Back to Journals » Psychology Research and Behavior Management » Volume 17
Resting-State Alpha Activity in the Frontal and Occipital Lobes and Assessment of Cognitive Impairment in Depression Patients
Authors Xie XM, Sha S, Cai H, Liu X, Jiang I, Zhang L, Wang G
Received 21 March 2024
Accepted for publication 12 August 2024
Published 17 August 2024 Volume 2024:17 Pages 2995—3003
DOI https://doi.org/10.2147/PRBM.S459954
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Bao-Liang Zhong
Xiao-Meng Xie,1 Sha Sha,1 Hong Cai,2 Xinyu Liu,1 Isadora Jiang,3 Ling Zhang,1 Gang Wang1
1The National Clinical Research Center for Mental Disorders & Beijing Key Laboratory of Mental Disorders Beijing Anding Hospital & the Advanced Innovation Center for Human Brain Protection, Capital Medical University, School of Mental Health, Beijing, People’s Republic of China; 2Unit of Medical Psychology and Behavior Medicine, School of Public Health, Guangxi Medical University, Nanning, Guangxi, People’s Republic of China; 3Bellarmine College of Liberal Arts, Loyola Marymount University, Los Angeles, CA, USA
Correspondence: Gang Wang, Beijing Anding Hospital, Beijing, People’s Republic of China, Email [email protected]
Background: Major depressive disorder (MDD) becomes one of the psychiatric disorders characteristic of a combination of cognitive, emotional, and somatic symptoms. Additionally, cognitive impairment has the most significant impact on functional results. However, the evaluation of cognitive level is still based on various subjective questionnaires as there is no objective standard assessment yet. This research focuses on resting-state alpha activity to identify cognition in MDD patients using electroencephalography (EEG) signals.
Methods: Ninety-two subjects were recruited: 44 patients with MDD and 48 healthy individuals as controls. Functional outcome and cognition were assessed using standardized instruments, and the EEG resting state signal of open and closed eyes was recorded. The comparison and correlation of cognitive levels with alpha power in the bilateral frontal region, bilateral central region, bilateral occipital region, and middle line was evaluated.
Results: The relative alpha power in MDD group was significantly lower than that in the control group (P < 0.05). Through correlation analysis, it was shown that the bilateral frontal and occipital alpha power of MDD patients in the closed-eyes state was positively correlated with information processing rate, verbal learning, working memory, and attention retention. The alpha power of the bilateral frontal region in the open-eyes state was positively correlated with information processing rate, working memory, and attention retention (P < 0.05).
Conclusion: The research indicates that the changes in frontal and occipital alpha activities may be a promising neurophysiological indicator of cognitive level to diagnose and treat response prediction.
Keywords: major depressive disorder, MDD, EEG, alpha power, cognition impairment
Introduction
Major depressive disorder (MDD) has been one of the most typical psychological disorders that influences about 5–6% of people all over the world.1 In accordance with the World Health Organization’s (WHO) report, MDD is the second major global cause of disease burden in 2020.2 One of the main challenges of treating MDD is to prevent relapse of MDD.3 On account of the essence of MDD as a dynamic disorder, repeated relapses are progressively independent from social and environmental pressures; besides, their probability and frequency gradually increase.4 The risk of reoccurrence is ascribed to the fact that the MDD patients are different from never-depressed persons in their cognition of vulnerability.5,6 MDD is related to cognitive deficits; besides, functional impairments are connected with the brain regions, like the frontal and temporal regions.7 According to recent research, MDD exhibits decreased left frontal activities, which is measured by increased interhemispheric alpha power/amplitude).8 Transcranial alternating current stimulation (tACS) has been shown to improve symptoms of major depression and is associated with a reduction in alpha oscillation power.9 Compared to subjects with no MDD, the MDD patients have shown less frontal activities. Hence, EEG alpha interhemispheric asymmetry is regarded as a risk marker.10 In addition to alpha oscillation power,11 several characteristics of alpha oscillation variation can also be used as potential biomarkers for MDD, including alpha peak frequency,12 and functional connectivity.13
