Back to Journals » Drug Design, Development and Therapy » Volume 18
Therapeutic Effects of Qingchang Tongluo Decoction on Intestinal Fibrosis in Crohn’s Disease: Network Pharmacology, Molecular Docking and Experiment Validation
Authors Li Y, Hu J, Au R, Cheng C, Xu F, Li W, Wu Y, Cui Y, Zhu L, Shen H
Received 9 January 2024
Accepted for publication 1 July 2024
Published 25 July 2024 Volume 2024:18 Pages 3269—3293
DOI https://doi.org/10.2147/DDDT.S458811
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Yanan Li,1,2,* Jingyi Hu,1,2,* Ryan Au,1– 3 Cheng Cheng,1,2 Feng Xu,1,2 Weiyang Li,1,2 Yuguang Wu,1,2 Yuan Cui,1,2 Lei Zhu,1,2 Hong Shen1,2
1Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, 210029, People’s Republic of China; 2Jiangsu Provincial Hospital of Chinese Medicine, Nanjing, 210029, People’s Republic of China; 3Academy of Chinese Culture and Health Sciences, Oakland, CA, 94612, USA
*These authors contributed equally to this work
Correspondence: Hong Shen; Lei Zhu, Jiangsu Provincial Hospital of Chinese Medicine (Affiliated Hospital of Nanjing University of Chinese Medicine), 155 Hanzhong Road, Qinhuai District, Nanjing, Jiangsu Province, People’s Republic of China, Tel\Fax +86-025- 86617141, Email [email protected]; [email protected]
Background: Qingchang Tongluo Decoction (QTF) is clinically used for the treatment of intestinal fibrosis in Crohn’s Disease (CD). However, the role of QTF in CD-associated fibrosis and its potential pharmacological mechanism remains unclear.
Purpose: The objective of this study was to elucidate the potential mechanism of QTF in treating CD-associated fibrosis, employing a combination of bioinformatics approaches — encompassing network pharmacology and molecular docking — complemented by experimental validation.
Methods: To investigate the material basis and potential protective mechanism of QTF, a network pharmacology analysis was conducted. The core components and targets of QTF underwent molecular docking analysis to corroborate the findings obtained from network pharmacology. In vitro, a colon fibrotic model was established by stimulating IEC-6 cells with 10 ng/mL of transforming growth factor(TGF-β 1). In vivo, an intestinal fibrosis model was induced in BALB/c mice by TNBS. The role of QTF in inhibiting the TGF-β 1/Smad signaling pathway was investigated through RT-qPCR, Western blotting, immunohistochemistry staining, and immunofluorescence staining.
Results: Network pharmacology analysis revealed that QTF could exert its protective effect. Bioinformatics analysis suggested that Flavone and Isoflavone might be the key components of the study. Additionally, AKT1, IL-6, TNF, and VEGFA were identified as potential therapeutic targets. Furthermore, experimental validation and molecular docking were employed to corroborate the results obtained from network pharmacology. RT-qPCR, Immunofluorescence, and Western blotting results demonstrated that QTF significantly improved colon function and inhibited pathological intestinal fibrosis in vivo and in vitro.
Conclusion: Through the application of network pharmacology, molecular docking, and experimental validation, QTF could be confirmed to inhibit the proliferation of intestinal fibroblasts associated with CD and reduce the expression of Collagen I and VEGFA. This effect is achieved through the attenuation of ECM accumulation, primarily via the inhibition of the TGF-β 1/Smad signaling pathway.
Keywords: Qingchang Tongluo Decoction, Crohn’s Disease, intestinal fibrosis, network pharmacology, Pharmacology
Graphical Abstract:
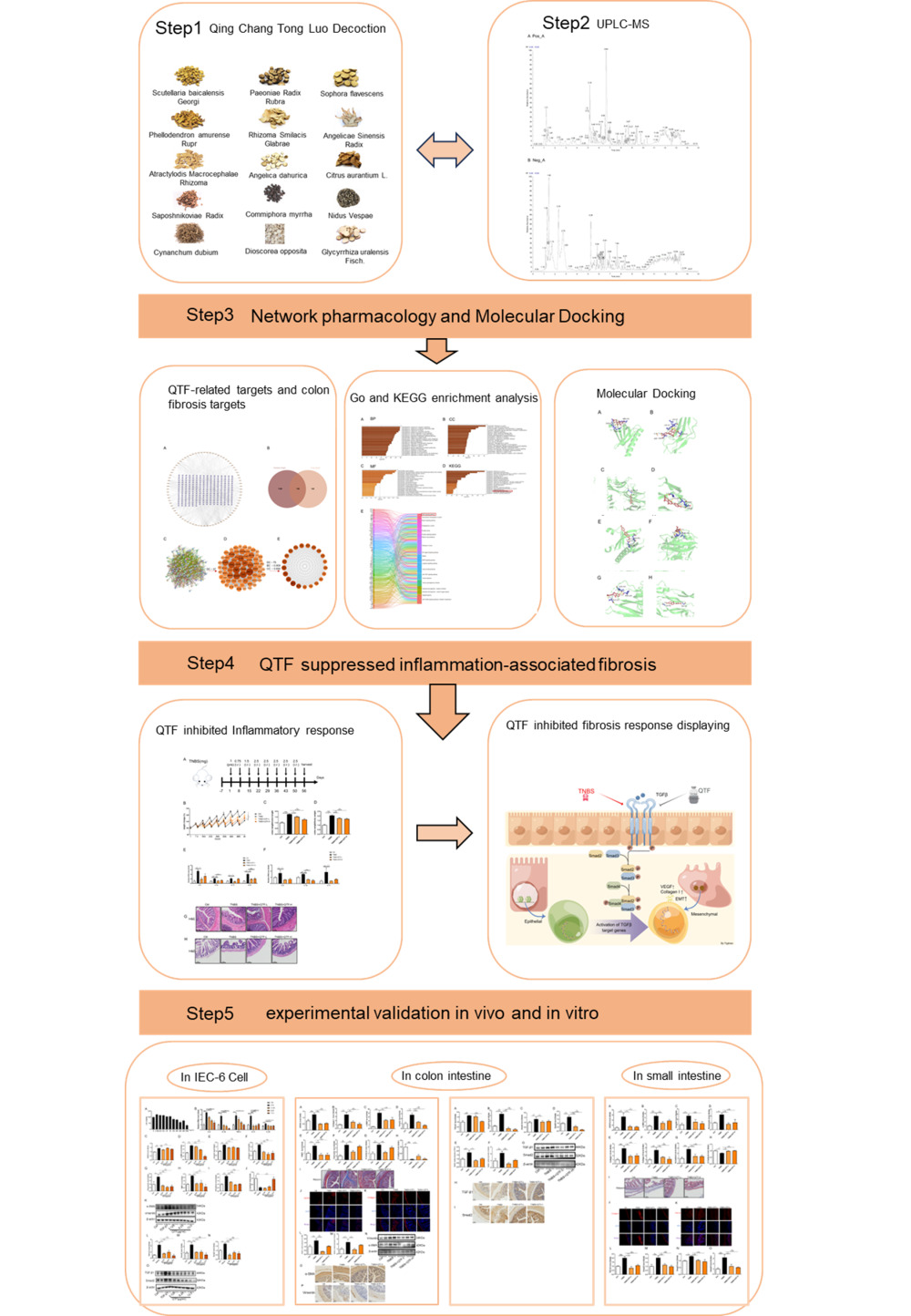
Introduction
Crohn’s Disease (CD) is a recurrent systemic inflammatory disorder predominantly impacting the gastrointestinal tract, with intestinal fibrosis representing major gastrointestinal complications.1 Inflammatory reactions trigger tissue healing, yet an inadequate equilibrium between the synthesis and breakdown of extracellular matrix (ECM) proteins can occasionally result in fibrosis.2 Although surgical intervention is one of the effective methods for treating fibrosis, epidemiological surveys show that the postoperative recurrence rate of fibrosis in 75% of CD patients who receive surgery is 70%-90%, ultimately requiring the need for further bowel resection.3,4 Intestinal fibrosis associated with CD significantly impairs patients’ quality of life and serves as an indicator of unfavorable treatment outcomes.5 Therefore, the methodology for alternative strategies to prevent intestinal fibrosis has become an important goal in the treatment of CD.
