Back to Journals » Cancer Management and Research » Volume 16
Guanine-Rich RNA Sequence Binding Factor 1 Deficiency Promotes Colorectal Cancer Progression by Regulating PI3K/AKT Signaling Pathway
Authors Huang J, Liu J , Lan J, Sun J, Zhou K , Deng Y, Liang L, Liu L, Liu X
Received 4 December 2023
Accepted for publication 11 April 2024
Published 11 June 2024 Volume 2024:16 Pages 629—638
DOI https://doi.org/10.2147/CMAR.S451066
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Jingzhan Huang,1,* Jialong Liu,1,* Jin Lan,1,* Jingbo Sun,1 Kun Zhou,1 Yunyao Deng,1 Li Liang,2 Lixin Liu,1,* Xiaolong Liu1
1Department of General Surgery, The Third Affiliated Hospital, Southern Medical University, Guangzhou, Guangdong, People’s Republic of China; 2Department of Pathology, School of Basic Medical Sciences, Southern Medical University, Guangzhou, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lixin Liu; Xiaolong Liu, Department of General Surgery, The Third Affiliated Hospital, Southern Medical University, 183 West Zhongshan Avenue, Guangzhou, Guangdong, 510630, People’s Republic of China, Email [email protected]; [email protected]
Background: Guanine-rich RNA sequence binding factor 1 (GRSF1), part of the RNA-binding protein family, is now attracting interest due to its potential association with the progression of a variety of human cancers. The precise contribution and molecular mechanism of GRSF1 to colorectal cancer (CRC) progression, however, have yet to be clarified.
Methods: Immunohistochemistry and Western Blot analysis was carried out to detect the expression of GRSF1 in CRC at both mRNA and protein levels and its subsequent effects on prognosis. A series of functional tests were performed to understand its influence on proliferation, migration, and invasion of CRC cells.
Results: The universal downregulation of GRSF1 in CRC was identified, indicating a correlation with poor prognosis. Our functional studies unveiled that the elimination of GRSF1 enhances tumour activities such as proliferation, migration, and invasion of CRC cells, while GRSF1 overexpression curtailed these abilities.
