Back to Journals » Journal of Inflammation Research » Volume 17
Therapeutic Potential of Essential Oils Against Ulcerative Colitis: A Review
Authors Li J, Zhang X, Luan F, Duan J, Zou J, Sun J , Shi Y, Guo D, Wang C, Wang X
Received 26 January 2024
Accepted for publication 25 April 2024
Published 31 May 2024 Volume 2024:17 Pages 3527—3549
DOI https://doi.org/10.2147/JIR.S461466
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Jinkai Li,* Xiaofei Zhang,* Fei Luan,* Jiawei Duan, Junbo Zou, Jing Sun, Yajun Shi, Dongyan Guo, Changli Wang, Xiao Wang
Key Laboratory of Basic and New Drug Research in Chinese Medicine, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiao Wang, Key Laboratory of Basic and New Drug Research in Chinese Medicine, Shaanxi University of Chinese Medicine, Xianyang, Shaanxi, 712046, People’s Republic of China, Email [email protected]
Abstract: Ulcerative colitis (UC) is a chronic non-sp ecific inflammatory disease of the colorectal mucosa. Researchers have associated UC onset with familial genetics, lifestyle behavior, inflammatory immune factors, intestinal microbiota, and the integrity of the intestinal mucosal barrier. The primary therapeutic interventions for UC consist of pharmacological management to control inflammation and promote mucosal healing and surgical interventions. The available drugs effectively control and decelerate the progression of UC in most patients; nonetheless, their long-term administration can exert adverse effects and influence the therapeutic effect. Plant essential oils (EOs) refer to a group of hydrophobic aromatic volatile substances. EOs have garnered considerable attention in both domestic and international research because of their anti-inflammatory, antibacterial, and antioxidant properties. They include peppermint, peppercorns, rosemary, and lavender, among others. Researchers have investigated the role of EOs in medicine and have elucidated their potential to mitigate the detrimental effects of UC through their anti-inflammatory, antioxidant, antidepressant, and anti-insomnia properties as well as their ability to regulate the intestinal flora. Furthermore, EOs exert minimal toxic adverse effects, further enhancing their appeal for therapeutic applications. However, these speculations are based on theoretical experiments, thereby warranting more clinical studies to confirm their effectiveness and safety. In this article, we aim to provide an overview of the advancements in utilizing natural medicine EOs for UC prevention and treatment. We will explore the potential pathogenesis of UC and examine the role of EOs therapy in basic research, quality stability, and management specification of inadequate EOs for UC treatment. We intend to offer novel insights into the use of EOs in UC prevention and management.
Keywords: ulcerative colitis, essential oils, active ingredients, signaling pathway
Graphical Abstract:
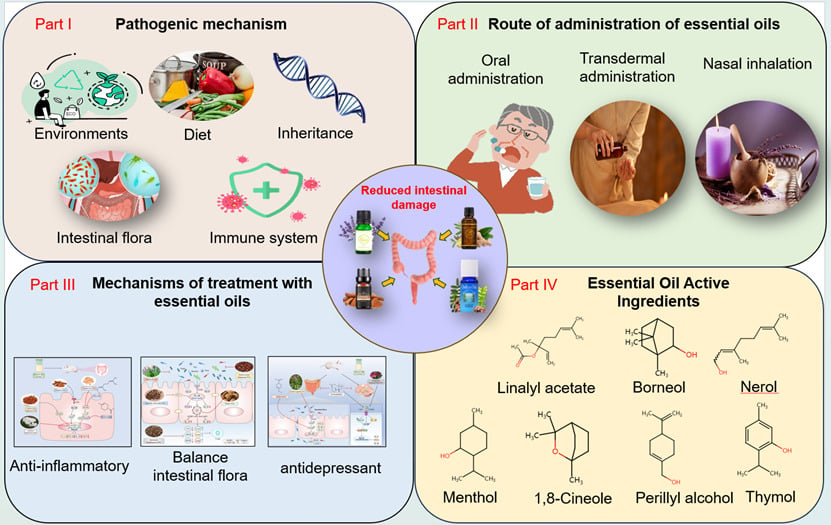
Introduction
In the clinical setting, patients with ulcerative colitis (UC) present with symptoms, such as abdominal pain, diarrhea, and blood in stool. Often, these symptoms are accompanied by heightened oxidative stress and aggregated cellular inflammatory factors. Furthermore, the course of UC is typically prolonged and prone to recurrence; the long-term chronic inflammatory state increases the risk of developing colon cancer and associated diseases.1 Data from surveys have indicated a strong correlation between UC prevalence and the level of economic development. Moreover, its incidence is rapidly increasing with the progress of social and economic development. For example, the prevalence of UC ranges from 5.50 to 24.30 per 10,000 globally. UC prevalence in developed countries, such as North America and Europe is approximately 24.30 per 10,000 and 19.20 per 10,000, respectively. By contrast, the prevalence in Asia and the Middle East is approximately 6.30 per 10,000. UC prevalence in mainland China is approximately 11.60 per 10,000, which may be underestimated.2 Inflammatory bowel disease is more common in industrialized and Western countries; UC has a higher prevalence than Crohn’s disease globally and in less developed countries.3
Clinical treatment of UC is based on reducing inflammatory lesions, controlling symptoms, reducing recurrence, and controlling acute exacerbations. The drugs commonly used for UC treatment include aminosalicylic acid (salicylazosulfapyridine, mesalazine), immunosuppressants (azathioprine (AZA)), steroids (methylprednisolone), and biologics (adalimumab, infliximab, bifidobacteria, and bacillus coagulans).4 However, these drugs are often expensive and display poor therapeutic efficacy. The long-term use of salicylates leads to adverse effects, such as nausea and vomiting. Excess glucocorticoid administration leads to Cushing’s syndrome. Moreover, immunosuppressant use can cause bone marrow suppression and opportunistic infections.5 Considering the adverse effects of traditional medicine use, researchers are investigating effective and inexpensive medicines. Flavonoids, terpenoids, and sulfur-containing compounds in medicinal plants have been identified as potential drugs for the treatment of experimental UC.6
Essential oils (EOs), also referred to as volatile oils or ethereal oils, are derived from plant parts, such as flowers, bracts, leaves, branches, roots, bark, fruits, seeds, and resins of herbaceous plants. EOs are extracted through processes, namely distillation, pressing, and solvent extraction. Primarily, they consist of terpenes and aromatic compounds, along with oxygen-containing derivatives, such as alcohols, aldehydes, ketones, phenols, ethers, and endolipids. Additionally, EOs may consist of nitrogen-containing and sulfur-containing compounds. The earliest use of EOs dates back >6000 years to ancient Egypt; archaeologists theorized that Cleopatra used sandalwood, rose, and neroli oils for bathing and anointing. The natives applied EOs all over their bodies after bathing to protect their skin as well as for antiseptic and antibacterial purposes. This is the earliest record of aromatherapy (“Aroma” stands for fragrance in Greek).7 The extraction, composition analysis, and functional properties of EOs have gained attention because of their antimicrobial, antiviral, antioxidant, and neuromodulatory effects.8–10 Researchers have demonstrated new advancements in UC treatment using EOs. Ghasemi-Pirbaluti et al11 demonstrated that the administration of menthol, the active ingredient of peppermint EO, improved the gross signs of UC in rats. It improved hematocrit levels and histopathology and significantly reduced the levels of interleukin (IL)-1β, IL-6, tumor necrosis factor alpha (TNF-α), and myeloperoxidase (MPO) in the colon. Additionally, menthol was effective in preventing acetic acid-induced UC in rats. In a therapeutic model of oxazolone-induced colitis, Agastache Mexicana EOs12 significantly reduced abdominal writhing in rats; limonene and thujone were the primary bioactive metabolites in this model. EOs of Atractylodis caryophyllus and Hainan sandalwood have demonstrated ameliorative effects in UC.13,14 EOs can stimulate the olfactory nerves, which in turn affects the central nervous system and endocrine system. Consequently, it regulates immune function and exhibits antioxidant and anti-inflammatory capacities in UC. However, this view is primarily based on theoretical experiments, thus necessitating additional clinical studies to determine the efficacy and safety of EOs in UC treatment. A limited number of reviews have reported on the utilization of EOs in UC management. This article presents a comprehensive synthesis of contemporary research on EOs for UC treatment, encompassing the application of plant EOs, their monomer components, and the comparative benefits of conventional therapies. We aim to establish a theoretical framework to utilize EOs in UC treatment and to furnish an enhanced theoretical foundation for future treatment endeavors.
Potential Pathogenesis of Ulcerative Colitis
Environmental Factors
In recent years, the incidence of UC has been increasing, especially in developing countries. Nutritional imbalance and dietary factors play an indispensable role in the changes of intestinal flora, the damage of mucosal barrier and the regulation of immune response. Many factors such as environmental hygiene and living habits also increase the risk of UC. In the psychological aspect, if people’s mental state is in a high-pressure, stressful situation for a long period of time, then the adrenal axis, mucosa, and pathogens will interact with each other, which will contribute to the activation of the activity of intestinal mucosal mast cells and the production of more hormones that cause intestinal inflammation, which will ultimately lead to the triggering of UC.15 Appendectomy can increase extraintestinal symptoms of UC.16 Histological analysis shows that aging can cause steatosis of appendiceal lymphoid tissue, and appendiceal lymphoid tissue in patients with UC is more prone to steatosis, and the inactivation of appendiceal lymphoid tissue degeneration can further exacerbate the onset and development of UC.17 Through the in-depth understanding of the intestinal flora and intestinal mucosal immune system, the appendix can provide a rich nutrient for the normal intestinal flora for the organism and protect the intestinal lumen from pathogenic bacteria.18
Dysbiosis of Intestinal Flora
More and more studies have found that intestinal flora dysbiosis plays an important role in the pathogenesis of UC, so this factor is also a focus of research in the study of the pathogenesis of UC. Gut flora is the most abundant microbiota in the human body, and most human gut microbiome consists of four phyla, ie, Sclerotinia, Anaplasma, Aspergillus, and Actinobacteria.19 Also, gut microbes produce metabolites with anti-inflammatory effects, such as short-chain fatty acids (SCFA), and through the metabolites, they provide nutrients and energy to the body and modulate the immune system thereby protecting the host.20,21 The gut microbiota is involved in the development and progression of UC by mediating a variety of relevant immune-inflammatory pathways. Disruption of the balance of beneficial and harmful flora in the gut microbiota, ie, dysbiosis, is associated with the development of colitis.22 When suffering from ulcerative colitis, the proportion of short-chain fatty acid-producing thick-walled bacterial phylum and Clostridium spp. in the intestine decreases, while probiotics are significantly reduced while pathogenic bacteria increase, and their release of enterotoxins can disrupt the integrity of the intestinal epithelium, leading to increased permeability of epithelial cells.23 At the same time, the reduced diversity of the intestinal flora, the decrease in SCFA, and the increase in the intestinal flora releasing inflammatory mediators activate the immune activity of the intestinal mucosa, resulting in a persistent localized inflammatory response.24 Although the pathogenesis of UC is not fully understood, intestinal microecological imbalance influences the development of UC at multiple levels.
