Back to Journals » Clinical Ophthalmology » Volume 18
The Effects of Low Viscosity Preservative-Free Chloroprocaine Ophthalmic Gel 3% versus BAK-Containing Tetracaine 0.5% on the Bactericidal Action of Povidone-Iodine
Received 12 December 2023
Accepted for publication 11 March 2024
Published 13 March 2024 Volume 2024:18 Pages 825—831
DOI https://doi.org/10.2147/OPTH.S454496
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Haroon Ilyas,1 Richard Costine2
1Brandon Eye Associates, Brandon, FL, 33511, USA; 2Harrow, Nashville, TN, 37205, USA
Correspondence: Richard Costine, Harrow, Nashville, TN, 37205, USA, Email [email protected]
Purpose: To evaluate if chloroprocaine ophthalmic gel 3% acts as a barrier to the bactericidal actions of povidone-iodine (PVI) which has been seen in other higher viscosity gel anesthetics.
Methods: This was a single site, prospective, randomized, patient-masked study evaluating the effects of preservative-free chloroprocaine ophthalmic gel 3% (IHEEZO®, Harrow, Nashville, TN) compared with tetracaine ophthalmic solution 0.5% and their effects on the bactericidal action of povidone-iodine 5%. The study comprised 82 patients who had both eyes cultured before and after application of randomized treatment and povidone-iodine.
Results: In terms of mean percent reduction in colony forming units, chloroprocaine with povidone-iodine was non-inferior to tetracaine with povidone-iodine, with a higher mean percent reduction in colony forming units in the chloroprocaine group (change [∆] = − 7.2; 90% CI, − 13.56 to 3.28).
Conclusion: Data collected in this study suggest that preservative-free chloroprocaine ophthalmic gel 3% does not act as a barrier to the bactericidal actions of povidone-iodine 5% and that the reduction in CFU from PVI is similar when compared with tetracaine 0.5% ophthalmic solution with PVI.
Keywords: anesthetics, cataract, cornea, infection, drug formulation
Introduction
Topical local anesthetics play a pivotal role in ophthalmology and are widely used throughout most ophthalmic procedures and surgeries. The first FDA approved topical ocular anesthetic products included solutions such as oxybuprocaine, proparacaine, and tetracaine. Reports of off-labeled use of urologic lidocaine 2% jelly for ophthalmic procedures showed a favorable profile which led to the development of a branded lidocaine 3.5% gel in 2008 (Akten Gel®, Thèa Pharma, Waltham, MA) indicated for ocular surface anesthesia during ophthalmic procedures.1–3 Surprisingly, studies leading to the FDA approval of these anesthetics did not involve patients undergoing an ophthalmic surgical procedure.
Chloroprocaine, first synthesized in 1946 from procaine, is a local anesthetic belonging to the amino-ester class, with systemic characteristics of rapid onset and a duration of action up to one hour, depending on dose and route of administration. Due to its rapid hydrolysis by pseudocholinesterase, the systemic toxicity of chloroprocaine is virtually non-existent leading to chloroprocaine being widely considered the local anesthetic with the safest toxicological profile.4 Preservative-free topical chloroprocaine ophthalmic gel 3% (IHEEZO®, Harrow, Nashville, TN) is the first topical ocular anesthetic that gained FDA approval based on a study involving a surgical intervention against an active comparator, tetracaine ophthalmic solution 0.5%, as well as in a well-controlled, randomized, multi-site study. This study involved chloroprocaine ophthalmic gel 3% as monotherapy in patients undergoing cataract surgery, without the addition of any pre- or intra-operative opioids and was approved in 2023 by the FDA for ocular surface anesthesia. In the study (n=338), chloroprocaine was shown to be equivalent to tetracaine in the defined primary endpoint, which was successful surface anesthesia, defined as patient-reported pain scores of 0 (no pain/discomfort) or 1 (occasional pressure sensations, less than 5 separate times throughout the procedure) on a pain scale up to 5 without the need for supplementation prior to intra-ocular lens insertion. There were no statistically significant differences between the two treatments, however, patients in the tetracaine arm reported higher pain scores with 9 patients reporting a score of 2 (occasional burning/stinging less than 5 times throughout the procedure), 1 patient reporting a score of 3 (occasional burning/stinging over 5 times throughout the procedure), 5 patients reporting a score of 4 (continuous sensations of burning/stinging, tolerable) and 1 patient reporting a 5 (severe or non-tolerable). In the chloroprocaine group, 12 patients reported a score of 2.5
