Back to Journals » Biologics: Targets and Therapy » Volume 18
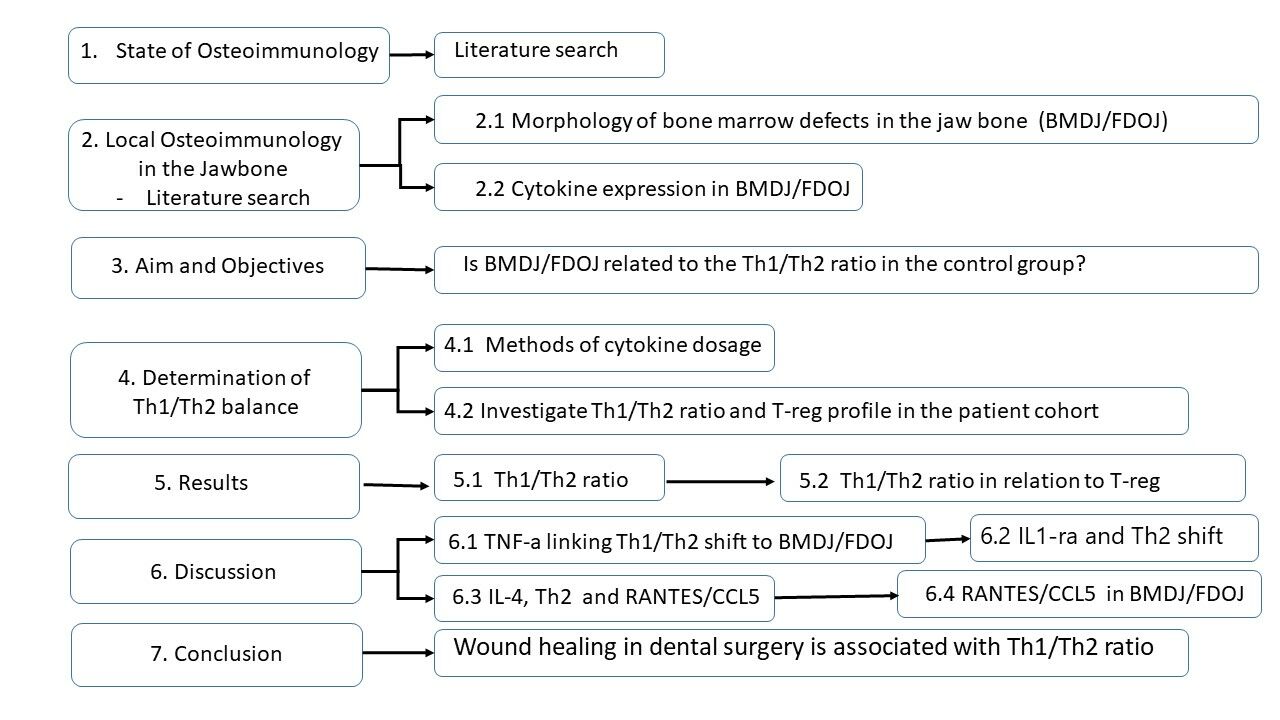
Osteoimmune Interaction and TH-1/TH-2 Ratio in Jawbone Marrow Defects: An Underestimated Association – Original Research
Authors Lechner J , von Baehr V, Notter F, Schick F
Received 17 November 2023
Accepted for publication 29 May 2024
Published 6 June 2024 Volume 2024:18 Pages 147—161
DOI https://doi.org/10.2147/BTT.S448587
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Shein-Chung Chow
Johann Lechner,1 Volker von Baehr,2 Florian Notter,1 Fabian Schick1
1Clinic for Integrative Dentistry, Munich, Germany; 2Department of Immunology and Allergology, Institute for Medical Diagnostics, Berlin, Germany
Correspondence: Johann Lechner, Gruenwalder Str. 10A, Munich, 81547, Germany, Tel +49 89 697 0129, Fax +49 89 692 5830, Email [email protected]
Introduction: Osteoimmunology recognizes the relationship between bone cells and immune cells. Chronic osteoimmune dysregulation is present in bone marrow defects of the jaw (BMDJ) as fatty-degenerative osteonecrosis (FDOJ). In comparison to samples from healthy jaw bone, the cytokine analysis of samples of BMDJ/FDOJ from 128 patients showed downregulated TNF-α and IL-6 expression and the singular overexpression of the chemokine RANTES/CCL5.
Aim and Objectives: This paper raises the question of whether the osteoimmune defects due to incomplete wound healing in BMDJ/FDOJ in 128 patients are related to dysregulation of the Th1/Th2 ratio and regulatory T cell (T-reg) expression in a control group of 197 BMDJ/FDOJ patients, each presenting with BMDJ/FJOD and one of seven different immune disorders.
Material and Methods: In the control group, serum concentrations of the cytokines IFN-y and IL-4 were determined after stimulated cytokine release and displayed as Th1/Th2 ratios.
Results: Data show a shift in Th2 in more than 80% (n = 167) of the control cohort of 197 chronically ill patients with concomitant BMDJ/FDOJ. In these 167 subjects, the Th1/Th2 ratio was < 6.1 demonstrating impaired immune regulation. Forty-seven subjects or 30% showed not only a shift in Th2 but also excessive T-reg overactivation with levels of > 1.900 pg/mL, indicating strongly downregulated immune activity.
