Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
The Use of Bronchial Rheoplasty in Emphysema Patients Previously Treated with Endoscopic Lung Volume Reduction: A Case Series
Authors Jensen K, Egenod T, Franzen DP, Perch M
Received 16 April 2024
Accepted for publication 22 July 2024
Published 5 August 2024 Volume 2024:19 Pages 1791—1797
DOI https://doi.org/10.2147/COPD.S469214
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Kristine Jensen,1 Thomas Egenod,2 Daniel P Franzen,3,4 Michael Perch1,5
1Department of Cardiology, Section for Lung Transplantation and Respiratory Medicine, Rigshospitalet Heart Center, Copenhagen, Denmark; 2Department of Interventional Pulmonology, Dupuytren University Hospital, Limoges, France; 3Department of Internal Medicine, Uster Hospital, Uster, Switzerland; 4Department of Pulmonology, University of Zurich, Zurich, Switzerland; 5Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark
Correspondence: Michael Perch, Department of Cardiology, Section for Lung Transplantation and Respiratory Medicine, Rigshospitalet, Heart Center, Inge Lehmanns Vej 7, Copenhagen, 2100, Denmark, Email [email protected]
Abstract: Endoscopic lung volume reduction (ELVR) is an established treatment option for patients with severe emphysema. Not all patients are candidates for this type of intervention, and in the context of significant airway secretions, they may be excluded from treatment. Bronchial Rheoplasty (BR) was developed to treat mucus hypersecretion by delivering nonthermal pulsed electric fields to the airway epithelium and submucosa. The literature to date demonstrates that patients treated with BR in clinical studies have a reduction in airway goblet cell hyperplasia as well as substantive clinical improvement in the setting of chronic bronchitis (CB). In this case series, we present four patients treated at three different institutions who had previously undergone ELVR with beneficial outcome. However, over time, these patients subsequently developed worsening clinical issues, including complaints of increased and thickened mucus, along with exacerbations in the setting of a loss of some ELVR-associated benefits. These patients then underwent exploratory treatment with BR with the intent of reducing their secretion burden and potentially restoring the efficacy associated with the initial placement of the airway valves. All BR procedures were well tolerated, and three of the four patients showed substantial improvement in their symptom burden. Airway examinations during the second of the two BR procedures also revealed what appeared to be less airway mucosal inflammation and a decrease in the quantity of airway secretions. Therefore, treatment with BR may have the potential to improve and restore the initial benefits associated with ELVR, thus enhancing long-term outcomes. Further clinical studies with sufficient follow-up are warranted to assess this in a larger cohort of patients, and to determine whether treatment with BR prior to ELVR may make more patients eligible for this treatment through reduction in their secretions and/or symptoms.
Keywords: bronchial rheoplasty, endobronchial valves, emphysema
Introduction
Since regulatory approval, endoscopic lung volume reduction (ELVR) for the treatment of severe emphysema continues to be integrated into various care pathways for this cohort of patients. Not all patients with severe emphysema, however, are candidates for this type of intervention and various cautions exist for ELVR in patients with comorbid disease entities such as bronchiectasis and chronic bronchitis (CB) with frequent exacerbations.1 Moreover, in patients with exuberant secretions of any etiology, thoughtful assessment of the various risks and benefits of ELVR in those settings is a further consideration in clinical practice. In the context of significant airway secretions, patients who might otherwise benefit from ELVR are often excluded from the therapy.2 In addition, there is a cohort of patients who, following ELVR, appear to develop more secretions, increased bacterial colonization of their airways, or in whom the secretions become more of a clinical issue.3–5 Should this occur, it may dilute the initial benefits associated with ELVR resulting in the return of dyspnea, more troublesome mucus and cough, reduction in FEV1, potential exacerbations, or even post-valvular pneumonia.2,3,6,7 Moreover, these issues may also necessitate repeated airway valve manipulation or even airway valve removal, which would also result in the loss of benefit.
Bronchial Rheoplasty (BR) was developed to treat the bronchial epithelium and submucosa in the setting of moderate-to-severe symptoms of CB in adults.8 A multi-center, single-arm trial demonstrated both procedural safety as well as clinical efficacy measured by a reduction of chronic obstructive pulmonary disease (COPD) Assessment Test (CAT) and St. George’s Respiratory Questionnaire (SGRQ) out to 1 year.9 Another notable finding from that trial included a decrease in the secretory component of the airway mucosa as measured by a reduction in goblet cell hyperplasia from airway mucosal biopsies pre- and post-intervention. More specifically, in a clinical study of biopsy specimens collected from the airways before and after treatment with BR, there was a mean reduction in goblet cell hyperplasia of 39% at 3-month follow-up. These cellular changes observed in airway biopsies were associated with statistically significant and clinically meaningful improvements in quality of life. Subsequent studies, including another multicenter, single-arm trial, further substantiated the initial findings demonstrating safety and clinical improvement.10 In fact, durable clinical responses in that trial were noted out to 2 years. A prospective, randomized, parallel group, double-blind, concurrently controlled, multicenter trial is currently ongoing to further assess the safety and effectiveness of BR for treating CB symptoms in adult COPD patients with moderate-to-severe CB (NCT04677465).
