Back to Journals » Infection and Drug Resistance » Volume 17
Visceral Leishmaniasis: A Case Confirmed by Metagenomic Next-Generation Sequencing from Northwestern China
Authors Li E , Zhu Q, Lv Z, Xie S, Zhang C, Li W , Mi L, Liu Q, Wang Y, Lu X
Received 3 April 2024
Accepted for publication 26 June 2024
Published 18 July 2024 Volume 2024:17 Pages 3153—3158
DOI https://doi.org/10.2147/IDR.S472172
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Ente Li,1,2,* Qingfeng Zhu,3,* Ziman Lv,1,2,* Songsong Xie,3 Chunju Zhang,4 Wei Li,5 Ligu Mi,1,2 Quan Liu,6 Yuanzhi Wang,1,2 Xiaobo Lu2
1Department of Basic Medicine, School of Medicine, Shihezi University, Shihezi City, Xinjiang Uygur Autonomous Region, 832002, People’s Republic of China; 2Key Laboratory for Prevention and Control of Emerging Infectious Diseases and Public Health Security, the Xinjiang Production and Construction Corps, Shihezi University, Shihezi City, Xinjiang Uygur Autonomous Region, 832002, People’s Republic of China; 3The First Affiliated Hospital of Shihezi University Medical College, Shihezi City, Xinjiang Uygur Autonomous Region, 832002, People’s Republic of China; 4Tumxuk Center for Disease Prevention and Control, Tumxuk City, Xinjiang Uygur Autonomous Region, 843806, People’s Republic of China; 5Department of Basic Medicine, School of Medicine, Tarim University, Alaer City, Xinjiang Uygur Autonomous Region, 843300, People’s Republic of China; 6Department of Infectious Diseases and Center of Infectious Diseases and Pathogen Biology, Key Laboratory of Organ Regeneration and Transplantation of the Ministry of Education, Key Laboratory of Zoonotic Diseases, The First Hospital of Jilin University, Changchun City, Jilin Province, 30061, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuanzhi Wang; Xiaobo Lu, Email [email protected]; [email protected]
Abstract: Visceral leishmaniasis (VL), also known as kala-azar. It is characterized by prolonged intermittent fever, anemia, splenomegaly, hepatomegaly, and skin darkening. VL is primarily endemic in regions, such as Brazil, East Africa, and India. However, Northern Xinjiang, which is located in northwestern China, is considered a low-incidence area for VL, contributing to its status as a neglected infectious disease. In this report, we present a case of VL caused by Leishmania donovani that was diagnosed using metagenomic next-generation sequencing (mNGS). This case underscores the diagnostic value of mNGS, particularly in regions with low incidence of VL.
Keywords: Visceral leishmaniasis, Leishmania donovani, metagenomic next-generation sequencing, northwestern China
Graphical Abstract:
Introduction
Visceral leishmaniasis (VL) is a vector-borne disease that is transmitted through the bites of infected female sandflies.1 The disease predominantly affects the reticuloendothelial system, leading to symptoms, such as fever, weight loss, anemia, hepatosplenomegaly, and hypergammaglobulinemia. A distinctive feature of VL is skin darkening (kala-azar).2 The diagnosis of VL relies on clinical manifestations, epidemiological history, serological tests, and parasitological confirmation.
VL is endemic in 96 countries and territories, with an estimated 50,000–90,000 new cases reported annually.3 Most cases were concentrated in Brazil, East Africa, and India. In China, 4822 VL cases of VL reported between 2004 and 2018.4 From 2017 to 2021, the primary endemic areas were Gansu Province (Gannan Tibetan Autonomous Prefecture), Shanxi Province (Yangquan City), and Shaanxi Province (Hancheng City).5 Northern Xinjiang, covering approximately 3.9×105 km2 of the Xinjiang Uygur Autonomous Region (XUAR, Northwestern China), is a low-incidence area for VL, with only eight cases reported from 2017 to 2021.5
In the field of infectious disease diagnostics, metagenomic next-generation sequencing (mNGS) is used to identify elusive pathogens. For instance, mNGS identified Leptospira santarosai as the cause of hydrocephalus and status epilepticus in a 14-year-old boy with severe immunodeficiency, despite negative clinical tests.6 Similarly, mNGS detected West Nile virus in a 14-year-old transplant girl with neurological complications.7 In this report, we present a case of VL without skin darkening, initially diagnosed using mNGS and subsequently confirmed through rK39 dipstick testing. The patient was treated with pentavalent antimony.