Immune Factors
Abnormal immune function is an important factor in the pathogenesis of UC. Imbalance of intestinal flora, overreaction of mucosal immune system and inability of mucosal defense mechanism to inhibit inflammatory stimuli will involve innate or adaptive immune response, which is mainly manifested in epithelial cell damage, intestinal barrier dysfunction, autoimmunity, abnormal expression of cytokines, and immune cell infiltration. When the local microenvironment of the intestinal tract changes or is stimulated by exogenous stimuli, it will activate the innate and adaptive immune mechanisms, promote the synthesis and secretion of immune molecules, and induce the activation of immune cells to initiate the immune defense response. UC is induced when the body is repeatedly attacked by pathogens resulting in the activation and expansion of specific T cells, disrupting the balance between T cell subsets and driving excessive inflammatory responses.25 With increasing research, the role of inflammatory vesicles in the immune system is gradually being recognized. Different inflammasomes are expressed in both immune and non-immune cells. Inflammatory vesicles act as protein sensors in response to pathogens and are the first participants in the innate immune system, recruiting innate inflammatory factors and subsequently activating adaptive immune responses.25–27
UC Pathogenesis and the Limitations of Marketed Drugs
The primary clinical manifestations of UC include abdominal pain, diarrhea, mucus, pus, and blood in stool, which may be accompanied by systemic reactions, such as fever, malnutrition, and other extraintestinal manifestations. The lesions primarily affect the mucosa and submucosa, usually beginning in the rectal mucosa, developing retrograde to the proximal segment, and spreading to the entire colon in severe cases. Moreover, recurrent and prolonged intestinal inflammation and ulcers in UC increase the risk of colorectal carcinogenesis, which not only reduces the quality of patient survival but also imposes a serious healthcare burden.28 The acute exacerbation of severe colitis occurs in approximately 15% of the patients with UC, of which 30% require colectomy.29 UC is typically treated using Western medicine with chemical drugs, such as glucocorticoid hormones, aminosalicylic acid preparations, and immunosuppressive agents. These drugs are preferred for mildly and moderately active patients. Furthermore, they are used as maintenance drugs for patients in remission; nonetheless, they exert substantial adverse effects, tend to make the patients dependent and generate poor clinical outcomes. 5-aminosalicylic acid (5-ASA) is a well-tolerated drug; the oral administration of 2 g to 3 g of 5-ASA daily is sufficient for most patients with mildly to moderately active UC. Additionally, clinicians use immunosuppressive drugs in UC treatment because they suppress the immune system. AZA, methyl methacrylate, and 6-mercaptopurine are commonly used for UC treatment. These drugs are clinically indicated only in patients who underwent failed glucocorticoid or aminosalicylic acid therapy or in patients who cannot tolerate the adverse effects of drugs. Thiopurines were identified in a Spanish database study. Thiopurine administration may cause nausea (8%), hepatotoxicity (4%), bone marrow toxicity (4%), pancreatitis (4%), non-melanoma skin cancer, and lymphoma.30 Biologics effectively treat UC and are well tolerated by individuals, with few adverse effects. Moreover, they provide an alternative approach to UC treatment. Infliximab is preferred for clinical use. Duan Zexing et al31 used infliximab to treat patients with moderate-to-severe UC; the clinical symptoms were significantly relieved after 8 weeks and the mucosal healing rate was high. Moreover, no serious adverse effects were observed during the study; nonetheless, the price of the drug was high. The clinical methods to improve intestinal flora in healthy individuals primarily consist of antimicrobial drugs, probiotics, prebiotics, and fecal transplantation. Probiotics are the most commonly used microecological agents in clinical practice. They refer to a group of biologically active microorganisms that are beneficial to the body when ingested appropriately. Probiotics can inhibit pathogenic bacteria, strengthen the intestinal mucosal barrier, and regulate immune response.
Researchers have reported on limited drugs with considerable efficacy, few adverse effects, and suitable prices. Among natural medicines, EOs with wide distribution, high content, low toxicity, and good anti-inflammatory effects can be used as an alternative for UC treatment in the future.
Routes of Essential Oils Administration
EOs therapy is implemented in numerous ways, including fumigation, bathing, massage, gua sha, inhalation, oral administration, and other methods, through the sense of olfaction, gustation, and tactile functions. EOs are absorbed through the skin, digestive, and respiratory systems; they regulate the central nervous system, circulatory, and endocrine systems to restore the coordination of the body and mind to eliminate depression, anxiety, boredom, anger, and fatigue-related emotions and feelings. Vegetable EOs have antibacterial and antiseptic functions; thus, they are widely used in food and medicine.32
Oral Route of Absorption
The advantages of drug absorption through the oral mucosa include convenient administration, rapid absorption, and entry to the internal jugular vein through numerous capillaries under the oral mucosa. Moreover, they reach the heart directly without passing through the liver. Wang et al33 reported that lavender EOs administered via gavage alleviated colonic mucosal injury in dextran sulfate sodium (DSS)-induced colitis experiments in mice. Furthermore, it reduced the inflammation of epidermal growth factor receptor, TNF-α, and IFN-γ levels of cytokines. Zhou et al34 used ginger volatile oil to gavage mice with peritonitis; ILs secreted by peritoneal macrophages were significantly reduced in the experimental group, resulting in controlled inflammatory response. Cinnamon EOs can treat renal diseases and rheumatic diseases. Wang et al35 administered cinnamon EOs to rats with hyperuricemia via gavage; the rats presented with reduced uric acid weakened hepatic xanthine oxidase activity, and increased superoxide dismutase and glutathione peroxidase levels, which improved renal injury. EOs can improve insomnia symptoms. Li et al36 reported that the oral administration of rosemary hydrosol significantly increased the serum levels of 5-hydroxytryptamine (5-HT), gamma-aminobutyric acid (GABA), and dopamine in rats with insomnia. Furthermore, it increased the levels of serotonin 1A receptor, cyclic adenosine monophosphate (cAMP)-dependent protein kinase, GABA, cAMP, and adenylyl cyclase 5 proteins in the hippocampus (HP), which elevated the duration of sleep.
Nasal Route of Administration
The nasal administration of EOs is a convenient route of drug administration. Its inherent advantages consist of a large surface area for absorption, rich vascularity of the nasal mucosa, and rapid entry of drugs into the circulatory system through the nasal cavity, which can bypass the hepatic first-pass effect. Wu et al37 demonstrated that EOs inhalation altered the brain metabolism in rats, with increased carbohydrate levels and decreased neurotransmitters, amino acids, alcohols, and fatty acid levels. By contrast, the nucleoside and lactic acid content increased in urine; these changes were considered beneficial to the anti-anxiety response in rats. According to Li et al38, reported the inhalation of lavender EOs could inhibit 5-HT-related receptors and block the Ca2+/ calcium/calmodulin-dependent protein kinase II/extracellular-signal regulated kinase1/2 pathway downstream of the cAMP signaling pathway in a rat model of xenophobia, thus exerting an antiemetic effect. Cui et al39 reported that aluminum trichloride induced anxiety in rats that inhaled and sniffed bergamot EOs, which increased the frequency of entry into the central region, walking distance, open-arm time, and frequency, γ-aminobutyric acid levels, and antioxidant activity in the HP and frontal cortex (FC). Furthermore, it decreased the levels of propylene glycol, a lipid peroxidation product, and inflammatory factors in the HP and FC. Thus, bergamot EOs may alleviate anxiety through antioxidant, anti-inflammatory, and GABA-modulatory effects. The nasal inhalation of EOs serves as a complementary alternative therapy in the neurological, digestive, respiratory, and cardiovascular systems. However, some aromatic EOs can cause neurotoxicity, hepatotoxicity, gastrointestinal irritation, phototoxicity, contact sensitization, and other adverse effects.
Transdermal Route of Administration
Transdermal administration is a common mode of essential oil delivery, which has the advantages of high bioavailability, low adverse effects and good patient compliance.40,41 Li et al42 human in the treatment of DNCB-induced atopic dermatitis with rosemary EOs, observed improved skin lesions in mice coated with rosemary essential oil, a significant reduction in serum IL-6, TNF-α, as well as in skin tissues with decreased expression of p-JAK1, CD4+ cells, IL-4, p-P65/P65, p-STAT3/STAT3 protein expression was reduced. Pires F Q et al43 utilized HP-β-CD and nanostructured lipid carrier (NLC) for encapsulation of EOs, which solved the problem of volatility and solubility of EOs while achieving the control of the drug release rate and improved the utilization of EOs for transdermal administration. Rafii et al44 found that massage with lavender and chamomile EOs had a favorable effect on anxiety and improved the quality of sleeping in burn patients. Nasiri et al45 had patients with osteoarthritis of the knee joints undergo self-massage with lavender oils for nine times over a three-week period and found that the severity of pain in patients of the intervention group There was a significant difference in the severity of pain immediately after the intervention and 1 week after the intervention, but not at the fourth week follow-up, probably because the effects of the EOs wore off over time. Transdermal drug delivery is simple to learn and easy to administer, is increasingly accepted by patients, and is widely used in clinical nursing practice. Because of its use of EOs as a medium for massage, in addition to the therapeutic effect of EOs themselves, it can also increase the comfort brought to patients by simple massage, which is conducive to the establishment of a good nurse-patient relationship, and it can be said that it is an important research direction for the future of non-pharmacological therapies.
