Back to Journals » Journal of Pain Research » Volume 17
Study on the Efficacy and Safety of the Huangqi Guizhi Wuwu Decoction in the Prevention and Treatment of Chemotherapy-Induced Peripheral Neuropathy: Meta-Analysis of 32 Randomized Controlled Trials
Authors Yang XR , Zhang XY, Xia YJ , Fu J, Lian XX, Liang XR, He YQ, Li ZH
Received 22 May 2024
Accepted for publication 30 July 2024
Published 8 August 2024 Volume 2024:17 Pages 2605—2628
DOI https://doi.org/10.2147/JPR.S466658
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Xin-Rong Yang,1 Xin-Yi Zhang,1 Yi-Jia Xia,1 Jin Fu,1 Xiao-Xuan Lian,2 Xin-Ru Liang,2 Ying-Qi He,2 Zhuo-Hong Li1
1Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China; 2Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China
Correspondence: Zhuo-Hong Li, Email [email protected]
Purpose: Chemotherapy-induced peripheral neuropathy (CIPN) still lacks efficient therapeutic drugs. This study aimed to systematically evaluate the effects of Huangqi Guizhi Wuwu Decoction (HGWD) alone or combined with positive drugs on CIPN prevention and treatment.
Methods: The PubMed, Embase, Web of Science, Cochrane, China National Knowledge Infrastructure (CNKI), Wan Fang Data, China Science and Technology Journal (VIP) and Chinese Biomedical (CBM) databases were searched for randomized controlled trials (RCTs) of HGWD for CIPN prevention and treatment. The search time ranged from database establishment to October 17, 2023. The Cochrane risk-of-bias assessment tool was used for quality assessment, Review Manager 5.3 and STATA 12.0 were used for meta-analysis, and GRADEprofiler was used for evidence level assessment.
Results: A total of 32 RCTs involving 1987 patients were included. The meta-analysis results revealed the following: 1. In terms of the total CIPN incidence, that in the HGWD group was lower than that in the blank control group. The incidence in both the HGWD and HGWD+positive drug groups was lower than that in the monotherapy-positive drug group. 2. In terms of the incidence of severe CIPN, that in the HGWD group was lower than that in the blank control and positive drug groups. There was no statistically significant difference between the HGWD+positive drug and positive drug groups. Sensitivity analysis revealed that the results of severe incidence in the HGWD group was lower than that in the positive drug group were unstable 3. HGWD did not increase the number of chemotherapy-related adverse events.
Conclusion: HGWD can safely and effectively prevent CIPN, reduce symptoms, improve quality of life and reduce the impact of chemotherapy drugs on sensory nerve conduction. However, more high-quality RCTs are needed to compare the efficacy of HGWD with that of positive control drugs in preventing severe CIPN.
Keywords: huangqi guizhi wuwu decoction, chemotherapy-induced peripheral neuropathy, chemotherapy-induced peripheral neurotoxicity, Chinese medicine, meta-analysis
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a common dose-limiting adverse reaction caused by chemotherapeutic drugs used in cancer treatment. In total, approximately 48% of patients receiving neurotoxic drugs develop CIPN, and the incidence of CIPN is related to the type and dose of chemotherapeutic drugs.1 At present, taxanes, platinum vincristine, thalidomide and bortezomib are known to cause neurotoxicity.2 The clinical manifestations of CIPN-related sensory nerve damage include typical abnormal sensations of gloves and socks, sensory disturbance, numbness and tingling, hypoesthesia, and other symptoms.3 Small fibrous nerve injury is characterized by burning pain, pain, hypothermia and other symptoms in the hands and feet;4 it can also involve motor and autonomic nerves to produce corresponding symptoms. When CIPN occurs, it is usually necessary to reduce the dose of chemical drugs or stop treatment to relieve symptoms, which seriously affects the efficacy of treatment. Even so, CIPN sometimes continues to progress and cause deterioration within an average of 2–6 months after stopping chemotherapy—a phenomenon known as “gliding”,5,6 which continues to affect the quality of life of patients and places a heavy financial burden on patients and the health care system.7
However, the prevention and treatment of CIPN are still challenging. The guidelines of the American Society of Clinical Oncology (ASCO) and the European Society of Medical Oncology (ESMO) do not recommend any drugs that can be used to prevent CIPN;8,9 only duloxetine is recommended for the treatment of neuropathic pain, whereas duloxetine needs to be used under close observation by doctors, with potentially unbearable side effects (nausea, insomnia and dizziness).10 A new systematic review revealed that placebo and duloxetine had similar efficacy.11 Other antidepressants, anticonvulsant drugs and nutritional supplements have shown poor efficacy in existing clinical studies. Therefore, there is an urgent need for safe and effective methods to prevent and control CIPN. Traditional Chinese medicine has played an important role in improving the clinical symptoms of patients with tumors, improving the efficacy of chemotherapy and reducing side effects.12,13 Huangqi Guizhi Wuwu Decoction (HGWD) is a classic traditional Chinese medicine used for the treatment of CIPN. The five herbal components of the basic HGWD prescription are shown in Table 1. The main purpose of this prescription is to tonify Qi, warm meridians, harmonize the blood and free the collateral vessels, which is consistent with the pathogenesis of CIPN-related blood arthralgia syndrome.14 AC591, a standardized extract of HGWD, can prevent oxaliplatin-induced peripheral neuropathy without reducing desirable antitumor activity, indicating that HGWD has great potential in the treatment of CIPN.15 However, the lack of large-sample studies and evidence-based research hinders its promotion and application. Therefore, the purpose of this study was to evaluate the efficacy and safety of HGWD for the treatment of CIPN through a meta-analysis of randomized controlled trials and to provide evidence-based medical support for the clinical use of the HGWD intervention.
 |
Table 1 The Five Herbal Components of the Basic HGWD Prescription |
Materials and Methods
This meta-analysis followed the registered protocol (number Inplasy2023120048). All procedures, including the study design, study search, information extraction, data analysis, and evidence interpretation, were fully compliant with the PRISMA 2020 Checklist (Supplementary Table S1).16
Search Strategy
We searched the PubMed, Embase, Web of Science, Cochrane Central Register of Controlled Trials, China National Knowledge Infrastructure (CNKI), Wan Fang Data, China Science and Technology Journal (VIP) and Chinese Biomedical Database (CBM) databases. All searches started from the establishment of the database and extended to October 17, 2023. The specific search strategy for all the databases is shown in Supplementary Table S2, and no language restrictions were applied to this search strategy. In addition, we manually searched the references of relevant articles to obtain as many studies as possible.
Inclusion and Exclusion Criteria
Inclusion Criteria
The inclusion criteria for the studies were strictly defined in the PICOS format.
- Participants: Patients were required to have malignant tumors confirmed by histopathology and/or cytology or imaging examination. Chemotherapy regimens involved the use of drugs currently known to cause chemotherapy-related peripheral neuropathy: platinum (eg, cisplatin, carboplatin and oxaliplatin), vincristine (eg, vincristine), paclitaxel (eg, paclitaxel and docetaxel), bortezomib and thalidomide. There were no restrictions on tumor type or stage, age, race or sex.
- Intervention: Patients were treated with HGWD alone or HGWD combined with positive control drugs on the basis of routine chemotherapy. The methods used for HGWD administration (oral or external washing or fumigation) and dosage forms used (for decoctions, granules or capsules) were not limited.
- Comparison: A blank control, placebo control or positive control drug (duloxetine or mecobalamin) was administered on the basis of routine chemotherapy.
