nanoparticles effectively inhibited the formation of biofilms by P. gingivalis/S. gordonii for a duration of 12 hours.148
Metformin (MET) is an antidiabetic drug that possesses properties that can reduce inflammation and prevent bone loss. Treatment of periodontitis in diabetic rats with MET-loaded PLGA (10 mg/kg) reduced bone loss by increasing osteocalcin immunoblotting and decreasing RANKL reduction, which implies an increase in mature osteoblasts and a decrease in the number of osteoclasts. Additionally, there were decreased levels of pro-inflammatory factors such as IL-1β and TNF-α. The study determined that the administration of MET-loaded PLGA resulted in a decrease in inflammation and bone loss in diabetic rats with periodontitis.68
In addition, PLGA was able to improve the photodynamic effect of methylene blue. The photodynamic effect of PLGA NPs loaded with methylene blue on bacteria was superior to that of free methylene blue. And the results of a 3-month clinical trial in patients with chronic periodontitis showed that SRP + aPDT improved the gingival bleeding index more than SRP alone.149
Other Organic NPs
In addition to the organic NPs described above, several nanostructures also have been used to treat periodontitis. Qiu et al developed a nano-platform (PEG-ss-PCL NPs) that can scavenge ROS. They achieved this by encapsulating N-acetylcysteine (NAC), a compound known for its ROS scavenging properties, within specifically designed polymeric NPs that can be cleaved by ROS. These NPs were used as a delivery system to transport NAC into cells. ROS in the inflammatory microenvironment could promote polymer degradation by disrupting thiocondone bonds, which then led to the release of encapsulated NAC. NAC eliminated all LPS-induced ROS, while PssL-NAC regulated ROS levels slightly higher than controls. However, this study demonstrated that a suitable level of intracellular ROS was a benefit for the osteogenic differentiation of hPDLSCs. PssL-NAC NPs could inhibit the LPS-induced apoptosis of hPDLSCs, and reverse the inhibitory effect of LPS on osteogenic differentiation of hPDLSCs. The results showed that the microenvironment controlled by PssL-NAC NPs was well suited for osteogenic differentiation, as seen by elevated levels of bone morphogenetic protein 2 (BMP2), Runx2, and protein kinase A system expression. Furthermore, the administration of PssL-NAC into the region affected by periodontitis reduced the damage to the tissues induced by the ligation of maxillary second molars. PssL-NAC exhibited superior efficacy in suppressing osteoclast activity and inflammation in periodontitis, thus promoting the repair of damaged tissues.56
Dendrimers are synthetic molecules that have a highly branching and spherical structure, and they exhibit low dispersibility. The macromolecules possess a very accurate and manipulable structure, allowing for customization of their molecular weights and exceptional capability for drug delivery.150 An innovative nanocarrier system that is self-assembled and capable of responding to two different stimuli has the ability to carry two different drugs. The compound consists of 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-Poly (ethylene glycol) (DSPE-PEG) loaded with alpha-lipoic acid (ALA). The DSPE-PEG is electrostatically adsorbed with Mino contained in a PAMAM structure, which has a hydrophilic shell. The nanocarriers controlled release of Mino and ALA in reaction to lipase stimulation caused by periodontal pathogens and low pH levels in the inflammatory microenvironment. In vitro and in vivo studies have shown that diabetic pathological conditions, prevent the growth of microbial colonies below the gum line while stimulating the development of bone cells and enhancing the process of bone loss in the gums.151
The liposome is a man-made spherical vesicle consisting of a bilayer structure mostly made up of phospholipids and related lipids that have both hydrophilic and hydrophobic properties. This shape allows for the separation of the inner core and the outer layers.152 Hu et al synthesized novel pH-activated nanoparticles (TMC-Lip-Dox NPs) using quaternary chitosan, liposomes, and Dox. TMC-Lip-Dox NPs demonstrated remarkable suppression of both planktonic bacteria and the production of biofilms and showed a strong affinity with fibroblasts in the human periodontal ligament. Animal investigations demonstrated that NPs exerted a potent inhibitory effect on biofilm formation and effectively stopped the resorption of alveolar bone.153
Nanoparticulate Composites: Association of NPs with Scaffolds
In periodontal tissue engineering, GTR/GBR membranes have been devoted to periodontal bone regeneration. The majority of these barriers consist of polytetrafluoroethylene and collagen, serving solely as physical obstacles that cannot fulfill the diverse needs of periodontal regeneration. Therefore, scholars have encapsulated active ingredients such as antibiotics and growth hormones in these membranes to obtain multifunctional materials for promoting periodontal bone regeneration.
Nanofibrous Structure
Nanofibers are ultrafine fibers with a fiber diameter of less than 1000 nm. The fabrication of nanofiber-based scaffolds in dentistry involves many techniques such as electrostatic spinning, emulsion method, hybrid method, coaxial process, and surface modification method.154 These techniques were employed to incorporate therapeutic compounds into the scaffolds, aiming to obtain the desired clinical outcomes. Among them, the electrostatic spinning method is widely used for its advantages of simple operation, wide range of applications, and relatively high production efficiency.
Over the past few years, electrostatically spun materials have been endowed with “smart” properties, expanding their range of applications to include bone regeneration.155,156 An inherent drawback of electrostatically spun scaffolds for bone regeneration and repair is their intrinsically feeble mechanical qualities, which restrict the potential clinical applications of these materials. Li et al designed an electrically conductive alginate/gelatin (AG) scaffold composed of polydopamine-mediated graphene oxide (PGO) and hydroxyapatite nanoparticles (PHA), which together can scavenge ROS, reduce inflammation, and modulate the immune system. Figure 9A to C illustrate the synthesis schematic of PHA, PGO, and the PGO-PHA-AG scaffold. The inclusion of PHA in the PHA-AG scaffolds resulted in an increase in compressive strength from around 30 kPa to 50 kPa. Similarly, the addition of PGO in the PGO-PHA-AG scaffolds led to a rise in compressive strength from roughly 0 kPa to 10 kPa as the PGO concentration was raised from 50 to 140 wt ‰. The presence of catechol groups on the surface of the PGOs contributes to their favorable mechanical properties. These groups interact with the scaffold network and enhance the density of crosslinks. Although the shape of the scaffold cannot be changed after crosslinking, the PGO-PHA-AG scaffolds can be cut into different shapes as needed in clinical applications due to their good elasticity and cutability. PHA endowed the scaffolds with osteoinductive ability, while PGO endowed the scaffolds with electrical conductivity. Conductive scaffolds encourage bone repair by activating Ca2+ channels and delivering endogenous electrical signals to cells (Figure 9E). Scaffolds made of polydopamine-mediated nanomaterials also have anti-inflammatory and ROS-scavenging properties. Additionally, via influencing glycolysis and the RhoA/ROCK pathways in macrophages, it has immunomodulatory properties, prevents M1 macrophage polarization, and stimulates M2 macrophages to produce cytokines linked to osteogenesis (Figure 9F). Electrical conductivity and immunomodulatory properties promote diabetic periodontal bone regeneration (Figure 9D and G). This study provides significant insights into the collective influence of electrical conductivity, osteoinductivity, and immune-modulation capacity on the process of bone regeneration. It also presents a new approach for developing biomaterials with immunomodulatory properties for tissue regeneration.157
 |
Figure 9 The Diagram depicts the process of creating the PGO-PHA-AG scaffold, which possesses several functions and has the potential to be used in the regeneration of periodontal bone in individuals with diabetes. (A-C) Schematic diagrams of PHA, PGO and PGO-PHA-AG scaffold synthesis. (D) ROS, M1 macrophages, and inflammatory cytokines are overexpressed in the local diabetic periodontal microenvironment. (E) The scaffold promoted cell adhesion and transferred the endogenous electrical signals to cells, activating Ca2+ channels. (F) PDA endowed the scaffold with an immunomodulatory activity that reduced M1 macrophage polarization and activated M2 macrophages polarization. (G) The conductivity and immunomodulatory activity synergistically promoted periodontal bone regeneration. Reproduced from Li Y, Yang L, Hou Y, et al. Polydopamine-mediated graphene oxide and nanohydroxyapatite-incorporated conductive scaffold with an immunomodulatory ability accelerates periodontal bone regeneration in diabetes. Bioact Mater. 2022;18:213–227. Published 2022 Elsevier. Available under https://creativecommons.org/licenses/by-nc-nd/4.0/.157 Abbreviations: HA, hydroxyapatite; DA, polydopamine; PHA, polydopamine-modified hydroxyapatite nanoparticles; GO, graphene oxide; PGO, polydopamine-mediated graphene oxide; ROS, reactive oxygen species; BMSCs, bone marrow mesenchymal stem cells. |
Sprio et al developed a novel multifunctional hybrid scaffold by integrating biomineralization processes, as well as tape casting and electrospinning techniques. The presence of the superparamagnetic apatite phase in the scaffold provides it with the ability to respond to remote magnetic signals and enhance the formation of new bone tissue. In this case, alveolar bone is developed by biomineralization. Alveolar bone develops through biomineralization. Col I can self-assemble in the presence of Ca2+, Fe2+/Fe3+, and PO43- ions for heterogeneous nucleation of iron-doped hydroxyapatite (FeHA) with a FeHA/Collde weight ratio of 70:30. Cementum is electrospun with FeHA mineralized biodegradable polymers to create extremely thin layers of mineralized fibers. The periodontal ligament is fabricated by pH-driven self-assembly and supramolecular organization of Col I nanofibers, followed by cross-linking reactions and freeze-drying processes to obtain fibrous scaffolds with mechanical properties. The periodontal ligament is fabricated by self-assembly and supramolecular organization of Col I nanofibers (driven by pH) followed by cross-linking reactions and freeze-drying to create fibrous scaffolds. A novel periodontal scaffold exhibits excellent cytocompatibility in vitro.158
Regulatory T cells (Tregs) are key regulators of the immune system’s ability to maintain tolerance to autoantigens and alleviate the symptoms of autoimmune diseases by reducing the activity of additional immune cells.159 Liu et al integrated poly (lactic acid) (PLLA) nanofiber sponge microspheres (NF-SMS), PLLA/ PEG co-functionalized MSNs, and PLGA microspheres (PLGA MS) into a multi-bio-delivery vehicle to enrich Tregs in situ. MSNs and PLGA MS were employed to effectively release IL-2/TGF-β and miR-10a, which served to attract T cells and promote their transformation into Tregs. On the other hand, PLLA NF-SMS acted as a scaffold that could be injected to facilitate the attachment and growth of these Tregs. Using the mouse periodontitis model, we administered PLLA NF-SMS through injection and biomolecular delivery. This led to an increase in Treg cells, their growth, and the application of Treg-mediated immunotherapy to combat bone loss.160
To address the constraints of existing scaffold materials with regard to their osteogenic and antibacterial characteristics, Qian et al created a new type of scaffold called PP-pDA-Ag-COL, which is made of electrospun PLGA/PCL and covered with collagen. The creation of this unique scaffold involved the electrospinning of a basic PLGA/PCL matrix, subsequent in situ reduction impregnation with AgNPs, followed by a coating of polydopamine, and finally a coating of Col I. PP-pDA-Ag-COL scaffold greatly improved the attachment of MC3T3 cells after 12 and 24 hours of incubation, in comparison to the control scaffold. Additionally, it exhibited the highest levels of β1 integrin expression at both the mRNA and protein levels. The ALP activity, BMP2, and Runx2 expression levels were markedly elevated in MC3T3 cells cultivated on PP-pDA-Ag-COL scaffolds in comparison to the control. Meanwhile, the scaffolds exhibited a larger antibacterial zone compared to the control group. PP-pDA-Ag-COL scaffolds showed a 31.8% improvement in alveolar bone regeneration and proved to be a successful treatment for periodontitis in a mouse model of the illness.161
Alipour et al fabricated PCL-PEG-PCL nanofibrous scaffolds bound to zeolites by electrospinning method. Both nanofiber scaffolds with or without zeolite showed bead-free structures with average diameters of 437 nm and 430 nm, respectively. The results showed that PCL-PEG-PCL/Zeolite nanofiber scaffolds could support better cell attachment, multiplication, and osteogenic differentiation of hDPSCs.162 In another research, Shoba et al prepared a bilayer functional gradient scaffold mimicking the periodontal extracellular matrix, functionalizing the polymeric scaffold with bromelain and magnesium-doped hydroxyapatite nanoparticles. The findings demonstrated that the scaffold supported the release of bromelain and hydroxyapatite nanoparticles doped with magnesium and improved angiogenesis, cell migration, proliferation, and antimicrobial potential—all crucial elements of the healing process. The in vivo experiments conducted on the Wistar rat model, along with the enhanced protein production of wound-healing markers, provided evidence for the scaffold’s efficacy in treating oral wounds.163
