Back to Journals » Drug Design, Development and Therapy » Volume 18
Remimazolam in General Anesthesia: A Comprehensive Review of Its Applications and Clinical Efficacy
Authors Zhang H , Li H, Zhao S, Bao F
Received 21 April 2024
Accepted for publication 24 July 2024
Published 5 August 2024 Volume 2024:18 Pages 3487—3498
DOI https://doi.org/10.2147/DDDT.S474854
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Honggang Zhang,1 Huiling Li,1 Shuangjun Zhao,2 Fangping Bao1,2
1Department of Anesthesiology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, People’s Republic of China; 2The Fourth Affiliated Hospital, International Institutes of Medicine, Zhejiang University School of Medicine, Yiwu, Zhejiang Province, People’s Republic of China
Correspondence: Fangping Bao, Email [email protected]
Abstract: Remimazolam is a novel ultra-short-acting benzodiazepine with a unique pharmacokinetic profile that makes it an attractive option for use in general anesthesia. This review paper provides an in-depth analysis of remimazolam’s applications in the field of general anesthesia, focusing on its pharmacological properties, clinical efficacy, safety profile, and potential advantages compared to other anesthetic agents. Remimazolam acts on GABAa receptors, offering rapid onset and recovery times due to its unique metabolic pathway involving tissue esterases. Clinical trials have demonstrated its efficacy in procedural sedation and general anesthesia, showing a favorable safety profile with minimal cardiovascular and respiratory depression. Compared to traditional anesthetics such as propofol, remimazolam presents distinct advantages, including predictable pharmacokinetics, reduced risk of prolonged sedation, and a reliable safety margin. These attributes position remimazolam as a promising agent in various clinical settings. The purpose of this review is to synthesize current evidence on remimazolam and discuss its potential to improve clinical outcomes in anesthesia practice.
Keywords: remimazolam, clinical efficacy, general anesthesia, safety profile, respiratory depression
Introduction
General anesthesia is a cornerstone of modern medical practice, enabling complex surgical and diagnostic procedures by inducing reversible unconsciousness while maintaining vital physiological parameters. The choice of anesthetic agents is of paramount importance, as it profoundly influences patient safety, surgical outcomes, and postoperative recovery.1 Within the spectrum of anesthetics, remimazolam, a relatively recent entrant, has garnered attention for its unique pharmacological properties and potential advantages in the realm of general anesthesia.2–4
Remimazolam belongs to the benzodiazepine class of drugs, sharing a common structural framework with compounds such as diazepam and midazolam.5 However, what sets remimazolam apart is its remarkable pharmacokinetic profile. It is characterized by a rapid onset of action, ultra-short duration, and predictable dose-response relationships.6 These distinctive features make it an intriguing choice for anesthetic induction and maintenance, allowing anesthesiologists to precisely tailor the depth and duration of anesthesia, potentially reducing postoperative recovery times and associated complications.
This review offers an extensive examination of remimazolam’s applications in general anesthesia, encompassing its pharmacological properties, clinical efficacy, safety profile, and potential advantages in comparison to traditional anesthetic agents. Furthermore, we explore ongoing research and clinical investigations, shedding light on the future prospects of remimazolam in anesthesia practice.
Structure and Pharmacological Profile of Remimazolam
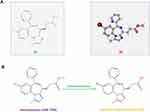
Remimazolam, chemically classified as C21H19BrN4O2, with an average mass of 439.31 Da, is a member of the benzodiazepine class of drugs and exhibits a pharmacological profile that sets it apart from its counterparts, rendering it an attractive option for general anesthesia (Figure 1A).
Remimazolam undergoes hepatic metabolism, primarily via esterase enzymes, resulting in inactive metabolites. Esterases in the body cleave the ester bonds in remimazolam, leading to the formation of inactive metabolite-CNS 7054 (Figure 1B). After ester hydrolysis, the resulting metabolites can undergo conjugation reactions with endogenous compounds, such as glucuronic acid. This conjugation facilitates the excretion of metabolites through the kidneys, further contributing to the drug’s elimination. The metabolism of remimazolam into these inactive metabolites is a crucial factor in its rapid clearance from the body, allowing for the quick emergence of patients from anesthesia and sedation.
One of the key distinguishing features of remimazolam is its rapid onset of action. Following intravenous administration, it achieves peak plasma concentrations within minutes, which is notably quicker than many other anesthetics. Kim et al reported that the volume of distribution at steady state (Vss) for remimazolam averaged 34.8 L, contrasting with midazolam’s 81.8 L. Furthermore, the elimination clearance of remimazolam was approximately three times greater than that of midazolam (70.3 vs 23.0 L/h).5 This rapid onset is advantageous for particular cases where swift induction of anesthesia is essential, such as in emergency surgeries or situations where minimizing patient anxiety is crucial. Remimazolam’s ultra-short duration of action is another hallmark of its pharmacological profile. Its effects wear off quickly, allowing for precise control over the depth and duration of anesthesia.7 The rapid metabolism of remimazolam contributes to its short duration of action and facilitates a smooth and quick emergence from anesthesia. The drug and its metabolites are predominantly eliminated through renal excretion. This characteristic can potentially reduce the risk of prolonged postoperative recovery and the associated complications that can arise with longer-acting agents.
Remimazolam also exhibits predictable dose-response relationships, which is a valuable attribute in anesthesia management. A pharmacodynamic analysis of intravenous bolus remimazolam study demonstrated that the onset of loss of consciousness and respiratory depression displayed steep dose-responses, with ED50/ED95 values of 0.11/0.19 and 0.14/0.27 mg/kg, respectively.8 Based on the ED95 estimates for the corresponding age groups, this study also suggests the optimal doses of 0.25 to 0.33 mg/kg, 0.19 to 0.25 mg/kg, and 0.14 to 0.19 mg/kg for patients aged <40, 60 to 80, and >80 years, respectively. With a clearly defined dose-response curve for remimazolam, its effects can be confidently predicted, as they closely correlate with the dosage administered. Titrating the anesthesia makes it convenient to attain the desired level of unconsciousness while minimizing the risk of over-sedation.
