Back to Journals » International Journal of Nanomedicine » Volume 19
Self-Nanoemulsifying Drug Delivery System (SNEDDS) Using Lipophilic Extract of Viscum album subsp. austriacum (Wiesb.) Vollm
Authors Pereira CFDA , Melo MNDO , de Campos VEB , Pereira IP, Oliveira AP, Rocha MS, Batista JVDC, Paes de Almeida V , Monchak IT , Ricci-Júnior E , Garrett R , Carvalho AGA, Manfron J , Baumgartner S, Holandino C
Received 10 April 2024
Accepted for publication 25 May 2024
Published 14 June 2024 Volume 2024:19 Pages 5953—5972
DOI https://doi.org/10.2147/IJN.S464508
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kamakhya Misra
Camila Faria de Amorim Pereira,1,* Michelle Nonato de Oliveira Melo,1,* Vania Emerich Bucco de Campos,2 Ivania Paiva Pereira,1 Adriana Passos Oliveira,1 Mariana Souza Rocha,1 João Vitor da Costa Batista,3,4 Valter Paes de Almeida,5 Irailson Thierry Monchak,5 Eduardo Ricci-Júnior,6 Rafael Garrett,7 Aline Gabrielle Alves Carvalho,7 Jane Manfron,5 Stephan Baumgartner,3,8,9 Carla Holandino1,3
1Multidisciplinary Laboratory of Pharmaceutical Sciences, Faculty of Pharmacy, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil; 2Department of Pharmacy, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, Brazil; 3Society for Cancer Research, Hiscia Institute, Arlesheim, Switzerland; 4Department of Pharmaceutical Sciences, Division of Pharmaceutical Technology, University of Basel, Basel, Switzerland; 5Postgraduate Program in Pharmaceutical Sciences, Universidade Estadual de Ponta Grossa, Ponta Grossa, Paraná, Brazil; 6Galenic Development Laboratory (LADEG), Department of Drugs and Medicines, Faculty of Pharmacy, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil; 7Metabolomics Laboratory, Chemistry Institute, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil; 8Institute of Integrative Medicine, University of Witten/Herdecke, Herdecke, Germany; 9Institute of Complementary and Integrative Medicine, University of Bern, Bern, Switzerland
*These authors contributed equally to this work
Correspondence: Carla Holandino, Multidisciplinary Laboratory of Pharmaceutical Sciences, Universidade Federal do Rio de Janeiro, Faculty of Pharmacy, Block B basement, Room 34, 373, Carlos Chagas Filho Avenue, Cidade Universitária, Rio de Janeiro, RJ, 21941-902, Brazil, Email [email protected] Stephan Baumgartner, Institute of Complementary and Integrative Medicine, University of Bern, Bern, Switzerland, Email [email protected]
Background and Purpose: Natural products are potential sources of anticancer components. Among various species, the lipophilic extract of the Viscum album subsp. austriacum (Wiesb.) Vollm. (VALE) has shown promising therapeutic potential. The present work aimed to qualify the plant source and characterize the extract’s chemical profile. In addition, a self-nanoemulsifying drug delivery system (SNEDDS) containing VALE (SNEDDS-VALE) was developed.
Methods: V. album subsp. austriacum histochemistry was performed, and the chemical profile of VALE was analyzed by GC-MS. After the SNEEDS-VALE development, its morphology was visualized by transmission electron microscopy (TEM), while its stability was evaluated by the average droplet size, polydispersity index (PdI) and pH. Lastly, SNEDDS-VALE chemical stability was evaluated by LC-DAD-MS.
Results: The histochemical analysis showed the presence of lipophilic compounds in the leaves and stems. The major compound in the VALE was oleanolic acid, followed by lupeol acetate and ursolic acid. SNEDDS was composed of medium chain triglyceride and Kolliphor® RH 40 (PEG-40 hydrogenated castor oil). A homogeneous, isotropic and stable nanoemulsion was obtained, with an average size of 36.87 ± 1.04 nm and PdI of 0.14 ± 0.02, for 14 weeks.
Conclusion: This is the first histochemistry analysis of V. album subsp. austriacum growing on Pinus sylvestris L. which provided detailed information regarding its lipophilic compounds. A homogeneous, isotropic and stable SNEDDS-VALE was obtained to improve the low water solubility of VALE. Further, in vitro and in vivo experiments should be performed, in order to evaluate the antitumoral potential of SNEDDS-VALE.
Keywords: Viscum album subsp. austriacum, mistletoe, lipophilic extract, oleanolic acid, SNEDDS
Graphical Abstract:
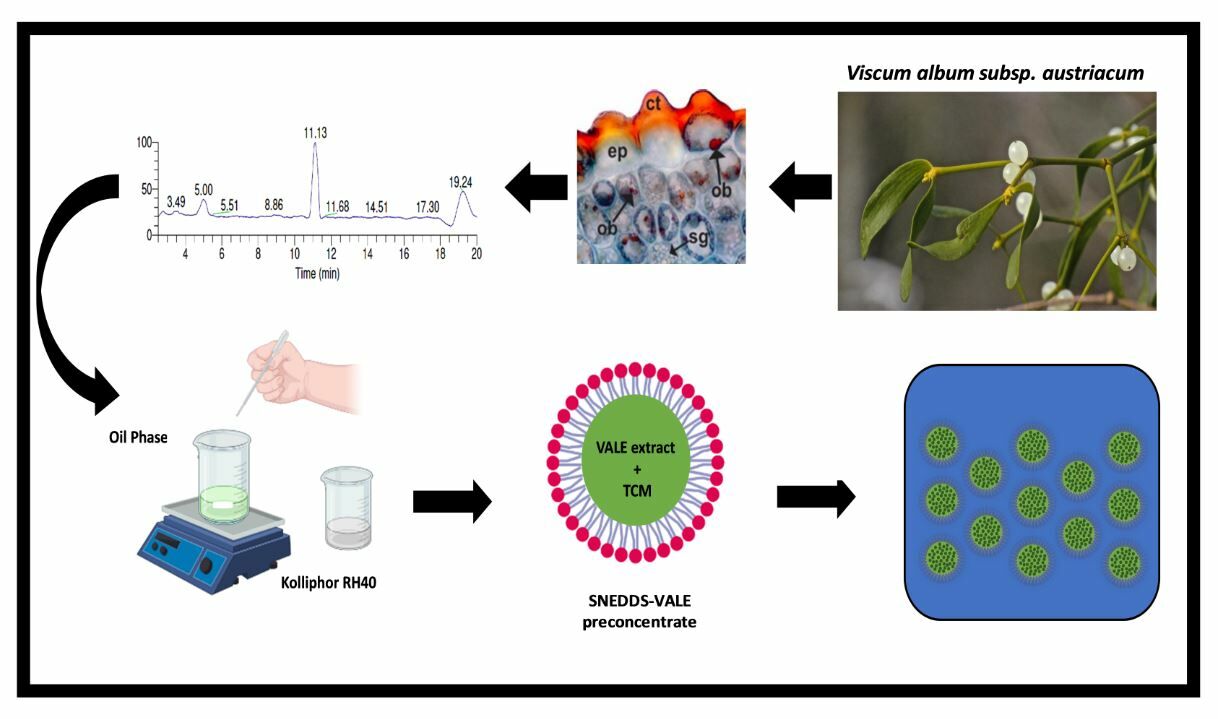
Introduction
Among several parasitic plants, the European mistletoe (Viscum album L.) is one of the most studied.1,2 This species has many distinct host trees, which leads to different chemical metabolic profiles,3–5 and biological activities.6 Medicines from the V. album have been used worldwide in complementary cancer therapy since 1917, mainly in central Europe.7 The commercial products are generally prepared by aqueous extractions that are rich in lectins and viscotoxins.8,9 These aqueous-soluble compounds are capable of inducing cytotoxic effects on tumor cell lines in addition to induce immunological response.8,10–12 However, a lipophilic extract from the V. album obtained by ultra-critical CO2 extraction (VALE) has also presented an interesting therapeutic potential.13–16
The main active components of VALE are considered to be the triterpenes betulinic acid (BA), oleanolic acid (OA), and ursolic acid (UA).13 These pentacyclic triterpenes have interesting activities in different biological conditions, considering their lipophilic behavior.17 The antiproliferative effect of the extract and its fractions (BA, OA, UA) were observed, in addition to their ability to promote apoptotic cell death in MOLT4, K562 and U932 leukemia cells.13 In the literature, the use of oleanolic acid resulted cytotoxic activity against A549 and H460 human non-small cell lung cancer cells and promoted autophagy, dependent of ROS, in HepG2 and SMC7721 hepatoma cell lines by suppression of the PI3K/Aκt1/mTOR signaling pathway.18,19 The therapeutic application of betulinic acid in the treatment of different cancers has also been described, including hepatoma, prostate, pancreatic, breast and leukemia cancer cell lines.20 Moreover, UA isolated from Oldenlandia diffusa inhibited the proliferation of doxorubicin-resistant human hepatoma cell line (R-HepG2) through apoptosis and loss of mitochondrial membrane potential.21 Considering the antitumor potential of VALE observed in vitro, clinical case studies were subsequently carried out, which demonstrated complete remission and strong improvement with topical treatment of an ointment with 10% VALE in basal cell carcinoma, actinic keratosis and squamous cell carcinoma.16,22
Despite the potential anticancer effects of VALE, it is challenging to deliver its active compounds topically or via the gastrointestinal tract using conventional pharmaceutical preparations. These challenges are mainly due to poor solubilization of the extract related to its lipophilic characteristics.17 In this sense, nanotechnology has been used to solve some of these barriers.23 The use of lipid-based nanocarriers, such as self-nanoemulsifying drug delivery systems (SNEDDS), constitutes an outstanding formulation approach for enhancing aqueous solubility.24–26 SNEDDS are isotropic mixtures of oil and surfactants that form a self-nanoemulsifying system in situ when exposed to an aqueous environment, producing an oil-in-water nanoemulsion with a droplet size of 20–200 nm.27 In this context, the development of a SNEDDS containing VALE is a promising alternative for the delivery of its active substances. It allows overcoming the limitations related to the administration of the extract, increasing its solubility and improving its oral absorption and bioavailability. Furthermore, other advantages of this system are its high stability and physical and chemical protection of the encapsulated active ingredients, favoring the therapeutic efficacy of VALE.