Besides the alpha band, the other bands, like the theta band, have also shown correlation through reported decreased frontal theta activities.14 Furthermore, hypo-activation in left frontal regions and hyperactivation in right frontal regions have been investigated.15 Regarding the cognitive level of MDD patients, an in-depth EEC investigation is needed, which involves characteristics like alpha interhemispheric asymmetry and spectral power of different frequency bands. Compared with other researches, EEG alpha interhemispheric asymmetry is significant to evaluate the cognitive level of the depressed patients.16 For example, it seems that the depressed patients have relatively higher left frontal alpha power compared to non-depressive individuals.17 Higher alpha power can be determined as active inhibition but not cognitive inertia.18 Therefore, this evidence increases the validity of the EEG alpha interhemispheric asymmetry feature to assess the cognitive level of individuals with depression. Wang et al had revealed distinct alpha oscillatory patterns in individuals with MDD, characterized by elevated high alpha oscillations and reduced low alpha oscillations compared to healthy controls.19 However, the integration of EEG and measurement of cognition is less explored, and its differences in brain regions of resting-state alpha activity remain uncertain.
This study fills the gap by evaluating the resting-state alpha activity and cognitive level among depressed patients (spectral power calculations for discrepant EEG frequency bands and EEG alpha interhemispheric asymmetry). We hypothesized that there would be multi-dimensional impairments in the cognitive level of patients with MDD in our research. Furthermore, we speculate a positive correlation between the alpha level in the resting state and the patient’s information processing rate, reasoning, problem solving, verbal learning, and working memory, etc., suggesting that the alpha level in the resting state may be used as a biological indicator for cognitive impairment in MDD patients.
Method
Study Sites and Participants
This research was implemented at the 300-bed Mental Disorders Centre of Beijing Anding Hospital from July 2022 to May 2023. In the psychiatric service, the whole patients were successively invited to engage in the investigation. Inclusion criteria included 1) ages between 18 and 60; 2) diagnoses of MDD in accordance with the International Classification of Diseases, Tenth Revision (ICD-10);20 3) an aggregate score ≥14 on the 17-item Hamilton Rating Scale for Depression (HAMD-17) and an aggregate score <6 on the Young Mania Rating Scale (YMRS);21 4) comprehending the assessment and offering written informed consent. Exclusion criteria included 1) serious medical or surgical history; 2) history of drug or alcohol dependence/abuse; 3) dementia diagnosis or other cognitive impairment. Using advertisements, healthy controls (HCs) were recruited from society. This research protocol was approved by the Biomedical Ethics Board of Beijing Anding Hospital.
Data Collection and Measurements
The fundamental clinical and socio-demographic data were gathered and recorded in a form. Instruments for psychopathology contained the Hamilton Depression Scale (HAMD),22 evaluating the severity of depression.
Function Assessment
Through the Global Assessment Functioning (GAF) test23,24 and the Functioning Assessment Short Test (FAST),24,25 functional status was assessed. Moreover, the GAF score ranged from 1 to 100 and measured psychological, social, and occupational dimensions, with higher scores indicating better functioning.26 The FAST involved 24 items and covered 6 aspects of functioning, cognitive functioning, occupational functioning, autonomy, interpersonal relationships, financial problem, and free time. Each item score ranged between 0 and 3 with a higher total score, which illustrated the higher severity of functional impairment.
Cognition Assessment
Working memory was measured using the Wechsler memory scale-III ss; the score was figured out through adding forward and backward spatial spans.
