Back to Journals » International Journal of Nanomedicine » Volume 19
Smart Nanoplatforms Responding to the Tumor Microenvironment for Precise Drug Delivery in Cancer Therapy
Authors Wang Y, Deng T, Liu X, Fang X, Mo Y, Xie N , Nie G, Zhang B, Fan X
Received 29 January 2024
Accepted for publication 20 May 2024
Published 19 June 2024 Volume 2024:19 Pages 6253—6277
DOI https://doi.org/10.2147/IJN.S459710
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kamakhya Misra
Yujie Wang,1 Tingting Deng,1 Xi Liu,2 Xueyang Fang,1 Yongpan Mo,3 Ni Xie,4 Guohui Nie,1 Bin Zhang,1 Xiaoqin Fan1,4
1Shenzhen Key Laboratory of Nanozymes and Translational Cancer Research, Department of Otolaryngology, Shenzhen Institute of Translational Medicine, The First Affiliated Hospital of Shenzhen University, Shenzhen Second People’s Hospital, Shenzhen, 518035, People’s Republic of China; 2Department of Nephrology, Shenzhen Longgang Central Hospital, Shenzhen, 518116, People’s Republic of China; 3Department of Breast Surgery, The First Affiliated Hospital of Shenzhen University, Shenzhen Second People’s Hospital, Shenzhen, 518035, People’s Republic of China; 4The Bio-Bank of Shenzhen Second People’s Hospital, The First Affiliated Hospital of Shenzhen University, Shenzhen Second People’s Hospital, Shenzhen, Guangdong, 518035, People’s Republic of China
Correspondence: Bin Zhang; Xiaoqin Fan, Email [email protected]; [email protected]
Abstract: The tumor microenvironment (TME) is a complex and dynamic entity, comprising stromal cells, immune cells, blood vessels and extracellular matrix, which is intimately associated with the occurrence and development of cancers, as well as their therapy. Utilizing the shared characteristics of tumors, such as an acidic environment, enzymes and hypoxia, researchers have developed a promising cancer therapy strategy known as responsive release of nano-loaded drugs, specifically targeted at tumor tissues or cells. In this comprehensive review, we provide an in-depth overview of the current fundamentals and state-of-the-art intelligent strategies of TME-responsive nanoplatforms, which include acidic pH, high GSH levels, high-level adenosine triphosphate, overexpressed enzymes, hypoxia and reductive environment. Additionally, we showcase the latest advancements in TME-responsive nanoparticles. In conclusion, we thoroughly examine the immediate challenges and prospects of TME-responsive nanopharmaceuticals, with the expectation that the progress of these targeted nanoformulations will enable the exploitation, overcoming or modulation of the TME, ultimately leading to significantly more effective cancer therapy.
Keywords: tumor microenvironment, stimulus-responsive, drug delivery, cancer therapy, intelligent biomedicine
Graphical Abstract:
Introduction
Malignant tumors are a leading cause of human mortality. In recent years, significant advancements have been made in tumor therapy, thanks to the development of various approaches. Notably, nanotechnology and targeted drug delivery systems have garnered considerable attention due to the favorable characteristics of nanoparticles, such as excellent permeability, enhanced loading capacity and high specificity. Consequently, nanoparticles have become widely adopted as effective carriers for delivering chemotherapeutic drugs, nucleic acids and genes to precise target sites for the treatment of oncological diseases. The tumor microenvironment (TME) plays a pivotal role in tumor development, consisting of tumor cells, tumor stromal cells, the extracellular matrix, as well as various cytokines and chemokines (Figure 1).1–5 Compared to the normal human internal environment, the TME exhibits distinct biochemical properties, including hypoxia, acidity, strong redox characteristics, abnormal enzyme metabolism, and immunosuppression.6 Of particular interest in recent research on nano-targeted agents are the weak acidic and strong redox properties, given their significant roles in tumor progression, metastasis and immune evasion.7 Metabolic differences between cancer cells and normal cells contribute to unique features within the tumor microenvironment, such as hypoxia, angiogenesis, acidosis, hypermetabolism and elevated redox state. Customizing nanomaterials that target the tumor microenvironment based on its specific characteristics holds paramount importance. These tailored nanocarriers can facilitate tumor stimulus-responsive drug delivery through size adjustments, surface charge conversion or other modifications of physicochemical properties. This adaptability ensures that the nanomaterials possess the necessary attributes for efficient accumulation in tumor tissues, penetration into tumor cells, facilitation of endocytosis and ultimately, controlled drug release.
Compared to the conventional clinical use of common nanomaterials like doxorubicin (DOX) liposomes and paclitaxel albumin nanoparticles, tumor microenvironment-responsive nanomaterials offer significant improvements in vivo drug delivery efficiency. They effectively exploit the enhanced permeability and retention effect (EPR), resulting in enhanced therapeutic effects and reduced side effects. As a result, tumor microenvironment-responsive nanomaterials hold immense potential for targeted drug delivery, emerging as a highly effective tool for tumor therapy. This review delves deeply into the realm of tumor microenvironment-responsive nano-delivery systems, with a keen focus on their capacity to respond to or modulate the tumor microenvironment. The study presents three dynamic targeting schemes: size contraction, surface charge conversion and ligand exposure strategies. Furthermore, recent advances in the development of targeted delivery vectors based on the tumor microenvironment were showcased, highlighting various examples of typical multi-stimulus-responsive drug delivery systems (DDSs). The potential applications of tumor microenvironment-responsive NPs in therapeutic industries are emphasized, accompanied by discussions on the feasibility of clinical translation for these nanomaterials. Additionally, the main challenges and potential future developments in this field are outlined. By employing tumor microenvironment-responsive nano-agents, medical professionals can significantly enhance drug delivery to target sites, leading to more effective treatments with fewer side effects. The ongoing exploration and refinement of these nanomaterials hold promising prospects for advancing cancer therapies in the future. Although there are review papers published on the topic “tumor microenvironment-responsive biomedical nanocomposites”, as a research hotspot, nanoplatforms responding to TME for precise drug delivery in cancer therapy have been continuously constructed and updated. Therefore, recent advances should be summarized in time reflecting the latest research achievements of this topic, which serves as the starting point of this review paper updating researchers of the constantly development in this field.
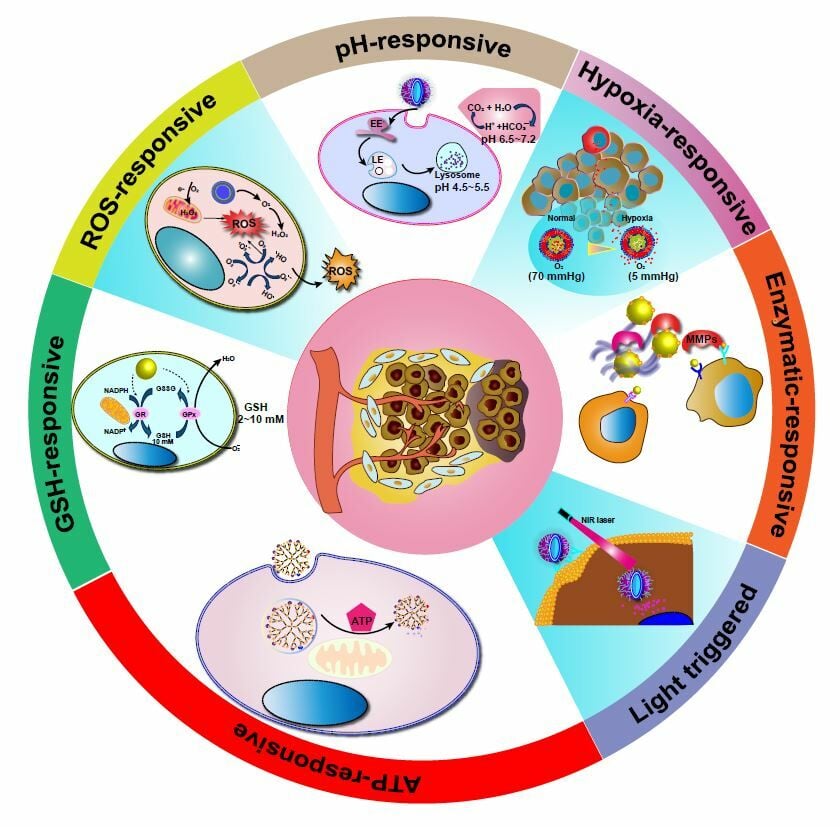
TME‐responsive Nanoplatforms
TME-responsive nanodrugs have demonstrated both safety and efficacy in achieving targeted and localized drug release within tumor tissues, leading to improved patient response rates to drug therapy while minimizing associated side effects. Figure 2 illustrates the stimulus-triggered activation of nanoparticles in the tumor microenvironment, enabling rapid drug release at the intended site, enhancing cell binding and internalization, or promoting drug diffusion throughout the tumor region. This comprehensive summary encompasses recent breakthroughs in TME-responsive nanodrugs, specifically highlighting their responses to signals within the TME, such as highly reactive oxygen species, weakly acidic and reducing environments, hypoxia, overexpressed enzymes, and high concentrations of adenosine triphosphate.
pH-Responsive Nanoplatforms
The Warburg effect, characterized by increased glucose uptake and aerobic glycolysis in cancer cells leading to elevated lactate production, was first proposed by Warburg in the 1930s.8 In most solid tumors, the pH of the extracellular tumor microenvironment ranges from 6 to 7, while that of intracellular endosomes and lysosomes falls within the range of 4 to 6.9,10 Figure 3 showcase that researchers have extensively explored the use of pH-sensitive drug delivery systems, making the tumor microenvironment an ideal platform for such applications, due to these acidic pH conditions.11
This review (Table 1) will discuss classic pH-sensitive drug delivery systems separately, based on their triggering mechanisms.12–20 However, it’s important to note that the acidic nature of the pH in solid tumors raises some controversies concerning the efficacy and safety of pH-inducible delivery. As the use of pH-inducible delivery systems expands in both therapeutic and diagnostic fields, there is an urgent need for further exploration of the tumor microenvironment and the development of pH-triggering biorthogonal chemistry.
 |
Table 1 Typical Examples of pH-Responsive Nanomedicine for Tumor Therapy |
“Protonated” chemical groups, such as carboxylic acid and amine groups, experience conformational changes due to proton transfer in different pH environments. These pH-dependent conformational changes make these “protonated” chemical groups highly suitable for controlled release systems. There are three main types of pH-sensitive delivery systems based on specific “protonated” chemical structures: biomolecules, inorganic materials and polymers.34
Biomolecules Based pH-Sensitive Delivering System
Endogenous proteins and DNA can be developed as pH-sensitive carriers. Ferritin, a classic carrier, undergoes a conformational change induced by α-helix under different pH environments. It self-assembles into a hollow cage structure by removing the E-helix, allowing it to encapsulate metal complexes. This “Ferritin” cargo vessel unloads the metal complexes by disassembling in an acidic environment. Leveraging the nature of ferritin, Jung’s group has developed ferritin nanocages that trap Fe (II)-DOX complexes. These nanocages remain stable at pH 6.0 and disassemble at pH 4.0–5.0 (the endosomal pH of tumor cells), enabling the release of cargos under acidic conditions.35 The pH (low) insertion peptide (pHLIP) can shuttle through the tumor cell membrane when triggered by acidity. When protonated, pHLIP’s hydrophobicity increases, and helix structures form spontaneously, facilitating rapid transmembrane shuttling through a non-endocytic pathway. Cheng’s group has reported a pHLIP-based platform to carry therapeutic nuclear acid analogs via glutathione (GSH)-responsive disulfide linkages with pHLIP.36 Upon entering tumor cells, GSH triggers the release of cargos. The efficacy of this pHLIP-carrier has been demonstrated in silencing miR-155 and inhibiting tumor growth in a mouse lymphoma model.36 The amine group of poly (L-histidine) acts as the trigger of a proton pump in lysosomes after protonation. Jia et al have developed poly(L-histidine)-based pH-sensitive micelles, which release the encapsulated drug into the cytoplasm after proton pump activation and an increase in lysosomal osmotic pressure.37 Oligodeoxynucleotides (ODNs) can also serve as acidic-stimulating carriers. ODNs tethered to gold nanoparticles (AuNPs) have been reported to co-deliver DOX and antisense ODNs to bcl-2 mRNA.38 In this system, AuNPs were coated with antisense ODNs and i-motif binding ODNs. A cytosine-rich i-motif sequence acted as the linker for AuNPs assembly. The C-G rich DNA duplexes provided a nest for the cargo DOX. Under acidic conditions (pH 5.0), the partial hybridization of cytosine and protonated cytosine from the i-motif sequence formed a unique tetrameric structure, leading to the disassembly of the gold clusters and subsequent release of DOX and exposure of antisense ODNs. The ODNs-AuNPs have demonstrated 2.5-fold higher tumor-specific accumulation and efficient tumor growth inhibition in A549-bearing nude mice xenografts. The biocompatibility of biomolecules makes them a preferable choice for in vivo drug delivery.