Discussion: BMDJ/FDOJ is characterized by a lack of Th1 cytokines and an excessive expression of RANTES/CCL5 and IL-1ra and, thus, the inversion of an acute inflammatory cytokine pattern. In contrast, abdominal fat contains a very high proportion of regulatory Th1 cells and produces an inflammatory immune response through the high overexpression of TNF-α and IL-6. The lack of Th1 activation in BMDJ/FDOJ areas inhibits normal wound healing and supports the persistence of BMDJ/FDOJ.
Conclusion: The Th1/Th2 ratio requires greater consideration, especially with respect to wound healing following dental surgical interventions, such as jaw surgery, implantation and augmentation, to avoid the emergence of the osteoimmune situation that is characteristic of BMDJ/FDOJ.
Keywords: osteoimmunology, T-cells, Th1/Th2 shift, jawbone, osteoclastogenesis, RANTES/CCL5
Graphical Abstract:
Introduction – The Evolving Field of Osteoimmunology
Bone is a dynamic organ with a balance between bone formation and bone resorption and there are complex interactions between the immune and skeletal systems. Immune cells participate in the regulation of bone homeostasis and are key regulators of wound healing and contribute to the early stages of angiogenesis.1 Therefore, the term “osteoimmunology” was coined to highlight the two-way communication between the bone and immune systems.2 The immune system controls bone formation, regulates bone resorption and acts as a key factor in bone homeostasis, and thus is involved in bone synthesis.3 Inversely, immune cell functions are influenced by the bone system.4 It has been concluded that the bone and immune systems are unified in a single entity: the osteoimmune system.5 Osteoimmunology is now a recognized scientific field of study examining the relationship between bone cells and immune cells. In this interdisciplinary field, bone destruction as observed in chronic inflammatory diseases has been found to be associated primarily with an imbalance between high levels of pro-osteoclastogenic cytokines (TNF-α, IL-17, and RANK-L), produced mainly by Th17 cells, and decreased production of anti-osteoclastogenic factors (Il-10, IL-4, and IFN-γ) by Th1 and Th2 cells and regulatory T cells (T-reg), leading to a dramatic increase in the differentiation of bone-resorbing osteoclasts as shown in Figure 1.6,7
 |
Figure 1 Control of bone cells by immune cells through cytokine-mediated differentiation of Th1/Th2 cells (see red circle). Notes: The enlarged detail from this image shows the Th1/Th2 balance, which plays a crucial role within the cytokine network of the bone remodelling cycle and the osteoimmune interaction. Undifferentiated naive T cells secrete specific cytokines after antigenic stimulus that polarize into Th1 or Th2 cells. The red circle emphasizes the primary cell control by Th1/Th-2 osteoimmune balance on the osteoclast. Adapted from Yang N, Liu Y. The Role of the Immune Microenvironment in Bone Regeneration. Int. J. Med. Sci. 2021;18:3697–3707 (https://creativecommons.org/licenses/by/4.0/).4. |
Immune cells also play an important role in post-injury bone regeneration.6 Bone healing after trauma requires an initial inflammatory phase involving mainly innate immune cells that clear damaged tissue and initiate the formation of scar tissue. In the following phase, this scar tissue is remodeled by osteoclasts to enable proper bone regeneration while inflammation is downregulated.7,8 As such, the persistence of inflammation leads to impaired bone healing. In osteoimmunological research, it is assumed that optimal bone preservation requires a balance between Th1 and Th2 cells, each fulfilling distinct immunological roles (Figure 1, adapted from Yang et al, Int J Med Sci 2021).4
At the interface between bone and the immune system, T cells are activated during inflammation and secrete various cytokines, which, in turn, are indispensable for the activation of resorption-driving osteoclasts, a process known as osteoclastogenesis.7 The field of osteoimmunology is mainly concerned with Th17 and T-reg, less with Th1, and very little with Th2 cells. In fact, Th1 and Th2 have been described as anti-osteoclastogenic T cells, although both are inflammatory T cells, however, engaged in different immune responses.8 Accordingly, bone destruction occurs when proinflammatory factors become prevalent and there is a preponderance of the Th1 cytokine TNF-α. In contrast, IFN-γ and IL-4 inhibit osteoclastogenesis. Of these three cytokines, only TNF-α promotes osteoclastogenesis. Indeed, both TNF-α and IL-1 are major drivers of inflammation and osteoclastogenesis and appear to be crucial in the first stage of healing.4 IFN-γ is also expressed during healing and has been reported to inhibit osteoclastogenesis and promote bone formation. However, the exact role of IFN-γ in bone healing remains unclear.6 IL-4 and IL-10 both inhibit bone resorption and are anti-inflammatory cytokines involved in the transition between the inflammatory phase and the regeneration phase.4,9 Thus, the balance between these five cytokines is a key parameter, the control of which is a major therapeutic target in bone healing and regeneration.8
In this research, we elucidate the role of the Th1 to Th2 balance in bone regeneration from the perspective of wound healing, with the aim of providing further prior insights into the immune mechanisms involved in the bone regeneration process and a new therapeutic target for improving the outcome for the treatment of bone injuries.
Local Osteoimmunology of the Jaw
As with any initial research in a new field, it is now up to clinicians to translate the findings from this research into a better understanding of the osteoimmune process and, where possible, new treatment approaches. Problems in wound healing in the jawbone arise when the homeostatic mechanism of bone resorption and formation is disturbed.
