Back to Journals » International Journal of Nanomedicine » Volume 19
The Advancing Role of Nanocomposites in Cancer Diagnosis and Treatment
Authors Andoh V , Ocansey DKW , Naveed H, Wang N, Chen L , Chen K , Mao F
Received 29 March 2024
Accepted for publication 12 June 2024
Published 19 June 2024 Volume 2024:19 Pages 6099—6126
DOI https://doi.org/10.2147/IJN.S471360
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. RDK Misra
Vivian Andoh,1,* Dickson Kofi Wiredu Ocansey,2,3,* Hassan Naveed,1 Naijian Wang,4 Liang Chen,1 Keping Chen,1 Fei Mao2
1School of Life Sciences, Jiangsu University, Zhenjiang, People’s Republic of China; 2Department of Laboratory Medicine, Lianyungang Clinical College, Jiangsu University, Lianyungang, Jiangsu, People’s Republic of China; 3Directorate of University Health Services, University of Cape Coast, Cape Coast, Central Region, CC0959347, Ghana; 4Key Laboratory of Medical Science and Laboratory Medicine of Jiangsu Province, School of Medicine, Jiangsu University, Zhenjiang, Jiangsu, People’s Republic of China
*The first two authors contributed equally to this work
Correspondence: Fei Mao, Department of Laboratory Medicine, Lianyungang Clinical College, Jiangsu University, Lianyungang, Jiangsu, 222006, People’s Republic of China, Tel/Fax +86 511 8503 8215, Email [email protected]
Abstract: The relentless pursuit of effective cancer diagnosis and treatment strategies has led to the rapidly expanding field of nanotechnology, with a specific focus on nanocomposites. Nanocomposites, a combination of nanomaterials with diverse properties, have emerged as versatile tools in oncology, offering multifunctional platforms for targeted delivery, imaging, and therapeutic interventions. Nanocomposites exhibit great potential for early detection and accurate imaging in cancer diagnosis. Integrating various imaging modalities, such as magnetic resonance imaging (MRI), computed tomography (CT), and fluorescence imaging, into nanocomposites enables the development of contrast agents with enhanced sensitivity and specificity. Moreover, functionalizing nanocomposites with targeting ligands ensures selective accumulation in tumor tissues, facilitating precise imaging and diagnostic accuracy. On the therapeutic front, nanocomposites have revolutionized cancer treatment by overcoming traditional challenges associated with drug delivery. The controlled release of therapeutic agents from nanocomposite carriers enhances drug bioavailability, reduces systemic toxicity, and improves overall treatment efficacy. Additionally, the integration of stimuli-responsive components within nanocomposites enables site-specific drug release triggered by the unique microenvironment of the tumor. Despite the remarkable progress in the field, challenges such as biocompatibility, scalability, and long-term safety profiles remain. This article provides a comprehensive overview of recent developments, challenges, and prospects, emphasizing the transformative potential of nanocomposites in revolutionizing the landscape of cancer diagnostics and therapeutics. In Conclusion, integrating nanocomposites in cancer diagnosis and treatment heralds a new era for precision medicine.
Keywords: nanocomposites, cancer, diagnosis, therapy, nanoparticles, theranostic
Graphical Abstract:
Introduction
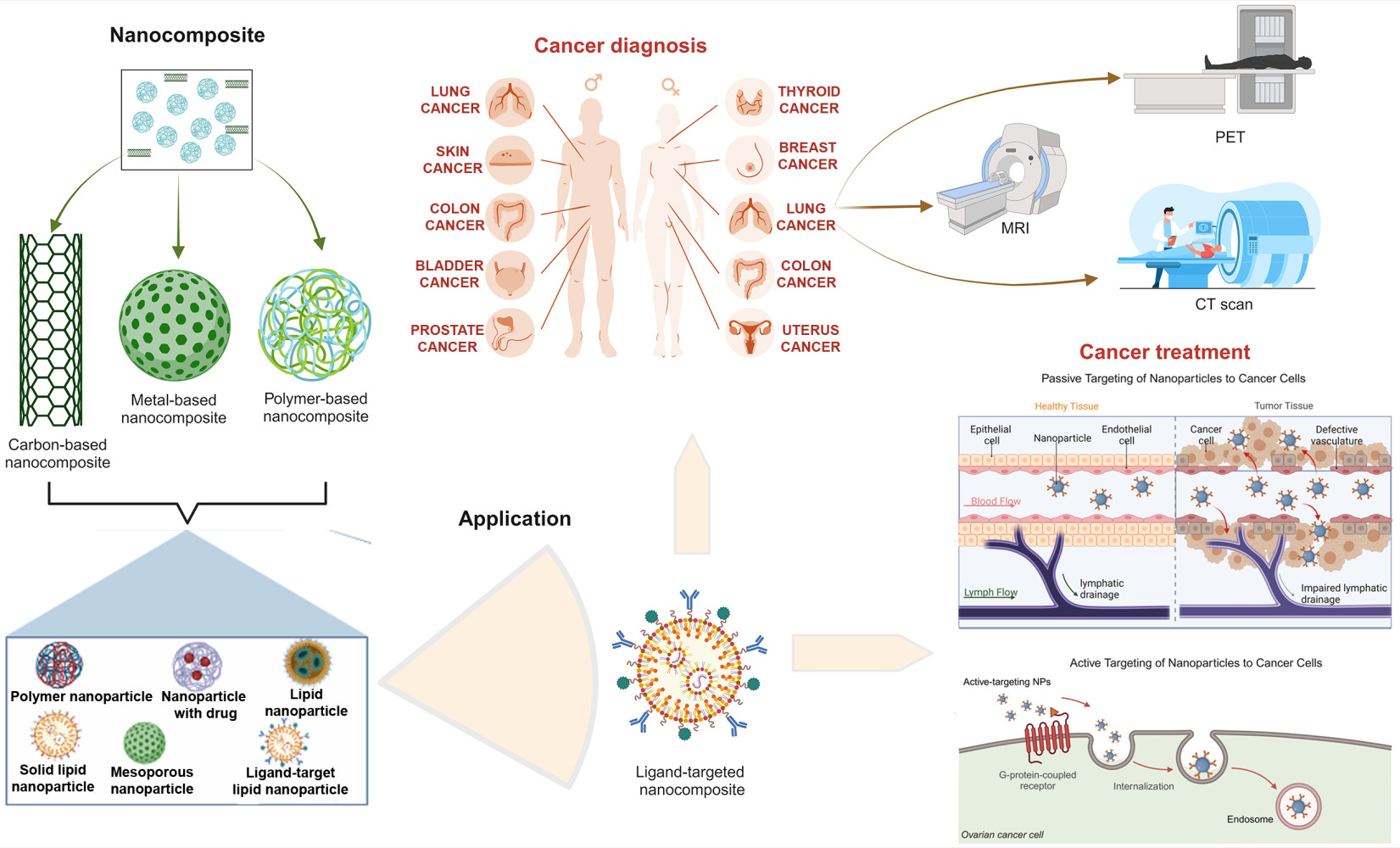
Nanocomposites belong to a broader class of intentionally produced materials called nanomaterials, which incorporate nanosized particles into a matrix of standard materials. These nanosized particles can have one, two, or three dimensions of less than 100 nanometers.1 Integrating nanoscale components imparts unique characteristics to the composite material, making nanocomposites valuable in various applications, including healthcare.2 The tunable properties of nanocomposites, such as biocompatibility, stimuli responsiveness, and functionality, have deemed them ideal platforms for diagnosing and treating chronic diseases, including cancer, diabetes, and cardiovascular disease.2,3 In the phase of increasing cancer cases and deaths, there is a growing global cancer burden and a need for effective theranostic strategies. Researchers aim to speed up cancer diagnosis with efforts to reduce waiting times and enhance the efficiency of the diagnostic process for patients.4 Nanocomposites have emerged as unique particles, promising to revolutionize cancer diagnosis and treatment.
In their specific application in cancer, nanocomposites play a crucial role in advancing cancer diagnosis and treatment as they are employed in various applications for precise and effective strategies in the fight against cancer.5 While nanomaterials contribute to precise applications for cancer detection and diagnosis, aiming to synthesize nanomaterials for improved efficacy,6 nanotechnology, which encompasses applications like drug delivery, has yielded promising results in cancer diagnosis and treatment and continues to contribute to advancements in the field.7 For instance, the application of molybdenum disulfide (MoS2)-based nanocomposites, including its biosensors, bioimaging, photoredox catalysis, and antibacterial properties, show promise in cancer diagnosis and therapy and contribute to the development of innovative approaches.5,7 Various nanocomposites, such as gold nanorods loaded, chitosan conjugated, and pluronic-based nanocarriers, serve as imaging agents for cancer cells and hyperthermic agents, showcasing their versatility in cancer diagnosis and treatment.8 Moreover, bio-nanotechnology, a subset of nanotechnology, is applied in tumor diagnosis and treatment, providing effective support through targeting and gene therapy.8,9 For example, nanocomposites of graphene oxide-silver nanoparticles and cisplatin strongly potentiate cisplatin-induced cytotoxicity, apoptosis, and autophagy in human cancer cells.10 A bio-graphene-based multifunctional nanocomposites also exhibited intracellular drug delivery in cervical cancer treatment.9
In diagnostics, nanocomposites serve as both imaging agents and biosensors. Metal-based nanomaterials and nanocomposites are utilized as contrast agents in cancer imaging, gaining attention for their unique properties in diagnostics.11 Bio-nanotechnology-based cell biosensors also exhibit high sensitivity and speed in detecting and distinguishing different types of tumor cells, contributing to tumor diagnosis.8 Nanoparticles serve as effective drug carriers for diseases like cancer due to their small size and increased stability, enhancing targeted drug delivery partly via encapsulating active pharmaceutical ingredients.12 Considering the growing interest and rapid data expansion in this field, we review the progress made in recent years. The synthesis and characterization of nanocomposites and their application in cancer diagnosis and treatment are explored. The advances made, challenges, and prospects are also discussed.
Synthesis and Characterization of Nanocomposites for Cancer Applications
The synthesis and characterization of nanocomposites for cancer applications involve a series of crucial steps determining their efficacy and safety. The synthesis typically involves the combination of nanoparticles with various polymers or other materials to form a composite structure. Various techniques can be employed for synthesis, including the sol-gel method, in-situ polymerization, and layer-by-layer (LbL) assembly.13,14 While the sol-gel method involves the hydrolysis and condensation of precursors to form a gel network that encapsulates nanoparticles, the in-situ polymerization involves the polymerization of monomers in the presence of nanoparticles, leading to their incorporation into the polymer matrix. The LbL assembly involves a sequential deposition of oppositely charged nanoparticles and polymers to form a multilayer structure.13–15 Characterization techniques play a pivotal role in evaluating the properties and performance of nanocomposites for cancer applications. Common characterization techniques include dynamic light scattering (DLS), scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FTIR)14–17 (Figure 1). Each of these techniques provides a unique assessment of the properties of the nanocomposite. DLS measures the particle size distribution of nanoparticles in the nanocomposite, SEM provides high-resolution images of the surface morphology and structure, TEM offers detailed information about the internal structure and morphology, and XRD determines the crystal structure and crystallinity of the nanocomposite. Moreover, FTIR is used to identify the functional groups in the nanocomposite.16,18 Researchers can design nanocomposites with optimized properties for cancer diagnosis, therapy, and theranostics by carefully tailoring the synthesis and characterization processes. These nanocomposites offer promising advancements in cancer treatment by enhancing drug delivery, targeting tumor cells, and improving therapeutic efficacy while minimizing side effects.
The synthesis and characterization of nanocomposites for cancer applications also involve developing advanced materials with unique properties tailored for diagnosis and treatment. Recent research highlights notable examples include smart supermagnetic nanocomposites,14 Yttrium oxide nanocomposites,19 ZnO–TiO2–Chitosan–Escin nanocomposites,15 branched magnetic nanocomposites,18 and Copper Oxide–Titanium Oxide–Chitosan–Amygdalin nanocomposites.17 Supermagnetic nanocomposites, based on iron oxide nanoparticles coated with Pluronic F127, have been synthesized for targeted drug delivery in cancer therapy,14 and polymer-gated and superparamagnetic nanoparticle embedded hollow mesoporous silica nanoparticles as a smart multifunctional nanocarrier for targeted and controlled drug delivery for cancer treatment.20 These studies emphasize nanocomposites’ smart design for potential cancer treatment applications. Other nanocomposites have been synthesized to carry bio-macromolecules and therapeutic agents,18,20 implicating the potential of these nanocomposites for cancer diagnostic and therapeutic applications.
Regardless of the progress made in this field, the synthesis and characterization of nanocomposites present several key challenges and considerations that must be addressed to ensure the successful development of these materials with desired properties. These challenges are broadly categorized into synthesis challenges (including uniform dispersion of nanoparticles, surface interactions, and scalability of synthesis methods)21–23 and characterization challenges (including accuracy in characterizing the size, shape, and distribution, interfacial characterization, and property evaluation).21,22,24 Addressing these challenges and considerations is crucial for advancing nanocomposite technology and developing materials with tailored properties for a wide range of applications.
Nanocomposites in Cancer Diagnosis
Nanocomposites have emerged as promising materials for cancer diagnosis due to their unique properties that can enhance sensitivity, specificity, and imaging resolution.25,26 These materials are composed of two or more phases at the nanoscale, with one phase typically being a nanoparticle and the other being a polymer or other matrix material. The nanoparticles can be tailored to interact with specific cancer biomarkers, while the matrix material provides stability and biocompatibility.7,27 The use of nanocomposites in cancer diagnosis offers several advantages over traditional diagnostic methods. Nanocomposites exhibit enhanced sensitivity as they can detect smaller amounts of cancer cells or biomarkers, leading to earlier detection of cancer7 and improved specificity as they can be targeted to specific cancer cells, reducing the risk of false positives.26 More importantly, nanocomposites possess multifunctional capabilities and combine diagnostic and therapeutic functions, enabling theranostic approaches.7 Several types of nanocomposites have been investigated for cancer diagnosis, including metal-, carbon-, and polymer-based nanocomposites.