The first aim of this study was to qualify the plant source and to provide the chemical quality control of the herbal extract. The subsequent aim was to use the characterized extract in the development of a suitable SNEDDS formulation to enhance the solubility and stability of VALE.
Materials and Methods
Reagents and Materials
Viscum album subsp. austriacum (Wiesb.) Vollm. was collected from Pinus sylvestris L., during the winter season, on a cultivated natural site that belongs to the Society for Cancer Research, Switzerland. The samples of plants were identified by Dr. Marcelo Guerra Santes (Universidade Estadual do Rio de Janeiro-UERJ), and a voucher (C.H.Quaresma 18.329) was provided by the Herbarium of the Faculdade de Formação de Professores, UERJ, Brazil. V. album lipophilic extract (VALE) was donated by Hiscia Institute, Switzerland. The extract was obtained by supercritical CO2 extraction method, from green organs (leaves and stems) of Viscum album subsp. austriacum. PEG-40 hydrogenated castor oil (Kolliphor® RH40), sorbitan monooleate (Span 80®), polysorbate 80 (TWEEN 80®), Pyridine, N,O-bis-(trimethylsilyl) trifluoroacetamide (BSTFA), trimethylchlorosilane (TMCS) and the commercial standards of stigmasterol, β-sitosterol, lupeol, ursolic acid, oleanolic acid, betulinic acid and saturated alkanes C7-C30 were purchased, (Sigma-Aldrich, St. Louis, MO, USA/São Paulo, Brazil) as well as medium chain triglycerides (MCT) (Farmácia Quintessência, Rio de Janeiro, Brazil), isopropyl myristate (Vetec Química Fina LTDA, Rio de Janeiro, Brazil), grape seed oil, sunflower oil, sesame oil, sweet almond oil, sorbitol and mineral oil (Ferquima Indústria e Comércio LTDA, Vargem Grande Paulista, São Paulo, Brazil).
Histochemical Analysis
Microchemical analyses of Viscum album subsp. austriacum harvested in the winter were performed using specific reagents and stains to detect, within cells and tissues, the distribution and accumulation of lipophilic compounds.28,29 Free-hand cross-sections of fresh material (leaves and stems) were exposed to Sudan III, which stains lipophilic compounds in red, and Sudan black, which stains lipophilic compounds in black.29 Besides, Nile blue sulfate was used to detect neutral fats in red-to-pink color and fatty acids in blue color.30 The microscopic procedures were conducted in the Laboratory of Pharmacognosy at the State University of Ponta Grossa (UEPG, Brazil).
VALE Extract Qualitative Analysis by TLC
Thin-layer chromatography (TLC) analyses were achieved by silica gel 60 F254 (250 μm thickness, SiliCycle, Quebec, Canada) using n-hexane and ethyl acetate (7:3
Phytochemical Characterization of VALE by GC-MS
VALE and the analytical standards were derivatized by silylation,32 converting hydroxyl and carboxyl groups in the extract molecules into trimethylsilyl (TMS). Cholesterol was added in a concentration of 200 μg.mL−1 as an internal standard. 125 μL of pyridine, 125 μL of N,O-bis-(trimethylsilyl) trifluoroacetamide (BSTFA), and 25 μL trimethylchlorosilane (TMCS) were added to 2 mg of VALE. Then the tube was heated (70°C) for 30 minutes. Subsequently, the derivatized sample was analyzed by gas chromatography-mass spectrometry (CG-MS). For quantitative analysis, β-sitosterol, stigmasterol, lupeol, oleanolic acid, betulinic acid, β-amyrin acetate, lupeol acetate, and ursolic acid calibration curves were prepared with the following concentrations: 10, 25, 50, 100, 150 and 200 μg.mL−1. Sample analysis was performed on a gas chromatograph (ThermoFisher Scientific, Trace 1300 Series GC) coupled to a triple-quadrupole mass spectrometer (ThermoFisher Scientific, TSQ8000) as follows: 1 μL of the sample was injected (ThermoFisher Scientific, Triplus RSH) using a split ratio of 1:200 and injector temperature at 250°C. The column oven temperature was programmed to start at 80°C, followed by a temperature gradient of 20°C/min up to 180°C, held for 5 min, which was then followed by a temperature gradient of 15°C/min up to 285°C, held for 12 min, totaling 29 min. A solvent delay time of 5.5 min was used to avoid a large solvent peak at the start of the run. A nonpolar HP-5 column (Agilent Technologies; 50m x 0.200 mm x 0.33μm) was used to separate the compounds. Helium was used as carrier gas at a flow rate of 1.2 mL/min, and the transfer line was maintained at 290°C. The electron ionization voltage was 70 eV, ion source was maintained at 250°C, and the quantitation of compounds was performed by selected reaction monitoring (SRM) acquisition mode as described in Table S1. Tracefinder Forensics v4.1 (Thermo Scientific) software was used for the identification and quantification of metabolites.
SNEDDS Pre-Formulation Study
Solubility Studies
As VALE has low solubility in water due to its chemical composition, the solubility study of this extract was evaluated at different concentrations (1 to 20%), by sequential weight addition of the following pharmaceutical oils in triplicate: Medium-chain triglyceride oil (MCT), Isopropyl myristate, Mineral, Sorbitol, Grape seed, Sunflower, Sesame and Sweet almond. Considering the nature of the extract, 0.1 g of it was added to a 5 mL glass stoppered vial and increasing parts of the solvent were added and homogenized by magnetic stirring, at room temperature for 15 min. Afterward, the vials containing the oil phase were left at 25°C for 24 h to reach equilibrium. Visual observation was done using the reference table “Description and relative solubility of USP and NF articles”, a qualitative parameter to determine the approximate solubility following the United States Pharmacopeia.33,34
Surfactant Selection
A preliminary hydrophilic-lipophilic-balance (HLB) scan test was performed, in which the chosen oil and a pair of surfactants or Smix (Tween 80®/Span 80®) with a known HLB value were used in a fixed proportion (1:3). The HLB range investigated was from 8 to 15, where oil phase and Smix were heated at 40 ±1.0°C and the continuous phase (water) was heated at 70 ± 2.0°C. The water phase (80.0% w/w) was slowly added into the oil phase (5% MCT /15% Smix w/w) under a magnetic agitation speed of 500 rpm. Emulsions were kept under mixing for 1 h, until reaching room temperature, 25°±2.0°C.35 The HLB number of the surfactant mixture was calculated using the weight fraction of the corresponding surfactants, as can be seen in the following equation:
Ternary Phase Diagram Construction
A ternary phase diagram (TPD) was constructed using the aqueous titration method to identify the right proportions of oil, surfactant and water contents necessary to develop spontaneous nanoemulsion drug delivery systems.36 The selected oil phase containing VALE and the surfactant were added in flasks at fixed ratios (9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, and 1:9) while predetermined and increasing proportions of water (variable component, 8 to 88%) were added to each flask. The aqueous phase titration comprises 99 formulations in which oil/surfactant ratio (OSR) range from 1:9 to 9:1. After each titration, samples were homogenized at 25±1.0 °C in a stirring plate and kept for 24 h to reach equilibrium. The formulation macroscopic aspects were then observed according to the variation in their viscosity and stability (presence of creaming, coalescence and/or phase separation). Such features were evaluated by visual inspection and classified as NE (nanoemulsion), PS (phase separation), or MA (macroemulsion). The results were plotted using OriginPro software (version 8.0), and the different regions generated were identified in the triplot graph. From the spontaneous nanoemulsification area (NE), an optimal formulation composition was selected to solubilize the VALE in order to create the self-nanoemulsifying drug delivery system (SNEDDS).24,36
SNEDDS Preparation
BLANK-SNEDDS (without VALE) and VALE-SNEDDS were prepared using the self-nanoemulsification method (low energy), through a two-step addition of the components.27 For the SNEDDS-VALE preparation, 150 mg of VALE was solubilized in 29% wt of MCT under constant magnetic stirring at 200 rpm for 30 min. The oil phase was then kept for 24 h protected from light to reach equilibrium. Then, 68 wt % of PEG-40 hydrogenated castor oil was added to the oil phase, followed by further agitation at 200 rpm for 1 h. The theoretical amount of VALE was 29 mg/g, and the SNEDDS was carried out at 25°C.
