Back to Journals » Drug Design, Development and Therapy » Volume 18
Revealing Changes in Celecoxib Nanostructured Lipid Carrier’s Bioavailability Using Hyaluronic Acid as an Enhancer by HPLC-MS/MS
Authors Zhu Y, Chen M, Yang C, Lu G, Huang S , Chen M, Wang Y, Ban J
Received 30 January 2024
Accepted for publication 18 June 2024
Published 29 July 2024 Volume 2024:18 Pages 3315—3327
DOI https://doi.org/10.2147/DDDT.S461969
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Yi Zhu,1,2,* Meiling Chen,3,* Chuangzan Yang,1,2 Geng Lu,1,2 Sa Huang,1,2 Meili Chen,3 Yufei Wang,4 Junfeng Ban1– 3
1Guangdong Pharmaceutical University, Guangzhou, People’s Republic of China; 2The Innovation Team for Integrating Pharmacy with Entrepreneurship, Guangdong Pharmaceutical University, Guangzhou, People’s Republic of China; 3Guangdong Laboratory Animals Monitoring Institute, Guangdong Provincial Key Laboratory of Laboratory Animals, Guangzhou, People’s Republic of China; 4Analytical and Testing Center of Guangzhou University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yufei Wang; Junfeng Ban, Email [email protected]; [email protected]
Purpose: Oral drug administration is the most common and convenient route, offering good patient compliance but drug solubility limits oral applications. Celecoxib, an insoluble drug, requires continuous high-dose oral administration, which may increase cardiovascular risk. The nanostructured lipid carriers prepared from drugs and lipid excipients can effectively improve drug bioavailability, reduce drug dosage, and lower the risk of adverse reactions.
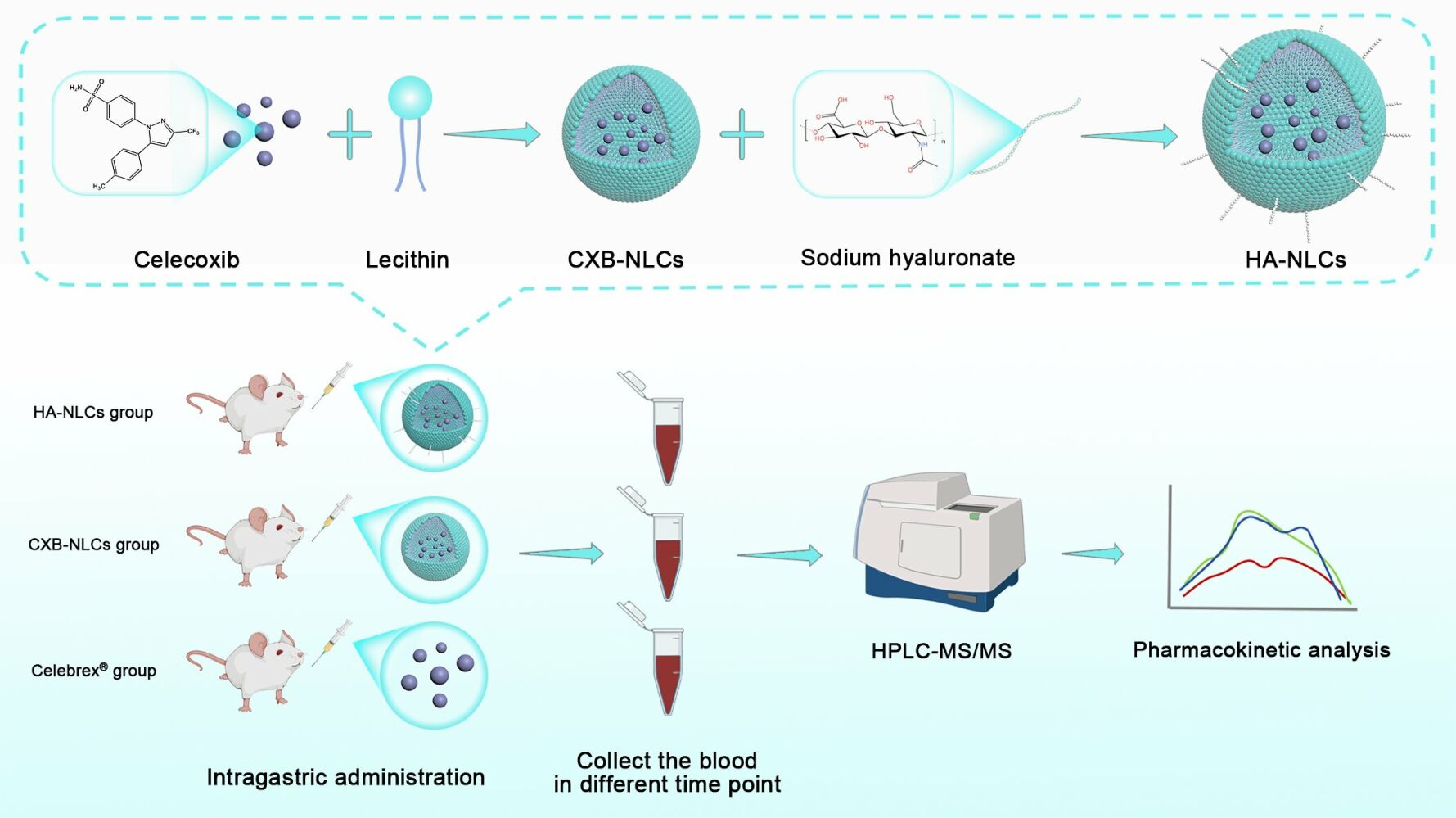
Methods: In this study, we prepared hyaluronic acid-modified celecoxib nanostructured lipid carriers (HA-NLCs) to improve the bioavailability of celecoxib and reduce or prevent adverse drug reactions. Meanwhile, we successfully constructed a set of FDA-compliant biological sample test methods to investigate the pharmacokinetics of HA-NLCs in rats.