Gold, silver, and iron oxide nanoparticles are commonly used metal-based nanoparticles in cancer diagnostics. These nanoparticles can be conjugated to antibodies or other targeting ligands to specifically bind to cancer cells. They serve as contrast agents and can be detected using various imaging techniques, such as computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET)25,26 (Figure 2). Interestingly, metal-based nanoparticles induce antitumor immune responses, contributing to their potential in cancer diagnostics by leveraging cellular and molecular mechanisms.28,29 Carbon nanotubes, graphene oxide, and fullerene derivatives have also been explored for cancer diagnosis as carbon-based nanomaterials. These nanomaterials have unique optical properties that can be used for fluorescence or photoacoustic imaging. They can also be used to deliver diagnostic agents or drugs to cancer cells.25,26 Polymeric nanocomposites can be designed with controlled size, shape, and surface properties, making them suitable for various diagnostic applications. They can encapsulate diagnostic agents, such as fluorescent dyes or radioactive isotopes, and release them in a controlled manner at the tumor site.7 Despite the promising potential of these nanocomposites in cancer diagnosis, further research is needed to address challenges such as biocompatibility, long-term safety, and scalable production.
Properties and Mechanisms of Nanocomposites in Cancer Diagnosis
High Surface Area
Nanoparticles have a large surface area-to-volume ratio, allowing them to interact with many molecules. This makes them ideal for carrying and delivering diagnostic agents. The high surface area, which arises from the presence of nanoscale features, contributes to various enhanced properties and functionalities in nanocomposite materials, including increased strength and stiffness, enhanced thermal conductivity, improved electrical conductivity, enhanced gas barrier properties, increased catalytic activity, and improved drug delivery systems.30,31 For example, in polymer-based nanocomposites such as carbon nanotube-polymer nanocomposites, the addition of nanoscale reinforcements like clay nanoparticles can significantly increase the strength and stiffness of the composite material. The high surface area of the nanoparticles allows for a more efficient load transfer within the matrix, leading to improved mechanical properties.32 Carbon nanotubes are known for their excellent thermal conductivity. When incorporated into a polymer matrix, the high surface area of individual nanotubes facilitates efficient heat transfer between them, resulting in a nanocomposite with enhanced thermal conductivity compared to the pure polymer.32,33 In addition, titanium dioxide (TiO2) nanocomposites: TiO2, a widely used photocatalyst, can be incorporated into nanocomposites to enhance their photocatalytic activity.34 TiO234 and zeolite-polymer nanocomposites (porous, crystalline aluminosilicates)35 have large surface areas, which provide more active sites for photocatalysis and improved separation and catalytic properties, respectively. These advancements make nanocomposites promising materials for various applications in healthcare, including cancer diagnosis.
Biocompatibility
Nanocomposites can be designed to be biocompatible, ensuring their safe use in the body. This is important for cancer diagnosis, as diagnostic agents need to be able to reach tumors without causing harm to healthy tissues. Several factors influence the Biocompatibility of nanocomposites, including the type of nanofiller, its size, shape, surface chemistry, and the distribution of the nanofiller within the matrix.28,29 Current data shows that biodegradable and biocompatible polymer nanocomposites are gaining traction in biomedical applications and are often used in diagnostic and drug delivery systems, showing compatibility with biological systems and reducing the risk of adverse reactions.36 Metal oxide nanocomposites, such as zinc oxide (ZnO) and titanium dioxide (TiO2),37 and carbon-based nanocomposites, such as carbon nanotubes and graphene,38 have been shown to be biocompatible and can be used in biosensors for detecting various analytes, including glucose, DNA, and proteins. Degradable nanocomposites are particularly appealing for diagnostic applications as the body can break them down after fulfilling their diagnostic purpose, minimizing the risk of long-term accumulation.39 In immunological applications, nanomaterials revolutionize medicine by enabling novel sensing, diagnostic, and therapeutic approaches. Their biocompatibility is crucial in applications that involve interaction with the immune system for targeted diagnostics or drug delivery.28 The biocompatibility of nanocomposites is an ongoing area of research, and new materials with improved biocompatibility are being developed. As our understanding of the factors that influence biocompatibility deepens, nanocomposites are poised to play an increasingly important role in various diagnostic applications, including cancer.
Targeted Delivery
Nanocomposites play a crucial role in cancer diagnosis through targeted delivery systems, offering enhanced precision and effectiveness of cancer detection, leading to earlier diagnosis and improved treatment outcomes. Nanoparticles can be conjugated to antibodies or other targeting ligands to specifically bind to cancer cells.40 This allows for the accumulation of diagnostic agents at the tumor site, improving sensitivity and reducing off-target effects. For instance, MoS2-based nanocomposites are employed for cancer diagnosis and imaging. These nanomaterials, integrated with targeting ligands, enable selective binding to cancer cells, improving the accuracy of diagnostic imaging.7 Additionally, magnetic nanoparticles can be manipulated using external magnetic fields, allowing for targeted delivery to specific regions of the body. Once internalized within tumor cells, nanocomposites can release diagnostic agents, such as fluorescent dyes or molecular probes, which can specifically bind to or interact with tumor-associated biomarkers.41,42 The emitted fluorescence or molecular signals can then be detected using various imaging techniques, such as fluorescence microscopy or bioluminescence imaging, providing precise information about the location and extent of tumor growth.43 With ongoing efforts to optimize design and characterization for specific diagnostic applications, this active area of research offers great promise for cancer diagnosis.
Enhanced and Targeted Imaging
Mechanisms contributing to enhanced and targeted imaging include enhanced permeability and retention effect, active targeting, and targeted delivery. Nanoparticles with small sizes can exploit the leaky vasculature of tumors, allowing them to extravasate into the tumor tissue and accumulate there.44 Nanocomposites in targeted imaging can incorporate various imaging agents, such as fluorescent dyes, radioisotopes, or magnetic nanoparticles, which can be used to visualize tumors using different imaging techniques, such as fluorescence imaging, positron emission tomography (PET) or magnetic resonance imaging (MRI). Moreover, Nanoparticles conjugated to antibodies can be used to target and image cancer cells using CT or MRI.45 Nanocomposites facilitate deeper tissue penetration and improved imaging capabilities, aiding in the precise diagnosis of cancer. The distinctive optical, magnetic, and chemical characteristics of nanoscale materials enable the development of imaging probes featuring enhanced contrast, heightened sensitivity, regulated biodistribution, improved spatial and temporal details, versatile functionality, and the ability to engage in multimodal imaging, spanning MRI, PET, SPECT, and ultrasound.25,46 This maturity of this property of nanocomposites holds the potential to lead to earlier and more accurate detection of tumors.
Bioresponsive Imaging
Nanocomposites can be designed to respond to specific changes in the tumor environment, such as pH, temperature, or hypoxia, and release imaging agents in response to these changes.47 Studies report the synthesis of a protease-triggered bioresponsive drug delivery platform for the targeted theranostics of malignancy. The exceptional ability of proteases for biological recognition and catalysis, along with the impressive physicochemical properties of nanomaterials, leads to the development of nano-drug delivery systems (nanoDDS) activated by enzymes. These systems exhibit theranostic functions, responding highly specifically to stimuli associated with the tumor phenotype.48 Similarly, a nanogel multienzyme mimics synthesized by biocatalytic atomic transfer radical polymerization (ATRP) and metal coordination for bioresponsive fluorescence imaging has been reported. Studies involving both cells and tumor-bearing mice confirm the achievement of effective biofluorescence imaging responsive to reactive oxygen species (ROS) using multienzyme-mimic nanogels (MPGs).49 These observations indicate that bioresponsive nanocomposites respond to specific biological cues in the tumor environment, altering their properties and enhancing their imaging capabilities, and hold great promise for cancer diagnosis.
Electrochemical Sensing
Nanocomposites can be used to create electrochemical biosensors that can detect cancer biomarkers in the blood or other bodily fluids.50 Graphene, a versatile nanomaterial, is employed in electrochemical biosensors for cancer diagnosis. These sensors demonstrate the potential for rapid, sensitive, and cost-effective detection of cancer biomarkers. For instance, graphene-assisted sensors have been explored for the detection of pancreatic cancer biomolecules such as the K-Ras gene, CEA, and microRNA.51 Nanocomposite applications have also been documented for the sensitive electrochemical detection of a broad range of cancer biomarkers, including circulating tumor cells, proteins, DNA mutations, and non-coding RNAs like miRNA, glycoproteins, and metabolites.52 Recent developments in electrochemical biosensors also utilize polymer nanocomposites (PNCs) containing metallic nanoparticles and carbon nanomaterials to enhance the sensor’s performance, improving diagnostic sensitivity and selectivity.53 Additionally, electrochemical sensing platforms offer label-free detection,54 providing a versatile and efficient means to detect cancer-related molecules.
Magnetic Separation
Magnetic separation of nanocomposites relies on the principle that magnetic nanoparticles (MNPs) can be manipulated using external magnetic fields. When exposed to a magnetic field, MNPs align with the field lines, allowing for their selective separation from other particles. In the context of cancer diagnosis, MNPs can be conjugated with biomolecules that specifically bind to tumor cells or biomarkers. Upon exposure to a magnetic field, these MNPs will accumulate at the site of the tumor, facilitating their isolation and characterization.55,56 Magnetic nanoparticles are widely employed in cancer diagnosis for tumor imaging. MRI utilizes the magnetic properties of nanoparticles to provide detailed and high-contrast images of tumors.56 Hybrid magnetic nanostructures are also applied in magnetic separation, diagnostics, and cancer drug delivery. These multifunctional nanocomposites enable magnetic separation to isolate cancer cells from complex biological samples efficiently, facilitating downstream analysis and diagnosis.57 Moreover, magnetic nanoparticles can be used to capture and isolate circulating tumor cells (CTCs) from the blood. For example, ferroplatinum iron oxide (Fe3O4–FePt) magnetic nanocomposites can capture and separate CTCs.58 This technology holds significance in cancer diagnosis and prognosis, allowing for the isolation and analysis of CTCs from blood samples. Magnetic separation offers the potential for multimodal imaging as the nanoparticles can be loaded with imaging agents, enabling simultaneous diagnosis and imaging and providing a more comprehensive understanding of the tumor. It also assists in isolating and detecting tumor-associated biomarkers, such as proteins, DNA, or RNA, for early cancer detection and prognosis.55,56,59 The features of nanocomposites and techniques in cancer diagnosis are illustrated in Figure 3.
Key Nanocomposites-Based Cancer Diagnostic Techniques
Application of Imaging Techniques
Nanocomposites can be used to enhance the contrast and sensitivity of imaging techniques such as magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET). For instance, iron oxide nanoparticles can be used as MRI contrast agents to improve the visualization of tumors.25 The co-application of ferumoxytol, an iron oxide nanoparticle, and gadolinium-contrasted MRI helps identify pseudoprogression in glioblastoma.60 Thus, ultrasmall superparamagnetic iron oxide nanoparticles are an emerging tool in MRI diagnostics of tumors and can add clinically useful information due to their distinct physicochemical features and biodistribution, while having a good safety profile.61 Magnetic nanoparticles conjugated with the radiotracer 67Ga-DOTATATE have been used for PET imaging of neuroendocrine tumors. These nanoparticles exhibit enhanced accumulation in tumor tissues and provide accurate tumor localization.62 A multimodal cancer-targeted imaging system capable of concurrent fluorescence imaging, radionuclide imaging, and MRI in vivo has been reported. The researchers demonstrated that the nanocomposite, MFR-AS1411, exhibits specific fluorescence signals and specifically targets cancer cells, providing a potential versatile imaging tool for specific cancer diagnosis.63 Similarly, a nanocomposite made up of poly(catechin) capped-gold nanoparticles (Au@PC NPs) and smaller nucleolin-binding aptamer (AS1411) conjugated gold NPs (AS1411-Au NPs), served as a targeting agent in LDI-MS (laser desorption/ionization mass spectrometry)-based tumor tissue imaging. The satellite-like AS1411-Au NPs/Au@PC NP nanocomposite showed enhanced multivalent binding with nucleolin molecules on tumor cell membranes and ultrahigh signal amplification, improving LDI-MS imaging.64 It is also reported that a nanocomposite consisting of magnetic PPy/Fe3O4-core and a gold nanoshell effectively enhances the contrast for both MRI and X-ray CT imaging in identifying cancer cells. It also provides an effective platform for multimodal imaging-guided cancer treatment.65
Optical imaging techniques, such as photoacoustic tomography (PAT) and fluorescence imaging, are also being explored for cancer diagnosis using nanocomposites.66 Photoacoustic and CT imaging have also been applied in tumor theranostics using a core-shell Au@Cu2-xSe heterogeneous metal nanocomposite.67 Fluorescent nanoparticles, such as quantum dots or upconversion nanoparticles, can emit light upon excitation, allowing for visualization of tumors. Nanocomposites can incorporate these fluorescent nanoparticles into their structure, enhancing their stability and targeting capabilities. For example, gold nanoparticles conjugated with fluorescent dyes have been used for fluorescence imaging of breast cancer cells.68 Other studies report precision delivery of theranostic agents via thermosensitive polymer dot nanocomposites for trimodal CT/photoacoustic/fluorescence imaging in tumors.69 In all, optical imaging offers a high potential for noninvasive detection of cancer in humans, where unspecific contrast agents such as indocyanine green (ICG) and omocyanine have been applied, whereas molecular probes for direct targeted imaging of tumors are still in preclinical research.70 Additionally, the safety of CD8 PET imaging using 89Zr-Df-IAB22M2C is confirmed in clinical studies, demonstrating the capability to observe the distribution of CD8+ immune cells throughout the entire body in both tumors and normal tissues.71 Additionally, it holds promise in tumor diagnostics and predicting early responses to immunotherapy.