Use of Essential Oils in UC Treatment and Its Complications
Anti-Inflammatory Effects
Inflammation refers to a local protective response of the infected cells and adjacent tissues to irritation, injury, or infection; it is characterized by pain, redness, swelling, and the loss of cell function in severe cases.46 Plant EOs have anti-inflammatory activity47 and are central to UC treatment (Figure 1). Shiso aldehyde, the active ingredient in Perilla frutescens EOs, exerts an inhibitory effect on inflammation. Uemura et al48 investigated the anti-inflammatory activity of perilla aldehyde in a mouse model of DSS-induced colitis; its administration inhibited the expression of DSS-induced colonic factor genes and matrix metalloproteinase 9 and improved intestinal inflammation. Nuclear factor kappa B (NF-κB) controls the gene regulation of numerous inflammatory factors. Moreover, NF-κB effectively controls UC inflammation.49 Ocimum basilicum EOs inhibit acetic acid-induced colitis in rats by reducing leukocyte aggregation and inhibiting myeloperoxidase production.50 Foeniculum vulgare EOs51 attenuates acetic acid-induced colitis in rats by inhibiting NF-κB. This anti-inflammatory effect may be attributed to its active ingredients, such as trans-anethole brain and D-limonene, which inhibit NF-κB activation(Table 1). Compound EOs can exert synergistic efficacy to alleviate UC. Shishenwan volatile oil, which is composed of tonicolium, nutmeg, Wuzhu, and Schisandra volatile oils, effectively improved UC symptoms in mice, with a significant increase in body mass and decreases in disease activity index (DAI) scores, inflammatory cell infiltration, and colonic mucosal injury.52
 |
Figure 1 Essential oils modulate different signaling pathways to exert anti-inflammatory effects. |
Effects on the Intestinal Flora
The intestinal microenvironment is primarily composed of intestinal microbiota and mucosal cells, which can participate in physiological and pathological activities through four aspects as follows: intestinal microbial, chemical, mechanical, and immune barriers.53 These barriers are central to maintaining intestinal health. Impaired intestinal barriers increase the permeability of the intestinal mucosa to harmful bacteria, causing an imbalance in the intestinal flora, and leading to intestinal inflammation.54 Intestinal inflammation is related to UC pathogenesis.55 Adherent and invasive Aspergillus fungi are involved in altered intestinal dysbiosis, exploiting host defenses and inducing pro-inflammatory changes. Eventually, they lead to dysbiosis of the intestinal flora.56 Additionally, Streptococcaceae and Enterobacteriaceae are abundant in UC models; their abundance has been associated with increased inflammatory responses. EOs may reduce UC severity by modulating the microenvironment of the parietal gut and restoring intestinal integrity (Figure 2). The oral administration of stigmasterol reduced the levels of Aspergillus phylum, Enterobacteriaceae, and Streptococcus pepticus, thereby modulating the intestinal flora to alleviate DSS-induced UC. Several studies have demonstrated decreased levels of Lactobacillus and Bifidobacterium and increased levels of Escherichia coli (E. coli) in patients with colitis and DSS-induced colitis in mice.57,58 The use of peppercorn EOs59 modulated changes in bacterial composition and significantly reduced DSS-induced elevated E. coli levels. Treatment with aromatic turmeric ketone reduced Helicobacter pylori and Desulfovibrio family levels. Aromatic turmeric ketone is effective in UC treatment; it prevents DSS-induced damage of the intestinal flora, maintains some beneficial bacteria, and reduces harmful bacteria.60 A diet rich in n-6 unsaturated fatty acids (PUFA) increases the microbiota in mice, which induces inflammation and exacerbates infections causing colitis. However, the addition of n-3 PUFA to a diet rich in n-6 PUFA converts the pro-inflammatory microbiota to anti-inflammatory microbiota, thereby preventing colitis. Some of the chief components of EOs can be added to the daily diet to maintain gut microbial balance and provide novel ideas for treatment (Table 1).
Antioxidant Effects
Reactive oxygen species (ROS) primarily refers to highly reactive oxygen-containing substances, such as superoxide anion (O2−), hydroxyl radical (OH−), and hydrogen peroxide (H2O2). Reactive oxygen radicals oxidize the biomolecules of intestinal epithelial cells, destroy the mechanical barrier of the intestinal mucosa, and cause pathophysiological processes, such as bacterial translocation, intrinsic immune damage, mitochondrial dysfunction, and iron death. Moreover, they promote the development of UC.61,62 Many EOs have both anti-inflammatory and antioxidant effects and can be used to treat UC (Figure 2). Bergamot lactone, the active ingredient of bergamot EOs, can reduce TNF-α, IL-1, and IL-6 levels in rats. Additionally, it can significantly reduce oxidative damage by attenuating the increase in malondialdehyde (MDA) and 8-hydroxydeoxyguanosine levels as well as by restoring the decrease in antioxidant enzyme levels.39 The EOs of Salvia miltiorrhiza63 significantly increased superoxide dismutases and glutathione peroxidase activities and decreased nitric oxide synthase levels in the colon tissues of UC rats, suggesting the antioxidant capacity of VOA to accelerate the scavenging of free radicals and attenuate the damage caused by oxygen free radicals and lipid peroxidation in colon tissues (Table 1). Perillyl alcohol displays anti-inflammatory and anti-tumor activities. Additionally, it exerts antioxidant effects, prevents NF-κB activation, and reduces the production of pro-inflammatory cytokines because of its ROS-scavenging properties.64
 |
Figure 2 Essential oils balance the gut microbiological environment. |
Antidepressant Effects
The development of the “biopsychosocial” medical model has directed attention toward the role of psychosocial factors in UC pathogenesis and treatment. UC has been recognized as a physical and mental disease. Patients with UC often present with numerous abnormal psychological states, predominantly characterized by anxiety and depression. Its pathogenesis may be related to the brain-intestinal axis. Moreover, these psychiatric factors affect the neuropituitary, endocrine, intestinal immunity, and other functions, causing changes in intestinal inflammation and UC occurrence and development.65 Aromatic olfaction therapy is widely used because of its ease of operation and beneficial effects on relieving bad moods. It is expected to become a useful supplement to traditional medical treatments. The aroma components of plant EOs positively affect mood regulation.66 Compared with chemical antidepressants, EOs of natural medicines can achieve the goal of multi-targeted depression treatment using pharmacological components (Figure 3). They can mimic the effect of antidepressants while reducing adverse effects, which is valuable for the development of new antidepressants and the prevention of UC.67 Citronellol and geraniol, the chief components of geranium EOs demonstrate some antidepressant effects.68 The etiology of UC is unclear; nonetheless, researchers have reported a bidirectional relationship between psychological co-morbidities and UC treatment, which coexist to influence the disease course. The use of plant EOs for aromatherapy can alleviate anxiety and depression. Moreover, clinicians should not ignore patients with UC along with depression and other psychological problems, particularly active intervention and treatment (Table 1).
Anti-Insomnia Effects
Insomnia refers to the subjective experience of patients who are dissatisfied with the duration or quality of sleep; it affects daytime social functioning. Insomnia is a common sleep disorder. Impaired daytime functioning is the chief reason for clinic visits. The purpose of treatment is to improve the quality of sleep and restore daytime functioning in patients. However, insomnia is not only a sleep problem; particularly, chronic insomnia poses the risk of major diseases (such as anxiety disorders, depression, hypertension, diabetes, dementia, and cancer) and is often co-morbid with somatic and psychiatric disorders.69 Some studies have demonstrated a bidirectional association between sleep deprivation and UC. Poor sleep quality may increase the severity of UC inflammation and the risk of recurrence.70 Thus, aromatherapy is used as a safe and effective non-pharmacological therapy to improve sleep quality in patients with insomnia (Figure 3). Chien et al administered lavender through inhalation for 12 weeks in 67 women with sleep disorders. These volunteers were aged from 45 to 55 years. After 12 weeks, they were instructed to inhale lavender EOs using water as a blank control, twice a week, 20 min each time. After 1 week, lavender EOs improved the condition of sleep disorders. Koo et al71 significantly prolonged the time of sodium pentobarbital-induced sleep in mice after inhaling 10h of essential oil of Acorus calamus. Changes in the content of γ-aminobutyric acid in mice’s brains may exert a sedative-hypnotic effect. Aromatic EOs therapy can effectively improve the sleep quality of patients with UC along with insomnia; thus, it is a safe and feasible therapeutic and nursing intervention (Table 1).
 |
Figure 3 Essential oils inhibit inflammation in ulcerative colitis through antidepressant effects. |
 |
Table 1 Mechanism of Action of Essential Oils in the Treatment of Ulcerative Colitis |
Mechanisms Underlying Anti-UC Action of Plant Essential Oils
Regulating the NF-κB Signaling Pathway
The NF-κB signaling pathway is a classical inflammatory signaling pathway that regulates the expression of inflammatory factors and cell surface receptors. Moreover, it participates in immune and inflammatory responses. Under unstimulated conditions, NF-κB is attached to NF-κB inhibitors (IκB), and its component heterodimers are retained in the cytoplasm in an inactive form.90 However, upon stimulation by inflammatory factors, NF-κB is released into the nucleus via the phosphorylated degradation of IκB and P65 and encodes various cytokines and chemokines, which initiate target gene expression, such as TNF-α, IL-1β, and others contributing to the activity of NF-κB signaling pathway.91 Amir et al92 demonstrated that Bunium persicum EOs (200 mg/kg and 400 mg/kg) reduced MPO and TNF-α activities in rat colon tissues. Additionally, they inhibited acetic acid-induced p-NF-κB protein expression; this anti-inflammatory activity may be attributed to cuminaldehyde, γ-terpinene, and p-cymene. According to Maged et al73 treatment with yarrow EOs in UC mice significantly increased the anti-inflammatory cytokine IL-10 and decreased the inflammatory cytokine IL-6 levels and NF-κB expression and TNF-α. Zhang et al59 reported that pepper EOs activated peroxisome proliferator-activated receptor gamma (PPAR-γ), and, thus, inhibited the expression of NF-κB, TNF-α, IL-1β, and IL-12 and reduced DSS-induced colon injury. Zheng et al93 demonstrated that Brucea javanica oil, which is rich in brusatol, reversed DSS-induced p65 phosphorylation in a dose-dependent manner, thereby inhibiting NF-κB activation.
Regulating the (TLR)4 Signaling Pathway
Toll-like receptor (TLR)4 is an important signaling pathway that mediates inflammatory responses in the body. Upon stimulation, TLR4 recognition of pathogen-associated molecular patterns is activated, allowing the recruitment of myeloid differentiation protein and triggering a signaling cascade that induces NF-κB activation.94 Furthermore, it regulates the expression of pro-inflammatory factors, such as IL-6 and IL-1β, which promote inflammatory responses.95 Li et al78 demonstrated that the oral administration of cinnamon EOs in mice with colitis downregulated TLR4 signaling, IL-6, and TNF-α, increased the anti-inflammatory cytokine IL-10, and displayed potential antimicrobial activity in inhibiting H. pylori infections. This phenomenon controlled the inflammatory process of the intestinal tract. Altered TLR4 signaling expression and aberrant immune responses promote intestinal inflammation in patients with inflammatory bowel disease, which is closely related to the balance of the intestinal microbiota.96 H. pylori spp. was significantly and positively correlated with TLR4 expression, IL-6, and TNF-α. Members of the H. pylori genus can activate the immune response and induce the release of pro-inflammatory cytokines by recognizing TLR4.97
Regulating the MAPK signaling Pathway
The mitogen-activated protein kinase (MAPK) signaling pathway is a central pathway in the eukaryotic signaling network and is involved in cell proliferation, differentiation, apoptosis, and stress response under normal and pathological conditions. MAPK refers to a group of evolutionarily conserved serine-threonine kinases, which can be divided into four subfamilies as follows: the extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinases (p38MAPK), Jun N-terminal kinase, and large mitogen-activated protein kinase 1 (BMK1, also termed ERK5), which represent the four classical MAPK pathways, respectively.98 Zhang99 demonstrated that the geranylgeranyl acetate in Salvia miltiorrhiza EOs exerts an inhibitory effect on inflammatory responses generated by intestinal mucosal injury in rats by blocking the activation-regulated p-p38MAPK protein signaling pathway. Ayman et al100 reported that the anti-colitis mechanism of action of geraniol operates through regulating Wnt/glycogen synthase kinase-3 beta/β-catenin, p38MAPK, NF-κB, and PPAR-γ signaling pathways, thus exerting antioxidant, anti-inflammatory, and immunosuppressive effects. According to Wang et al.101 Boswellia serrata EOs inhibited MAPK and NF-κB signaling pathways and improved body weight loss, diarrhea, and blood in stool in mice with DSS-induced colitis. Additionally, they reduced serum TNF-α and IL-6 levels.
