Back to Journals » International Journal of Nanomedicine » Volume 19
Self-Assembled Aggregated Structures of Natural Products for Oral Drug Delivery
Authors Zhong Q, Zeng J, Jia X
Received 8 March 2024
Accepted for publication 24 May 2024
Published 13 June 2024 Volume 2024:19 Pages 5931—5949
DOI https://doi.org/10.2147/IJN.S467354
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. RDK Misra
Qiyuan Zhong,1,* Jingqi Zeng,1,* Xiaobin Jia1,2
1School of Traditional Chinese Pharmacy, China Pharmaceutical University, Nanjing, 211198, People’s Republic of China; 2State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, 210009, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaobin Jia, School of Traditional Chinese Pharmacy, China Pharmaceutical University, 639 Longmian Avenue, Jiangning District, Nanjing, Jiangsu, 211198, People’s Republic of China, Email [email protected]
Abstract: The self-assembling aggregated structures of natural products have gained significant interest due to their simple synthesis, lack of carrier-related toxicity, and excellent biological efficacy. However, the mechanisms of their assembly and their ability to traverse the gastrointestinal (GI) barrier remain unclear. This review summarizes various intermolecular non-covalent interactions and aggregated structures, drawing on research indexed in Web of Science from 2010 to 2024. Cheminformatics analysis of the self-assembly behaviors of natural small molecules and their supramolecular aggregates reveals assembly-favorable conditions, aiding drug formulation. Additionally, the review explores the self-assembly properties of macromolecules like polysaccharides, proteins, and exosomes, highlighting their role in drug delivery. Strategies to overcome gastrointestinal barriers and enhance drug bioavailability are also discussed. This work underscores the potential of natural products in oral drug delivery and offers insights for designing more effective drug delivery systems.
Keywords: natural products, self-assembly, aggregated structures, supramolecules, oral drug delivery, gastrointestinal barrier
Graphical Abstract:
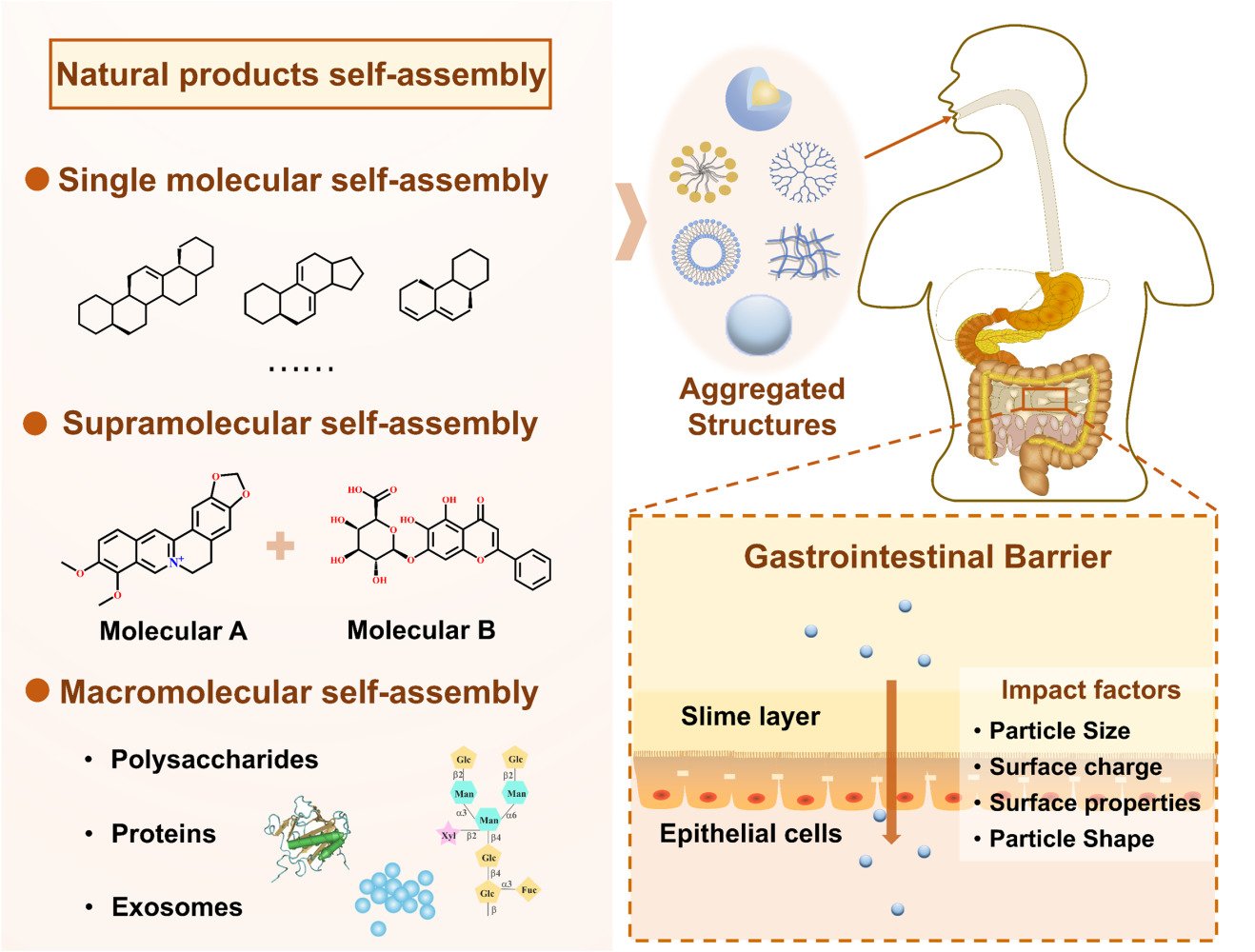
Introduction
Oral drug delivery is highly esteemed for its convenience and substantial patient compliance, rendering it a preferred method in contemporary healthcare. Nonetheless, the development of effective oral drug delivery systems is fraught with significant challenges, particularly in enhancing the solubility, permeability, and bioavailability of pharmaceutical agents. These challenges are inherently linked to the physicochemical properties of drug molecules, which are critical determinants of their absorption efficiency within the gastrointestinal tract, thereby influencing therapeutic effectiveness and safety.1
Historically, natural products have been pivotal in drug discovery, exemplified by breakthroughs such as penicillin and paclitaxel. These compounds have been instrumental in treating a wide array of diseases, including cancer and various infectious diseases.2 However, a major limitation in the utilization of natural products for drug discovery is their often-poor stability, solubility, or pharmacokinetics, with over 90% of these compounds being unsuitable for drug use.3 Nanotechnology offers solutions by providing physical protection to encapsulated drugs and optimizing their size for enhanced gastrointestinal penetration and circulation.4 However, nanoparticles synthesized from polymers, lipids, or inorganic materials often face challenges such as sensitivity to acidic pH and inadequate interaction with the intestinal epithelium. This results in reduced stability in the acidic stomach environment and necessitates surface modifications to target intestinal receptors.3
In response to these challenges, carrier-free self-assembled nanoplatforms have garnered significant interest. Self-assembly, defined as the natural and orderly organization of molecules into structured forms, is widespread in nature.5 Self-assembled aggregated structures play a crucial role in drug delivery by enhancing the stability and bioavailability of drugs within biological systems, such as reducing gastrointestinal degradation and improving permeability and absorption.6,7
Natural products can be categorized into small molecules and macromolecules, each exhibiting varied self-assembly behaviors that influence their performance in drug delivery. For instance, certain small molecules, such as terpenes (including betulin, betulinic acid, and oleanolic acid), can self-assemble, while steroids and glycoside compounds can form gel-like structures.8,9 The synthesis or structural modification of high-end nanodelivery systems might pose risks of toxicity and side effects in humans. In contrast, the self-assembly of natural products enables the construction of carrier-free nanoplatforms through non-covalent forces, avoiding the Introduction of toxic reagents and carrier toxicity during nanomedicine preparation.
Self-assembled aggregated structures provide an environmentally friendly and efficient synthetic approach. They are easily dispersible in aqueous media, simple to functionalize, have low production costs, and require no specialized equipment, demonstrating great potential in future applications. Additionally, these small molecule natural products have structures similar to endogenous components in the body and may be transported directly through intestinal epithelial transport proteins, eliminating the need for additional ligands.3 For example, dehydrotrametenolic acid is a kind of triterpenoid, which can form nanoparticles through hydrogen bonding self-assembly and serve as drug delivery systems. They penetrate the gastrointestinal tract by hijacking the apical sodium-dependent bile transporter (ASBT) -based intestinal transport system and is capable of efficiently delivering therapeutics for effective disease treatment.3 Therefore, gaining an in-depth understanding of the self-assembly behaviors of natural products is imperative for developing innovative drug delivery strategies and optimizing drug design.