Results: The pharmacokinetic analysis confirmed that HA-NLCs significantly enhanced drug absorption, resulting in an AUC0-t 1.54 times higher than the reference formulation (Celebrex®). Moreover, compared with unmodified nanostructured lipid carriers (CXB-NLCs), HA-NLCs enhance the retention time and improve the drug’s half-life in vivo.
Conclusion: HA-NLCs significantly increased the bioavailability of celecoxib. The addition of hyaluronic acid prolonged the drug’s in vivo duration of action and reduced the risk of cardiovascular adverse effects associated with the frequent administration of oral celecoxib.
Keywords: nanostructured lipid carriers, celecoxib, HPLC-MS/MS, pharmacokinetics
Graphical Abstract:
Introduction
Oral administration is the most common and convenient drug delivery route with favorable patient compliance. However, conventional oral preparations are adversely affected by gastrointestinal enzymes, acid or alkaline environments, and the first-pass effect of the liver. The drug’s solubility is critical in determining its efficacy. Low solubility hinders drug absorption, leading to poor bioavailability and reduced efficacy, which is especially problematic for insoluble drugs.1,2 Oral nano-drug delivery systems have the potential to address the aforementioned issues, thereby enhancing bioavailability, improving targeting, and drug stability while simultaneously mitigating adverse reactions, which are considered significant areas in pharmacy research.3 Celecoxib, an FDA-recommended non-steroidal anti-inflammatory drug in osteoarthritis clinical applications,4 is a selective cyclooxygenase 2 inhibitor that exerts analgesic and anti-inflammatory effects through prostaglandin inhibition. Despite its widely acknowledged efficacy, celecoxib faces significant pharmacokinetic limitations, including poor water solubility leading to reduced bioavailability and first-pass effect after oral administration5. Furthermore, it exhibits a high plasma protein binding rate, impeding its extensive distribution in various tissues and organs.6 The continuous administration of high doses increases the risk of adverse cardiovascular effects. Therefore, satisfying osteoarthritis patients’ compliance, while immensely improving oral absorption, reducing drug dosages and reducing drug toxicity is one strategy to develop a novel delivery system for celecoxib.
Nano formulations prepared by mixing drugs with lipid excipients can augment drug bioavailability. Nanostructured lipid carriers can interact with cells during oral administration through processes like endocytosis, transcytosis, and cell membrane fusion. These interactions help to overcome the epithelial barrier, leading to enhanced oral absorption, improved drug stability, and protection of drug release.7 Hyaluronic acid (HA) can directly enter the body, travel to the tissues, and the low molecular weight HA facilitates better penetration of the intestinal barrier during oral administration.8 The organic combination of the two may further improve the intestinal barrier permeability and bioavailability of the drug.
Pharmacokinetic analysis provides an effective characterization of in vivo changes in drugs. The Mitrovic team demonstrated the enhanced cellular uptake of loaded DK-I-60-3 oral lipid nanoparticles due to their increased affinity and bio-adhesion to the intestinal wall by pharmacokinetic studies.9 Furthermore, the mixed micelles formed by lipid degradates and bile salts in vivo were found to enhance drug permeability and absorption. Therefore, pharmacokinetic research on lipid-based drug formulations should broaden their focus beyond the free drug itself and consider the dynamic changes occurring within the drug-lipid excipient mixtures in vivo. This approach puts forward new requirements and challenges for the bio-analytical methods of lipid-based nano preparations. Common analytical methods for lipid-based nanoparticles include high-performance liquid chromatography (HPLC) and high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). HPLC-MS/MS synergize the advantages of chromatography and mass spectrometry with high sensitivity and specificity, allowing the qualitative and quantitative analysis of a wide range of sample components. Estella-Hermoso’s team conducted a comparative analysis of two analytical methods, HPLC-MS and HPLC-MS/MS, for the pharmacokinetic determination of lipid nanoparticle systems.10 The application of HPLC-MS/MS led to a substantial decrease in the duration required for analyzing the drug. Moreover, a three-fold decrease in retention time and a two-fold reduction in the asymmetry factor were also observed. Furthermore, HPLC-MS/MS exhibited a higher level of sophistication and achieved precision in the range of 0.0075~75 μg·mL−1, which is notably higher than the precision range of 0.1~75 μg·mL−1 obtained via HPLC-MS. These improvements are particularly advantageous for the clinical application of lipid nanoparticles, as they facilitate the monitors of drug blood concentrations and the minimization or avoidance of adverse reactions.
