Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Value of Lung Ultrasound Sonography B-Lines Quantification as a Marker of Heart Failure in COPD Exacerbation
Authors Lajili F , Toumia M, Sekma A, Bel Haj Ali K , Sassi S, Zorgati A, Yaakoubi H, Youssef R, Grissa MH, Beltaief K , Mezgar Z, Khrouf M, Chamtouri I, Bouida W, Boubaker H, Msolli MA, Dridi Z, Boukef R , Nouira S
Received 13 December 2023
Accepted for publication 7 July 2024
Published 1 August 2024 Volume 2024:19 Pages 1767—1774
DOI https://doi.org/10.2147/COPD.S447819
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Fadwa Lajili,1,2 Marwa Toumia,1,2 Adel Sekma,1,2 Khaoula Bel Haj Ali,1,2 Sarra Sassi,1,2 Asma Zorgati,3 Hajer Yaakoubi,3 Rym Youssef,3 Mohamed Habib Grissa,1,2 Kaouther Beltaief,1,2 Zied Mezgar,4 Mariem Khrouf,4 Ikram Chamtouri,5 Wahid Bouida,1,2 Hamdi Boubaker,1,2 Mohamed Amine Msolli,1,2 Zohra Dridi,6 Riadh Boukef,1,3 Semir Nouira1,2
1Research Laboratory LR12SP18, Monastir University, Monastir, 5019, Tunisia; 2Emergency Department, Fattouma Bourguiba University Hospital, Monastir, 5000, Tunisia; 3Emergency Department, Sahloul University Hospital, Sousse, 4011, Tunisia; 4Emergency Department, Farhat Hached University Hospital, Sousse, 4031, Tunisia; 5Department of Cardiology B, Fattouma Bourguiba University Hospital, Monastir, 5000, Tunisia; 6Department of Cardiology A, Fattouma Bourguiba University Hospital, Monastir, 5000, Tunisia
Correspondence: Semir Nouira, Emergency Department and Laboratory Research(LR12SP18), Fattouma Bourguiba University Hospital, Monastir, 5000, Tunisia, Tel +21673106046, Email [email protected]
Introduction: Identifying heart failure (HF) in acute exacerbation of chronic obstructive pulmonary disease (AECOPD) can be challenging. Lung ultrasound sonography (LUS) B-lines quantification has recently gained a large place in the diagnosis of HF, but its diagnostic performance in AECOPD remains poorly studied.
Purpose: This study aimed to assess the contribution of LUS B-lines score (LUS score) in the diagnosis of HF in AECOPD patients.
Patients and methods: This is a prospective cross-sectional multicenter cohort study including patients admitted to the emergency department for AECOPD. All included patients underwent LUS. A lung ultrasound score (LUS score) based on B-lines calculation was assessed. A cardiac origin of dyspnea was retained for a LUS score greater than 15. HF diagnosis was based on clinical examination, pro-brain natriuretic peptide levels, and echocardiographic findings. The LUS score diagnostic performance was assessed by receiver operating characteristic (ROC) curve, sensitivity, specificity, and likelihood ratio at the best cutoffs.
Results: We included 380 patients, mean age was 68± 11.6 years, sex ratio (M/F) 1.96. Patients were divided into two groups: the HF group [n=157 (41.4%)] and the non-HF group [n=223 (58.6%)]. Mean LUS score was higher in the HF group (26.8± 8.4 vs 15.3± 7.1; p< 0.001). The mean LUS score in the HF patients with reduced LVEF was 29.2± 8.7, and was 24.5± 7.6 in the HF patients with preserved LVEF. LUS score area under ROC curve for the diagnosis of HF was 0.71 [0.65– 0.76]. The best sensitivity (89% [85.9– 92,1]) was observed at the threshold of 5; the best specificity (85% [81.4– 88.6]) was observed at the threshold of 30. Correlation between LUS score and E/E’ ratio was good (R=0.46, p=0.0001).
Conclusion: Our results suggest that LUS score could be helpful and should be considered in the diagnostic approach of HF in AECOPD patients, at least as a ruling in test.
