Back to Journals » Journal of Multidisciplinary Healthcare » Volume 17
Risk Factors for Central Nervous System Infections After Craniotomy
Authors Liu Y , Liu J, Wu X, Jiang E
Received 29 April 2024
Accepted for publication 14 July 2024
Published 29 July 2024 Volume 2024:17 Pages 3637—3648
DOI https://doi.org/10.2147/JMDH.S476125
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Yufeng Liu,1,* Jie Liu,1,* Xiaoyan Wu,1 Enshe Jiang2,3
1Department of Cardiovascular Medicine, Luoyang Central Hospital affiliated to Zhengzhou University, Luoyang, Henan, 471000, People’s Republic of China; 2Department of Neurosurgery, The First Affiliated Hospital of Henan University, Kaifeng, Henan, 475004, People’s Republic of China; 3Institute of Nursing and Health, Henan University, Kaifeng, Henan, 475004, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Enshe Jiang, Email [email protected]
Abstract: The central nervous system (CNS) is less prone to infection owing to protection from the brain-blood barrier. However, craniotomy destroys this protection and increases the risk of infection in the brain of patients who have undergone craniotomy. CNS infection after craniotomy significantly increases the patient’s mortality rate and disability. Controlling the occurrence of intracranial infection is very important for post-craniotomy patients. CNS infection after craniotomy is caused by several factors such as preoperative, intraoperative, and post-operative factors. Craniotomy may lead to postsurgical intracranial infection, which is mainly associated with surgery duration, infratentorial (posterior fossa) surgery, cerebrospinal fluid leakage, drainage tube placement, unregulated use of antibiotics, glucocorticoid use, age, diabetes, and other systemic infections. Understanding the risk factors of CNS infection after craniotomy can benefit reducing the incidence of intracranial infectious diseases. This will also provide the necessary guidance and evidence in clinical practice for planning to control intracranial infection in patients with craniotomy.
Keywords: surgery duration, posterior fossa, cerebrospinal fluid leakage, drainage tube, antibiotics, glucocorticoids
Introduction
Central nervous system (CNS) infection is a severe post-operative complication of craniotomy, which refers to infectious inflammation caused by microbial invasion of the brain parenchyma, meninges, and blood vessels. The pathogenic microorganisms include viruses, bacteria, fungi, and/or parasites.1 Patients with intracranial infection after neurosurgical craniotomy often experience critical complications such as intracranial hypertension and disturbance of consciousness, which progress rapidly and can be deadly, with a high mortality rate. Surviving patients may also be left with long-term sequelae such as dementia, epilepsy, paralysis, and cranial nerve palsy, which pose a great threat to the lives of patients and significantly affect their quality of life.2,3
With the rapid development of antibiotics, the improvement of sterile conditions in the operating room, and the application of advanced medical equipment, CNS infection after neurosurgery has been markedly improved.4 However, the incidence of intracranial disease after neurosurgery is still as high as 10% because of the inherent characteristics of invasive procedures in the CNS and the increase in implants.5,6 Once intracranial infection occurs, it is difficult to diagnose and treat, affecting the surgical effect, prolonging hospitalization, increasing the economic burden, and likely leading to poor prognoses.7 A observational study showed that transcranial color-coded duplex sonography can be used for bedside monitoring of CNS infection for patients with post-craniectomy.8
Therefore, understanding the risk factors of CNS infection after neurosurgery and then guiding the adoption of corresponding measures can significantly reduce the injury caused by CNS infection after neurosurgery. In this narrative review, we discussed the risk factors of CNS infection for patients after craniotomy.
Surgery Duration
Many studies have pointed out that the surgery duration positively correlates with the CNS infection rate after craniotomy.9–11 With improved accuracy of craniocerebral surgery, the development of complex surgery, and the use of microscopes in modern neurosurgery, the process is more effective, and the trauma to the body is relatively minor. These inevitably prolong the surgery duration. Many studies show that surgery duration exceeding 4 h is one of the high-risk factors for post-operative CNS infection in patients.12–14
The extended surgery duration can easily lead to CNS infection. On the one hand, it may be related to bacteria in the operating room air. At present, most operating rooms are not sterile. As the surgery duration progresses, the microorganisms suspended in the air in the operating room are more likely to colonize the surgical instruments and the tissue surface in the working area, thereby putting the patient at risk of developing a post-operative CNS infection.4,15 On the other hand, the pathogen may originate from the patient’s scalp as the surgery duration continues. Although careful disinfection before surgery can remove bacteria on the scalp surface, it is difficult to kill the pathogens in the skin pores thoroughly.4 Many Staphylococcus bacteria grow in the hair follicles and sebaceous glands of the human scalp. These bacteria living in the deep part of the scalp can gradually migrate to the skin surface naturally. The longer the surgery duration, the more likely the hidden pathogens in the scalp will remobilize and contaminate the surgical area, thereby increasing the incidence of CNS infections.
In addition, the long operation time also leads to the occurrence of the CNS infections by following pathophysiological mechanisms: ① Mechanical damage to the tissue: the prolongation of the operation time increases the time for the tissue to be stretched during the operation, which increases the damage around the brain tissue to a certain extent. It reduces the resistance to infection in the local of the body. ② Tissue ischemia and hypoxia: Excessive operation time will cause insufficient blood supply to the tissue around the surgical wound, resulting in tissue ischemia and hypoxia. Ischemia and hypoxia make traumatized tissues susceptible to bacterial invasion and also weaken the body’s ability to remove pathogenic microorganisms. ③ Inflammatory response disorder: The existence of long-term surgical wounds causes the body to continue to be in a state of inflammatory response, release inflammatory mediators such as cytokines, etc, and put the body’s immune system in a hyperactive state. The long-term persistence of this hyperactive state may suppress the body’s immune function and weaken the defense against infection. ④ Decreased overall resistance in patients: Prolonged general anesthesia reduces the patient’s resistance, and the patient is more susceptible after surgery, making it easier for external bacteria to invade the body.16,17 Therefore, when the operation time is expected to be long, such patients should be regarded as high-risk patients with post-operative CNS infection, and preventive measures should be taken as much as possible before, during, and after surgery. It is better to optimize surgical planning and operating procedures to minimize operating time.
