Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Real-World Clinical Effectiveness of Glucagon-Like Peptide-1 Receptor Agonist on Mild-to-Moderate Diabetic Kidney Disease in Patients with Type 2 Diabetes: A Retrospective, Single-Arm Clinical Trial
Authors Cao Y , Zhao J , Ma Y , Cao S , Liu Y
Received 9 April 2024
Accepted for publication 26 July 2024
Published 1 August 2024 Volume 2024:17 Pages 2913—2921
DOI https://doi.org/10.2147/DMSO.S472968
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Yongsheng Cao,1 Jianqin Zhao,2 Yanjuan Ma,2 Shujie Cao,2 Ying Liu2
1Department of Neurology, Sunshine Union Hospital, Weifang, Shandong, People’s Republic of China; 2Department of Endocrinology, Sunshine Union Hospital, Weifang, Shandong, People’s Republic of China
Correspondence: Ying Liu, Department of Endocrinology, Sunshine Union Hospital, 9000 Yingqian Street, Weifang City, Shandong, 261000, People’s Republic of China, Email [email protected]
Background: Cardiovascular outcome trials indicate renal benefits of glucagon-like peptide-1 receptor agonists (GLP-1RAs); however, real-world efficacy and safety studies in Diabetic kidney disease (DKD) are scarce.
Methods: This retrospective, single-arm real-world trial involved adults with DKD treated with GLP-1RA for at least 6 months. The primary endpoint was hemoglobin A1c (HbA1c) levels after 6 months.
Results: This study included a total of 364 patients with DKD, 153 (42.0%) of whom were female. The median disease duration was 8.0 years, and the mean values of age, HbA1c level, body mass index, and the urinary albumin-to-creatinine ratio (UACR) were 52.1 years, 8.6%, 27.8 kg/m2, and 88.0 mg/g, respectively. Additionally, 73.6% and 26.4% of patients had mild and moderate DKD, respectively. Following 6 months of GLP-1RA treatment, the mean HbA1c level and UACR declined by 1.77% and 40.3%, respectively (both p < 0.001). Compared to their baseline values, patients exhibited significant improvements in 24-h urinary protein, estimated glomerular filtration rate (eGFR), fasting blood glucose, body weight, systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol, triglycerides, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol (all p < 0.001). Patients with a disease duration of < 10 years had more pronounced changes in the HbA1c level, UACR, and eGFR (all p < 0.001) than those with a disease duration of ≥ 10 years. Changes in SBP and DBP were more pronounced in patients also taking angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers (ACEis/ARBs) than in those not taking ACEis/ARBs, whereas the changes in UACR and eGFR did not significantly differ.
Conclusion: Six-month GLP-1RA treatment improves glucose, blood pressure, lipids, and body weight in patients with mild-to-moderate DKD while slowing down kidney disease progression. It independently reduces proteinuria beyond ACEi/ARB impact, with early use yielding faster outcomes, supporting evidence-based practice.
Keywords: diabetic kidney disease, type 2 diabetes, GLP-1RA, HbA1C, UACR
Introduction
According to the 2021 International Diabetes Federation Diabetes Atlas, 537 million adults aged 20–79 years, representing 10.5% of the global population in that age group, are currently living with diabetes. Diabetes is a major cause of death worldwide, and in 2021, an estimated 6.7 million adults aged 20–79 years died of diabetes or related complications.1 Diabetic kidney disease (DKD) is a chronic kidney disease secondary to diabetes and one of the most serious complications of diabetes.2 Approximately 30–40% of patients with diabetes have concomitant DKD,3 which is a major cause of end-stage renal disease (ESRD),4 with 30–50% of ESRD cases worldwide being caused by DKD.5
Currently, prevention and treatment of DKD focuses on glycemic and blood pressure control. Widely used renoprotective agents include renin-angiotensin-aldosterone system inhibitors, such as angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers (ACEis/ARBs). However, despite the availability of these treatments, disease progression in DKD is inevitable, and novel therapies are urgently needed.6 The new classes of glucose-lowering agents, including sodium-glucose transporter 2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP-1RAs), are believed to have renoprotective effects independent of their glucose-lowering effects.7 In addition, finerenone, a non-steroidal mineralocorticoid receptor antagonist, has been shown to reduce the urinary albumin-to-creatinine ratio (UACR) and improve DKD progression and heart disease associated with DKD.8
GLP-1RAs exert their glucose-lowering effects through multiple mechanisms, such as stimulating insulin secretion from pancreatic β-cells, inhibiting glucagon secretion from pancreatic α-cells, suppressing appetite, and delaying gastric emptying.9 In addition, GLP-1RA has antihypertensive, weight-lowering, and lipid-modifying effects.10 Several cardiovascular outcome trials have demonstrated renal benefits of GLP-1RA in addition to cardiovascular benefits.11 The LEADER, REWIND, and SUSTAIN-6 trials all showed that GLP-1RA reduced the risk of renal composite endpoint events in patients with type 2 diabetes mellitus (T2DM).12–14 Moreover, both the 2022 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines and the 2023 American Diabetes Association (ADA) guidelines recommend GLP-1RA as a second-line hypoglycemic agent for the treatment of DKD.15,16 However, the exact mechanism by which GLP-1RAs reduce DKD risk is not known.
