Back to Journals » International Journal of Nanomedicine » Volume 19
Pushing Forward the DNA Walkers in Connection with Tumor-Derived Extracellular Vesicles
Authors Liu Q, Zhang Q, Yao Z, Yi G, Kang Y, Qiu Y, Yang Y, Yuan H, Fu R, Sheng W, Cheng L, Wang W, Wang H, Peng C
Received 19 March 2024
Accepted for publication 15 May 2024
Published 19 June 2024 Volume 2024:19 Pages 6231—6252
DOI https://doi.org/10.2147/IJN.S464895
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. RDK Misra
Qingyi Liu,1,2 Qiongdan Zhang,1,2 Zhijian Yao,1,2 Gangqiang Yi,2 Yeonseok Kang,3 Yixing Qiu,1,2 Yupei Yang,1,2 Hanwen Yuan,1,2 Ronggeng Fu,2 Wenbing Sheng,1,2 Lidong Cheng,4 Wei Wang,1,2 Huizhen Wang,1,2 Caiyun Peng1,5
1TCM and Ethnomedicine Innovation & Development International Laboratory, School of Pharmacy, Hunan University of Chinese Medicine, Changsha, People’s Republic of China; 2School of Pharmacy, Hunan University of Chinese Medicine, Changsha, People’s Republic of China; 3College of Korean Medicine, Wonkwang University, Jeonbuk, Korea; 4Hunan Yirentang Chinese Herbal Pieces Co., Ltd, Changde, People’s Republic of China; 5Institute of Innovation and Applied Research in Chinese Medicine Hunan University of Chinese Medicine, Changsha, People’s Republic of China
Correspondence: Huizhen Wang; Caiyun Peng, Tel +86-151-1648-1295 ; +86-138-7480-7689, Email [email protected]; [email protected]
Abstract: Extracellular vesicles (EVs) are microparticles released from cells in both physiological and pathological conditions and could be used to monitor the progression of various pathological states, including neoplastic diseases. In various EVs, tumor-derived extracellular vesicles (TEVs) are secreted by different tumor cells and are abundant in many molecular components, such as proteins, nucleic acids, lipids, and carbohydrates. TEVs play a crucial role in forming and advancing various cancer processes. Therefore, TEVs are regarded as promising biomarkers for the early detection of cancer in liquid biopsy. However, the currently developed TEV detection methods still face several key scientific problems that need to be solved, such as low sensitivity, poor specificity, and poor accuracy. To overcome these limitations, DNA walkers have emerged as one of the most popular nanodevices that exhibit better signal amplification capability and enable highly sensitive and specific detection of the analytes. Due to their unique properties of high directionality, flexibility, and efficiency, DNA walkers hold great potential for detecting TEVs. This paper provides an introduction to EVs and DNA walker, additionally, it summarizes recent advances in DNA walker-based detection of TEVs (2018– 2024). The review highlights the close relationship between TEVs and DNA walkers, aims to offer valuable insights into TEV detection and to inspire the development of reliable, efficient, simple, and innovative methods for detecting TEVs based on DNA walker in the future.
Keywords: EVs, TEVs, DNA walker, detection
Graphical Abstract:
Introduction
Cancer is a serious disease that is often diagnosed in later stages, so early detection is crucial to improving survival rates. Scientists are now interested in using extracellular vesicles (EVs) as biomarkers in non-invasive liquid biopsy tests to detect various cancers. EVs are tiny particles released by cells and can be found in body fluids. And these EVs carry important biological components from their parent cells,1,2 including tumor-derived extracellular vesicles (TEVs) that contribute to cancer progression. However, detecting TEVs in the early stages of cancer can be challenging due to their low levels. Developing sensitive and accurate detection methods for TEVs is essential for improving early cancer diagnosis and treatment.
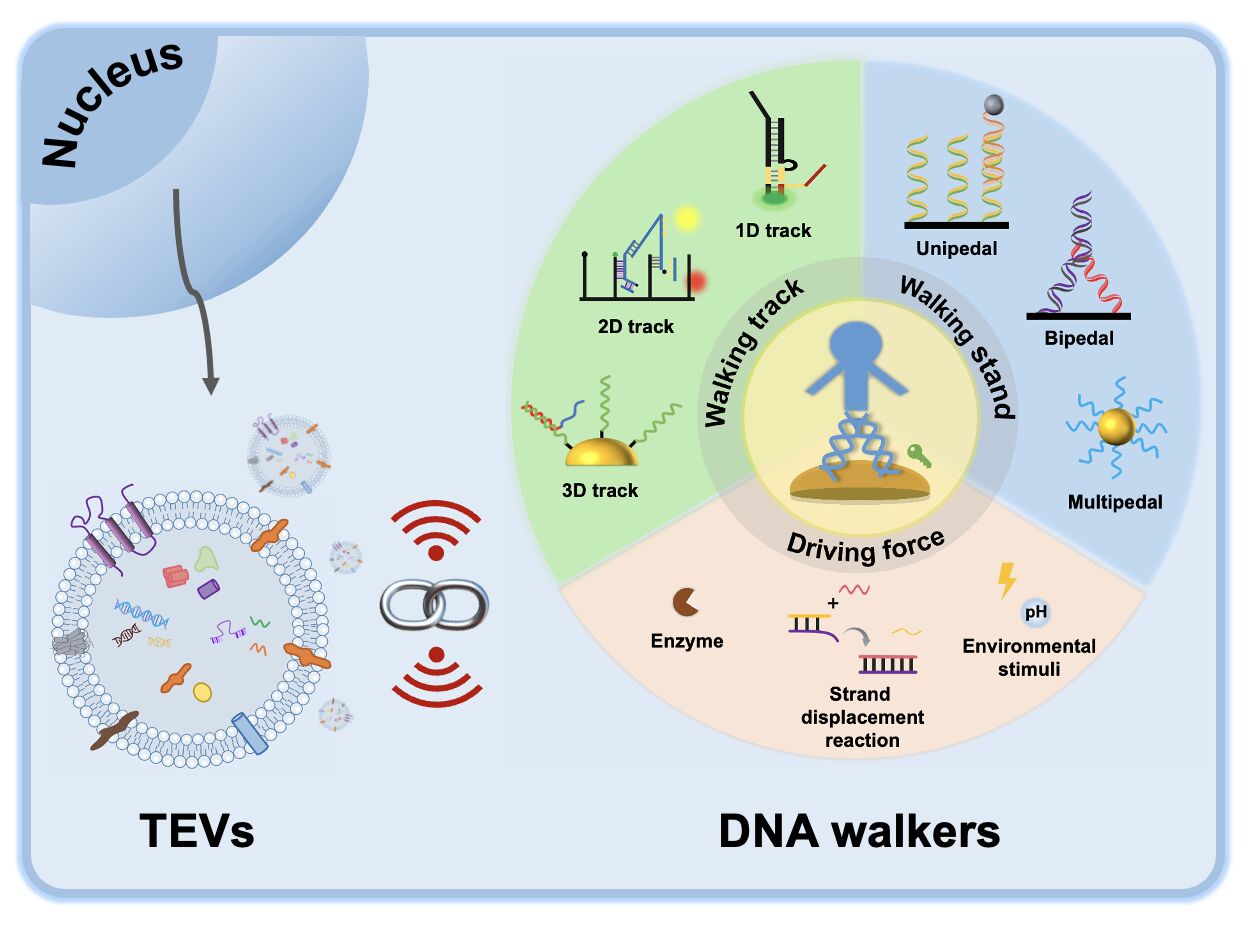
DNA walkers, a novel artificial nanomachine that consists of tracks, walking feet and driving forces, have emerged as a rapidly growing area of research biology.3 This is primarily attributed to their ability to be specifically designed and programmed, as well as their excellent controllability and exquisite predictability. DNA walkers move progressively and autonomously at the designing nanometre scale, making them highly suitable for various applications such as biosensing, material assembly and synthesis, and early cancer diagnosis.4
Previous reviews have summarized research developments on EVs and methods for their detection.5,6 Here, this review combines EVs, tumor and DNA walkers, providing a comprehensive summary of these DNA walker-based nanoplatforms for detecting TEVs, to assist in the accurate tumors diagnosis. These advancements are expected to have a profound impact on clinical TEV detection and offer valuable guidance for the development of more reliable, efficient, simple, and groundbreaking DNA walkers in the future.
Extracellular Vesicles
EVs are particles released from cells that are bounded by a lipid bilayer and cannot replicate on their own.7 EVs are generally split into three subtypes on the basis of their mechanism of biogenesis-exosomes, shed microvesicles, and apoptotic bodies.8 And they are often categorized based on their size into small, medium, and large EVs due to the difficulty of isolating EV subtypes. EVs play a crucial role in regulating intercellular communication by transporting various bioactive molecules, including DNAs, RNAs, lipids, metabolites, cytosolic and cell-surface proteins among cells. Tumor cells, unlike normal cells, often exhibit a higher level of EVs production, resulting in the accumulation of TEVs that carry valuable information about the tumors.9 Hence, TEVs have emerged as promising biomarkers for diagnosing various human diseases.
Extracellular Vesicles Biogenesis
The regulation of EVs biogenesis involves the coordination of molecular pathways and the influence of environmental stimuli, resulting in changes in the quantity, content, and eventually the dynamics of EVs inside a biological system. Therefore, it is crucial to understand the process of EVs biogenesis. This understanding will not only contribute to our knowledge of cellular communication but also aid in the development of novel assays and therapeutic strategies for various diseases.