Fibroblasts, myofibroblasts, and smooth muscle cells represent distinct types of intestinal mesenchymal cells that produce ECM and facilitate the recruitment of Collagen I.6 In addition, another important pathological feature is the proliferation of capillary endothelial cells caused by the accumulation of VEGFA.7 Specific growth factors like transforming growth factor TGF-β1 induce Collagen I and VEGFA expression and lead to the differentiation of myofibroblast-like cells.8,9 Meanwhile, other cell types such as endothelial cells are transformed into myofibroblasts promoting VEGFA expression under the repeated stimulation by pro-inflammatory factors, chemokines, and more. This leads to ECM deposition, lamina propria thickening, stricture formation, and intestinal obstruction.10 TGF-β1 is implicated in fibrotic processes in various human tissues and organs, including the lungs, kidneys, and the heart. Additionally, it is recognized as a significant contributor to intestinal fibrosis in CD, a conclusion supported by both clinical observations and experimental fibrosis models.11–14
This study utilizes network pharmacology to analyze and predict the potential mechanisms of drug intervention in diseases, offering a comprehensive, macroscopic perspective. The method of network pharmacology has been widely utilized in the innovation of Chinese herbal medicine.15 This approach offers fresh insights into the complex systems of Traditional Chinese Medicine (TCM) and provides scientific and technological support for clinical practices.16 Molecular docking, on the other hand, investigates the interactions between different molecules and predicts their binding affinities and modes.17
TCM is characterized by its multi-target effects, minimal side effects, and a high efficacy profile.18 Research has indicated that various TCM therapies can alleviate symptoms of CD, encompassing inflammation reduction, immune modulation, microbiota regulation, and facilitation of normal healing in damaged tissues.19,20 The findings of this study suggest that TCM may represent a promising therapeutic option for the prevention and treatment of CD-associated intestinal fibrosis. Within the framework of TCM theory, the primary pathogenesis of CD-related intestinal fibrosis is attributed to the retention of damp-toxin, stagnation of Qi and blood, and malnourishment of the intestinal network. Qingchang Tongluo Decoction (QTF) is an innovative derivation combining elements of Qingchang Huashi granule, Huangqin Tang, and Xianfang Huoming Yin. In the affiliated hospital of Nanjing University of Chinese Medicine, QTF has been utilized in the management of intestinal fibrosis associated with CD.21 QTF is composed of 15 TCM herbs (Table 1), including Scutellaria baicalensis Georgi [Labiatae, Scutellariae Radix, SR], Paeoniae Rubra [Paeoniaceae, Paeoniae Radix Rubra, PRR], Sophora flavescens Aiton [Legume, Sophorae Flavescentis Radix, SFR], Phellodendron amurense Rupr. [Rutaceae, Phellodendri Amurensis Cortex, PAC], Smilacis Glabrae [Smilacaceae, Smilacis Glabrae Rhizoma, SGR], Angelica Sinensis preparata [Apiaceae, Angelicae Sinensis Radix Preparata, ASP], Angelica dahurica [Apiaceae, Angelicae Dahuricae Radix, ADR], Citrus aurantium L. [Rutaceae, Aurantii Fructus, AF], Atractylodes Macrocephela preparata [Asteraceae, Atractylodis Macrocephalae Rhizoma Preparata, AMRP], Saposhnikovia [Apiaceae, Saposhnikoviae Radix, SR], Commiphora myrrha [Burseraceae, Myrrha, M], Nidus Vespae [Vespidae, Vespae Nidus, VN], Cynanchum dubium [Apocynaceae, Cynanchi Atrati Radix, CAR], Dioscorea opposita preparata [Dioscoreaceae, Dioscoreae Rhizoma Preparata, DRP], and Glycyrrhiza uralensis Fisch. [Legume, Glycyrrhizae Radix, GR]. The botanical nomenclature of the plant has been verified through the MPNS database (http://mpns.kew.org). Clinically, it has been confirmed that clearing intestinal dampness is effective in treating inflammatory bowel disease, and Invigorating blood circulation and dissolving blood stasis as a treatment for fibrosis.22,23 Although QTF is a clinical approach for treating CD, the efficacy and underlying mechanisms of QTF in treating CD-associated intestinal fibrosis remain unclear and warrant further investigation.
 |
Table 1 Composition of QTF |
This study investigated the active constituents, potential targets, and molecular mechanisms of QTF in the treatment of intestinal fibrosis, employing network pharmacology, molecular docking, and experimental validation.
Material and Methods
Reagents
The 2,4,6-Trinitrobenzenesulfonic acid (TNBS, 5%, P2297) was purchased from Sigma-Aldrich (USA). Isoflurane (R510-22-8) was acquired from Ruiwode Life Science (Shenzhen, China). Anhydrous ethanol (20210528) was obtained from Sinopharm Chemical Reagent. Olive oil (8001–25-0) was procured from Yuanye Bio-Technology (Shanghai, China); Rat intestinal epithelium cell (IEC-6, BNCC100548) were sourced from BeNa Culture Collection Co., Ltd. 1%TNBS Presensitization Solution (anhydrous ethanol: olive oil = 4:1); For Western blotting, β-actin (66009–1), anti-Vimentin (10366–1), anti-α-smooth muscle actin (α-SMA,55135–1), anti-TGF-β1 (21,898–1), anti-Smad2 (12,570–1) antibodies were secured from Proteintech, HRP-labeled goat anti-rabbit IgG (GB23303) and HRP-labeled goat anti-mouse IgG (GB23301) was acquired from Wuhan Servicebio Technology. Anti-Collagen I antibody (GB114197) and anti-VEGFA antibody (GB11034B) were obtained by way of Wuhan Servicebio Technology. Lipopolysaccharide (LPS,437627) was gained from Sigma-Aldrich (USA). TGF-β1 (HY-P73615) was derived from MedChemExpress. TRIzolTM Reagent (15596018) was supplied from Invitrogen; HiScript II RT SuperMix for qPCR (R323-01), ChamQ SYBR qPCR Master Mix (Q311-02), and the Cell Counting Kit (CCK)-8 cell viability assay kit were acquired from Vazyme Biotech (Nanjing, China); PCR Primers (QP-D09-01) were synthesized by General Biotech (Shanghai, China).
