Back to Journals » Integrated Pharmacy Research and Practice » Volume 13
Outcomes of Community Pharmacy Interventions on Patients with Medicines Under Additional Monitoring
Authors Aizpurua-Arruti X , Benrimoj SCI , Goyenechea E , Isla A , Oñatibia-Astibia A , Malet-Larrea A , Gastelurrutia MÁ , Cuevas O, Rodríguez-Castejón J , Domingo-Echaburu S , Solinís MÁ , García M , del Pozo-Rodríguez A
Received 9 May 2024
Accepted for publication 13 July 2024
Published 29 July 2024 Volume 2024:13 Pages 115—125
DOI https://doi.org/10.2147/IPRP.S466129
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Walid Al-Qerem
Xabier Aizpurua-Arruti,1,2 Shalom Charlie I Benrimoj,3 Estibaliz Goyenechea,1 Arantxa Isla,2,4 Ainhoa Oñatibia-Astibia,1 Amaia Malet-Larrea,1 Miguel Ángel Gastelurrutia,1,3 Olatz Cuevas,1 Julen Rodríguez-Castejón,2,4 Saioa Domingo-Echaburu,5 María Ángeles Solinís,2,4 Montserrat García,6 Ana del Pozo-Rodríguez2,4
1Official Pharmacist Association of Gipuzkoa, Donostia / San Sebastián, Spain; 2Pharmacy Practice Research Group, PharmaNanoGene Group, Faculty of Pharmacy, University of the Basque Country UPV/EHU, Vitoria-Gasteiz, Spain; 3Pharmaceutical Care Research Group, Faculty of Pharmacy, University of Granada, Granada, Spain; 4Bioaraba, Microbiology, Infectious Disease, Antimicrobial Agents, and Gene Therapy, Vitoria-Gasteiz, Spain; 5Osakidetza Basque Health Service, Debagoiena Integrated Health Organisation, Pharmacy Service, Arrasate, Spain; 6Basque Country Pharmacovigilance Unit, Osakidetza Basque Health Service, Galdakao-Usansolo Hospital, Galdakao, Spain
Correspondence: Ana del Pozo-Rodríguez, Pharmacy Practice Research Group, PharmaNanoGene Group, Faculty of Pharmacy, University of the Basque Country UPV/EHU, Vitoria-Gasteiz, Spain, Email [email protected]
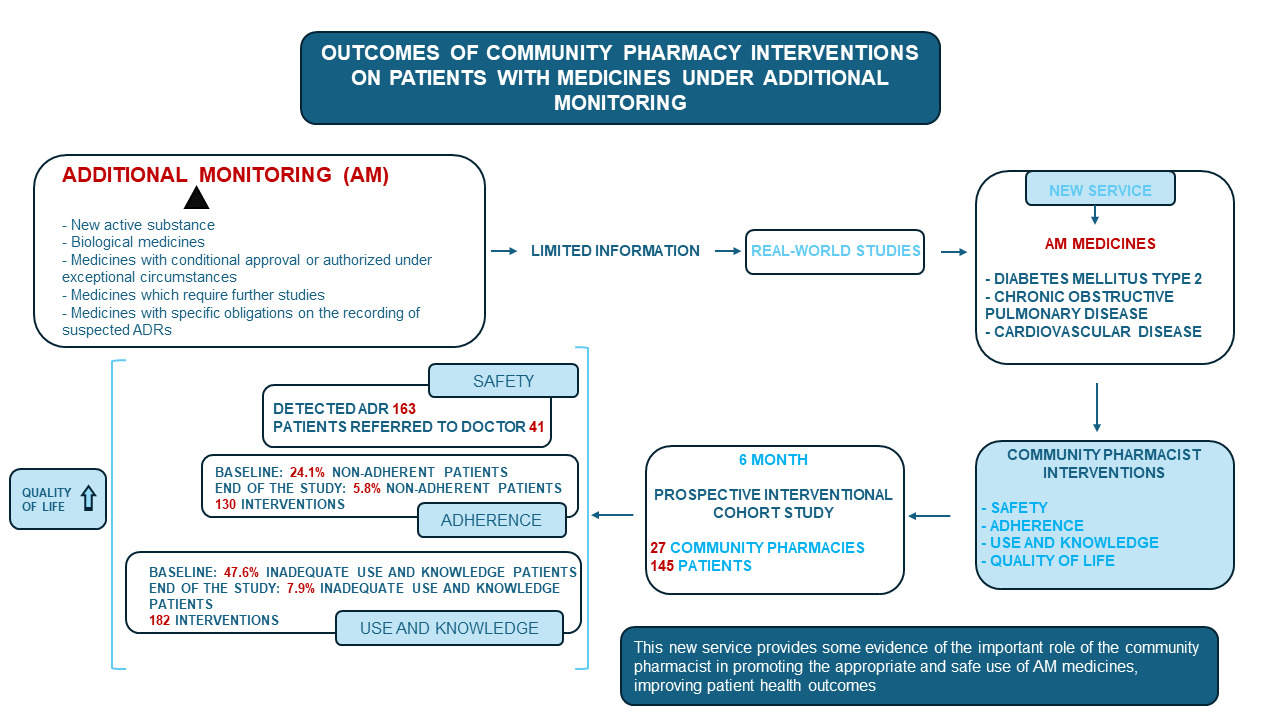
Purpose: Additional monitoring (AM) medicines include (i) medicines containing a new active substance; (ii) biological medicines; (iii) medicines with conditional approval or authorized in special situations; (iv) medicines which require further studies; (v) medicines that have specific requirements regarding the reporting of suspected adverse drug reactions (ADRs). When AM medicines are marketed, their most common ADRs are known, but safety information is limited because relatively rare ADRs are often not detected in clinical trials. Their AM status warrants real-world studies to identify other safety issues; however, such studies are lacking. Correct use and adherence to dosage regimen by patients are key factors for the evaluation of the safety and efficacy of medicines. The objective of this work was assessing the impact on safety, adherence, use and knowledge (U&K) about medicines and patient’s quality of life (QOL), of community pharmacist (CP)-led interventions in a new service focused on AM medicines targeted at three prevalent chronic diseases: diabetes mellitus type 2, chronic obstructive pulmonary disease and cardiovascular disease.
Patients and Methods: A prospective interventional cohort study was conducted with a 6-month follow-up in 27 community pharmacies (145 patients). Safety, adherence to treatment, patient U&K and QOL were assessed at follow-up visits (months 0, 3 and 6).