Morphology of Bone Marrow Defects of the Jaw
The death of local bone marrow cells due to chronic stimulation resulting from unfavourable factors such as inflammation of the jawbone may lead to chronic osteoimmune dysregulation. In previous publications, we defined the chronic inflammatory process that occurs in bone marrow defects of the jaw (BMDJ) as fatty-degenerative osteonecrosis of the jaw (FDOJ) which is associated with the chronic overexpression of the proinflammatory cytokine RANTES/CCL5.10,11 BMDJ/FDOJ is also primarily defined in the literature as “bone marrow edema”12,13 or silent or subclinical inflammation without the typical signs of acute inflammation. Figure 2 shows a tissue sample obtained during surgery to excise a bone marrow defect in the jaw. In earlier publications, we described the pathologically altered morphology of such areas.13–15
Collection and Processing of BMDJ/FDOJ Samples
The 128 BMDJ/FDOJ patients recruited to this study ranged in age from 36 to 73 years (median age 54). The female-to-male ratio was 86:42. The inclusion criteria were as follows: a proven diagnosis of BMDJ/FDOJ, as determined by two-dimensional orthopantomogram (2D-OPG) and three-dimensional digital volume tomography (3D-DVT) and additional trans-alveolar ultrasonography (TAU). Only the prior use of specific bone treatments served as exclusion criteria. Due to the presence of “silent inflammation”, the 128 patients enrolled in this study underwent removal of the abnormal BDJ/FDOJ areas as part of a routine operation. To shed light on the correlation between BMDJ/FDOJ, neuroinflammation, and neurodegeneration, these conspicuously altered medullar parts of the jawbone (BMDJ/FDOJ) were subjected to a postoperative cytokine analysis. We compared the values of seven cytokines in this group with the same cytokine pattern observed for 19 samples of healthy jawbones that were obtained from patients (age range: 33–72 years; average age, 51.4 years; female: male gender ratio, 10:9). The normal R/C level in healthy, medullary and maxillary spongy bones is 149.9 pg/mL.12–14
RANTES/CCL5 expression in BMDJ/FDOJ samples was analyzed using Human Cytokine/Chemokine Panel I (MPXHCYTO-60K; Millipore GmbH, Hesse, Germany) according to the manufacturer’s instructions using Luminex® 200™ and xPonent® software (Luminex Co, Austin, TX, USA). Concentrations were calculated according to a standard curve generated for the specific target and were expressed as pg/mL. When the concentrations were below the detection threshold, they were assumed to be 3.2 pg/mL.
Cytokine Expression in BMDJ/FDOJ
Drawing on our clinical experience with the notable fatty degenerative morphology of BMDJ/FDOJ, the authors had tissue samples that were readily excised from such defects examined for cytokine expression. The results of a panel of 27 cytokines measured in samples obtained from five patients showed the overexpression of both interleukin-1 receptor antagonist (IL-1ra) and the proinflammatory chemokine RANTES/CCL5. In contrast, TNF-α expression – which was expected to be high – showed vanishingly low values (Figure 3).12
 |
Figure 3 Multiplex analysis (in pg/mL) of 27 cytokines in five BMDJ/FDOJ samples showing the singular overexpression of IL-1ra and RANTES/CCL5 with values extrapolated beyond the range of the multiplex device. Notes: These results provided the initial impetus to further investigate the cytokine production in samples of BMDJ/FDOJ.13 |
This pilot study prompted us to further investigate 128 additional BMDJ/FDOJ samples with a reduced cytokine panel consisting of FGF-2, IL-1ra, IL-8, IL-6, IL-10, MCP-1, TNF-α and RANTES/CCL5 (Table 1 and Figure 4).9
 |
Table 1 Results of 128 BMDJ/FDOJ Samples Multiplex Analysis for Seven Cytokine Panel Consisting of FGF-2, IL-1ra, IL-8, IL-6, IL-10, MCP-1, TNF-α and RANTES/CCL5 |
The clinical BMDJ/FDOJ samples were provided by patients undergoing surgical treatment at the authors’ clinic. Each patient expressed an interest in determining whether chronic inflammation was present in the jawbone and, if so, associated with a pre-existing chronic immune disorder or systemic disease. It is clinically remarkable that, with the exception of cases of atypical facial pain and trigeminal neuralgia, BMDJ/FDOJ does not elicit a pain response in most patients. The absence of a painful inflammatory response may be explained by the decreased expression of TNF-α and Il-6, with TNF-α expression reduced by one-third and Il-6 expression reduced by one-tenth compared to the expression levels found in 19 samples of healthy bone marrow. Thus, the levels of these acute inflammatory mediators appear to be generally insufficient to drive acute and painful inflammation in most cases of BMDJ/FDOJ.14
Wound healing in bone begins with an inflammatory response, which is the normal response of vascularized living tissue to trauma.9 The consistent lack of proinflammatory cytokines in the fatty degenerative BMDJ/FDOJ samples is an indicator of impaired wound healing.13,14 This is so since inflammation is considered to be a useful mechanism in the early phase of bone healing. When the inflammatory response is prolonged, however, the beneficial effects are reversed and the process becomes detrimental.11 Fibrosis, for example, which is induced by the release of inflammatory cytokines from leukocytes, is one of the consequences of long-term inflammation. In the jaw bone samples that were investigated, the downregulation of angiogenic cytokines also results in the lack of angiogenesis, which is responsible for ischemia and hypoxia in the BMDJ/FDOJ areas concerned.5,15
Aim and Objectives
The loss of bone mass, as seen in osteoporosis and rheumatoid arthritis, is generally associated with the pattern of inflammatory mediators shown in Figure 4. The unexpected cytokine pattern that we previously measured in areas of impaired bone in samples of BMDJ/FDOJ raises the question of whether such dysregulated local inflammatory patterns and cytokine expression are associated with a systemic Th1/Th2 imbalance, as discussed in Introduction – The Evolving Field of Osteoimmunology. Is the predominant driver for BMDJ/FDOJ the impaired activation of immune cells, or rather, the impaired differentiation of osteoblastic cells?