Inorganic Materials-Based pH-Sensitive Delivering System
Several inorganic materials, namely zinc oxide (ZnO), calcium carbonate (CaCO3), and manganese dioxide (MnO2), have shown considerable sensitivity to acidity.21–23,39 Zhong’s research group has achieved a significant milestone by developing a cutting-edge ZnO-based nanoplatform, boasting tri-modality bioimaging and acid-inducible DOX release capabilities for cancer therapeutics and diagnosis. The ingenious design involves coating lanthanide-doped upconverting NPs (UCNPs) with a mesoporous silica layer, expertly embedding ZnO to facilitate controlled drug release.40 The ZnO-based acid-inducible nanoplatform has showcased remarkable drug release capabilities at pH 5.0, while its tri-modality imaging system has undergone comprehensive validation both in vitro and in vivo. Also, a study on a novel zinc oxide-functionalized up-conversion nanotherapeutic platform for pH-triggered on-demand drug release for multi-modality bioimaging and therapeutic platforms. Among this platform, zinc oxide effectively blocks the drug in the mesopores of the prepared formulations and promotes the dissolution of the drug in the acidic environment surrounding the tumor, thus enabling a drug-on-demand chemotherapeutic approach.23 Furthermore, the dual pH-responsive function of MnO2 nanosheets can be used for MRI and drug-responsive release of cancer. The pH-triggered fracture behavior can further promote the rapid cargo release in the weakly acidic microenvironment and combat the multidrug resistance of cancer cells, which paves the way for biomedical applications of two-dimensional multifunctional pH-responsive nanomaterials.22 Despite the inherent limitations regarding the scope of applications for acid-sensitive inorganic materials, their solubility variance between neutral and acidic environments firmly establishes these specific inorganic materials as viable alternatives for acid-sensitive diagnosis and therapeutics.
pH-Sensitive Polymers
Poly-L-histidine (polyHis) exhibits a pKa of approximately 7.0 and undergoes reversible transitions between hydrophilic and hydrophobic states. Bae’s research group has made significant strides by successfully developing mixed micelles comprising polyHis-b-PEG and poly (L-lactic acid) (PLLA)-b-PEG. These micelles gradually become destabilized under acidic conditions (pH < 7.0) due to the protonation of the polyHis segments within the micelle.41 In neutral or basic environments, the anticancer drug doxorubicin (DOX) can be encapsulated within the micelle through hydrophobic interactions with the deprotonated polyHis segments. Once these mixed micelles reach the tumor sites, they undergo dissociation, facilitating the release of DOX in the acidic environment. In xenograft models, these pH-sensitive micelles have demonstrated vastly improved antitumor efficacy compared to their pH-insensitive counterparts. Furthermore, polyHis polymers have found applications in triggering the release of targeting ligands in acidic tumor microenvironments, thereby preventing plasma protein binding at physiological pH. Hyeon’s group has pioneered a hybrid system that combines pH-sensitive polyHis polymers with metal oxides.42 In this system, the PEG-polyHis polymer is functionalized with catechol groups and chlorin e6 (Ce6). The catechol groups effectively anchor the polymers to the iron oxide surface, while Ce6 acts as a fluorescent tracer and photosensitizer for photodynamic therapeutic nanoparticles. Under physiological conditions, the hybrid superstructures remain inert, securely trapping multiple iron oxide nanoparticles and Ce6. However, upon exposure to an acidic environment, the hybrid’s charge gradually shifts from negative to positive, resulting in particle swelling, drug release, and cellular internalization. The photodynamic and fluorescence tracking capabilities of Ce6 have been convincingly demonstrated in both colorectal carcinoma xenografts and heterogeneous drug-resistant tumors. Cholesteryl hemisuccinate (CHEMS) possesses a pKa of around 5.8, and its carboxyl group undergoes protonation at acidic pH levels, leading to alterations in the spatial structure of liposomes and promoting drug release. Additionally, poly [2-(diisopropylamino) ethyl methacrylate] (PDPA) and its derivatives have pKa values ranging from 4.0 to 7.4, making them highly sensitive to pH changes in acidic endocytic organelles.43 Gao’s research group has achieved significant advancements in the field of controlled drug delivery by developing star-like nanoparticles (NPs) through the anchoring of PDPA-b-POEGMA on a β-CD core.24 Under normal physiological conditions (pH 7.4), the PDPA blocks facilitate the copolymers to effectively trap the drug cargo through hydrophobic interactions. However, in an acidic environment (pH<6), the drug release is triggered due to a hydrophobicity-hydrophilicity transition. This innovative approach holds great promise for precise and controlled drug delivery. Furthermore, the research group has successfully modified various PDPA derivatives with linear or cyclic side chains at the tertiary amines to enable the delivery of ovalbumin (OVA) for cancer immunotherapy.44 This signifies a significant step towards targeted cancer treatment using nanoscale drug carriers. Notably, the PC7A NPs, with a pKa of approximately 7.9, exhibited remarkable OVA-specific splenocyte cytotoxicity and enhanced efficacy, including improved lymph node accumulation and tumor inhibition, in xenograft models of C57BL/6 mice bearing B16F10 melanoma, MC38 colon, and TC-1 cancer cells.45 These findings highlight the potential of PC7A NPs as a promising platform for cancer immunotherapy. In addition to PDPA-based NPs, sulfonamide-based polymers have been explored as pH-sensitive micelles.46 The nearly neutral pKa of the secondary amine group in sulfonamide serves as a pH-responsive trigger. In a basic or neutral environment, the polymers aggregate due to electrostatic interactions between the negatively charged sulfonamide and the positively charged polymer. However, as the pH decreases, the cargos are efficiently released or flipped due to the elimination of electrostatic associations. This remarkable feature has been successfully demonstrated in various systems, including pH-sensitive sulfonamide-coated TAT peptide-decorated micelles25 and zwitterionic gold nanoparticles.26 By carefully and rationally modifying these easily prepared and multi-loaded polymers with pH-triggering functional groups, they can be further developed as potent carriers for imaging agents and drugs. This promising avenue holds great potential in enhancing diagnostic sensitivity and therapeutic efficacy, thus significantly advancing the field of nanomedicine.
Acid-Labile Chemical Groups
The utilization of pH-sensitive chemical bonds represents a well-established delivery strategy, particularly effective in the acidic tumor environment. In this approach, cargos (drugs or imaging agents) are typically attached to vesicles using various acid-labile moieties, such as hydrazine,27 acetal,28 or metal-organic frameworks (MOFs).47 These linkers can be easily hydrolyzed in acidic environments (pH 4.5–6.5) while remaining relatively stable under physiological conditions. This unique property allows for the specific release of cargos at acidic tumor sites. Numerous reports categorize acid-labile linkers into organic linkers and metal-organic complexes. The organic acid-labile linkers utilized in tumor microenvironment-induced delivery systems are listed in the Table 1. On the other hand, metal-organic complexes consist of organic ligands coordinated with metal ions to form one-, two-, or three-dimensional structures, making them ideal for encapsulating cargos. These complexes are usually stable and demonstrate acceptable biocompatibility in physiological environments. The coordination bonds, at the core of the metal-organic complexes, serve as the trigger for cargo release through acid-mediated bond breakage. For instance, Zhuang’s research group successfully encapsulated cargos (eg, fluorescein, camptothecin (CPT), and iron oxide NPs) in acidic-sensitive zeolitic imidazolate framework-8 (ZIF-8) nanospheres. This system demonstrated stability at pH 7.4 and efficiently released cargos within an acidic buffer (pH 6.0), showcasing the versatility of the ZIF-8 strategy.47 In a similar study, a pH-responsive Fe3O4@ZIF-8 nanocomposite carrier was prepared, which effectively accumulated in mouse tumor tissues to improve the anti-tumor effect.29 In other studies, researchers assembled DOX-encapsulated MOFs by sequentially coordinating therapeutic molecules with functional carboxyl groups with Zn2+ ions for the initial self-assembling, and then introducing another distinct organic linker for coordination complex reassembling.30 Moreover, Sun’s research group developed a yolk-like Fe3O4@Gd2O3 nanoplatform for acid-inducible T1-T2 dual-mode magnetic resonance imaging (MRI) and cisplatin delivery. By further optimizing the system with PEG and FA to prolong circulation and enhance targeting, both drug release efficacy and T1-T2 dual-mode MRI were significantly improved at pH 4.5.31
pH-Responsive Insertion Peptide
A class of peptides, known as pH Low Insertion Peptides (pHLIPs), can serve as a responsive delivery system in acidic tumor microenvironments by integrating into cellular membranes. These pHLIP peptides consist of a transmembrane sequence and two flanking sequences at each terminus. Under neutral or basic conditions, the peptides exist as disordered linear monomers in an aqueous solution. However, in an acidic environment, the transmembrane sequences, containing aspartate (Asp) and glutamate (Glu) residues, facilitate the insertion of the entire peptide into the lipid bilayer or cellular membrane through hydrophobic interactions. Protonation of the carboxyl groups of Asp and Glu residues, as well as the C-terminus, increases the peptide’s hydrophobicity. This protonation also promotes the formation of an interfacial helix, which aids the peptide’s insertion into the hydrophobic bilayer of the cellular membrane. Studies have demonstrated that the membrane insertion process is thermodynamically favorable, with the peptides exhibiting 30–50 times higher affinity for the bilayer membrane at acidic pH.48 Furthermore, the insertion process consists of two stages: the formation of the interfacial helix and the movement across the membrane, both of which can be completed within seconds to minutes.49,50 Therefore, the use of transmembrane peptide sequences as an inducible delivery system in acidic tumor microenvironments is both thermodynamically and kinetically advantageous.51 The pH-sensitive membrane insertion strategy has been validated by delivering various imaging cargos (eg, near-infrared (NIR) fluorescent dyes, positron emission tomography, and single-photon emission computed tomography) to tumor sites, resulting in significantly high tumor-specific fluorescent signals.52–54 Additionally, this peptide-induced delivery system has been employed to transport other polar cargos (eg, phalloidin toxin, peptide nucleic acids, and microRNAs) and nanoparticles into the cytosol of cancer cells.55 Considering the convenience, modifiability, low cost, and high biocompatibility of peptides as pH-responsive delivery agents, the application scope of the system can be further expanded.
Gas Generating Based Systems
Acid-promoted gas generation represents a novel and promising strategy for cargo release in delivery systems.56 Two extensively studied gas generators are the carbon dioxide gas generator (HCO3−) and the nitric oxide (NO)-releasing donor (NONOate). In one study, pH-sensitive liposomes were employed to encapsulate bicarbonate ions and the anticancer drug DOX, enabling the release of DOX from the liposomes upon triggering carbon dioxide generation in an acidic environment.32 Another study focused on developing injectable PLGA hollow microspheres (HM) armed with a NO-releasing donor (NONOate) and encapsulated with the anticancer agent irinotecan (CPT-11) or Cy5, a fluorescent agent. At pH 6.6, the NO generator released NO bubbles, reversing p-glycoprotein-mediated multidrug resistance (MDR), and disrupting the HM shell, leading to a burst release of the encapsulated CPT-11 or Cy5.33 The results demonstrated strong fluorescence intensity in MCF-7/ADR tumors and suppressed tumor volume, showcasing the feasibility of acidic tumor microenvironment-initiated release using the NO generator.