The Hopkins Verbal Learning Test-Revised (HVLT-R) is a word list learning test consisting of 12-item word lists presented in three learning tests. The memory score is three times the sum of learning test (0 to 36), on behalf of the learning and working memory.27 Sustained attention was assessed through the Continuous Performance Test-Identical Pairs version, measuring the capability of identifying and reacting to particular occasional incentives at stochastic intervals and impeding responses to non-target incentives.28 The rate of processing was determined via the Trail Making Test-Part A,29 the Category Fluency, the Brief Assessment of Cognition in Schizophrenia, and Symbol Coding subtest items. The verbal fluency score was evaluated via the Animal Naming in Category Fluency.30 The cognitive testing was performed by a trained neuropsychologist. Aside from the attention, greater performance on cognitive function trials was correlated to higher scores.
EEG Signal Acquisition and Data Processing
The EEG was acquired using a Neuracle ® equipment (Version NSM1FS-200801, Changzhou, Jiangsu province, China), and the 19-channel electroencephalography signals were recorded from the scalp of the participants during the experiment.
The subjects were required to keep themselves emotionally stable for five minutes. When participators seated themselves in a silent room, the resting EEG session was carefully recorded; they were requested to relax and alternate between open and closed eyes every five minutes.
Consistent with the regional division in classical resting-state EEG studies, the average value of the power of electrodes in six regions and the power at three sites in the midline were selected as the indicators of resting-state EEG. The six brain regions included the left frontal region (Fp1, F3, F7), right frontal region (Fp2, F4, F8), left central region (T3, C3), right central region (T4, C4), left occipital region (T5, P3, O1), and right occipital region (T6, P4, O2). The occipital area is generally considered to include the posterior temporal lobe and the parietal lobe, and we mainly collected EEG signals from the posterior temporal lobe.
The three sites on the middle line are Fz, Cz, and Pz. EEG signals were online referenced to the Cz electrode and constantly recorded at a rate of 1000 Hz. In the process of recording, electrode impedance was kept lower than 50 kΩ. All data were analyzed using Matlab (The MathWork, MA, USA) combined with EEGLAB toolbox. First, the channel sequence of the data was corrected using EEGLAB, the data were filtered between 0.1 and 30 Hz to ensure that the data collection method was correct for all participants, and then visual checks are performed to remove any bad channels/trials. Independent component analysis (ICA) was then performed to remove large artifacts such as sustained muscle activity or blinking and side eye movements. Finally, the channels removed in the previous analysis step are replaced with spherical interpolation, and the data are re-referenced as an average across all electrodes.
Statistical Analysis
All analyses were implemented with the SPSS version 21.0. The two groups were compared between demographic and clinical variables using chi-square tests, t-tests, and Mann–Whitney U-test accordingly. The effect of electrode location and in eyes open and eyes closed conditions was analyzed using 2-way analysis of variance (ANOVA). Pearson correlation analysis was performed between the alpha power with eyes closed and eyes open and the scores of cognitive constructs in the two groups; besides, p value <0.05 achieved statistical significance (two-tailed).
Results
Basic Demographic and Clinical Characteristics
Forty-four MDD patients and 48 HCs were implicated in this research. Table 1 showed the clinical and socio-demographic features of the samples. The mean HAMD-17 total score for the MDD patients was 22.45 ± 5.49. The total illness duration of the MDD patients was 106.43 ± 100.37 months, while the mean current episode duration was 5.30 ± 6.41 months. By comparison with HCs, the MDD patients might be married (P < 0.001), and more likely to maintain current smoking behaviors (P = 0.002).
 |
Table 1 Comparison of General Data Between MDD Group and Control Group |
Cognition and Alpha Levels in MDD Group and Control Group
In contrast to HCs (all P < 0.05) in Table 2, the MDD patients might have weaker neuropsychological and functional (GAF and FAST) results, like working memory, sustained attention, verbal fluency, processing rate, etc.