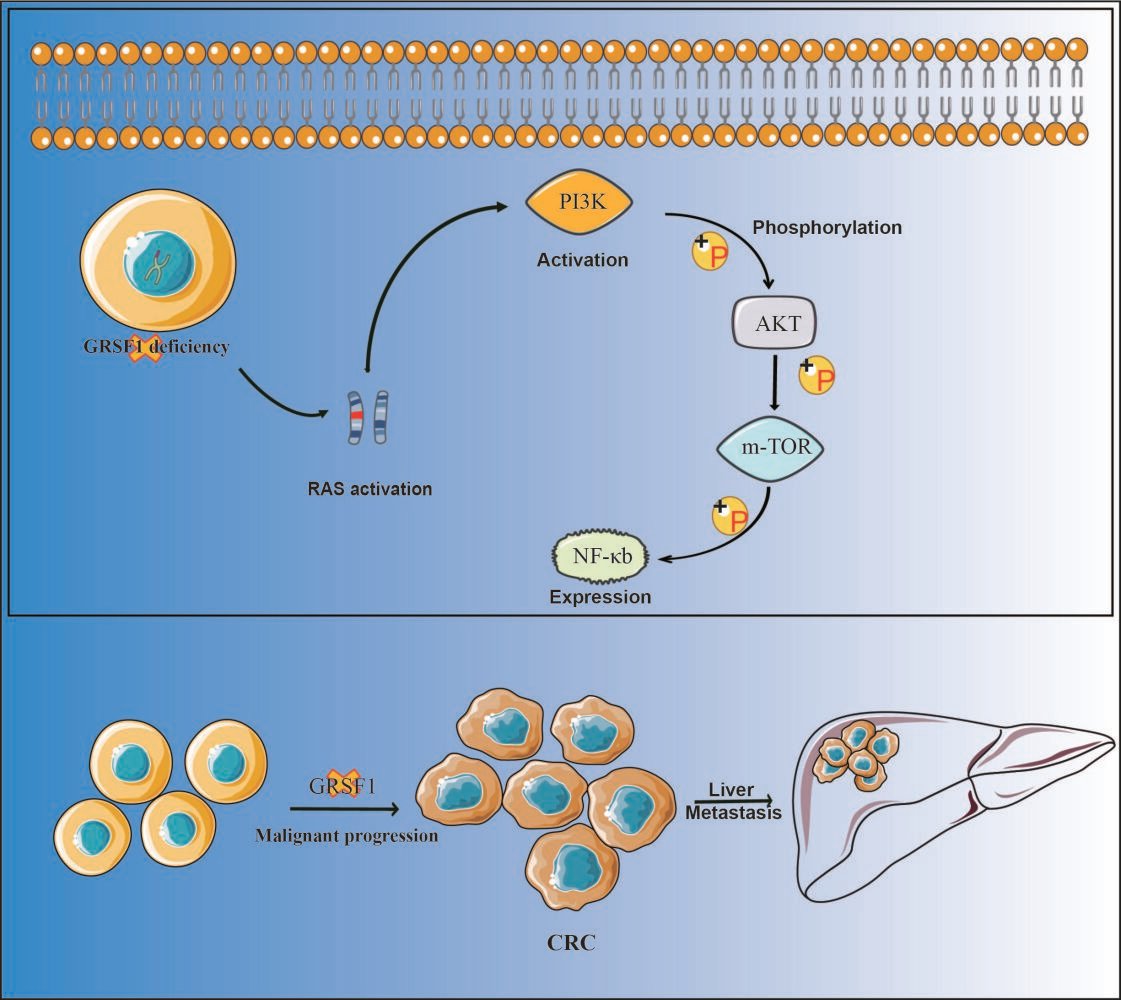
Conclusion: Notably, we uncovered that GRSF1 insufficiency modulates the PI3K/Akt signaling pathway and Ras activation in CRC. Therefore, our data suggest GRSF1 operates as a tumor suppressor gene in CRC and may offer promise as a potential biomarker and novel therapeutic target in CRC management.
Keywords: GRSF1, CRC, proliferation, metastasis, Ras/PI3K/Akt
Graphical Abstract:
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide with an increasing incidence among young adults.1 The prognosis of CRC patients remains poor due to the malignant progression and limited effective treatments. Therefore, it is urgent to elucidate the underlying mechanisms of CRC progression to identify novel therapeutic targets.
Guanine-rich RNA sequence binding factor 1 (GRSF1), a member of the heterogeneous nuclear ribonucleoproteins F/H family, is characterized by binding to RNAs containing the G-rich element2 in most eukaryotic cells. GRSF1 is located in the cytoplasm and nucleus3 and plays critical roles in the regulation of DNA hypermethylation and cellular senescence.4 Accumulating evidence suggested that GRSF1 plays an essential role in erythropoiesis, the regulation of redox homeostasis,5 posttranscriptional mitochondrial RNA processing,6 and muscle differentiation.7 Previous studies have found that GRSF1 is involved in malignant behavior and Epithelial-Mesenchymal Transition mediated metastasis via PI3K/AKT pathway in gastric cancer,8 The RNA-binding protein GRSF1 and miR-30e-5p competitively regulated YY1 by binding to its 3`UTR region to promote hepatocarcinogenesis9 or directly upregulating TMED5 and LMNB1 expression to activate classical WNT-CTNNB1/β-catenin signaling pathway in cervical cancer.10 These findings imply that GRSF1 might function in human tumors progression. However, the function and mechanism of GRSF1 in CRC remain unclear.