Although the general term “EVs” is commonly used to refer to all secreted membrane vesicles, they exhibit significant heterogeneity.10 Below we focus on the biogenesis of microvesicles and exosomes. First, cargo destined for secretion within EVs must be targeted to the site of production, either at the plasma membrane (for microvesicles), or at the limiting membrane of the multivesicular endosome (MVE) (for exosomes) containing intraluminal vesicles (ILVs).11 Second, the cargo is enriched in the formed vesicles through a stepwise mechanism of clustering and budding followed by division and vesicle release (Figure 1).11 Although the generation of microvesicles and exosomes occurs at different sites within the cell, the biogenesis of both entities involves common intracellular and sorting mechanisms.12
 |
Figure 1 The biogenesis of extracellular vesicles. Reprinted from Nat Rev Mol Cell Biol, volume 19(4); Van Niel G, d’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. . 213–228, Copyright 2018, with permission from Springer Nature.11 |
The Contents of the Extracellular Vesicles
EVs carry various biologically active molecules derived from their parental cells, such as RNAs, DNAs, proteins, lipids, and metabolites (Figure 2). Next, we focus on RNAs and proteins found in EVs and their relationship with malignancies.
 |
Figure 2 The contents of extracellular vesicles. |
RNA
Within the EVs, different types RNAs are present, mainly including microRNAs (miRNAs), messenger RNAs (mRNAs), and further studies discovered additional types of non-coding RNAs (ncRNAs), piwi-interacting RNAs (piRNAs) and transfer RNAs (tRNAs).13,14 The Vesiclepedia database (http://microvesicle.org) currently contains 3533 EVs studies. In addition, the Vesiclepedia database also integrates from 50,550 RNA entries, which provides a broader platform to obtain information on vesicle composition.15 RNAs in EVs have always attracted the attention of researchers, especially regarding TEVs. Since the first report of exosomal miRNA in 2007,16 numerous studies have unveiled the significance of tumor exosomal miRNAs in cancer biology. These miRNAs have been found to regulate tumor development through multiple pathways. For example, certain gastric cancer exosomes with a higher metastatic potential can inhibit tumor growth by releasing let-7 family miRNAs that transmit signals leading to tumor metastasis.17 Researchers have also explored the diagnostic potential of exosomal miRNAs. Madhavan et al identified highly expressed miRNAs (miR-4306, miR-4644, miR-3976, and miR-1246) within exosomes of pancreatic cancer patients compared to normal control tissues.18 In recent years, research on tumor-related ncRNAs has become a boom. Paramanantham et al discuss the function of exosomal ncRNA in lung tumor pathophysiology and also discuss the future clinical applications of exosomal-ncRNA as lung cancer biomarkers and therapeutic targets.19
Protein
The EVs are tiny vesicles that encapsulate a diverse array of molecules, including proteins among other constituents. The Vesiclepedia database (http://microvesicle.org) also integrates 566,911 protein entries.15 Proteomics research techniques have helped uncover the protein composition and functions of EVs originating from various sources. At the same time, they also have revealed various mechanisms action of TEVs in tumorigenesis and development. For example, Annexin II protein found in malignant tumor exosomes creates a favorable microenvironment for tumor metastasis.20 High expression of human epidermal growth factor receptor (EGFR), epithelial cell adhesion molecule (EpCAM) and Alix has been observed in serum exosomes derived from individuals diagnosed with non-small cell lung cancer.21 CD97 has shown potential as a diagnostic marker for gastric cancer, while human epidermal growth factor receptor 2 (HER-2) has been seen to have significant expression in the serum exosomes of gastric cancer patients.22,23 In addition, studies have shown that when breast cancer exosomes interacted with the human submandibular gland cell line human salivary gland (HSG) in vitro, the exosomal proteins and mRNA secreted by HSG were altered.24
Extracellular Vesicles and Tumor Development
Tumor cells present in the tumor microenvironment have the ability to secrete EVs.25 Studies have shown that TEVs participate in and affect many hallmarks of cancer,26 and also play a significant role in several aspects of cancer progression, including the formation of the tumor microenvironment, the growth of new blood vessels, the invasion and spread of cancer cells and resistance to drugs.27 Among them, the relationship between TEVs and the tumor microenvironment has attracted the focus of researchers, which can be observed from two important aspects: (1) Tumor microenvironment promotes TEVs generation. Such as hypoxia and alteration in pH levels, may stimulate the production and release of TEVs. (2) TEVs modulate the tumor microenvironment: TEVs carry various bioactive molecules can be taken up by neighboring distant cells within the tumor microenvironment. This process can lead to changes in the surrounding tumor microenvironment, promoting the transformation of target cells into cancerous cells and exhibiting tumor characteristics. For example, Wang et al found that Glioblastomas (GBMs) EVs significantly affected normal brain cells constituting the tumor microenvironment. EVs from different GBM induce brain cells to alter secretomes with pro-inflammatory or tumor microenvironment-modifying effects.28
It is also important to note that cancer cells have the ability to release EVs into the tumor microenvironment, which impacts many cells such as cancer-associated fibroblasts (CAF), cancer stem cells and other stromal cells. This interaction influences inter- and intracellular signaling, promoting cancer cell survival, proliferation, invasion, metabolic reorganization and tumor angiogenesis. Overall, TEVs have a significant impact on tumorigenesis,17 metastasis,29 progression,30 immune activation,31 immune escape,31 and even tumor diagnosis.32 For example, Chen et al reported that EVs secreted by metastatic melanoma carry PD-L1, which inhibits the function of CD8 T cells to promote tumor growth. Circulating exosomal PD-L1 levels in patients with metastatic melanoma show varying degrees of change during anti-PD-1 treatment. It is also found that a substantial increases in exosomal PD-L1 early in treatment differentiate clinical responders from non-responders.33
DNA Walkers
The field of DNA nanotechnology has witnessed continuous advancements in the development of DNA molecular machines. Researchers have been increasingly interested in utilizing DNA molecules with various structural and functional properties to construct nanomachines. Among these, the DNA walker stands out as a particularly fascinating molecular machine, known for its specificity, stability, biocompatibility and functionality.3 DNA walkers are a highly promising biological strategy for biosensing, often referred to as a “fourth-generation sensor”.34 A typical artificially-engineered DNA walker consists of three main elements: the walking strand, the walking track and the driving force (Figure 3).4 The driving force disrupts the initial equilibrium to convert light or chemical energy into mechanical energy, propelling the walking strand along the walking track. Then, by consuming fuel molecules, the reaction equilibrium is restored, thereby generating a signal. This cycle of breaking and restoring equilibrium leads to the amplification of sensing signals, which can detect the analytes with high sensitivity, thus being widely used in the field of biosensor-based detection. The following describes a detailed design principle of DNA walkers as well as summarizes their respective merits and demerits (Table 1), and its application in the field of biosensors is also discussed.
 |
Figure 3 The design principle diagram of DNA walkers. |
 |
Table 1 Merits and Demerits of Different DNA Walkers |
Design Principle of DNA Walkers
Walking Track
The walking track of the DNA walker is carefully planned, and it follows designed rules for the arrangement of substrate molecules and formation by immobilized substrate molecules. The walking track must be durable and have stable stators to ensure a secure connection between the walking strand and track. Additionally, it needs to possess precise mechanical, electrochemical, or optical properties for designing walker systems. The tracks can be categorized based on the movement way and range of the walking strand into one-dimensional (1D) tracks, two-dimensional (2D) tracks, and three-dimensional (3D) tracks.
In the 1D tracks, there are DNA double helix short tracks and carbon nanotubes. The short DNA double helix tracks have low thermodynamic stability when in solution. Due to the limited amount of substrate molecules attached to the track, the walking distance and frequency are restricted.35 Carbon nanotubes offer distinct advantages over the DNA double helix short track owing to their longer distance and the sufficient area they allow for walking without the need for precise control of the beginning location. For example, Cha et al used RNA-modified carbon nanotubes as linear walking tracks for the DNA walker, allowing the walking strand to move longer.39
Compared to 1D DNA walkers, 2D DNA walkers are superior signal amplifiers due to their planar structure and their rigid backbone and extensible directions that allows for greater freedom of movement on the DNA track. 2D DNA origami, gold electrode, and glassy carbon electrode are commonly used as flat surfaces to directly generate and boost electrochemical signals using DNA walking. For example, Yang et al utilized different DNA origami structures to directly see the photoinduced motions of individual molecules.40 This DNA walker is propelled by light and can go along a linear path on a single 2D DNA tile.
As researchers further study, the introduction of 3D DNA walkers by Ellington et al in 2015 has greatly influenced the advancement of new analytical methods for biomolecular analysis.41 3D DNA walkers usually rely on 3D DNA origami, gold nanoparticle, magnetic microparticle, composite material and cell membrane, which offer an increased specific surface area and enhanced enrichment effect.36 By expanding the area available for movement and increasing the speed at which reactions occur, this method surpasses the effectiveness of 1D and 2D DNA walkers that are built on flat structures like electrodes, DNA origami, and biochips. Therefore, they are an ideal tool for signal transduction and amplification and have been widely used in biological detection. Qi et al described a flexible single magnetic nanoparticle-constrained, click chemistry-driven digital 3D DNA walker for microRNA detection, where a DNA walking leg was attached to a single magnetic nanoparticle.42 The 3D DNA walker demonstrated a walking efficiency of less than 100 rounds for each swing arm, leading to the successful retrieval of an adequate amount of fluorophores on the magnetic nanoparticle, enabling precise quantification of digital microRNA.
Walking Strand
During the movement of the DNA walkers, the direct or indirect involvement of a target causes a change in the thermodynamic and kinetic equilibrium of the process, which affects the conversion of its energy into mechanical energy within a particular DNA strand, allowing it to move along the stable pathway. This is the travel paths of the DNA walker, and this is what defines of walking stand (also referred to ‘legs’). It can be categorized into unipedal, bipedal, and multipedal based on the number of free-moving walking stand. Therefore, the choice of DNA walker type should be based on the specific needs of the application and each type of DNA walker has unique advantages. In the case of unipedal DNA walkers, their use only one leg for its walking process. For example, Bath et al constructed a unipedal DNA walker that can walks directly on one leg.43
However, as the study progressed it was found bipedal and multipedal DNA walkers exhibit higher stability and higher velocity.37 For example, Wang et al designed an electrochemical biosensor based on a bipedal DNA walker, which ultimately contributes nearly 5-fold synergistic enhancement of the current signal compared to a unipedal walker, and successfully realizing the highly sensitive detection of target.44 In addition, Mao et al developed a multipedal DNA walker for the first time to achieve ultra-sensitive analysis of circulating tumor cell (CTCs).45 This gold nanoparticles based multipedal DNA walker also enables rapid analysis of CTCs and shows great potential in early clinical diagnosis and treatment of cancer.
Driving Force
The driving force categories are often chosen based on the characteristics of the targets. In order to enhance performance, a variety of driving forces are raised. Currently, the primary factors propelling DNA walkers are enzymatic (protein enzyme or DNAzyme) reactions, strand displacement reactions and environmental stimulus reactions. Next, we introduce these driving forces respectively.
A category of DNA walkers exists that are powered by enzymatic reactions resulting from the hydrolysis of covalent bonds in DNA substrates situated along their designated tracks. Enzyme-assisted DNA amplification methods exhibit enhanced efficiency and effectiveness in the detection of low-abundance targets. DNA walkers are often moved by enzymes like endonuclease, exonuclease, and DNAzyme as they cleave DNA substrates along designated tracks. For example, based on the “burning bridge mechanism”, Fan et al used exonuclease III (Exo III) to recognize the DNA hybridization track and gradually catalyze the removal of the single nucleotide at its blunt 3’ end to release the walking chain and completing the DNA walker.46
DNA strand displacement reaction refers to a reaction process in which a long single-stranded DNA replaces a short single-stranded DNA. Strand displacement reaction eliminate the need for enzymes, offering several benefits, including simplicity, stability, and cost-effectiveness. Common examples include catalytic hairpin assembly (CHA), hybridization chain reactions (HCR), and entropy-driven amplification (EDC), etc. Strand displacement reactions are crucial for propelling DNA walkers and enabling behaviors that rely on strand displacement reactions.38 Yang et al developed proximity-shifting DNA walkers induced by aptamer/target binding to detect thrombin.47 This reaction does not require the participation of enzymes but leads to the release and recycling of aptamer/protein complexes and the function of molecular machines through strand displacement, ultimately sensitively detecting thrombin.