Infratentorial (Posterior Fossa) Surgery
The site of intracranial surgery is often divided into supratentorial and infratentorial parts (posterior fossa). The incidence of CNS infection after infratentorial (posterior fossa) surgery is much higher than that of supratentorial surgery. A systematic review and meta-analysis showed that the incidence of surgical site infections after craniotomy following surgery for posterior fossa tumors was 9.67%.18 The following properties of the posterior fossa surgery are associated with the high risk of CNS infection.
The Location and Structure of the Posterior Fossa are Complex
The posterior fossa has essential structures, such as the brainstem, which plays a crucial role in cardiovascular activity, breathing, and other life activities.4,19 The posterior fossa surgery is complicated and requires utmost care compared to surgery in other parts of the brain. The complexity of its surgery prolongs the surgery duration to a certain extent and increases the risk of CNS infection. The volume of the posterior fossa is relatively tiny and the surgical site is deep. The situation of the patient will develop rapidly after the posterior fossa infection. It increases the risk of disease worsening. In addition, it is difficult to expose the surgical site of posterior fossa, because the muscle layer of the posterior fossa is thicker and the occipital skull is thicker than other parts. Therefore it is necessary to use retractors during the surgery to retract the skin and muscles to expose the surgical field and electrocoagulation hemostasis is carried out during the surgery. The tissue injury around the incision also increases the chance of post-operative infection. It is another risk factor for the increase in the infection of the CNS after surgery.4
Opening of Mastoid Air Cells
It is easy for posterior fossa surgery to cause the mastoid air cells to open in the mastoid part of the temporal bone during the surgery. The mastoid air cell is interconnected with the tympanic cavity of the middle ear through the mastoid antrum, which makes the intracranial structure open to the outside space so that external bacteria may take the opportunity to enter the intracranial space, thereby increasing the risk of post-operative CNS infection.20
Cerebrospinal Fluid Leakage Induced by Difficulty in Suturing
In the suturing process of posterior fossa surgery, post-operative infection is often caused by the inability to suture tightly.21 Posterior fossa surgery removes part of the occipital bone and severs the connection between the tuberosity and the muscle/ligament, separating the muscle and ligament from the periosteum and forming a cavity between the dura and the muscle layer. Because the scalp is thicker in this area and the subcutaneous layers are rich in fatty tissue, it is difficult to suture layer by layer. Moreover, it is difficult to suture the dura mater tightly because of contractures caused by electrocoagulation. In that case, it is easy to cause cerebrospinal fluid leakage in the incision, and pathogenic bacteria will enter the skull retrograde along the incision.22,23 Couple with the possibility of liquefaction necrosis of fat, it affects wound healing and provides an opportunity for bacterial colonization.
Difficulty in Post-Operative Incision Cleaning
Since the surgical area of the posterior fossa is mainly in the occipital region, it is easy to cause poor incision healing after the surgery owing to the scalp being compressed in the operation area following the long-term supine position. In addition, patients after posterior fossa surgery are often unconscious, which increases the difficulty of changing the dressing and cleaning the incision, leading to an increasing risk of post-operative infection due to insufficient cleaning.
Preventive Measures of Post-Operative Infection in the Posterior Fossa
In short, infratentorial surgery has a higher risk of post-operative CNS infection than surgery in other locations. It involves pathophysiological mechanisms such as regional anatomy and skull base anatomy. To prevent CNS infection after posterior fossa surgery, choosing an appropriate surgical approach and avoiding unnecessary opening of the mastoid air cells is necessary. When it cannot be avoided, bone wax or fatty tissue should be used to block the mastoid air cells to reduce surgical site contamination. At the end of the surgery, the dura mater, muscle layer, and scalp should be tightly sutured. When the dura mater contracture causes suture difficulty, artificial dura mater can be used for repair.24 It should be ensured that the muscle layer is close to the dura mater, the suture at the muscle layer must be firm, and no dead space should remain. Post-operative pressure bandaging reduces the incidence of CNS infection.