Case Presentation
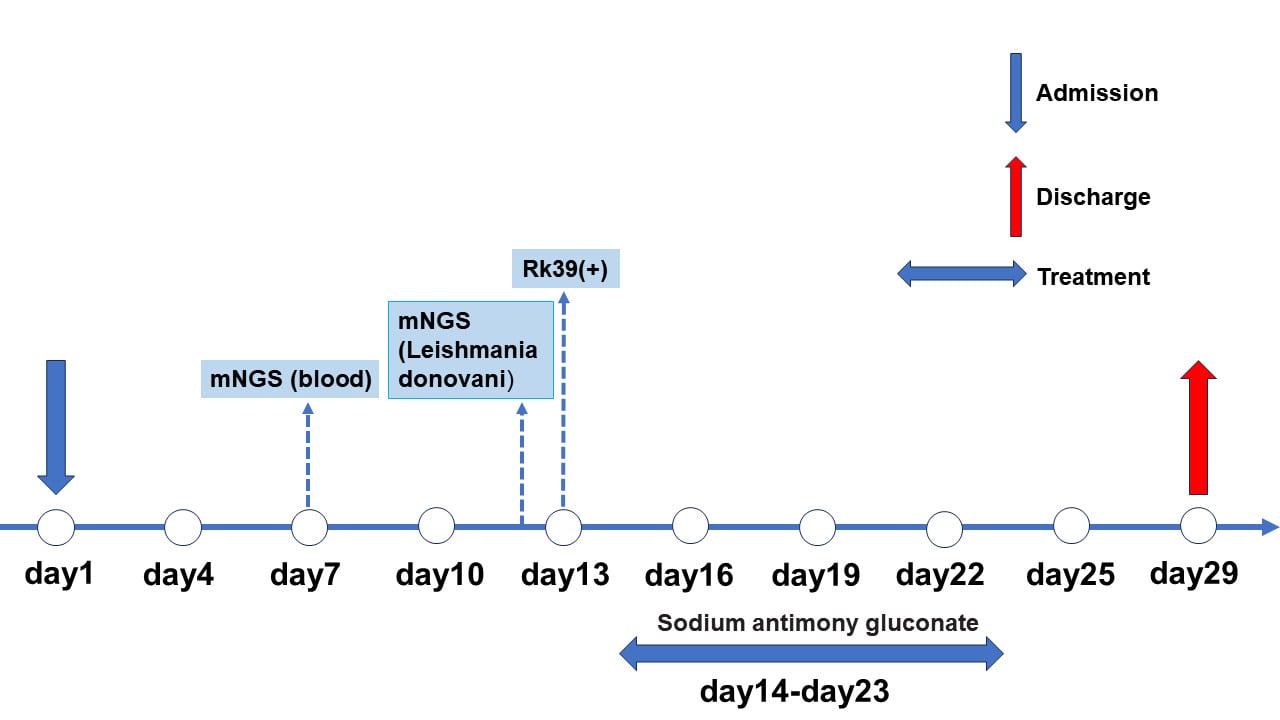
A 56-year-old male farmer in Jinghe County, Xinjiang Uygur Autonomous Region, northwestern China (Figure 1), presented with a sudden onset of fever and right upper abdominal pain on November 14, 2022. His maximum recorded body temperature was 40.0°C, accompanied by chills, weakness, dry throat, and dizziness. Notably, the patient had a medical history of coronary heart disease spanning a decade and type 2 diabetes mellitus for five years. He had resided in Jinghe County for over 40 years with no reported travel history to areas endemic for VL. The patient was then admitted to a local Water Management Station. The patient initially sought medical attention at two local hospitals, where he was diagnosed with suspected pneumonia. Treatment included a 15-day regimen of intravenous levofloxacin (0.5 g/day), ribavirin (0.5 g/day), and supportive care, which included oral potassium chloride oral solution, intravenous 20% mannitol, vitamin C and B6 injections. However, the response to this treatment was inadequate. Subsequently, on November 29, 2022, he presented to the Fever Clinic Department at the First Affiliated Hospital of Shihezi University.