Determination of Systemic Th1/Th2 Balance
In order to place the highly specific local BMDJ/FDOJ cytokine profile (see Local Osteoimmunology of the Jaw) in a systemic immunological context, we examined the Th1/Th2 profile in the serum of a second cohort of BMDJ/FDOJ patients. The selected cohort also presented with a concomitant chronic systemic inflammatory disorder, which provided insight into the systemic immunological regulation, ie immunocompetence, of this patient group.
Methods of Cytokine Analysis to Determine Th1/Th2 Ratios
For clarification, we used reliable laboratory testing procedures to perform cytokine analysis (IMD-Berlin). Serum concentrations of the cytokines IFN-y and IL-4 were determined after stimulated cytokine release. For this purpose, heparinised blood from the patient was mixed for 24 hours with the nonspecific stimulants concanavalin A (ConA; Sigma-Aldrich Chemie GmbH) and staphylococcal enterotoxin B (SEB; Sigma-Aldrich Chemie GmbH). Subsequently, the cytokines released into the cell culture supernatant were determined using a multiplex assay (MILLIPLEX MAP® Human Cytokine/Chemokine Magnetic Bead Panel; Merck-Millipore) and the Luminex 200™Analyzer.
Th1/Th2 Ratio and T-Reg Expression Level of the Study Cohort
To shift the focus from solely jaw bone metabolism to systemic immune regulation, we studied a cohort of 197 patients with chronic immune disorders (CID). The conditions were classified into the following categories: allergies (including food allergies, chronic rhinitis, and compromised immune system; n = 29); atypical facial and trigeminal pain (n = 47); neurodegenerative diseases (including migraine, tinnitus, multiple sclerosis, and amyotrophic lateral sclerosis; n = 57); tumors (breast, prostate, pancreatic, and colon cancers; n = 16); rheumatism (fibromyalgia and Lyme disease; n = 51); chronic fatigue syndrome (n = 56); and parasympathetic disorders (ie, disorders related to blood pressure, dizziness, and anxiety; n = 49). The mean age of patients was 54.05 years (range: 23–75 years). The female-to-male ratio was 89:225.
All of the patients in the selected cohort were diagnosed with BMDJ/FDOJ in one or more regions of the jaw using 2D and 3D radiography. Bone marrow defects such as BMDJ/FDOJ are difficult to detect in radiographs and there has thus been little research investigating this condition.16,17 Radiologically suspected BMDJ/FDOJ was also confirmed with a radiation-free measurement of bone density using a newly introduced trans-alveolar ultrasound (TAU) device.18,19
The present study was conducted as a retrospective case–control study and classified as such by the forensically accredited Institute for Medical Diagnostics, Nikolaistr. 22, D-12247 Berlin (IMD-Berlin), according to DIN EN 15198/DIN EN 17025. All patients provided their written informed consent (as outlined in the PLOS consent form) to participate and for the publication of their anonymized case details. This study was conducted in accordance with the Declaration of Helsinki.
The study presented here is patient-centered; the samples and data were obtained in the course of routine clinical practice and retrospectively evaluated. Institutional approval was not required for publication of this manuscript. The severity of the clinical pictures presented in the study cohort necessitated further insight into the immunological regulation of each patient group and the associated dynamics. We sought to examine the immunological status of each group. Accordingly, we sought to examine this by analyzing T-helper cells and determining the Th1/Th2 balance. Figure 5 shows the distribution of disease patterns in this patient cohort in number (n) and percentage (%). The mean age of the patients was 54.05 years (range: 23–75 years). The female-to-male ratio was 89:225.
Results
The Th1/Th2 cytokine profile was determined by first ascertaining the IFN-γ/IL-4 ratio and T-reg profile (Il-10). The Th1/Th2 and T-reg cytokine profiles, represented by the release of IFN-γ, IL-4 and IL-10, respectively, may help to clarify the regulation of systemic inflammation in these patients. The following analyses were conducted and provided the results presented below.20–22
Statistical Analysis
The quantitative data of the new research were analyzed using descriptive statistics, which were calculated using IBM SPSS, version 19 (IBM Corporation, Armonk, NY, USA). The median, the arithmetic mean value, and the data distribution were calculated. Differences between cohorts were computed with Student’s t-test or Spearman’s rho. The two-sided unpaired t-test was used to determine differences within groups, whereas Spearman’s coefficient was used to analyze correlations among the results of the cytokine profile analyses. The significance level was set at P<0.05.