- Outcomes: The main outcome measures included the total incidence of CIPN, the incidence of severe CIPN (grade III or above), the total effective rate and the total score on the European Organization for Cancer Treatment and Research Quality of Life-Peripheral Neurotoxicity Assessment scale for Chemotherapeutic Drugs-EORTC QLQ-CIPN20 scale. The secondary outcome indicators included the sensory nerve conduction velocity (SNCV) (median nerve and peroneal nerve) before and after treatment, the Karnofsky Performance Scale (KPS) reflecting the physical function of the patients, and adverse events related to treatment. The neurotoxicity scale was classified by Levi grade, WHO grade, or NCI grade. The total effective rate definitions were as follows: significant effect—peripheral neurotoxicity grade 0; effective—peripheral neurotoxicity grade decreased by more than 1 grade; ineffective—peripheral neurotoxicity grade had no change or aggravation).
- Study design: Only randomized controlled clinical trials (RCTs) were included in the evaluation of the efficacy of HGWD for the prevention and treatment of CIPN.
- The specific experimental group (treated with HGWD or HGWD combined with positive drugs on the basis of routine chemotherapy) and the control group (blank control or placebo control or positive drug control on the basis of routine chemotherapy) were included in the RCTs of HGWD combined with other therapies for the prevention and treatment of CIPN.
- Only studies of peripheral neurotoxicity as a safety index in the literature were included.
Exclusion Criteria
- Ongoing clinical trials; nonrandomized controlled trials; uncontrolled case studies; reviews; meta-analyses; discussions; conferences; animal or in vitro trials; and other nonclinical trials.
- Studies that failed to provide complete outcome data or mismatched outcome indicators or did not describe the scale classification of neurotoxicity.
- Repeatedly published literature.
- Studies that involved treatment with other drugs that could cause neurotoxicity.
- Patients with systemic diseases and other nonchemotherapy factors that cause neurological dysfunction, such as severe cervical spondylosis, severe lumbar disc compression, and severe diabetes.
- Studies involving participants with a history of hand and foot dermatosis and drug allergy.
- The intervention measures used in the experimental group were from the literature on HGWD combined with other traditional Chinese medicine or traditional Chinese treatment methods (such as acupuncture, acupoint massage, massage, auricular point pressing beans, etc).
Literature Selection and Data Extraction
Two researchers (Xin-Yi Zhang and Xin-Rong Yang) independently screened the literature and extracted the data. First, the search results were imported into Endnote21 to automatically delete duplicates, and then manual review was carried out to eliminate the remaining duplicates. Afterward, according to the title/abstract, studies on unrelated topics were excluded, and the full text was further obtained and read, excluding repetitive literature, ongoing clinical trials, reviews, meta-analyses, discussions, conferences, animal or in vitro trials, and other nonclinical trials. Finally, the studies for inclusion were identified, and the reasons for the inclusion and exclusion of the studies were recorded in detail. The characteristics included in the study were extracted and included the first author, the date of publication, the number of participants in the test group and the control group, sex, average age, type of cancer, chemotherapy regimen, detailed intervention and outcome indicators of the test group and the control group. In the process, the two researchers cross-checked the screening results of the literature and the extracted data, and the differences were resolved through consultation with a third researcher (Xin-Ru Liang). If the data were missing or unclear, we contacted the author for additional information.
Methodological Quality Assessment
The Cochrane Handbook of Systematic Review of Interventions was used to evaluate the risk of bias in the included RCTs via Review Manager 5.3 software.17 The methodological quality of each included study was assessed through seven aspects, namely, the randomization method, allocation concealment, the blinding method, outcome data integrity, the selection report, and other sources of bias. The literature quality was classified as high risk, low risk or unclear risk according to the bias of each item. The risk bias was assessed and cross-checked independently by two researchers (Xin-Yi Zhang and Xin-Rong Yang), and if there was a disagreement, the researcher discussed and decided with a third researcher (Xin-Ru Liang). After the results were confirmed, Review Manager 5.3 software was used to construct a bias risk graph.
Data Analysis
We used Review Manager 5.3 and Stata 12.0 for the data analysis. The EORTC QLQ-CIPN20 score, SNCV and KPS score were continuous variables. The changes in the intervention group and the control group at baseline and at the end of the trial are expressed as the weighted mean difference (MD) and 95% confidence interval (CI), respectively. If only the data after treatment were reported, the values at the end of the trial were compared. The total incidence of CIPN, incidence of severe CIPN, and total effective rate were calculated as dichotomous variables and are expressed as hazard ratios (RRs) with 95% CIs. For the results of multiple time points reported, only the data from the last time point were extracted. Heterogeneity was assessed by the I2 statistic and chi-square test. A fixed effects model was used for meta-analysis when there was low heterogeneity (P >0.05, I2 ≤50%), and a random effects model was used for high heterogeneity (P <0.05, I2 >50%). To explore the source of heterogeneity, meta-regression analysis (including ≥10 studies) was performed to explore the possible parameters that could have led to high heterogeneity. Sensitivity analysis was also conducted to determine whether the conclusions of the meta-analysis were stable.
Publication Bias
For studies with an outcome index ≥10, publication bias was evaluated via funnel plots and Begg’s and Egger’s tests (P <0.05 represented significant publication bias; otherwise, there was no significant bias). If the funnel chart is symmetrical, there is no publication bias; otherwise, there is publication bias. When publication bias existed, the influence of publication bias on the results of the meta-analysis was tested via the trim-and-fill method.
Evidence Strength
The strength of the evidence in this meta-analysis was assessed as high, moderate, low, or very low according to the Grading of Recommendation, Assessment, Development and Evaluation (GRADE) approach for each outcome measure.18
Results
Characteristics of the Included Studies
According to the prespecified screening criteria, 32 randomized controlled trials with a total of 1987 patients were included. The inclusion process is shown in (Figure 1).19–50 The included RCTs were published between 2006 and 2023, and 29 RCTs were positively compared20–22,24–43,45–50 (including 2 specific experimental and control groups in the multiarm trials of HGWD combined with moxibustion or reverse acupuncture),36,47 2 RCTs were compared in three arms,23,44 and 1 randomized crossover trial was included.19 On the basis of unified chemotherapy, 16 studies compared HGWD with the blank control,19,21–24,27,28,30,32,35–38,40,42,49 1 study compared HGWD with the placebo,45 13 studies compared HGWD with positive drugs (11 studies used mecobalamin,20,23,25,26,29,31,33,39,41,44,46 1 used duloxetine,48 and 1 used mecobalamin plus vitamin B6),43 and 4 studies compared HGWD combined with positive drugs with positive drugs alone (all studies used mecobalamin).34,44,47,50 Table 2 shows the specific characteristics of the included studies.
 |
Table 2 Basic Characteristics of the Included Studies |
 |
Figure 1 Flow diagram for the selection of trials. |
Risk Bias of the Included Studies
The risk bias of the included trials was evaluated accordingly. Fifteen studies described specific and correct methods of random allocation, and the quality of random sequence generation in these studies was evaluated as “low risk”.21–23,32,34–36,39,42–45,47,49,50 Qiu SA’s studies were grouped according to the order of patient visits,31 and Yu B and Zhou YQ’s studies were grouped according to the order of admission and were rated as “high risk”.26,33 The remaining studies were described only according to the “random principle” and were rated as “unclear” risk.19,20,24,25,27–30,33,38,40,41,46,48 Only Guo HL’s study described a reasonable allocation concealment method,47 with a rating of “low risk”, and all the other trials mentioned were rated as “unknown risk“.19–46,48–50 The studies of Guo HL and Ma J described a specific and reasonable double-blind method,45,47 the blinding factor was rated as “low risk”, and all the other trials mentioned were rated as “unknown risk”.19–44,46,48–50 The number of study dropouts of Cao SJ was too large,29 and the complete outcome data were rated as “high risk”. The remaining studies were rated as “low risk”.19–28,30–50 All the studies reported the outcome indicator data according to the scheduled plan,19–50 and the selective reporting items were rated as “low risk”. In addition, whether there were other biases could not be judged, and all the studies were rated as “unclear risk” in this item.19–50 The risk of bias of the included studies is shown in Figure 2.
 |
Figure 2 Risk of bias graph. |
The results of the Meta-Analysis
Owing to the different intervention measures and controls in the treatment group, the same intervention and control groups were combined.