Research on the aggregation structures of natural products is expanding, offering advantages that traditional nanotechnology lacks. Our focus is on analyzing the formation mechanisms of these aggregation structures. For small molecule compounds, we employ chemical informatics methods to digitally characterize various molecular properties and explore the structural and property characteristics of small molecules capable of assembly. Detailed data supporting these analyses can be found in Tables S1–S3. For macromolecular compounds, we summarize the structural characteristics and advantages of several typical natural carriers, such as polysaccharides, proteins, and exosomes. Additionally, we explore the factors and regulatory strategies affecting the oral absorption of aggregated structures, aiming to introduce new perspectives and methods for drug development.
Intermolecular Non-Covalent Interactions in Self-Assembled Aggregated Structures
As illustrated in Figure 1, the self-assembly process involves a variety of non-covalent interactions, including hydrogen bonding, electrostatic interactions, π-π interactions, hydrophobic interactions, and van der Waals forces. These interactions play a crucial role in the self-assembly process, driving molecules to spontaneously organize from a disordered state into an ordered structure. These interactions not only determine the formation of self-assembled aggregated structures but also influence their stability. Different types of self-assembled structures may be dominated by different intermolecular interactions. Therefore, a deep understanding of these molecular-level mechanisms is essential for the precise control and design of drug delivery systems. By accurately controlling these interactions, efficient systems that respond and release drugs under specific conditions can be designed.
 |
Figure 1 Types of intermolecular non-Covalent interactions driving the self-assembled Aggregated Structures. |
Hydrogen Bonding
Hydrogen bonding is a key intermolecular attractive interaction where a hydrogen atom is situated between two electronegative species, such as oxygen or nitrogen. Their energy varies, typically between 0.5 to 40 kcal/mol, allowing hydrogen bonds to rapidly form and break at ambient temperatures.10 Hydrogen bonding is particularly important in self-assembly, especially for molecules rich in oxygen functional groups like hydroxyl and carboxyl, which act not only as donors and acceptors of hydrogen bonds but also play a pivotal guiding role in the self-assembled structures. Fourier transform infrared (FTIR) spectroscopy is the most effective and extensive method for studying hydrogen bonds, and it can be observed that the stretching vibration of the group undergoes a red shift. In addition, nuclear magnetic resonance hydrogen spectroscopy, X-ray diffraction, etc. are also commonly used to characterize hydrogen bonds.
Electrostatic Interactions
Electrostatic interactions are the attractive forces between atoms or molecules with opposite charges. In polar solvents like water, these interactions are particularly significant, as water molecules can reduce the repulsion between charges, thereby enhancing the attraction. The electrostatic interaction between typical carboxyl and quaternary ammonium ions can lead to weak bonding strength of C=O double bonds and slow down the stretching vibration frequency, resulting in a peak redshift in FTIR spectra. Electrostatic interactions play a key role in the self-assembly of natural products as well as in the formation of liposomes and polyelectrolyte complexes.
π-π Interactions
π-π stacking interactions are weak interactions exhibited by π-electron conjugated systems arranged in a particular spatial configuration. Such interactions are common in self-assembly, particularly among molecules containing aromatic systems. For example, the π-π interactions in simple small molecule systems like benzene dimers primarily stem from the attraction between the electron clouds of aromatic systems.11 These interactions can be identified by spectroscopic methods, such as the ultraviolet and visible (UV) spectrum characteristics of molecules containing carbonyl and alkene double-bond functional groups.
Hydrophobic Interactions
The hydrophobic part of hydrophobic or amphiphilic molecules is isolated from polar water molecules as much as possible and aggregates together, and this force is called hydrophobic interactions. The magnitude of hydrophobic interactions is not measured simply by the magnitude of forces, but is characterized by changes in the local microenvironment caused by intermolecular interactions. Hydrophobic interactions are the main driving force for the self-assembly of amphiphilic molecules in water. The hydrophobic parts of these molecules tend to aggregate together to minimize their contact area with water, while exposing their hydrophilic parts to the aqueous environment.12 For instance, in self-assembling peptide nanotechnologies, hydrophobic interactions play a central role.
Van der Waals Forces
Van der Waals forces are weak interactions ubiquitously present between molecules, collectively influencing the stability of molecular aggregates. These forces include dipole-dipole interactions, dipole-induced dipole interactions, and London forces.13 Both molecular dynamics simulations and experimental methods, such as Two-Dimensional nuclear magnetic resonance nuclear overhauser effect spectroscopy (2D NMR NOESY) and infrared spectroscopy, have been employed to demonstrate that the van der Waals force, which is typically considered to be a dispersive interaction such as σ-σ and π-σ dispersion, plays a crucial role in driving self-assembly.14 Although individually weak, their multiple interactions can significantly impact molecular assembly. For example, the van der Waals forces between the multi-ring skeletons of terpene molecules play an important role in the assembly of these compounds.15
Types of Aggregated Structures and Their Applications in Drug Delivery
Aggregated structures play an essential role in the design and optimization of modern drug delivery systems. Leveraging their unique physicochemical properties and multifunctionality, aggregated structures have opened new realms in drug delivery. As shown in Figure 2, the transition from single molecules to complex aggregates encompasses a variety of forms, including micelles, hydrogels, liposomes, nano/microcapsules, and dendrimers. The diversity and flexibility of these structures provide a wide range of options for the characteristics and delivery needs of different drugs.
Micelles
Micelles are spontaneously formed by amphiphilic molecules with hydrophobic and hydrophilic groups, possessing a unique core-shell structure. This structure can effectively encapsulate poorly soluble drug molecules, providing superior drug encapsulation and delivery performance. The simplest form is micelles composed of surfactants, such as phospholipids, where molecules aggregate when their concentration exceeds the critical micelle concentration (CMC). In preclinical animal models, polymer micelles have optimized pharmacokinetic properties, significantly enhancing efficacy while maintaining high safety.16 Polymer micelle systems can also be used to deliver therapeutic compounds like small molecule drugs, proteins, and nucleic acids, further expanding their application range.17 For example, Zhang et al prepared super-antiresistant PTX micelles exhibited significantly increased cell uptake efficiency and permeability across caco-2 cell monolayers when compared with Taxol, achieving efficient oral administration.18
Hydrogels
Hydrogels are viscoelastic systems formed by encapsulating liquid in a three-dimensional network, characterized by significant hydrophilicity, flowability, and deformability. The physical properties of hydrogels, such as rheology, texture, water-holding capacity, and swelling rate, can be modulated by selecting different gelling agents, modifying gelation techniques, and adding other components, thereby altering the diffusion and release characteristics of bioactive components within.19 Nano-gels, as three-dimensional cross-linked aqueous materials, possess properties similar to natural tissues, able to resist shear forces in blood and serum proteins, offering new pathways for deep tumor penetration.20 Alginate hydrogel is commonly considered as a biocompatible platform for oral insulin administration that is stable in an acidic environment.21 Based on this, a novel carboxymethyl chitosan/sodium alginate nano hydrogel was constructed for oral delivery of insulin.22 The hydrogel has good pH sensitivity, biocompatibility and in vivo and in vitro controlled-release performance.