Due to its short duration of action and minimal accumulation in the body, remimazolam offers advantages in scenarios where repeated or prolonged administration of anesthesia is necessary, such as in lengthy surgical procedures.
Mechanism of Action with Remimazolam
Remimazolam shares the similar mechanism as like other benzodiazepines, acts on the gamma-aminobutyric acid (GABA) receptors, specifically GABAa receptors.9 GABAa receptors are widely distributed in the brain and play a fundamental role in regulating neuronal excitability. When activated, these receptors allow the flow of chloride ions, leading to hyperpolarization of the neuron, which inhibits its firing and reduces the overall excitability of the nervous system. When remimazolam binds to these receptors, it increases the affinity of GABA for its binding sites on the receptor. This, in turn, augments the inhibitory effects of GABA, leading to hyperpolarization of the neuron and a reduction in neuronal excitability (Figure 2). As a results, remimazolam could significantly impact the cerebral circulation function, leads to a reduction of blood flow velocity, oxygen saturation, arterial pressure, etc.10,11
Clinical Efficacy of Remimazolam
The clinical efficacy of remimazolam in various medical procedures and scenarios has been a subject of growing interest and investigation. Its unique pharmacological properties, including rapid onset, ultra-short duration, and predictable dose-response relationships, have contributed to its success in multiple clinical settings. This section delves into the clinical efficacy of remimazolam with a focus on its applications in anesthesia, procedural sedation, and its potential advantages compared to other anesthetic agents.
Anesthesia Induction: Remimazolam’s rapid onset of action allows for swift anesthesia induction, making it particularly useful in cases where a quick transition to an unconscious state is essential. Studies have demonstrated its efficacy in achieving the desired anesthetic depth within minutes, reducing patient discomfort and anxiety.5,12 A Phase IIa randomized study of remimazolam shows that a single dose of remimazolam led to successful procedures in 32%, 56%, and 64% of patients in the low (0.10 mg/kg), middle (0.15 mg/kg), and high (0.20 mg/kg) dose groups, respectively, compared to 44% of patients in the midazolam (0.075 mg/kg) dose group. The onset of sedation ranged from 1.5 to 2.5 minutes in the remimazolam dose groups, whereas it took 5 minutes for midazolam.12
Maintenance of Anesthesia: Remimazolam’s controllable sedation level and predictable dose-response relationships contribute to its efficacy in maintaining anesthesia throughout various surgical procedures. Its short duration of action allows for precise titration of anesthesia depth, ensuring that patients remain unconscious only for the necessary duration of surgery. A single ascending-dose study of remimazolam shows that administering a 6-mg initial loading dose of remimazolam, followed by 3-mg maintenance doses at intervals of more than 2 minutes, is recommended. According to predictions, recovery to a Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) score of 5 is anticipated within 16 minutes for 89% of the treated population after this loading/maintenance dose regimen.7
Hemodynamic Stability: Remimazolam’s pharmacological profile minimizes the risk of significant hemodynamic instability during anesthesia. Its controllable sedation level and reduced impact on blood pressure and heart rate are favorable characteristics that contribute to patient safety.
Undergoing a diagnostic or therapeutic colonoscopy, Wang et al reported that the occurrence of oxygen desaturation was notably higher in patients who received propofol compared to those administered remimazolam (6.7% vs 1.1%, p = 0.001). Similarly, the higher frequency of hypotension was in patients with propofol than with remimazolam (29.2% vs 10.6%, p < 0.0001).13 Similarly, a meta-analysis involving ten studies and 1813 patients suggests that while remimazolam showed a slightly lower success rate of sedation/general anesthesia (RR, 1.02; 95% CI: 1.01 to 1.03; P=0.004; N=1402), it demonstrated a decreased incidence of hypoxia, hypotension, and injection pain compared to propofol.14 In a systematically structured narrative review on the role of remimazolam in cardiac surgery, Ursoleo et al concluded that remimazolam usage was associated with potentially superior hemodynamic stability compared to other hypnotic drugs.15 Remimazolam also demonstrated potential safety, efficacy, and ease of use for both anesthesia induction and maintenance in cardiac surgery patients and high-risk cardiovascular patients undergoing non-cardiac surgery.
Emergence from Anesthesia: The ultra-short duration of action of remimazolam enables rapid emergence from anesthesia. Patients tend to awaken quickly and exhibit reduced postoperative drowsiness, contributing to a smoother recovery process and reducing the time spent in the PACU. A study conducted by Mao et al revealed that scores for physical comfort and emotional state were lower in the remimazolam group compared to the propofol group at both Postoperative Day 1 (POD1) and Postoperative Day 3 (POD3), as assessed by the five dimensions (physical comfort, emotional state, physical independence, psychological support, and pain) of the Quality of Recovery-15 (QoR-15) scale.16
Patient Satisfaction: Remimazolam’s quick onset and offset, coupled with reduced side effects such as postoperative cognitive dysfunction, contribute to higher patient satisfaction. Patients often report feeling more alert and comfortable during the immediate postoperative period.11,17
Special Populations: Remimazolam’s efficacy has been assessed in special patient populations, including pediatric and geriatric patients.18–21 Its controllability and predictable dose-response relationships have made it a viable option for tailored sedation in these groups, further expanding its clinical utility.