Harnessing chemical reactions under certain pH values for precise drug release at the tumor region provides a good paradigm for intelligent drug delivery. However, due to the irregular concentration of reductants such as GSH and reactive oxygen species (ROS) in TME, and redox is the most common reaction in physiological environment. Efficiently releasing drugs over redox-responsive and ROS-responsive nanoplatforms offer additionally smart strategies for precise drug delivery.
Glutathione-Responsive Nanomedicines
GSH is a tripeptide composed of glutamate, cysteine, and glycine, primarily found in the cytoplasm of living cells, where it plays a crucial role in maintaining physiological redox homeostasis. When exposed to oxidizing agents, GSH readily undergoes oxidation, forming oxidized glutathione (GSSG). In normal cells, the concentration of GSH typically ranges from approximately 1–10 mM within the intracellular space, while in the extracellular environment, it remains at only 1–10 μM.57 However, in cancer cells, the GSH level can increase significantly, reaching levels as high as 4–40 mM, which renders them more reductive compared to the extracellular milieu.58–60 Perry’s group has compiled data on GSH levels in various tumors, proving to be valuable for the development and optimization of GSH-mediated delivery systems.61 According to their report, colorectal tumors exhibit a GSH level of approximately 90 nmol/mg protein, which is at least four times higher than that observed in ovarian, head and neck, lung, brain, and breast tumors. This high GSH level suggests that GSH-induced delivery systems might be particularly effective for treating colorectal cancer.61 Several nanocarriers, such as zwitterionic dextran nanoparticles, single-walled carbon nanotubes, chitosan-based nanoparticles, PEGylation micelles, and mesoporous silica nanoparticles, have been utilized in GSH-triggered delivery systems.62 These carriers have been thoughtfully designed to incorporate GSH-responsive moieties, ensuring stability in the bloodstream and tumor tissue until triggered by GSH. The differences in intracellular and extracellular GSH concentrations can be exploited to fine-tune the stability and targeting efficiency of the delivery system. Moreover, this concentration difference contributes to prolonged circulation time and controlled intracellular molecular release of the GSH-sensitive delivery system. Due to the unique properties of GSH and the diverse reduction-mediated breakage of linkers, GSH has undergone extensive investigation in the development of GSH-responsive carriers.
Based on the functional moieties of GSH-responsive delivery systems, they can be categorized into five main groups: disulfide, diselenide/ditelluride, thioether/selenide/tellurium, metal-thiol-based linkers, and ferrocenium. These moieties, along with their mechanisms and applications, have been summarized in Table 2.63–75 In this review, we will delve into the specifics of each of these moieties to gain a better understanding of their potential in GSH-responsive delivery systems.
 |
Table 2 Summary of GSH Responsive Drug Delivery Systems |
Disulfide
Disulfide bridges provide a highly convenient linkage that can be incorporated into GSH-responsive carriers, such as nanogels, micelles, polymersomes, and mesoporous silica NPs. Disulfide bonds can be easily formed through cost-effective and robust chemistries, such as L-cystine,80 di-thiodiglycolic acid anhydride,81 and pyridyl disulfide chemistry.82 In many strategies, cargoes are packaged within GSH-responsive carriers using disulfide bridges, which can be readily broken through intracellular thiol-disulfide exchange in GSH-rich environments. For instance, a nanogel (STP-NG) was constructed with sarcoma-targeting polypeptide-modified disulfide linkages, encapsulating the cargo shikonin.76 The STP-NG system effectively delivered shikonin to osteosarcoma tumors, inhibiting osteosarcoma progression. Within 72 hours, shikonin was released from the nanogel with an efficacy of approximately 98%, thanks to a 10.0 mM GSH-mediated disulfide bond (S-S) breakage. Moreover, the STP-NG selectively aggregated inside the tumor, leading to the inhibition of orthotopic 143B osteosarcoma progression and pulmonary metastasis. Ling’s group developed poly (disulfide amide)-based micelles to precisely deliver cisplatin into solid tumors.64 In this system, the high disulfide density consumed the elevated concentration of cytosolic GSH within tumor cells, facilitating tumor-specific cisplatin release and minimizing off-site toxicity. The poly (disulfide amide)-cisplatin micelles exhibited a significantly higher tumor inhibition rate (83%) compared to control micelles (1.5%) and free cisplatin (1.5%) in the A2780CIS tumor-bearing athymic nude mice model. Optimization of micelle properties, such as particle size, Pt loading capacity, and drug release, can be achieved by incorporating hydrophobic chemical groups, such as aromatic groups or alkyl chain lengths. A stomatocyte nanomotor system of poly (ethylene glycol)-SS-polystyrene (PEG-SS-PS) polymersomes was designed to deliver DOX in response to GSH.71 Nanomotors are nanoscale devices that convert energy into movement, with specific chemical signaling molecules functioning as energy sources. In the PEG-SS-PS-based stomatocyte nanomotors, Pt NPs catalysts were packaged inside the polymers, and DOX was encapsulated into the lumen of the stomatocytes. The H2O2 gradients from the human body drove the nanomotors to disease regions, where concentrated GSH initiated disulfide bond breakage, leading to PEG-shell peeling. Consequently, the nanomotor lost mobility and released the encapsulated drug into the lesions. However, the large size of PEG-SS-PS-based stomatocyte nanomotors (around 350 nm) limits their applications in drug delivery. Du’s group designed a GSH-sensitive delivery system based on poly (γ-glutamic acid) (γ-PGA)-coated MSNPs.66 In this design, the γ-PGA shell was formed via electrostatic interaction with the nontoxic poly(ethylenimine) (PEI), which remained stable at a neutral pH environment. At pH 5.0, the protonated γ-PGA dissociated from the cationic PEI. The cargo DOX was modified with an amino group to conjugate with the nanoparticle via the disulfide bond, which is crucial for GSH-mediated drug release. With the dual stimulation of pH and GSH, the poly (γ-glutamic acid) (γ-PGA)-coated MSNPs effectively delivered DOX into the cytoplasm. It has been proven that the carbonate linkage of disulfide bonds is more suitable for a quick response to GSH than the carbamate linkage. In conclusion, the convenience of disulfide bond formation has made it the most commonly used moiety for GSH stimulation. However, further modifications and integration with other functional moieties are urgently needed in the development of GSH-sensitive delivery systems.
Ditelluride/Diselenide Bond
Ditelluride and diselenide bonds exhibit greater responsiveness to various redox environments compared to disulfide bonds, with their energy levels following the order of Te-Te (149 kJ/mol) < Se-Se (192 kJ/mol) < S-S (240 kJ/mol). Consequently, ditelluride bonds hold significant potential in the development of redox-stimulated delivery systems, particularly for highly sensitive carriers. Wang’s group was at the forefront of reporting ditelluride-containing poly(ether-urethane) nanoparticles as a GSH-mediated drug delivery system.77 Specifically, ditelluride acted as the trigger for GSH-mediated release, rapidly reducing to tellurol by GSH (10 mM) within 5 minutes. DOX was attached to the NPs through hydrophobic interactions. Upon GSH-mediated reduction, the nanoparticles dissociated, releasing the cargo DOX into the environment. The tumor site accumulation and anti-tumor effects of the ditelluride-containing poly(ether-urethane) nanoparticles were demonstrated in the 4T1 tumor mice model. Diselenide-bond-based delivery systems have also been explored, with some exhibiting a more sensitive response to ROS stimulation. The lower energy of ditelluride and diselenide bonds has provided them with an advantage in GSH-mediated delivery systems. However, further investigations are still required to optimize the structural linkages of ditelluride/diselenide bonds, synthesize ditelluride/diselenide bond-based macromolecules, and explore their applications.
Thioether/Selenide/Tellurium Thioether-Based
Thioether-containing polymers are commonly synthesized through the Michael addition of thiols with maleimide, enabling easy dissociation via the concentrated GSH-mediated retro-Michael reaction and thiol exchange. Additionally, a thioether succinimide crosslinked hydrogel was constructed by integrating maleimide-functionalized liposomes with aryl thiol-functionalized 4-arm PEG polymers. The hydrogel’s diverse domains create separate compartments for DOX and cytochrome c (cyto-c). Upon treatment with GSH (10 mM), DOX and cyto-c simultaneously released through different mechanisms. The hybrid systems’ structural integrity and functionalities facilitate both the burst release of cargos and controlled sequential targeted delivery. In addition to GSH-stimulated release, thioether/selenide/tellurium-based delivery systems are also sensitive to H2O2 stimuli. For example, Luo’s group developed a series of redox-responsive materials with diverse linkages, including thioether, dithioether, and ester bonds.71 Paclitaxel (PTX) was conjugated to oleic acid (OA) with long unsaturated alkyl chains via thioether, dithioether, and ester bond-based linkages. These polymers were then assembled into nanoparticles, which were coated with tocopheryl polyethylene glycol 2000 succinate (TPGS 2k) to extend circulation time. Among these linkages, PTX-S-OA demonstrated the best performance in terms of speed and selectivity in releasing free PTX from the nanoparticles in response to redox stimuli.71 This suggests that the sulfur atom near the ester bond with the attached drug enhances redox-sensitivity. Furthermore, the PEGylated PTX-S-OA nanoparticles, with a drug loading of approximately 57.4%, effectively inhibited the proliferation of human epidermoid carcinoma xenografts. Overall, these findings underscore the potential of thioether-containing and other redox-responsive delivery systems as promising platforms for targeted drug delivery applications.
GSH Responsive NPs of Metal Ions
Polyvalent metal ions exhibit GSH responsiveness through redox reactions. Common used metal ions include Fe3+/Fe2+, Cu2+/Cu+, and Mn4+/Mn2+. Fe3+ is synchronized with GSH redox to form Fe2+ ions, which can further undergo a Fenton reaction with endogenous H2O2 in tumors to generate hydroxyl radicals (˙OH), thus inducing iron-mutagenic response-based tumor therapy. At the same time, Fe3+ ions act as oxidants to deplete GSH to prevent 1O2 quenching.83 Other multivalent metal ions containing Cu2+/Cu+ and Mn4+/Mn2+ also have similar functions to those of iron ions. GSH-responsive composites were synthesized by anchoring negatively charged BSA-modified MnO2 NPs to the surface of reduced graphene oxide nanosheets (rGO NSs) modified in polyethyleneimine. The MnO2 NPs were reduced to Mn2+ ions by intracellular GSH, which converted H2O2 to ˙OH through a Fenton-like reaction, and the photo-thermal under irradiation of near-infrared light (808 nm) conversion generates high temperatures that increase the Fenton reaction rate, and rGO NSs further kill tumor cells by photothermal therapy.78 An iron-copper co-doped PANI nanoparticle (Fe-Cu@PANI) was prepared, in which the Cu(II) in the nanoparticle could undergo a redox reaction with endogenous GSH in tumor tissues leading to the etching of the nanoparticle. With the increase of the concentration of GSH, the size of the nanoparticle gradually decreased, inducing the absorption spectrum of the nanoparticle to red-shift from the visible region to the near-infrared region, and activating the photo-acoustic imaging of the tumors and the photo-thermal therapy (PTT) simultaneously.79
Thus, GSH-responsive NPs of metal ions are promising for cancer therapy by taking advantage of the synergistic effect of PTT and the photothermal/delivery effect. Metal-organic frameworks (MOFs) have emerged as a novel therapy for the treatment of malignant tumors. Thus, another study developed a GSH-sensitive MO material to encapsulate an inhibitor of the immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO), and s-nitrosothiol groups (SNAP), a nitric oxide (NO) donor, to obtain the nanomedicine BMS-SNAP-MOF. In the presence of high concentrations of GSH, this MOF was effective in producing NO. IDO inhibitors and NO can synergistically modulate the immunosuppressive tumor microenvironment by increasing CD8+ T cells and decreasing Treg cells, resulting in an efficient immunotherapeutic effect.84 These moieties, along with their mechanisms and applications, have been summarized in Table 2.