This study aimed to develop an HPLC-MS/MS biological sample assay to investigate the oral pharmacokinetics of lipid-based and surface hyaluronic acid-modified celecoxib nanostructured lipid carriers (HA-NLCs). This study also sought to reveal the potential enhancement of hyaluronic acid-modified nanostructured lipid carriers for improving the oral bioavailability of the drug by comparing the reference formulation (Celebrex®) with unmodified celecoxib nanostructured lipid carriers (CXB-NLCs).
Materials and Methods
Materials
Celecoxib (purity>98%) was purchased from Sigma-Aldrich Co (St Louis, MO, USA) and celecoxib-D7 was purchased from CFW Laboratories Inc. (purity = 98.2%, Newark, DE, USA). Celecoxib API was purchased from Jiangxi Tonghe Pharmaceutical Co., Ltd (Yichun, China). Celebrex® capsules, each containing 200 mg of celecoxib, were acquired from Pfizer (Seoul, Korea). Ultrapure water was prepared using the Lab Tower EDI 15 system (Thermo Scientific, Langenselbold, Germany). All other analytical grade chemical reagents used in this study were sourced from Hainan Yigao Instrument Co., LTD (Haikou, China).
Preparation and Characterization of Celecoxib Nanostructured Lipid Carriers
The high-pressure homogenization technique is commonly used to prepare nanostructured lipid carriers. In this study, CXB-NLCs and HA-NLCs were prepared using a combination of solvent volatilization and high-pressure homogenization as detailed in this report.11 To confirm the successful preparation of both, the study was carried out to determine the particle size, zeta-potential as well as the content and encapsulation rate accordingly.
The particle size, polydispersity index (PDI), and zeta-potential of CXB-NLCs and HA-NLCs were measured at (25 ± 1) °C using a laser particle sizer (Delsa Nano C, Beckman Coulter Inc, USA). To minimize the impact of multiple scattering phenomena on the measurement, the drug-loaded nanocarriers were diluted 20 times with deionized water in the experiments (n = 3).
The micromorphology of CXB-NLCs and HA-NLCs was examined using negative stain under a transmission electron microscope (TEM, Gemini SEM300, ZEISS, Germany). To prepare the sample for observation under a TEM, first, the sample is diluted 20 times. The diluted sample was then dropped onto a hydrophilic treated copper mesh supported by a 200-mesh carbon film. Allowing the surface liquid to be removed after 5 minutes, 2% phosphotungstic acid was dropped for staining, with a duration of 2 minutes. Following staining, any excess surface liquid is carefully absorbed using filter paper. Subsequently, the sample is left to dry naturally before proceeding with observation under the TEM.
1 mL of CXB-NLCs or HA-NLCs was added to a 10 mL volumetric flask, filled the flask with methanol, followed by sonication at 53 kHz for 10 minutes to determine the total concentration of celecoxib (C1). On the other hand, 200 μL of drug-loaded nanocarriers were added to a dextran gel microcolumn with a column height of 2 cm. Then, water was added to the microcolumn as eluent and centrifuged at 2000 rpm for 4 minutes. The nanostructured lipid carriers will elute preferentially, and the concentration of celecoxib in this carrier (C2) would be determined and calculated the encapsulation rate.
Pharmacokinetic Study and Sample Collection
Pharmacokinetics can be used to study the process of absorption, distribution, metabolism and excretion of drugs in the body. The study was used to investigate the temporal trajectory of celecoxib (as well as nanostructured lipid carriers) plasma concentrations in Sprague-Dawley (SD) rats. This was done to examine the effect of lipid nanotechnology on oral bioavailability of celecoxib and other pharmacokinetic properties. Female SD rats, aged 6 weeks and weighed about 100–120 g, were purchased from Guangdong Medical Laboratory Animal Center (SCXK 2022–0002) in the study. The rats were randomly divided into 3 groups (n=6/group) and fasted overnight with free access to water before the experiment. Celebrex®, CXB-NLCs, and HA-NLCs were administered to the three groups of rats by oral gavage at a dose of 5 mg·kg−1, and the drug suspension was shaken well before administration. The rats of Celebrex® group were gavaged with a suspension obtained by dispersing 200 mg Celebrex® capsules in 400 mL of ultrapure water. Blood samples were collected from the orbital vein at specific time intervals (0, 0.25, 0.5, 0.75, 1, 2, 4, 8, 10, and 24 h) after the administration to each rat. Following sample collection, whole blood was transferred to heparinized round-bottomed centrifuge tubes and centrifuged at 4000 r·min−1 and 4 °C for 15 minutes. Subsequently, the supernatant was transferred into another sharp-bottomed plastic centrifuge tube and stored in a refrigerator at −80 °C until future use.