To tackle the issue of insufficient release and effectiveness caused by the limited loading of bioadhesive nanoparticles in the oral cavity and their poor retention, Mahmoud et al developed a rapid-release platform consisting of polymer electrospun fibers (EFs) encapsulating BAR. To obtain a high loading of BAR and a fast release, PLGA, PLLA, and PCL were electrospun individually or in combination with polyethylene oxide (PEO). The combination of 10:90 PLGA: PEO EFs resulted in a 95% release of BAR after 4 hours. It also showed a dose-dependent suppression of biofilms, with an IC50 value of 1.3 μM, and disrupted established P. gingivalis/S. gordonii biofilms, with an IC50 value of 2 μM. Furthermore, it maintained high levels of cell viability.164 In another study, Shen et al fabricated a nanofibrous scaffold based on pure PLA and chitosan/polylactic acid blends by emulsion electrospinning. The results show that the average diameter of nanofibers is approximately 200 nm. The average diameter of chitosan nanoparticles was around 50 nm. Chitosan NPs were added to pure PLA nanofibers, resulting in improved mechanical characteristics and increased cell adhesion. Additionally, it stimulated the process of bone formation in BMSCs, leading to an increase in the expression of genes that indicate bone formation, such as BSP, OCN, Col I, and OPN. Furthermore, it improved the mineralization of the extracellular matrix. Nevertheless, it resulted in a significant upregulation of inflammatory mediators and TLR4 in hPDLCs.165 Abdelaziz et al prepared an electrospun nanofiber scaffold based on poly lactic acid/cellulose acetate (PLA/CA) or PCL polymers. The scaffolds were supplemented with varying amounts of Ag NPs (1–2% w/v) and HANPs (10–20% w/v). Experiments conducted in a controlled laboratory environment showed that the incorporation of HANPs into the scaffold resulted in a 50% increase in cell survival. The use of 10% HANPs enhanced the tensile characteristics, whereas a higher concentration of 20% had a detrimental effect. AgNPs greatly improved the antimicrobial activity, with a 40 mm zone of inhibition still present after 32 days. Furthermore, the degradation curve results showed that the nanofiber scaffolds lost about 40–70% of their mass within 8 weeks.166
Data from other studies explored the finer structure. Lian et al fabricated a novel multifunctional bilayer “GBR scaffold” by incorporating copper-containing MSNs (Cu@MSNs) into a PLGA/gelatin (PLGA/Gel, labeled PG) fiber matrix to construct a composite PG-Cu@MSNs fiber scaffold (Figure 10A and B). The scaffold combines solution electrospinning (SES) and solution electrospinning writing (SEW) methods for specific printers. The porous and loose SEW layer supports and promotes cell growth. In addition, the compact and dense SES layer resists interference from non-osteoblasts and stays away from non-physiologic barriers. The scaffold has superior mechanical properties to the Bio-GideⓇmembrane167 and can already release copper ions in a sustained manner to induce osteogenesis and exert antimicrobial activity (Figure 10C). In vitro, the PG-Cu@MSNs composite scaffold has good osteogenic and antimicrobial characteristics. In artificial saliva, the degradation rate of the obtained GBR scaffolds was around 40% in the initial month, 57% in the following month, and about 70% in the third month (Figure 10D). In addition, the effectiveness of PG-Cu@MSNs scaffolds in promoting bone regeneration was validated by conducting experiments on live rats with periodontal defects (Figure 10E and F).168
 |
Figure 10 (A) Schematic illustrations demonstrate the method of preparing and applying the bi-layered electrowritten PG-Cu@MSNs scaffold. (B) The diagram depicts the use of a two-layered GBR scaffold in the regeneration of maxillofacial and alveolar bone. (C) In vitro release behavior of Cu ions from the PG-Cu@MSNs composite scaffold. (D) In vitro degradation profiles of the bi-layered scaffolds incubated in artificial saliva. (E and F) Quantitative evaluation of CEJ-ABC distance, and the BV/TV values of the regenerated bone tissues. *P < 0.05, and **P < 0.01. Reprinted from Acta Biomaterialia, 118, Lian M, Han Y, Sun B, et al. A multifunctional electrowritten bi-layered scaffold for guided bone regeneration, 83-99. Copyright 2020 with permission from Elsevier.168 Abbreviations: PG, Poly (lactic-co-glycolic acid) and gelatin composite; CEJ-ABC distance, the vertical distance between the cemento−enamel junction and the alveolar bone crest; BV/TV, bone volume/tissue volume. |
Ekambaram et al prepared SPEEK, SPEEK + NH2-ZrO2 and SPEEK + NH2-ZrO2 + Cur nanoscaffolds using electrostatic spinning technique. The SPEEK + NH2-ZrO2 + Cur nanoscaffolds were immersed in PBS solution at pH 7.4, and the results showed that the nanofibers exhibited about 20% curcumin release within 0 ~ 5 h. The release increased sharply after 18 hours, reaching 70%, followed by sustained release. In addition, this nanofiber scaffold enhanced the survival of cells, the ability to combat bacteria, and the growth of cells, all of which are crucial for the process of mending periodontal wounds. The matrix, which is coupled with zirconia and functionalized with amines, is expected to promote favorable cell adhesion and perhaps induce electrical stimulation in cells. The incorporation of SPEEK with aminated zirconia and curcumin enhanced bioactivity, ultimately leading to higher cell survival and regenerative periodontal regeneration applications.169
Hydrogels
Hydrogels are three-dimensional polymer networks that are interconnected through physical interactions or covalent bonding. Hydrogels have found extensive application in various domains, including experimental research, biological studies, and clinical trials. These applications encompass areas such as contaminated wound healing, tissue regeneration engineering, and the development of cellular microenvironments.170–172 Undoubtedly, there exist numerous applications within the realm of dentistry. Hydrogels possess the benefits of having a high level of porosity, a large amount of water content, the ability to degrade, and the capacity to self-repair. These qualities allow hydrogels to imitate the natural cytoplasmic matrix. Moreover, hydrogels exhibit exceptional properties for drug administration and controlled release, as well as serving as a biotherapeutic framework for tissue regeneration. Precision in situ treatment of disease areas is crucial in the dentistry sector. Adequate therapeutic platforms are necessary for complex wound morphologies and medication delivery systems. Regarding the development and progression of periodontitis, effective management of early inflammation necessitates the precise and continuous delivery of medications to regulate the immune system. Conversely, in cases of severe periodontitis, a platform that promotes cell growth and specialization is needed to facilitate the regeneration of periodontal tissue. Hydrogels offer a superior option for attaining the aforementioned goals of inflammation control and tissue restoration.173
The targeted use of hydrogel-based pharmaceutical formulations in periodontal pockets and lesions has demonstrated the ability to administer therapeutic agents with precision and consistency. This can effectively aid in managing the progression of periodontitis. Hydrogel-loaded drugs are usually released slowly and continuously. Mou et al prepared serum albumin microspheres that contain 0.06% minocycline and 0.025% zinc oxide NPs (ZnONPs) and incorporated them into Carbopol 940® hydrogel. The hydrogel showed significant therapeutic effects and self-healing ability of the gingival tissue compared to 2% minocycline ointment (Bio-Gide® Perio). Minocycline had an encapsulation efficiency of 99.99%, a duration of release exceeding 72 hours, and qualities that are sensitive to changes in pH. The greatest bioadhesive force observed in vitro was 0.35 N. The hydrogel demonstrates a wide range of antibacterial resistance and favorable biocompatibility.174
To improve the release of two insoluble drugs, Aminu et al used triclosan (TCS) and flurbiprofen (FLB) to exploit a nanogel (NG) with dual action (antibacterial and anti-inflammatory effects). The authors prepared NPs using triclosan (an antibacterial drug) and PCL, while flurbiprofen (an anti-inflammatory drug) was directly loaded in chitosan hydrogels. The NPs and hydrogel loading system were entwined to form the NG. In vitro, the antibacterial effect of NG against E. coli and S. aureus exceeded that of the pure TCS and FLB mixture, with the diameter of the inhibitory circle being almost twice as large as that of the pure mixture. In vivo, the anti-inflammatory effect of NG was demonstrated in studies on rats with experimental periodontitis.175
Compared with typical hydrogel molding processes, smart hydrogels which are capable of adapting to varied external environments to satisfy different therapeutic needs are garnering more and more attention. Thermosensitive hydrogels are extensively researched intelligent hydrogels for dental purposes. When in the solution condition, these substances can conform to irregular and deeper wound defects in periodontal pockets to the greatest extent feasible. The gel solidifies when the surrounding temperature reaches a physiological level, which makes it easier to fill the bracket and extends the duration of drug release. In this study, Soe et al developed a thermo-responsive in situ gel containing asiaticoside (AS)/cyclodextrin (CD) complexes for enhancing the bioavailability of AS. Non-encapsulated formulations consisting of AS/hydroxypropyl β-CD (HPβCD) complexes and encapsulated formulations containing AS loaded sulfobutylether β-CD/chitosan NPs (SBEβCD/CS NPs) were prepared. When in contact with simulated saliva at body temperature, all formulations achieved a sol-to-gel transition within 50–100 s. The in vitro gel formation time was longer and the mechanical strength of the encapsulated formulations was higher compared to the non-encapsulated formulations. In vitro adhesion and release studies showed good adhesion and sustained release of AS from the nano-encapsulated in situ gels (plateau levels were not reached during the 48h of the experiment). These formulations did not exhibit cytotoxic effects on HPDCLs. SBEβCD/CS NPs containing low AS content could express the synthesis of Col I.176
In a separate study, the authors addressed the need for multifunctionality in periodontal therapy by adding drugs with multiple functions within the hydrogel. Johnson et al developed an antimicrobial agent-loaded hydrogel consisting of cellulose nanofibers (CNF) and κ-carrageenan oligosaccharides (CO) NPs. The authors chose two antimicrobial agents (surfactin and Herbmedotcin) as therapeutic agents. This material showed strong antimicrobial activity against S. mutans, P. gingivalis, P. nucleatum, and P. aeruginosa. Furthermore, in the presence of hydrogel, a notable increase in malondialdehyde (MDA) production, biofilm formation, and reduction of bacterial metabolic activity were observed. Furthermore, it decreased the synthesis of ROS, transcription factors, and cytokines in human gingival fibroblasts during inflammatory circumstances. To summarize, the hydrogel exhibits both antibacterial and anti-inflammatory characteristics.177 Li et al integrated the advantages of MOFs and hydrogels by incorporating dexamethasone-loaded zeolitic imidazolate frameworks-8 (DZIF) NPs into a photocrosslinking matrix of methacrylic polyphosphoester (PPEMA) and GelMA, resulting in an injectable nanocomposite hydrogel (Figure 11A-E). The injectable hydrogel was capable of being administered into deep periodontal pockets to attain elevated local concentrations without inducing antibiotic resistance (Figure 11B). The hydrogel has high antimicrobial activity, has a stable microenvironment construct that maintains cell viability, proliferation, proliferation, and osteogenesis, and downregulates inflammatory gene expression in vitro. The findings from an experiment conducted on rats with experimental periodontitis demonstrated that the nanocomposite hydrogel successfully decreased inflammation in the gums (Figure 11F) and mitigated the loss of bone caused by periodontitis (Figure 11G and H).178
 |
Figure 11 (A) Schematic illustration showing the synthesis processes for DZIF NPs. (B) Schematic showing nanocomposite hydrogel preparation by PPEMA (C) and GelMA (D). (E) Injection into periodontal pockets. The hydrogel decreased inflammation in the gums (F) and mitigated the loss of bone caused by periodontitis (G and H). *P < 0.05, and ***P < 0.001. Reprinted from Materials Today Bio, 16, Li N, Xie L, Wu Y, et al. Dexamethasone-loaded zeolitic imidazolate frameworks nanocomposite hydrogel with antibacterial and anti-inflammatory effects for periodontitis treatment, 100360. Copyright 2022 with permission from Elsevier.178 Abbreviations: DEX, dexamethasone; DZIF, dexamethasone-loaded zeolitic imidazolate frameworks-8; PPEMA, photocrosslinking methacrylic polyphosphoester; GelMA, methacrylic gelatin; HGFs, human gingival fibroblasts; ABC-CEJ, the vertical distance between the alveolar bone crest and the cemento−enamel junction in maxillary second molar. |
Some of the previously mentioned nanoparticles with ROS scavenging effects were also doped into hydrogels after modification to form antioxidant hydrogels for periodontitis treatment. Ou et al developed a triple antioxidant hydrogel by doping puerarin (PUE) and ferulic acid (FA) into PEG but acrylate (PEG-DA) hydrogel using PDA nanoparticles. The FA loadings for PDA/FA NPs and PDA/PUE/FA NPs were 9.45 ± 0.89% and 6.09 ± 2.31%, respectively. The PUE loadings for PDA/PUE NPs and PDA/PUE/FA NPs were 7.93 ± 1.77% and 5.67 ± 1.89%, respectively. An initial rapid release of the medication within 12 hours, followed by a consistent and sustained release. Furthermore, the drug release in PEG-DA hydrogels without PDA was much faster than the drug release profile of other hydrogels. The maximum clearance of -OH- and DPPH- by PEG-DA/PDA/PUE/FA hydrogels was 79.27±2.20 and 52.55±2.98%, respectively.179
