Back to Journals » Cancer Management and Research » Volume 16
The Efficiency and Safety of Triple-Drug Combination of Albumin-Bound Paclitaxel, Anlotinib and PD-1/L1 Inhibitors in the 2nd or Above Line of Advanced NSCLC: A Retrospective Cohort Study
Authors Li X , Wu D, Tang J, Wu Y
Received 9 May 2024
Accepted for publication 2 August 2024
Published 8 August 2024 Volume 2024:16 Pages 1003—1012
DOI https://doi.org/10.2147/CMAR.S472196
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Xiaobing Li,1 De Wu,2 Jing Tang,3 Yuebing Wu3
1Department of Thoracic Oncology, Hubei Cancer Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 2Department of Pathology, Hubei Cancer Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 3Department of Lymphoma, Hubei Cancer Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China
Correspondence: Yuebing Wu, Department of Lymphoma, Hubei Cancer Hospital, Tongji Medical College, Huazhong University of Science and Technology, 116# South Zhuodaoquan Road, Hongshan District, Wuhan, 430079, People’s Republic of China, Tel/Fax +86-27-87670351, Email [email protected]
Background: Existing research data indicates that albumin-bound paclitaxel (nab-ptx), anlotinib, and PD-1/L1 inhibitors have individually shown efficacy in second-line and subsequent treatments for advanced non-small cell lung cancer (NSCLC). This study seeks to investigate the potential of an optimized treatment regimen in this context by combining these three drugs and evaluating both efficacy and safety outcomes.
Patients and Methods: Between January 2020 and January 2022, we collected data from pre-treated advanced NSCLC patients who received a combination therapy of nab-ptx, anlotinib, and PD-1/L1 inhibitors as a second-line or later treatment. The primary endpoints for the study included the objective response rate (ORR), progression-free survival (PFS), disease control rate (DCR) and overall survival (OS), while adverse events (AEs) were also recorded.
Results: Our findings revealed that the ORR of this regimen in pretreated NSCLC patients was 35.71%, with mean PFS of 5.0 months and mean OS of 10.0 months. Further analysis suggested correlations between the efficacy of the regimen and factors such as PD-L1 expression levels, the occurrence of certain types of adverse events, and the status of NK cell activity. Additionally, the tolerable toxicity profile of this regimen indicates its potential applicability in the treatment of pretreated advanced NSCLC.
Conclusion: Our study displayed that triple-drug combination of nab-ptx, anlotinib and PD-1/L1 inhibitors showed promising efficiency and tolerated cytotoxicity in the 2nd or above line treatment of advanced NSCLC, indicating the potential of such regimen as an important option for second-line treatment of advanced NSCLC. However, due to limitations in patient numbers, its actual clinical value awaits further research confirmation.
Keywords: nab-ptx, anlotinib, PD-1/L1, NSCLC, triple drug combination
Introduction
It is well recognized that over the past decades,1,2 significant progress had been made in the treatment of advanced NSCLC, especially in the first-line treatment;3,4 however, in the 2nd or above line treatment, the development is slow obviously and there still lack of effective treatment mean.5 This underscores the urgent need for further research to enhance therapeutic efficacy and outcomes in these settings.
Due to the limited efficiency brought about by single drug, either chemotherapy or PD-1/L1 inhibitor, from the perspective of efficiency enhancement, drug combination would be reasonable.6,7 Actually, increasing evidences indicated that drug combination either chemotherapy plus anti-angiogenesis8,9 or chemotherapy plus PD-110–12 had shown promising efficiency with lower cytotoxicity, indicating that drug combination would probably become the standard treatment mean in the field of 2nd or above line treatment of advanced NSCLC. Moreover, the trend toward multiple drug combinations has already been established in first-line NSCLC treatment,13 and such modality even has some specific advantages toward these special populations such as liver metastasis,14,15 brain metastasis,16 pleural effusion et al17 importantly, latest evidence exhibited that multiple drug combination (pemetrexed, cisplatin, bevacizumab plus sintilimab) shows promising efficiency in overcoming drug resistance caused by EGFR-TKI treatment,18 which will further extend the application scope of multiple drug combination in clinic. However, the potential for overlapping toxicities with multiple drug combinations cannot be overlooked, emphasizing the need for optimized regimens. In the specific combination regimen, while retaining PD-1/L1 inhibitors, leveraging the significant advantages of the anti-tumor angiogenesis small-molecule inhibitor anlotinib,19 as well as the albumin-bound paclitaxel20 with higher penetration compared to traditional chemotherapy drugs, becomes an important choice for optimization. Based on these considerations, we explore the feasibility of a triple-drug combination (nab-ptx, anlotinib, and PD-1/L1 inhibitors) for second-line and subsequent treatments of advanced NSCLC, aiming to identify effective therapeutic strategies that further enhance treatment efficacy in this setting.