ROS Stimulated Delivery Systems
ROS, such as H2O2, O2•, •OH, ONOO−, and OCl−, play a crucial role in cellular metabolic pathways. Various pathological conditions, including cancers, inflammations, diabetes, and neurodegenerative diseases, have been linked to oxidative stress and inflammatory events resulting from an imbalance in ROS levels. Among these ROS, H2O2 stands out as one of the most prevalent. It is present in significant concentrations in both cancer tissues (50–100 μM) and normal tissues (20 nM). However, the physiological concentration of H2O2 in cancer tissues is not sufficient to trigger drug release. Consequently, exogenously generated ROS can be employed to enable H2O2-mediated delivery systems. Two primary types of exogenous and biocompatible ROS generators are commonly used: photodynamic therapy (PDT) and ROS-producing agent treatments (Figure 4).85 Through the amplification of ROS concentration, drug release can be facilitated via amphiphilicity transition and bond cleavage mechanisms, which will be discussed in detail below.
 |
Figure 4 Illustration of ROS-sensitive drug delivery systems. |
Amphiphilicity Transition Models
Polymers containing hydrophobic mono-sulfide, mono-selenium, or mono-tellurium groups can undergo easy conversion into hydrophilic oxidative products using H2O2 (Figure 5).86,87 This process disrupts the amphiphilicity of the NPs, leading to the dissociation of the NPs and subsequent release of their cargo. Yang’s group has developed a ROS-responsive delivery system that integrates 6-maleimidocaproic acid (MAL) with PTX through a mono-sulfide linkage. The resulting PTX-S-MAL NPs self-assemble into uniform-sized nanoparticles, releasing their cargo in response to H2O2 concentrations exceeding 1 mM.86 Duvall’s group reported a curcumin delivery system based on sulfide-containing poly (propylene sulfide) (PPS) NPs.71 In this case, the hydrophobic PPS-based NPs were converted to hydrophilic poly (propylene sulfoxide) and poly (propylene sulfone) with H2O2 treatment at concentrations ranging from 0.5 to 500 mM. Although the systems discussed above displayed a good morphological hydrophobic-to-hydrophilic transition at high concentrations of H2O2, their application in controlled release at tumor sites has been limited due to the physiological concentrations of ROS (50–100 μM). Therefore, it is necessary to explore more sensitive moieties to ROS at endogenous concentrations or simultaneous ROS accumulation. Wang’s group constructed an ultra-sensitive ROS-responsive system that functions at a concentration of 100 μM. The H2O2-mediated hydrophobic-to-hydrophilic transition induces cargo release, and this process can be reversed using mild reductants, such as vitamin C.88 Another group also reported ultra-sensitive polymeric micelles with diselenide linkages, which exhibited a response to H2O2 at 100 μM. The delivery efficacy of these micelles was demonstrated to be 3.73-fold and 1.69-fold higher in terms of drug amount when compared with free drugs and their non-crosslinked counterparts in PC3 tumor-bearing mice, resulting in an efficient inhibition of tumor size.89 However, the diselenide-bond-based mesoporous silica nanoparticles (MSNPs) did not exhibit a preference in releasing efficacy upon GSH treatment (5 mM). Considering the different responses of diselenide-bond-based MSNPs to H2O2 and GSH, exploring this aspect might offer a new option to overcome tumor redox heterogeneity. Additionally, the effects of thioether-/selenoether-based delivery systems have been demonstrated in 4T1 murine breast subcutaneous tumor models. The anti-tumor effect, circulation time, and tumor distribution of both CTX and pyropheophorbide have been prolonged, indicating the synergistic potency of the dual-functional delivery system.
 |
Figure 5 ROS-responsive nano-drug delivery system. |
Bond Cleavage
Linkage bond cleavage can also be utilized for the dissociation of NPs through H2O2-mediated bond cleavage. Once the linkers are broken, the structure of the NPs is disrupted, leading to the release of the cargo. Commonly, boronic acid and poly(thioketal) are designed as bond cleavage moieties, which can be cleaved by H2O2-mediated oxidation. Wang’s group incorporated aryl boronic acid into a system for reversible anticancer protein restoration.90 Specifically, they used RNase A as the model protein, where lysine residues are crucial for its activity. The lysine residues were capped with aryl boronic esters (NBC) via a carbamate ester linker, rendering RNase A inactive. Additionally, the NBC-capped lysine residues facilitated the surface charge transition of RNase A from positive to negative, promoting co-assembly with cationic lipid NPs through electrostatic binding. Cationic lipids protected the NBC from degradation during circulation and facilitated internalization into cell membranes through electrostatic interactions. However, NPs with a positively charged surface still face the risk of elimination by the RES systems. The NBC-based delivery system requires further optimization to maintain the advantages while improving the drawbacks. Another H2O2-cleavable linkage is the thioketal bond. Farokhzad’s group developed a novel ROS-triggered mitoxantrone (MTO) delivery system containing a poly(thioketal)-based poly-prodrug.91 This delivery system consists of three key modules: a PEG outer shell to prolong circulation time, surface-attached internalizing RGD (iRGD) for tumor targeting and internalization, and a polyprodrug inner core modified with thioketal groups responsible for drug release upon ROS stimulation. The thioketal linkage can be cleaved in response to H2O2, resulting in cargo release and cancer cell inhibition. Well-known ROS-producing agents, such as copper ions,92 α-tocopheryl succinate (α-TOS),93 and palmitoyl ascorbate,94 have been integrated with therapeutic anticancer drugs within nanostructures. The ROS-producing mechanisms are discussed below. Copper ions can generate H2O2 through a Fenton-like reaction. α-TOS, a vitamin E analogue, can rapidly produce ROS intracellularly by interacting with mitochondrial respiratory complex II and interfering with the electron transport chain. The ascorbate group of palmitoyl ascorbate can be oxidized to ascorbate radicals, which form superoxide radicals through electron transfer. These superoxide radicals contribute to the production of tumoricidal H2O2. Zhang’s group reported a ROS-sensitive delivery system using positive feedback MSNPs loaded with α-TOS and DOX.95 In this case, β-cyclodextrin crystalline (β-CD) was conjugated on the surface of MSNPs via the thioketal linker. The MSNPs were also coated with adamantane-conjugated PEG (AD-PEG) to prolong circulation time. Upon internalization into cell membranes, endogenous ROS induced the opening of limited pores on the MSNPs, leading to the simultaneous release of α-TOS. The generated α-TOS-induced ROS further mediated the breakage of the thioketal linkers, resulting in an explosive release of DOX. Moreover, the newly generated ROS facilitated the production of more α-TOS, reinforcing the ROS-mediated thioketal linker cleavage. The delivery system efficiently released DOX at 100 μM H2O2 in vitro and exhibited remarkable antitumor effects in human breast cancer mouse models. The integration of nanostructures and ROS-generating moieties has significantly improved the properties of ROS-responsive delivery systems. The application of ROS-mediated delivery systems can be further expanded by exploring more suitable ROS-sensitive moieties, discovering novel cargos, and rationally substituting functional modules.
Enzymatic Activation
The tumor microenvironment exhibits elevated secretion of various enzymes, including proteases, peptidases, and lipases, each with specific chemical properties. Produced by tumor cells and stromal cells, these enzymes not only promote tumor growth, angiogenesis, invasion, and metastasis but also offer potential for controlled delivery of therapeutics or diagnostic agents. Leveraging the unique enzymatic environment within tumors, the enzyme-stimulated delivery system allows for the precise release of drugs or imaging agents in response to the tumor microenvironment. Notably, solid tumors overexpress enzymes such as proteases (eg, matrix metalloproteinase and cathepsin B),96,97 peptidases (eg, aminopeptidase),98,99 and lipases (eg, phospholipase A2),100 with their respective cleavage sites listed in Table 3.101–105
 |
Table 3 Enzymatic Activation for Tumor Microenvironment Drug Delivery |
Cathepsin B, a tumor-specific protease, has long been recognized as a key regulator of the tumor microenvironment and has been utilized as a trigger for various delivery systems. For example, when loaded the cathepsin B-sensitive moiety (GFLGK) onto the branches of polyHPMA copolymers. This dual functional delivery system involved covalent conjugation of the drug (DOX) to the copolymer via a pH-sensitive hydrazine bond.95 Upon reaching the tumor site, cathepsin B cleaved the GFLGK moiety, leading to the release of the DOX-hydrazine-HPMA polymer into the tumor microenvironment. Subsequently, the acidic pH of the tumor microenvironment triggered the breakage of the hydrazine bond, inducing conformational changes in HPMA and causing the release of DOX. This HPMA-based dual functional (enzyme- and pH-sensitive) delivery platform was successfully applied in 4T1 tumor models, demonstrating highly effective tumor targeting and suppression efficacy. Matrix metalloproteinase-2 enzymes, another tumor-specific protease family, show promise as initiators of tumor-targeting delivery systems. Fan’s group developed a gelatin nanoparticle armed with polyamidoamine (PAMAM) dendrimer-loaded methotrexate (MTX) to enhance tumor targeting and antitumor effects.108 The gelatin nanoparticles were designed to be susceptible to the concentrated matrix metalloproteinase-2 enzymes in the tumor microenvironment, leading to their breakdown and subsequent release of PAMAM dendrimers into the tumor. This sequential drug release to the tumor site improved the antitumor effect while minimizing cytotoxicity to normal tissues. The smart nanoscale drug delivery and imaging platform (NDDIP) delivery system has been successfully applied by Chau and Rastegari’s group and Zhong’s group in tumor-specific release during cancer therapies.106,107 Enzyme-triggered nanocarriers not only facilitate drug delivery but also offer the potential for delivering imaging agents for fluorescence or radiography with high spatial resolution. Tumor-specific enzymes, as ignition triggers of the delivery system, have significant potential for broad development as completely biocompatible agents with minimal toxicity to normal tissues. When integrated with other tumor environment-specific strategies, tumor-specific enzymes become crucial in the fields of anticancer therapies and imaging.
Hypoxic Activation
The entire tumor tissue is often in a hypoxic state due to the abnormal vascular network inside solid tumors and the high oxygen consumption of malignant proliferating tumor cells. Tumor cells in the hypoxic region tend to proliferate slowly compared to their well-oxygenated counterparts, making them relatively resistant to traditional anti-cancer drugs. Additionally, hypoxia can promote the emergence of cell variants with lethal mutations or acquired resistance to traditional therapies.109 Therefore, there is an urgent need to develop more efficient delivery systems or drugs that can target hypoxic solid tumors. The hypoxic property of solid tumors holds the potential for a tumor-specific trigger. Hypoxia-activated delivery systems targeting solid tumors have attracted interest from both the industry and academic scientists. Lin et al constructed a selective photorelease system using chitosan nanoparticles, in which the coumarin phototrigger incorporated nitroimidazole as an electron acceptor. Only under unique tumor-hypoxic physiological conditions, the phototrigger can be unlocked by bioreduction, thereby releasing the drug load under excitation by either single-photon visible light or two-photon near-infrared light. This approach distinguishes highly anticancer effects on hypoxic tumor cells rather than healthy normal cells (Figure 6).110 Hypoxia-activated prodrugs (HAP) have been extensively studied in the field of hypoxia-targeted cancer therapy, with several HAP candidates now in clinical trials. One notable example is TH-302, which is in Phase 3 clinical trials under a Special Protocol Assessment with the United States Food and Drug Administration, developed by Threshold Pharmaceuticals.111 In this system, nitroimidazole serves as the hypoxia-responsive electron acceptor, leading to nanoparticle disassembling via a hydrophobic to hydrophilic transition. The second functional moiety is the coumarin-cage, which is conjugated with drugs in glycol-chitosan nanoparticles. Coumarin dyes are usually quenched via photoinduced electron transfer (PET) to the nitroimidazole electron acceptor in normal tissues. However, the reduction of the nitroimidazole to an amine eliminates the PET quenching and initiates the cleavage of the drug-coumarin linkage under hypoxic environments. In addition to excitation with short-wavelength lights (UV to blue excitation light sources), two-photon excitation at 800 nm has been shown to effectively break the drug-coumarin linker. Although excitation efficacy is sacrificed, penetration depth within tissues is greatly improved by excitation at 800 nm. Considering that hypoxia is usually deeper inside solid tumors, long-wavelength excitation could ensure tissue penetration and the efficacy of drug release within the tumor. The second well-studied strategy for hypoxia-mediated delivery systems is based on the azobenzene group, which is fragile under hypoxic conditions and becomes positively charged. Torchilin’s group has utilized the azobenzene group to construct hypoxia-sensitive siRNA nanoparticles. In this system, the azobenzene group serves as the linker between PEG and polyethyleneimine (PEI) polymer segments.112 When the nanocarriers enter the oxygen-deprived environment inside the solid tumor, the azobenzene bond is cleaved, and the PEG surface is removed. After hypoxia-mediated azobenzene cleavage, the remaining PEI/siRNA complex becomes positively charged, facilitating easy intracellular uptake. The efficacy of tumor targeting and release of PEI/siRNA nanocarriers has been demonstrated both in vitro and in vivo. The heterogeneity and complexity of solid tumors have made it challenging to deliver anticancer drugs or imaging agents into hypoxic regions. Nanoparticle systems have been considered powerful tools for the delivery of both small molecules and biomacromolecules. However, the insufficient diffusion inside solid tumors has limited their applications in cancer therapy and diagnosis. Therefore, hypoxia-responsive nanocarriers hold the potential to deliver anticancer drugs or imaging agents with high diffusion rates and tumor targeting efficacy.
 |
Figure 6 Schematic illustration of a phototrigger that specifically releases drugs under tumor-hypoxic physiological conditions. |
ATP- Responsive Drug Delivery Systems
As mentioned above, pH and redox species are utilized for stimulating drug release based on chemical reactions breaking the bonding linkage between the carrier and drug. In addition to these chemistry-related process, energy-related process might also be made the best of due to the drastic differences of ATP concentrations between TME and the microenvironment in normal tissues.