Administering anesthesia to pediatric patients presents unique challenges for anesthesiologists due to the significant physiological differences between children and adults.22–24 These differences necessitate a tailored approach to ensure safety and efficacy during surgical procedures. Several key factors contribute to the complexity of pediatric anesthesia: (1) Greater Oxygen Consumption: Pediatric patients have a higher metabolic rate, which leads to greater oxygen consumption compared to adults. This increased demand for oxygen requires careful monitoring and management to prevent hypoxemia during anesthesia. (2) Diminished Functional Residual Capacity: The functional residual capacity (FRC) in children is lower than in adults. A lower FRC means that pediatric patients have less oxygen reserve, making them more prone to rapid desaturation, especially during periods of apnea or airway obstruction. (3) Elevated CO2 Ratios: Children tend to have higher CO2 production relative to their body weight. This can lead to elevated CO2 levels if ventilation is not adequately managed during anesthesia. Maintaining appropriate ventilation is crucial to avoid respiratory acidosis and other complications. (4) Susceptibility to Perioperative Hypoxia: The combination of higher oxygen consumption, lower FRC, and elevated CO2 production makes pediatric patients particularly susceptible to perioperative hypoxia. (5) Pharmacokinetics and Pharmacodynamics: The pharmacokinetics (absorption, distribution, metabolism, and excretion of drugs) and pharmacodynamics (drug effects and mechanisms of action) of anesthetic agents can differ significantly between children and adults. Pediatric patients often require different dosing regimens and may respond differently to anesthetic agents. (6) Emotional and Psychological Factors: Children may experience heightened anxiety and fear related to medical procedures. This emotional stress can impact their physiological responses and necessitates a compassionate and patient-centered approach to anesthesia care.
In a clinical study involving 418 children, Kimoto et al found that remimazolam treatment (administered at a rate of 12 mg/kg/h for induction until desired effect and 1–2 mg/kg/h for maintenance) led to significant changes in mean arterial blood pressure (MAP) from baseline in a majority of patients. Specifically, 75.2% experienced a greater than 20% change in MAP, while 203 patients (49.3%) had a greater than 30% change. Only 5% of patients required ephedrine to address unanticipated hemodynamic variability. Notably, post-operative nausea and vomiting (PONV) occurred in 16 patients (13.8%), constituting the primary adverse events documented in the study.21 Another study on pediatric same-day painless a significantly longer induction time compared to propofol (3 mg/kg), but experienced significantly lower incidences of injection pain, intraoperative respiratory depression, hypotension, and bradycardia. The remimazolam group also showed more stable changes in MAP, (heart rate) HR, and perfusion index (PI) compared to the propofol group.25
A recent scoping review26 about remimazolam for anesthesia and sedation in pediatric patients further suggested that remimazolam is a safe and effective option for both sedation and general anesthesia in pediatric patients, particularly those with concurrent mitochondrial disorders, myopathic diseases, or at risk for malignant hyperthermia. Additionally, current evidence suggests that remimazolam may help reduce preoperative anxiety and postoperative delirium in children. Its favorable pharmacodynamic and pharmacokinetic profiles demonstrate potential safety, effectiveness, and ease of use in various perioperative pediatric contexts, making it suitable for integration into specific protocols such as intraoperative monitoring of evoked potentials and management of difficult intubation.
The elderly population often presents unique challenges due to physiological changes associated with aging, comorbidities, and increased sensitivity to anesthetic agents. In a study involving the elderly patients undergoing orthopedic surgery, the remimazolam group demonstrated a postoperative delirium incidence of 15.6%, compared to 12.4% in the propofol group (Risk ratio: 1.26; 95% CI: 0.72 to 2.21; Risk difference: 3.2%; 95% CI: −4.7% to 11.2%; p = 0.42). No significant differences were noted between the two groups regarding the delirium’s onset time, duration, and subtype. Furthermore, patients in the remimazolam group experienced a lower incidence of hypotension after induction and required fewer vasoactive drugs intraoperatively.20 Liu et al evaluated the effect of remimazolam tosilate on the incidence of hypoxemia during sedation in elderly patients undergoing gastrointestinal endoscopy. They found that the incidence of moderate hypoxemia was significantly reduced in the remimazolam group compared to the propofol group (2.8% vs 17.4%). The median lowest SpO2 during the examination was 98% (IQR, 96.0%–99.0%) in the remimazolam group, significantly higher than in the propofol group (96%, IQR, 92.0%–99.0%; p < 0.001). Additionally, there was a statistically significant difference in the incidence of hypotension between the two groups (2.8% vs 12.8%). However, there were no significant differences in the incidence of adverse events such as nausea, vomiting, dizziness, and prolonged sedation.27
In summary, remimazolam has demonstrated clinical efficacy across a spectrum of applications in anesthesia and procedural sedation. Its rapid onset, ultra-short duration, hemodynamic stability, and favorable recovery profiles contribute to its growing adoption in various medical settings. Comparative studies and research in special patient populations continue to provide evidence of its effectiveness and advantages over other anesthetic agents.
Safety and Adverse Effects of Remimazolam
Remimazolam, like any medication, comes with potential safety considerations and adverse effects. Understanding these aspects is crucial for healthcare providers to make informed decisions about its use in clinical settings. Here’s an extended discussion on the safety and adverse effects of remimazolam:
Respiratory Depression: Remimazolam, as a central nervous system depressant, can lead to respiratory depression, especially when administered at higher doses or in combination with other sedative agents.28,29 In a study involving elderly patients undergoing gastroscopy, the incidence of respiratory depression was significantly lower in the remimazolam tosilate (0.2 mg/kg) group compared to the propofol (1.5 mg/kg) group (9.8% vs 17.9%, p = 0.042).28 Similar findings were noted in a study involving 192 patients undergoing painless fiberoptic bronchoscopy, where the incidence of respiratory depression during the inspection was lower in the remimazolam besylate group compared to the propofol group (13.5% vs 39.6%, p < 0.01).29
It is essential for healthcare providers to carefully monitor patients’ respiratory status, particularly in situations where deep sedation or general anesthesia is required. Adequate ventilatory support should be readily available when using remimazolam to mitigate the risk of respiratory compromise.
Hypotension and Hypertension: Remimazolam may cause blood pressure changes, including both hypotension and, less commonly, hypertension.30,31 Post-induction hypotension was less prevalent in remimazolam group compared to propofol group (62.5% versus 82.9%; p = 0.04). Additionally, the decrease in mean blood pressure from baseline was 9.6 mmHg lower in the remimazolam group than in the propofol group before the initial intubation attempt.31 Consistently, another study demonstrated that the incidence of hypotension (50.9% vs 32.4%, p = 0.001) and hypotension requiring treatment (5.8% vs 1.7%, p= 0.031) was significantly higher in the propofol group compared to the remimazolam tosilate group.28
Bradycardia: Some patients may experience bradycardia when administered remimazolam.32,33 This is more likely in individuals who are predisposed to heart rhythm disturbances. Monitoring cardiac function and having appropriate interventions available to address bradycardia are essential precautions.