In the present study, the bioinformatic analysis showed that GRSF1 was downregulated in CRC tissues, and the low expression of GRSF1 was associated with a poor prognosis in CRC patients. Therefore, we speculate that GRSF1 may play as a tumor suppressor gene in CRC, and the deletion of GRSF1 can promote tumor progression and metastasis in vitro and in vivo. Our results indicated that GRSF1 may serve as a novel potential target for CRC treatment.
Method
Microarray
The Oncomine database sets consisting of 276 CRC and adjacent normal tissue samples were used for gene expression profiling on a public website (https://www.oncomine.org).
For the TCGA CRC microarrays, the intact and clinical data-matched samples were selected for data representation. The GRSF1 expression was classified into high and low expression groups. Meanwhile, the overall survival time, pathological stage, tumor T-stage, lymph node metastasis and distant metastasis in the clinical data were analyzed. Different subgroups were set according to the types of clinical data to assess the risk and prognosis associations between GRSF1 expression levels and CRC clinical data.
Clinical Samples and Cell Culture
Twenty-five cases of CRC and matched adjoining normal tissue were obtained from patients being treated for surgery at the Third Affiliated Hospital, Southern Medical University. Neither radiotherapy nor chemotherapy was administered prior to surgery. The research protocol and informed consents received approvals from the Ethics Committee of the Third Affiliated Hospital of Southern Medical University. All participants had a clear understanding of the purpose and process of the study and signed an informed consent form before participating in the study. All human CRC cell lines (HT29, HCT116, SW620, SW480, DLD1, RKO, and LOVO) were acquired from the Cell Bank of the Chinese Academy of Medical Sciences (Shanghai, China). Cells were cultivated in Dulbecco’s modified Eagle medium (DMEM; Gibco, Carlsbad, United States of America) with 10% fetal bovine serum (FBS; Thermo Scientific, Waltham, MA, United States), 100 IU/mL of penicillin G and 100 µg/mL of trichothecene (Invitrogen Life Technologies, Carlsbad, CA, United States of America) in with Dulbecco’s modified Eagle medium (DMEM; Gibco, Carlsbad, United States of America). The cells were cultivated in a refrigerated 5% CO2 humidified incubator at 37°C.
RNA Isolation, Reverse Transcription, and Quantitative Real-Time PCR
Total RNA was extracted using Trizol (Invitrogen, U.S.A.). Polyadenylation and reverse transcription (RT) of total RNA was carried out using the ThermoScriptTM RT-PCR system (Invitrogen). Real-time polymerase chain reaction (PCR) analysis was performed on an ABI 7500 HT system with SYBR Green PCR master mix (Applied Biosystems, United States). GAPDH was used as an endogenous control. All samples were normalized to the internal control and folding changes were calculated by relative quantification. Primer sequences are listed in Supplementary Table 1.
Western Blot
Cells were cleaved in RIPA lysis buffer contained protease inhibitor mixture in all samples to extract proteins. Proteins were quantified with BCA Protein Assay Kit (Pierce, KeyGEN BioTECH, China) before separated by SDS-PAGE gels and transferred to PVDF membranes. For blocking non-specific binding, membranes were incubated for 1 h at room temperature with 5% skim milk powder. The membranes were incubated overnight at 4°C with Rabbit Antibodies GAPDH, GRSF1, RAS, AKT (1:1000, Bioss) and Mouse Antibodies PI3K, NF-κb (1:1000, Proteintech), P-AKT and P-PI3K, (1:1000, Affinity) and then treated with HRP-conjugated Secondary Antibodies (anti-rabbit IgG/anti-mouse IgG, CST, 1:15,000). Following three washes with PBST, the membranes were visualized with ECL substrate and imaged with an enhanced chemiluminescence detection system (Tennon 5200, China) as described by the manufacturer.