Although clinical guidelines propose standardized treatment regimens derived from randomized controlled trial (RCT) results, disease progression and medication regimens in clinical practice often present a higher level of complexity.17 Real-world data on GLP-1RA use in treating DKD are limited. This study aimed to evaluate the efficacy and safety of GLP-1RA in DKD treatment in a real-life clinical context.
Materials and Methods
Study Design
The present study was a single-arm, retrospective, real-world trial. From August 2021 to June 2023, we screened 573 patients with DKD who had been taking GLP-1RA. Of these, 209 patients were excluded because they had been treated with GLP-1RA for less than 6 months. Finally, a total of 364 patients who received GLP-1RA therapy for at least 6 months were included in this study. We retrospectively collected patient data before and 6 months after treatment from the electronic medical record system. The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Sunshine Union Hospital (IRB No.: YGRHLLKY-0008). The requirement for informed consent was waived because of the retrospective study design and minimal harm to patients. This trial was registered with the Chinese Clinical Trial Registry under the registration number ChiCTR2300073482.
Inclusion and Exclusion Criteria
The present study included patients with T2DM who were treated at our institution between August 2021 and June 2023. The inclusion criteria were as follows: (1) age ≥ 18 years and clinical diagnosis of DKD and (2) treatment with GLP-1RA for at least 6 months. The exclusion criterion was the following: patients with type 1 diabetes mellitus. Based on the estimated glomerular filtration rate (eGFR), patients with DKD were categorized as having mild (eGFR ≥ 60 mL/min/1.73 m2), moderate (eGFR 30–59 mL/min/1.73 m2), or severe DKD (eGFR ≤ 29 mL/min/1.73 m2).
Data Collection and Assessments
Patient demographic characteristics, including age, sex, disease duration, body weight, body mass index (BMI), and baseline use of glucose-lowering, lipid-modifying, and antihypertensive medications, were collected at the initiation of GLP-1RA therapy. Laboratory measurements, including blood glucose, blood lipids, blood pressure, UACR, 24-h urinary protein, and eGFR, were collected before and after GLP-1RA treatment.
Outcomes
The primary endpoint was the hemoglobin A1c (HbA1c) level at 6 months of treatment. Secondary endpoints included UACR, 24-h urinary protein, eGFR, body weight, fasting blood glucose, blood lipids, and blood pressure at 6 months of treatment. Safety endpoints included gastrointestinal adverse reactions and hypoglycemic events.
Statistical Analysis
The Kolmogorov–Smirnov test was used to test whether continuous variables conformed to the normal distribution. Paired t-tests or paired Wilcoxon tests were used for comparisons before and after treatment. The chi-square test was used for comparing categorical variables. The independent samples t-test or Wilcoxon test was used for subgroup analysis. IBM SPSS Statistics 23.0 software (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Differences with a two-tailed p < 0.05 were considered statistically significant.