Methods
Study Design
The study adhered to the principles outlined in the Declaration of Helsinki (revised in 2013). This retrospective trial received approval from the Ethics Committee of Hubei Cancer Hospital, affiliated with Tongji Medical College (Wuhan, China) (ID number: HBCHEC2023159), and the patients or their guardians all signed informed consent before enrollment. In total, 42 patients with advanced NSCLC underwent treatment with a triple-drug combination consisting of PD-1/L1 inhibitors, nab-paclitaxel, and anlotinib in the second line of treatment or beyond.
Inclusion Criteria
Patients aged between 18 and 70 years with histologically confirmed advanced NSCLC were eligible for enrollment. Enrollment criteria included a prior lack of response or intolerance to at least one chemotherapeutic regimen, including both doublet platinum regimens. Patients who had previously undergone EGFR tyrosine kinase inhibitor (TKI) therapy were required to have confirmation of drug resistance. None of the patients had been previously treated with the drugs included in the protocol (namely nab-paclitaxel, anlotinib, and PD-1/L1 inhibitors) before disease progression. Progression to first or second-line chemotherapy or targeted therapy was determined based on computed tomography (CT) and magnetic resonance imaging (MRI) evaluations. Additional enrollment criteria included having at least one measurable lesion as defined by the Response Evaluation Criteria in Solid Tumors (RECIST), an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1, acceptable hematologic, hepatic, and renal function, and a life expectancy of more than 3 months.
Exclusion Criteria
The key exclusion criteria comprised symptomatic brain metastasis, cachexia, and a life expectancy of fewer than 3 months.
Gene or Biomarker Detection
Most patients underwent CT-guided needle aspiration for diagnosis. Upon pathological confirmation of diagnosis, a portion of the sample was utilized for multiple gene detection, including EGFR, ALK, C-Met, and K-RAS, using standard assays. The detection of PD-L1 was conducted according to the standard assay using the Dako 22C-3 antibody, with a cutoff value for PD-L1 set at 1%.
Lymph Cell Subpopulation Analysis by FCAM
Samples of ethylenediamine tetra-acetic acid (EDTA) anticoagulated peripheral blood (2 mL) were collected from patients with advanced NSCLC before initial treatment, with a second sample collected after subsequent treatment cycles. All samples were tested within 6 hours of collection. The CD3+/CD4+/CD8+ T-cell, CD19+ B-cell, and CD16+CD56+ natural killer (NK)-cell counts (cells/μL) were measured by multiple-color flow cytometry using human monoclonal anti-CD3-fluorescein isothiocyanate (FITC), anti-CD4-phycoerythrin (PE), anti-CD8-allophycocyanin (APC), anti-CD19-PE, anti-CD16-APC, and anti-CD56-PE antibodies (BD Multitest; Becton, Dickinson, and Co. Biosciences, Franklin Lakes, NJ, USA) following the manufacturer’s instructions. Cell analysis was conducted on a BD FACS Canto II flow cytometry system (BD Biosciences).
Drug Application
All participants had received one or more lines of treatment. Upon disease progression or development of drug resistance confirmed by clinical assessment, a modified regimen comprising a triple-drug combination of PD-1/L1 inhibitors, nab-paclitaxel, and anlotinib was administered, with adjustments made to the dosage. The dosage of nab-paclitaxel ranged from 100 to 200 mg/m2 every 3 weeks. The dosage for PD-1/L1 inhibitors (such as pembrolizumab, nivolumab, atezolizumab, camrelizumab, tislelizumab, sintilimab, among others) was administered as per the instructions. Anlotinib was orally administered for two consecutive weeks, followed by a one-week break, with each cycle lasting 3 weeks. Treatment was continued until disease progression or the onset of intolerable toxicities. In the treatment sequence, PD-1/L1 inhibitors were administered intravenously first, followed by nab-paclitaxel.