Keywords: chronic obstructive pulmonary disease, COPD, heart failure, dyspnea, lung ultrasound sonography
Introduction
Chronic obstructive pulmonary disease (COPD) represents a serious public health problem due to its frequency and severity. The evolution of COPD is marked by exacerbations that worsen the vital prognosis and accelerate the evolution to irreversible respiratory failure. COPD exacerbation factors are multiple, dominated by respiratory infections. The contribution of heart failure (HF) to acute exacerbations of COPD (AECOPD), presenting to the emergency department (ED), is not well established but seems to be substantial; its prevalence is estimated to be in the range of 20–30%.1–4 Identifying cardiac origin in AECOPD is challenging and necessary for appropriate management of these patients. The coexistence of several comorbidities and pathologies causing dyspnea makes etiological diagnosis more difficult. Conventional complementary tests such as chest X-ray and brain natriuretic peptide (BNP) lack specificity and/or sensitivity5 while cardiac ultrasound requires training and not always being available in the ED. Lung ultrasound (LUS) B-lines quantification is becoming an increasingly used tool in emergency medicine practice.6–9 LUS, an easily feasible and non-invasive tool performed by clinicians at the bedside with portable devices, might enhance the diagnosis accuracy and contribute to make rapidly the decision. However, its value in AECOPD patients has not been well evaluated. Indeed, in COPD patients, due to the obstructive syndrome and air trapping, the thorax becomes distended and hyperinflated making LUS more difficult to perform which could decrease the diagnostic yield of the examination. Even in the presence of interstitial syndrome, the number of B-lines in COPD patients would be underestimated as intrathoracic air content increases and lung density decreases.10
The objective of our study is to verify the validity of LUS B-lines quantification in the diagnosis of acute HF in COPD patients presenting to the emergency room with acute dyspnea.
Methods
Study Design and Setting
This is a prospective study carried out in the ED of three academic hospitals in Tunisia (Fattouma Bourguiba University Hospital Monastir, Sahloul University Hospital Sousse, and Farhat Hached University Hospital Sousse) from March 2022 to May 2023.
Study Population
We included patients aged 18 years and over consulting the ED for an acute exacerbation of COPD. Exacerbation is defined as an acute event characterized by worsening of usual respiratory symptoms, requiring modification of treatment.11
We excluded patients hemodynamically unstable (presence of peripheral signs of shock, use of vasoactive drugs), respiratorily unstable (respiratory distress, use of mechanical ventilation), and/or with altered consciousness (a Glasgow Coma Score (GCS) ≤13). Similarly, we excluded exacerbations of traumatic origin, and patients not consenting to the protocol. The study protocol was prepared in accordance with the revised Helsinki Declaration for Biomedical Research Involving Human Subjects and Guidelines for Good Clinical Practice. Also, the study protocol was approved by the Ethics committee of Monastir Medical Faculty and is registered at ClinicalTrials.gov (NCT05352490). For all included patients an informed consent was obtained.