Results
In this study of 364 patients with DKD, 153 (42.0%) were female. The median disease duration was 8.0 years. Patients had a mean age, HbA1c level, and BMI of 52.1 years, 8.6%, and 27.8 kg/m2, respectively, and a median UACR of 88.0 mg/g. Mild and moderate DKD were present in 73.6% and 26.4% of patients, respectively (Table 1). The numbers of patients treated with liraglutide, semaglutide, dulaglutide, and polyethylene glycol loxenatide (PEG-Loxe, only approved in China) were 49, 52, 104, and 159, respectively.
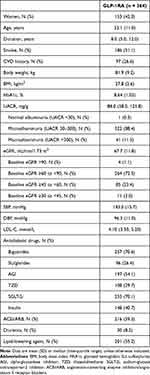 |
Table 1 Baseline Characteristics |
After six months of GLP-1RA treatment, the mean HbA1c level showed a significant decrease of –1.77% (SD: 0.93) from 8.64% (1.03) to 6.87% (0.90) (p < 0.001). Concurrently, the UACR was reduced by 40.3%, dropping from a median of 88.0 mg/g (IQR: 58.0, 125.8) to 50.0 mg/g (30.0, 87.0). Moreover, 24-h urinary protein and eGFR also demonstrated substantial improvements, with the former decreasing from 220.5 mg to 125.0 mg and the latter increasing from 67.7 mL/min/1.73 m2 to 81.8 mL/min/1.73 m2 (both p < 0.001). Compared to their baseline values, patients exhibited significant improvements in fasting blood glucose, body weight, systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol (TC), blood glucose, triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) (all p < 0.001) (Table 2).
 |
Table 2 Changes in Outcomes Over 6 Months of GLP-1RA Use |
Subgroup analysis indicated no effect of sex, SBP, DBP, ACEi/ARB use, or SGLT-2i use on changes in HbA1c, UACR, and eGFR after 6 months of GLP-1RA treatment. Patients with a disease duration of <10 years had more pronounced changes in the HbA1c level (–1.95% vs –1.49%, p < 0.001), UACR (–37.0 mg/g vs −28.0 mg/g, p = 0.036), and eGFR (15.6 mL/min/1.73 m2 vs 11.7 mL/min/1.73 m2, p < 0.001) than those with a disease duration of ≥10 years. Similarly, better outcomes were observed in patients with BMI ≥ 28 kg/m2, UACR > 300 mg/g, and eGFR ≥ 60 mL/min/1.73 m2 (Table 3).
 |
Table 3 Subgroup Analysis |
Regarding safety, after 6 months of GLP-1RA treatment, the incidence of gastrointestinal adverse reactions was 32.4%, with nausea, vomiting, and diarrhea occurring at rates of 16.2%, 7.7%, and 8.5%, respectively. The incidence of hypoglycemia stood at 7.1% (Table 4).
 |
Table 4 Gastrointestinal Adverse Reactions and Hypoglycemia During Treatment |
Discussion
The present study retrospectively evaluated the real-world therapeutic efficacy of GLP-1RA in patients with DKD. The results demonstrated that 6 months of GLP-1RA treatment significantly enhanced glycemic control and ameliorated disease progression in patients with DKD compared to their status before treatment.
The treatment of DKD should include comprehensive management, including lifestyle interventions, control of risk factors (eg, hyperglycemia, hypertension, disorders of lipid metabolism, and obesity), and diabetes education, to reduce the risk of adverse kidney events and death in patients with diabetes.18,19 The KDIGO guidelines recommend metformin and SGLT2i as the hypoglycemic agents of choice for the treatment of DKD, with a preference for GLP-1RA in cases of poor glycemic control.15 The patients included in the present study were already being treated with glucose-lowering medications at baseline, with metformin or SGLT2i being used in >70% of cases. The baseline rates of ACEi/ARB treatment and lipid-lowering agent treatment were 59.3% and 55.2%, respectively. At 6 months after the addition of GLP-1RA to the original treatment regimen, the risk factors of the patients (including blood glucose, blood pressure, blood lipids, and body weight) were significantly improved. Therefore, the present study provides real-world evidence of the efficacy of GLP-1RA in patients with DKD.