In addition, conformational changes induced by environmental stimuli such as chemical stimulus48 and light,49 can also provide energy for DNA walkers to facilitate the interaction between walking chains and tracks. For example, Yao et al developed a DNA walker controlled by alternating changes in pH.48 The start and stop of the walker, as well as its attachment and detachment from particles, can also be dynamically controlled by pH, with simple, programmable characteristics.
Applications of DNA Walkers
In order to further improve the detection sensitivity of various biological and chemical samples, various signal amplification methods have been used in biosensors in recent years, including nanomaterials, enzymatic reactions, DNA nanotechnology, etc.50,51 DNA walkers have opened up new and exciting opportunities for target detection and bioanalysis in modern life sciences and are considered very promising and have huge application potential in various fields.3 The evolution of DNA walkers from inception to the present day is of great existential significance. As illustrated in Figure 4, the concept of DNA walkers was first introduced by Seeman’s group in 2004,52 and soon after, the Pierce group demonstrated first DNA walker.53 Since then, DNA walkers have continued to evolve. Scientists have effectively utilized biosensors based on DNA walkers for biological detection over the past decade. In 2015, Wang et al designed a novel microRNA-responsive DNA walker biosensor based on strand displacement cascade reaction and enzyme cycle cleavage strategy to detect nucleic acids.54 Xu et al proposed a highly sensitive ferrocene-switched electrochemiluminescence sensor for protein (cardiac troponin I) detection using target transduction and DNA walking machine in 2015.55 In 2018, Qing et al designed a highly sensitive and efficient method for ultrasensitive electrochemical detection of copper ions (Cu2+) using a click chemical reaction to trigger a 3D DNA walker.56 Furthermore, in the same year, DNA walkers were successfully applied for detecting TEVs, demonstrating the versatility and potential of this technology. Dong et al propose a novel strategy for exosome detection based on aptamer recognition-induced multiple DNA release and followed by cyclic enzymatic amplification.57 In 2019, Mao et al developed for the first time a multipedal DNA walker for ultrasensitive detection of tumor circulating cells, which enables ultrahigh sensitivity of its analysis.45 In 2020, Li et al proposed a simple, sensitive, and efficient electrochemical biological detection method for bacteria using a multiplex amplification strategy of simultaneous amplification of 3D DNA walkers, RCA, and HCR.58 The journey of using DNA walkers to detect biological samples has been evolving, and using DNA walkers to detect low-abundance targets has always been on the minds of researchers. Different from previous reviews, this article mainly introduces the application of DNA walkers in the field of biosensors from the aspect of detecting TEVs. It is worth mentioning that we summarize the construction of universal biosensors for detecting TEVs through several DNA walker patterns and provide some key directions and trends.
 |
Figure 4 A timeline that marks key milestones in the development of synthetic DNA walkers for the purpose of testing biological samples. |
DNA Walkers for Detection of Tumor-Derived Extracellular Vesicles
Traditional detection methods for detection of EVs, including Western blotting,59 tracking and analysis of nanoparticles,60 flow cytometry,61 and enzyme-linked immunosorbent assay,62 have significant advantages but also have certain limitations. These limitations include the need for a substantial quantity of samples, expensive instruments, and limited sensitivity. Despite the use of optimization strategies such as signal amplification technology, the successful utilization of these techniques still requires complex sequence design, fine surface modification, and tedious sample preparation steps.63 These limitations hinder the effectiveness of EVs assays, especially for TEVs. Furthermore, TEVs are present in very low abundance in biofluids, and their signals are often masked by heterogeneous foreign bodies. Therefore, it is essential to come up with innovative approaches for the detection of TEVs that may capable of overcome these challenges.
Recently, new detection methods have been proposed for sensitive and effective TEV detection.64,65 There is a type of detection methods based on DNA walker that we cannot ignore, and this section introduces the application of innovative DNA walker-based techniques and reviews and summarizes the utilization of DNA walker signal amplification methods in detecting TEVs (Table 2). Since the researchers (example below) mentioned that these EVs (the term “exosomes” also refers to EVs) are associated with tumor cells, we abbreviate them as “TEVs”.
 |
Table 2 Summary of DNA Walker in the Detection of Tumor-Derived Extracellular Vesicles |
DNA Walkers Based on Exosomal Protein Recognition
To accurately detect TEVs, the strategy of studying recognition probes for TEVs surface protein markers is highly effective. The most widely used probes are protein-specific and rely on immunoaffinity reaction of antibodies and nucleic acid aptamers. In the following sections, we will briefly introduce these two specific recognition probes combined with DNA walkers for the detection of TEVs.
Antibody Recognition-Based DNA Walkers
Using antibodies as recognition elements and using immune recognition of proteins to detect EVs is currently one of the most commonly used methods and is also the most mature protein analysis method.85,86 To measure EVs, a sandwich structure between the EVs, the capture antibody, and the target antibody by binding them to a solid-phase carrier. Then a series of experiments were performed to achieve quantitative analysis of EVs. Researchers combine immunoaffinity reactions with electrochemical analysis,87,88 chromatographic analysis,89,90 surface-enhanced Raman scattering (SERS),91 and microfluidic detection platforms to establish a variety of EVs analysis method.92,93 At the same time, the application of antibodies as recognition elements combined with DNA walkers has been used to detect TEVs. As illustrated in Figure 5A, Cao et al enriched target exosomes using anti-CD63 functionalized immunobeads and identified them using DNA strands containing the CD63 aptamer region.66 First, dibenzocyclooctyne functionalized gold electrode (DBCO/GE) was prepared according to the previous method. Exosomes are first enriched on the surface of anti-CD63 antibody functionalized immunomagnetic beads (Anti-CD63/IMBs) through immune recognition. The inherent CD63 aptamer region is then used to bind to exosomes. After magnetic separation, probe a on the exosomes serves as a trigger to initiate cascade toehold-mediated strand displacement reaction (CTSDR)-dependent catalytic molecular machinery. The CTSDR process is roughly that probe a reacts with the toehold of azido-b1, thereby releasing b2 from the azido-b1/b2 duplex. The newly formed a/azido-b1 exposes an additional toehold that is partially complementary to the signaling probe MB-c, thereby probe a to be displaced and generating a new double-stranded azide-b1/MB-c. The released probe a then triggers a new cycle of b2 displacement from the azido-b1/b2 duplex. After multiple CTSDR cycles, a large number of azido-b1/MB-c duplexes are generated and can be transferred onto DBCO/GE to generate amplified electrochemical signals. The molecular machine demonstrates exceptional efficiency, with a linear range of 1×105 to 5×107 particles/mL when targeting exosomes. Its limit of detection (LOD) is 1.72×104 particles/mL surpasses that of most existing detection methods. While this example is based on the recognition of TEVs using a one antibody, the use of two antibodies for recognition of TEVs provides even greater selectivity. Ding et al proposed a novel strategy for the simultaneous determination of exosomal proteins, serum amyloid A-1 protein (SAA1) and coagulation factor V (FV) using DNAzyme walkers-triggered CRISPR-Cas12a/Cas13a.67 As illustrated in Figure 5B, first, antibody-walkers will be prepared, which can bind the captured SAA1 and FV to form a sandwich complex. Then, magnetic beads 2 (MBs2) orbitals obtained by coupling two orbitals (T1 and T2) to streptavidin modified MBs2 were added to the sandwich complex. In the presence of coenzyme Mg2+, DNAzyme walkers can specifically cleave tracks and release multiple P1 and P2. In addition, P1 and P2 can hybridize with crRNA to activate Cas 13a and Cas 12a, resulting in obvious fluorescent (FL) signals. The detection approach exhibits exceptional sensitivity, with a LOD as low as 30.00 pg/mL for SAA1 and 200.00 pg/mL for FV. More importantly, a deep learning (DL) model based on SAA1 and FV concentrations in plasma exosomes was also developed and applied to tumor diagnosis.
 |
Figure 5 (A) Schematic representation of amplified electrochemical detection of exosomes based on antibody recognition and DNAzyme walkers. Reprinted from Biosens Bioelectron, volume 141, Cao Y, Li L, Han B, Wang Y, Dai Y, Zhao J. A catalytic molecule machine-driven biosensing method for amplified electrochemical detection of exosomes. 111397, Copyright 2019, with permission from Elsevier.66 (B) Schematic representation of the two antibodies recognition-DNAzyme walkers and CRISPR-Cas12a/Cas13a bioassay for plasma exosomes detection. Reprinted from Biosens Bioelectron, volume 219, Ding L, Wu Y, L-e L, et al. Universal DNAzyme walkers-triggered CRISPR-Cas12a/Cas13a bioassay for the synchronous detection of two exosomal proteins and its application in intelligent diagnosis of cancer. 114827, copyright 2023, with permission from Elsevier.67 |
Aptamer Recognition-Based DNA Walkers
Given the problems of high antibody cost and harsh storage conditions in immunoaffinity reactions, exploring an antibody substitute with strong specificity, high stability and clinical application is crucial. Nucleic acid aptamers, as a new type of molecular recognition tool, are single-stranded oligonucleotide DNA or RNA fragments with a length of about 25 to 60, obtained from random single-stranded nucleic acid libraries by in vitro screening technology. It can specifically recognize ions, proteins, bacteria and cells and other target molecules.94,95 This in vitro screening technology was originally proposed by Gold’s research group in 1990.96 It is a kind of exponential enrichment ligand system evolution technology. Compared to antibodies, aptamers have the advantages of high specificity, strong affinity, good stability, low cost, and a wide range of target molecules. Therefore, as a promising molecular recognition tool, aptamers have been widely used in the field of biochemical analysis.97
Since there are many similar proteins on the surface of TEVs and tumor cells, many aptamers that target tumor cells can also be used for specific recognition studies of cell-derived exosomes. Moreover, DNA walker-based aptamer recognition can also be used to study the specific recognition of TEVs. For example, Wu et al developed a feasible and affordable methodology involving recognition of exosomes by single aptamer and a ratiometric DNA walker machine for ultrasensitive detection of TEVs in the urinary system and accurate classification of early urinary diseases.68 As illustrated in Figure 6A, this design introduces a cascade amplification strategy that combines DNA walking and rolling circle amplification (RCA) to construct a ratiometric 3D DNA machine based on AS-modified MNPs. uEV competitively binds to the CD63 aptamer, and with the assistance of Exo III, releases large amounts of walkers from the aptamer-walker hybrid complex. The released walkers trigger the 3D DNA machine to generate ratiometric fluorescence intensity as a signal output. This biosensor achieved a LOD of 9.9×103 particles/mL, exhibiting a linear range of 104−108 particles/mL.