Data Collection
After the consent of each patient included in the study, data from the clinical examination and complementary examinations were collected. Systematic collection of the following clinical data was performed including age, sex, body mass index (BMI), cardiovascular risk factors such as hypertension, diabetes, dyslipidemia, smoking, heart failure (HF), and baseline New York Heart Association (NYHA) dyspnea stage (Table 1). For all included patients, we also collected data from physical examination, ECG, standard biological tests, BNP level, and cardiac ultrasound data. Cardiac ultrasound was performed about 4 hours after admission. Quantitative echocardiographic measurements were based mainly on the measurement of LV ejection fraction (LVEF) and the ratio of the peak early mitral inflow velocity (E) over the early diastolic mitral annular velocity (E′). The E/E′ ratio was calculated as E wave divided by E′ velocities. Peak early diastolic tissue velocity (E′) was measured at the septal and lateral mitral annulus. Mitral inflow velocity was assessed by pulsed wave Doppler from the apical 4-chamber view, positioning the sample volume at the tip of the mitral leaflets. Deceleration time of the E wave was measured as the interval from the peak of the E wave to its extrapolation to the baseline. Flow through the mitral valve with increased velocity associated with slow distension of the ventricle during rapid filling induces an increase in the E/E’ ratio. This indicates an increase in filling pressure. An E/E’ wave ratio > 15 on mitral tissue Doppler indicates heart failure with preserved ejection fraction (diastolic heart failure). To perform the lung ultrasound, two trained senior emergency physicians performed B-lines quantification on a patient in the supine position using a 5-MHz convex probe device (Sonosite Inc., Bothell, WA, USA). Evaluations took 2 to 3 minutes for each technique, with the patient lying supine if tolerated or in a semi-recumbent position if needed, and was well tolerated. For each side of the chest, 4 zones have to be assessed; 2 anterior and 2 lateral. The operator was asked to calculate the LUS score which is the sum of the B-lines found in both sides (8 zones).12 B-line was defined as a vertical bright echogenic bundle with a narrow basis, spreading from the transducer to the deepest part of the screen. The B-lines score is suggestive of CHF when it is ≥15. The final leading diagnosis of dyspnea was assessed by two independent EM senior physicians after reviewing the entire medical record of each patient, based on clinical presentation, physical exam findings, and diagnostic tests’ results including chest X-ray, echocardiography, and brain natriuretic peptide. In the case of a disagreement, a third EM senior physician was consulted and given the responsibility of making a conclusive assessment. Informed consent was obtained from all the patients before the start of the protocol.
 |
Table 1 Baseline Patients’ Characteristics |
Statistical Analysis
Continuous normally distributed variables were presented as mean±SD and compared using the Student’s t-test. Normality was assessed using the Shapiro–Wilk test and visual inspection of quantile–quantile plots. Non-normally distributed data were presented as median and interquartile range (IQR) and compared using the Wilcoxon rank sum test. Categorical data were compared between groups using the χ2 test, or Fisher’s exact test. We used sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and air under receiver operating characteristic (ROC) curve to assess the discriminative value of LUS score in the diagnosis of HF. Correlation between change in LUS score and E/E’ wave ratio was examined using Spearman’s rank correlation coefficient. The difference was considered statistically significant for a value of p≤0.05. All data were entered, recorded, and analyzed by IBM SPSS-Statistics version 21.0 computer software.
Results
During the study period, we included 380 patients admitted to the emergency room for AECOPD. The patients were divided into two groups according to the final diagnosis of HF: heart failure group (HF), n=157 (41.4%); and non-heart failure group (non-HF), n=223 (58.6%). Baseline patients’ characteristics are summarized in Table 1. The mean age of our population was 68±11.6 years. It was significantly higher in the HF group (70±11 years) compared with the non-HF group (66.5±12.5) (p=0.04). The most frequent comorbidities were hypertension (44.5%), diabetes (35%), and chronic heart failure (17.9%). Patients in the HF group had more comorbidities with a higher frequency of hypertension, chronic heart failure, coronary artery disease, diabetes, and renal failure; the difference was statistically significant. The mean of the LVEF was 47.2±14.3% in HF patients and 58.6±12.5% in non-HF patients (p<0.001). LVEF was preserved (>45%) in 56 patients (35.6%) in the HF group. Mean LUS score was significantly higher in the HF group (26.8±8.4) compared to the non-HF group (15.3±7.1) (p<0.001). The mean LUS score in the HF subgroup with reduced LVEF (LVrEF) was 29.2±8.7, and was 24.5±7.6 in the HF subgroup with preserved LVEF (LVpEF); the difference was not statistically significant (p=0.23). More than half of patients (52.1%) had LUS score >15. The distribution of patients according to LUS score is shown in Figure 1. The area under the ROC curve for the diagnosis of HF was 0.71 [0.65–0.76]. (Figure 2). Table 2 shows the diagnostic performance of LUS score using different thresholds. For a threshold of 15 which appears to be associated with the best performance, the sensitivity and specificity of LUS score were 73% [68.5–77.5] and 62% [57.1–66.9] respectively; the positive predictive value was 58% [53–63] and the negative predictive value was 75% [70.6–79.4]. The best sensitivity 89% [85.9–92.1] was observed at the threshold of 5; the best specificity 85% [81.4–88.6] was observed at the threshold of 30. Correlation between the LUS score and E/E’ ratio was good (R=0.46, p=0.0001) (Figure 3).