Biosensor Application
Nanocomposites can be incorporated into biosensors to detect cancer biomarkers in biological fluids or tissues, such as proteins, nucleic acids, and metabolites. These biosensors can provide rapid, sensitive, and specific detection of cancer.45 Label-free biosensors based on nanostructure are believed to be superior to the traditional methods in detection speed, sensitivity, cost, and versatility.33 Biosensors in cancer diagnosis can be grouped into broader application scopes such as electrochemical biosensors, field effect transistor (FET) biosensors, and fluorescent biosensors. Electrochemical biosensors detect biomarkers by converting their interaction with bioreceptors into measurable electrical signals. For example, MoS2-based electrochemical sensors have successfully detected tumor markers like H2O2, carcinoembryonic antigen (CEA), circulating tumor cells (CTCs), and miRNA.72 A study reports that AuPtPd/rGO-modified GCE (glassy carbon electrode) nanocomposites serve as an excellent biosensor for monitoring the release of H2O2 from living cancer cells,73 providing a foundation for biological and biomedical applications such as early cancer diagnosis. A 3D nanocomposite that not only exhibits a large surface area and favorable microenvironment but also possesses remarkable stability, conductivity, and biocompatibility was employed as a biosensor for CEA detection. The AuNPs/PB-PEDOT modified immunosensor could detect a CEA range of 0.05–40 ng mL−1, with a detection limit of 0.01 ng mL−1.74 Since CEA, as a clinical tumor marker, can be expressed in lung, breast, ovarian, cystadenocarcinoma, and other cancers, monitoring its levels could help detect, diagnose, and manage these cancers.75 In other cancers, such as prostate cancer, detecting sarcosine content in blood or urine has been considered to provide a basis for the diagnosis. In exploring this concept, Ti3 C2 TX /Pt-Pd nanocomposite with excellent electrochemical performance and high stability was applied on a GCE with sarcosine oxidase (Sox) to form a sarcosine biosensor (GCE/Ti3 C2 TX /Pt-Pd/SOx). The biosensor, capable of detecting sarcosine concentration of 1–1000 µM with a low limit of detection of 0.16 µM and a sensitivity of 84.1 µA/mM cm2, exhibits potential in the early diagnosis of prostate cancer.76 Similar nanocomposite-based biosensor platforms that detect sarcosine in prostate cancer have been reported.77,78
Moreover, the combined utilization of a PEG polymer film and Au nanoparticle mixed interface enables high levels of sensitivity and effective assaying of the breast cancer susceptibility gene (BRCA1) in patient samples. The label-free DNA sensor shows feasible clinical application for breast cancer diagnosis via the BRCA1 gene.79 Similarly, a polymer nanocomposite-modified GCE poly (dopamine beta cyclodextrine-cetyl trimethylammonium bromide) fixed with silver NPs (P[DA-β-CD/CTAB])-AgNPs-GCE) was synthesized for BRCA1 biomarkers detection. The researchers observed satisfactory biosensor results in detecting BRCA1 in human plasma samples and MCF-7 cancer cell lysates,80 implicating the potential of the immunoassay to be applied in clinical analysis. Polymers can also be combined with nanomaterials to create biosensors with tailored properties for detecting a wide range of cancer biomarkers, including CTCs, DNA fragments, and proteins.81 Owing to the unique characteristics of graphene-based nanocomposites, such as excellent electrical and thermal conductivity, luminescence, and mechanical flexibility, these thin two-dimensional nanostructures have been widely employed as bases for the detection of biomolecules and cells, proofing a promising tool for the early diagnosis of cancer.82 In summary, nanocomposites-based biosensors are emerging as a powerful tool for cancer diagnosis due to their enhanced sensitivity, specificity, and miniaturization compared to conventional biosensors. These biosensors integrate nanomaterials with biorecognition elements, such as antibodies, aptamers, or enzymes, to selectively detect cancer biomarkers, providing the potential to enable earlier cancer detection, improve treatment outcomes, and reduce healthcare costs.
Theragnostic Application
Nanocomposites can be designed as theragnostic agents, combining diagnostic and therapeutic capabilities. These agents can target tumors, deliver diagnostic probes, and simultaneously release therapeutic drugs, enabling real-time monitoring of treatment response and improving therapeutic efficacy.8,83 A study reported that a nanocomposite of DNA-templated fluorescent silver nanoclusters (AgNCs) assembled around DNA-modified gold nanoparticle (AuNP) functions as a cancer cell-specific imaging and targeted therapeutic agent. The AuNP@(AS1411-AgNCs)n nanocomposite exhibits improved biostability, marked near-infrared fluorescence emission, and carries a high density of the first anti-cancer aptamer targeting nucleolin protein, AS1411.84 This demonstrates the highly specific cancer cell-targeted imaging and selective killing of nanocomposites, thus potential therapnostics in the clinic. Doan and colleagues synthesized IR783 conjugated chitosan-polypyrrole nanocomposites (IR-CS-PPy NCs) as a theragnostic photoacoustic/fluorescence dual-modal imaging agent. The nanocomposite portrayed marked biosafety and near-infrared (NIR) absorbance properties with enhanced photostability. IR-CS-PPy NCs demonstrated high-efficiency MDA-MB-231 breast cancer cell ablation under NIR laser irradiation and complete killing of tumor tissues without further recurrence.85 Thus, nanocomposites-based imaging systems could be effective theragnostic agents for imaging-guided cancer treatment. Another study found that Fe2O3@PPy-DOX-PEG nanocomposite can kill cancer cells and realize MRI in vivo. Mechanistically, acid stimulus and alternating magnetic field triggered the nanocomposite to release the loaded doxorubicin hydrochloride (DOX), resulting in a remarkable combination of therapeutic effects via chemotherapy and magnetic hyperthermia and realized MRI.86 It offers a potential alternative for the development of new nanocomposites for combination cancer therapy with MRI in vivo.
It is reported that a multifunctional nanoplatform, MFG-LPM NPs, exhibit not only MRI capacity in cancer diagnosis but also a high drug loading capacity, and integrates magnetic- and active-targeting drug delivery, light-controlled drug release, MRI, and photothermal and chemotherapy in cancer theranostics. In vivo, an MRI of tumor-bearing mice shows the nanocomposite’s remarkable performance as a T2 contrast agent. In addition, in vitro cytotoxicity studies demonstrate a synergistic effect with high cancer cell-killing efficacy87 presenting MFG-LPM NPs as an excellent theranostic agent that collectively combines multiple functions for effective MRI-guided cancer diagnosis and treatment. Fluorescent nanoparticles conjugated with a tumor-specific antibody have been used to image and target prostate cancer cells. The nanoparticles accumulate in tumor cells and emit fluorescence, allowing for the visualization of tumors.88,89 Theranostic nanoparticles are still under development, but they have the potential to revolutionize cancer diagnosis and treatment by providing more effective and personalized applications. The application of nanocomposites in cancer diagnosis is summarized in Table 1.
 |
Table 1 The Diagnostic Application of Nanocomposites in Cancer |
Flow Cytometry Application
The integration of nanocomposites into flow cytometry has significantly enhanced its capabilities in cancer diagnostics. Flow cytometry is a valuable technique for evaluating the effectiveness of nanocomposite-based strategies. This technique is potent in analyzing the physical and chemical attributes of cells, facilitating the comprehension of the efficacy of novel cancer therapies and their response to varying environments.7 It often uses commercial fluorescent beads that vary in size from hundreds of nanometers to a few micrometers to enhance the sensitivity and specificity of cancer cell detection. Estévez et al designed an innovative technique for rapid and precise identification of cancer cells by employing dye-doped silica nanoparticles. Their method significantly enhanced the sensitivity of detection in flow cytometry investigations, surpassing current methods by 10 to 100 times.90 Researchers have investigated the use of gold nanoparticles combined with other biomolecules to specifically target cancer cells. Subsequently, flow cytometry is employed to examine the fluorescence signals, enabling the detection and quantification of these cells,91 Molybdenum disulfide (MoS2) nanocomposites have been created for the purpose of cancer diagnostics, as they possess the unique capability to selectively react to the tumor microenvironment. These nanocomposites have the ability to be combined with targeted agents and fluorescent markers, allowing for accurate identification and measurement of cancer cells utilizing flow cytometry.7 MoS2-based nanomaterials can be genetically modified to selectively target cancer cells, minimizing harm to healthy cells and enhancing the safety and effectiveness of diagnostic methods.
Flow cytometry analyzes the cellular uptake, distribution, and targeting efficacy of functional nanocomposites, including those combining polymers and metallic nanoparticles, in cancer cells.92 Thus, nanoparticles can be engineered to transport therapeutic substances, enabling the use of both diagnostic and therapeutic functions in a single application. This not only aids in the identification of cancer cells but also facilitates the direct delivery of treatment to the specific cells, hence improving treatment results.
Nanocomposites in Cancer Treatment
Nanocomposite-Based Treatment Models/Strategies
The development of efficacious therapeutics and remedies for cancer, which ranks among the leading causes of mortality globally, is of the utmost importance. The potential of nanocomposites to facilitate the development of tailored cancer treatments lies in their distinctive characteristics. Nanocomposites have been found to possess a wide range of applications in cancer treatment, including surgery, radiation therapy, chemotherapy, etc. Nevertheless, it is imperative to thoroughly analyze the original tumor before selecting the appropriate course of treatment. Implementing nanoparticles as drug carriers has facilitated enhancements in drug kinetics, such as modulating drug and tissue interactions and prolonging circulation time.93 Nanoparticles serve as passive or active vehicles by transporting drug loads, enabling them to remain undigested and preventing their elimination from the body (Figure 4). Nanomedicines incorporating nanoparticles exhibit enhanced therapeutic efficacy by selectively targeting tumor sites, immune cells, and lymph nodes.94,95 Concentrating therapeutic efforts within the tumor microenvironment reduces the risk of systemic exposure and unintended side effects. At the same time, the effectiveness of the treatment in the local environment is optimized. This targeted approach contributes to improved outcomes in cancer immunotherapy and surgical interventions, offering a promising avenue for advanced treatments with minimal side effects. Thus, the constraints of cancer treatment have been reduced due to the emergence of nanocarriers.
 |
Figure 4 Targeting of nanoparticles (NPs) to cancer cells. |
Drug Delivery Systems
Drug delivery systems (DDSs) have been investigated as an alternative method to systemic drug administration due to their ability to deliver therapeutic quantities of the drug directly to the site of the tumor in a controlled manner. DDS offers two primary benefits: first is the provision of localized drug delivery that is sustained and uninterrupted, and second is the achievement of substantial drug concentrations exclusively in the tumor microenvironment while minimizing drug levels in the bloodstream and extra-tumor tissues. Moreover, multiple material-related factors impede the effective implementation of drug delivery systems in cancer treatment, such as host immune response against the delivery system and inadequate control over drug release.96 Research has demonstrated that DDS utilizing nanocomposites augments the therapeutic effectiveness of anticancer medications while concurrently mitigating adverse effects. A range of DDSs, including liposomes, carbon nanotubes, and polymeric nanoparticles, have demonstrated efficacy as carriers in cancer therapy. For example, M-MSN(Dox/Ce6)/PEM/P-gp shRNA nanocomposites exhibited a pH-responsive drug release profile. Combined photodynamic therapy and chemotherapy achieved synergistic anti-tumor effects in tumor-bearing Balb/c mice, demonstrating that tumor-bearing animals injected intravenously with these nanocomposites exhibited significant tumor uptake.97 Nanoscale graphene oxide (nGO) encapsulated Fe3O4, conjugated with folic acid (FA), and loaded with doxorubicin (DOX) (FA@Fe3O4@nGO-DOX) was a stable material used for targeted drug delivery.98 Moreover, several challenges that have been effectively addressed in experimental and preclinical settings involving human and animal models, including insufficient tumor penetration capability, inappropriate and nonspecific tissue accumulation, premature drug release into tissues, and uncontrolled drug release at the target site, may be surmounted by employing DDSs.99 Furthermore, they also enhance the medication’s duration of action and effectiveness.
Photothermal Therapy (PTT)
With the advent of photothermal therapy (PTT), treating cancers and tumors has seen significant success. PPT employs light of specific wavelengths facilitated by photothermal absorbers.5 The increased heat produced by near-infrared (NIR) radiation, which induces cancer cell death, has led to the increased utilization of PTT as an alternative treatment.100,101 PTT is a controllable and minimally invasive method. It employs materials with high photothermal conversion efficiencies to raise the temperature of the targeted malignant sites, resulting in cancer cell death. Metal nanoparticles such as copper, gold, silver, graphene, and molybdenum have been shown to exhibit PTT.5 For instance, the Ion Oxide decorated MoS2 (MoS2-IO-(d)PEG) nanocomposite was successfully employed to ablate 4T1 murine breast tumors.100 The problem of low drug transdermal efficiency and poor efficacy of monotherapy was solved using PTT in combination with chemodynamic therapy (CDT) and chemotherapy (CT). The constructed BPMN-CuS/DOX system significantly inhibited melanoma.102 Also, an anticancer drug, nanocomposites of the poly(N-isopropylacrylamide) (PNIPAM) containing graphene oxide (GO), PNIPAM/GO- and PNIPAMAAM/GO-based nanogels were effectively transported to cancer cells using PNIPAM/GO-DOX through the photothermal effects of NIR irradiation.103
Photodynamic Therapy (PDT)
Photodynamic therapy (PDT), like PTT, uses optical interference. However, unlike PTT, PDT uses a therapeutic approach for triggering cell apoptosis/or necrosis that depends on singlet oxygen or other reactive oxygen species (ROS) created by photosensitizer (PS) molecules when exposed to specific light irradiation, leading to cell death.104 Composite nanosystems, which combine polymer nanocarriers with embedded plasmonic metal nanoparticles and photosensitizers, are considered the most effective method for PDT in oncology. For biomedical applications, metal nanostructures must possess robust and adjustable surface plasmon resonance (SPR), exhibit little toxicity, be easily administered, and be conveniently linked to bioconjugates for targeted cancer cell interactions. Due to their robust SPR, gold nanoparticles (GNPs) are consistently evaluated for their potential application in PDT.105 In PDT, the ability of GNPs to absorb light and convert it into heat can be utilized to either release a chemical payload by inducing heating or generate ROS to cause cellular necrosis or apoptosis at targeted tumor sites.106 For instance, by integrating lactate oxide (LOx) and catalase (CAT) into iron oxide (Fe3O4) nanoparticle/indocyanine green (ICG) co-loaded hybrid nanogels, toxic ROS were produced by endogenous hydrogen peroxide (H2O2) at the tumor site, resulting in the death of cancer cells even without the presence of external oxygen (O2).107 In addition, a porphyrin-based nanoscale metal-organic framework (NMOF), DBP-UiO, was used as PS for PDT. This chlorin PS functionalized with DBC-UiO demonstrates a redshift of 13 nm and an 11-fold increase in the extinction coefficient of the lowest-energy Q band. This results in more efficient singlet oxygen production (1O2) and considerably improves the efficacy of PDT.108
Gene Therapy
In emergent cancer therapies, gene therapy (GT) represents a method by which targeted tumor cells are injected with genes that either overexpress oncogenic proteins or promote apoptosis. Gene therapy encompasses the processes of gene transduction, gene stability maintenance, and complete gene expression. The precision of gene replacement strategies is enhanced by the targeted and efficient delivery of therapeutic genes made possible by nano-delivery systems.109 In gene therapy, nanocomposite delivery systems provide targeted drug delivery and enhanced treatment efficacy, offering several benefits in cancer treatment, including heightened specificity, diminished adverse effects, and improved therapeutic efficacy.110 The material must possess two critical characteristics for gene delivery applications: compact size and the capacity to interact with genetic material, forming a durable complex.111 Efforts have been devoted to developing carriers that effectively compact and safeguard oligonucleotides for gene therapy. In cancer treatment, microRNA (miRNA)-based therapies have demonstrated encouraging outcomes; furthermore, the application of nanocarriers to deliver miRNA can augment their therapeutic effectiveness. In various preclinical cancer treatment studies, nanocomposites of polymers and lipids have been assessed for their ability to inhibit dysregulated cytokines and impede tumor growth. Several primary categories of nanocarriers have been employed to transport miR34a in the context of cancer therapy. A recent study used a lipid-based nanoparticle formulation to deliver a small molecule inhibitor of the protein CK2 to treat pancreatic cancer. The lipid-based nanoparticles were found to have the CK2 inhibitor to the tumor site in an effective manner and to inhibit CK2 activity to a significant degree, exhibiting no discernible toxicity or intolerance in the mice.112 In another study, researchers employed mesoporous silica nanoparticle (MSN)-based drug delivery systems to treat breast cancer.113 They used MSNs loaded with the chemotherapy drug doxorubicin (DOX) and conjugated them with a tumor-targeting peptide called iRGD.