Cognitive Impairment: Like other sedative drugs, remimazolam can cause cognitive impairment, drowsiness, and difficulty with coordination and memory.34,35 The Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) test results of remimazolam group at 3 days postoperative and 7 days postoperative indicated significantly higher cognitive function impaired compared to the saline group (p < 0.05).35 Patients who receive remimazolam should be cautioned against engaging in activities that require alertness and coordination, such as driving, for a period of time after the drug’s administration.
Paradoxical Reactions: In rare cases, individuals may exhibit paradoxical reactions to remimazolam, which can include agitation, aggression, or disinhibition. This is more common in certain patient populations, such as children and the elderly.36
Drug Interactions: Remimazolam can interact with other medications, such as opioids, anesthetics, or sedatives, potentially intensifying their effects.37,38 For example, cross-sectional analysis of the isobologram showed that remimazolam and propofol exhibit synergistic clinical effects, especially when the ratio of remimazolam and propofol dose was 1:7 (mg/kg).38 Healthcare providers must carefully review a patient’s medication history and consider potential interactions when using remimazolam in combination with other drugs.
Prolonged Sedation: Although remimazolam is known for its rapid offset of action, individual responses can vary. In some cases, patients may experience prolonged sedation, especially if the drug accumulates due to prolonged infusion or specific patient factors.6,39 Providers should be prepared for this possibility and have plans in place for the management of prolonged sedation, including airway support.
Postoperative Nausea and Vomiting (PONV): Like many anesthetics and sedatives, remimazolam can occasionally lead to PONV.40,41 Previously reported incidences of PONV with remimazolam ranged from 3.7% to 27%, varying based on the type of surgery and patient demographics.42,43 Preventive measures, such as antiemetic medications, may be administered to minimize this risk.
It’s essential for healthcare providers to be well-trained in the use of remimazolam, closely monitor patients during its administration, and have the necessary equipment and medications available to manage potential adverse effects. Patient selection and individualized dosing are crucial to ensure safety and efficacy when using remimazolam in clinical practice.
Clinical Applications of Remimazolam
Remimazolam has demonstrated versatility in various clinical applications owing to its unique pharmacological properties. Here are some of its key clinical applications:
Procedural Sedation: Remimazolam has been employed for procedural sedation in endoscopic examinations, radiological interventions, and minor surgical procedures. Studies have demonstrated its effectiveness in providing a controlled level of sedation while allowing for rapid recovery.39,44 Remimazolam exhibits an onset of action ranging from 1 to 3 minutes, which is faster compared to midazolam’s onset of 3 to 5 minutes.45 According to Antonik et al, remimazolam induced sedation rapidly, with onset occurring within 1 minute infusion at doses of 0.075 mg/kg or higher. Peak sedation was observed within 1 to 4 minutes post-dosing, and sedation depth increased with dose. Time to full alertness ranged from 5.5 to 31.5 minutes across different doses, compared to 40 minutes for midazolam at a dose of 0.075 mg/kg.46 Additionally, the half-life of remimazolam is approximately 7 to 8 minutes, significantly shorter than that of midazolam.47 This has clinical significance as it simplifies the administration of precise maintenance doses.
Ambulatory Surgery: In ambulatory or day surgery settings, remimazolam offers the advantage of achieving a suitable level of sedation for short procedures with the benefit of minimal postoperative drowsiness.48,49 This makes it a valuable option for patients who need to return to their normal activities quickly. In a day surgery involving 115 patients, Luo et al found that smaller proportions of patients who received remimazolam (26.3%) and remimazolam + flumazenil (31.6%) experienced hypotension during anesthesia maintenance compared to those who received propofol (68.4%). Consequently, the remimazolam group required less ephedrine and phenylephrine for management. Additionally, serum triglyceride levels were lower, and injection pain was significantly less frequent in the remimazolam groups, with or without flumazenil, compared to the propofol group (5.3% vs 0% vs 18.4%).49
Cardiac Surgery: Remimazolam has been investigated in the context of cardiac surgery, particularly for procedures like cardiac catheterization and electrophysiological studies.50,51 Its pharmacokinetic profile allows for precise control of anesthesia depth, which can be crucial in these settings. In a case report involving an elderly patient undergoing cardiopulmonary bypass (CPB) surgery, Saito et al successfully used remimazolam for induction (6.0 mg/kg/h) and maintenance (0.6–1.0 mg/kg/h) of general anesthesia, maintaining the bispectral index value within the range of 36 to 48 throughout the CPB period.50 Moreover, in another study involving patients with comorbid cardiac conduction abnormalities, successful sedation was achieved using a remimazolam-based regimen (2.5–4 mg with an infusion at 10 to 15 μg/kg/min), supplemented with premedication and additional sedatives as necessary. The sedation protocol exhibited no adverse effects on hemodynamic or conduction function, and all patients experienced rapid recovery, being discharged from the PACU within 60 minutes.51
Colonoscopy: The use of remimazolam in colonoscopy procedures has shown promise in achieving optimal sedation levels, reducing patient discomfort, and facilitating the examination process. Its rapid onset and quick offset are advantageous in this context.52,53 A Phase III study evaluating the efficacy and safety of remimazolam (initial single dose of 5 mg; 2.5 mg maintenance dose, with a maximum of 5 doses per 15-minute window) in colonoscopy suggested that patients administered remimazolam required less fentanyl, experienced faster recovery of neuropsychiatric function, were ready for discharge earlier, and felt back to normal sooner compared to those receiving either placebo or midazolam.54 In another dose study of remimazolam tosylate for colonoscopy, procedural success rates were 80.49%, 87.18%, and 95.00% with doses of 0.1 mg/kg, 0.15 mg/kg, and 0.2 mg/kg, respectively. However, there were no significant differences in induction time or time to recovery among the three groups. Incidence of adverse events, including hypotension, hypoxemia, and bucking, was similar across all three dosage groups.53
Neurosurgery: Remimazolam’s short duration of action and controllable sedation make it a viable option for certain neurosurgical procedures.17,55 Its high clearance rate (70.3 ± 13.9 L/h), low volume of distribution (mean Vss, 34.8 ± 9.4 L) and short terminal elimination half-life (45 ± 9 min) make remimazolam benefit for prompt awakening and neurological assessment, which is valuable in these patients.46,55
These clinical applications highlight the versatility of remimazolam in providing sedation and anesthesia in a range of medical procedures. Its unique pharmacological characteristics contribute to its growing role in improving patient comfort and postoperative outcomes across various healthcare settings.