 |
Figure 6 (A) Schematic representation of the EV detection and profiling EV proteins and multidisease classification based on single aptamer recognition and Exo III-assisted DNA machine. Reprinted from Wu N, Zhang X-Y, Xia J, Li X, Yang T, Wang J-H. Ratiometric 3D DNA machine combined with machine learning algorithm for ultrasensitive and high-precision screening of early urinary diseases. Acs Nano. 2021;15(12):19522–19534. Copyright © 2021 American Chemical Society.68 (B) Schematic representation of the tumor exosome detection based on two aptamer recognition and Exo III-assisted ratiometric electrochemical DNA walker. Reprinted from Zhao L, Sun R, He P, Zhang X. Ultrasensitive detection of exosomes by target-triggered three-dimensional DNA walking machine and exonuclease III-assisted electrochemical ratiometric biosensing. Anal Chem. 2019;91(22):14773–14779. Copyright © 2019 American Chemical Society.69 (C) Schematic representation of a homogeneous electrochemical sensing platform based on two aptamer recognition and DNAzyme walker for accurate exosomes detection. Reprinted from Sensors and Actuat B Chem, volume 404, Zhang M, Zhang T, Mei W, et al. A homogeneous electrochemical sensing platform based on DNAzyme walker for accurate detection of breast cancer exosomes. 135252, Copyright 2024, with permission from Elsevier.70 |
Using two nucleic acid aptamers that recognize TEVs can significantly improve the accuracy and sensitivity of TEV detection.98 For example, Zhao et al constructed a method using CD63 aptamer and EpCAM aptamer to recognize tumor exosomes and combined them with DNA walker to achieve an ultra-sensitive electrochemical detection method of tumor exosomes.69 As illustrated in Figure 6B, when exosomes are present, the binding of CD63 aptamer on the magnetic bead (MB) and the EpCAM aptamer on the swing arm helps the recognition process. Then, the Mg2+ cofactor triggers the catalytic activity of DNAzyme, resulting in the release of P1 strands. Hairpin-DNA with methylene blue modification at the 3’-end (MB-DNA) is bound to the gold electrode surface through AuS bonds, where mercaptohexanol (MCH) was used to block unbound sites. When the P1 strands are introduced, a blunt end is formed at the 3’-end due to the hybridization of P1 strands with the MB-DNA on the electrode. Then, digestion of Exo III is triggered, which results in the release of MB molecules and recycling of P1 strands. After cyclic shearing, a large number of short chains are left on the electrode, and these short chains can hybridize with ferrocene-modified Fc-DNA. Therefore, electrochemical ratiometric determination based on the decrease in the oxidation peak current of MB accompanied by the increase in the differential pulse voltammetry value of Fc was achieved. Under optimal conditions, this method achieved a LOD of 1.3×104 particles/mL with high selectivity. Another recent example, Zhang et al improved the accuracy of DNA walker detection of tumor exosomes through double recognition of MUC1 protein and HER2 protein in breast cancer exosomes.70 As illustrated in Figure 6C, the aptamer sensor induces enzymatic cleavage through double recognition, while the DNA walker’s continuous movement leads to the cleavage of a large number of substrate strands. This results in the release of large amounts of DNA fragments containing enzymes that catalyze the production of hydrogen peroxide (H2O2) by oxidizing excess glucose. The screen-printed carbon electrode (SPCE) is used to cause the redox reaction of H2O2, and the detection of exosomes can be achieved by reading the changes in the current signal. Under optimal conditions, this method achieved a LOD of 3.63×104 particles/mL with high accuracy.
DNA Walkers Based on Multiple Dimensional Tracks
To detect TEVs, we will introduce the use of 2D and 3D DNA walkers, which have greater potential for application compared to 1D DNA walkers due to their low processivity. This will result in improved signal amplification and more accurate bioanalysis.
Two-Dimensional (2D) DNA Walkers
2D DNA walkers have more mobility on the DNA track than 1D DNA walkers because of their planar structure. This technique is highly effective for detecting TEVs, as demonstrated by Feng et al who utilized 2D DNA walker to develop an innovative electrochemiluminescence (ECL) aptasensor.71 The purpose of this aptasensor was to enable the highly-sensitive detection of TEVs. As illustrated in Figure 7A, in this design, two types of DNA were covalently bonded-together on the surface of an electrode surface modified with RuSi NPs through amide bonds. Upon the addition of exosomes, the free swing arm that was removed from the double-stranded DNA by the conjugation of CD63 aptamer and CD63 protein on the exosome surface hybridized with anchor DNA and formed the cleavage site for Nb.BbvCI. Simultaneously, the released swing arm hybridized independently with another anchor DNA, generating a new cleavage site. This process triggered the continuous movement of the swing arm through the nicking reaction of Nb.BbvCI, resulting in the accumulation of numerous single-stranded DNAs on the electrode surface. Finally, a noticeable quenching effect on ECL reactions was obtained after the addition of glucose oxidase (GOD) and a Ru(bpy)32+-TPrA system. The LOD for this aptasensor was determined to be 60 particles/µL of exosomes. Similarly, Dong et al also described a methodological approach based on 2D DNA walker for the detection of tumor exosomes.57 As illustrated in Figure 7B, initially, the capture of exosomes was achieved using aptamer-magnetic bead bioconjugates, resulting in the release of three types of messenger DNAs (mDNAs). Following a magnetic separation, the mDNAs that were provided were able to form hybrid complexes with probe DNAs that were immobilized on a gold electrode. The outcome of the Exo III digestion led to the “turn off” of the electrochemical signal. The methodology successfully achieved a detection limit of up to 70 particles/µL under optimal conditions, which is below the LOD of most currently available methods.
 |
Figure 7 (A) Schematic representation of the 2D DNA walking machine for ECL detection of tumor exosomes. Reprinted from Feng QM, Ma P, Cao QH, Guo YH, Xu JJ. An aptamer-binding DNA walking machine for sensitive electrochemiluminescence detection of tumor exosomes. Chem Commun. 2019;56(2):269–272.71 (B) Schematic representation of the tumor exosome detection based on 2D DNA walker and Exo III-assisted amplification technology. Reprinted from Dong H, Chen H, Jiang J, Zhang H, Cai C, Shen Q. Highly sensitive electrochemical detection of tumor exosomes based on aptamer recognition-induced multi-DNA release and cyclic enzymatic amplification. Anal Chem. 2018;90(7):4507–4513. Copyright © 2018 American Chemical Society.57 (C) Schematic representation of the DNAzyme-assisted APCD biosensors based on 3D DNA walker for simultaneous detection of exosomal microRNAs. Reprinted from Zhang X, Wei X, Qi J, et al. Simultaneous detection of bladder cancer exosomal MicroRNAs based on inorganic nanoflare and DNAzyme walker. Anal Chem. 2022;94(11):4787–4793. Copyright © 2022 American Chemical Society.72 (D) Schematic representation of homogeneous electrochemical strategy for exosomal microRNA detection based on Pt−S bond-mediated 3D DNA nanomachine. Reprinted from Yang L, Guo H, Gao Q, et al. Integrating reliable Pt–S bond-mediated 3D DNA nanomachine with magnetic separation in a homogeneous electrochemical strategy for exosomal MicroRNA detection with low background and high sensitivity. Anal Chem. 2023;95(48):17834–17842. Copyright © 2023 American Chemical Society.73 (E) Schematic representation of self-serviced-track 3D DNA walker for sensitive and wash-free detection of tumor Exos via glycoprotein profiling. Reprinted from Wang H, Zeng J, Huang J, et al. A self‐serviced‐track 3D DNA walker for ultrasensitive detection of tumor exosomes by glycoprotein profiling. Angew Chem. 2022;134(19):1. © 2022 Wiley-VCH GmbH.74 |
Three-Dimensional (3D) DNA Walkers
Compared with 1D DNA walkers and 2D DNA walkers, 3D walkers can assemble more tracks on the surface and exhibit higher walking efficiency, which has now attracted the attention of more researchers. The following section describes the use of a 3D DNA walker for the detection of TEVs. For example, Zhang et al introduced a fluorescent biosensor based on 3D DNA walker that uses inorganic nanoflowers and walkers propelled by DNAzyme for simultaneous detection of microRNAs associated with tumor exosomes.72 As illustrated in Figure 7C, the biosensor was constructed using carbon dot (CD)-labelled substrates and DNAzyme strand-modified Au nanoparticles (AuNP) (referred to as APCD). Upon the presence of a target microRNA, the DNAzyme is activated, leading to cleavage of the CD-labelled substrate and subsequent movement along the AuNP, resulting in fluorescence recovery. The APCD biosensor exhibits high sensitivity and specificity, reaches the LOD of single microRNAs at femtomolar levels (10 fM), and has a broad linear range from 50 fM to 10 nM. The biosensor was also used for simultaneous analysis of bladder cancer-associated exosomal microRNA-133b and microRNA-135b in clinical serum samples, showing consistent results with qRT-PCR and suggesting its potential for diagnosing bladder cancer and other cancers. Also, Yang et al used Pt-S bond-mediated 3D DNA nanomachines in an electrochemical strategy to detect exosomal microRNA.73 As illustrated in Figure 7D and 3D3 DNA nanomachines were prepared by simultaneously anchoring protected DNA/walker DNA (PDNA/WDNA) and DNA1/DNA2 duplexes on the Fe3O4@Pt surface via Pt−S bonds. When the target and Exo III are present, target microRNA and WDNA are released and hybridize with another PDNA and DNA2, respectively, realizing the circulation of target DNA and walker DNA, ultimately leading to more single strands DNA1 existing on the surface of Fe3O4@Pt. To increase the detection signal, methylene blue (MB) was encapsulated in liposomes and functionalized with DNA3 to obtain MB-loaded liposome (liposome-MB-DNA3) nanocarriers. Therefore, in the presence of target microRNA, a large amount of liposome-MBDNA3 will be captured on the Fe3O4@Pt surface. After incubation with TritonX-100, a large number of MB molecules in the liposomes were released, resulting in a significant enhancement of the electrochemical signal, successfully achieving highly sensitive detection of exosomal microRNA-155 with a LOD was 0.28 fM. Moreover, Wang et al used target exosomes as a 3D platform to build a self-serviced-track DNA walker (STDW) for the wash-free detection of tumor exosomes.74 This detection method relied on the utilization of exosomal glycoprotein and was made possible by the implementation of split aptamer-recognition-initiated autonomous running. As illustrated in Figure 7E, the hairpin H1 and H2 are anchored to the exosome surface via cholesterol-lipid interactions. When probes a and b are added, targeting the exosome surface proteins with their cleaved aptamer sequences, the result is the aggregation and recombination of these two probes. This aggregation induces the cleaved CHA trigger sequence to come close and form the complete trigger sequence (T). Through toehold-mediated strand displacement, the T sequence then opens the adjacent hairpin H1, exposing a new toehold region. The resulting new toehold region continues to open the neighboring hairpin H2 strand, forming an intermediate hybridization product (T-H1-H2) through the process of strand migration. As the DNA strand displacement reaction continues, the subtable T-H1-H2 product eventually generates a thermodynamically stable double-stranded hybrid (H1-H2), in which the Cy3 of the hairpin H1 and the Cy5 of the hairpin H2 come into close proximity to each other, generating a fluorescence resonance energy transfer (FRET) signal. At the same time, the released T participates in the next cycle and generates a large number of FRET signals after several rounds of the “binding and competing” mode, ultimately realizing the sensitive detection of the target exosome. The LOD of the STDW was determined to be 1 particle/μL in buffer, owing to its advantageous characteristics of high selectivity and sensitivity.