 |
Figure 1 Source location of the visceral leishmaniasis case in this report. |
Upon admission, the patient’s vital signs included body temperature of 38.5°C, heart rate of 80 beats per minute, respiratory rate of 20 breaths per minute, and blood pressure of 137/70 mmHg. Physical examination revealed soft abdominal tissue with tenderness in the right upper abdominal region but no rebound pain. The liver and spleen were not palpable below the rib cage. The patient reported right upper abdominal pain with no indication of pathological signs or normal physiological reflexes. However, during the course of the illness, the patient developed abdominal pain, hepatomegaly, and jaundice. Thorough physical examination and ultrasound studies revealed a significant hepatomegaly.
Blood cell analysis and biochemical variable studies revealed leukopenia (3.10×10.09/L, normal reference range: 3.50–9.50×109/L) and mild anemia (hemoglobin, 99.0 g/L; normal reference range: 130–175 g/L). The liver function tests indicated elevated levels of alanine aminotransferase (168 U/L, normal reference range: 0–50.0 U/L), aspartate aminotransferase (281 U/L, normal reference range: 17.0–59.0 U/L), alkaline phosphatase (646 U/L, normal reference range: 38.0–126 U/L), and glutamyl transpeptidase (586 U/L, normal reference range:15.0–73.0 U/L). Renal function test results were within the normal range. Elevated C-reactive protein (52.3 mg/L, normal reference range: <10.0 mg/L) were observed. His procalcitonin level was elevated levels (0.21 ng/mL, normal reference range:<0.05ng/mL). Blood culture results were negative for bacteria and fungi as well as serological tests for coronavirus disease 2019, hepatitis B, hepatitis C, acquired immune deficiency syndrome, and syphilis. Abdominal ultrasonography revealed thickening of the gallbladder wall and hepatomegaly and splenomegaly.
Two pairs of primers, outer (P221: 5′-GGTTCCTTTCCTGATTTACG-3′ and P332: 5′-GGCCGGTAAAGGCCGAATAG-3′) and inner (P223: 5′-TCCCATCGCA ACCTCGGTT-3′ and P333: 5′-AAGCGGGCGCGGTGCTG-3′) primers were employed. The blood sample from the patient tested negative by nested PCR.8 Because conventional diagnostic methods fail to identify the causative agent, metagenomic next-generation sequencing (mNGS) was performed on the patient’s blood sample. Using the Illumina Hiseq 3000 sequencing instrument, the sample was detected with a read length of 76nt. Surprisingly, out of a total of 54,053,249 sequence reads, the raw data detected by the metagenomic (mNGS) approach were aligned with the genome of Leishmania donovani (GenBank accession: GGCA_000227135.2) using BOWTIE2. Metagenomic next-generation sequencing (mNGS) of the patient’s blood sample indicated a coverage of 0.06% of the L. donovani whole genome of L. donovani (Figure 2). The initial NCBI database alignment of 306 sequences revealed similarities to L. donovani, albeit with potential overlap with other Leishmania species (such as L. infantum). Furthermore, after additional alignment with the NCBI and TriTrypDB databases, 20 sequences were identified as being exclusively similar to L. donovani, excluding other Leishmania species. In addition, 20 L. donovani species-specific sequences were identified (Table S1). Phylogenetic analysis using the putative chaperone protein DNAj gene fragment (corresponding to read 2 in Table S1) revealed that it was clustered with L. donovani within the same clade, with L. infantum, L. chagasi, and L. major as sister clades (Figure 3).9,10 No other pathogenic bacteria, fungi, viruses or parasites were detected. Subsequently, a positive result was obtained using an rK39 dipstick (InBios International, Seattle, WA, USA). Unfortunately, no L. donovani amastigotes were observed in blood smears stained using the Rysh-Giemsa method (December 15, 2022, 2 days after pentavalent antimony was used) and bone marrow smears stained using the HE method (December 2, 2022, 3 days after admission).
 |
Figure 2 The result of the metagenomic next-generation sequencing (mNGS) of the patient’s blood sample indicated a coverage of 0.06% of the Leishmania donovani whole genome. |
The raw metagenomic sequence reads generated in this study were deposited in the Sequence Read Archive (SRA) (http://www.ncbi.nlm.nih.gov/sra) under BioProject ID PRJNA1031801 with the specific BioSample number SAMN37972829.