Total Incidence of CIPN
- Chemotherapy+HGWD vs chemotherapy
A total of 14 studies with 957 subjects were included.19,21–24,27,28,30,32,35–38,40 The heterogeneity test revealed that 14 studies had high heterogeneity (chi2 =52.53, P <0.00001, I2 =75%). Therefore, the random effects model was used for data analysis. Meta-analysis revealed a statistically significant difference in the incidence of peripheral nerve toxicity between the treatment group and the control group (RR =0.57, 95% CI [0.47, 0.69]; P <0.00001) (Figure 3A). The funnel plot of publication bias was asymmetric (Figure 4A). Begg’s and Egger’s tests revealed significant publication bias (P <0.05). The trim-and-fill method was used for further analysis. The heterogeneity test revealed a value of 37.910 (P =0.000). A random effects model was used. The combined effect indicator results were logRR =−0.546, 95% CI [−0.716, −0.377]. After two iterations, the number of missing studies was estimated to be 0 by using the linear method. The meta-analysis was repeated, and the results were stable Publication bias had little effect on the results. Sensitivity analysis revealed that the meta-analysis results were stable (Figure 5A), and the results showed that HGWD could effectively prevent the occurrence of CIPN.
A total of 11 studies with 575 subjects were included.20,23,25,26,29,31,33,39,41,43,44 A heterogeneity test revealed that 11 studies had high heterogeneity (chi2 =39.68, P <0.0001, I2 =75%). Therefore, the random effects model was used for data analysis. Meta-analysis revealed a statistically significant difference in the incidence of peripheral nerve toxicity between the treatment group and the control group (RR =0.58, 95% CI [0.43, 0.79]; P =0.0005) (Figure 3B). The funnel plot of publication bias was asymmetric (Figure 4B). Begg’s (P =0.043) and Egger’s (P =0.001) analyses revealed that there was significant publication bias. The trim-and-fill method was used for further analysis. The heterogeneity test yielded Q =23.44 and P =0.01. A random effects model was used, and the combined effect indicator results were logRR =−0.494, 95% CI [−0.730, −0.258]. After two iterations, the number of missing studies was estimated to be 0 by using the linear method, the meta-analysis was repeated, the results were stable, and publication bias had little effect on the results. Sensitivity analysis confirmed that the results of the meta-analysis were stable (Figure 5B). The results showed that HGWD could prevent CIPN more effectively than positive drug intervention.
Four studies with 248 subjects were included.34,44,47,50 A heterogeneity test revealed that there was no significant heterogeneity among the four studies (chi2 =3.05, P =0.38, I2 =2%). Therefore, a fixed effects model was used for the data analysis. Meta-analysis revealed a statistically significant difference in the incidence of peripheral nerve toxicity between the treatment group and the control group (RR =0.69, 95% CI [0.58, 0.84]; P =0.0001) (Figure 3C). Sensitivity analysis confirmed that the results of the meta-analysis were stable (Figure 5C). The results showed that HGWD combined with positive drug intervention could prevent CIPN more effectively than treatment with positive drugs alone.
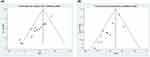 |
Figure 4 Funnel plots of the total incidence of CIPN.(A) Chemotherapy+HGWD vs chemotherapy.(B) Chemotherapy+HGWD vs chemotherapy+positive drugs. |
Incidence of Severe CIPN
A total of 15 studies with 1017 subjects were included.19,21–24,27,28,30,32,35–38,40,42 The heterogeneity test revealed that 15 studies had no significant heterogeneity (chi2 =3.61, P =1.00, I2 =0%). Therefore, a fixed effects model was used for the data analysis. Meta-analysis revealed a statistically significant difference in the incidence of severe peripheral nerve toxicity between the treatment group and the control group (RR =0.15, 95% CI [0.08, 0.26]; P <0.00001) (Figure 6A). The funnel plot of publication bias was asymmetric (Figure 7A), but there was no significant publication bias according to Begg’s (P =1.000) or Egger’s test (P =0.424). Sensitivity analysis revealed that the meta-analysis results were stable (Figure 8A). The results showed that HGWD intervention could effectively prevent the occurrence of severe CIPN.
A total of 11 studies with 575 subjects were included.20,23,25,26,29,31,33,39,41,43,44 A heterogeneity test revealed that 11 studies had no significant heterogeneity (chi2 =3.46, P =0.94, I2 =0%). Therefore, a fixed effects model was used for the data analysis. Meta-analysis revealed a statistically significant difference in the incidence of severe peripheral nerve toxicity between the treatment group and the control group (RR =0.45, 95% CI [0.24, 0.82]; P =0.01) (Figure 6B). The funnel plot of publication bias was asymmetric (Figure 7B), but Begg’s (P =0.721) and Egger’s tests (P =0.276) revealed no publication bias. Sensitivity analysis revealed that one study was to be eliminated in turn, and the meta-analysis was performed again. After merging, when the studies of Xu XR were eliminated, the 95% CI intersected with the invalid line, indicating that the meta-analysis results were unstable (Figure 8B). Therefore, existing studies could not prove that HGWD could prevent the occurrence of severe CIPN more effectively than positive drug intervention.
Three studies with 198 subjects were included.34,44,50 The heterogeneity test revealed that there was no significant heterogeneity among the three studies (chi2 =0.33, P =0.85, I2 =0%). Therefore, a fixed effects model was used for the data analysis. Meta-analysis revealed no significant difference in the incidence of severe peripheral nerve toxicity between the treatment group and the control group (RR =0.40, 95% CI [0.13, 1.21]; P =0.10) (Figure 6C). Sensitivity analysis confirmed that the results of the meta-analysis were stable (Figure 8C). These results could not prove that HGWD combined with positive drug intervention had a better effect than the positive drug alone in the treatment of severe CIPN.
 |
Figure 7 Funnel plots of the Incidence of severe CIPN.(A) Chemotherapy+HGWD vs chemotherapy.(B) Chemotherapy+HGWD vs chemotherapy+positive drugs. |
Total Effective Rates
Only one study with 60 subjects was included in the descriptive analysis.46 The total effective rate was 96.7% (29/30) in the HGWD group and 60.0% (18/30) in the mecobalamin group (P <0.01).
Three studies with 188 subjects were included,34,47,50 and the heterogeneity test revealed that there was no significant heterogeneity among the three studies (chi2 =1.22, P =0.54; I2 =0%). Therefore, a fixed effects model was used for the data analysis. The meta-analysis revealed that the total effective rates in the treatment group and the control group were significantly different (RR =3.85, 95% CI [2.13, 6.98], P <0.00001) (Figure 9A), and the sensitivity analysis revealed that the meta-analysis results were stable (Figure 9B). The probability of HGWD combined with positive drugs improving the curative effect by one or more grades was 3.85 times greater than that of the positive control drug alone.
 |
Figure 9 (A) Forest plot of the total effective rate. (B) Sensitivity analysis of the total effective rate. |
EORTC Qlq-Cipn20
Only one study with 46 subjects was included.49 Descriptive analysis revealed that the EORTC QLQ-CIPN20 score was significantly lower in the HGWD group than in the control group after 6 months of treatment (P <0.05).
Only one study was included,48 with 56 subjects, and the positive drug used was duloxetine. Descriptive analysis was carried out. From the 4th to 7th month after the start of chemotherapy, the EORTC QLQ-CIPN20 score of the HGWD group was significantly lower than that of the positive drug group, and the difference between the groups was statistically significant (P <0.05).