Results: The number of detected ADRs was 163 with 41 patients referred to the doctor. At baseline, 24.1% of the patients were non-adherent, mainly due to unintentional causes. After six months and 130 interventions by CPs on adherence, a significant reduction to lower than 5.8% was achieved. The inadequate U&K of medicines also decreased, from 47.6% to 7.9% after 182 interventions. Also, the patient’s QOL improved.
Conclusion: A new patient-centered pharmacy service provides some evidence on the important role of CP in assisting the proper and safe use of AM medicines, improving patient health outcomes.
Keywords: community pharmacy, medicines under additional monitoring, safety, adherence, use and knowledge, pharmaceutical care
Graphical Abstract:
Introduction
The concept of additional monitoring (AM) of medicines was introduced as part of the pharmacovigilance legislation by the European Union (EU) in 2010 and came into effect in July 2012.1 AM medicines include2 (i) medicines that contain a new active substance; (ii) biological medicines, such as vaccines or plasma-derived medicines; (iii) medicines with a conditional approval or medicines authorized under exceptional circumstances; (iv) medicines for which further studies are needed; (v) medicines authorized with specific obligations on the recording of suspected adverse drug reactions (ADRs). A black inverted triangle (▼) identifies AM medicines in the summary of product specifications and prospectus. In a similar way, the Black Triangle Scheme is used in Australia3 and UK.4 These strategies enable prompt identification by patients and healthcare professionals.5,6 When medicines under AM are marketed, their most common ADRs have been previously identified in clinical trials, but information about their safety may consider limited because the number of patients included in those trials is small compared to the total number of users that will eventually receive it, and relatively uncommon ADRs are often not detected. Therefore, their status of medicines under AM warrants real-world studies to identify further safety problems. However, studies on real-world data of medicines under AM are lacking.
The main goal of AM is to collect information to further inform ADRs, which are directly linked to patient health outcomes, as they tend to worsen patients’ quality of life (QOL).7,8 In many cases, ADRs are associated with poor adherence to therapy and inappropriate medicine use.9,10 Correct use and adherence to dosage regimen by patients are key factors for the evaluation of safety and effectiveness of a medicine.11
Community pharmacists (CP) are the most accessible healthcare providers and are crucial to strengthen communication with patients, in order to solve non-adherence problems and to improve health promotion and knowledge about medication.12 Several authors highlight the relationship between limited knowledge of the pathology and pharmacological treatment and lack of adherence.13,14 Appropriate patient counselling and education has been shown to improve medication adherence and disease.15,16
The aim of the present work is to analyze the impact on the safety of therapy, adherence, the use and knowledge about medicines (U&K) and QOL of the patient’s, of pharmacist-led interventions in the context of a new pharmacy service. The study was focused on chronic pathologies for which most of the medicines under AM are prescribed in the Basque Country (Spain): diabetes mellitus type 2 (DM2), chronic obstructive pulmonary disease (COPD) and cardiovascular disease (CVD).