Effects of the Chief Essential Oils Components on UC
Terpenes
Terpenoids, such as monoterpenes and sesquiterpenes, are the key components of secondary metabolites of plants. They are characterized by numerous structural types and biological activities.102
β-stilbene is a natural bicyclic sesquiterpene, which can be extracted from EOs of plants with low toxicity. Moreover, it exerts numerous pharmacological effects, including anti-inflammatory,103 antibacterial,104 anxiolytic,105,106 antitumor,106 and antioxidant effects.107 The intestinal symptoms of DSS-induced colitis in mice were improved by the inhibition of iron death.108 Wu et al109 investigated Rsl3-induced macrophage iron death in the colon of UC mice; β-stigmasterol decreased pro-iron death factors, inhibited lipid peroxidation and inflammation, and increased anti-iron death factors, suggesting its protective effect on UC.
Perillyl alcohol also termed dihydrocumyl alcohol, is a naturally occurring monocyclic terpenoid derived from the mevalonate pathway in plants. Perillyl alcohol is effective in cancer treatment, such as hepatocellular carcinoma, breast cancer, and lung cancer.110,111 Perillyl alcohol can inhibit the proliferation and Notch-1 protein expression of colon cancer SW480 cells and downregulate the Rspo3 gene and protein expression, suggesting its use as a therapeutic agent for Rspo3 protein high-expressing colon cancers. This in turn provides a novel direction for the prevention and treatment of colon cancer.112 Eswara Rao Puppala et al113 demonstrated that perillyl alcohol significantly reduced the protein expression of NF-κB, STAT3, and TLR-4 in colon tissues as well as reduced the expression of TNF-α, IL-1β, IL-6, and JAK2. Additionally, it increased the levels of dopamine and 5-HT in the cerebral cortex and HP in dextran sulfate-sodium-induced UC experiments in mice. Simultaneously, it significantly reduced adrenaline levels in the cerebral cortex and HP and cortisol levels. Perillyl alcohol can be used as a novel treatment for psychological stress-induced UC.
Olive bittersweet (oleuropein, Ole) is an active ingredient extracted from the leaves of Olea europaea. It exerts strong antioxidant, antibacterial, and antiviral effects.114 Giner et al115 investigated the use of Ole in the oral treatment of DSS-induced UC mice; Ole inhibited the accumulation of neutrophils and the colonic tissue expression of matrix metalloproteinase-9 expression as well as the ability of hydroxytyrosol (active metabolite) to reduce lipopolysaccharide-induced moderate reductions in IL-1β, IL-6, and TNF-α mRNA expression in macrophages. Ole has the potential as a therapeutic agent for UC.
1.8-eudesmus (1,8-cineole) is a natural monoterpene found in aromatic plants of the genera Eucalyptus, Rosaceae, and Salvia officinalis. It has an odor similar to that of camphor, good transdermal penetration, low toxicity to humans and animals, and environmental friendliness. 1.8-cineole possesses several biological and pharmacological activities, including the amelioration of pancreatitis,116 antiproliferative and anticholesterogenic properties,117,118 the amelioration of nonalcoholic steatohepatitis,118 antibacterial, insecticidal, and anti-inflammatory effects. Santos et al119 investigated the possible mechanism of action of 1.8-cineole on tissue damage in rats with 2.4,6-Trinitrobenzene sulfonic acid (TNBS) colitis; it decreased MPO activity, increased glutathione (GSH) in the colon, and exerted an anti-inflammatory effect.
The chained monoterpene carbohydrate β-laurene is an essential component of plant EOs. It is a raw material for fragrances, which can be easily obtained from turpentine, the second major component of β-pinene, by thermal cracking. β-Laurene possesses good analgesic, anti-inflammatory, and antioxidant properties. According to Almarzooqi et al120 the administration of β-laurose in DSS-treated mice restored the colon length, decreased DAI and MPO enzyme activity, and inhibited pro-inflammatory mediators. Its mechanism of action involves the inhibition of MAPKs and NF-κB pathways to limit inflammation.
p-Cymene is a natural monoterpene present in citrus peel oil and cinnamon leaves; it is used as a flavoring agent in foods and has antioxidant and antimicrobial properties121. Formiga et al122 investigated p-Cymene for the treatment of TNBS-induced colitis in rats. It reduced MPO activity in the colon, displayed antioxidant properties, lowered MDA levels, and restored GSH levels. Additionally, p-Cymene reduced IL-1β and TNF-α in serum, restored IL-10 levels in colon tissues, and downregulated NF-κB transcription, thereby reducing intestinal inflammation (Table 2).
Monophenols
Carvacrol, also termed carvacrol, is a monoterpene phenol abundant in aromatic plants; it displays strong antibacterial, anti-inflammatory, and antioxidant pharmacological and health-promoting effects with promising applications.123 EOs of Origanum onites L. reduced colonic damage in 2,4,6-trinitrobenzene sulfonic acid-induced colitis, and carvacrol was identified as a major component.74 Souza et al124 investigated acetic acid- or other drug-induced UC mice, with oxidative damage as one of the key features. The use of carvacrol in UC treatment suppressed TNF-α and IL-1β levels and reduced oxidative damage through the increase of antioxidant enzymes in mice. These findings suggest the potential of carvacrol for UC treatment.
Muscimol, also termed thymol, has the pungent and herbaceous aroma of Thymus spp. It was first extracted from the EOs of muskgrass and Chenpi. Muscimol has antiseptic and bactericidal properties and is widely used in food and medicine. Medically, it is used as an antiseptic, fungicide, and liver function reagent.125 The therapeutic benefits of muscimol have been reported in acute and chronic gastric ulcers in rats.126 The drug prednisolone displays potent immunosuppressive properties for the treatment of UC; however, it leads to substantial adverse effects.127 Unlike prednisolone, muscimol is a safe and natural phenol with considerable immunomodulatory capacity as well as antimicrobial and antioxidant properties.128–130Tahmasebi et al131 treated acetic acid-induced UC rats using muscimol; the muscimol-treated group demonstrated strong antioxidant properties, compared with the positive drug group. Muscimolol reduced nitric oxide levels and malondialdehyde concentrations, inhibited the NF-κB pathway, and reduced IL-6, TNF-α, and IL-1β inflammatory factors.
Nerolidol is present in plants, such as orange leaves, roses, lavender, lemon, and grapefruit.132 Either as a constituent of plant EOs or alone, nerolidol inhibits the growth of microorganisms and is safe to use.133 González-Ramírez et al134 reported that the administration of nerolidol ameliorated the pathological features of colitis, prevented gastric damage, and exerted an immunomodulatory effect in the oxazolone model (Table 2).
Coumarins
Bergamottin, a member of the furanocoumarin family of plant secondary metabolites, inhibits the growth of colon cancer cells135 and blocks NF-κB signaling activation, exhibiting anti-inflammatory capacity.136 Adakudugu et al137 studied acetic acid-induced UC in rats; bergamottin promoted intestinal ulcer wound healing, reduced pro-inflammatory cytokine expression (TNF-α and IL-6), and decreased ROS expression, thereby reducing oxidative stress. Coumarins antagonize vitamin K, which in turn exhibits anticoagulant effects.138 Researchers have reported a close relationship between inflammation and coagulation.139 The anti-inflammatory effects of bergamottin in UC treatment may be attributed to its anticoagulant activity. Bergamottin is a potential alternative method for UC treatment in the future (Table 2).
 |
Table 2 Mechanism of Action of the Chief Active Components of Essential Oils in the Treatment of Ulcerative Colitis |
Summaries and Perspectives
The incidence of UC, classified as an inflammatory bowel disease, has been increasing progressively. Patients with UC endure considerable pain and urgently require medication with minimal adverse effects and high efficacy. EOs possess aromatic properties that enable rapid therapeutic effects and targeted delivery to the affected area. Additionally, EOs exert diverse pharmacological effects, including anti-inflammatory, anti-tumor, anti-oxidant, and anti-bacterial activities, thereby demonstrating substantial potential for advancement in UC treatment. This article provides a comprehensive overview of various EOs and their potential efficacy in alleviating and managing UC through anti-inflammatory, antioxidant, antidepressant, and anti-insomnia properties as well as their ability to restore the intestinal flora balance. Notably, terpenes have emerged as the primary active constituents in UC treatment. Despite the efficacy of EOs in UC management, certain issues about the fundamental research, quality stability, and standardization of management hinder their clinical application. The majority of compound investigations on EOs were confined to in vitro studies, with limited in vivo research. Researchers should conduct additional animal experiments to substantiate their efficacy while exploring drug formulations and clinical trials for validation. EOs consumed orally have been associated with varying degrees of adverse effects. Some EOs are safe at low concentrations, whereas some exhibit toxicity at high concentrations, manifesting as lethal doses.149 Adokoh et al150 investigated the oral toxicity of EOs extracts of Citrus aurantium by using the following methods: acute toxicity single-dose and repeated-dose studies. They observed necrosis, edema, and inflammation in the liver, spleen, and kidneys through histopathological studies. Low levels of hemoglobin and high levels of liver enzymes in the subchronic phase confirmed the mild toxicity of Melissa officinalis EOs. The oral administration of Melissa officinalis L. EOs resulted caused substantial changes in mice behavior and elucidated the biochemical parameters of hepatic and renal functions.151 When administered orally at >1 g/kg doses, different pathological changes were detected in the stomach, duodenum, liver, and kidneys. Chaihu EOs caused liver injury in rats at doses ranging from 0.19 mL/kg to 0.42 mL/kg. Additionally, the hepatocytes demonstrated irreversible changes, such as edema, steatosis, and eosinophilia.152 The oral administration of 300 mg/kg of Croton EOs in mice for 7 days resulted in renal tubular injury, proteinuria, collagen deposition, macrophage infiltration, and nephrotoxicity.153
First, researchers should achieve a comprehensive understanding of the toxic effects of EOs, elucidate the underlying toxic substances, toxic processes, and mechanisms of action, establish relevant quality standards, or utilize contemporary preparation techniques to effectively regulate the quality and stability of EOs. Furthermore, enhancing the management of EOs is crucial. From a clinical perspective, clinicians should judiciously select the appropriate route of administration, rigorously control the dosage or purity, and carefully consider the applicable population to mitigate the potential risks. Research on the safety of EOs is long-term and extensive and can provide a more reliable basis for the use of drugs and healthcare. Different EOs have varying degrees of toxicity, and the cross-dose between the efficacy dose and the toxicity dose has not yet been explored.