Droplet Size, Polydispersity and pH Determination
Droplet size and SNEDDS polydispersity index were carried out by the dynamic light scattering (DLS) method (Zetasizer Nano S90, Malvern Panalytical, Spectris Company, Egham, UK). The samples were diluted 1:100 v/v in distilled water and analyzed at room temperature (25°C). All measurements were made in triplicate and data were represented as average size in nanometers and PdI (mean ± SD).37 For pH determination, the pre-concentrated samples were diluted 1:10 v/v and verified using a pH meter at 25°C (Digimed pHmeter, São Paulo, Brazil).38
Transmission Electron Microscopy Analysis
The morphology of SNEDDS-VALE and SNEDDS-BLANK-were evaluated by transmission electron microscope (TEM), at 80 K and at 80-kV magnification (Nano Imaging Lab facilities, Basel University, Swiss Nanoscience Institute, Switzerland). Both samples (10 μL) were diluted 1:10 with double distilled water and taken in a Formvar-coated grid, following negative stain with 10 μL of uranyl acetate 2.0% (w/v), for 1 min. After 1 min, the excess was dried using filter paper and the grids were left to dry at room temperature (25 ± 1°C) before being submitted to analysis by TEM (CM100, Philips).39
Physical Stability Study
The VALE-SNEDDS was evaluated for its physical stability in triplicate by three different methodologies (a-c). At the end of each procedure, the samples were visually evaluated for instability presence regarding creaming, cracking or phase separation. a) Heating and cooling cycle: the samples were submitted to six cycles, at 4°C and 40°C, at minimum of 48 h of storage, in each temperature per cycle. In the absence of any of the phenomena described above, the samples would proceed to the centrifugation tests; b) Centrifugation: SNEDDS-VALE samples were centrifuged at two consecutive cycle parameters: 5,000 rpm for 30 min and 10,000 rpm for 30 min. The samples were submitted to the freeze-thaw cycle when instability absence was detected; c) Freezing and thawing cycle: six cycles at 25°C and −20°C were applied, in a minimum of 48 h of storage, in each temperature per cycle. The last stability test consisted of the droplet size and polydispersity index measurements of all samples by DLS technique.40–42
Accelerated Stability Study
A short-term stability assessment was performed by preparing a new formulation batch in the same conditions as described in the SNEDDS preparation section. The stability was studied by dynamic light scattering (Zetasizer Nano ZS90). The SNEDDS-VALE was stored in glass vials and submitted to different temperatures (25 ± 5°C and 40 ± 5°C) for 14 weeks. At predetermined temperatures, droplet size and polydispersity index (PdI) were measured immediately and after 1, 3, 6 and 14 weeks. The formulation was diluted 100-fold with distilled water, and the measurements were taken in triplicate to evaluate the stability over a higher period of time.34,43,44
Robustness to Dilution
Under physiological conditions, the SNEDDS emulsification process occurs spontaneously and gradually.45 The dilution robustness test aims to verify the performance of the SNEDDS preconcentrate in the formation of a stable nanoemulsion, under biological mimetic conditions (pH variations and different dilutions). For the robustness dilution test, SNEDDS-VALE was subjected to the following dilutions, ie, 1:50, 1:100, and 1:500, in three different solvents media (distilled water; hydrochloric acid buffer at pH 1.2; phosphate buffer at pH 6.8). The droplet size and PdI parameters were evaluated by the DLS method.46,47
Oleanolic Acid Content by HPLC-DAD-MS
The samples SNEDDS-VALE, SNEDDS-BLANK and VALE free extract were dissolved in n-propanol at 10 mg/mL (in relation to the VALE extract) and the oleanolic acid chemical standard in the same solvent at 2 mg/mL. The chromatographic profile and the quantitative HPLC analysis were performed using a UHPLC-MS (Dionex Ultimate 300 coupled Thermo LCQ Fleet Ion Trap mass spectrometer, Thermo Fisher, San Jose, CA, USA) equipped with a quaternary pump, a column oven, and a DAD detector. Analyses were performed on a reverse-phase C18 column (250 mm × 4.6 mm × 5.0 μm; Kromasil, Akzo Nobel) equipped with a precolumn (3 × 10 mm). The flow rate was 1.0 mL/min and an isocratic condition of acetonitrile (ACN) in 0.1% aqueous formic acid (85:15) in 15 min was used with some adjustments.48 The quantification of oleanolic acid was performed at 210 nm and its concentration was calculated by a calibration curve obtained via linear regression (Excel® software). Data acquisition and processing were carried out shortly after (Xcalibur® software). The oleanolic acid quantification of the pre-concentrated samples was performed after 24 h and 12 months of storage at 25°C.
Results
Histochemistry Aspects of Viscum album subsp. austriacum
Viscum album subsp. austriacum (Figure 1a and b) is a hemiparasite growing on the branches of conifer trees such as Pinus sylvestris. Its growth is dichasium with several articulated, glabrous and green stems (Figure 1c), green to yellowish-green leaves (Figure 1d) and globular, whitish and translucent berries (Figure 1e).49 The characterization of chemical compounds found in plant tissues can be facilitated by light microscopy-based microchemical investigations carried out using certain reagents and stains.50–53 Besides, the use of dyes allows us to localize the distribution and accumulation of secondary metabolites, inside certain cells and tissues, such as lipophilic substances.54 For this purpose, Viscum album subsp. austriacum leaves and stems were subjected to histochemical tests to highlight the compartmentalization sites of lipophilic compounds. The samples of vegetative organs analyzed reacted positively with Sudan III and Sudan black, showing lipophilic compounds in cuticles of the leaves [lamina (Figure 2a) and petiole (Figure 2b)] and stems (Figure 2c). Besides, oil bodies are found in the lamina epidermis of the leaves (Figure 2a), ground parenchyma of the petiole (Figure 2b), in the epidermis of the stems (Figure 2c), mesophyll of the leaves (Figure 2d), and phloem and xylem parenchyma cells of the leaves (Figure 2e–j). The same structure is spread in all tissues such as cortical, vascular and pith of the stems (Figure 2k–m). These oil bodies (Figure 2g, j and m) also stain with Nile blue, indicating the presence of neutral fat in these structures.
VALE Extract Qualitative Analysis by TLC
Typical triterpenes compounds at white light, after revelation with anisaldehyde-sulphuric acid reagent, were observed for the VALE using TLC (Supplementary Material, Figure S1). VALE extract showed different blue-violet and red-violet color bands, which are characteristics for triterpenes.31 VALE exhibited one main blue-violet spot with Rf-value similar to the oleanolic acid standard (Rf 0.57) and other zones of blue-violet and red-violet color with less intensity. These are probably attributed to other pentacyclic triterpenes markers, once it was localized next to ursolic and betulinic acid standards (Rf 0.66 and Rf 0.73, respectively).
Phytochemical Characterization of VALE
The GC-MS analysis showed a chromatogram composed of eight main peaks (Figure 3) that were identified according to the MS/MS fragmentation and chemical standards. The confirmation ions used for Viscum album lipophilic extract (VALE) were analyzed by selected reaction monitoring mode (Supplementary Material, Table S1). Considering these main compounds present in the VALE, six of them were terpenes (lupeol, oleanolic acid, betulinic acid, β-amyrin acetate, lupeol acetate, and ursolic acid) and 2 phytosterols (β-sitosterol and stigmasterol). Each compound was quantified using a calibration curve and data is shown in Table 1. The silylation process improved the ionization of β-sitosterol, stigmasterol, lupeol, oleanolic acid, betulinic acid, and ursolic acid. The major compound in the VALE was the oleanolic acid, with 59.1 ± 5.77 mg/g followed by lupeol acetate (33.9 ± 2.59 mg/g) and ursolic acid (31.5 ± 2.74 mg/g).
 |
Table 1 Quantitative Analysis of Identified Compounds in the VALE Extract |
SNEDDS-VALE Pre-Formulation Study
Among the pharmaceutical oils tested, only the medium chain triglycerides (MCT) and isopropyl myristate were able to solubilize VALE, as shown in Table 2. The range of VALE solubility in both solvents has an estimated concentration between 33 and 100 mg of VALE per g of oil (33–100 mg/g). Oils with moderate chain length, such as MCT, efficiently generate nanoemulsifying systems.55 Their lipophilicity and concentration used in the formulation are important parameters to get the nanoemulsion with a proper range of droplet size (20–200 nm).56
 |
Table 2 VALE Apparent Solubility in Different Pharmaceutical Oils |
The HLB study of MCT varied from 8 to 15 (ideal for o/w emulsions) and showed the presence of translucent (HLB = 13) and slightly bluish formulations (HLB 14 and 15), respectively (Supplementary Material, Table S2 and Figure S2). The results corroborate the HLB presented in the literature for MCT regarding an o/w system, which may differ depending on the origin of the oil, since the composition of fatty acids in MCT presents a range of variation between caprylic acid (50 to 65%) and capric acid (30 to 45%).57
Based on the HLB study, PEG-40 hydrogenated castor oil presented a theoretically required HLB value close to the oil of choice (14–16). Furthermore, this surfactant demonstrated compatibility with other components of the formulation (extract enriched in triterpenes and MTC) and is known for its emulsification efficiency in the development of nanoemulsions containing at least one of those components.55,58,59 Thus, MCT and PEG-40 hydrogenated castor oil were chosen as the oil phase and the emulsifying agent respectively.