Cerebrospinal Fluid Leakage
Many studies have pointed out that cerebrospinal fluid leakage after craniotomy is one of the main risk factors for CNS infection.25–27 Bacterial invasion and local inflammatory response are the main pathophysiological mechanisms leading to infection. The cerebrospinal fluid leakage connects the body’s brain tissue with the outside world, allowing the pathogenic bacteria to enter the intracranial space, leading to CNS infection.23 Once intracranial infection occurs, it will increase intracranial pressure, further aggravating cerebrospinal fluid leakage. Cerebrospinal fluid leakage can stimulate an inflammatory response in surrounding tissue, leading to swelling and blood circulation changes. This inflammatory response provides a suitable environment for bacteria to reproduce and survive, thus forming a vicious circle. Therefore, the longer the leakage time, the greater the probability of infection. After craniotomy, common cerebrospinal fluid leakage includes incisional cerebrospinal fluid leakage, cerebrospinal fluid rhinorrhea, and cerebrospinal fluid otorrhea.28
Incisional Cerebrospinal Fluid Leakage
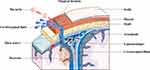
Incisional cerebrospinal fluid leakage is usually caused by insufficient suture of the dura mater and muscle during the surgery, residual dead space in the incision, poor incision healing, incision expansion owing to increased intracranial pressure, and a loosely closed scalp incision after drainage tube extraction. For these reasons, the cerebrospinal fluid leaks out from the dura mater and the subcutaneous tissue gap.29,30
The patient may have subcutaneous effusion near the surgical incision when incisional cerebrospinal fluid leakage occurs. The subcutaneous effusion is connected with the gap on the dura mater caused by the loose suture, which affects the local metabolic environment and likely reduces the circulating speed of the cerebrospinal fluid at the surgical site. This increases the protein level in the cerebrospinal fluid, which is conducive to bacterial reproduction.31 On the other hand, after subcutaneous effusion accumulation, local puncture and drainage are often required, increasing the chance of bacteria entering the brain, which is another risk factor for intracranial infection caused by incisional cerebrospinal fluid leakage (Figure 1).31
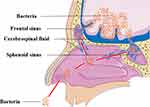
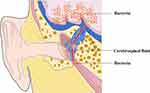
Cerebrospinal Fluid Rhinorrhea and Cerebrospinal Fluid Otorrhea
Cerebrospinal fluid rhinorrhea and cerebrospinal fluid otorrhea are usually caused by the opening of the paranasal sinuses and mastoid air cells during the craniotomy, which is not tightly sealed with bone wax, and the dura meter is not tightly sutured when the skull is closed.27,29,32 At this time, cerebrospinal fluid can flow out through the paranasal sinuses and mastoid air cells, resulting in cerebrospinal fluid rhinorrhea and otorrhea. Then, the channels between the intracranial and extracranial regions are formed, and pathogenic bacteria may enter the brain, causing infection of the CNS (Figures 2 and 3).
Preventive Measures of Post-Operative Infection by Cerebrospinal Fluid Leakage
Tight suturing of the dura mater and muscle layer during the surgery is the most effective measure to prevent post-operative cerebrospinal fluid incision leakage. When the dura mater is severely damaged and cannot be tightly sutured, artificial dura mater can be used for repair. As a foreign body, it should be noted that artificial dura mater may aggravate the intracranial infection in open traumatic brain injury.32 For the parts where the tissue is loose and can quickly form subcutaneous effusion, a local compression bandage should be placed after the surgery to prevent the occurrence of subcutaneous effusion. These measures will reduce the incidence of intracranial infection after craniotomy. To prevent cerebrospinal fluid rhinorrhea and otorrhea after surgery, the barrier among the cranial cavity, the paranasal sinuses, and the mastoid air cells should be repaired before the skull is closed.32 Once cerebrospinal fluid rhinorrhea and otorrhea occur, the nostril and ear canal should not be blocked. The cerebrospinal fluid should be thoroughly drained to promote the early closure of the external channel and cranial cavity. It needs to be repaired if it is necessary.33
At the same time, monitoring of the patient’s condition post craniotomy should be strengthened. As soon as a sudden increase in intracranial pressure or signs of meningeal irritation is found, lumbar cistern catheterization should be performed immediately for continuous drainage to reduce the risk of cerebrospinal fluid leakage, thereby reducing the risk of CNS infection. Once cerebrospinal fluid leaks, the patient should quickly be placed in a semi-recumbent or supine position. Lumbar puncture or lumbar cistern catheterization should drain the cerebrospinal fluid, reduce intracranial pressure, and promote healing of the leak.30,33 When the above treatments fail, exploration and revision surgery must be performed.34
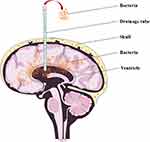
Drainage Tube
Drainage after craniotomy is another risk factor for CNS infection.35 Common drainage methods include external ventricular drainage, subdural drainage, and epidural drainage. Once bacteria invade the intracranial space during drainage after craniotomy, they can easily proliferate and reproduce because the self-defense ability of the cerebroventricular system is weak, and the cerebrospinal fluid is a nutrient-rich bacterial culture medium (Figure 4). After craniotomy, it is necessary to drain blood and fluid from the operative cavity, epidural space, and other parts to reduce intracranial pressure and prevent post-operative brain herniation and hydrocephalus. Drainage tubes are needed in the cerebral ventricle, operative cavity, subdural and epidural space, and subcutaneous space.36 However, while the drainage tube is used to draw out blood and fluids, it also bridges the internal and external environments of the skull. The drainage tube directly provides a channel from the external environment to the intracranial space, bypassing the blood-brain barrier. Extracranial pathogens can enter the brain easily through the channel and increase the possibility of intracranial infection.37
Intracranial infection caused by the drainage tube includes the following: ① Scalp flora: Conventional scalp disinfection before craniotomy cannot effectively kill all bacteria. After placing the drainage tube, the bacteria on the scalp can enter the skull directly through the drainage tube. In addition, intracranial infection is more likely to occur, especially when the drainage tube leaks.35 ② Drainage fluid backflow: When the drainage tube is placed too high, it may lead to poor drainage, and the drainage fluid that may be contaminated in the drainage tube can enter the brain retrogradely.13 ③ Biofilm formation by bacterial growth: The formation of biofilm on the surface of the drainage tube is also one of the risk factors. Post-operative drainage tube placement also provides favorable conditions for the construction of biofilm by bacterial growth.38–40 When the drainage tube is implanted into the body, its surface is immediately surrounded by various fluids such as blood and multiple other glycoproteins, mucopolysaccharides, ions, and components. They will penetrate and attach to the surface within a few minutes, forming a conditioned membrane. This conditioned membrane will then cover the substrate surface like a mesh, allowing bacteria to attach to it.41,42 Once bacteria form a biofilm, it is difficult to eliminate, increasing the risk of intracranial infection.43 ④Foreign body: the human body rejects the drainage tube as a foreign body, which may show a local inflammatory response and cause endogenous infection. ⑤Prolonged drainage time: This will also increase the chance of intracranial infection.44,45 With prolonged placement of an indwelling drainage tube, the chance of retrograde infection due to bacterial growth increases, and pathogenic bacteria can repeatedly invade the intracranial space.13 ⑥ Lack of strict aseptic operation: Irregular sterile operation during post-operative drainage may lead to intracranial infection. ⑦ Extubation: When the drainage tube is removed, there may be leakage from the skin drainage orifice. If the cerebrospinal fluid leaks after extubation, it may lead to the invasion of bacteria and increase the risk of intracranial infection.