Immunohistochemical Analysis
The paraffin-embedded CRC and normal tissue samples were sliced into 4 μm sections. The sections were then baked at 60°C for 1.5 hours, de-paraffined in xylene, and rehydrated using a series of fractionated alcohols. Tissue sections were then incubated with 3% hydrogen peroxide for 15 minutes at room temperature to deplete the endogenous peroxidase activity. Sections were then boiled in EDTA antigen repair solution for 10–15 minutes. After blocking with 5% BSA for 1 hour, GRSF1, p-NF-κb, p-AKT, p-PI3K (1:150, Affinity) and Ki67 (1:100, ZSGB Bio) were stained at 4°C for overnight. The corresponding secondary antibodies were applied at RT for 1 hour. Immunohistochemistry (IHC) was detected after DAB staining for the target moieties. Sections were finally counterstained with hematoxylin.
Transfection with siRNA Against GRSF1
Small interference RNA (siRNA) directed to human GRSF1 mRNA was denoted as si-GRSF1 (GTGGATGCCTTAATGAAGA). The negative control (NC) RNA duplex of siRNA was denoted as NC and did not have any homology with human genomic sequences. HCT116 (3 × 105) and RKO cells (1.5 × 105) were incubated in 6-well plates for 15 h and then transfected with 100 pmol of RNA duplex and 5 μL Lipo6000 (Beyotime, lipo6000, China) according to the manufacturers’ instructions. Cells were harvested after 48 or 72 h for further experiments, included Western blotting and transwell assays.
Wound Healing Assay
Pre-transfected HCT116 and RKO cells were seeded into 24-well plates and allowed to attach for 24 hours. A straight-line wound was created by scratching the cell monolayer using a 10 microlitre pipette tip in the eastward direction. After washing away cell debris with PBS, photographs were taken. Following a 48-hour waiting period for cell migration, the wound healing process was documented and imaged using a microscope. The percentage of wound healing was analyzed using Image J.
Statistical Analysis
Each experiment was conducted simultaneously at least three times. For statistical analysis, Prism 8.0 (GraphPad Software, San Diego, CA, United States) was applied. Student’s t-test was used to assess the significance of variances between groups. p < 0.05 was considered a statistically significant difference. All data are given as mean ± standard deviation (SD).
Results
GRSF1 Expression is Downregulated in CRC
To investigate the new potential predictive factors of prognosis in CRC, the RNA expression of CRC tissues and adjacent normal tissues were compared through microarray data derived from Oncomine datasets. We found that GRSF1 was downregulated significantly in CRC tissues compared with matching normal tissue (Figure 1A–F). To further confirm the result, RT-PCR was applied in 25 paired CRC tissues and adjacent normal tissues. In addition, we detected GRSF1 in 9 pairs of CRC tissues by Western blot. These results were consistent with the microarray data, GRSF1 was downregulated both at mRNA level (Figure 1G) and protein level (Figure 1H and I) in CRC tissues compared with corresponding adjacent normal colorectal tissues. The immunohistochemical examination of human tissue samples also demonstrated a diminished expression level of GRSF1 in colorectal cancer tissues. Thereby, we speculated that GRSF1 may function as a tumor suppressor gene in CRC. To further explore the biological function of GRSF1 in CRC, we detected the endogenous expression of GRSF1 by Western blot in CRC cell lines (Figure 1K), and we found that GRSF1 was overexpressed in RKO and HCT-116 cells and expressed at a low level in SW480 cells. These results were thus adopted to use in subsequent experiments.
GRSF1 Loss Correlates with CRC Clinicopathological Characteristics and Poor Prognosis
To investigate the significance of GRSF1 expression in CRC, we analyzed the correlations between GRSF1 expression and clinicopathological features of 278 CRC samples derived from TCGA datasets. As summarized the chi-square test, GRSF1 expression was significantly correlated with clinical stage (Figure 2A) and metastasis (Figure 2B). Of note, the tumor has a higher clinical stage with lower expression of GRSF1. Notably, the expression of GRSF1 was lower in the samples with positive lymph node biopsy, but it does not show significant statistical differences (Figure 2C, p = 0.063). Moreover, survival analysis of CRC patients from the GEPIA database was shown. GRSF1 loss was closely correlated with the short cumulative overall survival of CRC patients (Figure 2D and E). Taken together, these results suggested that GRSF1 may serve as a novel detectable molecule to supplement the clinical classification of CRC or predict distant organ metastasis.