Only one study with 56 subjects was included in the descriptive analysis.45 From the fourth month after the start of treatment to the end of the trial, the EORTC QLQ-CIPN20 score in the HGWD group was significantly lower than that in the placebo group (P <0.05).
Karnofsky Performance Scale (KPS)
Two studies with 106 subjects were included.36,49 The heterogeneity test revealed that there was no significant heterogeneity between the two studies (chi2 =1.70, P =0.19, I2 =41%). Therefore, a fixed effects model was used for the data analysis. The meta-analysis revealed that the KPS scores significantly differed between the treatment group and the control group (MD =7.09, 95% CI [4.53, 9.66], P <0.00001) (Figure 10), indicating that HGWD could improve the quality of life of patients.
Only one study with 56 subjects was included in the descriptive analysis.45 From the fourth month after the start of treatment to the end of the trial, the KPS score in the HGWD group was significantly greater than that in the placebo group (P <0.05).
SNCV of the Median Nerve (MN) Before and After Treatment
A total of 162 subjects were included in 3 studies.21,23,42 A heterogeneity test revealed that 3 studies had significant heterogeneity (chi2 =5.41, P =0.07, I2 =63%). Therefore, the random effects model was used for data analysis. Meta-analysis revealed that the difference in the SNCV of the median nerve between the treatment group and the control group before and after treatment was statistically significant (MD =6.44, 95% CI [3.87, 9.01]; P <0.00001) (Figure 11A). One study was removed in turn, and the meta-analysis was performed again. The results of the sensitivity analysis were stable after merging, indicating that HGWD could prevent the slowing of the SNCV in the MN.
Three studies with 171 subjects were included,23,33,39 and the heterogeneity test revealed that there was no significant heterogeneity among the three studies (chi2 =1.50, P =0.47; I2 =0%). Therefore, a fixed effects model was used for the data analysis. Meta-analysis revealed that the difference in the SNCV of the MN between the treatment group and the control group before and after treatment was statistically significant (MD =5.56, 95% CI [4.19, 6.92]; P <0.00001) (Figure 11B). One study was removed in turn, and the meta-analysis was carried out again. After consolidation, the sensitivity analysis results were stable, which proved that HGWD was more effective than the other drugs at improving the slowing of the SNCV in the MN.
Only one study with 78 subjects was included in the descriptive analysis.50 There was no significant difference in the SNCV of the MN between the two groups before treatment (P >0.05). After treatment, the SNCV of the MN increased in both groups (P <0.05), and the SNCV of the MN in the HGWD combined with mecobalamin group was greater than that in the mecobalamin alone group (P <0.05).
SNCV of the Peroneal Nerve Before and After Treatment
Only one study with 60 subjects was included in the descriptive analysis.21 There was no significant difference in the SNCV of the peroneal nerve between the two groups before treatment (P >0.05). After treatment, the SNCV of the peroneal nerve in the two groups decreased significantly (P <0.01), but the decrease in the HGWD group was less than that in the blank control group (P <0.01).
Two studies with 125 subjects were included.33,39 The heterogeneity test revealed that there was no significant heterogeneity between the two studies (chi2 =1.10, P =0.29, I2 =9%). Therefore, a fixed effects model was used for the data analysis. The meta-analysis revealed that the difference in the SNCV of the peroneal nerve between the treatment group and the control group before and after treatment was statistically significant (MD =4.81, 95% CI [3.34, 6.29], P <0.00001) (Figure 12), which suggested that HGWD was more effective than positive control drugs for improving SNCV slowing.
Only one study with 78 subjects was included in the descriptive analysis.50 There was no significant difference in the SNCV between the two groups before treatment (P >0.05). After treatment, the SNCV in the peroneal nerve increased in both groups (P <0.05), and the SNCV in the peroneal nerve in the HGWD combined with mecobalamin group was greater than that in the mecobalamin alone group (P <0.05).
 |
Figure 10 Forest plot of the Karnofsky performance score (KPS) for chemotherapy+HGWD vs chemotherapy. |
 |
Figure 11 Forest plot of the sensory nerve conduction velocity (median nerve). (A) Chemotherapy+HGWD vs chemotherapy. (B) Chemotherapy+HGWD vs chemotherapy+positive drugs. |
 |
Figure 12 Forest plot of Sensory nerve conduction velocity (peroneal nerve) for chemotherapy+HGWD group vs the chemotherapy+positive drug group. |
Adverse Events Related to Treatment
Among the 32 studies, 25 did not mention adverse events;19–30,33,35–41,43,44,46,47,50 6 reported adverse events;31,32,42,45,48,49 and 1 reported no adverse reactions.34 The common adverse reactions in the HGWD intervention group and the control group were cytopenia, nausea and vomiting, and no serious adverse reactions were reported. Overall, the incidence and severity of adverse reactions in the HGWD intervention group were lower than those in the control group of patients treated with conventional treatment. Adverse events are presented in Table 3.
 |
Table 3 Adverse Events Related to Treatment |
Meta-Regression Analysis
The “chemotherapy+HGWD vs chemotherapy” group in the outcome index of the total incidence of CIPN was subjected to meta-regression to explore the source of heterogeneity, and the results revealed that age was the factor influencing the heterogeneity. However, some studies of the “chemotherapy+HGWD vs chemotherapy+positive drugs” type did not report the average age, and all the patients were receiving oral drugs. According to our meta-regression, the year of publication, sample size, cancer type and chemotherapy regimen were not sources of heterogeneity, as shown in Tables 4 and 5. In addition, differences in the dose and cycle of chemotherapy drugs and the dose and treatment time of HGWD may also be potential reasons for the high heterogeneity.
 |
Table 4 Meta-Regression Analysis (chemotherapy+HGWD Vs Chemotherapy) |
 |
Table 5 Meta-Regression Analysis (chemotherapy+HGWD Vs Chemotherapy+positive Drugs) |
The Quality of Evidence
Most of the studies included in all outcome indicators had random allocation methods, assigning the ambiguous risks of hidden and blind methods, so all the evidence was reduced by one level; in terms of inconsistency, the evidence of heterogeneous outcome indicators was downgraded by one level; in terms of inaccuracy, the number of patients included in the outcome indicators was less than 400, the evidence was downgraded by one grade, and those without statistical meaning were downgraded by another level. The publication bias was determined by the trim-and-fill method, in which the grade was not reduced if there was no significant effect. All the final evidence levels are provided in Supplementary Table S3, Supplementary Table S4 and Supplementary Table S5. The level of evidence for most outcome measures was low.
Discussion
Pathogenesis of CIPN and the Pharmacological Effects of HGWD
The pathogenesis of CIPN has not yet been fully elucidated. At present, the pathogenesis of CIPN caused by different chemotherapeutic drugs likely differs, and CIPN induced by various drugs can be caused by multiple factors. The main mechanism of CIPN induced by taxanes is disruption of microtubules and interference with axonal transport, resulting in axonal degeneration. Platinum drugs cause CIPN because platinum complexes accumulate in the dorsal root ganglion (DRG).51 Vinblastine binds to tubulin, leading to destabilization of microtubule polymers, dysfunction of axonal transport, distal axonal lesions, and ultimately CIPN. It has been hypothesized that the mechanism by which thalidomide causes CIPN is through the downregulation of TNF-α and the inhibition of NF-κB. This leads to dysregulation of neurotrophins and their receptors, thereby accelerating neuronal cell death. Additionally, thalidomide-induced antiangiogenic effects may lead to secondary ischemia and hypoxia in nerve fibers, ultimately leading to ischemic neuropathy.52 Bortezomib is a protease inhibitor that induces sphingolipid metabolism dissimple in the dorsal horn of the spinal cord, thereby increasing the expression levels of sphingosine-1-phosphate, sphingosine-1-phosphate receptor 1 and dihydrogenS1P (DH-SIP). S1P and DH-S1P in turn bind to S1PR1 receptors that are highly expressed on astrocytes, increasing presynaptic glutamine release, which in turn leads to CIPN.53 In addition, mitochondrial dysfunction, oxidative stress, abnormal cytokine secretion and abnormal immune cell function, which subsequently cause neuroinflammation, are also common mechanisms of CIPN induced by a variety of drugs.