 |
Table 2 Performance of the LUS Score in the Diagnosis of Heart Failure Using Different Thresholds |
 |
Figure 1 Distribution of patients according to lung ultrasound score intervals. Patients without heart failure (non-HF group) and patients with heart failure (HF group). |
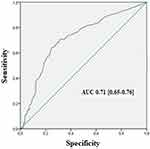 |
Figure 2 Receiver operating characteristic curve of lung ultrasound score in the diagnosis of heart failure in acute exacerbation of COPD. Note: Area under curve=0.71 [0.65–0.76]. |
 |
Figure 3 Correlation curve between lung ultrasound score and the ratio of the peak early mitral inflow velocity (E) over the early diastolic mitral annular velocity (E′). (R=0.46, p=0.0001). |
Discussion
Our study showed that discriminatory power of the LUS score in the diagnosis of HF in AECOPD is acceptable. At a cutoff of 5, LUS score had a good sensitivity; and at a cutoff of 30, LUS score had a good specificity. More precisely, a LUS score below 5 can help to exclude HF while patients with LUS score over 30 are more likely to have HF. LUS score values were not different between HF patients with LVrEF and those with LVpEF; they were correlated with the E/E’ ratio which is considered a surrogate parameter of left ventricular filling pressure.13
Heart failure (HF) is frequently associated with COPD as both conditions share the same cardiovascular risk factors. Many factors have been discussed to explain the frequent association of COPD and HF. It is essentially based on the concept of the propagation of pulmonary inflammation to the systemic circulation. COPD patients have low-grade systemic inflammation that promotes systemic atherosclerosis and coagulation contributing to the development of ischemic heart disease.14 A recent study of 450 COPD patients explored by cardiac magnetic resonance (CMR) demonstrated the existence of myocardial fibrosis caused by myocardial inflammation which was more severe when CMR is performed closer to the onset of the exacerbation.15 The association of AECOPD and heart failure represents a diagnostic challenge. The best strategy to detect HF in a COPD exacerbation has not yet been determined. In fact, the standard etiological approach, often based on clinical, chest radiography and biology, although very informative, is accompanied by diagnostic errors that limit its application in emergency practice. This leads to a sometimes excessive delay of urgent treatment and worsens the prognosis.16–18 Making rapidly the distinction between patients with and without HF with noninvasive testing is an important goal for emergency physicians. While the sensitivity of BNP in the diagnosis of HF is generally good, its specificity is reduced in many clinical situations. In particular, BNP levels over 500 pg/mL can be observed in cases of right ventricular dilatation.19–22 Tung et al, showed that in COPD patients with a history of HF, the specificity of BNP is only 47%.23 Recently, it was shown that dynamic CT scan can accurately delineate cardiac pathologies (coronary artery disease and heart failure with reduced ejection fraction) in AECOPD patients;24 however, CT scan could hardly been performed systematically with regard to the risk of irradiation and renal failure. Ultrasound assessment of left ventricular function is therefore an important part of the investigation of patients with COPD, especially when the diagnosis of chronic pulmonary heart disease is made. Nevertheless, trans-thoracic echocardiography is often influenced by poor acoustic windows in COPD patients with emphysematous lung. In this context, LUS has been proposed and it was shown that B-line calculation had good accuracy to detect HF in patients admitted to the ED for acute dyspnea.25–27 However, LUS findings could be masked because the pulmonary acoustic window of a COPD patient is considered unfavorable due to the large amount of trapped air and pulmonary hyperinflation.28 To our knowledge, no similar studies have been performed in AECOPD patients except a recent study including a low sample size of hospitalized patients with AECOPD and showing a low sensitivity (17%) for a positive LUS to detect concurrent HF.10 Another study including a limited cohort of COPD patients (n=53) showed that LUS has moderate sensitivity and specificity in patients with high BNP levels (>100 ng/L).29 Including larger sample size, our study demonstrated that LUS was associated with a low negative likelihood ratio (0.34) at a cutoff of 5, and a good positive likelihood ratio (2.68) at a cutoff of 30. The good correlation between LUS and E/E’ is another support to the validity of our results.