Key Nanocomposite-Based Materials in Cancer Treatment
Nanocomposites, including carbon-based nanocomposites,114 polymeric nanocomposites,115 metal-based nanocomposites,11 lipid-based nanocomposites,116 hybrid nanocomposites,117 and ceramic nanocomposites,118 have garnered significant interest for treating prominent cancers (Figure 5 and Table 2). The emergence of these materials has enabled the comprehension of cellular mechanisms within living cells and the development of technologies that aid in the timely detection and management of diverse diseases. The ultimate goal of cancer therapy, as is well known, is to selectively target tumor cells while avoiding harm to adjacent healthy tissues. Nanocarriers enhance the therapeutic efficacy of anticancer medications and facilitate selective accumulation at their intended site.
 |
Table 2 Types of Nanocomposites Used in Cancer Treatment |
Nanocomposite-based drug delivery systems have been demonstrated to enhance the therapeutic efficacy of anticancer treatments and reduce side effects. Nanocomposites offer several advantages over small-molecule drugs or medications. These include precise or targeted delivery to specific cells or tissues, minimizing off-target effects.129 In terms of size and composition, they are in the nanometer size range and have unique compositions that confer them superiority over conventional materials.129 Moreover, tailored applications130 allow for the customization of polymeric nano-biocomposites for sustained and targeted drug delivery applications, providing customized solutions for specific medical needs. Nanocomposites have superior nanostructures, enhancing drug transport effectiveness.131 They ensure site-specific delivery and precise medicines for treating chronic human diseases.132 Additionally, nanocomposites have a high capacity to encapsulate a large amount of drug internally or on their capacity, which exhibits an increased capacity to encapsulate a large amount of drug internally or on their surface, enhancing drug delivery efficiency.133 As a result, numerous varieties of nanocarriers, including polymers, micelles, dendrimers, mesoporous silica nanoparticles, liposomes, and nanosized metal-organic frameworks (nMOFs), have been developed for cancer treatment.95
Carbon-Based Nanocomposites
Carbon-based nanocomposites integrate a carbon-based substance (such as carbon nanotubes, graphene, or fullerenes) with another substance at the nanoscale. The combination frequently yields improved characteristics in comparison to the individual constituents. They possess exceptional mechanical strength, electrical conductivity, thermal conductivity, barrier characteristics, and chemical resistance. These devices are cleverly developed and constructed to improve the effectiveness of drug delivery in cancer treatment. Carbon-based nanocarriers are the most commonly used in the delivery field due to their unique properties, which include binding to the cell membrane, which provides the optimal environment for DOX release, and triggering cell membrane ruffling, which induces cell stress and ultimately results in methuosis, apoptosis, and ROS production.134 Carbon nanotubes, due to their hydrophobic hollow interior, facilitate the loading of water-insoluble drugs, making them promising for delivering tumor-treating medications. Carbon nanotubes (CNTs) are classified as single-walled carbon nanotubes (SWCNTs) or multi-walled carbon nanotubes (MWCNTs)135 In addition, they are sensitive to PET and MRI. Graphene and its nanocomposites have recently been widely used in biomedicine for cancer therapy. Also, graphene, which is lipophilic, can help in membrane barrier penetration. As nanocarriers, reduced graphene oxide (rGO) and MWCNTs enhance cellular permeability and loading capacity.136
Polymeric Nanocomposites
Polymeric nanocomposites are a class of nanocomposite materials in which at least one of the phases consists of a polymer. Nanoparticles, usually smaller than 100 nm, are dispersed within a polymer matrix to form them. These blends frequently result in substantial character enhancements compared to conventional polymer materials. Polymeric nanocomposites have notable characteristics, including facile synthesis, biocompatibility, non-toxicity, non-immunogenicity, biodegradability, and tailored drug administration, rendering them favored theranostic tools.137 Some commonly used polymeric nanoparticles are polymersomes, polymeric micelle, and dendrimers. Polymersomes are polymeric self-assembled vesicles prepared using amphiphilic block copolymers with a hydrophobic bilayer encapsulating an aqueous core.138 As such, polymersomes are gaining popularity as theranostic nanoparticles due to their expanding applications as platforms for simultaneous drug delivery and imaging. Polymeric micelles, another amphiphilic block copolymer, have been used as delivery vehicles for hydrophobic agents.138 PLA-based micelles were created to load Ruthenium (Ru) for improved cancer treatment. The micelles consisted of MPEG-SS-PMLA, which is a combination of poly(ethylene glycol) and phenyl-functionalized poly(lactic acid) connected by a disulfide connection. The MPEG-SS-PMLA contained approximately 83% Ru content, which was attributed to the π-π bonding between the phenyl ring and the Ru complex. At a concentration of 10 mmol/L, under the influence of GSH, 70% of Ru was released, leading to apoptosis in MCF-7 cells.139 Dendrimers are tree-like, three-dimensional macromolecules that are exceptionally branched. Their considerable surface area facilitates the affixed of many functional groupings, imparting versatility across various applications. Recently, there has been considerable interest in cancer research regarding dendrimer-based drug delivery. This interest stems from the branched structure and abundant functional group terminations present in dendrimer polymers, which enhance the efficiency of drug encapsulation and conjugation. Consequently, numerous biologically active molecules, including chemotherapeutic medications and MRI contrast agents, were successfully delivered via dendrimers.140,141
Metal-Based Nanocomposites
Metal-based nanocomposites combine a metal or metal oxide with other materials at the nanoscale. Their properties can be customized by varying the type of metal, the matrix material, and the fabrication process. They exhibit enhanced strength, toughness, stiffness, thermal and electrical conductivity, and resistance to corrosion, wear, and heat.142 There has been a growing fascination with magnetic nanoparticles, which can potentially be used for disease diagnosis and treatment. The primary concept is to regulate the targeted distribution of these nanoparticles solely through utilizing the magnetic field. Photothermal therapeutic agents can utilize metal particles. For example, functionalized gold nanoparticles (AuNPs) are highly suitable for delivering novel therapeutics due to their remarkable biocompatibility and predictable bioavailability.143,144 The predominant application of iron oxide nanoparticles is in cancer therapies based on magnetic spin, in which oxygen radicals are generated to detect the presence of cancer. Furthermore, these nanoparticles can induce local toxicity ROS and reactive nitrogen species for tumor therapy and are remotely controllable via an external electromagnetic field. This type of treatment produces fewer adverse effects in healthy and normal tissues. The capability of remotely controlling iron oxide nanoparticles laden with antitumor drugs confers additional benefits compared to conventional antitumor drugs.11
Lipid-Based Nanocomposites
Lipid-based nanocomposites, also known as lipid-based nanoparticles, are materials that blend lipids (oils or fats) with other elements to form structures possessing distinctive functions and properties. Lipids are endogenous to the body, rendering them inherently biocompatible and reducing the likelihood of eliciting unpleasant reactions. They can be engineered to selectively target particular cells or tissues, augmenting treatments’ effectiveness and minimizing adverse reactions. Additionally, they can encapsulate and safeguard pharmaceuticals or other compounds, allowing for regulated discharge either gradually or at particular locations within the body. Liposomes have emerged as the preferred drug delivery vehicle due to their extensive clinical history and firmly established attributes. For instance, stealth liposomes coated with polyethylene glycol (PEG) demonstrated improved blood circulation time for 2–3 days following intravenous administration.145
Hybrid Nanocomposites
Hybrid nanocomposites are materials formed by synthesizing two or more distinct components at the nanoscale, often involving one organic and one inorganic component. This combination frequently leads to improved characteristics in the resulting hybrid material, which neither component could individually attain.146 Titanate nanotube (TiONt) nanohybrids were created to combat prostate cancer using intratumoral (IT) injection. Before use, the surface of TiONts was pre-coated with a siloxane called APTES. This coating allowed for the attachment of both dithiolated diethylenetriaminepentaacetic acid-modified gold nanoparticles (Au@DTDTPA NPs) and a heterobifunctional polymer called PEG3000. The purpose of this coating was to greatly enhance the stability of the suspension and the compatibility of TiONts for specific biomedical applications.127
Ceramic Nanocomposites
Ceramic nanocomposites are advanced composite materials that merge ceramics with reinforcing elements at the nano-scale, enabling the customization of material properties to suit specific applications. This combination yields materials with enhanced characteristics, including decreased weight compared to conventional ceramics, greater strength, dimensional stability, and heightened resistance to heat, wear, and tear.147,148 For instance, bioceramics are ceramic materials compatible with living tissues, making them suitable for medical and dental implants. Bioactive magnesium silicate ceramics are predominantly assessed in bone tissue engineering. This is owing to their exceptional mechanical characteristics and capacity to induce cellular adhesion, proliferation, and differentiation, consequently facilitating more rapid bone regeneration than traditional bioceramics. As a result of their superior mechanical properties and accelerated bone regeneration, magnesium silicate-based bioactive ceramics (Mg2SiO4) have been evaluated in bone tissue engineering for their ability to stimulate cell adhesion, differentiation, and proliferation.149 Calcium-phosphate ceramics have been explored as a delivery medium for antibiotics, proteins, steroids, hormones, anticoagulants, and anticancer medications.150 Also, the sustained release capabilities of hydroxyapatite (HA) and tri-calcium phosphate (TCP) porous blocks for anticancer medications, such as methotrexate and cisplatin, have been assessed.96
Improving Targeted Cancer Therapies
Nanomaterials typically have dimensions ranging from 1–100 nm. Because of their small sizes, they can interact distinctively with tissues and cells, making them viable candidates for cancer therapy and drug delivery. These nanoscale features give them distinct optical, electrical, and magnetic capabilities. The integration of nanotechnology and cancer treatment presents an opportunity to enhance the effectiveness of therapeutic agents, mitigate unintended harm to benign cells, and improve patient prognoses.151 Additionally, it is possible to modify nanomaterials to target a moiety specifically. Targeted delivery is a significant advantage of nanomaterial-based cancer therapy over free drugs. Targeted drug delivery systems reduce cell toxicity by delivering drugs selectively and directly into cells. Nanomaterial-based drug delivery systems have reflected cancer treatment and management benefits by demonstrating precise targeting, good pharmacokinetics, reduced side effects, and drug resistance.152,153 Nanoparticle drug delivery systems, characterized by their ability to achieve accurate targeting and exhibit favorable pharmacokinetics, play a significant role in enhancing the efficacy of cancer treatment.154 Furthermore, their modifications are designed to selectively affect cancer cells, the immune system, and the tumor microenvironment (TME), expanding the range of potential cancer treatments.
Diverse forms of nanotechnology, such as lipid-based nanomaterials, polymeric nanoparticles, liposomes, carbon nanomaterials, etc., have been utilized in cancer therapy. These materials have improved drug delivery to cancer cells, reduced toxicity to healthy cells, and improved patient outcomes.155 Using intricate design and modification, nano-drugs can effectively address the drawbacks associated with conventional chemical therapy by virtue of their enhanced specificity, bioavailability, reduced cytotoxicity towards normal tissue, increased loading capacity, prolonged half-life, and distinctive drug release patterns. Research highlights the potential of carbon nanomaterials in cancer treatment, including graphene and fullerene, demonstrating continuous advancements in this domain.156–158 Recently, there has been a growing interest in applying graphene-related nanomaterials to photothermal therapy. Graphene oxide (GO) has been shown in multiple studies as a drug nanocarrier capable of enhancing cancer-targeting drug release profiles, facilitating chemotherapy cellular uptake and accumulation in cancer cells, and mitigating chemotherapy’s adverse effects on normal cells.159 Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibited immune responses as an immune checkpoint blockade in cancer immunotherapy.160 Notwithstanding the vast array of nanomaterials developed to target cancer, only a restricted selection of liposomes and polymer nanoformulations have obtained clinical approval. For instance, long-circulating polymeric nanoparticles have been developed with diblock copolymers composed of PEG and biodegradable polymers like PLGA. Liposomes have been enhanced by the drug delivery field to transport chemotherapeutic agents to tumors effectively, resulting in an improved therapeutic index and decreased adverse effects.161
Progress and Advancements
Recent Advances in Nanocomposite-Based Cancer Diagnosis
Cancer diagnosis plays a pivotal role because it entails the specific identification of a tumor within the body and the assessment of its size in order to determine whether it is in its early stages or a case of recurrence. Nanocomposites have emerged as a frontiering domain in the realm of cancer diagnosis, presenting novel methodologies to augment both detection and therapeutic interventions. In recent years, substantial progress has been made in utilizing nanocomposites for cancer diagnosis. Using nanocomposites in diagnostic applications introduces novel approaches that improve accuracy and sensitivity.