 |
Table 2 Comparison of Function and Cognitive Level Between MDD Group and Control Group |
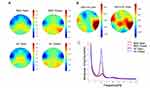
The effect of electrode location and in eyes open and eyes closed conditions was analyzed using 2-way ANOVA pairwise comparison. In terms of the closed-eyes state, the comparative alpha power of bilateral frontal, right central, and bilateral occipital regions in the MDD patients was remarkably lower than that in the HCs (all P < 0.001). On the contrary, in terms of the open-eyes state, the comparative alpha power of the bilateral frontal and bilateral central regions in the MDD patients was lower than that of the HCs. As results, the difference achieved statistical significance (all P < 0.001) in Table 3 and Figure 1.
 |
Table 3 Comparison of Closed and Open-Eyes Alpha Power Between MDD Group and Control Group |
Correlation Analysis Between Cognitive Levels and Resting-State Alpha Power
As for the closed-eyes state, the alpha relative power of the bilateral occipital regions was positively correlated with working memory, information processing rate, verbal fluency, and sustained attention (P < 0.05, Table 4 and Figure S1); the alpha relative power of the bilateral frontal regions was positively correlated with verbal fluency and sustained attention (P < 0.05); the alpha relative power of the right central region was positively correlated with information processing rate (P < 0.05). In most brain regions apart from the left central region, there were relations between resting alpha power and scores of GAF and FAST, which reflect patient functional outcomes (P < 0.05, Table 4).
 |
Table 4 Correlation Analysis Between Alpha Power and Cognitive Dimensions in MDD Patients with Closed and Open Eyes |
In the open-eyes state, the alpha relative power of the right frontal and occipital regions was positively correlated with working memory, information processing rate, verbal fluency, and sustained attention (P < 0.05, Table 4 and Figure S1); the alpha relative power of the left frontal region was positively correlated with working memory, information processing rate, and sustained attention (P < 0.05, Table 4 and Figure S1); the alpha relative power of the left central region was proactively connected to information processing rate and working memory (P < 0.05, Table 4). In the bilateral frontal and central region, there was a relation between resting alpha power and scores of GAF and FAST (P < 0.05, Table 4).
Discussion
Commonly, functional and cognitive impairments are related to affective disorders like MDD, which is consistent with most of the literature.31 Functional impairments are heavily impacted by cognitive impairments as one of the clinical features of MDD, including emotional and somatic symptoms.32 Perhaps, cognitive impairments exist during the early phases of MDD,33,34 and during the premorbid stage before the onset of disease.35 At all stages of MDD, the persistent overreaction of the prelimbic network results in dysfunction and increased vulnerability to emotional dysregulation.36 Previous studies37–39 have confirmed the correlation between alpha power and cognition. Studies40–42 have discovered that event-associated alpha energy is associated with semantic or episodic memory, while performance is associated with working memory. Previous literature have pointed out that individuals with higher resting alpha energy and lower theta energy perform better in cognition and memory, which are potentially helpful indicators of cognitive load.43
Alpha power is generally believed to be generated by intercortical interactions driven by thalamic rhythm points. The reduction of alpha power indicates the weakening of cortical inhibition, which enhances the release of subcortical signals and results in impulsive excitability and inattention in behavior.44,45 Alpha power is connected to both inhibitory function and distant coordination of gamma oscillations.46,47 Correlation analysis illuminated that in terms of MMD patients, bilateral frontal and occipital alpha power in closed-eyes states was positively correlated with verbal learning, information processing rate, attention retention, and working memory. The results in the open-eyes states further verified the above findings. The alpha power of bilateral frontal regions was positively correlated with information processing rate, working memory, and attention retention, indicating stronger cognitive ability in MDD patients with higher resting alpha power levels.