Extraction of Celecoxib from
Celecoxib plasma concentrations were determined using the internal standard method.12 In brief, after the plasma samples were returned to room temperature, 50 μL of rat plasma was transferred into an EP tube. Subsequently, 30 ng of celecoxib-D7 isotopic internal standard was added into a 0.1% methanol formate solution and vortexed for 10 min. This solution was then centrifuged at 13,000 rpm for 10 min. About 100 μL of the supernatant was transferred, and the drug concentrations were determined by HPLC/MS-MS.
Statistical Analysis
The pharmacokinetic parameters of each celecoxib formulation were analyzed by a non-compartment model analysis using DSA2.0 software. The GraphPad Prism 9.3.1 software was used to for one-way ANOVA analysis and plotted drug plasma concentration curve. Moreover, p < 0.05 was considered to be statistically significant.
Analyze Conditions
Chromatographic Conditions
The Agilent Eclipse Plus C18 RRHD column (2.1×50 mm, 1.8 μm) was used for HPLC. The mobile phases were 0.01% formic acid in water (A) and acetonitrile (B). A gradient elution was used with the following conditions: 0–0.8 min, 10.0% B; 0.80–1.00 min, 10–30% B; 1.00–1.50 min, 30%; B; 1.50–2.00 min, 30–95% B; 2.00–4.50 min, 95% B; 4.50–5.00 min, 10%. The mobile-phase flow rate was 0.32 mL·min−1. The column temperature was set at 40 °C. The injection volume was 2.00 μL and the total analysis time was 3 min.
Mass Spectrometry Conditions
The HPLC-MS/MS system was constructed both of the Shimadzu Nexera XR ultra-high liquid chromatography HPLC (Kyoto, Japan) and the AB sciex API 4000 plus triple quadruple mass spectrometer (Toronto, Canada). It was also equipped with a Turbo V electrospray ionization (ESI) probe, two LC-20 AD pumps, a DGU-20A3R degassing unit, a SIL-20A autosampler and a CTO-20A column heating box. Control of the HPLC-MS/MS system and implementation of data acquisition and analysis was done through the AB-SCIEX software. The ESI interface was run in negative ion mode with Multi-Reactive Ion Model Monitoring (MRM) selected, wherein the spray voltage was set to –4500 V, the ionization temperature was set to 500 °C, the collision air pressure was set to 3 psi, the air curtain air pressure was set to 30 psi, the spray air pressure was set to 50 psi and the auxiliary heating air pressure was set to 45 psi.
Solution Preparation
Standard Stock and Internal Standard Solutions
10 mg of celecoxib standard was dissolved in methanol to obtain a 1 mg·mL−1 stock solution, which was then serially diluted with methanol to obtain 10, 25, 50, 100, 1000, 10,000, 20000 ng·mL−1 standard working solution. Celecoxib-D7 was prepared in methanol containing 0.1% formic acid to obtain 1 mg·mL−1 stock solution and then further diluted to 200 μg·mL−1 internal standard solution. The standard stock solution, standard working solutions and internal standard solution were stored at −20 °C prior to use.
Calibration Standard and Quality Control Sample Preparation
Plasma calibration standards were prepared at concentrations of 1, 2.5, 5, 10, 100, 1000, and 2000 ng·mL−1, respectively. 5 μL of standard working solution of various concentrations was mixed with 45 μL of blank plasma. Similarly, the lower limit of quantification (LLOQ) sample (2.5 ng·mL−1) and three plasma quality control (QC) samples (6 ng·mL−1, 120 ng·mL−1 and 1600 ng·mL−1) were obtained by adding the celecoxib standard working solution to the blank plasma containing celecoxib-D7.
Method Validation of Plasma Samples
Our analytical methods were validated for specificity, linearity, accuracy, precision, matrix effects, extraction effects and stability. This was done in compliance with the recommended criteria published in the FDA’s Guidance on Validation of Bioanalytical Methods.13
Specificity
HPLC-MS/MS was performed on blank plasma samples, celecoxib, plasma samples containing the internal standard and oral plasma samples. These were acquired from the Celebrex® group, the CXB-NLCs group, and the HA-NLCs group, respectively. The chromatogram outputs were used to detect possible endogenous interference.
Linearity and Lower Limit of Quantification (LLOQ)
Plasma calibration standard samples (n=6) were assayed and a standard curve for celecoxib constructed. The peak area ratio of celecoxib to internal standard was used as the y-axis, and celecoxib concentration as the x-axis. Subsequently, a linear equation was fitted through linear regression, utilizing a weighted regression method (1/X2). It was imperative that the correlation coefficient exceeds 0.999. The lower limit of quantitation (LLOQ) was defined as the lowest concentration on the calibration curve that maintains a signal-to-noise ratio (S/N) of ≥10 with precision and accuracy within 20%.
Precision and Accuracy
Assessment of the intra- and inter-day accuracy and precision, the low, medium, and high concentration QC samples and the LLOQ were repeated six times over three consecutive days. Accuracy values were evaluated using the percentage relative error (RE), which measures the percentage deviation of the mean output concentration and the actual concentration. Meanwhile, precision values were determined by calculating the relative standard deviation (RSD) at each concentration.