Efficiency Evaluation and Adverse Event (AE) Monitoring
All patients underwent a minimum of 2 cycles of treatment and were subsequently monitored every 6 weeks using lung CT and MRI scans, with RECIST v1.1 as the primary criterion. The primary study endpoints included objective response rate (ORR), progression-free survival (PFS), overall survival (OS) and disease control rate (DCR). ORR was calculated as the ratio of complete response (CR) plus partial response (PR) patients to the total number of patients, while DCR was defined as the ratio of non-progressive disease (PD) patients to the total. PFS was delineated as the duration from the initiation of ICI therapy to the onset of disease progression or any-cause death, whichever came first. In instances where neither progression nor death occurred, the date of the last imaging examination was employed. OS was characterized as the time from the commencement of combination therapy to any-cause mortality or the date of the last follow-up if no deaths were recorded. Safety assessments were conducted by evaluating the incidence of treatment-related adverse events (AEs), graded according to the National Cancer Institute’s Terminology Criteria for Adverse Events, version 4.0. Data encompassed all events, whether immune- or non-immune mediated, reported from the first dose to 30 days after the last dose of the drug. Safety analyses included all patients who received at least one dose of the drug. No treatment-related grade 5 events (deaths) were reported at the time of the database lock. Additionally, AEs necessitating drug discontinuation or requiring immunomodulatory medications were documented.
Statistical Analysis
All statistical analyses were conducted using SPSS 13.0 (IBM Corp., Chicago, IL, USA). Two-tailed tests were employed at a significance level of α=0.05, with P < 0.05 considered statistically significant. Subgroup comparisons of count data were executed using the chi-square test or Fisher’s exact test. The relationship between variables and survival was evaluated through Kaplan–Meier curves and the Log rank test for subgroup differences in survival. Corresponding figures were generated using Graph Prism 5.0 (GraphPad Software, San Diego, CA, USA). A P value <0.05 was deemed statistically significant.
Results
Patient Characteristics
A total of 42 patients were enrolled in the study, comprising 26 males and 16 females, with a median age of 65 years. The general performance status of the patients ranged from 0 to 2, with 7 cases presenting brain metastasis and 5 cases with liver metastasis. Most patients had a performance status of 0–1, while a minority had a status of 2 (16.67%). Among the female patients, the majority were non-smokers, whereas most male patients had a history of smoking. Based on pre-treatment PD-L1 expression detection, 15 patients exhibited high expression of PD-L1 (≥1%), while 19 patients had low expression of PD-L1 (<1%). The primary pathological types included adenocarcinoma or squamous cell carcinoma, with 28 cases of adenocarcinoma and 14 cases of squamous cell carcinoma (refer to Table 1).
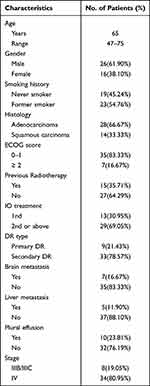 |
Table 1 Baseline Clinical Characteristics of the Study Cohort |
Previous Treatment
All participants had received at least one line of chemotherapy treatment. The first-line treatment regimens included gemcitabine, docetaxel, or pemetrexed combined with platinum. Priority regimens for adenocarcinoma comprised the combination of pemetrexed and platinum (cisplatin or carboplatin), while for squamous cell carcinoma, the combination of gemcitabine and platinum (cisplatin or carboplatin) was prioritized. Among the patients, a total of 15 had undergone radiotherapy, with a treatment interval of not less than 3 weeks before enrollment.