If early periodontal inflammation is not well managed, unregulated immune reactions and proliferating bacteria can lead to the destruction of periodontal tissue. The patient’s needs cannot be adequately addressed by antibacterial and anti-inflammatory medications alone. It is crucial to have the ability to enhance the growth, specialization, and restructuring of periodontal tissues. Xu et al proposed an injectable sodium alginate hydrogel composite (CTP-SA) for GTR. Cu2O and PDA-coated TiO2(TiO2@PDA) NPs were incorporated into this hydrogel. CTP-SA converted from liquid to solid state after injection, which could automatically match the site of the metaphyseal defect. Furthermore, CTP-SA demonstrated a wide range of antibacterial effectiveness when exposed to BL irradiation. Furthermore, the emission of ROS upon exposure to BL excitation accelerated the oxidation of Cu+ to Cu2+. The stimulation of freshly generated Cu2+ and the photothermal response of CTP-SA under NIR light simultaneously enhance the osteogenic effect of this hydrogel. In conclusion, with this dual-light (BL and NIR) non-invasive modulation, CTP-SA can switch between antimicrobial and osteogenic modes to cater to the specific needs of the patient, thus enabling customized GTR procedures.180
Qu et al designed and fabricated metformin (MF)-loaded MSN-containing GelMA photo-crosslinked hydrogels. GelMA doped with 1.5 mg/mL of MSNs-COOH showed the greatest compression modulus and swelling ratio. The introduction of MSNs-COOH (at concentrations of up to 3 mg/mL) into GelMA did not have any impact on the viability of the cells. MF in MSN-COOH / gel MA greatly enhanced cell growth. The expression of osteogenesis-related genes, with the exception of OCN, was significantly increased in modified hydrogels (MSN-COOH and MF-MSNS-COOH) compared to GelMA.181
Wu et al uniformly introduced ZnONPs into chitin hydrogel (ChT-1%ZnO) using a one-step dissolution regeneration technique with an alkali/urea solution. Following a 5-week period of immersion, the remaining weight of ChT-1%ZnO in a solution containing 150 μg/mL of lysozyme was determined to be 52%. ChT-1%ZnO showed significant antibacterial efficacy against P. gingivalis and S. aureus after 6 h, 12 h, and 24 h. Furthermore, the osteogenesis-promoting effects of ChT-1%ZnO were observed in laboratory conditions and subsequently evaluated in a live rat model with periodontal defects. At 8 weeks postoperatively, the ChT-1%ZnO group showed a value of 1.608 mm for osteoid-enamel bonding, which was statistically different compared to the membrane-free group (1.825 mm) and the ChT group (1.685 mm).182
Films and Membranes
Tissue engineering techniques can reconstruct new microenvironments and lead to the formation of functional tissues.183 Scaffolds (biomaterials, stem cells, and scaffold fabrication methods) and controlled drug delivery strategies (biomolecules, drug release methods, and their appropriate concentrations) are two important factors that must be talked about in tissue engineering.184–186 In most of the studies, polymers have been used to provide the essential features of complex structures and layered scaffolds of periodontal tissues.187 Depending on the main polymer components, experimental studies have been divided into three main categories: natural (collagen, hyaluronic acid, chitosan, and lignin), synthetic polymers including PCL, PEG, acrylates, PTFE, PLGA, and combinations of natural and synthetic polymers. Table 1 shows the application of nanocomposites (a combination of nanoparticles and membranes) in the treatment of periodontitis.
 |
Table 1 Nanoparticulate Composites (Association of NPs with Membranes) in Periodontal Tissue Engineering |
Vale et al developed a novel antimicrobial free-standing (FS) film using chitosan, hyaluronic (HA), and catechol-functionalized hyaluronic acid (HA-DN). HA-DN provided wet bonding properties. Silver doped bioactive glass (BG) NPs (Ag-BGs) were incorporated into the composite film to promote bactericidal properties and bioactivity. Bioactivity tests showed the formation of a bone-like apatite layer after immersion in simulated body fluids. Furthermore, these FS membranes exhibited significant antibacterial effects after 16 hours of direct contact with S. aureus and E. coli cultures.188 In another study, Shah et al developed a novel three-layer functionally graded chitosan membrane (FGM) by a lyophilization method. Gradient gradations of chitosan, BG, and Pluronic F127 were incorporated into the membrane, where each layer has a distinct surface function. The lowest layer is designed to mimic the structure of alveolar bone and consists of 50% weight percent BG. SEM analysis shows that the surface of the bottom FGM exhibits a porous structure containing embedded BG particles. The middle layer consists of 25% weight percent of BG, whereas the upper layer is composed of a non-porous material devoid of BG and exhibits interconnected structures. Contact angle measurements verified that the surface coated with BG exhibited hydrophilic properties (≈00), whereas the opposing surface had hydrophobic characteristics (910 ± 3.840). Osteoblasts and fibroblasts both exhibit optimal adhesion at contact angles.189
Soltani Dehnavi et al synthesized nanocomposite films using PCL, PEG, and BG nanopowder by solvent casting method for the regeneration of periodontal tissues in vitro. Although the percentage of copper incorporated into the BGs was insignificant, this insignificant amount produced significant cytotoxic effects on cells. The study indicated that hybrid membranes containing 7 wt% copper-free BGs showed optimal mechanical properties, biodegradable properties, a more wettable surface, higher proliferation rates of adipose-derived stem cells (ADSCs), superior ALP activity, and excellent bone mineralization capacity.190
Using an electrospinning technique, Ghavimi et al created an asymmetric GBR membrane containing curcumin and aspirin. The prepared GBR membrane had monodisperse nanosized fibers, a homogeneous network-like morphology, and a negative surface charge. The release curve results indicate that the initial release concentration of aspirin was high (20% from the first to the fifth day), in contrast to a lower concentration at a later stage, but aspirin continued to be released until the 30th day. The membrane was also antimicrobial effective against all bacteria tested, including S. aureus, E. coli, and Enterococcus faecalis. Curcumin and aspirin enhanced osteogenic capacity at both the transcriptional and translational stages. During animal trials, the test area exhibited rapid ossification within only 28 d, but the commercial membrane area showed no signs of bone formation. Additionally, there was a layer of soft tissue present above the newly formed bone region on the test side.191
Toledano-Osorio et al fabricated a non-absorbable composite nanofibrous membrane using NanomyP® (Granada, Spain) functionalized with Zn or Dox. The results showed that doping membranes with Dox and Zn increased ALP activity by 70% and 30%, respectively. Dox-doped membrane upregulated several antigenic markers with immunomodulatory potential, such as CD54, CD80, CD86, and HLA-DR.192 In another study, Dox-doped membrane exhibited upregulation of BMP-2, ALP, OPG, TGF-β1, and TGF-βR1. Zinc-functionalized membrane induced a significant increase in the expression of Col I, ALP, and TGF-β1. In addition, both films greatly decreased the expression of RANKL. However, mechanical testing of the composite film was not performed in either article.193
Nardo et al developed an antibacterial coating of AgNPs on polytetrafluoroethylene (PTFE) membranes using a green and simple method. The antibacterial activity of the PTFE-DOPA-Ag membrane was demonstrated in vitro against S. aureus and E. coli. In vitro cytometric assays using NIH 3T3 fibroblasts showed limited viability and proliferation despite cell adhesion to PTFE-DOPA-Ag membranes, again demonstrating the antibacterial activity of PTFE-DOPA-Ag membranes.194
Pouroutzidou et al created the drug-carrying composite fibrous membranes (PLGA/MOX-MSNs) utilizing an electrostatic spinning process. While the encapsulation of moxifloxacin (MOX) has a protective impact on red blood cells, the usage of MSNs as MOX nanocarriers in fibrous PLGA membranes appears to promote drug release by establishing a more regulated and longer release profile and boosting its hemolysis activity. In addition, improved fiber dispersion, more porosity, and larger fiber diameter were produced by the increase in polymer concentration. The tensile and elongation strength, however, was constrained by the presence of MSNs. Additionally, as MSNs concentration increased, hydrophilicity and degradation rate also rose. However, without the inclusion of MSNs, the drug’s rate of membrane encapsulation was greater. Additionally, the neat polymeric fibers that formed following the addition of the medication reduced fiber cross-linking while the MSNs added after MOX encapsulation had no effect on the morphology of the fibers. As they regulate the release of moxifloxacin and ensure antibacterial efficacy with adjustable degradation rates against a variety of periodontal pathogens, the suggested composite electrostatic spinning membranes may be a successful alternative method for periodontitis treatment. Such barrier membranes could stimulate osteogenic and angiogenic potential while overcoming the drawbacks of hydrophobicity and constrained bioactivity.195
Craciunescu et al developed a multifunctional three-dimensional hybrid biomaterial containing extracellular matrix components, collagen, chondroitin sulfate, and fibronectin, functionalized with AgNPs. The material has a microstructure of interconnected pores, with two-dimensional culture conditions, and exhibits significantly higher cell viability and proliferation within the three-dimensional hybrid biomaterial, and no genotoxic effects. In vivo experiments showed that cells and blood vessels colonized the hybrid material and synthesized a new extracellular matrix. The use of silver nanoparticles enhanced the anti-inflammatory efficacy of the hybrid biomaterials. The antibacterial activity of the silver nanoparticles persisted even after being incorporated into the polymer scaffold, effectively suppressing the development of F. nucleatum and P. gingivalis.196
He et al prepared gelatin (Gln) and chitosan composite GBR films containing hydroxyapatite NPs (nHAp) and antimicrobial peptide (Pac-525)-loaded PLGA microspheres (AMP@PLGA-MS) using sequential layer-by-layer electrospinning and electrospraying techniques, which should have osteogenic and antimicrobial activities. The diameter of nHAp-containing fibers was 359 ± 174 nm, in contrast to 409 ± 197 nm for electrospun fibers without nHAp. nHAp involvement and chemical cross-linking were both able to improve their tensile strength. The Gln/CS composite membranes exhibited excellent biocompatibility in vitro, characterized by favorable cell adhesion, diffusion, and proliferation. Furthermore, membranes composed of Gln/CS and containing nHAp have the ability to enhance the process of osteogenic differentiation in BMSCs. The GBR membranes, which incorporated AMP@PLGA-MS, demonstrated prolonged and controlled release of Pac-525 in laboratory conditions. These membranes exhibited bactericidal effects within a week and maintained antibacterial activity against S. aureus and E. coli for a duration of 1 month.197
In another study, He et al developed a novel asymmetric barrier membrane composed primarily of agarose hydrogel. The hydrothermal process was used to prepare hollow carbonated hydroxyapatite (CHA), which was then precipitated in agarose and exhibited an asymmetric structure. The antimicrobial agent ε-poly-lysine (ε-PLL) was used to provide the membrane with a durable antibacterial effect. Increasing the amount of CHA additive resulted in improved biocompatibility and enhanced mechanical qualities of the barrier membranes. We have shown the osteoconductivity and antibacterial characteristics of the membranes through both in vitro and in vivo experiments.198
To address the problem of treatment failure due to bacterial colonization of GTR membranes, Prado-Prone et al fabricated composite membranes with topical antimicrobial properties by electrospinning technique. In this study, acetic acid was used as the only common solvent to improve the miscibility of PCL and Gel in the electrospun solution, resulting in homogeneous fiber films. The composite films showed adequate mechanical properties and degradability. The mechanical strength was better at 55:45 than at 70:30 for the same ZnO-NPs content; the higher the percentage of ZnO-NPs was for the same PCL to Gel ratio, the higher the mechanical strength was. The presence of ZnO-NPs in the composite membrane significantly reduced the growth of S. aureus and its biofilm, but this inhibition was independent of the concentration of ZnO-NPs, and the cytotoxicity increased with the increase of the concentration. The composite films containing 1 and 3 wt% ZnO-NPs were biocompatible with human osteoblasts and HGFs.199
Conclusion
Advances in nanotechnology provide promising solutions for the treatment of inflammatory diseases such as periodontitis. The development and approval of immunomodulatory nanomedicines by the FDA marks a breakthrough in this field. However, there are some criterions should be addressed when preparing NPs for the treatment of periodontitis.