The patient was treated with 5% glucose injection (500 mL) and sodium antimony gluconate (equivalent to 0.6 g of pentavalent antimony) (Shandong Xinhua Pharmaceutical Company Ltd, Zibo, Shandong, China) through intravenous infusion for ten consecutive days. During treatment, the patient’s fever subsided, right upper abdominal pain was relieved, liver function normalized, and the gallbladder wall, liver, and spleen returned to normal. Within seven days of treatment, all signs were normal, and the patient recovered completely. The patient was discharged on October 26, 2022. After a year of rigorous follow-up, the patient’s condition remained stable without any signs of recurrence.
Discussion
This report presents an unusual case of visceral leishmaniasis (VL) in a low-incidence area of China, where the patient did not exhibit typical clinical features or had an epidemiological history of the disease. The diagnosis was established using metagenomic next-generation sequencing (mNGS) and further confirmed using the rK39 dipstick test, followed by treatment with pentavalent antimony. This case highlights the potential of mNGS in identifying L. donovani infections when leishmaniasis is often neglected.
VL is a neglected tropical disease caused by protozoan parasites belonging to the genus Leishmania, which are transmitted through the bites of infected Phlebotomus or Lutzomyia.1 In Jinghe County, located in a temperate zone, three sandfly species have been identified: P. mongolensis, P. alexandri and P. caucasicus.11 It has been confirmed that P. alexandri can transmit L. donovani.12
The conventional methods for diagnosing VL include microscopy, culture, serology, and molecular techniques. Microscopy and culture are regarded as the gold standard methods for detecting Leishmania parasites in clinical specimens, such as blood, bone marrow, spleen, or lymph node aspirates. However, these methods entail invasive procedures. In the realm of academic research, the process of aspirating materials from the bone marrow, spleen, or lymph nodes biosafety facilities, are time-consuming, and require skilled practitioners for accurate execution. In this study, both blood and bone marrow smears yielded negative results, possibly because of the stage of infection.13 Serological methods, such as the rK39 dipstick and enzyme-linked immunosorbent assays, are commonly employed for the initial diagnosis of VL because of their simplicity, rapidity, cost-effectiveness, high sensitivity, and specificity.14 In this study, local healthcare providers did not consider leishmaniasis as a potential diagnosis until the results of mNGS became available.
mNGS is a cutting-edge technique that employs high-throughput sequencing and bioinformatics analysis to identify the genetic sequences of microorganisms in clinical samples without the need for prior knowledge or specific primers.15 Previously, a case of VL was reported in Sichuan, China, and the diagnosis was confirmed using metagenomic sequencing.16 In the present study, 20 species-specific sequences of L. donovani were extracted from 306 Leishmania genus-specific reads. These findings provide clinicians with an important basis in the presence of Leishmania infection. Given the numerous advantages of mNGS over conventional diagnostic methods for VL, including the absence of specialized invasive equipment requirements, simplicity of execution for healthcare practitioners, and rapid result delivery (within 24 to 48 h), mNGS should be considered as a routine diagnostic method for infectious pathogens, especially in distant or low-incidence regions.
Conclusion
In summary, we present a rare case of VL caused by L. donovani in a low incidence region. The patient was initially diagnosed through metagenomic next-generation sequencing (mNGS) and subsequently confirmed with an rK39 dipstick, followed by successful treatment with pentavalent antimony. This case highlights the potential of mNGS for enhancing the accuracy and timeliness of VL diagnosis and treatment. These findings underscore the importance of mNGS as a routine diagnostic method in clinical practice. However, the current costs of mNGS are high and preference for identification of parasites rather than DNA, given the toxicity associated with treatment.