Liposomes
Liposomes have a spherical-vesicle structure made up of bilayers of phospholipids and steroids or other surfactants. Liposomes can be created by hydrating dry phospholipids, and they form spontaneously when certain lipids are dispersed in the media where they are prepare. These structures, surrounded by one or more lipid bilayers enclosing an aqueous core, facilitate the effective encapsulation and intercellular transport of drugs. The composition of liposomes can be adjusted to improve their pharmacokinetic properties and the ability to release active ingredients in targeted organs or cells.23,24 Sometimes, modifications are added to the surface of liposomes to achieve better intestinal wall penetration. Using glycerylcaldityl tetraether and a cyclic polyarginine as cell-penetrating peptides in the liposomal formulation, oral bioavailability of vancomycin was increased about five-fold, achieving therapeutic effects in methicillin-resistant Staphylococcus aureus(MRSA) infected mice after oral administration of liposomal vancomycin when compared to the free drug.25
Nano/Micro-Capsules
Nano/micro-capsules, composed of various amphiphilic materials, can have multi-lamellar structures or composite material shells. Polyethylene glycol (PEG) and polyvinyl alcohol are commonly used biocompatible polymers for synthesizing nanocapsules. They facilitate drug release by diffusion and can be eliminated from the bloodstream via the reticuloendothelial system.26 Materials like poly(lactic-co-glycolic acid) (PLGA), especially when modified with PEG for improved hydrophilicity and prolonged blood circulation time, are of particular interest for their excellent carrier capability for both hydrophilic and hydrophobic drugs.27 For encapsulation of hydrophilic drug, it is convenient to carry out functionalization of the PLGA nanoparticles with hydrophilic molecules such as polyethylene glycol (PEG) to potentiate the transport and drug release properties of this system.28 Natural polysaccharide alginate has been widely utilized in the preparation of microcapsules along with high potential as Active Pharmaceutical Ingredient delivery carriers.29 Zhang et al encapsulated methotrexate(MTX)-loaded human serum albumin nanoparticles into calcium alginate chitosan microcapsules, which could efficiently protect nanoparticles from degradation in gastric acid and protease to target accumulate in arthritic joints and intestinal inflammation lesions, while inhibiting the severe gastrointestinal toxicity caused by the oral administration of free MTX.30
Dendrimers
Dendrimers are notable for their branched structure and spherical geometry, formed by monomer subunits radiating from a central core. Dendrimers can improve the bioavailability of drugs by increasing their solubility in water, changing of surface charge, and by reducing toxicity. Polyamidoamine (PAMAM) dendrimers, the first synthesized class, are the most studied dendrimers in biomedical applications. PAMAM dendrimers can be synthesized by divergent method using methyl acrylate (MA) and ethylenediamine (EDA) as raw materials.31 They possess peptide bonds inside branches and are usually prepared by divergent synthesis.32 Hyperbranched polymer is a vital subclass of dendritic polymers, and natural hyperbranched polysaccharides, as one of them, can be used as an ideal delivery of pharmacological agents.33–35 Santos et al fabricated trans-resveratrol -encapsulated dextran-based layer-by-layer nanoparticles for enhanced oral bioavailability in Wistar Han rats, which represented excellent stability in plasma as well as in GI fluids.33
Carbon Nanotubes and Quantum Dots
Carbon nanotubes and quantum dots, as integral components of advanced nano delivery systems, exhibit improved solubility and biocompatibility.36 Carbon nanotubes, with their unique physical, chemical, and mechanical properties, demonstrate superior drug loading capacity and potential for targeted delivery in drug delivery systems. Quantum dots are renowned for their unique optical properties, particularly excelling in applications of biological imaging and drug tracking.37 The varied synthesis techniques of these nanostructures reflect the continuous evolution and innovation in the field of nanotechnology.38,39
Self-Assembly of Natural Small Molecules
At present, the structure-activity relationship of natural products lags far behind the research on their biological application and pharmacological research. We look forward to summarizing the relationship between molecular structure and self-assembly performance in order to predict more self-assembled aggregates and expand their applications. To unravel the molecular principles behind the phenomenon of self-assembly, we focused on the rules of small molecule self-assembly within natural products. Understanding these principles is crucial for deepening knowledge of self-assembly mechanisms within biological systems and guiding the optimization of drug design and delivery systems.
In exploring the self-assembly rules of small molecules in natural products, we considered two basic scenarios: single small molecular self-assembly and supramolecular self-assembly. The first type we taking saponins as an example, their amphiphilic structure endows them with high surface activity and self-assembly capabilities, making them a classic representative of natural biosurfactants.40 Supramolecular self-assembly involves the self-assembly between two different small molecules, such as the interactions between berberine and baicalin.41 This type of self-assembly demonstrates the diversity of intermolecular interactions and provides new research directions for explaining the synergistic effects of multiple components.
We conducted extensive literature research, systematically collecting and analyzing cases of self-assembly. Based on the review and summary of existing studies,14,42 we engaged in thorough literature tracing and verification and expanded the search to include the latest research findings. Our focus was on instances of small molecules driven by non-covalent forces forming complex structures in solvents, compiling 54 relevant publications, research sourced from Web of Science between 2010 and 2024. This comprehensive study gathered 66 instances of self-assembly, including 37 cases of single small molecule assembly and 29 cases of supramolecular assembly, covering 59 different small molecules. Detailed information of small molecule self-assembly will be presented in Tables S1–S3.
This work not only deepens our understanding of the phenomenon of self-assembly in nature but also provides a theoretical foundation for the design and development of novel drug delivery systems based on natural products. By summarizing these cases of self-assembly, we can more accurately predict the self-assembly behavior of small molecules under specific conditions, offering valuable guidance for the development of more efficient and targeted drug delivery strategies.
Single Molecular Self-Assembly
Characteristics of Terpenoids
Terpenoids are commonly found in single molecular self-assembly phenomena due to their unique structural characteristics. Molecular descriptors convert the chemical information encoded in molecular symbol representations into useful numbers or the results of some standardized experiments. By meticulously analyzing the structural descriptors of these molecules, we have identified key factors in the self-assembly capabilities of terpene small molecules.
As shown in Figure 3a, we performed a principal component analysis on the collected terpenoids self-assembling small molecules, dividing them into two major categories, with the second category predominantly composed of glycoside small molecules. This suggests a fundamental difference in solubility between the two categories. By comparing the topological polar surface area (TPSA, the area of the polar portion on the molecular surface, which is positive correlation with the water solubility of molecules) of both groups, we observed significant differences in solubility (Figure 3b).
Further examining the structure of these terpenoids, we noticed their common feature of multi-ring aliphatic skeletons (Figure 3c). Using the “NumAliphaticCarbocycles” descriptor (Represents the number of non aromatic carbon rings in the molecule), we quantified the structural conditions for self-assembling terpene small molecules. Specifically, for molecules with lower solubility (TPSA < 200), the structure should contain at least three non-aromatic carbon rings; for those with higher solubility (TPSA ≥ 200), at least four non-aromatic carbon rings are required (Figure 3d). This rule applies to 90% of the literature collection samples, quantifying the self-assembly rules of single terpene small molecules.
Self-Assembly Mechanisms of Terpenoids
The polycyclic alkane structure of terpenoids, combined with their polar hydroxyl and non-polar methyl groups, forms a rigid and planar skeleton, which significantly enhances their ability as self-assembly units. The polycyclic alkane skeleton of the molecule represents the hydrophobic regions, while the oxygen-containing functional groups act as hydrogen bond donors/acceptors, forming the polar sites of the molecule. In order to achieve a good hydrophilic/hydrophobic equilibrium of the overall molecular aggregates in aqueous solutions, molecules with high solubility and multiple polar oxygen-containing functional groups require a larger number of polycyclic alkane skeletons, which leads to the formation of aggregation structures.
Case Analysis
Among a wide variety of natural small molecules, apart from triterpenoids, diterpenoids are also one of the most abundant ingredients with better biological activity. For monoterpenes and diterpenes with fewer rings, a more detailed case-by-case analysis of self-assembly capability is required. For instance, dehydroabietic acid, 15-hydroxy-dehydroabietic acid contain benzene rings, which form advanced structures through π-π interactions of the benzene rings.43 Sesquiterpene compounds such as dihydroartemisinin form nanoparticles through hydrophobic interactions and hydrogen bonding, overcoming their poor solubility in water.44 Other categories of natural product small molecules, like flavonoids, anthraquinones, and alkaloids, also exhibit self-assembly capabilities. For example, puerarin forms hydrogels through C=O⋯π interactions,45 and rhein self-assembles into stable higher-order structures through π-π stacking and electrostatic interactions of carboxylic acids.46 These low molecular weight gels hold great potential in drug delivery. Although predicting their self-assembly behavior is challenging, research in this field continues to progress.
Supramolecular Self-Assembly
Supramolecular self-assembly is a complex process involving interactions between molecules of different structures. These molecules, though individually may not meet the criteria for self-assembly, can form supramolecular structures when interacting via non-covalent forces. This process is often based on complementary binding between compounds, such as the interactions between nitrogen-containing alkaloids and carboxylic organic acids, forming high dipole moments and orderly assembling through electrostatic and dipole-dipole interactions.47 This includes the assembly of nitrogen-containing alkaloids with carboxylic organic acids. By analyzing 30 cases of supramolecular assembly, we focused on 12 instances where individual molecules do not meet the criteria for single molecular self-assembly, involving 15 natural small molecules.
As shown in Figure 4a, berberine is currently reported to engage in supramolecular self-assembly with many compounds, where the electrostatic interaction between the negative centers of compounds and the positive center of berberine is the main driving force. Further, we used molecular descriptors to elucidate the rules of supramolecular self-assembly. Considering that supramolecular assembly is based on the pairing of two types of compounds, we calculated the difference in molecular descriptors for each pair to describe their characteristics. Specifically, after analyzing 12 sets of molecular descriptors, we noted that the relative standard deviation (RSD) of the difference between MaxEStateIndex and MinEStateIndex descriptors is the smallest, indicating these indices as stable indicators of the electronic state distribution. These two descriptors belong to the EState index, which is calculated based on the electronic properties of atoms and is used to quantify the electronic state of atoms in molecules. We found that when a pair of compounds has a MaxEStateIndex and MinEStateIndex difference greater than 1, these two small molecules are highly likely to have the potential to assemble into a supramolecule (Figure 4b).