GRSF1 Inhibits CRC Proliferation, Migration, and Invasion in vitro
To investigate the biological behaviors of GRSF1 in CRC cells, we constructed the GRSF1 shRNA-expression lentivirus. RKO and HCT116 cell lines with high endogenous GRSF1 expression were chosen to silence GRSF1. SW480 cell line with low expression was selected for overexpression. Transfection efficiency was confirmed by Western blot. (Figure 3A). Next, the cell counting kit-8 (CCK8) assay results showed that the activity were significantly increased compared with control cells after GRSF1 knockdown, while these abilities were significantly inhibited in SW480/GRSF1 cells (Figure 3B). Transwell assay results showed that the migration and invasion abilities of GRSF1 knockdown cells were significantly enhanced compared to control cells. In contrast, these abilities were inhibited in the GRSF1 overexpression cells (Figure 3C and D). The wound healing assay findings hit notable increase in migration of CRC cells after GRSF1 silence (Figure 3E and F), compared to controls. These results collectively proved that GRSF1 inhibits CRC cell activity, migration, and invasion ability.
GRSF1 Inhibits CRC Tumor Growth and Liver Metastasis in vivo
The roles of GRSF1 on tumor growth and metastasis were also investigated in vivo. For the subcutaneous tumor model, the size of subcutaneous tumors derived from the GRSF1 knockdown group was significantly increased compared with the control group (Figure 4A). IHC staining revealed a significant increase in the number of cells with positive proliferation marker Ki67 (Figure 4C). Meanwhile, the number of mice developing liver metastasis also increased in the knockdown group (n=7) than in the control group (n=2) (Figure 4B). H&E staining results also support the above views. These results suggested that the knockdown of GRSF1 accelerates CRC cell growth and metastasis in vivo.
GRSF1 Inhibits RAS/PI3K/AKT/NF-κb Signaling Pathway Activation
To elucidate the molecular mechanisms associated with GRSF1 in regulating CRC cell proliferation, migration, and invasion, we made pathway prediction analysis by LinkedOmics and the result showed that GRSF1 was enriched in the Ras signaling pathway (Figure 5A). It is confirmed that Ras/PI3K/AKT pathway was complex regulation progress that can regulate several biological processes including CRC progression and metastasis.11 The protein expression levels of Ras/PI3K/Akt signaling pathway-associated molecules were detected to determine the potential involvement of the Ras signaling pathway. Western blot experiments demonstrated that the GRSF1 knockdown group promoted Ras activation and affected the phosphorylation level of its downstream molecule PI3K/AKT/NF-κb signaling pathway (Figure 5B). Related molecules also showed the same trend in the subcutaneous tumor model with immunohistochemical staining (Figure 5C). These results suggested that knockdown of GRSF1 expression in CRC cells activates RAS and promotes phosphorylation of the PI3K/AKT/NF-κB signaling pathway.