The renoprotective effects of GLP-1RA have been reported in numerous studies. A meta-analysis revealed that GLP-1RA reduced the risk of the renal composite endpoint by 17% in patients with T2DM, primarily by reducing proteinuria.20 An RCT revealed that treatment with dulaglutide 1.5 mg for 26 weeks reduced the UACR by 27.7%.21 Another RCT showed that 16 weeks of treatment with exenatide alone or in combination with dapagliflozin reduced the UACR by 15.6% and 39.6%, respectively.22 In line herewith, the patients in the present study, all of whom had mild-to-moderate DKD, had a 40.3% (–35.5 mg/g) reduction in the UACR, with a baseline rate of SGLT2i treatment of 70.1%.
In a retrospective study in Spanish patients with moderate-to-severe DKD, semaglutide caused a 51% reduction in the UACR after 12 months of treatment.23 In a real-world study conducted in Japan, the UACR significantly decreased (from 60.8 to 47.7 mg/g) in patients with microalbuminuria after 1 year of liraglutide treatment.26 However, real-world evidence of GLP-1RA treatment in patients with mild to moderate DKD was lacking, particularly in the Chinese population. The current study, including Chinese patients with mild-to-moderate DKD, fills these gaps.
The KDIGO guidelines recommend ACEi or ARB treatment for patients with diabetes, comorbid hypertension, and proteinuria.15 However, studies have shown that treatment with ACEis or ARBs achieves the ADA-recommended control goal (a 30% reduction in urinary albumin) in only approximately 50% of patients.24 In the present study, 59.3% of patients were taking an ACEi or ARB at baseline, and this subgroup of patients had a 41.5% (–36.5 mg/g) reduction in the UACR after GLP-1RA treatment. Another 41.7% of patients were not taking ACEis or ARBs at baseline, and their UACR was reduced by 35.8% (–31.5 mg/g) after GLP-1RA treatment. This result suggests that GLP-1RA reduces proteinuria independently of ACEis or ARBs.
The duration of diabetes is a risk factor for the progression of DKD.25 In this study, the HbA1c level and UACR were more significantly improved in patients with a disease duration of <10 years than in those with a disease duration of ≥10 years, suggesting that GLP-1RA treatment should be administered in the early disease stages for optimal efficacy.
Some limitations of the present study are worth mentioning. First, this was a non-controlled study, and multiple interventions were used concomitantly with GLP-1RA treatment. Second, the data were collected retrospectively, which may introduce uncertainty due to confounding factors; therefore, the results should be interpreted with caution.
Conclusions
In summary, real-world GLP-1RA treatment for 6 months significantly improved risk factors (including blood glucose, blood pressure, blood lipids, and body weight) and kidney disease progression in patients with mild-to-moderate DKD. GLP-1RA reduced proteinuria independently of ACEis or ARBs, and earlier application of GLP-1RA resulted in earlier benefits. These results provide meaningful support for evidence-based clinical practice.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank Yale Duan for assistance with statistical analyses.
Funding
This study was funded by Jiangsu Hansoh Pharmaceutical Group Co., Ltd.
Disclosure
The authors declare that they have no conflicts of interest to disclose.