Materials and Methods
Study Design and Procedure
An interventional cohort prospective study with 6-month follow-up was conducted in 27 community pharmacies of Gipuzkoa (Basque Country, Spain) between June 2019 and June 2021. The start date and end date of each pharmacy’s participation in the study varied due to COVID-19, but in all cases, participation lasted 6 months. Despite coincidence with COVID-19 pandemic, face-to-face communication was used throughout the study.
Pharmacist Training
All pharmacies in the regions of Bidasoaldea, Donostialdea, Tolosaldea and Alto Deba (n = 154) were mailed by the official college of pharmacy and 27 pharmacies provided names of CP that were interested in participation. CP received face-to-face training sessions with a step-by-step description of the new pharmaceutical service, including the critical points in the interviews and specific training for interventions and data collection. Practice change facilitator (PCF) oversaw the service delivery by the CP, providing support for service provision and resolving barriers.17
Description of the New Service
The service was divided into two stages (Figure 1).
 |
Figure 1 Flowchart of the service. Abbreviations: ADR, Adverse Drug Reaction; V0, visit 0; V3, visit at month 3; V6, visit at month 6. |
Patient Identification and Recruitment
In order to be included in the study, participants had to be 18 years or older, as well as, being patients with DM2, COPD or CVD in treatment with antidiabetics, bronchodilators or antithrombotic classified as medicines under AM from the list specified in Table S1. The exclusion criteria were pregnancy, participation in other pharmaceutical care programs at the time of recruitment, and inability to communicate with health professionals. Patients who satisfied the inclusion criteria were informed about the service and those who agreed to participate, signed the consent form, and were scheduled for visits (follow-up stage).
Patient Follow-Up Stage
Patients’ assessment using the service model was carried out face to face in the private care area. The follow-up stage consisted of three interviews over 6 months: at baseline (V0), 3 months later (V3) and at the end of the study (V6). The safety, the adherence to treatment and the patients´ U&K of the medicine were assessed throughout the service (from V0 to V6), and the QOL was tested at the starting (V0) and ending points (V6).
The Template for Intervention Description and Replication (TIDieR)18 was used to report the intervention in a standardized way (Table S2).
Data Collection
A data collection notebook was prepared for each patient. It included a copy of the informed consent, baseline data and the data recorded in each interview on safety, non-adherence, type of non-adherence, U&K about the medicine, and a description of the interventions.
Outcome Measures
Safety
To assess the safety, CP asked the patient about unusual new symptoms detected since started taking the medication. Using WHO-UMC system for standardized case causality assessment,19 CP detected an ADR related to the medicine under study, whether these had been previously identified or not in the literature, it was documented, and advice was given to the patient on how to proceed. In the case in which the CP considered that there may be necessary to change the dose, pharmaceutical form or medication, a referral was made to the doctor.
CP also reported to the Spanish pharmacovigilance system through the Pharmacovigilance Unit in the Basque Country.
Adverse Drug Reaction (ADR) Monitoring
To compare the notifications of ADR before the participation in the study and during the study, the Pharmacovigilance Unit of the Basque Country provided pre-study and in-study ADR notifications from pharmacies included and not included in the study in the Basque Country.
ADR notifications related to COVID-19 vaccinations were not considered.
Adherence to Treatment
The adherence to treatment was assessed by the Morisky-Green-Levine Medication Assessment Questionnaire (MGL MAQ).20,21 Patients were classified as adherent (questions were answered as NO, YES, NO, NO) or non-adherent (≥1 questions answered contrarily). The cause of non-adherence was analyzed by the CP depending on the patient’s responses and was classified as intentional (patient deliberately decided not to be adherent), unintentional (patient was no adherent to the medication for reasons beyond his/her control) or mixed (patient had intentional and unintentional causes) (Table S3). CP intervened at V0 and V3 according to the type and cause of non-adherence (Table S4), and in the following interviews, it was assessed the resolution or not of the lack of adherence.
Use and Knowledge (U&K) of Medicine
The U&K of the medicine was assessed by a validated questionnaire22 of five standard questions:
- do you know what the medicine is used for?
- do you know how much to take? (dose/pattern)
- do you know how long to take?
- do you know how to use?
- do you know the precautions for use?