Discussion
Liver metastasis is one of the most common causes of mortality in CRC patients.12 According to the specific subsets of colorectal cancer (CRC), the use of targeted drugs holds promise for extending survival. However, the toxicity and stringent screening requirements associated with these drugs present challenges for their widespread adoption in clinical practice.13 Thus, finding valuable biomarkers has a positive effect on the prognosis of CRC and may lead to the development of targeted therapeutic options.14
In our study, we found that the expression of GRSF1 was down-regulated in CRC tissues. The low expression of GRSF1 was correlated with poor prognosis of CRC patients. Our research demonstrated that GRSF1 knockdown accelerates CRC cell proliferation, migration, and invasion. Furthermore, in vivo assay demonstrated that GRSF1 knockdown promotes tumor growth and distant metastasis. Contrary to the previous research, GRSF1 contributes to breast cancer,15 cervical cancer,16,17 and hepatocellular carcinoma18 cell proliferation, migration, or invasion in vitro. Our study suggested that GRSF1 may function as a tumor suppressor gene in CRC. Filling the gap in the specific mechanism of GRSF1 in CRC cells. RAS (KRAS, NRAS, and HRAS) was the most frequently mutated gene in cancers.19 Sufficient data indicated that The crosstalk between Ras and phosphatidylinositol-3-kinase (PI3K)/ protein kinase B (PKB; also known as AKT) signaling pathway has practical clinical significance in cancer therapy.20 The PI3K/ AKT signaling pathway is involved in regulating cell growth, migration, and invasion activities in CRC.21–23 This pathway can also be taken as a targeting effect in malignant progression or liver metastasis of colorectal carcinoma.24,25 Furthermore, the study by CZ. et al demonstrated that, PI3K/ AKT signaling pathway is considered to be a vital mechanism for the development of multidrug resistance during cancer therapy.26,27 PI3K is known to activate ABC transporters, including ABCB1, which has been linked to tumor resistance and malignant progression. Subsequently, we propose that CRC GRSF1 deficiency fosters malignant progression in CRC by inducing the upregulation of ABC transporters through PI3k activation. Activation of this pathway relies on PI3K/AKT molecular phosphorylation.28,29 We found that GRSF1 knockdown was associated with the activation of Ras. Numerous studies have demonstrated that the Ras/PI3K/AKT pathway was complex regulation progress that can regulate several biological processes.30 Our study indicated that knockdown of GRSF1 expression in CRC activates RAS and promotes phosphorylation levels of its downstream PI3K/AKT/NF-κB signaling pathway molecules. NF-κB, as a downstream of PI3K/Akt signaling pathway, has been reported to mediate Th17 cytokines, IL-6, and TNF-α co-activation to promote CRC cell growth.31 IHC assays also showed significant activity of NF-κB. Hence, we suggest that GRSF1 may be involved in CRC cell proliferation, migration, invasion, and liver metastasis by regulating the Ras/PI3K/AKT/NF-κB pathway, which is needed to be further confirmed by rescue assays in future research.
Collectively, our study clarified that down-regulation of GRSF1 was correlated with a poor prognosis in CRC patients. GRSF1 functions as a tumor suppressor gene in CRC and actives Ras/PI3K/AKT/NF-κB signaling pathways to regulate CRC cell proliferation, migration, and invasion. Thus, GRSF1 could serve as a novel prognostic biomarker and a potential therapeutic target for CRC treatment.
Data Sharing Statement
Datasets used and/or analyzed data are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the ethics committee of The Third Affiliated Hospital, Southern Medical University. Informed consent was obtained from the study participants prior to the start of the study. Our research complies with the Declaration of Helsinki.