Therefore, disinfection and debridement of the surgical area should be done in a timely manner when the drainage tube is placed after surgery. The drainage tube is soaked in antibiotics during the operation to prevent biofilm formation.46,47 The post-operative drainage tube should be placed above the incision, slightly below the level of the cerebral ventricle, to ensure smooth drainage and prevent the drainage fluid from flowing back into the cranial cavity. Care should be taken to strictly follow the aseptic operation procedures in the post-operative drainage tube. The drainage device’s wound and connection ends should be regularly disinfected, and the wound dressing should be changed in time. The T-tube connector should be soaked when the cerebrospinal fluid is extracted and the drug is injected through the drainage tube. The drainage tube must be clamped to prevent retrograde infection when replacing the drainage bag or discharging fluid. The suitable measures should be taken as regular follow-up and monitoring of patients after surgery to detect signs of infection early and take timely intervention measures.
Irregular Use of Antibiotics
Due to severe complications caused by post-operative infection, many studies in the 1980s and 1990s believed that prophylactic antibiotics could reduce the infection rate of the CNS after craniotomy.48–53 Prophylactic antibiotics gradually became the mainstream treatment strategy. The preoperative prophylactic use of various antibiotics became popular.14 However, drug-resistant bacteria are constantly emerging due to the unreasonable application of the antibiotics. Even if antibiotics are used prophylactically in the perioperative period, infection still occurs, and the effect of antibiotics is significantly reduced.54
Preoperative use of antibiotics to prevent post-operative infection is necessary for contaminated incisions. However, there has been controversy over whether prophylactic antibiotics should be used in clean neurosurgery. Therefore, clean neurosurgery must be considered with the patient’s situation in practice. If the patient has risk factors for post-operative CNS infection, underlying diseases, and low immunity before surgery, prophylactic antibiotics may be used.
For clean neurosurgery requiring prophylactic antibiotics, the timing of administration, selection of antimicrobials, dosage, and additional medication during surgery are vital factors.55 An error in any of these points can render antibiotics ineffective, indirectly leading to post-operative CNS infection and even promoting the emergence of drug-resistant bacteria. Once post-operative infection occurs, the effect of anti-infective treatment will significantly reduce.54 For clean neurosurgery, the main pathogenic bacteria that may lead to post-operative infection, primarily Gram-positive cocci, mainly come from the scalp. Staphylococcus aureus accounts for a substantial proportion of gram-positive cocci.14,56,57 Therefore, only a single drug is required for the prophylactic application of antibiotics before clean neurosurgery. It is better to choose the drug which is narrow-spectrum, gram-positive bacteria targeted, inexpensive, and minimal in side effects. It is not necessary to cover all the flora. In most cases, a standard dose is sufficient. Supplemental drugs during surgery: Prophylactic antibiotics should cover at least the period from skin incision to the closure of the surgical incision to ensure a high drug concentration in blood during surgery. The serum half-life of commonly used cephalosporins is 1–2 h. Therefore, if the process is extended to more than 3 h or the blood loss exceeds 1500 mL, a second dose should be added, even a third dose can be used if necessary. If ceftriaxone which has a 7–8 h half-life, is used, no additional dosage is required.58,59
Glucocorticoids
Some patients with intracranial tumors require long-term chemotherapy, immunosuppressive agents, and glucocorticoids. The pathophysiological mechanisms involves in inhibiting the production and function of various immune cells, inflammatory mediators and cytokines in the immune system. Patients with immunosuppressive therapies are considered a high risk population for CNS infections and the incidence of CNS infection are associated with the dose of the immunosuppressants such as corticosteroids.60 These drugs will reduce the patient’s immune function, making the body vulnerable to pathological invasion and inducing CNS infection.61–63
In some cases, glucocorticoids are required for patients who have undergone neurosurgical craniotomy. Glucocorticoids can reduce endothelial damage, scavenge free radicals, inhibit cell peroxidation, maintain cell membrane stability, and minimize cerebrospinal fluid secretion; for patients with malignant intracranial tumors such as glioma and advanced brain metastases, the routine use of glucocorticoids can significantly reduce the edema around cancer before and after surgery, improve cerebrospinal fluid circulation, and protect the small blood vessels of the brain tissue, reduce the lesion, and improve the symptoms of hydrocephalus.64,65
However, if glucocorticoids have been used long-term, there will be noticeable side effects, such as glucose metabolism disorders, hyperglycemia in patients, immunosuppression, reduced immunity, and/or delayed wound healing, leading to an increase in the infection rate after surgery.66 Therefore, the indications and use time for applying glucocorticoids should be strictly controlled. Generally, short-term sufficient pulse therapy should be used to avoid side effects caused by the long-term, high-dose application.
Patients Related Risk Factors
Old Age
Age is also one of the risk factors for CNS infection after neurosurgical craniotomy.57 The pathophysiological mechanisms mainly involve in the following aspects. Degenerative changes occur in various tissues and organs among aged patients. The function of multiple organs is gradually weakened, autoimmunity is slowly reduced, and anti-infection ability is weak. In addition, the stress stimulation caused by neurosurgery can heighten the risk of CNS infection among older patients. Nevertheless, older patients often have underlying diseases such as diabetes, hypertension, and respiratory system disease, which make them more prone to intracranial infection after craniotomy than younger patients.67 Elderly patients often require long-term medication to manage their chronic diseases, such as anticoagulants, immunosuppressants, etc. These drugs may affect the immune system, increasing the risk of infection. The neurological function of elderly patients may have suffered a certain degree of decline, including reduced blood supply and tissue repair ability. This may lead to difficulty in post-operative wound healing, and the tissue around the wound is susceptible to infection.