A ternary phase diagram was constructed in order to identify the nanoemulsifying region and the right proportion of oily phase, surfactant and water (Figure 4). In the TPD study, all oil/surfactant ratios (OSR) were prepared containing the herbal constituent since its presence directly influenced the formation of the self-emulsification region.27 Among the 99 generated formulations, a percentage of 32% were macroscopically classified as nanosystems in the TPD (Figure 4).
The OSR of 1:9 and 2:8 generated stable nanosystems, despite the higher amount of water. However, at lower water concentrations (≤56%) a transparent gel-like system with high viscosity was observed for both proportions (1:9 and 2:8), which is probably due to the initial swelling of surfactant chains by water addition, promoting a phase equilibrium consistent with a w/o nanoemulsion system. Additionally, the amount of oil present at those proportions was not enough to solubilize a considerable fraction of lipophilic extract, attributing the major solubilization function of the extract to the surfactant. The 3:7 OSR also originated stable translucent nanoemulsions independent of the water addition and with a lower surfactant concentration. The other proportions of oil/surfactant (4:6; 5:5; 6:4; 7:3; 8:2; 9:1) presented an initial w/o nanoemulsion regions (4:6 and 5:5) that were progressively altering its phase behavior for macroemulsions at surfactant concentrations <42%. Below this percentage, the occurrence of physical destabilization resulted in the formation of turbid macro-emulsified systems containing larger droplet size, that are mostly governed by the Ostwald ripening phenomenon and led to phase break under increase in volumetric alteration.60 After the phase behavior composition study, the optimum formulation considering the nanoemulsion region in the phase diagram presented the proportions of oil phase and surfactant corresponding to 32% and 68%, respectively. Regarding the oily phase, 9% of its total is represented by the presence of the lipophilic extract of VALE (2.9% in total formulation).
Droplet Size, Polydispersity, pH Determination of SNEDDS-VALE
Mean droplet size and PdI values of the SNEDDS-VALE obtained by DLS are shown in Figure 5A and B. After diluting samples (1:100 v/v in distilled water), mean droplet size and PdI were 37.00 ± 1.04 nm and 0.12 ± 0.10, respectively. The samples presented a narrow size distribution in function of volume and intensity parameters. Transmission electron microscopy (TEM) highlights the morphological aspects of the SNEDDS-VALE, such as: droplets with uniform spherical shape homogeneously dispersed, with nanometric sizes varying from 30 to 50 nm (Figure 5C and D). The particle size observed by TEM is in accordance with dynamic light scattering (DLS) in which concerns the size distribution and average size of the droplets of SNEDDS-VALE. Besides, no variations were observed in relation to the pH of the SNEDDS-VALE comparing the 24 h time value (5.0 ± 0.01) with the final pH verified at the end of the physical stability test (5.03 ± 0.02). Together, these data indicate a high stability of the self-nanoemulsifying system.
Physical Stability Study
In order to check the stability of the formulation and classify it as a nanoemulsifying system, SNEDDS-VALE was exposed to different stages of stress, centrifugation study, heating and cooling cycle, and freezing and thawing cycle, as described before. The results obtained are shown in Table 3. At the end of each stress process, instability phenomena such as phase separation, turbidity, creaming, or cracking were not observed. Additionally, SNEDDS-VALE presented small droplet size and PdI values consistent with monodisperse systems and nanoemulsion parameters.61 The DLS results (mean droplet size, PdI) and pH were obtained in triplicate (sample 1, 2 and 3) using three different assays (Table 3).
 |
Table 3 Physical Stability Results of SNEDDS-VALE: Droplet Size (D.nm), PdI and pH. Results are Expressed as Mean ± SD (n = 3) |
Accelerated Stability Studies
It was shown that SNEDDS-VALE was able to keep its hydrodynamic diameter and PdI parameters after 14 weeks of storage at 25°C and 40°C (75 ± 5% RH) (Figure 6). There was no phase separation, presence of precipitates or color alteration during the stability assay.
 |
Figure 6 Average hydrodynamic diameter (HD.nm) and polydispersity index (PdI) of SNEDDS-VALE submitted to accelerated stability assay at temperatures of 25 °C (A) and 40 °C (B) for 14 weeks. |
Robustness to Dilution
The SNEDDS-VALE chosen through the phase diagram proved to be stable during the physical stability test and, therefore, it was considered eligible for the dilution robustness test. The dispersibility studies of the SNEDDS-VALE in the presence of distilled water, HCl buffer (pH 1.2), and phosphate buffer (pH 6.8) after 24 h are presented in Table 4. No significant differences were observed in relation to the average size as a function of the variation of the pH value. Furthermore, the formulation maintained its physical (size and PdI) and visual (monodisperse and isotropic) characteristics even after 24 h in the tested media, without signs of instability phenomena such as flocculation or phase separation.
Chemical Stability of the Oleanolic Acid Content in VALE Extract and SNEDDS-VALE
UHPLC‑DAD‑MS analysis of the VALE used to produce the SNEDDS formulation revealed a major metabolite at the same retention time (11.1 min) of the oleanolic acid reference compound (Figure 7). The molecular mass (455 amu according to ESI‑MS) and blue-violet band on TLC (Figure S1, Supplementary Material) after staining with vanillin sulphuric acid reagent suggested to be triterpene. The major peak (11.1 min) of VALE was increased after its fortification with the oleanolic acid reference compound (data not shown).
The quantification of oleanolic acid, the chemical VALE marker, was done by a UV detector. The calibration curve of the oleanolic acid was prepared and generated the following linear regression, y = 3,000,000x - 843,201, with R2 of 0.9996. In this analysis, the oleanolic content in VALE was 11.7%.
The chromatograms of VALE and SNEDDS-VALE showed the presence of a peak in the same retention time of the oleanolic acid (11.1 min) by HPLC analysis (Figure 7). It was also possible to see that the SNEDDS-BLANK did not present this peak demonstrating no influence of the SNEDDS excipients in VALE chemical compounds. Besides, the SNEDDS-VALE presented a content of 120.64 ± 0.36 mg OA/ g VALE after 24 h preparation, and 116.6 ± 3.28 mg OA/ g VALE when stored at room temperature for 365 days. It means there was no statistical difference of this majority compound over time (t-test, p <0.05).
Discussion
Histochemical analysis of the Viscum album subsp. austriacum was used as the first evaluation of the starting plant material for VALE production. Optical microscopy using standard lipophilic reagents was performed for the first time in this subspecies and showed the localization of lipophilic compounds in plant organs, (leaves and stems) describing the tissues in which they are present. Thus, the identification of the lipophilic content in the leaves and stems of this subspecies led to the choice of these organs as plant raw material to obtain the VALE extract. The identification through histochemical analysis of tissues and organs in which lipophilic content is present may also be used as a quality parameter for plant raw materials associated with other microscopic analysis (morpho-anatomy) of the same subspecies.54,62,63 It may help confirm the subspecies identity and investigate the plant parts (organs) that will be used to acquire the extract since these factors directly influence the lipophilic constituents of the VALE. Almeida et al49 also found oily bodies scattered in the organs of V. album subsp. album using the same dyes, corroborating the results above, since this method allows the comparison between different plant organs and subspecies.64 Studies involving the anatomy, micromorphology and histochemistry of Viscum album are still limited in the literature, and their continuation will allow a more precise identification of this species and, perhaps, a future differentiation regarding its subspecies.49,65,66
VALE is a complex mixture whose components present a high hydrophobic profile.13 The chemical characterization study identified OA as the major compound in the VALE (59.1 ± 5.77 mg/g), as well as the presence of the other pentacyclic triterpenes as betulinic and ursolic acid.