ATP plays a pivotal role in cellular signaling and metabolism. Intracellularly, ATP concentrations vary significantly, ranging from 1 to 10 mM, while extracellularly, they remain below 0.4 mM. Interestingly, in some tumor tissues, the ATP concentration is substantially higher compared to normal tissues, displaying a 1.2-fold increase in cancer cells.113 Recent reports have illuminated four commonly used delivery systems that rely on ATP-sensitive moieties: Zinc-dipicolylamine (TDPA-Zn2+),114 phenylboronic acid-sugar-functional polymers,115 ATP-consuming enzymes,116 and ATP aptamers.117 In the following sections, we will explore the application of these modules in ATP-sensitive delivery systems.
Zinc-Dipicolylamine (TDPA-Zn2+) Based
Zinc-dipicolylamine (TDPA-Zn2+) possesses a unique ability to coordinate with oligo-aspartate containing polypeptides, however, this coordination can be disrupted by intracellular ATP. TDPA-Zn2+ exhibits a higher binding affinity for ATP compared to the oligo-aspartate containing polypeptides. Taking advantage of this property, TDPA-Zn2+ was employed to coordinate peptides into upconversion nanoparticles with a mesoporous-silica shell (UCNP@MSN), and these functional nanoparticles were utilized for ATP-stimulated drug delivery.114 In this case, the UCNP@MSNs could be excited at 980 nm, resulting in a distinct emission in the UV-NIR region. The emission of UCNPs in the UV-visible region was quenched due to the multivalent coordination of the UCNP shell, peptide, and TDPA-Zn2+, but exhibited strong NIR emission. DOX served as both the encapsulated cargo and the acceptor for luminescence resonance energy transfer (LRET) from the UCNPs (donor) in the UV-visible range. The TDPA-Zn2+-peptide-UCNP complex with DOX trapped inside was applied in tumor models, demonstrating efficient drug release and a difference in luminescence and UV-Vis emission. Besides the therapeutic effect of the released DOX, the changes in LRET and UV-Vis emission could also be utilized to dynamically monitor drug release.
Phenylboronic Acid-Sugar-Functional Polymers
The unique chemical properties of phenylboronic acid (PBA) make it an exceptionally attractive moiety for on-demand (pH or/and ATP-sensitive) delivery systems. Its ability to undergo reversible ester formation with diols provides excellent circulation stability and controlled release characteristics. Moreover, PBA-ATP conjugation demonstrates favorable thermodynamic stability, readily forming a bond with ATP modified with a cis-diol moiety in its ribose ring and PBA with diols. Additionally, PBA acts as a potential ligand for sialyl epitopes overexpressed on the surface of cancer cells. Furthermore, PBA exhibits sensitivity to pH below its pKa, enabling a seamless transition to a hydrophilic state. Naito’s group has published a study on a PBA-functionalized polyion complex micelle that effectively encapsulates the therapeutic agent “siRNA” as cargo.115 In this system, the micelle scaffold was constructed using cationic poly (ethylene glycol)-block-poly(L-lysine) (PEG-b-pLys) with terminal lysine residues, to which 3-fluoro-4-carboxyphenyl boronic acid (FPBA) was conjugated via carbodiimide chemistry. The functionalized cationic polymers were then packaged with siRNA and assembled into micelles. These siRNA-encapsulated micelles remained stable at extracellular ATP levels but disassembled at the intracellular concentration of ATP, facilitating the release of siRNA into the cytosol. Similarly, a comparable strategy was employed to successfully deliver an anti-angiogenic gene (pDNA) into MCF-7-xenografted mice using PBA-galactose grafted poly(ethylenimine) (PEI) copolymers.118 Furthermore, a cationic PEI-PBA-alginate structure (CrossPPA)/siRNA NPs strategy proved effective in delivering and releasing siRNA into 4T1 tumor-bearing mice.119 The feasibility and efficacy of PBA-based delivery and release have been demonstrated in multiple delivery systems and cargos.
ATP Consuming Enzymes Based
ATP-consuming enzymes can also serve as triggers for ATP-sensitive delivery systems, based on the binding or hydrolysis of ATP to responsive proteins. One such enzyme is the chaperone GroEL, which can capture denatured proteins and hydrolyze ATP to ADP. Aida’s group utilized GroEL to construct an ATP-responsive delivery system. In this system, GroEL contains cysteine residues that coordinate with merocyanine (MC)-Mg2+-MC, resulting in the formation of nanotubes (NB).116 Cargos were then encapsulated within the NBs. The conformation of ATP-bound GroEL differed from apo-GroEL, leading to the disruption of MC-Mg2+-MC interaction. Consequently, the NBs would dissociate, releasing the cargos. Furthermore, this system could be further modified with boronic acid to improve cell membrane permeability. The delivery study of the system in the HeLa xenograft model indicated that the GroEL-based delivery system exhibited preferential accumulation at the tumor site and intracellular ATP-induced cargo release. In addition to utilizing natural ATP protein carriers, another approach involves a polymer that responds to ATP, composed of poly(ethylene oxide)-b-polymethacrylate (PEO45-b-PHM126) and β-CD, serving as the backbone and macrocyclic pendant, respectively.120 Short bi-guanidine spacers crosslinked the copolymer to form a pocket-like polymer receptor, acting as the ATP habitat. The ATP/polymer complexes formed, resulting in a decrease in ATP in the hydrophilic site. Additionally, the ATP/polymer hybrid transitions between an aggregate and disassembled state in response to phosphatase. The integration of the ATP-binding polymer and phosphatase into the multifunctional polymer offers novel options for ATP-sensitive delivery systems.
ATP Aptamers-Based
ATP aptamers are single-stranded DNA (ssDNA) molecules with a high affinity for ATP, typically identified through in vitro screening from a large pool of random ssDNA sequences. These aptamers are characterized by rich “GC” pairs and negatively charged surfaces, enabling the encapsulation of multiple anticancer agents and cationic polymers. Due to their short sequences (around 30 bases), they can be easily modified to specifically respond to ATP, making ATP aptamers the most popular module for ATP-sensitive delivery systems. Moreover, ATP aptamers have the ability to form DNA duplexes through complementary base pairing, offering potential as habitat nanocarriers. These unique physical and chemical features have established ATP aptamers as popular modules for ATP-sensitive delivery systems for a considerable time.117 An ATP aptamer-based dual delivery system was developed to simultaneously deliver DOX and siRNA. In this system, GC-rich DNA duplexes provided a cavity for DOX, while cationic PEI condensed the negatively charged DOX duplexes and miRNA. Ultimately, the ternary nanocomplex of PEI/DOX-Duplex/siRNA formed, displaying synergistic effects in cancer treatment.121 Additionally, ATP aptamers serve as ATP-sensitive gatekeepers for drug release in porous materials.122 For example, Chen’s group constructed a MOF nanoparticle with Zr4+ and triphenyl dicarboxylic acid (TPDC) terminated with an amino group. ATP aptamers were conjugated to the MOF nanoparticle through DBCO modification and the azide group from the terminal amino group.123 Another ingenious design by Gu et al involved a nanogel with a DNA duplex core coated with HA, responding to ATP stimuli. The DNA duplex, rich in “GC” pairs and negatively charged, formed a cationic core complex with the positively charged protamine (a cell-penetrating peptide) via electrostatic interaction.124 The core complex acted as the cavity for DOX and mediated DOX release in response to the formation of aptamer/ATP complexes. Anionic HA formed the outer shell and facilitated complex intracellular delivery. The binding of HA to tumor-specific overexpressed receptors (eg, CD44 and RHAMM) enhanced tumor targeting. The system’s high selectivity for intra- and extracellular environments resulted in enhanced anti-tumor effects, demonstrated in both in vitro and in vivo experiments. The same group also developed an advanced co-delivery system by introducing exogenous ATP to accelerate drug release. Similar to the previous design, the system consisted of a negatively charged duplex loaded with DOX and coated with the positively charged protamine peptide. A second liposome, featuring fusogenic dioleoyl phosphatidylethanolamine (DOPE), was integrated into the delivery system, acting as the releasing accelerator. Once the dual-liposome system was recognized by tumor cell membrane receptors and internalized, the acidity of endocytic vesicles triggered a releasing cascade, accelerating DOX release in the cytosol. This dual-functional system combined targeting, ATP responsivity, therapeutic agents, and exogenous ATP generation.125 Its dual functionality and excellent anti-cancer efficacy were demonstrated in cancer xenograft nude mice, outperforming the control and the original design. Another ATP-sensitive delivery system was composed of a CaCO3 or SiO2-based core and an aptamer-modified polymer shell.126 The CaCO3 or SiO2-based core could form microcapsules after dissolving with EDTA. Meanwhile, aptamer-modified polymers crosslinked different polymer layers to the shell, releasing the drug upon ATP stimulation. Considering the diversity of sequence-specific carriers, ease of modification, and the urgent need for selectively delivered payloads, further investigation of more ATP aptamer-based delivery systems and the expansion of their application fields are crucial.
Conclusion and Perspectives
Nanocarriers within a specific size range of 20 to 200 nm demonstrate active transcytosis or passive targeting to tumor sites through the enhanced permeability and retention effect (EPR).127 This characteristic leads to improved therapeutic efficacy and reduced toxic side effects. Tumors possess distinct microenvironmental characteristics, including high levels of L-Glutathione (GSH), low pH values, and hypoxia. Stimulus-responsive nano-drug delivery systems, which are specifically engineered to respond to the TME, exhibit enhanced accumulation at the tumor site via the EPR effect. As a result, the drugs exhibit selective release in response to the tumor microenvironment, leading to enhanced utilization of chemotherapeutic agents by tumor cells. This mechanism ultimately improves the effectiveness of therapy while simultaneously reducing the occurrence of adverse effects. This comprehensive review provides an overview of nanocarriers developed for TME responses, specifically acidity and hypoxia, and highlights their biomedical applications. Despite the promising potential of TME-responsive nanoplatforms, their practical application still faces challenges, particularly concerning the safety of nanomaterials, which limits their clinical use. To address these concerns, advanced strategies involving biomimetic nanoparticles, such as exosomes, cell membrane-encapsulated nanoparticles (CNPs), and virus-like particles (VLPs), have emerged, showing promise in enhancing the safety of carriers. Notably, exosomes play a pivotal role as signaling mediators in regulating the TME, and several studies have explored their engineering for targeted drug delivery in cancer therapeutics.128–131 Most of the current TME-responsive nanocarriers only target individual elements of the tumor microenvironment and lack the ability to target the entire tumor microenvironment at the same time, thus there is a need to develop multilevel responsive nanodelivery systems in the future. With the deeper understanding of TME, synergistic multifunctional tumor stimulation-responsive drug delivery systems targeting the microenvironmental response in effective combination with other therapeutic modalities, such as chemotherapy, photothermal therapy, and immunotherapy, have also attracted much attention. These innovative multimodal combination therapy nanoplatforms can effectively realize targeted drug delivery and smart release function, which is an important direction for the development of responsive nanodrug delivery systems in the future.