Second, most studies on pharmacological activity are based on EOs that have not been isolated and purified; the complex chemical composition leads to the selection of EOs for UC treatment. Nonetheless, their mechanism of action has not been elucidated. Terpenoids are the key EOs components and are effective against diseases, such as artemisinin, an anti-malarial sesquiterpene, and cyclic sesquiterpene chamomile lactone, with anti-Leishmania amazonensis activity against malaria. Chamomile lactone, the cyclic sesquiterpene, works against Amazonian Leishmania protozoa. Therefore, researchers should investigate the pharmacological effects of terpenoids in plant EOs, particularly the complex and diverse structure of sesquiterpenes. Additionally, they should explore whether the different types of carbon skeletons correspond to different pharmacological activities.154
The pharmacological effects of EOs on UC are focused on evaluating their antibacterial, antioxidant, and anti-inflammatory functions. Additionally, preliminary explorations of EOs compounding in anti-UC studies have provided ideas for the identification of novel pharmacological functions. Plants consist of numerous active EOs with characteristic components; however, their application is limited and has not been exploited. Therefore, researchers should explore the following four areas: (1) EOs and their individual compounds to determine the pharmacological effects and mechanism of action, analysis of the internal effective component groups, and individual components of the pharmacodynamic function; (2) varying synergistic effects of EOs, the efficacy of traditional Chinese medicine through histological research, the study of a single flavor, drug pairs or a combination of drug components, the content of changes and the mechanism of change, and the new pharmacological and pharmacodynamic effects of the drug; (3) because of the relative safety, reliability, and efficiency, EOs in the field of food application research, rational selection in the development of natural spices, natural antiseptic preservatives, the development of spices, and composite preservatives warrant research; (4) considering the differences in origin, parts, and extraction methods of different EOs, researchers should investigate the cultivation standards, medicinal standards, and safety of medicinal herbs, establish scientific evaluation methods, and guide their rational use. These steps will help them promote the development and research on EOs as well as their comprehensive application and industrialization.
In conclusion, this article presents a review of the existing EOs and their active ingredients, which will facilitate the treatment of UC and drug development.
Abbreviations
UC, Ulcerative colitis; EOs, Essential oils; IBD, inflammatory bowel disease; NF-κB, nuclear factor kappa-B; TNF-α, tumor necrosis factor; IL-6, Interleukin-6; IL-1β, Interleukin-1β; PPARγ, peroxisome proliferators-activated receptors; MPO, myeloperoxidase; SCFA, short-chain fatty acids; TLR4, Toll Like Receptor 4; NLRP3, Nucleotide- binding oligomerization domain, leucine- rich repeat and pyrin domain- containing 3; ROS, reactive oxygen species; NO, nitrous Oxide; DSS, dextran sulfate sodium salt; TNBS, trinitro-benzene-sulfonic acid; DAI, disease activity index; NOS, nitric oxide synthase; DNCB, 2, 4-dinitrochlorobenzene; 5-HT, 5-Hydroxytryptamine; MMP-9, Matrix metalloproteinase-9; MAPK, mitogen-activated protein kinase; MDA, malondialdehyde; SOD, super oxide dismutase; 8-OHdG, 8-hydroxydeoxyguanosine; GSH-Px, glutathione peroxidase.
Data Sharing Statement
No data was used for the research described in the article.
Acknowledgments
The authors gratefully acknowledge the financial supports by the National Natural Science Foundation of China (81703720, 82074026 and 82274105), the National Key Research and Development Program (2021YFD1601004), Project of Shaanxi Provincial Department of science and technology (2022JM-555), Key R & D program of Xianyang City (2021ZDYF-SF-0015), Shaanxi Administration of Traditional Chinese Medicine (ZYJXG-Y23005), Shaanxi Provincial Administration of Traditional Chinese Medicine “Qin Medicine” Development Key Research Project (2021-02-22-014), Key Research and Development Programme of Xianyang Municipality: Research on Ginger-Braised Zu Shi Ma Concoction Process and Quality Markers Based on Toxicity-Efficacy Integration(L2023-ZDYF-SF-019), Shaanxi University of Chinese Medicine School-level Projects(2021GP29), Research on standardised cultivation and standards of Astragalus (ZYBZH-C-QIN-45), Shaanxi Provincial University Youth Innovation Team of Aromatic Chinese Medicine Industrialization Key Technology, and Shaanxi Provincial University Engineering Research Center of Chinese Medicine Aromatic Industry.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Liu C, Yan X, Zhang Y, et al. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J Nanobiotechnology. 2022;20(1):206. doi:10.1186/s12951-022-01421-w
2. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54.e42. doi:10.1053/j.gastro.2011.10.001
3. Talley NJ, Abreu MT, Achkar JP, et al. An evidence-based systematic review on medical therapies for inflammatory bowel disease. Am J Gastroenterol. 2011;106:S2–25. doi:10.1038/ajg.2011.58
4. Si-Yu C, Sheng-Jie Y, Wei-Wei W, Bing W, Tong Z, Yi-Qiong P. Progress in active compounds effective on ulcerative colitis from Chinese medicines. Chinese J Nat Med. 2019;17(02):81–102. doi:10.1016/S1875-5364(19)30012-3
5. Zixing Y, Xiaobin Z, Lixing Z, Zhenwen L, Mui LY. Research progress of Chinese and western medicine in the treatment of ulcerative colitis. Pharmacognosy. 2019;31(02):260–262.
6. Santana MT, Cercato LM, Oliveira JP, Camargo EA. Medicinal Plants in the Treatment of Colitis: evidence from Preclinical Studies. Planta Med. 2017;83(7):588–614. doi:10.1055/s-0043-104933
7. Guo ZX. Aromatherapy - Health and Therapeutic Benefits of Essential Oils. Sci Chem Daily Use. 2005;1(6):44–48.
8. Shelef LA. Antimicrobial effects of spices1. J Food Saf. 1984;6(1):29–44. doi:10.1111/j.1745-4565.1984.tb00477.x
9. Ceylan E, Fung DYC. Antimicrobial activity of spices 1. J Rapid Methods Automation Microbiol. 2004;12(1):1–55. doi:10.1111/j.1745-4581.2004.tb00046.x
10. Burt S. Essential oils: their antibacterial properties and potential applications in foods--a review. Int J Food Microbiol. 2004;94(3):223–253. doi:10.1016/j.ijfoodmicro.2004.03.022
11. Ghasemi-Pirbaluti M, Motaghi E, Bozorgi H. The effect of menthol on acute experimental colitis in rats. Eur J Pharmacol. 2017;805:101–107. doi:10.1016/j.ejphar.2017.03.003
12. Adriana Estrella GR, María Eva GT, Alberto HL, et al. Limonene from Agastache mexicana essential oil produces antinociceptive effects, gastrointestinal protection and improves experimental ulcerative colitis. J Ethnopharmacol. 2021;280:114462. doi:10.1016/j.jep.2021.114462
13. Xiaolan L, Yongzhong Z, Junling Z, Xiaobo N, Lin J, Huili L. Ameliorative effect of volatile oil of Atractylodes macrocephala on ulcerative colitis in rats. Tianjin Pharmaceuticals. 2020;48(10):956–960.
14. Jin Z. The effect of volatile oil of Hainan salvia on experimental ulcerative colitis and its safety evaluation [bachelor’s degree]. 2009.
15. Khalili H, Chan SSM, Lochhead P, Ananthakrishnan AN, Hart AR, Chan AT. The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2018;15(9):525–535. doi:10.1038/s41575-018-0022-9
16. Manguso F, Sanges M, Staiano T, et al. Cigarette smoking and appendectomy are risk factors for extraintestinal manifestations in ulcerative colitis. Am J Gastroenterol. 2004;99(2):327–334. doi:10.1111/j.1572-0241.2004.04039.x
17. Takeuchi T. Factors Involved in the Degeneration of Lymphoid Tissue in the Appendix. Kurume Med J. 2020;65(4):123–127. doi:10.2739/kurumemedj.MS654006
18. Laurin M, Everett ML, Parker W. The cecal appendix: one more immune component with a function disturbed by post-industrial culture. Anat Rec (Hoboken). 2011;294(4):567–579. doi:10.1002/ar.21357
19. Guo XY, Liu XJ, Hao JY. Gut microbiota in ulcerative colitis: insights on pathogenesis and treatment. J Dig Dis. 2020;21(3):147–159. doi:10.1111/1751-2980.12849
20. Wu Y, Tang L, Wang B, Sun Q, Zhao P, Li W. The role of autophagy in maintaining intestinal mucosal barrier. J Cell Physiol. 2019;234(11):19406–19419. doi:10.1002/jcp.28722
21. Leslie JL, Vendrov KC, Jenior ML, Young VB. The Gut Microbiota Is Associated with Clearance of Clostridium difficile Infection Independent of Adaptive Immunity. mSphere. 2019;4(1). doi:10.1128/mSphereDirect.00698-18
22. Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146(6):1489–1499. doi:10.1053/j.gastro.2014.02.009
23. Lee JG, Han DS, Jo SV, et al. Characteristics and pathogenic role of adherent-invasive Escherichia coli in inflammatory bowel disease: potential impact on clinical outcomes. PLoS One. 2019;14(4):e0216165. doi:10.1371/journal.pone.0216165
24. Vieira-Silva S, Sabino J, Valles-Colomer M, et al. Quantitative microbiome profiling disentangles inflammation- and bile duct obstruction-associated microbiota alterations across PSC/IBD diagnoses. Nat Microbiol. 2019;4(11):1826–1831. doi:10.1038/s41564-019-0483-9
25. Cronkite DA, Strutt TM. The Regulation of Inflammation by Innate and Adaptive Lymphocytes. J Immunol Res. 2018;2018:1467538. doi:10.1155/2018/1467538
26. Lang T, Lee JPW, Elgass K, et al. Macrophage migration inhibitory factor is required for NLRP3 inflammasome activation. Nat Commun. 2018;9(1):2223. doi:10.1038/s41467-018-04581-2
27. Rivera A, Siracusa MC, Yap GS, Gause WC. Innate cell communication kick-starts pathogen-specific immunity. Nat Immunol. 2016;17(4):356–363. doi:10.1038/ni.3375
28. Curtius K, Kabir M, Al Bakir I, et al. Multicentre derivation and validation of a colitis-associated colorectal cancer risk prediction web tool. Gut. 2022;71(4):705–715. doi:10.1136/gutjnl-2020-323546
29. Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53 Suppl 5(Suppl 5):V1–16. doi:10.1136/gut.2004.043372
30. Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–s106. doi:10.1136/gutjnl-2019-318484
31. Zexing D, Hing LC, Keung LW. A short-term study of infliximab in the treatment of moderate-to-severe ulcerative colitis. Chine J Modern Med. 2013;23(26):79–82.