This study was limited by a possible selection bias from the convenience sampling methodology. Our results do not apply to all COPD exacerbations because severe patients were not included in this study. So, extrapolation of the study results to this population is not allowed. LUS was performed about 4 hours after admission, during which the patient could be improved by the treatment. This would be responsible for a decrease in the sensitivity of the test.
In summary, our study suggests that the diagnostic performance of LUS remains good for identifying HF in COPD patients in exacerbation. Our results suggest that LUS B-lines assessment should be considered as an early diagnostic tool in the ED to diagnose and initiate targeted management of patients with AECOPD with HF. Integration of LUS to clinical assessment and to already widely used biomarkers can limit misdiagnoses of HF in patients with AECOPD.
Data Sharing Statement
The de-identified data that support the findings of this study are available on request from the corresponding author. The data are not publicly available.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rutten FH, Moons KGM, Cramer MM, et al. Recognising heart failure in elderly patients with stable chronic obstructive pulmonary disease in primary care: cross sectional diagnostic study. BMJ. 2005;331(7529):1379. doi:10.1136/bmj.38664.661181.55
2. Pellicori P, Cleland JGF, Clark AL. Chronic obstructive pulmonary disease and heart failure: a breathless conspiracy. Cardiol Clin. 2022;40(2):171–182. doi:10.1016/j.ccl.2021.12.005
3. MacDonald MI, Shafuddin E, King PT, Chang CL, Bardin PG, Hancox RJ. Cardiac dysfunction during exacerbations of chronic obstructive pulmonary disease. Lancet Respir Med. 2016;4(2):138–148. doi:10.1016/S2213-2600(15)00509-3
4. Bafadhel M, Criner G, Dransfield MT, et al. Exacerbations of chronic obstructive pulmonary disease: time to rename. Lancet Respir Med. 2020;8(2):133–135. doi:10.1016/S2213-2600(19)30414-X
5. Rutten FH, Cramer M-JM, Lammers J-WJ, Grobbee DE, Hoes AW. Heart failure and chronic obstructive pulmonary disease: an ignored combination? Eur J Heart Fail. 2006;8(7):
6. Bekgoz B, Kilicaslan I, Bildik F, et al. BLUE protocol ultrasonography in emergency department patients presenting with acute dyspnea. Am J Emerg Med. 2019;37:2020–2027. doi:10.1016/j.ajem.2019.02.028
7. Mebazaa A, Yilmaz MB, Levy P, et al. Recommendations on pre-hospital & early hospital management of acute heart failure: a consensus paper from the heart failure association of the European society of cardiology, the European society of emergency medicine and the society of academic emergency. Eur J Heart Fail. 2015;17(6):544–558. doi:10.1002/ejhf.289
8. Ang S, Andrus P. Lung ultrasound in the management of acute decompensated heart failure. CCR. 2012;8(2):123–136. doi:10.2174/157340312801784907
9. Staub LJ, Mazzali Biscaro RR, Kaszubowski E, Maurici R. Lung ultrasound for the emergency diagnosis of pneumonia, acute heart failure, and exacerbations of chronic obstructive pulmonary disease/asthma in adults: a systematic review and meta-analysis. J Emerg Med. 2019;56(1):53–69. doi:10.1016/j.jemermed.2018.09.009
10. Johannessen Ø, Uthaug Reite F, Bhatnagar R, Øvrebotten T, Einvik G, Myhre PL. Lung ultrasound to assess pulmonary congestion in patients with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2023;18:693–703. doi:10.2147/COPD.S396855
11. Chiem AT, Chan CH, Ander DS, Kobylivker AN, Manson WC. Comparison of expert and novice sonographers’ performance in focused lung ultrasonography in dyspnea (FLUID) to diagnose patients with acute heart failure syndrome. Acad Emerg Med. 2015;22(5):564–573. doi:10.1111/acem.12651