References
1. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabet Res Clin Pract. 2022;183:109119. doi:10.1016/j.diabres.2021.109119
2. Mohandes S, Doke T, Hu H, et al. Molecular pathways that drive diabetic kidney disease. J Clin Invest. 2023;133(4):e165654. doi:10.1172/JCI165654
3. Bonner R, Albajrami O, Hudspeth J, et al. Diabetic kidney disease. Prim Care. 2020;47(4):645–659. doi:10.1016/j.pop.2020.08.004
4. Mima A, Nomura A, Fujii T. Current findings on the efficacy of incretin-based drugs for diabetic kidney disease: a narrative review. Biomed Pharmacother. 2023;165:115032. doi:10.1016/j.biopha.2023.115032
5. Ruiz-Ortega M, Rodrigues-Diez RR, Lavoz C, et al. Special issue “Diabetic Nephropathy: diagnosis, Prevention and Treatment”. J Clin Med. 2020;9(3):813. doi:10.3390/jcm9030813
6. Cherney DZI, Bakris GL. Novel therapies for diabetic kidney disease. Kidney Int Suppl. 2018;8(1):18–25. doi:10.1016/j.kisu.2017.10.005
7. Khurana N, James S, Coughlan MT, et al. Novel therapies for kidney disease in people with diabetes. J Clin Endocrinol Metab. 2022;107(1):e1–e24. doi:10.1210/clinem/dgab639
8. Filippatos G, Anker SD, Pitt B, et al. Finerenone and heart failure outcomes by kidney function/albuminuria in chronic kidney disease and diabetes. JACC Heart Fail. 2022;10(11):860–870. doi:10.1016/j.jchf.2022.07.013
9. Andreasen CR, Andersen A, Knop FK, et al. Understanding the place for GLP-1RA therapy: translating guidelines for treatment of type 2 diabetes into everyday clinical practice and patient selection. Diabetes Obes Metab. 2021;23(S3):40–52. doi:10.1111/dom.14500
10. Ard J, Fitch A, Fruh S, et al. Weight loss and maintenance related to the mechanism of action of glucagon-like peptide 1 receptor agonists. Adv Ther. 2021;38(6):2821–2839. doi:10.1007/s12325-021-01710-0
11. Caruso I, Cignarelli A, Giorgino F. Heterogeneity and similarities in GLP-1 receptor agonist cardiovascular outcomes trials. Trends Endocrinol Metab. 2019;30(9):578–589. doi:10.1016/j.tem.2019.07.004
12. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi:10.1056/NEJMoa1607141
13. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi:10.1056/NEJMoa1603827
14. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394(10193):131–138. doi:10.1016/S0140-6736(19)31150-X
15. Kidney Disease: Improving Global Outcomes Diabetes Work, G. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022;102(5S):S1–S127. doi:10.1016/j.kint.2022.06.008
16. ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic Approaches to Glycemic Treatment: standards of Care in diabetes-2023. Diabetes Care. 2023;46(Supplement_1):S140–S157. doi:10.2337/dc23-S009
17. Mulder R, Singh AB, Hamilton A, et al. The limitations of using randomised controlled trials as a basis for developing treatment guidelines. Evid Based Ment Health. 2018;21(1):4–6. doi:10.1136/eb-2017-102701
18. Gupta S, Dominguez M, Golestaneh L. Diabetic kidney disease: an update. Med Clin North Am. 2023;107(4):689–705. doi:10.1016/j.mcna.2023.03.004
19. Yamazaki T, Mimura I, Tanaka T, et al. Treatment of diabetic kidney disease: current and future. Diabetes Metab J. 2021;45(1):11–26. doi:10.4093/dmj.2020.0217
20. Giugliano D, Scappaticcio L, Longo M, et al. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovasc Diabetol. 2021;20(1):189. doi:10.1186/s12933-021-01366-8
21. Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605–617. doi:10.1016/S2213-8587(18)30104-9
22. Van Ruiten CC, van der Aart-van der Beek AB, IJzerman RG, et al. Effect of exenatide twice daily and dapagliflozin, alone and in combination, on markers of kidney function in obese patients with type 2 diabetes: a prespecified secondary analysis of a randomized controlled clinical trial. Diabetes Obes Metab. 2021;23(8):1851–1858. doi:10.1111/dom.14410
23. Aviles Bueno B, Soler MJ, Perez-Belmonte L, et al. Semaglutide in type 2 diabetes with chronic kidney disease at high risk progression-real-world clinical practice. Clin Kidney J. 2022;15(8):1593–1600. doi:10.1093/ckj/sfac096
24. Curovic VR, Jongs N, Kroonen MYAM, et al. Optimization of albuminuria-lowering treatment in diabetes by crossover rotation to four different drug classes: a randomized crossover trial. Diabetes Care. 2023;46(3):593–601. doi:10.2337/dc22-1699
25. Hui D, Sun Y, Xu S, et al. Analysis of clinical predictors of kidney diseases in type 2 diabetes patients based on machine learning. Int Urol Nephrol. 2023;55(3):687–696. doi:10.1007/s11255-022-03322-1
26. Osonoi T, Saito M, Osonoi Y, et al. Liraglutide improves estimated glomerular filtration rate slopes in patients with chronic kidney disease and type 2 diabetes: a 7-year retrospective analysis. Diabetes Technol Ther. 2020;22(11):828–834. doi:10.1089/dia.2020.0070
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