32. Cooke B, Ernst E. Aromatherapy: a systematic review. Br J Gen Pract. 2000;50(455):493–496.
33. Wang Y, Zou J, Jia Y, et al. The Mechanism of Lavender Essential Oil in the Treatment of Acute Colitis Based on ”Quantity-Effect Weight Coefficient Network Pharmacology. Front Pharmacol. 2021;12:644140. doi:10.3389/fphar.2021.644140
34. Zhou HL, Deng YM, Xie QM. The modulatory effects of the volatile oil of ginger on the cellular immune response in vitro and in vivo in mice. J Ethnopharmacol. 2006;105(1–2):301–305. doi:10.1016/j.jep.2005.10.022
35. Wang X, Zhou P, Shi H, et al. Cinnamon essential oil based on NLRP3 inflammasome and renal uric acid transporters for hyperuricemia. Food Bioscience. 2023;56:103285. doi:10.1016/j.fbio.2023.103285
36. Li T, Wang W, Guo Q, et al. Rosemary (Rosmarinus officinalis L.) hydrosol based on serotonergic synapse for insomnia. J Ethnopharmacol. 2024;318(Pt B):116984. doi:10.1016/j.jep.2023.116984
37. Wu Y, Zhang Y, Xie G, et al. The metabolic responses to aerial diffusion of essential oils. PLoS One. 2012;7(9):e44830. doi:10.1371/journal.pone.0044830
38. Li J et al. (2022). Study of the Mechanism of Antiemetic Effect of Lavandula angustifolia Mill. Essential Oil Based on Ca2+/CaMKII/ERK1/2 Pathway. Drug Des Devel Ther, 16 2407–2422. 10.2147/DDDT.S366597
39. Cui Y, Che Y, Wang H. Bergamot essential oil attenuate aluminum-induced anxiety-like behavior through antioxidation, anti-inflammatory and GABA regulation in rats. Food Chem Toxicol. 2020;145:111766. doi:10.1016/j.fct.2020.111766
40. Xia N et al. (2024). Deciphering the antidepressant effects of Rosa damascena essential oil mediated through the serotonergic synapse signaling pathway. Journal of Ethnopharmacology, 328 118007 10.1016/j.jep.2024.118007
41. Duan J et al. (2024). Therapeutic effects and mechanism of action of lavender essential oil on atopic dermatitis by modulating the STAT3/RORγt pathway. Arabian Journal of Chemistry, 17(2), 105525 10.1016/j.arabjc.2023.105525
42. Li J, Duan J, Wang Y, et al. The JAK/STAT/NF-κB signaling pathway can be regulated by rosemary essential oil, thereby providing a potential treatment for DNCB-induced in mice. Biomed Pharmacother. 2023;168:115727. doi:10.1016/j.biopha.2023.115727
43. Pires FQ, da Silva JKR, Sa-Barreto LL, Gratieri T, Gelfuso GM, Cunha-Filho M. Lipid nanoparticles as carriers of cyclodextrin inclusion complexes: a promising approach for cutaneous delivery of a volatile essential oil. Colloids Surf B Biointerfaces. 2019;182:110382. doi:10.1016/j.colsurfb.2019.110382
44. Rafii F, Ameri F, Haghani H, Ghobadi A. The effect of aromatherapy massage with lavender and chamomile oil on anxiety and sleep quality of patients with burns. Burns. 2020;46(1):164–171. doi:10.1016/j.burns.2019.02.017
45. Nasiri A, Mahmodi MA, Nobakht Z. Effect of aromatherapy massage with lavender essential oil on pain in patients with osteoarthritis of the knee: a randomized controlled clinical trial. Complementary Therapies in Clinical Practice. 2016;25:75–80. doi:10.1016/j.ctcp.2016.08.002
46. Huang MY, Liao MH, Wang YK, Huang YS, Wen HC. Effect of lavender essential oil on LPS-stimulated inflammation. Am J Chin Med. 2012;40(4):845–859. doi:10.1142/S0192415X12500632
47. Rahman H, Vakati K, Eswaraiah MC. In-Vivo and In-Vitro Anti-Inflammatory Activity of Aquilaria agallocha Oil. Int J Med. 2012.
48. Uemura T, Yashiro T, Oda R, et al. Intestinal Anti-Inflammatory Activity of Perillaldehyde. J Agric Food Chem. 2018;66(13):3443–3448. doi:10.1021/acs.jafc.8b00353
49. Christian F, Smith EL, Carmody RJ. The Regulation of NF-κB Subunits by Phosphorylation. Cells. 2016;5(1):12. doi:10.3390/cells5010012
50. Rashidian A, Roohi P, Mehrzadi S, Ghannadi AR, Minaiyan M. Protective Effect of Ocimum basilicum Essential Oil Against Acetic Acid-Induced Colitis in Rats. J Evid Based Complementary Altern Med. 2016;21(4):Np36–42. doi:10.1177/2156587215616550
51. Rezayat SM, Dehpour AR, Motamed SM, et al. Foeniculum vulgare essential oil ameliorates acetic acid-induced colitis in rats through the inhibition of NF-kB pathway. Inflammopharmacology. 2018;26(3):851–859. doi:10.1007/s10787-017-0409-1
52. Mengxue W, Qingqing J, Jiaqi H, Duanyong L, Xiaoying H, Haimei Z. Regulation of follicular helper T-cell subsets in mice with ulcerative colitis by volatile oil from Shishenwan. N Chine Medicines Clin Pharmacol. 2022;33(03):293–299.
53. Qiongge Z, Kai W, Zuowu X, Qingqing S, Erhao L. Exploring the mechanism of Chinese medicine against ulcerative colitis based on the intestinal microenvironment. Chine J Exp Formulary. 2023;29(07):222–229.
54. Oshitani N, Watanabe K, Nakamura S, Fujiwara Y, Higuchi K, Arakawa T. Dislocation of tight junction proteins without F-actin disruption in inactive Crohn’s disease. Int J Mol Med. 2005;15(3):407–410.
55. Elinav E, Strowig T, Kau AL, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145(5):745–757. doi:10.1016/j.cell.2011.04.022
56. Mukhopadhya I, Hansen R, El-Omar EM, Hold GL. IBD-what role do Proteobacteria play? Nat Rev Gastroenterol Hepatol. 2012;9(4):219–230. doi:10.1038/nrgastro.2012.14
57. Håkansson Å, Tormo-Badia N, Baridi A, et al. Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice. Clin Exp Med. 2015;15(1):107–120. doi:10.1007/s10238-013-0270-5
58. Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015;37(1):47–55. doi:10.1007/s00281-014-0454-4
59. Zhang Z, Shen P, Liu J, et al. In Vivo Study of the Efficacy of the Essential Oil of Zanthoxylum bungeanum Pericarp in Dextran Sulfate Sodium-Induced Murine Experimental Colitis. J Agric Food Chem. 2017;65(16):3311–3319. doi:10.1021/acs.jafc.7b01323
60. Li C, Zhang W, Wu X, et al. Aromatic-turmerone ameliorates DSS-induced ulcerative colitis via modulating gut microbiota in mice. Inflammopharmacology. 2022;30(4):1283–1294. doi:10.1007/s10787-022-01007-w
61. Krzystek-Korpacka M, Kempiński R, Bromke MA, Neubauer K. Oxidative Stress Markers in Inflammatory Bowel Diseases: systematic Review. Diagnostics. 2020;10(8).
62. Pereira C, Grácio D, Teixeira JP, Magro F. Oxidative Stress and DNA Damage: implications in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21(10):2403–2417. doi:10.1097/MIB.0000000000000506
63. Yi Z, Jin Z, Guobiao C, Chun L. Therapeutic effect of volatile oil of Hainan salvia on ulcerative colitis induced by 2,4-dinitrochlorobenzene and acetic acid in rats. Chine J Pharmacol Toxicol. 2009;23(05):388–394.
64. Puppala ER, Jain S, Saha P, et al. Perillyl alcohol attenuates rheumatoid arthritis via regulating TLR4/NF-κB and Keap1/Nrf2 signaling pathways: a comprehensive study onin-vitro and in-vivo experimental models. Phytomedicine. 2022;97:153926. doi:10.1016/j.phymed.2022.153926
65. Tomás CC, Oliveira E, Sousa D, et al. Proceedings of the 3rd IPLeiria’s International Health Congress: Leiria, Portugal. 6-7 May 2016. BMC Health Serv Res. 2016;16 Suppl 3(Suppl 3):200. doi:10.1186/s12913-016-1423-5
66. Li J, Wang X, Xun S, et al. Study of the Mechanism of Antiemetic Effect of Lavandula angustifolia Mill. Essential Oil Based on Ca(2+)/CaMKII/ERK1/2 Pathway. Drug Des Devel Ther. 2022;16:2407–2422. doi:10.2147/DDDT.S366597
67. Zhang Y, Long Y, Yu S, et al. Natural volatile oils derived from herbal medicines: a promising therapy way for treating depressive disorder. Pharmacol Res. 2021;164:105376. doi:10.1016/j.phrs.2020.105376
68. Du JP, Liu YT, Wu YJ, Wu HH, Xu YT. Fast-onset antidepressant potentials of essential oil of herbs. Zhongguo Zhong Yao Za Zhi. 2017;42(10):2006–2016. doi:10.19540/j.cnki.cjcmm.2017.0086
69. Sutton EL. Insomnia. Ann Intern Med. 2021;174(3):ITC33–ITC48. doi:10.7326/AITC202103160
70. Hao G, Zhu B, li Y, Wang P, Li L, Hou L. Sleep quality and disease activity in patients with inflammatory bowel disease: a systematic review and meta-analysis. Sleep Med. 2020;75:301–308. doi:10.1016/j.sleep.2020.08.032
71. Koo BS, Park KS, Ha JH, Park JH, Lim JC, Lee DU. Inhibitory effects of the fragrance inhalation of essential oil from Acorus gramineus on central nervous system. Biol Pharm Bull. 2003;26(7):978–982. doi:10.1248/bpb.26.978
72. Bastani M, Mousavi Z, Asgarpanah J, Assar N, Najafizadeh P. Biochemical and Histopathological Evidence for Beneficial Effects of Pelargonium graveolens Essential Oil on the Rat Model of Inflammatory Bowel Disease. Res J Pharm. 2019;6:77–84.
73. Mohamed ME, Elsayed SA, Madkor HR, Eldien HMS, Mohafez OM. Yarrow oil ameliorates ulcerative colitis in mice model via regulating the NF-κB and PPAR-γ pathways. Intest Res. 2021;19(2):194–205. doi:10.5217/ir.2020.00021
74. Dundar E, Olgun EG, Isiksoy S, Kurkcuoglu M, Baser KH, Bal C. The effects of intra-rectal and intra-peritoneal application of Origanum onites L. essential oil on 2,4,6-trinitrobenzenesulfonic acid-induced colitis in the rat. Exp Toxicol Pathol. 2008;59(6):399–408. doi:10.1016/j.etp.2007.11.009
75. Yanqin W, Xingyao L. Effects of volatile oil of Chenpi on serum TNF-α and T-cell subsets in rats with ulcerative colitis. Gansu Chine Med. 2007;1(6):72–73.