Acknowledgments
The authors thank Professor Qingling Zhang of the Guangdong Provincial Key Laboratory of Artificial Intelligence in Medical Image Analysis and Application in Guangdong Province for her instructions on pathological analyses.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This project was supported by grants of Guangdong Basic and Applied Basic Research Foundation (XL. L, 2022A1515012290); Guangzhou Science & Technology Project (LX.L 202201011772, and JB. S, 2024A04J4807); President Foundation of The Third Affiliated Hospital, Southern Medical University (XL. L, YM202208, YP202217 and JB. S, YQ202214).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
2. Dumoulin B, Ufer C, Stehling S, et al. Identification of the COMM-domain containing protein 1 as specific binding partner for the guanine-rich RNA sequence binding factor 1. Biochim Biophys Acta Gen Subj. 2020;1864(11):129678. doi:10.1016/j.bbagen.2020.129678
3. Dumoulin B, Ufer C, Kuhn H, et al. Expression regulation, protein chemistry and functional biology of the guanine-rich sequence binding factor 1 (GRSF1). J Mol Biol. 2021;433(13):166922. doi:10.1016/j.jmb.2021.166922
4. Kim SJ, Chun M, Wan J, et al. GRSF1 is an age-related regulator of senescence. Sci Rep. 2019;9(1):5546. doi:10.1038/s41598-019-42064-6
5. Yin W, Yang L, Kong D, et al. Guanine-rich RNA binding protein GRSF1 inhibits myoblast differentiation through repressing mitochondrial ROS production. Exp Cell Res. 2019;381(1):139–149. doi:10.1016/j.yexcr.2019.05.004
6. Jourdain AA, Koppen M, Wydro M, et al. GRSF1 regulates RNA processing in mitochondrial RNA granules. Cell Metab. 2013;17(3):399–410. doi:10.1016/j.cmet.2013.02.005
7. Driscoll RK, Krasniewski LK, Cockey SG, et al. GRSF1 deficiency in skeletal muscle reduces endurance in aged mice. Aging. 2021;13(11):14557–14570. doi:10.18632/aging.203151
8. Wang B, Wang L, Lu Y, et al. GRSF1 promotes tumorigenesis and EMT-mediated metastasis through PI3K/AKT pathway in gastric cancer. Biochem Biophys Res Commun. 2021;555:61–66. doi:10.1016/j.bbrc.2021.03.121
9. Han L, Huang C, Wang X, et al. The RNA-binding protein GRSF1 promotes hepatocarcinogenesis via competitively binding to YY1 mRNA with miR-30e-5p. J Exp Clin Cancer Res. 2022;41(1):17. doi:10.1186/s13046-021-02217-w
10. Yang Z, Sun Q, Guo J, et al. GRSF1-mediated MIR-G-1 promotes malignant behavior and nuclear autophagy by directly upregulating TMED5 and LMNB1 in cervical cancer cells. Autophagy. 2019;15(4):668–685. doi:10.1080/15548627.2018.1539590
11. Yoshikawa Y, Takano O, Kato I, et al. Ras inhibitors display an anti-metastatic effect by downregulation of lysyl oxidase through inhibition of the Ras-PI3K-Akt-HIF-1α pathway. Cancer Lett. 2017;410:82–91. doi:10.1016/j.canlet.2017.09.017
12. Li C, Sun YD, Yu GY, et al. Integrated omics of metastatic colorectal cancer. Cancer Cell. 2020;38(5):734–47.e9. doi:10.1016/j.ccell.2020.08.002
13. Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325(7):669–685. doi:10.1001/jama.2021.0106
14. Jung G, Hernández-ILLÁN E, Moreira L, et al. Epigenetics of colorectal cancer: biomarker and therapeutic potential. Nat Rev Gastroenterol Hepatol. 2020;17(2):111–130. doi:10.1038/s41575-019-0230-y
15. Wang Y, Li C. lncRNA GHET1 promotes the progression of triple-negative breast cancer via regulation of miR-377-3p/GRSF1 signaling axis. Comput Math Methods Med. 2022;2022:8366569. doi:10.1155/2022/8366569
16. Song G, Wang R, Guo J, et al. miR-346 and miR-138 competitively regulate hTERT in GRSF1- and AGO2-dependent manners, respectively. Sci Rep. 2015;5(1):15793. doi:10.1038/srep15793