DNA Walkers Based on Different Types of Driving Forces
Driving force is an indispensable part of DNA walker. And common driving force of DNA walker is introduced in detail above. This section mainly discusses DNA walkers driven by enzymatic reaction and strand displacement reaction for the detection of TEVs.
Enzymatic Based DNA Walkers
Enzyme based DNA walker methods exhibit enhanced efficiency and effectiveness in the detection of TEVs. For example, Pang et al developed a dual-SERS biosensor for the detection of microRNA-10b in exosomes.75 As illustrated in Figure 8A, the biosensor was assisted by duplex-specific nuclease (DSN) and utilized Fe3O4@Ag-DNA-Au@Ag@DTNB (SERS tag) conjugates. DSN is the abbreviation for thermostable nuclease. This enzyme can selectively degrade DNA in double-stranded DNA and DNA-RNA hybrids but has little effect on single-stranded nucleic acid molecules. In the presence of target microRNA, it can form a hybrid with the complementary DNA probes. DSN was then introduced to cleave the DNA probe of the DNA-microRNA duplex selectively. This causes the release of SERS tags from the Fe3O4@Ag composition, thus initiating a reduction in SERS intensity. Subsequently, the microRNA that has been released has the capability to enter the cycle and declutter other DNA and SERS tags. By virtue of the dual-SERS enhancement shown by the Fe3O4@Ag-SERS tag conjugates, together with the capability for signal amplification by recycling, it is possible to attain a detection limit of 1 aM. In addition, this capability holds promise for point-of-care clinical cancer diagnosis. Furthermore, Guo et al developed a regenerable electrochemical biosensor assisted by DNAzymes for exosomes detection.76 DNAzymes are specific DNA sequences that possess catalytic activity similar to nuclease, allowing them to cleave specific substrates in the presence of cofactors.99 As illustrated in Figure 8B, the biosensor employed two probes: one with cholesterol at the 5’ terminal for binding to the lipid bilayer of exosomes, and the other with CD63 aptamer at the 5’ terminal for specific recognition of the CD63 protein. The two probes exhibited 3’ terminal ends with a Pb2+-dependent DNAzyme tail sequence. In the presence of Pb2+, the DNAzyme that is reliant on Pb2+ exhibited a continuous cleavage of the track DNA, resulting in the release of intermediate DNA strands (T). Following this, a large number of T strands that are dependent on exosomes were generated. Utilizing the aforementioned T strands, the stem-loop structure of H-MB was initiated, resulting in a rod-like structure with double-stranded S:H-MB:T. This sequestered MB from the electrode surface resulted in a significant decrease in the current response (referred to as the “off” state), enabling the detection of exosomes. This method allows for the quantitative detection of exosomes in a broad concentration range, spanning from 5.0×104 to 1×108 particles/mL. Additionally, it achieves a LOD of 1.6×104 particles/mL.
 |
Figure 8 (A) Schematic representation of SERS based on DSN-assisted DNA walker for exosomal microRNA detection. Reprinted from Biosens Bioelectron, volume 130, Pang Y, Wang C, Lu L, Wang C, Sun Z, Xiao R. Dual-SERS biosensor for one-step detection of microRNAs in exosome and residual plasma of blood samples for diagnosing pancreatic cancer. 204–213, Copyright 2019, with permission from Elsevier.75 (B) Schematic representation of the DNAzyme-assisted DNA walker regenerable electrochemical biosensor for highly sensitive detection of tumor exosomes. Reprinted from Sensors and Actuat B Chem, volume 349, Guo Y, Liu S, Yang H, Wang P, Feng Q. Regenerable electrochemical biosensor for exosomes detection based on the dual-recognition proximity binding-induced DNA walker. 130765, Copyright 2021, with permission from Elsevier.76 (C) Schematic representation based on aptamer recognition powered by CHA for ultrasensitive detection of tumor exosomes. Reprinted from Wang H, Wan K, Zhou Y, et al. A three-dimensional multipedal DNA walker for the ultrasensitive detection of tumor exosomes. Chem Commun. 2020;56(85):12949–12952.77 (D) Schematic representation of the aptamer-recognized DNA molecular machines powered by EDC for the sensitive detection of tumor exosomes. Reprinted from Jin D, Peng -X-X, Qin Y, et al. Multivalence-actuated DNA nanomachines enable bicolor exosomal phenotyping and PD-L1-guided therapy monitoring. Anal Chem. 2020;92(14):9877–9886. Copyright © 2020 American Chemical Society.78 |
Strand Displacement Reactions Based DNA Walkers
To enhance the detection performance of TEVs, a promising approach, a combined detection involving DNA walkers and strand displacement reaction amplification technology, comes to the fore. As mentioned earlier, common strand displacement reactions include CHA, HCR and EDC. Currently, CHA-driven DNA walkers and EDC-driven DNA walkers are the most widely used for TEV detection.
For example, Wang et al demonstrated the construction of a CHA-driven DNA walker designed for ultrasensitive detection of tumor exosomes.77 As illustrated in Figure 8C, micro meter-scale sepharose beads (MB) are first captured by partial hybridization with anchored DNA (aDNA). MB is used here to capture a large number of PTK7 aptamers. After adding the target tumor-derived exosomes (EXs), a “spider”-like multipedal DNA walker was constructed through the stable binding between the PTK7 receptor and the PTK7 aptamer. This design enables the DNA walker to easily attach more DNA “legs” through aptamer recognition, eliminating the need for multiple isolation and washing steps, as well as tedious chemical modifications and subsequent release processes. In addition, powered by CHA, a multipedal DNA walker can activate directed autonomous motion, generating a large number of signaling molecules and providing a strong amplified signal. By harnessing the properties of the multipedal DNA walker, this method achieved ultrasensitive detection of exosomes with the LOD of 1 particle μL−1. It is approximately 66-fold more sensitive than non-catalyzed reactions. Furthermore, it establishes that this approach is exceeding practical and dependable for use in clinical diagnosis. As a low-background, high-efficiency DNA signal amplification technique, the EDC reaction has attracted much attention in the field of TEVs research. For example, Jin et al introduce a bioinspired exosome-activated DNA molecular machine (ExoADM) which utilizes EDC-driven multivalent cyclic amplification to facilitate the detection and phenotyping of circulating exosomes with exceptional sensitivity.78 As illustrated in Figure 8D, in this work, the initiator (purple sequence) is first enclosed by the CD63 aptamer (green sequence). When the target exosome is present, CD63 aptamer specifically recognizes CD63 protein and releases the initiator, which then initiates branch migration through two sticky ends (toehold 1 and toehold 2), and ultimately achieves signal amplification. The results showed that this self-supplied ExoADM has high detection sensitivity, with a detection limit of 33 particles/μL for the target. At the same time, ExoADM can be utilized for two-color phenotyping by integrating it with another DNA molecular machine, allowing for the concurrent monitoring of exosomal PD-L1 and CD-63. Additionally, the ExoADM method can effectively differentiate between cancer patients and healthy individuals.
DNA Walkers Based on Different Numbers of Legs
As mentioned earlier, DNA walkers can be classified into unipedal, bipedal and multipedal DNA walkers. In the following sections, we introduce these types of DNA walkers, which are useful for detecting TEVs.
Unipedal DNA Walkers
Unipedal DNA walkers are capable of walking with one leg (refers to the walking strand of DNA walker) and are widely used in TEV detection due to the simplest formation. For example, Guo et al constructed a multi-signal amplified electrochemical sensing platform for based on a unipedal DNA walker for the detection of tumor exosome.79 As illustrated in Figure 9A, in this method, exosomes are enriched on CD63 aptamer-functionalized magnetic beads and then recognized by the EpCAM aptamer. Next, the P chain reacts with the S:M:W complex, triggering entropy-driven DNA assembly and releasing a large number of signal transduction probes (W). Powered by the CHA, the DNA walker is activated, introducing a significant amount of electroactive methylene blue (MB) onto the electrode (GCE) surface. Thus, the detection of exosomes is translated into the measurement of MB currents with good linearity in the range of 100–75,000 particles μL−1 and the LOD was 40 particles/μL. In fact, considerable research efforts on single-foot walking devices have been devoted to the development from a 2D track to 3D track. For example, Yu et al developed a unipedal-3D DNA motor (it refers to DNA walker) powered by Nt.BbvCI.80 As illustrated in Figure 9B, the design involved a 3D DNA motor constructed with gold nanoparticle (GNP) tracks. These tracks contained substrate strands labeled with fluorescein and engine strands bound by aptamers and aptamer-bound motor strands. The motor strand, locked by the aptamer, would be unlocked upon recognition of the target protein on exosomes, initiating the activation of the DNA motor. Driven by Nt.BbvCI, the motor strands autonomously traversed the GNP track. During the operation of the DNA motor, DNA strands labeled with fluorescein were cleaved off, resulting in the restoration of fluorescence. The suggested method for the high-sensitivity detection of exosomes achieved a LOD of around 8.2 particles/μL with a wide dynamic range including five orders of magnitude.