Electrochemical biosensors have recently attracted much attention due to their extraordinarily high selectivity, sensitivity, and affordability in early cancer diagnosis. Nanocomposite biosensors are being developed for non-invasive cancer detection using readily available fluids such as blood or saliva, holding enormous promise for early diagnosis. Biosensors comprised of nanocomposites, including graphene and MoS2, have been developed to provide label-free diagnosis of cancer cells and biomarkers with exceptional precision.7,162 Also, functionalized nanoparticles have a high affinity for specific cancer biomarkers, such as proteins and nucleic acids, allowing for sensitive detection and accuracy, which is vital in improving patient outcomes.91 Nanocomposite-based microfluidic devices can efficiently capture and analyze rare circulating tumor cells (CTCs) from blood, enabling early detection of metastasis and treatment monitoring.163 Functionalized DNA nanostructures have a significant role in cancer diagnosis. These structures function as adaptable platforms for biosensors and other diagnostic instruments, demonstrating the potential of DNA-based nanomaterials in oncology. Chen and Seeman presented 3-D organized DNA in 1991,164 with the DNA tetrahedron gradually becoming a typical 3D structure after development and simplification; it still plays essential roles in detection and analysis applications and drug carriers. More recently, a group of researchers synthesized DNA-templated silver nanoclusters (DNA AgNCs) by utilizing a multi-branched linear (MBL) DNA structure that was generated through a triggered hybridization chain reaction (HCR). The DNA AgNCs, assembled using the MBL structure and attached to aptamers targeting cancer cells, demonstrated a detection sensitivity approximately 20 times higher than DNA AgNCs attached to individual aptamers.165
Recent Advances in Nanocomposite-Based Cancer Treatment
Nanomaterial-based cancer treatment has demonstrated advantages over free drugs, especially in targeted delivery.166 In the past decade, there has been exciting improvement in nanocomposite-based cancer treatment. As a result of their substantial surface-area-to-volume ratios and nanoscale dimensions, nanocarriers can positively impact the basic properties and biological functions of their payloads. Nanocomposites can improve the effectiveness of immunotherapy and decrease the overall exposure of the drugs to the body. Favorable characteristics of nanocomposites often encompass their capacity to transport multiple loads, safeguard therapeutic agents throughout delivery, profoundly penetrate malignant tissue, and enable targeted administration to specific sites.166 This approach has the potential to overcome drug resistance and improve treatment outcomes. Lipid-mediated and polymer-mediated nanoformulations of anti-cancer medications, combined with nanotherapy in drug delivery, have substantially enhanced targeted drug delivery, augmented therapeutic efficacy, and minimized adverse effects on healthy cells.163 Nanoparticles can be modified by targeting ligands that selectively identify and adhere to cancer cells, transporting the therapeutic payload directly to the tumor location while limiting harm to healthy organs. The latest advancements in nanosystems based on layered double hydroxides (LDHs) demonstrate their considerable potential as effective instruments in the field of cancer therapy. These nanocomposites provide diversity and efficacy in therapeutic drug delivery.167 As carriers for delivering therapeutic genes to cancer cells, gene therapy nanocomposites present a promising avenue for personalized medicine and treating cancer-associated genetic mutations. This technology exhibits significant promise in advancing innovative cancer therapies with enduring impacts.163 Stimulus-responsive nanocomposites are likewise engineered to exploit particular stimuli in the tumor microenvironment, such as pH, temperature, or enzymatic activity, to liberate their therapeutic substratum. By implementing a controlled release mechanism, the effectiveness of the substance can be increased while potential adverse effects are reduced.163 As ongoing research in this domain advances, it is foreseeable that further groundbreaking and efficacious therapeutics will surface, presenting a glimmer of hope for enhanced patient prognoses and, conceivably, cancer cures.
Regulatory Considerations for the Clinical Translation of Nanocomposites
Although the clinical translation of nanocomposites for therapeutic applications opens up new avenues for customized medicine and better patient outcomes due to the unique features and possible hazards connected with nanoparticles, it also needs careful consideration of numerous regulatory challenges. Firstly, safety and efficacy should be considered. Concerns, including the thorough characterization of the nanocomposite, assessment of its potential toxicity, mechanisms of biodistribution, and clearance methods, are crucial to consider. Various regulatory bodies, including the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA), have established precise criteria for evaluating the safety and effectiveness of nanomedicines. These requirements frequently necessitate comprehensive information regarding the manufacturing process, quality control protocols, and characterization of the physicochemical properties of the nanocomposite.168 In addition, manufacturing and quality control are essential. Another area to be considered is the risk-benefit assessment. The ability to reliably and at scale create nanocomposites for clinical studies and possible commercialization is critical. Regulatory bodies need stringent quality control methods to assure batch-to-batch uniformity and reduce any variability in the medicinal product. Furthermore, it is essential to ascertain the nanocomposite’s precise dimensions, shape, surface characteristics, and composition to maintain consistent quality and monitor potential safety issues. Regulatory authorities may demand extensive documentation of the manufacturing process and batch release criteria.168,169
Regulatory bodies balance the potential benefits of a nanocomposite-based therapy against the reported hazards, considering aspects such as disease severity, current treatment choices, and possible adverse effects. This evaluation aids in determining the regulatory pathway and approval requirements for clinical trials and market authorization. Strategies for risk reduction are another area to contemplate. It is critical for regulatory approval to identify and mitigate any risks connected with the nanocomposite, such as immunological response, off-target effects, or environmental impact. Also, ethical behavior is an essential aspect to consider. Informed permission is necessary for involvement in clinical trials employing nanocomposites. Patients should be fully informed about the therapy’s possible dangers and benefits, including the uncertainty related to nanomaterials. Building trust and acceptance requires open communication; therefore, transparency and communication with the patients, healthcare professionals, and the general public regarding nanocomposite-based medicines’ development and potential hazards.170 The regulatory framework for nanocomposites in clinical translation is complicated, but it is critical for guaranteeing the safety and efficacy of these exciting novel medicines. Researchers and developers can boost the likelihood of successful clinical translation and deliver the benefits of nanocomposite-based drugs to patients by proactively addressing these factors and collaborating with regulatory regulators.
Ongoing Clinical Trials and Their Outcomes
Current clinical studies in cancer nanotechnology investigate several strategies, such as nanomedicines and nanocomposites. Cancer treatment utilizing nanocomposites is advancing rapidly, with several active clinical trials evaluating the safety and efficacy of these innovative medications. Nevertheless, the intricate structure of nanomaterials poses difficulties in their manufacturing and quality control processes, which often require optimization based on additional clinical discoveries. Preliminary results from ongoing clinical trials exploring the application of nanocomposite-based cancer therapy demonstrate promising outcomes in terms of safety, tolerability, and potential efficacy. Research emphasizes the importance of these trials in enhancing cancer diagnosis and therapies. Significantly, specific trials prioritize the use of combination immunotherapy to augment treatment results. Although pre-clinical studies have shown encouraging outcomes, additional research is necessary to convert these findings into practical use in clinical settings.
A new era for the use of nanoparticles in various chemotherapies began with the FDA’s 1995–1996 approval of liposomal and PEG-conjugated doxorubicin and 2005’s approval of albumin-nanoparticle conjugated paclitaxel.171 Nanoparticles, particularly those made of polymers responsive to ultrasound, demonstrate efficacy in detecting and treating cancer. Also, several liposomal nanoparticle (NP) therapies have progressed in clinical trials for various cancers. For instance, Onivyde (liposomal irinotecan) has completed Phase I/II trials for metastatic breast cancer. BIND-014 (PSMA-targeted polymeric NPs) has completed Phase I/II trials for KRAS-positive/squamous cell NSCLC and metastatic castration-resistant prostate cancer. Vyxeos (liposomal NPs co-delivering cytarabine and daunorubicin) is FDA-approved and in Phase I/II trials evaluating combinations with other drugs for leukemia.116 The efficacy and safety of combining motolimod, pegylated liposomal doxorubicin, and durvalumab (an immune checkpoint inhibitor) were investigated in Trial NCT02431559 (completed Phase I/II) (data collected from ClinicalTrials.gov). The study focused on participants with platinum-resistant ovarian cancer who had undergone at least two rounds of chemotherapy before the trial. The trial results demonstrated that the combination exhibited favorable tolerability and superior efficacy compared to monotherapies. These trials have shown that combining immune checkpoint inhibitors, chemotherapy drugs, and nanocomposites may extend the time patients with various cancers live without their disease worsening.
Challenges and perspectives
Challenges and Future Directions of Nanocomposite-Based Cancer Diagnosis
While nanocomposites have enormous potential to revolutionize cancer diagnostics, they also face distinct challenges and fascinating prospects. Concerns about reliability are a fundamental challenge in assuring the dependability of nanotechnology-based systems in cancer diagnosis. Consistent and precise results are critical for practical application.172 Although nanoparticle-based drug delivery systems show promise in cancer treatment, precision targeting needs further improvement to improve efficacy and avoid off-target effects.154 In addition, current research in MoS2-nanocomposites focuses on biosensors, bioimaging, and therapeutic applications. Overcoming these obstacles is critical for progressing cancer diagnosis and therapy.7 Future directions include creating safe nano-based materials for cancer detection; thus, the safety of these materials is critical for clinical applications. Innovative nanomaterials for cancer theranostics encounter obstacles that must be addressed before they can be successfully integrated into clinical practice.42 Addressing reliability difficulties, improving precise targeting, producing safe nanomaterials, and overcoming theranostics challenges will pave the road for nanocomposite-based cancer diagnostics to become more reliable and successful. However, to tackle the obstacles successfully and take full advantage of the promising prospects of nanocomposite-based cancer diagnostics, researchers, clinicians, and engineers must work together collaboratively.
Challenges and Future Directions of Nanocomposite-Based Cancer Treatment
Unique and effective ways are being explored for targeted drug delivery, increased therapeutic efficacy, and fewer side effects in nanocomposite-based cancer treatment. However, significant difficulties must be overcome before these therapies are routinely used in clinical practice. For instance, maintaining high sensitivity while designing nanocomposites that can reliably distinguish cancerous from healthy cells remains a challenge. False positive and negative results may result in inappropriate treatment decisions and misdiagnoses. The extensive diversity among cancer types underscores the need to create adaptable nanocomposites that detect distinct tumor attributes and mutations. One-size-fits-all strategies are improbable to achieve success. Moreover, incorporating nanocomposites into established imaging modalities such as ultrasound or MRI necessitates meticulous deliberation regarding compatibility, safety, and signal amplification. These factors can be challenging to balance. Before their widespread clinical application, prospective long-term safety concerns regarding the biocompatibility and toxicity of nanocomposites must be exhaustively addressed. Not forgetting the imperative to ensure the accessibility and practical implementation of nanocomposite-based diagnostic instruments in healthcare settings necessitates the development of scalable and cost-effective manufacturing processes.
Nanocomposites have immense potential to transform the field of cancer diagnostics and treatment. The present state of research offers valuable insights into the prospects of utilizing nanocomposites in clinical development within this field. Further refinement of these therapies, optimization of delivery systems, and investigation of synergistic combinations with other treatment modalities will be the focus of ongoing and forthcoming clinical trials. Future endeavors are centered around improving nanocomposites for specific cancer treatment, aiming to increase treatment accuracy and minimize adverse effects. Nanoparticles are proposed as a remedy to address drug resistance, a prevalent obstacle in chemotherapy. The objective of nanocomposite design is to enhance the efficacy of treatment. The exploration of nanocomposites, specifically the use of MnO2 nanoparticles to generate oxygen and alleviate tumor hypoxia, is being studied to hinder the proliferation and migration of cancer cells. Scientists are developing customized treatments using nanomaterials, considering specificity, cytotoxicity, and drug capacity parameters.7,25,151,166,173 With the advancement of research, it is foreseeable that in the near future, nanocomposite-based cancer treatments will become more productive and secure.
Conclusion
The rise in cancer cases has led to the rapid advancement of many diagnostic and therapeutic agents, including proteins, peptides, and small molecule cytotoxic drugs, hence broadening the range of cancer diagnosis and treatment options. The advancement of nanotechnology has led to many self-adaptive nanomaterials designed explicitly for bioactive particle delivery. Thus, nanotechnology-based therapy and diagnosis represent an innovative strategy aimed at advancing the clinical management of cancer while mitigating the limitations associated with presently accessible models. By strategically creating nanoparticles, researchers can deliver drugs to specific cell populations, the tumor site, or even difficult-to-access tissue compartments, thereby reducing the potential for adverse effects on healthy tissues and organs. The fabrication of responsive and multifunctional composite systems is facilitated by functionalizing inorganic nanoparticles using biocompatible polymers and biomolecules, whether naturally occurring or synthetic. Targeted nanocomposites ensure drug delivery to the desired tissue, cell, or organ (subcellular targeting), enhancing accuracy, efficacy, and adverse effect mitigation. By minimizing drug elimination from the system, these nanosystems employ both passive and active delivery to tumor areas. Despite the limited number of human clinical trials that have commenced thus far, nanocomposite materials composed of functionalized semiconductors and metal nanoparticles can fundamentally transform the methods employed in detecting and treating cancer. It is crucial to acknowledge that although obstacles and ambiguities exist, continuous research and optimization endeavors concerning the utilization of nanocomposites present a prospect to tackle these issues and facilitate the development of cancer diagnoses and treatments that are both safer and more efficient.
Funding
This research was funded by the National Natural Science Foundation of China (31861143051, 31872425, and 32250410288).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Malhotra BD, Ali Md A. Nanocomposite Materials. In: Nanomaterials for Biosensors. Elsevier; 2018:145–159. doi:10.1016/B978-0-323-44923-6.00005-4
2. Gutierrez AM, Frazar EM, Klaus MV, Paul P, Hilt JZ. Hydrogels and hydrogel nanocomposites: enhancing healthcare through human and environmental treatment. Adv Healthc Mater. 2022;11(7). doi:10.1002/adhm.202101820
3. Kannan P, Maduraiveeran G. Carbon nanocomposites-based electrochemical sensors and biosensors for biomedical diagnostics. Curr Med Chem. 2023;30. doi:10.2174/0929867330666230425163520
4. NHS Englang. Widespread clinical support for reforming NHS cancer standards to speed up diagnosis for patients. Cancer. 2023;2023:1.