It was considered that the patient did not have an appropriate U&K when at least one of the answers to the questionnaire was NO. In those cases, the CP intervention consisted of providing verbal and/or written explanation with the aim of correcting information (Table S5). As part of the written explanations, patient information leaflets were provided with information about the treatment, its proper use and the most frequent ADR (example in Appendix 1). The CP used placebo devices to accompany the explanations in a practical way.
Quality of Life
The QOL was analyzed at V0 and V6 by EQ visual analogue scale (EQ VAS) of the EQ-5D-5L questionnaire.23 It is a vertical scale on which patients self-assess their level of health, where the highest point (100) corresponds to “The best health you can imagine” and the lowest (0) to “The worst health you can imagine”.
Ethics Approval
This study was approved by the Spanish Agency of Medicines and Medical Devices (AEMPS) as a post authorization prospective follow-up study24 (EPA-SP, AIR-ENO-2019-01). The protocol was previously accepted by the Basque Clinical Research Ethics Committee (EPA2019016) and was in agreement with the Helsinki Declaration.
Statistical Analyses
The data distribution was analyzed by the Kolmogorov–Smirnov and Shapiro–Wilk tests. Paired t-tests, Student’s test or ANOVA were chosen for repeated measures analysis.
To analyze the frequency distribution and the relationship between groups Chi-square (χ2) and Fisher’s exact tests were used.
Analyses were performed in the intention-to-treat (ITT) population. For the ITT analyses, values were calculated based on the multiple imputation system. Patient lost during the follow-up was taking into account for all variables. Patients were deemed to have fulfilled the study if they attended all scheduled visits.
CP reporting of adverse reactions to the Spanish Pharmacovigilance system was noted with the notification ratios per pharmacy were compared between the participant pharmacies and the rest of pharmacies of the Basque Country. Notification ratios were calculated by dividing the number of notifications 6 months before and during the study by the number of pharmacies included (n = 27) or not included in the study in the Basque Country (n = 812). Data are provided for 6-month periods.
Statistical analysis was conducted using IBM SPSS Statistics 26 software. A two-tailed p value of less than 0.05 was considered statistically significant.
Results
Participant Recruitment and Baseline Characteristics
The 27 community pharmacies recruited 145 patients (Table 1). Eleven patients did not complete the study with seven dropping out after V0 and four after V3. The demographic data between patients who remained in the study and those who dropped out did not show significant differences (p>0.05). The duration of V0 (baseline) assessment was approximately between 45min-1h. In V3 and V6, the visits lasted 20 minutes.
 |
Table 1 Baseline Characteristics of the Studied Patients |
Evaluation of Safety
A total of 163 ADRs were detected (Table 2). If the detected ADR was related to lack of adherence or inappropriate U&K, pharmaceutical advice was based on the interventions summarized in Tables S4 and S5. Additionally, in 41 of the 163 cases of detected ADR, the intervention from the community pharmacy was a referral to doctor.
Using WHO-UMC system for standardized case causality assessment, of the 163 detected ADRs, CP notified 54 ADRs to the Spanish pharmacovigilance system of medicines for human use, specifically 48 related to the medicines under study.
Data on notifications were compared with the notifications made by the rest of the pharmacies located in the Basque Country (Table 3). The 27 participant pharmacies notified 54 ADRs (48 of them related to the medicines under study) during the 6-months follow-up period, which is a much higher value than the observed in the previous 6-month period; 3 (0 notifications related to the medicines under study). The notification rate during the implementation of the service was significantly higher from the 27 participant pharmacies than from the other 812 pharmacies (1.78 vs 0.01; p<0.001).
Evaluation of Adherence and Community Pharmacist’s Interventions on Adherence
Adherent patients accounted for 75.9% of the total, and significant differences were not detected among subgroups (p > 0.05), either when analyzing age, gender, time from the start of the treatment and total number of prescribed drugs. The percentage of total non-adherent patients significantly decreased (Figure 2AI) from 24.1% to 11.9% in the first 3 months (p < 0.001), and to 5.8% at the endpoint of the study (p < 0.001).
A decrease in non-adherent patients was also observed in all subgroups (from 28.8% (V0) to 7.4% (V6) in DM2 (p<0.001), from 13.7% (V0) to 5.0% (V6) in CVD (p<0.001) and from 32.1% (V0) to 4.2% (V6) in COPD, although in this case differences were not statistically significant (p>0.05)). No significant differences were found segmenting by pharmaceutical form.