76. Rashidian A, Akbarzadeh D, Asgarpanah J, Dehpour A. Bunium persicum essential oil reduced acetic acid-induced rat colitis through suppression of NF-κB pathway. Avicenna J Phytomed. 2021;11(5):505–514. doi:10.22038/AJP.2021.18037
77. Rashidian A, Mehrzadi S, Ghannadi AR, Mahzooni P, Sadr S, Minaiyan M. Protective effect of ginger volatile oil against acetic acid-induced colitis in rats: a light microscopic evaluation. J Integr Med. 2014;12(2):115–120. doi:10.1016/S2095-4964(14)60011-X
78. Li AL, Ni WW, Zhang QM, et al. Effect of cinnamon essential oil on gut microbiota in the mouse model of dextran sodium sulfate-induced colitis. Microbiol Immunol. 2020;64(1):23–32. doi:10.1111/1348-0421.12749
79. Yu X, Yang G, Jiang H, et al. Patchouli oil ameliorates acute colitis: a targeted metabolite analysis of 2,4,6-trinitrobenzenesulfonic acid-induced rats. Exp Ther Med. 2017;14(2):1184–1192. doi:10.3892/etm.2017.4577
80. Murad HA, Abdallah HM, Ali SS. Mentha longifolia protects against acetic-acid induced colitis in rats. J Ethnopharmacol. 2016;190:354–361. doi:10.1016/j.jep.2016.06.016
81. Qian Z, Ruiyuan Z, Hongdan D, et al. Antioxidant activity of volatile oil contained in Chinese medicine Houpao before and after processing and sweating and its efficacy in the treatment of ulcerative colitis. Chin J Trad Chin Med. 2023;43(1):1–17. doi:10.19852/j.cnki.jtcm.2023.01.001
82. Ostovan M, Fazljou SMB, Khazraei H, Araj Khodaei M, Torbati M. The Anti-Inflammatory Effect of Pistacia Lentiscus in a Rat Model of Colitis. J Inflamm Res. 2020;13:369–376. doi:10.2147/JIR.S259035
83. He J, Zhang Y, Ouyang K, et al. Extraction, Chemical Composition, and Protective Effect of Essential Oil from Chimonanthus nitens Oliv. Leaves on Dextran Sodium Sulfate-Induced Colitis in Mice. Oxid Med Cell Longev. 2022;2022:9701938. doi:10.1155/2022/9701938
84. Mohammed MA, Ibrahim BMM, Abdel-Latif Y, et al. Pharmacological and metabolomic profiles of Musa acuminata wastes as a new potential source of anti-ulcerative colitis agents. Sci Rep. 2022;12(1):10595. doi:10.1038/s41598-022-14599-8
85. Balaha M, Kandeel S, Elwan W. Garlic oil inhibits dextran sodium sulfate-induced ulcerative colitis in rats. Life Sci. 2016;146:40–51. doi:10.1016/j.lfs.2016.01.012
86. Zheng X, Mai L, Wang T, et al. Brusatol-Enriched Brucea javanica Oil Ameliorated Dextran Sulfate Sodium-Induced Colitis in Mice: involvement of NF-κB and RhoA/ROCK Signaling Pathways. Biomed Res Int. 2021;2021:5561221. doi:10.1155/2021/5561221
87. Takashima T, Sakata Y, Iwakiri R, et al. Feeding with olive oil attenuates inflammation in dextran sulfate sodium-induced colitis in rat. J Nutr Biochem. 2014;25(2):186–192. doi:10.1016/j.jnutbio.2013.10.005
88. Miao F, Shan C, Ma T, Geng S, Ning D. Walnut oil alleviates DSS-induced colitis in mice by inhibiting NLRP3 inflammasome activation and regulating gut microbiota. Microb Pathog. 2021;154:104866. doi:10.1016/j.micpath.2021.104866
89. Wang J et al. (2024). Rosemary essential oil microemulsion prevents DSS-induced intestinal injury in mice by modulating IL-17 signaling pathway. Journal of Functional Foods, 116 106180 10.1016/j.jff.2024.106180
90. Ho YC, Lee SS, Yang ML, et al. Zerumbone reduced the inflammatory response of acute lung injury in endotoxin-treated mice via Akt-NFκB pathway. Chem Biol Interact. 2017;271:9–14. doi:10.1016/j.cbi.2017.04.017
91. Ji J, Xiang P, Li T, et al. NOSH-NBP, a Novel Nitric Oxide and Hydrogen Sulfide- Releasing Hybrid, Attenuates Ischemic Stroke-Induced Neuroinflammatory Injury by Modulating Microglia Polarization. Front Cell Neurosci. 2017;11:154. doi:10.3389/fncel.2017.00154
92. Rashidian A, Akbarzadeh D, Asgarpanah J, Dehpour A. Bunium persicum essential oil reduced acetic acid-induced rat colitis through suppression of NF-κB pathway. Avicenna J Phytomed. 2021;11(5):505.
93. Zheng X, Mai L, Wang T, Xu Y, Su Z, Chen J, Zeng H, Xie Y. (2021). Brusatol-Enriched Brucea javanica Oil Ameliorated Dextran Sulfate Sodium-Induced Colitis in Mice: Involvement of NF-κB and RhoA/ROCK Signaling Pathways. Biomed Res Int, 2021 5561221 10.1155/2021/5561221
94. Chamanara M, Rashidian A, Mehr SE, et al. Melatonin ameliorates TNBS-induced colitis in rats through the melatonin receptors: involvement of TLR4/MyD88/NF-κB signalling pathway. Inflammopharmacology. 2019;27(2):361–371. doi:10.1007/s10787-018-0523-8
95. Li R, Guo Y, Zhang Y, Zhang X, Zhu L, Yan T. Salidroside Ameliorates Renal Interstitial Fibrosis by Inhibiting the TLR4/NF-κB and MAPK Signaling Pathways. Int J Mol Sci. 2019;20(5).
96. Frosali S, Pagliari D, Gambassi G, Landolfi R, Pandolfi F, Cianci R. How the Intricate Interaction among Toll-Like Receptors, Microbiota, and Intestinal Immunity Can Influence Gastrointestinal Pathology. J Immunol Res. 2015;2015:489821. doi:10.1155/2015/489821
97. Hu Y, Liu JP, Zhu Y, Lu NH. The Importance of Toll-like Receptors in NF-κB Signaling Pathway Activation by Helicobacter pylori Infection and the Regulators of this Response. Helicobacter. 2016;21(5):428–440. doi:10.1111/hel.12292
98. Akasaka E, Takekoshi S, Horikoshi Y, et al. Protein oxidative damage and heme oxygenase in sunlight-exposed human skin: roles of MAPK responses to oxidative stress. Tokai J Exp Clin Med. 2010;35(4):152–164.
99. Ting Z. Study on the protective effect and mechanism of the volatile oil of Saxifrage on drug-induced intestinal mucositis [bachelor’s degree]. 2017.
100. Soubh AA, Abdallah DM, El-Abhar HS. Geraniol ameliorates TNBS-induced colitis: involvement of Wnt/β-catenin, p38MAPK, NFκB, and PPARγ signaling pathways. Life Sci. 2015;136:142–150. doi:10.1016/j.lfs.2015.07.004
101. Wang Y, Wang X, Tang T, et al. Basis with RNA-Seq and WGCNA to explore the effect of Frankincense essential oil on dextran sodium sulfate-induced ulcerative colitis through MAPK/NF-κB signaling. Fitoterapia. 2023;172:105744. doi:10.1016/j.fitote.2023.105744
102. Barbosa HM, Albino AM, Cavalcante FSA, Lima RA. ABORDAGEM FITOQUÍMICA DE METABÓLITOS SECUNDÁRIOS EM Solanum acanthodes (SOLANACEAE) HOOK. South Am J Basic Educ. 2017;4.
103. Medeiros R, Passos GF, Vitor CE, et al. Effect of two active compounds obtained from the essential oil of Cordia verbenacea on the acute inflammatory responses elicited by LPS in the rat paw. Br J Pharmacol. 2007;151(5):618–627. doi:10.1038/sj.bjp.0707270
104. Sabulal B, Dan M, Aj J, et al. Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: chemical characterization and antimicrobial activity. Phytochemistry. 2006;67(22):2469–2473. doi:10.1016/j.phytochem.2006.08.003
105. Galdino PM, Nascimento MV, Florentino IF, et al. The anxiolytic-like effect of an essential oil derived from Spiranthera odoratissima A. St. Hil. leaves and its major component, β-caryophyllene, in male mice. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38(2):276–284. doi:10.1016/j.pnpbp.2012.04.012
106. Legault J, Pichette A. Potentiating effect of beta-caryophyllene on anticancer activity of alpha-humulene, isocaryophyllene and paclitaxel. J Pharm Pharmacol. 2007;59(12):1643–1647. doi:10.1211/jpp.59.12.0005
107. Calleja MA, Vieites JM, Montero-Meléndez T, et al. The antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br J Nutr. 2013;109(3):394–401. doi:10.1017/S0007114512001298
108. Xu M, Tao J, Yang Y, et al. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death Dis. 2020;11(2):86. doi:10.1038/s41419-020-2299-1
109. Wu YT, Zhong LS, Huang C, et al. β-Caryophyllene Acts as a Ferroptosis Inhibitor to Ameliorate Experimental Colitis. Int J Mol Sci. 2022;23(24):16055. doi:10.3390/ijms232416055
110. Gerhäuser C, Klimo K, Heiss E, et al. Mechanism-based in vitro screening of potential cancer chemopreventive agents. Mutat Res. 2003;523-524:163–172. doi:10.1016/S0027-5107(02)00332-9
111. Yuri T, Danbara N, Tsujita-Kyutoku M, et al. Perillyl alcohol inhibits human breast cancer cell growth in vitro and in vivo. Breast Cancer Res Treat. 2004;84(3):251–260. doi:10.1023/B:BREA.0000019966.97011.4d
112. Jianfeng R, Qisheng Z, Hui W. Effect of perillyl alcohol on apoptosis of colon cancer SW480 cells and its mechanism study. Oncol Pharmacol. 2019;9(03):395–399.