Nanomaterials must meet biosafety standards when applied to periodontal tissue to ensure that they do not cause any harm to healthy tissue structures. In order to achieve the goal of inhibiting bacterial growth using the nanoparticles themselves or loaded drugs, the drug release concentration must also exceed the minimum inhibitory concentration. This requires nanomaterials to be able to control the rate and concentration of drug release to ensure that the antimicrobial effect is sustained over the course of treatment. Many studies have evaluated the dispersion effect of NPs on biofilms, but this dispersion effect has not been confirmed in clinical trials.200
As discussed above, periodontal tissue destruction is associated with hyperinflammation of the host. NPs can alleviate this chronic inflammation by delivering active pharmaceutical ingredients, thereby facilitating the process of tissue repair. A moderate inflammatory response can stimulate the local immune system, promote wound healing and neovascularization, and remove waste material from the injured site.201 However, it is important to note that both the absence of any inflammation and the presence of excessive inflammation can adversely affect the tissue.202 Therefore, how to produce and maintain the optimal “ideal” level in the tissue environment, which can effectively resist external invasion without causing obvious damage to the normal tissue structure is a big challenge.
The application of NPs in periodontal tissue regeneration necessitates excellent biological absorption characteristics. If the material does not degrade on its own, the patient may need to undergo a secondary operation to remove the remaining material, which increases the patient’s suffering and recovery time. Hence, it is crucial to identify nanoparticle materials capable of self-degradation at an appropriate time. Moreover, the development of reliable techniques to track the distribution of NPs and assess their effects on the organism will help to understand their mechanisms of action and optimize therapeutic strategies.
Unfortunately, at present, there are few clinical studies on NPs in the treatment of periodontitis, and most of them are still in the preclinical stage. This indicates there are some challenges hinder the transition from academic success to clinical implementation. First, while encouraging preclinical results demonstrate the effectiveness of nano-formulations for the treatment of periodontitis, it is critical to conduct rigorous clinical trials to evaluate their performance under “real-world” conditions, which is absent in most currently researches. Second, the long-term toxicity of some NPs is unknown. Although numerous studies have explored the short-term toxicity of their NPs, there is a limited understanding of the accumulation in crucial organs or tissues might cause unforeseen toxic effects. Therefore, investigating the behavior of NPs (absorption, distribution, metabolism, and excretion) within the human body over extended periods, and monitoring the related potential side effects remain a challenge but need to be addressed. Third, and most important, strengthen the interdisciplinary collaboration between chemist and dentist is essential for the clinical usage of NPs for periodontitis treatment. The dentist provides crucial perspectives on clinical challenges and offers valuable requirements, while chemist specializes in synthesizing superior NPs accordingly.
Anyhow, advances in nanotechnology have provided us with an exciting new tool. We believe that safe and effective NPs will become a widely available option for patients suffering from periodontitis in the future. With the continued efforts of chemists and dentists, we may see a paradigm shift towards more effective treatments that offer hope to those affected by chronic inflammation-related diseases.
Acknowledgments
This work was supported by the Jilin Provincial Department of Finance backbone talent project (jcsz2023481-10). The graphical abstract was Created with BioRender.com.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gasmi Benahmed A, Gasmi A, Noor S, et al. Hallmarks of Periodontitis. Curr Med Chem. 2024;31:1–19. doi:10.2174/0109298673302701240509103537
2. Trindade D, Carvalho R, Machado V, Chambrone L, Mendes JJ, Botelho J. Prevalence of periodontitis in dentate people between 2011 and 2020: a systematic review and meta‐analysis of epidemiological studies. J Clin Periodontol. 2023;50(5):604–626. doi:10.1111/jcpe.13769
3. Chen K, Ma S, Deng J, Jiang X, Ma F, Li Z. Ferroptosis: a New Development Trend in Periodontitis. Cells. 2022;11(21):3349. doi:10.3390/cells11213349
4. Mohd-Said S, Mohd-Norwir NA, Ariffin AN, et al. Validation of a Simplified Digital Periodontal Health Screening Module for General Dental Practitioners. Healthcare. 2022;10(10):1916. doi:10.3390/healthcare10101916
5. Eke PI, Wei L, Borgnakke WS, et al. Periodontitis prevalence in adults ≥ 65 years of age, in the USA. Periodontol 2000. 2016;72(1):76–95. doi:10.1111/prd.12145
6. Hajishengallis G. Interconnection of periodontal disease and comorbidities: evidence, mechanisms, and implications. Periodontol 2000. 2022;89(1):9–18. doi:10.1111/prd.12430
7. Kapila YL. Oral health’s inextricable connection to systemic health: special populations bring to bear multimodal relationships and factors connecting periodontal disease to systemic diseases and conditions. Periodontol 2000. 2021;87(1):11–16. doi:10.1111/prd.12398
8. Nibali L, Gkranias N, Mainas G, Di Pino A. Periodontitis and implant complications in diabetes. Periodontol 2000. 2022;90(1):88–105. doi:10.1111/prd.12451
9. Parsegian K, Randall D, Curtis M, Ioannidou E. Association between periodontitis and chronic kidney disease. Periodontol 2000. 2022;89(1):114–124. doi:10.1111/prd.12431
10. Herrera D, Sanz M, Shapira L, et al. Association between periodontal diseases and cardiovascular diseases, diabetes and respiratory diseases: consensus report of the Joint Workshop by the European Federation of Periodontology (EFP) and the European arm of the World Organization of Family Doctors (WONCA Europe). J Clin Periodontol. 2023;50(6):819–841. doi:10.1111/jcpe.13807
11. Xu B, Han YW. Oral bacteria, oral health, and adverse pregnancy outcomes. Periodontol 2000. 2022;89(1):181–189. doi:10.1111/prd.12436
12. Koziel J, Potempa J. Pros and cons of causative association between periodontitis and rheumatoid arthritis. Periodontol 2000. 2022;89(1):83–98. doi:10.1111/prd.12432
13. Radaic A, Ganther S, Kamarajan P, Grandis J, Yom SS, Kapila YL. Paradigm shift in the pathogenesis and treatment of oral cancer and other cancers focused on the oralome and antimicrobial‐based therapeutics. Periodontol 2000. 2021;87(1):76–93. doi:10.1111/prd.12388
14. Aimetti M. Scaling and Root Planing in Nonsurgical Periodontal Treatment. Dental Abstracts. 2022;67(3):217–219. doi:10.1016/j.denabs.2022.05.042
15. Cobb CM, Sottosanti JS. A re-evaluation of scaling and root planing. J Periodontol. 2021;92(10):1370–1378. doi:10.1002/JPER.20-0839
16. Brayer WK, Mellonig JT, Dunlap RM, Marinak KW, Carson RE. Scaling and Root Planing Effectiveness: the Effect of Root Surface Access and Operator Experience. J Periodontol. 1989;60(1):67–72. doi:10.1902/jop.1989.60.1.67
17. Vinel A, Al Halabi A, Roumi S, et al. Non-surgical Periodontal Treatment: SRP and Innovative Therapeutic Approaches. Adv Exp Med Biol. 2022;1373:303–327 doi:10.1007/978-3-030-96881-6_16.