Despite the significant progress in stimuli-responsive nano drug delivery system particles for tumor therapy, serious challenges still exist. From animal experiments to clinical applications, a series of problems still need to be solved, especially the animal model does not simulate human characteristics, but of course we can use tumor-like organ models to make up for the shortcomings of the traditional tumor models, which can better reproduce the in vivo characteristics of primary tumors and become a reliable model for simulating human tumors. Secondly, individual differences of patients, different stages of disease development and heterogeneity of tumor cells may produce different drug delivery efficiencies. Since the expression and distribution of specific stimulus sources in TME are highly heterogeneous and dynamically variable, which may lead to the occurrence of unexpected stimulus response processes. In addition, the biosafety of nanodelivery systems greatly affects their clinical translation. When designing TME-responsive nano-delivery systems, biocompatibility, scaled-up production during clinical translation, quality controllability, and production cost should be prioritized. The activation of anti-tumor immune response of the body is systemic, and when multiple combined delivery is performed, not only the generation of therapeutic efficacy should be focused on, but also the possible toxic side effects, such as cytokine storm, should also be considered. In conclusion, although facing the above problems, it is believed that with the development of tumor immunology, pathophysiology, materials science and other interdisciplinary disciplines, the combination of TME-responsive nano-delivery systems will bring a major breakthrough in the clinical treatment of tumors in the near future.
Abbreviations
TME, the tumor microenvironment; DOX, doxorubicin; EPR, enhanced permeability and retention effect; ODNs, oligodeoxynucleotides; ZnO, zinc oxide; CaCO3, calcium carbonate; Ce6, chlorin e6; CHEMS, cholesteryl hemisuccinate; NPs, nanoparticles; OVA, ovalbumin; MOFs, metal-organic frameworks; CPT, camptothecin; OA, oleic acid; MTO, mitoxantrone; HAP, Hypoxia-activated prodrugs; LRET, luminescence resonance energy transfer; PBA, phenylboronic acid.
Acknowledgments
The study was supported by the National Natural Science Foundation of China (Project no.81902777, 82002936), Guangdong Basic and Applied Basic Research Foundation (2024A1515010627), Shenzhen Science and Technology Innovation Committee (Project no. JCYJ20210324103005014, ZDSYS201707281114196, KCXFZ20201221173413038, JCYJ20190806163209126, JCYJ20180508152528735 and LCYSSQ20220823091403007), Medical-Engineering Interdisciplinary Research Foundation of ShenZhen University (Project no.2023YG003), Shenzhen Otorhinolaryngology Clinical Research Center (Project no.20220819120540004-010).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bejarano L, Jordāo MJC, Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 2021;11(4):933–959. doi:10.1158/2159-8290.CD-20-1808
2. de Visser KE, Joyce JA. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41(3):374–403. doi:10.1016/j.ccell.2023.02.016
3. Qin X, Li T, Li S, et al. The tumor biochemical and biophysical microenvironments synergistically contribute to cancer cell malignancy. Cell Mol Immunol. 2020;17(11):1186–1187. doi:10.1038/s41423-019-0282-5
4. Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79(18):4557–4566. doi:10.1158/0008-5472.CAN-18-3962
5. Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–550. doi:10.1038/s41591-018-0014-x
6. Jin MZ, Jin WL. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 2020;5(1):166. doi:10.1038/s41392-020-00280-x
7. Gong F, Yang N, Wang X, et al. Tumor microenvironment-responsive intelligent nanoplatforms for cancer theranostics. Nano Today. 2020;32:100851. doi:10.1016/j.nantod.2020.100851
8. Liberti MV, Locasale JW. The warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41(3):211–218. doi:10.1016/j.tibs.2015.12.001
9. Wike-Hooley JL, Haveman J, Reinhold HS. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984;2(4):343–366. doi:10.1016/S0167-8140(84)80077-8
10. Koltai T. The Ph paradigm in cancer. Eur J Clin Nutr. 2020;74(S1):14–19. doi:10.1038/s41430-020-0684-6
11. Kato Y, Ozawa S, Miyamoto C, et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013;13(1):89. doi:10.1186/1475-2867-13-89
12. Jiang Y, Zhou Y, Zhang CY, Fang T. Co-delivery of paclitaxel and doxorubicin by ph-responsive prodrug micelles for cancer therapy. Int J Nanomed. 2020;15:3319–3331. doi:10.2147/IJN.S249144
13. Dechsri K, Suwanchawalit C, Chitropas P, et al. Rapid microwave-assisted synthesis of pH-sensitive carbon-based nanoparticles for the controlled release of doxorubicin to cancer cells. AAPS Pharm Sci Tech. 2023;24(5):135. doi:10.1208/s12249-023-02593-w
14. Xia F, Hou W, Zhang C, et al. pH-responsive gold nanoclusters-based nanoprobes for lung cancer targeted near-infrared fluorescence imaging and chemo-photodynamic therapy. Acta Biomater. 2018;68:308–319. doi:10.1016/j.actbio.2017.12.034
15. Feng Q, Shen Y, Fu Y, et al. Self-assembly of gold nanoparticles shows microenvironment-mediated dynamic switching and enhanced brain tumor targeting. Theranostics. 2017;7(7):1875–1889. doi:10.7150/thno.18985
16. Zhou P, Qin J, Zhou C, et al. Multifunctional nanoparticles based on a polymeric copper chelator for combination treatment of metastatic breast cancer. Biomaterials. 2019;195:86–99. doi:10.1016/j.biomaterials.2019.01.007
17. Wang D, Wang T, Liu J, et al. Acid-activatable versatile micelleplexes for PD-L1 blockade-enhanced cancer photodynamic immunotherapy. Nano Lett. 2016;16(9):5503–5513. doi:10.1021/acs.nanolett.6b01994
18. Hsu CW, Hsieh MH, Xiao MC, Chou YH, Wang TH, Chiang WH. pH-responsive polymeric micelles self-assembled from benzoic-imine-containing alkyl-modified PEGylated chitosan for delivery of amphiphilic drugs. Int J Biol Macromol. 2020;163:1106–1116. doi:10.1016/j.ijbiomac.2020.07.110
19. Park S, Lee WJ, Park S, Choi D, Kim S, Park N. Reversibly pH-responsive gold nanoparticles and their applications for photothermal cancer therapy. Sci Rep. 2019;9(1):20180. doi:10.1038/s41598-019-56754-8
20. Zhai L, Luo C, Gao H, Du S, Shi J, Wang F. A dual pH-responsive DOX-encapsulated liposome combined with glucose administration enhanced therapeutic efficacy of chemotherapy for cancer. Int J Nanomed. 2021;16:3185–3199. doi:10.2147/IJN.S303874
21. Zhao Y, Luo Z, Li M, et al. A preloaded amorphous calcium carbonate/doxorubicin@silica nanoreactor for pH-responsive delivery of an anticancer drug. Angew Chem Int Ed Engl. 2015;54(3):919–922. doi:10.1002/anie.201408510
22. Chen Y, Ye D, Wu M, et al. Break-up of two-dimensional MnO2 nanosheets promotes ultrasensitive ph-triggered theranostics of cancer. Adv Mater. 2014;26(41):7019–7026. doi:10.1002/adma.201402572
23. Wang Y, Song S, Liu J, Liu D, Zhang H. ZnO-functionalized upconverting nanotheranostic agent: multi-modality imaging-guided chemotherapy with on-demand drug release triggered by pH. Angew Chem Int Ed Engl. 2015;54(2):536–540. doi:10.1002/anie.201409519
24. Shi X, Ma X, Hou M, et al. pH-Responsive unimolecular micelles based on amphiphilic star-like copolymers with high drug loading for effective drug delivery and cellular imaging. J Mater Chem B. 2017;5(33):6847–6859. doi:10.1039/C7TB01477E
25. Sethuraman VA, Lee MC, Bae YH. A biodegradable pH-sensitive micelle system for targeting acidic solid tumors. Pharm Res. 2008;25(3):657–666. doi:10.1007/s11095-007-9480-4
26. Piao JG, Gao F, Li Y, et al. pH-sensitive zwitterionic coating of gold nanocages improves tumor targeting and photothermal treatment efficacy. Nano Res. 2018;11(6): 31933204 doi:10.1007/s12274-017-1736-7.