QTF Extraction Process
All herbal ingredients were procured from Jiangsu Province Hospital of Chinese Medicine (China). The composition and ratios of QTF are detailed in Table 1. The herbal components were immersed in distilled water (W: V, 1:10) for 1 h. Subsequently, the mixture underwent two 40 min extraction cycles with equal volumes of distilled water. After the dual extraction process, the solution was further evaporated and concentrated to achieve a final concentration of 13.45 g/kg for the QTF-L group or 26.90 g/kg for the QTF-H group. QTF extracts utilized for cell studies were filtered through a 0.22 µm pore size membrane to eliminate biological impurities. All concentrated QTF extracts were preserved at −80°C until use.
UPLC-MS Analysis of QTF
Aliquots of QTF samples were thawed and prepared for UPLC-MS analysis as described in a previous publication.24 A 5 μL aliquot of prepared QTF was filtered through a 0.22 µm filter and analyzed using a UPLC-ESI-Q-orbitrap-MS system, consisting of the Shimadzu Nexera X2 LC-30AD and the Q Exactive™ Plus Hybrid Quadrupole-Orbitrap™ Mass Spectrometer by Thermo Scientific, San Jose, USA. ACQUITY UPLC® high strength silica (HSS) T3 columns (2.1×100 mm, 1.8μm) from Waters, Milford, MA, USA, were employed for liquid chromatography separation with a flow rate of 0.3 mL/min. The mobile phases consisted of A: 0.1% formic acid in water and B: 100% acetonitrile, following an elution gradient from 0% B for 2 min, linearly increasing to 48% B in 4 min, and reaching 100% B in 4 min, maintained for 2 min. Raw MS data were processed in MS-DIAL, encompassing retention time correction, peak alignment, and peak area extraction. QTF constituents were identified by matching peak information against Shanghai BIO PROFILE Co., Ltd’s in-house secondary mass spectrometry database.
Network Pharmacology Analysis
Network pharmacology analysis was employed to further explore the protective mechanism of QTF against diseases. Active chemical components from the 15 herbs in QTF were identified using UPLC-MS. The TCMSP database (https://www.tcmsp-e.com/) was utilized to gather target components and therapeutic targets were retrieved from the OMIM database (https://omim.org/), Genecards database (https://www.genecards.org), TTD database (https://db.idrbla.net/ttd/), and Drugbank database (https://go.drugbank.com). Target names were converted to gene symbols using the UniProKB database (https://www.uniprot.org) and subsequently input into the STRING database (https://string-db.org) for protein-protein interaction (PPI) analysis. The interaction networks were visualized using Cytoscape software (https://www.cyto-scape.org/). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was conducted using the Metascape databases (https://metascape.org/).
Molecular Docking
Based on the results obtained from network pharmacology, the top two components of QTF and their corresponding core protein targets were subjected to molecular docking to assess the affinity of their interactions. The molecular structures of QTF’s active compounds in Mol2 format were acquired from TCMSP and processed using AutoDockTools 1.5.7, subsequently saving them in pdbqt format. The 3D structures of AKT1, IL-6, TNF, and VEGFA were retrieved from the PDB database (https://www.rcsb.org). Using Pymol, water and organic molecules were removed and replaced with hydrogen atoms in the target proteins, which were then imported into AutoDockTools 1.5.7 to designate as receptors and saved as pdbqt files.25 Molecular docking was performed using AutoDockTools 1.5.7, with the results visualized using Pymol.26
Animal
All female BALB/c mice were obtained from Sibefu Biotechnology Co., Ltd (License no. SCXK2019-0010) and were accommodated at the Experimental Animal Center of Jiangsu Provincial Hospital of Traditional Chinese Medicine (License no. SYXK2017-0069). These mice were kept in specific pathogen-free (SPF) environments and provided with unrestricted access to a standard diet and sterilized water. All experimental procedures were approved by the Animal Ethics Committee of Jiangsu Province Hospital of Traditional Chinese Medicine (License no. 2022DW-05-01) in accordance with the guidelines of the Guide for the Care and Use of Laboratory Animals.
Establishment and Treatment of the Experimental Model
The colitis induction method used in this study was based on the original model described by Morris et al but modified accordingly.27 A total of 29 healthy female BALB/C mice, maintained under SPF conditions, were randomly allocated into four groups as follows: control group (Ctrl n=5), model group (TNBS, n=8), low-dose QTF group (TNBS+QTF-L, 13.45 g/kg, n=8), and high-dose QTF group (TNBS+QTF-H, 26.90 g/kg, n=8). On the morning of day 1, after acclimation was complete, a 1% TNBS pre-sensitization solution was applied to a 1.5 cm*1.5 cm shaved area on the posterior aspect of the trunk. On the morning of day 8, enemas were performed after a 12 h fasting period. The enema solution was prepared as a mixture of 40% anhydrous ethanol and various dosages of TNBS, with a single injection being 100 µL. TNBS was given once a week for a total of seven times, at the following dosages: 0.75 mg, 1.5 mg, 2.5 mg, 2.5 mg, 2.5 mg, 2.5 mg, and 2.5 mg. To perform the enema, mice were anesthetized using an isoflurane vaporizer and maintained in a surgical plane of anesthesia as confirmed by a negative pedal withdrawal reflex test. The mice were inverted and the enema syringe was inserted rectally about 4–6 cm deep before injecting the TNBS solution. Enemas were retained for 1 min with the mice in an inverted position.
Control group: normal access to food and water; Model group: after inducing colitis, saline solution was administered by oral gavage once a day; QTF groups: Commencing from the day of the initial enema, a daily oral gavage of 0.2 mL of QTF was administered to each group. This intragastric treatment regimen continued for a total duration of 49 days.
Histology
Approximately 10% of the distal colon and ileum tissues were fixed in 4% paraformaldehyde for 24 h. Subsequently, these specimens were embedded in paraffin, sectioned at a thickness of 4 µm, and underwent hematoxylin-eosin staining using standard procedures for subsequent histological analysis. Tissue damage was analyzed as described in a previous study.28 Masson’s trichrome staining was conducted to identify the collagen layer within paraffin-embedded tissues and quantify the extent of tissue fibrosis. The remaining colon and ileum specimens were preserved at −80°C until required for RNA and protein extraction.29
Real-Time Quantitative PCR (qRT-PCR)
Colon and ileum tissues as well as cells were subjected to total RNA extraction using TRIzol reagent (Invitrogen), followed by reverse transcription into cDNA utilizing HiScript II RT SuperMix for PCR (Vazyme). mRNA levels were assessed using ChamQ SYBR Green qPCR Master Mix (Vazyme). The expression levels of each target gene were standardized to β-actin using the 2-ΔΔt method, as presented in Table 2.
 |
Table 2 The Sequences of Primers are Shown in Here |
Western Blotting
Total proteins were extracted from both colon tissues and cells and were subsequently separated based on their size using either 10% or 12% gradient SDS-PAGE (Bis-Tris Midi Gel, Invitrogen). Proteins were then transferred to blotting membranes before being blocked and incubated with α-SMA, Vimentin, TGF-β1, Smad2, and β-actin primary antibodies. Blotting membranes were incubated with HRP-labeled secondary antibodies and developed with HRP substrate. Using the ChemiDocTM XRS+ system, immunoreactive bands were visualized and analyzed. β-actin was used as a loading control to normalize protein levels. Then the intensity was calculated by ImageJ. Raw data from Western blotting are shown in Supplementary Material 2.