Clinical Outcomes of Remimazolam Compared to Other Anesthetic Agents
The comparison of remimazolam with other anesthetic agents entails a thorough evaluation of their clinical outcomes. This analysis concentrates on assessing patient responses and results. The following is a broad comparison with commonly employed anesthetic agents. It is crucial to acknowledge that the selection of anesthetic is influenced by individual patient characteristics and procedural necessities.
Remimazolam Vs Propofol
Propofol is a potent intravenous anesthetic agent widely used for the induction and maintenance of general anesthesia during surgical procedures. It belongs to the class of short-acting hypnotic agents and is known for its rapid onset and offset of action. Propofol is administered intravenously and acts on the GABA receptors in the central nervous system, enhancing inhibitory neurotransmission and leading to sedation and anesthesia.
While propofol and remimazolam share a similar mechanism of action, remimazolam demonstrates a significant improvement in clinical outcomes during anesthesia surgeries. Here, we have compiled a summary of the clinical outcomes of propofol and remimazolam based on review papers that include meta-analyses (Table 1).
 |
Table 1 Clinical Outcomes of Remimazolam Vs Propofol by Meta Analysis |
The meta-analysis comparing remimazolam and propofol in various surgical contexts reveals noteworthy clinical outcomes. Remimazolam induction demonstrated no significant difference56 or a lower success rate of sedation14,60 compared with propofol, with lesser incidence of injection pain,14,56–60 and a reduction in respiratory depression, post-induction hypotension, and postoperative nausea/vomiting in mixed surgeries and endoscopy procedures. There are no significant difference on eye opening, time to recovery, and discharge time between the two agents.14,56–58 Interestingly, when compared with propofol, remimazolam displayed a significantly reduced incidence of bradycardia within the realm of mixed surgeries,57 but no statistical significance in the endoscopy surgeries.58,60 These findings highlight a potential distinctive effect of remimazolam in mitigating bradycardia in different surgical procedures.
Overall, these findings underscore the potential advantages of remimazolam in improving patient outcomes, though considerations for individual case characteristics and procedural nuances remain crucial for informed clinical decisions.
Remimazolam Vs Dexmedetomidine
Dexmedetomidine is a highly selective alpha-2 adrenergic agonist that has found applications in general anesthesia.61 Its unique pharmacological profile sets it apart from traditional sedative agents. In the context of general anesthesia, dexmedetomidine is often utilized as an adjunctive agent rather than a primary anesthetic. It provides sedation, anxiolysis, and analgesia through its action on central alpha-2 receptors, resulting in a reduction of norepinephrine release and sympathetic outflow.
The available clinical data comparing remimazolam and dexmedetomidine are quite limited. In a study focused on lower extremity surgery under spinal anesthesia, Kim et al observed that patients in the remimazolam group reached the target sedation level more promptly.62 Conversely, the dexmedetomidine group displayed heightened occurrences of bradycardia and hypertension. Respiratory depression was more frequent in the remimazolam group. Patients administered remimazolam exhibited a speedier recovery, shorter stays in the PACU, and expressed higher satisfaction scores. Moreover, hypotension in the PACU were more prevalent in dexmedetomidine group.
Another RCT study with orthopedic surgery demonstrated that the time required to reach the initial target sedation was notably shorter in remimazolam besylate than in dexmedetomidine group.63 Patients receiving remimazolam spent a greater percentage of time within the target sedation range than those receiving dexmedetomidine. Interestingly, individuals in the remimazolam group had a lengthier period for delirium resolution in comparison to the dexmedetomidine group. Although oversedation was more prevalent with remimazolam besylate, it was linked to a lower incidence of hypotension.
Regarding postoperative cognitive function, Liao et al reported that at 3 and 7 days after surgery, there were no statistically significant differences observed in the incidence of postoperative cognitive dysfunction (POCD), as well as in the MMSE and MoCA scores between remimazolam group and dexmedetomidine groups.35
Future Directions
As remimazolam continues to establish its role in general anesthesia, ongoing research and advancements in its application are anticipated. Several future directions hold promise for refining its use and expanding its clinical utility:
Optimization of Dosing Protocols: Future research may focus on refining dosing protocols to achieve an optimal balance between rapid onset, precise sedation levels, and ultra-short duration. This involves tailoring dosages for specific procedures and patient populations to enhance efficiency and safety.
Special Populations and Pediatric Use: Investigations into remimazolam’s safety and efficacy in special populations, including pediatric patients, are likely to expand. Optimizing dosing strategies for vulnerable groups and ensuring a favorable safety profile will be essential for broader applicability.
Comparison Studies with Established Agents: Continued comparative studies with established anesthetic agents, such as propofol and midazolam, will provide valuable insights into the relative advantages and limitations of remimazolam. These studies will contribute to evidence-based decision-making in choosing the most appropriate agent for specific clinical scenarios.
Combination Therapies: Exploration of remimazolam in combination with other agents for synergistic effects or to mitigate potential side effects may be an avenue for future research. Combinations that enhance sedation while minimizing undesirable effects could further improve patient outcomes.