Previous studies have shown that HGWD can affect the TLR4/NF-κB and PI3K/Akt-Nrf2 pathways and inhibit paclitaxel-induced inflammatory and oxidative responses in the peripheral nervous system and that HGWD does not interfere with the antitumor activity of paclitaxel in both in vitro and in vivo models.54 Li M et al reported that HGWD could affect the TNF-α/IL-1β/IL-6/MAPK/NF-κB pathway to antagonize nerve cell injury in oxaliplatin-induced peripheral neuropathy (OIPN) model rats.55 Wu AP’s study revealed that HGWD could also prevent CIPN by preventing oxidative stress and inhibiting mitochondrial damage induced by the P53‒Bax pathway in DRG cells.56 Huo JG reported that HGWD could downregulate the expression of NR2B in the L4‒6 spinal cord of rats and upregulate the protein level of pNF-H in the DRG to improve CIPN.57 Gu ZC demonstrated that HGWD might alleviate the chronic neurotoxicity of OIPN by slightly downregulating the expression of the platinum transfer protein OCT2 mRNA in dorsal root ganglion cells and mainly upregulating the expression of the platinum transfer protein ATP7A mRNA.58 Other studies have shown that HGWD can also alleviate chronic OIPN by regulating intestinal flora homeostasis.59 However, studies on the effects of HGWD on CIPN induced by other chemotherapeutic drugs are lacking, and the underlying mechanism is still unclear.
Comprehensive Evaluation of the Efficacy of HGWD
HGWD is widely used in China to treat pain and numbness symptoms caused by a variety of neurological diseases, including diabetic peripheral neuropathy, and has good curative effects; however, research on the treatment of CIPN is in the initial stage.60,61 A network meta-analysis revealed that HGWD was more effective than ganglioside, vitamin E, omega-3 fatty acids, calcium and magnesium infusions, and glutathione in preventing CIPN, which showed that it has unique clinical value.62 Our study revealed that although HGWD can reduce the incidence of CIPN and even severe CIPN, the included studies relied mainly on doctors to use the mainstream clinical grading scale for the diagnosis and evaluation of CIPN, and the symptoms of CIPN are based on the subjective feelings of patients. The grading scale may underestimate symptoms and have poor sensitivity, which means that the true incidence of CIPN may be higher, which would reduce the reliability of the results.63 Only three studies have used the reliable and sensitive EORTC QLQ-CIPN20 total score, with the questionnaire directly completed by patients,64 showing that the CIPN20 total score of the HGWD group was significantly lower than that of the placebo, duloxetine and blank control groups from 4 and 6 months after treatment. A prospective cohort study revealed that the total CIPN20 score of the HGWD group was lower than that of the chemotherapy-alone group at all monitoring time points (at the 2nd/4th/6th cycle of chemotherapy) (P <0.5), and the average time of first occurrence of CIPN in the HGWD group was also later than that of the chemotherapy-alone group (P <0.5).65 A study using a separate subscale of the CIPN20 revealed that the CIPN20 sensory and motor scores of the HGWD group were lower than those of the placebo group at weeks 4 and 12 compared with baseline (P <0.5), but the difference in the autonomic score was not significant (P >0.5).66 This finding is consistent with our conclusion that HGWD can reduce clinical symptoms and increase tolerance to chemotherapy.
In addition, few studies have evaluated secondary outcome measures. Only three blank and placebo-controlled studies evaluated the KPS score, and the results showed that HGWD may improve the quality of life of patients. Zhu Q’s cohort study revealed that the KPS score of the HGWD group was significantly greater than that of the chemotherapy-alone group at the 4th/6th cycle of chemotherapy (P <0.5);65 however, the retrospective study of Xu WR compared HGWD with mecobalamin, and there was no statistically significant difference in the KPS score between the two groups after treatment (P >0.5).67 It is unclear whether HGWD has advantages in improving quality of life. Nerve conduction study (NCS) is an important method used in the diagnosis of CIPN. Previous studies have shown that the amplitudes of the sensory nerve action potential (SNAP) and SNCV in CIPN patients are significantly reduced.68,69 Only 7 of the included studies used the SNCV as a detection index. Data analysis suggested that HGWD improved the decrease in the SNCV more than mecobalamin did. A retrospective study by Li DH et al also revealed that the SNCV of the median nerve and common peroneal nerve in the HGWD group and the mecobalamin group decreased after chemotherapy but were still greater in the HGWD group than in the mecobalamin group (P <0.5),70 which preliminarily confirm our findings.
Mecobalamin, a neurotrophic agent, also has certain benefits in the treatment of CIPN. Our meta-analysis compared the efficacy of HGWD and mecobalamin many times and revealed that HGWD has slightly greater advantages in general and that the combination of these two drugs has synergistic effects. However, in the prevention of more serious CIPN above grade 3, owing to the unstable results of the sensitivity analysis, we cautiously believe that the advantages of HGWD over mecobalamin have a certain tendency but are not clear, and the combination of medications does not have a better effect. The results of this study are the first systematic and comprehensive evaluation of the effects of HGWD in the treatment of CIPN caused by all drugs, which compensates for the lack of previous studies and achieved good results. The first four meta-analyses were limited to oxaliplatin-related CIPN. Tian Jet al’s meta-analysis included only 6 studies due to its early age;71 Chen SS’s research conclusion is similar to our research results, but owing to the lack of research samples, it is not suitable for meta-regression to explore heterogeneity. Instead, she chose to directly eliminate some studies with high heterogeneity from the meta-analysis, which may have led to bias.72 Wang Het al did not analyze other outcome indicators, such as the nerve conduction velocity or KPS score.73 Notably, our study makes up for several limitations of Yu J’s study.74 We nearly doubled the sample size and report new findings regarding the prevention of severe CIPN. The synergistic efficacy of combined drugs was explored in many ways, supporting the evidence that HGWD improves nerve conduction in the upper and lower limbs. Importantly, Yu J selected the wrong effect size for the synthesis of CIPN incidence data and nerve conduction velocity outcomes, and there was an error in data extraction: RRs should be used for the synthesis of drug RCTs rather than odds ratios (ORs). It is generally believed that ORs should be used in case‒control studies. There are very large differences between ORs and RRs, heterogeneity is easily masked, and the interpretation of RRs in the meta-analysis results of RCTs is clearer and more reasonable. The measurement method and unit value of the nerve conduction velocity are the same, so the weighted mean difference (WMD) should be selected instead of the standardized mean difference (SMD). It is hoped that more guideline-making groups will pay attention to the progress of interventions in the field of traditional Chinese medicine.