While the viscosity of gel formulations increases residency time of the local anesthetic on the ocular surface, which results in increased drug exposure in the deeper tissues and reduces its systemic absorption, in recent years, there have been reports of increased endophthalmitis rates linked to the use of higher viscosity topical medications around the time of povidone-iodine (PVI) application in various ophthalmic procedures. It is theorized that the higher viscosity of these medications may act as a barrier to PVI, preventing free molecular iodine from reaching the bacterial cell membrane and acting as a cytotoxic agent.6
Two individual in vitro studies evaluating lidocaine gel before the application of PVI resulted in a decreased effectiveness of antisepsis and increased microbial survivability. The investigators proposed that the gel barrier prevented the PVI from coming in contact with the bacteria.7,8 One retrospective analysis of 154,198 anti-VEGF injections found that use of lidocaine jelly and tetracaine gel (Tetravisc, now discontinued) was an independent risk factor for endophthalmitis after injection (p<0.001).9 Another retrospective analysis analyzing 15,920 cataract surgeries also found that use of lidocaine 2% gel prior to PVI prep was a potential risk factor for endophthalmitis.10 These findings have led some clinicians to apply PVI prior to any viscous gel anesthetics. However, this often leads to patient dissatisfaction due to the burning/stinging associated with PVI on an unanesthetized eye.11
With the advantages and disadvantages of gels in mind, the developers of chloroprocaine ophthalmic gel 3% aimed to create a low viscosity gel formulation. Chloroprocaine ophthalmic gel 3% was developed with a viscosity ranging from 1200–2000 cP. In comparison, other commercially available ophthalmic gels such as lidocaine ophthalmic gel 3.5% has a viscosity between 4000–9000 cP and lidocaine jelly’s viscosity ranges between 12,000–14,000 cP.2,12–15 In contrast, tetracaine ophthalmic solution 0.5%, one of the most widely used topical ocular anesthetics, has a viscosity between 10–60 cP,16 close to that of human tears.17
The investigators hypothesized that the low viscosity gel vehicle of chloroprocaine ophthalmic gel 3% will not act as a barrier to PVI and thus not have the associated increased risk of endophthalmitis following application commonly seen in higher viscosity ophthalmic gel anesthetics.
Methods
This was a single site, prospective, randomized, patient-masked study evaluating the effects of chloroprocaine HCl ophthalmic gel 3% compared with tetracaine ophthalmic solution 0.5% and their effects on the bactericidal action of povidone-iodine 5% (PVI).
The trial was conducted between June 2023 and October 2023 at one center in the United States after obtaining IRB approval from Sterling IRB and in accordance with the relevant guidelines of the Declaration of Helsinki and the International Conference on Harmonization guidelines on Good Clinical Practice (ICH E6). Signed informed consent was obtained from all patients and the study was registered on Clinicaltrials.gov identifier NCT05934253.
The primary objective of this study was to evaluate the mean percent change from baseline in colony forming units (CFU) before and after application of chloroprocaine or tetracaine, and PVI. After signing informed consent, patients were screened.
Inclusion criteria included patients at least 18 years of age with the ability to comprehend and sign a statement of informed consent. Exclusion criteria included: any ocular surgeries (intraocular, oculoplastic, corneal, or refractive) within the past three months or at any time that the investigator’s clinical judgement deemed it would interfere with outcome measures, clinically significant ocular trauma, diagnosis of lagophthalmos or other severe eyelid abnormalities, active ocular inflammation, active ocular infection, ocular infection within the last three months, moderate to severe (grade 2–4) allergic, vernal, or giant papillary conjunctivitis, patients under the age of 18, pregnant or breastfeeding, and monocular patients.
Patients that signed informed consent and met both inclusion and exclusion criteria immediately had their eyes randomized based on a predetermined randomization key which assigned treatment based on enrollment number. One eye was randomized to receive chloroprocaine ophthalmic gel 3% and the other to tetracaine ophthalmic solution 0.5%. Patients were masked from which eye received which intervention. All interventions were performed by a Fellowship trained Corneal specialist wearing a N95 surgical mask and surgical gloves to reduce risks of error and contamination.