Extended Applications in Ambulatory Settings: Investigations into the use of remimazolam in ambulatory surgical settings may expand. Its rapid onset, controllability, and minimal residual effects make it an attractive option for procedures that require brief sedation with swift recovery.
Pharmacogenomics and Personalized Medicine: Future research may delve into the role of pharmacogenomics in determining individual responses to remimazolam. Tailoring dosages based on genetic factors could lead to more personalized and precise anesthesia management.
Neurological and Cardiac Procedures: The exploration of remimazolam’s application in specialized procedures, such as neurosurgery and certain cardiac interventions, may expand. Its unique pharmacokinetic profile could offer advantages in scenarios where rapid awakening and neurological assessments are crucial.
Real-world Evidence and Long-term Safety: Accumulating real-world evidence on the long-term safety profile of remimazolam will be essential. Monitoring for rare adverse events and conducting post-marketing surveillance will contribute to a comprehensive understanding of its safety in diverse clinical settings.
Development of Reversal Agents: Research efforts may focus on the development of reversal agents for remimazolam to provide an additional layer of safety. Having an effective antidote could offer a rapid means of reversing its effects in emergency situations.
Conclusion
In conclusion, remimazolam offers a unique pharmacokinetic profile that makes it a compelling option for use in general anesthesia. The future directions in remimazolam application in general anesthesia are poised to involve a combination of refining existing practices, exploring novel applications, and leveraging advancements in personalized medicine. The ongoing evolution of remimazolam’s role in anesthesia will likely be shaped by collaborative efforts among researchers, clinicians, and regulatory bodies striving to enhance patient outcomes and safety.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Brown EN, Pavone KJ, Naranjo M. Multimodal general anesthesia: theory and practice. Anesth Analg. 2018;127(5):1246–1258. doi:10.1213/ANE.0000000000003668
2. Sneyd JR, Rigby-Jones AE. Remimazolam for anaesthesia or sedation. Curr Opin Anaesthesiol. 2020;33(4):506–511. doi:10.1097/ACO.0000000000000877
3. Zhou J, Leonowens C, Ivaturi VD, et al. Population pharmacokinetic/pharmacodynamic modeling for remimazolam in the induction and maintenance of general anesthesia in healthy subjects and in surgical subjects. J Clin Anesth. 2020;66:109899. doi:10.1016/j.jclinane.2020.109899
4. Shirozu K, Nobukuni K, Tsumura S, et al. Neurological sedative indicators during general anesthesia with remimazolam. J Anesth. 2022;36(2):194–200. doi:10.1007/s00540-021-03030-7
5. Kim KM. Remimazolam: pharmacological characteristics and clinical applications in anesthesiology. Anesth Pain Med. 2022;17(1):1–11. doi:10.17085/apm.21115
6. Hu Q, Liu X, Wen C, Li D, Lei X. Remimazolam: an updated review of a new sedative and anaesthetic. Drug Des Devel Ther. 2022;16:3957–3974. doi:10.2147/DDDT.S384155
7. Wiltshire HR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase i single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part II. population pharmacokinetic and pharmacodynamic modeling and simulation. Anesth Analg. 2012;115:284–296.
8. Chae D, Kim H-C, Song Y, Choi YS, Han DW. Pharmacodynamic analysis of intravenous bolus remimazolam for loss of consciousness in patients undergoing general anaesthesia: a randomised, prospective, double-blind study. Br J Anaesth. 2022;129(1):49–57. doi:10.1016/j.bja.2022.02.040
9. Garcia P, Kolesky S, Jenkins A. General anesthetic actions on GABAA receptors. Curr Neuropharmacol. 2010;8(1):2–9. doi:10.2174/157015910790909502
10. Soejima T, Ueda K, Hasegawa S, et al. Change in cerebral circulation during the induction of anesthesia with remimazolam. J Anesth. 2023;37(1):92–96. doi:10.1007/s00540-022-03135-7
11. Gao J, Yang C, Ji Q, Li J. Effect of remimazolam versus propofol for the induction of general anesthesia on cerebral blood flow and oxygen saturation in elderly patients undergoing carotid endarterectomy. BMC Anesthesiol. 2023;23(1):153. doi:10.1186/s12871-023-02095-z
12. Borkett KM, Riff DS, Schwartz HI, et al. A phase iia, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg. 2015;120(4):771–780. doi:10.1213/ANE.0000000000000548
13. Wang X, Hu X, Bai N, et al. Safety and efficacy of remimazolam besylate in patients undergoing colonoscopy: a multicentre, single-blind, randomized, controlled, phase iii trial. Front Pharmacol. 2022;13:900723. doi:10.3389/fphar.2022.900723
14. Zhang J, Cairen Z, Shi L, et al. Remimazolam versus propofol for procedural sedation and anesthesia: a systemic review and meta-analysis. Minerva Anestesiol. 2022;88(12). doi:10.23736/S0375-9393.22.16817-3.