Through the analysis of supramolecular self-assembly cases, we not only proposed a method to quantitatively determine the potential of supramolecular assembly but also provided a valuable approach for discovering and designing new supramolecular assembly systems in the fields of drug delivery and material science.
Undeniably, as most scholars are concerned, further in-depth research is needed to determine the stability of these aggregated structures formed through non covalent interactions and their fate in vivo. A few self-assembled aggregates have obvious macroscopic morphological changes, such as the formation of visible gel or nano precipitation, which are relatively stable. However, most aggregation structures at the nanoscale are fragile, exhibiting molecular exchange, migration and rearrangement among other dynamic instabilities, and are prone to dissociation upon drying.48 In terms of improving the stability of small molecule nanocomponents, fluoroalkylation could enhance the self-assembly stability of amphiphilic polymers by utilizing the unique fluorination effect.49 Scientists found that replacing hydrogen atoms with fluorine atoms in carbon chains greatly improved the self-assembling capability and colloidal stability of nanoassemblies due to the fluoro-mediated hydrophobic characteristic and halogen bond.50 Besides, A reliable strategy for enhancing mechanical properties of molecular materials is to incorporate hydrogen bonding domains into the molecular design.51 For example, the collective hydrogen bonding between aromatic amides (aramids) in Kevlar (poly(p-phenylene terephthalamide)) leads to its renowned strength and impact resistance.52 In addition, compared to small molecules, peptides offer precise control of the nanostructure through a range of molecular interactions where physical stability can be engineered in and, to a certain extent, decoupled from size via rational design.53 Therefore, the in-depth research on self-assembled materials in the biomedical field will gradually overcome this challenge and achieve more efficient applications.
Self-Assembly of Natural Macromolecules
The self-assembly release system of natural macromolecules is an innovative approach widely applied in drug delivery and therapeutic fields. This technique involves encapsulating drugs in nanoparticles and systematically releasing them to targeted sites through meticulously designed release systems. It exhibits advantages like efficient drug transport, reduced risk of side effects, and extended drug action time.54 Self-assembly release systems based on natural macromolecules show immense potential in the medical field, bringing revolutionary changes to drug therapies.
These natural macromolecules include polysaccharides, proteins, and exosomes, playing critical roles within biological systems. For instance, polysaccharides like hyaluronic acid and chitosan are widely used in constructing drug delivery systems due to their biocompatibility and biodegradability.55 Natural proteins like collagen and silk fibroin are used in preparing biomaterials and drug delivery carriers due to their unique bioactivity and cell affinity.56 By understanding the self-assembly mechanisms of these molecules, researchers can precisely design and regulate self-assembly release systems to meet specific drug and therapeutic needs.
Polysaccharides
Natural polysaccharides are extensively used in areas like drug carriers, drug delivery components, bioactive materials, and formulating agents to enhance the stability and delivery efficiency of drugs. Interactions between these macromolecules and small molecule active ingredients can improve the solubility of insoluble components, thereby enhancing the oral bioavailability of water-insoluble drugs.57,58 For example, the self-assembly behavior of polysaccharides in water might be related to the hydrogen bonding interactions of their uronic acid residues, leading to aggregation in aqueous solutions.59 Figure 5 shows the structure and mechanism of natural polysaccharide self-assembly, revealing its operational principles and application potential in drug delivery.
 |
Figure 5 Mechanism and structure of natural polysaccharide self-assembly, illustrating the basic characteristics of polysaccharide self-assembly and its multifunctional applications in drug delivery. |
Some natural polysaccharides exhibit the characteristics of hyperbranched polymers,60 such as plant glycogen. Phytoglycogen nanoparticles possess the properties of high level of water retention, low viscosity, and exceptional stability of aqueous dispersion. This highly branched structure can effectively accommodate and disperse insoluble phytochemicals, thereby improving the solubility and permeability of drugs.61–63
Polysaccharides can also be used to construct nanogels for drug delivery.64,65 For instance, Codonopsis pilosula polysaccharide spontaneously forms physical gels with random three-dimensional networks in alcohol-water mixtures.66 However, most polysaccharide gels require crosslinkers to maintain structural stability,67 such as genipin crosslinking free amino groups on chitosan to form stable hydrogels with lower toxicity and higher biocompatibility.68
Grafting hydrophobic molecules onto the main chain of natural polysaccharides can form amphiphilic polysaccharides, which self-assemble into micelles in aqueous solutions.69 For example, after hydrophobic modification of Angelica sinensis polysaccharide with deoxycholic acid, amphiphilic conjugates were prepared that self-assembled into stable nanoparticles.70 The self-assembly behavior of such hydrophobically modified polysaccharides is still being explored, changing the proportion of hydrophobic groups may form structures similar to phospholipid bilayers,71 further assembling into vesicular structures.
Polysaccharides can also be used to prepare nano/microcapsules of different structures,72 offering broad application potential. These capsules can be used for targeted delivery, sustained release, or protecting sensitive active ingredients.
Proteins
Proteins, with their excellent biocompatibility, degradability, emulsifying properties, gel-forming ability, foam stability, and hydration capacity, are widely used as natural carriers. Proteins such as collagen, casein, zein from corn, and soy protein have been successfully applied in substance delivery. Zein: Positively charged zein is suitable for delivering negatively charged drugs, food, and nutrients.73 Casein: Due to its hydrophobic core and hydrophilic surface, casein shows remarkable stability in aqueous dispersions and can effectively load nonpolar drugs.74 Functional Proteins: For example, ferritin and silk fibroin, these biodegradable and biocompatible proteins containing numerous functional groups, can serve as anchors for forming networked structures, thereby enhancing the stability of dispersed micelles.56,75
In the extraction process of traditional Chinese medicine slices, the conventional decoction process may lead to component self-assembly into aggregated structures, which helps to improve the drug absorption efficiency.76,77 For instance, in Coptidis Rhizoma extract, the naturally formed aggregated structures of proteins can alter the morphology of berberine hydrochloride, prompting its transition from crystalline to amorphous form, thereby enhancing berberine’s solubility and permeability.78
Overall, the application of proteins in drug delivery offers new possibilities in enhancing drug efficacy, reducing side effects, and enhancing drug bioavailability. These properties play an important role in drug development and clinical therapy, especially in the development of precision medicine and targeted therapies.
Exosomes
In exploring the application of natural macromolecules in self-assembly, our focus is not limited to polysaccharides and proteins but also extends to exosomes. While polysaccharides and proteins are widely used in drug delivery systems for their unique biocompatibility, biodegradability, and functionality, exosomes serve as an emerging natural nanocarrier, complementing certain aspects of polysaccharides and proteins. The uniqueness of exosomes lies in their complex biochemical composition and naturally formed nanoscale size, showing unique potential in intercellular communication and drug delivery.79
Exosomes originate from the multivesicular bodies of eukaryotic plant cells and are released to the extracellular space through the fusion of the cytoplasmic membrane. These vesicles are rich in bioactive lipids, proteins, RNA, and other pharmacologically active molecules, playing a key role in intercellular communication, information transfer, and maintaining internal balance within organisms.80 Exosomes exhibit various physiological activities such as anti-inflammatory, gut microbiota regulation, antitumor, and antiviral effects.81,82 Their low immunogenicity, high cellular uptake rate, enhanced gastrointestinal stability, and targeting capability make them ideal natural nanocarriers. In the field of drug delivery, exosomes have been extensively studied to overcome various biological barriers and achieve drug delivery to specific target organs like the intestines and the brain.83,84
Exosomes play a significant role in miRNA regulation and are used as a carrier for miRNA delivery in cancer treatment. miRNA, susceptible to nucleases, needs to cross various biological barriers and be effectively delivered to the cytoplasm for stable protein expression. When taken orally, exosomes serve as a protective transport carrier for miRNA, maintaining stability in the acidic environment of the stomach and reaching the small intestine through specific transport mechanisms to enter intestinal cells and release their contents.85,86
In summary, as innovative natural nanocarriers, exosomes show immense application potential in the field of drug delivery. They provide new avenues for enhancing the efficiency and specificity of drug delivery in future medical research and clinical applications.
Factors and Strategies Impacting the Penetration of Aggregated Structures Through Gastrointestinal Barrier
The main challenge faced by oral drug delivery systems stems from the harsh environment within the gastrointestinal tract and the physical absorption barrier of the intestinal mucosa. While these physiological barriers are crucial for the body’s health, they also limit the effective absorption of drugs. Drugs need to overcome the stomach’s acidic environment to reach the small intestine, their primary absorption site. Two major barriers in the small intestine, the mucosal layer and intestinal epithelial cells, further complicate drug delivery. The mucosal layer, composed of water, mucins, DNA, proteins, lipids, and cell debris, has viscoelastic properties, providing protection for epithelial cells and preventing external threats and enzyme degradation.87
Drug carriers, like polymer nanoparticles, may adhere to the mucus, hindering their easy traversal through the mucosal layer. If nanoparticles are absorbed through the transcellular route in the small intestinal epithelial monolayer, they need to undergo a polar transfer from the luminal side to the inside of the cell, and then be excreted from the basal side.88 The physicochemical properties of the aggregated structures profoundly impact their transportation process in the body, thus affecting the therapeutic effectiveness and safety of nanomedicine.