12. Frassi F, Gargani L, Tesorio P, et al. Prognostic value of extravascular lung water assessed with ultrasound lung comets by chest sonography in patients with dyspnea and/or chest pain. J Card Fail. 2007;13(10):830–835. doi:10.1016/j.cardfail.2007.07.003
13. Pieske B, Tschöpe C, de Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European society of cardiology (ESC). Eur Heart J. 2019;40(40):3297–3317. doi:10.1093/eurheartj/ehz641
14. Horodinschi R, Bratu OG, Dediu GN, Pantea Stoian A, Motofei I, Diaconu CC. Heart failure and chronic obstructive pulmonary disease: a review. Acta Cardiologica. 2020;75:97–104. doi:10.1080/00015385.2018.1559485
15. Lagan J, Schelbert EB, Naish JH, et al. Mechanisms underlying the association of chronic obstructive pulmonary disease with heart failure. JACC. 2021;14(10):1963–1973. doi:10.1016/j.jcmg.2021.03.026
16. Tinè M, Bazzan E, Semenzato U, et al. Heart Failure is highly prevalent and difficult to diagnose in severe exacerbations of COPD presenting to the emergency department. J Clin Med. 2020;9(9):2644. doi:10.3390/jcm9082644
17. Wai Wong C, Tafuro J, Azam Z, et al. Misdiagnosis of heart failure: a systematic review of the literature. J Card Fail. 2021;27(9):925–933. doi:10.1016/j.cardfail.2021.05.014
18. Peacock WF, Emerman C, Costanzo MR, Diercks DB, Lopatin M, Fonarow GC. Early vasoactive drugs improve heart failure outcomes. Congest Heart Fail. 2009;15(6):256–264. doi:10.1111/j.1751-7133.2009.00112.x
19. Le Jemtel TH, Padeletti M, Jelic S. Diagnostic and therapeutic challenges in patients with coexistent chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol. 2007;49(2):
20. Chhabra SK, Gupta M. Coexistent chronic obstructive pulmonary disease- heart failure: mechanisms, diagnostic and therapeutic dilemmas. Indian J Chest Dis Allied Sci. 2010;52(4):
21. Van der Molen T. Co-morbidities of COPD in primary care: frequency, relation to COPD, and treatment consequences. Prim Care Respir J J Gen Pract Airw Group. 2010;19:
22. Hannink JDC, van Helvoort HAC, Dekhuijzen PNR, Heijdra YF. Heart failure and COPD: partners in crime? Respirol Carlton Vic. 2010;15:
23. Tung RH, Camargo CA, Krauser D, et al. Amino-terminal pro brain natriuretic peptide for the diagnosis of acute heart failure in patients with previous obstructive airway disease. Ann Emerg Med. 2006;48(1):
24. Leong P, MacDonald MI, King PT, et al. Treatable cardiac disease in hospitalized COPD exacerbations. ERJ Open Res. 2021;7(1):00756–2020. doi:10.1183/23120541.00756-2020
25. Al Deeb M, Barbic S, Featherstone R, Dankoff J, Barbic D. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: a systematic review and meta-analysis. Acad Emerg Med. 2014;21(8):843–852. doi:10.1111/acem.12435
26. Martindale JL, Wakai A, Collins SP, et al. Diagnosing acute heart failure in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2016;23(3):223–242. doi:10.1111/acem.12878
27. McGivery K, Atkinson P, Lewis D, et al. Emergency department ultrasound for the detection of B-lines in the early diagnosis of acute decompensated heart failure: a systematic review and meta-analysis. CJEM. 2018;20(3):343–352. doi:10.1017/cem.2018.27
28. Corradi F, Brusasco C, Brusasco V. ¿Cuándo, dónde y cómo utilizar la ecografía en pacientes con enfermedad pulmonar obstructiva crónica? Archivos de Bronconeumología. 2017;53(5):229–230. doi:10.1016/j.arbres.2016.10.019
29. Sriram KB, Singh M. Lung ultrasound B-lines in exacerbations of chronic obstructive pulmonary disease. Intern Med J. 2017;47(3):
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