15. Ursoleo JD, Licheri M, Barucco G, et al. Remimazolam for anesthesia and sedation in cardiac surgery and for cardiac patients undergoing non-cardiac surgery: a systematic-narrative hybrid review. Minerva Anestesiol. 2024. doi:10.23736/S0375-9393.24.17943-6
16. Mao Y, Guo J, Yuan J, Zhao E, Yang J. Quality of recovery after general anesthesia with remimazolam in patients’ undergoing urologic surgery: a randomized controlled trial comparing remimazolam with propofol. Drug Des Devel Ther. 2022;16:1199–1209. doi:10.2147/DDDT.S359496
17. Sato T, Kato Y, Yamamoto M, Nishiwaki K. Novel anesthetic agent remimazolam as an alternative for the asleep-awake-asleep technique of awake craniotomy. JA Clin Rep. 2020;6(92). doi:10.1186/s40981-020-00398-5
18. Nakayama J, Ogihara T, Yajima R, Innami Y, Ouchi T. Anesthetic management of super-elderly patients with remimazolam: a report of two cases. JA Clin Rep. 2021;7(1):71. doi:10.1186/s40981-021-00474-4
19. Shioji N, Everett T, Suzuki Y, Aoyama K. Pediatric sedation using dexmedetomidine and remimazolam for magnetic resonance imaging. J Anesth. 2022;36(1):1–4. doi:10.1007/s00540-021-02957-1
20. Yang -J-J, Lei L, Qiu D, et al. Effect of remimazolam on postoperative delirium in older adult patients undergoing orthopedic surgery: a prospective randomized controlled clinical trial. Drug Des Devel Ther. 2023;17:143–153. doi:10.2147/DDDT.S392569
21. Kimoto Y, Hirano T, Kuratani N, Cavanaugh D, Mason KP. Remimazolam as an adjunct to general anesthesia in children: adverse events and outcomes in a large cohort of 418 cases. J Clin Med. 2023;12(12):3930. doi:10.3390/jcm12123930
22. Sbaraglia F, Cuomo C, Della Sala F, et al. State of the art in pediatric anesthesia: a narrative review about the use of preoperative time. J Pers Med. 2024;14(2):182. doi:10.3390/jpm14020182
23. Monaco F, D’Andria Ursoleo J, Lerose CC, et al. Anaesthetic management of paediatric patients undergoing electrophysiology study and ablation for supraventricular tachycardia: a focused narrative review. J Clin Anesth. 2024;93:111361. doi:10.1016/j.jclinane.2023.111361
24. Bai C, Xu M, Guo Y, Jin Y, Zhao X. Clinical application and research progress of remimazolam for pediatric patients. Drug Des Devel Ther. 2024;18:1221–1229. doi:10.2147/DDDT.S453440
25. Chu T, Zhou S, Wan Y, et al. Comparison of remimazolam and propofol combined with low dose esketamine for pediatric same-day painless bidirectional endoscopy: a randomized, controlled clinical trial. Front Pharmacol. 2024;15:1298409. doi:10.3389/fphar.2024.1298409
26. Pieri M, D’Andria Ursoleo J, Di Prima AL, et al. Remimazolam for anesthesia and sedation in pediatric patients: a scoping review. J Anesth. 2024. doi:10.1007/s00540-024-03358-w
27. Liu F, Cheng X, Wang Y, et al. Effect of remimazolam tosilate on the incidence of hypoxemia in elderly patients undergoing gastrointestinal endoscopy: a bi-center, prospective, randomized controlled study. Front Pharmacol. 2023;14:1131391. doi:10.3389/fphar.2023.1131391
28. Hu B, Jiang K, Shi W, et al. Effect of remimazolam tosilate on respiratory depression in elderly patients undergoing gastroscopy: a multicentered, prospective, and randomized study. Drug Des Devel Ther. 2022;16:4151–4159. doi:10.2147/DDDT.S391147
29. Zhang L, Yu L, Xu L, et al. Effectiveness of remimazolam besylate combined with alfentanil for fiberoptic bronchoscopy with preserved spontaneous breathing: a prospective, randomized, controlled clinical trial. Eur Rev Med Pharmacol Sci. 2023;27(13):6071–6080. doi:10.26355/eurrev_202307_32961
30. Yokose M, Takaki R, Mihara T, et al. Hypotension after general anesthesia induction using remimazolam in geriatric patients: protocol for a double-blind randomized controlled trial. PLoS One. 2022;17(9):e0275451. doi:10.1371/journal.pone.0275451
31. Song SW, Kim S, Park J-H, Cho YH, Jeon Y-G. Post-induction hypotension with remimazolam versus propofol in patients routinely administered angiotensin axis blockades: a randomized control trial. BMC Anesthesiol. 2023;23(1):219. doi:10.1186/s12871-023-02188-9
32. Zhang X, Li S, Liu J. Efficacy and safety of remimazolam besylate versus propofol during hysteroscopy: single-centre randomized controlled trial. BMC Anesthesiol. 2021;21(1):156. doi:10.1186/s12871-021-01373-y
33. Liu X, Ding B, Shi F, et al. The efficacy and safety of remimazolam tosilate versus etomidate-propofol in elderly outpatients undergoing colonoscopy: a prospective, randomized, single-blind, non-inferiority trial. Drug Des Devel Ther. 2021;15:4675–4685. doi:10.2147/DDDT.S339535
34. Tan Y, Ouyang W, Tang Y, et al. Effect of remimazolam tosilate on early cognitive function in elderly patients undergoing upper gastrointestinal endoscopy. J Gastroenterol Hepatol. 2022;37(3):576–583. doi:10.1111/jgh.15761
35. Liao YQ, Min J, Wu ZX, Hu Z. Comparison of the effects of remimazolam and dexmedetomidine on early postoperative cognitive function in elderly patients with gastric cancer. Front Aging Neurosci. 2023;15:1123089. doi:10.3389/fnagi.2023.1123089
36. Zhu H, Su Z, Huai X, et al. Efficacy and safety of remimazolam tosylate for sedation during upper gastrointestinal endoscopy: study protocol for a multicenter randomized controlled trial. Trials. 2022;23(1):995. doi:10.1186/s13063-022-06935-0
37. Kops MS, Pesic M, Petersen K-U, Schmalix WA, Stöhr T. Impact of concurrent remifentanil on the sedative effects of remimazolam, midazolam and propofol in cynomolgus monkeys. Eur J Pharmacol. 2021;890:173639. doi:10.1016/j.ejphar.2020.173639
38. Lyu S, Deng Q, Lin W, Wu X. Randomized controlled trial for anesthesia during gastroscopy: interactions between remimazolam and propofol in combination with sufentanil. Int J Clin Pharm. 2023;45:857–863. doi:10.1007/s11096-023-01568-y
39. Noor N, Legendre R, Cloutet A, et al. A comprehensive review of remimazolam for sedation. Health Psychol Res. 2021;9(1). doi:10.52965/001c.24514.