Therefore, for older patients, primary disease should be well controlled before surgery, and complete preoperative preparations should be made, including sputum guidance, breathing training, and nutritional support. Post-operative preparations include nutritional support, improving immunity, and reducing complications. This will reduce the post-operative incidence of systemic infections in the CNS.
Diabetes
Poor preoperative glycemic control in diabetic patients is significantly associated with an increased risk of CNS infection after neurosurgical craniotomy.68,69 Diabetes, as a chronic disease, impacts the infection of the CNS after craniotomy. The pathophysiological mechanisms leading to CNS infection after craniotomy are multifaceted: ① Hyperglycemia causes metabolic disorder of sugar, protein, and fat in the body; decreased protein synthesis; and increased breakdown. The tissue repair, regeneration ability, and resistance to invasion by foreign bacteria are weakened. More fat is decomposed than synthesized during metabolism, which increases blood lipid levels. Lipids are deposited in blood vessels’ inner walls, resulting in vascular injury. The vascular lumen becomes stenosed or occluded. The blood flow becomes slower. The tissue oxygen supply is reduced, which may lead to poor incision healing. This will increase the possibility of bacteria invading the brain through the surgical incision.70 ② Diabetes may weaken the phagocytic sterilization ability of centrocytes and decrease lymphocyte reactivity, thus significantly reducing the body’s immune function.70 ③ After the blood sugar level rises, blood and tissue fluid become a suitable medium that will benefit the growth and reproduction of bacteria. ④ Diabetes can damage the nervous system, including peripheral neuropathy and abnormal function of the central nervous system. This affects the nervous system’s ability to repair and regenerate, making wound healing difficult after craniotomy and increasing the risk of infection.
Therefore, for patients with diabetes, blood sugar levels should be actively controlled and treated before surgery. A customized diabetes diet plan should be formulated to control blood sugar reasonably. The patient’s immune status and blood circulation should be assessed. Immunomodulatory therapy and improving blood circulation can control the patient’s disease. Care for the patient’s stable blood sugar levels, blood pressure levels, and fluid balance should be taken during the operation. After the operation, the patient’s body temperature, inflammation indicators and wound healing were closely observed.
Combination with Other System Infections
The post-operative CNS infection rate will be increased when the patients with craniotomy have other system infections such as infection of respiratory and urinary systems before surgery. Possibly because the surgery disrupts the integrity of the blood-brain barrier, bacteria from other body parts can spread through the blood into the brain. For example, in patients with pulmonary infection, tracheal cannulation during surgery can easily damage the tracheal mucosa, destroying its barrier function. Pathogenic bacteria can enter the brain through the blood, causing CNS infection. In addition, lung infection leads to low immunity and hypoxia, reducing the resistance of intracranial tissues to bacteria. The intracranial infection leads to a more severe disturbance of consciousness, forming a vicious circle. Therefore, to prevent CNS infection after craniotomy, it is necessary to control the overall situation, comprehensively evaluate the patient’s status, especially the prevention and control of respiratory system infection, reduce the invasion of opportunistic pathogens, and reduce post-operative CNS infection risk.
Conclusion
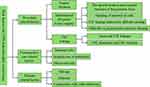
CNS infection after neurosurgical craniotomy is a severe postoperative complication that affects the surgical outcome and patient prognosis. Risk factors for CNS infection after neurosurgery craniotomy involve procedure, post-operative care and patients related factors, including surgery duration, infratentorial (posterior fossa surgery) site, cerebrospinal fluid leakage, drainage tube placement, inappropriate use of antibiotics, glucocorticoid use, age, and diabetes, and combined with other systemic infections (Figure 5). Studying these risk factors is significant in formulating individualized prevention plans for the patient’s surgery. Adequate preventive measures should be taken to reduce the incidence of CNS infection after craniotomy and improve the clinical prognosis of patients.