Efficiency
All 42 participants received triple combination therapy consisting of PD-1/L1 inhibitors, nab-paclitaxel, and anlotinib. Preliminary results revealed that in the second-line treatment of advanced NSCLC patients, the overall response rate (ORR) for this regimen was 35.71%, with no complete responses (CR), 15 partial responses (PR), 13 cases of stable disease (SD), and 14 cases of progressive disease (PD). The disease control rate (DCR) was 66.67% (refer to Table 2). The median progression-free survival (mPFS) was 5.0 months, while the median overall survival (mOS) was 10.0 months (refer to Figure 1A and B). In subgroup analysis, patients with higher PD-L1 expression (≥1%) exhibited greater efficacy compared to those with lower PD-L1 expression (<1%) (mPFS 6.0 vs 4.0 months, HR = 0.11, 95% CI: 0.04–0.29, P < 0.0001; mOS 11.0 vs 9.0 months, HR = 0.22, 95% CI: 0.09–0.53, P = 0.0006) (refer to Figure 1C and D). Additionally, we observed a correlation between the occurrence of specific adverse events (AEs) such as hand-foot syndrome (HFS), hypertension, and proteinuria, and treatment efficacy. Patients experiencing these specific AEs demonstrated improved efficacy compared to those without them (mPFS 6.0 vs 4.5 months, HR = 0.21, 95% CI: 0.09–0.49, P = 0.0002; mOS 11.0 vs 9.0 months, HR = 0.18, 95% CI: 0.08–0.40, P < 0.0001) (refer to Figure 1E and F).
 |
Table 2 Clinical Activity of Anlotinib, Nab-Ptx and PD-1/L1 Inhibitors in Advanced NSCLC |
Lymph Cell Subpopulation Changes
We also conducted an analysis on the effect of treatment on lymphocyte subpopulations before and after treatment. No significant difference was observed in the total count of baseline lymphocytes, including CD4+ T cells, CD8+ T cells, B cells, and NK cells. However, an increase in the NK cell ratio appeared to be associated with treatment efficacy. Compared to patients without an increase in NK cells, those with increased NK cells demonstrated better efficacy (mPFS 6.0 vs 5.0 months, HR = 0.11, 95% CI: 0.05–0.27, P < 0.0001; mOS 12.0 vs 9.0 months, HR = 0.20, 95% CI: 0.09–0.44, P = 0.0001) (refer to Figure 1G and H). These findings suggest that the synergistic mechanism of this regimen may be linked to the activation of NK cells in peripheral blood.
Toxicity
In addition to assessing efficacy, we meticulously documented the type and severity of adverse events (AEs) throughout the treatment duration. The primary toxicities associated with this regimen encompassed leukopenia, neutropenia, anemia, thrombocytopenia, peripheral neuropathy, vomiting, nausea, fatigue, and decreased appetite, among others. The incidence of severe side effects was approximately 49.98%. Notably, immune-related AEs were also recorded, with hypothyroidism, hyperthyroidism, rash, and itching being commonly observed. The occurrence of grade 3 or 4 immune-related AEs was 26.19% (refer to Table 3). Severe immune-related toxicities included interstitial pneumonia and infusion reactions. Specifically, one patient experienced third-degree interstitial pneumonia, which was successfully managed with high-dose hormone therapy.
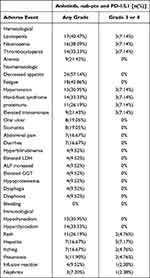 |
Table 3 Adverse Events of Anlotinib, Nab-Ptx and PD-1/L1 Inhibitors in Advanced NSCLC |
Discussion
There is no doubt that significant progress had been made in the treatment of advanced NSCLC, represented by molecular target therapy3 and immune checkpoint blockade.4 Although these new drugs have brought about significant improvements in the efficacy of advanced NSCLC both in the long term and in the short term, it is not hard to imagine that the occurrence of drug resistance would be inevitable.21,22 Additionally, most of these progresses had been focused mainly on the first-line treatment of advanced NSCLC. However, progress in the second-line and subsequent treatments has been slow and lacks effective options, highlighting the need for further improvements in therapeutic efficacy in this area.5,23
Since the limited efficiency of single drug had been widely reported either single chemotherapy or PD-1/L1 inhibitors alone, therefore, it is reasonable to try combination therapy in second-line therapy of advanced NSCLC. Actually, increasingly evidences indicate that compared to single drug, the efficiency brought about by drug combination such as chemotherapy plus anti-angiogenesis (pemetrexed plus apatinib)9 or chemotherapy plus PD-1/L1 inhibitors (paclitaxel plus pembrolizumab)10,11 is remarkable. Besides promising efficiency improvement, the toxicity caused by such regimen also seems to be tolerated, indicating that drug combination had the potential to become a new standard treatment option for the 2nd or above line treatment of advanced NSCLC.7 However, from the perspective of practical clinic application, there still exists room for improving the efficiency and optimizing the