The system received a CE Certification (July 2019, 35782) for ablation of bronchial epithelium and mucosa and the treatment of symptoms due to chronic bronchitis in patients with moderate-to-severe chronic bronchitis, which is characterized by excess cough and sputum. Herein, we present four cases of patients who underwent ELVR for severe emphysema (Table 1) and had an associated clinical benefit following treatment. Subsequently, these patients developed increasing complaints of mucus and cough, resulting in the mitigation of the benefits associated with ELVR. These patients subsequently underwent exploratory treatment with BR in an attempt to manage those issues.
 |
Table 1 Patient Demographics |
Methods
Participants and Data Collection
Patients were consented for the BR procedure (RheOx, Galvanize Therapeutics, Inc., Redwood City, CA) at three sites in Denmark, France, and Switzerland, and site ethics committee approval was obtained at each site in order to publish data from patients included in this case series. Patients with emphysema and chronic bronchitis that had undergone prior ELVR treatment and subsequently developed excessive mucus hypersecretion were identified as candidates for the BR procedure. To mitigate mucus hypersecretion, BR was performed roughly 2 years after initial airway valve placement (with a range of 5 months to 5 years, see Table 2) with the standard treatment approach: a first bronchoscopy in which treatment was delivered to the right-sided airways followed a month later by a second bronchoscopy in which treatment was delivered to the left-sided airways. Patient follow-up was according to routine care at each institution. In the context of this case series, patient response data were collected retrospectively, and thus not all metrics were available for each patient included in this case series.
 |
Table 2 Summary Data |
Results
Case 1
A 72-year-old male patient with CB had previously undergone ELVR of the left upper lobe, which was subsequently followed by complete lobar atelectasis and an associated FEV1 increase from 25% to 49% predicted. Shortly thereafter, he noted a gradual, albeit steady, increase in airway secretions, to the extent that significant daily mucus production became a regular phenomenon. Moreover, the patient developed recurrent respiratory infections and was sputum culture-positive for stenotrophomonas maltophilia. Despite bronchoscopic revision of the airway valves and two courses of antibiotic therapy, resulting in clearance of the bacteria, mucus hypersecretion persisted, with bacterial infections on and off.
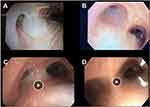
To mitigate mucus hypersecretion, BR was performed roughly 2 years after initial airway valve placement (Figure 1), and the BR procedures were performed without difficulty, were well tolerated, and without significant adverse events. CAT, 6-minute walk test (6 MWT), and the Pulmonary Function Test (PFT) results before and then 6 months after bronchial rheoplasty are presented in Table 2.
Case 2
A 66-year-old woman with severe emphysema along with a component of CB underwent ELVR in the RUL and RML approximately 5 years ago, followed by complete lobar atelectasis. However, she subsequently complained of increasing amounts of mucus and cough and presented with two non-severe COPD exacerbations that were treated on an outpatient basis. It should be noted that the patient also received a long-term course of azithromycin therapy to address the additional symptom burden and secretions, but without success. Roughly 4 weeks after the second of the two exacerbations, and once she had returned to her clinical baseline, she was treated with BR (Figure 1). The BR procedures were performed without difficulty, well tolerated and without significant adverse events. Three months of follow-up clinical data demonstrated a substantial improvement in CAT (Table 2). She had no exacerbations since treatment with BR, and her exertional dyspnea was equivalent to that of mMRC 2 and was stable.
Interestingly, the pattern of improvement in these first two cases was relatively similar to the majority of the improvement in four of the eight domains measured by CAT. Specifically, these four domains were mucus, cough, sleep, and confidence in leaving home (Table 3).
 |
Table 3 CAT Results for Cases 1 and 2 |
Case 3
A 63-year-old woman with emphysema and CB presented with complaints of dyspnea and excessive mucus hypersecretion. She underwent ELVR with successful atelectasis of the left lower lobe and improvement in dyspnea. However, after a COPD exacerbation one-year post-ELVR, the patient complained of increasing dyspnea and cough. A chest CT taken at that time revealed complete lobar atelectasis in regions of the lung distal to the location of the airway valves. At bronchoscopy, roughly 5 months after ELVR, she demonstrated copious “sticky”, mucoid secretions throughout the airways and, as such, she was thought to be a potential candidate for BR. The BR procedures were performed again without difficulty, were well tolerated, and without significant adverse events. Two months after the second BR treatment, the patient noted a substantial reduction in her bronchitis symptoms, along with a clear improvement in her productive cough and an improvement in her mMRC from 3 prior to BR to 2 post-treatment (Table 2).