27. Yang X, Grailer JJ, Rowland IJ, et al. Multifunctional stable and pH-responsive polymer vesicles formed by heterofunctional triblock copolymer for targeted anticancer drug delivery and ultrasensitive MR imaging. ACS Nano. 2010;4(11):6805–6817. doi:10.1021/nn101670k
28. Schlossbauer A, Dohmen C, Schaffert D, Wagner E, Bein T. pH-responsive release of acetal-linked melittin from SBA-15 mesoporous silica. Angew Chem Int Ed Engl. 2011;50(30):6828–6830. doi:10.1002/anie.201005120
29. Wu W, Yu X, Sun J, et al. Zeolitic Imidazolate Framework (ZIF-8) decorated iron oxide nanoparticles loaded doxorubicin hydrochloride for osteosarcoma treatment - in vitro and in vivo preclinical studies. Int J Nanomed. 2023;18:7985–7999. doi:10.2147/IJN.S438771
30. Zheng H, Zhang Y, Liu L, et al. One-pot synthesis of metal-organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J Am Chem Soc. 2016;138(3):962–968. doi:10.1021/jacs.5b11720
31. Sun X, Du R, Zhang L, et al. A pH-responsive yolk-like nanoplatform for tumor targeted dual-mode magnetic resonance imaging and chemotherapy. ACS Nano. 2017;11(7):7049–7059. doi:10.1021/acsnano.7b02675
32. Liu J, Ma H, Wei T, Liang XJ. CO2 gas induced drug release from pH-sensitive liposome to circumvent doxorubicin resistant cells. Chem Commun. 2012;48(40):4869–4871. doi:10.1039/c2cc31697h
33. Chung MF, Liu HY, Lin KJ, Chia WT, Sung HW. A pH-Responsive carrier system that generates NO bubbles to trigger drug release and reverse P-glycoprotein-mediated multidrug resistance. Angew Chem Int Ed Engl. 2015;54(34):9890–9893. doi:10.1002/anie.201504444
34. Wei D, Sun Y, Zhu H, Fu Q. Stimuli-responsive polymer-based nanosystems for cancer theranostics. ACS nano. 2023;17(23):23223–23261. doi:10.1021/acsnano.3c06019
35. Ahn B, Lee S-G, Yoon HR, et al. Four-fold channel-nicked human ferritin nanocages for active drug loading and pH-responsive drug release. Angew Chem Int Ed Engl. 2018;57(11):2909–2913. doi:10.1002/anie.201800516
36. Cheng CJ, Bahal R, Babar IA, et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature. 2015;518(7537):107–110. doi:10.1038/nature13905
37. Jia N, Ye Y, Wang Q, et al. Preparation and evaluation of poly(l-histidine) based pH-sensitive micelles for intracellular delivery of doxorubicin against MCF-7/ADR cells. Asian J Pharm Sci. 2017;12(5):433–441. doi:10.1016/j.ajps.2017.05.007
38. Kim J, Lee YM, Kang Y, Kim WJ. Tumor-homing, size-tunable clustered nanoparticles for anticancer therapeutics. ACS Nano. 2014;8(9):9358–9367. doi:10.1021/nn503349g
39. Xiao X, Liang S, Zhao Y, et al. Core-shell structured 5-FU@ZIF-90@ZnO as a biodegradable nanoplatform for synergistic cancer therapy. Nanoscale. 2020;12(6):3846–3854. doi:10.1039/C9NR09869K
40. Zheng C, Wang Y, Phua SZF, Lim WQ, Zhao Y. ZnO-DOX@ZIF-8 core-shell nanoparticles for pH-responsive drug delivery. ACS Biomater Sci Eng. 2017;3(10):2223–2229. doi:10.1021/acsbiomaterials.7b00435
41. Lee ES, Shin HJ, Na K, Bae YH. Poly(L-histidine)-PEG block copolymer micelles and pH-induced destabilization. J Control Release. 2003;90(3):363–374. doi:10.1016/S0168-3659(03)00205-0
42. Ling D, Park W, Park SJ, et al. Multifunctional tumor pH-sensitive self-assembled nanoparticles for bimodal imaging and treatment of resistant heterogeneous tumors. J Am Chem Soc. 2014;136(15):5647–5655. doi:10.1021/ja4108287
43. Licciardi M, Tang Y, Billingham NC, Armes SP, Lewis AL. Synthesis of novel folic acid-functionalized biocompatible block copolymers by atom transfer radical polymerization for gene delivery and encapsulation of hydrophobic drugs. Biomacromolecules. 2005;6(2):1085–1096. doi:10.1021/bm049271i
44. Jiang X, Wang J, Zheng X, et al. Intratumoral administration of STING-activating nanovaccine enhances T cell immunotherapy. J Immunother Cancer. 2022;10(5):e003960. doi:10.1136/jitc-2021-003960
45. Luo M, Wang H, Wang Z, et al. A STING-activating nanovaccine for cancer immunotherapy. Nat Nanotechnol. 2017;12(7):648–654. doi:10.1038/nnano.2017.52
46. Han SK, Na K, Bae YH. Sulfonamide based pH-sensitive polymeric micelles: physicochemical characteristics and pH-dependent aggregation. Colloids Surface A. 2003;214(1–3):49–59. doi:10.1016/S0927-7757(02)00389-8
47. Zhuang J, Kuo CH, Chou LY, Liu DY, Weerapana E, Tsung CK. Optimized metal-organic-framework nanospheres for drug delivery: evaluation of small-molecule encapsulation. ACS Nano. 2014;8(3):2812–2819. doi:10.1021/nn406590q
48. Reshetnyak YK, Andreev OA, Segala M, Markin VS, Engelman DM. Energetics of peptide (pHLIP) binding to and folding across a lipid bilayer membrane. Proc Natl Acad Sci U S A. 2008;105(40):15340–15345. doi:10.1073/pnas.0804746105
49. Andreev OA, Engelman DM, Reshetnyak YK. Targeting diseased tissues by pHLIP insertion at low cell surface pH. Front Physiol. 2014;5:97. doi:10.3389/fphys.2014.00097
50. Andreev OA, Karabadzhak AG, Weerakkody D, Andreev GO, Engelman DM, Reshetnyak YK. pH (low) insertion peptide (pHLIP) inserts across a lipid bilayer as a helix and exits by a different path. Proc Natl Acad Sci U S A. 2010;107(9):4081–4086. doi:10.1073/pnas.0914330107
51. Andreev OA, Dupuy AD, Segala M, et al. Mechanism and uses of a membrane peptide that targets tumors and other acidic tissues in vivo. Proc Natl Acad Sci U S A. 2007;104(19):7893–7898. doi:10.1073/pnas.0702439104
52. Macholl S, Morrison MS, Iveson P, et al. In vivo pH imaging with (99m)Tc-pHLIP. Mol Imaging Biol. 2012;14(6):725–734. doi:10.1007/s11307-012-0549-z
53. Daumar P, Wanger-Baumann CA, Pillarsetty N, et al. Efficient (18) F-labeling of Large 37-amino-acid pHLIP peptide analogues and their biological evaluation. Bioconjug Chem. 2012;23(8):1557–1566. doi:10.1021/bc3000222
54. Vāvere AL, Biddlecombe GB, Spees WM, et al. A novel technology for the imaging of acidic prostate tumors by positron emission tomography. Cancer Res. 2009;69(10):4510–4516. doi:10.1158/0008-5472.CAN-08-3781
55. An M, Wijesinghe D, Andreev OA, Reshetnyak YK, Engelman DM. pH-(low)-insertion-peptide (pHLIP) translocation of membrane impermeable phalloidin toxin inhibits cancer cell proliferation. Proc Natl Acad Sci U S A. 2010;107(47):20246–20250. doi:10.1073/pnas.1014403107
56. Yang Z, Chen H. Recent deveolpment of multifunctional responsive gas-releasing nanoplatforms for tumor therapeutic application. Nano Res. 2023;16(3):3924–3938. doi:10.1007/s12274-022-4473-5
57. Cui C-Y, Li B, Su X-C. Real-time monitoring of the level and activity of intracellular glutathione in live cells at atomic resolution by 19F-NMR. ACS Cent. Sci. 2023;9(8):1623–1632. doi:10.1021/acscentsci.3c00385
58. Raza A, Hayat U, Rasheed T, Bilal M, Iqbal HMN. Redox-responsive nano-carriers as tumor-targeted drug delivery systems. Eur J Med Chem. 2018;157:705–715. doi:10.1016/j.ejmech.2018.08.034
59. Niu B, Liao K, Zhou Y, et al. Application of glutathione depletion in cancer therapy: enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials. 2021;277:121110. doi:10.1016/j.biomaterials.2021.121110
60. Ding Y, Pan Q, Gao W, Pu Y, Luo K, He B. Reactive oxygen species-upregulating nanomedicines towards enhanced cancer therapy. Biomater. Sci. 2023;11(4):1182–1214. doi:10.1039/d2bm01833k
61. Perry RR, Mazetta JA, Levin M, Barranco SC. Glutathione levels and variability in breast tumors and normal tissue. Cancer. 1993;72(3):783–787. doi:10.1002/1097-0142(19930801)72:3<783::AID-CNCR2820720325>3.0.CO;2-U
62. Taleghani AS, Nakhjiri AT, Khakzad MJ, et al. Mesoporous silica nanoparticles as a versatile nanocarrier for cancer treatment: A review. J Mol Liq. 2021;328:115417. doi:10.1016/j.molliq.2021.115417
63. Sierra-Mondragon E, Molina-Jijon E, Namorado-Tonix C, Rodríguez-Muñoz R, Pedraza-Chaverri J, Reyes JL. All-trans retinoic acid ameliorates inflammatory response mediated by TLR4/NF-κB during initiation of diabetic nephropathy. J Nutr Biochem. 2018;60:47–60. doi:10.1016/j.jnutbio.2018.06.002
64. Ling X, Chen X, Riddell IA, et al. Glutathione-Scavenging poly(disulfide amide) nanoparticles for the effective delivery of Pt(IV) prodrugs and reversal of cisplatin resistance. Nano Lett. 2018;18(7):4618–4625. doi:10.1021/acs.nanolett.8b01924
65. Tu Y, Peng F, White PB, Wilson DA. Redox-sensitive stomatocyte nanomotors: destruction and drug release in the presence of glutathione. Angew Chem Int Ed Engl. 2017;56(26):7620–7624. doi:10.1002/anie.201703276
66. Du X, Xiong L, Dai S, Qiao SZ. γ-PGA-coated mesoporous silica nanoparticles with covalently attached prodrugs for enhanced cellular uptake and intracellular GSH-responsive release. Adv Healthc Mater. 2015;4(5):771–781. doi:10.1002/adhm.201400726
67. Sun B, Luo C, Yu H, et al. Disulfide bond-driven oxidation- and reduction-responsive prodrug nanoassemblies for cancer therapy. Nano Lett. 2018;18(6):3643–3650. doi:10.1021/acs.nanolett.8b00737
68. Wang Y, Zhu L, Wang Y, et al. Ultrasensitive GSH-responsive ditelluride-containing poly(ether-urethane) nanoparticles for controlled drug release. ACS Appl Mater Interfaces. 2016;8(51):35106–35113. doi:10.1021/acsami.6b14639
69. Criado-Gonzalez M, Mecerreyes D. Thioether-based ROS responsive polymers for biomedical applications. J Mater Chem B. 2022;10(37):7206–7221. doi:10.1039/D2TB00615D
70. Jing F, Guo Q, Xu W, Qu H, Sui Z. Docetaxel prodrug self-assembled nanosystem: synthesis, formulation and cytotoxicity. Bioorg Med Chem Lett. 2018;28(4):826–830. doi:10.1016/j.bmcl.2017.07.041
71. Luo C, Sun J, Liu D, et al. Self-assembled redox dual-responsive prodrug-nanosystem formed by single thioether-bridged paclitaxel-fatty acid conjugate for cancer chemotherapy. Nano Lett. 2016;16(9):5401–5408. doi:10.1021/acs.nanolett.6b01632
72. Cao W, Wang L, Xu H. Selenium/tellurium containing polymer materials in nanobiotechnology. Nano Today. 2015;10(6):717–736. doi:10.1016/j.nantod.2015.11.004
73. Hu S, Jiang H, Zhu J, et al. Tumor-specific fluorescence activation of rhodamine isothiocyanate derivatives. J Control Release. 2021;330:842–850. doi:10.1016/j.jconrel.2020.10.057
74. Chang Y, Yang K, Wei P, et al. Cationic vesicles based on amphiphilic pillar[5]arene capped with ferrocenium: a redox-responsive system for drug/siRNA co-delivery. Angew Chem Int Ed Engl. 2014;53(48):13126–13130. doi:10.1002/anie.201407272
75. Mao H, Xie Y, Ju H, et al. Design of tumor microenvironment-responsive drug-drug micelle for cancer radiochemotherapy. ACS Appl Mater Interfaces. 2018;10(40):33923–33935. doi:10.1021/acsami.8b11159
76. Li S, Zhang T, Xu W, et al. Sarcoma-targeting peptide-decorated polypeptide nanogel intracellularly delivers shikonin for upregulated osteosarcoma necroptosis and diminished pulmonary metastasis. Theranostics. 2018;8(5):1361–1375. doi:10.7150/thno.18299
77. Wang X, Cai X, Hu J, et al. Glutathione-triggered “off-on” release of anticancer drugs from dendrimer-encapsulated gold nanoparticles. J Am Chem Soc. 2013;135(26):9805–9810. doi:10.1021/ja402903h
78. Ma B, Guo S, Nishina Y, Bianco A. Reaction between graphene oxide and intracellular glutathione affects cell viability and proliferation. ACS Appl Mater Interfaces. 2021;13(3):3528–3535. doi:10.1021/acsami.0c17523
79. Wang S, Zhang L, Zhao J, He M, Huang Y, Zhao S. A tumor microenvironment-induced absorption red-shifted polymer nanoparticle for simultaneously activated photoacoustic imaging and photothermal therapy. Sci Adv. 2021;7(12):eabe3588 doi:10.1126/sciadv.abe3588.