Extraction Effects (EE) and Matrix Effects (ME)
The extraction effect of celecoxib is the percentage of the peak area ratio of the pre-extraction spiked sample found in the peak area ratio of the pure standard solution. It was quantified over the average of six replicates at the three QC concentrations. Matrix effects were determined by comparing the peak area ratio of spiked extracted samples to pure standard solutions at three QC concentrations (n=6). For this, six samples were analyzed in parallel for each group. Ideally, the EE% and ME% should be kept within 85 ~ 115%.
Stability
The stability of the low, medium, and high QC samples was assessed under various storage conditions. These include: (a) 6 hours of bench-top stabilization at room temperature, (b) 12 hours of stabilization under the autosampler tray, (c) three freeze-thaw cycles from −80 °C to room temperature, and (d) storage at or below −20 °C for 10 days. Each QC samples underwent analysis in triplicate to evaluate the stability of the sample content under each condition. The RE and RSD remained within 15% for the samples indicating high stability in all instances.
Results and Discussion
Characterization of Celecoxib Nanostructured Lipid Carriers
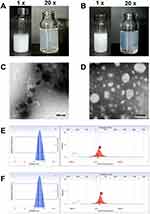
Both CXB-NLCs and HA-NLCs prepared in the study showed a milky white color, and a light blue milky light could be seen on the surface after dilution (Figure 1A and B), which indicated that both of them had nanoproperties.14 The microstructures of the two were further observed by TEM. The results showed that the CXB-NLCs were irregularly square and appeared black (Figure 1C), whereas the HA-NLCs had smooth and rounded surfaces and were white (Figure 1D). The average particle size of CXB-NLCs was (98.20 ± 14.36) nm, PDI was (0.251 ± 0.001), and zeta-potential was (−24.19 ± 3.54) mV (Figure 1E, n=3) as determined by the laser particle sizer, while the average particle size of HA-NLCs was (93.57 ± 10.89) nm, PDI was (0.251 ± 0.003), and zeta-potential was (−32.98 ± 1.36) mV (Figure 1F, n=3). Both two exhibited negatively charged surfaces, favoring intestinal epithelial uptake, with HA-NLCs showing greater stability. In the content and encapsulation rate assay, the content of CXB-NLCs was (535.85 ± 8.52) μg·mL−1 and the encapsulation rate was (84.36 ± 1.21)%, while the content and encapsulation rate of HA-NLCs were (495.76 ± 9.13) μg·mL−1 and (89.67 ± 0.62)%, respectively.
HPLC-MS/MS and Chromatographic Optimization
During the oral pharmacokinetic experiments, celecoxib plasma concentrations within the quantitative range of 1~2000 ng·mL−1 were determined. The commonly used high-performance liquid chromatography-ultraviolet detection (HPLC-UV) has a high detection limit, which made it difficult to detect the samples in this study and prone to serious errors. Therefore, we decided to shift to HPLC-MS/MS system to detect the drug content of samples. In HPLC-MS/MS system, HPLC first separates the substances from the sample to be measured and subsequently converts the ions of the substance to be measured into an electrical signal, which is then analyzed by a mass spectrometry peak. The combination of the separation capability of HPLC and the high sensitivity and selectivity of MS greatly improve the detection capability.15
The small, ultra-dense bonded Agilent Eclipse Plus C18 RRHD column (2.1 × 50 mm, 1.8 μm) is often an effective choice. It exhibits excellent peak shape for samples in the pH range of 2 to 9 and reduces adsorption for alkaline analyses. This column is particularly efficacious in the analysis of hydrophobic compounds like celecoxib, which contains a sulfonamide group and a pyrazole ring and displays good solubility in methanol.12,13 Appropriate acidic conditions could improve the extraction and separation of celecoxib and reduce the trailing problem. In this study, celecoxib in plasma was extracted using methanol containing 0.1% formic acid and separated using water with 0.01% formic acid and acetonitrile as the mobile phase, which was able to produce symmetrical peaks with good separation.
Celecoxib-D7 was chosen as an internal standard given the fact that it is known to significantly improve matrix effects, whilst not interfering with the analyte.16 Celecoxib and celecoxib-D7 tend to lose electrons and are positively charged. This means that they can show better responsiveness and sensitivity in ESI and MRM modes. In the HPLC-MS/MS system, the ESI source ionizes the sample and detects it via MRM mode, using a single ion channel for celecoxib and celecoxib-D7 to achieve optimal selectivity, accelerating data acquisition.17 The maximum parent and daughter ion abundances of celecoxib and celecoxib-D7 exhibited highly selective negative ionization mode jumps. Figure 2 shows the daughter ion mass spectra and ion structures of celecoxib and celecoxib-D7 in negative ionization mode with their respective ion pairs: m/z 380.0➔ 316.0 (DP, −135; CE, −31) and m/z 387.1➔ 323.1 (DP, −130; CE, −30).
 |
Figure 2 Sub-ion patterns and chemical structures of celecoxib (A) and celecoxib-D7 (B). |
Method Validation
The study methodology was validated according to the FDA requirements.13 Figure 3 depicted dual-channel images of celecoxib and celecoxib-D7 in an HPLC-MS/MS system for various plasma samples, including blank plasma samples, plasma samples containing celecoxib-D7, oral plasma samples from the Celebrex® group, the CXB-NLCs group, and the HA-NLCs group. Notably, celecoxib’s retention time was 3.05 min and no interference peaks impacting its quantification were observed.