Immunohistochemistry and Immunofluorescence
For the detection of α-SMA, Vimentin, TGF-β1, Smad2, VEGFA, and Collagen I, mouse colons were fixed in Carnoy’s fixative without flushing the luminal content, followed by paraffin embedding and sectioning at 3 µm thickness. After dewaxing and rehydration, the specimens were subjected to antigen retrieval by steaming in citrate buffer (pH 6) (Solarbio, China) at 100°C for 20 min. The sections were blocked with 5% BSA at room temperature for 1.5 h. Staining was performed using anti-α-SMA antibody (1:100), anti-Vimentin antibody (1:5000), anti-TGF-β1 antibody (1:400), anti-Smad2 antibody (1:500), anti-VEGFA antibody (1:400), and anti-Collagen I antibody (1:500), followed by overnight incubation at 4°C. The secondary antibody was detected using a FITC-conjugated goat anti-rabbit secondary antibody or goat anti-mouse antibody and incubated for 1 h at room temperature. DAPI was applied at room temperature for 10 min for nuclei staining. Finally, coverslips were mounted using an antifade mounting medium (Servicebio, China).
Cell Culture and Cell Viability Assay
IEC-6 cells were cultured under sterile conditions in Dulbecco’s Modified Eagle Medium (C3110-0500, VivaCell Biosciences), mixed with 10% fetal bovine serum (C04001-500, VivaCell Biosciences) and 1% penicillin-streptomycin-amphotericin B, and incubated at 37°C with a 5% CO2 atmosphere in a cell culture flask.
IEC-6 cells were seeded at a density of 10^4 cells per well in 96-well plates, with each well containing 100 µL of medium. The cells were subsequently treated with varying concentrations of QTF (ranging from 0 to 1600 µg/mL) for 24 h. Following this incubation period, the Cell Counting Kit-8 (CCK-8) reagent was introduced for an additional 1 to 4 h, and the metabolic activity was assessed by quantifying the absorbance of light at 450 nm.
Cell Treatment
For experiments, the cells were distributed into 6-well plates, with each well containing 3 × 10^5 cells, and then separated into 5 groups (n=3): control group (Ctrl), LPS-treated group (LPS), QTF-treated groups with concentrations of 3.125 µg/mL, 6.25 µg/mL, and 12.5 µg/mL. In the LPS-treated groups, when cell confluence reached 75%, lipopolysaccharide (LPS, Sigma, USA) at a concentration of 1 µg/mL was employed to induce cell injury for 24 h. Simultaneously, QTF was added to inhibit LPS-induced injury. A similar grouping method was employed for TGF-β1-induced EMT in cells. TGF-β1 (MedChemExpress, HY-P73615) was used to induce fibrosis/EMT in IEC-6 cells. When cell confluence reached 50%, IEC-6 cells were incubated with TGF-β1 (10 ng/mL) to induce EMT for 24 h. After one day, cells were cultured with different doses (3.125 µg/mL, 6.25 µg/mL, 12.5 µg/mL) of QTF for 24 h. Subsequently, cell samples were collected for subsequent protein and mRNA measurements.
Statistical Analysis
GraphPad Prism (Version 8.0.1; GraphPad Software, Inc.; San Diego, CA, USA) served as the primary software for statistical analysis in this study. The results underwent analysis for normality and homogeneity using the Shapiro–Wilk test. The statistical significance of experimental results was assessed using a one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test, where statistical significance was defined as P < 0.05. All graphs were generated using GraphPad Prism, and the data were presented as the mean ± standard error (SEM).
Results
The Chemical Components of QTF
To pinpoint the prominent chemical constituents within QTF, UPLC-MS analysis was conducted. Figure 1A and B illustrated the total ion chromatograms for both positive ESI mode and negative ESI mode, respectively. A total of 494 compounds were either identified or tentatively characterized by comparing their retention times, precise mass, and MS/MS fragment data with reference standards or information documented in the literature. The representative compounds from each herb presented in QTF were distinctly labeled and detailed in Table 3. Additionally, the chemical structures and extracted ion chromatography (EIC) findings could be found in Supplementary Figure 1.
 |
Table 3 Chemical Characterization of Bioactive Compounds in QTF |
 |
Figure 1 Analysis of QTF components by UPLC-MS. (A and B) The total ion chromatograms of QTF in (A) positive ESI mode and (B) negative ESI mode. |
Potential Inflammation-Related Intestinal Fibrosis Therapeutic Mechanisms of QTF Through Network Pharmacological Analysis
Network pharmacology analysis was performed to elucidate the underlying mechanism behind QTF’s protective effect against fibrosis. Based on the UPLC- MS results, a total of 94 active ingredients were identified, and 278 potential fibrosis-related targets from relevant databases were retrieved (Figure 2A and Supplementary Table 1). Through a comparison of QTF targets and those associated with intestinal fibrosis, we found that QTF contained 136 targets with its active ingredients (Figure 2B). The top 2 constituents were Flavone (MOL000008) and Isoflavone (MOL000392) shown in Table 4. Genes corresponding to these target proteins were entered into the STRING database to construct a PPI network, which encompassed 136 nodes and 2963 edges (Figure 2C). PPI network analysis suggested that VEGFA could potentially be the most critical target for QTF’s anti-fibrosis therapy (Figure 2D). Subsequently, a hub network comprising 21 nodes and 209 edges was identified based on median values of BC, CC, and double degree (Figure 2E). As a result, the top 4 targets, including VEGFA, AKT1, IL-6, and TNF, were predicted to be the key targets of QTF for fibrosis therapy (Table 5).
 |
Table 4 Top 2 Compounds Information of QTF Network |
 |
Table 5 Top 4 Targets Information of PPI Network |
KEGG signaling pathway and GO enrichment analyses of the 136 intersecting genes identified earlier revealed that the targeted genes were associated with biological processes, including responses to inorganic substances, positive regulation of cell migration, and the remarkable regulation of smooth muscle cell proliferation (Figure 3A). Cellular components were mainly enhanced in the pathways in cancer, lipid and atherosclerosis, IL-4, and IL-13 signaling (Figure 3B). For molecular functions, these targets were mainly included in kinase binding, RNA polymerase II-specific DNA-binding transcription factor binding, and signaling receptor activator activity (Figure 3C). The results of KEGG found that these targets were closely related to “JAK-STAT signaling pathway”, “NF-kappa B signaling pathway” and “VEGF signaling pathway” (Figure 3D and E). Utilizing network pharmacology analysis, it has been elucidated that QTF can ameliorate inflammation-associated intestinal fibrosis through the inhibition of inflammatory pathways and VEGF signaling.