There were a total of 130 interventions on non-adherent patients (Figure 2AII). Among the 35 non-adherent patients at V0, 4 (11.4%) were non-adherent due to intentional causes, 26 (74.3%) due to unintentional causes and 5 (14.3%) due to mixed causes. In patients with mixed non-adherence, only 35.1% of the cases were resolved after the first intervention, although at the end of the study the 95.5% of the cases of non-adherent patients were resolved. The proportion of adherent patients was 94.2% six months after their inclusion in the study, as compared to the baseline 75.9% (p<0.001).
Evaluation of U&K and Community Pharmacist’s Intervention on U&K
CP found that 52.4% of the patients used and knew their treatment correctly. There were no differences among the pathology subgroups (p>0.05), either when analyzing age, gender, time from the start of the treatment and total number of prescribed drugs. A significant reduction (p<0.001) in the percentage of patients who had inadequate U&K was observed (Figure 2BI) from 47.6% at baseline to 19.2% in the first 3 months, and to 7.9% at the endpoint of the study (p<0.001).
This reduction was also observed when considering the pathology subgroups (from 50.0% (V0) to 9.2% (V6) in DM2 (p<0.001), from 43.1% (V0) to 6.3% (V6) in CVD (p<0.001) and from 50.0% (V0) to 7.9% (V6) in COPD (p<0.01)).
No significant differences were found segmenting by pharmaceutical form.
The 87% of the cases associated with U&K detected at V0 were resolved in the next visit, as well as 84% of the cases detected at V3 with 182 interventions (Figure 2BII). About 92.1% of patients showed a correct U&K after six months of study, a significantly higher percentage compared to baseline (52.4%, p<0.001).
Quality of Life
Self-rated QOL values communicated by the patients significantly improved from 70.1 ± 14.8 at V0 to 73.7 ± 13.9 at V6 (p < 0.001). This variable also improved (p < 0.001) when considering pathology subgroups: 69.6 ± 16.1 (V0) vs 73.7 ± 14.6 (V6) in DM2; 68.8 ± 14.3 (V0) vs 72.8 ± 12.3 (V6) in CVD; 73.4 ± 12.0 (V0) vs 75.4 ± 15.2 (V6) in COPD.
Discussion
A new service on patients in treatment with medicines under AM has been evaluated in 27 Spanish pharmacies and contributed to significantly improve patient safety, adherence, U&K and QOL. There are very few studies on real-world settings in the literature that describe data collected on the safety of AM.25,26 These studies are extremely important to obtain information after commercialization for regulatory authorities and healthcare specialists.2 The Strengthening Collaboration for Operating Pharmacovigilance in Europe (SCOPE) questionnaire reveals that nearly 60% of Member States (MS) fail to recognize ADR reports for medicines under AM in their databases.27
The philosophy of real-world studies on AM medicines is to augment information on safety of these medications. The new service encouraged the identification of ADR and the monitoring of adherence and U&K with essentially four outcomes; the provision of advice on how to manage ADR, improving adherence and knowledge on the use of the medications and if required the referral of patients to general medical practitioners. These outcomes, related to pharmacovigilance are important, since international studies show that healthcare providers possess a restricted knowledge related to pharmacovigilance, and their viewpoints on ADR significantly impact their reporting frequencies.28,29 In particular, there is evidence of underreporting among CP worldwide.30–34 Additionally, it has been also demonstrated an association between ADR knowledge and notification frequencies. Herdeiro et al showed an almost 6-fold raise in the number of ADR notifications when they received a short pharmacovigilance training of 1 hour.35 A similar situation was observed in our study, with higher notification rates of ADR as compared to the rest of pharmacies of the Basque Country.
Adherence is a key factor that contributes to the safe and effective use of medicines under AM. It is estimated that only 50% of patients with chronic diseases are adherent to their medication.36,37 However, the higher percentage of adherent patients in our study (75.9% at baseline) may be related to various factors. The patients included in our study perceived a high QOL, with values near 70 at V0, and is consistent with the higher adherence observed as previously reported by Fernández-Lázaro et al38 It is noteworthy that, even in the case of a group of patients with high adherence rate at baseline, the interventions made by CP resulted in an improved adherence, reaching 95% after six months of follow-up. Appropriate patient education about their pharmacological treatment is closely related with patient’s adherence.39 The improvement of the U&K about the medicines, from around 50% of patients at V0 to more than 90% after pharmacists’ interventions would also have contributed to the increase in adherence. Interestingly, 65% of patients that reported unintentional non-adherence showed gaps in their knowledge about the use and precautions of prescribed medicines. With this new service, patients have the opportunity to acquire, comprehend, and apply fundamental health data to properly choose the best options to promote and maintain their good health.40–42
In spite of the promising results reported in the present work, CP usually encounter several barriers that hinder their participation in this type of services, such as, lack of specific training and knowledge, time required to perform the service or non-remuneration.43,44 Studies like this would help to break down barriers so that community pharmacies would be more involved in providing pharmaceutical care services to their patients.