Limitations
Before the conclusions of this meta-analysis can be recommended to clinicians, it is important to fully consider the limitations of our study. First, all the included studies were conducted in China, which may have led to geographical bias. Second, most of the included RCTs had a small sample size, and the overall methodological quality was not high. Third, the existing studies of HGWD intervention in CIPN have focused mostly on taxanes and platinum drugs, whereas studies of other neurotoxic drugs are rare. Fourth, different chemotherapy regimens involve different drug types, doses and chemotherapy cycles. Fifth, although the components of HGWD in the included studies are generally similar, some researchers have improved HGWD according to the theoretical rules and clinical diagnosis and treatment characteristics of traditional Chinese medicine, so the types, doses, and treatment times of the herbs in various studies are not exactly the same, and there are certain differences. The improved and increased herbs are herbs that can tonify Qi and blood, activate blood and resolve stasis or dispel wind to free the collateral vessels. See Supplementary Table S6 and Supplementary Table S7 for differences in the main components. Although this can increase clinical efficacy, it negatively affects the reliability of the meta-analysis results. We call for relevant trials in the future to use the basic HGWD formula as much as possible and to strictly follow the authoritative list of RCT report specifications for traditional Chinese medicine to improve overall quality.75 Finally, most of the drugs used in the existing studies were mecobalamin. Only one study suggested that HGWD may have an advantage over duloxetine in alleviating CIPN symptoms. However, whether HGWD or duloxetine has more advantages remains to be determined. Therefore, the results should be interpreted cautiously. Further multicenter large-sample and rigorous clinical studies are needed to verify and update the results.
Conclusion
In summary, HGWD can effectively prevent the occurrence of CIPN, improve the symptoms and quality of life of patients with CIPN, improve the effect of chemotherapeutic drugs on the sensory nerve conduction velocity, and is safe. In the above aspects, HGWD or HGWD combined with positive drugs offers more advantages than positive drugs alone, but it has not been proven that these agents can also be used to prevent the occurrence of severe CIPN. More rigorous multicenter, large-sample, double-blind randomized controlled trials are needed to evaluate the efficacy and safety of using HGWD in combination with other positive control drugs.
Data Sharing Statement
Data is contained within the article and Supplementary Material.
Acknowledgments
Thank you for the support of the research platform of Chengdu University of Traditional Chinese Medicine.
Funding
This research was funded by the Scientific Research Practice and Innovation Project of Chengdu University of Traditional Chinese Medicine from 2023 to 2024 (ky-2024041).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155(12):2461–2470. doi:10.1016/j.pain.2014.09.020
2. Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol. 2010;6(12):657–666. doi:10.1038/nrneurol.2010.160
3. Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941–1967. doi:10.1200/jco.2013.54.0914
4. Timmins HC, Li T, Kiernan MC, Horvath LG, Goldstein D, Park SB. Quantification of Small Fiber Neuropathy in Chemotherapy-Treated Patients. J Pain. 2020;21(1–2)::44–58. doi:10.1016/j.jpain.2019.06.011
5. Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 2012;82(1):51–77. doi:10.1016/j.critrevonc.2011.04.012
6. Flatters SJL, Dougherty PM, Colvin LA. Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): a narrative review. Br J Anaesth. 2017;119(4):737–749. doi:10.1093/bja/aex229
7. Pike CT, Birnbaum HG, Muehlenbein CE, Pohl GM, Natale RB. Healthcare costs and workloss burden of patients with chemotherapy-associated peripheral neuropathy in breast, ovarian, head and neck, and nonsmall cell lung cancer. Chemother Res Pract. 2012;2012:913848. doi:10.1155/2012/913848
8. Jordan B, Margulies A, Cardoso F, et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO-EONS-EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann Oncol. 2020;31(10):1306–1319. doi:10.1016/j.annonc.2020.07.003
9. Loprinzi CL, Lacchetti C, Bleeker J, et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J Clin Oncol. 2020;38(28):3325–3348. doi:10.1200/jco.20.01399
10. Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309(13):1359–1367. doi:10.1001/jama.2013.2813
11. Chow R, Novosel M, So OW, et al. Duloxetine for prevention and treatment of chemotherapy-induced peripheral neuropathy (CIPN): systematic review and meta-analysis. BMJ Supp Palli Care. 2023;13(1):27–34. doi:10.1136/spcare-2022-003815
12. Liu X, Li M, Wang X, et al. Effects of adjuvant traditional Chinese medicine therapy on long-term survival in patients with hepatocellular carcinoma. Phytomedicine. 2019;62:152930. doi:10.1016/j.phymed.2019.152930
13. Pan J, Jia Y, Shi J, Yao R, Guo J. The efficacy and safety of compound kushen injection for adults with esophageal cancer: a meta-analysis of randomized controlled trials. J Ethnopharmacol. 2024;322:117604. doi:10.1016/j.jep.2023.117604
14. Cao P, Huo JG. Treatment of chemotherapy-induced Peripheral Neuropathy from Blood arthralgia. Guangming Tradit Chin Med. 2013;28(01):12–13+15. Chinese. doi:10.3969/j.issn.1003-8914.2013.01.007
15. Cheng X, Huo J, Wang D, et al. Herbal Medicine AC591 Prevents Oxaliplatin-Induced Peripheral Neuropathy in Animal Model and Cancer Patients. Front Pharmacol. 2017;8:344. doi:10.3389/fphar.2017.00344
16. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi:10.1136/bmj.n160
17. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi:10.1136/bmj.d5928
18. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi:10.1136/bmj.39489.470347.AD
19. Li Y, Cui HJ, Huang JC, Wu XQ. Clinical study of Jiawei Huangqi Guizhi Wuwu Decoction in preventing and treating peripheral neuro-sensory toxicity caused by oxaliplatin. Chin J Integr Med. 2006;12(1):
20. Hu GY, Jiang J. Observation on Jiajian Huangqi Guizhi Wuwu Decoction in the treatment of oxaliplatin induced neurotoxicity. Mod J Integr Med. 2010;19(11):1350–1351. Chinese. doi:10.3969/j.issn.1008-8849.2010.11.031
21. Huang ZB, Huang ZM, Chen GQ, et al. Clinical study on external bath of ”Huangqi Guizhi Decoction” in relieving peripheral neurotoxicity induced by Oxaliplatin. Shanghai J Tradit Chin Med. 2010;44(05):40–42. Chinese. doi:10.16305/j.1007-1334.2010.05.016
22. Lin HM, Luo K. Nursing observation on Jiajian Huangqi Guizhi Wuwu decoction in the treatment of peripheral neurotoxicity caused by oxaliplatin. Chin Gen Pract Nurs. 2011;9(6). Chinese. doi:10.3969/j.issn.1674-4748.2011.06.010
23. Liu H, Zhou ZY, Wu LY, et al. Clinical research on the effects of ”Huangqi Guizhi Wuwu Decoction” on peripheral neurotoxicity induced by oxaliplatin. Shanghai J Tradit Chin Med. 2011;(3):2011480967. Chinese. doi:10.16305/j.1007-1334.2011.03.030