OA is well known in the literature for its therapeutic potential, in which evidence from in vitro and in vivo studies points to its anticancer, antibacterial, antidiabetic, anti-inflammatory, antioxidant, immunoregulatory, hepatoprotective and anti-atherosclerotic effects, through a complex targeting of cell signaling pathways.67–74 The other pentacyclic triterpenes present in VALE share most of the pharmacological properties of OA, as they have great structural similarity. However, the particularities of each molecule may influence the intensity of the effect evaluated, resulting in greater or lesser bioactivity.17,75,76
The presence of the above-mentioned pentacyclic triterpenes has been already described by Urech et al13 in the Viscum album. Moreover, according to Kuonen et al15 and Estko et al,14 oleanolic acid is the main compound of VALE, corroborating our results. The phytochemical characterization by GC-MS showed for the first time the presence of the phytosterols, β-sitosterol and stigmasterol in the VALE. Furthermore, the technique allowed us to quantify and confirm the identity of other terpenes in VALE composition, such as lupeol, lupeol acetate and β-amyrin acetate, reinforcing the lipophilic character of this extract. Some of these components, especially OA, are well known for their low water solubility and permeability, being classified by the Biopharmaceutics Classification System (BCS) as IV category.77,78 Thus, the formulation of the VALE in a nanocarrier system, such as SNEDDS, would overcome its lipophilic pharmaceutical limitations.58,79,80
In our pre-formulation study, only the medium chain triglycerides (MCT) and isopropyl myristate were able to solubilize VALE at a 1:20 proportion. Among the advantages of using MCT in a SNEDDS is its ease of absorption and digestion, favoring the lymphatic transport of the digested MCT product together with endogenous lipoproteins.81,82 Besides, since MCT is synthetically originated from a natural source and presents extensive use in pharmaceutical technology, including the development of drug delivery systems containing pentacyclic triterpenes,55,83,84 it was chosen as the oil phase for the development of present SNEDDS.
PEG-40 hydrogenated castor oil is a biocompatible and nonionic surfactant known by presenting an HLB between 14 and 16. The presence of a large number of ethylene oxide groups gives a strong emulsification ability when compared to other agents.85 In the SNEDDS formulation process, the surfactant affinity for the oil phase and its HLB value are important parameters to be considered, since it influences the nanoemulsification process and stability, especially when using a low-energy input method to obtain a nanosystem.27,86 Based on our study, PEG-40 hydrogenated castor oil presented an HLB value close to the oil of choice. Furthermore, this surfactant is frequently used in the literature in association with MCT or extracts rich in pentacyclic triterpenes in the development of lipid nanosystems due to its emulsification efficiency and high compatibility with these components.55,58,59 These factors guided its choice as an emulsifying agent for the development of SNEDDS-VALE.
In the next step, after oil phase and surfactant selection, the proportion of each constituent in the SNEDDS was defined following the TPD. In SNEDDS systems, the minimum concentration of surfactant capable of generating stable nanoemulsions with low droplet sizes (20–200 nm) and polydispersity values (<0.3) are desirable.27 High surfactant concentrations may promote in vivo precipitation phenomena, affecting the bioavailability.45 In this sense, the ideal proportion selected by the TPD (OSR 3:7) used the lowest concentration of surfactant capable of generating a macroscopic stable nanoemulsion system containing an oil phase amount that could completely solubilize the VALE extract.
Although SNEDDS-VALE may contain a high amount of PEG-40 hydrogenated castor oil according to some authors, the percentage in which this excipient is present in the formulation allows it to act in a dual role, being the nanoemulsifying agent and also an extract solubilizer, facilitating the complete incorporation of it into the system. Furthermore, similar proportions of PEG-40 hydrogenated castor oil close to the used in SNEDDS-VALE were observed in studies involving the development of SNEDDS and self-microemulsifying systems (SMEDDS) even with the addition of cosolvents in its composition.85,87,88 PEG-40 hydrogenated castor oil also presents less toxicity compared to other surfactants of the same class, being considered safe and well tolerated for the nanosystem purpose since it is widely used in nanoemulsified delivery systems containing anticancer agents.27,89,90
The study of the TPD allowed the classification of formulations based on their macroscopic appearance in order to determine the theoretical limit of each component. However, this technique may be linked to observational errors since the time required for the system to reach equilibrium, especially near to the phase limits, could be longer than the time predetermined for the assay (24 h). In order to identify and exclude the presence of undesirable metastable systems in the nanoemulsion region, the physical stability study was performed and no alterations in pH, PdI and droplet size parameters were observed at the end of the assay.91
A system’s kinetic stability is one of the main features of a nanoemulsion. Due to their very small droplet size, its kinetics destabilization is very slow and solely governed by the Ostwald ripening process.60 However, unlike conventional nanoemulsions, SNEDDS goes through the process of nanoemulsification in the body, once in contact with biological fluids it should not be influenced by factors such as sample dilution or temperature variations.92 To ensure a satisfactory performance in situ, SNEDDS must be evaluated using a methodology that mimics the variables present in the physiological environment.93 It is known that the presence of electrolytes can directly influence the stability of formulations due to their ability to interact with the components of the formulation, especially the surfactant.27,94 Besides, the nanoemulsification process remains under the influence of factors such as the volume of biological fluids and gastrointestinal motility.95 Our results indicate that the formulation maintained its colloidal stability when nanoemulsified in the USP gastrointestinal mimetic buffers, pH 1.2 and 6.8.40 In addition, the characterization showed a nanometric droplet size and a monodisperse system coherent with other works for the oral administration route. The comparison between the blank and loaded formulation was made to determine whether the presence of VALE would affect the process of obtaining nanoemulsions in terms of average droplet size and distribution. The absence of significant changes in these parameters indicates that the presence of VALE, at the concentration used in the present study, did not interfere with the interaction among the nanoemulsion ingredients, neither with the interfacial film generation. Besides, the high stability to this system could be attributed to the nanometric size of the droplets generated.96,97 As expected, the spherical morphology visualized by TEM confirmed nanodroplets in a uniform distribution as previously presented by DLS results. Considering the character of a self-nanoemulsifying drug delivery system, particle size and its homogeneity is very critical, once it will impact factors such as drug release profile, cellular uptake and biodistribution.98
In addition, to evaluate the chemical stability of SNEDDS-VALE, the content of VALE majority marker was done by HPLC-UV (Figure 5) and the analytical method demonstrated to be specific for the quantification of oleanolic acid in SNEDDS-VALE. In this analysis, the oleanolic content in the VALE was 11.7% and similar results were also described by other authors that found a content ranging from 9.3% to 10% of oleanolic acid in the same extract.14,15 Chemical standardization of an herbal product is important for quality control and to assure its biological activities.2,3,6 The constant quality of the herbal material and in-process controls during the production procedure in each and every step leads to the desired batch-to-batch consistency.99
The accelerated stability conducted at 25°C and 40°C for fourteen weeks showed no alterations in the droplet size and PdI values highlighting the stability of the SNEDDS-VALE under these conditions. Furthermore, at the end of one year, the oleanolic acid content was approximately 100% of the start point indicating no significant losses of the chemical marker in the formulation. Wang et al,100 using an accelerated stability study, showed that the content of free oleanolic acid (control) in a solid dispersion formulation did not suffer changes or deterioration after 6 months of evaluation. However, Xi et al80 demonstrated that oleanolic acid was not chemically stable after 15 days at 60°C in a SNEDDS presenting a reduction of 10%, emphasizing its thermal instability and the need to avoid storage of oleanolic acid formulations at high temperatures. According to our data and the above-mentioned authors, the oleanolic acid seems to be stable at room temperature in both nano and oral classical formulations. In short, VALE delivery through a nanosystem, may be promising in terms of improving the oral bioavailability and chemical stability of its phytocomponents when compared to classical systems.101
Conclusion
This is the first histochemical analysis of leaves and stems of V. album subsp. austriacum growing in Pinus sylvestris. The features found in this study help the species identification and support the quality control of plant material. A homogeneous, isotropic and stable SNEDDS-VALE was obtained to improve the low water solubility of this lipophilic extract encouraging its biological uses. The primary lipophilic components of VALE, namely oleanolic acid, present promising therapeutic potential and further in vivo experiments should be done, to evaluate the anticancer potential of the developed SNEDDS-VALE.
Acknowledgments
The authors are grateful to Hiscia Institute for the VALE donation, to Laboratório de Farmacotécnica Experimental, Instituto de Tecnologia em Fármacos, Fundação Oswaldo Cruz, Brazil for the support in the viscosity assay, to Metabolomics Laboratory, Chemistry Institute, Universidade Federal do Rio de Janeiro, Brazil for the VALE chemical screening. We also acknowledge the C-Labmu Center at the Universidade Estadual de Ponta Grossa (UEPG) for the SEM and EDS facilities, and Nano Imaging Lab (University of Basel, Swiss Nanoscience Institute) for the TEM analysis.