 |
Figure 9 (A) Schematic representation of the stepwise preparation of the electrochemical biosensor based on unipedal DNA walkers for tumor exosome detection. Reprinted from Anal Chim Acta, volume 1135, Guo Y, Cao Q, Feng Q. Catalytic hairpin assembly-triggered DNA walker for electrochemical sensing of tumor exosomes sensitized with Ag@ C core-shell nanocomposites. 55–63, Copyright 2020, with permission from Elsevier.79 (B) Schematic illustration of the Nb.BbvCI-assisted unipedal DNA walkers for tumor exosome detection. Reprinted from Biosens Bioelectron, volume 167, Yu Y, Zhang WS, Guo Y, et al. Engineering of exosome-triggered enzyme-powered DNA motors for highly sensitive fluorescence detection of tumor-derived exosomes. 112482, Copyright 2020, with permission from Elsevier.80 (C) Schematic representation of the bipedal DNA walker-based ratiometric electrochemical biosensor for the detection of exosomal microRNA-21. Reprinted from Biosens Bioelectron, volume 102, Zhang J, Wang -L-L, Hou M-F, et al. A ratiometric electrochemical biosensor for the exosomal microRNAs detection based on bipedal DNA walkers propelled by locked nucleic acid modified toehold mediate strand displacement reaction. 33–40, copyright 2018, with permission from Elsevier.81 (D) Schematic representation of the multipedal DNA walking strategy based on Fe3O4@AuNPs for tumor exosome detection. Reprinted from Miao P, Tang Y. A multipedal DNA walker for amplified detection of tumor exosomes. Chem Commun. 2020;56(37):4982–4985.82 |
Bipedal and Multipedal DNA Walkers
Bipedal DNA walkers consist of two legs and multipedal DNA walkers consist of more free legs that can walk alternately. In theory, they have the potential to walk along DNA tracks for longer periods, resulting in higher signal amplification efficiency. For example, Zhang et al designed a biosensor employing a bipedal DNA walker to detect exosomal microRNA-21 from tumor exosomes at attomolar levels.81 As illustrated in Figure 9C, the bipedal DNA walker is released through a terminal-mediated strand displacement reaction when the target is present. The DNA walker driven by CHA then continuously walks along the surface of the electrode, resulting in the generation of a large number of H1-H2 duplexes. This leads to the close proximity of ferrocene (FC) and methylene blue (MB) to each other leading to the generation of a ratiometric electrochemical signal. The sensor demonstrated a notable level of sensitivity, as shown by a LOD of 67 aM when using microRNA-21 within the breast cancer cell-derived exosome as a target model. Also, researchers have studied many forms of multipedal DNA walkers. For example, Miao et al have developed a highly sensitive and selective method based on multipedal DNA walkers for detecting tumor exosomes.82 As illustrated in Figure 9D, Fe3O4@AuNPs (a synthetic nanomaterial) were subjected to modification using probe A, which contains CD63 aptamer for the purpose of capturing exosomes. Subsequently, the walker chain of probe B, which also included the CD63 aptamer sequence, was introduced to facilitate the specific binding with the exosomes. Upon the introduction of probe C and Nb.BbvCI nicking endonuclease (NEase), probe A underwent cleavage and was released as a linker between exosomes and Fe3O4@AuNPs. Simultaneously, probe C was liberated to aid in the cleavage of probe A catalyzed by NEase. The surface of the electrode was subjected to functionalization using probe D which was modified with MB at 5’ end. The probes B, D and E were designed with partial complementary sequences, enabling hybridization and formation of three-way junction structures between the exosomes and the electrode. Ultimately, a substantial quantity of probe D was released from the electrode surface via NEase-catalyzed digestion, resulting in a substantial attenuation of MB’s electrochemical signal. The quantification of exosomes was analyzed in the linear range of 10 to 2000 particles/mL, with a LOD of 6 particles/mL.
Other DNA Walkers
With the development of DNA walker, its use in TEV detection is constantly being updated, and many novel, stable, and excellent-sensitivity DNA walker biosensors have surfaced. For example, Guo et al proposed a strategy for exosome detection based on intramolecular electrochemiluminescence resonance energy transfer (ECL-RET) and DNA tetrahedral nanostructure (DTN)-corbelled DNA walker.83 As illustrated in Figure 10A, the design based on the combination of Zr12-adb nanoplates (NPs) and Ru(bpy)32+ into one nanostructure with high ECL-RET efficiency significantly enhances the ECL response. When target exosomes are introduced, they can bind to aptamers on DTNs, and then the swing arms are released and hybridize to the orbital strands. The formed Mg2+-dependent DNAzyme promotes the cleavage of orbital DNA, releases short DNA fragments labeled with Fc, and achieves the recovery of ECL intensity, thereby enabling the detection of exosomes. Impressively, the DNA walker with six orbital strands supported by DTN exhibits enhanced walking speed and high response efficiency, and the LOD was 185 particles/μL for the biosensor, exhibiting excellent sensing performance for exosome detection. In addition, Chen et al designed an endogenous gated DNA walker (E-DNA walker) nanoprobe for precise detection of TEV microRNAs while enabling imaging of sensitizing microRNAs in TEVs.84 As illustrated in Figure 10B, it prepares E-DNA walker nanoprobes by affixing fluorophore (FAM)-labeled chimeric substrates and DNAzyme preprobe (E-Dz) onto AuNPs. First, it needs to be mentioned that APE1 is an important DNA repair enzyme that is reported to be secreted into the TEVs, targeting and cleaving the phosphodiester bond of the flipped AP site.100,101 This design utilizes endogenous APE1 as an endogenous activator. Upon entry into the TEVs, secreted APE1 cleaves the short-stranded fragment, and the short-stranded fragment of the cleavage is then dissociated from the parental E-Dz preprobe, followed by the formation of an accessible DNAzyme probe (T-Dz). After the catalytic core of the T-Dz probe is split into two parts by introducing a target-binding sequence, it is hybridized with microRNA, the split T-Dz is linked into an active conformation, and the chimeric substrate is cleaved with the help of the cofactor Mg2+. Afterwards, the FAM-labeled fragment was detached from the walking system and the fluorescence was restored, thus achieving the LOD of the microRNA-21 is 0.57pM. It is worth mentioning that the strict dual activation of this experiment ensures the accurate identification of TEVs.
 |
Figure 10 (A) Schematic representation of tumor exosome detection using DNA tetrahedral nanostructure-corbelled DNA walker. Reprinted from Sensors and Actuat B Chem, volume 410, Guo L, Zhou Y, Feng Q, Yin H. DNA tetrahedral nanostructure-corbelled DNA walker for intramolecular electrochemiluminescence resonance energy transfer sensing of tumor exosomes. 135735, copyright 2022, with permission from Elsevier.83 (B) Schematic representation of prescreening microRNA detection in EVs based on endogenous gated DNA walker. Reprinted from Chen W, Sun J, Mao Y, Tang Y, Wang J, Liu Z. Endogenously Gated DNA walking machine for prescreened MicroRNA detection in extracellular vesicles. Anal Chem. 2024;2004:1. Copyright © 2024 American Chemical Society.84 |
Conclusions and Perspectives
This review extensively covers the introduction of EVs, their association with tumors, and highlights the utility of DNA walkers in detecting TEVs. DNA walkers, known for their programmability and versatility, provide a powerful tool for sensitive TEV detection. It could help TEVs with liquid biopsies while providing a bright path for clinical detection and monitoring of cancer. Despite the existence of potential, challenges still persist, particularly in detecting low levels of TEVs at early cancer stages and addressing heterogeneity within populations of TEVs. To improve DNA walker design for enhanced detection, focus on: (1) Enhancing recognition using multiple aptamers to address heterogeneity of TEVs. (2) Improving accuracy and walking efficiency by refining nanostructures, more walking legs, or optimizing driving force. (3) Integrating DNA walkers with portable devices like thermal readers and smartphones for user-friendly, fast, and real-time clinical testing.
The integration of tumor diagnostic techniques using TEVs into clinical practice is not yet mature and still has a long way to go. Several issues still require consideration: (1) Current limitations in molecular protein profiling diversity hinder its use as a guide in routine clinical practice. (2) Understanding composition networks like proteins and RNAs within TEVs is crucial for identifying related functional mechanisms. (3) The role of TEVs as primitive particles in generating the first protocell remains uncertain. (4) Faster, more sensitive TEV detection methods and standardization are essential for seamless integration into clinical diagnostic workflows. Therefore, machine learning integrated with clinical sample quantification is crucial for analyzing a large number of clinical samples in cancer research. It simplifies vast TEVs databases to accelerate data analysis, leading to a deeper understanding of TEVs variations. Utilizing machine learning models trained on vast TEVs data can lead to more precise predictions in clinical settings, potentially becoming a dominant trend in the future.
In summary, we expect advancements in TEV detection research and look forward to new breakthroughs in DNA walker from principle to the application of TEVs.
Funding
This work was supported by the Hunan Provincial Natural Science Foundation (Grant No. 2024JJ8237) and Key R&D Plan of Hunan Provincial Department of Science and Technology (Grant No. 2023SK2046) and Opening Funding of Traditional Chinese Medicine and Ethnomedicine Innovation & Development International Laboratory (Grant No. 2022GJSYS07) and National Natural Science Foundation of China (Grant No. 82304890) and the Changsha Natural Science Foundation (Grant No. Kq2208193).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Christianson HC, Svensson KJ, Van Kuppevelt TH, Li J-P, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci. 2013;110(43):17380–17385. doi:10.1073/pnas.1304266110
2. Krishnan SKSG, Greening D, Rai A, et al. Extracellular vesicles: their role in cancer biology and epithelial-mesenchymal transition. European Disability Forum. 2017;474(1):21–45.
3. Song L, Zhuge Y, Zuo X, Li M, Wang F. DNA walkers for biosensing development. Adv Sci. 2022;9(18):2200327. doi:10.1002/advs.202200327
4. Xu M, Tang D. Recent advances in DNA walker machines and their applications coupled with signal amplification strategies: a critical review. Anal Chim Acta. 2021;1171:338523. doi:10.1016/j.aca.2021.338523
5. Ferguson S, Yang KS, Weissleder R. Single extracellular vesicle analysis for early cancer detection. Trends Mol Med. 2022;28(8):681–692. doi:10.1016/j.molmed.2022.05.003
6. Zhang Q, Wang H, Liu Q, et al. Exosomes as powerful biomarkers in cancer: recent advances in isolation and detection techniques. Int j Nanomed. 2024;19:1923–1949. doi:10.2147/IJN.S453545
7. Welsh JA, Goberdhan DC, O’Driscoll L, et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J Extracell Vesicles. 2024;13(2):e12404. doi:10.1002/jev2.12404
8. Marar C, Starich B, Wirtz D. Extracellular vesicles in immunomodulation and tumor progression. Nat Immunol. 2021;22(5):560–570. doi:10.1038/s41590-021-00899-0
9. Khan M, Nickoloff E, Abramova T, et al. Embryonic stem cell–derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circulat Res. 2015;117(1):52–64. doi:10.1161/CIRCRESAHA.117.305990
10. Teng F, Fussenegger M. Shedding light on extracellular vesicle biogenesis and bioengineering. Adv Sci. 2021;8(1):2003505. doi:10.1002/advs.202003505
11. Van Niel G, d’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. doi:10.1038/nrm.2017.125
12. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289.