17. Guo J, Lv J, Liu M, et al. miR-346 Up-regulates Argonaute 2 (AGO2) protein expression to augment the activity of other MicroRNAs (miRNAs) and contributes to cervical cancer cell malignancy. J Biol Chem. 2015;290(51):30342–30350. doi:10.1074/jbc.M115.691857
18. Han L, Huang C, Wang X, et al. Correction: the RNA-binding protein GRSF1 promotes hepatocarcinogenesis via competitively binding to YY1 mRNA with miR-30e-5p. J Exp Clin Cancer Res. 2022;41(1):181. doi:10.1186/s13046-022-02392-4
19. Moore AR, Rosenberg SC, Mccormick F, et al. Author Correction: RAS-targeted therapies: is the undruggable drugged? Nat Rev Drug Discov. 2020;19(12):902. doi:10.1038/s41573-020-0089-1
20. Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. 2011;36(6):320–328. doi:10.1016/j.tibs.2011.03.006
21. Abdel-Wahab BA, Alqhtani H, Walbi IA, et al. Piclamilast mitigates 1,2-dimethylhydrazine induced colon cancer in rats through modulation of Ras/PI3K/Akt/mTOR and NF-κβ signaling. Chem Biol Interact. 2021;350:109686. doi:10.1016/j.cbi.2021.109686
22. Kumar S, Agnihotri N. Piperlongumine, a piper alkaloid targets Ras/PI3K/Akt/mTOR signaling axis to inhibit tumor cell growth and proliferation in DMH/DSS induced experimental colon cancer. Biomed Pharmacother. 2019;109:1462–1477. doi:10.1016/j.biopha.2018.10.182
23. Tan X, Gong L, Li X, et al. Promethazine inhibits proliferation and promotes apoptosis in colorectal cancer cells by suppressing the PI3K/AKT pathway. Biomed Pharmacother. 2021;143:112174. doi:10.1016/j.biopha.2021.112174
24. Pan S, Ren F, Li L, et al. MiR-328-3p inhibits cell proliferation and metastasis in colorectal cancer by targeting Girdin and inhibiting the PI3K/Akt signaling pathway. Exp Cell Res. 2020;390(1):111939. doi:10.1016/j.yexcr.2020.111939
25. Sanaei MJ, Baghery Saghchy Khorasani A, Pourbagheri-Sigaroodi A, et al. The PI3K/Akt/mTOR axis in colorectal cancer: oncogenic alterations, non-coding RNAs, therapeutic opportunities, and the emerging role of nanoparticles. J Cell Physiol. 2022;237(3):1720–1752. doi:10.1002/jcp.30655
26. Zhang L, Li Y, Wang Q, et al. The PI3K subunits, P110α and P110β are potential targets for overcoming P-gp and BCRP-mediated MDR in cancer. Mol Cancer. 2020;19(1):10. doi:10.1186/s12943-019-1112-1
27. Zhang L, Li Y, Hu C, et al. CDK6-PI3K signaling axis is an efficient target for attenuating ABCB1/P-gp mediated multi-drug resistance (MDR) in cancer cells. Mol Cancer. 2022;21(1):103. doi:10.1186/s12943-022-01524-w
28. Sun F, Wang J, Sun Q, et al. Interleukin-8 promotes integrin β3 upregulation and cell invasion through PI3K/Akt pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38(1):449. doi:10.1186/s13046-019-1455-x
29. Kearney AL, Norris DM, Ghomlaghi M, et al. Akt phosphorylates insulin receptor substrate to limit PI3K-mediated PIP3 synthesis. Elife. 2021;10. doi:10.7554/eLife.66942
30. Zhang J, Liu W, Dong H, et al. RETRACTED ARTICLE: k-Ras G12V/Y40C -PI3K/AKT pathway regulates H1.4 S35ph through PKA to promote the occurrence and development of osteosarcoma cancer. Artif Cells Nanomed Biotechnol. 2019;47(1):2048–2057. doi:10.1080/21691401.2019.1617726
31. De Simone V, Franzè E, Ronchetti G, et al. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2015;34(27):3493–3503. doi:10.1038/onc.2014.286
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
A 5`-tRNA Derived Fragment NamedtiRNA-Val-CAC-001 Works as a Suppressor in Gastric Cancer
Zheng J, Li C, Zhu Z, Yang F, Wang X, Jiang P, Yan F
Cancer Management and Research 2022, 14:2323-2337
Published Date: 4 August 2022
The Progress of Platelets in Breast Cancer
Wang L, Zhang K, Feng J, Wang D, Liu J
Cancer Management and Research 2023, 15:811-821
Published Date: 11 August 2023