Limitations and Strengths
While the main limitation of the present work is the number of total patients, statistical power of the study when nonsignificance was reported exceeded 80%, indicating a high likelihood of detecting significant effects if they exist. Thus, the study suggested that the new service, through several interventions per patient, improved safety, adherence, U&K and QOL. A randomized control trial would be required to substantiate this finding.
It could be also considered the risk of biases associated with inclusion in the study of different classes of medications with incomparable complexity. However, the results of adherence and U&K were analyzed separating by pharmaceutical form, and no significant differences were found.
Although the pharmacies and patients recruited were the ones that demonstrated interest in participating, clear and rigorous inclusion criteria were established to ensure that the sample was as representative as possible of the general population of pharmacies and patients. Also, participating pharmacies represent a diversity of settings, including both urban and rural areas and different pharmacy sizes, to capture a variety of operational contexts and pharmacy practices.
Another point to take into consideration is the fact that the proposed new service was focused on medicines under AM or those that include the inverted triangle on their packaging. This concept is not used in all regions of the world, but the service design could not be limited to these types of medicines, as CP-led interventions in safety, adherence and U&K may also be of interest in other types of medicines.
Conclusion
A new patient-centered pharmacy service was evaluated in real-world setting to assess and improve safety, adherence, U&K and QOL of patients in treatment with antidiabetics, bronchodilators and antithrombotics classified as medicines under AM. Results show that, besides improving the ADR notifications, more than 90% of patients were adherent and had good U&K about the treatments as well as better self-perceived QOL at the end of the study. Altogether, this work offers some evidence about the important role of the CP as healthcare providers in assisting the proper and safe use of medicines in improving patient health outcomes.
Abbreviations
AM, additional monitoring; ADR, adverse drug reaction; U&K, use and knowledge; QOL, quality of life; CP, community pharmacists; EU, European union; DM2, diabetes mellitus type 2; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; PCF, practice change facilitator; TIDieR, Template for Intervention Description and Replication; EQ-VAS, EQ visual analogue scale; AEMPS, Spanish Agency of Medicines and Medical Devices; ITT, intention-to-treat; SCOPE, Strengthening Collaboration for Operating Pharmacovigilance in Europe; MS, member states.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We gratefully acknowledge all community pharmacists and patients that participated in the study.
Funding
This work was supported by Distribuidora Farmacéutica de Gipuzkoa (DFG), the University of the Basque Country UPV/EHU (US18/24 and GIU20/048) and the Department of Education of the Basque Government (IT1587-22). The supporting sources had no restrictions regarding publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Regulation (eu) no 1027/2012 of the European Parliament and of the Council of; 2012. Available from: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:316:0038:0040:EN:PDF.
2. European Medicines Agency. Medicines Under Additional Monitoring. Available from: https://www.ema.europa.eu/en/human-regulatory-overview/post-authorisation/pharmacovigilance-post-authorisation/medicines-under-additional-monitoring.
3. Australian Government. Therapeutic Goods Administration. The Black Triangle scheme. Available from: https://www.tga.gov.au/how-we-regulate/monitoring-safety-and-shortages/report-adverse-event-or-incident/report-adverse-events-medicines-and-biologicals/black-triangle-scheme#:~:text=How%20the%20Black%20Triangle%20Scheme,Product%20Information%20(PI)%20documents.
4. UK Government. The Black Triangle Scheme. Available from: https://www.gov.uk/drug-safety-update/the-black-triangle-scheme-or#:~:text=A%20Black%20Triangle%20symbol%20is,a%20new%20route%20of%20administration.
5. European Medicines Agency. Guideline on Good Pharmacovigilance Practices Module X—Additional Monitoring; 2013. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-module-x-additional-monitoring_en.pdf.
6. European Commission. Regulation (EC) No 726/2004 of the European Parliament and of the Council of 31 March 2004 laying down Community Procedures for the Authorisation and Supervision of Medicinal Products for Human and Veterinary Use and Establishing a European Medicines Agency. Off J Eur Union. 2004;50:1–33.
7. Coleman JJ, Pontefract SK. Adverse drug reactions. Clin Med. 2016;16(5):481–485. doi:10.7861/clinmedicine.16-5-481
8. World Health Organization. Medication Safety in Polypharmacy; 2019. Available from: https://www.who.int/publications/i/item/WHO-UHC-SDS-2019.11.