Funding
This research was partially supported by Brazilian Governmental Agency, Faperj (Code: 201.004/2022. SEI - 260003 / 003766/2022, BBP), and by Leading House for the Latin American Region – Seed Money Grant (Agreement number SMG 1928).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Song C, Wei X-Y, Qiu Z-D, et al. Exploring the resources of the genus Viscum for potential therapeutic applications. J Ethnopharmacol. 2021;277:114233. doi:10.1016/j.jep.2021.114233
2. Melo MNDO, Batista JVDC, Peñaloza EMC, et al. A scoping review of genus viscum: biological and chemical aspects of alcoholic extracts. Plants. 2023;12(9):1–35. doi:10.3390/plants12091811
3. Jäger T, Holandino C, Melo M, et al. Metabolomics by UHPLC-Q-TOF reveals host tree-dependent phytochemical variation in Viscum album L. Plants. 2021;10(8):1726. doi:10.3390/plants10081726
4. Moyo B, Tavengwa N, Madala N. Application of the UHPLC-q-TOF-MS and molecular networking in revealing chemo-taxonomical markers of Viscum combreticola Engl. and Viscum album L. Biochem Syst Ecol. 2023;111:104720. doi:10.1016/j.bse.2023.104720
5. Holandino C, Melo MNDO, Oliveira AP, et al. Phytochemical analysis and in vitro anti-proliferative activity of Viscum album ethanolic extracts. BMC Complement Med Ther. 2020;20(1):215–226. doi:10.1186/s12906-020-02987-4
6. Melo MNDO, Ochioni AC, Zancan P, et al. Viscum album mother tinctures: harvest conditions and host trees influence the plant metabolome and the glycolytic pathway of breast cancer cells. Front Pharmacol. 2022;13:1027931. doi:10.3389/fphar.2022.1027931
7. Nazaruk J, Orlikowski P. Phytochemical profile and therapeutic potential of Viscum album L. Nat Prod Res. 2016;30(4):373–385. doi:10.1080/20469047.2015.1109238
8. Urech K, Schaller G, Ziska P, Giannattasio M. Comparative study on the cytotoxic effect of viscotoxin and mistletoe lectin on tumor cells in culture. Phyther Res. 1995;9:49–55. doi:10.1002/ptr.2650090112
9. Urech K, Baumgartner S. Chemical constituents of Viscum album L.: implications for the pharmaceutical preparation of mistletoe. Transl Res Biomed. 2015;4:11–23. doi:10.1159/000375422
10. Kelter G, Schierholz JM, Fischer IU, Fiebig HH. Cytotoxic activity and absence of tumor growth stimulation of standardized mistletoe extracts in human tumor models in vitro. Anticancer Res. 2007;27(1 A):223–234. doi:10.1016/j.phymed.2007.07.020
11. Valentiner U, Pfüller U, Baum C, Schumacher U. The cytotoxic effect of mistletoe lectins I, II and III on sensitive and multidrug resistant human colon cancer cell lines in vitro. Toxicology. 2002;171(2–3):187–199. doi:10.1016/S0300-483X(01)00581-9
12. Schaller G, Urech K, Giannattasio M. Cytotoxicity of different viscotoxins and extracts from the European subspecies of Viscum album L. Phyther Res. 1996;10(6):473–477. doi:10.1002/(SICI)1099-1573(199609)10:6<473::AID-PTR879>3.0.CO;2-Q<473::AID-PTR879>3.0.CO;2-Q
13. Urech K, Scher JM, Hostanska K, Becker H. Apoptosis inducing activity of viscin, a lipophilic extract from Viscum album L. J Pharm Pharmacol. 2005;57(1):101–109. doi:10.1211/0022357055083
14. Estko M, Baumgartner S, Urech K, et al. Tumour cell derived effects on monocyte/macrophage polarization and function and modulatory potential of Viscum album lipophilic extract in vitro. BMC Complement Altern Med. 2015;15(1):130. doi:10.1186/s12906-015-0650-3
15. Kuonen R, Weissenstein U, Urech K, et al. Effects of lipophilic extract of Viscum album L. and oleanolic acid on migratory activity of NIH / 3T3 fibroblasts and on HaCat keratinocytes. Evid Based Complement Altern Med. 2013;2013:718105. doi:10.1155/2013/718105
16. Königsberger K, Urech K, Reif M, Baumgartner S, Martin D, Tröger W. Viscum album lipophilic extract in actinic keratosis, cutaneous squamous cell carcinoma and basal cell carcinoma: a retrospective case series. Complement Med Res. 2024;1–24. doi:10.1159/000537979
17. Ren Y, Kinghorn AD. Natural product triterpenoids and their semi-synthetic derivatives with potential anticancer activity. Planta Med. 2019;85(11–12):802–814. doi:10.1055/a-0832-2383
18. Shi Y, Song Q, Hu D, Zhuang X, Yu S, Teng D. Oleanolic acid induced autophagic cell death in hepatocellular carcinoma cells via PI3K/Akt/mTOR and ROS-dependent pathway. Korean J Physiol Pharmacol. 2016;20(3):237–243. doi:10.4196/kjpp.2016.20.3.237
19. Lúcio KA, da Rocha GG, Monção-Ribeiro LC, Fernandes J, Takiya CM, Gattass CR. Oleanolic acid initiates apoptosis in non-small cell lung cancer cell lines and reduces metastasis of a B16F10 melanoma model in vivo. PLoS One. 2011;6(12):e28596. doi:10.1371/journal.pone.0028596
20. Saneja A, Arora D, Kumar R, Dubey RD, Panda AK, Gupta PN. Therapeutic applications of betulinic acid nanoformulations. Ann N Y Acad Sci. 2018;1421(1):5–18. doi:10.1111/nyas.13570
21. Yang L, Liu X, Lu Z, et al. Ursolic acid induces doxorubicin-resistant HepG2 cell death via the release of apoptosis-inducing factor. Cancer Lett. 2010;298(1):128–138. doi:10.1016/j.canlet.2010.06.010
22. Mösch P, Urech K, Odeh M, Kunz M, Kunz C. Clinical experiences in the topical treatment of skin lesions with Viscum album, Extractum resinosum 10%, Unguentum – a retrospective case series study. Phytomedicine. 2015;22:S28. doi:10.1016/j.phymed.2015.05.050
23. Khan KU, Minhas MU, Badshah SF, Suhail M, Ahmad A, Ijaz S. Overview of nanoparticulate strategies for solubility enhancement of poorly soluble drugs. Life Sci. 2022;291:120301. doi:10.1016/j.lfs.2022.120301
24. Liu H, Mei J, Xu Y, et al. Improving the oral absorption of nintedanib by a self-microemulsion drug delivery system: preparation and in vitro/in vivo evaluation. Int J Nanomed. 2019;14:8739–8751. doi:10.2147/IJN.S224044
25. Alghananim A, Özalp Y, Mesut B, Serakinci N, Özsoy Y, Güngör S. A solid ultra fine self-nanoemulsifying drug delivery system (S-snedds) of deferasirox for improved solubility: optimization, characterization, and in vitro cytotoxicity studies. Pharmaceuticals. 2020;13(8):162. doi:10.3390/ph13080162
26. Khan AW, Kotta S, Ansari SH, Sharma RK, Ali J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: design, characterization, in vitro and in vivo evaluation. Drug Delivery. 2015;22(4):552–561. doi:10.3109/10717544.2013.878003
27. Date AA, Desai N, Dixit R, Nagarsenker M. Self-nanoemulsifying drug delivery systems: formulation insights, applications and advances. Nanomedicine. 2010;5(10):1595–1616. doi:10.2217/nnm.10.126
28. Sass JE. Botanical Microtechnique.
29. Pearse AGE. Histochemistry: Theoretical and Applied.
30. Cain AJ. The use of Nile blue in the examination of lipoids. J Cell Sci. 1947;S3–88(3):383–392. doi:10.1242/jcs.s3-88.3.383
31. Hildebert W, Bladt S. Drugs containing triterpenes. In: Plant Drug Analysis: A Thin Layer Chromatography Atlas.
32. Isca VMS, Seca AML, Pinto DCGA, Silva H, Silva AMS. Lipophilic profile of the edible halophyte Salicornia ramosissima. Food Chem. 2014;165:330–336. doi:10.1016/j.foodchem.2014.05.117
33. United States Pharmacopeia and National Formulary. Monograph components - Description and Solubility <5.30>. In: USP–NF. Rockville MD: USP: United States Pharmacopeial Convention; 2021.