13. Lötvall J, Hill AF, Hochberg F, et al. Minimal Experimental Requirements for Definition of Extracellular Vesicles and Their Functions: A Position Statement from the International Society for Extracellular Vesicles. Taylor & Francis; 2014:26913.
14. Mohankumar S, Patel T. Extracellular vesicle long noncoding RNA as potential biomarkers of liver cancer. Brief Funct Genom. 2016;15(3):249–256. doi:10.1093/bfgp/elv058
15. Chitti SV, Gummadi S, Kang T, et al. Vesiclepedia 2024: an extracellular vesicles and extracellular particles repository. Nucleic Acids Res. 2024;52(D1):D1694–D1698. doi:10.1093/nar/gkad1007
16. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi:10.1038/ncb1596
17. Skog J, Würdinger T, Van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi:10.1038/ncb1800
18. Shi M, Jiang Y, Yang L, Yan S, Wang YG, Lu XJ. Decreased levels of serum exosomal miR‐638 predict poor prognosis in hepatocellular carcinoma. J Cell Biochem. 2018;119(6):4711–4716. doi:10.1002/jcb.26650
19. Paramanantham A, Asfiya R, Das S, McCully G, Srivastava A. Extracellular Vesicle (EVs) associated non-coding RNAs in lung cancer and therapeutics. Int J Mol Sci. 2022;23(21):13637. doi:10.3390/ijms232113637
20. Maji S, Chaudhary P, Akopova I, et al. Exosomal annexin II promotes angiogenesis and breast cancer metastasis. Mol Cancer Res. 2017;15(1):93–105. doi:10.1158/1541-7786.MCR-16-0163
21. Sandfeld-Paulsen B, Aggerholm-Pedersen N, Baek R, et al. Exosomal proteins as prognostic biomarkers in non-small cell lung cancer. Mol Oncol. 2016;10(10):1595–1602. doi:10.1016/j.molonc.2016.10.003
22. Li C, Liu D-R, G-G L, et al. CD97 promotes gastric cancer cell proliferation and invasion through exosome-mediated MAPK signaling pathway. World J Gastroenterol. 2015;21(20):6215. doi:10.3748/wjg.v21.i20.6215
23. Baran J, Baj-Krzyworzeka M, Weglarczyk K, et al. Circulating tumour-derived microvesicles in plasma of gastric cancer patients. Cancer Immunol Immunother. 2010;59:841–850. doi:10.1007/s00262-009-0808-2
24. Lau CS, Wong DT. Breast cancer exosome-like microvesicles and salivary gland cells interplay alters salivary gland cell-derived exosome-like microvesicles in vitro. PLoS One. 2012;7(3):e33037. doi:10.1371/journal.pone.0033037
25. Salmond N, Williams KC. Isolation and characterization of extracellular vesicles for clinical applications in cancer–time for standardization? Nanoscale Adv. 2021;3(7):1830–1852. doi:10.1039/D0NA00676A
26. Kanada M, Bachmann MH, Contag CH. Signaling by extracellular vesicles advances cancer hallmarks. Trends Cancer. 2016;2(2):84–94. doi:10.1016/j.trecan.2015.12.005
27. Yang N, Li S, Li G, et al. The role of extracellular vesicles in mediating progression, metastasis and potential treatment of hepatocellular carcinoma. Oncotarget. 2017;8(2):3683. doi:10.18632/oncotarget.12465
28. Wang M, Graner A, Knowles B, et al. A tale of two tumors: differential, but detrimental, effects of glioblastoma extracellular vesicles (EVs) on normal human brain cells. bioRxiv;2024.
29. Zhang H, Deng T, Liu R, et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat Commun. 2017;8(1):15016. doi:10.1038/ncomms15016
30. Dd Y, Wu Y, Shen H, et al. Exosomes in development, metastasis and drug resistance of breast cancer. Cancer Sci. 2015;106(8):959–964. doi:10.1111/cas.12715
31. Wolfers J, Lozier A, Raposo G, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nature Med. 2001;7(3):297–303. doi:10.1038/85438
32. Melo SA, Luecke LB, Kahlert C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523(7559):177–182. doi:10.1038/nature14581
33. Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–386. doi:10.1038/s41586-018-0392-8
34. Wang Z-G, Elbaz J, Willner I. DNA machines: bipedal walker and stepper. Nano Lett. 2011;11(1):304–309. doi:10.1021/nl104088s
35. Mason SD, Tang Y, Li Y, Xie X, Li F. Emerging bioanalytical applications of DNA walkers. TrAC Trend Analy Chem. 2018;107:212–221. doi:10.1016/j.trac.2018.08.015
36. Ma H, Chen L, Lv J, Yan X, Li Y, Xu G. The rate-limiting procedure of 3D DNA walkers and their applications in tandem technology. Chem Commun. 2023;59(69):10330–10342. doi:10.1039/D3CC02597G
37. Ma Y, Chen R, Zhang R, Liang J, Ren S, Gao Z. Application of DNA‐fueled molecular machines in food safety testing. Compr Rev Food Sci Food Saf. 2024;23(1):e13299. doi:10.1111/1541-4337.13299
38. Dai Y, Furst A, Liu CC. Strand displacement strategies for biosensor applications. Trends Biotechnol. 2019;37(12):1367–1382. doi:10.1016/j.tibtech.2019.10.001
39. Cha T-G, Pan J, Chen H, et al. A synthetic DNA motor that transports nanoparticles along carbon nanotubes. Nature Nanotechnol. 2014;9(1):39–43. doi:10.1038/nnano.2013.257
40. Yang Y, Goetzfried MA, Hidaka K, et al. Direct visualization of walking motions of photocontrolled nanomachine on the DNA nanostructure. Nano Lett. 2015;15(10):6672–6676. doi:10.1021/acs.nanolett.5b02502
41. Jung C, Allen PB, Ellington AD. A stochastic DNA walker that traverses a microparticle surface. Nature Nanotechnol. 2016;11(2):157–163. doi:10.1038/nnano.2015.246
42. Qi Y, Zhai Y, Fan W, Ren W, Li Z, Liu C. Click chemistry-actuated digital DNA walker confined on a single particle toward absolute microRNA quantification. Anal Chem. 2020;93(3):1620–1626. doi:10.1021/acs.analchem.0c04073
43. Bath J, Green S, Turberfield A. A free-running DNA motor powered by a nicking enzyme. Angew Chem. 2005;44(28):4358–4361. doi:10.1002/anie.200501262
44. Wang X, Meng J, Zhang H, et al. Electrochemical biosensor based on bipedal DNA walker for highly sensitive amplification detection of apurinic/apyrimidinic endonuclease 1. Sensors and Actuat B Chem. 2023;381:133425. doi:10.1016/j.snb.2023.133425
45. Miao P, Tang Y. Gold nanoparticles-based multipedal DNA walker for ratiometric detection of circulating tumor cell. Anal Chem. 2019;91(23):15187–15192. doi:10.1021/acs.analchem.9b04000
46. Qu X, Zhu D, Yao G, et al. An exonuclease III‐powered, on‐particle stochastic DNA walker. Angew Chem. 2017;129(7):1881–1884. doi:10.1002/ange.201611777
47. Yang J, Dou B, Yuan R, Xiang Y. Aptamer/protein proximity binding-triggered molecular machine for amplified electrochemical sensing of thrombin. Anal Chem. 2017;89(9):5138–5143. doi:10.1021/acs.analchem.7b00827
48. Yao D, Bhadra S, Xiong E, Liang H, Ellington AD, Jung C. Dynamic programming of a DNA walker controlled by protons. ACS nano. 2020;14(4):4007–4013. doi:10.1021/acsnano.9b08166
49. Dong JX, Zhang SM, Li YL, et al. Photocontrollable DNA walker-based molecular circuit for the tunable detection of microRNA-21 using metal–organic frameworks as label-free fluorescence tags. Anal Chem. 2023;95(45):16744–16753. doi:10.1021/acs.analchem.3c03913
50. Guo Y, Tang Y, Tan Y, Li Y, Xiang Y. Nanomaterials for fluorescent detection of hemoglobin. Crit Rev Anal Chem. 2024;2024:1–15.
51. Chen Y, Yan X, Yang W, et al. A signal transduction approach for multiplexed detection of transcription factors by integrating DNA nanotechnology, multi-channeled isothermal amplification, and chromatography. J Chromatogr A. 2020;1624:461148. doi:10.1016/j.chroma.2020.461148
52. Sherman WB, Seeman NC. A precisely controlled DNA biped walking device. Nano Lett. 2004;4(7):1203–1207. doi:10.1021/nl049527q
53. Shin J-S, Pierce NA. A synthetic DNA walker for molecular transport. J Am Chem Soc. 2004;126(35):10834–10835. doi:10.1021/ja047543j
54. Wang L, Deng R, Li J. Target-fueled DNA walker for highly selective miRNA detection. Chem Sci. 2015;6(12):6777–6782. doi:10.1039/C5SC02784E
55. Xu Z, Dong Y, Li J, Yuan R. A ferrocene-switched electrochemiluminescence “off–on” strategy for the sensitive detection of cardiac troponin I based on target transduction and a DNA walking machine. Chem Commun. 2015;51(76):14369–14372. doi:10.1039/C5CC04745E
56. Qing M, Xie S, Cai W, et al. Click chemistry reaction-triggered 3D DNA walking machine for sensitive electrochemical detection of copper ion. Anal Chem. 2018;90(19):11439–11445. doi:10.1021/acs.analchem.8b02555
57. Dong H, Chen H, Jiang J, Zhang H, Cai C, Shen Q. Highly sensitive electrochemical detection of tumor exosomes based on aptamer recognition-induced multi-DNA release and cyclic enzymatic amplification. Anal Chem. 2018;90(7):4507–4513. doi:10.1021/acs.analchem.7b04863
58. Li Y, Liu H, Huang H, et al. A sensitive electrochemical strategy via multiple amplification reactions for the detection of E. coli O157: H7. Biosens Bioelectron. 2020;147:111752. doi:10.1016/j.bios.2019.111752
59. Kowal EJ, Ter-Ovanesyan D, Regev A, Church GM. Extracellular vesicle isolation and analysis by Western blotting. Extracell Vesicl. 2017;2017:143–152.