The linear fit of the concentration range was determined to be y = 0.00167x + 0.00182, with a high correlation coefficient, r = 0.9992, indicating a strong linear relationship between the peak area ratio of celecoxib to internal standard and the concentration of celecoxib. This finding was established within the target linear concentration range of 1~2000 ng·mL−1. The lower limit for quantification in plasma samples was determined to be 2.5 ng·mL−1.
The accuracy of QC samples at three concentrations ranged from 97.24% to 109.94%, as shown in Table 1. The intra-day precision and inter-day precision RSD ranged from 2.67% to 6.37%, remaining within the 15% target threshold. Meanwhile, the intra-day precision for LLOQ was 8.78%, the inter-day precision was 10.19%, and the accuracy ranged from 87.05% to 92.94%. These values fall within the target range and comply with FDA standards, indicating the feasibility and reproducibility of the extraction method for determining celecoxib in rat plasma.
 |
Table 1 The Examination of the Precision and Accuracy of Celecoxib in Plasma |
Table 2 shows the matrix effects and extraction effects of celecoxib determined at low, medium and high QC sample concentrations. The extraction effects were (99.90 ± 3.06)%, (94.91 ± 4.13)%, (92.18 ± 5.03)% with RSD of 3.06%, 4.36% and 5.45%, respectively. The RSD of the extraction effects remained in the range of 15% and was close to 100% at all concentrations. Overall, the method was considered effective for efficient and reproducible extraction of celecoxib from rat plasma samples. While the mean matrix effect and RSD in plasma were (100.18 ± 9.66)%, (92.12 ± 1.43)%, (87.27 ± 8.05)% and 9.64%, 1.55% and 9.23%, respectively, which also complied with the assay.
 |
Table 2 The Results of Matrix Effect and Extraction Effect (n = 6) |
The stability of celecoxib in plasma was assessed through a series of stability tests using QC samples at low, medium, and high concentrations. The accuracy of all the samples, as summarized in Table 3, fell within a range of 94.77% to 111.67%. This indicated the stability of celecoxib in plasma to be within acceptable limits under all four conditions.
 |
Table 3 Stability of Celecoxib Under Four Storage Conditions (n = 6) |
Pharmacokinetics of HA-NLCs
The study’s findings, as depicted in Figure 4, provide a comprehensive understanding of the oral pharmacokinetic changes observed in CXB-NLCs and HA-NLCs compared to the reference formulation, Celebrex®. The utilization of lipid nanotechnology demonstrates a notable impact on oral absorption, as evidenced by the analysis of time-blood concentration curves. Both CXB-NLCs and HA-NLCs exhibit heightened drug concentrations compared to the Celebrex® group. CXB-NLCs and HA-NLCs reached the peak drug concentration at 2 h, but the concentration of CXB-NLCs decreased more rapidly, which was related to the susceptibility of the nanostructured lipid carriers to be captured by opsonization effects.18 Of particular interest was the unique behavior observed in the HA-NLCs group, characterized by a slow change in concentration and the presence of double peaks in the curve. This suggested the hyaluronic acid modification to protract nanostructured lipid carriers degradation, indicating the existence of alternative transporter pathways or cyclic processes.19 The lower molecular weight of HA has strong enterocyte permeability and lymphatic transport capacity, and the modification of the surface of the nanostructured lipid carriers enhances the intestinal permeability. Meanwhile, the stomach and small intestine have no naturally occurring HA digestive enzymes, which makes HA easy to enter the body directly and achieve distribution20. However, it is noteworthy that cecum contents and intestinal flora were able to degrade HA on the surface of HA-NLCs, while this process did not affect lipid metabolism.16,21 Therefore, when the HA on the carrier surface completes its degradation in vivo, HA-NLCs likewise show a faster metabolic elimination. The metabolic process of HA does not affect lipid metabolism but accelerates the elimination of surface modifiers of HA-NLCs, resulting in faster metabolic elimination in vivo.