Th1/Th2 Ratio
The normal reference range for the Th1 (IFN-γ pg/mL)/Th2 (IL-4 pg/mL) ratio is 6.1 to 21 (Figure 6). Among the cohort of 197 patients with CID and BMDJ/FDO, 167 had an IFN-γ expression level below this range (2.09 ± 1.61 pg/mL). Only 27 patients had IFN-γ levels of approximately normal value (9.36 ±2.71 pg/mL), while three had significantly higher IFN-γ levels (23.98 ± 2.57 pg/mL). These results demonstrate that the majority of patients had a low Th1/Th2 response.
Th1/Th2 Ratio Compared to T-Reg Expression
The normal reference range of T-reg is 760 to 1900 pg/mL. From the cohort of 197 patients with CID and BMDJ/FDOJ, one group with an underexpression of Th1 cytokines and corresponding underexpression of T-reg comprised 55 patients. This group of patients had a Th1/Th2 ratio with a mean value (MV) of 1.57 (±1.52) and an associated underexpressed T-reg with a mean value (MV) of 481.11 (±214.48) pg/mL. From the same cohort of 197 patients, a second group with an underexpression of Th1 cytokines and corresponding overexpression of T-reg comprised 47 patients. This group had a Th1/Th2 ratio with a mean value (MV) of 2.19 (±1.77) and an associated overexpressed T-reg with a mean value (MV) of 2527.28 (±790.28) pg/mL (Figure 7).
Summary of Results
The analysis of the Th1/Th2 ratio and T-reg profile of the patient cohort with BMDJ/FDOJ is shown in Figure 8 compared to the normal values for the Th1/Th2 ratio and normal range of T-reg.
Our studies and data show a shift in the Th1/Th2 balance towards a Th2 response that is associated with inflammation-induced immune depletion in more than 80% (n = 167) of the total cohort of 197 patients with CID and concomitant BMDJ/FDOJ. In these 167 subjects, the Th1/Th2 ratio was <6.1 with a T-reg expression of <1900, demonstrating impaired immune regulation with suspected chronic inflammation. In addition, 47 subjects or 30% of the patient cohort showed not only a shift in Th2 but also excessive T-reg with values of >1900, indicating the downregulation of immune activity.
Discussion
Is Low TNF-αthe Link Between Downregulation of the Th1/Th2 Ratio and Inflammation of the Jaw?
According to our data, about 80% of the patient cohort with chronic immune disorders (CID) showed downregulation of the Th1 cytokine response and upregulation of the Th2 response, as well as concomitant marked chronic BMDJ/FDOJ, which may contribute to the severity of CID. Since each subject in the cohort of 197 CID patients presented with clinically verified BMDJ/FDOJ, the question arises as to the nature of the relationship between the local Th2 shift that occurs in BMDJ/FDOJ and the systemic Th2 shift that was observed in this study?
When the cytokine pattern detected in 128 BMDJ/FDOJ samples, as shown in Figure 4, is examined for proinflammatory and anti-inflammatory factors, we find a clear deficit of proinflammatory factors. Figure 9 shows the local Th1/Th2 imbalance present in chronic BMDJ/FDOJ.
In BMDJ/FDOJ samples examined postoperatively, we demonstrated, using scientifically recorded data, that TNF-α and, especially, IL-6 - which is typically active in the transition from innate immunity to mechanisms of acquired immunity during inflammatory processes - show significantly reduced expression in the cytokine pattern associated with BMDJ/FDOJ. On average, TNF-α is downregulated to one-third and IL-6 to one-tenth of the levels found in healthy cancellous bone. This feature of BMDJ/FDOJ13 appears to impede an appropriate inflammatory defense response. At the same time, the lack of acute medullary tissue reaction may lead to the non-painful, cryptic symptomatology of BMDJ/FDOJ. This contrasts with the singular overexpression of the proinflammatory chemokine RANTES/CCL5 found in BMDJ/FDOJ, without the development of the classic markers of inflammation.
The absence of typical inflammatory patterns is also characteristic of Th2 upregulation and Th1 downregulation. The 7-cytokine profile of 128 BMDJ/FDOJ samples shows comparable cytokine patterns to those seen in the systemic downregulation of Th2 responses found in the CID cohort: on average, TNF-α is downregulated to one-third, and IL-6 to one-tenth, of the expression found in healthy jaw bone. In our studies, there was a 3.5-fold overexpression of IL-1ra in BMDJ/FDOJ areas and enhanced local Th2 upregulation (see Figure 10).
 |
Figure 10 In addition to the systemic immune imbalance found in the study cohort (see Figure 8), there was also a local Th2 shift and corresponding low IFN-y in BMDJ/FDOJ areas. Notes: Dysregulation in BMDJ/FDOJ is defined by chronic downregulation of inflammatory activity, ie, low expression of TNF-α and IL-6, and induction of Th2 upregulation, ie, high expression of IL-ra. |
Upregulation of IL1-Ra and Th2 Shift
Every immune response is regulated by pro- and anti-inflammatory cytokines. The key proinflammatory cytokines IL-1 and TNF-αare the main signaling agents and activators of the Th1 response. These cytokines are released by tissue macrophages when exposed to an inflammatory stimulus (such as bacteria, fungi, autoantigens, and other foreign substances). The main proinflammatory effects of IL-1 and TNF-α are stimulation of T and B cells and macrophages, activation of the vascular endothelium, and induction of the acute-phase response.