80. Wu J, Zhao L, Xu X, et al. Hydrophobic cysteine poly(disulfide)-based redox-hypersensitive nanoparticle platform for cancer theranostics. Angew Chem Int Ed Engl. 2015;54(32):9218–9223. doi:10.1002/anie.201503863
81. Zhang S, Guan J, Sun M, et al. Self-delivering prodrug-nanoassemblies fabricated by disulfide bond bridged oleate prodrug of docetaxel for breast cancer therapy. Drug Deliv. 2017;24(1):1460–1469. doi:10.1080/10717544.2017.1381201
82. Bulmus V, Woodward M, Lin L, Murthy N, Stayton P, Hoffman A. A new pH-responsive and glutathione-reactive, endosomal membrane-disruptive polymeric carrier for intracellular delivery of biomolecular drugs. J Control Release. 2003;93(2):105–120. doi:10.1016/j.jconrel.2003.06.001
83. Cheng H, Wang X, Liu X, et al. An effective NIR laser/tumor-microenvironment co-responsive cancer theranostic nanoplatform with multi-modal imaging and therapies. Nanoscale. 2021;13(24):10816–10828. doi:10.1039/D1NR01645H
84. Du L, He H, Xiao Z, et al. GSH-responsive metal-organic framework for intratumoral release of NO and IDO inhibitor to enhance antitumor immunotherapy. Small. 2022;18(15):e2107732. doi:10.1002/smll.202107732
85. Yu M, Cao R, Ma Z, Zhu M. Development of “smart” drug delivery systems for chemo/PDT synergistic treatment. J Mat Chem B. 2023;11(7):1416–1433. doi:10.1039/D2TB02248F
86. Yang B, Wang K, Zhang D, et al. Polydopamine-modified ROS-responsive prodrug nanoplatform with enhanced stability for precise treatment of breast cancer. RSC Adv. 2019;9(16):9260–9269. doi:10.1039/C9RA01230C
87. Xu H, Cao W, Zhang X. Selenium-containing polymers: promising biomaterials for controlled release and enzyme mimics. Acc Chem Res. 2013;46(7):1647–1658. doi:10.1021/ar4000339
88. Wang L, Fan F, Cao W, Xu H. Ultrasensitive ROS-responsive coassemblies of tellurium-containing molecules and phospholipids. ACS Appl Mater Interfaces. 2015;7(29):16054–16060. doi:10.1021/acsami.5b04419
89. Deepagan VG, Kwon S, You DG, et al. In situ diselenide-crosslinked polymeric micelles for ROS-mediated anticancer drug delivery. Biomaterials. 2016;103:56–66. doi:10.1016/j.biomaterials.2016.06.044
90. Wang L, Cao W, Yi Y, Xu H. Dual redox responsive coassemblies of diselenide-containing block copolymers and polymer lipids. Langmuir. 2014;30(19):5628–5636. doi:10.1021/la501054z
91. Xu X, Saw PE, Tao W, et al. ROS-responsive polyprodrug nanoparticles for triggered drug delivery and effective cancer therapy. Adv Mat. 2017;29(33):1700141 doi:10.1002/adma.201700141.
92. da Silva DA, De Luca A, Squitti R, et al. Copper in tumors and the use of copper-based compounds in cancer treatment. J Inorg Biochem. 2022;226:111634. doi:10.1016/j.jinorgbio.2021.111634
93. Daund V, Chalke S, Sherje AP, Kale PP. ROS responsive mesoporous silica nanoparticles for smart drug delivery: A review. J Drug Delivery Sci Technol. 2021;64:102599. doi:10.1016/j.jddst.2021.102599
94. Sun Y, Wang Z, Zhang P, et al. Mesoporous silica integrated with Fe3O4 and palmitoyl ascorbate as a new nano-Fenton reactor for amplified tumor oxidation therapy. Biomater Sci. 2020;8(24):7154–7165. doi:10.1039/D0BM01738H
95. Hu J-J, Lei Q, Peng M-Y, Zheng D-W, Chen Y-X, Zhang X-Z. A positive feedback strategy for enhanced chemotherapy based on ROS-triggered self-accelerating drug release nanosystem. Biomaterials. 2017;128:136–146. doi:10.1016/j.biomaterials.2017.03.010
96. Zhang X, Wang X, Zhong W, Ren X, Sha X, Fang X. Matrix metalloproteinases-2/9-sensitive peptide-conjugated polymer micelles for site-specific release of drugs and enhancing tumor accumulation: preparation and in vitro and in vivo evaluation. Int J Nanomed. 2016;11:1643–1661. doi:10.2147/IJN.S101030
97. Grünwald B, Vandooren J, Locatelli E, et al. Matrix metalloproteinase-9 (MMP-9) as an activator of nanosystems for targeted drug delivery in pancreatic cancer. J Control Release. 2016;239:39–48. doi:10.1016/j.jconrel.2016.08.016
98. Wickström M, Larsson R, Nygren P, Gullbo J. Aminopeptidase N (CD13) as a target for cancer chemotherapy. Cancer Sci. 2011;102(3):501–508. doi:10.1111/j.1349-7006.2010.01826.x
99. Yang L, Li Y, Ding Y, Choi KS, Kazim AL, Zhang Y. Prolidase directly binds and activates epidermal growth factor receptor and stimulates downstream signaling. J Biol Chem. 2013;288(4):2365–2375. doi:10.1074/jbc.M112.429159
100. Linkous A, Geng L, Lyshchik A, Hallahan DE, Yazlovitskaya EM. Cytosolic phospholipase A2: targeting cancer through the tumor vasculature. Clin Cancer Res. 2009;15(5):1635–1644. doi:10.1158/1078-0432.CCR-08-1905
101. Li H, Yu SS, Miteva M, et al. Matrix metalloproteinase responsive, proximity-activated polymeric nanoparticles for siRNA delivery. Adv Funct Mater. 2013;23(24):3040–3052. doi:10.1002/adfm.201202215
102. Secret E, Kelly SJ, Crannell KE, Andrew JS. Enzyme-responsive hydrogel microparticles for pulmonary drug delivery. ACS Appl. Mater. Interfaces. 2014;6(13):10313–10321. doi:10.1021/am501754s
103. Gondi CS, Rao JS. Cathepsin B as a cancer target. Expert Opin Ther Targets. 2013;17(3):281–291. doi:10.1517/14728222.2013.740461
104. Hsu CW, Olabisi RM, Olmsted-Davis EA, Davis AR, West JL. Cathepsin K-sensitive poly(ethylene glycol) hydrogels for degradation in response to bone resorption. J Biomed Mater Res A. 2011;98(1):53–62. doi:10.1002/jbm.a.33076
105. Bughda R, Dimou P, RR D, Klampatsa A. Fibroblast Activation Protein (FAP)-Targeted CAR-T Cells: launching an attack on tumor stroma. Immunotargets Ther. 2021;10:313–323. doi:10.2147/ITT.S291767
106. Rastegari B, Karbalaei-Heidari HR, Zeinali S, Sheardown H. The enzyme-sensitive release of prodigiosin grafted β-cyclodextrin and chitosan magnetic nanoparticles as an anticancer drug delivery system: synthesis, characterization and cytotoxicity studies. Colloids Surf B Biointerfaces. 2017;158:589–601. doi:10.1016/j.colsurfb.2017.07.044
107. Chau Y, Zhong J. Enzyme-sensitive biomaterials for drug delivery. Comprehensive Biotechnol. 2019;5:501–519 doi:10.1016/B978-0-444-64046-8.00310-4.
108. Fan Y, Yuan S, Huo M, et al. Spatial controlled multistage nanocarriers through hybridization of dendrimers and gelatin nanoparticles for deep penetration and therapy into tumor tissue. Nanomedicine. 2017;13(4):1399–1410. doi:10.1016/j.nano.2017.01.008
109. Chen Z, Han F, Du Y, Shi H, Zhou W. Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2023;8(1):70. doi:10.1038/s41392-023-01332-8
110. Lin Q, Bao C, Yang Y, et al. Highly discriminating photorelease of anticancer drugs based on hypoxia activatable phototrigger conjugated chitosan nanoparticles. Adv Mater. 2013;25(14):1981–1986. doi:10.1002/adma.201204455
111. Li Y, Zhao L, Li XF. The hypoxia-activated prodrug TH-302: exploiting hypoxia in cancer therapy. Front Pharmacol. 2021;12:636892. doi:10.3389/fphar.2021.636892
112. Joshi U, Filipczak N, Khan MM, Attia SA, Torchilin V. Hypoxia-sensitive micellar nanoparticles for co-delivery of siRNA and chemotherapeutics to overcome multi-drug resistance in tumor cells. Int J Pharm. 2020;590:119915. doi:10.1016/j.ijpharm.2020.119915
113. Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994;140(1):1–22. doi:10.1007/BF00928361
114. Lai J, Shah BP, Zhang Y, Yang L, Lee KB. Real-time monitoring of ATP-responsive drug release using mesoporous-silica-coated multicolor upconversion nanoparticles. ACS Nano. 2015;9(5):5234–5245. doi:10.1021/acsnano.5b00641
115. Naito M, Ishii T, Matsumoto A, Miyata K, Miyahara Y, Kataoka K. A phenylboronate-functionalized polyion complex micelle for ATP-triggered release of siRNA. Angew Chem Int Ed Engl. 2012;51(43):10751–10755. doi:10.1002/anie.201203360
116. Shen HK, Morishita K, Hashim PK, et al. ATP-responsive nanoparticles covered with biomolecular machine “Chaperonin GroEL”. Angew Chem Int Ed Engl. 2023;62(31):e202304894. doi:10.1002/anie.202304894
117. Sameiyan E, Bagheri E, Dehghani S, et al. Aptamer-based ATP-responsive delivery systems for cancer diagnosis and treatment. Acta Biomater. 2021;123:110–122. doi:10.1016/j.actbio.2020.12.057
118. Kim J, Lee YM, Kim H, Park D, Kim J, Kim WJ. Phenylboronic acid-sugar grafted polymer architecture as a dual stimuli-responsive gene carrier for targeted anti-angiogenic tumor therapy. Biomaterials. 2016;75:102–111. doi:10.1016/j.biomaterials.2015.10.022
119. Zhou Z, Zhang Q, Zhang M, et al. ATP-activated decrosslinking and charge-reversal vectors for siRNA delivery and cancer therapy. Theranostics. 2018;8(17):4604–4619. doi:10.7150/thno.26889
120. Duan H, Li L, Zou K, Deng Y, Chen G. Cyclodextrin-integrated PEO-Based composite solid electrolytes for high-rate and ultrastable all-solid-state lithium batteries. ACS Appl Mater Interfaces. 2021;13(48):57380–57391. doi:10.1021/acsami.1c18589
121. Zhang J, Wang Y, Chen J, et al. Inhibition of cell proliferation through an ATP-responsive co-delivery system of doxorubicin and Bcl-2 siRNA. Int J Nanomed. 2017;12:4721–4732. doi:10.2147/IJN.S135086
122. Chen WH, Yu X, Cecconello A, Sohn YS, Nechushtai R, Willner I. Stimuli-responsive nucleic acid-functionalized metal-organic framework nanoparticles using pH- and metal-ion-dependent DNAzymes as locks. Chem Sci. 2017;8(8):5769–5780. doi:10.1039/C7SC01765K
123. Ye Z, Jiang Y, Li L, Wu F, Chen R. Rational design of MOF-based materials for next-generation rechargeable batteries. Nanomicro Lett. 2021;13(1):203. doi:10.1007/s40820-021-00726-z
124. Mo R, Jiang T, DiSanto R, Tai W, Gu Z. ATP-triggered anticancer drug delivery. Nat Commun. 2014;5:3364. doi:10.1038/ncomms4364
125. Qian C, Chen Y, Zhu S, et al. ATP-responsive and near-infrared-emissive nanocarriers for anticancer drug delivery and real-time imaging. Theranostics. 2016;6(7):1053–1064. doi:10.7150/thno.14843
126. Chen W-H, Yu X, Liao W-C, et al. ATP-responsive aptamer-based metal–organic framework Nanoparticles (NMOFs) for the controlled release of loads and drugs. Adv Funct Mat. 2017;27:1702102 doi:10.1007/s12274-017-1736-7.
127. Ren H, Hu Q, Sun Y, et al. Surface chemistry mediates the tumor entrance of nanoparticles probed using single-molecule dual-imaging nanodots. Biomater Sci. 2023;11(21):7051–7061. doi:10.1039/D3BM01171B
128. Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183–3195. doi:10.7150/thno.52570
129. Liang Y, Iqbal Z, Lu J, et al. Cell-derived nanovesicle-mediated drug delivery to the brain: principles and strategies for vesicle engineering. Mol Ther. 2023;31(5):1207–1224. doi:10.1016/j.ymthe.2022.10.008
130. Liu Q, Li D, Pan X, Liang Y. Targeted therapy using engineered extracellular vesicles: principles and strategies for membrane modification. J Nanobiotechnol. 2023;21(1):334. doi:10.1186/s12951-023-02081-0
131. Xu X, Xu L, Wen C, Xia J, Zhang Y, Liang Y. Programming assembly of biomimetic exosomes: an emerging theranostic nanomedicine platform. Mater Today Bio. 2023;22:100760. doi:10.1016/j.mtbio.2023.100760
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