Recent findings also indicate that reduced alpha power in MDD patients may represent a discontinuity in the function of the fronto-parieto-occipital effective connectivity, which relates to initiating and regulating cognitive control.48,49 Clinically, MDD patients lack sufficient cognitive control and often suffer from continuously thinking about personal feelings and problems, a process known as emotional rumination.50 In addition, the EEG pattern of reduced alpha energy may reflect reduced metabolism in the forebrain or thalamus. This implies that the central nervous system is in a state of low arousal, thereby reducing the level and ability to respond to external stimuli,51 in turn also affecting cognitive function, as manifested in anhedonia in MDD patients.52 The origin of alpha oscillations is complex and reflects the various mechanisms of cortico-cortical pathways53 or thalamic-cortical pathways.54 The dynamic modulation of alpha oscillations is influenced by the neurotransmitter systems in the thalamus and cortex, and these alterations are reflected in individuals with MDD,19 where shift toward high alpha oscillations and diminished lower alpha oscillations can be observed in relation to relaxation or cognitive engagement.
This research should be cautiously interpreted due to the following limitations. At the beginning, the sample size was comparatively small. Next, this was a cross-sectional study, not providing more definite conclusions about the progressions of the alpha–cognition relationship. From the viewpoint of the methodology, a bigger sample would be utilized for analysis of all potential combinations among depression, cognition, and their correlation to neural indexes. Third, this study mainly focused on the analysis of the correlation between alpha activity and cognition in MDD patients and did not involve other concussion bands. In future studies, more bands of brain electrical activity should be analyzed in order to more accurately identify the disease characteristics of MDD based on different EEG patterns.
Conclusion
In summary, the MDD patients presented multidimensional cognitive impairment and variation in resting alpha activity. The frontal and occipital changes in alpha activity in MDD patients suggest reduced activation in these regions, which affects emotional and cognitive states, providing promising neurophysiological indicators for diagnosis of MDD and predicting treatment responses, which is advantageous for future clinical practice.
Data Sharing Statement
The data of the investigation will be made publicly available if necessary.
Ethics Approval
This study involving human participants was reviewed and approved by the Human Research and Ethics Committee of Beijing Anding Hospital, Capital Medical University. All the study procedures were carried out in accordance with relevant guidelines. This study was performed in line with the principles of the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was supported by high-level public health technical personnel construction project (elite-02-37), the Advanced Innovation Center for Human Brain Protection Project (3500-12020137).
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Ann Rev Public Health. 2013;34:119–138. doi:10.1146/annurev-publhealth-031912-114409
2. Sagar R, Dandona R, Gururaj G, et al. The burden of mental disorders across the states of India, the global burden of disease study 1990–2017. Lancet Psychiatry. 2020;7(2):148–161. doi:10.1016/S2215-0366(19)30475-4
3. Keune PM, Bostanov V, Hautzinger M, Kotchoubey B. Mindfulness-based cognitive therapy (MBCT), cognitive style, and the temporal dynamics of frontal EEG alpha asymmetry in recurrently depressed patients. Biolog Psychol. 2011;88(2–3):243–252. doi:10.1016/j.biopsycho.2011.08.008