Acknowledgments
We thank the company Charlesworth Author Services for its language editing. We thank the support of the funding from the Postgraduate Cultivating Innovation and Quality Improvement Action Plan of Henan University [grant number YJSJG2022XJ059] and Henan Provincial Medical Science and Technology Research Plan Joint Construction Project [grant number LHGJ20200959].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shi ZH, Xu M, Wang YZ, et al. Post-craniotomy intracranial infection in patients with brain tumors: a retrospective analysis of 5723 consecutive patients. Br J Neurosurg. 2017;31(1):5–9. doi:10.1080/02688697.2016.1253827
2. Fang C, Zhu T, Zhang P, Xia L, Sun C. Risk factors of neurosurgical site infection after craniotomy: a systematic review and meta-analysis. Am J Infect Control. 2017;45(11):e123–e134. doi:10.1016/j.ajic.2017.06.009
3. Heth JA. Neurosurgical aspects of central nervous system infections. Neuroimaging Clin N Am. 2012;22(4):791–799. doi:10.1016/j.nic.2012.05.005
4. Walcott BP, Redjal N, Coumans JV. Infection following operations on the central nervous system: deconstructing the myth of the sterile field. Neurosurg Focus. 2012;33(5):E8. doi:10.3171/2012.8.FOCUS12245
5. McClelland S, Hall WA. Postoperative central nervous system infection: incidence and associated factors in 2111 neurosurgical procedures. Clin Infect Dis. 2007;45(1):55–59. doi:10.1086/518580
6. Wang LY, Cao XH, Shi LK, Ma ZZ, Wang Y, Liu Y. Risk factors for intracranial infection after craniotomy: a case-control study. Brain Behav. 2020;10(7):e01658. doi:10.1002/brb3.1658
7. Ruan L, Wu D, Li X, et al. Analysis of microbial community composition and diversity in postoperative intracranial infection using high‑throughput sequencing. Mol Med Rep. 2017;16(4):3938–3946. doi:10.3892/mmr.2017.7082
8. Robba C, Simonassi F, Ball L, Pelosi P. Transcranial color-coded duplex sonography for bedside monitoring of central nervous system infection as a consequence of decompressive craniectomy after traumatic brain injury. Intensive Care Med. 2019;45(8):1143–1144. doi:10.1007/s00134-018-5405-4
9. Han C, Song Q, Ren Y, Luo J, Jiang X, Hu D. Dose-response association of operative time and surgical site infection in neurosurgery patients: a systematic review and meta-analysis. Am J Infect Control. 2019;47(11):1393–1396. doi:10.1016/j.ajic.2019.05.025
10. Karhade AV, Cote DJ, Larsen AM, Smith TR. Neurosurgical Infection Rates and Risk Factors: a National Surgical Quality Improvement Program Analysis of 132,000 Patients, 2006–2014. World Neurosurg. 2017;97:205–212. doi:10.1016/j.wneu.2016.09.056
11. Abu Hamdeh S, Lytsy B, Ronne-Engstrom E. Surgical site infections in standard neurosurgery procedures- a study of incidence, impact and potential risk factors. Br J Neurosurg. 2014;28(2):270–275. doi:10.3109/02688697.2013.835376
12. Korinek AM, Korinek A-M, Laisne M-J. Risk factors for neurosurgical site infections after craniotomy: a prospective multicenter study of 2944 patients. The French Study Group of Neurosurgical Infections, the SEHP, and the C-CLIN Paris-Nord. Service Epidemiologie Hygiene et Prevention. Neurosurgery. 1997;41(5):1073–1079; discussion1079–1081. doi:10.1097/00006123-199711000-00010
13. Yao J, Liu D. Logistic Regression Analysis of Risk Factors for Intracranial Infection After Multiple Traumatic Craniotomy and Preventive Measures. J Craniofac Surg. 2019;30(7):1946–1948. doi:10.1097/SCS.0000000000004972
14. Korinek AM, Golmard JL, Elcheick A, et al. Risk factors for neurosurgical site infections after craniotomy: a critical reappraisal of antibiotic prophylaxis on 4578 patients. Br J Neurosurg. 2005;19(2):155–162. doi:10.1080/02688690500145639
15. Shea KG, Styhl AC, King HA, Hammons J, Clapp M. Surgical Site Infection Reduction Program: challenges and Opportunities. J Pediatr Orthop. 2015;35(5 Suppl 1):S51–54. doi:10.1097/BPO.0000000000000549
16. Boavista Barros Heil L, Leme Silva P, Ferreira Cruz F, Pelosi P, Rieken Macedo Rocco P. Immunomodulatory effects of anesthetic agents in perioperative medicine. Minerva Anestesiol. 2020;86(2):181–195. doi:10.23736/S0375-9393.19.13627-9
17. Ackerman RS, Luddy KA, Icard BE, Pineiro Fernandez J, Gatenby RA, Muncey AR. The Effects of Anesthetics and Perioperative Medications on Immune Function: a Narrative Review. Anesth Analg. 2021;133(3):676–689. doi:10.1213/ANE.0000000000005607
18. Lee KS, Borbas B, Plaha P, Ashkan K, Jenkinson MD, Price SJ. Incidence and risk factors of surgical site infection after cranial surgery for patients with brain tumors: a systematic review and meta-analysis. World Neurosurg. 2024;185:e800–e819. doi:10.1016/j.wneu.2024.02.133
19. Singh R, Kumar R, Kumar A. Vascular anomalies of posterior fossa and their implications. J Craniofac Surg. 2017;28(8):2145–2150. doi:10.1097/SCS.0000000000003867
20. Wiatr M, Skladzien J, Tomik J, Strek P, Przeklasa-Muszynska A. Bony wall damage in the region of the middle and posterior cranial fossa observed during otosurgery. Med Sci Monit. 2012;18(6):BR215–220. doi:10.12659/msm.882871
21. Heymanns V, Oseni AW, Alyeldien A, et al. Sandwich wound closure reduces the risk of cerebrospinal fluid leaks in posterior fossa surgery. Clin Pract. 2016;6(2):824. doi:10.4081/cp.2016.824
22. Lepski G, Reis B, de Oliveira A, Neville I. Recursive partitioning analysis of factors determining infection after intracranial tumor surgery. Clin Neurol Neurosurg. 2021;205:106599. doi:10.1016/j.clineuro.2021.106599