60. Coughlan C, Bruce KD, Burgy O, et al. Exosome isolation by ultracentrifugation and precipitation and techniques for downstream analyses. Current Protoc Cell Biol. 2020;88(1):e110. doi:10.1002/cpcb.110
61. Nolan JP, Jones JC. Detection of platelet vesicles by flow cytometry. Platelets. 2017;28(3):256–262. doi:10.1080/09537104.2017.1280602
62. Goetzl EJ, Schwartz JB, Abner EL, Jicha GA, Kapogiannis D. High complement levels in astrocyte‐derived exosomes of Alzheimer disease. Ann Neurol. 2018;83(3):544–552. doi:10.1002/ana.25172
63. Huang Z, Lin Q, Yang B, et al. Cascade signal amplification for sensitive detection of exosomes by integrating tyramide and surface-initiated enzymatic polymerization. Chem Commun. 2020;56(84):12793–12796. doi:10.1039/D0CC04881J
64. Gu C, Bai L, Pu L, Gai P, Li F. Highly sensitive and stable self-powered biosensing for exosomes based on dual metal-organic frameworks nanocarriers. Biosens Bioelectron. 2021;176:112907. doi:10.1016/j.bios.2020.112907
65. Liu X, Gao X, Yang L, Zhao Y, Li F. Metal–organic framework-functionalized paper-based electrochemical biosensor for ultrasensitive exosome assay. Anal Chem. 2021;93(34):11792–11799. doi:10.1021/acs.analchem.1c02286
66. Cao Y, Li L, Han B, Wang Y, Dai Y, Zhao J. A catalytic molecule machine-driven biosensing method for amplified electrochemical detection of exosomes. Biosens Bioelectron. 2019;141:111397. doi:10.1016/j.bios.2019.111397
67. Ding L, Wu Y, L-e L, et al. Universal DNAzyme walkers-triggered CRISPR-Cas12a/Cas13a bioassay for the synchronous detection of two exosomal proteins and its application in intelligent diagnosis of cancer. Biosens Bioelectron. 2023;219:114827. doi:10.1016/j.bios.2022.114827
68. Wu N, Zhang X-Y, Xia J, Li X, Yang T, Wang J-H. Ratiometric 3D DNA machine combined with machine learning algorithm for ultrasensitive and high-precision screening of early urinary diseases. Acs Nano. 2021;15(12):19522–19534. doi:10.1021/acsnano.1c06429
69. Zhao L, Sun R, He P, Zhang X. Ultrasensitive detection of exosomes by target-triggered three-dimensional DNA walking machine and exonuclease III-assisted electrochemical ratiometric biosensing. Anal Chem. 2019;91(22):14773–14779. doi:10.1021/acs.analchem.9b04282
70. Zhang M, Zhang T, Mei W, et al. A homogeneous electrochemical sensing platform based on DNAzyme walker for accurate detection of breast cancer exosomes. Sensors and Actuat B Chem. 2024;404:135252. doi:10.1016/j.snb.2023.135252
71. Feng Q-M, Ma P, Cao Q-H, Guo Y-H, J-J X. An aptamer-binding DNA walking machine for sensitive electrochemiluminescence detection of tumor exosomes. Chem Commun. 2019;56(2):269–272. doi:10.1039/C9CC08051A
72. Zhang X, Wei X, Qi J, et al. Simultaneous detection of bladder cancer exosomal MicroRNAs based on inorganic nanoflare and DNAzyme walker. Anal Chem. 2022;94(11):4787–4793. doi:10.1021/acs.analchem.1c05588
73. Yang L, Guo H, Gao Q, et al. Integrating reliable Pt–S bond-mediated 3D DNA nanomachine with magnetic separation in a homogeneous electrochemical strategy for exosomal MicroRNA detection with low background and high sensitivity. Anal Chem. 2023;95(48):17834–17842. doi:10.1021/acs.analchem.3c03914
74. Wang H, Zeng J, Huang J, et al. A self‐serviced‐track 3D DNA walker for ultrasensitive detection of tumor exosomes by glycoprotein profiling. Angew Chem. 2022;134(19):1.
75. Pang Y, Wang C, Lu L, Wang C, Sun Z, Xiao R. Dual-SERS biosensor for one-step detection of microRNAs in exosome and residual plasma of blood samples for diagnosing pancreatic cancer. Biosens Bioelectron. 2019;130:204–213. doi:10.1016/j.bios.2019.01.039
76. Guo Y, Liu S, Yang H, Wang P, Feng Q. Regenerable electrochemical biosensor for exosomes detection based on the dual-recognition proximity binding-induced DNA walker. Sensors and Actuat B Chem. 2021;349:130765. doi:10.1016/j.snb.2021.130765
77. Wang H, Wan K, Zhou Y, et al. A three-dimensional multipedal DNA walker for the ultrasensitive detection of tumor exosomes. Chem Commun. 2020;56(85):12949–12952. doi:10.1039/D0CC04360E
78. Jin D, Peng -X-X, Qin Y, et al. Multivalence-actuated DNA nanomachines enable bicolor exosomal phenotyping and PD-L1-guided therapy monitoring. Anal Chem. 2020;92(14):9877–9886. doi:10.1021/acs.analchem.0c01387
79. Guo Y, Cao Q, Feng Q. Catalytic hairpin assembly-triggered DNA walker for electrochemical sensing of tumor exosomes sensitized with Ag@ C core-shell nanocomposites. Anal Chim Acta. 2020;1135:55–63. doi:10.1016/j.aca.2020.08.036
80. Yu Y, Zhang WS, Guo Y, et al. Engineering of exosome-triggered enzyme-powered DNA motors for highly sensitive fluorescence detection of tumor-derived exosomes. Biosens Bioelectron. 2020;167:112482. doi:10.1016/j.bios.2020.112482
81. Zhang J, Wang -L-L, Hou M-F, et al. A ratiometric electrochemical biosensor for the exosomal microRNAs detection based on bipedal DNA walkers propelled by locked nucleic acid modified toehold mediate strand displacement reaction. Biosens Bioelectron. 2018;102:33–40. doi:10.1016/j.bios.2017.10.050
82. Miao P, Tang Y. A multipedal DNA walker for amplified detection of tumor exosomes. Chem Commun. 2020;56(37):4982–4985. doi:10.1039/D0CC01817A
83. Guo L, Zhou Y, Feng Q, Yin H. DNA tetrahedral nanostructure-corbelled DNA walker for intramolecular electrochemiluminescence resonance energy transfer sensing of tumor exosomes. Sensors and Actuat B Chem. 2024;410:135735. doi:10.1016/j.snb.2024.135735
84. Chen W, Sun J, Mao Y, Tang Y, Wang J, Liu Z. Endogenously Gated DNA walking machine for prescreened MicroRNA detection in extracellular vesicles. Anal Chem. 2024;2004:1.
85. Logozzi M, De Milito A, Lugini L, et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One. 2009;4(4):e5219. doi:10.1371/journal.pone.0005219
86. Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Bioch et Biophy Acta. 2012;1820(7):940–948. doi:10.1016/j.bbagen.2012.03.017
87. Liu J, Zhan Z, Liang T, Xie G, Aguilar ZP, Xu H. Dual-signal amplification strategy: universal asymmetric tailing-PCR triggered rolling circle amplification assay for fluorescent detection of Cronobacter spp. in milk. Journal of Dairy Science. 2020;103(4):3055–3065. doi:10.3168/jds.2019-17590
88. Zhao M, Y-P L, Geng X-R, et al. Expression level of MiRNA-126 in serum exosomes of allergic asthma patients and lung tissues of asthmatic mice. Curr Drug Metabol. 2019;20(10):799–803. doi:10.2174/1389200220666191011114452
89. He F, Liu H, Guo X, Yin B-C, Ye B-C. Direct exosome quantification via bivalent-cholesterol-labeled DNA anchor for signal amplification. Anal Chem. 2017;89(23):12968–12975. doi:10.1021/acs.analchem.7b03919
90. Vaidyanathan R, Naghibosadat M, Rauf S, et al. Detecting exosomes specifically: a multiplexed device based on alternating current electrohydrodynamic induced nanoshearing. Anal Chem. 2014;86(22):11125–11132. doi:10.1021/ac502082b
91. Zong S, Wang L, Chen C, et al. Facile detection of tumor-derived exosomes using magnetic nanobeads and SERS nanoprobes. Anal. Methods. 2016;8(25):5001–5008. doi:10.1039/C6AY00406G
92. Kang Y-T, Kim YJ, Bu J, Cho Y-H, Han S-W, Moon B-I. High-purity capture and release of circulating exosomes using an exosome-specific dual-patterned immunofiltration (ExoDIF) device. Nanoscale. 2017;9(36):13495–13505. doi:10.1039/C7NR04557C
93. Zhang P, He M, Zeng Y. Ultrasensitive microfluidic analysis of circulating exosomes using a nanostructured graphene oxide/polydopamine coating. Lab on Chip. 2016;16(16):3033–3042. doi:10.1039/C6LC00279J
94. Im H, Shao H, Park YI, et al. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nature Biotechnol. 2014;32(5):490–495. doi:10.1038/nbt.2886
95. Rahbari M, Rahbari N, Reissfelder C, Weitz J, Kahlert C. Exosomes: novel implications in diagnosis and treatment of gastrointestinal cancer. Langenbecks Arch Surg. 2016;401:1097–1110. doi:10.1007/s00423-016-1468-2
96. Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. science. 1990;249(4968):505–510. doi:10.1126/science.2200121
97. Fang X, Tan W. Aptamers generated from cell-SELEX for molecular medicine: a chemical biology approach. Acc Chem Res. 2010;43(1):48–57. doi:10.1021/ar900101s
98. Yang L, Yin X, An B, Li F. Precise capture and direct quantification of tumor exosomes via a highly efficient dual-aptamer recognition-assisted ratiometric immobilization-free electrochemical strategy. Anal Chem. 2020;93(3):1709–1716. doi:10.1021/acs.analchem.0c04308
99. Gong L, Zhao Z, Y-F L, et al. DNAzyme-based biosensors and nanodevices. Chem Commun. 2015;51(6):979–995. doi:10.1039/C4CC06855F
100. Liu T-C, Lin C-T, Chang K-C, et al. APE1 distinguishes DNA substrates in exonucleolytic cleavage by induced space-filling. Nat Commun. 2021;12(1):601. doi:10.1038/s41467-020-20853-2
101. Mangiapane G, Parolini I, Conte K, et al. Enzymatically active apurinic/apyrimidinic endodeoxyribonuclease 1 is released by mammalian cells through exosomes. J Biol Chem. 2021;2021:296.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