In the case of IL-1, these proinflammatory effects are mediated by binding to the IL-1 receptor. The IL-1 receptor antagonist (IL-1ra), which is also released by macrophages following a time delay, functions as a competitive inhibitor of IL-1 through binding to the IL-1 receptor thereby hindering further IL-1 signaling]. Thus, IL-1ra, which is highly elevated in the cytokine profile of BMDJ/FDOJ, is the antagonistic, anti-inflammatory counterpart of IL-1. In BMDJ/FDOJ, therefore, Th2 upregulation is further amplified locally. If the release of IL-1ra increases at the same time, there is no further induction of inflammation with increased Th1 activity.
Upregulation of IL-4 (Th2) and RANTES/CCL5 Overexpression
Studies found in the literature demonstrate a strong reciprocal relationship between RANTES/CCL5 and the upregulation of IL-4. One study, for example, determined that
RANTES increased IL-4 production in the presence of IL-4, whereas it suppressed IL-4 production in the absence of IL-4. The enhancing effect of RANTES/CCL5 was positively correlated with the donors’ plasma IgE levels.
The authors of the study concluded that “RANTES may induce IgE synthesis by increasing IL-4 production in individuals predisposed to high IgE responses”.23 This link between RANTES/CCL5 and both IgE synthesis and BMDJ/FDOJ suggests an association that remains unclear.
Systemic Consequences of Local RANTES/CCL5 Overexpression in BMDJ/FDOJ
What is the interaction between the RANTES/CCL5 signaling pathway and systemic immune dysregulation in the BMDJ/FDOJ cohort presenting with seven distinct clinical pictures of CID?
To test our working hypothesis, our team conducted a brief review of PubMed indexed publications for “RANTES CCL5 AND Allergy” and obtained 582 results. An excerpt from one of these publications, for example, stated: “Patients with chronic spontaneous urticaria (CSU) had significantly higher levels of CCL5/RANTES in comparison”.24 Under “RANTES CCL5 AND Trigeminal Pain”, 14 papers were found and one of these, for example, stated: “High chemokine levels may stimulate trigeminal nerve activation”.25 The search “RANTES CCL5 AND Neurodegenerative Diseases” yielded 54 hits, including a publication stating: “The results of the meta-analysis showed higher peripheral concentrations of IL-6, tumor necrosis factor, IL-1β, IL-2, IL-10, C-reactive protein, and RANTES in patients with Parkinson’s Disease”.26,27 “RANTES CCL5 AND Tumors” yielded 1758 hits, including a paper with the following statement: “CCR5 and the CCL5 ligand have been detected in hematologic malignancies, lymphomas, and a large number of solid tumors”.28,29 “RANTES CCL5 AND Rheumatoid Arthritis” yielded 10 results and one of these papers stated: “The present study suggests that there are molecular mechanisms underlying the development of RA, such as CCL5. CCL5 may have a negative impact on the development of RA”.30 “RANTES and CFS” (chronic fatigue syndrome/neuroinflammation) yielded 94 hits, including one publication stating: “The protein levels of the chemokine CCL5/RANTES were remarkably increased in the astrocytes of injured rat spinal cord”.31 Finally, “RANTES CCL5 AND Blood Pressure/Anxiety” provided 51 results of which one paper stated: “The chemokines CCL2 and CCL5 have long been associated with hypertension as they strongly influence diapedesis. Data suggest differential, context-specific effects of these chemokines on blood pressure”.32
These indications of the inflammatory role of RANTES/CCL5 in chronic immune disorders and the demonstrated overexpression of RANTES/CCL5 in BMDJ/FDOJ suggest associations that remain hitherto unrecognized in mainstream dentistry and medicine. It may also be worthwhile to further consider the presence of adipose tissue in BMDJ/FDOJ that is not present in other inflammatory diseases (eg, arthritis). Research indicates that this tissue may affect immune regulation as adipocytes are known to produce cytokines (adipokines) that modulate the immune response. In particular, adipose tissue is known to contain a high proportion of T-reg.11 BMDJ/FDOJ is a chronic osteoimmune disorder that may be considered an additional burden, further contributing to the progression of many immune and inflammatory diseases.33
Surgical Implications in Dental Practice
The extremely low local TNF-α and IL-6 cytokine expression found in BMDJ/FDOJ impairs the normal healing process by inhibiting an acute inflammatory response that would prevent the development of fatty degenerative morphology. In the context of BMDJ/FDOJ resection, it is essential to determine the patient’s immunological status in advance in order to define the conditions for successful patient-oriented surgical outcomes. In the preceding section, we discussed the importance of the Th2 shift to the systemic response to inflammation. However, the question remains as to the extent to which BMDJ/FDOJ may be the cause or consequence of such an immunological imbalance.
Summary
The data presented in our study cohort show that the majority of CID patients exhibit Th1 downregulation and a Th2 shift. The data also indicate that beyond the osteoimmune interactions previously presented in the medical literature,5–9,34 there appears to be a dysregulated cytokine pattern in BMDJ/FDOJ that almost completely inverts the classic panel of proinflammatory cytokines associated with Th1 activation.
In the jawbone, this inversion of an acute inflammatory cytokine pattern leads to BMDJ/FDOJ which is characterized by a lack of Th1 cytokines and, particularly, by the excessive overexpression of the proinflammatory chemokine RANTES/CCL5 and, to a lesser extent, IL-1ra.