40. Suzuki Y, Kawashima S, Makino H, Doi M, Nakajima Y. Comparison of postoperative nausea and vomiting between remimazolam and propofol: a propensity score-matched, retrospective, observational, single-center cohort study. Korean J Anesthesiol. 2023;76(2):143–151. doi:10.4097/kja.22441
41. Kim E-J, Kim C-H, Yoon J-Y, et al. Comparison of postoperative nausea and vomiting between remimazolam and propofol in patients undergoing oral and maxillofacial surgery: a prospective randomized controlled trial. BMC Anesthesiol. 2023;23(1):132. doi:10.1186/s12871-023-02091-3
42. Hari Y, Satomi S, Murakami C, et al. Remimazolam decreased the incidence of early postoperative nausea and vomiting compared to desflurane after laparoscopic gynecological surgery. J Anesth. 2022;36(2):265–269. doi:10.1007/s00540-022-03041-y
43. Song SW, Jang YN, Yoon M-W, Jeon Y-G. Quality of recovery in patients administered remimazolam versus those administered an inhalant agent for the maintenance of general anesthesia: a randomized control trial. BMC Anesthesiol. 2022;22(1):226. doi:10.1186/s12871-022-01770-x
44. Lee A, Shirley M. Remimazolam: a review in procedural sedation. Drugs. 2021;81(10):1193–1201. doi:10.1007/s40265-021-01544-8
45. Wesolowski AM, Zaccagnino MP, Malapero RJ, Kaye AD, Urman RD. Remimazolam: pharmacologic considerations and clinical role in anesthesiology. Pharmacother J Hum Pharmacol Drug Ther. 2016;36(9):1021–1027. doi:10.1002/phar.1806
46. Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase i single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part I. safety, efficacy, and basic pharmacokinetics. Anesth Analg. 2012;115:274–283.
47. Goudra BG, Singh PM. The future of its sedative potential. Saudi J Anaesth. 2014;8(3):388–391. doi:10.4103/1658-354X.136627
48. Choi SR, Kim TH, Eom DW, Jung JW, Park SY. Monitored anesthesia care with remimazolam for gynecological day surgeries: case reports. Clin Case Rep. 2022;10(11):e6536. doi:10.1002/ccr3.6536
49. Luo W, Sun M, Wan J, et al. Efficacy and safety of remimazolam tosilate versus propofol in patients undergoing day surgery: a prospective randomized controlled trial. BMC Anesthesiol. 2023;23(1):182. doi:10.1186/s12871-023-02092-2
50. Saito K, Ohno S, Maeda M, Hirata N, Yamakage M. Remimazolam anesthesia for cardiac surgery with cardiopulmonary bypass: a case report. JA Clin Rep. 2021;7(21). doi:10.1186/s40981-021-00424-0
51. Kalsotra S, Khan S, McKee C, Tobias JD. Remimazolam as the primary agent for sedation during cardiac catheterization in three patients with comorbid cardiac conduction abnormalities. Cardiol Res. 2023;14(1):86–90. doi:10.14740/cr1477
52. Ul-Haque I, Shaikh TG, Ahmed SH, et al. Efficacy of remimazolam for procedural sedation in American Society of Anesthesiologists (ASA) I to IV patients undergoing colonoscopy: a systematic review and meta-analysis. Cureus. 2022. doi:10.7759/cureus.22881
53. Zheng X, Ji J, Cheng H, et al. Efficacy and safety of different doses of remimazolam tosylate for colonoscopy: single-center, prospective, randomized, double-blind, parallel trial. Ann Transl Med. 2022;10(22):1244. doi:10.21037/atm-22-5133
54. Rex DK, Bhandari R, Desta T, et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88(3):427–437.e6. doi:10.1016/j.gie.2018.04.2351
55. Teixeira MT, Brinkman NJ, Pasternak JJ, Abcejo AS. The role of remimazolam in neurosurgery and in patients with neurological diseases: a narrative review. J Neurosurg Anesthesiol. 2023. doi:10.1097/ANA.0000000000000917
56. Ko -C-C, Hung K-C, Illias AM, et al. The use of remimazolam versus propofol for induction and maintenance of general anesthesia: a systematic review and meta-analysis. Front Pharmacol. 2023;14:1101728. doi:10.3389/fphar.2023.1101728
57. Chang Y, Huang Y-T, Chi K-Y, Huang Y-T. Remimazolam versus propofol for procedural sedation: a meta-analysis of randomized controlled trials. PeerJ. 2023;11:e15495. doi:10.7717/peerj.15495
58. Zhao M, Hu H-F, Li X-L, et al. The safety and efficacy between remimazolam and propofol in intravenous anaesthesia of endoscopy operation: a systematic review and meta-analysis. Int J Surg. 2023;109(11):3566–3577. doi:10.1097/JS9.0000000000000638
59. Wu X, Wang C, Gao H, et al. Comparison of remimazolam and propofol about safety outcome indicators during general anesthesia in surgical patients: a systematic review and meta-analysis. Minerva Anestesiol. 2023;89(6). doi:10.23736/S0375-9393.23.17034-9.
60. Zhu X, Wang H, Yuan S, et al. Efficacy and safety of remimazolam in endoscopic sedation—a systematic review and meta-analysis. Front Med. 2021;8:655042. doi:10.3389/fmed.2021.655042
61. Giovannitti JA, Thoms SM, Crawford JJ. Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesth Prog. 2015;62:31–38. doi:10.2344/0003-3006-62.1.31
62. Kim H, Kim Y, Bae J, et al. Comparison of remimazolam and dexmedetomidine for intraoperative sedation in patients undergoing lower extremity surgery under spinal anesthesia: a randomized clinical trial. Reg Anesth Pain Med. 2023;49:104415. rapm-2023-. doi:10.1136/rapm-2023-104415
63. Deng Y, Qin Z, Wu Q, et al. Efficacy and safety of remimazolam besylate versus dexmedetomidine for sedation in non-intubated older patients with agitated delirium after orthopedic surgery: a randomized controlled trial. Drug Des Devel Ther. 2022;16:2439–2451. doi:10.2147/DDDT.S373772
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.