5. Dhas N, Kudarha R, Garkal A, et al. Molybdenum-based hetero-nanocomposites for cancer therapy, diagnosis and biosensing application: current advancement and future breakthroughs. J Control Release. 2021;330:257–283. doi:10.1016/j.jconrel.2020.12.015
6. Deshwal A, Kukreja EA, Shrivastava N, Sheikh FA, Amna T, Tripathi RM. Nanomaterial Applications in Cancer Therapy and Diagnosis. In: Interaction of Nanomaterials with Living Cells. Springer Nature Singapore; 2023:471–484. doi:10.1007/978-981-99-2119-5_16
7. Wang J, Sui L, Huang J, et al. MoS2-based nanocomposites for cancer diagnosis and therapy. Bioact Mater. 2021;6(11):4209–4242. doi:10.1016/j.bioactmat.2021.04.021
8. Li J, Yao M, Shao Y, Yao D. The application of bio-nanotechnology in tumor diagnosis and treatment: a view. Nanotechnol Rev. 2018;7(3):257–266. doi:10.1515/ntrev-2018-0011
9. Vasanthakumar A, Rejeeth C, Vivek R, et al. Design of bio-graphene-based multifunctional nanocomposites exhibits intracellular drug delivery in cervical cancer treatment. ACS Appl Bio Mater. 2022;5(6):2956–2964. doi:10.1021/acsabm.2c00280
10. Yuan YG, Gurunathan S. Combination of graphene oxide–silver nanoparticle nanocomposites and cisplatin enhances apoptosis and autophagy in human cervical cancer cells. Int J Nanomed. 2017;12:6537–6558. doi:10.2147/IJN.S125281
11. Kumar S, Shukla MK, Sharma AK, et al. Metal‐based nanomaterials and nanocomposites as promising frontier in cancer chemotherapy. MedComm. 2023;4(2). doi:10.1002/mco2.253
12. Mundekkad D, Cho WC. Nanoparticles in Clinical Translation for Cancer Therapy. Int J Mol Sci. 2022;23(3):1685. doi:10.3390/ijms23031685
13. Chauhan S, Al-Dayan N, Kumar R, et al. Synthesis and characterization of novel bimetallic-semi-aromatic polyester nanocomposite for possible biomedical use. Mater Lett. 2022;306:130943. doi:10.1016/j.matlet.2021.130943
14. Carrera Espinoza MJ, Lin KS, Weng MT, Kunene SC, Lin YS, Wu CM. Synthesis and characterization of supermagnetic nanocomposites coated with pluronic F127 as a contrast agent for biomedical applications. Pharmaceutics. 2023;15(3):740. doi:10.3390/pharmaceutics15030740
15. Elderdery AY, Alhamidi AH, Elkhalifa AME, et al. Synthesis and characterization of ZnO–TiO 2 –chitosan–escin metallic nanocomposites: evaluation of their antimicrobial and anticancer activities. Green Processing and Synthesis. 2022;11(1):1026–1039. doi:10.1515/gps-2022-0086
16. Mariadoss AVA, Saravanakumar K, Sathiyaseelan A, Wang MH. Preparation, characterization and anti-cancer activity of graphene oxide--silver nanocomposite. J Photochem Photobiol B. 2020;210:111984. doi:10.1016/j.jphotobiol.2020.111984
17. Elderdery AY, Alzahrani B, Hamza SMA, Mostafa-Hedeab G, Mok PL, Subbiah SK. 2022. Synthesis, Characterization, and Antiproliferative Effect of CuO-TiO2-Chitosan-Amygdalin Nanocomposites in Human Leukemic MOLT4 Cells. In: Rethinam S, editor. Bioinorg Chem Appl. Vol. 2022. 1–13. doi:10.1155/2022/1473922
18. Tarhan T, Tural B, Tural S. Synthesis and characterization of new branched magnetic nanocomposite for loading and release of topotecan anti-cancer drug. J Anal Sci Technol. 2019;10(1):30. doi:10.1186/s40543-019-0189-x
19. Ţucureanu V, Obreja CA, Pachiu C, Brîncoveanu O, Matei A. Synthesis and Characterization of Nanocomposites Based on Carbon Materials and Transitional Oxides. In: IOCN 2023. MDPI; 2023:8. doi:10.3390/IOCN2023-14453
20. Asghar K, Qasim M, Dharmapuri G, Das D. Thermoresponsive polymer gated and superparamagnetic nanoparticle embedded hollow mesoporous silica nanoparticles as smart multifunctional nanocarrier for targeted and controlled delivery of doxorubicin. Nanotechnology. 2020;31(45):455604. doi:10.1088/1361-6528/ab8b0e
21. Viswanathan V, Laha T, Balani K, Agarwal A, Seal S. Challenges and advances in nanocomposite processing techniques. Mater Sci Eng R Rep. 2006;54(5–6):121–285. doi:10.1016/j.mser.2006.11.002
22. Naghib SM, Zare Y, Rhee KY. A facile and simple approach to synthesis and characterization of methacrylated graphene oxide nanostructured polyaniline nanocomposites. Nanotechnol Rev. 2020;9(1):53–60. doi:10.1515/ntrev-2020-0005
23. Pacioni NL, Molina Torres MA, Núñez RN. Synthesis and Characterization of Nanomaterials for Biomedical Applications. In: Nanoengineering Materials for Biomedical Uses. Springer International Publishing; 2019:13–34. doi:10.1007/978-3-030-31261-9_2
24. Timerbaev AR, Kuznetsova OV, Keppler BK. Current trends and challenges in analysis and characterization of engineered nanoparticles in seawater. Talanta. 2021;226:122201. doi:10.1016/j.talanta.2021.122201
25. Alrushaid N, Khan FA, Al-Suhaimi EA, Elaissari A. Nanotechnology in cancer diagnosis and treatment. Pharmaceutics. 2023;15(3):1025. doi:10.3390/pharmaceutics15031025
26. Jin C, Wang K, Oppong-Gyebi A, Hu J. Application of nanotechnology in cancer diagnosis and therapy - a mini-review. Int J Med Sci. 2020;17(18):2964–2973. doi:10.7150/ijms.49801
27. Pourmadadi M, Rajabzadeh-Khosroshahi M, Eshaghi MM, et al. TiO2-based nanocomposites for cancer diagnosis and therapy: a comprehensive review. J Drug Deliv Sci Technol. 2023;82:104370. doi:10.1016/j.jddst.2023.104370
28. Kyriakides TR, Raj A, Tseng TH, et al. Biocompatibility of nanomaterials and their immunological properties. Biomed Mater. 2021;16(4):042005. doi:10.1088/1748-605X/abe5fa
29. Chiticaru EA, Damian CM, Pilan L, Ioniță M. Label-Free DNA biosensor based on reduced graphene oxide and gold nanoparticles. Biosensors. 2023;13(8):797. doi:10.3390/bios13080797
30. Ashraf MA, Peng W, Zare Y, Rhee KY. Effects of size and aggregation/agglomeration of nanoparticles on the interfacial/interphase properties and tensile strength of polymer nanocomposites. Nanoscale Res Lett. 2018;13(1):214. doi:10.1186/s11671-018-2624-0
31. Harmer MA, Farneth WE, Sun Q. High Surface Area Nafion Resin/silica nanocomposites: a new class of solid acid catalyst. J Am Chem Soc. 1996;118(33):7708–7715. doi:10.1021/ja9541950
32. Xavier JR, Sadagopan Pandian V. Carbon nanotube‐based polymer nanocomposites: evaluation of barrier, hydrophobic, and mechanical properties for aerospace applications. Polym Eng Sci. 2023;63(9):2806–2827. doi:10.1002/pen.26407
33. Yang W, Ratinac KR, Ringer SP, Thordarson P, Gooding JJ, Braet F. Carbon nanomaterials in biosensors: should you use nanotubes or graphene?. Angew Chem Int Ed. 2010;49(12):2114–2138. doi:10.1002/anie.200903463
34. Abla F, Elsayed Y, Abu Farha N, et al. Fabrication of High Surface Area TiO2-MoO3 Nanocomposite as a photocatalyst for organic pollutants removal from water bodies. Catalysts. 2023;13(2):362. doi:10.3390/catal13020362
35. Cheng S, Yang M, Fu J, Wang R, He J, Li Q. Surface‐coated polymer nanocomposites containing z‐aligned high‐k nanowires as high‐performance dielectrics at elevated temperatures. IET Nanodielectr. 2023;6(4):237–245. doi:10.1049/nde2.12060
36. ScienceDirect. Biodegradable and Biocompatible Polymer Nanocomposites- Processing, Characterization, and Applications. Kalim D, Mayank P, eds.. Elsevier; 2023. doi:10.1016/C2021-0-00952-4
37. Mahajan H, Cho S. Novel Au nanorod/Cu 2 O composite nanoparticles for a high-performance supercapacitor. RSC Adv. 2022;12(15):9112–9120. doi:10.1039/D2RA00812B
38. Bardhan N. Nanomaterials in diagnostics, imaging and delivery: applications from COVID-19 to cancer. MRS Commun. 2022;12(6):1119–1139. doi:10.1557/s43579-022-00257-7
39. Liu H, Jian R, Chen H, et al. Application of biodegradable and biocompatible nanocomposites in electronics: current status and future directions. Nanomaterials. 2019;9(7):950. doi:10.3390/nano9070950
40. Wang Y, Gu H. Core–shell‐type magnetic mesoporous silica nanocomposites for bioimaging and therapeutic agent delivery. Adv Mater. 2015;27(3):576–585. doi:10.1002/adma.201401124
41. Verma M, Fatima S, Ansari IA. Phytofabricated nanoparticle formulation for cancer treatment: a comprehensive review. Curr Drug Metab. 2022;23(10):818–826. doi:10.2174/1389200223666220427101427
42. Kashyap BK, Singh VV, Solanki MK, Kumar A, Ruokolainen J, Kesari KK. Smart nanomaterials in cancer theranostics: challenges and opportunities. ACS Omega. 2023;8(16):14290–14320. doi:10.1021/acsomega.2c07840
43. Khan FA, Albalawi R, Pottoo FH. Trends in targeted delivery of nanomaterials in colon cancer diagnosis and treatment. Med Res Rev. 2022;42(1):227–258. doi:10.1002/med.21809
44. Xie W, Liu Y, Lin J. Advances in organic–inorganic nanocomposites for cancer imaging and therapy. Nanotechnol Rev. 2023;12(1). doi:10.1515/ntrev-2023-0133
45. Qamar SUR. Nanocomposites: potential therapeutic agents for the diagnosis and treatment of infectious diseases and cancer. Colloid Interface Sci Commun. 2021;43:100463. doi:10.1016/j.colcom.2021.100463
46. Gao L, Zhang Y, Zhao L, et al. An artificial metalloenzyme for catalytic cancer-specific DNA cleavage and operando imaging. Sci Adv. 2020;6(29). doi:10.1126/sciadv.abb1421
47. Zhang X, Chen X, Zhao Y. Nanozymes: versatile platforms for cancer diagnosis and therapy. Nano-micro Lett. 2022;14(1):95. doi:10.1007/s40820-022-00828-2
48. Li Y, Zhang C, Li G, et al. Protease-triggered bioresponsive drug delivery for the targeted theranostics of malignancy. Acta Pharm Sin B. 2021;11(8):2220–2242. doi:10.1016/j.apsb.2021.01.017
49. Qi M, Pan H, Shen H, et al. Nanogel multienzyme mimics synthesized by biocatalytic ATRP and metal coordination for bioresponsive fluorescence imaging. Angew Chem Int Ed. 2020;59(29):11748–11753. doi:10.1002/anie.202002331
50. Sadeghi M, Sadeghi S, Naghib SM, Garshasbi HR. A Comprehensive review on electrochemical nano biosensors for precise detection of blood-based oncomarkers in breast cancer. Biosensors. 2023;13(4):481. doi:10.3390/bios13040481
51. Xu Z, Peng M, Zhang Z, et al.. Graphene-assisted electrochemical sensor for detection of pancreatic cancer markers. Front Chem. 2021:9. doi:10.3389/fchem.2021.733371
52. Fu L, Zheng Y, Li X, Liu X, Lin CT, Karimi-Maleh H. Strategies and applications of graphene and its derivatives-based electrochemical sensors in cancer diagnosis. Molecules. 2023;28(18):6719. doi:10.3390/molecules28186719
53. Mohammadpour-Haratbar A, Zare Y, Rhee KY. Electrochemical biosensors based on polymer nanocomposites for detecting breast cancer: recent progress and future prospects. Adv Colloid Interface Sci. 2022;309:102795. doi:10.1016/j.cis.2022.102795
54. Sanko V, Kuralay F. Label-Free Electrochemical Biosensor Platforms for Cancer Diagnosis: recent Achievements and Challenges. Biosensors. 2023;13(3):333. doi:10.3390/bios13030333
55. Yigit MV, Moore A, Medarova Z. Magnetic Nanoparticles for Cancer Diagnosis and Therapy. Pharm Res. 2012;29(5):1180–1188. doi:10.1007/s11095-012-0679-7
56. Wu M, Huang S. Magnetic nanoparticles in cancer diagnosis, drug delivery and treatment (Review). Mol Clin Oncol. 2017. doi:10.3892/mco.2017.1399
57. Govindan B, Sabri MA, Hai A, Banat F, Haija MA. A review of advanced multifunctional magnetic nanostructures for cancer diagnosis and therapy integrated into an artificial intelligence approach. Pharmaceutics. 2023;15(3):868. doi:10.3390/pharmaceutics15030868
58. Liu C, Yang B, Chen X, et al. Capture and separation of circulating tumor cells using functionalized magnetic nanocomposites with simultaneous in situ chemotherapy. Nanotechnology. 2019;30(28):285706. doi:10.1088/1361-6528/ab0e25
59. Alromi D, Madani S, Seifalian A. Emerging application of magnetic nanoparticles for diagnosis and treatment of cancer. Polymers. 2021;13(23):4146. doi:10.3390/polym13234146
60. Barajas RF, Hamilton BE, Schwartz D, et al. Combined iron oxide nanoparticle ferumoxytol and gadolinium contrast enhanced MRI define glioblastoma pseudoprogression. Neuro Oncol. 2019;21(4):517–526. doi:10.1093/neuonc/noy160