23. Krishnan SS, Nigam P, Manuel A, Vasudevan MC. Bone sandwich closure technique for posterior fossa craniectomy. J Neurol Surg B Skull Base. 2020;81(1):8–14. doi:10.1055/s-0039-1678602
24. Hale AT, Gannon SR, Zhao S, et al. Graft dural closure is associated with a reduction in CSF leak and hydrocephalus in pediatric patients undergoing posterior fossa brain tumor resection. J Neurosurg Pediatr. 2019:1–7.doi: 10.3171/2019.9.PEDS1939
25. Korinek AM, Baugnon T, Golmard JL, van Effenterre R, Coriat P, Puybasset L. Risk factors for adult nosocomial meningitis after craniotomy: role of antibiotic prophylaxis. Neurosurgery. 2006;59(1):126–133; discussion126–133. doi:10.1227/01.NEU.0000220477.47323.92
26. Kourbeti IS, Vakis AF, Ziakas P, et al. Infections in patients undergoing craniotomy: risk factors associated with post-craniotomy meningitis. J Neurosurg. 2015;122(5):1113–1119. doi:10.3171/2014.8.JNS132557
27. Kono Y, Prevedello DM, Snyderman CH, et al. One thousand endoscopic skull base surgical procedures demystifying the infection potential: incidence and description of postoperative meningitis and brain abscesses. Infect Control Hosp Epidemiol. 2011;32(1):77–83. doi:10.1086/657635
28. Azad T, Mendelson ZS, Wong A, Jyung RW, Liu JK. Fat graft-assisted internal auditory canal closure after retrosigmoid transmeatal resection of acoustic neuroma: technique for prevention of cerebrospinal fluid leakage. J Clin Neurosci. 2016;24:124–127. doi:10.1016/j.jocn.2015.08.016
29. Lin J, Zhang Y, Peng R, et al. Preoperative imaging and microscopic navigation during surgery can avoid unnecessarily opening the mastoid air cells through craniotomy using the retrosigmoid approach. World Neurosurg. 2019;121:e15–e21. doi:10.1016/j.wneu.2018.08.181
30. James HE, Postlethwait R. Primary treatment with temporary subcutaneous peritoneal shunts for postoperative spinal cerebrospinal fluid fistulas. J Neurosurg. 2006;104(5 Suppl):299–301. doi:10.3171/ped.2006.104.5.299
31. Ding W, Chen H, Xiang Y, et al. Revision surgery technique in the treatment of refractory subcutaneous cerebrospinal fluid collection combined with intracranial infection following posterior fossa surgery. Cureus. 2020;12(9):e10610. doi:10.7759/cureus.10610
32. Yanagawa T, Hatayama T, Harada Y, et al. Preoperative risk assessment for predicting the opening of mastoid air cells in lateral suboccipital craniotomy for microvascular decompression. Clin Neurol Neurosurg. 2020;189:105624. doi:10.1016/j.clineuro.2019.105624
33. Hussein M, Abdellatif M. Continuous lumbar drainage for the prevention and management of perioperative cerebrospinal fluid leakage. Asian J Neurosurg. 2019;14(2):473–478. doi:10.4103/ajns.AJNS_265_18
34. Mangus BD, Rivas A, Yoo MJ, et al. Management of cerebrospinal fluid leaks after vestibular schwannoma surgery. Otol Neurotol. 2011;32(9):1525–1529. doi:10.1097/MAO.0b013e318232e4a4
35. Sneh-Arbib O, Shiferstein A, Dagan N, et al. Surgical site infections following craniotomy focusing on possible post-operative acquisition of infection: prospective cohort study. Eur J Clin Microbiol Infect Dis. 2013;32(12):1511–1516. doi:10.1007/s10096-013-1904-y
36. Atkinson R, Fikrey L, Jones A, Pringle C, Patel HC. Cerebrospinal fluid infection associated with silver-impregnated external ventricular drain catheters. World Neurosurg. 2016;89:505–509. doi:10.1016/j.wneu.2016.01.034
37. Lallani SB, Hyte M, Trieu E, Reyes-Sacin C, Doan N. Use of an intracranial drain as a conduit for treatment of an intracranial streptococcus intermedius abscess. Cureus. 2021;13(4):e14613. doi:10.7759/cureus.14613
38. Stevens NT, Tharmabala M, Dillane T, Greene CM, Jp O, Humphreys H. Biofilm and the role of the ica operon and aap in Staphylococcus epidermidis isolates causing neurosurgical meningitis. Clin Microbiol Infect. 2008;14(7):719–722. doi:10.1111/j.1469-0691.2008.02012.x
39. Ledwoch K, Robertson A, Lauran J, Norville P, Maillard JY. It’s a trap! The development of a versatile drain biofilm model and its susceptibility to disinfection. J Hosp Infect. 2020;106(4):757–764. doi:10.1016/j.jhin.2020.08.010
40. Renz N, Ozdirik B, Finger T, Vajkoczy P, Trampuz A. Infections after cranial neurosurgery: prospective cohort of 103 episodes treated according to a standardized algorithm. World Neurosurg. 2018;116:e491–e499. doi:10.1016/j.wneu.2018.05.017
41. Leid JG, Shirtliff ME, Costerton JW, Stoodley P. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect Immun. 2002;70(11):6339–6345. doi:10.1128/IAI.70.11.6339-6345.2002
42. Conen A, Fux CA, Vajkoczy P, Trampuz A. Management of infections associated with neurosurgical implanted devices. Expert Rev Anti Infect Ther. 2017;15(3):241–255. doi:10.1080/14787210.2017.1267563
43. Jost GF, Wasner M, Taub E, Walti L, Mariani L, Trampuz A. Sonication of catheter tips for improved detection of microorganisms on external ventricular drains and ventriculo-peritoneal shunts. J Clin Neurosci. 2014;21(4):578–582. doi:10.1016/j.jocn.2013.05.025
44. Jamjoom AAB, Joannides AJ, Poon MT, et al. Prospective, multicentre study of external ventricular drainage-related infections in the UK and Ireland. J Neurol Neurosurg Psychiatry. 2018;89(2):120–126. doi:10.1136/jnnp-2017-316415
45. Hoefnagel D, Dammers R, Ter Laak-Poort MP, Avezaat CJ. Risk factors for infections related to external ventricular drainage. Acta Neurochir. 2008;150(3):209–214; discussion214. doi:10.1007/s00701-007-1458-9