QTF Treatment Ameliorates TNBS-Induced Colitis in Mice
Initially, the potential therapeutic benefits of QTF in a TNBS-induced colitis model were explored (Figure 4A). The changes in mouse body weight, colon length, colon weight, and histopathology were monitored throughout the experiment. TNBS was administered rectally once a week, following a modified protocol recently described by Wirtz et al.30 Compared with the TNBS group, QTF treatment elevated the body weight of mice from the fourth TNBS instillation to the end of the experiment while decreasing both the colon weight/colon length ratios and the colon weight/body weight ratios (Figure 4B–D).
Given the pivotal role of proinflammatory cytokines in colitis onset, the influence of QTF on these factors was assessed. QTF treatment significantly lowered the levels of interleukin (IL), including IL-6, IL-1β, IL-17, and IL-23, in colonic and ileum tissue (Figure 4E and F). Additionally, QTF notably inhibited the infiltration of inflammatory cells and preserved the architecture of intestinal mucosa (Figure 4G and H). Collectively, these findings suggest that QTF treatment mitigated TNBS-induced colitis.
QTF Can Regulate the Expression of TGF-β1-Induced Fibrosis Factors in IEC-6 Cells
To comprehend the mechanism underlying the impact of QTF, in vitro experiments were conducted to assess the influence of QTF on a pivotal profibrotic pathway, namely the TGF-β1/Smad/VEGF pathway, which serves as a central mediator of fibrogenesis. Firstly, the CCK-8 assay was utilized to assess cell viability with QTF treatment. The results showed that QTF was not cytotoxic at concentrations of 0–400 µg/mL in IEC-6 cells. QTF concentrations of 3.125 6.25, and 12.5 µg/mL were selected for subsequent experiments considering safety and toxicity parameters (Figure 5A). The pro-inflammatory cytokines mRNA expression was detected to evaluate inflammation levels in the LPS-treated IEC-6 cells. IEC-6 cells treated with QTF led to a significant decrease in the levels of IL-6, IL-1β, TNF-α, IL-17, and IL-23 compared to the LPS group (Figure 5B).
In fibrotic processes, various fibrosis marker proteins, including VEGFA, α-SMA, and Vimentin, were released, when activated fibroblasts directly contribute to fibrogenesis. To verify the role of QTF in affecting the expression of fibrosis factors, TGF-β1 protein activated the fibrotic state in IEC-6 cells. The expression levels of VEGFA, α-SMA, Vimentin, Collagen I, Fibronectin, N-cadherin, and TIMP1 were downregulated in the QTF group (Figure 5C–I). In addition, E-cadherin expression levels were downregulated in the TGF-β1 group compared to the control group, which was upregulated in the QTF groups compared to the TGF-β1 group (Figure 5J). The same result was reached that protein expression levels for α-SMA and Vimentin in IEC-6 cells (Figure 5K). These findings indicate that QTF can ameliorate the expression of fibrosis-related factors induced by TGF-β1 in IEC-6 cells.
qRT-PCR and Western blotting analyses were employed to estimate the expression of TGF-β1 and Smads associated with IBD fibrosis. Following QTF treatment, there was a substantial decrease in the mRNA expression levels of TGF-β1, Smad2, Smad3, and Smad4 (Figure 5L - N). In terms of protein expression, the levels of TGF-β1 and Smad2 in the TGF-β1 group were notably superior to those in the control group, but they decreased in the QTF groups (Figure 5O). These findings provide further evidence that QTF can modulate the TGF-β1-induced expression of fibrosis-related factors in IEC-6 cells by suppressing the TGF-β1/Smad signaling pathway.
QTF Reduces Chronic Colitis-Associated Intestinal Fibrosis by Downregulating the TGF-β1/Smad/VEGF Signaling Pathway
To evaluate the impact of QTF on chronic colitis complications, the mice model of intestinal fibrosis through weekly incremental TNBS induction was established. During the fibrosis process, activated fibroblasts release various fibrosis marker proteins, including VEGFA, α-SMA, Collagen I, and Vimentin, which directly contribute to fibrogenesis. We investigated whether QTF could restrain the activation of fibroblasts and the subsequent expression of fibrosis markers. The assessment of marker protein expression was carried out using qRT-PCR, immunohistochemistry, immunofluorescence, and Western Blotting. Compared to the control group, the expression levels of VEGFA, Collagen I, α-SMA, Vimentin, TIMP1, Fibronectin, and N-cadherin were significantly increased in the TNBS model group. These fibrosis markers were noticeably decreased after QTF treatment (Figure 6A–G). In contrast, E-Cadherin expression was upregulated after QTF treatment (Figure 6H). Additionally, Masson’s trichrome staining revealed substantial Collagen deposition in TNBS-treated mice, which was essentially reduced with QTF treatment (Figure 6I).
Immunofluorescence staining was employed to observe the distribution pattern of VEGFA and Collagen I deposition in colonic tissue. In comparison with the TNBS group, QTF treatment groups had visibly less VEGFA and Collagen I deposition (Figure 6J and K). The disparities in α-SMA and Vimentin protein expression levels in colon tissue were assessed. The results revealed that the α-SMA and Vimentin protein levels in the TNBS model group were elevated in comparison to the control group, whereas both QTF treatment groups exhibited a contrasting trend (Figure 6L–P). The experimental results show that QTF can inhibit fibrosis and decrease the deposition of excess Collagen in colon tissue in a TNBS-induced IBD fibrosis mice model.
According to previous studies, the mechanisms of intestinal fibrosis involved the accumulation of ECM and deposition of Collagen through the TGF-β1 signaling pathway.31 Western Blotting, Immunohistochemistry, and qRT-PCR were used to assess TGF-β1 and various Smad signaling proteins, which take part in the TGF-β1 pathway. The mRNA expression levels of TGF-β1, Smad2, Smad3, and Smad4 were notably elevated in the TNBS model group when contrasted with the control group. However, in the QTF treatment groups, these expression levels were decreased (Figure 7A–D). Additionally, Western blotting and Immunohistochemistry analyses further demonstrated the protein expression of TGF-β1 and Smad2 (Figure 7E–I).
Similarly, we observed that mRNA expression levels of fibrosis-related factors such as VEGFA in the small intestine of mice (Figure 8A–H) and protein expression of Collagen (Figure 8I and J) align with those in the colon. Additionally, verification on mice small intestine showed that expression levels of TGF-β mRNA and proteins (Figure 8K and L) and Smads-related mRNA (Figure 8M–O) correspond similarly to those in the colon. These results show that treatment with QTF was much less susceptible to TNBS-induced fibrosis and that QTF functions via the TGF-β1/Smad pathway to alleviate TNBS-induced fibrosis in colonic or in small intestine tissue in mice.