18. Slots J. Primer on etiology and treatment of progressive/severe periodontitis: a systemic health perspective. Periodontol 2000. 2020;83(1):272–276. doi:10.1111/prd.12325
19. Lu H, He L, Jin D, Zhu Y, Meng H. Effect of adjunctive systemic antibiotics on microbial populations compared with scaling and root planing alone for the treatment of periodontitis: a pilot randomized clinical trial. J Periodontol. 2022;93(4):570–583. doi:10.1002/JPER.20-0764
20. Kang Y, Sun B, Chen Y, et al. Dental Plaque Microbial Resistomes of Periodontal Health and Disease and Their Changes after Scaling and Root Planing Therapy. mSphere. 2021;6(4):e0016221. doi:10.1128/mSphere.00162-21
21. Cortellini P, Stalpers G, Mollo A, Tonetti MS. Periodontal regeneration versus extraction and dental implant or prosthetic replacement of teeth severely compromised by attachment loss to the apex: a randomized controlled clinical trial reporting 10‐year outcomes, survival analysis and mean cumulative cost of recurrence. J Clin Periodontol. 2020;47(6):768–776. doi:10.1111/jcpe.13289
22. Gao Y, Wang S, Shi B, et al. Advances in Modification Methods Based on Biodegradable Membranes in Guided Bone/Tissue Regeneration: a Review. Polymers. 2022;14(5):871. doi:10.3390/polym14050871
23. Wang H, Lin F, Wu Y, et al. Carrier-Free Nanodrug Based on Co-Assembly of Methylprednisolone Dimer and Rutin for Combined Treatment of Spinal Cord Injury. ACS Nano. 2023;17(13):12176–12187. doi:10.1021/acsnano.3c00360
24. Zhang X, Zhang P, Xiao C, Chen X. ROS-Responsive Self-Degradable DNA Nanogels for Targeted Anticancer Drug Delivery. ACS Macro Lett. 2023;12(10):1317–1323. doi:10.1021/acsmacrolett.3c00442
25. Zeng S, Tang Q, Xiao M, et al. Cell membrane-coated nanomaterials for cancer therapy. Mater Today Bio. 2023;20:100633. doi:10.1016/j.mtbio.2023.100633
26. Xiong Y, Rao Y, Hu J, Luo Z, Chen C. Nanoparticle-Based Photothermal Therapy for Breast Cancer Noninvasive Treatment. Adv Mater. 2023;2305140. doi:10.1002/adma.202305140
27. He X, Chen F, Chang Z, et al. Silver Mesoporous Silica Nanoparticles: fabrication to Combination Therapies for Cancer and Infection. Chem Rec. 2022;22(4):e202100287. doi:10.1002/tcr.202100287
28. Wang H, Xiao CS, Chen XS. Polymeric nanomedicines for controlled release of sulfur dioxide. Acta Polymerica Sinica. 2024;55(1):1–12 doi:10.11777/j.issn1000-3304.2023.23169.
29. Kumaar NR, Nair SC. Nanomaterials: an intra-periodontal pocket drug-delivery system for periodontitis. Therapeutic Delivery. 2023;14(3):227–249. doi:10.4155/tde-2023-0001
30. Xu T, Xie K, Wang C, Ivanovski S, Zhou Y. Immunomodulatory nanotherapeutic approaches for periodontal tissue regeneration. Nanoscale. 2023;15(13):5992–6008. doi:10.1039/D2NR06149J
31. Yan R, Liu J, Dong Z, Peng Q. Nanomaterials-mediated photodynamic therapy and its applications in treating oral diseases. Biomaterials Adv. 2023;144:213–218. doi:10.1016/j.bioadv.2022.213218
32. Carrouel F, Viennot S, Ottolenghi L, Gaillard C, Bourgeois D. Nanoparticles as Anti-Microbial, Anti-Inflammatory, and Remineralizing Agents in Oral Care Cosmetics: a Review of the Current Situation. Nanomaterials. 2020;10(1):140. doi:10.3390/nano10010140
33. Emmanuel R, Palanisamy S, Chen SM, et al. Antimicrobial efficacy of green synthesized drug blended silver nanoparticles against dental caries and periodontal disease causing microorganisms. Mater Sci Eng C Mater Biol Appl. 2015;56:374–379. doi:10.1016/j.msec.2015.06.033
34. Liang Y, Wang B, Yu Q, Wang W, Ge S, Shao J. Ebselen Optimized the Therapeutic Effects of Silver Nanoparticles for Periodontal Treatment. Int J Nanomed. 2023;18:8113–8130. doi:10.2147/IJN.S434579
35. Bai B, Gu C, Lu X, et al. Polydopamine functionalized mesoporous silica as ROS-sensitive drug delivery vehicles for periodontitis treatment by modulating macrophage polarization. Nano Res. 2021;14(12):4577–4583. doi:10.1007/s12274-021-3376-1
36. Nasiri K, Masoumi SM, Amini S, et al. Recent advances in metal nanoparticles to treat periodontitis. J Nanobiotechnol. 2023;21(1):283. doi:10.1186/s12951-023-02042-7
37. Mascarenhas R, Hegde S, Manaktala N. Chitosan nanoparticle applications in dentistry: a sustainable biopolymer. Review. Front Chem. 2024;12:1362482. doi:10.3389/fchem.2024.1362482
38. Higino T, França R. Drug-delivery nanoparticles for bone-tissue and dental applications. Biomed Phys Eng Express. 2022;8(4):042001. doi:10.1088/2057-1976/ac682c
39. Kumaar NR, Nair SC. Nanomaterials: an intra-periodontal Pocket drug-delivery System for Periodontitis. Therapeutic Delivery. 2023;14(3):227–249 doi:10.4155/tde-2023-0001.
40. Fang L, Zhou H, Cheng L, Wang Y, Liu F, Wang S. The application of mesoporous silica nanoparticles as a drug delivery vehicle in oral disease treatment. Front Cell Infect Microbiol. 2023;13:1124411. doi:10.3389/fcimb.2023.1124411
41. Dastidar GD, Ash A, Saha D, Chakraborty P, Tribedi P. Periodontal Film: a Potential Treatment Strategy for Periodontitis. Drug Deliv Lett. 2022;12(3):184–195. doi:10.2174/2210303112666220617110030
42. Li J, Wang Y, Tang M, et al. New insights into nanotherapeutics for periodontitis: a triple concerto of antimicrobial activity, immunomodulation and periodontium regeneration. J Nanobiotechnol. 2024;22(1):19. doi:10.1186/s12951-023-02261-y
43. Ji S, Choi YS, Choi Y. Bacterial invasion and persistence: critical events in the pathogenesis of periodontitis? J Periodontal Res. 2014;50(5):570–585. doi:10.1111/jre.12248
44. Darveau RP. Consensus Report Periodontal Diseases: epidemiology and Diagnosis. Ann Periodontol. 1996;1(1):216–222. doi:10.1902/annals.1996.1.1.216
45. Hajishengallis G, Chavakis T, Lambris JD. Current understanding of periodontal disease pathogenesis and targets for host‐modulation therapy. Periodontol 2000. 2020;84(1):14–34. doi:10.1111/prd.12331
46. Hajishengallis G. New developments in neutrophil biology and periodontitis. Periodontol 2000. 2019;82(1):78–92. doi:10.1111/prd.12313
47. Darveau RP. Porphyromonas gingivalis neutrophil manipulation: risk factor for periodontitis? Trends Microbiol. 2014;22(8):428–429. doi:10.1016/j.tim.2014.06.006
48. Kim H, Hong J-S, Yun P-Y, et al. Exploration of the interplay between spatially distinct microbial habitats through comparative analysis. J Oral Microbiol. 2023;15(1):2229693. doi:10.1080/20002297.2023.2229693
49. Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol. 2014;35(1):3–11. doi:10.1016/j.it.2013.09.001
50. Preshaw PM, Bissett SM. Periodontitis and diabetes. Br. Dent. J. 2019;227(7):577–584. doi:10.1038/s41415-019-0794-5
51. Albandar JM, Susin C, Hughes FJ. Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: case definitions and diagnostic considerations. J Clin Periodontol. 2018;45(S20):S171–S189. doi:10.1111/jcpe.12947
52. Chapple ILC, Hirschfeld J, Kantarci A, Wilensky A, Shapira L. The role of the host—Neutrophil biology. Periodontol 2000. 2023. doi:10.1111/prd.12490
53. Nasiri K, Masoumi SM, Amini S, et al. Recent advances in metal nanoparticles to treat periodontitis. J Nanobiotechnology. 2023;21(1):283 doi:10.1186/s12951-023-02042-7.
54. Herrera D, van Winkelhoff AJ, Matesanz P, Lauwens K, Teughels W. Europe’s contribution to the evaluation of the use of systemic antimicrobials in the treatment of periodontitis. Periodontol 2000. 2023. doi:10.1111/prd.12492
55. Tian M, Chen G, Xu J, et al. Epigallocatechin gallate-based nanoparticles with reactive oxygen species scavenging property for effective chronic periodontitis treatment. Chem Eng J. 2022;433:132197. doi:10.1016/j.cej.2021.132197
56. Qiu X, Yu Y, Liu H, et al. Remodeling the periodontitis microenvironment for osteogenesis by using a reactive oxygen species-cleavable nanoplatform. Acta Biomater. 2021;135:593–605. doi:10.1016/j.actbio.2021.08.009
57. Oves M, Aslam M, Rauf MA, et al. Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenix dactylifera. Mater Sci Eng C Mater Biol Appl. 2018;89:429–443. doi:10.1016/j.msec.2018.03.035
58. Sun X, Wang L, Lynch CD, et al. Nanoparticles having amphiphilic silane containing Chlorin e6 with strong anti-biofilm activity against periodontitis-related pathogens. J Dent. 2019;81:70–84. doi:10.1016/j.jdent.2018.12.011
59. Mukherjee A, Itohiya H, Matsushima Y, et al. Organic resolution function and effects of platinum nanoparticles on bacteria and organic matter. PLoS One. 2019;14(9):e0222634. doi:10.1371/journal.pone.0222634
60. Liang G, Shi H, Qi Y, et al. Specific Anti-biofilm Activity of Carbon Quantum Dots by Destroying P. gingivalis Biofilm Related Genes. Int J Nanomed. 2020;15:5473–5489. doi:10.2147/IJN.S253416
61. Wu Y, Li C, van der Mei HC, Busscher HJ, Ren Y. Carbon Quantum Dots Derived from Different Carbon Sources for Antibacterial Applications. Antibiotics. 2021;10(6):623. doi:10.3390/antibiotics10060623
62. Yu Y, Zhao S, Gu D, et al. Cerium oxide nanozyme attenuates periodontal bone destruction by inhibiting the ROS–NFκB pathway. Nanoscale. 2022;14(7):2628–2637. doi:10.1039/D1NR06043K
63. Bao X, Zhao J, Sun J, Hu M, Yang X. Polydopamine Nanoparticles as Efficient Scavengers for Reactive Oxygen Species in Periodontal Disease. ACS Nano. 2018;12(9):8882–8892. doi:10.1021/acsnano.8b04022
64. Madhumathi K, Rubaiya Y, Doble M, Venkateswari R, Sampath Kumar TS. Antibacterial, anti-inflammatory, and bone-regenerative dual-drug-loaded calcium phosphate nanocarriers—in vitro and in vivo studies. Drug Deliv Transl Res. 2018;8(5):1066–1077. doi:10.1007/s13346-018-0532-6
65. Steckiewicz KP, Cieciórski P, Barcińska E, et al. Silver Nanoparticles as Chlorhexidine and Metronidazole Drug Delivery Platforms: their Potential Use in Treating Periodontitis. Int J Nanomed. 2022;17:495–517. doi:10.2147/IJN.S339046
66. Lu M, Wang Q, Chang Z, et al. Synergistic bactericidal activity of chlorhexidine-loaded, silver-decorated mesoporous silica nanoparticles. Int J Nanomed. 2017;12:3577–3589. doi:10.2147/IJN.S133846
67. Martin V, Ribeiro IAC, Alves MM, et al. Understanding intracellular trafficking and anti-inflammatory effects of minocycline chitosan-nanoparticles in human gingival fibroblasts for periodontal disease treatment. Int J Pharm. 2019;572:118821. doi:10.1016/j.ijpharm.2019.118821
68. Pereira A, Brito G, Lima M, et al. Metformin Hydrochloride-Loaded PLGA Nanoparticle in Periodontal Disease Experimental Model Using Diabetic Rats. Int J Mol Sci. 2018;19(11):3488. doi:10.3390/ijms19113488
69. Zhang Y, Wang P, Wang Y, et al. Gold Nanoparticles Promote the Bone Regeneration of Periodontal Ligament Stem Cell Sheets Through Activation of Autophagy. Int J Nanomed. 2021;16:61–73. doi:10.2147/IJN.S282246
70. Liu X, He X, Jin D, et al. A biodegradable multifunctional nanofibrous membrane for periodontal tissue regeneration. Acta Biomater. 2020;108:207–222 doi:10.1016/j.actbio.2020.03.044.