46. Cui Z, Wang B, Zhong Z, et al. Impact of antibiotic- and silver-impregnated external ventricular drains on the risk of infections: a systematic review and meta-analysis. Am J Infect Control. 2015;43(7):e23–32. doi:10.1016/j.ajic.2015.03.015
47. Sonabend AM, Korenfeld Y, Crisman C, Badjatia N, Mayer SA, Connolly ES. Prevention of ventriculostomy-related infections with prophylactic antibiotics and antibiotic-coated external ventricular drains: a systematic review. Neurosurgery. 2011;68(4):996–1005. doi:10.1227/NEU.0b013e3182096d84
48. Quartey GR, Polyzoidis K. Intraoperative antibiotic prophylaxis in neurosurgery: a clinical study. Neurosurgery. 1981;8(6):669–671. doi:10.1227/00006123-198106000-00005
49. Geraghty J, Feely M. Antibiotic prophylaxis in neurosurgery. A randomized controlled trial. J Neurosurg. 1984;60(4):724–726. doi:10.3171/jns.1984.60.4.0724
50. Blomstedt GC, Kytta J. Results of a randomized trial of vancomycin prophylaxis in craniotomy. J Neurosurg. 1988;69(2):216–220. doi:10.3171/jns.1988.69.2.0216
51. Bullock R, van Dellen JR, Ketelbey W, Reinach SG. A double-blind placebo-controlled trial of perioperative prophylactic antibiotics for elective neurosurgery. J Neurosurg. 1988;69(5):687–691. doi:10.3171/jns.1988.69.5.0687
52. van Ek B, Dijkmans BA, van Dulken H, van Furth R. Antibiotic prophylaxis in craniotomy: a prospective double-blind placebo-controlled study. Scand J Infect Dis. 1988;20(6):633–639. doi:10.3109/00365548809035664
53. Gaillard T, Gilsbach JM. Intra-operative antibiotic prophylaxis in neurosurgery. A prospective, randomized, controlled study on cefotiam. Acta Neurochir. 1991;113(3–4):103–109. doi:10.1007/BF01403193
54. Lallemand S, Thouverez M, Bailly P, Bertrand X, Talon D. Non-observance of guidelines for surgical antimicrobial prophylaxis and surgical-site infections. Pharm World Sci. 2002;24(3):95–99. doi:10.1023/a:1016122202439
55. Goede WJ, Lovely JK, Thompson RL, Cima RR. Assessment of prophylactic antibiotic use in patients with surgical site infections. Hosp Pharm. 2013;48(7):560–567. doi:10.1310/hpj4807-560
56. Ma YF, Wen L, Zhu Y. Prospective study evaluating post-operative central nervous system infections following cranial surgery. Br J Neurosurg. 2019;33(1):80–83. doi:10.1080/02688697.2018.1519112
57. Bokop Fotso C, Abaver DT, Muballe D, Vasaikar S, Apalata T. Postoperative infections: aetiology, incidence and risk factors among neurosurgical patients in Mthatha, South Africa. S Afr Med J. 2020;110(5):403–408. doi:10.7196/SAMJ.2020.v110i5.13779
58. Mellin-Olsen J, McDougall RJ, Cheng D. WHO Guidelines to prevent surgical site infections. Lancet Infect Dis. 2017;17(3):260–261. doi:10.1016/S1473-3099(17)30078-6
59. Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283. doi:10.2146/ajhp120568
60. de Amorim JC, Torricelli AK, Frittoli RB, et al. Mimickers of neuropsychiatric manifestations in systemic lupus erythematosus. Best Pract Res Clin Rheumatol. 2018;32(5):623–639. doi:10.1016/j.berh.2019.01.020
61. McCutcheon BA, Ubl DS, Babu M, et al. Predictors of surgical site infection following craniotomy for intracranial neoplasms: an analysis of prospectively collected data in the American college of surgeons national surgical quality improvement program database. World Neurosurg. 2016;88:350–358. doi:10.1016/j.wneu.2015.12.068
62. Schipmann S, Akalin E, Doods J, Ewelt C, Stummer W, Suero Molina E. When the infection hits the wound: matched case-control study in a neurosurgical patient collective including systematic literature review and risk factors analysis. World Neurosurg. 2016;95:178–189. doi:10.1016/j.wneu.2016.07.093
63. Hughes MA, Parisi M, Grossman S, Kleinberg L. Primary brain tumors treated with steroids and radiotherapy: low CD4 counts and risk of infection. Int J Radiat Oncol Biol Phys. 2005;62(5):1423–1426. doi:10.1016/j.ijrobp.2004.12.085
64. Karschnia P, Le Rhun E, Vogelbaum MA, et al. The evolving role of neurosurgery for central nervous system metastases in the era of personalized cancer therapy. Eur J Cancer. 2021;156:93–108. doi:10.1016/j.ejca.2021.07.032
65. Jessurun CAC, Hulsbergen AFC, Lamba N, Nandoe Tewarie RDS, Smith TR, Broekman MLD. Practice variation in perioperative steroid dosing for brain tumor patients: an international survey. World Neurosurg. 2022;159:e431–e441. doi:10.1016/j.wneu.2021.12.067
66. Gaston RG, Kuremsky MA. Postoperative infections: prevention and management. Hand Clin. 2010;26(2):265–280. doi:10.1016/j.hcl.2010.01.002
67. Johans SJ, Garst JR, Burkett DJ, et al. Identification of preoperative and intraoperative risk factors for complications in the elderly undergoing elective craniotomy. World Neurosurg. 2017;107:216–225. doi:10.1016/j.wneu.2017.07.177
68. Buchanan IA, Donoho DA, Patel A, et al. Predictors of surgical site infection after nonemergent craniotomy: a nationwide readmission database analysis. World Neurosurg. 2018;120:e440–e452. doi:10.1016/j.wneu.2018.08.102
69. Malone DL, Genuit T, Tracy JK, Gannon C, Napolitano LM. Surgical site infections: reanalysis of risk factors. J Surg Res. 2002;103(1):89–95. doi:10.1006/jsre.2001.6343
70. Moura J, Madureira P, Leal EC, Fonseca AC, Carvalho E. Immune aging in diabetes and its implications in wound healing. Clin Immunol. 2019;200:43–54. doi:10.1016/j.clim.2019.02.002
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.