 |
Figure 4 Plasma concentration vs time profiles of Celebrex® (●), CXB-NLCs (■), HA-NLCs (▲) in SD rats (mean ± SD, n = 6). |
The study aimed to analyze the pharmacokinetic parameters of the two nanostructured lipid carriers in comparison to the reference formulation using DAS software (Table 4). The results revealed the AUC0-t to be (18371.69 ± 5585.27) h∙ng∙mL−1 and (19985.26 ± 2207.79) h∙ng∙mL−1 in the CXB-NLCs group and HA-NLCs group, respectively. These values were 1.42-fold and 1.54-fold higher than that of the Celebrex® group. Additionally, the Cmax was 1.65-fold and 1.60-fold higher than that of Celebrex® group, suggesting an improvement in celecoxib bioavailability. Similar to previous studies,16 the half-life of celecoxib was shortened by CXB-NLCs. Common nanostructured lipid carriers improved absorption but was found to be accompanied by accelerated drug metabolism, leading to a shorter half-life of celecoxib. When ordinary lipid carriers enter the circulation, they rapidly become protein-bound, resulting in the formation of a protein corona. This protein corona accelerates the recognition and clearance of the carriers by the mononuclear phagocyte system.22 HA modification partially reduced the degradation of the lipid carriers and prolong the half-life, which began to significantly decline after 8 h.
 |
Table 4 The Pharmacokinetic Parameters of Celecoxib in Different Forms (n = 6) |
The low solubility of celecoxib makes it difficult to absorb the large amount of undissolved drug by the intestinal barrier, thus its oral application needs to be accomplished with high frequency and multiple doses, which increases the risk of cardiovascular adverse effects of the drug. Maru’s23 research revealed that commercially available celecoxib led to elevated levels of cardiac biomarkers troponin I and CK-MB in rats when administered through gavage, resulting in slight myocardial damage and an increased cardiac risk. In this study, CXB-NLCs and HA-NLCs can improve celecoxib solubility and facilitate drug transport through the intestinal barrier using the carriers. At the same time, the nanometer particle size facilitates intestinal permeation absorption, potentially lowering the required frequency and dosage of drug administration in practical applications.24 Additionally, the interaction between HA and CD44 can enhance drug delivery and retention at the inflamed joint site.25 Kang’s report revealed that HA nanoparticles can suppress joint inflammation and safeguard cartilage function, presenting a novel idea for the further development of this study.26 In conclusion, the modification of the nanostructured lipid carriers with hyaluronic acid further enhanced the oral bioavailability of celecoxib in rats. This modification not only improved the absorption of the drug but also prolonged its likely pharmacological activity, which to a certain extent reduces the dosage and frequency of the drug in patients, thus reducing the risks of possible cardiovascular side-effects of celecoxib.18,19
Conclusion
In this study, we developed an analytical method based on HPLC/MS-MS to analyze drug-lipid mixtures in biological samples, which satisfied the FDA’s bioanalytical method validation standards. This bio-sample analytical method proved effective in determining the oral pharmacokinetics of hyaluronic acid modified nanostructured lipid carriers, HA-NLCs. Hyaluronic acid modification of lipid carriers enhances drug transport and intestinal absorption, prolongs drug circulation time in the body, and effectively reduces the dosage and frequency of drugs in patients, thereby reducing the risk of cardiovascular adverse effects associated with celecoxib.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval
The experiment was approved prior to commencement by the Guangdong Pharmaceutical University Institutional Animal Ethics and Use Committee, which ensured that animal experiments were in compliance with the National Institutes of Health’s Guidelines for the Ethics and Use of Laboratory Animals.
Acknowledgments
We like to thank the Natural Science Foundation of Guangdong Province (Grant No 2023A1515010470) and the National Natural Science Foundation of China (81941010) for financial support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Elbrink K, Van Hees S, Roelant D, Loomans T, Holm R, Kiekens F. The influence on the oral bioavailability of solubilized and suspended drug in a lipid nanoparticle formulation: in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2022;179:1–10. doi:10.1016/j.ejpb.2022.08.010
2. Padhye T, Maravajjala KS, Swetha KL, Sharma S, Roy A. A comprehensive review of the strategies to improve oral drug absorption with special emphasis on the cellular and molecular mechanisms. J Drug Delivery Sci Technol. 2021;61:102178. doi:10.1016/j.jddst.2020.102178
3. Sultana A, Zare M, Thomas V, Kumar TSS, Ramakrishna S. Nano-based drug delivery systems: conventional drug delivery routes, recent developments and future prospects. Med Drug Discover. 2022;15:100134. doi:10.1016/j.medidd.2022.100134