61. Iv M, Telischak N, Feng D, Holdsworth SJ, Yeom KW, Daldrup-Link HE. Clinical applications of iron oxide nanoparticles for magnetic resonance imaging of brain tumors. Nanomedicine. 2015;10(6):993–1018. doi:10.2217/nnm.14.203
62. Fortunati E, Argalia G, Zanoni L, Fanti S, Ambrosini V. New PET radiotracers for the imaging of neuroendocrine neoplasms. Curr Treat Options Oncol. 2022;23(5):703–720. doi:10.1007/s11864-022-00967-z
63. Hwang DW, Ko HY, Lee JH, et al. A nucleolin-targeted multimodal nanoparticle imaging probe for tracking cancer cells using an aptamer. J Nucl Med. 2010;51(1):98–105. doi:10.2967/jnumed.109.069880
64. Tseng YT, Harroun SG, Wu CW, Mao JY, Chang HT, Huang CC. Satellite-like gold nanocomposites for targeted mass spectrometry imaging of tumor tissues. Nanotheranostics. 2017;1(2):141–153. doi:10.7150/ntno.18897
65. Han L, Zhang Y, Zhang Y, Shu Y, Chen XW, Wang JH. A magnetic polypyrrole/iron oxide core/gold shell nanocomposite for multimodal imaging and photothermal cancer therapy. Talanta. 2017;171:32–38. doi:10.1016/j.talanta.2017.04.056
66. Zeng Z, Ouyang J, Sun L, Zeng C, Zeng F, Wu S. Activatable nanocomposite probe for preoperative location and intraoperative navigation for orthotopic hepatic tumor resection via MSOT and aggregation-induced near-IR-I/II fluorescence imaging. Anal Chem. 2020;92(13):9257–9264. doi:10.1021/acs.analchem.0c01596
67. Zhang L, Jiang C, Li B, et al. A core-shell Au@Cu2-xSe heterogeneous metal nanocomposite for photoacoustic and computed tomography dual-imaging-guided photothermal boosted chemodynamic therapy. J Nanobiotechnology. 2021;19(1):410. doi:10.1186/s12951-021-01159-x
68. Okamoto A, Funakoshi Y, Oe M, et al. Identification of breast cancer stem cells using a newly developed long-acting fluorescence probe, C5S-A, targeting ALDH1A1. Anticancer Res. 2022;42(3):1199–1205. doi:10.21873/anticanres.15586
69. Men X, Chen H, Sun C, et al. Thermosensitive polymer dot nanocomposites for trimodal computed tomography/Photoacoustic/Fluorescence imaging-guided synergistic chemo-photothermal therapy. ACS Appl Mater Interfaces. 2020;12(46):51174–51184. doi:10.1021/acsami.0c13252
70. Ebert B, Grosenick D. Optical imaging of breast tumors and of gastrointestinal cancer by laser-induced fluorescence. Molec Imag Oncol. 2013;18:331–350. doi:10.1007/978-3-642-10853-2_11
71. Farwell MD, Gamache RF, Babazada H, et al. CD8-targeted PET imaging of tumor infiltrating T cells in patients with cancer: a phase I first-in-human study of 89 Zr-Df-IAB22M2C, a radiolabeled anti-CD8 minibody. J Nucl Med. 2021. doi:10.2967/jnumed.121.262485
72. Grieshaber D, MacKenzie R, Vörös J, Reimhult E. Electrochemical biosensors - sensor principles and architectures. Sensors. 2008;8(3):1400–1458. doi:10.3390/s80314000
73. Dong W, Ren Y, Bai Z, et al. Trimetallic AuPtPd nanocomposites platform on graphene: applied to electrochemical detection and breast cancer diagnosis. Talanta. 2018;189:79–85. doi:10.1016/j.talanta.2018.06.067
74. Yang T, Gao Y, Liu Z, Xu J, Lu L, Yu Y. Three-dimensional gold nanoparticles/prussian blue-poly(3,4-ethylenedioxythiophene) nanocomposite as novel redox matrix for label-free electrochemical immunoassay of carcinoembryonic antigen. Sens Actuators B Chem. 2017;239:76–84. doi:10.1016/j.snb.2016.08.001
75. Azizi-Lalabadi M, Jafari SM. Bio-nanocomposites of graphene with biopolymers; fabrication, properties, and applications. Adv Colloid Interface Sci. 2021;292:102416. doi:10.1016/j.cis.2021.102416
76. Ran B, Chen C, Liu B, Lan M, Chen H, Zhu Y. A Ti 3 C 2 T X /Pt–Pd based amperometric biosensor for sensitive cancer biomarker detection. Electrophoresis. 2022;43(20):2033–2043. doi:10.1002/elps.202100218
77. Uhlirova D, Stankova M, Docekalova M, et al. A rapid method for the detection of sarcosine using SPIONs/Au/CS/SOX/NPs for prostate cancer sensing. Int J Mol Sci. 2018;19(12):3722. doi:10.3390/ijms19123722
78. Narwal V, Kumar P, Joon P, Pundir CS. Fabrication of an amperometric sarcosine biosensor based on sarcosine oxidase/chitosan/CuNPs/c-MWCNT/Au electrode for detection of prostate cancer. Enzyme Microb Technol. 2018;113:44–51. doi:10.1016/j.enzmictec.2018.02.010
79. Wang W, Fan X, Xu S, Davis JJ, Luo X. Low fouling label-free DNA sensor based on polyethylene glycols decorated with gold nanoparticles for the detection of breast cancer biomarkers. Biosens Bioelectron. 2015;71:51–56. doi:10.1016/j.bios.2015.04.018
80. Hasanzadeh M, Feyziazar M, Solhi E, et al. Ultrasensitive immunoassay of breast cancer type 1 susceptibility protein (BRCA1) using poly (dopamine-beta cyclodextrine-Cetyl trimethylammonium bromide) doped with silver nanoparticles: a new platform in early stage diagnosis of breast cancer and effici. Microchem J. 2019;145:778–783. doi:10.1016/j.microc.2018.11.029
81. Malhotra BD, Kumar S, Pandey CM. Nanomaterials based biosensors for cancer biomarker detection. J Phys Conf Ser. 2016;704:012011. doi:10.1088/1742-6596/704/1/012011
82. Balaji A, Zhang J. Electrochemical and optical biosensors for early-stage cancer diagnosis by using graphene and graphene oxide. Cancer Nanotechnol. 2017;8(1):10. doi:10.1186/s12645-017-0035-z
83. Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 2021;14(1):85. doi:10.1186/s13045-021-01096-0
84. Zhu YJ, Li WJ, Hong ZY, Tang AN, Kong DM. Stable, polyvalent aptamer-conjugated near-infrared fluorescent nanocomposite for high-performance cancer cell-targeted imaging and therapy. J Mater Chem B. 2017;5(46):9229–9237. doi:10.1039/C7TB02218B
85. Doan VHM, Nguyen VT, Mondal S, et al. Fluorescence/photoacoustic imaging-guided nanomaterials for highly efficient cancer theragnostic agent. Sci Rep. 2021;11(1):15943. doi:10.1038/s41598-021-95660-w
86. Zhou J, Li J, Ding X, et al. Multifunctional Fe 2 O 3 @PPy-PEG nanocomposite for combination cancer therapy with MR imaging. Nanotechnology. 2015;26(42):425101. doi:10.1088/0957-4484/26/42/425101
87. Nan X, Zhang X, Liu Y, Zhou M, Chen X, Zhang X. Dual-targeted multifunctional nanoparticles for magnetic resonance imaging guided cancer diagnosis and therapy. ACS Appl Mater Interfaces. 2017;9(11):9986–9995. doi:10.1021/acsami.6b16486
88. Du D, Fu HJ, Ren Wei W, Li XL, Guo LH. PSA targeted dual-modality manganese oxide–mesoporous silica nanoparticles for prostate cancer imaging. Biomed Pharmacother. 2020;121:109614. doi:10.1016/j.biopha.2019.109614
89. Li H, Li K, Zeng Q, et al. Novel vinyl-modified RGD conjugated silica nanoparticles based on photo click chemistry for in vivo prostate cancer targeted fluorescence imaging. RSC Adv. 2019;9(44):25318–25325. doi:10.1039/C9RA04513A
90. Estévez MC, O’Donoghue MB, Chen X, Tan W. Highly fluorescent dye-doped silica nanoparticles increase flow cytometry sensitivity for cancer cell monitoring. Nano Res. 2009;2(6):448–461. doi:10.1007/s12274-009-9041-8
91. Chinen AB, Guan CM, Ferrer JR, Barnaby SN, Merkel TJ, Mirkin CA. Nanoparticle Probes for the Detection of Cancer Biomarkers, Cells, and Tissues by Fluorescence. Chem Rev. 2015;115(19):10530–10574. doi:10.1021/acs.chemrev.5b00321
92. Subramanian AP, Jaganathan SK, Supriyanto E. Overview on in vitro and in vivo investigations of nanocomposite based cancer diagnosis and therapeutics. RSC Adv. 2015;5(89):72638–72652. doi:10.1039/C5RA11912J
93. Namiot ED, Sokolov AV, Chubarev VN, Tarasov VV, Schiöth HB. Nanoparticles in clinical trials: analysis of clinical trials, FDA approvals and use for COVID-19 vaccines. Int J Mol Sci. 2023;24(1):787. doi:10.3390/ijms24010787
94. Lu J, Tai Z, Wu J, et al. Nanomedicine-induced programmed cell death enhances tumor immunotherapy. J Adv Res. 2023. doi:10.1016/j.jare.2023.09.018
95. Zhong Fang X, Sun X. Nanomedicines based on nanoscale metal-organic frameworks for cancer immunotherapy. Acta Pharmacol Sin. 2020;41(7):928–935. doi:10.1038/s41401-020-0414-6
96. El-Ghannam A, Ricci K, Malkawi A, et al. A ceramic-based anticancer drug delivery system to treat breast cancer. J Mater Sci Mater Med. 2010;21(9):2701–2710. doi:10.1007/s10856-010-4121-6
97. Yang H, Chen Y, Chen Z, et al. Chemo-photodynamic combined gene therapy and dual-modal cancer imaging achieved by pH-responsive alginate/chitosan multilayer-modified magnetic mesoporous silica nanocomposites. Biomater Sci. 2017;5(5):1001–1013. doi:10.1039/C7BM00043J
98. Li D, Deng M, Yu Z, et al. Biocompatible and Stable GO-Coated Fe 3 O 4 nanocomposite: a robust drug delivery carrier for simultaneous tumor MR imaging and targeted therapy. ACS Biomater Sci Eng. 2018;4(6):2143–2154. doi:10.1021/acsbiomaterials.8b00029
99. Gai S, Yang G, Yang P, et al. Recent advances in functional nanomaterials for light–triggered cancer therapy. Nano Today. 2018;19:146–187. doi:10.1016/j.nantod.2018.02.010
100. Liu T, Shi S, Liang C, et al. Iron oxide decorated MoS 2 Nanosheets with Double PEGylation for chelator-free radiolabeling and multimodal imaging guided photothermal therapy. ACS Nano. 2015;9(1):950–960. doi:10.1021/nn506757x
101. Li Y, Liu X, Pan W, Li N, Tang B. Photothermal therapy-induced immunogenic cell death based on natural melanin nanoparticles against breast cancer. Chem. Commun. 2020;56(9):1389–1392. doi:10.1039/C9CC08447A
102. Tao J, Wang B, Dong Y, et al. Photothermal and acid-responsive fucoidan-cus bubble pump microneedles for combined CDT/PTT/CT treatment of melanoma. ACS Appl Mater Interfaces. 2023;15(34):40267–40279. doi:10.1021/acsami.3c08368
103. Baipaywad P, Ryu N, Im SS, et al. Facile preparation of poly(N -isopropylacrylamide)/graphene oxide nanocomposites for chemo-photothermal therapy. Des Monomers Polym. 2022;25(1):245–253. doi:10.1080/15685551.2022.2111854
104. Osaki T, Yokoe I, Sunden Y, et al. Efficacy of 5-aminolevulinic acid in photodynamic detection and photodynamic therapy in veterinary medicine. Cancers. 2019;11(4):495. doi:10.3390/cancers11040495
105. Kutsevol N, Naumenko A, Harahuts Y, et al. New hybrid composites for photodynamic therapy: synthesis, characterization and biological study. Appl Nanosci. 2019;9(5):881–888. doi:10.1007/s13204-018-0768-y
106. Vines JB, Yoon JH, Ryu NE, Lim DJ, Park H. Gold nanoparticles for photothermal cancer therapy. Front Chem. 2019;7. doi:10.3389/fchem.2019.00167
107. Qin X, Wu C, Niu D, et al. Peroxisome inspired hybrid enzyme nanogels for chemodynamic and photodynamic therapy. Nat Commun. 2021;12(1):5243. doi:10.1038/s41467-021-25561-z
108. Lu K, He C, Lin W. A chlorin-based nanoscale metal–organic framework for photodynamic therapy of colon cancers. J Am Chem Soc. 2015;137(24):7600–7603. doi:10.1021/jacs.5b04069
109. Roma-Rodrigues C, Rivas-García L, Baptista PV, Fernandes AR. Gene therapy in cancer treatment: why go nano?. Pharmaceutics. 2020;12(3):233. doi:10.3390/pharmaceutics12030233
110. Iqbal MJ, Javed Z, Sadia H, et al. Targeted therapy using nanocomposite delivery systems in cancer treatment: highlighting miR34a regulation for clinical applications. Cancer Cell Int. 2023;23(1):84. doi:10.1186/s12935-023-02929-3
111. Putnam D. Polymers for gene delivery across length scales. Nat Mater. 2006;5(6):439–451. doi:10.1038/nmat1645
112. Hadji H, Bouchemal K. Effect of micro- and nanoparticle shape on biological processes. J Control Release. 2022;342:93–110. doi:10.1016/j.jconrel.2021.12.032
113. Sheikh A, Alhakamy NA, Md S, Kesharwani P. Recent progress of RGD modified liposomes as multistage rocket against cancer. Front Pharmacol. 2022;12. doi:10.3389/fphar.2021.803304
114. Díez-Pascual AM. Carbon-based polymer nanocomposites for high-performance applications II. Polymers. 2022;14(5):870. doi:10.3390/polym14050870
115. Wu Y, Wang H, Gao F, Xu Z, Dai F, Liu W. An Injectable supramolecular polymer nanocomposite hydrogel for prevention of breast cancer recurrence with theranostic and mammoplastic functions. Adv Funct Mater. 2018;28(21). doi:10.1002/adfm.201801000