regimen. Considering the multiple drug combination (chemotherapy, anti-angiogenesis and PD-1/L1 inhibitors) had become the main trend for the first-line treatment of advanced NSCLC, the use of multidrug combinations in second-line treatment would also be a useful attempt.8,24 Actually, the latest study displayed that multiple drug combination, namely, pemetrexed, cisplatin, bevacizumab and sintilimab had produced remarkable efficiency toward these advanced NSCLC patients suffering from EGFR-TKI drug resistance.18 However, the cytotoxicity should also not be ignored, no matter the AEs brought about by platinum doublet chemotherapy or the anti-angiogenesis inhibitor (bevacizumab), especially under the condition that many patients are in poor physical condition in the later line treatment, indicating that although the efficiency was promising, however, such regimen needs to be further optimized to improve patient tolerance.7 Under above consideration, we firstly observed the triple-drug combination of nab-paclitaxel,25 anlotinib23 and PD-1/L1 inhibitors in the 2nd or above line treatment of advanced NSCLC.5 The efficacy and safety of any of the drugs included in this regimen have been confirmed in NSCLC. Consistent with our expectation, such regimen resulted in about 36% ORR, the mean PFS and OS were 5m and 10m, respectively, and the DCR reached as higher as 67%, implying the improved efficiency compared to previous drug combination. Additionally, we further discover that the occurrence of some types of adverse event (hypertension, proteinuria, and hand-foot syndrome) and expression level of PD-L1 correlated with the efficiency. Besides promising efficiency, we also found that this regimen did not significantly increase the side effects of treatment. The irAEs incidence of grade 3 and above was about 26%. Most of which can be effectively relieved with corresponding support treatment, indicating that the toxicity of such regimen can be tolerated. Although it is difficult to clarify the mechanism of action due to the application of triple-drug combination,26,27 our further subgroup analysis indicated that the increase of NK cell ratio seems to be correlated with the efficiency of such regimen.26,28 This implies that the regimen’s mechanism might involve enhancing immune activation to improve immunotherapy efficacy, consistent with findings from prior studies.29,30 Nonetheless, further research is needed to fully elucidate its mechanism,31 which is currently underway.
To sum up, as far as we know, this is the first study to report the promising efficiency and lower cytotoxicity of triple-drug combination (nab-paclitaxel, anlotinib and PD-1/L1 inhibitors) in the 2nd or above line treatment of advanced NSCLC through upregulating NK cell ratio. While this regimen shows promise as a treatment option, several challenges remain unresolved. These include elucidating its exact mechanism of action, identifying biomarkers to prioritize patient populations, and optimizing the combination of multiple drugs.32,33 Nevertheless, with wider adoption of the triple-drug combination and further accumulation of clinical experience, its potential will continue to be explored. This holds the promise of benefiting an increasing number of patients with advanced NSCLC in the near future.
Conclusions
The combination of anlotinib, albumin-bound paclitaxel, and PD-1/L1 inhibitors represents a promising therapeutic strategy in the second-line treatment of advanced NSCLC. This regimen offers synergistic benefits in terms of efficacy by targeting multiple pathways involved in tumor progression and immune suppression. With careful management of safety considerations and ongoing research efforts, this approach holds potential to further improve outcomes and redefine standards of care for patients with advanced NSCLC.
Data Sharing Statement
The data produced in this study are accessible upon reasonable request from the corresponding author.
Ethics Approval
The authors bear full responsibility for ensuring the accuracy and integrity of all aspects of the study. Any queries regarding the precision or honesty of any part of the work were duly investigated and addressed. The study adhered to the principles outlined in the Declaration of Helsinki (revised in 2013). Approval for this retrospective trial was obtained from the Ethics Committee of Hubei Cancer Hospital Affiliated with Tongji Medical College (Wuhan, China) (Approval No. HBCHEC2023159). Prior to participation, patients or their guardians provided informed consent by signing consent forms. For patients with communication barriers, the informed consent form prior to enrollment will be signed by their legal guardian on behalf of the patient.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was supported by Natural Science Foundation of Hubei Province (No. 2019CFC929).