The right eye (OD) was always the first treatment eye. The lower eyelid was carefully everted, and the conjunctival membrane and eyelid margin was then gently swabbed. The swab was then plated in a zigzag streaking pattern in blood agar culture plates that contained 5% sheep’s blood in a tryptic soy agar medium. Two drops of the randomized treatment were immediately applied to the eye after the swab. To simulate real world situations where there is often a gap of time between application of anesthetics, PVI and start of procedure, two minutes passed before PVI 5% was applied to the ocular surface. After an additional three minutes, one final drop of treatment was applied. The conjunctiva and lid margins were then swabbed again following the same procedure as the initial swab (see Figure 1). All steps were then repeated for the left eye (OS) with the other randomized anesthetic. Patients were exited from the study at the completion of these steps for their OS.
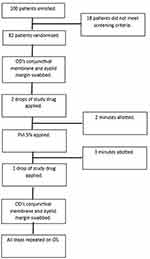 |
Figure 1 Study interventions. Description of study protocol that patients underwent. Notes: OD – right eye; OS – left eye; PVI – povidone iodine. |
Cultures were sent to a regional independent pathology lab where they tested each plate for the presence of aerobic cultures. The pathogens were identified and the number of colony forming units (CFU) was identified. Results were analyzed by the investigator, who had discretion to void samples if they deemed the results to be contaminated or if the initial culture, prior to application of PVI, grew zero colonies.
Treatment-related adverse events (TEAE) were not part of this study as they have been documented in each product’s package insert.5
Statistical Analysis
Descriptive statistics (mean, standard deviation (SD), median, minimum, and maximum) of actual values and changes in CFUs from baseline to post PVI installation were recorded by treatment group. Baseline was defined as the culture obtained before any treatment installation. The distribution of the change from baseline in CFUs will be assessed for normality. The non-inferiority of the chloroprocaine treatment group compared with the tetracaine group was evaluated using two-sided 90% confidence intervals for the between-group difference in the mean change in CFUs.
The frequency and percentage of patients with no CFUs post PVI installation was also assessed by treatment group. Two-sided 90% confidence intervals will be calculated for the between-group difference in the percentage of patients with a CFU of 0. A Chi-square test will also be performed to compare the two treatments with respect to the proportion of patients with no CFUs post treatment. Confidence intervals were used to assess the similarity of chloroprocaine to tetracaine.
All analyses were performed using Statistical Analysis System (SAS®) Software version 9.4.
Results
A total of 100 patients were enrolled in the study, of which 18 were dropped as screening failure. The subsequent 82 patients were randomized based on a pre-set randomization key. 36/82 (44%) were randomized to chloroprocaine in the OD and tetracaine in the OS. 77% of subjects were female and the average age of participants was 37 years of age. 44% self-identified as Hispanic, 36.5% as Caucasian, 17% as Black/African American, and 2.5% as Asian/Pacific Islander. All 82 subjects who were randomized completed the study.
A total of six samples were deemed to be contaminated and excluded from analysis (three chloroprocaine group, three tetracaine group). No imputation method was applied during the analysis since no missing values were observed in the collected data. The primary endpoint for this study was the mean percent reduction in colony forming units (CFU) after PVI application from baseline.
In the chloroprocaine group, the average baseline CFU was 41.9. The average post PVI CFU was 8.7. The mean reduction in CFU from baseline and post PVI was 79.3% for the chloroprocaine group. In the tetracaine group, the average baseline CFU was 38.9. The average post PVI CFU was 10.8. The mean reduction in CFU from baseline and post PVI was 72.1% for the tetracaine group. The mean change from the baseline in CFU after PVI application was not statistically significant between chloroprocaine and tetracaine treated groups (change [∆] = −7.2; 90% CI, −13.56 to 3.28).