116. Loo YS, Zahid NI, Madheswaran T, Mat Azmi ID. Recent advances in the development of multifunctional lipid-based nanoparticles for co-delivery, combination treatment strategies, and theranostics in breast and lung cancer. J Drug Deliv Sci Technol. 2022;71:103300. doi:10.1016/j.jddst.2022.103300
117. Hu L, Xiong C, Wei G, et al. Stimuli-responsive charge-reversal MOF@polymer hybrid nanocomposites for enhanced co-delivery of chemotherapeutics towards combination therapy of multidrug-resistant cancer. J Colloid Interface Sci. 2022;608:1882–1893. doi:10.1016/j.jcis.2021.10.070
118. Gamal-Eldeen AM, Abdel-Hameed SAM, El-Daly SM, Abo-Zeid MAM, Swellam MM. Cytotoxic effect of ferrimagnetic glass-ceramic nanocomposites on bone osteosarcoma cells. Biomed Pharmacother. 2017;88:689–697. doi:10.1016/j.biopha.2017.01.113
119. Wu Q, Chu M, Shao Y, Wo F, Shi D. Reduced graphene oxide conjugated with CuInS2/ZnS nanocrystals with low toxicity for enhanced photothermal and photodynamic cancer therapies. Carbon N Y. 2016;108:21–37. doi:10.1016/j.carbon.2016.06.070
120. Kalluru P, Vankayala R, Chiang CS, Hwang KC. Nano-graphene oxide-mediated In vivo fluorescence imaging and bimodal photodynamic and photothermal destruction of tumors. Biomaterials. 2016;95:1–10. doi:10.1016/j.biomaterials.2016.04.006
121. Tang X, Liang Y, Feng X, Zhang R, Jin X, Sun L. Co-delivery of docetaxel and Poloxamer 235 by PLGA–TPGS nanoparticles for breast cancer treatment. Mater Sci Eng C. 2015;49:348–355. doi:10.1016/j.msec.2015.01.033
122. Gao X, Huang N, Shi H, et al.. Enhancing the anti-colon cancer activity of quercetin by self-assembled micelles. Int J Nanomed. 2015:2051. doi:10.2147/IJN.S75550
123. He Y, Wan J, Yang Y, et al. Multifunctional Polypyrrole‐Coated Mesoporous TiO 2 nanocomposites for photothermal, sonodynamic, and chemotherapeutic treatments and dual‐modal ultrasound/Photoacoustic Imaging of Tumors. Adv Healthc Mater. 2019;8(9). doi:10.1002/adhm.201801254
124. Nejadshafiee V, Naeimi H, Goliaei B, et al. Magnetic bio-metal–organic framework nanocomposites decorated with folic acid conjugated chitosan as a promising biocompatible targeted theranostic system for cancer treatment. Mater Sci Eng C. 2019;99:805–815. doi:10.1016/j.msec.2019.02.017
125. Dong X, Yin W, Zhang X, et al. Intelligent MoS 2 Nanotheranostic for Targeted and Enzyme-/pH-/NIR-responsive drug delivery to overcome cancer chemotherapy resistance guided by PET Imaging. ACS Appl Mater Interfaces. 2018;10(4):4271–4284. doi:10.1021/acsami.7b17506
126. Wang D, Xu Z, Yu H, et al. Treatment of metastatic breast cancer by combination of chemotherapy and photothermal ablation using doxorubicin-loaded DNA wrapped gold nanorods. Biomaterials. 2014;35(29):8374–8384. doi:10.1016/j.biomaterials.2014.05.094
127. Loiseau A, Boudon J, Oudot A, et al. Titanate nanotubes engineered with gold nanoparticles and docetaxel to enhance radiotherapy on xenografted prostate tumors. Cancers. 2019;11(12):1962. doi:10.3390/cancers11121962
128. Chan MH, Hsieh MR, Liu RS, Wei DH, Hsiao M. Magnetically guided theranostics: optimizing magnetic resonance imaging with sandwich-like kaolinite-based iron/platinum nanoparticles for magnetic fluid hyperthermia and chemotherapy. Chem. Mater. 2020;32(2):697–708. doi:10.1021/acs.chemmater.9b03552
129. Kaurav H, Manchanda S, Dua K, Kapoor DN. Nanocomposites in controlled & targeted drug delivery systems. Nano Hybrids Composit. 2018;20:27–45. doi:10.4028/www.scientific.net/NHC.20.27
130. Rahim M, Mas Haris MRH, Saqib NU. An overview of polymeric nano-biocomposites as targeted and controlled-release devices. Biophys Rev. 2020;12(5):1223–1231. doi:10.1007/s12551-020-00750-0
131. Dahiya MS, Tomer VK, Duhan S. Metal–ferrite nanocomposites for targeted drug delivery. In: Applications of Nanocomposite Materials in Drug Delivery. Elsevier; 2018:737–760. doi:10.1016/B978-0-12-813741-3.00032-7
132. Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71. doi:10.1186/s12951-018-0392-8
133. Yusuf A, Almotairy ARZ, Henidi H, Alshehri OY, Aldughaim MS. Nanoparticles as drug delivery systems: a review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers. 2023;15(7):1596. doi:10.3390/polym15071596
134. Ashrafizadeh M, Saebfar H, Gholami MH, et al. Doxorubicin-loaded graphene oxide nanocomposites in cancer medicine: stimuli-responsive carriers, co-delivery and suppressing resistance. Expert Opin Drug Deliv. 2022;19(4):355–382. doi:10.1080/17425247.2022.2041598
135. Seifalian A. A new era of cancer treatment: carbon nanotubes as drug delivery tools. Int J Nanomed. 2011;2963. doi:10.2147/IJN.S16923
136. Pattnaik S, Swain K, Lin Z. Graphene and graphene-based nanocomposites: biomedical applications and biosafety. J Mater Chem B. 2016;4(48):7813–7831. doi:10.1039/C6TB02086K
137. Sharma A, Shambhwani D, Pandey S, et al. Advances in lung cancer treatment using nanomedicines. ACS Omega. 2023;8(1):10–41. doi:10.1021/acsomega.2c04078
138. Mohammadi M, Ramezani M, Abnous K, Alibolandi M. Biocompatible polymersomes-based cancer theranostics: towards multifunctional nanomedicine. Int J Pharm. 2017;519(1–2):287–303. doi:10.1016/j.ijpharm.2017.01.037
139. He M, Zhang Z, Jiao Z, et al. Redox-responsive phenyl-functionalized polylactide micelles for enhancing Ru complexes delivery and phototherapy. Chin. Chem. Lett. 2023;34(3):107574. doi:10.1016/j.cclet.2022.05.088
140. Abbasi E, Aval SF, Akbarzadeh A, et al. Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett. 2014;9(1):247. doi:10.1186/1556-276X-9-247
141. Umoren SA, Solomon MM, Saji VS. Polymeric Materials in Corrosion Inhibition. Elsevier; 2022; doi:10.1016/C2020-0-00555-4
142. Zare EN, Jamaledin R, Naserzadeh P, et al. Metal-based nanostructures/PLGA nanocomposites: antimicrobial activity, cytotoxicity, and their biomedical applications. ACS Appl Mater Interfaces. 2020;12(3):3279–3300. doi:10.1021/acsami.9b19435
143. Zhao W, Li J, Zhong C, Zhang X, Bao Y. Green synthesis of gold nanoparticles from Dendrobium officinale and its anticancer effect on liver cancer. Drug Deliv. 2021;28(1):985–994. doi:10.1080/10717544.2021.1921079
144. Yang Y, Yan Q, Liu Q, et al. An ultrasensitive sandwich-type electrochemical immunosensor based on the signal amplification strategy of echinoidea-shaped Au@Ag-Cu2O nanoparticles for prostate specific antigen detection. Biosens Bioelectron. 2018;99:450–457. doi:10.1016/j.bios.2017.08.018
145. Cattel L, Ceruti M, Dosio F. From conventional to stealth liposomes a new frontier in cancer chemotherapy. Tumori J. 2003;89(3):237–249. doi:10.1177/030089160308900302
146. Umoren SA, Solomon MM, Saji VS. Hybrid Nanocomposites for Nanotechnology. Merhari L, ed.. Springer US;2009. doi:10.1007/978-0-387-30428-1
147. Kamalan Kirubaharan AM, Kuppusami P. Corrosion behavior of ceramic nanocomposite coatings at nanoscale. In: Corrosion Protection at the Nanoscale. Elsevier; 2020:295–314. doi:10.1016/B978-0-12-819359-4.00016-7
148. Palmero P. Structural ceramic nanocomposites: a review of properties and powders’ synthesis methods. Nanomaterials. 2015;5(2):656–696. doi:10.3390/nano5020656
149. Diba M, Goudouri OM, Tapia F, Boccaccini AR. Magnesium-containing bioactive polycrystalline silicate-based ceramics and glass-ceramics for biomedical applications. Curr Opin Solid State Mater Sci. 2014;18(3):147–167. doi:10.1016/j.cossms.2014.02.004
150. El-Ghannam A. Bone reconstruction: from bioceramics to tissue engineering. Expert Rev Med Devices. 2005;2(1):87–101. doi:10.1586/17434440.2.1.87
151. Chehelgerdi M, Chehelgerdi M, Allela OQB, et al. Progressing nanotechnology to improve targeted cancer treatment: overcoming hurdles in its clinical implementation. Mol Cancer. 2023;22(1):169. doi:10.1186/s12943-023-01865-0
152. Dadwal A, Baldi A, Kumar Narang R. Nanoparticles as carriers for drug delivery in cancer. Artif Cells Nanomed Biotechnol. 2018;46(sup2):295–305. doi:10.1080/21691401.2018.1457039
153. Hu CMJ, Aryal S, Zhang L. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther Deliv. 2010;1(2):323–334. doi:10.4155/tde.10.13
154. Gavas S, Quazi S, Karpiński TM. Nanoparticles for cancer therapy: current progress and challenges. Nanoscale Res Lett. 2021;16(1):173. doi:10.1186/s11671-021-03628-6
155. Piktel E, Niemirowicz K, Wątek M, Wollny T, Deptuła P, Bucki R. Recent insights in nanotechnology-based drugs and formulations designed for effective anti-cancer therapy. J Nanobiotechnology. 2016;14(1):39. doi:10.1186/s12951-016-0193-x
156. Zhao CY, Cheng R, Yang Z, Tian ZM. Nanotechnology for Cancer Therapy Based on Chemotherapy. Molecules. 2018;23(4):826. doi:10.3390/molecules23040826
157. Jadia R, Scandore C, Rai P. Nanoparticles for effective combination therapy of cancer. Int J Nanotechnol Nanomed. 2016;1(1):1.
158. Wang Y, Li J, Li X, Shi J, Jiang Z, Zhang CY. Graphene-based nanomaterials for cancer therapy and anti-infections. Bioact Mater. 2022;14:335–349. doi:10.1016/j.bioactmat.2022.01.045
159. Li R, Wang Y, Du J, et al. Graphene oxide loaded with tumor-targeted peptide and anti-cancer drugs for cancer target therapy. Sci Rep. 2021;11(1):1725. doi:10.1038/s41598-021-81218-3
160. Sanaei MJ, Pourbagheri-Sigaroodi A, Kaveh V, Sheikholeslami SA, Salari S, Bashash D. The application of nano-medicine to overcome the challenges related to immune checkpoint blockades in cancer immunotherapy: recent advances and opportunities. Crit Rev Oncol Hematol. 2021;157:103160. doi:10.1016/j.critrevonc.2020.103160
161. van Leent MMT, Priem B, Schrijver DP, et al. Regulating trained immunity with nanomedicine. Nat Rev Mater. 2022;7(6):465–481. doi:10.1038/s41578-021-00413-w
162. Parihar A, Khan R. yttrium functionalized reduced graphene oxide nanocomposite-based aptasensor for ultrasensitive detection of a breast cancer biomarker. ACS Appl Nano Mater. 2023. doi:10.1021/acsanm.3c03234
163. Tiwari H, Rai N, Singh S, et al. Recent advances in nanomaterials-based targeted drug delivery for preclinical cancer diagnosis and therapeutics. Bioengineering. 2023;10(7):760. doi:10.3390/bioengineering10070760
164. Chen J, Seeman NC. Synthesis from DNA of a molecule with the connectivity of a cube. Nature. 1991;350:631–633. doi:10.1038/350631a0
165. Wu Q, Liu C, Liu Y, Cui C, Ge J, Tan W. Multibranched linear DNA-controlled assembly of silver nanoclusters and their applications in aptamer-based cell recognition. ACS Appl Mater Interfaces. 2022;14(13):14953–14960. doi:10.1021/acsami.1c24547
166. Zhu R, Zhang F, Peng Y, Xie T, Wang Y, Lan Y. Current progress in cancer treatment using nanomaterials. Front Oncol. 2022;12. doi:10.3389/fonc.2022.930125
167. Wen J, Yang K, Huang J, Sun S. Recent advances in LDH-based nanosystems for cancer therapy. Mater Des. 2021;198:109298. doi:10.1016/j.matdes.2020.109298
168. Metselaar JM, Lammers T. Challenges in nanomedicine clinical translation. Drug Deliv Transl Res. 2020;10(3):721–725. doi:10.1007/s13346-020-00740-5
169. Snodin DJ, McCrossen SD. Guidelines and pharmacopoeial standards for pharmaceutical impurities: overview and critical assessment. Regul Toxicol Pharmacol. 2012;63(2):298–312. doi:10.1016/j.yrtph.2012.03.016
170. Zhao R, Keen L, Kong X. Clinical Translation and Safety Regulation of Nanobiomaterials. In: Nanobiomaterials. Wiley; 2018:459–479. doi:10.1002/9783527698646.ch19
171. Sharma P, Otto M. Multifunctional nanocomposites modulating the tumor microenvironment for enhanced cancer immunotherapy. Bioact Mater. 2024;31:440–462. doi:10.1016/j.bioactmat.2023.08.022
172. Sharaf M, Alhamad AA, Ltaief OO, Amor IB. Challenges of nanomaterials-based cancer therapy: a future destination. Int j Surg. 2023;109(6):1819–1820. doi:10.1097/JS9.0000000000000412
173. Naeimi R, Najafi R, Molaei P, Amini R, Pecic S. Nanoparticles: the future of effective diagnosis and treatment of colorectal cancer?. Eur J Pharmacol. 2022;936:175350. doi:10.1016/j.ejphar.2022.175350
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