4. Solomon DA, Keller MB, Leon AC, et al. Multiple recurrences of major depressive disorder. Am J Psychiatry. 2000;157(2):229–233. doi:10.1176/appi.ajp.157.2.229
5. Werner-Seidler A, Moulds ML. Autobiographical memory characteristics in depression vulnerability, Formerly depressed individuals recall less vivid positive memories. Cognition & Emotion. 2011;25(6):1087–1103. doi:10.1080/02699931.2010.531007
6. de Jonge M, Bockting CL, van Oppen P, et al. The association between the number of previous episodes and modifiable vulnerability factors in remitted patients with recurrent depression. PLoS One. 2018;13(11):e0206495. doi:10.1371/journal.pone.0206495
7. Rigucci S, Serafini G, Pompili M, Kotzalidis GD, Tatarelli R. Anatomical and functional correlates in major depressive disorder, the contribution of neuroimaging studies. World J Biol Psychiatry. 2010;11(2):165–180. doi:10.3109/15622970903131571
8. Mumtaz W, Xia L, Ali SSA, Yasin MAM, Hussain M, Malik AS. Electroencephalogram (EEG)-based computer-aided technique to diagnose major depressive disorder (MDD). Biom Signal Proc Control. 2017;31:108–115. doi:10.1016/j.bspc.2016.07.006
9. Alexander ML, Alagapan S, Lugo CE, et al. Double-blind, randomized pilot clinical trial targeting alpha oscillations with transcranial alternating current stimulation (tACS) for the treatment of major depressive disorder (MDD). Transl Psychiatry. 2019;9(1):106. doi:10.1038/s41398-019-0439-0
10. Mahato S, Paul S. Classification of depression patients and normal subjects based on electroencephalogram (EEG) signal using alpha power and theta asymmetry. J Med Syst. 2019;44(1):28. doi:10.1007/s10916-019-1486-z
11. Liu S, Liu X, Yan D, et al. Alterations in patients with first-episode depression in the eyes-open and eyes-closed conditions, a resting-state EEG study. IEEE Trans Neural Syst Rehabil Eng. 2022;30:1019–1029. doi:10.1109/TNSRE.2022.3166824
12. Zhou P, Wu Q, Zhan L, et al. Alpha peak activity in resting-state EEG is associated with depressive score. Front Neurosci. 2023;17:1057908. doi:10.3389/fnins.2023.1057908
13. Huang SS, Yu YH, Chen HH, et al. Functional connectivity analysis on electroencephalography signals reveals potential biomarkers for treatment response in major depression. BMC Psychiatry. 2023;23(1):554. doi:10.1186/s12888-023-04958-8
14. Arns M, Etkin A, Hegerl U, et al. Frontal and rostral anterior cingulate (rACC) theta EEG in depression, implications for treatment outcome? Eur Neuropsychopharmacol. 2015;25(8):1190–1200. doi:10.1016/j.euroneuro.2015.03.007
15. Wang X-L, M-Y D, Chen T-L, et al. Neural correlates during working memory processing in major depressive disorder. Prog Neuro Psychopharmacol Biol Psychiat. 2015;56:101–108. doi:10.1016/j.pnpbp.2014.08.011
16. Kaiser AK, Gnjezda M-T, Knasmüller S, Aichhorn W. Electroencephalogram alpha asymmetry in patients with depressive disorders, current perspectives. Neuropsychiatr Dis Treat. 2018;14:1493–1504. doi:10.2147/NDT.S137776
17. Bruder GE, Fong R, Tenke CE, et al. Regional brain asymmetries in major depression with or without an anxiety disorder, a quantitative electroencephalographic study. Biol. Psychiatry. 1997;41(9):939–948. doi:10.1016/S0006-3223(96)00260-0
18. Cooper NR, Croft RJ, Dominey SJ, Burgess AP, Gruzelier JH. Paradox lost? Exploring the role of alpha oscillations during externally vs. internally directed attention and the implications for idling and inhibition hypotheses. Int J Psychophysiol. 2003;47(1):65–74. doi:10.1016/S0167-8760(02)00107-1
19. Wang B, Li M, Haihambo N, et al. Characterizing Major Depressive Disorder (MDD) using alpha-band activity in resting-state electroencephalogram (EEG) combined with MATRICS Consensus Cognitive Battery (MCCB). J Affect Disord. 2024;355:254–264. doi:10.1016/j.jad.2024.03.145
20. World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders, clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization; 1992.
21. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania, reliability, validity and sensitivity. Br J Psychiat. 1978;133(5):429–435. doi:10.1192/bjp.133.5.429
22. Miller IW, Bishop S, Norman WH, Maddever H. The modified Hamilton rating scale for depression, reliability and validity. Psychiatry Res. 1985;14(2):131–142. doi:10.1016/0165-1781(85)90057-5
23. Jones SH, Thornicroft G, Coffey M, Dunn G. A brief mental health outcome scale-reliability and validity of the Global Assessment of Functioning (GAF). Br J Psychiatry. 1995;166(5):654–659. doi:10.1192/bjp.166.5.654
24. Zhang Y, Long X, Ma X, et al. Psychometric properties of the Chinese version of the Functioning Assessment Short Test (FAST) in bipolar disorder. J Affect Disord. 2018;238:156–160. doi:10.1016/j.jad.2018.05.019
25. Rosa AR, Sánchez-Moreno J, Martínez-Aran A, et al. Validity and reliability of the Functioning Assessment Short Test (FAST) in bipolar disorder. Clin Pract Epidemiol Ment Health. 2007;3:5. doi:10.1186/1745-0179-3-5
26. Grootenboer EMV, Giltay EJ, Lem RVD, Veen TV, Njavd W, Zitman FG. Reliability and validity of the Global Assessment of Functioning Scale in clinical outpatients with depressive disorders. J Evaluat Clin Pract. 2012;18(2):502–507. doi:10.1111/j.1365-2753.2010.01614.x
27. Brandt J, Benedict R. Hopkins verbal learning test. Rev Profess Man. 2001;2001:1.
28. Keilp JG, Herrera J, Stritzke P, Cornblatt BA. The continuous performance test, identical pairs version (CPT-IP), III. Brain functioning during performance of numbers and shapes subtasks. Psychiatry Res. 1997;74(1):35–45. doi:10.1016/S0925-4927(96)02881-8
29. Sánchez-Cubillo I, Periáñez J, Adrover-Roig D, Rodríguez-Sánchez J, Ríos-Lago M, Tirapu J. Construct validity of the Trail Making Test, role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J Int Neuropsychol Soc. 2009;15:438–450. doi:10.1017/S1355617709090626
30. Benito-Cuadrado MM, Esteba-Castillo S, Böhm P, Cejudo-Bolívar J, Peña-Casanova J. Semantic verbal fluency of animals, a normative and predictive study in a Spanish population. J Clin Exp Neuropsychol. 2002;24(8):1117–1122. doi:10.1076/jcen.24.8.1117.8376
31. Castellano S, Torrent C, Petralia MC, et al. Clinical and neurocognitive predictors of functional outcome in depressed patients with partial response to treatment, one year follow-up study. Neuropsychiatr Dis Treat. 2020;16:589–595. doi:10.2147/NDT.S224754
32. Pan Z, Park C, Brietzke E, et al. Cognitive impairment in major depressive disorder. CNS Spectr. 2019;24(1):22–29. doi:10.1017/S1092852918001207
33. Bora E, Pantelis C. Meta-analysis of cognitive impairment in first-episode bipolar disorder, comparison with first-episode schizophrenia and healthy controls. Schizophr Bull. 2015;41(5):1095–1104. doi:10.1093/schbul/sbu198
34. Lee RS, Hermens DF, Scott J, et al. A meta-analysis of neuropsychological functioning in first-episode bipolar disorders. J Psychiatr Res. 2014;57:1–11. doi:10.1016/j.jpsychires.2014.06.019
35. Martino DJ, Samamé C, Ibañez A, Strejilevich SA. Neurocognitive functioning in the premorbid stage and in the first episode of bipolar disorder, a systematic review. Psychiatry Res. 2015;226(1):23–30. doi:10.1016/j.psychres.2014.12.044
36. Kurtz MM, Gerraty RT. A meta-analytic investigation of neurocognitive deficits in bipolar illness, profile and effects of clinical state. Neuropsychology. 2009;23(5):551–562. doi:10.1037/a0016277
37. Bays BC, Visscher KM, Le Dantec CC, Seitz AR. Alpha-band EEG activity in perceptual learning. J Vis. 2015;15(10):7. doi:10.1167/15.10.7