Factors Affecting the Traversal of Aggregated Structures Through the Gastrointestinal Barrier
As shown in Figure 6, key parameters like particle size, surface charge, and hydrophilicity/hydrophobicity significantly impact overcoming the oral gastrointestinal barrier. Particle size affects the efficiency of aggregated structures in traversing the intestinal barrier, while surface charge and hydrophilicity/hydrophobicity determine the interactions of aggregated structures with mucus and intestinal epithelial cells. Understanding how these parameters affect the mechanism by which natural product self-assembled aggregated structures penetrate the gastrointestinal barrier is crucial for designing more efficient oral drug delivery systems.
Particle Size
Particle size is a key parameter affecting the ability of nanomedicine to traverse the gastrointestinal barrier. The mucosal layer acts as a size filter, generally allowing smaller aggregated structures to penetrate more easily than larger particles.89,90 Larger aggregated structures (500 nm to 5 μm) encounter difficulties in traversing the mucosal layer, while smaller particles show higher absorption rates.91 However, very small particles (eg, 50 nm) have lower absorption rates, suggesting the existence of a lower size limit, below which particle size no longer significantly affects absorption.92 Therefore, considering particle size to adapt to the permeability characteristics of the mucosal layer and intestinal epithelial cell layer is crucial in drug design.
Surface Charge
The surface charge of aggregated structures significantly impacts their ability to cross the gastrointestinal barrier.93 It is a key determinant of patterns of interaction with membranes, cell surfaces and plasma protein. The components of mucus, such as sialic acid moieties and sulfates, impart a negative electrostatic charge to the mucus layer.94 Therefore, negatively charged aggregated structures traverse the mucosal layer more easily than positively charged particles. Negatively charged aggregated structures can trigger the dissociation of tight junction proteins and enhance paracellular permeability.95 Regardless of the charge, aggregated structures with a low charge amount show higher transport levels, indicating that surface charge has an important impact on the transport pathway of aggregated structures.
Surface Hydrophilicity/Hydrophobicity
The surface hydrophilicity/hydrophobicity of aggregated structures is influenced by their surface functional groups. As the mucosal layer is overall hydrophilic, hydrophobic particles have limited diffusion ability within it.96 PEG is an effective mucosal permeation enhancer. It is shown that low molecular weight PEG and high surface coverage of PEG could increase the hydrophilic of drug and minimize mucoadhesion by reducing hydrophobic or electrostatic interactions promotes cell uptake of aggregated structures.88 In the everted intestinal sac model constructed from the isolated small intestine of rats, PEGylated nanoparticles have a higher permeability than non-PEGylated nanoparticles, which can be explained as the efficient modification of PEG to the particle surface allowed nanoparticles to rapidly penetrate through highly viscoelastic small intestinal mucus by moving through openings between mucin mesh fibers.97 However, non-PEGylated nanoparticles show higher uptake rates in intestinal epithelial cells, which may be attributed to the lipophilic nature of the cell membrane.88
Particle Shape
The geometric shape of aggregated structures significantly impacts their interaction with biological targets, thereby affecting formulation efficacy. Rod-shaped and disc-shaped aggregated structures show greater transport through intestinal cells compared to spherical ones.98 This may be due to the larger contact surface area provided by rod and disc shapes for interaction and adhesion with the cell membrane, increasing the likelihood of interaction with cell receptors.99
Self-Assembly Strategy for Aggregated Structure Characteristics
In designing oral drug delivery systems, focusing on the physicochemical characteristics of aggregated structures is crucial. Smaller sizes help reduce mucus layer capture, a low amount of negative charge avoids adhesion of mucins in the mucus layer, and moderate hydrophilicity aids in mucosal permeation. These characteristics together facilitate more effective drug diffusion in the small intestine and entry into the bloodstream or lymphatic circulation through submucosal and epithelial cell barriers, thereby enhancing drug absorption. Considering these factors in drug design enhances the ability of aggregated structures to traverse the small intestinal mucosal layer, improving drug delivery efficiency.
In traversing the intestinal epithelial cell barrier, appropriate size, positive surface charge, and certain hydrophobic characteristics significantly enhance adhesion and phagocytosis by intestinal epithelial cells, thus promoting drug absorption. Positively charged particles are more likely to interact with the cell membrane, while moderate hydrophobicity helps the particles to cross the cell membrane. This strategy is particularly suitable for drugs that need to be absorbed via intracellular pathways. By adjusting these characteristics of aggregated structures, drug delivery and release can be more effectively controlled, achieving precise therapeutic effects.
Optimize the preparation method to achieve suitable physical properties such as size and shape, and control the molecular weight and dosage of the modified polymer used to obtain suitable surface properties. This dual strategy offers a comprehensive approach to optimizing oral drug delivery systems, focusing not only on drug transport within the body but also on interactions with biological barriers. Through this method, the challenges of oral drug delivery can be more effectively overcome, enhancing drug bioavailability and therapeutic efficacy.
Self-Assembly Strategy Involving Endogenous Components
In drug delivery systems, utilizing endogenous components for self-assembly can enhance the efficiency and specificity of drug delivery. This strategy leverages natural pathways within the body, such as specific receptors or transport mechanisms in the intestines, and enhances drug absorption and permeation through self-assembly processes in the gastrointestinal environment.
The self-assembly behavior of bile salts provides new insights into the behavior of oral drugs in the gastrointestinal tract, aiding in the optimization of drug design and delivery strategies.100,101 For example, sodium deoxycholate, as an endogenous surfactant, can spontaneously form micelle structures under suitable conditions, thus acting as an absorption enhancer to improve the drug’s ability to traverse biological barriers.102,103 The self-assembly characteristics of bile salts help to enhance the bioavailability of poorly soluble drugs, thereby increasing the efficiency of oral drug delivery.
Natural small molecules may undergo supramolecular self-assembly with proteins in the digestive system, such as trypsin and pepsin, affecting their catalytic activity.104–106 Additionally, after entering the bloodstream, natural small molecules may self-assemble with proteins like albumin and globulin, affecting the drug’s distribution and stability in the blood. For example, the binding of flavonoid compounds with plasma proteins through hydrophobic interactions and hydrogen bonding influences their bioavailability.107
Utilizing self-assembly strategies involving endogenous components not only enhances the naturalness and biocompatibility of drug delivery but also reduces reliance on foreign substances, lowering potential toxicity and side effects. This approach offers new directions and possibilities for the design and optimization of drug delivery systems. When researching the application of endogenous component self-assembly, it’s necessary to consider how factors like temperature, heating time, and pH might affect the stability of the nanomedicine. Therefore, in-depth research into these factors to ensure the stability and efficacy of the formulation is essential.
Conclusion
Self-assembly is the process where molecules spontaneously form aggregated structures induced by non-covalent forces under certain conditions. This characteristic endows some natural products with extraordinary microstructures, functions, applications, and advantages, freeing them from the constraints of toxic additives and complex synthesis. The discovery of self-assembled aggregated structures marks a breakthrough in natural product research, suggesting that the value of natural products extends beyond drug discovery to include their potential as valuable nanomaterials for drug delivery. Molecules with low solubility and bioavailability, which typically restrict drug formation, can achieve better absorption through self-assembly into aggregated structures.
This review presents a detailed examination of self-assembled aggregated structures in oral drug delivery, emphasizing the role of intermolecular non-covalent interactions and various aggregated structures like micelles, hydrogels, and liposomes. By focusing on the self-assembly behaviors of natural small molecules and their supramolecular aggregates, we have compiled numerous literature cases and summarized them using chemical informatics methods. Molecular descriptors were used to quantitatively analyze the structure and properties of assembled molecules, determining specific conditions favorable for assembly. This approach helps identify more small molecules with assembly potential, providing a strong theoretical foundation for understanding how these structures enhance drug bioavailability and offering novel insights into designing carrier-free nanoplatforms for drug delivery.