Molecular Docking Analysis
Based on the PPI network analysis and experimental findings, Flavone (MOL000008) and Isoflavone (MOL000392) were selected for molecular docking with the four primary targets: AKT1 (PDB ID:1UNQ), IL-6 (PDB ID: 1BQU), TNF (PDB ID: 2AZ5), and VEGFA (PDB ID: 6ZFL). It is generally acknowledged that the stability of the binding conformation correlates inversely with the binding energy between the ligand and receptor.32 The binding energy results from the molecular docking are presented in Table 6, demonstrating effective docking between the compound ligands and protein receptors (Figure 9).
 |
Table 6 The Binding Energy of Compound and Targets (Kcal/Mol) |
Discussion
Fibrosis associated with CD poses a substantial health challenge, marked by a multifaceted pathogenesis. The lack of efficacious therapeutic strategies for this condition has contributed to heightened morbidity and mortality worldwide.33 Research has identified significant advantages of TCM in managing inflammation-related diseases. TCM may improve symptoms, alleviate pain, reduce the adverse effects of conventional treatments, and enhance quality of life. Thus, it constitutes an integral part of the therapeutic regimen for CD. Within the TCM framework, the pathogenesis of CD-associated intestinal fibrosis is attributed to the retention of damp-toxin, stagnation of qi and blood, and malnourishment of the intestinal collaterals. QTF is designed to dispel dampness and detoxify, invigorate blood and resolve stasis, and nourish blood to unblock collaterals. In this study, the active compounds and mechanisms underlying QTF for the treatment of colitis-associated intestinal fibrosis were revealed using a combination of network pharmacology, molecular docking, and in vivo and in vitro experiments.
The extraction of active compounds from TCM plays a pivotal role in elucidating their therapeutic mechanisms. UPLC-MS analysis identified 494 compounds with high accuracy, which were selected as potential active compounds of QTF. Network pharmacology represents a robust approach to predicting pharmacological mechanisms. Previous research has demonstrated that the candidate compounds identified in TCM can be considered active compounds for network pharmacology analysis. In this study, we subjected the 94 compounds identified as potential active compounds of QTF, along with their respective targets, to network pharmacology analysis. The comprehensive analysis of the 278 targets associated with these 94 compounds and the 1236 fibrosis-related genes revealed that pathways such as “JAK-STAT signaling pathway”, “NF-kappa B signaling pathway” and “VEGF signaling pathway” might be implicated in the therapeutic mechanisms of QTF in the treatment of intestinal fibrosis. Utilizing network pharmacology analysis, it has been elucidated that QTF can ameliorate inflammation-associated intestinal fibrosis through the inhibition of inflammatory pathways and VEGF signaling. The principal components, namely Flavone and Isoflavone, in conjunction with the key targets, including AKT1, IL-6, TNF, and VEGFA, are likely to elucidate the anti-fibrotic mechanism of QTF.
Accordingly, a consensus appears to exist regarding the mechanism by which CD leads to intestinal fibrosis: intestinal damage can be triggered by specific detrimental factors, leading to a direct inflammatory response. As can be seen from Figure 4, the anti-inflammatory action of QTF was confirmed. Of interest, the TNBS+QTF-L group exhibited better expression of inflammatory factors compared to the TNBS+QTF-H group in Figure 4, indicating that the efficacy of the drug is not directly proportional to the dosage. Several reasons may account for this phenomenon. Firstly, interactions between drugs and other substances in the body can affect absorption, distribution, and metabolism, thus influencing efficacy of drugs.34 Secondly, many drugs exhibit non-linear concentration-response curves, where increasing the dose may no longer significantly enhance efficacy.35 Lastly, some drugs have a narrow therapeutic window, being ineffective at low doses and potentially causing toxicity at high doses.36 Regardless, achieving efficacy at lower drug doses is preferable. This not only meets therapeutic requirements but also addresses resource limitations. Because anti-inflammatory agents have minimal effect on fibrosis once it has started,37 we believe that QTF’s anti-inflammatory properties alone do not fully explain its protective effects. As a consequence, it is crucial to clarify the mechanisms that control fibrogenesis.
Wound healing is a physiological process initiated by inflammation, and its outcome may entail tissue restoration with the return to normal morphology and function or replacement by fibrotic tissue. This outcome is contingent on the balance between the production and degradation of extracellular matrix proteins.38 Surgical interventions are typically employed to manage these complications.5 In the context of CD development and exacerbation, TNBS-induced acute colitis can progress to severe chronic inflammation, characterized by irregular epithelial structure, thickened intestinal walls, substantial infiltration of mononuclear cells, and persistent collagen deposition.39 Numerous studies have highlighted the pivotal role of the inflammatory response in intestinal fibrosis development and progression.40 Moreover, the TGF-β1/Smad/VEGF signaling pathway is recognized as a master regulator of intestinal fibrosis. Consequently, the progression from CD to fibrosis is believed to involve various detrimental factors that inflict damage upon the intestinal, triggering an immediate inflammatory response.41 Ultimately, this leads to the deposition of ECM proteins into the normal intestinal structures, culminating in the formation of fibrotic tissue.40 In the present study, we not only observed the inflammatory changes induced by TNBS in mice (Figure 4) but also assessed alterations in multiple fibrosis-related factors at both the mRNA and protein levels (Figures 6–7). These findings corroborate the ameliorative effects of QTF on intestinal fibrosis.
In recent clinical studies of patients with colitis, it has been found that fibrosis is common in the small intestine of CD patients, and its incidence is far higher than that of any other intestinal sites.5 However, most fundamental research regarding CD rarely explores the progression of fibrosis related to the small intestine.42 We hypothesized that the factors leading to fibrosis of the small intestine will also change when TNBS is used to induce IBD in a mouse model. As shown in Figure 8, In TNBS-induced mice, the levels of these factors in the small intestine were notably elevated, but they showed a decrease following QTF treatment. In addition, IEC-6 cells were used to further confirm that QTF regulates the deposition of collagen and the expression of fibrosis factors (Figure 5).