4. Haartmans MJJ, Timur UT, Emanuel KS, et al. Evaluation of the anti-inflammatory and chondroprotective effect of celecoxib on cartilage ex vivo and in a rat osteoarthritis model. Cartilage. 2022;13(3):194760352211155. doi:10.1177/19476035221115541
5. Gong L, Thorn CF, Bertagnolli MM, Grosser T, Altman RB, Klein TE. Celecoxib pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012;22(4):310–318. doi:10.1097/FPC.0b013e32834f94cb
6. Begum MY, Osmani RAM, Alqahtani A, et al. Development of stealth liposomal formulation of celecoxib: in vitro and in vivo evaluation. PLoS One. 2022;17(4):e0264518. doi:10.1371/journal.pone.0264518
7. Haddadzadegan S, Dorkoosh F, Bernkop-Schnürch A. Oral delivery of therapeutic peptides and proteins: technology landscape of lipid-based nanocarriers. Adv Drug Deliv Rev. 2022;182:114097. doi:10.1016/j.addr.2021.114097
8. Kobayashi T, Chanmee T, Itano N. Hyaluronan: metabolism and function. Biomolecules. 2020;10(11):1525. doi:10.3390/biom10111525
9. Mitrović JR, Divović-Matović B, Knutson DE, et al. High amount of lecithin facilitates oral delivery of a poorly soluble pyrazoloquinolinone ligand formulated in lipid nanoparticles: physicochemical, structural and pharmacokinetic performances. Int J Pharm. 2023;633:122613. doi:10.1016/j.ijpharm.2023.122613
10. Estella-Hermoso De Mendoza A, Campanero MA, Mollinedo F, Blanco-Príeto MJ. Comparative study of A HPLC–MS assay versus an UHPLC–MS/MS for anti-tumoral alkyl lysophospholipid edelfosine determination in both biological samples and in lipid nanoparticulate systems. J Chromatogr B. 2009;877(31):4035–4041. doi:10.1016/j.jchromb.2009.10.020
11. Qin Z, Lv G, Wang T, et al. The delivery of nanoparticles improves the pharmacokinetic properties of celecoxib to open a therapeutic window for oral administration of insoluble drugs. Biomed Chromatogr. 2023;37(2):e5552. doi:10.1002/bmc.5552
12. Lou Y, Sun Z, Chai Y, et al. Simultaneous quantification of donafenib, sorafenib, and their N-oxide metabolites in rat plasma using a HPLC-MS/MS method. J Chromatogr B. 2023;1229:123871. doi:10.1016/j.jchromb.2023.123871
13. Meesters R, Voswinkel S. Bioanalytical method development and validation: from the USFDA 2001 to the USFDA 2018 guidance for industry. J Appl Bioanal. 2018;4(3):67–73.
14. Singh Y, Meher JG, Raval K, et al. Nanoemulsion: concepts, development and applications in drug delivery. J Control Release. 2017;252:28–49. doi:10.1016/j.jconrel.2017.03.008
15. Zhao L, Li F. UHPLC-MS strategies and applications for bioanalyses related to pharmacokinetics and drug metabolism. Trends Anal Chem. 2014;63:170–179. doi:10.1016/j.trac.2014.08.006
16. Dongari N, Sauter ER, Tande BM, Kubátová A. Determination of Celecoxib in human plasma using liquid chromatography with high resolution time of flight-mass spectrometry. J Chromatogr B. 2014;955–956:86–92. doi:10.1016/j.jchromb.2014.02.012
17. Ma Y, Gao S, Hu M. Quantitation of celecoxib and four of its metabolites in rat blood by UPLC-MS/MS clarifies their blood distribution patterns and provides more accurate pharmacokinetics profiles. J Chromatogr B. 2015;1001:202–211. doi:10.1016/j.jchromb.2015.07.026
18. Le N, Cao V, Nguyen T, Le TTH, Tran TT, Hoang Thi TT. Soy lecithin-derived liposomal delivery systems: surface modification and current applications. Int J Mol Sci. 2019;20(19):4706. doi:10.3390/ijms20194706
19. Lu Y, Wu L, Lin M, et al. Double layer spherical nanoparticles with hyaluronic acid coating to enhance oral delivery of exenatide in T2DM rats. Eur J Pharm Biopharm. 2023;191:205–218. doi:10.1016/j.ejpb.2023.09.003
20. Zheng X, Wang B, Tang X, et al. Absorption, metabolism, and functions of hyaluronic acid and its therapeutic prospects in combination with microorganisms: a review. Carbohydr Polym. 2023;299:120153. doi:10.1016/j.carbpol.2022.120153
21. Park MS, Shim WS, Yim SV, Lee KT. Development of simple and rapid LC–MS/MS method for determination of celecoxib in human plasma and its application to bioequivalence study. J Chromatogr B. 2012;902:137–141. doi:10.1016/j.jchromb.2012.06.016
22. Scioli Montoto S, Muraca G, Ruiz ME. Solid lipid nanoparticles for drug delivery: pharmacological and biopharmaceutical aspects. Front Mol Biosci. 2020;7. doi:10.3389/fmolb.2020.587997
23. Maru S, Verma J, Wilen CE, Rosenholm JM, Bansal KK. Attenuation of celecoxib cardiac toxicity using Poly(δ-decalactone) based nanoemulsion via oral route. Eur J Pharm Sci. 2023;190:106585. doi:10.1016/j.ejps.2023.106585
24. Banerjee A, Qi J, Gogoi R, Wong J, Mitragotri S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J Control Release. 2016;238:176–185. doi:10.1016/j.jconrel.2016.07.051
25. Chiesa E, Greco A, Riva F, et al. CD44-targeted carriers: the role of molecular weight of hyaluronic acid in the uptake of hyaluronic acid-based nanoparticles. Pharmaceuticals. 2022;15(1):103. doi:10.3390/ph15010103
26. Kang LJ, Yoon J, Rho JG, et al. Self-assembled hyaluronic acid nanoparticles for osteoarthritis treatment. Biomaterials. 2021;275:120967. doi:10.1016/j.biomaterials.2021.120967
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.