Disclosure
The authors have no conflicts of interest to declare for this work.
References
1. Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health. 2019;85(1). doi:10.5334/aogh.2419
2. Chen P, Liu Y, Wen Y, Zhou C. Non-small cell lung cancer in China. Cancer Commun. 2022;42(10):937–970. doi:10.1002/cac2.12359
3. Alexander M, Kim SY, Cheng H. Update 2020: management of non-small cell lung cancer. Lung. 2020;198(6):897–907. doi:10.1007/s00408-020-00407-5
4. Nasser NJ, Gorenberg M, Agbarya A. First line Immunotherapy for non-small cell lung cancer. Pharmaceuticals. 2020;13(11):373. doi:10.3390/ph13110373
5. Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase iii trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35(35):3924–3933. doi:10.1200/JCO.2017.74.3062
6. Elkrief A, Yu H. Combination regimens in EGFR-mutated lung cancer: can we get ORIENT-ed? Lancet Oncol. 2022;23(9):1113–1114. doi:10.1016/S1470-2045(22)00449-1
7. Grant MJ, Herbst RS, Goldberg SB. Selecting the optimal immunotherapy regimen in driver-negative metastatic NSCLC. Nat Rev Clin Oncol. 2021;18(10):625–644. doi:10.1038/s41571-021-00520-1
8. Tozuka T, Kitazono S, Sakamoto H, et al. Addition of ramucirumab enhances docetaxel efficacy in patients who had received anti-PD-1/PD-L1 treatment. Lung Cancer. 2020;144:71–75. doi:10.1016/j.lungcan.2020.04.021
9. Tang J, Li XY, Liang JB, Wu D, Peng L, Li X. Apatinib plus chemotherapy shows clinical activity in advanced NSCLC: a retrospective study. Oncol Res. 2019;27(6):635–641. doi:10.3727/096504018X15288447760357
10. Yin C, Zou GR, He Y, et al. Efficiency and toxicity of nab-paclitaxel and camrelizumab in the second or above line treatment of advanced non-small cell lung cancer: a retrospective cohort study. J Thoracic Dis. 2023;15(4):1838–1847. doi:10.21037/jtd-23-387
11. Nassabein R, Gaudreau PO, Belkaid W, Florescu M, Blais N. A phase I/II study of pembrolizumab in combination with nab-paclitaxel in patients with unresectable stage III or stage IV non small-cell lung carcinoma (NSCLC). Cancer Treat Res Commun. 2021;28:100421. doi:10.1016/j.ctarc.2021.100421
12. Arrieta O, Barrón F, Ramírez-Tirado LA, et al. Efficacy and safety of pembrolizumab plus docetaxel vs docetaxel alone in patients with previously treated advanced non-small cell lung cancer: the prolung phase 2 randomized clinical trial. JAMA Oncol. 2020;6(6):856–864. doi:10.1001/jamaoncol.2020.0409
13. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. New Engl J Med. 2018;378(24):2288–2301. doi:10.1056/NEJMoa1716948
14. Kawachi H, Tamiya M, Tamiya A, et al. Association between metastatic sites and first-line pembrolizumab treatment outcome for advanced non-small cell lung cancer with high PD-L1 expression: a retrospective multicenter cohort study. Invest New Drugs. 2020;38(1):211–218. doi:10.1007/s10637-019-00882-5
15. Funazo T, Nomizo T, Kim YH. Liver metastasis is associated with poor progression-free survival in patients with non-small cell lung cancer treated with nivolumab. J Thorac Oncol. 2017;12(9):e140–e1. doi:10.1016/j.jtho.2017.04.027
16. Hendriks LEL, Henon C, Auclin E, et al. Outcome of patients with non-small cell lung cancer and brain metastases treated with checkpoint inhibitors. J Thorac Oncol. 2019;14(7):1244–1254. doi:10.1016/j.jtho.2019.02.009
17. Alessi JV, Elkrief A, Ricciuti B, et al. Clinicopathologic and genomic factors impacting efficacy of first-line chemoimmunotherapy in advanced NSCLC. J Thorac Oncol. 2023;18(6):731–743. doi:10.1016/j.jtho.2023.01.091