38. Clayton MS, Yeung N, Cohen Kadosh R. The many characters of visual alpha oscillations. Eur J Neurosci. 2018;48(7):2498–2508. doi:10.1111/ejn.13747
39. Foster JJ, Sutterer DW, Serences JT, Vogel EK, Awh E. Alpha-band oscillations enable spatially and temporally resolved tracking of covert spatial attention. Psychol Sci. 2017;28(7):929–941. doi:10.1177/0956797617699167
40. Matsuoka T, Shimode T, Ota T, Matsuo K. Event-related alpha-band power changes during self-reflection and working memory tasks in healthy individuals. Front Hum Neurosci. 2020;14:570279. doi:10.3389/fnhum.2020.570279
41. Shen L, Jiang Y, Wan F, Ku Y, Nan W. Successful alpha neurofeedback training enhances working memory updating and event-related potential activity. Neurobiol Learn Mem. 2023;205:107834. doi:10.1016/j.nlm.2023.107834
42. Xia J, Mazaheri A, Segaert K, et al. Event-related potential and EEG oscillatory predictors of verbal memory in mild cognitive impairment. Brain Commun. 2020;2(2):fcaa213. doi:10.1093/braincomms/fcaa213
43. Raufi B, Longo L. An evaluation of the EEG alpha-to-theta and theta-to-alpha band ratios as indexes of mental workload. Front Neuroinf. 2022;16:861967. doi:10.3389/fninf.2022.861967
44. Klimesch W. α-band oscillations, attention, and controlled access to stored information. Trends Cognit Sci. 2012;16(12):606–617. doi:10.1016/j.tics.2012.10.007
45. Ronnqvist KC, McAllister CJ, Woodhall GL, Stanford IM, Hall SD. A multimodal perspective on the composition of cortical oscillations. Front Hum Neurosci. 2013;7:132. doi:10.3389/fnhum.2013.00132
46. Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations, the inhibition-timing hypothesis. Brain Res Rev. 2007;53(1):63–88. doi:10.1016/j.brainresrev.2006.06.003
47. Miller EK, Lundqvist M, Bastos AM. Working Memory 2.0. Neuron. 2018;100(2):463–475. doi:10.1016/j.neuron.2018.09.023
48. Feffer K, Lee HH, Wu W, et al. Dorsomedial prefrontal rTMS for depression in borderline personality disorder, A pilot randomized crossover trial. J Affective Disorders. 2022;301:273–280. doi:10.1016/j.jad.2021.12.038
49. Keller AS, Ball TM, Williams LM. Deep phenotyping of attention impairments and the ‘inattention biotype’ in major depressive disorder. Psychological Medicine. 2020;50(13):2203–2212. doi:10.1017/S0033291719002290
50. Tement S, Pahor A, Jaušovec N. EEG alpha frequency correlates of burnout and depression, The role of gender. Biol Psychol. 2016;114:1–12. doi:10.1016/j.biopsycho.2015.11.005
51. Foster JJ, Awh E. The role of alpha oscillations in spatial attention, limited evidence for a suppression account. Curr Opin Psychol. 2019;29:34–40. doi:10.1016/j.copsyc.2018.11.001
52. Gheza D, Bakic J, Baeken C, De Raedt R, Pourtois G. Abnormal approach-related motivation but spared reinforcement learning in MDD, Evidence from fronto-midline Theta oscillations and frontal Alpha asymmetry. Cogn Affect Behav Neur. 2019;19(3):759–777. doi:10.3758/s13415-019-00693-4
53. Zazio A, Miniussi C, Bortoletto M. Alpha-band cortico-cortical phase synchronization is associated with effective connectivity in the motor network. Clin Neurophysiol. 2021;132(10):2473–2480. doi:10.1016/j.clinph.2021.06.025
54. Hindriks R, van Putten MJ. Thalamo-cortical mechanisms underlying changes in amplitude and frequency of human alpha oscillations. Neuroimage. 2013;70:150–163. doi:10.1016/j.neuroimage.2012.12.018
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.