Data Sharing Statement
The metagenome raw sequence reads generated in this study were deposited and are available in the Sequence Read Archive (SRA) (http://www.ncbi.nlm.nih.gov/sra) under BioProject ID PRJNA1031801 with the specific BioSample number SAMN37972829. The other original contributions presented in this study are included in the article and further inquiries can be directed to the corresponding author.
Ethics Statement
Permission to use the information in the medical records of the patients was granted by the ethical committee of The First Affiliated Hospital of Shihezi University (Number: KJ2023-505-02). The patient signed the informed consent forms.
Consent Statement
The authors certify that they have obtained all appropriate patient consent forms. The patient signed a consent form for the publication of case details and images.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Key project of Natural Science Foundation of Xinjiang Uygur Autonomous Region (2022B03014), National Key Research & Development Program of China (2022YFC2304000), National Natural Science Foundation of China (82260410, 82260414 and 82260399), Scientific and Technological Projects in Key Areas of the Corps (2022AB014), High-Level Talent Initiative Foundation of Shihezi University (RCZK202369).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018;392(10151):951–970. doi:10.1016/S0140-6736(18)31204-2
2. Sanyal RK. Leishmaniasis in the Indian subcontinent. In: Chang KP, Bray RS, editors. Leishmaniasis. Amsterdam, the Netherlands: Elsevier Science Publishers; 1985:443–467.
3. World Health Organization. Leishmaniasis. Geneva: World Health Organization; 2011. Available from: http://www.who.int/mediacentre/factsheets/fs375/en/.
4. Zhou ZB, Li YY, Zhang Y, Li SZ. Prevalence of visceral leishmaniasis in China in 2019. Chin J Parasitol Parasitic Dis. 2020;38(5):602–606.
5. Yang H, Sun J, Zheng C, Shi Y, Yin W, Zhou S. Epidemiological characteristics of visceral leishmaniasis in China, 2017–2021. Dis Surveill. 2023;38(6):676–678.
6. Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi:10.1093/molbev/msw054
7. Wilson MR, Naccache SN, Samayoa E, et al. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med. 2014;370(25):2408–2417. doi:10.1056/NEJMoa1401268
8. Wilson MR, Zimmermann LL, Crawford ED, et al. Acute West Nile virus meningoencephalitis diagnosed via metagenomic deep sequencing of cerebrospinal fluid in a renal transplant patient. Am J Transplant. 2017;17(3):803–808. doi:10.1111/ajt.14058
9. Deepachandi B, Weerasinghe S, Soysa P, Karunaweera N, Siriwardana Y. A highly sensitive modified nested PCR to enhance case detection in leishmaniasis. BMC Infect Dis. 2019;19(1):623. doi:10.1186/s12879-019-4180-3
10. Solana JC, Bernardo L, Moreno J, Aguado B, Requena JM. The astonishing large family of HSP40/DnaJ proteins existing in Leishmania. Genes. 2022;13(5):742. doi:10.3390/genes13050742
11. Xiong G, Jin C, Chai J, Zou X. The North Xinjiang Sandfly Fauna. Endemic Dis Bull. 1988;1:67–74.
12. Studentsky L, Orshan L, Akad F, et al. Leishmania donovani transmission cycle associated with human infection, Phlebotomus alexandri Sand Flies, and Hare Blood Meals, Israel. Emerg Infect Dis. 2023;29(5):945–955. doi:10.3201/eid2905.221657
13. Turganbayi, Ayishamugu, Xiao L, et al. Analysis of 50 cases of Visceral leishmaniasis. Chin J Misdiagnostics. 2006;15:2988–2989.
14. Maalej IA, Chenik M, Louzir H, et al. Comparative evaluation of ELISAs based on ten recombinant or purified Leishmania antigens for the serodiagnosis of Mediterranean visceral leishmaniasis. Am J Trop Med Hyg. 2003;68(3):312–320. doi:10.4269/ajtmh.2003.68.312
15. Miao Q, Ma Y, Wang Q, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. 2018;67(suppl_2):S231–S240. doi:10.1093/cid/ciy693
16. Gao H, Wang J, Zhang S, et al. A case report of two kala-azar cases in China diagnosed by metagenomic next-generation sequencing. Front Microbiol. 2022;13:922894. doi:10.3389/fmicb.2022.922894
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