9. Chiatti C, Bustacchini S, Furneri G, et al. The economic burden of inappropriate drug prescribing, lack of adherence and compliance, adverse drug events in older people: a systematic review. Drug Saf. 2012;35(Suppl 1):73–87. doi:10.1007/BF03319105
10. Carow F, Rieger K, Walter-Sack I, et al. Objective assessment of nonadherence and unknown co-medication in hospitalized patients. Eur J Clin Pharmacol. 2012;68(8):1191–1199. doi:10.1007/s00228-012-1229-2
11. Jimmy B, Jose J. Patient medication adherence: measures in daily practice. Oman Med J. 2011;26(3):155–159. doi:10.5001/omj.2011.38
12. Showande SJ, Laniyan MW. Patient medication counselling in community pharmacy: evaluation of the quality and content. J Pharm Policy Pract. 2022;15(1):103. doi:10.1186/s40545-022-00502-3
13. Llorca CV, Cortés Castell E, Ribera Casado JM, et al. Factors Associated with Non-Adherence to Drugs in Patients with Chronic Diseases Who Go to Pharmacies in Spain. Int J Environ Res Public Health. 2021;18(8):4308. doi:10.3390/ijerph18084308
14. Kobue B, Moch S, Watermeyer J. “It’s so hard taking pills when you don’t know what they’re for”: a qualitative study of patients’ medicine taking behaviours and conceptualisation of medicines in the context of rheumatoid arthritis. BMC Health Serv Res. 2017;17(1). doi:10.1186/s12913-017-2246-8
15. Calvo Hernáez B, Gastelurrutia Garralda MÁ, Urionagüena de la Iglesia A, Isla Ruiz A, Del Pozo Rodríguez A, Solinís Aspiazu MÁ. Oferta de servicios de atención farmacéutica: clave para un nuevo modelo de servicios de salud [Supply of pharmaceutical care services: the key to a new model of health services]. Aten Primaria. 2022;54(1):102198. doi:10.1016/j.aprim.2021.102198
16. World Health Organization (WHO). Adherence to long-term therapies. Evidence for action; 2003. Available from: https://apps.who.int/iris/handle/10665/42682?locale-attribute=es&.
17. Gastelurrutia MA, Benrimoj SI, Castrillon CC, et al. Facilitators for practice change in Spanish community pharmacy. Pharm World Sci. 2009;31(1):32–39. doi:10.1007/s11096-008-9261-0
18. Hoffmann T, Glasziou P, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348(mar07 3):g1687. doi:10.1136/bmj.g1687
19. World Health Organization (WHO). The use of the WHO-UMC system for standardised case causality assessment. Available from: https://www.who.int/docs/default-source/medicines/pharmacovigilance/whocausality-assessment.pdf.
20. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi:10.1097/00005650-198601000-00007
21. Val Jiménez A, Amorós Ballestero G, Martínez Visa P, et al. Descriptive study of patient compliance in pharmacologic antihypertensive treatment and validation of the Morisky and Green test. Aten Primaria. 1992;10(5):767–770.
22. García Delgado P, Gastelurrutia Garralda MA, Baena Parejo MI, Fisac Lozano F, Martínez Martínez F. Validación de un cuestionario para medir el conocimiento de los pacientes sobre sus medicamentos [Validation of a questionnaire to assess patient knowledge of their medicines]. Aten Primaria. 2009;41(12):661–668. Romanian. doi:10.1016/j.aprim.2009.03.011
23. Williams A. The EuroQol Instrument. In: Kind P, Brooks R, Rabin R, editors. EQ-5D Concepts and Methods. Netherlands: Springer; 2005:1–17.
24. Kiri VA. A pathway to improved prospective observational post-authorization safety studies. Drug Saf. 2012;35(9):711–724. doi:10.1007/BF03261968
25. Carvalho da Silva SP, Jesus M, Roque F, et al. Active Pharmacovigilance Study: a Follow-Up Model of Oral Anti-Cancer Drugs under Additional Monitoring. Curr Oncol. 2023;30(4):4139–4152. doi:10.3390/curroncol30040315
26. Mendes D, Rigueiro G, Silva RS, et al. Intensive safety monitoring program of antineoplastic medicines: a pilot study in a Portuguese oncology hospital. J Oncol Pharm Pract. 2020;26(1):133–140. doi:10.1177/1078155219849277
27. European Medicines Agency. Identification, Management and Raising Awareness of ADR Reports for Drugs Subject to Additional Monitoring. Available from: https://www.ema.europa.eu/en/documents/other/scope-training-identification-management-raising-awareness-adr-reports-drugs-subject-additional_en.pdf.