To date, the cytokine pattern associated with BMDJ/FDOJ, ie, that inverts the classic inflammatory cytokine pattern, has not been found in any other organ fat and appears to be reserved for those degenerated jaw bone areas. In contrast, abdominal fat, for example, contains a very high proportion of regulatory Th1 cells and is capable of producing many cytokines that modulate the immune response through the high overexpression of TNF-α and IL-6. The lack of Th1 activation in BMDJ/FDOJ areas inhibits the normal healing of these pathologic changes in cancellous bone marrow and leads to the corresponding cryptic and clinically asymptomatic chronicity of the clinical picture. In the literature, the resulting chronic RANTES/CCL5 overexpression has also been linked to wide-ranging symptoms common to the group of chronic immune disorders of the patients in our study cohort”.27–33 The extent to which the issues discussed here concerning osteoimmunology and BMDJ/FDOJ relate to the interactions of RANK/RANKL, OPG and RANTES/CCL5 requires further investigation.
Conclusion
While the new interdisciplinary field of osteoimmunology presents broadly consistent accounts of the associated processes between bone cells and immune cells,35 these do not appear to apply to the jawbone in a surprisingly large number of clinical cases. The unique nature of the osteoimmune situation in BMDJ/FDOJ, as described above (see Local Osteoimmunology of the Jaw), requires greater consideration of the systemic immune response especially with respect to dental surgical interventions such as jaw surgery, implantation and augmentation to avoid the induction of the dysregulated Th2 response that is characteristic of BMDJ/FDOJ. Indeed, the hitherto unrecognized cause of many failed dental interventions may be linked to this specific osteoimmune state. Further clinical elucidation of the interaction of the local osteoimmune balance with systemic T-cell activation is necessary to provide a clear scientific statement and appropriate therapeutic advice.
Acknowledgments
The authors would like to thank Professor C Blin for her scientific advice on osteoimmunology. This manuscript was edited, with additional translation from German to English, by Natasha Gabriel.
Disclosure
Johann Lechner reports non-financial support from Digital Dental and Healthcare Technology (DDHT), during the conduct of the study. Johann Lechner is the holder of a patent used in the new TAU apparatus, CaviTAU®. The authors report no other conflicts of interest in this work.
References
1. Su L, Zheng J, Wang Y, Zhang W, Hu D. Emerging progress on the mechanism and technology in wound repair. Biomed Pharmacother. 2019;117:109191. doi:10.1016/j.biopha.2019.109191
2. Arron JR, Choi Y. Bone versus immune system. Nature. 2000;408:535–536. doi:10.1038/35046196
3. Yasuda H, Shima N, Nakagawa N, et al. A novel molecular mechanism modulating osteoclast differentiation and function. Bone. 1999;25:109–113. doi:10.1016/S8756-3282(99)00121-0
4. Yang N, Liu Y. The Role of the Immune Microenvironment in Bone Regeneration. Int J Med Sci. 2021;18:3697–3707. doi:10.7150/ijms.61080
5. Okamoto K, Nakashima T, Shinohara M, et al. Osteoimmunology: the Conceptual Framework Unifying the Immune and Skeletal Systems. Physiol Rev. 2017;97:1295–1349. doi:10.1152/physrev.00036.2016
6. Sato K, Suematsu A, Okamoto K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203(12):43–47. doi:10.1038/35005552 PMID: 17088434; PMCID: PMC2118166.
7. Barbul A, Breslin RJ, Woodyard JP, Wasserkrug HL, Efron G. The effect of in vivo T helper and T suppressor lymphocyte depletion on wound healing. Ann Surg. 1989;209(4):479–483. doi:10.1097/00000658-198904000-00015 PMID: 2522759; PMCID: PMC1493975.
8. Efron JE, Frankel HL, Lazarou SA, Wasserkrug HL, Barbul A. Wound healing and T-lymphocytes. J Surg Res. 1990;48(5):460–463. doi:10.1016/0022-4804(90)90013-r PMID: 2352421.
9. Zheng ZW, Chen YH, Wu DY, et al. Development of an accurate and proactive immunomodulatory strategy to improve bone substitute material-mediated osteogenesis and angiogenesis. Theranostics. 2018;8(19):5482–5500. doi:10.7150/thno.28315 PMID: 30555559; PMCID: PMC6276091.
10. Kong YY, Feige U, Sarosi I, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402(6763):43–47. doi:10.1038/35005552
11. Boyle W, Simonet W, Lacey D. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi:10.1038/nature01658
12. Lechner J, Mayer W. Immune messengers in neuralgia inducing cavitational osteonecrosis (NICO) in jaw bone and systemic interference. Eur J Int Med. 2010;2(2):71–77.
13. Lechner J, von Baehr V. RANTES and fibroblast growth factor 2 in jawbone cavitations: triggers for systemic disease? Int J Gen Med. 2013;6:277–290. doi:10.2147/IJGM.S43852
14. Lechner J, von Baehr V. Chemokine RANTES/CCL5 as an unknown link between wound healing in the jawbone and systemic disease: is prediction and tailored treatments in the horizon? EPMA Journal. 2015;6:10. doi:10.1186/s13167-015-0032-4
15. Lechner J, Schuett S, von Baehr V. Aseptic-avascular osteonecrosis: local “silent inflammation” in the jawbone and RANTES/CCL5 over expression. Clin Cosmetic Invest Dentistry. 2017;9:99–109. doi:10.2147/CCIDE.S149545
16. Patel S, Dawood A, Mannocci F, Wilson R, Pitt Ford T. Detection of periapical bone defects in human jaws using cone beam computed tomography and intraoral radiography. Int Endod J. 2009;42:507–515. doi:10.1111/j.1365-2591.2008.01538.x
17. Dotti R, Müller DM, Benini A. Clinical Appearence, Etiology, Pathogenesis, Diagnosis and Treatment of Avascular Bone Necrosis – Review of Present Literature) Praxis. Bern: © Verlag Hans Huber; 2002:163–176.