In our exploration, we also introduced natural polysaccharides and derived polysaccharides as nano delivery systems, the drug delivery potential of proteins with different structures, and the application of novel plant exosome carriers. These natural products offer better safety and avoid carrier toxicity. Overcoming the gastrointestinal barrier remains a significant challenge for oral administration. By examining factors such as particle size and surface properties, we proposed new strategies for optimizing drug delivery systems. Additionally, the involvement of intrinsic endogenous components in the absorption process of drug molecules, forming specific aggregated structures in the gastrointestinal environment, can promote drug absorption, providing new insights into the gastrointestinal absorption of oral drugs. This perspective offers new directions and possibilities for the design and optimization of drug delivery systems.
In summary, assemblies based on natural products are at the forefront of chemical and material science development. They are invaluable for studying molecular interactions, developing new materials, and enhancing the oral absorption of drugs. Our findings significantly contribute to drug development by leveraging the unique properties of natural products. This study not only advances the understanding of oral drug delivery mechanisms but also paves the way for future innovations in drug delivery systems, promising more effective and safer therapeutic options.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Grant No. 82230117, China). The funders of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. During the preparation of this work, the author(s) used OpenAI’s ChatGPT for text generation and information retrieval. Following the use of this tool, the author(s) reviewed and edited the content as necessary and take full responsibility for the content of the publication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas: took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this paper.
References
1. Zhang L, Wang S, Zhang M, et al. Nanocarriers for oral drug delivery. J Drug Target. 2013;21(6):515–527. doi:10.3109/1061186X.2013.789033
2. Ekiert HM, Szopa A. Biological activities of natural products. Molecules. 2020;25(23):5769. doi:10.3390/molecules25235769
3. Yang X, Ma C, Chen Z, et al. Single small molecule-assembled nanoparticles mediate efficient oral drug delivery. Nano Res. 2019;12(10):2468–2476. doi:10.1007/s12274-019-2470-0
4. Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–951. doi:10.1038/nbt.3330
5. Fan Y, Liu Y, Wu Y, et al. Natural polysaccharides based self-assembled nanoparticles for biomedical applications – a review. Int J Biol Macromol. 2021;192:1240–1255. doi:10.1016/j.ijbiomac.2021.10.074
6. Yadav S, Sharma AK, Kumar P. Nanoscale self-assembly for therapeutic delivery. Front Bioeng Biotechnol. 2020;8:500966. doi:10.3389/fbioe.2020.00127
7. Wang J, Zhao H, Qiao W, et al. Nanomedicine-cum-carrier by co-assembly of natural small products for synergistic enhanced antitumor with tissues protective actions. ACS Appl Mater Interfaces. 2020;12(38):42537–42550. doi:10.1021/acsami.0c12641
8. Bag BG, Majumdar R. Self-assembly of renewable nano-sized triterpenoids. Chem Rec. 2017;17(9):841–873. doi:10.1002/tcr.201600123
9. Zhi K, Zhao H, Yang X, et al. Natural product gelators and a general method for obtaining them from organisms. Nanoscale. 2018;10(8):3639–3643. doi:10.1039/C7NR08368H
10. Desiraju GR. A bond by any other name. Angew Chem Int Ed Engl. 2011;50(1):52–59. doi:10.1002/anie.201002960
11. Cai W, Xu D, Qian L, et al. Force-induced transition of π-π stacking in a single polystyrene chain. J Am Chem Soc. 2019;141(24):9500–9503. doi:10.1021/jacs.9b03490
12. Gao Y, Wang L, Zhang X, et al. Advances in self-assembled peptides as drug carriers. Pharmaceutics. 2023;15(2):482. doi:10.3390/pharmaceutics15020482
13. Berland K, Chakraborty D, Thonhauser T. van der Waals density functional with corrected C6 coefficients. Phys Rev B. 2019;99(19):195418. doi:10.1103/PhysRevB.99.195418
14. Hou Y, Zou L, Li Q, et al. Supramolecular assemblies based on natural small molecules: union would be effective. Mater Today Bio. 2022;15:100327. doi:10.1016/j.mtbio.2022.100327
15. Zhi K, Wang J, Zhao H, et al. Self-assembled small molecule natural product gel for drug delivery: a breakthrough in new application of small molecule natural products. Acta Pharm Sin B. 2020;10(5):913–927. doi:10.1016/j.apsb.2019.09.009
16. Hwang D, Ramsey JD, Kabanov AV. Polymeric micelles for the delivery of poorly soluble drugs: from nanoformulation to clinical approval. Adv Drug Deliv Rev. 2020;156:80–118. doi:10.1016/j.addr.2020.09.009
17. Cabral H, Miyata K, Osada K, et al. Block copolymer micelles in nanomedicine applications. Chem Rev. 2018;118(14):6844–6892. doi:10.1021/acs.chemrev.8b00199
18. Zhang T, Luo J, Fu Y, et al. Novel oral administrated paclitaxel micelles with enhanced bioavailability and antitumor efficacy for resistant breast cancer. Colloids Surf B Biointerfaces. 2017;150:89–97. doi:10.1016/j.colsurfb.2016.11.024
19. Mao L, Lu Y, Cui M, et al. Design of gel structures in water and oil phases for improved delivery of bioactive food ingredients. Crit Rev Food Sci Nutr. 2020;60(10):1651–1666. doi:10.1080/10408398.2019.1587737
20. Ma X, Li SJ, Liu Y, et al. Bioengineered nanogels for cancer immunotherapy. Chem Soc Rev. 2022;51(12):5136–5174. doi:10.1039/d2cs00247g
21. Wu H, Nan J, Yang L, et al. Insulin-loaded liposomes packaged in alginate hydrogels promote the oral bioavailability of insulin. J Control Release. 2023;353:51–62. doi:10.1016/j.jconrel.2022.11.032
22. Zhang H, Gu Z, Li W, et al. pH-sensitive O-carboxymethyl chitosan/sodium alginate nanohydrogel for enhanced oral delivery of insulin. Int J Biol Macromol. 2022;223:433–445. doi:10.1016/j.ijbiomac.2022.10.274
23. Nisini R, Poerio N, Mariotti S, et al. The multirole of liposomes in therapy and prevention of infectious diseases. Front Immunol. 2018;9:155. doi:10.3389/fimmu.2018.00155
24. He H, Lu Y, Qi J, et al. Adapting liposomes for oral drug delivery. Acta Pharm Sin B. 2019;9(1):36–48. doi:10.1016/j.apsb.2018.06.005
25. Werner J, Umstätter F, Hertlein T, et al. Oral delivery of the vancomycin derivative FU002 by a surface-modified liposomal nanocarrier. Adv Healthc Mater. 2024;13(14):e2303654. doi:10.1002/adhm.202303654
26. Arredondo-Ochoa T, Silva-Martínez GA. Microemulsion based nanostructures for drug delivery. Front Nanotechnol. 2021;3:753947. doi:10.3389/fnano.2021.753947
27. Noori Koopaei M, Khoshayand MR, Mostafavi SH, et al. Docetaxel loaded PEG-PLGA nanoparticles: optimized drug loading, in-vitro cytotoxicity and in-vivo antitumor effect. Iran J Pharm Res. 2014;13(3):819–833.