71. Wang J, Fan X, Han X, et al. Ultrasmall Inorganic Mesoporous Nanoparticles: preparation, Functionalization, and Application. Adv. Mater. 2024:2312374. doi:10.1002/adma.202312374
72. Yao X, Hao L, Wang T, Xiong F, Shen Q, Huang W. Facile Preparation of Polysarcosine-Tethered Inorganic Nanospheres by Using Unimolecular Polypept(o)ides as Template. Small Methods. 2024;2301424. doi:10.1002/smtd.202301424
73. Liu X, Li J, Yan N, Jiang W. Uniform Nanorods with Regioselective Distribution of Inorganic Nanoparticles Templated by 2D Block Copolymer Nanosheets. ACS Macro Lett. 2022;11(4):549–554. doi:10.1021/acsmacrolett.1c00791
74. Kesharwani P, Ma R, Sang L, et al. Gold nanoparticles and gold nanorods in the landscape of cancer therapy. Mol Cancer. 2023;22(1):98. doi:10.1186/s12943-023-01798-8
75. Zhang S, Zhou H, Kong N, et al. l-cysteine-modified chiral gold nanoparticles promote periodontal tissue regeneration. Bioact Mater. 2021;6(10):3288–3299. doi:10.1016/j.bioactmat.2021.02.035
76. Zhou J, Zhang Y, Li L, Fu H, Yang W, Yan F. Human β-defensin 3-combined gold nanoparticles for enhancement of osteogenic differentiation of human periodontal ligament cells in inflammatory microenvironments. Int J Nanomed. 2018;13:555–567. doi:10.2147/IJN.S150897
77. Miao Y, Yang T, Yang S, Yang M, Mao C. Protein nanoparticles directed cancer imaging and therapy. Nano Convergence. 2022;9(1):2. doi:10.1186/s40580-021-00293-4
78. Ji Y, Lv R, Wang H, et al. Functional surface modifications impact on the in vitro/in vivo toxicity and intracellular internalization behavior of mesoporous silica nanoparticles. Colloids Surf. A. 2024;689:133675. doi:10.1016/j.colsurfa.2024.133675
79. Yin IX, Zhang J, Mei ML, Li Q, Chu CH. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int J Nanomed. 2020;15:2555–2562. doi:10.2147/IJN.S246764
80. Xu Y, Zheng B, He J, Cui Z, Liu Y. Silver nanoparticles promote osteogenic differentiation of human periodontal ligament fibroblasts by regulating the RhoA–TAZ axis. Cell Biol Int. 2019;43(8):910–920. doi:10.1002/cbin.11180
81. Maťátková O, Michailidu J, Miškovská A, Kolouchová I, Masák J, Čejková A. Antimicrobial properties and applications of metal nanoparticles biosynthesized by green methods. Biotechnol. 2022;58:107905 doi:10.1016/j.biotechadv.2022.107905.
82. Rathinam VAK, Zhao Y, Shao F. Innate immunity to intracellular LPS. Nat Immunol. 2019;20(5):527–533. doi:10.1038/s41590-019-0368-3
83. Prapaipittayakhun J, Boonyuen S, Zheng ALT, Apinyauppatham K, Arpornmaeklong P. Biologic effects of biosynthesized Oroxylum indicum/silver nanoparticles on human periodontal ligament stem cells. OpenNano. 2023;9:100117. doi:10.1016/j.onano.2022.100117
84. Page Faulk W, Malcolm Taylor G. Communication to the editors. Immunochemistry. 1971;8(11):1081–1083. doi:10.1016/0019-2791(71)90496-4
85. Ni C, Zhou J, Kong N, et al. Gold nanoparticles modulate the crosstalk between macrophages and periodontal ligament cells for periodontitis treatment. Biomaterials. 2019;206:115–132. doi:10.1016/j.biomaterials.2019.03.039
86. Yin Y, Tian B-M, Li X, et al. Gold nanoparticles targeting the autophagy–lysosome system to combat the inflammation-compromised osteogenic potential of periodontal ligament stem cells: from mechanism to therapy. Biomaterials. 2022;288:121743. doi:10.1016/j.biomaterials.2022.121743
87. Yang C-B, Liu J, Tong BC-K, et al. TFEB, a master regulator of autophagy and biogenesis, unexpectedly promotes apoptosis in response to the cyclopentenone prostaglandin 15d-PGJ2. Acta Pharmacol. Sin. 2021;43(5):1251–1263. doi:10.1038/s41401-021-00711-7
88. Li L, Jiang H, Chen R, et al. Human β-defensin 3 gene modification promotes the osteogenic differentiation of human periodontal ligament cells and bone repair in periodontitis. Int J Oral Sci. 2020;12(1):13. doi:10.1038/s41368-020-0078-6
89. Li L, Zhang Y, Wang M, et al. Gold Nanoparticles Combined Human β-Defensin 3 Gene-Modified Human Periodontal Ligament Cells Alleviate Periodontal Destruction via the p38 MAPK Pathway. Front Bioeng Biotechnol. 2021;9:631191. doi:10.3389/fbioe.2021.631191
90. Chi M, Qi M, L A, et al. Novel Bioactive and Therapeutic Dental Polymeric Materials to Inhibit Periodontal Pathogens and Biofilms. Int J Mol Sci. 2019;20(2):278. doi:10.3390/ijms20020278
91. Deng X, Liang S, Cai X, et al. Yolk–Shell Structured Au Nanostar@Metal–Organic Framework for Synergistic Chemo-photothermal Therapy in the Second Near-Infrared Window. Nano Lett. 2019;19(10):6772–6780. doi:10.1021/acs.nanolett.9b01716
92. Ejima H, Richardson JJ, Caruso F. Metal-phenolic networks as a versatile platform to engineer nanomaterials and biointerfaces. Nano Today. 2017;12:136–148. doi:10.1016/j.nantod.2016.12.012
93. Wang H, Wang D, Huangfu H, et al. Branched AuAg nanoparticles coated by metal–phenolic networks for treating bacteria-induced periodontitis via photothermal antibacterial and immunotherapy. Mater Des. 2022;224:111401. doi:10.1016/j.matdes.2022.111401
94. Dong Z, Lin Y, Xu S, et al. NIR-triggered tea polyphenol-modified gold nanoparticles-loaded hydrogel treats periodontitis by inhibiting bacteria and inducing bone regeneration. Mater Des. 2023;225:111487. doi:10.1016/j.matdes.2022.111487
95. Wu T, Sun J, Lei J, et al. An efficient treatment of biofilm-induced periodontitis using Pt nanocluster catalysis. Nanoscale. 2021;13(42):17912–17919. doi:10.1039/D1NR05198A
96. Gao L, Zhuang J, Nie L, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2(9):577–583. doi:10.1038/nnano.2007.260
97. Natalio F, André R, Hartog AF, et al. Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nat Nanotechnol. 2012;7(8):530–535. doi:10.1038/nnano.2012.91
98. Ren S, Zhou Y, Fan R, et al. Constructing biocompatible MSN@Ce@PEG nanoplatform for enhancing regenerative capability of stem cell via ROS-scavenging in periodontitis. Chem Eng J. 2021;423:130207. doi:10.1016/j.cej.2021.130207
99. Shubayev VI, Pisanic TR, Jin S. Magnetic nanoparticles for theragnostics. Adv Drug Deliv Rev. 2009;61(6):467–477. doi:10.1016/j.addr.2009.03.007
100. Santos LF, Silva AS, Mano JF. Magnetic‐Based Strategies for Regenerative Medicine and Tissue Engineering. Adv Healthc Mater. 2023;12(25):e2300605. doi:10.1002/adhm.202300605
101. Peng W, Ren S, Zhang Y, et al. MgO Nanoparticles-Incorporated PCL/Gelatin-Derived Coaxial Electrospinning Nanocellulose Membranes for Periodontal Tissue Regeneration. Front Bioeng Biotechnol. 2021;9:668428. doi:10.3389/fbioe.2021.668428
102. Mangalampalli B, Dumala N, Perumalla Venkata R, Grover P. Genotoxicity, biochemical, and biodistribution studies of magnesium oxide nano and microparticles in albino Wistar rats after 28‐day repeated oral exposure. Environ Toxicol: Int J. 2017;33(4):396–410. doi:10.1002/tox.22526
103. Verron E, Bouler JM, Guicheux J. Controlling the biological function of calcium phosphate bone substitutes with drugs. Acta Biomater. 2012;8(10):3541–3551. doi:10.1016/j.actbio.2012.06.022
104. George SM, Nayak C, Singh I, Balani K. Multifunctional Hydroxyapatite Composites for Orthopedic Applications: a Review. ACS Biomater Sci Eng. 2022;8(8):3162–3186. doi:10.1021/acsbiomaterials.2c00140
105. Xiang M, Zhu M, Yang Z, et al. Dual-Functionalized Apatite Nanocomposites with Enhanced Cytocompatibility and Osteogenesis for Periodontal Bone Regeneration. ACS Biomater Sci Eng. 2020;6(3):1704–1714. doi:10.1021/acsbiomaterials.9b01893
106. Paludan SR, Bowie AG. Immune Sensing of DNA. Immunity. 2013;38(5):870–880. doi:10.1016/j.immuni.2013.05.004
107. Crump KE, Oakley JC, Xia-Juan X, et al. Interplay of Toll-Like Receptor 9, Myeloid Cells, and Deubiquitinase A20 in Periodontal Inflammation. Infect Immun. 2016;85(1):e00814–16. doi:10.1128/IAI.00814-16
108. Kim PD, Xia-Juan X, Crump KE, et al. Toll-Like Receptor 9-Mediated Inflammation Triggers Alveolar Bone Loss in Experimental Murine Periodontitis. Infect Immun. 2015;83(7):2992–3002. doi:10.1128/IAI.00424-15
109. Kumagai Y, Takeuchi O, Akira S. TLR9 as a key receptor for the recognition of DNA. Adv Drug Deliv Rev. 2008;60(7):795–804. doi:10.1016/j.addr.2007.12.004
110. Tu Z, Zhong Y, Hu H, et al. Design of therapeutic biomaterials to control inflammation. Nat Rev Mater. 2022;7(7):557–574. doi:10.1038/s41578-022-00426-z
111. Huang H, Pan W, Wang Y, et al. Nanoparticulate cell-free DNA scavenger for treating inflammatory bone loss in periodontitis. Nat Commun. 2022;13(1):5925. doi:10.1038/s41467-022-33492-6
112. Hoang Thi TT, Cao VD, Nguyen TNQ, Hoang DT, Ngo VC, Nguyen DH. Functionalized mesoporous silica nanoparticles and biomedical applications. Mater Sci Eng C Mater Biol Appl. 2019;99:631–656. doi:10.1016/j.msec.2019.01.129
113. Panáček A, Kvítek L, Smékalová M, et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat Nanotechnol. 2017;13(1):65–71. doi:10.1038/s41565-017-0013-y
114. Graves J, Thomas M, Ewunkem J. Antimicrobial Nanomaterials: why Evolution Matters. Nanomaterials. 2017;7(10):283. doi:10.3390/nano7100283
115. M-m L, Ge Y, Qiu J, et al. Redox/pH dual-controlled release of chlorhexidine and silver ions from biodegradable mesoporous silica nanoparticles against oral biofilms. Int J Nanomed. 2018;13:7697–7709. doi:10.2147/IJN.S181168
116. Li D, Qiu Y, Zhang S, Zhang M, Chen Z, Chen J. A Multifunctional Antibacterial and Osteogenic Nanomedicine: QAS-Modified Core-Shell Mesoporous Silica Containing Ag Nanoparticles. Biomed Res Int. 2020;2020:4567049. doi:10.1155/2020/4567049