Steroids are widely utilized for the treatment of various fibrotic conditions. However, there are no precise drugs tailored for the treatment of intestinal fibrosis. Furthermore, the precise molecular targets responsible for the anti-fibrotic effects of steroids have remained elusive.43 This study unequivocally demonstrates that QTF leads to the downregulation of the TGFβ1-Smad-VEGF signaling pathway, resulting in the inhibition of fibrotic factors such as VEGFA, α-SMA, Vimentin, and Collagen I (Figures 5–8). Ultimately, our screening identified two core biologically active compounds, Flavone and Isoflavone, along with four representative targets: AKT1, IL-6, TNF, and VEGFA. The results of molecular docking indicate the effective binding of these compounds to their respective protein targets, suggesting a stable interaction (Figure 9). The activation of TGF-β1-mediated signaling pathways underlies the pathogenesis of stricture-related complications in both CD patients and animal models (Figure 10). In this context, this study offers a novel and clinically significant insight into the management of these complications. We have explored the mechanism through which QTF suppresses the development of TNBS-induced colitis and have concluded that it also holds promise for the treatment of intestinal fibrosis, as supported by several independent observations.
 |
Figure 10 Signaling pathway displaying how QTF regulates CD-induced intestinal fibrosis through inhibiting the TGF-β1/Smad/VEGF pathway. |
The higher incidence of fibrosis in CD is probably the result of transmural bowel inflammation, which exposes ECM-producing mesenchymal cells to fibrotic mediators.44 Biopsy samples obtained from tissues with or without strictures have indicated that pathogenic mechanisms identified in myofibroblast cell lines may also contribute to the development of intestinal strictures in CD patients.45 In clinical practice, surgical intervention is often the primary choice for patients with fibrosis; however, the post-operative recurrence rate is substantial. In terms of pharmaceutical treatments, biological agents are commonly employed, but their efficacy is often limited, with high recurrence rates, ultimately failing to slow down the fibrotic progression.46 Consequently, treatments such as QTF may offer more promising outcomes compared to current therapeutic options. QTF alleviates intestinal fibrosis by targeting the classical fibrosis pathway involving TGF-β1-Smad-VEGF. Nevertheless, there may be other molecular mechanisms associated with intestinal fibrosis in CD. This raises an intriguing question that should be addressed in future studies: how does QTF interact functionally with the diverse molecular mechanisms underlying CD-associated fibrosis to inhibit its pathogenesis?
Conclusion
In conclusion, QTF can ameliorates inflammation-associated intestinal fibrosis by inhibiting the TGF-β1-Smad-VEGF pathway, impacting both colonic and small intestinal fibrosis. Experimental studies of this novel medicine will provide more options for the treatment of fibrosis and advance our understanding of the mechanisms behind alternative treatment strategies.
Abbreviations
α-SMA, α-smooth muscle actin; ANOVA, one-way analysis of variance; CCK, cell counting kit. CD, Crohn’s disease; ECM, extracellular matrix; EIC, extracted ion chromatography; ESI, electrospray ionization; H&E, hematoxylin and eosin; IEC-6, Rat small intestinal crypt cell; IF, immunofluorescence; LPS, lipopolysaccharide; MS, mass spectrometry; QTF, Qingchang Tongluo Decoction; TCM, traditional Chinese medicine; TGF-β1, transforming growth factor-β1; TNBS, 2,4,6-Trinitrobenzenesulfonic acid; UPLC-MS, ultra-high performance liquid chromatography and mass spectrometry; VEGFA, Vascular endothelial growth factor A.
Ethical Approval
All procedures in this study were approved by the Animal Ethics Committee of Jiangsu Province Hospital of Traditional Chinese Medicine, under the license number 2022DW-05-01. All databases utilized in this study are publicly accessible and contain data available for unrestricted reuse under open licenses. In accordance with the guidelines provided by the National Science and Technology Ethics Committee of China, as stated in their official document (https://www.gov.cn/zhengce/zhengceku/2023-02/28/content_5743658.htm), the utilization of legally obtained public data is exempt from ethical review. Consequently, the aspect of this research involving public databases was granted an ethics approval waiver by the Ethics Committee of the Affiliated Hospital of Nanjing University of Traditional Chinese Medicine.
Acknowledgments
This work was supported by the Jiangsu Provincial Medical Innovation Center for Digestive Diseases of Chinese Medicine [Su Health Science and Education (2022) No.15], State Administration of Traditional Chinese Medicine High-level Chinese Medicine Key Discipline Programme (Chinese Medicine Spleen and Gastroenterology) [Letter of Human Education in Chinese Medicine (2023) No. 85], and the Postgraduate 579 Research & Practice Innovation Program of Jiangsu Province [grant nos. 580 KYCX22_1938] to F. X.
Author Contributions
All authors have made substantial contributions to the work reported, encompassing the conception, study design, execution, acquisition of data, analysis, and interpretation. Each author has participated in drafting, revising, or critically reviewing the manuscript; has given final approval for the version to be published; concurs with the journal to which the article has been submitted; and agrees to be accountable for all aspects of the work, ensuring the accuracy and integrity of the article.
Disclosure
All authors affirm that they do not have any conflicts of interest to disclose in this work.
References
1. Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380(9853):1590. doi:10.1016/s0140-6736(12)60026-9
2. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–1040. doi:10.1038/nm.2807
3. Louis E, Collard A, Oger FA, et al. Behaviour of crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49(6):777–782. doi:10.1136/gut.49.6.777
4. Spinelli A, Correale C, Szabo H, et al. Intestinal fibrosis in crohn’s disease: Medical treatment or surgery? Curr Drug Targets. 2010;11(2):242–248. doi:10.2174/138945010790309984
5. D’Alessio S, Ungaro F, Noviello D, et al. Revisiting fibrosis in inflammatory bowel disease: The gut thickens. Nat Rev Gastroenterol Hepatol. 2022;19(3):169–184. doi:10.1038/s41575-021-00543-0
6. Stenke E. Bourke BKnaus U. Crohn’s strictures-moving away from the knife. Front Pediatr. 2017;5:141. doi:10.3389/fped.2017.00141
7. Powell DW, Pinchuk IV, Saada JI, et al. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol. 2011;73:213–237. doi:10.1146/annurev.physiol.70.113006.100646
8. Koumas L, Smith TJ, Feldon S, et al. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol. 2003;163(4):1291–1300. doi:10.1016/s0002-9440(10)63488-8
9. Rieder F, Fiocchi C. Intestinal fibrosis in ibd--a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. 2009;6(4):228–235. doi:10.1038/nrgastro.2009.31
10. Valatas V, Filidou E, Drygiannakis I, et al. Stromal and immune cells in gut fibrosis: The myofibroblast and the scarface. Ann Gastroenterol. 2017;30(4):393–404. doi:10.20524/aog.2017.0146
11. Imai J, Hozumi K, Sumiyoshi H, et al. Anti-fibrotic effects of a novel small compound on the regulation of cytokine production in a mouse model of colorectal fibrosis. Biochem Biophys Res Commun. 2015;468(4):554–560. doi:10.1016/j.bbrc.2015.10.123
12. Lawrance IC, Rogler G, Bamias G, et al. Cellular and molecular mediators of intestinal fibrosis. J Crohns Colitis. 2017;11(12):1491–1503. doi:10.1016/j.crohns.2014.09.008
13. Liu J, Deng T, Wang Y, et al. Calycosin inhibits intestinal fibrosis on ccd-18co cells via modulating transforming growth factor-β/smad signaling pathway. Pharmacology. 2019;104(1–2):81–89. doi:10.1159/000500186
14. Speca S, Rousseaux C, Dubuquoy C, et al. Novel pparγ modulator ged-0507-34 levo ameliorates inflammation-driven intestinal fibrosis. Inflamm Bowel Dis. 2016;22(2):279–292. doi:10.1097/mib.0000000000000618
15. Li S, Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chin J Nat Med. 2013;11(2):110–120. doi:10.1016/s1875-5364(13)60037-0
16. Luo TT, Lu Y, Yan SK, et al. Network pharmacology in research of Chinese medicine formula: methodology, application and prospective. Chin J Integr Med. 2020;26(1):72–80. doi:10.1007/s11655-019-3064-0
17. Vakser IA. Protein-protein docking: From interaction to interactome. Biophys J. 2014;107(8):1785–1793. doi:10.1016/j.bpj.2014.08.033
18. Wang K, Chen Q, Shao Y, et al. Anticancer activities of tcm and their active components against tumor metastasis. Biomed Pharmacother. 2021;133:111044. doi:10.1016/j.biopha.2020.111044
19. Huang S, He J, Chen Y, et al. Effect of huangqin decoction on regulating intestinal flora in colitis mice characterized as inhibition of the nod2-dependent pathway. Pharm Biol. 2022;60(1):108–118. doi:10.1080/13880209.2021.2017981
20. Yuan S, Li Y, Li J, et al. Traditional Chinese medicine and natural products: Potential approaches for inflammatory bowel disease. Front Pharmacol. 2022;13:892790. doi:10.3389/fphar.2022.892790
21. Shen H, Zhu L, Liu Y, et al. A Traditional Chinese Medicine Compound Composition for the Treatment of Crohn’s Disease and Its Preparation. Jiangsu Province; 2023: 15.