24. Wang YA. Clinical observation and study on Jiawei Huangqi Guizhi Wuwu Decoction in the Prevention and treatment of peripheral neuropathy caused by oxaliplatin. Zhejiang J Integr Med. 2011;21(9):2011760003. Chinese. doi:10.3969/j.issn.1005-4561.2011.09.031
25. Xu HJ. Clinical observation on the prevention of oxaliplatin neurotoxicity by Jiawei Huangqi Guizhi Wuwu Decoction. Clin J Tradit Chin Med. 2011;23(08):669–670. Chinese. doi:10.16448/j.cjtcm.2011.08.006
26. Zhou YQ, Liu H. Observation on the therapeutic effect of Jiajian Huangqi Guizhi Wuwu Decoction in preventing and treating peripheral neurotoxicity induced by vincristine chemotherapy. Chin J Inf Tradit Chin Med. 2011;18(2):2011379145. Chinese. doi:10.3969/j.issn.1005-5304.2011.02.038
27. He YY. Clinical observation and study on Jiawei Huangqi Guizhi Wuwu Decoction in the treatment of peripheral neuropathy caused by oxaliplatin. J Shandong Univ Tradit Chin Med. 2012;36(01):42–43. Chinese. doi:10.16294/j.cnki.1007-659x.2012.01.017
28. Wang F, Wang YH. Observation on the Prevention and Treatment of Acute Peripheral Neurotoxicity of Oxaliplatin with Jiawei Huangqi Guizhi Wuwu Decoction. Clin J Tradit Chin Med. 2012;24(4):2012556145. Chinese. doi:10.16448/j.cjtcm.2012.04.033
29. Cao SJ. Observation on the efficacy of modified Huangqi Guizhi Wuwu Decoction in preventing and treating oxaliplatin induced peripheral neurotoxicity. Guangming Tradit Chin Med. 2013;28(01):88–89. Chinese. doi:10.3969/j.issn.1003-8914.2013.01.041
30. Li DM, Wang R, Xie J. Clinical Study on Huangqi Guizhi Wuwu Decoction Treating the Neuro-sensory Toxicity Caused by Oxaliplatin. J Nanjing Univ Tradit Chin Med. 2014;30(2):2014326560. Chinese. doi:10.14148/j.issn.1672-0482.2014.02.011
31. Qiu SA, Jiang XQ. Observation on the therapeutic effect of Huangqi Guizhi Wuwu Decoction on oxaliplatin induced neurotoxicity and hematotoxicity. Shangdong Med J. 2014;54(06):88–89. Chinese. doi:10.3969/j.issn.1002-266X.2014.06.035
32. Wu J, Xing LJ, Xing YY, Xiang P, Song ZJ. Clinical study on the prevention and treatment of chemotherapy side effects caused by oxaliplatin with Huangqi Guizhi Wuwu Decoction. Yanbian Med J. 2014. 36:73–75. Chinese
33. Yu B, Su ZX, Yuan Y, Wu HM. Observation on the therapeutic effect of Jiajian Huangqi Guizhi Wuwu Decoction in preventing and treating neurotoxicity caused by TP regimen chemotherapy. J Chengdu Univ Tradit Chin Med. 2014;37(02):18–20. Chinese. doi:10.13593/j.cnki.51-1501/r.2014.02.018
34. Shen J, He SL, Sun XJ, Hu NH, Cai YY. Clinical Study on External Bath of Modified Huangqi Guizhi Wuwu Decoction for Peripheral Neurotoxicity Induced by Oxaliplatin. Chin J Inf Tradit Chin Med. 2015;22(11):2016101774. Chinese. doi:10.3969/j.issn.1005-5304.2015.11.004
35. Wu GN, Yao XQ, Wu XY, Liu FK. Observation on the therapeutic effect of Jiajian Huangqi Guizhi Wuwu Decoction on postoperative chemotherapy induced peripheral neurotoxicity. Sichuan J Tradit Chin Med. 2015;33(12):2016207093.
36. Wu TT, Jin Y, Zhong Y, et al. Efficacy of Huangqi Guizhi Wuwu decoction combined with needle warming moxibustion on peripheral neurotoxicity and immunologic function of patients with malignant cancer after chemotherapy. Shangdong Med J. 2015;(33):2017467365. Chinese. doi:10.3969/j.issn.1002-266X.2015.33.001
37. Yu ZQ. Observation on the therapeutic effect of Huangqi Guizhi Wuwu Decoction hand and foot bath on the prevention and treatment of oxaliplatin neurotoxicity. Health Way. 2015;14(09):223. Chinese. doi:10.3969/j.issn.1671-8801.2015.09.225
38. Mu DC. Clinical efficacy analysis of Jiajian Huangqi Guizhi Wuwu Decoction in preventing chemotherapy-induced peripheral neuropathy. Asia-Pac Trad Med. 2016;12(23):2017152213. Chinese. doi:10.11954/ytctyy.201623066
39. Xu XR. The preventive and therapeutic effects of Jiajian Huangqi Guizhi Wuwu Decoction on neurotoxicity caused by TP regimen chemotherapy in ovarian cancer. Shanxi J Tradit Chin Med. 2016;37(04):396–397. Chinese. doi:10.3969/j.issn.1000-7369.2016.04.005
40. Yan JF, Sun MK, Lin SF, Liu ZH, Tao L. Clinical study on the prevention and treatment of peripheral neurotoxicity caused by oxaliplatin with Jiajian Huangqi Guizhi Wuwu Decoction fumigation and washing. Asia-Pac Trad Med. 2016;12(19):125–126. Chinese. doi:10.11954/ytctyy.201619055
41. Xu CX, Hu XM, Xu SH. Clinical observation of modified Huangqi Guizhi Wuwu Decoction in the prevention and treatment of peripheral neurotoxicity induced by oxaliplatin. Shanghai J Tradit Chin Med. 2017;51(1):2017232574. Chinese. doi:10.16305/j.1007-1334.2017.01.015
42. Yang Y. [Clinical Observation on the Prevention of Peripheral Neurotoxicity Caused by Oxaliplatin with Huangqi Guizhi Wuwu Decoction]. Master. Nanjing: Nanjing University of Chinese Medicine; 2017.
43. Gong SX, Xue Q, You JL. Observation on the therapeutic effect of Jiawei Huangqi Guizhi Wuwu Decoction on peripheral neurotoxicity and hand foot syndrome caused by XELOX chemotherapy in patients with colorectal cancer. Guid J Tradit Chin Med Pharm. 2018;24(2):2018212985. Chinese. doi:10.13862/j.cnki.cn43-1446/r.2018.02.036
44. Ma XZ, Zhang SY, Tang Y, He XH, Fan JX. A Study on the Combination of Huangqi Guizhi Wuwu Decoction and Mecobalamin in the Treatment of Peripheral Neurotoxicity after TP Chemotherapy. Contemp Med. 2018;24(4):2018222489. Chinese. doi:10.3969/j.issn.1009-4393.2018.04.024
45. Ma J, Wei GL, Zhu LJ, et al. A multicenter, randomized, double-blind, controlled clinical study on the prevention and treatment of oxaliplatin neurotoxicity with Jiawei Huangqi Guizhi Wuwu formula granules. World Sci Technol Mod Tradit Chin Med Mater Med. 2019;21(07):1495–1504. Chinese. doi:10.11842/wst.2019.07.031
46. Sun P, Chen J, Feng ZM, et al. Clinical Analysis on Treatment of Neurotoxicity after Chemotherapy with Fumigation with Huangqi Guizhi Wuwu Decoction. Liaoning J Tradit Chin Med. 2020;(5):2020612134. Chinese. doi:10.13192/j.issn.1000-1719.2020.05.043
47. Guo HL. Clinical Effect of Huangqi Guizhi Wuwu Decoction Fumigation Combined with Moxibustion on Peripheral Neurotoxicity Induced by Oxaliplatin of Malignant Tumor Patients. J Zhejiang Univ Tradit Chin Med. 2021;45(5):2021337757. Chinese. doi:10.16466/j.issn1005-5509.2021.05.016
48. Dai HQ, Ding XL, Gu ZC. Clinical observation and study on Jiawei Huangqi Guizhi Wuwu Decoction in the treatment of peripheral neuropathy caused by oxaliplatin. Med Health. 2022;10.
49. Chen C, Ma HL, Gu YX, et al. Clinical effect of modified Huangqi Guizhi Wuwu Decoction in the treatment of peripheral neurotoxicity induced by Oxaliplatin. Chin Med Herald. 2023;20(10):122–125. Chinese. doi:10.20047/j.issn1673-7210.2023.10.27
50. Qi LX, Guo ZF, Zhou LJ, Huang PC. Effects of Modified Huangqi Guizhi Wuwu Decoction on Chemotherapy-Induced Peripheral Neuropathy in Patients with Malignant Tumor. Inf Tradit Chin Med. 2023;40(3):2023228442.