34. de Campos VEB, Cerqueira-Coutinho CS, Capella FNC, Soares BG, Holandino C, Mansur CRE. Development and in vitro assessment of nanoemulsion for delivery of ketoconazole against Candida albicans. J Nanosci Nanotechnol. 2017;17(7):4623–4630. doi:10.1166/jnn.2017.13445
35. de Morais JM, Dos Santos ODH, Delicato T, Gonçalves RA, da Rocha-Filho PA. Physicochemical characterization of canola oil/water nano-emulsions obtained by determination of required HLB number and emulsion phase inversion methods. J Dispers Sci Technol. 2006;27(1):109–115. doi:10.1081/DIS-200066829
36. Barradas TN, de Campos VEB, Senna JP, et al. Development and characterization of promising o/w nanoemulsions containing sweet fennel essential oil and non-ionic sufactants. Colloids Surf a Physicochem Eng Aspects. 2015;480:214–221. doi:10.1016/j.colsurfa.2014.12.001
37. Matos APDS, Lopes DCDXP, Peixoto MLH, et al. Development, characterization, and anti-leishmanial activity of topical amphotericin B nanoemulsions. Drug Deliv Transl Res. 2020;10(6):1552–1570. doi:10.1007/s13346-020-00821-5
38. Annisa R, Mutiah R, Yuwono M, Hendradi E. The Development Formulation of Eleutherine Palmifolia Extract-Loaded Self Nanoemulsifying Drug Delivery System (Snedds) using D-optimal mixture design approach. Int J Appl Pharm. 2023;15(5):269–276. doi:10.22159/ijap.2023v15i5.47645
39. de Campos VEB, Teixeira CAA, da Veiga VF, Ricci-Júnior E, Holandino C. L-Tyrosine-loaded nanoparticles increase the antitumoral activity of direct electric current in a metastatic melanoma cell model. Int J Nanomed. 2010;5:961–971. doi:10.2147/IJN.S13634
40. Syukri Y, Fitriani H, Pandapotan H, Nugroho BH. Formulation, characterization and stability of ibuprofen-loaded Self-Nano Emulsifying Drug Delivery System (SNEDDS). Indones J Pharm. 2019;30(2):105–113. doi:10.14499/indonesianjpharm30iss2pp105
41. Ujilestari T, Martien R, Ariyadi B, Dono ND, Zuprizal. Self-nanoemulsifying drug delivery system (SNEDDS) of Amomum compactum essential oil: design, formulation, and characterization. J Appl Pharm Sci. 2018;8(6):14–21. doi:10.7324/JAPS.2018.8603
42. Karavasili C, Andreadis II, Tsantarliotou MP, et al. Self-Nanoemulsifying Drug Delivery Systems (SNEDDS) containing rice bran oil for enhanced fenofibrate oral delivery: in vitro digestion, ex vivo permeability, and in vivo bioavailability studies. AAPS Pharm Sci Tech. 2020;21(6):208. doi:10.1208/s12249-020-01765-2
43. de Oliveira ECV, Carneiro ZA, De albuquerque S, Marchetti JM. Development and evaluation of a nanoemulsion containing ursolic acid: a promising trypanocidal agent. AAPS Pharm Sci Tech. 2017;18(7):2551–2560. doi:10.1208/s12249-017-0736-y
44. Kontogiannidou E, Meikopoulos T, Gika H, et al. In vitro evaluation of self-nano-emulsifying drug delivery systems (SNEDDS) containing room temperature ionic liquids (RTILs) for the oral delivery of amphotericin B. Pharmaceutics. 2020;12(8):699. doi:10.3390/pharmaceutics12080699
45. Buya AB, Beloqui A, Memvanga PB, Préat V. Self-nano-emulsifying drug-delivery systems: from the development to the current applications and challenges in oral drug delivery. Pharmaceutics. 2020;12(12):1194. doi:10.3390/pharmaceutics12121194
46. Patel J, Kevin G, Patel A, Raval M, Sheth N. Design and development of a self-nanoemulsifying drug delivery system for telmisartan for oral drug delivery. Int J Pharm Investig. 2011;1(2):112–118. doi:10.4103/2230-973x.82431
47. Patel J, Patel A, Raval M, Sheth N. Formulation and development of a self-nanoemulsifying drug delivery system of irbesartan. J Adv Pharm Technol Res. 2011;2(1):9–16. doi:10.4103/2231-4040.79799
48. Tietbohl LAC. Avaliação da atividade antiproliferativa de extratos de Myrciaria floribunda e suas nanoformulações [Evaluation of the antiproliferative activity of Myrciaria floribunda extracts and their nanoformulations]; 2018: 125.Portuguese
49. de Almeida VP, Monchak IT, Batista JVDC, et al. Investigations on the morpho-anatomy and histochemistry of the European mistletoe: viscum album L. subsp. album. Sci Rep. 2023;13(1):4604. doi:10.1038/s41598-023-29799-z
50. O’Brien TP, Feder N, McCully ME. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma. 1964;59(2):368–373. doi:10.1007/BF01248568
51. Kraus JE, de Sousa HC, Rezende MH, Castro NM, Vecchi C, Luque R. Astra blue and basic fuchsin double staining of plant materials. Biotech Histochem. 1998;73(5):235–243. doi:10.3109/10520299809141117
52. Berlyn GP, Miksche JP. Botanical Microtechnique and Cytochemistry. The Iowa State University Press; 1976.
53. Fuchs CH. Fuchsin staining with NaOH clearing for lignified elements of whole plants or plants organs. Stain Technol. 1963;38(3):141–144. doi:10.3109/10520296309067156
54. de Souza DM, Sa RD, Araújo EL, Randau KP. Anatomical, phytochemical and histochemical study of Solidago chilensis Meyen. An Acad Bras Cienc. 2018;90(2 Suppl 1):2107–2120. doi:10.1590/0001-3765201720160280
55. Miastkowska M, Sliwa P. Influence of terpene type on the release from an O/W nanoemulsion: experimental and theoretical studies. Molecules. 2020;25(12):2747. doi:10.3390/molecules25122747
56. Hasan NMY. Role of medium-chain fatty acids in the emulsification mechanistics of self-micro-emulsifying lipid formulations. Saudi Pharm J. 2014;22(6):580–590. doi:10.1016/j.jsps.2014.02.005
57. Traul KA, Driedger A, Ingle DL, Nakhasi D. Review of the toxicologic properties of medium-chain triglycerides. Food Chem Toxicol. 2000;38(1):79–98. doi:10.1016/S0278-6915(99)00106-4
58. Hayati F, Salsabila T, Chabib L, Fawwazi MHAF. Self Nano Emulsifying Drug Delivery System (SNEDDS) activity of pegagan (Centella asiatica L) extraction on Zebrafish caudal fins regeneration. J Res Pharm. 2022;26(7):1946–1954. doi:10.29228/jrp.288
59. Qu D, He J, Liu C, Zhou J, Chen Y. Triterpene-loaded microemulsion using Coix lacryma-jobi seed extract as oil phase for enhanced antitumor efficacy: preparation and in vivo evaluation. Int J Nanomed. 2014;9:109–119. doi:10.2147/IJN.S54796
60. Wooster TJ, Golding M, Sanguansri P. Impact of oil type on nanoemulsion formation and Ostwald ripening stability. Langmuir. 2008;24(22):12758–12765. doi:10.1021/la801685v
61. Tadros T, Izquierdo P, Esquena J, Solans C. Formation and stability of nano-emulsions. Adv Colloid Interface Sci. 2004;108–109:303–318. doi:10.1016/j.cis.2003.10.023
62. Amantayeva M, Kozhanova K, Kadyrbayeva G, et al. Macroscopical, microscopical and histochemical analysis of Eryngium karatavicum Iljin Growing on the Territory of South Kazakhstan. Plants. 2023;12(14):2714. doi:10.3390/plants12142714
63. Elkiran O. Comparative Anatomical Study of Viscum album subsp. album L. and Viscum album subsp. austriacum (Wiesb.) Vollman. Ejons Int J Math Eng Nat Sci. 2022;6(21):12–17.
64. Erst AA, Petruk AA, Zibareva LN, Erst AS. Morphological, histochemical and biochemical features of cultivated Rhodiola rosea (Altai Mountains Ecotype). Contemp Probl Ecol. 2021;14(6):701–710. doi:10.1134/S1995425521060135
65. Mehrvarz SS, Shavvon RS, Golmohammadi N. Notes on the genus Viscum (Viscaceae) in Iran: a new combination based on morphological evidence. African J Agric Res. 2012;7(11):1694–1702. doi:10.5897/ajar11.1382
66. Khan MA, Sharif T, Ahmad M, Zafar M, Tareen RB. Anatomical characterization of parasitic plants of Pakistan. Pak J Bot. 2009;41(6):2661–2669.