18. Lechner J, Zimmermann B, Schmidt M. Focal Bone-Marrow Defects in the Jawbone Determined by Ultrasonography—Validation of New Trans-Alveolar Ultrasound Technique for Measuring Jawbone Density in 210 Participants. In: Ultrasound in Medicine & Biology. Elsevier Published; 2021:12. doi:10.1016/j.ultrasmedbio.2021.07.012
19. Lechner J, Zimmermann B, Schmidt M, von Baehr V. Ultrasound Sonography to Detect Focal Osteoporotic Jawbone Marrow Defects: clinical Comparative Study with Corresponding Hounsfield Units and RANTES/CCL5 Expression. Clin Cosmet Investig Dent. 2020;12:205–216. doi:10.2147/CCIDE.S247345
20. Gallimore AM, Simon AK. Positive and negative influences of regulatory T cells on tumour immunity. Oncogene. 2008;27:5586–5593. doi:10.1038/onc.2008.269
21. Dagklis A, Fazi C, Sala C. The immunoglobulin gene repertoire of low-count chronic lymphocytic leukemia (CLL)-like monoclonal B lymphocytosis is different from CLL: diagnostic implications for clinical monitoring. Blood. 2009;114(1):26. doi:10.1182/blood-2008-09-176933
22. Kilpatrick RD, Rickabaugh T, Hultin LE, et al. Homeostasis of the naive CD4+ T cell compartment during aging. J Immunol. 2008;180:1499–1507. doi:10.4049/jimmunol.180.3.1499
23. Schmidt-Bleek K, Kwee BJ, Mooney DJ, Duda GN. Boon and Bane of Inflammation in Bone Tissue Regeneration and Its Link with Angiogenesis. Tissue Eng Part B Rev. 2015;21:354–364. doi:10.1089/ten.teb.2014.0677
24. Puxeddu I, Panza F, Pratesi F, et al. CCL5/RANTES, sVCAM-1, and sICAM-1 in chronic spontaneous urticaria. Int Arch Allergy Immunol. 2013;162(4):330–334. doi:10.1159/000354922
25. Bruno PP, Carpino F, Carpino G, Zicari A. An overview on immune system and migraine. Eur Rev Med Pharmacol Sci. 2007;11(4):245–248.
26. Qin XY, Zhang SP, Cao C, Loh YP, Cheng Y. Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: a systematic review and meta-analysis. JAMANEUROL. 2016;73(11):1316–1324. doi:10.1001/jamaneurol.2016.2742
27. Lechner J, von Baehr V, Schick F. RANTES/CCL5 Signaling from Jawbone Cavitations to Epistemology of Multiple Sclerosis - Research and Case Studies. Degener Neurol Neuro Dis. 2021;11:41–50. doi:10.2147/DNND.S315321
28. Aldinucci D, Colombatti A. The inflammatory chemokine CCL5 and cancer progression. Mediators Inflamm. 2014;292376:11125197371542014. doi:10.1155/2014/292376
29. Lechner J, Schulz T, Lejeune B, von Baehr V. Jawbone Cavitation Expressed RANTES/CCL5: case Studies Linking Silent Inflammation in the Jawbone with Epistemology of Breast Cancer. Breast Canc. 2021;13:225–240. doi:10.2147/BCTT.S295488
30. Huang Y, Zheng S, Wang R, Tang C, Zhu J, Li J. CCL5 and related genes might be the potential diagnostic biomarkers for the therapeutic strategies of rheumatoid arthritis. Clin Rheumatol. 2019;38(9):2629–2635. doi:10.1007/s10067-019-04533-1
31. Zhou Y, Guo W, Zhu Z, et al. Macrophage migration inhibitory factor facilitates production of CCL5 in astrocytes following rat spinal cord injury. J Neuroinflam. 2018;15(1):253. doi:10.1186/s12974-018-1297-z
32. Rudemiller NP, Crowley SD. The role of chemokines in hypertension and consequent target organ damage. Pharmacol Res. 2017;119:404–411. doi:10.1016/j.phrs.2017.02.026
33. Lechner J, Rudi T, von Baehr V. Osteoimmunology of tumor necrosis factor-alpha, IL-6, and RANTES/CCL5: a review of known and poorly understood inflammatory patterns in osteonecrosis. Clin Cosm Investl Den. 2018;10:251–262. doi:10.2147/CCIDE.S184498
34. Madel MB, Ibáñez L, Wakkach A, et al. Immune Function and Diversity of Osteoclasts in Normal and Pathological Conditions. Front Immunol. 2019;10:1408. doi:10.3389/fimmu.2019.01408
35. Choukroun E, Surmenian J, Simonpieri A, Choukroun J. Oxidative Stress and Osteoimmunology: the two Missing Pieces of the Oral Osseointegration Puzzle. Immun Res They J. 2021;3(1):119.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