18. Lu S, Wu L, Jian H, et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): first interim results from a randomised, double-blind, multicentre, Phase 3 trial. Lancet Oncol. 2022;23(9):1167–1179. doi:10.1016/S1470-2045(22)00382-5
19. Wang P, Fang X, Yin T, Tian H, Yu J, Teng F. Efficacy and safety of anti-PD-1 plus anlotinib in patients with advanced non-small-cell lung cancer after previous systemic treatment failure-A retrospective study. Front Oncol. 2021;11:628124. doi:10.3389/fonc.2021.628124
20. Xu H, Wang W, Yin J, Song C, Li L, Sun Z. Efficacy and Safety of the PD-1 inhibitor combined with albumin-bound paclitaxel and nedaplatin in preoperative neoadjuvant therapy of unresectable stage iii lung squamous cell carcinoma. Drug Des Devel Ther. 2022;16:4269–4277. doi:10.2147/DDDT.S388777
21. Kluger H, Barrett JC, Gainor JF, et al. Society for Immunotherapy of Cancer (SITC) consensus definitions for resistance to combinations of immune checkpoint inhibitors. J ImmunoTher Cancer. 2023;11(3):e005921. doi:10.1136/jitc-2022-005921
22. Rizvi N, Ademuyiwa FO, Cao ZA, et al. Society for Immunotherapy of Cancer (SITC) consensus definitions for resistance to combinations of immune checkpoint inhibitors with chemotherapy. J ImmunoTher Cancer. 2023;11(3):e005920. doi:10.1136/jitc-2022-005920
23. Han B, Li K, Wang Q, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 2018;4(11):1569–1575. doi:10.1001/jamaoncol.2018.3039
24. Brueckl WM, Reck M, Rittmeyer A, et al. Efficacy of docetaxel plus ramucirumab as palliative second-line therapy following first-line chemotherapy plus immune-checkpoint-inhibitor combination treatment in patients with non-small cell lung cancer (NSCLC) UICC stage IV. Transl Lung Cancer Res. 2021;10(7):3093–3105. doi:10.21037/tlcr-21-197
25. Yoneshima Y, Morita S, Ando M, et al. Phase 3 trial comparing nanoparticle albumin-bound paclitaxel with docetaxel for previously treated advanced NSCLC. J Thorac Oncol. 2021;16(9):1523–1532. doi:10.1016/j.jtho.2021.03.027
26. Van Damme H, Dombrecht B, Kiss M, et al. Therapeutic depletion of CCR8 + tumor-infiltrating regulatory T cells elicits antitumor immunity and synergizes with anti-PD-1 therapy. J ImmunoTher Cancer. 2021;9(2):e001749. doi:10.1136/jitc-2020-001749
27. Stankovic B, Bjørhovde HAK, Skarshaug R, et al. Immune cell composition in human non-small cell lung cancer. Front Immunol. 2018;9:3101. doi:10.3389/fimmu.2018.03101
28. Sivori S, Pende D, Quatrini L, et al. NK cells and ILCs in tumor immunotherapy. Mol Aspect Med. 2021;80:100870. doi:10.1016/j.mam.2020.100870
29. Glorieux C, Xia X, You X, et al. Cisplatin and gemcitabine exert opposite effects on immunotherapy with PD-1 antibody in K-ras-driven cancer. J Adv Res. 2022;40:109–124. doi:10.1016/j.jare.2021.12.005
30. Yan X, Yao C, Fang C, et al. Rocaglamide promotes the infiltration and antitumor immunity of NK cells by activating cGAS-STING signaling in non-small cell lung cancer. Int J Bio Sci. 2022;18(2):585–598. doi:10.7150/ijbs.65019
31. Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens JHM. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol. 2019;30(2):219–235. doi:10.1093/annonc/mdy551
32. Johnson PC, Gainor JF, Sullivan RJ, Longo DL, Chabner B. Immune checkpoint inhibitors - the need for innovation. New Engl J Med. 2023;388(16):1529–1532. doi:10.1056/NEJMsb2300232
33. Peters S, Reck M, Smit EF, Mok T, Hellmann MD. How to make the best use of immunotherapy as first-line treatment of advanced/metastatic non-small-cell lung cancer. Ann Oncol. 2019;30(6):884–896. doi:10.1093/annonc/mdz109
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