67. Alvarado HL, Calpena AC, Garduño-Ramírezc ML, et al. Nanoemulsion strategy for ursolic and oleanic acids isolates from Plumeria obtusa improves antioxidant and cytotoxic activity in melanoma cells. Anticancer Agents Med Chem. 2018;18(6):847–853. doi:10.2174/1871520618666180111151846
68. Liu YB, Wang XA, Xiang SS, et al. Oleanolic acid induces mitochondrial-dependent apoptosis and G0/G1 phase arrest in gallbladder cancer cells. Drug Des Devel Ther. 2015;9:3017–3030. doi:10.2147/DDDT.S84448
69. Tang ZY, Li Y, Tang YT, Ma XD, Tang ZY. Anticancer activity of oleanolic acid and its derivatives: recent advances in evidence, target profiling and mechanisms of action. Biomed Pharmacother. 2022;145:112397. doi:10.1016/j.biopha.2021.112397
70. Jiang Q, Wang D, Han Y, Han Z, Zhong W, Wang C. Modulation of oxidized-LDL receptor-1 (LOX1) contributes to the antiatherosclerosis effect of oleanolic acid. Int J Biochem Cell Biol. 2015;69:142–152. doi:10.1016/j.biocel.2015.10.023
71. Wang X, Liu R, Zhang W, et al. Oleanolic acid improves hepatic insulin resistance via antioxidant, hypolipidemic and anti-inflammatory effects. Mol Cell Endocrinol. 2013;376(1–2):70–80. doi:10.1016/j.mce.2013.06.014
72. Gutiérrez-Rebolledo GA, Siordia-Reyes AG, Meckes-Fischer M, Jiménez-Arellanes A. Hepatoprotective properties of oleanolic and ursolic acids in antitubercular drug-induced liver damage. Asian Pac J Trop Med. 2016;9(7):644–651. doi:10.1016/j.apjtm.2016.05.015
73. Sung HY, Kang SW, Kim JL, et al. Oleanolic acid reduces markers of differentiation in 3T3-L1 adipocytes. Nutr Res. 2010;30(12):831–839. doi:10.1016/j.nutres.2010.10.001
74. Wang J, Ren H, Xu QL, et al. Antibacterial oleanane-type triterpenoids from pericarps of Akebia trifoliata. Food Chem. 2015;168C:623–629. doi:10.1016/j.foodchem.2014.07.105
75. Castellano JM, Ramos-Romero S, Perona JS. Oleanolic acid: extraction, characterization and biological activity. Nutrients. 2022;14(3):623. doi:10.1016/b978-0-08-013368-3.50007-8
76. Dzubak P, Hajduch M, Vydra D, et al. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat Prod Rep. 2006;23(3):394–411. doi:10.1039/b515312n
77. Eloy JO, Saraiva J, de Albuquerque S, Marchetti JM. Preparation, characterization and evaluation of the in vivo trypanocidal activity of ursolic acid-loaded solid dispersion with poloxamer 407 and sodium caprate. Brazilian J Pharm Sci. 2015;51(1):101–109. doi:10.1590/S1984-82502015000100011
78. Xia X, Liu H, Lv H, Zhang J, Zhou J, Zhao Z. Preparation, characterization, and in vitro/vivo studies of oleanolic acid-loaded lactoferrin nanoparticles. Drug Des Devel Ther. 2017;11:1417–1427. doi:10.2147/DDDT.S133997
79. Pratiwi L, Fudholi A, Martien R, Pramono S. Self-nanoemulsifying drug delivery system (Snedds) for topical delivery of mangosteen peels (Garcinia Mangostana L.): formulation design and in vitro studies. J Young Pharm. 2017;9(3):341–346. doi:10.5530/jyp.2017.9.68
80. Xi J, Chang Q, Chan CK, et al. Formulation development and bioavailability evaluation of a self-nanoemulsified drug delivery system of oleanolic acid. AAPS Pharm Sci Tech. 2009;10(1):172–182. doi:10.1208/s12249-009-9190-9
81. Chouhan N, Mittal V, Kaushik D, Khatkar A, Raina M. Self Emulsifying Drug Delivery System (SEDDS) for phytoconstituents: a review. Curr Drug Deliv. 2014;12(2):244–253. doi:10.2174/1567201811666141021142606
82. Preeti, Sambhakar S, Saharan R, et al. Exploring LIPIDs for their potential to improves bioavailability of lipophilic drugs candidates: a review. Saudi Pharm J. 2023;31(12):101870. doi:10.1016/j.jsps.2023.101870
83. Alvarado HL, Abrego G, Souto EB, et al. Nanoemulsions for dermal controlled release of oleanolic and ursolic acids: in vitro, ex vivo and in vivo characterization. Coll Surf B Biointerf. 2015;130:40–47. doi:10.1016/j.colsurfb.2015.03.062
84. Jyotshna GAC, Bawankule DU, Verma AK, Shanker K. Nanoemulsion preconcentrate of a pentacyclic triterpene for improved oral efficacy: formulation design and in-vivo antimalarial activity. J Drug Deliv Sci Technol. 2020;57:101734. doi:10.1016/j.jddst.2020.101734
85. Tang H, Xiang S, Li X, Zhou J, Kuang C, Xu B. Preparation and in vitro performance evaluation of resveratrol for oral self-microemulsion. PLoS One. 2019;14(4):e0214544. doi:10.1371/journal.pone.0214544
86. Yukuyama MN, Kato ETM, de Araujo GLB, et al. Olive oil nanoemulsion preparation using high-pressure homogenization and D-phase emulsification – a design space approach. J Drug Deliv Sci Technol. 2019;49:622–631. doi:10.1016/j.jddst.2018.12.029
87. Yang X, Gao P, Jiang Z, Luo Q, Mu C, Cui M. Preparation and evaluation of Self-emulsifying Drug Delivery System (SEDDS) of Cepharanthine. AAPS Pharm Sci Tech. 2021;22(8):255. doi:10.1208/s12249-021-02085-9
88. Liu W, Tian R, Hu W, et al. Preparation and evaluation of self-microemulsifying drug delivery system of baicalein. Fitoterapia. 2012;83(8):1532–1539. doi:10.1016/j.fitote.2012.08.021
89. de Oliveira MC, Bruschi ML. Self-emulsifying systems for delivery of bioactive compounds from natural origin. AAPS Pharm Sci Tech. 2022;23(5):134. doi:10.1208/s12249-022-02291-z
90. Ujhelyi Z, Kalantari A, Vecsernyés M, et al. The enhanced inhibitory effect of different antitumor agents in self-microemulsifying drug delivery systems on human cervical cancer HeLa cells. Molecules. 2015;20(7):13226–13239. doi:10.3390/molecules200713226
91. Shafiq-un-Nabi S, Shakeel F, Talegaonkar S, et al. Formulation development and optimization using nanoemulsion technique: a technical note. AAPS Pharm Sci Tech. 2007;8(2):E12–E17. doi:10.1208/pt0802028
92. Anton N, Vandamme TF. Nano-emulsions and micro-emulsions: clarifications of the critical differences. Pharm Res. 2011;28(5):978–985. doi:10.1007/s11095-010-0309-1
93. Parmar N, Singla N, Amin S, Kohli K. Study of cosurfactant effect on nanoemulsifying area and development of lercanidipine loaded (SNEDDS) self nanoemulsifying drug delivery system. Coll Surf B Biointerf. 2011;86(2):327–338. doi:10.1016/j.colsurfb.2011.04.016
94. Morakul B. Self-nanoemulsifying drug delivery systems (SNEDDS): an advancement technology for oral drug delivery. Pharm Sci Asia. 2020;47(3):205–220. doi:10.29090/psa.2020.03.019.0121
95. Xue X, Cao M, Ren L, Qian Y, Chen G. Preparation and Optimization of Rivaroxaban by Self-Nanoemulsifying Drug Delivery System (SNEDDS) for enhanced oral bioavailability and no food effect. AAPS Pharm Sci Tech. 2018;19(4):1847–1859. doi:10.1208/s12249-018-0991-6
96. Karimi M, Karimian KA, Heli H. A nanoemulsion-based delivery system for imatinib and in vitro anticancer efficacy. Brazilian J Pharm Sci. 2020;56:1–9. doi:10.1590/S2175-97902020000118973
97. Demisli S, Mitsou E, Pletsa V, Xenakis A, Papadimitriou V. Development and study of nanoemulsions and nanoemulsion‐based hydrogels for the encapsulation of lipophilic compounds. Nanomaterials. 2020;10(12):1–19. doi:10.3390/nano10122464
98. Danaei M, Dehghankhold M, Ataei S, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):57. doi:10.3390/pharmaceutics10020057
99. Batista JVDC, Matos APS, Oliveria AP, et al. Thermoresponsive hydrogel containing viscum album extract for topic and transdermal use: development, stability and cytotoxicity activity. Pharmaceutics. 2022;14(1):37. doi:10.3390/pharmaceutics14010037
100. Wang W, Cui C, Li M, Zhang Z, Lv H. Study of a novel disintegrable oleanolic acid-polyvinylpolypyrrolidone solid dispersion. Drug Dev Ind Pharm. 2017;43(7):1178–1185. doi:10.1080/03639045.2017.1301950
101. Feng A, Yang S, Sun Y, Zhang L, Bo F, Li L. Development and evaluation of oleanolic acid dosage forms and its derivatives. Biomed Res Int. 2020;2020:1308749. doi:10.1155/2020/1308749
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.