Case 4
A 60-year-old patient with both emphysema and CB presented with dyspnea and excessive mucus hypersecretion. She then underwent ELVR, resulting in improvement of dyspnea, but little improvement in the productive cough. In the spring of 2022, the patient experienced a COPD exacerbation. Further workup at that time included a chest CT scan that demonstrated atelectasis in the ELVR-treated area. On airway examination, the airway valves appeared to be positioned appropriately but copious, severe, “sticky”, mucoid secretions and bronchitis were noted and thus she was thought to be an appropriate candidate for BR. The BR procedures were again performed without difficulty, were well tolerated, and without significant adverse events. Roughly 6 weeks after the second BR treatment, the patient showed no significant changes in her baseline symptoms, including her mMRC (Table 2).
Discussion
ELVR is increasingly being used to treat patients with severe emphysema. While providing clinical benefit, issues that may be associated with the implantation of airway valves include foreign-body reactions, an increase in secretions, and bacterial colonization. Each of these factors may undermine the benefits associated with ELVR3,11 and are typically discussed and reviewed with the patient prior to airway valve implantation. Inhalation therapy is also recommended for patients treated with ELVR, but this treatment is aimed at dilating the airways, and currently, there are no approved pharmaceutical treatments directed specifically at the mucus hypersecretion associated with the chronic bronchitis phenotype of COPD. BR was developed to treat mucus hypersecretion in that context. In addition to the symptom-based improvements noted in previous trials with BR, additional findings, including histological changes in the airway mucosa pre- and post-treatment, have been described.9
In this case series, we present four patients who had previously undergone ELVR with beneficial outcome but subsequently developed worsening issues presumed to be associated with increasing airway secretions. As a result, these patients underwent exploratory BR treatment, in this context, in an attempt to address both the secretion burden and to restore some initial benefits associated with ELVR, such as FEV1 improvement. In all cases, the BR procedures were well tolerated and without any complications. Of note, there were no changes to the patients’ inhalational therapies post-intervention. In three of the four cases, significant symptomatic benefits were described, with the onset of clinical improvement typically 1 to 4 weeks after the first of the two procedures. More specifically, a significant decrease in both the perceived amount secretions along with a decrease in the “thickness” of the expectorated secretions were described in the three patients who had an improvement in their symptom burden. All of these patients are now at least 6 months out from initial treatment, and as noted above, three of the four patients continued to note substantial symptomatic improvement. Furthermore, two of the four patients are now more than 12 months out from the initial treatment, with one patient being nearly 2 years out from the initial treatment, and both continue to note clinical benefit.
Validated methods for objectively assessing sputum amounts have yet to be established, and thus no means to quantify the benefit provided by BR in terms of mucus secretions are available. However, in addition to symptomatic improvements, airway examinations in the majority of this patient cohort at the time of the second procedure demonstrated qualitative visual changes to the airways themselves, as well as to the amount and quality of the airway secretions. More specifically, at the time of the second bronchoscopy, the treated side of the airway tree tended to demonstrate less airway mucosal induration, along with a decrease in both the amount and “thickness” of airway secretions in those distributions as well.
This case series is an initial feasibility report on the exploratory application of BR in patients who developed increased secretions following ELVR. Additional clinical studies with appropriate follow-up are warranted to further define the associated benefit in this setting. Given the published evidence demonstrating an increased bacterial load in the bronchial secretions following airway valve implantation,4,5 an assessment of whether BR impacts the local microbiome and airway colonization is also warranted, including whether patients with reduced airway secretions have a decreased frequency of exacerbations.
As BR was not available at the time of ELVR for this patient cohort nor were the airways behind the valves treated in this group, additional considerations for future use of BR in this setting include treating the airway tree “behind” the valves with BR, as well as delivering BR prior to ELVR. While speculative, the potential may exist to improve the duration of ELVR and thus ensure more effective long-term outcomes, whether it be through a change in either mucus quantity and quality or through a change in the local microbiome or both. More specifically, it may be that those patients who would benefit from ELVR but who are not initial candidates as a consequence of copious airway secretions might then have a reduction in their secretions and/or symptoms post-treatment with BR and thus be “shifted” into the group of patients considered for valve placement. In addition to both the timing of treatment with BR in this patient cohort and the patient-reported outcome metrics, additional outcome measures, such as imaging assessment or analysis of the airway microbiome, should be considered as part of the overall assessment.