117. Casarrubios L, Gómez-Cerezo N, Feito MJ, Vallet-Regí M, Arcos D, Portolés MT. Ipriflavone-Loaded Mesoporous Nanospheres with Potential Applications for Periodontal Treatment. Nanomaterials. 2020;10(12):2573. doi:10.3390/nano10122573
118. Xue P, Li B, An Y, et al. Decreased MORF leads to prolonged endoplasmic reticulum stress in periodontitis-associated chronic inflammation. Cell Death Differ. 2016;23(11):1862–1872. doi:10.1038/cdd.2016.74
119. Fergie N, Todd N, McClements L, McAuley D, O’Kane C, Krasnodembskaya A. Hypercapnic acidosis induces mitochondrial dysfunction and impairs the ability of mesenchymal stem cells to promote distal lung epithelial repair. FASEB J. 2019;33(4):5585–5598. doi:10.1096/fj.201802056R
120. Dong Z, Wu L, Hong H. Mitochondrial Dysfunction in the Pathogenesis and Treatment of Oral Inflammatory Diseases. Int J Mol Sci. 2023;24(20):15483. doi:10.3390/ijms242015483
121. Luo S, Xu T, Zheng Q, et al. Mitochondria: an Emerging Unavoidable Link in the Pathogenesis of Periodontitis Caused by Porphyromonas gingivalis. Int J Mol Sci. 2024;25(2):737. doi:10.3390/ijms25020737
122. Zhai Q, Chen X, Fei D, et al. Nanorepairers Rescue Inflammation‐Induced Mitochondrial Dysfunction in Mesenchymal Stem Cells. Adv Sci (Weinh). 2021;9(4):e2103839. doi:10.1002/advs.202103839
123. Pouroutzidou GK, Liverani L, Theocharidou A, et al. Synthesis and Characterization of Mesoporous Mg- and Sr-Doped Nanoparticles for Moxifloxacin Drug Delivery in Promising Tissue Engineering Applications. Int J Mol Sci. 2021;22(2):577. doi:10.3390/ijms22020577
124. Feng W, Han C, Li F. Upconversion‐Nanophosphor‐Based Functional Nanocomposites. Adv Mater. 2013;25(37):5287–5303. doi:10.1002/adma.201301946
125. Zhou J, Liu Q, Feng W, Sun Y, Li F. Upconversion Luminescent Materials: advances and Applications. Chem Rev. 2014;115(1):395–465. doi:10.1021/cr400478f
126. Zhang T, Ying D, Qi M, et al. Anti-Biofilm Property of Bioactive Upconversion Nanocomposites Containing Chlorin e6 against Periodontal Pathogens. Molecules. 2019;24(15):2692. doi:10.3390/molecules24152692
127. Qi M, Li X, Sun X, et al. Novel nanotechnology and near-infrared photodynamic therapy to kill periodontitis-related biofilm pathogens and protect the periodontium. Dent Mater. 2019;35(11):1665–1681. doi:10.1016/j.dental.2019.08.115
128. Hu D, Zhang C, Sun C, et al. Carvacrol combined with NIR light-responsive nano-drug delivery system with specific anti-bacteria, anti-inflammation, and immunomodulation for periodontitis. Nano Res. 2023;16(5):7199–7215. doi:10.1007/s12274-022-5349-4
129. Abdelhamid HN. Zeolitic Imidazolate Frameworks (ZIF-8) for Biomedical Applications: a Review. Curr Med Chem. 2021;28(34):7023–7075. doi:10.2174/0929867328666210608143703
130. Li W, Cao Z, Yu L, et al. Hierarchical drug release designed Au @PDA-PEG-MTX NPs for targeted delivery to breast cancer with combined photothermal-chemotherapy. J Nanobiotechnology. 2021;19(1):143. doi:10.1186/s12951-021-00883-8
131. Liu Y, Chen X, Ma Q. A novel amplified electrochemiluminescence biosensor based on Au NPs@PDA@CuInZnS QDs nanocomposites for ultrasensitive detection of p53 gene. Biosens Bioelectron. 2018;117:240–245. doi:10.1016/j.bios.2018.06.023
132. Ding X, Wang Z, Yu Q, et al. Superoxide Dismutase‐Like Regulated Fe/Ppa@PDA/B for Synergistically Targeting Ferroptosis/Apoptosis to Enhance Anti‐Tumor Efficacy. Adv Healthc Mater. 2023;12(29):e2301824. doi:10.1002/adhm.202301824
133. Yang X, Chen Y, Guo J, et al. Polydopamine Nanoparticles Targeting Ferroptosis Mitigate Intervertebral Disc Degeneration Via Reactive Oxygen Species Depletion, Iron Ions Chelation, and GPX4 Ubiquitination Suppression. Adv Sci (Weinh). 2023;10(13):e2207216. doi:10.1002/advs.202207216
134. Sánchez MC, Toledano-Osorio M, Bueno J, et al. Antibacterial effects of polymeric PolymP-n Active nanoparticles. An in vitro biofilm study. Dent Mater. 2019;35(1):156–168. doi:10.1016/j.dental.2018.11.015
135. Li X, Qi M, Li C, et al. Novel nanoparticles of cerium-doped zeolitic imidazolate frameworks with dual benefits of antibacterial and anti-inflammatory functions against periodontitis. J Mater Chem B. 2019;7(44):6955–6971. doi:10.1039/C9TB01743G
136. Van Den Bulcke AI, Bogdanov B, De Rooze N, Schacht EH, Cornelissen M, Berghmans H. Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules. 2000;1(1):31–38. doi:10.1021/bm990017d
137. Liu Y, Li T, Sun M, et al. ZIF-8 modified multifunctional injectable photopolymerizable GelMA hydrogel for the treatment of periodontitis. Acta Biomater. 2022;146:37–48. doi:10.1016/j.actbio.2022.03.046
138. Abd El-Hack ME, El-Saadony MT, Shafi ME, et al. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: a review. Int J Biol Macromol. 2020;164:2726–2744. doi:10.1016/j.ijbiomac.2020.08.153
139. Shivakumar P, Gupta MS, Jayakumar R, Gowda DV. Prospection of chitosan and its derivatives in wound healing: proof of patent analysis (2010–2020). Int J Biol Macromol. 2021;184:701–712. doi:10.1016/j.ijbiomac.2021.06.086
140. Alvarez Echazú MI, Olivetti CE, Peralta I, et al. Development of pH-responsive biopolymer-silica composites loaded with Larrea divaricata Cav. extract with antioxidant activity. Colloids Surf B Biointerfaces. 2018;169:82–91. doi:10.1016/j.colsurfb.2018.05.015
141. Xu S, Zhou Q, Jiang Z, et al. The effect of doxycycline-containing chitosan/carboxymethyl chitosan nanoparticles on NLRP3 inflammasome in periodontal disease. Carbohydr Polym. 2020;237:116163. doi:10.1016/j.carbpol.2020.116163
142. Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers. 2011;3(3):1377–1397. doi:10.3390/polym3031377
143. Tabatabaei Mirakabad FS, Nejati-Koshki K, Akbarzadeh A, et al. PLGA-Based Nanoparticles as Cancer Drug Delivery Systems. Asian Pac J Cancer Prev. 2014;15(2):517–535. doi:10.7314/APJCP.2014.15.2.517
144. Daep CA, Novak EA, Lamont RJ, Demuth DR, Camilli A. Structural Dissection and In Vivo Effectiveness of a Peptide Inhibitor of Porphyromonas gingivalis Adherence to Streptococcus gordonii. Infect Immun. 2011;79(1):67–74. doi:10.1128/IAI.00361-10
145. Mahmoud MY, Demuth DR, Steinbach-Rankins JM. BAR-encapsulated nanoparticles for the inhibition and disruption of Porphyromonas gingivalis–Streptococcus gordonii biofilms. J Nanobiotechnology. 2018;16(1):69. doi:10.1186/s12951-018-0396-4
146. Mahmoud MY, Steinbach-Rankins JM, Demuth DR. Functional assessment of peptide-modified PLGA nanoparticles against oral biofilms in a murine model of periodontitis. J Control Release. 2019;297:3–13. doi:10.1016/j.jconrel.2019.01.036
147. Wright CJ, Burns LH, Jack AA, et al. Microbial interactions in building of communities. Mol Oral Microbiol. 2012;28(2):83–101. doi:10.1111/omi.12012
148. Desai H, Mahmoud MY, Tan J, Minooei F, Demuth DR, Steinbach-Rankins JM. Assessment of CafA Targeted BAR-Encapsulated Nanoparticles against Oral Biofilms. Pharmaceutics. 2020;12(9):835. doi:10.3390/pharmaceutics12090835
149. De Freitas LM, Calixto GM, Chorilli M, et al. Polymeric Nanoparticle-Based Photodynamic Therapy for Chronic Periodontitis in Vivo. Int J Mol Sci. 2016;17(5):769. doi:10.3390/ijms17050769
150. Huang D, Wu D. Biodegradable dendrimers for drug delivery. Mater Sci Eng C Mater Biol Appl. 2018;90:713–727. doi:10.1016/j.msec.2018.03.002
151. Wang L, Li Y, Ren M, et al. pH and lipase-responsive nanocarrier-mediated dual drug delivery system to treat periodontitis in diabetic rats. Bioact Mater. 2022;18:254–266. doi:10.1016/j.bioactmat.2022.02.008
152. Large DE, Abdelmessih RG, Fink EA, Auguste DT. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv Drug Deliv Rev. 2021;176:113851. doi:10.1016/j.addr.2021.113851
153. Hu F, Zhou Z, Xu Q, et al. A novel pH-responsive quaternary ammonium chitosan-liposome nanoparticles for periodontal treatment. Int J Biol Macromol. 2019;129:1113–1119. doi:10.1016/j.ijbiomac.2018.09.057
154. Jiang S, Lv L-P, Landfester K, Crespy D. Nanocontainers in and onto Nanofibers. Acc Chem Res. 2016;49(5):816–823. doi:10.1021/acs.accounts.5b00524
155. Hiwrale A, Bharati S, Pingale P, Rajput A. Nanofibers: a current era in drug delivery system. Heliyon. 2023;9(9):e18917. doi:10.1016/j.heliyon.2023.e18917
156. He C, Lv Q, Liu Z, et al. Random and aligned electrostatically spun PLLA nanofibrous membranes enhance bone repair in mouse femur midshaft defects. J Biomater Appl. 2023;37(9):1582–1592. doi:10.1177/08853282221144220
157. Li Y, Yang L, Hou Y, et al. Polydopamine-mediated graphene oxide and nanohydroxyapatite-incorporated conductive scaffold with an immunomodulatory ability accelerates periodontal bone regeneration in diabetes. Bioact Mater. 2022;18:213–227. doi:10.1016/j.bioactmat.2022.03.021
158. Sprio S, Campodoni E, Sandri M, et al. A Graded Multifunctional Hybrid Scaffold with Superparamagnetic Ability for Periodontal Regeneration. Int J Mol Sci. 2018;19(11):3604. doi:10.3390/ijms19113604
159. Sakaguchi S, Sakaguchi N, Shimizu J, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2002;182(1):18–32. doi:10.1034/j.1600-065X.2001.1820102.x
160. Liu Z, Chen X, Zhang Z, et al. Nanofibrous spongy microspheres to distinctly release miRNA and growth factors to enrich regulatory T cells and rescue periodontal bone loss. ACS Nano. 2018;12(10):9785–9799. doi:10.1021/acsnano.7b08976
161. Qian Y, Zhou X, Zhang F, Diekwisch TGH, Luan X, Yang J. Triple PLGA/PCL Scaffold Modification Including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration. ACS Appl Mater Interfaces. 2019;11(41):37381–37396. doi:10.1021/acsami.9b07053