28. Khabbaz HJA. Healthcare Professionals’ Knowledge, Attitude and Perception toward Adverse Drug Reaction Reporting in Saudi Arabia: a Systematic Review. J Pharm Res Int. 2023;35(28):36–48. doi:10.9734/jpri/2023/v35i287448
29. Cervantes-Arellano MJ, Castelán-Martínez OD, Marín-Campos Y, Chávez-Pacheco JL, Morales-Ríos O, Ubaldo-Reyes LM. Educational interventions in pharmacovigilance to improve the knowledge, attitude and the report of adverse drug reactions in healthcare professionals: systematic Review and Meta-analysis. Daru. 2024;32(1):421–434. doi:10.1007/s40199-024-00508-z
30. Lozano R, Vera E, Lozano MC, Madurga M, Serna A. Knowledge and attitude about Pharmacovigilance practices of pharmacy professionals of community pharmacy and hospital pharmacy in Spain. Rev Esp Salud Pública. 2020;94:e202007068.
31. Albayrak A, Karahalil B. Pharmacist’s Knowledge and Behaviors Toward Pharmacovigilance and Adverse Drug Reactions Reporting Process in Türkiye. Turk J Pharm Sci. 2022;19(6):694–700. doi:10.4274/tjps.galenos.2022.59422
32. Ferreira-da-silva R, Alves JM, Vieira C, et al. Motivation and Knowledge of Portuguese Community Pharmacists Towards the Reporting of Suspected Adverse Reactions to Medicines: a Cross-Sectional Survey. J Community Health. 2023;48(2):295–308. doi:10.1007/s10900-022-01168-3
33. Li R, Curtain C, Bereznicki L, et al. Community pharmacists’ knowledge and perspectives of reporting adverse drug reactions in Australia: a cross-sectional survey. Int J Clin Pharm. 2018;40(4):878–889. doi:10.1007/s11096-018-0700-2
34. Abu Assab M, Alhamad H, Albahar F, Abu Dayyih W, Echarif S, Abu Assab H. Pharmacovigilance Concept Knowledge, Perspectives and Attitudes: a Cross-Sectional Study Among Community Pharmacists. Inquiry. 2024;61:469580241246464. doi:10.1177/00469580241246464
35. Herdeiro MT, Polonia J, Gestal-Otero JJ, Figueiras A. Improving the reporting of adverse drug reactions: a cluster randomized trial among pharmacists in Portugal. Drug Saf. 2008;31(4):335–344. doi:10.2165/00002018-200831040-00007
36. Brown MT, Bussell J, Dutta S, et al. Medication Adherence: truth and Consequences. Am J Med Sci. 2016;351(4):387–399. doi:10.1016/j.amjms.2016.01.010
37. Walsh CA, Cahir C, Tecklenborg S, et al. The association between medication non-adherence and adverse health outcomes in ageing populations: a systematic review and meta-analysis. Br J Clin Pharmacol. 2019;85(11):2464–2478. doi:10.1111/bcp.14075
38. Fernández-Lázaro CI, García-González JM, Adams DP, et al. Adherence to treatment and related factors among patients with chronic conditions in primary care: a cross-sectional study. BMC Family Prac. 2019;20(1). doi:10.1186/s12875-019-1019-3
39. Taibanguay N, Chaiamnuay S, Asavatanabodee P, et al. Effect of patient education on medication adherence of patients with rheumatoid arthritis: a randomized controlled trial. Patient Prefer Adherence. 2019;13:119–129. doi:10.2147/PPA.S192008
40. Rowlands G. Health literacy. Hum Vaccin Immunother. 2014;10(7):2130–2135. doi:10.4161/hv.29603
41. Al-Taie A. Reported patients’ attitudes and practices for knowledge of prescribed medications with chronic disease conditions:A cross-sectional study. Biomed Biotechnol Res J. 2020;4(4):349–354. doi:10.4103/bbrj.bbrj_174_20
42. Nutbeam D, McGill B, Premkumar P. Improving health literacy in community populations: a review of progress. Health Promot Int. 2018;33(5):901–911. doi:10.1093/heapro/dax015
43. Sun Q, Wan C, Xu Z, Huang Y, Xi X. Association of pharmaceutical care barriers and role ambiguity and role conflict of clinical pharmacists. Front Pharmacol. 2023;14:1103255. doi:10.3389/fphar.2023.1103255
44. van Mil JWF, de Boer WO, Tromp THFJ. European barriers to the implementation of pharmaceutical care. Int J Pharm Pract. 2001;9(3):163–168. doi:10.1111/j.2042-7174.2001.tb01044.x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