113. Puppala ER, Aochenlar SL, Shantanu PA, et al. Perillyl alcohol attenuates chronic restraint stress aggravated dextran sulfate sodium-induced ulcerative colitis by modulating TLR4/NF-κB and JAK2/STAT3 signaling pathways. Phytomedicine. 2022;106:154415. doi:10.1016/j.phymed.2022.154415
114. Kikusato M, Muroi H, Uwabe Y, Furukawa K, Toyomizu M. Oleuropein induces mitochondrial biogenesis and decreases reactive oxygen species generation in cultured avian muscle cells, possibly via an up-regulation of peroxisome proliferator-activated receptor γ coactivator-1α. Anim Sci J. 2016;87(11):1371–1378. doi:10.1111/asj.12559
115. Giner E, Andújar I, Recio MC, Ríos JL, Cerdá-Nicolás JM, Giner RM. Oleuropein ameliorates acute colitis in mice. J Agric Food Chem. 2011;59(24):12882–12892. doi:10.1021/jf203715m
116. Lima PR, de Melo TS, Carvalho KM, et al. 1,8-cineole (eucalyptol) ameliorates cerulein-induced acute pancreatitis via modulation of cytokines, oxidative stress and NF-κB activity in mice. Life Sci. 2013;92(24–26):1195–1201. doi:10.1016/j.lfs.2013.05.009
117. Rodenak Kladniew B, Polo M, Montero Villegas S, Galle M, Crespo R, García de Bravo M. Synergistic antiproliferative and anticholesterogenic effects of linalool, 1,8-cineole, and simvastatin on human cell lines. Chem Biol Interact. 2014;214:57–68. doi:10.1016/j.cbi.2014.02.013
118. Kennedy-Feitosa E, Okuro RT, Pinho Ribeiro V, et al. Eucalyptol attenuates cigarette smoke-induced acute lung inflammation and oxidative stress in the mouse. Pulm Pharmacol Ther. 2016;41:11–18. doi:10.1016/j.pupt.2016.09.004
119. Santos FA, Silva RM, Campos AR, De Araújo RP, Lima Júnior RC, Rao VS. 1,8-cineole (eucalyptol), a monoterpene oxide attenuates the colonic damage in rats on acute TNBS-colitis. Food Chem Toxicol. 2004;42(4):579–584. doi:10.1016/j.fct.2003.11.001
120. Almarzooqi S, Venkataraman B, Raj V, et al. β-Myrcene Mitigates Colon Inflammation by Inhibiting MAP Kinase and NF-κB Signaling Pathways. Molecules. 2022;27(24):8744. doi:10.3390/molecules27248744
121. Cao XL, Sparling M, Dabeka R. p-Cymene, a natural antioxidant, in Canadian total diet foods: occurrence and dietary exposures. J Sci Food Agric. 2019;99(12):5606–5609. doi:10.1002/jsfa.9854
122. Formiga RO, Alves Júnior EB, Vasconcelos RC, et al. p-Cymene and Rosmarinic Acid Ameliorate TNBS-Induced Intestinal Inflammation Upkeeping ZO-1 and MUC-2: role of Antioxidant System and Immunomodulation. Int J Mol Sci. 2020;21(16). doi:10.3390/ijms21165870
123. Sharifi-Rad M, Berkay Yılmaz Y, Antika G, et al. Phytochemical constituents, biological activities, and health-promoting effects of the genus Origanum. Phytother Res. 2021;35(1):95–121. doi:10.1002/ptr.6785
124. de Santana Souza MT, Teixeira DF, de Oliveira JP, et al. Protective effect of carvacrol on acetic acid-induced colitis. Biomed Pharmacother. 2017;96:313–319. doi:10.1016/j.biopha.2017.10.017
125. Lee K-W. Essential oils in broiler nutrition. Int J Poultry Sci. 2004;3(12):738–752. doi:10.3923/ijps.2004.738.752
126. Ribeiro AR, Diniz PB, Pinheiro MS, Albuquerque-Júnior RL, Thomazzi SM. Gastroprotective effects of thymol on acute and chronic ulcers in rats: the role of prostaglandins, ATP-sensitive K(+) channels, and gastric mucus secretion. Chem Biol Interact. 2016;244:121–128. doi:10.1016/j.cbi.2015.12.004
127. Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270(5234):286–290. doi:10.1126/science.270.5234.286
128. Gholijani N, Amirghofran Z. Effects of thymol and carvacrol on T-helper cell subset cytokines and their main transcription factors in ovalbumin-immunized mice. J Immunotoxicol. 2016;13(5):729–737. doi:10.3109/1547691X.2016.1173134
129. Marchese A, Orhan IE, Daglia M, et al. Antibacterial and antifungal activities of thymol: a brief review of the literature. Food Chem. 2016;210:402–414. doi:10.1016/j.foodchem.2016.04.111
130. Wang HF, Yih KH, Yang CH, Huang KF. Anti-oxidant activity and major chemical component analyses of twenty-six commercially available essential oils. J Food Drug Anal. 2017;25(4):881–889. doi:10.1016/j.jfda.2017.05.007
131. Tahmasebi P, Abtahi Froushani SM, Afzale Ahangaran N. Thymol has beneficial effects on the experimental model of ulcerative colitis. Avicenna J Phytomed. 2019;9(6):538–550. doi:10.22038/AJP.2019.13383
132. Tisserand R, Young R. 2 - Essential oil composition. In: Tisserand R, Young R, editors. Essential Oil Safety.
133. Yuting X, Zhaobin C, Wenjie C. Observations on the bactericidal effect of nerolidol. Chine J Sterilisation. 2020;37(05):339–341.
134. Adriana Estrella G, María Eva G, Alberto H, María Guadalupe V, Azucena C, Sandra O, Noé A, Francisco Javier L. (2021). Limonene from Agastache mexicana essential oil produces antinociceptive effects, gastrointestinal protection and improves experimental ulcerative colitis. J Ethnopharmacol, 280 114462 10.1016/j.jep.2021.114462
135. Zhang H, Yang S, Lin T. Bergamottin exerts anticancer effects on human colon cancer cells via induction of apoptosis, G2/M cell cycle arrest and deactivation of the Ras/Raf/ERK signalling pathway. Arch Med Sci. 2022;18(6):1572–1581. doi:10.5114/aoms.2019.86226
136. Ko JH, Nam D, Um JY, Jung SH, Sethi G, Ahn KS. Bergamottin Suppresses Metastasis of Lung Cancer Cells through Abrogation of Diverse Oncogenic Signaling Cascades and Epithelial-to-Mesenchymal Transition. Molecules. 2018;23(7). doi:10.3390/molecules23071601
137. Adakudugu EA, Ameyaw EO, Obese E, et al. Protective effect of bergapten in acetic acid-induced colitis in rats. Heliyon. 2020;6(8):e04710. doi:10.1016/j.heliyon.2020.e04710
138. Wu S, Liu S, Davis CH, Stafford DW, Kulman JD, Pedersen LG. A hetero-dimer model for concerted action of vitamin K carboxylase and vitamin K reductase in vitamin K cycle. J Theor Biol. 2011;279(1):143–149. doi:10.1016/j.jtbi.2011.03.030
139. Esmon CT. Reprint of Crosstalk between inflammation and thrombosis. Maturitas. 2008;61(1):122–131. doi:10.1016/j.maturitas.2008.11.008
140. Shin YK, Kwon S, Hsieh YS, Han AY, Seol GH. Linalyl acetate restores colon contractility and blood pressure in repeatedly stressed-ulcerative colitis rats. Environ Health Prev Med. 2022;27(1):27. doi:10.1265/ehpm.22-00041
141. González-Ramírez AE, González-Trujano ME, Orozco-Suárez SA, Alvarado-Vásquez N, López-Muñoz FJ. Nerol alleviates pathologic markers in the oxazolone-induced colitis model. Eur J Pharmacol. 2016;776:81–89. doi:10.1016/j.ejphar.2016.02.036
142. Juhás S, Cikos S, Czikková S, et al. Effects of borneol and thymoquinone on TNBS-induced colitis in mice. Folia Biol (Praha). 2008;54(1):1–7.
143. Qu C, Yuan ZW, Yu XT, et al. Patchouli alcohol ameliorates dextran sodium sulfate-induced experimental colitis and suppresses tryptophan catabolism. Pharmacol Res. 2017;121:70–82. doi:10.1016/j.phrs.2017.04.017
144. Yeom JE, Kim S-K, Park S-Y. Regulation of the Gut Microbiota and Inflammation by β-Caryophyllene Extracted from Cloves in a Dextran Sulfate Sodium-Induced Colitis Mouse Model. Molecules. 2022;27(22):7782. doi:10.3390/molecules27227782
145. Toden S, Theiss AL, Wang X, Goel A. Essential turmeric oils enhance anti-inflammatory efficacy of curcumin in dextran sulfate sodium-induced colitis. Sci Rep. 2017;7(1):814. doi:10.1038/s41598-017-00812-6
146. Kim Y-R, Han A-R, Kim J-B, Cao S, Jin CH. Isoegomaketone From Perilla frutescens Ameliorates Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice. Nat Prod Commun. 2022;17(6):1934578X221105694. doi:10.1177/1934578X221105694
147. Li L, He Y, Wang N, et al. Atractylone in the Atractylodes macrocephala Rhizoma Essential Oil and Its Anti-Inflammatory Activity. Molecules. 2023;28(21):657.
148. Bento AF, Marcon R, Dutra RC, et al. β-Caryophyllene inhibits dextran sulfate sodium-induced colitis in mice through CB2 receptor activation and PPARγ pathway. Am J Pathol. 2011;178(3):1153–1166. doi:10.1016/j.ajpath.2010.11.052
149. Sinha S, Jothiramajayam M, Ghosh M, Mukherjee A. Evaluation of toxicity of essential oils palmarosa, citronella, lemongrass and vetiver in human lymphocytes. Food Chem Toxicol. 2014;68:71–77. doi:10.1016/j.fct.2014.02.036
150. Adokoh CK, Asante DB, Acheampong DO, et al. Chemical profile and in vivo toxicity evaluation of unripe Citrus aurantifolia essential oil. Toxicol Rep. 2019;6:692–702. doi:10.1016/j.toxrep.2019.06.020
151. Stojanović NM, Randjelović PJ, Mladenović MZ, et al. Toxic essential oils, part VI: acute oral toxicity of lemon balm (Melissa officinalis L.) essential oil in BALB/c mice. Food Chem Toxicol. 2019;133:110794. doi:10.1016/j.fct.2019.110794
152. Hibiscus S, Qian Y. Hepatotoxicity of Chai Hu volatile oil in rats: a study of the ”dosage-temporal-toxicity” relationship. Pharmacol Clin Chine Med. 2011;27(03):49–51.
153. Silva KF, Peruchetti DB, Sirtoli GM, et al. High Doses of Essential Oil of Croton Zehntneri Induces Renal Tubular Damage. Plants (Basel). 2021;10(7). doi:10.3390/plants10071400
154. Ying HT, Weiyi X, Cheng L, Zhengrong Z. Progress on the composition and pharmacological activity of the essential oils of Platycodon grandiflorus L. Nat Products Res Dev. 2023;35(09):1624–1636.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