162. Alipour M, Aghazadeh M, Akbarzadeh A, Vafajoo Z, Aghazadeh Z, Raeisdasteh Hokmabad V. Towards osteogenic differentiation of human dental pulp stem cells on PCL-PEG-PCL/zeolite nanofibrous scaffolds. Artif Cells Nanomed Biotechnol. 2019;47(1):3431–3437. doi:10.1080/21691401.2019.1652627
163. Shoba E, Lakra R, Kiran MS, Korrapati PS. 3 D nano bilayered spatially and functionally graded scaffold impregnated bromelain conjugated magnesium doped hydroxyapatite nanoparticle for periodontal regeneration. J Mech Behav Biomed Mater. 2020;109:103822. doi:10.1016/j.jmbbm.2020.103822
164. Mahmoud MY, Sapare S, Curry KC, Demuth DR, Steinbach-Rankins JM. Rapid Release Polymeric Fibers for Inhibition of Porphyromonas gingivalis Adherence to Streptococcus gordonii. Front Chem. 2020;7:926. doi:10.3389/fchem.2019.00926
165. Shen R, Xu W, Xue Y, et al. The use of chitosan/PLA nano-fibers by emulsion eletrospinning for periodontal tissue engineering. Artif Cells Nanomed Biotechnol. 2018;46(sup2):419–430. doi:10.1080/21691401.2018.1458233
166. Abdelaziz D, Hefnawy A, Al-Wakeel E, El-Fallal A, El-Sherbiny IM. New biodegradable nanoparticles-in-nanofibers based membranes for guided periodontal tissue and bone regeneration with enhanced antibacterial activity. J Adv Res. 2021;28:51–62. doi:10.1016/j.jare.2020.06.014
167. Oortgiesen DAW, Plachokova AS, Geenen C, et al. Alkaline phosphatase immobilization onto Bio‐Gide® and Bio‐Oss® for periodontal and bone regeneration. J Clin Periodontol. 2012;39(6):546–555. doi:10.1111/j.1600-051X.2012.01877.x
168. Lian M, Han Y, Sun B, et al. A multifunctional electrowritten bi-layered scaffold for guided bone regeneration. Acta Biomater. 2020;118:83–99. doi:10.1016/j.actbio.2020.08.017
169. Ekambaram R, Paraman V, Raja L, Suresh MK, Dharmalingam S. Design and development of electrospun SPEEK incorporated with aminated zirconia and curcumin nanofibers for periodontal regeneration. J Mech Behav Biomed Mater. 2021;123:104796. doi:10.1016/j.jmbbm.2021.104796
170. Liang Y, He J, Guo B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano. 2021;15(8):12687–12722. doi:10.1021/acsnano.1c04206
171. Tu Y, Chen N, Li C, et al. Advances in injectable self-healing biomedical hydrogels. Acta Biomater. 2019;90:1–20. doi:10.1016/j.actbio.2019.03.057
172. Gutierrez AM, Frazar EM, Klaus X, Paul MV, Hilt JZ. Hydrogels and Hydrogel Nanocomposites: enhancing Healthcare through Human and Environmental Treatment. Adv Healthc Mater. 2021;11(7):e2101820. doi:10.1002/adhm.202101820
173. Zheng H, Zhou Y, Zheng Y, Liu G. Advances in hydrogels for the treatment of periodontitis. J Mater Chem B. 2023;11(31):7321–7333. doi:10.1039/D3TB00835E
174. Mou J, Liu Z, Liu J, Lu J, Zhu W, Pei D. Hydrogel containing minocycline and zinc oxide-loaded serum albumin nanopartical for periodontitis application: preparation, characterization and evaluation. Drug Delivery. 2019;26(1):179–187. doi:10.1080/10717544.2019.1571121
175. Aminu N, Chan S-Y, Yam M-F, Toh S-M. A dual-action chitosan-based nanogel system of triclosan and flurbiprofen for localised treatment of periodontitis. Int J Pharm. 2019;570:118659. doi:10.1016/j.ijpharm.2019.118659
176. Soe HMSH, Luckanagul JA, Pavasant P, Jansook P. Development of in situ gel containing asiaticoside/cyclodextrin complexes. Evaluation in culture human periodontal ligament cells (HPLDCs). Int J Pharm. 2020;586:119589. doi:10.1016/j.ijpharm.2020.119589
177. Johnson A, Kong F, Miao S, et al. Therapeutic effects of antibiotics loaded cellulose nanofiber and κ-carrageenan oligosaccharide composite hydrogels for periodontitis treatment. Sci Rep. 2020;10(1):18037. doi:10.1038/s41598-020-74845-9
178. Li N, Xie L, Wu Y, et al. Dexamethasone-loaded zeolitic imidazolate frameworks nanocomposite hydrogel with antibacterial and anti-inflammatory effects for periodontitis treatment. Mater Today Bio. 2022;16:100360. doi:10.1016/j.mtbio.2022.100360
179. Ou Q, Zhang S, Fu C, et al. More natural more better: triple natural anti-oxidant puerarin/ferulic acid/polydopamine incorporated hydrogel for wound healing. J Nanobiotechnology. 2021;19(1):237. doi:10.1186/s12951-021-00973-7
180. Xu Y, Zhao S, Weng Z, et al. Jelly-Inspired Injectable Guided Tissue Regeneration Strategy with Shape Auto-Matched and Dual-Light-Defined Antibacterial/Osteogenic Pattern Switch Properties. ACS Appl Mater Interfaces. 2020;12(49):54497–54506. doi:10.1021/acsami.0c18070
181. Qu L, Dubey N, Ribeiro JS, et al. Metformin-loaded nanospheres-laden photocrosslinkable gelatin hydrogel for bone tissue engineering. J Mech Behav Biomed Mater. 2021;116:104293. doi:10.1016/j.jmbbm.2020.104293
182. Wu T, Huang L, Sun J, et al. Multifunctional chitin-based barrier membrane with antibacterial and osteogenic activities for the treatment of periodontal disease. Carbohydr Polym. 2021;269:118276. doi:10.1016/j.carbpol.2021.118276
183. Ding T, Kang W, Li J, Yu L, Ge S. An in situ tissue engineering scaffold with growth factors combining angiogenesis and osteoimmunomodulatory functions for advanced periodontal bone regeneration. J Nanobiotechnology. 2021;19(1):247. doi:10.1186/s12951-021-00992-4
184. Yu X, Wang Y, Zhang M, et al. 3D printing of gear-inspired biomaterials: immunomodulation and bone regeneration. Acta Biomater. 2023;156:222–233. doi:10.1016/j.actbio.2022.09.008
185. Yu M, Luo D, Qiao J, et al. A hierarchical bilayer architecture for complex tissue regeneration. Bioact Mater. 2022;10:93–106. doi:10.1016/j.bioactmat.2021.08.024
186. Farokhi M, Mottaghitalab F, Shokrgozar MA, Ou K-L, Mao C, Hosseinkhani H. Importance of dual delivery systems for bone tissue engineering. J Control Release. 2016;225:152–169. doi:10.1016/j.jconrel.2016.01.033
187. Hasani-Sadrabadi MM, Sarrion P, Nakatsuka N, et al. Hierarchically Patterned Polydopamine-Containing Membranes for Periodontal Tissue Engineering. ACS Nano. 2019;13(4):3830–3838. doi:10.1021/acsnano.8b09623
188. Vale AC, Pereira P, Barbosa AM, Torrado E, Mano JF, Alves NM. Antibacterial free-standing polysaccharide composite films inspired by the sea. Int J Biol Macromol. 2019;133:933–944. doi:10.1016/j.ijbiomac.2019.04.102
189. Shah AT, Zahid S, Ikram F, et al. Tri-layered functionally graded membrane for potential application in periodontal regeneration. Mater Sci Eng C Mater Biol Appl. 2019;103:109812. doi:10.1016/j.msec.2019.109812
190. Soltani Dehnavi S, Mehdikhani M, Rafienia M, Bonakdar S. Preparation and in vitro evaluation of polycaprolactone/PEG/bioactive glass nanopowders nanocomposite membranes for GTR/GBR applications. Mater Sci Eng C Mater Biol Appl. 2018;90:236–247. doi:10.1016/j.msec.2018.04.065
191. Ghavimi MA, Bani Shahabadi A, Jarolmasjed S, Memar MY, Maleki Dizaj S, Sharifi S. Nanofibrous asymmetric collagen/curcumin membrane containing aspirin-loaded PLGA nanoparticles for guided bone regeneration. Sci Rep. 2020;10(1):18200. doi:10.1038/s41598-020-75454-2
192. Toledano-Osorio M, Manzano-Moreno FJ, Toledano M, et al. Doxycycline-Doped Polymeric Membranes Induced Growth, Differentiation and Expression of Antigenic Phenotype Markers of Osteoblasts. Polymers. 2021;13(7):1063. doi:10.3390/polym13071063
193. Toledano-Osorio M, Manzano-Moreno FJ, Toledano M, et al. Doxycycline-doped membranes induced osteogenic gene expression on osteoblastic cells. J Dent. 2021;109:103676. doi:10.1016/j.jdent.2021.103676
194. Nardo T, Chiono V, Carmagnola I, et al. Mussel-inspired antimicrobial coating on PTFE barrier membranes for guided tissue regeneration. Biomed Mater. 2021;16(3):035035. doi:10.1088/1748-605X/abf27e
195. Pouroutzidou GK, Lazaridou M, Papoulia C, et al. Electrospun PLGA Membranes with Incorporated Moxifloxacin-Loaded Silica-Based Mesoporous Nanocarriers for Periodontal Regeneration. Nanomaterials (Basel). 2022;12(5):850. doi:10.3390/nano12050850
196. Craciunescu O, Seciu A-M, Zarnescu O. In vitro and in vivo evaluation of a biomimetic scaffold embedding silver nanoparticles for improved treatment of oral lesions. Mater Sci Eng C Mater Biol Appl. 2021;123:112015. doi:10.1016/j.msec.2021.112015
197. He Y, Jin Y, Wang X, et al. An Antimicrobial Peptide-Loaded Gelatin/Chitosan Nanofibrous Membrane Fabricated by Sequential Layer-by-Layer Electrospinning and Electrospraying Techniques. Nanomaterials. 2018;8(5):327. doi:10.3390/nano8050327
198. He Z, Zhou X, Wang Y, et al. Asymmetric barrier membranes based on polysaccharide micro-nanocomposite hydrogel: synthesis, characterization, and their antibacterial and osteogenic activities. Carbohydr Polym. 2021;273:118525. doi:10.1016/j.carbpol.2021.118525
199. Prado-Prone G, Silva-Bermudez P, Bazzar M, et al. Antibacterial composite membranes of polycaprolactone/gelatin loaded with zinc oxide nanoparticles for guided tissue regeneration. Biomed Mater. 2020;15(3):035006. doi:10.1088/1748-605X/ab70ef
200. Das V, Vinod V, Biswas L, Kumar A, Biswas R. An update on possible alternative therapeutics for future periodontal disease management. J Appl Microbiol. 2023;134(1):lxac039. doi:10.1093/jambio/lxac039
201. Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44(3):450–462. doi:10.1016/j.immuni.2016.02.015
202. Moon S, Hong J, Go S, Kim B-S. Immunomodulation for Tissue Repair and Regeneration. Tissue Eng Regen Med. 2023;20(3):389–409. doi:10.1007/s13770-023-00525-0
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