28. Palacio J, Monsalve Y, Villa-Pulgarin JA, et al. Preparation and evaluation of PLGA-PEG/Gusperimus nanoparticles as a controlled delivery anti-inflammatory drug. J Drug Delivery Sci Technol. 2022;77:103889. doi:10.1016/j.jddst.2022.103889
29. Uyen NTT, Hamid ZAA, Tram NXT, et al. Fabrication of alginate microspheres for drug delivery: a review. Int J Biol Macromol. 2020;153:1035–1046. doi:10.1016/j.ijbiomac.2019.10.233
30. Zhang F, Du Y, Zheng J, et al. Oral Administration of Multistage Albumin Nanomedicine Depots (MANDs) for targeted efficient alleviation of chronic inflammatory diseases. Adv Funct Mater. 2023;33(9):2211644. doi:10.1002/adfm.202211644
31. Liu S, Liang H, Sun T, et al. A recoverable dendritic polyamidoamine immobilized TEMPO for efficient catalytic oxidation of cellulose. Carbohydr Polym. 2018;202:563–570. doi:10.1016/j.carbpol.2018.09.016
32. Pedziwiatr-Werbicka E, Milowska K, Dzmitruk V, et al. Dendrimers and hyperbranched structures for biomedical applications. Eur Polym J. 2019;119:61–73. doi:10.1016/j.eurpolymj.2019.07.013
33. Santos AC, Veiga FJ, Sequeira JAD, et al. First-time oral administration of resveratrol-loaded layer-by-layer nanoparticles to rats - a pharmacokinetics study. Analyst. 2019;144(6):2062–2079. doi:10.1039/C8AN01998C
34. Chen L, Ge MD, Zhu YJ, et al. Structure, bioactivity and applications of natural hyperbranched polysaccharides. Carbohydr Polym. 2019;223:115076. doi:10.1016/j.carbpol.2019.115076
35. Nickels JD, Atkinson J, Papp-Szabo E, et al. Structure and hydration of highly-branched, monodisperse phytoglycogen nanoparticles. Biomacromolecules. 2016;17(3):735–743. doi:10.1021/acs.biomac.5b01393
36. Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354(6348):56–58. doi:10.1038/354056a0
37. Pardo J, Peng Z, Leblanc RM. Cancer targeting and drug delivery using carbon-based quantum dots and nanotubes. Molecules. 2018;23(2):378. doi:10.3390/molecules23020378
38. Wang B, Wang S, Wang Y, et al. Highly fluorescent carbon dots for visible sensing of doxorubicin release based on efficient nanosurface energy transfer. Biotechnol Lett. 2016;38(1):191–201. doi:10.1007/s10529-015-1965-3
39. Ahirwar S, Mallick S, Bahadur D. Electrochemical method to prepare graphene quantum dots and graphene oxide quantum dots. ACS Omega. 2017;2(11):8343–8353. doi:10.1021/acsomega.7b01539
40. Liao Y, Li Z, Zhou Q, et al. Saponin surfactants used in drug delivery systems: a new application for natural medicine components. Int J Pharm. 2021;603:120709. doi:10.1016/j.ijpharm.2021.120709
41. Wang P, Guo W, Huang G, et al. Berberine-based heterogeneous linear supramolecules neutralized the acute nephrotoxicity of aristolochic acid by the self-assembly strategy. ACS Appl Mater Interfaces. 2021;13(28):32729–32742. doi:10.1021/acsami.1c06968
42. Li Z, Xu X, Wang Y, et al. Carrier-free nanoplatforms from natural plants for enhanced bioactivity. J Adv Res. 2023;50:159–176. doi:10.1016/j.jare.2022.09.013
43. Cheng J, Fu S, Qin Z, et al. Self-assembled natural small molecule diterpene acids with favorable anticancer activity and biosafety for synergistically enhanced antitumor chemotherapy. J Mater Chem B. 2021;9(11):2674–2687. doi:10.1039/D0TB02995E
44. Li Y, Zhang W, Shi N, et al. Self-assembly and self-delivery of the pure nanodrug dihydroartemisinin for tumor therapy and mechanism analysis. Biomater Sci. 2023;11(7):2478–2485. doi:10.1039/D2BM01949C
45. Pang Z, Wei Y, Wang N, et al. Gel formation of puerarin and mechanistic study during its cooling process. Int J Pharm. 2018;548(1):625–635. doi:10.1016/j.ijpharm.2018.07.038
46. Zheng J, Fan R, Wu H, et al. Directed self-assembly of herbal small molecules into sustained release hydrogels for treating neural inflammation. Nat Commun. 2019;10(1):1604. doi:10.1038/s41467-019-09601-3
47. Wang L, Gao G, Zhou Y, et al. Tough, adhesive, self-healable, and transparent ionically conductive zwitterionic nanocomposite hydrogels as skin strain sensors. ACS Appl Mater Interfaces. 2019;11(3):3506–3515. doi:10.1021/acsami.8b20755
48. Christoff-Tempesta T, Cho Y, Kim DY, et al. Self-assembly of aramid amphiphiles into ultra-stable nanoribbons and aligned nanoribbon threads. Nat Nanotechnol. 2021;16(4):447–454. doi:10.1038/s41565-020-00840-w
49. Zhang C, Liu T, Wang W, et al. Tuning of the aggregation behavior of fluorinated polymeric nanoparticles for improved therapeutic efficacy. ACS Nano. 2020;14(6):7425–7434. doi:10.1021/acsnano.0c02954
50. Wang X, Yang B, Li L, et al. Probing the fluorination effect on the self-assembly characteristics, in vivo fate and antitumor efficacy of paclitaxel prodrug nanoassemblies. Theranostics. 2021;11(16):7896–7910. doi:10.7150/thno.61337
51. Sherrington DC, Taskinen KA. Self-assembly in synthetic macromolecular systems via multiple hydrogen bonding interactions. Chem Soc Rev. 2001;30(2):83–93. doi:10.1039/b008033k
52. Dobb MG, Johnson DJ, Saville BP. Supramolecular structure of a high-modulus polyaromatic fiber (Kevlar 49). J Polym Sci. 1977;15(12):2201–2211.
53. König N, Szostak SM, Nielsen JE, et al. Stability of nanopeptides: structure and molecular exchange of self-assembled peptide fibers. ACS Nano. 2023;17(13):12394–12408. doi:10.1021/acsnano.3c01811
54. An J, Liu M, Din ZU, et al. Toward function starch nanogels by self-assembly of polysaccharide and protein: from synthesis to potential for polyphenol delivery. Int J Biol Macromol. 2023;247:125697. doi:10.1016/j.ijbiomac.2023.125697
55. Mohammed ASA, Naveed M, Jost N. Polysaccharides; classification, chemical properties, and future perspective applications in fields of pharmacology and biological medicine (A Review of Current Applications and Upcoming Potentialities). J Polym Environ. 2021;29(8):2359–2371. doi:10.1007/s10924-021-02052-2
56. Yu B, Sun Z, Li X, et al. Research progress of novel drug delivery systems of Chinese medicine monomers based on natural silk fibroin: a mini-review. Curr Drug Deliv. 2023;20(3):211–222. doi:10.2174/1567201819666220413111439
57. Wang B, Huang B, Yang B, et al. Structural elucidation of a novel polysaccharide from Ophiopogonis Radix and its self-assembly mechanism in aqueous solution. Food Chem. 2023;402:134165. doi:10.1016/j.foodchem.2022.134165
58. Yang B, Wu X, Zeng J, et al. A multi-component nano-co-delivery system utilizing astragalus polysaccharides as carriers for improving biopharmaceutical properties of astragalus flavonoids. Int J Nanomed. 2023;18:6705–6724. doi:10.2147/IJN.S434196
59. Zhao Y, Wan P, Wang J, et al. Polysaccharide from vinegar baked radix bupleuri as efficient solubilizer for water-insoluble drugs of Chinese medicine. Carbohydr Polym. 2020;229:115473. doi:10.1016/j.carbpol.2019.115473
60. Zhang M, Ma H, Wang X, et al. Polysaccharide-based nanocarriers for efficient transvascular drug delivery. J Control Release. 2023;354:167–187. doi:10.1016/j.jconrel.2022.12.051
61. Xue J, Luo Y. Properties and applications of natural dendritic nanostructures: phytoglycogen and its derivatives. Trends Food Sci Technol. 2020;107:432–444. doi:10.1016/j.tifs.2020.11.013
62. Chen H, Yao Y. Phytoglycogen improves the water solubility and Caco-2 monolayer permeation of quercetin. Food Chem. 2017;221:248–257. doi:10.1016/j.foodchem.2016.10.064
63. Xie Y, Yao Y. Octenylsuccinate hydroxypropyl phytoglycogen enhances the solubility and in-vitro antitumor efficacy of niclosamide. Int J Pharm. 2018;535(1–2):157–163. doi:10.1016/j.ijpharm.2017.11.004
64. Yan J, Wang Y, Zhang X, et al. Snakegourd root/Astragalus polysaccharide hydrogel preparation and application in 3D printing. Int J Biol Macromol. 2019;121:309–316. doi:10.1016/j.ijbiomac.2018.10.008
65. Yang L, Han Z, Chen C, et al. Novel probiotic-bound oxidized Bletilla striata polysaccharide-chitosan composite hydrogel. Mater Sci Eng C Mater Biol Appl. 2020;117:111265. doi:10.1016/j.msec.2020.111265
66. Zhao T, Dong S, Shao S, et al. Injectable hydroethanolic physical gels based on Codonopsis pilosula polysaccharide for sustained anticancer drug delivery. Int J Biol Macromol. 2023;230:123178. doi:10.1016/j.ijbiomac.2023.123178