The percentage of patients with no colonies (CFU=0) post PVI application was the same in both groups (32 [43.2%], 90% CI, −13.0% to 13.0%), which suggests non-inferiority between chloroprocaine and tetracaine in its effect on the bactericidal action of PVI. The vast majority of isolates were Staphylococcus aureus (93.2%). (See Table 1.)
 |
Table 1 Summary of Results |
Discussion
The purpose of this in vivo study was to compare the effects of chloroprocaine ophthalmic gel 3% with PVI compared with a low viscosity tetracaine ophthalmic solution while simulating real world practices. Previous studies have shown that high viscosity gel vehicles act as a barrier to PVI and may increase the risk of endophthalmitis following ophthalmic procedures. Tetracaine ophthalmic solution 0.5% was selected as the comparator because it is one of the most commonly used topical ophthalmic anesthetics and because of its viscosity being close to human tears, thus not causing a potential barrier effect to povidone-iodine. One concern when selecting tetracaine ophthalmic solution is that it contains benzalkonium chloride (BAK), a preservative used in approximately 70% of ophthalmic formulations,18 while chloroprocaine ophthalmic gel 3% is preservative-free. BAK has been shown in many studies to cause cytotoxic damage to conjunctival, corneal epithelial, and corneal endothelial cells.18,19 However, the proven antimicrobial activity of this preservative continues to make it a popular addition to new formulations. One study evaluated the antibacterial activity of BAK in ophthalmic tetracaine vs preservative-free anesthetics and found that preservative-free anesthetic solutions did not interfere with bacterial development in culture media while BAK-containing solutions inhibited development of gram-positive bacteria S. aureus, but not P. aeruginosa.20 Approximately 40–80% of all endophthalmitis cases are caused by cataract surgery; of these cases, 70% are the result of coagulase-negative staphylococci, 10% are from Staphylococcus aureus, and 9% are from streptococci. The second most common cause of endophthalmitis occurs after intravitreal injection, with coagulase-negative staphylococci and streptococci being the primary pathogens.21
Given the difference in viscosity and that chloroprocaine gel is preservative-free while tetracaine solution contains BAK, the researchers expected the results to be similar, with a skew towards tetracaine. However, despite these differences, chloroprocaine ophthalmic gel 3% showed a greater average percent reduction in CFU post PVI application compared with tetracaine ophthalmic solution 0.5%. The authors theorize that this lower viscosity gel could act as a conduit to PVI, rather than a barrier seen in higher viscosity gels, reducing its drainage from the eye, and thus allowing for more contact time with the surface of the eye.
One area of note is that only about 3 out of 4 eyes in both groups showed 10 or less CFU present post PVI application and only 34% of subjects showed zero CFU post PVI application in both groups. A similar study conducted by Ferguson et al, comparing PVI 5% with PVI 1% evaluated change in bacterial colonies before and one minute after application of PVI. This study found that PVI 5% decreased median CFU from 100 to 40 (60% decrease) while PVI 1% decreased median CFU from 120 to 100 (16.7% decrease).22 Further studies are needed to evaluate the time kill action of PVI in the ophthalmic setting to find the optimal time it should be utilized prior to start of the procedure as well as the associated risk of minor bacterial colonies.
Conclusion
Data collected in patients during this study suggest that preservative-free chloroprocaine ophthalmic gel 3% does not act as a barrier to the bactericidal actions of povidone-iodine 5% and that the reduction in CFU from PVI is similar when compared with low viscosity tetracaine 0.5% ophthalmic solution with PVI.
Data Sharing Statement
Deidentified patient data not presented in this manuscript will not be shared with any parties. Requests for additional information may be made to the corresponding author. These data will be made available for a minimum of three years.
Funding
This study was funded by Harrow.
Disclosure
Haroon Ilyas served as the primary investigator for this study and was reimbursed by Harrow for his time and expertise. Richard Costine is an employee of Harrow.
References
1. Akten Gel (lidocaine HCl ophthalmic gel 3.5%) [Package Insert]. Akorn Pharmaceuticals; 1972.
2. Page MA, Fraunfelder FW. Safety, efficacy, and patient acceptability of lidocaine hydrochloride ophthalmic gel as a topical ocular anesthetic for use in ophthalmic procedures. Clin Ophthalmol. 2009;3:601–609. doi:10.2147/opth.s4935
3. Busbee BG, Alam A, Reichel E. Lidocaine hydrochloride gel for ocular anesthesia: results of a prospective, randomized study. Ophthalmic Surg Lasers Imaging. 2008;39(5):386–390. doi:10.3928/15428877-20080901-03