51. Fujita S, Hirota T, Sakiyama R, Baba M, Ieiri I. Identification of drug transporters contributing to oxaliplatin-induced peripheral neuropathy. J Neurochem. 2019;148(3):373–385. doi:10.1111/jnc.14607
52. Islam B, Lustberg M, Staff NP, Kolb N, Alberti P, Vinca Alkaloids AAA. thalidomide and eribulin-induced peripheral neurotoxicity: From pathogenesis to treatment. J Peripher Nerv Syst. 2019;24(2):S63–s73. doi:10.1111/jns.12334
53. Chen Z, Doyle TM, Luongo L, et al. Sphingosine-1-phosphate receptor 1 activation in astrocytes contributes to neuropathic pain. Proc Natl Acad Sci U S A. 2019;116(21):10557–10562. doi:10.1073/pnas.1820466116
54. Lv Z, Shen J, Gao X, et al. Herbal formula Huangqi Guizhi Wuwu decoction attenuates paclitaxel-related neurotoxicity via inhibition of inflammation and oxidative stress. Chin Medicine. 2021;16(1):76. doi:10.1186/s13020-021-00488-1
55. Li M, Li Z, Ma X, et al. Huangqi Guizhi Wuwu Decoction can prevent and treat oxaliplatin-induced neuropathic pain by TNFα/IL-1β/IL-6/MAPK/NF-kB pathway. Aging (Albany NY). 2022;14(12):5013–5022. doi:10.18632/aging.203794
56. Wu AP, Wu LL, Zhang HY, Wei GL, Ma J. Prevention of modified Huangqi Guizhi Wuwu decoction on inhibiting mitochondrial apoptosis mechanism of oxaliplatin peripheral neurotoxicity. Med J West China. 2023;35(2):2023163689. Chinese. doi:10.3969/j.issn.1672-3511.2023.02.006
57. Huo JG, Hu Y, Yang J, et al. Effect of Huangqi Guizhi Wuwu Decoction on Chemotherapy-induced Peripheral Never Injury in Rats. J Tradit Chin Med. 2012;53(23):2031–2034. Chinese. doi:10.13288/j.11-2166/r.2012.23.025
58. Gu ZC. [The Protective Effects of Huangqiguizhiwuwu Decoction on Oxaliplatin-Induced Peripheral Neuropathy Based on the Mechanism of Platinum accumulation]. Master. Nanjing: Nanjing University of Chinese Medicine; 2016.
59. Zhang Z, Ye J, Liu X, et al. Huangqi Guizhi Wuwu decoction alleviates oxaliplatin-induced peripheral neuropathy via the gut-peripheral nerve axis. Chin Med. 2023;18(1):114. doi:10.1186/s13020-023-00826-5
60. Zheng Y, Yang F, Han L, et al. Efficacy of Chinese Herbal Medicine in the Treatment of Moderate-Severe Painful Diabetic Peripheral Neuropathy: a Retrospective Study. J Diabetes Res. 2019;2019:4035861. doi:10.1155/2019/4035861
61. Pang B, Zhao TY, Zhao LH, et al. Huangqi Guizhi Wuwu Decoction for treating diabetic peripheral neuropathy: a meta-analysis of 16 randomized controlled trials. Neural Regeneration Research. 2016;11(8):1347–1358. doi:10.4103/1673-5374.189202
62. Liang CL, Zhang Y, Chen QY, Zhuo PP. Efficacy of Multiple Drugs in Preventing Chronic Peripheral Neuropathy Induced by Platinum and Taxane: a Network Meta-analysis. Cancer Res Prev Treat. 2022;49(02):128–140. Chinese. doi:10.3971/j.issn.1000-8578.2022.21.0675
63. Cho Y, Ruddy KJ, Lavoie Smith EM. Evaluation of Chemotherapy-Induced Peripheral Neuropathy. In: Lustberg M, Loprinzi C, editors. Diagnosis, Management and Emerging Strategies for Chemotherapy-Induced Neuropathy: A MASCC Book. Springer International Publishing; 2021:53–93.
64. Li T, Park SB, Battaglini E, et al. Assessing chemotherapy-induced peripheral neuropathy with patient reported outcome measures: a systematic review of measurement properties and considerations for future use. Qual Life Res. 2022;31(11):3091–3107. doi:10.1007/s11136-022-03154-7
65. Zhu Q. [The Clinical Study of Huangqi Guizhi Wuwu Decoction for Prevention and Treatment of Paclitaxel-Induced Peripheral Neuropathy]. Chengdu: Chengdu University of Traditional Chinese Medicine; 2021.
66. Chai Y, Zhao F, Ye P, et al. A Prospective, Randomized, Placebo-Controlled Study Assessing the Efficacy of Chinese Herbal Medicine (Huangqi Guizhi Wuwu Decoction) in the Treatment of Albumin-Bound Paclitaxel-Induced Peripheral Neuropathy. Article J Clin Med. 2023;12(2). doi:10.3390/jcm12020505
67. Xu WR, Yu MW, Fu Q. Retrospective Study on Modified Huangqi Guizhi Wuwu Decoction in Treating Oxaliplatin-Induced Peripheral Neuropathy. J Guangzhou Univ Tradit Chin Med. 2022;39(1):2022206474. Chinese. doi:10.13359/j.cnki.gzxbtcm.2022.01.005
68. Argyriou AA, Park SB, Islam B, et al. Neurophysiological, nerve imaging and other techniques to assess chemotherapy-induced peripheral neurotoxicity in the clinical and research settings. J Neurol Neurosurg Psychi. 2019;90(12):1361–1369. doi:10.1136/jnnp-2019-320969
69. Matsuoka A, Mitsuma A, Maeda O, et al. Quantitative assessment of chemotherapy-induced peripheral neurotoxicity using a point-of-care nerve conduction device. Cancer Sci. 2016;107(10):1453–1457. doi:10.1111/cas.13010
70. Li DH, Wang AH, Yu J, Sun X, Li R. Effects of Modified Huangqi Guizhi Wuwu Decoction on the Prevention and Treatment of Neurotoxicity and Nerve Conduction Velocity Induced by Chemotherapy in Ovarian Cancer Patients. Med Innov Chin. 2020;17(11):82–85. Chinese. doi:10.3969/j.issn.1674-4985.2020.11.020
71. Tian J, Yao XQ, Wu XY, et al. Systematic Review and Meta Analysis on Efficacy of Huangqi Guizhi Wuwu Decoction for Oxaliplatin-induced Peripheral Neurotoxicity. Chin J Exp Tradit Med Form. 2013;19(22):2014147485. Chinese. doi:10.11653/syfj2013220325
72. Chen SS. [Prevention of Oxaliplatin-Included Peripheral Neurotoxicity by Huangqi Guizhi Wuwu Decoction: A Systematic Review and Meta-Analysis]. Nanjing: Nanjing University Of Chinese Medicine; 2018.
73. Wang H, Wei XC, Zhu LQ, Wang CG, Deng Q, Li X. Systematic evaluation of efficacy and safety of Huangqi Guizhi Wuwu Decoction for preventing oxaliplatin-induced peripheral neurotoxicity. Int J Biomed Eng. 2020;43(1):2020656956. Chinese. doi:10.3760/cma.j.issn.1673-4181.2020.01.004
74. Yu J, Chen S, Wei G, et al. Efficacy and Safety of Huangqi Guizhi Wuwu Decoction for Oxaliplatin-Induced Peripheral Neurotoxicity: a Systematic Review and Meta-Analysis. Altern Ther Health Med. 2024;30(1):446–453.
75. Cheng CW, Wu TX, Shang HC, et al. CONSORT Extension for Chinese Herbal Medicine Formulas 2017: recommendations, Explanation, and Elaboration. Ann Intern Med. 2017;167(2):112–121. doi:10.7326/m16-2977
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