Conclusion
In summary, four patients were treated with BR who had previously undergone ELVR. All BR procedures were well tolerated, and three of the four patients had substantive improvements in their symptom burden. Further clinical studies with sufficient follow-up are warranted to assess this in a larger cohort of patients and to determine whether treatment with BR prior to ELVR may increase the number of patients eligible for this treatment by reducing their secretions and/or symptoms.
Abbreviations
ELVR, endoscopic lung volume reduction; CB, chronic bronchitis; FEV1, forced expiratory volume in 1 second; BR, bronchial rheoplasty; COPD, chronic obstructive pulmonary disease; CAT, COPD assessment test; SGRQ, St. George’s respiratory questionnaire; LUL, left upper lobe; RUL, right upper lobe; RML, right middle lobe; LLL, left lower lobe; 6MWT, 6-minute walking time; PFT, pulmonary function test; FVC, forced vital capacity; TLC, total lung capacity; RV, residual volume; DLCO, diffusing lung capacity of the lungs for carbon monoxide; mMRC, modified medical research council dyspnea scale; CT, computed tomography.
Written Informed Consent for Publication
Informed consent was obtained from all subjects.
Acknowledgments
Substantial contribution to drafting and presentation of data:
Mary Sophy Yohannan, MBBS; Beryl A. Hatton, PhD.
Disclosure
Dr Michael Perch reports personal fees from AstraZeneca, personal fees from PulmonX, grants from PulmonX, grants from Therakos, personal fees from Takeda, personal fees from Zambon, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Sciurba FC, Ernst A, Herth FJF, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363(13):1233–1244. doi:10.1056/NEJMoa0900928
2. Valipour A. Valve therapy in patients with emphysematous type of chronic obstructive pulmonary disease (COPD): from randomized trials to patient selection in clinical practice. J Thorac Dis. 2018;10(Suppl 23). doi:10.21037/jtd.2018.08.86
3. Koster TD, Klooster K, Ten Hacken NHT, Van Dijk M, Slebos DJ. Endobronchial valve therapy for severe emphysema: an overview of valve-related complications and its management. Expert Rev Respir Med. 2020;14(12):1235–1247. doi:10.1080/17476348.2020.1813571
4. Sarmand N, Gompelmann D, Kontogianni K, Polke M, Herth FJ, Eberhardt R. New bacterial growth in bronchial secretions after bronchoscopic valve implantation. Int J Chron Obstruct Pulmon Dis. 2018;13:565–570. doi:10.2147/COPD.S148196
5. Trudzinski FC, Seiler F, Wilkens H, et al. Microbiological airway colonization in COPD patients with severe emphysema undergoing endoscopic lung volume reduction. Int J Chron Obstruct Pulmon Dis. 2017;13:29–35. doi:10.2147/COPD.S150705
6. Burnier M, Fricker AF, Hayoz D, Nussberger J, Brunner HR. Pharmacokinetic and pharmacodynamic effects of YM087, a combined V1/V2 vasopressin receptor antagonist in normal subjects. Eur J Clin Pharmacol. 1999;55(9):633–637. doi:10.1007/s002280050685
7. Franzen D, Straub G, Freitag L. Complications after bronchoscopic lung volume reduction. J Thorac Dis. 2018;10(Suppl 23). doi:10.21037/jtd.2018.06.66
8. Krimsky W, Neal Ii RE, Kim V. Airway mucosal remodeling: mechanism of action and preclinical data of pulsed electric fields for chronic bronchitis and mucus hypersecretion. Respir Int Rev Thorac Dis. 2023;1–13. doi:10.1159/000534370
9. Valipour A, Fernandez-Bussy S, Ing AJ, et al. Bronchial rheoplasty for treatment of chronic bronchitis. twelve-month results from a multicenter clinical trial. Am J Respir Crit Care Med. 2020;202(5):681–689. doi:10.1164/rccm.201908-1546OC
10. Sciurba FC, Dransfield MT, Kim V, et al. Bronchial rheoplasty for chronic bronchitis: 2-year results from a US feasibility study with rheox. BMJ Open Respir Res. 2023;10(1):e001710. doi:10.1136/bmjresp-2023-001710
11. Slebos DJ, Shah PL, Herth FJF, Valipour A. Endobronchial valves for endoscopic lung volume reduction: best practice recommendations from expert panel on endoscopic lung volume reduction. Respiration. 2017;93(2):138–150. doi:10.1159/000453588
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.