22. He HH, Shen HZheng K. observation of the curative effect of qingchang huashi recipe for treating active ulcerative colitis of inner-accumulation of damp-heat syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32(12):1598–1601.
23. Zhao ZM, Zhu CW, Huang JQ, et al. Efficacy and safety of fuzheng huayu tablet on persistent advanced liver fibrosis following 2 years entecavir treatment: a single arm clinical objective performance criteria trial. J Ethnopharmacol. 2022;298:115599. doi:10.1016/j.jep.2022.115599
24. Cheng C, Hu J, Li Y, et al. Qing-chang-hua-shi granule ameliorates dss-induced colitis by activating nlrp6 signaling and regulating th17/treg balance. Phytomed. 2022;107:154452. doi:10.1016/j.phymed.2022.154452
25. Seeliger D, de Groot BL. Ligand docking and binding site analysis with pymol and autodock/vina. J Comput Aided Mol Des. 2010;24(5):417–422. doi:10.1007/s10822-010-9352-6
26. Ye M, Luo G, Ye D, et al. Network pharmacology, molecular docking integrated surface plasmon resonance technology reveals the mechanism of toujie quwen granules against coronavirus disease 2019 pneumonia. Phytomed. 2021;85:153401. doi:10.1016/j.phymed.2020.153401
27. Morris GP, Beck PL, Herridge MS, et al. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96(3):795–803.
28. Hu J, Huang H, Che Y, et al. Qingchang huashi formula attenuates dss-induced colitis in mice by restoring gut microbiota-metabolism homeostasis and goblet cell function. J Ethnopharmacol. 2021;266:113394. doi:10.1016/j.jep.2020.113394
29. Wang R, Wang D, Wang H, et al. Therapeutic targeting of nrf2 signaling by maggot extracts ameliorates inflammation-associated intestinal fibrosis in chronic dss-induced colitis. Front Immunol. 2021;12:670159. doi:10.3389/fimmu.2021.670159
30. Wirtz S, Popp V, Kindermann M, et al. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc. 2017;12(7):1295–1309. doi:10.1038/nprot.2017.044
31. Yun SM, HKim KS, H E. The molecular mechanism of transforming growth factor-β signaling for intestinal fibrosis: a mini-review. Front Pharmacol. 2019;10:162. doi:10.3389/fphar.2019.00162
32. Liu J, Liu J, Tong X, et al. Network pharmacology prediction and molecular docking-based strategy to discover the potential pharmacological mechanism of huai hua san against ulcerative colitis. Drug Des Devel Ther. 2021;15:3255–3276. doi:10.2147/dddt.S319786
33. Singh A, Koenen BKirby DF. Bariatric surgery and its complications in inflammatory bowel disease patients. Inflamm Bowel Dis. 2020;26(8):1155–1165. doi:10.1093/ibd/izz246
34. Liu X. Transporter-mediated drug-drug interactions and their significance. Adv Exp Med Biol. 2019;1141:241–291. doi:10.1007/978-981-13-7647-4_5
35. C OS, Doak AK, Ganesh AN, et al. Colloidal drug formulations can explain ”bell-shaped” concentration-response curves. ACS Chem Biol. 2014;9(3):777–784. doi:10.1021/cb4007584
36. Habet S. Narrow therapeutic index drugs: clinical pharmacology perspective. J Pharm Pharmacol. 2021;73(10):1285–1291. doi:10.1093/jpp/rgab102
37. Latella G, Sferra R, Speca S, et al. Can we prevent, reduce or reverse intestinal fibrosis in ibd? Eur Rev Med Pharmacol Sci. 2013;17(10):1283–1304.
38. Malik TA. Inflammatory bowel disease: historical perspective, epidemiology, and risk factors. Surg Clin North Am. 2015;95(6):1105. doi:10.1016/j.suc.2015.07.006
39. Scheibe K, Kersten C, Schmied A, et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology. 2019;156(4):1082–1097.e11. doi:10.1053/j.gastro.2018.11.029
40. Gordon IO, Agrawal N, Goldblum JR, et al. Fibrosis in ulcerative colitis: Mechanisms, features, and consequences of a neglected problem. Inflamm Bowel Dis. 2014;20(11):2198–2206. doi:10.1097/mib.0000000000000080
41. Lovisa S, Genovese GDanese S. Role of epithelial-to-mesenchymal transition in inflammatory bowel disease. J Crohns Colitis. 2019;13(5):659–668. doi:10.1093/ecco-jcc/jjy201
42. Bettenworth D, Bokemeyer A, Baker M, et al. Assessment of crohn’s disease-associated small bowel strictures and fibrosis on cross-sectional imaging: a systematic review. Gut. 2019;68(6):1115–1126. doi:10.1136/gutjnl-2018-318081
43. Maul J, Zeitz M. Ulcerative colitis: immune function, tissue fibrosis and current therapeutic considerations. Langenbecks Arch Surg. 2012;397(1):1–10. doi:10.1007/s00423-011-0789-4
44. Rams H, Rogers A, IGhandur-Mnaymneh L. Collagenous colitis. Ann Intern Med. 1987;106(1):108–113. doi:10.7326/0003-4819-106-1-108
45. Latella G, Sferra R, Vetuschi A, et al. Prevention of colonic fibrosis by boswellia and scutellaria extracts in rats with colitis induced by 2,4,5-trinitrobenzene sulphonic acid. Eur J Clin Invest. 2008;38(6):410–420. doi:10.1111/j.1365-2362.2008.01955.x
46. Henderson NC, Rieder FWynn TA. Fibrosis: From mechanisms to medicines. Nature. 2020;587(7835):555–566. doi:10.1038/s41586-020-2938-9
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.