67. Wang H, Deng H, Gao M, et al. Self-assembled nanogels based on ionic gelation of natural polysaccharides for drug delivery. Front Bioeng Biotechnol. 2021;9:703559. doi:10.3389/fbioe.2021.703559
68. Ding L, Shan X, Zhao X, et al. Spongy bilayer dressing composed of chitosan-Ag nanoparticles and chitosan-Bletilla striata polysaccharide for wound healing applications. Carbohydr Polym. 2017;157:1538–1547. doi:10.1016/j.carbpol.2016.11.040
69. Gong H, Li W, Sun J, et al. A review on plant polysaccharide based on drug delivery system for construction and application, with emphasis on traditional Chinese medicine polysaccharide. Int J Biol Macromol. 2022;211:711–728. doi:10.1016/j.ijbiomac.2022.05.087
70. Zhang Y, Cui Z, Mei H, et al. Angelica sinensis polysaccharide nanoparticles as a targeted drug delivery system for enhanced therapy of liver cancer. Carbohydr Polym. 2019;219:143–154. doi:10.1016/j.carbpol.2019.04.041
71. Cai G, Jiang H, Chen Z, et al. Synthesis, characterization and self-assemble behavior of Chitosan-O-poly(ε-caprolactone). Eur Polym J. 2009;45(6):1674–1680. doi:10.1016/j.eurpolymj.2009.03.007
72. Meng Q, Zhong S, Gao Y, et al. Advances in polysaccharide-based nano/microcapsules for biomedical applications: a review. Int J Biol Macromol. 2022;220:878–891. doi:10.1016/j.ijbiomac.2022.08.129
73. Kasaai MR. Zein and Zein-based nano-materials for food and nutrition applications: a review. Trends Food Sci Technol. 2018;79:184–197. doi:10.1016/j.tifs.2018.07.015
74. Cuggino JC, Picchio ML, Gugliotta A, et al. Crosslinked casein micelles bound paclitaxel as enzyme activated intracellular drug delivery systems for cancer therapy. Eur Polym J. 2021;145:110237. doi:10.1016/j.eurpolymj.2020.110237
75. Liu Y, Yang R, Liu J, et al. Fabrication, structure, and function evaluation of the ferritin based nano-carrier for food bioactive compounds. Food Chem. 2019;299:125097. doi:10.1016/j.foodchem.2019.125097
76. Lin X, Huang X, Tian X, et al. Natural small-molecule-based carrier-free self-assembly library originated from traditional Chinese herbal medicine. ACS Omega. 2022;7(48):43510–43521. doi:10.1021/acsomega.2c04098
77. Gao Y, Dong Y, Guo Q, et al. Study on supramolecules in Traditional Chinese Medicine Decoction. Molecules. 2022;27(10):3268. doi:10.3390/molecules27103268
78. Zhao J, Zhao Q, Lu JZ, et al. Natural nano-drug delivery system in coptidis rhizoma extract with modified berberine hydrochloride pharmacokinetics. Int J Nanomed. 2021;16:6297–6311. doi:10.2147/IJN.S323685
79. Cao M, Diao N, Cai X, et al. Plant exosome nanovesicles (PENs): green delivery platforms. Mater Horiz. 2023;10(10):3879–3894. doi:10.1039/D3MH01030A
80. Dad HA, Gu TW, Zhu AQ, et al. Plant Exosome-like Nanovesicles: emerging Therapeutics and Drug Delivery Nanoplatforms. Mol Ther. 2021;29(1):13–31. doi:10.1016/j.ymthe.2020.11.030
81. Li Y, Buckhaults P, Li S, et al. Temporal efficacy of a sulforaphane-based broccoli sprout diet in prevention of breast cancer through modulation of epigenetic mechanisms. Cancer Prev Res. 2011;11(8):451–464. doi:10.1158/1940-6207.CAPR-17-0423
82. Gao C, Zhou Y, Chen Z, et al. Turmeric-derived nanovesicles as novel nanobiologics for targeted therapy of ulcerative colitis. Theranostics. 2022;12(12):5596–5614. doi:10.7150/thno.73650
83. Niu W, Xiao Q, Wang X, et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021;21(3):1484–1492. doi:10.1021/acs.nanolett.0c04753
84. Wang B, Zhuang X, Deng ZB, et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther. 2014;22(3):522–534. doi:10.1038/mt.2013.190
85. Del Pozo-Acebo L, López de Las Hazas MC, Tomé-Carneiro J, et al. Therapeutic potential of broccoli-derived extracellular vesicles as nanocarriers of exogenous miRNAs. Pharmacol Res. 2022;185:106472. doi:10.1016/j.phrs.2022.106472
86. You JY, Kang SJ, Rhee WJ. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact Mater. 2021;6(12):4321–4332. doi:10.1016/j.bioactmat.2021.04.023
87. Murgia X, Loretz B, Hartwig O, et al. The role of mucus on drug transport and its potential to affect therapeutic outcomes. Adv Drug Deliv Rev. 2018;124:82–97. doi:10.1016/j.addr.2017.10.009
88. Guo S, Liang Y, Liu L, et al. Research on the fate of polymeric nanoparticles in the process of the intestinal absorption based on model nanoparticles with various characteristics: size, surface charge and pro-hydrophobics. J Nanobiotechnology. 2021;19(1):32. doi:10.1186/s12951-021-00770-2
89. Bandi SP, Kumbhar YS, Venuganti VVK, et al. Effect of particle size and surface charge of nanoparticles in penetration through intestinal mucus barrier. J Nanopart Res. 2020;22(3):62. doi:10.1007/s11051-020-04785-y
90. Li Q, Liu CG, Yu Y. Separation of monodisperse alginate nanoparticles and effect of particle size on transport of vitamin E. Carbohydr Polym. 2015;124:274–279. doi:10.1016/j.carbpol.2015.02.007
91. Xu M, Qi Y, Liu G, et al. Size-dependent in vivo transport of nanoparticles: implications for delivery, targeting, and clearance. ACS Nano. 2023;17(21):20825–20849. doi:10.1021/acsnano.3c05853
92. Win KY, Feng SS. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;6(15):2713–2722. doi:10.1016/j.biomaterials.2004.07.050
93. Dawson M, Wirtz D, Hanes J. Enhanced viscoelasticity of human cystic fibrotic sputum correlates with increasing microheterogeneity in particle transport. J Biol Chem. 2003;278(50):50393–50401. doi:10.1074/jbc.M309026200
94. Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86(1):245–278. doi:10.1152/physrev.00010.2005
95. Wang J, Kong M, Zhou Z, et al. Mechanism of surface charge triggered intestinal epithelial tight junction opening upon chitosan nanoparticles for insulin oral delivery. Carbohydr Polym. 2017;157:596–602. doi:10.1016/j.carbpol.2016.10.021
96. Boegh M, Nielsen HM. Mucus as a barrier to drug delivery – understanding and mimicking the barrier properties. Basic Clin Pharmacol Toxicol. 2015;116(3):179–186. doi:10.1111/bcpt.12342
97. Yuan H, Chen CY, Chai GH, et al. Improved transport and absorption through gastrointestinal tract by PEGylated solid lipid nanoparticles. Mol Pharm. 2013;10(5):1865–1873. doi:10.1021/mp300649z
98. Banerjee A, Qi J, Gogoi R, et al. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J Control Release. 2016;238:176–185. doi:10.1016/j.jconrel.2016.07.051
99. Muro S, Garnacho C, Champion JA, et al. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol Ther. 2008;16(8):1450–1458. doi:10.1038/mt.2008.127
100. Gelli R, Tempesti P, Ridi F, et al. Formation and properties of amorphous magnesium-calcium phosphate particles in a simulated intestinal fluid. J Colloid Interface Sci. 2019;546:130–138. doi:10.1016/j.jcis.2019.03.060
101. Lu J, Ormes JD, Lowinger M, et al. Impact of endogenous bile salts on the thermodynamics of supersaturated active pharmaceutical ingredient solutions. Cryst Growth Des. 2017;17(3):1264–1275. doi:10.1021/acs.cgd.6b01664
102. Hanafy AF, Abdalla AM, Guda TK, et al. Ocular anti-inflammatory activity of prednisolone acetate loaded chitosan-deoxycholate self-assembled nanoparticles. Int J Nanomed. 2019;14:3679–3689. doi:10.2147/IJN.S195892
103. Mo Y, Yang Y, Zeng J, et al. Enhancing the biopharmacological characteristics of asperosaponin VI: unveiling dynamic self-assembly phase transitions in the gastrointestinal environment. Int J Nanomed. 2023;18:7335–7358. doi:10.2147/IJN.S436372
104. Li X, Geng M. Probing the binding of procyanidin B3 to trypsin and pepsin: a multi-technique approach. Int J Biol Macromol. 2016;85:168–178. doi:10.1016/j.ijbiomac.2015.12.075
105. Li X, Liu H, Wu X, et al. Exploring the interactions of naringenin and naringin with trypsin and pepsin: experimental and computational modeling approaches. Spectrochim Acta A Mol Biomol Spectrosc. 2021;258:119859. doi:10.1016/j.saa.2021.119859
106. Martinez-Gonzalez AI, Díaz-Sánchez ÁG, Rosa LA, et al. Polyphenolic compounds and digestive enzymes: in vitro non-covalent interactions. Molecules. 2017;22(4):669. doi:10.3390/molecules22040669
107. Jiao Q, Wang R, Jiang Y, et al. Study on the interaction between active components from traditional Chinese medicine and plasma proteins. Chem Cent J. 2018;12(1):48. doi:10.1186/s13065-018-0417-2
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