4. Covino BG. Pharmacology of LA agents. Br J Anaesth. 1986;58(7):701–716. doi:10.1093/bja/58.7.701
5. Iheezo (chloroprocaine HCl ophthalmic gel 3%) [Package Insert]. Harrow; 1955.
6. Ziaei P, Resnick J, Stella N, et al. Novel combined lidocaine/povidone iodine delivery system for preintravitreal injection. J Ocul Pharmacol Ther. 2022;38(4):319–325. doi:10.1089/jop.2021.0095
7. Boden J, Myers M, Lee T, et al. Effect of lidocaine gel on povidone-iodine antisepsis and microbial survival. J Cataract Refract Surg. 2008;34(10):1773–1775. doi:10.1016/j.jcrs.2008.05.056
8. Doshi R, Leng T, Fung A. Povidone-iodine before lidocaine gel anesthesia achieves surface antisepsis. Ophthalmic Surg Lasers Imaging. 2011;42(4):346–349. doi:10.3928/15428877-20110210-02
9. Stem M, Rao P, Lee I, et al. Predictors of endophthalmitis after intravitreal injection: a multivariable analysis based on injection protocol and povidone iodine strength. Ophthalmol Retina. 2019;3(1):3–7. doi:10.1016/j.oret.2018.09.013
10. Miller J, Scott I, Flynn H, et al. Acute-onset endophthalmitis after cataract surgery (2000–2004): incidence, clinical setting, and visual acuity outcomes after treatment. Am J Ophthalmol. 2005;139(6):983–987. doi:10.1016/j.ajo.2005.01.025
11. Rae SM, Edelhauser HF. The corneal toxicity of presurgical skin antiseptics. Am J Ophthalmol. 1984;97(2):221–232.
12. Shah H, Reichel E, Busbee B. A novel lidocaine hydrochloride ophthalmic gel for topical ocular anesthesia. Local Reg Anesth. 2010;3:57–63. doi:10.2147/lra.s6453
13. Barequet IS, Soriano ES, Green WR, et al. Provision of anesthesia with single application of lidocaine 2% gel. J Cataract Refract Surg. 1999;25(5):626–631. doi:10.1016/S0886-3350(99)00004-8
14. Bardocci A, Lofoco G, Perdicaro S, et al. Lidocaine 2% gel versus lidocaine 4% unpreserved drops for topical anesthesia in cataract surgery: a randomized controlled trial. Ophthalmology. 2003;110(1):144–149. doi:10.1016/S0161-6420(02)01562-2
15. Soliman MM, Macky TA, Samir MK. Comparative clinical trial of topical anesthetic agents in cataract surgery: lidocaine 2% gel, bupivacaine 0.5% drops, and benoxinate 0.4% drops. J Cataract Refract Surg. 2004;30(8):1716–1720. doi:10.1016/j.jcrs.2003.12.034
16. Prather WC, Stoecker JF, Vehige JG, et al. Clinical performance of a new mid-viscosity artificial tear for dry eye treatment. Invest Ophthalmol Visual Sci. 2002;42:3152.
17. Tiffany JM. The Viscosity of Human Tears. Int Ophthalmol. 1991;15(6):371–376. doi:10.1007/BF00137947
18. Goldstein M, Silva F, Blender N, et al. Ocular benzalkonium chloride exposure: problems and solutions. Eye. 2022;36(2):361–368. doi:10.1038/s41433-021-01668-x
19. Liu H, Routley I, Teichmann KD. Toxic endothelial cell destruction from intraocular benzalkonium chloride. J Cataract Refract Surg. 2001;27(11):1746–1750. doi:10.1016/S0886-3350(01)01067-7
20. Dantas PE, Uesugui E, Nishiwaki-Dantas MC, et al. Antibacterial activity of anesthetic solutions and preservatives: an in vitro comparative study. Cornea. 2000;19(3):353–354. doi:10.1097/00003226-200005000-00019
21. Mahabadi N, Gurnani B, Craig N, et al. Bacterial Endophthalmitis. StatPearls Publishing LLC; 2023.
22. Ferguson AW, Scott JA, McGavigan J, et al. Comparison of 5% povidone iodine solution against 1% povidone iodine solution in preoperative cataract surgery antisepsis: a prospective randomized double blind study. Br J Ophthalmol. 2003;87(2):163–167. doi:10.1136/bjo.87.2.163
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
